Abstract
This paper provides an overview of the significance of precise thermal analysis in the context of lithium-ion battery systems. It underscores the requirement for additional research to create efficient methodologies for modeling and controlling thermal properties, with the ultimate goal of enhancing both the safety and performance of Li-ion batteries. The interaction between temperature regulation and lithium-ion batteries is pivotal due to the intrinsic heat generation within these energy storage systems. A profound understanding of the thermal behaviors exhibited by lithium-ion batteries, along with the implementation of advanced temperature control strategies for battery packs, remains a critical pursuit. Utilizing tailored models to dissect the thermal dynamics of lithium-ion batteries significantly enhances our comprehension of their thermal management across a wide range of operational scenarios. This comprehensive review systematically explores diverse research endeavors that employ simulations and models to unravel intricate thermal characteristics, behavioral nuances, and potential runaway incidents associated with lithium-ion batteries. The primary objective of this review is to underscore the effectiveness of employed characterization methodologies and emphasize the pivotal roles that key parameters—specifically, current rate and temperature—play in shaping thermal dynamics. Notably, the enhancement of thermal design systems is often more feasible than direct alterations to the lithium-ion battery designs themselves. As a result, this thermal review primarily focuses on the realm of thermal systems. The synthesized insights offer a panoramic overview of research findings, with a deeper understanding requiring consultation of specific published studies and their corresponding modeling endeavors.
1. Introduction
Lithium battery systems encompass a diverse array of configurations, as illustrated in Figure 1. Among these configurations, lithium-ion batteries emerge as a highly promising storage technology for reducing greenhouse gas emissions within the transportation sector. With their exceptional power and energy densities, lithium-ion batteries offer a well-suited solution for an extensive range of renewable energy storage applications. The inherent versatility of lithium-ion battery technology makes it adaptable to various usage scenarios, each with distinct specifications. In addition to their role in the renewable energy sector, lithium-ion batteries have gained significant traction in military, aerospace, and residential domains due to their exceptional safety profile, unwavering reliability, substantial power capacities, and extended operational lifespans [1,2,3]. The intricate interplay depicted in Figure 1 sheds light on the diverse technologies and applications seamlessly accommodated by lithium-ion batteries.
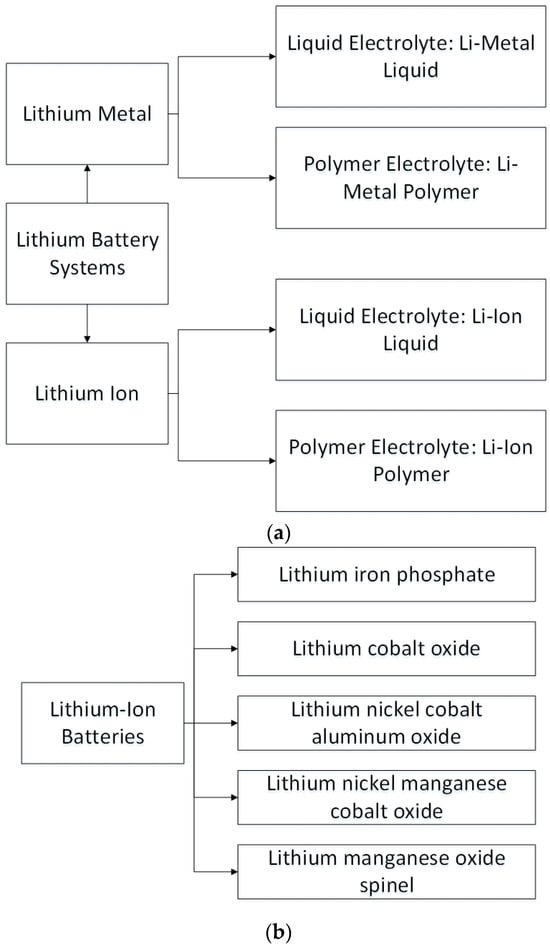
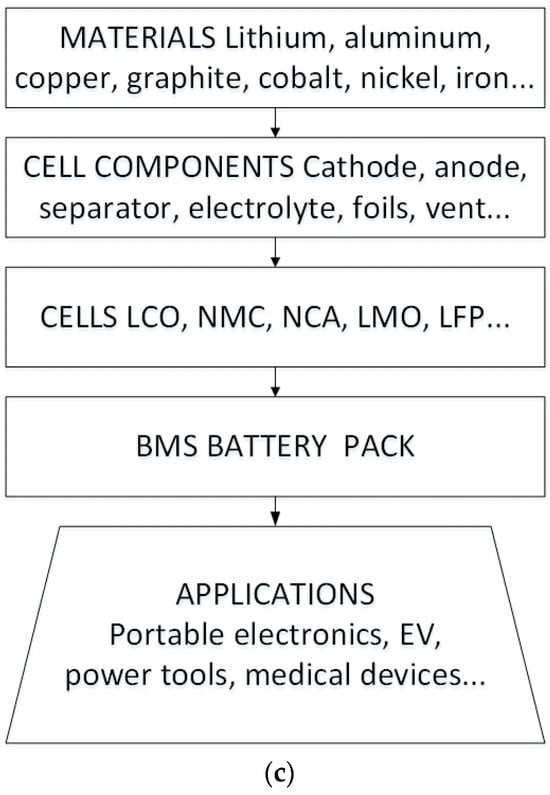
Figure 1.
Categorization of lithium battery systems according to (a) The nature of their electrolyte, (b) Their cathode chemistry, and (c) The construction configuration of lithium-ion battery manufacturing [4,5,6,7,8].
Dafen Chen et al. conducted an extensive comparative investigation encompassing various cooling methodologies for lithium-ion batteries [9]. These methodologies included direct liquid cooling, indirect liquid cooling, and active air-cooling mechanisms. By combining numerical simulations with experimental data obtained from laboratory tests, the study yielded significant insights. The results highlighted the limited effectiveness of air-cooling due to its relatively lower thermal conductivity and heat capacity, leading to only a modest reduction in maximum temperature. While air-based systems required less power for circulation, they were less efficient at rapidly dissipating heat during quick discharges. Indirect liquid cooling, while effective, introduced added weight due to the higher density of aluminum compared to mineral oil. Notably, the investigation also explored fin cooling, utilizing water/glycol as the coolant fluid. The analysis extended to the characterization of temperature distributions across the battery surface, contingent on varying velocities and flow directions for air-cooling and direct liquid cooling. Importantly, integrating experimental data from battery calorimeters assumes paramount importance in the thermal analysis of lithium-ion batteries. This necessity arises from the pronounced temperature elevation and heat dissipation gradients experienced by these batteries during charging and discharging, arising from both irreversible and reversible heat generation. A potential solution involves developing a dynamic, time-dependent heat generation model for lithium-ion batteries, incorporating data from battery calorimeters. This model could effectively address the challenge of non-uniform temperature distribution and heat generation on battery cell surfaces. Strategies involving modifications to the battery cell’s geometry, such as spatially separating negative and positive tabs or increasing tab size to enhance contact between electrode current collectors and external tabs, emerge as potential measures. Furthermore, exploring the configuration of current collectors within lithium-ion battery cells can lead to the selection of an optimal design if direct alterations by manufacturers prove unfeasible [10,11,12,13,14,15,16].
Another significant study by M. Shadman Rad et al. focused on the thermal modeling of lithium-ion batteries [17]. The researchers developed thermal models based on heat generation data derived from various experiments. Using an experimental setup consistent with contemporary simulation laboratories, the thermal model analyzed heat generation and temperature changes within a lithium-ion battery cell. The resulting model-calculated heat generation and temperature values were meticulously compared against experimental data to validate the model’s accuracy. In a similar vein, Cong Zhu et al. developed a customized thermal model specifically tailored to the complex configuration and geometry of lithium-ion batteries [18]. Validating the model through comparisons with laboratory measurements, the study confirmed the model’s ability to predict the batteries’ thermal behavior. Panchal et al. [19] delved into a thermal analysis of lithium-ion batteries, revealing temperature fluctuations along the battery cell’s surface, particularly under high current rates. This phenomenon originated from significant heat dissipation driven by notable temperature gradients. Collectively, previous investigations have aimed to elucidate diverse strategies for managing and evaluating the thermal characteristics of lithium-ion batteries across various cycling and operational scenarios. Literature has explored numerous configurations and assemblies to unravel the thermal intricacies of these batteries. Effective thermal management is crucial to mitigate temperature escalation, preempt further exothermic reactions, regulate heat generation, and prevent thermal runaway. While numerous simulations have attempted to predict the thermal properties of lithium-ion batteries and propose enhancements to their thermal behavior, comprehensive comparative analyses and investigations into diverse thermal configurations remain limited. This study serves as a valuable resource for both researchers and manufacturers, providing insights into the multifaceted thermal behavior of lithium-ion batteries across diverse conditions. It underscores the urgency for more extensive research into thermal characteristics, paralleling the substantial progress made in understanding the mechanics and mechanisms of these batteries. Consequently, a thorough assessment of the current state and recent advancements in thermal modeling research for lithium-ion batteries emerges as a critical undertaking.
