Abstract
The placer deposit at Barrytown, New Zealand, has been worked for gold and is known for high levels of ilmenite that has not been exploited. Other heavy minerals are present but have not been well characterized, which is the purpose of this research. Sand grains were separated into the density fractions and the heavier fractions analyzed by laser ablation ICP-MS for elemental composition and by scanning electron microscopy (SEM) EDS in whole grains and polished sections. Grain size distributions were determined from SEM images of polished grain mounts. Elemental associations have been identified with different minerals. A wide range of ore minerals, or potential useful industrial minerals, have been shown to be present largely as individual sand grains. These include gold, ilmenite, garnet, zircon, monazite, allanite, uraninite, thorite, cassiterite, wolframite, scheelite, and columbite. The ilmenite contains many inclusions, consisting of silicates and phosphates and 100–400 ppm Nb. Scandium is found to be present in zircon at 100–600 ppm along with 3000 ppm Y. Monazite is depleted in Eu relative to chondrite and contains Ga and Ge at 1000–3000 ppm. Because the sand grains are mostly individual minerals, it is suggested that separation may be possible using a combination of density, electrostatic and magnetic methods to obtain almost pure mineral fractions. This knowledge should inform decisions on potential exploitation of the resource.
1. Introduction
The placer deposits (beach sands) of heavy minerals at Barrytown on the West Coast of the South Island of New Zealand have been known for many years [1,2,3]. However, there has been very little published on the detailed mineral compositions including solid solution components and the nature and extent of inclusion impurities.
This gap in knowledge has been partly filled with a comprehensive recent study on the garnet composition in this and related deposits [4]. Some studies have been done to investigate the mineralogy of the ilmenite, but little has been done on the other potentially valuable minerals since a wide-ranging study in the 1940s from the South Island, New Zealand, beach sand deposits and gold dredge concentrates [5]. The ilmenite has a relatively low titanium content of 45–47% TiO2 because of the relatively high levels of inclusions, although the ilmenite matrix is closed to stoichiometric FeTiO3, with 52.6% TiO2, 47.3% FeO [6]. These inclusions consist mainly of feldspars, garnet, quartz, rutile, zircon, mica, and apatite [6]. The iron within the ilmenite is mostly as Fe2+ with little present as Fe3+ and the ilmenite is highly soluble in hydrochloric acid [7]. The garnet has been reported to be mostly almandine [6]. The zircon has a high degree of purity with >66% ZrO2 and also contains up to 3% HfO2 with a low level of radioactivity [6].
The placer deposit at Barrytown is a Holocene deposit formed within the last 10,000 years with the oldest beach dated at around 6500 years [8]. The ilmenite and other heavy minerals, such as gold, monazite, zircon, and garnet, are derived from the metamorphic rocks, particularly schist, of the Southern Alps [9,10,11] (Figure 1). Their origin is to the south, as erosional debris, and they have subsequently been transported northward [4,6] by the longshore drift current that runs up the coast [12] and then further concentrated by wave action. Therefore, there is finer sand at Barrytown than at some of the beaches further south which are closer to the source [4].
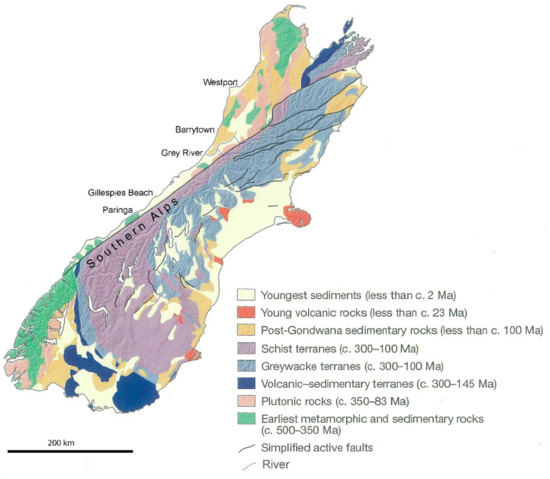
Figure 1.
Simplified geological map of the South Island of New Zealand (adapted from [13] with permission). For a range of other relevant and detailed maps, including longshore drift currents, the reader is directed to the recent work of Ritchie et al. [4], which is not duplicated here.
Gold has been mined since 1867 at Canoe Creek, which runs across the Barrytown Flats, with mining of the beach sands for gold starting not long afterwards [14], and continuing in some form to the present day. Ilmenite was identified as a mineral present in large quantities in the region around 1948 [15], although “blacksand leads”, as they were known, have been recognized by gold dredgers well prior to this. Serious commercial interest in exploiting the ilmenite resource has existed for many years. In 1966, the Carpentaria Exploration Company began an exploration program with extensive drilling to evaluate ilmenite, zircon, and monazite. This was discontinued in 1976 [16]. In 1982, the Grampian Mining Company, later called Fletcher Titanium Products Ltd., began another extensive drilling program of the Barrytown Flats [6]. This continued until 1990 when North Broken Hill Peko Limited (known as Westland Ilmenite) continued investigating the resource [17]. Subsequently there were a variety of other entities interested in the resource with Barrytown JV Ltd. formed in 2018 to continue exploration of the Barrytown placer deposits. However, despite this interest over a long period, other than gold, the placer minerals at Barrytown have not been exploited.
