Abstract
In mammals, the suprachiasmatic nucleus (SCN), located in the hypothalamus serves as the master biological clock and precisely regulates circadian rhythms through a complex network structure. As a central pacemaker, the SCN has two primary functions: one is to synchronize the daily rhythms in physiological and behavioral activities; the other is to entrain the endogenous rhythms to the external light–dark cycle. A deep understanding of the SCN network structure is crucial for elucidating the functional mechanisms of the biological clock system. In this review, we systematically summarized the impact of the SCN network structure on functional regulation under light stimulation based on mathematical models. Studies have shown that the coupling between the light-sensitive subgroups in the left and right nuclei of the SCN can enhance the entrainment ability. As an integrated network structure, the SCN may have the characteristics of the small-world network or the scale-free network, as these properties are more conducive to the realization of functions. Additionally, the higher-order coupling mechanism within the SCN can effectively expand the entrainment range. These theoretical research results offer new insights into the relationship between the SCN network and functions and provide crucial theoretical guidance and validation directions for subsequent experimental research.
1. Introduction
Due to the rotation of the Earth, almost all living organisms exhibit circadian rhythms in both behavior and physiology, characterized by periodic changes with a cycle close to 24 h [1,2]. In mammals, the circadian rhythm is coordinated by the suprachiasmatic nucleus (SCN), the central circadian rhythm pacemaker in the brain, which enables the behavioral and physiological rhythms within our body to remain synchronized with the external 24 h light–dark cycle [3,4]. The circadian rhythms of the SCN demonstrate a high degree of stability, whether in continuous darkness or continuous light environments or upon exposure to a natural light–dark cycle [5,6,7]. Therefore, the SCN has two primary functions in the regulation of circadian rhythms: one is to act as an endogenous clock, playing a synchronization role; the other is to act as a phase regulator, playing an entrainment role [8,9,10]. The synergistic effects of these two functions constitute the key physiological basis for organisms to adapt to the Earth’s rotational environment.
As an endogenous clock, the SCN generates and maintains stable circadian rhythm signals through internal molecular oscillation mechanisms and complex neuronal network, thereby synchronizing the periodic changes in various physiological and behavioral activities of the organisms [8]. This autonomous timing system exhibits remarkable robustness, as the SCN can still generate robust endogenous rhythmic oscillations even when animals are removed from any environmental cycle and placed in continuous darkness or continuous light conditions [11]. This endogenous rhythm typically operates with a period close to but not exactly equal to 24 h, which is thus referred to as the free-running period (FRP). Under conditions of continuous darkness or continuous light, the FRPs of different species exhibit significant differences [12]. For example, the FRP of the deer mouse is approximately 22.9 h, that of the southern flying squirrel is approximately 23.8 h, and that of human beings is approximately 24.2 h [5,12]. The differences in the FRPs among species provide important theoretical entry points for in-depth research into the intrinsic regulatory mechanisms and network structural characteristics of the biological clock system.
As the core calibrator of the biological rhythm system, the SCN entrains the body’s rhythms to the 24 h light–dark cycle of the external environment by integrating light signals [9]. The SCN can not only entrain the internal rhythms to the natural 24 h light–dark cycle but also to the non-24 h external light–dark cycles [13]. This flexibility demonstrates that the SCN has powerful rhythmic regulation ability and can adapt to various environmental cycles. In the experiment, the external light–dark period can be artificially altered to be longer or shorter than 24 h [14]. If the artificial light-dark periods become too short or too long, the SCN will not be successfully entrained by these artificial periods. Specifically, the shortest external artificial period that the SCN can successfully entrain is called the lower limit of entrainment (LLE), while the longest external artificial period is called the upper limit of entrainment (ULE) [15]. The range between the LLE and the ULE is called the entrainment range of the SCN [16]. This range is a key indicator for an organism’s ability to adapt to the external environmental changes, reflecting the flexibility and rhythmic plasticity of the SCN [17]. That is to say, a wider entrainment range indicates that the biological clock has larger flexibility and adaptability to the external environmental changes [13]. There are significant differences in the entrainment ranges among different species, and some organisms can adapt to a wider range of the external light–dark cycles [18]. For example, the deer mouse can be entrained from 22.5 h to 25.1 h, the southern flying squirrel can be entrained from 23.5 h to 24.9 h, and the humans can be entrained from 20.5 h to 29.0 h. The range of periods that organisms can be entrained is limited, which restricts their ability to synchronize with external environmental cycles [19]. When the period of the external light–dark cycle is set to a value outside the entrainment range of a species, animals will fail to synchronize with the external cycle and may exhibit more complex behavioral patterns [16].
The SCN neuronal oscillators form a tightly interconnected network through synaptic connections, neuropeptide release, and electrical coupling (such as the gap junctions), and this structure provides the foundation for synchronization and entrainment [20]. Anatomical studies have shown that the SCN is a bilateral symmetrical structure located in the anterior hypothalamus that consists of two structurally symmetrical nuclei, each containing approximately 10,000 neuronal oscillators [21,22]. These neuronal oscillators form a heterogeneous functional network through a complex coupling mechanisms, but the exact network topology of the SCN remains unclear [23]. Based on functional heterogeneity and network characteristics, the neuronal oscillators in a single nucleus of the SCN can be further divided into two distinct subgroups, namely the ventrolateral (VL) subgroup and the dorsomedial (DM) subgroup [24,25]. These two subgroups exhibit significant differences in the expression of neurotransmitters and neuropeptides [22]. Specifically, the VL subgroup is rich in neuronal oscillators expressing vasoactive intestinal peptide (VIP) and gastrin-releasing peptide (GRP), which display high sensitivity to external light stimulation [26]. In contrast, the DM subgroup is predominantly composed of neuronal oscillators expressing arginine vasopressin (AVP), which are insensitive to external light stimulation [25]. Although the SCN expresses a variety of different neuropeptides, gamma-aminobutyric acid (GABA) is expressed in almost all the SCN neuronal oscillators [26]. Recent research has revealed that the traditionally “light-insensitive” DM neuronal oscillators actually possess a certain light-response ability, but their sensitivity is significantly lower than that of the VL neuronal oscillators [27]. This discovery revised the previous understandings of the SCN light-signal processing mechanism and offered a new research direction for a deeper comprehension of the light regulation of circadian rhythms.
The influence of external light stimulation on the behavioral rhythms of mammals involves the regulation of the collective behavior or function of the SCN neuronal oscillators. When exposed to a 22 h artificial light–dark cycle, a desynchronization (dissociation) phenomenon occurs between the VL and DM subgroups within each nucleus of the SCN [28]. In other words, behavioral activity exhibits two periodic components, one of which is approximately 22 h and the other is approximately 24 h [28,29]. Research has found that the 22 h periodic component was controlled by the VL subgroup, while the 24 h periodic component was controlled by the DM subgroup [30]. In jet lag experiments (the light–dark cycle was advanced or delayed), the VL subgroup immediately synchronized to the new environmental phase (time), whereas the DM subgroup initially retained the previous environmental phase and gradually transitioned to the VL subgroup phase [31,32,33]. Therefore, during the process of jet lag recovery, the VL and the DM subgroups lose synchronization and the rhythmic amplitude of the SCN network decreases. These findings also support that the VL subgroup is sensitive to the light information, while the DM subgroup is not, and the coupling between the VL and the DM subgroups is asymmetric, with the VL subgroup dominating over the DM subgroup [21]. In addition, experimental studies have demonstrated that alternating light–dark waveforms significantly affect the behavioral rhythms of mammals [16,34,35]. Prolonged exposure to the light during the nighttime can disrupt the circadian rhythms of organisms [36]. Under a longer photoperiod, the synchronization of individual neuronal oscillators was poor, but the amplitude was not less than that under a shorter photoperiod [32]. In addition, the extension of the photoperiod led to the entrainment range of the SCN first increasing and then decreasing [37]. Therefore, for both diurnal and nocturnal animals, appropriate light intensity and duration are crucial, as they not only reduce the occurrence of related diseases but also enhance the animals’ adaptability to external environment changes [38]. These findings provided important theoretical evidence for understanding the regulatory mechanisms of the light environment on biological rhythms.