2. Purpose of the Battery Materials
The primary aim of battery components is to facilitate the effective and dependable storage and discharge of electrical energy within batteries. Batteries are indispensable elements in a broad array of devices and applications, ranging from compact consumer gadgets like smartphones and laptops to larger systems such as electric vehicles and grid energy storage. Battery materials assume a pivotal role in determining the performance, capacity, and lifespan of batteries. The following are the key objectives of battery materials:
Energy Retention: Battery materials store electrical energy in a chemical state, enabling its controlled release when needed. This capacity for energy storage is crucial for powering portable electronic devices and electric vehicles.
Power Generation and Backup: Batteries have the capability to store energy generated from renewable sources like solar panels and wind turbines, ensuring a consistent power supply even during periods of insufficient sunlight or wind. They also serve as backup power sources in the event of grid failures.
Mobility for Electronic Devices: In consumer electronics such as smartphones, laptops, and tablets, battery materials empower these devices to be mobile and function without the constant need for a power connection.
Electric Vehicles: Battery materials, such as lithium-ion, are utilized in electric vehicle batteries to stockpile energy for propulsion. The efficiency and capacity of these materials directly impact the range and performance of electric cars.
Grid-Scale Energy Storage: Large-scale battery systems employ advanced materials to store surplus energy during periods of low demand and release it during peak demand, thereby stabilizing the electrical grid and facilitating the integration of renewable energy sources.
Energy Efficiency: Battery materials can enhance the energy efficiency of various systems by accumulating energy during periods of low demand and discharging it during peak demand, reducing the necessity for constant energy generation.
Environmental Advantages: Battery materials can play a pivotal role in diminishing greenhouse gas emissions by enabling the adoption of electric vehicles and renewable energy sources, which possess a smaller carbon footprint compared to fossil fuels.
Diminishing Dependence on Fossil Fuels: Through the provision of energy storage solutions, battery materials contribute to reducing our dependence on fossil fuels, aiding in the mitigation of climate change and the reduction of air pollution.
Augmenting Energy Accessibility: Batteries with efficient materials can extend electricity to remote or off-grid areas, improving the living conditions of underserved populations.
Research and Innovation: The continuous development of new battery materials represents an ongoing field of research and innovation, motivated by the need for more efficient, longer-lasting, and safer energy storage solutions.
Battery materials are vital for the efficient storage and release of electrical energy, enabling a diverse range of applications and promoting the transition to cleaner and more sustainable energy systems. Advances in battery materials continue to exert a profound impact on technology and the environment.
3. The Reasons for Choosing Lithium-Ion Batteries
Lithium-ion (Li-ion) batteries have gained widespread popularity in various applications due to their numerous beneficial features. Below are some compelling reasons for selecting lithium-ion batteries:
Impressive Energy Density: Li-ion batteries boast a remarkable energy density, enabling them to store substantial energy within a compact and lightweight package. This makes them particularly suitable for portable electronic devices, electric vehicles (EVs), and scenarios where size and weight are crucial factors.
Reusability: Li-ion batteries are rechargeable, allowing for multiple uses throughout their lifespan. This translates to cost-effectiveness compared to disposable single-use batteries.
Extended Cycle Life: Li-ion batteries typically endure a longer cycle life when compared to many other rechargeable battery types, capable of enduring hundreds to thousands of charge and discharge cycles before experiencing significant capacity degradation.
Low Self-Discharge Rate: Li-ion batteries exhibit a relatively low self-discharge rate, ensuring they retain their charge for extended periods when not in active use. This feature is valuable for devices that may remain idle for prolonged periods between uses.
Swift Charging: Li-ion batteries can be charged rapidly, especially when compared to certain other battery technologies. This rapid charging capability is essential for applications like smartphones and electric vehicles.
Versatility: Lithium-ion batteries come in various shapes and sizes, making them adaptable and suitable for a wide array of applications, ranging from small consumer electronics to large-scale energy storage systems.
Minimal Memory Effect: Unlike some other rechargeable battery types, Li-ion batteries exhibit a minimal memory effect. Consequently, they do not require complete discharge before recharging, making them more user-friendly.
Elevated Voltage Output: Li-ion batteries provide a relatively high nominal voltage. This elevated voltage output is advantageous for many applications, facilitating efficient device operation.
Reliability and Safety: While Li-ion batteries can present safety concerns if mishandled, they feature enhanced safety measures compared to older battery technologies. Manufacturers have integrated various safety mechanisms, including thermal protection and pressure relief valves, to mitigate the risk of thermal runaway and other safety issues.
Environmental Considerations: Li-ion batteries are considered more environmentally friendly than certain other battery chemistries, such as lead-acid batteries, due to their lower toxicity and potential for recycling. Recycling programs for Li-ion batteries are becoming increasingly widespread.
It is important to note that while Li-ion batteries offer numerous advantages, they are not without limitations, including environmental concerns, the potential for thermal runaway, and the finite lifespan of the cells. However, ongoing research and development endeavors are aimed at addressing these challenges and further enhancing Li-ion battery technology.
4. Impact of Temperature on Lithium-Ion Batteries
The impact of temperature on lithium-ion batteries’ performance degradation is vividly depicted in Figure 2. This deterioration primarily results from the intricate interplay of battery materials and the chemical reactions occurring within. Thermal fluctuations have the potential to induce variations in the kinetics of electrochemical reactions taking place within the battery matrix. Moreover, temperature significantly influences fundamental parameters, including the ionic conductivities of both electrolytes and electrodes. The consequences of extreme temperature conditions become even more complex when compared to milder thermal environments. The ambient temperature, influenced by variables such as seasonal changes, meteorological factors, and broader climate influences, displays location-specific fluctuations based on vehicular geography. This modulation of environmental temperature emerges as a pivotal factor determining battery longevity. Calendar life, a crucial metric representing battery lifespan, is influenced by the dynamic interplay between state of charge and temperature. Locations with elevated ambient temperatures often experience noticeable declines in battery capacity during storage conditions. Additionally, it is noteworthy that temperature also significantly affects battery cycle life, exerting a substantial influence on this critical parameter [20,21].
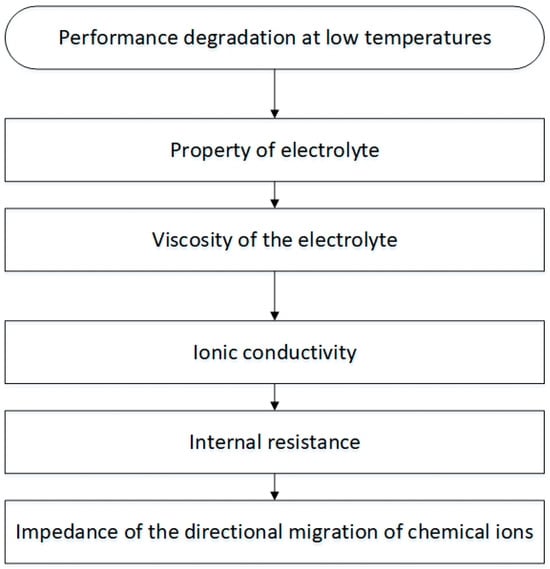
Figure 2.
The performance degradation of lithium-ion batteries at low temperatures [20].
Presenting a comprehensive view of the battery’s journey, Figure 3 encapsulates the battery lifecycle. This encompassing trajectory begins with the production phase, navigates through intricate design considerations, and extends to second-life applications and the integration of electric vehicles (EVs). Thus, the profound impact of temperature fluctuations on lithium-ion batteries unravels a nuanced tapestry where diverse parameters and intricate interactions converge to shape the battery’s performance, longevity, and overall lifecycle.
Figure 3.
Battery life cycle [21].
5. Electrochemical-Thermal Behavior of Lithium-Ion Batteries
The comprehensive spectrum of electrochemical and thermal models developed to elucidate the intricate behaviors of lithium-ion batteries is represented in Table 1. This compilation underscores the diversity inherent in these models, all crafted with the overarching goal of simulating and understanding the multifaceted thermal and electrochemical dynamics exhibited by lithium-ion batteries across various environmental temperatures and current profiles. The crux of lithium-ion battery modeling lies in the formulation of complex sets of equations meticulously designed to capture the battery’s dynamic response and performance. These efforts have primarily aimed to foster the development and deployment of electrochemical-thermal battery simulation models, often involving the integration of heat transfer experiments and simulations, further enhanced by techniques like computational fluid dynamics and a range of battery-centric experiments.