The size and composition of the Barrytown placer deposit has been estimated for gold, ilmenite, zircon, and to a lesser extent for garnet and monazite. There is an estimated 155,000 oz gold for the northern half of the resource at a level of 116–141 mg/m3 [17]. Ilmenite has been estimated at 12.77 M tonnes ilmenite at 2% cut off grade from 112 M tonnes mineral sand (11.4% average grade) with 133 M tonnes overburden [16] (Figure 2). Elsewhere it is reported as 6.9 M tonnes of ilmenite at an average grade of 13.8% ilmenite [3].
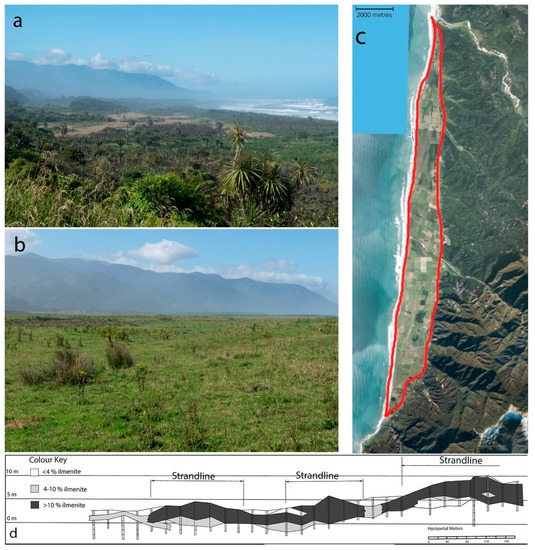
Figure 2.
(a) Barrytown flats North, looking south (January 2019); (b) Barrytown flats South, looking south (January 2019); (c) aerial photo of Barrytown deposit (LINZ 2015–2106 summer); (d) sketch of cross section of deposit adapted from MR4295-3 Westland Ilmenite [18].
Zircon is reported as a mean grade of 0.22% from reasonably extensive drill cores [19]. Monazite has not been analyzed in detail and estimates vary from 0.1–0.2% [6,19]. Garnet has been reported to be present at approximately twice the quantity of ilmenite [20]. Titanite is present with one estimate at a grade of 1–3% [6].
Placer deposits of heavy minerals, including those with high levels of ilmenite, are found in other parts of the world [21,22,23,24].
The purpose of this research is to identify the principal heavy minerals; quantify the mineral compositions; identify the solid solution components of potential economic interest; describe the inclusion impurities; and provide observations on the monominerallic nature of individual grains. From this we may speculate on potential methods and ease of exploitation of the deposit.
2. Materials and Methods
Unconsolidated sand was obtained from the Barrytown deposit. The ilmenite was sampled from several hundred tonnes of ilmenite separated in 1989 as part of an extensive drilling and exploration program and cannot be identified to a specific location in the deposit. The heavy and superheavy mineral fractions were taken from about 500 m from the shore about 3 km north of the Barrytown township at a depth ranging from 10–30 m which is the location of the second strandline (thus, of the middle age of the deposit). For density separation of the heavy fraction of the sand, a wave (shaker) table (Action Mining Services Inc., Sandy, OR, USA) with three density fractions separated was used, which are described here as superheavy (ca. >4.6 g·cm−3), heavy (4–5), and middling (3–4). These fractions were subsampled for each geochemical analysis or SEM and LA-ICP-MS. The grains were mounted in epoxy resin and polished for use in both SEM and LA-ICP-MS.
A subsample of the mineral concentrates were dried at 120 °C then calcined at 980 °C, ground then fused with a flux containing 35.3% lithium tetraborate and 64.7% lithium metaborate (12:22 flux from XRF Scientific, Perth, Australia) to form standard fused discs for analysis. An ilmenite fraction was from that prepared by Fletcher Titanium in 1989.
2.1. SEM/EDS
SEM mounts were prepared by setting heavy sand fractions in moulds with Epo-tek 301-1b resin (Epoxy Technology Inc Billerica, MA, USA) and hand polished successively down to 1 μm diamond paste. The polished face was carbon coated and imaged using an FEI Quanta 200 scanning electron microscope (ThermoFisher, Hillsboro, OR, USA). with xT microscope Version 3.0.7 imaging software and EDAX Genesis (Ametek, Mahwah, NJ, USA). Genesis Spectrum Version 5.21 software (Ametek, Mahwah, NJ, USA). A spot size of 5.0 was used, with 2000–3000 counts per second (CPS) and a dead time of 30%. The collection time used was 100 s.
2.2. LA-ICP-MS
Trace element measurements were made on 205 individual mineral crystals using a RESOlution-SE Compact 193 nm excimer laser ablation (LA) (Australian Scientific Instruments, Fyshwick, ACT, Australia). system in conjunction with an Agilent 8900 Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) (Agilent Technologies, Santa Clara, CA, USA). Data was collected by pulsing the laser at 10 Hz with a 30 µm spot size and energy density of 5 J/cm2 for 45 s for each spot analysis. A minimum of 20 spot analyses per mineral density fraction were carried out and background counts (gas background, measured with the laser off) were collected for 30 s between samples. National Institute of Standards and Technology (NIST) glass standards 610 and 612 were analyzed every 10 spots to account for any instrumental drift. Ultra-high purity helium was used as the carrier gas to deliver the ablated sample from the LA system to the ICP-MS. The ICP-MS was optimized to maximum sensitivity prior to experimental analysis. Operating conditions included forward (reflected power) 1500 W, plasma gas flow 14 L/min (Ar), auxiliary gas flow 0.90 L/min, carrier gas flow 1.05 L/min, sampling depth 4.0 mm, pulse count detector mode, peak hopping sweep mode, dwell time 0.01–0.1 s, one point per peak.
