Abstract
Carbon nanotubes (CNTs), due to mechanical, electrical, and surface area properties and their ability to adapt to different nanocomposite structures, are very substantial in supercapacitor electrodes. In this review, we have summarized high-performance, flexible, and symmetry CNT supercapacitors based on the CNTs/graphene, CNTs/metal, and CNTs/polymer electrodes. To present recent developments in CNT supercapacitors, we discuss the performance of supercapacitors based on electrical properties such as specific capacitance (SC), power and energy densities, and capacitance retention (CR). The comparison of supercapacitor nanocomposite electrodes and their results are reported for future researchers.
1. Introduction
Supercapacitors are capable energy storage systems, as they offer fast charge/discharge, high cycle stability, high power density, and stable electrical properties. Supercapacitors in several industries are used for energy harvesting and high-power systems in electric vehicles. Recently, supercapacitors based on CNTs composite electrodes have received more attention due to the electrical and chemical properties of CNTs [1,2,3,4,5,6,7]. Growth in the publication of CNT-based supercapacitors is shown in Figure 1.
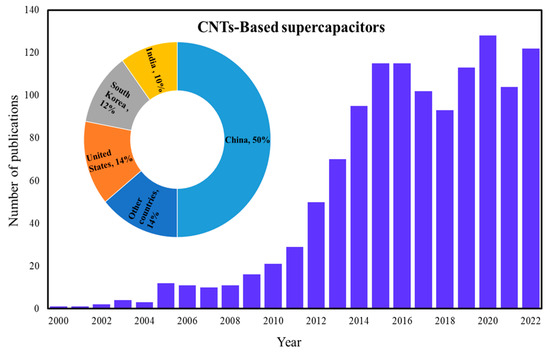
Figure 1.
Growth in CNT-based supercapacitor publications.
The SC is a crucial parameter in the calcification of supercapacitors. The SC can be obtained by cyclic voltammetry and charge/discharge methods. From the cyclic voltammetry method, the SC can be obtained from the following formula:
where m is the mass of the active materials’ electrode, S is the voltage scan rate (mV/s), is the potential window, I is the current in the CV curve, and m is the mass of the electrode materials. From the charge/discharge method, the SC can be obtained from the following formula [8]:
Figure 2 shows the SC of CNT-based supercapacitors that was published in 2022. As shown in Figure 2, 14% of supercapacitors achieved a SC higher than 1000 F/g and 65% lower than 500 F/g.
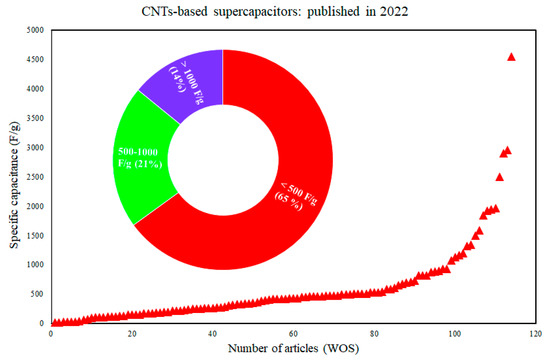
Figure 2.
Specific capacitance of CNTs–based supercapacitors as a function of the number of articles.
The co-occurrence keywords analysis of the CNT-based supercapacitor by VOSviewer from the Scopus for 2022 publications is shown in Figure 3. As can be observed from Figure 3, the electrode nanocomposite based on the CNTs/graphene, CNTs/metal, and CNTs/polymer are more highlighted in the co-occurrence keywords analysis. Other characterizations such as symmetric [9,10,11], flexible [12,13], wearable electronic [14,15], cellulose [16,17,18], cycling stability [19,20], pseudocapacitor [21,22], solid-state supercapacitor [23,24,25], activated carbon [26,27,28], polymers [29,30,31], MXene [32,33], and CNTs [34,35,36,37,38,39,40,41,42,43,44,45,46] were mentioned in Figure 3.
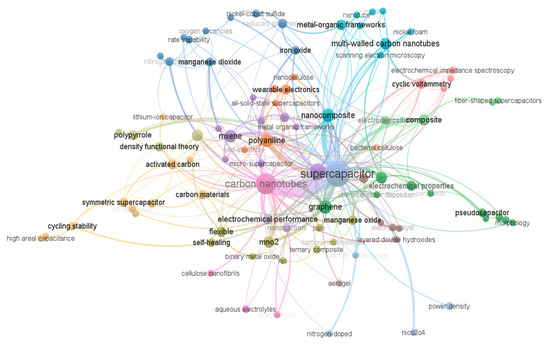
Figure 3.
Co-occurrence keywords analysis of CNTs–based supercapacitor by VOSviewer.
Nanomaterials are widely used for industrial applications such as supercapacitors, batteries, antibacterial activity, nano-membranes, and sensors [47,48,49,50,51,52,53,54,55,56]. Graphene, due to its highly tunable surface area, outstanding electrical conductivity, good chemical stability, and excellent mechanical behavior, is promising for applications in supercapacitors. The recent development of graphene-based supercapacitors from zero-dimensions to three-dimensions was reported in Ref. [57]. Lu et al. [58] reported a symmetric supercapacitor based on the CNTs/reduced graphene oxide (rGO) electrodes at different temperatures. They demonstrated that the specific capacitances of the symmetric CNT/rGO supercapacitors reached 107.8 and 128.2 F/g at 25 °C and 80 °C, and 80.0 and 144.6 F/g at −20 °C and 60 °C. The CR of CNT/rGO supercapacitors remained almost unchanged after 20,000 cycles. Flexible supercapacitors are used in portable and wearable electronics. The flexible supercapacitors are based on CNTs/graphene, CNTs/GO, and CNTs/SnS2 compared in Ref. [59]. The paper presented the areal and volumetric capacitance of the symmetric CNT/SnS2 supercapacitors, which were 533 mF/cm2 and 63 mF/cm3, respectively, whereas CNT/GO-based electrodes display a high-rate capability. Zhang et al. [60] investigated the fabrication of the supercapacitor based on the CNT/graphene/FeCo electrode. They stated that the specific capacitance of the CNT/graphene/FeCo supercapacitor was 560.26 F/g and that the supercapacitor exhibits better electron conductivity and electrochemical performance than pure graphene. The electrical properties of supercapacitors based on the CNTs/COOH/Fe3O4 and CNT/COOH/Fe3O4/rGO structures were compared in Ref. [61]. The paper demonstrated that the specific capacitance, energy, and power densities of the CNT/COOH/Fe3O4 and CNT/COOH/Fe3O4/rGO supercapacitors were 167.29 F/g, 41.65 Wh/kg, and 1333 W/kg and 448.10 F/g, 34.02 Wh/kg, and 1282 W/kg, respectively. The cyclic stability of the CNT/COOH/Fe3O4 supercapacitor was 69% up to 2000 cycles, and for the CNT/COOH/Fe3O4/rGO supercapacitor, it had 93% cyclic stability after 2000 cycles. With the explosive growth of portable electronic devices and electric vehicles, the consideration of renewable energy and the development of sustainable green energy storage devices are more highlighted. In another study, a supercapacitor based on the CNT/rGO-aminoindle structure was reported in Ref. [62]. The paper mentioned using redox-active 7-aminoindole (7-Ai) effectively, the agglomeration of rGO nanosheets was reduced, and a rapid diffusion and transport of ions/electrons from network channels constructed by CNTs happened. The specific capacitance of CNT/rGO-aminoindle supercapacitor, power, and energy densities was 183.86 mAh/g, 17.8 kW/kg, and 163.63 Wh/kg, respectively, with a cycling stability of 87.12% for 16,000 cycles. Juang et al. [63] produced graphene sheets via an amino-assisted liquid phase exfoliation method and proposed that the electrical conductivity of functionalized CNT/graphene buckypaper was 87.500 S/m, which is six times higher than unfunctionalized CNT/graphene buckypaper. They stated that for functionalized graphene/CNT buckypaper, the capacitance was 359.6 mF/cm3 with no capacitance change after 10,000 cycles. A study investigated the preparation of a composite consisting of polypyrrole-derived highly defective CNTs and highly conductive rGO [64]. The highly defective structures serve as effective electrochemically active sites to enhance ion adsorption. Meanwhile, the CNTs and rGO structures act as rapid electrons/ions’ dual transport channels. The combination of CNTs and rGO structures with defective structures synergistically promotes fast energy storage/release. The paper demonstrated that the carbon composite with an optimized structure had a specific capacitance of 160.6 F/g. Li et al. [65] fabricated a supercapacitor with the CNT/rGO/PANI structure, and PANI was grown vertically on CNT/rGO fiber. This structure enabled fast transport for electrons and rapid diffusion for ions. For the CNT/rGO/PANI supercapacitor, the volumetric capacitance of 50.2 F/cm3 with 62.5% CR was achieved A supercapacitor based on the CNT/graphene nanoribbons/molybdenum disulfide (MoS2) electrode displayed a specific capacitance of 282 F/g [66]. Mendoza et al. [67] compared three types of flexible supercapacitors with structures of CNTs, CNTs/Boron nitride (BN)/graphene, and CNT/Boron nitride (BN)/graphene/lithium titanate (Li2TiO3). They reported that the specific capacitance of CNTs, CNTs/BN/graphene, and CNT/BN/graphene/Li2TiO3 supercapacitors was 329.7 F/g, 890.2 F/g, and 1662.2 F/g, respectively. On the other hand, the added BN/graphene and BN/graphene/Li2TiO3 increased the capacitance by ~4 times. The fabrication of graphene fiber as an electrode for CNT/rGO fiber supercapacitors, using GO/polyacrylonitrile (PAN) fibers, is researched in Ref. [68]. The paper described a specific capacitance increase from 34 F/g to 141 F/g with a CR of 96% after 2500 cycles that was due to the improved inaccessibility and pseudo-capacitance provided by oxygen-containing functional groups. CNT/graphene nanocomposites have drawn scientific attention as effective electrodes for supercapacitors. A supercapacitor based on the CNTs/graphene electrode and 1 M NaCl as the electrolyte showed SC of 179 F/g [69]. The advancement of multifunctional materials and their carbonaceous hybrids as supercapacitor electrodes are of critical importance. Das et al. [70] reported a supercapacitor based on the ternary germanium selenide (Ge4Se9) with CNTs and reduced graphene oxide (RGO) and 1 M H2SO4 as the electrolyte. They observed that the SC of the supercapacitor was 440 F/g with 98% coulombic efficiency after 5000 cycles. A study investigated a supercapacitor constructed by the tungsten trioxide (WO3)/CNT/RGO electrode and demonstrated the SC of 691.38 F/g at 5 mV/s [71]. Ngo et al. [72] fabricated polydopamine/CNT/graphene oxide nanocomposites as the flexible symmetric electrode for the supercapacitor and demonstrated the SC of 217.4 F/g. The supercapacitors based on the CNTs/MnO2 nanocomposite electrode have been developed to fabricate high-performance supercapacitors [73,74,75,76,77,78]. The dispersion of MnO2 and conductive additives in an electrode are key factors in increasing the efficiency of the electrode. The high theoretical capacitance of MnO2 offered it as a promising electrode for supercapacitors. Yesilbag et al. [76] compared the supercapacitors with CNTs and CNT/MnO2 electrodes and demonstrated that the SC of supercapacitors were 53.7 F/g and 497 F/g, respectively. This review aimed to describe the supercapacitors based on the CNTs, graphene, metals, and polymer electrodes. Furthermore, the SC, energy, and power densities were investigated for CNT-based supercapacitors. The developments of CNT-based supercapacitors for 2022 publications (Scopus) were also compared.