Table 1.
Compilation of Electrochemical and Thermal Models for Lithium-ion Batteries.
In a significant contribution, Baghdadi et al. [22] introduced a precisely tailored, dynamic electro-thermal model calibrated specifically for high-power utilization of lithium batteries. The model’s parameters were derived from empirical insights obtained through experimental investigations, with many parameters dependent on current magnitude, temperature, and battery state of charge (SOC). Validation across a variety of operational conditions, including static and dynamic current profiles, demonstrated the model’s accuracy. Impressively, simulation results showed close agreement with extensive test data spanning twenty hours. Another noteworthy effort by Song et al. [23] involved the development of a mathematical model for the comprehensive exploration of heat transfer and thermal management aspects inherent to lithium polymer batteries. Building upon an existing electrochemical model, this enhanced version incorporated temperature-dependent parameters such as the diffusion coefficient of lithium ions, ionic conductivity of lithium ions, and transference number of lithium ions to provide a deeper understanding of thermal intricacies within the lithium polymer framework. Experimental validation, coupled with analyses of discharge behaviors and heat generation rates within lithium polymer cells, enabled a thorough comparison of experimental observations with model-derived outcomes, accompanied by a detailed discussion of diverse thermal management strategies. Similarly, Choi S.S. et al. [25] conducted a comprehensive investigation into the factors influencing the cycle life of lithium-ion cells. The insights gained emphasized the substantial impact of charge conditions on cycle life, with discharge conditions exhibiting relatively lesser sensitivity. Charging cells at rates exceeding the 1C rate, prolonged float-charge periods above 4.2 V, and high charge cut-off voltages were identified as factors negatively affecting cycle life. Interestingly, unlike other battery types, the depth of discharge displayed a limited correlation with cycle life enhancement. The association between degradation rate and charge voltage, along with the duration of exposure to high charge voltage, implicated electrochemical oxidation as a fundamental degradation mechanism.
Conversely, Thomas E.V. et al. [26] embarked on a meticulously designed accelerated aging study, thoroughly exploring the interplay between aging duration, temperature, and state-of-charge (SOC) in shaping lithium-ion cell performance. Through an extended monitoring process involving a hybrid pulse power characterization test at low current regimes over a 44-week period, a notable empirical model of power fade was established. This model comprehensively encapsulated two simultaneous degradation processes—one rapid and temperature-accelerated, and the other proceeding at a slower pace influenced by temperature and SOC. Addressing temperature-related challenges, Smart M.C. et al. [27] systematically explored the profound impact of electrolyte composition on low-temperature lithium-ion cell performance. By carefully selecting ester solvents for incorporation into multi-component electrolyte formulations, the study leveraged favorable physicochemical attributes such as low viscosity, high permittivity, and low melting points. The compatibility of these formulations with diverse electrode compositions, including LiCoO2 and LiNiCoAlO2, was demonstrated. The study also delved into lithium intercalation and deintercalation ease within Li-carbon cells at varying temperatures, employing conventional electrochemical techniques to uncover insights into surface film attributes.
Expanding into the realm of abuse testing, Spotnitz R. et al. [28] conducted a comprehensive synthesis of published studies investigating abuse testing involving lithium-ion cells and their components, complemented by the application of modeling techniques. These studies meticulously identified specific exothermic reactions and estimated heats of reaction for each, subsequently leading to model development. These models, enriched with estimated kinetic parameters and designed to address high-rate batteries, comprehensively captured cell behavior under diverse abuse conditions such as high temperatures, short-circuits, overcharging, nail penetration, and physical crushing. Notably, these models shed light on the role of fluorinated binders in thermal runaway, revealing the binder’s minimal contribution to this phenomenon. In summary, the orchestrated symphony of electrochemical and thermal models, exemplified in the table, aligns with a collective effort to decipher the intricate dynamics inherent to lithium-ion batteries. These models span a wide landscape, encompassing diverse perspectives and insights, thereby contributing to a deeper understanding of these pivotal energy storage systems.
In a distinct exploration, Rao L., Newman J. et al. [29] introduced an innovative energy balance framework for insertion battery systems, built upon enthalpy potentials as a foundational cornerstone. This pioneering approach facilitated the calculation of heat-generation rates through an inventive methodology. Additionally, an alternate model based on localized heat generation within an electrochemical cell was formulated, yielding consistent outcomes. The authors also introduced the concept of the effective open-circuit potential of an insertion battery, enhancing characterization during the open-circuit state of galvanostatic discharge. Specific simulation efforts focused on heat generation within a lithium cell during galvanostatic discharge, analyzing the interplay between the shape of the open-circuit potential and ohmic losses within the porous cathode. Remarkably, this study revealed that a single reaction could give the illusion of two reactions due to the presence of twin plateaus within the open-circuit potential. The cessation of current within electrochemical systems triggers heat generation, a consequence of the relaxation of concentration gradients. This phenomenon, referred to as the heat of mixing, results from this relaxation.
Thomas K.E. et al. [30] delved into this inquiry, providing two methodologies—a computational approach and an analytical approximation—to quantify the heat of mixing. While typically negligible within materials with robust transport properties ensuring satisfactory battery performance, exceptions arise, particularly with materials like lithium insertion electrodes engaged in insertion reactions. In such contexts, the entropy of reaction undergoes significant variations depending on the state of charge, introducing an entropy of reaction that manifests as a reversible heat effect comparable in magnitude to resistive heating. In a separate investigation, Kim U.S. et al. [31] conducted a comprehensive thermal analysis to examine the thermal performance of a lithium-polymer battery. This study delved into how electrode arrangement influences thermal behavior, considering factors such as electrode aspect ratio, positioning of current-collecting tabs, and discharge rates. Utilizing the finite element method, the study predicted potential and current density distribution across the battery’s electrodes during discharge, subsequently enabling calculations of temperature distribution within the lithium-polymer battery. Impressively, the temperature distributions derived from the model closely matched experimental measurements from batteries with different electrode types under various discharge rates. Expanding their horizons, Kim U.S. et al. [32] introduced a modeling approach to upscale a lithium-ion polymer battery (LIPB). Validation, confirmed by comparing experimental discharge curves with modeling results, demonstrated that parameters used for modeling small-scale LIPBs could be extended to larger scales, contingent on the consistency of electrode materials, composition, and manufacturing processes. Using the finite element method, the distribution of potential and current density across the LIPB electrodes during discharge was predicted and then utilized for calculations of temperature distributions within the LIPB. Notably, the temperature distributions derived from the model exhibited commendable agreement with corresponding experimental measurements.
Furthermore, Smith K.A. et al. [33] employed empirical correlations to examine the influence of temperature-dependent electrode film impedance growth (thermal stress) and cycling-dependent capacity fade (mechanical stress) on cell degradation and performance decline. This study aimed to quantify the non-uniform imbalance and performance deterioration that traverse the cell’s lifespan as degradation evolves. The results were compared with those from a 1D electrochemical/lumped thermal model. Simulations spanned varying temperatures, cycling intensities, and states-of-life, providing insight into diverse scenarios where the internal reaction field was influenced by temperature, potential, and degradation state. Shifting the focus, Yang F. et al. [34] centered their investigation on the interplay between long-term coulombic efficiency (CE) and battery degradation—an area still shrouded in mystery. This study, driven by cycle life tests on commercially available lithium-ion batteries, explored the behavior of long-term CE and its connection with capacity degradation. Through incremental capacity (IC) analysis, the study uncovered the underlying mechanisms of battery aging. The paper offered not only experimental observations but also profound discussions on battery degradation, aging mechanisms, and the evolution of CE. This inquiry unveiled two distinct degradation patterns, highlighting the link between active material loss and battery degradation and emphasizing the electrochemical interplay between the evolution of CE and capacity degradation.
To ensure accurate estimation of cell thermal characteristics, Bernardi D. et al. [35] devised a comprehensive energy balance equation for battery systems. Critical for battery system design and thermal management, this equation incorporates a range of factors driving temperature changes within cells, encompassing electrochemical reactions, phase transitions, mixing effects, and Joule heating. This versatile framework addresses multifaceted effects comprehensively while considering simplifications and practical scenarios. Demonstrating the equation’s practical utility, mathematical models of cell discharge with varying reaction mechanisms were analyzed. These examples illustrated how the energy equation facilitates the analysis of diverse term contributions, highlighting the intricate nature of heat generation processes within cells while emphasizing the benefits of adopting such a comprehensive energy equation for such analyses.