Data processing was carried out using the software lolite (Version 3.32) [25]. Background counts were subtracted from the raw data and the data was standardized to NIST 612 glass. NIST 610 glass was used as a secondary standard. GeoReM, a data base for reference materials and isotopic standards that are of mineralogical interest [26], was used for the NIST glass values.
2.3. Optical Microscopy
Optical images were captured for sand grains using an Olympus BX53 microscope with a XC50 camera using a mixture of transmitted and reflected illumination.
2.4. Grain Size Measurements
The grain size of each of the density fractions was measured from SEM images, where images had been taken at a magnification to capture over a hundred grains per image, before processing through ImageJ. A magnification of 50 x was used. The software was calibrated from the scale on the SEM image, and before processing using the Analyse Particles function. Circularity was set to 0.0–1.0 and “Show Outlines” was selected. Grain size values were exported and further processed in Excel.
3. Results
3.1. Optical Microscopy of Barrytown Sand
The superheavy (Figure 3) and heavy fraction of the Barrytown sand has a diverse appearance ranging from opaque to translucent grains with a variety of colors. They are largely subhedral grains.
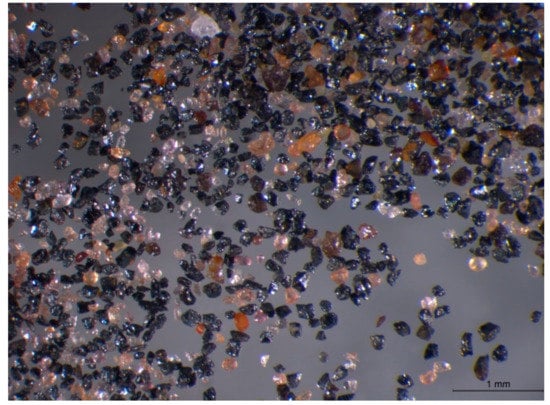
Figure 3.
Optical image of Barrytown superheavy fraction.
3.2. Grain Size Range
The superheavy fraction has a broad range of grain size with some fine grains (Figure 4a) while the heavy fraction and the ilmenite have a narrower size range (Figure 4b,c). For the ilmenite fraction, where grain size was determined by both SEM and a Malvern particle sizer, there is good agreement between the two sizing methods.
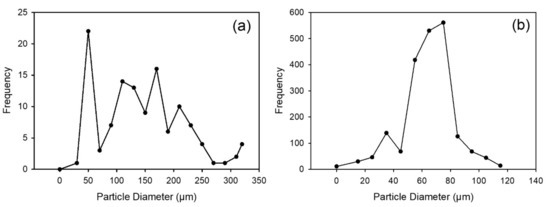
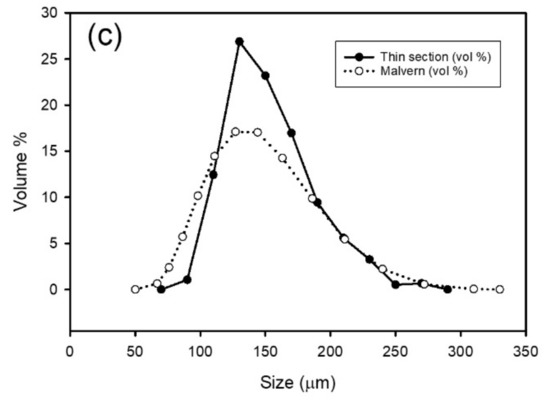
Figure 4.
Grain size distribution from SEM images (a) superheavy fraction; (b) heavy fraction; (c) ilmenite fraction with a comparison obtained by using a Malvern particle size analyzer.
3.3. Laser Ablation Analysis of Fused Glass Discs
Three mineral fractions were fused and analyzed by LA-ICP-MS. These were an “ilmenite” fraction, as well as a heavy and a superheavy fraction.
The “ilmenite” fraction contained Fe 29.5 wt %, Ti 26.7 wt %, Mn 1.15 wt %, Si 5.00 wt %, Al 1.22 wt %, V 138 ppm, Cr 199 ppm Cr, Nb 350 ppm, and Au 125 ppm. This fraction is not pure ilmenite but likely includes some other separate mineral grains, such as garnet, and the analysis also represents the mineral inclusions within the ilmenite.
The heavy and superheavy fractions show a high level of zirconium (zircon) with the heavy fraction containing ca. 40% ilmenite. Other elements of interest that can be noted in the full sample analyses for all three fractions include gold which is found at the highest levels in the “ilmenite”, followed by the heavy then superheavy fractions. Niobium is present in the “ilmenite” and heavy fractions (350, 323 ppm) with less presence in the superheavy fraction (199 ppm). Tantalum is present in the “ilmenite” fraction at 33 ppm but more so in the heavy (40 ppm) and superheavy (115 ppm) fractions. The Ta concentrations are not correlated with the Nb contents as might be expected if these were present as a columbite–tantalite source. Chromium is present in both the heavy and superheavy fractions equally (970 and 938 ppm, respectively) and less in the ilmenite fraction (199 ppm). Sn, rare earths (including Y), Ta, W, Th, and U are present in the heavy and superheavy fractions with higher levels in the superheavy fraction.
