Abstract
The selective separation of chalcopyrite from galena (lead sulfide) through the flotation method is still a challenging task in the field of mineral engineering. Mercaptoacetic acid, though a common depressant of many gangue (commercially worthless) minerals, has shown to be less selective in the flotation separation of chalcopyrite and galena. This resent study therefore systematically investigated the selectivity of different mercapto acids (especially three types: 3-mercaptopropionic acid, 3-mercaptoisobutyric acid and mercaptoacetic acid) on the separation of chalcopyrite and galena by making the use of flotation experiments and first principle calculations. The calculation results demonstrated that the sulfhydryl and carboxyl groups existing on the molecular structure of three mercapto acids are the reactive and chelating centers to metal ions on sulfide mineral surfaces. Mercapto acids have higher binding energies to Cu2+ by 300–400 kJ/mol compared to Pb2+, indicating a higher affinity towards chalcopyrite. The order of reactivity and chelating ability noted was as follows: 3-mercaptopropionic acid > 3-mercaptoisobutyric acid > mercaptoacetic acid. Flotation results further showed that the selectivity of 3-mercaptopropionic acid or 3-mercaptoisobutyric acid was better than mercaptoacetic acid. The good agreement between the first principle calculations and the flotation results demonstrated that the former reagent could be served as a most selective depressant in the improved flotation separation of chalcopyrite and galena.
1. Introduction
Froth flotation is a widely used physicochemical process for separating valuable minerals from gangues [1,2]. The depression of galena in the selective flotation of chalcopyrite is the general phenomenon that is mostly preferred for the separation of chalcopyrite and galena [3]. A high grade of metal concentrate can be obtained by using this method. As a higher recovery rate is more important to ensure a greater economic benefit, therefore, more xanthate is often used in the flotation process to obtain a higher recovery of copper concentrate [4,5]. The increased copper recovery causes the reduction in copper grade as more lead is floated. In order to minimize the loss of valuable metals, both minerals are first floated simultaneously by using the large amount of xanthate, and then lead is recovered from copper concentrate that had been floated with xanthate.
Moreover, considering the relatively low lead content and yield, these copper-lead concentrates are more suitable to be used in the flowsheet of depressing copper and floating lead. To improve separation efficiency and selectivity, a selective depressant is prerequisite.
With an increasing emphasis on minimizing the impact and downstream treatment of metallurgical processes, a focus on utilizing less harmful and easier to handle reagents is occurring [6,7]. In recent years, the organic depressants that have been reported, such as dextrin, O, O-bis(2,3- dihydroxypropyl) dithiophosphate, sodium pyrophosphate/mixed sodium pyrophosphate/sodium citrate, and high-molecular polymers, have, in general, been costly and potentially environmentally hazardous when discharged into the tailings [3,8,9]. Consequently, the efficient and clean separation of chalcopyrite and galena remains a challenge. Small-molecule organic depressants have been considered as more favorable flotation reagents, owing to their environmental friendliness and cost-effectiveness [8,10]. Small-molecule organic depressants have a simpler structure than that of large-molecule organic depressants [11,12]. Moreover, small-molecule organic depressants can effectively improve their selectivity as well as their depression ability. Small-molecule organic depressants are classified into different types (e.g., phosphorus-containing, sulfur-containing and benzene-ring-containing), based on their molecular structure and different affinity groups [3]. From this point of view, the molecular structure of small-molecule organic depressants is strongly related to their inhibitory properties. It is inferred that organic depressants with different molecular structures and molecular weights will exhibit various inhibitory effects on different sulfide ores.
In recent decades, the importance of computational chemistry in the study of the physical and chemical properties of chemical systems and reactions, has been recognized to be comparable to that of experimental chemistry, because of the remarkable advances in computer power, mathematical algorithms and robust computer programs [13,14]. Among the most useful computational chemistry methods, the density functional theory (DFT) is widely used to characterize the electronic properties and atomic structures of various chemical systems, owing to its excellent computational accuracy and efficiency [15,16]. For foam flotation, DFT enjoys considerable use in the study of the structure–reactivity relationship of flotation collectors and in interface chemistry reactions that reveal the flotation mechanism [17,18].
There is a general scientific consensus that stronger mineral/metal-selective reagents need to be designed to meet the requirements of the process for separating valuable minerals from refractory ores. Such reagents can be designed only by determining the structural criteria by which a compound is highly selective for a particular mineral/metal, although there have been some reports on the flotation of the various mercapto acids mentioned above. To the best of the authors’ knowledge, there has never been a comparative study on their structure–property relationship. Therefore, it is of great theoretical significance to study the relationship between the structure and reactivity of flotation depressants for the design of novel high-performance flotation depressants.