6. Thermal Characteristics of Lithium-Ion Batteries
Lithium-ion batteries, known for their nonhomogeneous composition, exhibit diverse heating patterns on the surface of battery cells. This intricate interplay poses significant challenges for effective thermal modeling and the design of efficient thermal management systems tailored to various lithium-ion battery applications. As illustrated in Table 2, researchers have employed a plethora of methods to scrutinize the thermal attributes of lithium-ion batteries. Furthermore, extensive laboratory-based experimental characterizations have been conducted on diverse lithium-ion batteries operating under varied conditions. This line of inquiry predominantly seeks to unravel the relationship between surface temperature gradients and the thermal dynamics of lithium-ion battery cells. Parameters including power, open-circuit voltage, capacity, entropic heat coefficient, heat capacity, internal resistance, temperature, and battery heat generation have been meticulously determined across diverse load currents and an expansive temperature range. The insights garnered from these experimental results are pivotal for refining thermal modeling approaches for these batteries.

Table 2.
Different methods for thermal analysis and characteristics of lithium-ion batteries.
The intricacies embedded in the thermal modeling of lithium-ion batteries necessitate a nuanced approach, as the solution varies depending on pack topologies, battery cell designs, and specific application contexts. In essence, a tailored thermal modeling system is indispensable for each unique lithium-ion battery instance. Key aspects such as the entropic heat coefficient, internal resistance, battery heat generation, and thermal models serve as foundational elements enabling the simulation of diverse lithium-ion batteries, unlocking insights into their thermal dynamics.
In a parallel pursuit, Bazinski, S.J. et al. [37] meticulously explored the influence of reversible (entropic) heat sources on the thermal behavior of lithium-ion batteries, particularly during the initial charge and discharge stages. The entropic coefficient (EC) emerged as a pivotal factor shaping the magnitude and direction of this reversible heat. The researchers identified varying EC values for a lithium-iron phosphate battery, revealing the significant impact of cell temperature on EC, particularly at extreme state-of-charge (SOC) levels. Employing curve fitting of experimental data, a correlation emerged linking EC to temperature and SOC. To validate this, calorimetric data from test cells were integrated to demonstrate the impact of reversible heating on the overall heat generation rate within the cell. This synergistic fusion of calorimetric data and EC measurement facilitated the assessment of irreversible heat generation within the cell. This exploration not only offers insights into the relationship between temperature, SOC, and EC but also sheds light on the processes of reversible and irreversible heat generation inherent to lithium-ion batteries.
In a different vein, Kumaresan, K. et al. [38] developed a thermal model to predict the discharge performance of a lithium-ion cell across varying operating temperatures. The model’s predictions were validated with experimental data from lithium-ion pouch cells. This thermal model incorporated a parameter set tailored to the lithium-ion cell, accounting for concentration and temperature dependencies. These parameters were determined through a comparative analysis of model predictions and experimental discharge profiles encompassing diverse temperatures and discharge rates. The concentration and temperature dependencies of these parameters were subsequently correlated using empirical formulations. The study also examined the implications of incorporating the temperature dependencies of various parameters within the model into simulated discharge profiles. The integration of temperature-dependent parameters enhanced the model’s predictive accuracy for the discharge performance of lithium-ion cells operating across different temperatures.
Taking a different approach, Botte, G.G. et al. [39] used a mathematical model integrating an anode (carbon) decomposition reaction to predict the temperature trajectory of a lithium-ion cell under medium- and high-rate discharge conditions. The investigation explored the influence of distinct design parameters and the activation energy associated with the anode (carbon) decomposition reaction on the projected temperature within a LixC6/LiyNiO2 cell. Model predictions highlighted the critical role of particle size in the negative electrode as a crucial parameter for accurately predicting the cell’s temperature. On a similar trajectory, Srinivasan, V. et al. [40] aimed to enhance the understanding of Li-ion cell thermal behavior through a two-dimensional, first principles-based thermal-electrochemical modeling approach. The model encompassed reversible, irreversible, and ohmic heats within matrix and solution phases, incorporating temperature-dependent transport, kinetic, and mass-transfer parameters based on Arrhenius expressions. Experimental data on the entropic contribution of manganese oxide spinal and carbon electrodes were integrated to assess the significance of this term in overall heat generation. Through simulations, the study estimated thermal and electrical energy, along with active material utilization, at distinct rates, providing a comprehensive exploration of the interplay between temperature and electrochemistry. Moreover, the paper explored the prospect of using experimental data instead of an electrochemical model to deduce heat generation rates. Discrepancies between local and lumped thermal models were analyzed, and the feasibility of using a heat generation rate established under specific thermal conditions for other scenarios was evaluated. Model simulations offered valuable insights into the appropriateness of various approximations when developing comprehensive thermal models for Li-ion cells.
In a distinct study, Al Hallaj, S. et al. [42] utilized a simplified one-dimensional thermal mathematical model with lumped parameters to simulate temperature profiles within lithium-ion cells. The model seamlessly integrated experimentally derived heat-generation parameters specific to the Sony (US18650) cell. Simulation outcomes were impeccably aligned with temperature measurements for discharge rates spanning C/2, C/3, and C/6, although slight deviations were noted for the C/1 discharge rate. The model’s capabilities extended to simulating temperature profiles under diverse operational scenarios and cooling rates for scaled-up cylindrical lithium-ion cells with capacities of 10 and 100 Ah. Profound insights emerged—cooling rate had a substantial impact on cell temperature across all discharge rates. Notably, a noticeable temperature gradient within the cell occurred only at higher cooling rates, where the Biot number exceeded 0.1. Conversely, at lower cooling rates, the cell’s behavior resembled that of a lumped system with a uniform temperature distribution. Pioneering the establishment of temperature thresholds for scale-up using the simplified model, commercial lithium-ion cells with various open circuit potentials (OCV) were tested in an accelerated rate calorimeter (ARC) to determine onset-of-thermal-runaway (OTR) temperatures. Specifically, Sony (US18650) cells with OCVs of 4.06, 3.0, and 2.8 V were examined, yielding measured OTR temperatures of 104 °C, 109 °C, and 144 °C, respectively. A significant finding emerged—a sharp OCV decrease, indicative of an internal short circuit, occurred at temperatures near the separator material’s melting point across all OCV values.
In a parallel endeavor, Smith, K. et al. [43] employed a thermal model to dissect the limitations of pulse power and thermal dynamics in a Li-ion hybrid-electric vehicle (HEV) battery pack. The pack, housing 72 cells with a nominal voltage of 276 V and a capacity of 6 Ah, underwent scrutiny. High-rate pulse discharges, operational at approximately 25 °C, consistently reached their minimum voltage threshold of 2.7 V per cell due to active material Li depletion or saturation on electrode surfaces, highlighting solid-state diffusion as the limiting factor. In contrast, the maximum voltage threshold of 3.9 V per cell, designed to prevent lithium deposition on the negative electrode during charging, was considered overly conservative for high-rate pulses initiated from states-of-charge (SOCs) below 100%. The investigation revealed an intriguing insight—the maximum pulse charge rate, originating from a 50% SOC, could increase by up to 50% without risking lithium deposition, challenging the necessity for an excessively cautious maximum voltage threshold. While adhering to minimum and maximum voltage limits, the battery pack aligned with the power assist mode pulse power requirements of the Partnership for a New Generation of Vehicles (PNGV) at temperatures exceeding 16 °C. However, it fell short of achieving the desired energy output target.
In another venture, Verbrugge, M.W. et al. [44] proposed a technique to address current and temperature distributions in large-scale battery modules with three-dimensional configurations. Simulations focused on a specific module comprising cells with a lithium metal anode, polymer electrolyte, and vanadium oxide cathode. The findings highlighted the nonlinear correlation between power output and system temperature, primarily influenced by temperature’s impact on electrochemical reaction rates and ionic conductivity. The study also explored the estimation of physicochemical parameters, some of which were not readily available in existing literature but played a pivotal role as model inputs.
In a parallel investigation, Chen, Y. M.W. et al. [45] embarked on the mathematical modeling of heat generation and transport within lithium/polymer-electrolyte batteries, with a focus on their deployment in electric vehicles. Findings revealed that thermal management remains inconsequential for batteries operating at low discharge rates. However, at high discharge rates, battery temperature can rise significantly, particularly if the cell stack’s thickness exceeds a specific threshold. Interestingly, it was found that enhancing cooling conditions does not have a significant impact on increasing heat dissipation within large-scale battery systems due to the limited thermal conductivity of the polymer material. Model predictions can guide the design of appropriate battery structures and the selection of suitable cooling strategies to achieve the desired operational temperature range for a given discharge rate.