3.4. Laser Ablation Analysis of Individual Mineral Grains
3.4.1. Monazite and Allanite
Monazite is a common mineral in the superheavy and heavy fractions. It contains the full range of rare earth elements, including the other Group 3 elements Y and Sc (Figure 5a). A chondrite normalized plot of rare earth elements (Figure 5b) follows a standard form but shows a large negative Eu anomaly. Gallium and Ge are also found in monazite with up to 1000 ppm of each and they are highly correlated with each other (Figure 6). Most of the Ga and Ge in the heavy mineral fraction are contained in the monazite, although up to 60 ppm Ga is found in the garnet (but more commonly 5–20 ppm Ga).
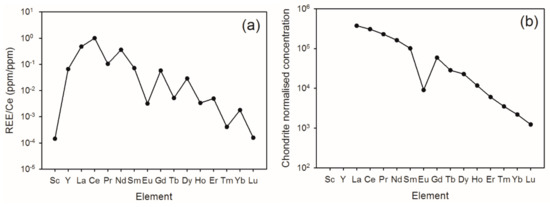
Figure 5.
(a) Group 3 and rare earth element normalized composition of monazites (average of 18 grains); (b) chondrite normalized concentrations of the rare earth elements (from (a)) showing the Eu anomaly.
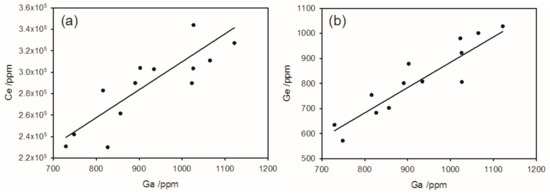
Figure 6.
(a) Gallium and (b) germanium in monazite.
Allanite is also a rare earth containing mineral, but is a lighter mineral which was not found in the heavy or superheavy fractions However, some allanite was observed in the middling fraction. Analysis by LA-ICP-MS showed allanite to be relatively low in Y (466 ppm) but high in the lanthanides (e.g., La 3.4%, Ce 5.7%, Nd 1.6%). The allanite does not exhibit an anomaly in the Eu concentration. No xenotime was found by LA-ICP-MS.
3.4.2. Zircon (and Hf, Sc, Y)
Zircon is an abundant mineral in the heavy and superheavy fractions. It contains hafnium, in proportion to the zirconium (Figure 7a), as is normally observed in zircon. No baddeleyite was identified by the LA-ICP-MS analyses. However, this mineral was observed with EDS.
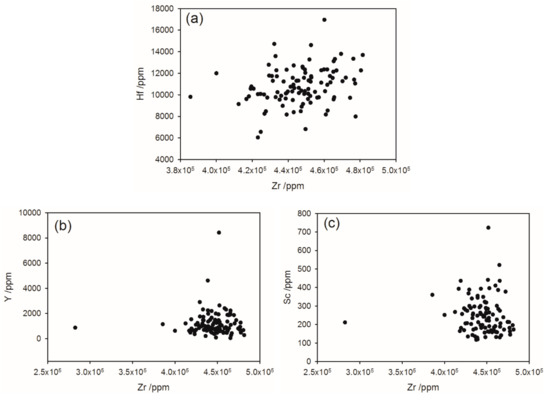
Figure 7.
Zircon analysis with the correlation between Zr and (a) Hf; (b) Y, (c) Sc.
Yttrium is present in zircon generally in the range 100–8000 ppm (Figure 7b). This is less than the Y concentration of 1.8–6.1% in monazite, but makes a significant contribution to the total Y content of the heavy mineral fractions.
Scandium is present in zircon at levels of 100–700 ppm (Figure 7c). This accounts for most of the Sc in the heavy fractions. Scandium is also present in garnet at variable levels from 6 to 267 ppm. There is very little scandium in the monazite.
3.4.3. Thorite (Th, U)
Thorium and uranium are closely associated with each other throughout all the minerals analyzed here. These elements are present in thorite where the concentration of Th and U is about equal (Figure 8). These elements are also present in monazite at levels of around 1% for Th and 1–10% ppm for U. Both Th and U are also in significant concentrations in zircon (10–1000 ppm) and also present at lower levels (0.001–100 ppm) in ilmenite and magnetite.
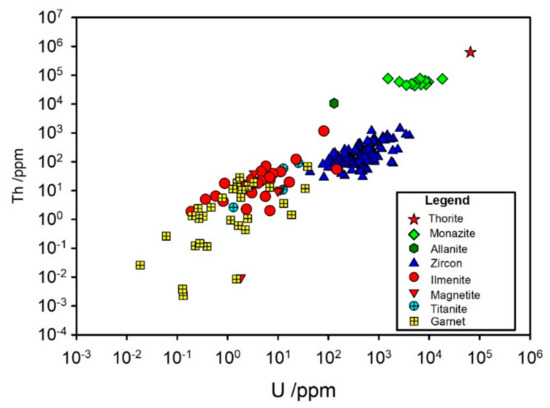
Figure 8.
Th and U content of individual mineral grains.
3.4.4. Ilmenite, Magnetite (and Other Ti-Fe Minerals)
The deposit is known to be rich in ilmenite and some ilmenite grain analyses are included here. A hand magnet did show the presence of some magnetite in the heavy and superheavy fractions but none in the “ilmenite” fraction. The ilmenite usually contains inclusions, and the analyses here are laser spot analyses focused mainly on the ilmenite matrix, and therefore may underestimate the levels of elements found in the inclusions. A log–log plot of Fe verse Ti composition of the suite of mineral grains analyzed (Figure 9) shows a cluster of high Ti, high Fe representing ilmenite. The plot shows other clusters, e.g., high Ti with lower Fe may be titanomagnetite; high Ti with lower Fe could be titanite. There are some nearly pure iron oxide minerals too, with 100–5000 ppm Ti which almost certainly is magnetite.