2. CNT/Graphene-Based Supercapacitors
CNTs, due to their unique electrical, mechanical, and chemical stability properties, can be used for the development of supercapacitors, as electrodes create a large interface between the electrode and electrolyte. To obtain a higher energy density, CNTs should be composites with other nanomaterials and conductive polymers [79]. Figure 4 shows the number of publications of CNT/graphene-based supercapacitors in different years. A comparison of the SC of CNT/graphene-based supercapacitors that was published in 2022 is also plotted in Figure 4.
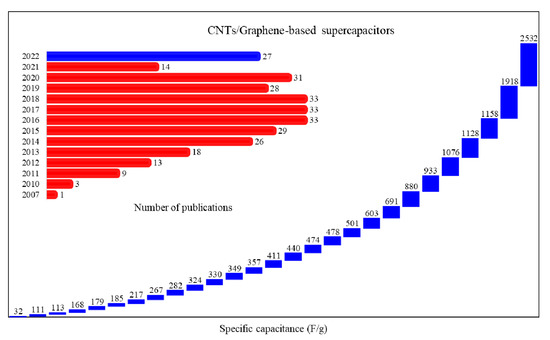
Figure 4.
Comparison of SC of CNT/graphene-based supercapacitors as a function of the number of publications.
As can be observed in Figure 4, the SC of different publications in 2022 are from 113 F/g to 2532 F/g. Yang et al. [80] reported a supercapacitor with nanocomposite electrodes based on the CNTs/graphene and Ti3C2Tx MXene. They demonstrated that the SC of the supercapacitor at the 3000 mV/s was 349 F/g with a CR of 97.1% after 100,000 cycles. In another study, a supercapacitor based on nanocomposite electrodes with the combination of CNTs, reduced graphene oxide (rGO), and ZrO2 nanoparticles, was investigated [81]. That study demonstrated that the CNT/rGO/ZrO2 nanocomposite electrode offers the SC of 357 F/g at 1A/g with a CR of 97.1% after 5000 cycles, and the structure of CNTs/rGO creates an excellent conductive network. Wang et al. [82] described a flexible supercapacitor with a CNT/rGO electrode and demonstrated a SC of 267.8 F/g. Recently, paper supercapacitors based on the carbon nanomaterials’ electrode and polymer electrolyte were used. Figure 5 shows a schematic of paper-based supercapacitors.
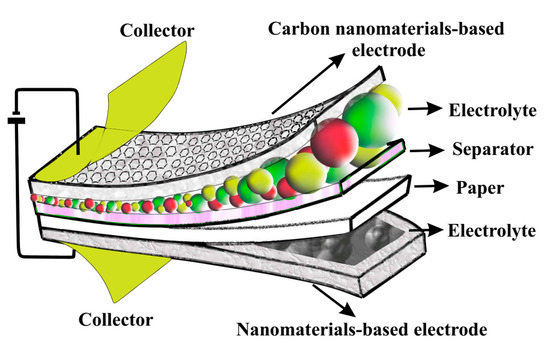
Figure 5.
Schematic of typical symmetry paper-based supercapacitor.
The comparison of CNT/graphene electrode-based supercapacitors is shown in Table 1. Liu et al. [83] described a hybrid supercapacitor with polyhedron-CNTs/graphene as the negative electrode and CNT/NiCo2O4/CoP core-sell polyhedron as the positive electrode. The study demonstrated that the polyhedron-CNT film with porous structure and high conductivity achieved an SC of 1918.4 F/g, and energy and power densities of 68.6 Wh/kg and 800 W/kg at 1 A/g, respectively. The asymmetric supercapacitor based on the CNT/rGO/FeO/NiFe electrode was investigated in Ref. [84]. They reported that the SC, and energy and power densities of the supercapacitor were 411.9 F/g, 41.4 Wh/kg, and 5600 W/kg, respectively, at a scan rate of 5 mV/s and CR of 102.2% after 5000 cycles.

Table 1.
Comparison of CNT/graphene electrode-based supercapacitors.
Qiu et al. [85] used nanocomposites based on CNTs, GO/nanoribbon@graphitic, carbon nitride/Ni and Co-layered double hydroxide/Ni foam (CNTs-GONRs@g-C3N4/Ni–Co-LDH/NF) for the supercapacitor electrode. They proposed that the SC of the supercapacitor at 1A/g was 2532.80 F/g and energy and power densities were 77.61 Wh/kg and 850 W/kg, respectively. Chen et al. [86] considered a nanocomposite based on CNTs/graphene/NiCo2O4/ZnCo2O4 as the electrode for an energy storage device. They indicated that the SC of the supercapacitor was 1128.6 F/g at 1A/g with a CR of 86.1% after 6000 cycles at 10A/g. That nanocomposite demonstrated an energy density of 68.6 Wh/kg and a power density of 800 W/kg at 1 A/g. Transition metal sulfides due to high theoretical capacity have become electrodes in supercapacitors. Zhang et al. [87] proposed a supercapacitor electrode based on the CNTs/graphene/N-Co3S4. They demonstrated that the CNT/graphene/N-doped -Co3S4 supercapacitor has energy and power densities of 37.69 Wh/kg and 8000 W/kg, respectively. In another study, the supercapacitor based on the CNT/graphene/N-doped-MnO2 electrode exhibited energy and power densities of 75.3 Wh/kg and 18,100 W/kg, respectively [88]. Hybrid nanostructures have been used to design flexible supercapacitors. However, the performance and low cost of supercapacitors are interesting characteristics. Pathak et al. [89] reported the supercapacitor based on the MXene (Ti3C2Tx) as the negative electrode and CNT/graphene/NiCo2S4/MXene nanocomposites as the positive electrode. They proposed that the SC of the supercapacitor was 1076 F/g. Transition metal oxides, CNTs, and graphene have been composited as possible electrodes for high-performance supercapacitors. The supercapacitor with CNT/graphene/Mn0.06Co2.94O4 structure displayed a high SC of 933 F/g [90]. The process for preparing ionic gel and CO2 plasma treatment to modify electrodes based on CNT/graphene composites is presented in Ref [91]. The paper demonstrated that the SC of the supercapacitor was 603 F/g, and the energy and power densities of the supercapacitor were 24.03 μWh/c and 2.16 mW/cm, respectively. Rustamaji et al. [92] designed and fabricated a supercapacitor based on the AC/CNT/graphene electrode. They reported that the SC of the AC/CNT/graphene supercapacitor was 32.13 F/g, and the power and energy densities of the supercapacitor were 69 W/kg and 6.6 Wh/kg, respectively. The supercapacitor based on the CNT/rGO/TiO2 electrode demonstrated the SC of 168 F/g and energy and power densities of 15 Wh/kg and 337.5 W/kg, respectively [93].