In another comprehensive overview, Gomadam, P.M. et al. [46] reviewed the mathematical models developed at the University of South Carolina for lithium and nickel battery systems. This encompassing survey covered models tailored for Li/Li-ion batteries, including simulations of single electrode particles, individual electrodes, full cells, and battery sets operating across diverse scenarios such as constant current discharge, pulse discharge, impedance, and cyclic voltammetry. Additionally, the review included models designed for nickel battery systems, elucidating complete cell performance and the behavior of nickel hydroxide as an active material. The robustness of these models, substantiated through recurrent comparisons with experimental data, showcased their accuracy in predicting real-world outcomes.
Shen et.al [47] introduced a modified air cooling system featuring a non-vertical, Z-shaped structure. They investigated how this innovative design affected the thermal properties of lithium iron phosphate power batteries. This system departs from the traditional Z-shaped cooling arrangement by tilting battery packs at different angles, resulting in a non-vertical airflow channel structure. When compared to the conventional Z-shaped air cooling system, the highest temperature within the battery pack decreased from an initial 38.15 °C to 34.14 °C, representing a 10.5% reduction. Moreover, the temperature variation decreased from an initial 2.59 °C to 1.97 °C, marking a 23.9% decrease. This modified air-cooled battery thermal management system improves the heat exchange rate between the battery pack and the surrounding air, leading to enhanced cooling performance and temperature uniformity. The outcomes of this study serve as a foundation for the development of a modified Z-shaped air cooling system, contributing to the safety improvements in electric vehicles and providing valuable insights for the further advancement of Battery Thermal Management Systems (BTMS).
Wu et.al [48] introduced an electrochemical-thermal model (ETM) designed to evaluate the heat generation characteristics of cylindrical Lithium-ion Batteries (LIBs). This model considers various discharge rates and the ratio of negative to positive electrode capacity (N/P ratio). To provide a comprehensive assessment of LIB thermal properties, the proposed ETM was validated using experimental data acquired at ambient temperatures of 25 °C and 35 °C. Subsequently, the study examined the distribution patterns of heat generation characteristics in LIBs under various conditions through numerical analysis. A notable aspect of this investigation was the thorough exploration of how different discharge rates and N/P ratios affect the heat generation in batteries. The results highlighted the significant role of heat generation in the negative electrode and emphasized the importance of considering the impact of the reversible term on the overall heat generation in LIB cells, particularly at lower discharge rates. Additionally, the research suggested that selecting the appropriate N/P ratio can improve the total heat generation of LIBs, offering advantages for optimizing performance in the early stages of battery design and thermal management.
Chen, S.C. et al. [49] developed a comprehensive three-dimensional thermal model to understand the thermal behavior of a lithium-ion battery. The model ingeniously incorporated the layered structure of cell stacks, battery pack casing, and the space between these elements to provide a detailed analysis of heat dissipation. It included location-dependent convection and radiation at boundaries to accurately represent distinct heat dissipation characteristics across all surfaces. The study also proposed a simplified thermal model that achieved comparable calculation speed to a one-dimensional model, with a maximum error of less than 0.54 K. Both models effectively captured the asymmetric temperature distribution within the battery and even predicted temperature anomalies on the surface when a metal case was used. Insights gained emphasized the importance of factors such as the metal battery case, contact layer, and heat-spreader effects in battery system design.
In the study conducted by Zhu and their team [50], a series of experiments were carried out using a cone calorimeter to investigate Lithium-ion Battery (LIB) packs of varying sizes (1 × 1, 1 × 2, 2 × 2, 2 × 3, 3 × 3) and at different states of charge (SOC) levels (100%, 50%, and 0%). The research examined several fire-related parameters, such as the heat release rate (HRR), mass loss, and concentrations of CO, CO2, and O2. Interestingly, the study observed similar combustion patterns characterized by intermittent jets for LIB packs at both 50% SOC and 100% SOC. The findings revealed a consistent positive correlation between the total mass loss (TML) and the peak value of HRR (pHRR), described by a power function, in relation to the surface area of the exposed heat source. Notably, for battery packs with a 100% SOC, the pHRR of the 3 × 3 cell module increased significantly, approximately by a factor of 8, reaching 12 kW. The study also assessed the presence of the toxic gas carbon monoxide (CO) by determining the fractional effective dose (FED). It was found that for battery packs with sizes smaller than 2 × 3, the FED remained below 1 for packs at both 50% SOC and 100% SOC. This research offers valuable insights into predicting the progression and fire risk associated with larger-scale battery fires and provides potential strategies for mitigating thermal runaway (TR) hazards in accident scenarios.
Chen, S.-C. et al. [51] developed a two-dimensional thermal model specifically tailored for spirally wound cells, aiming to establish a standardized simulation methodology for these battery configurations. The model carefully considered the geometric attributes and boundary conditions of the spiral architecture to avoid distorted results caused by improper approximations of the spiral geometry. While this versatile model architecture offered precision, it came at the expense of computational time. Simulations performed on lithium batteries exposed to natural convection revealed that peak temperatures clustered in a circular region near the liquid-filled hollow core rather than at its exact center. Additionally, radiation emerged as a significant contributor to heat dissipation, accounting for up to 53.6% of the total when surface emissivity approached unity. Introducing airflow parallel to the cylinder axis proved effective in maintaining surface temperatures, although internal temperatures remained elevated for batteries with a larger radius. Airflow perpendicular to the cylinder axis, while slightly less effective than parallel flow, still contributed to reduced heat dissipation. Ensuring temperature uniformity required a battery case with high thermal conductivity. Chen, Y. et al. [52] developed a three-dimensional model to simulate and compare heat generation and transport in a lithium polymer electrolyte battery during galvanostatic discharges and under a dynamic power profile, such as the Simplified Federal Urban Driving Schedule (SFUDS). The study aimed to achieve and maintain operational temperature and temperature uniformity within the battery through well-designed thermal management. The findings highlighted the crucial role of anisotropic thermal conductivity and emphasized its importance in battery design. The study offered insights into designing laminated cell stacks to achieve uniform operational temperatures, especially when cooling channels or electric heaters were applied to the stack’s extremities. Under the SFUDS power profile, the time-averaged heat generation rate was low, necessitating high-performance insulation materials to maintain desired operational temperatures. The thermal model served as a toolkit for evaluating different configurations of cooling channels and electric heaters, optimizing heating intensities, and selecting insulating materials. Chen, Y. et al. [53] conducted a thermal analysis of lithium polymer electrolyte batteries, aiming to understand the relationship between battery thermal behavior and various design parameters. The study aimed to guide the preservation of operational temperature by designing appropriate cell stack structures and selecting suitable cooling and insulating systems. The analysis explored the effects of stack size and different cooling/insulating conditions on battery temperature across a range of discharge rates. These investigations provided valuable insights for maintaining desired operational temperatures. The study also calculated temperature distributions within cell stacks for different cell designs, including variations in component thicknesses and current collector materials. This analysis not only identified optimal cell structures from a heat transfer perspective but also discussed the thermal properties of lithium polymer electrolyte batteries with different positive electrode materials, such as V6O13, TiS2, and redox polymers. The study shed light on the thermal conductivity of batteries influenced by varying electrode compositions. Lastly, Du, S. et al. [54] conducted a study focusing on irreversible heat generation in lithium-ion batteries and its implications for electronic device development. The primary factors contributing to internal irreversible heat generation in Li-ion batteries are polarization and ohmic heat generation. The study developed a thermo-electrochemical coupling model that integrated dynamic parameters and the electric double layer to uncover the mechanisms behind this phenomenon. Results revealed a key insight—irreversible heat production increases significantly with discharge rate, with polarization heat production being the dominant factor. Ohmic heat production primarily contributes to electrolyte heating, while heating at the negative active material is much smaller compared to the positive active material. Calculations demonstrated that the ratio of ohmic heat production to total irreversible heat production rises as the discharge rate increases, helping to balance the influence of polarization heating. The study further investigated the role of particle size in irreversible heat production and polarization heat production at the positive and negative electrodes. The findings underscored the greater impact of particle size at the negative electrode on these factors within the battery. In summary, this study highlighted the crucial role of irreversible heat generation in li-ion batteries, revealing polarization heat production’s dominance and the relatively smaller contribution of ohmic heat production from negative active materials. It also emphasized the influence of electrode particle size on irreversible heat production and polarization heat production, shedding light on an often overlooked but essential aspect of li-ion battery dynamics.