Chalcopyrite, as a typical copper sulfide mineral, has a good natural floatability. There is a series of organic and inorganic depressants that have been used for the depression of chalcopyrite. Sulfur-, phosphorus- and nitrogen-containing organic and inorganic small molecule inhibitors have been widely used to selectively depress the flotation of chalcopyrite. Among others, thioglycolic acid is a relatively new one that has been widely used in real ore flotation plants. Thioglycolic acid contains two polar groups: the carboxylic acid group (–COOH) and the thioglycolic acid group (–SH) [19,20]. Many scholars have carried out a lot of research on the depression mechanism of thioglycolic acid on chalcopyrite [20,21]. There are two main viewpoints: first, the bonds of thioglycolic acid molecules are adsorbed on the mineral surface to replace the adsorbed xanthate, and a chemisorption layer is formed by –SH and –COOH. The subsequent multimolecular layer is formed by the –S–S-bond generated by hydrogen bonding and oxidation. The other is that the –SH group in the molecular structure of thioglycolic acid is reductive and minerophilic, which makes physical or chemical adsorption with minerals [21]. With the help of the –COOH group, a hydrophilic film can be formed between minerals and agents to increase the hydrophilicity of the mineral surface [22,23]. 3-mercaptopropionic acid and 3-mercaptoisobutyric are types of unique compounds that contain S- and COO-donor atoms. Owing to their unique chelating ability to coordinate metal ions, they have received extensive attention in the flotation technology [24].
Given its special binding affinity to Cu2+ ions, some preliminary studies suggested that these compounds may perform well in hydrometallurgical processes, such as the flotation recovery of copper oxide minerals. In the view of the study of the chelation of 3-mercaptopropionic acid/3-mercaptoisobutyric with metals for chalcopyrite and galena, the chelation of 3-mercaptopropionic acid/3-mercaptoisobutyric acids with Cu2+ and Pb2+ ions were investigated by using computational and experimental methods. Mercaptoacetic acid was also investigated for comparison.
2. Methodology
2.1. Calculations
2.1.1. Calculation Methods
The calculations were performed using the Gaussian 09 software package (D.01 fRONT: 6.1 version, Gaussian, Inc, Washington, DC, USA), utilizing a three-parameter hybrid functional (B3LYP). The 6–31+G basis set was used for all nonmetallic elements (C, O, S and H), and the LANL2DZ effective core potential basis set was used for the Cu2+ and Pb2+ metal ions. Water as a solvent was simulated by the conductor-like polarizable continuum model. The different conformations and configurations of the depressant and its complexes were fully optimized with the metal ions to obtain the geometry. The lowest energy structure was selected from the possible structures of a given molecule for further evaluation. Vibrational, translational and rotational frequencies were calculated to ensure that there were no phantom frequencies, and to confirm that the optimized molecule corresponds to the minimum value on the corresponding potential energy surface. The natural bond orbital program was used to analyze the hybridization and binding states of molecules.
2.1.2. Chemical Reactivity
This section clarifies the details regarding the reactivity of the individual depressants, as well as the most widely used chemical reactivity indices, including the group electronegativity (χ). The ability of the polar group of depressants is related to the group electronegativity. A smaller electronegativity corresponds to a stronger solidophile ability, that is, a stronger depression ability. Electronegativity can be described as
where EHOMO and ELUMO are the energies of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), respectively, of an N-electron system.
2.1.3. Interaction Energy of Metal-Ligand Complexes
With the change in the Gibbs free energy, the interaction energy or binding energy of the metal-ligand complex can be calculated by following equations:
where EML2 is the total energy of the model, ECOO– is the energy of the depressant ion resulting from the removal of an H from the carboxyl group, ES– is the energy of the depressant ion resulting from the removal of an H from the mercapto group, and EM(II) is the energy of the divalent metal ion.
2.2. Experimental
2.2.1. Mineral Samples and Chemical Reagents
Representative samples of chalcopyrite and galena were obtained from Yunnan province, China. The large crystals of the two minerals were crushed into small fractions with a jaw crusher.
The hand-sorted, small and highly pure mineral crystals were further crushed in a roll crusher and then dry-sieved. An average particle size of ~38–74 μm was used in the flotation experiments. The purities of the chalcopyrite and galena samples were 97.52% and 95.53%, according to the X-ray diffraction results shown in Figure 1 and the chemical analysis results listed in Table 1.