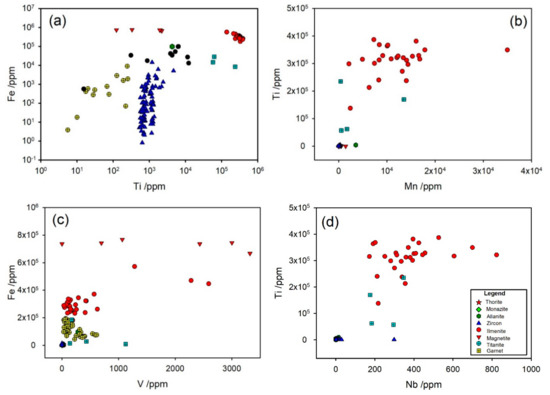
Figure 9.
Mineral analysis (where ilmenite, magnetite, and titanite have the highest concentrations of the elements represented in the plots) with correlation between (a) Ti and Fe; (b) Mn and Ti; (c) V and Fe, and (d) Nb and Ti. The legend applies to all plots in this figure.
The ilmenite matrix also contains a solid solution of some other elements and minor concentrations (Figure 9). These include Mn at 0.2–1.5%, Nb 100–400 ppm, and Ta 10–40 ppm. Cr is present in the ilmenite but is rather variable with the range 10–100 ppm.
Vanadium is found in the highest levels (2000–3000 ppm) in some magnetite and ilmenite but with a wide range of concentrations (Figure 9c).
3.4.5. Garnet
The garnets analyzed here fall into two groups. There is one group that has the composition (Fe,Mn,Ca)3Al2(SiO4)3 with a range of Mn-Fe substitution in the X position. Another tighter group has the composition (Ca,Fe)2Al3(SiO4)3 with Ca-Fe substitution in the X position (grossular-almandine) (Figure 10). The garnets are low (<1%) in Mg.
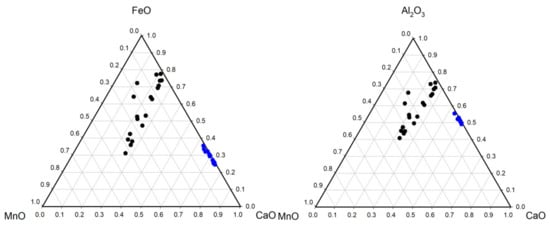
Figure 10.
Ternary plots of the composition of the normalized major element oxides of garnet (excluding Si and O). The blue squares and black circles are used to distinguish the same two groups across the two plots.
The garnet also contains 40–1100 ppm Y and a very variable amount of the lanthanides including 0–1700 ppm Ce.
3.5. SEM and EDS
The ore minerals are observed with SEM electron backscatter imaging (Figure 11). The image shown here illustrates a range of these heavy minerals present, although this does not represent the relative abundances of these minerals in the sample.
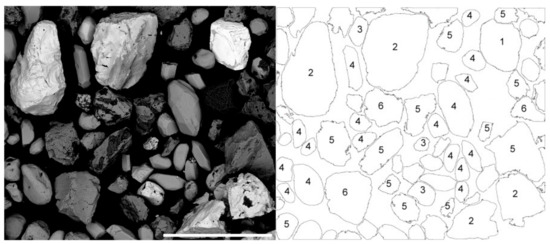
Figure 11.
SEM electron backscatter image for a selection of sand grains from the Barrytown superheavy fraction with components identified from an EDS analysis of each individual grain. Key: 1 uraninite, 2 cassiterite, 3 monazite, 4 zircon, 5 ilmenite, 6 magnetite. Scale bar = 400 µm.
Ilmenite makes up a large portion of the placer deposit. The ilmenite is subhedral and contains numerous inclusions (Figure 12a–c). These inclusions are mostly silicates and aluminosilicates such as feldspars with some phosphates such as apatite. Most of the inclusions are anhedral, but occasional euhedral feldspar inclusions are observed.
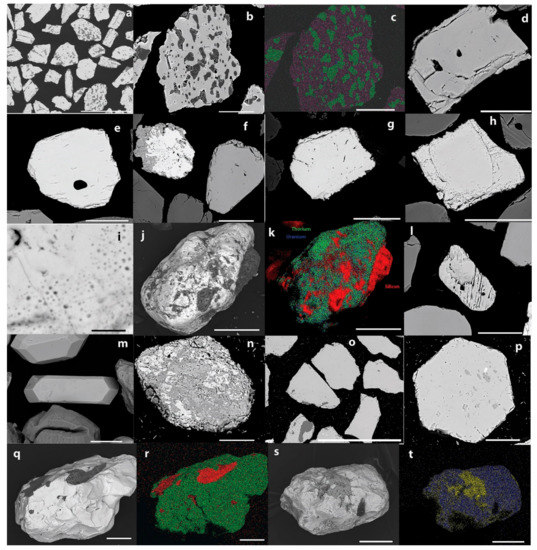
Figure 12.
SEM backscatter and elemental maps of a range of mineral grains in polished section or whole grains. (a) A selection of ilmenite grains; (b,c) ilmenite with inclusions of apatite and silicates, Si (green), P (blue), and Ti (purple); (d,e) monazite; (f) titanite (upper left) and allanite (right); (g,h,i) thorite showing areas of radiation damage; (j,k) thorite with inclusions of silicate, Th (green) U (blue), Si (red); (l) scheelite; (m) zircon; (n) a mixed titanium rock fragment, titanite (dark gray), rutile (light gray), ilmenite (white); (o) garnet with inclusions of quartz and ilmenite; (p) garnet with an inclusion of zircon, and inclusions without Fe and Mn; (q,r) cassiterite with silicate inclusion; (s,t) columbite, with magnetite inclusion, Nb (blue), Fe (yellow); Scale bar: (a), (o) = 300 µm; (i) = 2 µm; others = 50 µm.