In addition to the common light–dark cycle, constant light, as a special environmental condition, exerts complex and significant effects on the behavioral rhythms of mammals. When exposed to continuous light conditions, the behavioral activity of mammals may exhibit two antiphasic oscillatory components with 24 h periodicity, and the phase difference is stable at 12 h [39,40]. This phenomenon is referred to as “splitting”. Further experimental findings have shown that these two antiphasic components are regulated by the left and the right nuclei in the SCN [41]. The left and the right nuclei in the SCN contribute equally to the regulation of mammalian behavioral rhythms, suggesting the possibility of symmetric coupling between these two nuclei [42]. However, the exact coupling mechanism between the left and right SCN is not yet entirely clear. In addition, experimental studies have demonstrated that under constant light conditions, wild-type mice exhibit arrhythmic behavior and low-amplitude rhythmic activities with prolonged periods [40,43,44]. This phenomenon suggests that constant light may weaken the behavioral rhythms of mammals. In contrast, Hughes et al. found in VIP-VPAC2-signal-deficient mice that prolonged exposure to constant light could improve the synchrony between the SCN neuronal oscillators, indicating that constant light may have a potential role in enhancing rhythmic synchronization under certain specific conditions [45]. These findings provide an important theoretical basis for understanding the bidirectional regulation of biological rhythms by constant light.
The collective behavior of the SCN is not only influenced by the properties of individual neuronal oscillators, but also by the network topology structure, thereby affecting the functions of the SCN. The SCN neuronal oscillators form a complex network structure through synaptic connections and gap coupling, which determines the synchronization efficiency of rhythm signals, phase coordination, and resistance to external disturbances [23]. Under pathological conditions or environmental disturbances (such as shift work or a cross-time-zone flight), abnormal alterations in the network topology (such as connection loss or decreased coupling strength) can significantly reduce the anti-interference ability of the SCN, leading to rhythm desynchronization, amplitude attenuation, and even complete loss of rhythm. In addition, the SCN network is not an all-to-all network. Abel et al., through mutual information analysis on SCN slice data containing approximately 400 neuronal oscillators, found that the SCN connectivity network exhibits Newman–Watts (NW) small-world network characteristics, where the node degree follows an exponential distribution and has a shorter average path length and a larger average clustering coefficient [46]. Further research revealed the presence of the hub nodes in this NW network, which are primarily distributed in the central core region of the SCN, while the connections in the surrounding shell region are relatively sparse. This suggests that the network may also possess Barabási–Albert (BA) scale-free network properties. However, the network constructed by this method is an undirected network. To explore the directed network structure of the SCN, McBride et al., based on information theory methods, discovered that the in-degree distribution of the SCN neuronal oscillators is relatively uniform, but that there are significant differences in out-degree, with nodes having higher out-degrees primarily concentrated in the core region [47]. Gu et al. further analyzed the experimental data based on the transfer entropy method and found that the SCN slice network has BA network properties, where the node degree satisfies the power-law distribution and has a short average path length [48]. In addition, they measured the synchronization degree and the disassortativity coefficient of the network structure and found that the SCN network exhibited disassortativity in both the in-degree and the out-degree of the nodes. This disassortative network structure facilitates synchronization between the SCN neuronal oscillators, which means that more heterogeneous coupling in the SCN network is crucial for the normal function of the SCN. Research from Vasalou et al. revealed that the SCN has the topological structure characteristics of the NW network, indicating significant heterogeneity in the degree distribution of the neuronal oscillators [49]. However, compared to the regular network, this NW network requires fewer connections to achieve the same functionality as the SCN network, which is consistent with the research results of Gu et al. Therefore, an in-depth analysis of the network topology structure of the SCN not only helps to reveal the dynamical mechanisms of the emergence of collective rhythms in the biological clock system but also provides a theoretical basis for understanding the pathogenesis of circadian rhythm disorders and developing therapeutic strategies targeted at network regulation.
Unfortunately, due to the limitations of the current experimental techniques, the exact network structure of the SCN remains difficult to precisely resolve. Therefore, some studies have resorted to the numerical simulations based on circadian clock network models to examine the impact of the different possible SCN network structures on function [50,51,52]. In the mathematical models describing the SCN neuronal network, three representative models are mainly applied, namely the Goodwin model, the Kuramoto model, and the Poincaré model [15]. The Goodwin model precisely characterizes the transcription–translation oscillation process of core clock genes at the molecular level through negative feedback mechanisms in gene regulatory networks, providing a theoretical basis for understanding the generation mechanism of the intrinsic rhythm in individual neurons [23]. The Kuramoto model, from the perspective of population dynamics, reveals the key characteristic of how the SCN neuronal clusters achieve rhythm synchronization through synaptic connections through the coupling phase oscillators [53]. The Poincaré model adopts the phase space analysis method to characterize both the amplitude and phase features of neuronal oscillations through the dynamical changes of two state variables, thereby providing a more comprehensive theoretical framework for studying the overall dynamic behavior of SCN networks [15]. Researchers have adopted the appropriate models for analysis based on specific modeling requirements, and these complementary modeling methods provide systematic research tools for understanding the complex working mechanisms of the SCN biological clock networks.
In this review, we revisited the relationship between the network structure and functions of the biological clock under the light stimulation based on mathematical models. The remainder of this review was organized as follows. In Section 2, mathematical models commonly applied in the circadian rhythm field to describe the SCN network were introduced. Based on mathematical models, the influence of the SCN network structure on biological clock functions under light stimulation was reviewed in Section 3. Finally, discussions and conclusions were presented in Section 4 and Section 5.
2. Model Comparison
In the field of circadian rhythms, the network composed of SCN neuronal oscillators is typically described by the Goodwin model, the Kuramoto model, and the Poincaré model [15]. The Goodwin model is the simplest biophysical model, in which a single SCN neuronal oscillator is described by a genetic negative feedback loop of three variables capable of capturing the molecular mechanisms of transcriptional regulation [23]. The Kuramoto model is a simple model that simulates a neuronal oscillator with just one variable and is based on the phase information of the neuronal oscillator, with emphasis on the interactions between the different neuronal oscillators [53]. The Poincaré model is an abstract model that describes the oscillatory behavior of neuronal oscillators. It adopts a more general description of neuronal oscillators [15]. A neuronal oscillator is described by two variables and takes into account some basic characteristics required by the neuronal oscillator, including phase, period, and amplitude information.
2.1. Goodwin Model
The Goodwin model has been widely applied to simulate the circadian rhythm behavior of the SCN neuronal oscillators [23]. The networked Goodwin model describes how multiple SCN neuronal oscillators interact to form a synchronized circadian rhythm network, and the mathematical expression can be represented as
where the subscript i denotes the i-th neuronal oscillator in the SCN. Each individual SCN neuronal oscillator i is characterized by the four variables, namely the mRNA of the circadian clock gene X, the concentration of the circadian clock protein Y, the transcription inhibitor Z, and the concentration of the neurotransmitter V. The parameter N represents the total number of neuronal oscillators in the SCN. The N neuronal oscillators are coupled through the local or the global mean field mediated by the neurotransmitters, such as the VIP.
The network structure was described by an adjacency matrix E, where the elements were and i,j = 1, 2, …, N. If = 1, it indicates that there is a direct connection from the j-th neuronal oscillator to the i-th neuronal oscillator; if = 0, it indicates that there is no direct connection from the j-th neuronal oscillator to the i-th neuronal oscillator. In the SCN network, it was assumed that the neuronal oscillators can be connected to themselves through autocrine activation, i.e., = 1, where i = 1, 2, …, N [46]. The degree of the node i was defined as , i.e., the number of neighbors of the node i. D is the average node degree of the SCN network, i.e., . The parameter g is the coupling strength between the neuronal oscillators, denoting the sensitivity of the neuronal oscillator i to the mean field .
The light-sensitive term represents the sensitivity of the SCN neuronal oscillator i to the light information from the retinal hypothalamic tract. The parameter varies among the neuronal oscillators in the different subgroups. It was generally assumed that only the neuronal oscillators located in the VL subgroup of the SCN were sensitive to the light information, while the neuronal oscillators located in the DM subgroup of the SCN were not sensitive to the light information. Therefore, the light-sensitive term can be expressed as
where the parameter p represents the proportion of the number of light-sensitive neuronal oscillators to the total number of SCN neuronal oscillators. The parameter is the light intensity, and the parameter T corresponds to the period of the external light–dark cycle.
The values of other parameters were set as follows [54]: nM/h, nM, , nM/h, nM, /h, nM/h, nM, /h, nM/h, h, /h, nM/h, nM, nM/h, and nM.