Drake, S.J. et al. [55] introduced an innovative method for measuring the heat generation rate of Li-ion cells at high discharge rates, reaching up to 9.6C. This approach involves simultaneous measurements of cell temperature and surface heat flux, providing insights into heat stored and lost from the cell. Unlike calorimetry-based methods, this in-situ approach allows measurements in laboratory or field settings. Prior to heat generation measurements, a preliminary test measures the temperature gradient within the cell under identical ambient conditions. This data is used to correct temperature discrepancies within the cell during subsequent heat generation measurements. The paper also introduces a method to measure the internal cell temperature, providing more precise temperature data for heat generation analysis. By comparing heat generation measurements with established theoretical models, the study demonstrates the agreement between experimental data and theoretical projections. This validation confirms the effectiveness of the proposed measurement method and reinforces trust in the theoretical models used to understand heat generation in Li-ion cells. The paper also briefly discusses the potential benefits of actively cooling the cell, highlighting the advantages of cooling in managing heat generation and improving cell performance. Active cooling strategies are identified as valuable tools for mitigating excessive heat generation and enhancing the comprehensive thermal management of Li-ion cells. Gümüşsu, E. et al. [56] introduced a three-dimensional computational fluid dynamics (CFD) model to investigate the thermal performance of lithium-ion batteries under natural convection. This model encompasses the entire flow field surrounding the battery and internal conduction, to predict the battery’s temperature during discharge. The model relies solely on electrical performance parameters, granting it predictive power in thermal analysis. By comparing macro-scale thermophysical properties such as specific heat and thermal conductivity, the study reveals the significant role of specific heat in moderating the battery’s temperature, while the influence of thermal conductivity remains comparatively limited. Interestingly, the study finds that experimental data can be closely predicted even without considering the entropic term in heat generation calculations. The discrepancy between experimental and predicted battery surface temperatures remains within 3 °C across all discharge rates, regardless of the battery’s operational history. This developed CFD model serves as a versatile platform for exploring the thermal behavior of lithium-ion batteries across various packaging configurations, encompassing both natural and forced convection conditions. It facilitates a nuanced exploration of battery thermal management and optimization strategies, ultimately resulting in improved performance and safety for lithium-ion batteries. Xiao, M. et al. [57] conducted an experimental investigation using a calorimeter to enhance an electrochemical thermal model with additional terms. Calorimetric measurements were compared with model predictions to assess the model’s accuracy and reliability. The inclusion of these supplementary heat source terms, validated through experimental measurements, enhances the electrochemical thermal model’s ability to provide a comprehensive understanding of heat generation in batteries. This augmentation enhances battery thermal management and elevates safety considerations in practical applications. Abdul-Quadir, Y. et al. [58] introduced a method for discerning heat generation in individual battery cells during charge and discharge, a crucial element in effective battery thermal management. This method accounts for overpotential resistances through four distinct measurement techniques, incorporating the contribution of entropic heat generation within the cell. The authors conducted calorimeter tests to directly quantify heat generation within the battery cell, and the accuracy of the proposed method was validated through a comparison of calculated and measured heat generation values. The study highlights a strong agreement between overpotential resistances obtained from various techniques, except for direct current resistance measured using impedance spectroscopy. These findings instill confidence in the proposed method’s capability to accurately estimate heat generation, making it an essential tool for precise heat generation estimation. Eddahech, A. et al. [59] conducted a series of tests using an accelerating rate calorimeter to explore the thermal behavior of high-power lithium-ion cells during charge and discharge cycles at various current rates. The study focused on characterizing cell heat capacity, quantifying cell entropy, and understanding the impact of state-of-charge fluctuations and charge-discharge current rates on battery heat generation. These insights provide a deeper understanding of the cells’ heat generation and thermal characteristics, contributing to a more comprehensive grasp of their thermal behavior. Nieto, N. et al. [60] developed a thermal model tailored for a large Li-ion pouch cell with a capacity of 10.5 Ah. This model was based on experimental measurements of internal resistance and the entropic heat coefficient. Adiabatic calorimetry data were used to validate the thermal model’s accuracy. The study covered higher discharge rates and broader temperature operation ranges compared to previous research. The results demonstrated the thermal model’s reasonable prediction error for discharge processes conducted at moderate and elevated rates. The paper also discussed the strengths and limitations of the thermal model, offering insights into its practical applicability and key considerations for designing thermal management systems. Overall, the thermal model proved to be an effective predictor of heat generation behavior in large-format Li-ion pouch cells. Vertiz et al. [61] conducted a combined approach of calculated and experimental methods to explore the fundamental thermal characteristics of a commercially available high-capacity (14 Ah) pouch cell using LiFePO4/graphite chemistry. This investigation involved dual comparative analyses. Firstly, it compared heat generation predictions from Newman’s model with experimental heat measurements. Secondly, it established a correlation between empirical thermal behavior and the response of a 1D electro-thermal model. This research methodology allowed for a comprehensive assessment of the cell’s thermal behavior and validated the accuracy of theoretical predictions against experimental data.
7. Thermal Runaway of Lithium-Ion Batteries
Figure 4 provides a comprehensive illustration of the thermal effects observed in lithium-ion batteries, accompanied by an informative portrayal of the conventional thermal runaway process inherent in these batteries [62,63]. Inadequate thermal management of lithium-ion batteries can lead to a phenomenon known as thermal runaway. Figure 4b offers a detailed depiction, elucidating the typical progression of thermal runaway in lithium-ion batteries. This process unfolds in distinct stages. During the initial phase, the solid electrolyte interface layer undergoes decomposition, triggering self-heating within the cell. This self-heating serves as a catalyst for subsequent reactions, ultimately resulting in the melting of the separator. Initially, this leads to a minor micro-short circuit, which then escalates into a more significant internal short circuit, intensifying self-heating and progressing to the second stage. In the second phase, exothermic reactions between the electrolyte and the cathode begin. The culmination of this escalating sequence occurs in the final stage, characterized by the decomposition of both the electrode and the electrolyte, resulting in a full-fledged thermal runaway [63].
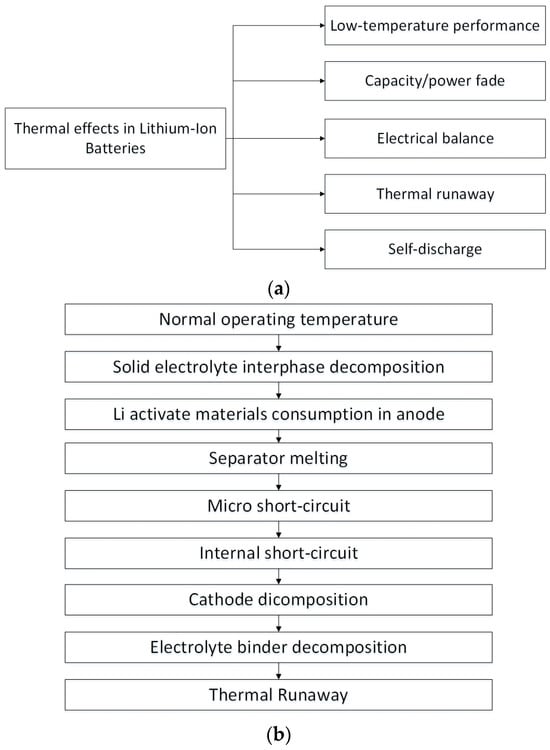
Figure 4.
(a) Thermal effects in lithium-ion batteries [62], (b) Illustrative drawing of a conventional thermal runaway process [63].
Thermal runaway occurs as a result of internal reactions within a battery, causing a rapid increase in internal temperature. This temperature rise, in a cyclic manner, accelerates reactions, creating a loop that further intensifies the temperature increase. This unchecked cycle of reactions and rising temperatures can lead to the generation of smoke, the ignition of fires, and, in extreme cases, even explosions. Table 3 provides an inclusive overview of various studies that delve into the complexities of the thermal runaway mechanism in lithium-ion batteries. The rise in pressure and temperature within the battery is driven by exothermic reactions occurring across the cathode, anode, and electrolyte. In a bid to ensure safety in commercial applications such as electric vehicles, many countries have established mandatory standards that batteries must meet before entering the market. However, it is important to emphasize that despite the existence of these standards, incidents related to thermal runaway still occur. These incidents do not arise due to a lack of standards but rather stem from inherent malfunctions and adverse conditions that batteries might encounter throughout their lifecycle in specific applications (as illustrated in Figure 5). It is vital to acknowledge that while internal malfunctions are possible, their likelihood is typically low [64,65].

