1. Introduction
The fermentation stage represents a pivotal process, exerting a profound influence on the final quality and flavor profile of beer [
1]. In traditional beer brewing, fermentation predominantly depends on empirical knowledge and manual operations. This conventional methodology frequently leads to extended fermentation cycles, inconsistent product quality, and suboptimal production efficiency. With the continuous surge in global beer demand, these traditional approaches are no longer sufficient to meet the escalating requirements for both quantity and quality. To tackle these challenges, contemporary brewing technologies have come to the fore, encompassing continuous fermentation processes [
2] and high-gravity brewing with dilution [
3,
4]. These technological advancements have significantly bolstered the efficiency and consistency of beer production. They not only enable more precise control over the fermentation process but also contribute to enhanced product quality.
Over the past few decades, the beer production industry has witnessed a remarkable expansion, leading to a proportional increase in the volumes of fermenters. Medium-sized breweries commonly employ fermenters with capacities ranging from 30 to 300 m
3. In contrast, larger breweries may utilize fermenters with capacities of up to 500 m
3 [
5], and some of the largest brewing operations even deploy tanks with a capacity of as much as 1000 m
3. Although these large-scale fermenters significantly enhance production capacity, they also pose new challenges in terms of temperature control and monitoring. To facilitate effective cleaning and sterilization, conical fermenters are generally designed without complex internal structures. This design feature, however, limits the installation of temperature measurement equipment, which is usually restricted to the tank wall. The fermentation system operates like a “black box”, where operators have to adjust cooling valves based solely on limited wall temperature data while observing subsequent changes in wall temperature. This information asymmetry often results in delayed responses and suboptimal control strategies. Furthermore, the cooling jackets used for regulating fermentation temperature need to be positioned at an appropriate distance from temperature sensors to avoid interference, further complicating the precise monitoring of temperature changes throughout the fermentation process. Research findings indicate that temperatures vary at different depths, including the wall surface, 0.5 m within the wall, and 1.0 m within the wall [
6]. These variations are influenced by both the fermentation stage and the height within the fermenter. For instance, during the maturation phase, temperature differences on the cone wall can reach up to 10 °C. Radial temperature difference primarily due to the complex dynamic characteristics of industrial-scale fermentation systems, including significant thermal inertia, time delays, and nonlinearity [
7].
Despite recent advancements in control systems and simulation techniques, such as intelligent proportional–integral–derivative (PID) methods [
8,
9] and fuzzy control algorithms [
10], they struggle to effectively regulate these complex dynamic systems because they lack direct insight into the internal spatial conditions within the tank. Therefore, developing tools capable of providing comprehensive internal spatial information is essential for addressing these operational challenges, highlighting the urgent need for simulation methods in practical applications.
In this context, simulation has emerged as a crucial tool for deepening our understanding and optimizing these complex systems. Computational Fluid Dynamics (CFD) first found its footing in the aerospace sector, where it was applied to aircraft design for aerodynamic shape optimization and flight performance enhancement [
11,
12,
13]. It later extended to the turbomachinery domain, playing a key role in internal flow field analysis for turbines and related mechanical systems [
14,
15,
16]. Beyond these, CFD has been applied to biomedical hemodynamics [
17,
18,
19,
20] for analyzing cardiovascular flow dynamics and disease mechanisms, as well as environmental pollutant dispersion modeling [
21,
22,
23,
24] to predict the transport of contaminants in air and water. These applications across complex systems, involving fluid flow, heat transfer, mass transfer, chemical reactions, biochemical reactions, and multiphase flows, help people solve complex problems and understand phenomena.
Likewise, in the fields of bioprocessing and fermentation, the application of CFD has also deepened, addressing key challenges for specific fermentation subjects. For instance, in biogas fermentation, researchers utilize CFD to simulate and optimize the mixing of material fluids inside the reactor to improve gas production efficiency [
25]. When producing biohydrogen from molasses, CFD is used to optimize the hydrodynamic characteristics of continuous stirred-tank reactors (CSTR) and horizontal reactors, thereby enhancing the hydrogen production rate [
26,
27]. For the cultivation of lactic acid bacteria, CFD can accurately predict the pH gradients formed within the fermenter due to metabolic acid production and the addition of alkali [
28]. On a microscopic scale, CFD can even simulate the simultaneous extraction and fermentation process occurring in a single sugar beet cossette [
29]. Furthermore, in large-scale aerobic fermentations such as those with
S. cerevisiae, CFD combined with the Euler–Lagrange method is used to analyze the substrate concentration gradients experienced by microorganisms, providing a critical basis for designing scale-down experimental systems that can faithfully reflect industrial conditions [
30]. However, in the critical industrial sector of alcoholic fermentation, while foundational CFD studies exist, key research gaps pertinent to different fermentation types remain. In the field of red wine fermentation, Zenteno et al. [
31] made an early attempt by simplifying the process into several “compartments,” yielding a low-resolution engineering model. A significant advancement was made by Miller et al. [
32], who developed a high-fidelity model coupling CFD and Finite Element Analysis (FEA). The focus of Miller’s work was to address the complexities of a multiphase (solid–liquid) system arising from the solid “cap” of grape skins and seeds. Their key contribution was the experimental determination of the cap’s thermal properties, enabling an accurate prediction of spatial distributions for temperature, biomass, and productivity in red wine fermentations.
While the work of Miller et al. provides a robust framework for multiphase alcoholic fermentations, industrial beer fermentation presents a distinct set of challenges that have not yet been adequately addressed. First, unlike the multiphase system of red wine, beer fermentation is a single-phase (liquid) process. The heterogeneity within it does not originate from a solid phase but is driven by thermal asymmetry induced by external cooling on very large-scale (often >300 m3) fermenters. Second, existing models for alcoholic fermentation, including that of Miller, have not integrated a detailed yeast population balance model that describes cell proliferation, division, and apoptosis—a biological process critical to the overall reaction kinetics.
Consequently, there remains a pressing need for a multiphysics model specifically tailored for industrial-scale beer fermentation that couples computational fluid dynamics (CFD), fermentation kinetics, and yeast population dynamics. From a practical engineering standpoint, the means for monitoring the internal state of fermenters in industrial production are extremely limited, relying primarily on a few temperature sensors located on the tank wall. This often leads to control strategies that are delayed and suboptimal. Consequently, there is an urgent need for a high-fidelity simulation tool capable of revealing the spatiotemporal distribution of key internal parameters (e.g., temperature, velocity, and species concentrations) to overcome the limitations of the “black box” operational paradigm.
To address the aforementioned research gaps, this study sets the following specific objectives:
To develop and establish a multiphysics model that couples computational fluid dynamics (CFD), fermentation kinetics, and yeast population dynamics to simulate the industrial-scale beer fermentation process.
To utilize the model to reveal the spatiotemporal distribution patterns of key parameters within industrial fermenters, such as temperature and velocity fields, as well as ethanol and sugar concentrations, thereby providing a theoretical basis for understanding the complex phenomena inside the “black box.”