3. CNT/PANI-Based Supercapacitors
Polymers due to electrochemical properties were used in both supercapacitors’ electrodes and electrolytes [94,95,96]. Conducting polymers such as polyaniline (PANI) have recently been widely used in the electrode material composite of supercapacitors for the fabrication of a lightweight, flexible, symmetry, and high-performance energy storage device. Panasenko et al. [97] reported the supercapacitor based on the CNT/PANI electrode with a SC of 541 F/g. The supercapacitor is based on the CNT/PANI/rGO electrode with a SC of 478 F/g presented in Ref. [98]. The micro-supercapacitors with CNT/PANI/MXene electrodes showed the SC of 414 F/g and cycling stability of 90.4% after 10,000 cycles [99]. Electrode material based on the PANI composite is ideal for flexible supercapacitors. However, the formation of PANI agglomeration leads to a decrease in the SC and cycling stability. The supercapacitor with the CNT/PANI/Sb composite electrode demonstrated the SC of 416 F/g [100]. A comparison of the SC, power, and energy densities of CNT/PANI-based supercapacitors published in 2022 is mentioned in Table 2. In Ref. [101], the supercapacitor based on the CNT/PANI/MnO2 electrodes is developed for energy storage applications and exhibited a high SC of 532 F/g at a current density of 1 A/g. Moreover, the paper demonstrated 80% CR after 4000 cycles and had energy and power densities of 11.8 Wh/kg and 3785 W/kg, respectively. MXene, a successful nanomaterial with a two-dimensional structure, has displayed a capable application on energy storage devices. Due to the susceptibility of MXene to oxidation as a cathode electrode, there is a need for research into developing these electrodes. Wu et al. [102] introduced a supercapacitor with a CNT/PANI@MXene electrode and reported the SC of 463 F/g at 5 mV s−1, and 92% CR after 10,000 cycles. They mentioned the supercapacitor with the composite cathode, and the MXene/CNTs anode achieved energy and power densities of 10 Wh/kg and 2808 W/kg, respectively. In a study, CNTs were embedded in polydimethylsiloxane for outstanding adhesion and integration and then PANI was deposited on its surface [103]. The study proposed that for the CNT/PANI/Polydimethylsiloxane composite electrode, the SC, power, and energy densities of the supercapacitor were 265 F/g, 25.5 Wh/kg, and 126.6 W/kg, respectively. In composite electrodes (nanomaterials/PANI), the electrochemical enhancement of PANI is related to the dispersion state of the nanomaterials in the polymer. Wang et al. [104] demonstrated the fabrication of a symmetric supercapacitor based on the CNT-lignosulfonate/PANI/molybdenum disulfide-lignosulfonate (CNTs-LS/MoS2-LS/PANI) electrode. They demonstrated that, in comparison to the supercapacitor based on the pure PANI, the performance of the supercapacitor with nanocomposites’ electrode enhanced. They reported that the SC, power, and energy densities of the supercapacitor with the CNT-LS/MoS2-LS/PANI nanocomposite electrode were 458.9 F/g, 265.3 W/kg, and 10.9 Wh/kg with 86% CR after 10,000 cycles. Fabrication of a symmetrical supercapacitor was based on the CNT/PANI/graphene/graphite electrode with a high surface area, described in Ref. [105]. The paper demonstrated the SC of 880 F/g at a current density of 1.5 A/g, an energy density of 68.4 mWh/g, a power density of 895.4 mW/g, and 80.6% CR after 10,000 cycles. Xu et al. [106] demonstrated a flexible, symmetric supercapacitor based on the CNT/PANI-Carboxymethylcellulose (CMC) electrode with a gravimetric SC of 348.8 F/g with 89.2% CR after 5000 cycles. Furthermore, they exhibited that the energy and power densities of symmetric supercapacitors with the CMC-PANI/CNT electrode were 99.89 μW h/cm2 and 400.02 μW/cm2, respectively. The supercapacitor with the CNT/vinyltrimethoxysilane/PANI flexible electrode was introduced in Ref. [107] and displayed the highest SC and energy density of 531.3F/g and 26.5 Wh/kg, respectively, at the current density of 1 A/g.

Table 2.
Comparison of CNT/PANI electrode-based supercapacitors.
4. CNTs/Metal-Based Supercapacitors
Increasing energy demands are causing the growth of new materials for supercapacitors’ electrodes and electrolytes. Recently, CNT/metal nanocomposites were widely investigated for the development of supercapacitor electrode materials such as CNTs/HCNFs/MOF, CNTs/NiPMo12, CNT/BT/Zn-N, CNTs@NHP/Co@LDH-CoZnAl, CNTs/ZnCo2O4, CNTs/NiO/MnO2, CNTs/Ti3C2TX, CNTs/KCl, CNTs/V2CTx MXene, CNTs/NiC32N8H16, CNTs/MnS, CNTs/MnO2, CNTs/Ni–Va and CNTs/ZnWO4/Ni foam, CNTs/NiO/MnO2, CNTs/ZnO/C, CNTs/NiCo2S4@N, CNTs@Bi2S3/MoS2, CNTs/Cu2P2O7, and CNTs/MXene with a SC of 712 F/g, 815.6 F/g, 252 F/g, 869.6 F/g, 888 F/g, 23 F/g, 270.5 F/g, 334.4 F/g, 1842 F/g, 12 F/g, 8 F/g, 1298 F/g, 386 F/g, 1493 F/g, 4552 F/g, 1320 F/g, 650 F/g, 254 F/g, 1338 F/g, 465 F/g, and 401.4 F/g, respectively [108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127]. A comparison of SC, power, and energy densities of CNT/metal-based supercapacitors published in 2022 is mentioned in Table 3.

Table 3.
Comparison of CNT/metal electrode-based supercapacitors.
Metals that are organic due to chemical and physical properties such as porosity were proposed for supercapacitors’ electrode material. A comparison of supercapacitors based on CNT/Ni electrode structures is reported in Table 3 [128,129,130,131,132]. Sun et al. [128] synthesized the CNT/Ni composite electrode for the supercapacitor electrode and reported that the SC of the supercapacitor was 97 F/g and 83.2% retention after 5000 cycles. They reached 32.6 Wh/kg and 476.5 W/kg energy density and power density, respectively, for CNT/Ni supercapacitors. The hierarchical electrode materials are attractive for energy storage applications. The supercapacitor is based on the CNT/NiCo2O4/Ni/C electrode structure described in Ref. [129]. The paper mentioned that the SC of a supercapacitor with the hierarchical electrode material was 1945.9 F/g with an energy density of 58.31 Wh/kg at a power density of 749.7 W/kg. One of the disadvantages of supercapacitors is related to the low energy density that can be improved by pseudocapacitive materials from redox reactions. A supercapacitor electrode based on the CNT/NiCoO2 mesoporous structure presented the SC of 1587 F/g and 92% CR after 5000 cycles, with an energy density of 41.8 Wh/kg at a power density of 412 W/kg [130]. Geioushy et al. [131] introduced the CNT/NiS2/NiCo2S4 nanostructure as electrode material for energy storage applications. They achieved the SC of 1587 F/g and 83% CR after 10,000 cycles. The composition of the binary metal oxide with CNTs is considered for increased energy storage in recent reports. The supercapacitor with the CNT/NiFe2O4 electrode structure demonstrated energy and power densities of 23.39 Wh/kg and 466.66 W/kg, respectively [132]. Supercapacitors with long-cycle stability and flexible electrodes are in demand for portable electronic equipment. Yao et al. [133] investigated the flexible quasi-solid-state supercapacitor based on the CNT/Co-S/carbon nanofibers’ electrode and stated that the SC of the supercapacitor was 416.5 F/g and 96.9% CR after 10,000 cycles. Due to the tunable structure and stability properties of the binary metal, oxides were used for electrode materials’ construction. The supercapacitor with the gel polymer electrolyte and the CNT/Co3V2O8 electrode was proposed in Ref. [134]. The paper detailed that the SC of the supercapacitor was 120.17 F/g and 95.26% CR after 3000 cycles. Despite the capability of supercapacitors in energy storage, there is a big difference in energy densities between batteries and supercapacitors. Houpt et al. [135] introduced a hybrid framework with the CNT/ZIF/MoS2 electrode material. They stated that the power density was 3682 W/kg for the supercapacitor with a SC of 262 F/g. A supercapacitor based on the metal–organic CNT/ZnCoS electrode demonstrated the SC of 2957.6 F/g with a 96% CR after 10,000 cycles [136]. Metal–oxygen material such as CNTs/MnO2 was used for the supercapacitor electrode and achieved the SC of 253.86 F/g with 78.26% CR after 6000 cycles, with energy and power densities of 32 Wh/kg and 413.7 W/kg, respectively [137]. The improvement of supercapacitors’ performance is related to the electrode materials and structural design. The CNT/Ti3C2/MnCo2S4 composite electrode with positively charged CNTs and negatively charged Ti3C2 was investigated in Ref. [138]. The paper mentioned that the symmetric supercapacitor demonstrated the SC of 823 F/g and energy and power densities of 49.5 Wh/kg and 350 W/kg respectively. Pseudocapacitive materials due to their good redox processes and low cost are proper for supercapacitor fabrication. A supercapacitor based on the CNT/Bi-Fe-P electrode exhibited an energy density and power densities of 81.5 Wh/kg and 890.2 W/kg, respectively, with 85.6% CR after 8000 cycles [139]. Problems in flexible energy storage devices are related to mechanical properties, cyclic stability, and small capacitance. A flexible symmetric supercapacitor based on the CNT/cerium selenide nanopebbles’ electrode and poly (vinyl alcohol) (PVA)-LiClO4 gel electrolyte was proposed in Ref. [140]. The study achieved energy and power densities in a range of 36.3–14.5 Wh/kg and 2800–5600 W/kg, respectively. There is sodium due to an abundance in the Earth’s crust, leading to low-cost energy storage and renewable energies. In combination with niobium pentoxide and CNTs, sodium ions can make intercalation into niobium pentoxide and electrostatic adsorption into CNTs.