Table 3.
Different studies about the thermal runaway mechanism of lithium-ion batteries.
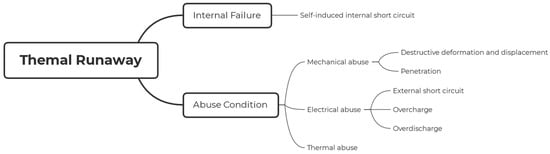
Figure 5.
Thermal runaway mechanism.
Abuse conditions, encompassing mechanical, electrical, and thermal abuse, pose a higher likelihood of occurrence and can lead to hazardous situations in lithium-ion batteries.
- Mechanical Abuse: Mechanical abuse transpires when batteries undergo displacement, deformation, or penetration, resulting in separator destruction. Such events can give rise to internal short circuits, electrolyte leakage, and eventual fires. The simulation of mechanical abuse and the evaluation of thermal runaway risks for large-format Li-ion batteries have been emphasized, underscoring the necessity to assess battery safety under mechanical abuse scenarios.
- Electrical Abuse: Electrical abuse comprises external short circuits, overcharging, and over-discharging conditions, each of which can lead to detrimental consequences if not managed effectively.
Researchers like S. Santhanagopalan et al. [66] have developed thermal models grounded in electrochemical principles to study internal short circuits in lithium-ion cells. Such models offer insights into various experimental observations. The simulations mimic different short-circuit situations that may occur within lithium-ion cells, calculating the resulting power generation. Parameters like state of charge (SOC) and initial cell temperature are examined to understand the behavior of short-circuited cells. Experimental tests are conducted to validate the model’s predictions, aiding in the design of safer cells and enhancing safety measures. R.A. Leising et al. [68] performed extensive analysis on prismatic lithium-ion batteries under short-circuit and overcharging conditions. Internal thermocouples were used to assess thermal profiles during these extreme scenarios. Results highlighted the importance of internal temperature measurements, as differences were noted between internal and surface temperatures. The study demonstrated the significance of cathode material quantity in overcharging-induced cell rupture. T.G. Zavalis et al. [69] employed a coupled electrochemical-thermal model to examine the potential hazards of thermal runaway stemming from exothermic side reactions in short-circuited lithium-ion battery cells. This study emphasized the pivotal role of mass transport in the electrolyte and electric resistance in determining temperature increase rates under different short-circuit scenarios. H. Maleki et al. [70] investigated the thermal stability of lithium-ion cells under internal short circuit (ISCr) conditions using a combination of experimental methods and thermal modeling. They examined the influence of ISCr location, cell capacity, and state of charge on ISCr events. The study highlighted the limitations of certain experimental approaches in simulating high-risk ISCr events and emphasized the importance of ISCr location in its consequences. T. Yamauchi et al. [71] introduced a model for lithium secondary batteries that elucidates charging/discharging behavior and temperature increases during internal short circuits. Their model suggests that the cumulative generation of Joule heat due to high currents during internal short circuits contributes to thermal runaway. In essence, research endeavors focusing on abuse conditions and safety considerations offer valuable insights into the complex interactions that can lead to hazardous outcomes in lithium-ion batteries. These insights play a crucial role in enhancing battery design, manufacturing, and management strategies to ensure safety and prevent accidents.
Certainly, the information you provided highlights several studies that delve into the mechanical aspects of lithium-ion batteries, specifically focusing on the effects of external forces, mechanical failures, and their interactions with electrical and thermal responses. These studies contribute to the overall understanding of the behavior and safety of lithium-ion batteries under different mechanical abuse conditions. The research conducted by these teams sheds light on various aspects:
- Impact and Crush Testing of Battery Packs (Y. Xia et al. [72]): This study employed finite element modeling to comprehensively investigate the effects of ground impact on lithium-ion battery packs in electric vehicles. By developing global and detailed FE models, they assessed the structural response and potential failure modes of battery packs subjected to ground forces. The research provided insights for designing protective structures to withstand ground impact and highlighted the importance of evaluating battery safety under mechanical abuse conditions.
- Mechanical-Electrical-Thermal Interactions (C. Zhang et al. [73] and C. Zhang et al. [74]): These studies developed models that simultaneously consider mechanical, electrical, and thermal aspects to understand the behavior of lithium-ion batteries under external crushing. The models incorporated detailed mechanical material properties and accurately predicted mechanical deformation, separator failure, and short-circuit initiation. The studies emphasized the significance of test conditions and electrical contacts in influencing the electrical-thermal behavior of cells following a short circuit.
- Separator Failure Under Loading (Y. Xia et al. [75] and E. Sahraei et al. [76]): These studies specifically focused on separator failure under various loading conditions. Y. Xia et al. [75] investigated the influence of loading conditions on separator failure through numerical simulations and found that torsion significantly increased strain, leading to the initiation of internal short circuits. E. Sahraei et al. [76] performed tests and finite element modeling on different types of battery cells, including separator failure, validating their models’ predictions against experimental observations.
- Deformation and Failure Analysis (M.Y. Ali et al. [77] and W.-J. Lai et al. [78]): These studies analyzed the mechanical behavior of battery components and modules under different loading conditions. M.Y. Ali et al. [77] developed computational models to simulate component and cell behavior under constrained compression, capturing various phenomena observed in experiments. W.-J. Lai et al. [78] conducted comprehensive tests on module components and investigated mechanical behavior through tensile and constrained compression tests, providing insights into module behavior and validation through finite element analysis.
Overall, these studies contribute valuable insights into the mechanical responses and failure modes of lithium-ion batteries under different abuse conditions, aiding in the design of safer battery systems and enhancing the understanding of battery safety considerations.
The information you’ve provided highlights additional studies that have contributed to the understanding of the mechanical behavior and failure mechanisms of lithium-ion battery cells under different loading conditions. These studies further demonstrate the importance of accurate modeling and testing for predicting mechanical responses and enhancing battery safety. Let us discuss these studies in more detail:
- Mechanical Abuse Testing and Fracture Prediction (L. Greve et al. [79]): This study conducted a comprehensive quasi-static mechanical abuse test program on cylindrical lithium-ion battery cells with a 0% state of charge. The focus was on various load cases, and the results revealed that macroscopic jelly roll fracture is a primary cause of internal short circuits. To predict these fracture events and short circuits, a macro-mechanical finite element crash simulation model was developed using the Mohr and Coulomb criterion. The model accurately predicted punch displacement at the point of fracture and the locations of internal short circuits, emphasizing the reliability of the criterion in predicting fracture events and their effects on battery safety.
- Finite Element Modeling of Mechanical Behavior (E. Sahraei et al. [80]): This study developed a finite element model for individual battery cells, utilizing shell elements for the aluminum and copper foils and solid elements for the active material, binder, and separator. The simulations correlated well with experimental results for various loading scenarios. The presence of a thin pouch enclosure was found to significantly influence deformation and failure mechanisms. The developed model serves as a foundation for modeling battery modules and packs at different length scales, enabling better optimization of strength-to-weight ratios and safety assessments.
- Mechanical Testing and Modeling of Shell Casings (X. Zhang et al. [81]): In this study, a comprehensive test program was conducted on empty shell casings of 18,650 lithium-ion cylindrical cells. Plasticity and fracture models were developed to predict the behavior of the shell casings under various loading conditions. The models accurately predicted plastic behavior and fracture initiation and propagation within the casings. The study highlighted the importance of considering the metallic shell casing in mechanical modeling, as it significantly contributes to the mechanical resistance of the cells under different loading scenarios.
These studies collectively contribute to a deeper understanding of the mechanical behavior and failure mechanisms of lithium-ion battery cells. By utilizing advanced modeling techniques and conducting comprehensive mechanical abuse testing, researchers are able to predict fracture events, understand failure mechanisms, and enhance battery safety considerations.
8. Safety of Lithium-Ion Batteries
Figure 6 illustrates a diagram indicating the stages leading toward thermal runaway. Ensuring the safety of lithium-ion (Li-ion) batteries is of paramount importance, given their widespread use in various applications. Thermal safety is a critical aspect, and addressing potential issues like thermal runaway is crucial to preventing accidents and ensuring the safe operation of Li-ion batteries. Let us delve deeper into the challenges and research efforts related to the safety of Li-ion batteries:
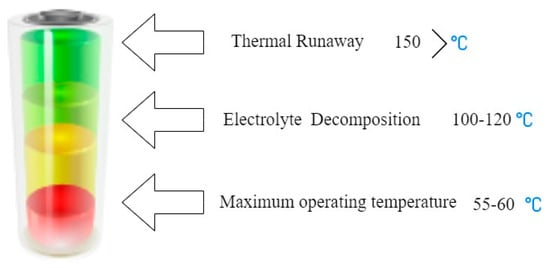
Figure 6.