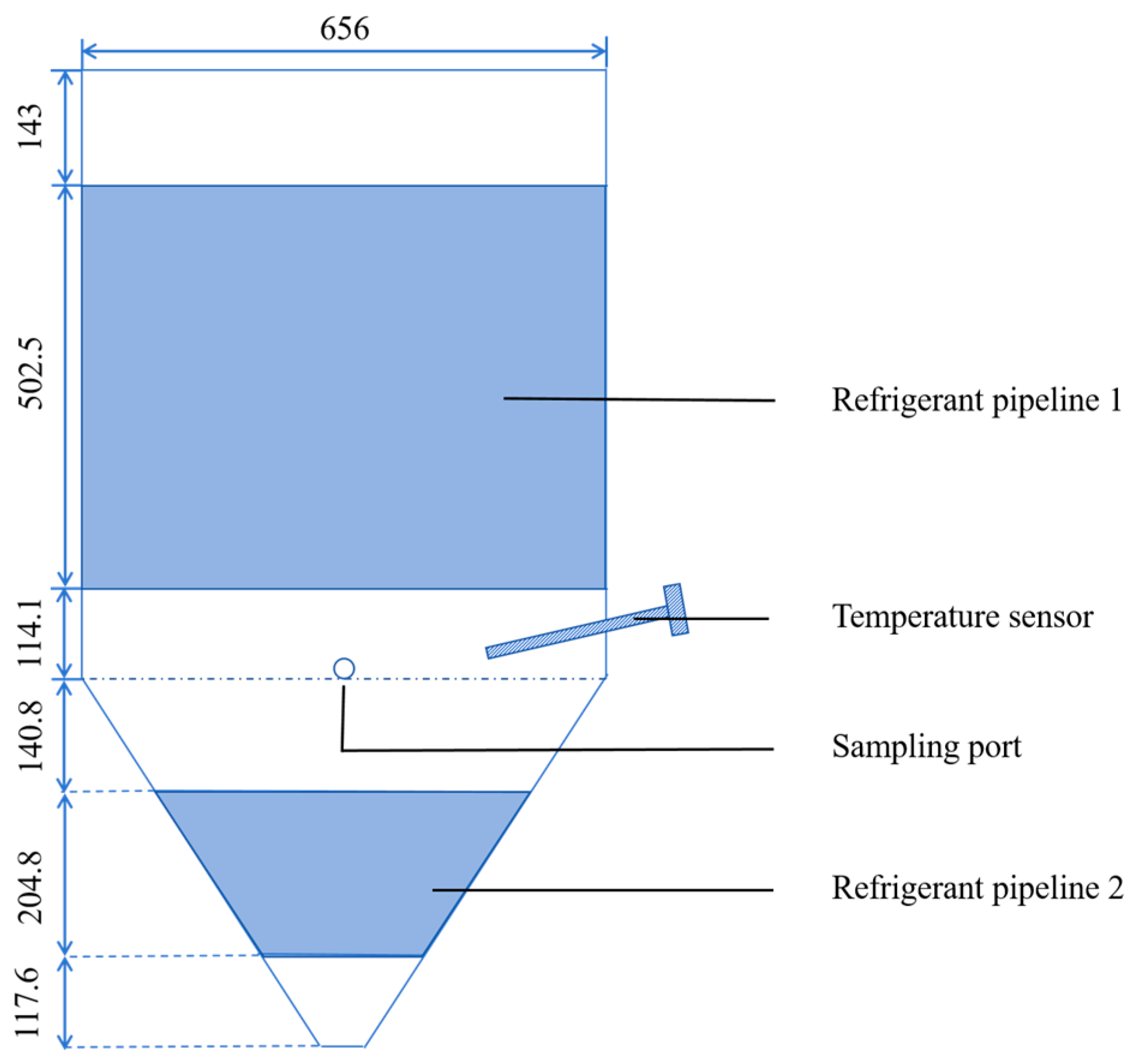
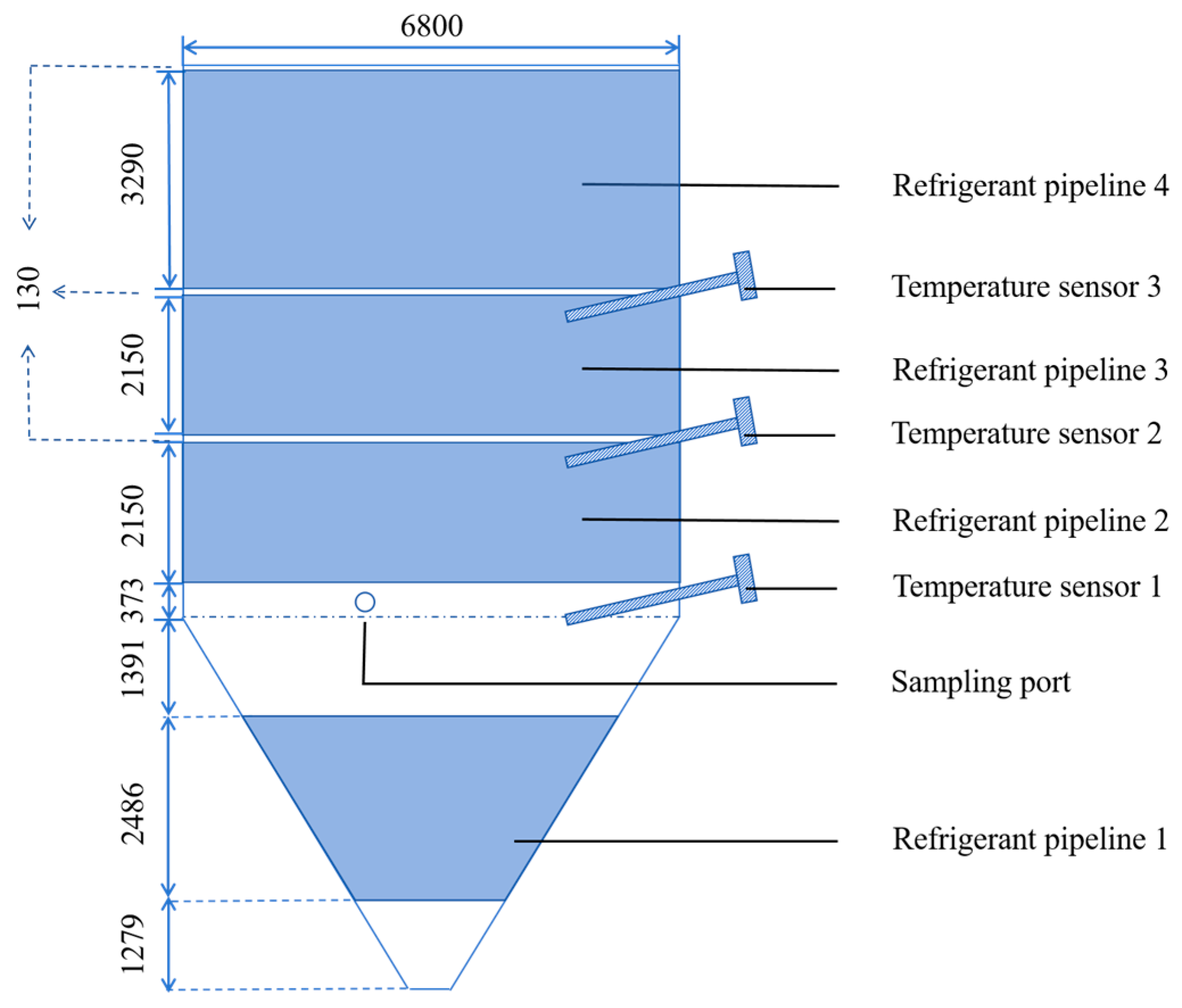
To comprehensively validate the accuracy and reliability of the established model against experimental data from both lab-scale (0.3 m3) and industrial-scale (375 m3) fermenters, ensuring its effectiveness for practical applications.
To achieve the aforementioned objectives, it is essential to understand the complex physicochemical mechanisms within the fermenter. The intrinsic complexity of industrial-scale beer fermentation stems from a multiphysics coupling process driven by biochemical reactions. The mechanistic linkage is as follows: First, the sugar metabolism by yeast is an exothermic reaction that not only converts high-density sugars into lower-density ethanol but also generates a significant heat source within the wort. Second, the release of heat and the change in composition collectively lead to changes in the local density and viscosity of the wort. In the gravitational field of a large conical tank, these local density differences create buoyancy forces, which in turn drive large-scale natural convection. Finally, this natural convection is the primary mode of mass and energy mixing and transport within the tank. However, it interacts with the forced cooling from the tank walls and the continuous internal heat generation, ultimately leading to the formation of non-uniform spatial gradients of temperature, species concentration, and yeast population density. Evidently, a strongly nonlinear, bidirectional coupling exists between fluid flow, heat transfer, mass transfer, and yeast population dynamics. This provides a sound theoretical basis for developing a coupled flow-heat transfer-mass transfer-population model that can integrate these interactions.
In this study, a multiphysics coupled simulation model is proposed. It combined CFD with fermentation reaction kinetics, establishes a theoretical framework for fermentation reactions. This model incorporates fundamental equations, such as Navier–Stokes equations, energy transport equation, species transport equation, reaction equation, Michaelis-Menten kinetics equation, and population balance equation. The reliability of the model will be validated through lab-scale (0.3 m3) and industrial-scale (375 m3) experiments.
The relevance of this study to the journal Symmetry lies in its in-depth analysis of symmetry and symmetry-breaking phenomena within an industrial-scale beer fermentation process. Geometrically, the conical fermenter possesses axial symmetry. In an idealized scenario, the fermentation process—including temperature and species distribution—would also exhibit this symmetry, leading to a spatially uniform state.
However, our research demonstrates that the crucial process of thermal regulation introduces a fundamental break in this symmetry. The cooling jackets, located on the periphery of the tank, create asymmetric thermal boundary conditions. This thermal asymmetry is the primary driver of the complex dynamics observed. It induces non-uniform temperature gradients and density variations within the wort, which in turn give rise to buoyancy-driven natural convection. The resulting fluid motion is characterized by large-scale, asymmetric circulation patterns and vortices, as revealed by our CFD simulations.
Therefore, the core of our work is to model and understand how these deviations from a symmetric state govern the efficiency of heat transfer, the uniformity of the fermentation reaction, and ultimately, the quality of the final product. By studying the asymmetric temperature and flow fields that emerge from a symmetrically designed system, we provide a basis for optimizing control strategies to manage these effects. This focus on the transition from a symmetric system to a complex, asymmetric dynamic state makes our paper a compelling contribution to the scope of Symmetry.
The primary contributions and novelties of this work are summarized as follows:
Development of a Comprehensive Multiphysics Model: This study pioneers a model that, for the first time, couples Computational Fluid Dynamics (CFD) with fermentation kinetics and yeast population dynamics specifically for industrial-scale beer fermentation. This integrated approach provides a more holistic and accurate representation of the process than previously possible.
Validation Against Industrial-Scale Experimental Data: Unlike many simulation studies limited to lab-scale or theoretical validation, our model’s reliability is rigorously verified using data from both a 0.3 m3 pilot-scale and a 375 m3 industrial-scale fermenter, bridging the crucial gap between numerical simulation and real-world industrial practice.