Real et al. [141] investigated a flexible, sodium-ion pseudocapacitor with the CNT/Nb2O5 electrode and demonstrated a SC of 192 F/g and 96% CR after 10,000 cycles. A supercapacitor based on the CNT/Nitrogen–Boron–Carbon electrode reached energy density and power density in a range between 11.67–7.74 Wh/kg and 300–1485 W/kg, respectively [142]. Due to large-volume oscillations, the low inherent conductivity and stability of the bare SnS2 are not expected for supercapacitor devices. A supercapacitor with CNT/SnS2-BN electrode material stated the SC of 87 F/g [143]. A comparison of Table 1, Table 2 and Table 3 in the specific capacitance, power density, and energy density for supercapacitors based on the CNTs/metal, CNTs/graphene, and CNT/PANI is plotted in Figure 6. As can be observed from Figure 6 for CNT/metal-based supercapacitors, the energy density is higher, and for CNT/graphene-based supercapacitors, the power density is highlighted. The CNT/metal-based supercapacitors achieved an energy density of about 80 Wh/kg, and the CNT/graphene-based supercapacitor reached a power density of 9000 W/kg.
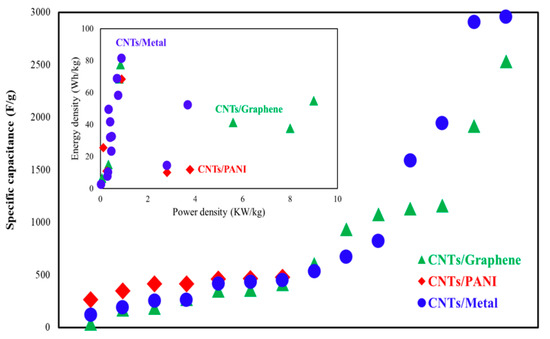
Figure 6.
Comparison of specific capacitance, power density, and energy density for supercapacitors based on the CNTs/metal, CNTs/graphene, and CNTS/PANI.
5. Conclusions
There is a continued need for supercapacitors in several industries for energy storage systems due to fast charge/discharge, high cycle stability, and high-power density. In this study, a recent development in CNT-based supercapacitors was reported. The comparison of supercapacitors based on the CNTs/graphene, CNTs/metal, and CNTs/polymer electrodes was investigated in this review. Results demonstrated that for the CNT/metal-based supercapacitors, the energy density is higher and for CNT/graphene-based supercapacitors, the power density is highlighted. The CNT/metal-based supercapacitors achieved an energy density of about 80 Wh/kg, and the CNT/graphene-based supercapacitor reached a power density of 9 kW/kg.
Author Contributions
G.B.P. and L.F.A. writing—review and project administration and H.A. and S.S. investigation and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was financially supported by the Iran National Science Foundation (INSF) and grant number (INSF: 97003234).
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research work was financially supported by East Tehran Branch, Islamic Azad University and the Iran National Science Foundation (INSF) and grant number (INSF: 97003234).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cevik, E.; Asiri, S.M.M.; Qahtan, T.F.; Bozkurt, A. Fabrication of high mechanical stability electrodes and bio-electrolytes for high-performance supercapacitor application. J. Alloys Compd. 2022, 913, 165230. [Google Scholar] [CrossRef]
- Jalalah, M.; Rudra, S.; Aljafari, B.; Irfan, M.; Almasabi, S.S.; Alsuwian, T.; Khazi, M.I.; Nayak, A.K.; Harraz, F.A. Sustainable synthesis of heteroatom-doped porous carbon skeleton from Acacia auriculiformis bark for high-performance symmetric supercapacitor device. Electrochim. Acta 2022, 414, 140205. [Google Scholar] [CrossRef]
- Keum, K.; Park, D.; Park, M.; Lee, Y.; Lee, H.; Jeong, H.; Kim, J.W.; Kim, D.-W.; Ha, J.S. All vanadium-based Li-ion hybrid supercapacitor with enhanced electrochemical performance via prelithiation. J. Alloys Compd. 2022, 914, 165288. [Google Scholar] [CrossRef]
- Mandal, M.; Subudhi, S.; Nayak, A.K.; Alam, I.; Subramanyam, B.V.R.S.; Maheswari, R.P.; Patra, S.; Mahanandia, P. In-situ synthesis of mixed-phase carbon material using simple pyrolysis method for high-performance supercapacitor. Diam. Relat. Mater. 2022, 127, 109209. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, R.A.; Koo, B.H.; Alsalme, A. Systematic study of physicochemical and electrochemical properties of carbon nanomaterials. RSC Adv. 2022, 12, 15593–15600. [Google Scholar] [CrossRef]
- Ye, T.; Wu, H.; Shao, Y.; Ye, Z.; Li, G.; Wang, J.; Chen, K. A Study on the Effect of Graphene/Carbon Nanotubes on the Enhanced Capacitance of IrO2-ZnO-G(CNT)/TiElectrodes. Energy Fuels 2022, 36, 3259–3271. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Raza, M.A.; Chishti, U.N.; Hussnain, A.; Maqsood, M.F.; Iqbal, M.Z.; Iqbal, M.J.; Latif, U. Role of Carbon Nanomaterials on Enhancing the Supercapacitive Performance of Manganese Oxide-Based Composite Electrodes. Arab. J. Sci. Eng. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghorannevis, M.; Pour, G.B. High performance supercapacitors based on the carbon nanotubes, grapheme and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef]
- Zhou, L.; Song, F.; Yi, J.; Xu, T.; Chen, Q. Nitrogen-Oxygen Co-Doped Carbon-Coated Porous Silica/Carbon Nanotube Composites: Implications for High-Performance Capacitors. ACS Appl. Nano Mater. 2022, 5, 2175–2186. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Liu, Y.; Xia, Y.; Liu, X.; Gao, Y.; Wu, S.; Li, T.; Zada, A.; Ye, F. Flexible Ti3C2Tx/Carbon Nanotubes/CuS Film Electrodes Based on a Dual-Structural Design for High-Performance All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 9158–9172. [Google Scholar] [CrossRef]
- Lyu, W.; Yan, C.; Chen, Z.; Chen, J.; Zuo, H.; Teng, L.; Liu, H.; Wang, L.; Liao, Y. Spirobifluorene-Based Conjugated Microporous Polymer-Grafted Carbon Nanotubes for Efficient Supercapacitive Energy Storage. ACS Appl. Energy Mater. 2022, 5, 3706–3714. [Google Scholar] [CrossRef]
- Filimonenkov, I.S.; Urvanov, S.A.; Kazennov, N.V.; Tarelkin, S.A.; Tsirlina, G.A.; Mordkovich, V.Z. Carbon nanotube cloth as a promising electrode material for flexible aqueous supercapacitors. J. Appl. Electrochem. 2022, 52, 487–498. [Google Scholar] [CrossRef]
- Kazari, H.; Pajootan, E.; Hubert, P.; Coulombe, S. Dry Synthesis of Binder-Free Ruthenium Nitride-Coated Carbon Nanotubes as a Flexible Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2022, 14, 15112–15121. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ling, H.; Huang, Q.; Yang, Y.; Wang, X. Interface Engineering on Cellulose-Based Flexible Electrode Enables High Mass Loading Wearable Supercapacitor with Ultrahigh Capacitance and Energy Density. Small 2022, 18, 2106356. [Google Scholar] [CrossRef]
- Jiang, L.; Hong, H.; Hu, J.; Yan, X. Development of flexible supercapacitors with coplanar integrated multi-walled carbon nanotubes/textile electrode and current collectors. J. Mater. Sci. Mater. Electron. 2022, 33, 5297–5310. [Google Scholar] [CrossRef]
- Alvarenga, D.F.; Junior, M.G.; Santos, M.C.G.; Pinto, P.S.; da Cunha, T.H.R.; Dias, M.C.; Lavall, R.L.; Ortega, P.F.R. Tuning carbon nanotube-based bucky paper properties by incorporating different cellulose nanofibrils for redox supercapacitor electrodes. J. Energy Storage 2022, 52, 104848. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, L.; Lyu, S.; Wang, S. Flexible and Freestanding MoS2 Nanosheet/Carbon Nanotube/Cellulose Nanofibril Hybrid Aerogel Film for High-Performance All-Solid-State Supercapacitors. ACS Omega 2022, 7, 14390–14399. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-C.; Yu, H.-Y.; Ouyang, Z.; Qi, D.; Zhou, Y.; Ju, A.; Li, Z.; Cao, Y. Chain-ring covalently interconnected cellulose nanofiber/MWCNT aerogel for supercapacitors and sensors. Nanoscale 2022, 14, 5163–5173. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, L.; Li, W.; Du, X.; Lin, Z.; Zhang, P. Carbon Nanotube prepared by catalytic pyrolysis as the electrode for supercapacitors from polypropylene wasted face masks. Ionics 2022, 28, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.S.; Dubey, P. Amorphous MnOx Nanostructure/Multiwalled Carbon Nanotube Composites as Electrode Materials for Supercapacitor Applications. ACS Appl. Nano Mater. 2022, 5, 8566–8582. [Google Scholar] [CrossRef]