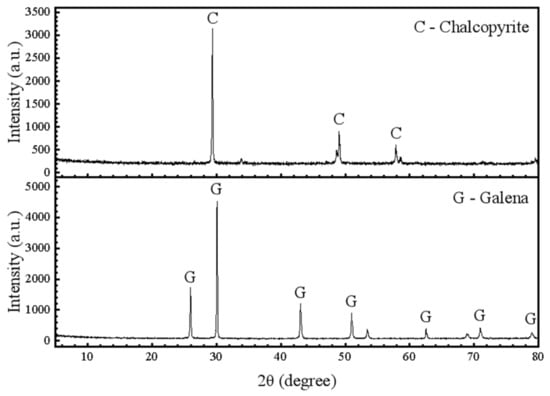
Figure 1.
X-ray diffractometry (XRD) spectra of the chalcopyrite and galena samples used in this study.

Table 1.
Chemical composition of the chalcopyrite and galena samples (wt%).
Commercial 3-mercaptopropionic acid, 3-mercaptoisobutyrate and mercaptoacetic acid were of analytical grade reagents that were purchased from Sigma Aldrich Division, St. Louis, MO, USA. Analytical grade HCl and NaOH were used to adjust the pH of flotation slurry, and the deionized water was used in all experiments.
2.2.2. Flotation Tests
Micro-flotation experiments were conducted in an XFG5-35 flotation machine (Wuhan Rock Crush and Grand Equipment Manufacture Co., Wuhan, China), in which 2.0 g of each mineral sample with particle sizes of −0.074 mm +0.038 mm were dispersed in a 40 mL flotation cell. First, the pulp pH was adjusted to the desired value by the addition of dilute solutions of HCl or NaOH. Thereafter, the depressant was added into the slurry with 3 min of stirring, and finally, the pulp was conditioned with butyl xanthate for 3 min, and floated for 5 min. Flotation recovery was calculated based on the froth and sink products’ weight after drying. Each flotation test was repeated three times, and the results are presented as averages of the repeated tests.
3. Results and Discussion
3.1. Reagent Molecular Structure-Reaction Relationship
Gaussian software was used to calculate the quantum chemistry of the three depressant molecules. To investigate the structure–reaction relationship of the reagent molecules, the molecular frontier orbital energy, Mullikan charge and electrostatic potential were calculated and determined.
The frontier orbital theory was proposed by Kenichi Fukui in 1952. The main idea of the theory is that among the numerous orbitals that constitute molecules, the frontier orbital determines the molecular properties, and these are determined by the HOMO and LUMO.
The HOMO is the most active highest energy orbital, and thus, is prone to electronic transition. Meanwhile, the LUMO has the lowest energy of all of the unoccupied orbitals, and easily accepts electrons. The highest orbital energy of the reagent molecule (EHOMO) can reflect its activity. Small EHOMO has more reactive molecules, and thus, can easily stick to the cations on the surface of minerals. The solidophile ability of the polar group of the depressant is related to the electronegativity of the group. The smaller electronegativity represents the stronger the solidophile ability, and the stronger the depressive efficiency of any reagent. The group electronegativity χ can be represented by (|EHOMO|+|ELUMO|)/2, and B3LYP/6-31+G is adopted to optimize the geometric structure. The front orbital and electrostatic potential electron density distributions of the three depressant molecules obtained are shown in Figure 2 and Figure 3, respectively.
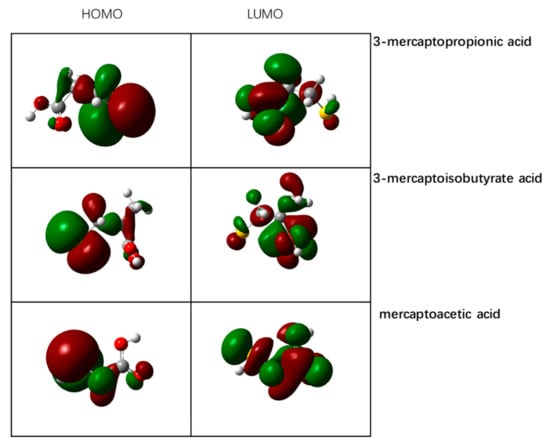
Figure 2.
Frontier orbital electron density distribution of the three depressant molecules.
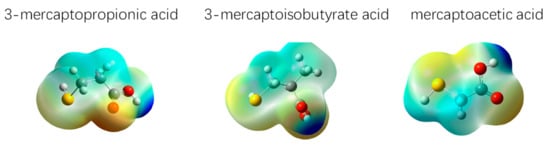
Figure 3.