Revelation of Internal Spatiotemporal Dynamics: The model provides an unprecedented high-fidelity visualization of the “black box,” revealing the complex, asymmetric spatiotemporal distributions of temperature, velocity, and species concentrations. This offers critical insights into the internal transport phenomena that are impossible to obtain from wall sensors alone.
A Theoretical Basis for Process Optimization: By elucidating the mechanisms behind spatial heterogeneity and symmetry-breaking driven by thermal control, this research provides a robust theoretical tool for optimizing control strategies, improving fermentation uniformity, and ultimately enhancing final product consistency and quality.
5. Conclusions
The study develops a multi-physics coupled simulation model for the beer fermentation process by integrating CFD technology with a fermentation reaction model. This research initiative is specifically aimed at addressing the challenges related to temperature monitoring and control in large-scale beer fermenters, with the overarching goals of enhancing beer quality and improving production efficiency. Through a series of lab-scale and industrial-scale experiments, the key components of the model are rigorously validated, enabling the simulation and in-depth analysis of the beer fermentation process under diverse operating conditions. The primary achievements of this study are summarized as follows:
- (1)
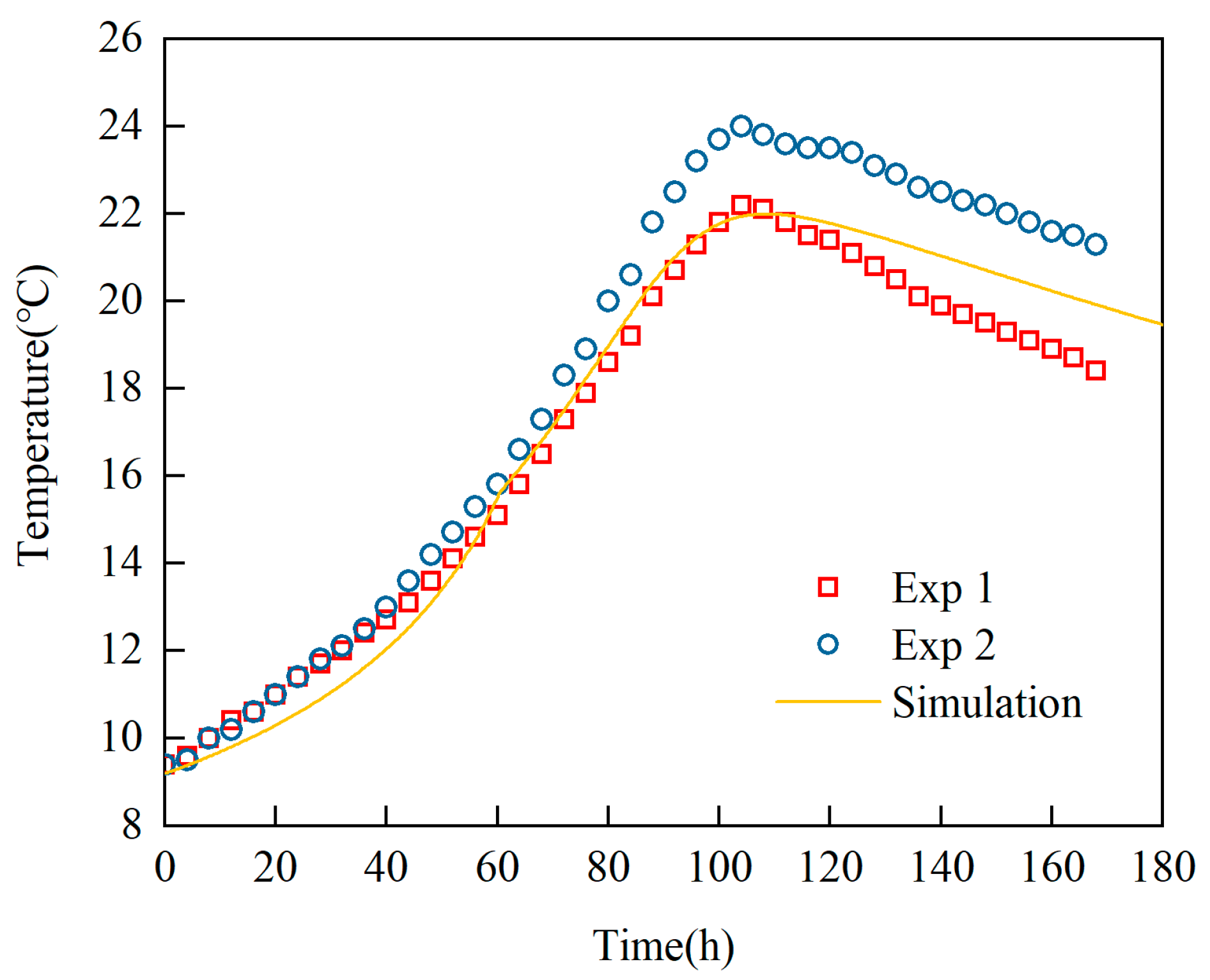
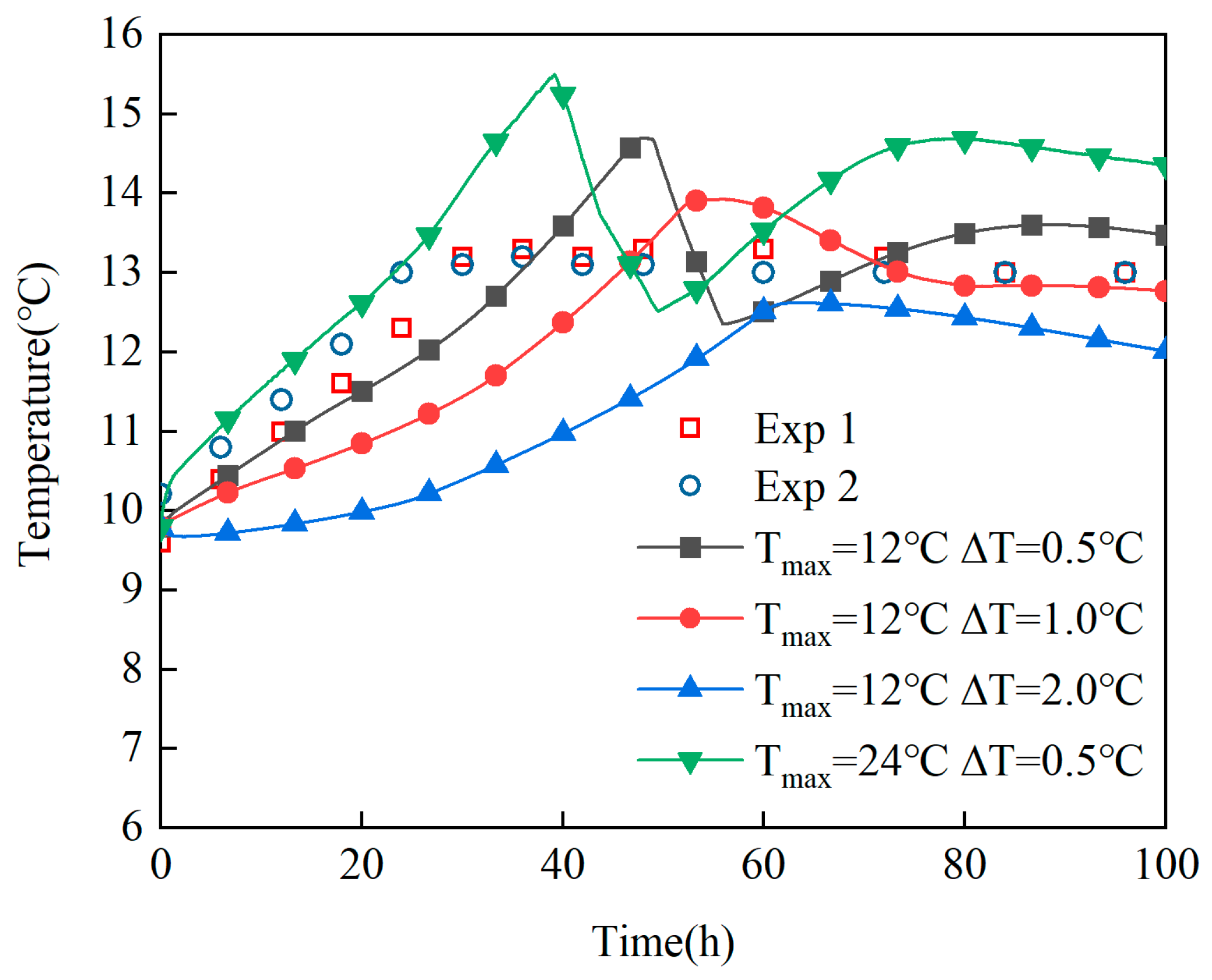
Model Establishment and Validation: Both laboratory and industrial experiments have confirmed the reliability of the heat generation equation within the fermentation reaction model. Based on the PID algorithm commonly used for temperature control in actual production, a dynamic function is incorporated to simulate the boundary conditions of cooling jackets. This approach successfully results in the development of a coupled simulation model that is well-suited for industrial-scale fermenter operations.