Monazite ranges in form from subhedral to anhedral and is largely without inclusions (Figure 12d,e). The distribution of the different rare earth elements appeared uniform throughout the monazite grains (EDS maps not shown). Rare earth containing allanite was also observed in the middling fraction of Barrytown sand. However, it did not appear to be an abundant mineral (Figure 12f). It contains Ce and La but not significant Y.
Thorite is more abundant than uraninite in the Barrytown placer deposit. The grains are subhedral and show regions of partial metamictization (Figure 12g–k). Much of the thorite is present as clean grains without inclusions. However, some is present in association with other minerals such as intergrowths with monazite (not shown).
Zircon is present mostly as euhedral columnar grains, but subhedral and anhedral zircon is also present (Figure 12m). The zircon generally does not contain inclusions and each grain has a uniform composition (EDS maps not shown).
Both scheelite (not shown) and wolframite (not shown) were observed in the superheavy fractions. These minerals have been noted at Barrytown previously [5]. The scheelite is more abundant and is in the form of subhedral grains with few inclusions.
The main Ti-bearing mineral presented in the Barrytown deposit is ilmenite. However, there are smaller amounts of titanite and rutile. No rutile was observed as separate mineral grains. However, rutile was observed as intergrowths or an alteration product with ilmenite or with titanite and ilmenite.
Garnet is present as subhedral to euhedral crystals containing minor inclusions including quartz, ilmenite, and zircon (Figure 12o–p). Subsequent to this analysis being performed, a comprehensive study of garnet from this deposit and deposits in neighboring localities has been published [4]. These authors found Fe-Al rich garnet dominant in this deposit with approximately equal Mn and Ca of varying amount. Here we found one group with this analysis, but another group, that we assigned to garnet, based on chemical composition alone, that is deficient in Mn but rich in Ca (Figure 10).
Cassiterite is present in the superheavy fraction of the Barrytown sand. The grains all have a distinctive surface with the appearance similar to a glass fracture with concave portions. Cassiterite has been noted from Barrytown and throughout the West Coast previously [5]. The cassiterite mostly contains some inclusions (of a variety of minerals) but the inclusions make up only a small portion of each grain (Figure 12q–r).
Columbite is present as occasional mineral grains, generally with inclusions of magnetite (Figure 12s–t). Columbite can be found in the complete range of solution with tantalite. However, in the Barrytown deposit only, the columbite end member was found, without tantalum. However, only a limited number of mineral grains have been sampled in this research.
4. Discussion
The major heavy mineral present in the Barrytown placer deposit is garnet, present at perhaps 20% [20]. The common garnets in the placer deposits on the West Coast are normally referred to as almandine and have a dark red or sometimes pinkish appearance. The garnets analyzed here fall into two groups. There is one group that has the composition (Fe,Mn,Ca)3Al2(SiO4)3 with a range of Mn-Fe substitution in the X position with up to 6 wt % Mn present. The other group has the composition (Ca,Fe)2Al3(SiO4)3 with Ca-Fe substitution in the X position (grossular-almandine) [27]. Based only on the composition it seems likely that the Fe is present in these minerals in the X position, and therefore is present at Fe2+. The composition of the garnet, with significant Ca, is suggestive of a largely schist origin of these garnets [28,29], and therefore transport from south of deposit, most likely south of the Grey River since the Grey River drains a predominantly plutonic area (Figure 1). It has been observed that the garnets are of decreasing size in beach deposits from Paringa to Westport (south to north) up the coast [4]. This is also suggestive of the transport of the garnet up the coast with sorting.
The second most abundant economic mineral is ilmenite, with around 12.8 M tonnes ilmenite at 2% cut off grade from 112 M tonnes mineral sand (11.4% average grade) [3,16]. The inclusions consist of silicates and phosphates, with Si making up 5.0 wt % and Al 1.2 wt % of the analysis of a bulk sample of ilmenite, which would suggest inclusions represent about 7–8 wt % of the ilmenite grains, and this is consistent with the SEM images of cross sections of ilmenite grains. The ilmenite is largely free of alteration products such as “leucoxene” or rutile. The high level of silicate inclusions in the ilmenite is characteristic of ilmenite derived from schist rather than from granite [9]. This is consistent with transport from south of the Grey River, where the rivers feed from steep eroding mountains of the Haast Schist of the Torlesse terrane (within the Schist terrane of Figure 1), rather than a more local source.
Zircon is present in the deposit at around 0.22% [19]. At this level of abundance the zircon content is less than that in recently proposed mines that rely mainly on zircon for economics. Two such proposed mines are the Dubbo Zirconia Project, NSW, Australia, with a resource of 75 Mt with 2.8% zircon and the Thunderbird Mineral Sands Project, Dampier Peninsula, WA, Australia, with 1050 Mt with 0.92% zircon with leucoxene and ilmenite, neither of which appear to be operational at the time of publication.