Distribution diagram of the electrostatic potential electron density of the three depressants.
Figure 2 shows that the HOMO and LUMO orbitals of the three depressant molecules, and thus, the active centers of the three depressants, are distributed on the sulfhydryl and carboxyl groups. In order to further investigate the electrostatic interaction potential of each part of the entire molecule, and to study the charge distribution characteristics of the depressant molecules, the electrostatic potential distribution of the depressant molecules is mapped to the surface of the total electron density. This will enable the calculation of the electrostatic potential at each point on the surface of equal electron density. Figure 3 shows the map of the electrostatic potential of the depressant molecules on the surface of total electron density.
In Figure 3, the blue region represents the region with strong, positive charge, whereas the red region represents the region with strong, negative charge, which is related to the electrophilic reaction. In general, the nucleophilic center of the depressant molecule forms covalent bonds by transferring or sharing free electrons with the metal cations on the mineral surface. The nucleophilic center of the depressant is generally the p electron in the covalent double bond, which has heteroatoms with lone-pair electrons and groups with negative charges. These can easily form covalent bonds.
As seen in Figure 3, among the three depressants, the O atom in the –COO group is the main nucleophilic reactive center atom. In addition, the S atom of the –SH bond is also the nucleophilic center. The O atom in the –COO group and the S atom of the –SH bond are the active atoms of the depressant molecule, and are also bonded. The quantum chemical parameters of the three depressant molecules are summarized in Table 2.

Table 2.
Quantum chemical parameters of the three depressant molecules.
As seen from the Mulliken charge results in Table 2, the negative charge of the bonded S atom is less than that of the O atom, indicating that the O atom contributes more energy to the HOMO orbital of the depressant molecule than the S atom does. The order of the sum of charges of the bonded S and O atoms is 3-mercaptopropionic acid > 3-mercaptoisobutyrate > mercaptoacetic acid. The above analysis results show that the S and O bonded atoms in 3-thiothiopropionic acid molecules have a higher electron supply capacity than those of the S and O bonded atoms in the 3-thiothioisobutyric acid and thiothioacetic acid molecules.
Table 2 shows that the EHOMO orbital energies of the three depressants are −0.24598 eV, −0.24720 eV, −0.25017 eV, respectively, and the order of magnitude is 3-mercaptopropionic acid > 3-mercaptoisobutyric acid > mercaptoacetic acid. This indicates that 3-mercaptopropionic acid has the highest electron supply capacity to metal ions, followed by 3-mercaptoisobutyric acid, and the lowest is mercaptoacetic acid. The order of electronegativity of the three depressants is as follows: 3-mercaptopropionic acid < 3-mercaptoisobutyric acid < mercaptoacetic acid. This further indicates that 3-mercaptopropionic acid has greater depression ability compared with 3-mercaptoisobutyric acid or mercaptoacetic acid.
3.2. Reagent Ionic Structure-Reaction Relationship
Similar to the analysis method of the depressant molecules, quantum chemical calculations were performed on the three depressant ions at the same level using the Gaussian software, including the frontier orbital energy, Mullikan charge and electrostatic potential, in order to investigate the structure–reaction relationship of the depressant ions. The electron density distributions of the front orbital and electrostatic potential of the three depressant ions are shown in Figure 4 and Figure 5, respectively. In Figure 3, the first and second columns show the HOMO/LUMO orbital formed by the removal of hydrogen atoms from the carboxyl group of the depressant molecule. Meanwhile, the third and fourth columns show the HOMO/LUMO orbital formed by the removal of hydrogen atoms from the sulfhydryl group of the depressant molecule. In Figure 5, the first column shows the electrostatic potential electron density distribution of the ions generated by the removal of hydrogen atoms from the carboxyl group by the depressant molecule. The second column shows the electrostatic potential electron density distribution of the ions generated by the removal of hydrogen atoms from the sulfhydryl group by the depressant molecule.

Figure 4.
Frontier orbital electron density distribution of the three depressant ions.
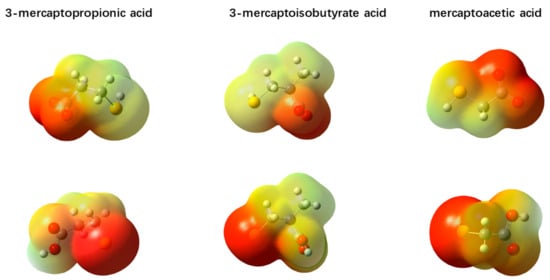
Figure 5.