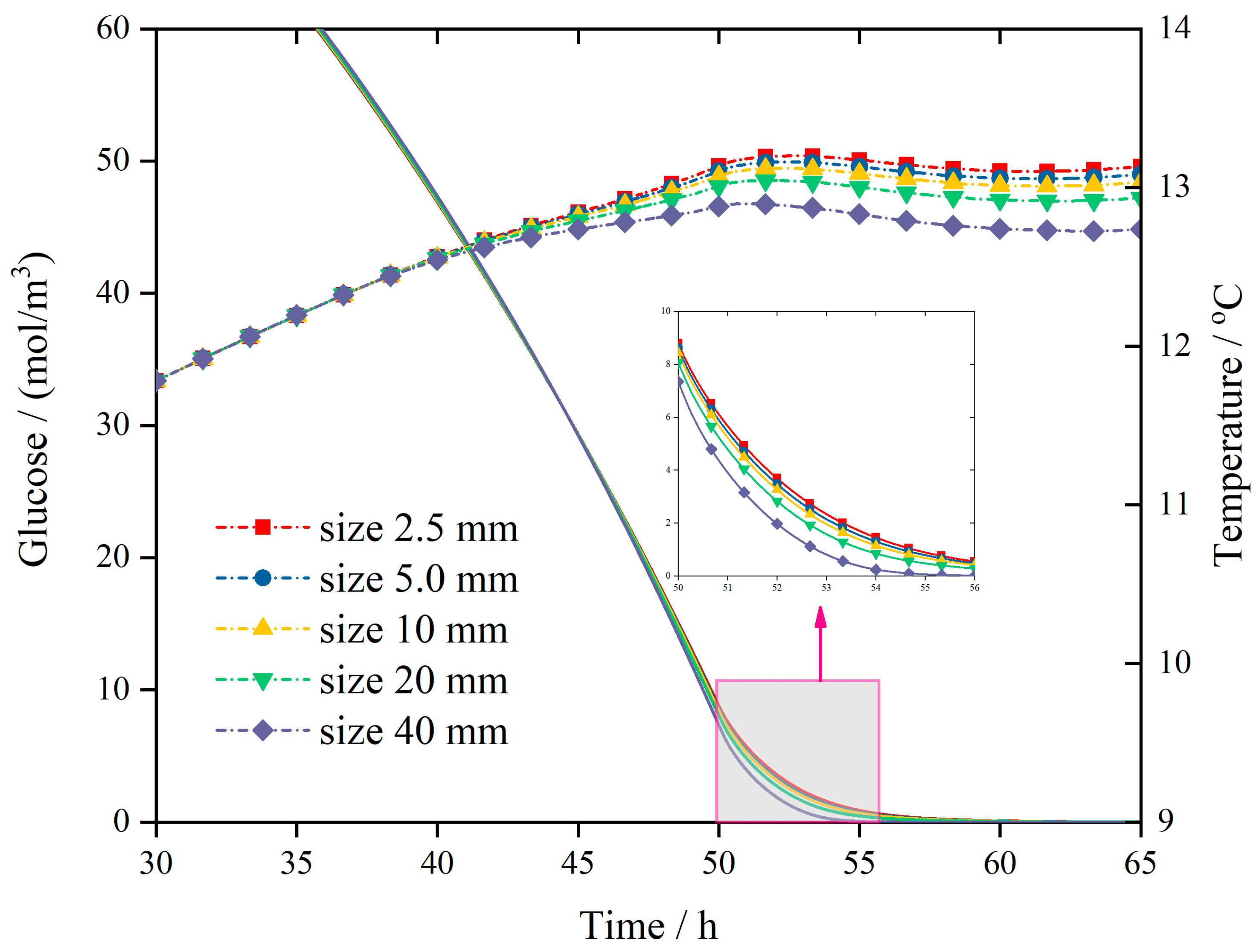
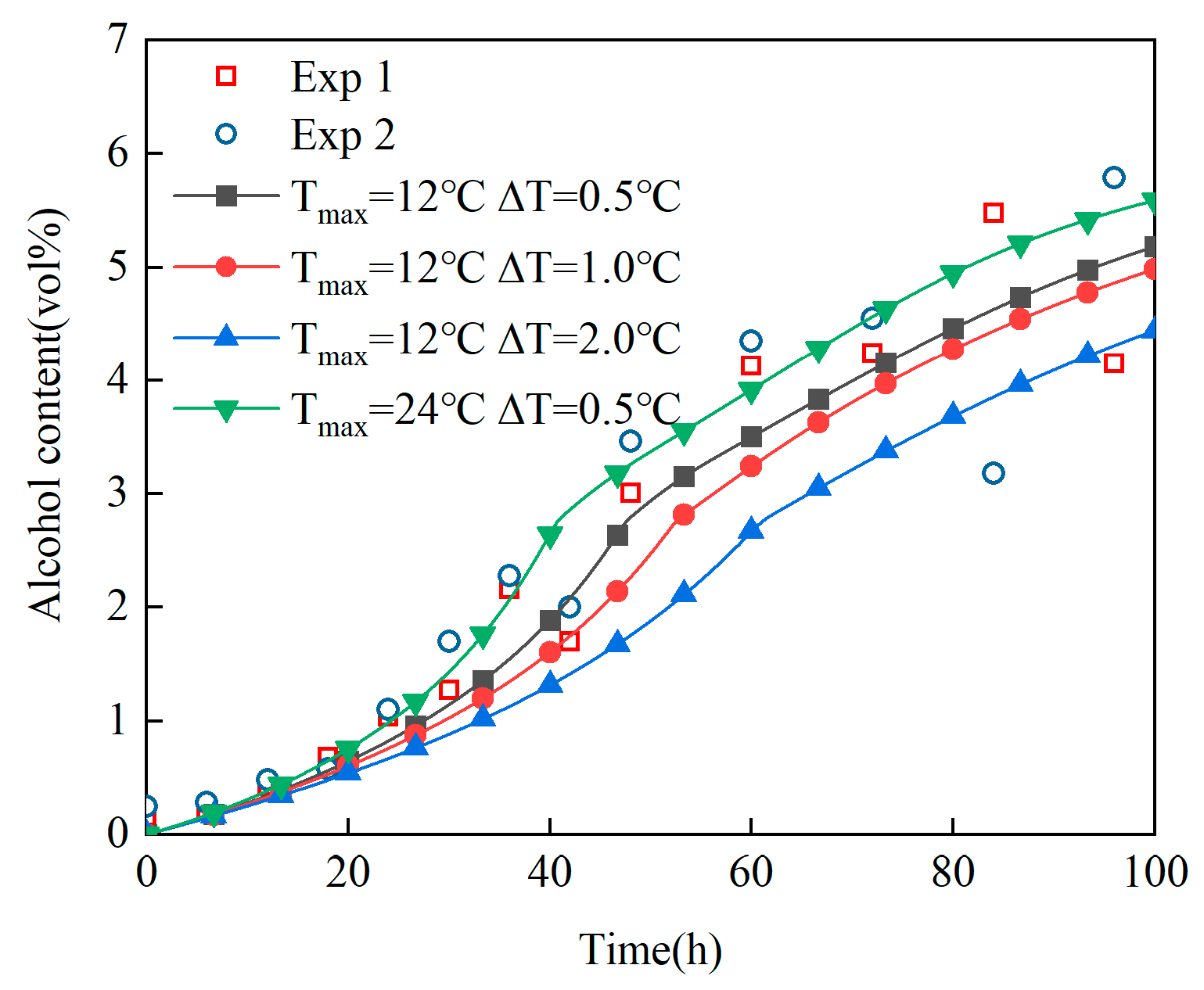
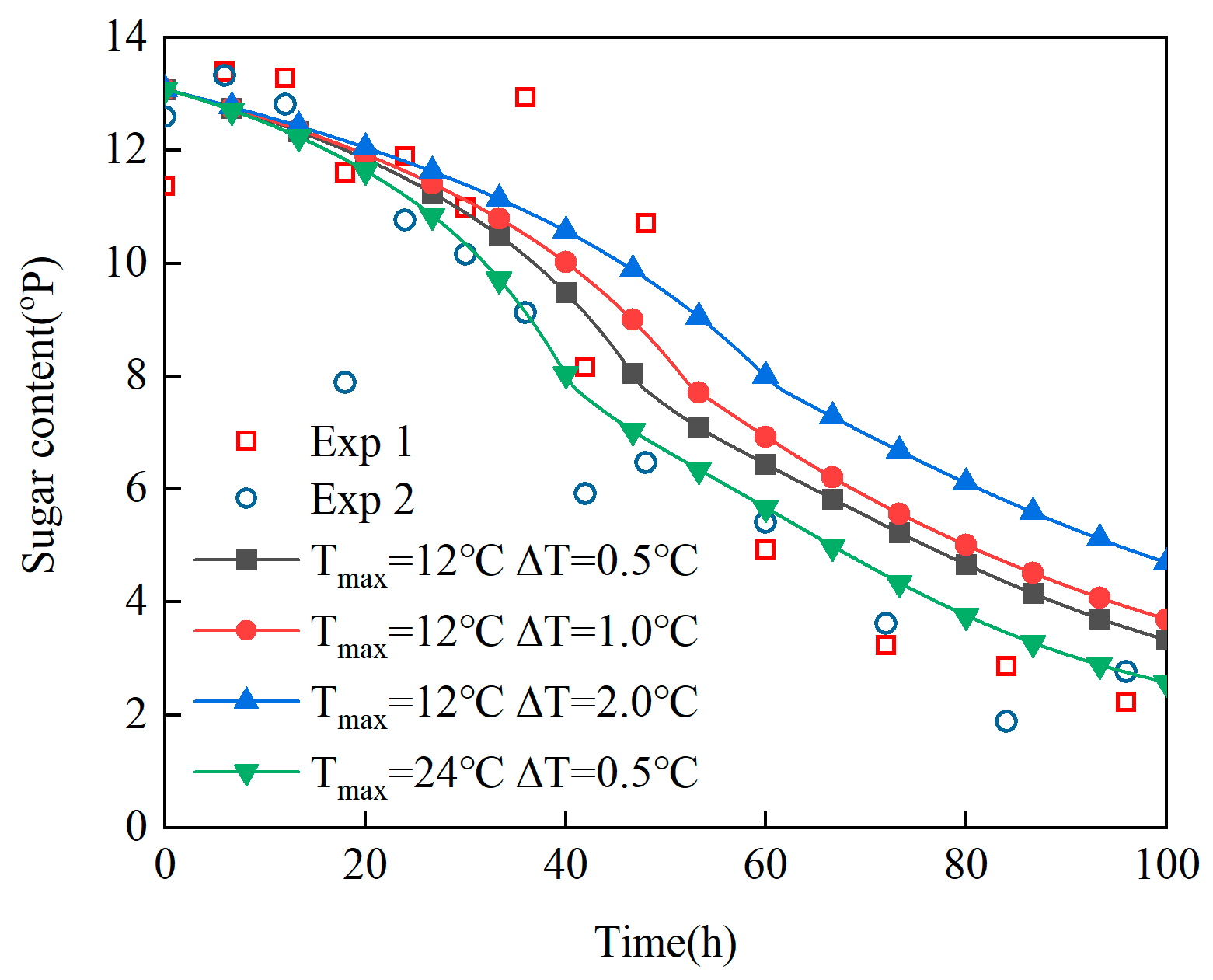
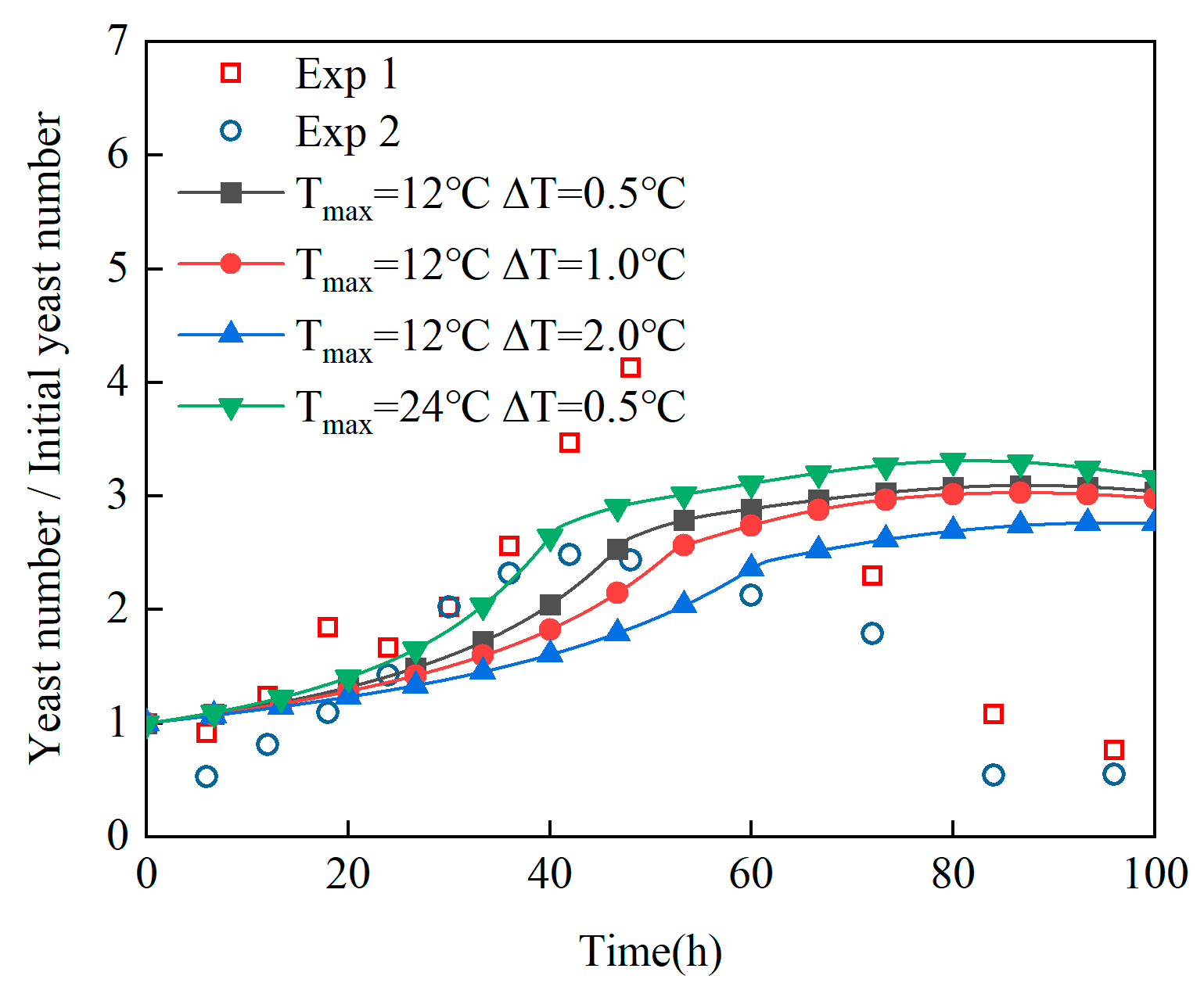
- (2)
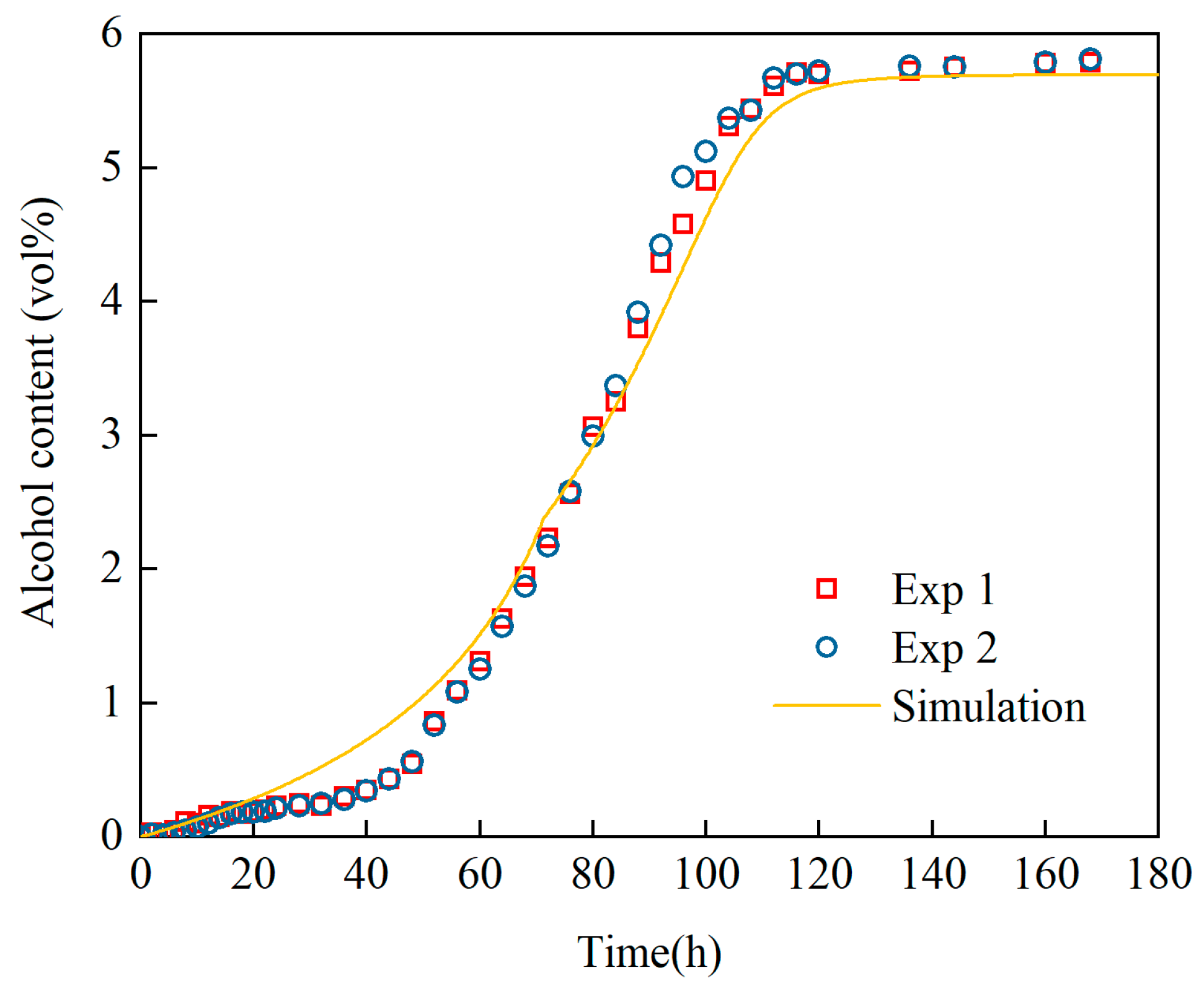
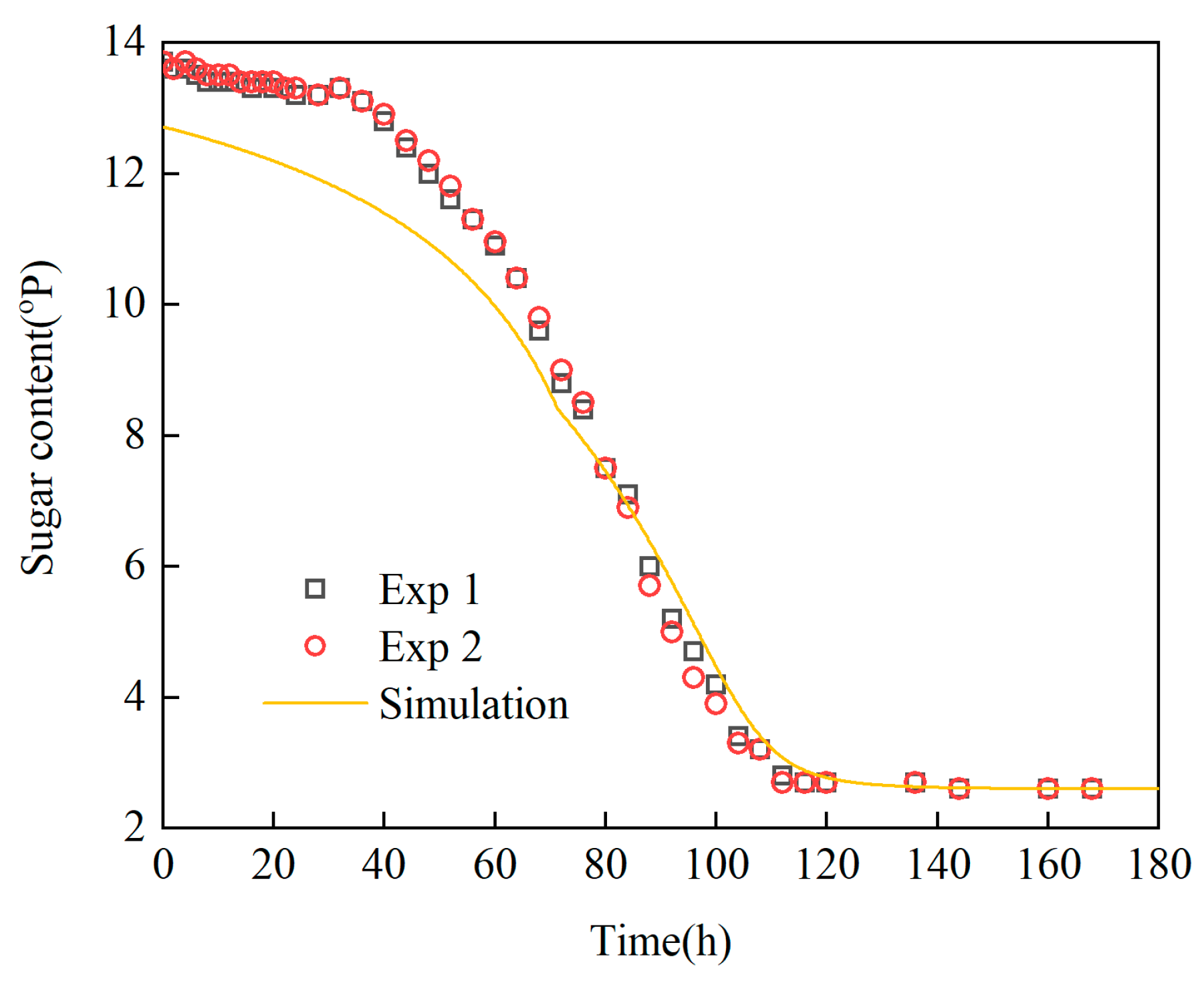
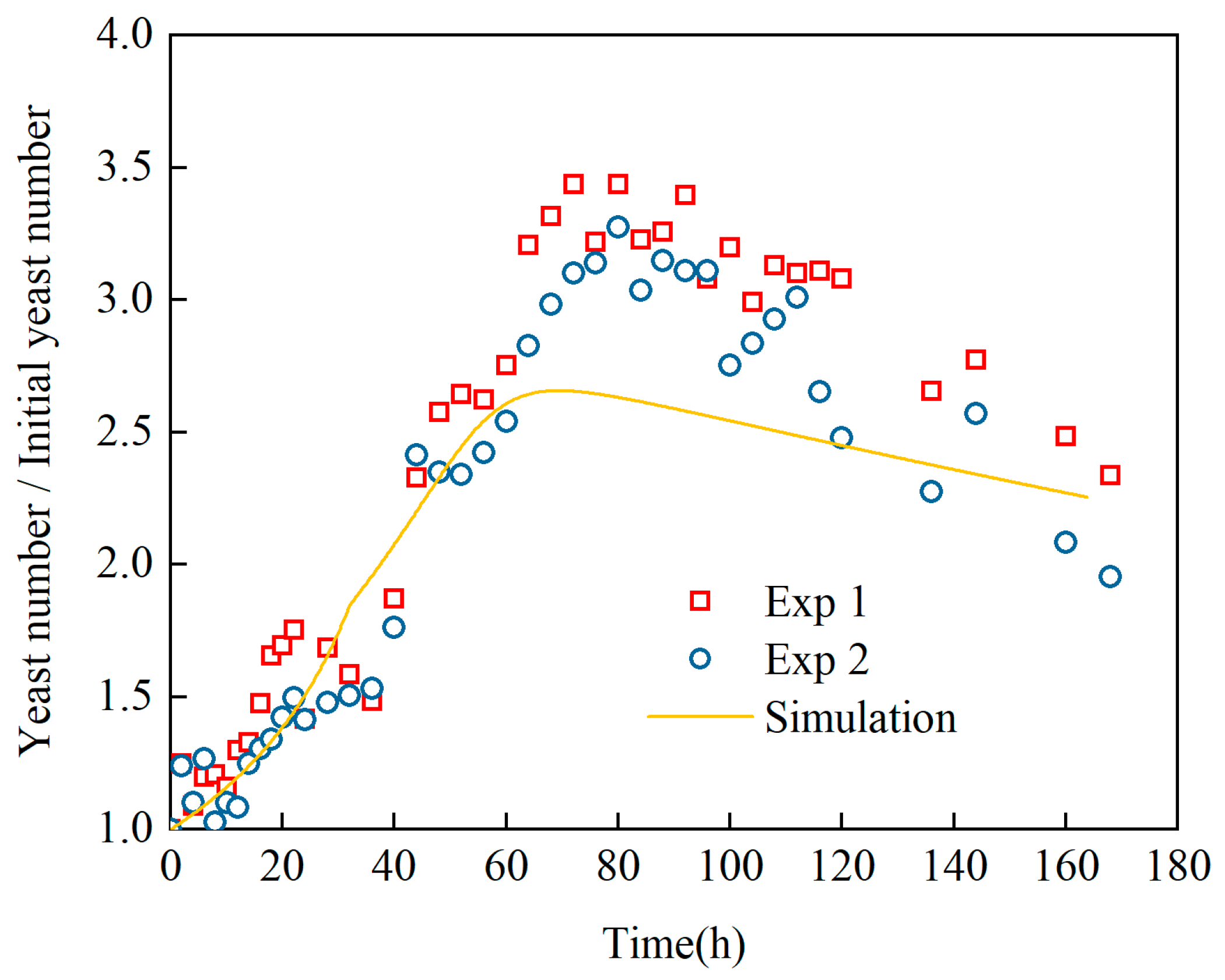
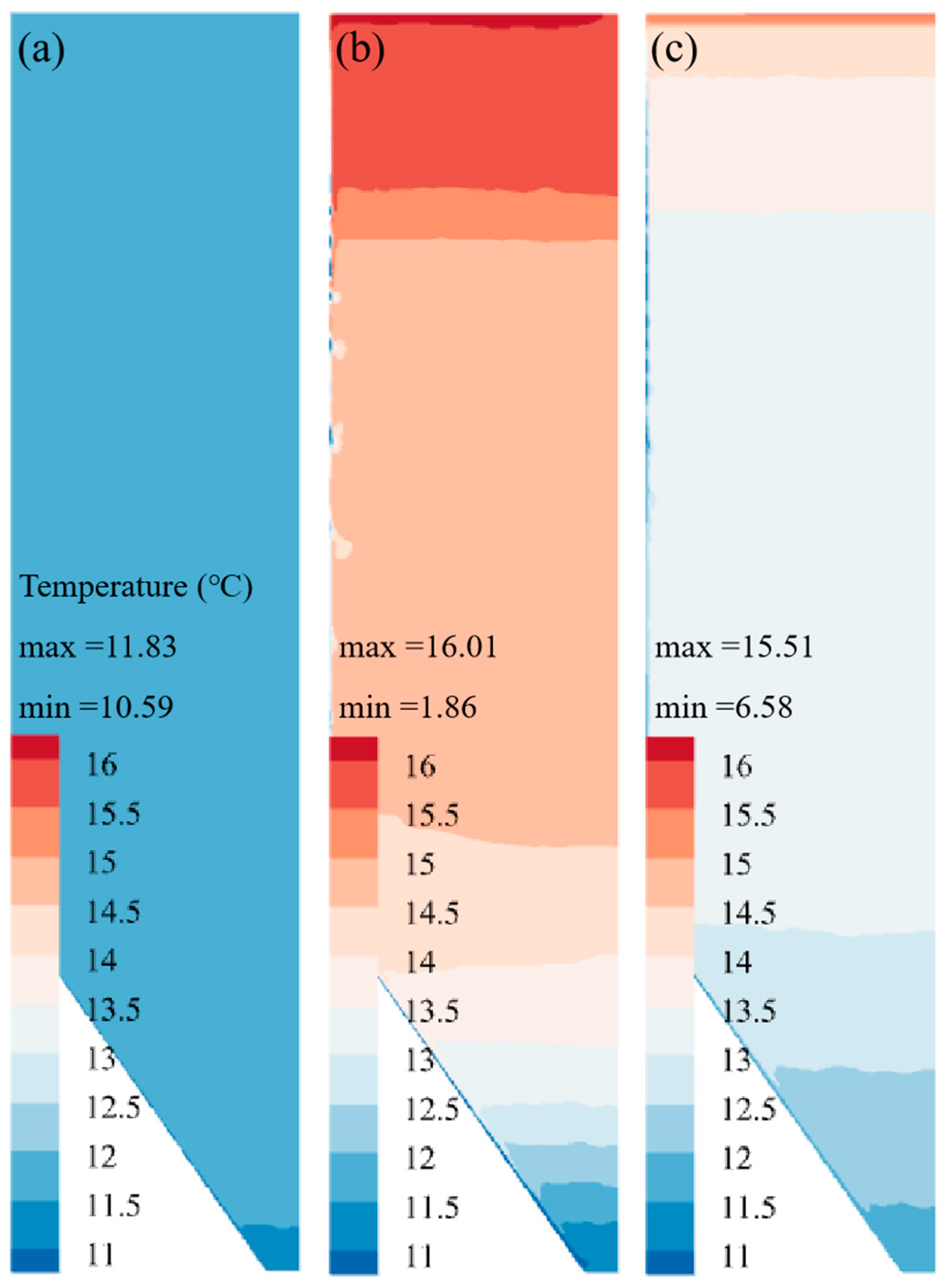
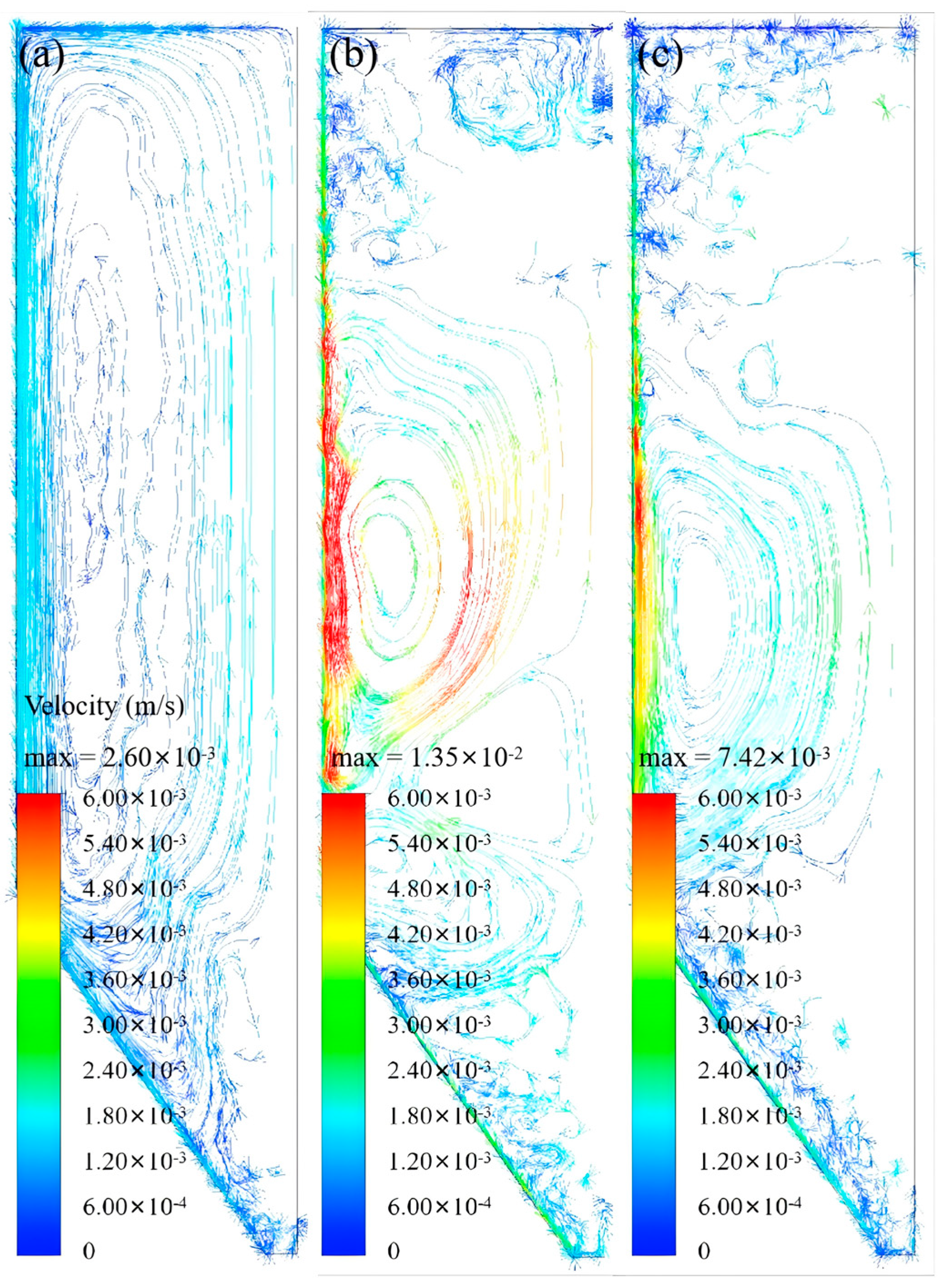
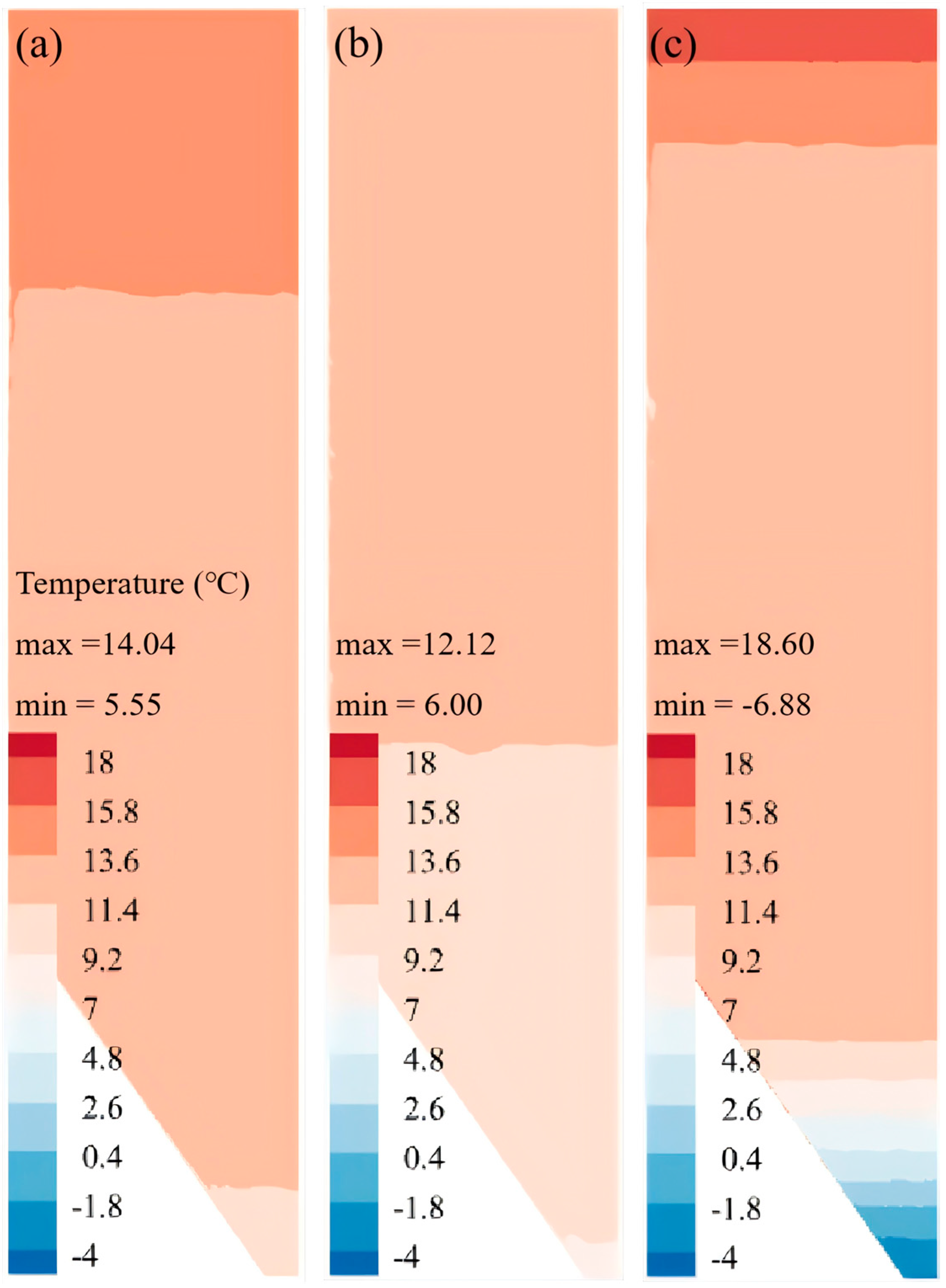
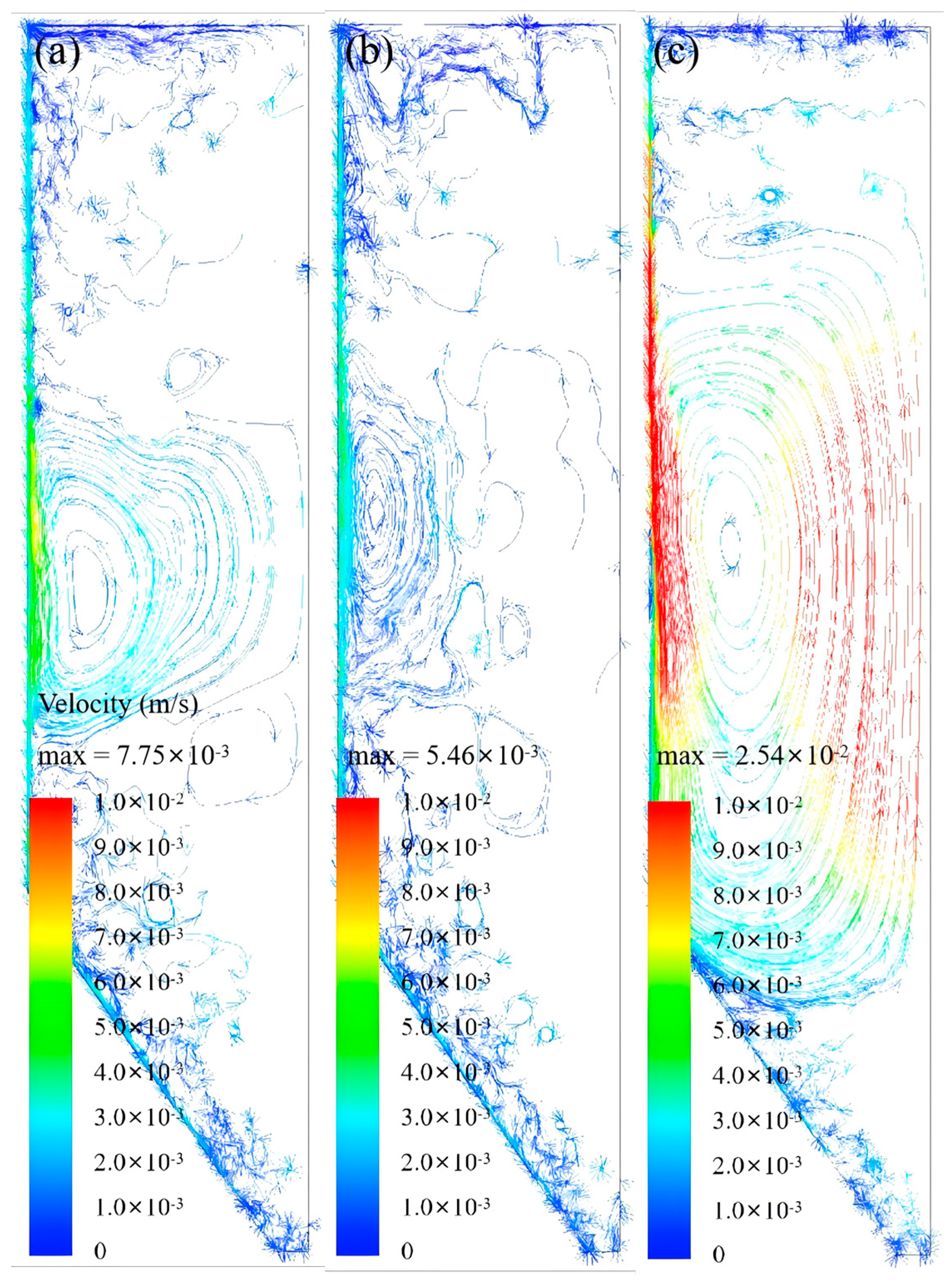
Revelation of Fermentation Process Dynamic Characteristics: The results of lab-scale experiments show that during the uncontrolled fermentation stages, parameters such as temperature, alcohol concentration, sugar content, and yeast concentration exhibit distinct temporal evolution patterns. Notably, the increase in temperature follows an approximately quadratic function curve, which indicates that the rate of