2.2. Kuramoto Model
The classical Kuramoto model provides a mathematical framework for describing the SCN network, which contains both the intrinsic periods and the actual phases of the neuronal oscillators [53]. The expression is as follows:
where the variable represents the relative phase of the i-th neuronal oscillator. The parameters , g, and N represent the intrinsic period of the neuronal oscillators, the coupling strength between the neuronal oscillators, and the number of the SCN neuronal oscillators, respectively. The parameter is the external light input and depends on the region where the neuronal oscillator i is located. If the neuronal oscillator i was located in the VL subgroup, i.e., , it was assumed that the parameter ; if the neuronal oscillator i was located in the DM subgroup, i.e., , it was assumed that the parameter . The parameter is the light intensity, T is the external light–dark period, t is the time, and p is the proportion of the number of SCN neuronal oscillators sensitive to the light information to the total number of SCN neuronal oscillators.
The classical Kuramoto model can be further extended to the higher-order Kuramoto model to simulate the SCN network composed of the coupled neuronal oscillators [55]. The generalized model is formulated as follows:
where the subscript . The parameters and represent the first-order coupling strength and the second-order coupling strength, respectively. The network structure was described by the adjacency matrices E and C, where the elements were and , respectively, and i, j, l = 1, 2, …, N [56]. If the neuronal oscillators i and j were connected by a link, then = 1; otherwise, = 0. If the neuronal oscillators i, j, and l belonged to the common 2-simplex, then = 1; otherwise, = 0. For each neuronal oscillator i, we represented the 1-simplex degree and the 2-simplex degree as the number of the different 1-simplexes and 2-simplexes to which the neuronal oscillator i belongs, respectively, where and are the degree of the average 1-simplex and 2-simplex of the entire network.
2.3. Poincaré Model
The Poincaré model consisting of N neuronal oscillators can be applied to simulate the SCN networks exposed to external light–dark cycles [57]. In the Poincaré model, the dynamic characteristics of each neuronal oscillator are characterized by the two state variables, x and y, which encode both the amplitude and the phase information. The coupling of all the neuronal oscillators is mediated through the local or the global mean field , thus forming a heterogeneous or an all-to-all network. The networked Poincaré model is formally described as follows:
where the subscript i denotes the i-th neuronal oscillator. The parameters , A, , and N represent the amplitude relaxation rate, the intrinsic amplitude, the intrinsic period, and the number of the neuronal oscillators in the SCN, respectively. The parameter is the local or global mean field of and determined by the average value of the of all the neighbors of the neuronal oscillator i. The adjacency matrix describes the topology of the SCN network.
The parameter is the actual amplitude of the i-th SCN neuronal oscillator, and the expression is
The coupling term and the light input term are expressed as and , respectively, where g represents the coupling strength between the neuronal oscillators and represents the external light intensity or the light sensitivity of the neuronal oscillators. In the light–dark cycle, the key parameter depends on the region where the neuronal oscillator i is located. If the neuronal oscillator i was located in the VL subgroup, i.e., , then the parameter ; if the neuronal oscillator i was located in the DM subgroup, i.e., , then the parameter . Here, the parameter represents the frequency of the external light–dark cycles, t represents the external time, and p represents the proportion of the number of neuronal oscillators in the SCN that can directly receive the light information to the total number of neuronal oscillators in the SCN. Under constant light conditions, the light term is given by the parameter , where is the constant light intensity and L is the sensitivity of the SCN neuronal oscillators to the light information.
2.4. Simulation Details
The fourth-order Runge–Kutta method was adopted for numerical simulation with a time step of 0.01 h. To eliminate transient effects, the initial 1,000,000 time steps were discarded and the subsequent 200,000 time steps were retained for analysis. The initial values of the variables X, Y, Z, V, x, and y were randomly selected from a uniform distribution between 0 and 1, while the initial phases were drawn from a uniform distribution of 0 to . The number of neuronal oscillators in the numerical simulations was taken as N = 200.
The circadian rhythm synchronization of the SCN network can be quantitatively characterized by the synchronization order parameter R of the neuronal oscillators [51,58]. Specifically, the temporal variation of the synchronization order parameter R for the SCN neuronal oscillators can be expressed as
or
where represents the phase angle of the j-th neuronal oscillator. The notation denotes the average value varying over the time and is applied to eliminate the influence of instantaneous fluctuations. The value range of the synchronization order parameter R is strictly defined within the interval of [0, 1]. R = 0 indicates a completely desynchronized state, where the neuronal oscillators exhibit a random phase distribution, while R = 1 indicates a completely synchronized state where the phase between the neuronal oscillators is the same.
In the coupled network model, when an individual neuronal oscillator j reaches a stable oscillating state, the oscillation period can be quantitatively characterized by the time interval between the continuous peaks or troughs of the variable [59]. For an SCN network composed of N neuronal oscillators, we defined the output period T of the SCN network as the average period of the N neuronal oscillators, and the mathematical expression given by [21]
In mathematical models, if the period T of the external light–dark cycle and the entrainment period of the neuronal oscillators in the SCN network satisfy the following relationship [15]
where = 0.00001 h, then it is defined that the SCN is synchronized with or entrained by the external light–dark period. The entrainment range is typically represented by the LLE or the ULE, provided that the LLE and the ULE are symmetrical in the frequency domain relative to the FRP of the SCN. Unless otherwise specified, this review applies the LLE to denote the entrainment range of the SCN. A smaller value for the LLE indicates a larger entrainment range for the SCN, and vice versa.
3. Results
In this section, based on mathematical models, it was explored how SCN neuronal oscillators, under light stimulation, affect biological clock function within different network structures: the motif structure, the all-to-all network, the NW network, the BA network, and the higher-order interaction network.
3.1. Motif Structure
Previous studies have predominantly focused on the overall coupling between the left and the right nuclei within the SCN or the coupling between the VL subgroup and the DM subgroup in a single nucleus of the SCN [18,49]. However, the coupling between the different subgroups in the left and the right nuclei of the SCN is also very important. Currently, experiments have not yet definitively revealed the specific connection structure among the four subgroups of the SCN [60]. By simultaneously considering all possible connections among the four subgroups within the SCN, the seven typical motif connection structures were proposed based on the mathematical models [50]. The connection structure of each motif is shown in Figure 1, where each circle represents a subgroup, i.e., the VL subgroup or the DM subgroup in a single nucleus of the SCN. The black lines denote the connections between the different subgroups. Note that only the VL subgroups receive the external light signal. To distinguish the bilateral structure of the SCN, the subscripts R and L represent the right and the left nuclei of the SCN, respectively. Through analyzing their differential impacts on the entrainment range, the potential coupling mechanisms among the four subgroups within the SCN were revealed. This research provided a new theoretical framework for understanding the relationship between the topological structure and the functional regulation of the SCN network.
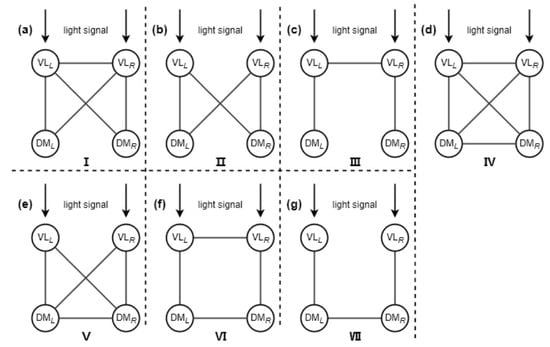
Figure 1.
Schematic diagram of the motif structures of the four subgroups within the SCN. This figure is modified from Ref. [50].
By considering the four distinct neuronal subgroups in the left and the right nuclei of the SCN as an interconnected network structure, the relationship between the seven motif connection structures and the entrainment range of the SCN was theoretically investigated through the Poincaré model (Figure 2). Both the numerical simulation results and the theoretical analysis indicate that the SCN exhibits a larger entrainment range (when approaches 0.2, the smallest value of the LLE is approximately 20.67 h) in the motif structures that include connections between the two VL subgroups across the left and the right nuclei within the SCN. In contrast, the SCN displays a smaller entrainment range in the motif structures that involve connections between the two DM subgroups across the left and the right nuclei within the SCN. This discovery suggests that the SCN exhibits remarkable plasticity in inter-nuclear connectivity, dynamically modulating the coupling strengths between the left and the right nuclei (either enhancing them or weakening them) to adapt to changes in the external environment, such as jet lag adjustment after traveling across time zones, adapting to changes in the light–dark cycle, and coping with the functional alterations in the SCN that occur with aging.