The zircon in the Barrytown deposit contains 2.4 wt % Hf, which is within the typical range found in zircon. Scandium is present in zircon at levels of 100–700 ppm. This accounts for most of the Sc in the heavy fractions. While Sc is a valuable element used in alloys of aluminum that retain strength after welding, the level of Sc in the deposit is not high and may be difficult to extract from the zircon. For comparison, a prospected deposit, at Nyngan, NSW, Australia, is claimed to be economic at an average grade of 409 ppm with reserves estimated to be 1.44 million tonnes containing about 590 tonnes of scandium using an effective cutoff grade of 155 ppm scandium [30]. Yttrium is also present in the Barrytown zircon in the range 100–3000 ppm and makes a significant contribution to the total Y content of the heavy mineral fractions.
The lanthanides (rare earth elements) are contained mostly within monazite. However, allanite is also present. The lanthanides follow the usual abundance found in primary sources, with a decreasing abundance at a higher atomic number. A chondrite normalized plot of the rare earth elements follows a standard form but shows a large anomaly in the Eu concentration indicating that the Eu has likely been incorporated into plagioclase phenocrysts, substituting for the similar size Ca2+ ion, and was thus depleted at the time the monazite crystallized. This analysis is normally done on whole rock samples, so the results here indicate that the rare earths are largely incorporated in the monazite. A similar analysis of Group 3 elements Sc and Y using data from [31] does not fit this trend, indicating that the Sc and Y are not concentrated into the monazite, but rather are distributed in other minerals.
The grade of monazite has been estimated to be around 0.1–0.2% [6,19] which may be economic to extract as a co-product. Ga and Ge are also found in the monazite up to 2500 ppm of each. Most of the Ga and Ge in the heavy mineral fraction is contained in the monazite, although a little Ga is associated with Al. Th and U are present in the monazite at levels of 20–200 ppm for Th and 100–10,000 ppm for U, which may create health and regulatory concerns if extraction of the rare earth content of monazite were to be considered.
Thorite is present in the deposit, although the grade in the deposit is not known. The thorite in the deposit is rich in U containing approximately equal U and Th by weight. Some radiation damage can be seen in parts of the thorite grains leading to a degree of metamictization.
It is not typical for thorite to be so rich in U, and nor is it for monazite to contain more U than Th [32]. The closest known uranium deposit is at Uranium Point in the Buller River. However, alluvial material from this region enters the coast north of Barrytown and will be transported north and not be deposited at Barrytown. High levels of U have been reported from well south of Barrytown, at Gillespie Beach. However, the Barrytown deposit has been formed by coastal currents transporting the material northwards, but Gillespies Beach is 200 km to the south of Barrytown (Figure 2), a considerable distance for transport of heavy minerals, and not considered to be deposited from the same source.
Tin is present in the deposit as cassiterite. The cassiterite is generally free of inclusions, and it may be able to be produced as a co-product. However, the grade is likely to be low.
Some columbite has been observed as the almost pure Nb variety low in Ta. The abundance of this mineral in the deposit has not been determined. However, this could also be produced as a co-product in a relatively pure form.
Tungsten is present in two minerals, wolframite and scheelite. These exist as relatively pure minerals with limited inclusions and may also be able to be separated by simple physical processes as a co-product if present in sufficient quantity.
Gold in the deposit was not investigated for this study, although it is at levels that may be considered economic.
5. Conclusions
A wide range of ore minerals are present in the Barrytown placer deposit with many of these as grains of sand consisting of single minerals relatively free of inclusions. The dominant heavy minerals are garnet and ilmenite. Other heavy minerals present include zircon, monazite, allanite, uraninite, thorite, cassiterite, wolframite, scheelite, columbite, and gold. Elemental associations have been identified for elements of significance and solid solution compositions identified. Scandium is present mostly in zircon. Gallium and germanium are present in monazite. Uranium and thorium are present in a range of minerals, at the highest levels in thorite and monazite, but also in zircon. Niobium is present in columbite and is also present in solid solution in ilmenite. This investigation suggests that because of the nature of the mineral structures separation, it should be possible using a combination of density, as electrostatic and magnetic methods to obtain nearly pure mineral fractions.