- Xiao, J.; Tong, H.; Jin, F.; Gong, D.; Chen, X.; Wu, Y.; Zhou, Y.; Shen, L.; Zhang, X. Heterostructure NiS2/NiCo2S4 nanosheets array on carbon nanotubes sponge electrode with high specific capacitance for supercapacitors. J. Power Sources 2022, 518, 230763. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Y.; Fang, H.; Xu, Y.; Sun, W.; Chen, S.; Wang, Y.; Lv, L.-P. Redox-Active Tetramino-Benzoquinone π-πStacking and H-Bonding onto Multiwalled Carbon Nanotubes toward a High-Performance Asymmetric Supercapacitor. ACS Appl. Energy Mater. 2022, 5, 8112–8122. [Google Scholar] [CrossRef]
- Zeng, M.-J.; Li, X.; Li, W.; Zhao, T.; Wu, J.; Hao, S.-M.; Yu, Z.-Z. Self-supported and hierarchically porous activated carbon nanotube/carbonized wood electrodes for high-performance solid-state supercapacitors. Appl. Surf. Sci. 2022, 598, 153765. [Google Scholar] [CrossRef]
- Yao, M.; Ji, X.; Ou, X.; Wu, P.; Cheng, S. Self-standing ultrathin NiCo2S4@carbon nanotubes and carbon nanotubes hybrid films as battery-type electrodes for advanced flexible supercapacitors. J. Power Sources 2022, 543, 231829. [Google Scholar] [CrossRef]
- Tripathi, H.S.; Dutta, A.; Sinha, T.P. Tailoring structural and electrochemical properties in Sr2+ incorporated nanostructured BiFeO3 for enhanced asymmetric solid-state supercapacitor. Electrochim. Acta 2022, 421, 140505. [Google Scholar] [CrossRef]
- Yang, T.; Cao, Y.; Yu, Y.; Liu, D.; Ma, Y.; Fu, H.; Yang, Z.; Liu, D.; Zhang, Y. Kinetic enhanced bio-derived porous carbon tile laminate paper for ultrahigh-rate supercapacitors. J. Power Sources 2022, 525, 231148. [Google Scholar] [CrossRef]
- Hsieh, C.-E.; Chang, C.; Gupta, S.; Hsiao, C.-H.; Lee, C.-Y.; Tai, N.-H. Binder-free CoMn2O4/carbon nanotubes composite electrodes for high-performance asymmetric supercapacitor. J. Alloys Compd. 2022, 897, 163231. [Google Scholar] [CrossRef]
- Feng, T.; Jiao, H.; Li, H.; Wang, J.; Zhang, S.; Wu, M. High Performance of Electrochemically Deposited NiCo2S4/CNT Composites on Nickel Foam in Flexible Asymmetric Supercapacitors. Energy Fuels 2022, 36, 2189–2201. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kim, W.-B.; Kumar, S.; Yoon, T.-H.; Shim, J.-J.; Lee, J.-S. Redox-active supercapacitor electrode from two-monomer-connected precursor (Pyrrole: Anthraquinonedisulfonicacid: Pyrrole) and sulfonated multi-walled carbon nanotube. Electrochim. Acta 2022, 415, 140243. [Google Scholar] [CrossRef]
- Liu, F.; Chuan, X.; Li, B.; Qi, P. One-step carbonization synthesis of in-situ nitrogen-doped carbon tubes using fibrous brucite as the template for supercapacitors. Mater. Chem. Phys. 2022, 281, 125811. [Google Scholar] [CrossRef]
- Chakraborty, S.; Simon, R.; Vadakkekara, A.; Mary, N.L. Microwave assisted synthesis of poly(ortho-phenylenediamine-co-aniline) and functionalised carbon nanotube nanocomposites for fabric-based supercapacitors. Electrochim. Acta 2022, 403, 139678. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, J.; Zhao, X.; Kong, N.; Tao, J.; Zhou, J. Understanding the Effect of Pore Size on Electrochemical Capacitive Performance of MXene Foams. Small 2022, 18, 2202203. [Google Scholar] [CrossRef]
- Sharma, A.; Rout, C.S. 1T metallic vanadium disulfide hybridized with MXene and functionalized-MWCNT as a remarkable electrode for high power density asymmetric supercapacitor applications. Int. J. Energy Res. 2022, 46, 24537–24553. [Google Scholar] [CrossRef]
- Kariper, İ.A.; Korkmaz, S.; Karaman, C.; Karaman, O. High energy supercapacitors based on functionalized carbon nanotubes: Effect of atomic oxygen doping viavarious radiation sources. Fuel 2022, 324, 124497. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Julistian, A.; Saravanan, L.; Chen, P.-R.; Xu, B.-C.; Xie, P.-J.; Lo, A.-Y. Hydrothermal synthesis of cuo/ruo2/mwcnt nanocomposites with morphological variants for high efficient supercapacitors. Catalysts 2022, 12, 23. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, P.; Zhong, Y.; Zheng, J.; Deng, K.; Lv, X.; Li, H.; Tian, W.; Ji, J. N, S co-doped branched carbon nanotubes with hierarchical porous structure and electron/ion transfer path ways for supercapacitors and lithium-ion batteries. Carbon 2022, 198, 91–100. [Google Scholar] [CrossRef]
- Pour, G.B.; Nazarpour, H.; Aval, L.F. Polyvinylpyridine-based electrodes: Sensors and electrochemical applications. Ionics 2020, 26, 549–563. [Google Scholar] [CrossRef]
- Alkhawaldeh, A.K.; Rheima, A.M.; Kadhim, M.M.; sabri Abbas, Z.; Abed, Z.T.; mohamed dashoor Al-Jaafari, F.; Jaber, A.S.; Hachim, S.K.; Ali, F.K.; Mahmoud, Z.H. Nanomaterials as Transmitters of Non-Viral Gene Vectors: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100372. [Google Scholar] [CrossRef]
- Li, K.; Teng, H.; Sun, Q.; Li, Y.; Wu, X.; Dai, X.; Wang, Y.; Wang, S.; Zhang, Y.; Yao, K.; et al. Engineering active sites on nitrogen-doped carbon nanotubes/cobaltosic oxide heterostructure embedded inbiotemplate for high-performance supercapacitors. J. Energy Storage 2022, 53, 105094. [Google Scholar] [CrossRef]
- Zhang, D.; Xiang, Q. Electrophoretic co-deposition of Bi2O3–multiwalled carbon nanotubes coating as supercapacitor electrode. J. Am. Ceram. Soc. 2022, 105, 5638–5648. [Google Scholar] [CrossRef]
- Peng, W.; Su, Z.; Wang, J.; Li, S.; Chen, K.; Song, N.; Luo, S.; Xie, A. MnCoOx-multi-walled carbon nanotubes composite with ultra-high specific capacitance for supercapacitors. J. Energy Storage 2022, 51, 104519. [Google Scholar] [CrossRef]
- Liu, J.; He, X.; Guo, F.; Liu, B.; Sun, Z.; Zhang, L.; Chang, H. Vanadium nitride nanoparticle decorated N-doped carbon nanotube/N-doped carbon nanosheet hybrids via a C3N4 self-sacrificing method for electrochemical capacitors. RSC Adv. 2022, 12, 15354–15360. [Google Scholar] [CrossRef] [PubMed]
- Pajootan, E.; Ye, M.; Zhang, M.; Niroumandrad, S.; Omanovic, S.; Coulombe, S. Plasma-functionalized multi-walled carbon nanotubes directly grown on stainless steel meshes as supercapacitor electrodes. J. Phys. D Appl. Phys. 2022, 55, 194001. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.-J.; Lin, P.-H.; Chao, L.-C.; Lee, K.-Y. Supercapacitor characteristics of MoS2 and MoOx coated onto honeycomb-shaped carbon nanotubes. J. Vac. Sci. Technol. B 2022, 40, 032401. [Google Scholar] [CrossRef]
- Wenjuan, Y.; Igor, Z. Colloidal Processing of Mn3O4-Carbon Nanotube Nanocomposite Electrodes for Supercapacitors. Nanomaterials 2022, 12, 803. [Google Scholar] [CrossRef]
- Pour, G.B.; Aval, L.F.; Mirzaee, M. Cnts supercapacitor based on the PVDF/PVA gel electrolytes. Recent Pat. Nanotechnol. 2020, 14, 163–170. [Google Scholar] [CrossRef]
- Moustafa, H.; Karmalawi, A.M.; Youssef, A.M. Development of dapsone-capped TiO2 hybrid nanocomposites and their effects on the UV radiation, mechanical, thermal properties and antibacterial activity of PVA bionanocomposites. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100482. [Google Scholar] [CrossRef]
- Moustafa, H.; Isawi, H.; Abd El Wahab, S.M. Utilization of PVA nano-membrane based synthesized magnetic GO-Ni-Fe2O4 nanoparticles for removal of heavymetals from water resources. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100696. [Google Scholar] [CrossRef]
- Moustafa, H.; Darwish, N.A.; Youssef, A.M. Facile coating of carbon nanotubes by different resins for enhancing mechanical, electrical properties andadhesion strength of NR/Nylon 66 systems. J. Adhes. 2021, 97, 801–820. [Google Scholar] [CrossRef]
- Eyssa, H.M.; Afifi, M.; Moustafa, H. Improvement of the acoustic and mechanical properties of sponge ethylene propylene diene rubber/carbonnanotube composites crosslinked by subsequent sulfur and electron beam irradiation. Polym. Int. 2023, 72, 87–98. [Google Scholar] [CrossRef]
- Raya, I.; Kzar, H.H.; Mahmoud, Z.H.; Al Ayub Ahmed, A.; Ibatova, A.Z.; Kianfar, E. A review of gas sensors based on carbon Nanomaterial. Carbon Lett. 2022, 32, 339–364. [Google Scholar] [CrossRef]
- Majdi, H.S.; Latipov, Z.A.; Borisov, V.; Yuryevna, N.O.; Kadhim, M.M.; Suksatan, W.; Khlewee, I.H.; Kianfar, E. Nano and Battery Anode: A Review. Nanoscale Res. Lett. 2021, 16, 177. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasset, W.K.; Jasim, S.A.; Bokov, D.O.; Oleneva, M.S.; Islamov, A.; Hammid, A.T.; Mustafa, Y.F.; Yasin, G.; Alguno, A.C.; Kianfar, E. Comparison and evaluation of the performance of graphene-based biosensors. Carbon Lett. 2022, 32, 927–951. [Google Scholar] [CrossRef]