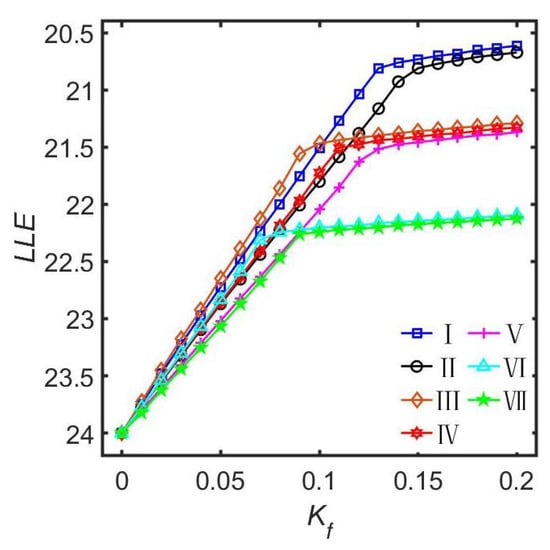
Figure 2.
Comparison diagram of the relationship between the LLE and the light intensity for seven motif structures. The coupling strength g is 0.10. This figure is modified from Ref. [50].
3.2. All-to-All Network
In theoretical modeling research, in order to simplify the analysis process, the SCN network is usually assumed to be an all-to-all network approximated by the global mean field [13,17,18]. In this model, each neuronal oscillator is coupled with all other neuronal oscillators, while also allowing for autocrine (self-activating) connections to itself [46]. Therefore, for an all-to-all network comprising N neuronal oscillators, the degree of each neuronal oscillator is equal to the total number of neuronal oscillators N. Mathematical models based on the all-to-all network assumption have successfully unveiled the impact of various key factors on biological clock functions under light stimulation, such as the heterogeneity in light sensitivity between the SCN subgroups, the different light waveforms, the network dynamics differences under strong and weak coupling states, and the adaptive coupling regulation.
3.2.1. Light Sensitivity Heterogeneity
It has been reported that there are significant differences in the sensitivity to light stimulation between the neuronal oscillators located in the VL subgroup and the DM subgroup of the SCN [25,26,27,61]. Zhu et al. systematically investigated the influence of the light sensitivity difference between the two subgroups of the neuronal oscillators in the SCN on the entrainment range based on the Poincaré model [13]. The results of the numerical simulations indicated that the relationship between the entrainment range and the sensitivity difference was affected by the coupling strength between the two subgroups of the neuronal oscillators. When the coupling strength is significantly smaller than the light intensity, the relationship between the two subgroups is parabolic and the maximum entrainment range is obtained when the two subgroups of neuronal oscillators have the same light sensitivity. When the coupling strength is significantly larger than the light intensity, the relationship between the two subgroups shows a monotonic trend and the maximum entrainment range occurs when the light sensitivity difference between the two subgroups of the neuronal oscillators is the largest. Furthermore, Zhou et al. investigated how this light-sensitive heterogeneity affects the FRP of the biological clock under constant light conditions based on the Poincaré model [62]. The results of the numerical simulations indicated that the FRP of the SCN network is prolonged with the increase in the heterogeneity degree of the light sensitivity of the neuronal subgroups. In addition, they discovered the existence of a critical threshold for the heterogeneity degree. If the heterogeneity degree is smaller than this threshold, the periods of the neuronal oscillators in both the VL subgroup and the DM subgroup remain consistent, which means that the SCN maintains synchronized. The higher the heterogeneity degree is, the larger value of the FRP is. If the heterogeneity degree is larger than this threshold, the periods of the neuronal oscillators between the two subgroups are different. The period of the neuronal oscillators located in the VL subgroup prolongs with the increase in the heterogeneity degree, while the period of the neuronal oscillators located in the DM subgroup shortens with the increase in the heterogeneity degree, leading to a loss of synchronization between the two subgroups. The discovery of this critical threshold may explain the experimental phenomenon observed under the constant light conditions, namely that some animals are able to maintain stable circadian rhythms while others exhibit a complete lose of rhythmicity [63].
3.2.2. The Light Waveforms
Experimental studies have demonstrated that alternating the light–dark waveforms significantly affects the behavioral rhythms of mammals [16,34,35]. In terms of research on the theoretical mechanism, Zheng et al. analyzed the effects of different light–dark schemes on the entrainment capacity of the SCN based on the Poincaré model (Figure 3) [37]. The numerical simulation results revealed that when the maximum light intensity, the minimum light intensity, and the total light exposure per cycle are all the same, the neuronal oscillators receiving more light during the daytime exhibit the largest entrainment range (Figure 4). In addition, extension of the photoperiod leads to a smaller entrainment range for the SCN (Figure 5). Research has shown that under long photoperiod (LP) conditions, the phases of the SCN neuronal oscillators are more dispersed and their synchronization degree is significantly lower than that under the short photoperiod (SP) conditions [64,65]. This smaller synchronization degree leads to a decrease in the entrainment capacity of the SCN under LP conditions [66]. Gu et al. provided a possible explanation for this phenomenon based on the Kuramoto model [67]. They proposed that quantifying the ratio of the number of directed connections between the VL and the DM neuronal oscillators to the total number of connections can characterize the directed network structure under the different photoperiods, i.e., the asymmetric coupling between the VL subgroup and the DM subgroup. Specifically, the ratio significantly increases under SP conditions and decreases under LP conditions. Model analysis further revealed that under the SP conditions, the increase in this ratio is positively correlated with the improvement in the synchronization degree between the neuronal oscillators.
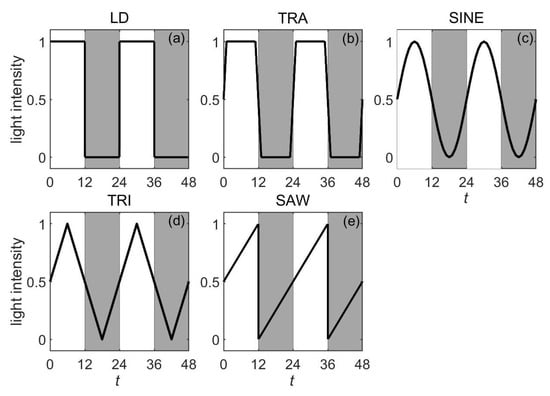
Figure 3.
Five light–dark schemes within a 24 h cycle. (a) LD. (b) TRA. (c) SINE. (d) TRI. (e) SAW. For simplicity, the LD, the TRA, the SINE, the TRI, and the SAW represent the rectangular scheme, the trapezoidal scheme, the sinusoidal scheme, the triangular scheme, and the sawtooth scheme, respectively. Note that the white background corresponds to the daytime and the grey background corresponds to the nighttime. This figure is modified from Ref. [37].
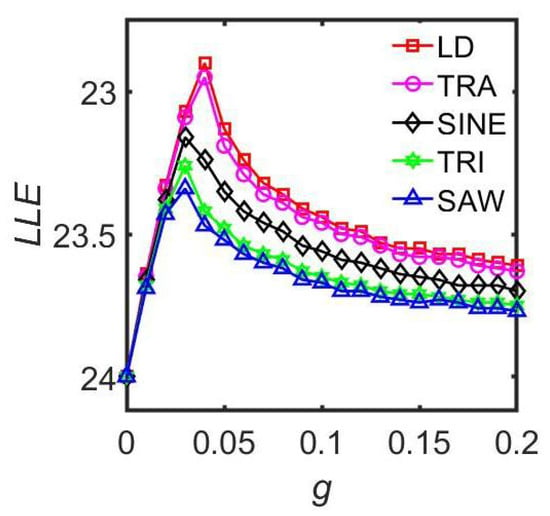
Figure 4.
Comparison of the relationship between the LLE and the coupling strength g among the five light–dark schemes. The light intensity is 0.10. This figure is modified from Ref. [37].
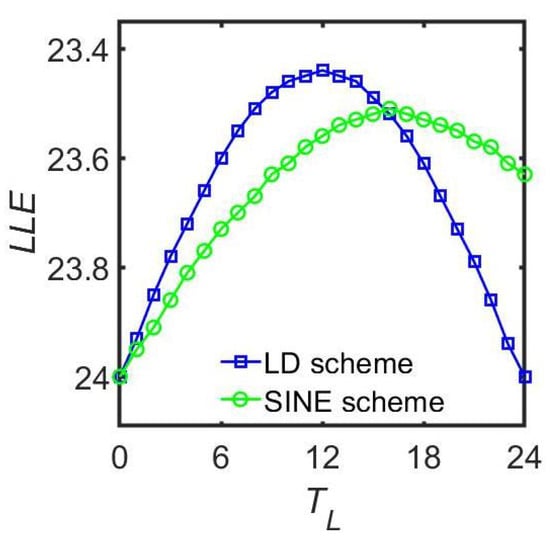
Figure 5.