fermentation reactions progresses in a relatively linear manner over time. Moreover, the findings from industrial-scale simulations demonstrate a high degree of consistency between the average temperature changes observed during the primary fermentation process and the corresponding experimental records. It is also observed that variations in the parameters of the control algorithm have a significant impact on both the temperature distribution and flow patterns within the fermenter. Larger temperature discrepancies lead to more pronounced convective effects and complex temperature profiles. A central finding is that this behavior is governed by a fundamental symmetry-breaking mechanism, where asymmetric cooling disrupts the thermal uniformity of the geometrically symmetric fermenter.
- (3)
Demonstration of the Advantages of Coupled Simulation: In contrast to traditional experimental methods, which typically only provide time-variant changes in variables, the proposed simulation approach is capable of offering detailed spatial distribution information for various parameters within the fermenter. This includes the temperature distribution and the flow path lines of wort at different fermentation stages. Such comprehensive data serves as an invaluable basis for optimizing fermenter design, configuring cooling systems, and formulating control strategies, thereby enhancing the stability of the beer fermentation process and improving overall product quality.
In summary, by elucidating the mechanisms of thermal symmetry-breaking and its impact on large-scale convection, the multi-physics coupled simulation model developed in this study provides a critical tool for understanding and controlling the ‘black box’ of industrial