Distribution diagram of electrostatic potential electron density of three depressant ions.
As seen in Figure 4, the HOMO and LUMO orbitals of depressant ions are distributed on the mercapto and carboxyl groups, indicating the locations of the active centers of the three depressant ions, just as with the depressant molecules.
In the electrostatic potential distribution diagram (Figure 5) of the three depressant ions, the red region, denoting strong negative charge, is related to the nucleophilic reaction. As seen in Figure 5, the nucleophilic centers of the three depressants are mainly distributed on the sulfhydryl group and carboxyl group, which are mainly composed of an S atom and O atom on the –COO group, respectively. In the ion type of the first column, the nucleophilic center is located on the O atom of the –COO group, which is also the nucleophilic reactive center where the O atom is the bonded atom. In the ion type of the second column, the nucleophilic center is located on the S atom of the sulfhydryl group.
As seen in Table 3, the EHOMO orbital energies of the three depressant ions are −0.18904 eV, −0.18892 eV and −0.20040 eV, respectively. The order of magnitude is: mercaptoacetic acid < 3-mercaptopropionic acid < 3-mercaptoisobutyric acid. This indicates that 3-mercaptoisobutyric acid and 3-mercaptopropionic acid have higher electron supply capacities to metal ions than mercaptoacetic acid. Therefore, 3-mercaptopropionic acid and 3-mercaptopropionic acid are more active than mercaptoacetic acid. The order of electronegativity of the three depressant ions is as follows: mercaptoacetic acid > 3-mercaptopropionic acid > 3-mercaptoisobutyric acid. This also indicates that among the depressant ions produced by removing hydrogen atoms on the carboxyl group, 3-mercaptopropionic acid and 3-mercaptoisobutyric acid have the best depression performance. Meanwhile, the EHOMO orbital energies of the three depressant ions produced by removing hydrogen atoms from the sulfhydryl group are −0.16358 eV, −0.16346 eV, and −0.17134 eV, respectively. The order of size is mercaptoacetic acid < 3-mercaptopropionic acid < 3-mercaptoisobutyric acid, which indicates that 3-mercaptoisobutyric acid and 3-mercaptopropionic have higher electron supply capacities to metal ions than mercaptoacetic acid. The order of electronegativity of the three depressants is as follows: mercaptoacetic acid > 3-mercaptoisobutyric acid > 3-mercaptopropionic acid. This also indicates that 3-mercaptoisobutyric acid and 3-mercaptopropionic acid exhibit better depression ability than mercaptoacetic acid.

Table 3.
Quantum chemical parameters of the three depressant ions.
As can be seen from the Mulliken charge results in Table 3, in the first type of depressant ions, produced by removing hydrogen atoms on the carboxyl group, the Mulliken charge of the –COO group in the three depressant ions is greater than the Mulliken charge of the S atom, indicating that the contribution of the O atom to the HOMO orbital energy of the depressant ion is greater than that of the S atom. In the second type of depressant ions, produced by removing hydrogen atoms from the sulfhydryl groups, the Mulliken charge of S in the three depressant ions is greater than that of the –COO group, indicating that the contribution of the S atom to the HOMO orbital energy of the depressant ions is greater than that of the O atom. Moreover, the Mulliken charges of the S and O atoms in the three depressant ions were all greater than those in the corresponding depressant molecules. To compare the negative charge of the entire ionic structure with that of the molecular structure, the HOMO orbital energy must be considered.
Compared with the molecular form of the depressant, the three ion depressants, with HOMO energy EHOMO and LUMO energy ELUMO, have larger orbits. This indicates that the ionic of the depressant is greater than the molecular of the depressant, regardless of whether the depressant is capable of providing or receiving an electron. It also indicates that the reactivity of the ionic form of the depressant is higher than that of the molecular form of the depressant in the aqueous solution system. According to the solution chemical properties of the three depressants, the three depressants mainly exist in the form of ions in the aqueous solution, and specifically, in the form of carboxyl dehydrogenation ions, indicating that using frontier orbital theory to study the flotation and depression performance of depressants is feasible.
Metal Ion Complexes
The optimized structures of the three depressants and Cu2+ and Pb2+ complexes are shown in Figure 6. A is the complex formed with metal ions after removing one H from the carboxyl group of two depressant molecules. B is the complex formed with metal ions after removing one H from the sulfhydryl groups of two depressant molecules. C is the complex formed with metal ions after removing one H from the carboxyl group of two depressant molecules. Label 1 shows the action on the divalent iron ion, and label 2 represents the action on the divalent copper ion.