Comparison in the relationship between the maximum LLE and the different photoperiods in the rectangular scheme and the sinusoidal scheme. The coupling strength g is 0.10, and the light intensity is 0.10. This figure is modified from Ref. [37].
3.2.3. The Coupling Strength
There are significant discrepancies in the existing research on the impact of the coupling strength on the entrainment range of the biological rhythms. Some studies suggest that enhancing coupling reduces the entrainment range, while others argue that it increases the entrainment range [15,32,68]. To clarify this controversy, Gu et al. combined the Poincaré model and the Goodwin model to test the entrainment characteristics of neuronal oscillators with different proportions responding to external stimuli [25]. When all the neuronal oscillators received the external input, a monotonic relationship was observed between the coupling strength and the entrainment range. When only a subset of the neuronal oscillators responded to the light stimulation, they found that when the coupling was weak, the entrainment range of the SCN was positively correlated with the coupling strength. However, when the coupling strength was strong and larger than a critical value, the entrainment range was negatively correlated with the coupling strength. To investigate the mechanisms for enhancing or weakening mammalian behavioral rhythms under constant light, Xu et al. conducted systematic research based on the Poincaré model [58]. Through numerical simulation analysis, it was found that constant light can significantly enhance the synchronization between SCN neuronal oscillators under weak coupling conditions, whereas it weakens the synchronization under strong coupling conditions. In addition, higher light intensity led to prolonged rhythmic periods and decreased amplitudes.
3.2.4. The Adaptive Coupling
Existing research has predominantly focused on the constant coupling strength between the SCN neuronal oscillators [13,37,50]. However, the actual SCN network has been found to be a plastic neural network in which the coupling strength between the neuronal oscillators is highly dynamic [69,70]. Based on an improved Kuramoto model, Zheng et al. investigated the impact of adaptive coupling between the SCN neuronal oscillators on the entrainment range of the master clock network, providing a possible theoretical explanation for neurotransmitter-mediated coupling plasticity (Figure 6) [71]. The numerical simulation results demonstrated that compared with a network with constant coupling strength, an SCN network with adaptive coupling strength can entrain to a wider range of the external light–dark conditions under a strong light exposure. This implies that the adaptive coupling mechanism significantly enhances the flexibility of the circadian rhythm system in responding to the external environment changes. Therefore, this plasticity regulation may offer crucial adaptive advantages for organisms to cope with challenges such as jet lag and shift work.
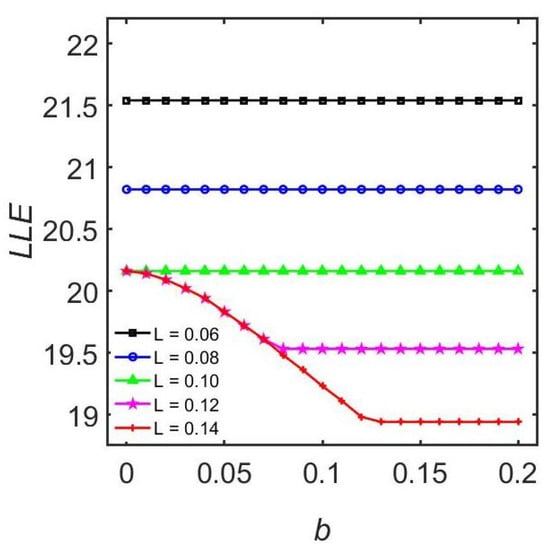
Figure 6.
The influence of adaptation strength b on entrainment range. The fixed coupling strength a is 0.10. This figure is modified from Ref. [71].
3.3. Small-World Network
In traditional theoretical research, the SCN is usually idealized as an all-to-all network to simplify model complexity. Recently, experiments have found that the network structure of the SCN exhibits the characteristics of a NW network, although the specific network topology details remain to be elucidated. Some theoretical studies have explored the impact of the NW network structure on SCN function and found that it can effectively improve the synchronization degree of the neuronal oscillators and the amplitude of the rhythmic oscillations [49,72,73,74].
Vasalou et al. constructed a multicellular SCN neuronal oscillator model based on an NW network architecture to investigate the influence of the network topology structure on circadian rhythm behavior [49]. In this model, the local connections between the VIP generation neuronal oscillator and its four nearest neighbors were enhanced by introducing random long-range couplings, and this design significantly shortened the average path length between the neuronal oscillators. The research findings indicated that the NW network structure achieved the optimal balance between the number of connections and the amplitude and the synchronization control of the circadian rhythm, thereby effectively enhancing the synchronization capability of the SCN network. Furthermore, by systematically adjusting the value of the network connection probability p and setting three typical lighting conditions (constant darkness (DD), constant light (LL), and 12 h of light followed by 12 h of darkness (LD)), they conducted an in-depth analysis of the synergistic effects of network topology and lighting patterns on synchronization behavior. Under the DD conditions, the cellular population could achieve a high synchronization degree when the value of p was within or above the characteristic range of the NW network. Regardless of the value of p applied, the LD lighting period generated a relatively large synchronization capability. In contrast, under the LL conditions, regardless of the value of p applied, the cellular population failed to establish a synchronization rhythm. This behavior has been supported by experiments, which have shown that continuous exposure to a strong light can disrupt the circadian rhythm synchronization of the SCN population [75]. Their research revealed that the primary advantage of the NW network is its ability to effectively promote rhythmic synchronization among sparsely connected cellular populations even in the absence of light-signal input.
Hafner et al. systematically investigated the influence of SCN network topology structures on synchronization and entrainment characteristics by constructing a multi-neuronal-oscillator model of the SCN [72]. The research findings revealed that compared with randomly connected networks and locally connected networks, BA networks could generate oscillatory rhythms with larger amplitudes. It was particularly worth noting that the BA networks with NW characteristics demonstrated stronger phase adjustment capabilities and could adapt more quickly to phase delays or phase advances in the light–dark cycle (such as jet lag). The research uncovered an important phenomenon where a certain degree of the cellular heterogeneity not only does not impair the synchronization performance of the network but could actually promote the resynchronization process after jet lag. When simulating the two functional regions of the SCN, they coupled the networks with different topological structures and observed that the coupled network structures could effectively filter out pulse-like disturbances during the process of light entrainment. These findings suggested that the complex heterogeneous structure of the SCN not only reduced the sensitivity of the network to short-term entrainment perturbations, but also enhanced its adaptability to long-term environmental changes.
Bodenstein et al. delved into the pivotal role of the network’s topological structure in circadian rhythm regulation by establishing a mathematical model of the structural dynamics of the SCN neuronal oscillators [73]. The study specifically focused on the effects of the long-range connections between neuronal oscillators with NW network characteristics. The numerical simulation results demonstrated a significant correlation between the proportion of long-range connections between the cells and the seasonal variations in daily rhythms. The proportion of long-range connections between the cells could alter the phase distribution pattern of the neuronal populations, thereby modulating the duration of the behavioral activity period. Specifically, the higher density of long-range connections in winter promoted better synchronization of the neuronal oscillators, resulting in a narrow activity phase, while the lower density of long-range connections in summer led to a significant broadening of the activity phase. This model successfully explained the experimental phenomenon that the biological clock is more sensitive to the light-induced phase shift in winter, attributing this to the dynamic properties of highly synchronized neural networks. This research provided a theoretical explanation for the regulatory mechanism underlying seasonal dynamic changes in the biological clock from the perspective of network structure plasticity, indicating that the SCN achieves adaptive responses to environmental photoperiod changes by adjusting its network topology structure.
To investigate the effects of molecular noise and intercellular connectivity on the synchronization of the circadian rhythm network of the SCN, Šimonka et al. established a stochastic multicellular model of the SCN neuronal oscillators [74]. The circadian rhythm pacemaker was modeled as a realistic NW neuronal oscillator network and was found to exhibit remarkable robustness against relatively high levels of intrinsic fluctuation. The research findings indicated that even under a very high level of stochasticity, the SCN neuronal oscillator network could be well synchronized with the light–dark cycle, confirming that intercellular coupling is a crucial factor in ensuring the coherence of rhythmic oscillations. Furthermore, their results revealed that the neuronal activity patterns exhibited a distinct local clustering phenomenon and that this spatial pattern was more pronounced under short photoperiod conditions than under long photoperiod conditions. The local synchronization behavior was also influenced by the topological structure of the SCN network.