- Behzadi Pour, G.; Fekri Aval, L.; Esmaili, P. Performance of gas nanosensor in 1–4 per cent hydrogen concentration. Sens. Rev. 2019, 39, 622–628. [Google Scholar] [CrossRef]
- Behzadi, G.; Fekri, L.; Golnabi, H. Effect of the reactance term on the charge/discharge electrical measurements using cylindrical capacitive probes. J. Appl. Sci. 2011, 11, 3293–3300. [Google Scholar] [CrossRef]
- Behzadi Pour, G.; Fekri Aval, L. Influence of oxide film surface morphology and thickness on the properties of gas sensitive nanostructure sensor. Indian J. Pure Appl. Phys. 2019, 57, 743–749. [Google Scholar]
- Smaisim, G.F.; Abed, A.M.; Al-Madhhachi, H.; Hadrawi, S.K.; Al-Khateeb, H.M.M.; Kianfar, E. Graphene-Based Important Carbon Structures and Nanomaterials for Energy Storage Applications as Chemical Capacitors and Supercapacitor Electrodes: A Review. BioNanoScience 2023, 13, 219–248. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, X.; Wang, T.; Huang, X.; Dou, J.; Wu, D.; Yu, J.; Wu, S.; Chen, X. S/N-codoped carbon nanotubes and reduced graphene oxide aerogel based supercapacitors working in a wide temperature range. J. Colloid Interface Sci. 2023, 638, 709–718. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Karam, Z.; Alshaya, A.; Ghodhbane, M.; Ashraf, J.M.; Giannini, V.; Busa, C. Synergistic effect of two-dimensional additives on carbon nanotube film electrodes towards high-performance all-solid-state flexible supercapacitors. J. Energy Storage 2023, 57, 106257. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Z.; Huang, Y.; Xiong, B.; Li, X.; Yang, H. Compositing carbon nanotubes and FeCo metals with multiple electrochemical exfoliation graphene substrate for enhancing supercapacitor electrode performance. Mater. Lett. 2023, 343, 134377. [Google Scholar] [CrossRef]
- Thoravat, S.S.; Patil, V.S.; Kundale, S.S.; Dongale, T.D.; Patil, P.S.; Jadhav, S.A. In situ Fe3O4 loaded sonochemically functionalized multi-walled CNTs and RGO based composites as electrode materials for supercapacitors. Synth. Met. 2023, 294, 117312. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Kang, H.; Yang, B.; Sun, C.; Li, Z. Redox-active 7-aminoindole and carbon nanotubes co-modified reduced graphene oxide for Zn-ion hybridcapacitors with excellent energy density and super-long cycling stability. J. Power Sources 2023, 562, 232789. [Google Scholar] [CrossRef]
- Juang, R.-H.; Guo, J.-S.; Huang, Y.-J.; Chen, I.-W.P. Experimental and theoretical investigations of covalent functionalization of 1D/2D carbon-based buckypaper viaaryl diazonium chemistry for high-performance energy storage. Carbon 2023, 205, 402–410. [Google Scholar] [CrossRef]
- Kong, L.; Hou, L.; Liu, M.; Chen, W.; Xu, X.; Zhou, X.; Liu, Z.; Shao, G. Highly defective N-doped carbon/reduced graphene oxide composite cathode material with rapid electrons/ionsdual transport channels for high energy density lithium-ion capacitor. Electrochim. Acta 2023, 443, 141704. [Google Scholar] [CrossRef]
- Li, M.; Xu, B.; Zheng, L.; Zhou, J.; Luo, Z.; Li, W.; Ma, W.; Mao, Q.; Xiang, H.; Zhu, M. Highly stable polyaniline array@ partially reduced graphene oxide hybrid fiber for high-performance flexible supercapacitors. Carbon 2023, 203, 455–461. [Google Scholar] [CrossRef]
- Prashant, H.D.; Sajan Raj, S.L.; Saraswathi, R.; Kavibharathy, K.; Kumaresan, L.; Chenrayan, S.; Vediappan, K. One-dimensional curved graphene nanoribbons assisted MoS2 nanosheets enhanced electrode material for high-performance supercapacitor. Mater. Lett. 2023, 331, 133507. [Google Scholar] [CrossRef]
- Mendoza-Jiménez, R.; Oliva, J.; Mtz-Enriquez, A.I.; Etafo, N.O.; Rodriguez-Gonzalez, V. Enhancement of capacitance and widening of the operating voltage window of flexible supercapacitors by adding Li2TiO3/BN on their electrodes. Ceram. Int. 2023, 49, 20980–20988. [Google Scholar] [CrossRef]
- Wang, S.; Cao, K.; Xu, L.; Tong, Y. Improving electrochemical properties of carbon nanotubes/reduced graphene oxide composite fibers by chemical modification. Appl. Phys. A Mater. Sci. Process. 2023, 129, 56. [Google Scholar] [CrossRef]
- Fikry, M.; Abbas, M.; Sayed, A.; Nouh, A.; Ibrahim, A.; Mansour, A.S. Using a novel graphene/carbon nanotubes composite for enhancement of the supercapacitor electrode capacitance. J. Mater. Sci. Mater. Electron. 2022, 33, 3914–3924. [Google Scholar] [CrossRef]
- Das, J.K.; Padhy, A.; Parida, S.; Pathi, R.M.; Behera, J.N. Tetra germanium nonaselenide enwrapped with reduced graphene oxide and functionalized carbon nanotubes (Ge4Se9/RGO/FCNTs) hybrids for improved energy storage performances. Dalton Trans. 2022, 51, 11526–11535. [Google Scholar] [CrossRef]
- Nasreen, F.; Anwar, A.W.; Majeed, A.; Ahmad, M.A.; Ilyas, U.; Ahmad, F. High performance and remarkable cyclic stability of a nanostructured RGO-CNT-WO3 supercapacitor electrode. RSC Adv. 2022, 12, 11293–11302. [Google Scholar] [CrossRef]
- Ngo, H.L.; Bui, T.H.; Van Thuan, D.; Pham, H.P.; Cao, X.T.; Sharma, A.K.; Nguyen, T.B.; Le, C.L.; Anh, T.H.; Hoang, S.M.T.; et al. Polydopamine-modified MWCNT/graphene oxide hybrid 3D carbon nano-structure for flexible symmetric supercapacitor electrodes. Appl. Nanosci. 2022, 13, 3853. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Isaikina, O.Y.; Novotortsev, R.Y.; Stolbov, D.N.; Xia, H.; Savilov, S.V. Application of MnO2/MWCNT composite in supercapacitors. Mater. Today Proc. 2022, 60, 1008–1011. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on Carbon Fibers Modified with Carbon Nanotubes for Ultrafast Flexible Supercapacitors in Ionic Liquid Electrolytes with Wide Voltage Windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]
- Yang, W.; Liang, W.; Zhitomirsky, I. Application of Rhamnolipids as Dispersing Agents for the Fabrication of Composite MnO2-Carbon Nanotube Electrodes for Supercapacitors. Molecules 2022, 27, 1659. [Google Scholar] [CrossRef] [PubMed]
- Yesilbag, Y.O.; Tuzluca Yesilbag, F.N.; Huseyin, A.; Salih, A.J.S.; Ertugrul, M. MnO2 nanosheets synthesized on nitrogen-doped vertically aligned carbon nanotubes as a supercapacitor electrode material. J. Alloys Compd. 2022, 925, 166570. [Google Scholar] [CrossRef]
- Ragupathi, H.; Arockiaraj, M.A.; Choe, Y. A novel β-MnO2 and carbon nanotube composite with potent electrochemical properties synthesized using amicrowave-assisted method for use in supercapacitor electrodes. New J. Chem. 2022, 46, 15358–15366. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Ma, G.; Ding, Y.; Wang, J.; Ye, Z.; Peng, X.; Li, D. Novel Synthesis and Characterization of Flexible MnO2/CNT Composites Co-deposited on Graphite Paper as Supercapacitor Electrodes. J. Electron. Mater. 2022, 51, 2982–2994. [Google Scholar] [CrossRef]
- Satiye, K.; İshak, A.K.; Ceren, K.; Onur, K. MWCNT/Ruthenium hydroxide aerogel supercapacitor production and investigation of electrochemical performances. Sci. Rep. 2022, 12, 12862. [Google Scholar] [CrossRef]
- Yang, X.; Yao, Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. 3D Macroporous Oxidation-Resistant Ti3C2Tx MXene Hybrid Hydrogels for Enhanced Supercapacitive Performances with Ultralong Cycle Life. Adv. Funct. Mater. 2022, 32, 2109479. [Google Scholar] [CrossRef]
- Jose, J.; Vigneshwaran, J.; Baby, A.; Viswanathan, R.; Jose, S.P.; Sreeja, P.B. Dimensionally engineered ternary nanocomposite of reduced graphene oxide/multiwalled carbonnanotubes/zirconium oxide for supercapacitors. J. Alloys Compd. 2022, 896, 163067. [Google Scholar] [CrossRef]
- Wang, S.; Cao, K.; Xu, L.; Zhao, D.; Tong, Y. Carbon nanotubes/reduced graphene oxide composites as electrode materials for supercapacitors. Appl. Phys. A Mater. Sci. Process. 2022, 128, 81. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Chen, X.; Ying, Y.; Shi, W. In-situ generated NiCo2O4/CoP polyhedron with rich oxygen vacancies interpenetrating by P-doped carbonnanotubes for high performance supercapacitors. J. Colloid Interface Sci. 2022, 608, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.; Lee, C.; Tai, N. High retention supercapacitors using carbon nanomaterials/iron oxide/nickel-iron layered double hydroxides as electrodes. J. Energy Storage 2022, 46, 103805. [Google Scholar] [CrossRef]