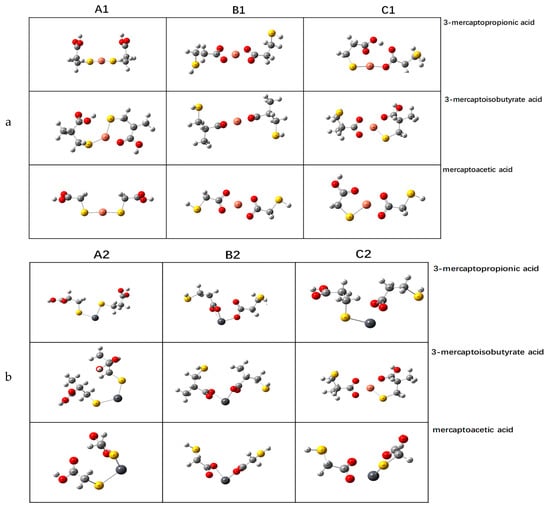
Figure 6.
Optimized structures of Cu2+ and Pb2+ complexes with 3-mercaptopropionic acid, 3-mercaptoisobutyric acid, and mercaptoacetic acid ligands. (a) Label 1 shows the action on the divalent iron ion; (b) Label 2 shows the action on the divalent copper ion.
The results show that the binding energies of Cu2+, Pb2+ and several configurations of the three depressants are negative, indicating that the simulated reactions are possible. Through the above analysis, the three depressants mainly exist in the form of carboxyl dehydrogenation ions in water; hence, we mainly analyze the class B configuration. From the data in Table 4, for copper ions, the binding energy of 3-mercaptopropionic acid is more negative, indicating the worst harvest effect, followed by 3-mercaptoisobutyric acid and mercaptoacetic acid. For lead ions, 3-mercaptoisobutyric acid was the best depressant, followed by 3-mercaptopropionic acid; mercaptoacetic acid was the worst.

Table 4.
Total energy of optimized models and their binding energies.
3.3. Flotation Separation Results
The effect of pH value on the flotation recovery of chalcopyrite and galena with butyl xanthate (BX) and Pine oil as collector and frother, respectively is illustrated in Figure 7. As can be seen from the figure, chalcopyrite and galena are highly floatable minerals with similar flotation recovery of more than 80% at pH 4–8. However, the flotation recovery of galena decreased rapidly when the pH value was above 8. It should be noted that the flotation recovery of galena was extremely low in the absence of a collector, indicating that galena has poor natural hydrophobicity. It can be inferred from the results shown Figure 7 that it is quite difficult to separate galena from chalcopyrite without using depressants.
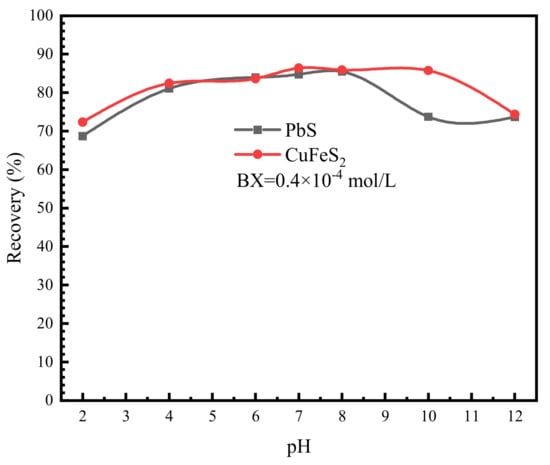
Figure 7.
Effect of pH value on flotation recovery of chalcopyrite and galena.
Flotation behaviors of chalcopyrite and galena in the presence of mercaptoacetic acid, 3-mercaptopropionic acid and 3-mercaptoisobutyric acid are shown in Figure 8. Flotation behaviors of both minerals were investigated as a function of depressant concentration at pH 6 with 0.04 mol/L dosage of BX. As illustrated in Figure 8a, chalcopyrite recovery decreased rapidly from 83.65% to 34.32% with the increasing of mercaptoacetic acid concentration, while the recovery of galena reduced slightly from 83.95% to approximately 78%. In Figure 8b, chalcopyrite recovery decreased dramatically from 83.65% to 3.32% with the increasing of 3-mercaptopropionic acid concentration, while the recovery of galena reduced gradually from 83.95% to approximately 75%. In Figure 8c, chalcopyrite recovery decreased drastically from 83.65% to 10.02% with the increasing of 3-mercaptoisobutyric acid concentration, while the recovery of galena decreased gradually from 83.95% to approximately 73%.