3.4. Scale-Free Network
Zhou et al. investigated the effect of SCN network topology on the FRP for the selected values of the average node degree D when exposed to constant light conditions through the Poincaré model [51]. Four typical network structures were considered, including the nearest-neighbor coupled network, the NW network, the Erdős–Rényi (ER) random network, and the BA network. The research results have helped researchers to understand how the network structure of the SCN influences the FRP. The results indicated that the FRP in the BA network is the longest among the four networks, owing to the fact that the BA network has the most heterogeneous structural characteristics compared to the other three networks. This result was independent of the average node degree D of the SCN network.
Furthermore, Zhou et al. examined the relationship between the synchronization degree R and the average node degree D of each network. When D ranges from 10 to 50, the synchronization degree R of the BA network is approximately 0.9, while for the nearest-neighbor network, the NW network, or the ER network, R is approximately 1. Therefore, the neuronal oscillations are highly synchronized and the variation in the average node degree of the SCN network does not affect the primary finding that the FRP in the BA network is longer than that in the other three network structures. According to Aschoff’s rule, different animals exhibit distinct FRPs under constant light conditions [76]. A possible explanation proposed by Zhou et al.’s research is that animals with longer FRPs may possess SCN network structures containing more hub nodes (e.g., the BA network), leading to higher sensitivity to light stimulation. In contrast, animals with the shorter FRPs may have SCN network structures with fewer or even no hub nodes (e.g., the nearest-neighbor network), which results in relatively lower sensitivity to light stimulation. This hypothesis offered a new network topological perspective for explaining the differences in FRPs among different species.
3.5. Higher-Order Interaction Network
Previous studies have predominantly focused on the first-order interactions between the coupled neuronal oscillators in the SCN network. However, the presence of higher-order interactions in the network has garnered significant attention. Although no experiments have yet definitively demonstrated the existence of higher-order interactions in the SCN network, an approach based on the model was adopted in advance of the experimental verification to explore the influence of the presence of the second-order coupling strength in the SCN network on its entrainment range [52]. This investigation aimed to uncover dynamical phenomena that cannot emerge under the first-order coupling.
Based on the improved Kuramoto model, the influence of the second-order coupling strength between SCN neuronal oscillators on the relationship between the entrainment range and the first-order coupling strength was systematically studied (Figure 7). Both the numerical simulations and the theoretical analyses showed that when all the neuronal oscillators in the SCN receive the external light information, the introduction of second-order coupling strength has no effect on the entrainment range of the SCN. However, in the case where only a portion of the neuronal oscillators in the SCN were sensitive to the external light information, if the value of the first-order coupling strength was small, introducing second-order coupling strength could expand the entrainment range of the SCN. Furthermore, when the proportion of the number of neuronal oscillators receiving the external light to the total number of neuronal oscillators in the SCN was small, or the light intensity was low, and the value of the second-order coupling strength between the neuronal oscillators was relatively large, the entrainment range depended on the different initial values of the SCN neuronal oscillator phase. This dependence was not observed in previous studies that focused exclusively on the first-order coupling strength between the coupled neuronal oscillators.
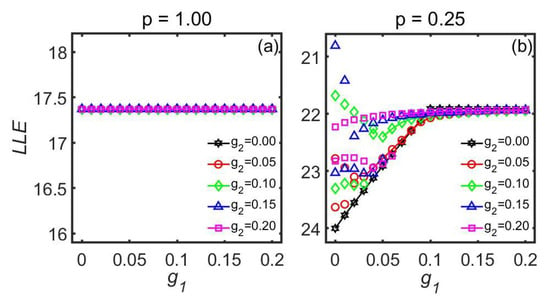
Figure 7.
The influence of different second-order coupling strengths on the relationship between the LLE and first-order coupling strength . (a) p = 1.00. (b) p = 0.25. The light intensity is 0.10. The parameter p represents the proportion of the number of light-sensitive neuronal oscillators to the total number of SCN neuronal oscillators. This figure is modified from Ref. [52].
4. Critical Discussion
As the master biological clock in mammals, the SCN achieves two major functions through its unique network structures: one is to synchronize endogenous rhythms; the other is to entrain to the external light–dark cycle. Based on mathematical models, this review systematically explored the influence of the network structure of the SCN on its circadian rhythm functions under light stimulation. The theoretical research findings have shown that there is a close correlation between the SCN network structure and its functions.
In the field of circadian rhythms, two types of models are mainly used to study the functions of biological clocks, namely biophysical models (mechanism models) and general models (phenomenon models) [15]. These two models describe the characteristics of the SCN neuronal oscillators, internal coupling mechanisms, network structures, and the received external lighting conditions from different perspectives. Biophysical models (such as the Goodwin model) describe the internal dynamics of SCN neuronal oscillators through detailed molecular and cellular mechanisms, accurately reflecting molecular processes such as transcription–translation negative feedback loops [23]. General models (such as the Kuramoto and Poincaré models) adopt simplified mathematical frameworks to describe the oscillatory behavior of SCN neuronal oscillators, with a greater focus on the analysis of synchronization behavior at the network level. Among them, the Kuramoto model only considers the phase information of neuronal oscillators, which means the model has the highest computational efficiency but ignores amplitude changes, while the Poincaré model simultaneously characterizes the phase and amplitude characteristics through two variables, providing richer dynamical information while maintaining computational efficiency [15,53]. In this review, we selected the corresponding models for analysis based on the modeling requirements. For example, when studying the effects of higher-order interactions and adaptive coupling on the function of the biological clock, the Kuramoto model is the most suitable, as these phenomena are essentially phase dependent [52,71]. In Table 1, we systematically summarized the mathematical models applied to different network structures under light stimulation and marked the key references. Note that “NA” indicates that the corresponding model was not applied in the relevant literature. It should be noted that although there are differences in the mathematical expression and computational complexity among different models, we found through cross-validation that these models show a high degree of consistency in the key conclusions of this review.

Table 1.
The application of different network structures in different mathematical models under light stimulation.
This review reveals the significant correlation between the SCN network structure and its functions through mathematical models, but there are also several limitations that need attention. Firstly, the current understanding of the precise network structure of the SCN is limited, and the application of mathematical models only provides the possibility for exploring the potential network structures of the SCN. This simplified model assumption may affect the accuracy of network dynamics simulations. Secondly, the experimental validation is insufficient, and some network topology predictions (such as NW or BA network features) still require data support from experiments like neuroimaging, optogenetic network dissection, and high-resolution SCN connectomics. Future research may require a combination of experimental data to further validate and optimize the network models. Finally, the heterogeneity of neuronal oscillators and their interaction mechanisms with network structures also need to be taken into account.
These limitations highlight the direction for future research, such as developing network models that integrate multi-omics data, applying cutting-edge imaging techniques to analyze SCN connectomes, and establishing cross-species research paradigms to bridge the gap between theoretical modeling and empirical verification, thereby deepening the understanding of biological clock regulation mechanisms and providing new ideas for the treatment of related diseases.
5. Conclusions
This study systematically reveals the correlation mechanism between the SCN network structure and its core functions through mathematical models. Specifically, the model analysis revealed that the coupling between the subgroups of the light-sensitive neuronal oscillators in the left and the right nuclei of the SCN could significantly enhance the ability of the biological clock to entrain to external light signals. This discovery provided a new theoretical perspective for explaining how the SCN effectively achieves synchronization with the external environment. It is particularly notable that the theoretical research has suggested that the SCN may possess the characteristics of NW networks or BA networks and that these topological structures might serve as important structural foundations for the SCN to efficiently perform its biological clock functions. The model results also indicated that there might be a higher-order coupling mechanism within the SCN. This complex interaction not only enriches the network dynamics behavior but, more importantly, it can effectively expand the entrainment range of the biological clock.
These theoretical research findings not only deepen our understanding of the relationship between the network structure and functions of the SCN, but more importantly, offer a clear theoretical framework and verification direction for subsequent experimental research. Through the organic combination of mathematical models and experimental research, it is expected a more refined topological structure for the biological clock system will be revealed.