- Qiu, H.; Ma, Q.; Sun, X.; Han, X.; Jia, G.; Zhang, Y.; He, W. Construction of orderly self-growing nanosheet arrays on nickel foam by introducing novel carbon composite materials for high-performance supercapacitors. Appl. Surf. Sci. 2022, 578, 152019. [Google Scholar] [CrossRef]
- Chen, X.; Xin, N.; Li, Y.; Sun, C.; Li, L.; Ying, Y.; Shi, W.; Liu, Y. Novel 2D/2D NiCo2O4/ZnCo2O4@rGO/CNTs self-supporting composite electrode with high hydroxyl ion adsorption capacity for asymmetric supercapacitor. J. Mater. Sci. Technol. 2022, 127, 236–244. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.; Chen, H.; Liu, H.; Yang, L.; Chen, Y.; Li, H. ZIF-67 derived in-situ grown N–Co3S4-GN/CNT interlinked conductive networks for high-performance especially cycling stable supercapacitors. Carbon 2022, 194, 10–22. [Google Scholar] [CrossRef]
- Gong, D.; Tong, H.; Xiao, J.; Wu, Y.; Chen, X.; Zhou, Y.; Zhang, X. Enhanced Reaction Kinetics of N-MnO2 Nanosheets with Oxygen Vacancies via Mild NH3·H2O Bath Treatment for Advanced Aqueous Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 7490–7502. [Google Scholar] [CrossRef]
- Pathak, M.; Rout, C.S. Hierarchical NiCo2S4 nanostructures anchored on nanocarbons and Ti3C2Tx MXene for high-performance flexible solid-state asymmetric supercapacitors. Adv. Compos. Hybrid Mater. 2022, 5, 1404–1422. [Google Scholar] [CrossRef]
- Zawar, S.; Ali, G.; Mustafa, G.M.; Patil, S.A.; Ramay, S.M.; Atiq, S. Mn0.06Co2.94O4 nano-architectures anchored on reduced graphene oxide as highly efficient hybrid electrodes for supercapacitors. J. Energy Storage 2022, 50, 104298. [Google Scholar] [CrossRef]
- Moreto, J.A.; Silva, P.H.S.; Moraes Moura, G.; da Silva, C.C.; Ferreira, D.C.; da Cunha, T.H.R.; Silva, G.G.; Rouxinol, F.; de Siervo, A.; Gelamo, R.V. The effect of plasma treatment on flexible self-standing supercapacitors composed by carbon nanotubes and multilayer graphene composites. J. Mater. Sci. 2022, 57, 8779–8799. [Google Scholar] [CrossRef]
- Rustamaji, H.; Prakoso, T.; Devianto, H.; Widiatmoko, P.; Nurdin, I. Design, Fabrication, and Testing of Supercapacitor Based on Nanocarbon Composite Material. ASEAN J. Chem. Eng. 2022, 22, 19–32. [Google Scholar] [CrossRef]
- Setyoputra, A.D.; Ruffa, H.; Sutanto, H.; Subagio, A. The Characterisation of MWCNT-rGO-TiO2 Nanocomposite as Potential Electrode Material for Hybrid Supercapacitor. Int. J. Electrochem. Sci. 2022, 17, 22053. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Li, Y.; Deng, C.; Liang, B. Potassium citrate-derived carbon nanosheets/carbon nanotubes/polyaniline ternary composite for supercapacitor electrodes. Electrochim. Acta 2022, 403, 139571. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Chang-Jian, C.-W.; Huang, T.-Y.; Chen, Y.-L.; Huang, J.-H.; Wu, N.-J.; Hsu, S.-C.; Chen, C.-P. High-performance supercapacitor based on a ternary nanocomposites of NiO, polyaniline, and Ni/NiO-decorated MWCNTs. J. Taiwan Inst. Chem. Eng. 2022, 134, 104318. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Samy, M.M.; Mansoure, T.H.; Sharma, S.U.; Tsai, M.-S.; Chen, J.-H.; Lee, J.-T.; Kuo, S.-W. Dispersions of 1,3,4-Oxadiazole-Linked Conjugated Microporous Polymers with Carbon Nanotubes as a High-Performance Electrode for Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 3677–3688. [Google Scholar] [CrossRef]
- Panasenko, I.V.; Bulavskiy, M.O.; Iurchenkova, A.A.; Aguilar-Martinez, Y.; Fedorov, F.S.; Fedorovskaya, E.O.; Mikladal, B.; Kallio, T.; Nasibulin, A.G. Flexible supercapacitors based on free-standing polyaniline/single-walled carbon nanotube films. J. Power Sources 2022, 541, 231691. [Google Scholar] [CrossRef]
- Dai, H.; Li, R.; Su, S.; Cui, Y.; Lin, Y.; Zhang, L.; Zhu, X. Preparation and Characterization of PANI/MWCNT/RGO Ternary Composites as Electrode Materials for Supercapacitors. J. Electron. Mater. 2022, 51, 1409–1420. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Y.; Cao, M. Constructing MXene-PANI@MWCNTs heterojunction with high specific capacitance towards flexible micro-supercapacitor. Nanotechnology 2022, 33, 295401. [Google Scholar] [CrossRef]
- Jiang, Y.; Ou, J.; Luo, Z.; Chen, Y.; Wu, Z.; Wu, H.; Fu, X.; Luo, S.; Huang, Y. High Capacitive Antimonene/CNT/PANI Free-Standing Electrodes for Flexible Supercapacitor Engaged with Self-Healing Function. Small 2022, 18, 2201377. [Google Scholar] [CrossRef]
- Jasna, M.; Muraleedharan Pillai, M.; Abhilash, A.; Midhun, P.S.; Jayalekshmi, S.; Jayaraj, M.K. Polyaniline wrapped carbon nanotube/exfoliated MoS2 nanosheet composite as a promising electrode for high power supercapacitors. Carbon Trends 2022, 7, 100154. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Qiu, J.; Geng, B.; Du, M.; Zheng, Q. Solvent-assisted self-assembly to fabricate a ternary flexible free-standing polyaniline@MXene-CNTs electrode forhigh-performance supercapacitors. J. Alloys Compd. 2022, 921, 166062. [Google Scholar] [CrossRef]
- Balboni, R.D.C.; Maron, G.K.; Masteghin, M.G.; Tas, M.O.; Rodrigues, L.S.; Gehrke, V.; Alano, J.H.; Andreazza, R.; Carreño, N.L.V.; Silva, S.R.P. An easy to assemble PDMS/CNTs/PANI flexible supercapacitor with high energy-to-power density. Nanoscale 2022, 14, 2266–2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, T.; Chen, Y.; Liu, G. Lignosulfonate functionalized nanomaterials for enhancement of the electrochemical performance of polyaniline. Appl. Surf. Sci. 2022, 593, 153457. [Google Scholar] [CrossRef]
- Dadashi, R.; Bahram, M.; Faraji, M. Fabrication of a solid-state symmetrical supercapacitor based on polyaniline grafted multiwalled carbon nanotube deposit onto Created Vertically Oriented Graphene Nanosheets on graphite sheet. J. Energy Storage 2022, 52, 104775. [Google Scholar] [CrossRef]
- Xu, H.; Cui, L.; Pan, X.; An, Y.; Jin, X. Carboxymethylcellulose-polyaniline/carbon nanotube (CMC-PANI/CNT) film as flexible and highly electrochemical active electrode for supercapacitors. Int. J. Biol. Macromol. 2022, 219, 1135–1145. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Xu, X.; Yang, H.; Zhou, Y.; Yang, D.; Zhang, Y.; Li, J. Grape-clustered polyaniline grafted with carbon nanotube woven film as a flexible electrode material for supercapacitors. J. Appl. Polym. Sci. 2022, 139, e52785. [Google Scholar] [CrossRef]
- Kim, T.; Subedi, S.; Dahal, B.; Chhetri, K.; Mukhiya, T.; Muthurasu, A.; Gautam, J.; Lohani, P.C.; Acharya, D.; Pathak, I.; et al. Homogeneous Elongation of N-Doped CNTs over Nano-Fibrillated Hollow-Carbon-Nanofiber: Mass and Charge Balance in Asymmetric Supercapacitors Is No Longer Problematic. Adv. Sci. 2022, 9, 2200650. [Google Scholar] [CrossRef]
- Zhuo, J.-L.; Wang, Y.-L.; Wang, Y.-G.; Xu, M.-Q.; Sha, J.-Q. Surfactant-assisted fabrication and supercapacitor performances of a 12-phosphomolybdate-pillared metal-organic framework containing a helix and its SWNT nanocomposites. CrystEngComm 2022, 24, 579–586. [Google Scholar] [CrossRef]
- Shi, L.; Yang, W.; Zha, X.; Zeng, Q.; Tu, D.; Li, Y.; Yang, Y.; Xu, J.; Chen, F. Metal-organic frameworks-derived porous carbon nanotube for high performance supercapacitor electrode materials. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129862. [Google Scholar] [CrossRef]
- Habibi, R.; Mehrpooya, M.; Ganjali, M. Synthesis of ternary CoZnAl layered double hydroxide and Co-embedded N-doped carbon nanotube hollow polyhedron nanocomposite as a bifunctional material for ORR electrocatalyst and supercapacitor electrode. J. Energy Storage 2022, 54, 105377. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Daphine, S.; Yuvakkumar, R.; Kungumadevi, L.; Ravi, G.; Al-Sehemi, A.G.; Velauthapillai, D. ZnCo2O4/CNT composite for efficient supercapacitor electrodes. Ceram. Int. 2022, 48, 24745–24750. [Google Scholar] [CrossRef]