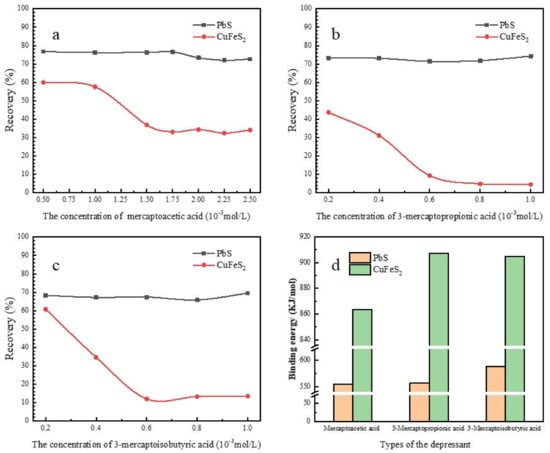
Figure 8.
Effect of concentration of mercaptoacetic acid (a), 3-mercaptopropionic acid (b), and 3-mercaptoisobutyric acid (c) on flotation recovery of galena and chalcopyrite, and the binding energy of Cu2+ and Pb2+ complexes with these three depressants (d).
It can be seen that, with increasing dosage of the three depressants, the recovery of chalcopyrite decreased sharply, whereas the recovery of galena decreased slightly. Thus, the separation of chalcopyrite from galena could be achieved by using these three depressants. Figure 8 shows that 3-mercaptopropionic acid exhibited the strongest depression effect on the flotation of chalcopyrite among the three reagents at a pH of 6, followed by 3-mercaptoisobutyric acid. By comparing the binding energies of Cu2+ and Pb2+ complexes with these three depressants, as shown in Figure 8d, it can be concluded that all of these results agreed with the DFT calculation results.
4. Conclusions
In this research, the effects of three organic depressants (specially three mercapto acids: 3-mercaptopropionic acid, 3-mercaptoisobutyric acid and mercaptoacetic acid) on the flotation behaviors of chalcopyrite and galena were investigated by making the use of flotation experiments and first principle calculations. According to the solution chemistry of the three depressants, the three depressants mainly exist in the form of ions in the aqueous solution, and specifically, in the form of carboxyl dehydrogenation ions, indicating that using the frontier orbital theory to study the flotation and depression performance of depressants is feasible. The DFT calculation results showed that the three depressants exhibited a better depression ability on the surface of chalcopyrite than that on galena through the analysis of frontier orbitals energies and atom charges. From the HOMO energies and electron densities of the reactive centers, the following order of depression ability on the chalcopyrite surface was theoretically obtained: 3-mercaptopropionic acid > 3-mercaptoisobutyric acid > mercaptoacetic acid. The flotation test results indicated that the DFT calculations could be used to predict the adsorption strength of organic molecules on mineral surfaces. This study therefore provides a straightforward method for using DFT calculations to design a depressant for chalcopyrite or other minerals to achieve the desired results.
Based on this research, the separation of mixed pure minerals and actual sulfide ore will be carried out next, which not only includes chalcopyrite and galena, but also sphalerite and pyrite. In addition, the inhibitor is planned to be used in the flotation separation of chalcopyrite and molybdenite.