Author Contributions
Conceptualization H.C.W. and R.G.H., investigation H.C.W. and R.G.H., methodology H.C.W. and R.G.H., visualization H.C.W. and R.G.H., writing—original draft H.C.W. and R.G.H., writing—review & editing, H.C.W. and R.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Bret Moynihan and Rob Rose from Greymouth provided the Barrytown sand samples and separated these into the superheavy, heavy, and middling fractions and provided fruitful discussions. The historical separated ilmenite samples came from Westland Ilmenite. Niki Minards and Pani Vijayan assisted with the SEM/EDS analysis. Steve Cameron, Kirsty Vincent, and Amanda French at Waikato University assisted with the laser ablation ICP-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mann, G.; James, D. The evaluation and development of the Barrytown ilmenite deposit as a source of titanium dioxide pigment. In Monograph Series; Australasian Institute of Mining and Metallurgy: Victoria, Australia, 1989; Volume 13, pp. 169–172. [Google Scholar]
- Christie, A.B.; Barker, R.G.; Brathwaite, R.L. Mineral Resource Assessment of the West Coast Region, New Zealand; GNS Science Report; GNS Science: Lower Hutt, New Zealand, 2010. [Google Scholar]
- Christie, A.B.; Brathwaite, R.L. Mineral commodity report 16—Titanium. N.Z. Min. 1998, 23, 15–25. [Google Scholar]
- Ritchie, T.W.; Scott, J.M.; Craw, D. Garnet Compositions Track Longshore Migration of Beach Placers in Western New Zealand. Econ. Geol. 2019, 114, 513–540. [Google Scholar] [CrossRef]
- Hutton, O.C. Studies of heavy detrital minerals. Bull. Geol. Soc. Am. 1950, 61, 635–710. [Google Scholar] [CrossRef]
- Newman, N.A. Barrytown ilmenite evaluation—Borehole reports. In The Geology, Mineralogy and Provinance of the Barrytown Beachsand Deposit: A Review; MR1434; Ministry of Economic Development: Wellington, New Zealand, 1989. [Google Scholar]
- Haverkamp, R.G.; Kruger, D.; Rajashekar, R. The digestion of New Zealand ilmenite by hydrochloric acid. Hydrometallurgy 2016, 163, 198–203. [Google Scholar] [CrossRef]
- Suggate, R.P. The postglacial shorelines and tectonic development of the Barrytown coastal lowland, North Westland, New Zealand. N. Z. J. Geol. Geophys. 1989, 32, 443–450. [Google Scholar] [CrossRef]
- McPherson, R.I. Geology of Quaternary ilmenite-bearing coastal deposits at Westport. N. Z. Geol. Surv. Bull. 1978, 87, 1–95. [Google Scholar]
- Roser, B.P.; Cooper, A.F. Geochemistry and terrane affiliation of haast schist from the western Southern Alps, New Zealand. N. Z. J. Geol. Geophys. 1990, 33, 1–10. [Google Scholar] [CrossRef]
- Grapes, R.; Watanabe, T. Paragenesis of titanite in metagraywackes of the Franz Josef-Fox Glacier area, southern Alps, New Zealand. Eur. J. Miner. 1992, 4, 547–555. [Google Scholar] [CrossRef]
- Brodie, J.W. Coastal Surface Currents Around New Zealand. N. Z. J. Geol. Geophys. 1960, 3, 235–252. [Google Scholar] [CrossRef]
- Isacc, M.J. Maps that Changed New Zealand—Overview. In A Continent on the Move, 2nd ed.; Geoscience Society of New Zealand: Wellington, New Zealand, 2015; pp. 25–29. [Google Scholar]
- Murray-North. Environmental Impact Assessment—Westland Ilmenite Limited, Barrytown Mineral Sands, West Coast 31-1064, 32-3198, 32-3199, 32-3200, 32-3201; MR3188; Ministry of Economic Development: Wellington, New Zealand, 1991.
- Nicholson, D.S.; Cornes, J.J.S.; Martin, W.R.B. Ilmenite deposits in New Zealand. N. Z. J. Geol. Geop. 1958, 1, 611–616. [Google Scholar] [CrossRef]
- Caffyn, P.H. Ilmenite Reserves at Barrytown, New Zealand; MR1327; Ministry of Economic Development: Wellington, New Zealand, 1976.
- McOnie, A.; Bull, V. Work on EP40760 Barrytown to 30 Sept 2007; MR4265; Ministry of Economic Development: Wellington, New Zealand, 2007.
- R.W. Westland Ilmenite, Barrytown Ilmenite Grade YG Line; MR4295-3; Ministry of Economic Development: Wellington, New Zealand, 1990.
- Lee, G. Investigation into Barrytown Mineral Sand Resources; MR4291; Ministry of Economic Development: Wellington, New Zealand, 1989.
- McOnie, A.; Bull, V. Karamea Project PP39349 Progress Report to October 2008; MR4431; Ministry of Economic Development: Wellington, New Zealand, 2008.
- Els, G.; Eriksson, P. Placer formation and placer minerals. Ore Geol. Rev. 2006, 28, 373–375. [Google Scholar] [CrossRef]
- Hou, B.; Keeling, J.; Van Gosen, B.S. Geological and Exploration Models of Beach Placer Deposits, Integrated from Case-Studies of Southern Australia. Ore Geol. Rev. 2017, 80, 437–459. [Google Scholar] [CrossRef]
- Roy, P.S. Heavy mineral beach placers in Southeastern Australia: Their nature and genesis. Econ. Geol. 1999, 94, 567–588. [Google Scholar] [CrossRef]
- Garzanti, E.; Dinis, P.; Vermeesch, P.; Andò, S.; Hahn, A.; Huvi, J.; Limonta, M.; Padoan, M.; Resentini, A.; Rittner, M.; et al. Sedimentary processes controlling ultralong cells of littoral transport: Placer formation and termination of the Orange sand highway in southern Angola. Sedimentology 2018, 65, 431–460. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. IMA report: Nomenclature of the garnet supergroup. Am. Miner. 2013, 98, 785–810. [Google Scholar] [CrossRef]
- Mason, B. Garnet compositions in metamorphic rocks and granites of Westland, New Zealand. J. R. Soc. N. Z. 1981, 11, 35–43. [Google Scholar] [CrossRef]
- Smale, D. Distribution and provenance of heavy minerals in the South Island: A review. N. Z. J. Geol. Geophys. 1990, 33, 557–571. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; USGS: Reston, VA, USA, 2018; p. 204.
- Schmitt, R.A.; Smith, R.H.; Lasch, J.E.; Mosen, A.W.; Olehy, D.A.; Vasilevskis, J. Abundances of the fourteen rare-earth elements, scandium, and yttrium in meteoritic and terrestrial matter. Geochim. Cosmochim. Acta 1963, 27, 577–622. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Das, S.K.; Vijayan, V.; Sengupta, D.; Saha, S.K. Geochemical studies of monazite sands of Chhatrapur beach placer deposit of Orissa, India by PIXE and EDXRF method. Nucl. Instrum. Methods Phys. Sect. Res. B 2003, 211, 145–154. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).