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Highly compressible binder-free sponge supercapacitor electrode based on flower-like NiO/MnO2/CNT. J. Alloys Compd. 2022, 913, 165053. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, R.; Yang, D.; Lu, S.; Wang, J.; Liu, C.; Wang, S. A Supercapacitor Electrode Synthesis Strategy: Proton Acid-Treated Ti3C2Tx Film with Single-walled Carbon Nanotubes as a Reinforcement. ChemistrySelect 2022, 7, e202200690. [Google Scholar] [CrossRef]
- Lv, S.; Ma, L.; Shen, X.; Tong, H. Potassium chloride-catalyzed growth of porous carbon nanotubes for high-performance supercapacitors. J. Alloys Compd. 2022, 906, 164242. [Google Scholar] [CrossRef]
- Zahra, S.A.; Anasori, B.; Iqbal, M.Z.; Ravaux, F.; Al Tarawneh, M.; Rizwan, S. Enhanced electrochemical performance of vanadium carbide MXene composites for supercapacitors. APL Mater. 2022, 10, 060901. [Google Scholar] [CrossRef]
- Gyulasaryan, H.T.; Azizbekyan, G.G.; Sisakyan, N.S.; Chilingaryan, G.N.; Grapov, D.V.; Kukuts, Y.M.; Sharoyan, E.G.; Manukyan, A.S. Electrode Material for Supercapacitors Based on Products of Solid Phase Pyrolysis of Metal-Phthalocyanines. J. Contemp. Phys. 2022, 57, 76–80. [Google Scholar] [CrossRef]
- Tamilselvan, A.; Kundu, M. Ex-situ synthesis of MnS nanoparticles imbedded with carbon nanotubes as a high-performance electrode material for supercapacitors. Mater. Today Proc. 2022, 68, 146–151. [Google Scholar] [CrossRef]
- Teng, S.; Shi, S.; Wang, G.; Xiang, Y.; Wan, G. Ozone-activated CNTs to induce uniform coating of MnO2 as high-performance supercapacitor electrodes. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 1163–1169. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, J.; Cai, S.; Zhang, K.; Zhan, H.; Huang, S.; Chen, L.; Sun, X. One-Step Preparation of NiV-LDH@CNT Hierarchical Composite for Advanced Asymmetrical Supercapacitor. Adv. Eng. Mater. 2022, 24, 2101174. [Google Scholar] [CrossRef]
- Tourchi Moghadam, M.T.; Seifi, M.; Jamali, F.; Azizi, S.; Askari, M.B. ZnWO4-CNT as a superior electrode material for ultra-high capacitance supercapacitor. Surf. Interfaces 2022, 32, 102134. [Google Scholar] [CrossRef]
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Outstanding supercapacitor performance with intertwined flower-like NiO/MnO2/CNT electrodes. Mater. Res. Bull. 2022, 149, 111745. [Google Scholar] [CrossRef]
- Otun, K.O.; Xaba, M.S.; Zong, S.; Liu, X.; Hildebrandt, D.; El-Bahy, S.M.; Alotaibi, M.T.; El-Bahy, Z.M. ZIF-8-derived ZnO/C decorated hydroxyl-functionalized multi-walled carbon nanotubes as a new compositeelectrode for supercapacitor application. Colloids Interface Sci. Commun. 2022, 47, 100589. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Vikraman, D.; Yadav, H.M.; Kim, H.-S.; Sivasamy, A.; Kim, H.S. Fabrication of NiCo2S4 accumulated on metal organic framework nanostructured with multiwalled carbon nanotubes composite material for supercapacitor application. Ceram. Int. 2022, 48, 29102–29110. [Google Scholar] [CrossRef]
- Sakthivel, P.; Anandha babu, G.; Karuppiah, M.; Asaithambi, S.; Balaji, V.; Pandian, M.S.; Ramasamy, P.; Mohammed, M.K.A.; Navaneethan, N.; Ravi, G. Electrochemical energy storage applications of carbon nanotube supported heterogeneous metal sulfide electrodes. Ceram. Int. 2022, 48, 6157–6165. [Google Scholar] [CrossRef]
- Agarwal, A.; Majumder, S.; Sankapal, B.R. Multi-walled carbon nanotubes supported copper phosphate microflowers for flexible solid-state supercapacitor. Int. J. Energy Res. 2022, 46, 6177–6196. [Google Scholar] [CrossRef]
- Li, K.; Zhang, P.; Soomro, R.A.; Xu, B. Alkali-Induced Porous MXene/Carbon Nanotube-Based Film Electrodes for Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 4180–4186. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Chen, L.; Chu, M.; Dong, Y.; Liu, D.; Liu, P.; Qu, D.; Duan, J.; Li, X. MOF(Ni)/CNT composites with layer structure for high capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128802. [Google Scholar] [CrossRef]
- Yang, R.; Bai, X.; Guo, X.; Song, K.; Jia, L.; Chen, X.; Wang, J. Hierarchical NiCo2O4 nanostructured arrays decorated over the porous Ni/C as battery-type electrodes for supercapacitors. Appl. Surf. Sci. 2022, 586, 152574. [Google Scholar] [CrossRef]
- Wang, B.Q.; Gong, S.H.; Sun, Q.S.; Liu, F.; Wang, X.C.; Cheng, J.P. Carbon nanotubes refined mesoporous NiCoO2 nanoparticles for high−performance supercapacitors. Electrochim. Acta 2022, 402, 139575. [Google Scholar] [CrossRef]
- Geioushy, R.A.; Attia, S.Y.; Mohamed, S.G.; Li, H.; Fouad, O.A. High-performance electrode materials for supercapacitor applications using Ni-catalyzed carbon nanostructures derived from biomass waste materials. J. Energy Storage 2022, 48, 104034. [Google Scholar] [CrossRef]
- Sivakumar, M.; Muthukutty, B.; Panomsuwan, G.; Veeramani, V.; Jiang, Z.; Maiyalagan, T. Facile synthesis of NiFe2O4 nanoparticle with carbon nanotube composite electrodes for high-performance asymmetric supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129188. [Google Scholar] [CrossRef]
- Yao, M.; Guo, C.; Zhang, Y.; Zhao, X.; Wang, Y. In situ encapsulation of metal sulfide into hierarchical nanostructured electrospun nanofibers as self-supported electrodes for flexible quasi-solid-state supercapacitors. J. Mater. Chem. C 2022, 10, 542–548. [Google Scholar] [CrossRef]
- Fahimi, Z.; Moradlou, O. High-performance solid-state asymmetric supercapacitor based on Co3V2O8/carbon nanotube nanocomposite and gel polymer electrolyte. J. Energy Storage 2022, 50, 104697. [Google Scholar] [CrossRef]
- Houpt, D.; Ji, J.; Yang, D.; Choi, J.H. High-Performance Supercapacitor Electrodes Based on Composites of MoS2 Nanosheets, Carbon Nanotubes, andZIF-8 Metal–Organic Framework Nanoparticles. ACS Appl. Nano Mater. 2022, 5, 1491–1499. [Google Scholar] [CrossRef]
- Chen, H.-C.; Hou, L.-Y.; He, C.; Laing, P.-J.; Huang, C.-Y.; Kuo, W.-S. Metal-Organic Framework-Assisted Synthesis of Three-Dimensional ZnCoS Effloresced Nanopillars@CNT Paper for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors with Ultrahigh Specific Capacitance. ACS Appl. Energy Mater. 2022, 5, 8262–8272. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, D.; Xu, C.; Li, Z.; Bi, S.; Xu, H.; Dou, H.; Zhang, X. MnO2/carbon nanotube free-standing electrode recycled from spent manganese-oxygen battery as high-performance supercapacitor material. J. Mater. Sci. 2022, 57, 8818–8827. [Google Scholar] [CrossRef]
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational design of Ti3C2/carbon nanotubes/MnCo2S4 electrodes for symmetric supercapacitors with high energy storage. Appl. Surf. Sci. 2022, 581, 152432. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhi, M.; Hong, Z. Bi-Fe chalcogenides anchored carbon matrix and structured core–shell Bi-Fe-P@Ni-P nanoarchitectures with appealing performances for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1352–1363. [Google Scholar] [CrossRef]
- Pandit, B.; Sankapal, B.R. Cerium Selenide Nanopebble/Multiwalled Carbon Nanotube Composite Electrodes for Solid-State Symmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 3007–3017. [Google Scholar] [CrossRef]
- Real, C.G.; Thaines, E.H.N.S.; Pocrifka, L.A.; Freitas, R.G.; Singh, G.; Zanin, H. Freestanding niobium pentoxide-decorated multiwalled carbon nanotube electrode: Charge storage mechanism insodium-ion pseudocapacitor and battery. J. Energy Storage 2022, 52, 104793. [Google Scholar] [CrossRef]
- Ren, X.; Yuan, Z.; Ma, Y.; Zhang, C.; Qin, C.; Jiang, X. Nitrogen-/Boron-Doped Carbon from Poplar Powder and Carbon Nanotube Composite as Electrode Material for Supercapacitors. Energy Fuels 2022, 36, 2841–2850. [Google Scholar] [CrossRef]
- Maity, C.K.; Sahoo, S.; Verma, K.; Nayak, G.C. SnS2@Conducting Energy Level-Induced Functionalized Boron Nitride for an Asymmetric Supercapacitor. Energy Fuels 2022, 36, 2248–2259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).