Author Contributions
Conceptualization, H.T. and W.S.; Methodology, R.L.; Software, R.X.; Validation, H.T., L.W. and F.J.; Formal Analysis, L.W.; Investigation, R.L.; Resources, J.J.; Data Curation, R.L.; Writing—Original Draft Preparation, R.L.; Writing—Review and Editing, R.L. and R.X.; Visualization, R.L. and Z.G.; Supervision, W.S.; Project Administration, W.S.; Funding Acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully appreciate the financial support from the National Natural Science Foundation of China (51974365, 51704329, 51704040), the National Key R&D Program of China (2018YFC1901901), and Key Laboratory of Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral Resources (2018TP1002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, B.; Yin, W.; Zhu, Z.; Wang, D.; Han, H.; Fu, Y.; Sun, H.; Chu, F.; Yao, J. A new model for the degree of entrainment in froth flotation based on mineral particle characteristics. Powder Technol. 2019, 354, 358–368. [Google Scholar] [CrossRef]
- Yin, W.; Yang, B.; Fu, Y.; Chu, F.; Yao, J.; Cao, S.; Zhu, Z. Effect of calcium hypochlorite on flotation separation of covellite and pyrite. Powder Technol. 2019, 343, 578–585. [Google Scholar] [CrossRef]
- Piao, Z.; Wei, D.; Liu, Z.; Liu, W.; Gao, S.; Li, M. Selective depression of galena and chalcopyrite by O, O-bis (2, 3-dihydroxypropyl) dithiophosphate. Trans. Nonferrous Met. Soc. China 2013, 23, 3063–3067. [Google Scholar] [CrossRef]
- Drzymala, J.; Kapusniak, J.; Tomasik, P. Removal of lead minerals from copper industrial flotation concentrates by xanthate flotation in the presence of dextrin. Int. J. Miner. Process. 2003, 70, 147–155. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y. Effect of calcium ions and citric acid on the flotation separation of chalcopyrite from galena using dextrin. Miner. Eng. 2000, 13, 1405–1416. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Wei, D. Research progress of flotation separation and separation depressants of copper-molybdenum mixed concentrate. Met. Mine 2018, 4, 35–41. [Google Scholar]
- Valdivieso, A.L.; Cervantes, T.C.; Song, S.; Cabrera, A.R.; Laskowski, J. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector. Miner. Eng. 2004, 17, 1001–1006. [Google Scholar] [CrossRef]
- Liu, R.; Qin, W.; Fen, J.; Wang, X.; Bin, P.; Yang, Y.; Lai, C. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Trans. Nonferrous Met. Soc. China 2016, 26, 265–271. [Google Scholar] [CrossRef]
- Qiu, T.; Song, Y.; Qiu, X.; Li, X. Performance of organic depressants in scheelite flotation system. Chin. J. Nonferrous Met. 2017, 27, 1527–1534. [Google Scholar]
- Liu, J.; Wang, Y.; Luo, D.; Zeng, Y. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation. Miner. Eng. 2018, 121, 31–38. [Google Scholar] [CrossRef]
- Piao, Z.; Wei, D.; Liu, Z. Effects of small molecule organic depressants on the flotation behavior of chalcopyrite and galena. J. Northeast. Univ. 2013, 34, 884–888. [Google Scholar]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Grimme, S.; Schreiner, P.R. Computational chemistry: The fate of current methods and future challenges. Angew. Chem. Int. Ed. 2018, 57, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Frank, D.P.; Matthias, F.B. Special collection: Computational chemistry. Chemistryopen 2019, 8, 814–816. [Google Scholar]
- Mao, Y.; Wang, H.; Hu, P. Theory and applications of surface micro-kinetics in the rational design of catalysts using density functional theory calculations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1321. [Google Scholar] [CrossRef]
- Alipour, M. Dipole moments of molecules with multi-reference character from optimally tuned range-separated density functional theory. J. Comput. Chem. 2018, 39, 1508–1516. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Wang, Z.; Han, H.; Yang, Y.; Liu, R.; Hu, Y.; Tang, H.; Sun, W. Self-assembly of mixed dodecylamine-dodecanol molecules at the air/water interface based on large-scale molecular dynamics. J. Mol. Liq. 2019, 276, 867–874. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Chen, X.; Zhou, Z.; Yang, H.; Yang, B.; Xu, B.; Liu, D. Bimetallic pbncun (n = 2–14) clusters were investigated by density functional theory. Comput. Theor. Chem. 2017, 1106, 21–27. [Google Scholar] [CrossRef]
- Poling, G.W.; Liu, Q. Flotation depression of chalcopyrite with thioglycolic acid. Trans. Inst. Min. Met. Sect. C Miner. Process. Extr. Metall. 1987, 96, C7–C12. [Google Scholar]
- Huang, H.; Wang, J.; Geng, Z.; Gao, Z. A study of the chalcopyrite depression mechanism by thioglycolic acid. Conserv. Util. Min. Resour. 2015, 6, 22–26. [Google Scholar]
- Chen, J.; Feng, Q.; Lu, Y. Research on the interaction of tga with chalocopyrite and sphalerite. Conserv. Util. Min. Resour. 2002, 5, 22–24. [Google Scholar]
- Bala, T.; Prasad, B.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of different metal ions with carboxylic acid group: A quantitative study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Yao, C.; Wang, Y. Sorption behaviour and mechanism of ytterbium (III) on imino-diacetic acid resin. Hydrometallurgy 2006, 82, 190–194. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Ma, C. Metal complexation and biodegradation of edta and s, s-edds: A density functional theory study. J. Phys. Chem. A 2010, 114, 443–454. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).