Author Contributions
Conceptualization, J.F., W.Z. and C.G.; methodology, J.F. and W.Z.; investigation, W.Z. and C.G.; writing—original draft preparation, W.Z.; writing—review and editing, J.F., W.Z. and C.G.; visualization, W.Z.; supervision, J.F. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 12275179) and the Natural Science Foundation of Shanghai (Grant No. 21ZR1443900).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SCN | suprachiasmatic nucleus |
| FRP | free-running period |
| LLE | lower limit of entrainment |
| ULE | upper limit of entrainment |
| VL | ventrolateral |
| DM | dorsomedial |
| VIP | vasoactive intestinal peptide |
| AVP | arginine vasopressin |
| GABA | gamma-aminobutyric acid |
| NW | Newman–Watts |
| BA | Barabási–Albert |
| LP | long photoperiod |
| SP | short photoperiod |
| DD | constant darkness |
| LL | constant light |
| ER | Erdős–Rényi |
References
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian rhythms disrupted by light at night and mistimed food intake alter hormonal rhythms and metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef]
- Evans, J.A.; Gorman, M.R. In Synch but Not in Step: Circadian Clock Circuits Regulating Plasticity in Daily Rhythms. Neuroscience 2016, 320, 259–280. [Google Scholar] [CrossRef]
- Finger, A.M.; Kramer, A. Mammalian circadian systems: Organization and modern life challenges. Acta Physiol. 2021, 231, e13548. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, Precision, and Near-24-Hour Period of the Human Circadian Pacemaker. Science 1999, 284, 2177. [Google Scholar] [CrossRef]
- Srivastava, M.; Varma, V.; Abhilash, L.; Sharma, V.K.; Sheeba, V. Circadian Clock Properties and Their Relationships as a Function of Free-Running Period in Drosophila melanogaster. J. Biol. Rhythm. 2019, 34, 231. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2021, 157, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Leise, T.L.; Castanon-Cervantes, O.; Davidson, A.J. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron 2013, 80, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.; Jagannath, A. Disrupted sleep and circadian rhythms in schizophrenia and their interaction with dopamine signaling. Front. Neurosci. 2020, 14, 636. [Google Scholar] [CrossRef]
- Gu, C.G.; Li, J.H.; Zhou, J.; Yang, H.J.; Wang, M. Strengthen the circadian rhythms by the mathematical model of the SCN. Eur. Phys. J. Spec. Top. 2022, 231, 827–832. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Isejima, H.; Matsuo, T. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 2003, 302, 1408–1412. [Google Scholar] [CrossRef]
- Daido, H. Why circadian rhythms are circadian: Competitive population dynamics of biological oscillators. Phys. Rev. Lett. 2001, 87, 048101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhou, J.; Jia, M.T.; Yang, H.J.; Gu, C.G. Entrainment range affected by the difference in sensitivity to light-information between two groups of SCN neurons. Chin. Phys. B 2020, 29, 068702. [Google Scholar] [CrossRef]
- Maywood, E.S.; Chesham, J.E.; Winsky-Sommerer, R.; Smyllie, N.J.; Hastings, M.H. Circadian chimeric mice reveal an interplay between the suprachiasmatic nucleus and local brain clocks in the control of sleep and memory. Front. Neurosci. 2021, 15, 639281. [Google Scholar] [PubMed]
- Abraham, U.; Granada, A.E.; Westermark, P.O.; Heine, M.; Kramer, A.; Herzel, H. Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 2010, 6, 438. [Google Scholar] [CrossRef]
- Usui, S.; Takahashi, Y.; Okazaki, T. Range of entrainment of rat circadian rhythms to sinusoidal light-intensity cycles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 278, R1148–R1156. [Google Scholar] [CrossRef]
- Li, Y.N.; Androulakis, I.P. Light entrainment of the SCN circadian clock and implications for personalized alterations of corticosterone rhythms in shift work and jet lag. Sci. Rep. 2021, 11, 17929. [Google Scholar] [CrossRef]
- Gu, C.G.; Yang, H.J.; Wang, M. Ratio between sensitive strength to light information and coupling strength affects entrainment range of suprachiasmatic nucleus. Commun. Theor. Phys. 2018, 70, 771–776. [Google Scholar] [CrossRef]
- Weinert, D.; Gubin, D. The impact of physical activity on the circadian system: Benefits for health, performance and wellbeing. Appl. Sci. 2022, 12, 9220. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.; Honma, S. Roles of neuropeptides, VIP and AVP, in the mammalian central circadian clock. Front. Neurosci. 2021, 15, 650154. [Google Scholar]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Schlaeger, L.; Olejniczak, I.; Lehmann, M.; Schmidt, C.X.; Astiz, M.; Oster, H.; Pilorz, V. Estrogen-mediated coupling via gap junctions in the suprachiasmatic nucleus. Eur. J. Neurosci. 2024, 59, 1723–1742. [Google Scholar] [CrossRef] [PubMed]
- Gonze, D.; Bernard, S.; Waltermann, C.; Kramer, A.; Herzel, H. Spontaneous synchronization of coupled circadian oscillators. Biophys. J. 2005, 89, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Rohling, J.H.T.; Vanderleest, H.T.; Michel, S.; Vansteensel, M.J.; Meijer, J.H. Phase resetting of the mammalian circadian clock relies on a rapid shift of a small population of pacemaker neurons. PLoS ONE 2011, 6, e25437. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.G.; Ramkisoensing, A.; Liu, Z.H.; Meijer, J.H.; Rohling, J.H.T. The proportion of light-responsive neurons determines the limit cycle properties of the suprachiasmatic nucleus. J. Biol. Rhythm. 2014, 29, 16–27. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Cambras, T.; Díez-Noguera, A.; Campuzano, A.; Oda, G.A.; Yamazaki, S.; de la Iglesia, H.O. Coupling between subregional oscillators within the suprachiasmatic nucleus determines free-running period in the rat. J. Biol. Rhythm. 2022, 37, 620–630. [Google Scholar] [CrossRef]
- Myung, J.; Pauls, S.D. Encoding seasonal information in a two-oscillator model of the multi-oscillator circadian clock. Eur. J. Neurosci. 2018, 48, 2718–2727. [Google Scholar] [CrossRef]
- De la Iglesia, H.O.; Cambras, T.; Schwartz, W.J.; Díez-Noguera, A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr. Biol. 2004, 14, 796–800. [Google Scholar] [CrossRef]
- Tsuno, Y.; Peng, Y.B.; Horike, S.I.; Wang, M.; Matsui, A.; Yamagata, K.; Sugiyama, M.; Nakamura, T.J.; Daikoku, T.; Maejima, T. In vivo recording of suprachiasmatic nucleus dynamics reveals a dominant role of arginine vasopressin neurons in circadian pacesetting. PLoS Biol. 2023, 21, e3002281. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Wotus, C.; Liu, T.C.; Friesen, W.O.; Borjigin, J.; Oda, G.A.; de la Iglesia, H.O. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc. Natl. Acad. Sci. USA 2009, 106, 17540–17545. [Google Scholar] [CrossRef]
- Lee, H.S.; Nelms, J.L.; Nguyen, M.; Silver, R.; Lehman, M.N. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat. Neurosci. 2003, 6, 111–112. [Google Scholar] [CrossRef]
- VanderLeest, H.T.; Rohling, J.H.T.; Michel, S.; Meijer, J.H. Phase Shifting Capacity of the Circadian Pacemaker Determined by the SCN Neuronal Network Organization. PLoS ONE 2009, 4, e4976. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Ikegami, K.; Minami, Y.; Kanazawa, Y.; Koinuma, S.; Sujino, M.; Shigeyoshi, Y. Slow shift of dead zone after an abrupt shift of the light-dark cycle. Brain Res. 2019, 1714, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Boulos, Z.; Macchi, M.; Terman, M. Twilight transitions promote circadian entrainment to lengthening light-dark cycles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1996, 271, R813–R818. [Google Scholar] [CrossRef] [PubMed]
- Boulos, Z.; Macchi, M.M.; Terman, M. Twilights widen the range of photic entrainment in hamsters. J. Biol. Rhythm. 2002, 17, 353–363. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Zheng, W.X.; Gu, C.G.; Yang, H.J.; Rohling, J.H.T. A modeling approach shows the effects of different light-dark schemes on the entrainment ability of the suprachiasmaticnucleus. Nonlinear Dyn. 2023, 111, 12625–12638. [Google Scholar] [CrossRef]