fermentation. It serves as a powerful foundational tool intended to support future optimization and design studies, such as optimizing cooling jacket zoning, temperature control trajectories, and sensor placement. Its importance in advancing technological progress in the beer fermentation industry cannot be overstated.
However, the current model has certain limitations. The most significant of these is the omission of the effects of carbon dioxide (CO2) evolution during fermentation. The buoyancy-driven flow induced by rising CO2 bubbles could significantly impact local fluid velocities and turbulence intensity, thereby altering heat and mass transfer efficiencies. Additionally, the model incorporates other simplifications, such as the Boussinesq approximation for density variations, a simplified free-surface treatment, and uncertainties in kinetic parameters. In future work, we will prioritize addressing the impact of CO2 evolution by introducing more advanced multiphase flow models (e.g., the Volume of Fluid method or an Euler–Lagrange approach) to capture the gas–liquid dynamics more accurately.
Future research will focus on integrating these more advanced numerical techniques to effectively handle the increasingly complex scenarios encountered in industrial production. For instance, Large Eddy Simulation (LES) could be employed to resolve buoyancy-driven turbulence with higher fidelity, or Bayesian calibration methods could be used to quantify and reduce the uncertainty in kinetic parameters. The application of these methods will help address practical challenges such as non-isothermal wort charging, dynamic variations in coolant supply temperatures, and the differing fermentation characteristics of various yeast strains.