- Madahi, P.G.; Ivan, O.; Adriana, B.; Diana, O.; Carolina, E. Constant light during lactation programs circadian and metabolic systems. Chronobiol. Int. 2018, 35, 1153–1167. [Google Scholar] [CrossRef]
- De la Iglesia, H.O.; Meyer, J.; Carpino, A.; Schwartz, W.J. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 2000, 290, 799–801. [Google Scholar] [CrossRef]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005, 8, 267–269. [Google Scholar] [CrossRef]
- Leise, T.L.; Goldberg, A.; Michael, J.; Montoya, G.; Solow, S.; Molyneux, P.; Vetrivelan, R.; Harrington, M.E. Recurring circadian disruption alters circadian clock sensitivity to resetting. Eur. J. Neurosci. 2020, 51, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.G.; Li, J.H.; Zhou, J.; Yang, H.J.; Rohling, J.H.T. Network structure of the master clock is important for its primary function. Front. Physiol. 2021, 12, 678391. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 1976, 106, 223. [Google Scholar] [CrossRef]
- Moriya, T.; Yoshinobu, Y.; Kouzu, Y.; Katoh, A.; Gomi, H.; Ikeda, M.; Yoshioka, T.; Itohara, S.; Shibata, S. Involvement of glial fibrillary acidic protein (GFAP) expressed in astroglial cells in circadian rhythm under constant lighting conditions in mice. J. Neurosci. Res. 2000, 60, 212–218. [Google Scholar] [CrossRef]
- Hughes, A.T.L.; Croft, C.L.; Samuels, R.E.; Takumi, T.; Piggins, H.D. Constant light enhances synchrony among circadian clock cells and promotes behavioral rhythms in VPAC2-signaling deficient mice. Sci. Rep. 2015, 5, 14044. [Google Scholar] [CrossRef]
- Abel, J.H.; Meeker, K.; Granados-Fuentes, D.; St John, P.C.; Wang, T.J.; Bales, B.B.; Doyle, F.J.; Herzog, E.D.; Petzold, L.R. Functional network inference ofthe suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2017, 113, 4512–4517. [Google Scholar] [CrossRef]
- McBride, D.; Petzold, L. Model-based inference of a directed network of circadian neurons. J. Biol. Rhythm. 2018, 33, 515–522. [Google Scholar] [CrossRef]
- Gu, C.G.; Gu, X.W.; Wang, P.; Ren, H.G.; Weng, T.F.; Yang, H.J.; Rohling, J.H.T. Disassortative network structure improves the synchronization between neurons in the suprachiasmatic nucleus. J. Biol. Rhythm. 2019, 34, 515–524. [Google Scholar] [CrossRef]
- Vasalou, C.; Herzog, E.D.; Henson, M.A. Small-world network models of intercellular coupling predict enhanced synchronization in the suprachiasmatic nucleus. J. Biol. Rhythm. 2009, 24, 243–254. [Google Scholar] [CrossRef]
- Zheng, W.X.; Gu, C.G.; Yang, H.J.; Rohling, J.H.T. Motif structure for the four subgroups within the suprachiasmatic nuclei affects its entrainment ability. Phys. Rev. E 2022, 105, 014314. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, C.G.; Song, Y.X.; Xu, Y. Free running period affected by network structures of suprachiasmatic nucleus neurons exposed to constant light. Chin. Phys. B 2023, 32, 098701. [Google Scholar] [CrossRef]
- Zheng, W.X.; Gu, C.G.; Xu, Y.; Yang, H.J. Entrainment range affected by the second-order interactions between coupled neuron oscillators in the suprachiasmatic nucleus. Chaos Solitons Fractals 2023, 172, 114051. [Google Scholar] [CrossRef]
- Liu, C.; Weaver, D.R.; Strogatz, S.H.; Reppert, S.M. Cellular Construction of a Circadian Clock: Period Determination in the Suprachiasmatic Nuclei. Cell 1997, 91, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.C.W.; Westermark, P.O.; Kramer, A.; Herzel, H. Global parameter search reveals design principles of the mammalian circadian clock. BMC Syst. Biol. 2008, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Xu, C.; Zheng, Z.G. Phase transition and scaling in Kuramoto model with high-order coupling. Nonlinear Dyn. 2021, 103, 2721–2732. [Google Scholar] [CrossRef]
- Skardal, P.S.; Arenas, A. Higher order interactions in complex networks of phase oscillators promote abrupt synchronization switching. Commun. Phys. 2020, 3, 218. [Google Scholar] [CrossRef]
- Schmal, C.; Myung, J.; Herzel, H.; Bordyugov, G. A theoretical study on seasonality. Front. Neurol. 2015, 6, 94. [Google Scholar] [CrossRef]
- Xu, Y.; Gu, C.G.; Qu, D.Q.; Wang, H.Y.; Rohling, J.H.T. Light-induced synchronization modulation: Enhanced in weak coupling and attenuated in strong coupling among suprachiasmatic nucleus neurons. Phys. Rev. E 2025, 111, 014401. [Google Scholar] [CrossRef]
- Gonze, D. Modeling circadian clocks: From equations to oscillations. Cent. Eur. J. Biol. 2011, 6, 699–711. [Google Scholar] [CrossRef]
- Michel, S.; Marek, R.; vanderLeest, H.T.; vanSteensel, M.J.; Schwartz, W.J.; Colwell, C.S.; Meijer, J.H. Mechanism of bilateral communication in the suprachiasmatic nucleus. J. Neurosci. 2013, 37, 964–971. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Chang, Y.T.; Hattar, S.; Chen, S.K. Architecture of retinal projections to the central circadian pacemaker. Proc. Natl. Acad. Sci. USA 2016, 113, 6047–6052. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, C.G.; Zhu, B.; Yang, H.J.; Rohling, J.H.T. Poincaré model shows how heterogeneity in light sensitivity can alter circadian clock function. Commun. Nonlinear Sci. Numer. Simul. 2022, 111, 106462. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Zeman, M. Differential Effects of Constant Light and Dim Light at Night on the Circadian Control of Metabolism and Behavior. Int. J. Mol. Sci. 2020, 21, 5478. [Google Scholar] [CrossRef] [PubMed]
- VanderLeest, H.T.; Houben, T.; Michel, S.; Deboer, T.; Albus, H.; Vansteensel, M.J.; Block, G.D.; Meijer, J.H. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 2007, 17, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.H.; Michel, S.; vanderLeest, H.T.; Rohling, J.H.T. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur. J. Neurosci. 2010, 32, 2143–2151. [Google Scholar] [CrossRef]
- Ramkisoensing, A.; Gu, C.G.; Gastelaars, H.M.D.V.; Michel, S.; Deboer, T.; Rohling, J.H.T.; Meijer, J.H. Enhanced Phase Resetting in the Synchronized Suprachiasmatic Nucleus Network. J. Biol. Rhythm. 2014, 29, 4–15. [Google Scholar] [CrossRef]
- Gu, C.G.; Tang, M.; Yang, H.J. The synchronization of neuronal oscillators determined by the directed network structure of the suprachiasmatic nucleus under different photoperiods. Sci. Rep. 2016, 6, 28878. [Google Scholar] [CrossRef]
- Colwell, C.S.; Michel, S.; Itri, J.; Rodriguez, W.; Tam, J.; Lelievre, V.; Hu, Z.; Liu, X.; Waschek, J.A. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R939–R949. [Google Scholar] [CrossRef]
- Freeman, G.M.; Krock, R.M.; Aton, S.J.; Thaben, P.; Herzog, E.D. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron 2013, 78, 799–806. [Google Scholar] [CrossRef]
- Zhou, J.X.; Wang, H.L.; Ouyang, Q. Network rewiring and plasticity promotes synchronization of suprachiasmatic nucleus neurons. Chaos 2022, 32, 023101. [Google Scholar] [CrossRef]
- Zheng, W.X.; Gu, C.G.; Yang, H.J.; Wang, H.Y.; Rohling, J.H.T. Adaptive coupling between neurons widens the entrainment range of the suprachiasmatic nucleus. Phys. Rev. E 2024, 110, 034212. [Google Scholar] [CrossRef]
- Hafner, M.; Koeppl, H.; Gonze, D. Effect of Network Architecture on Synchronization and Entrainment Properties of the Circadian Oscillations in the Suprachiasmatic Nucleus. PLoS Comput. Biol. 2012, 8, e1002419. [Google Scholar] [CrossRef]
- Bodenstein, C.; Gosak, M.; Schuster, S.; Marhl, M.; Perc, M. Modeling the seasonal adaptation of circadian clocks by changes in the network structure of the suprachiasmatic nucleus. PLoS Comput. Biol. 2012, 8, e1002697. [Google Scholar] [CrossRef] [PubMed]
- Šimonka, V.; Fras, M.; Gosaka, M. Stochastic simulation of the circadian rhythmicity in the SCN neuronal network. Physica A 2015, 424, 1–10. [Google Scholar] [CrossRef]
- Ohta, H.; Mitchell, A.C.; McMahon, D.G. Constant light disrupts the developing mouse biological clock. Pediatr. Res. 2006, 60, 304–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aschoff, J. Exogenous and Endogenous Components in Circadianrhythms. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 11–28. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).