A diagram indicating the stages leading toward TR [82].
Challenges in Ensuring Safety:
- Thermal Runaway: Thermal runaway is a key safety concern for Li-ion batteries. It involves a self-accelerating process in which the battery’s internal temperature rises uncontrollably due to exothermic reactions, potentially leading to smoke, fire, or explosions. Managing and preventing thermal runaway is a critical challenge in battery design and operation.
- Predictive Modeling: Developing accurate predictive models for thermal runaway is complex due to the intricate interplay of electrochemical, thermal, and mechanical factors within the battery. Creating models that can reliably predict and simulate the onset and progression of thermal runaway is an ongoing challenge.
- Detection and Mitigation: Detecting the early signs of thermal runaway is vital for timely intervention. Developing effective methods for detecting temperature increases, gas emissions, and other indicators of thermal runaway is essential. Additionally, devising strategies to mitigate the consequences of thermal runaway is crucial for minimizing damage and ensuring safety.
Research Efforts and Strategies:
- Thermal Runaway Modeling: Researchers have been actively working on developing sophisticated models that simulate thermal runaway processes. These models integrate factors such as heat generation, heat dissipation, and internal cell behavior to predict the conditions that can lead to thermal runaway. Accurate models aid in designing safer battery chemistries and architectures.
- Thermal Management: Effective thermal management strategies are crucial to preventing excessive heat buildup within batteries. Strategies include using advanced cooling methods, optimizing electrode materials, and designing efficient pathways for heat dissipation to maintain safe operating temperatures.
- Advanced Materials: Researchers are exploring new materials that have improved thermal stability and reduced reactivity, aiming to minimize the likelihood of thermal runaway. Materials like solid-state electrolytes and advanced electrode materials can contribute to enhanced battery safety.
- State-of-Health Monitoring: Developing monitoring systems that continuously assess the battery’s health and performance can provide early warnings of potential safety issues. Monitoring parameters such as internal resistance, voltage, and temperature changes can help predict and prevent thermal runaway.
- Safety Standards and Regulations: Governments and international organizations are setting safety standards and regulations for the design, manufacture, and use of Li-ion batteries. Compliance with these standards ensures that batteries meet certain safety criteria and reduces the risk of accidents.
- Battery Design Optimization: Battery pack design and configuration play a significant role in safety. Designing robust cell enclosures, separators, and thermal management systems helps contain and dissipate heat effectively.
- Advanced Testing Techniques: Researchers are developing advanced testing methods that subject batteries to various abuse conditions, including mechanical, electrical, and thermal abuse. These tests help identify failure mechanisms and design batteries that are more resilient to abuse.
In conclusion, ensuring the safety of Li-ion batteries requires a multidisciplinary approach involving materials science, electrochemistry, thermal engineering, and more. Research efforts continue to address challenges related to thermal runaway prediction, early detection, mitigation strategies, and regulatory frameworks. By understanding the stages leading to thermal runaway and actively researching solutions, the field is moving toward safer and more reliable Li-ion batteries for a wide range of applications.
Absolutely, the safety of lithium-ion (Li-ion) batteries in electric vehicles is a critical concern, given their pivotal role in these technologies. The research efforts and studies you’ve highlighted underscore the multi-faceted nature of battery safety and the ongoing work to address various challenges. Let us further discuss these key points:
Harmonization of Battery Testing Standards:
- The rapid proliferation of Li-ion batteries in electric vehicles has led to the need for consistent safety testing standards across different markets. Variations in regulations and standards can result in inefficiencies and inconsistencies in battery testing.
- Lin et al. [83] conducted a comprehensive analysis comparing Chinese, German, United Nations, and European regulations. By identifying discrepancies and suggesting improvements, they aim to promote harmonization among these regulations. This would lead to standardized testing methodologies that can improve efficiency and reliability in battery testing.
Nonflammable Electrolytes and Safety Considerations:
- Safety concerns related to the flammability of liquid electrolytes have driven researchers to explore nonflammable alternatives. However, as demonstrated by Liu et al. [84], the complexity of battery chemistry can lead to unexpected safety outcomes.
- Liu et al.’s study emphasizes the importance of considering the interactions between different components within the battery system. Even though a nonflammable electrolyte was used, the interaction with other elements led to unforeseen exothermic reactions, highlighting the need for a comprehensive approach to safety evaluation.
Temperature Prediction and Battery Safety:
- Battery temperature management is crucial for maintaining safety and performance, particularly in electric vehicles. The study by Hong et al. [85] introduces an innovative approach for real-time temperature prediction using clustering-based data partitioning and neural networks.
- Accurate temperature prediction aids in the early detection of thermal anomalies, helping prevent thermal runaway incidents. The study’s methodology leverages real-world data to create a reliable model adaptable to various climates and operational conditions.
Fault Diagnosis and Risk Assessment:
- As Li-ion batteries age and experience various operating conditions, the potential for faults increases. Detecting and diagnosing faults is essential for maintaining battery safety and performance.
- Zhang et al. [86] provide an overview of fault diagnosis methods tailored for Li-ion batteries. These methods encompass statistical analysis, models, signal processing, knowledge-based approaches, and data-driven techniques.
- Risk assessment of thermal abuse, as presented by Xia et al. [87], is crucial for understanding the potential consequences of various internal, external, and random factors. Their approach integrates models, stochastic considerations, and risk evaluation to predict thermal safety boundaries and assess risk probabilities.
In conclusion, the safety of Li-ion batteries in electric vehicles is a multifaceted challenge that requires a holistic approach. Researchers are addressing various aspects, including harmonizing testing standards, exploring new electrolytes, predicting temperatures, diagnosing faults, and assessing risk probabilities. The ongoing efforts in research and development aim to enhance the safety, reliability, and performance of Li-ion batteries, supporting their continued adoption in electric vehicles and other applications.
9. Thermal Management of Lithium-Ion Batteries
C. Zhang et al. [88] achieved temperature control of a lithium-ion battery (TAFEL-LAE895 100 Ah ternary) in electric cars by combining heat pipes (HP) and a thermoelectric cooler (TEC). The utilization of heat pipes, with their high thermal conductivity, increased temperature loss. However, the initial design fell short of dissipating heat effectively at high rates. Consequently, a thermoelectric cooler was incorporated to enhance the battery’s heat dissipation at rates exceeding the average. Simulations indicate that this innovative approach will effectively prolong the battery’s lifespan through temperature regulation.
To reduce the temperature of lithium-ion batteries, T. Talluri et al. [89] incorporated commercial phase change materials (PCMs) with different thermal properties. The researchers examined the effect of expanded graphite on temperature loss and performed statistical analysis on single-pouch battery data. The findings indicated that the inclusion of expanded graphite enhanced heat dissipation due to its high thermal conductivity. When PCMs were used, the battery’s temperature during discharge could be lowered by approximately 11 degrees Celsius compared to cases without expanded graphite. Accurate temperature analysis was conducted through cold and warm soaking methods. The utilization of expanded graphite resulted in a reduction of approximately 4 and 2 h in the equilibrium time during cold and hot soaking, respectively, demonstrating improved temperature control performance after implementing PCMs. Consequently, based on the simulated results, expanded graphite emerged as the optimal choice for PCMs in this research.
According to a study by R. Zhao et al. [90], the higher the battery capacity, the higher the probability of thermal runaway in nail locations. This conclusion was the result of simulations on three commercial lithium-ion batteries and their thermal runaway comparisons with the help of a model called electrochemical thermal coupling and the nail penetration test. In addition, researchers found that during nail diffusion, the thermal behavior of these batteries depends on the internal resistance as well as the nail’s dimensions. Therefore, after examining the three proposed solutions to stop the thermal runaway, contact resistance enhancement was introduced as the best solution in this research. Researchers claimed that if contact is increased, enough time can be provided for the heat to dissipate.
The thermal management of the battery encompasses three cooling methods: air cooling (the simplest), liquid cooling, and phase change material (PCM). R. D. Jilte et al. [91] observed that the localized temperature zone within lithium battery cells is influenced by the module’s position. In certain specific areas of the battery, temperature increases of up to 7 degrees Celsius were recorded, leading to the formation of a temperature gradient and compromising thermal uniformity within the battery cell. In this study, the heat generation during discharge was simulated using a user-defined function (UDF). The simulation results revealed a temperature profile in the cooling air currents across the width and depth of the battery. The highest local temperature was observed in the middle of the module’s depth.
Based on the findings presented by H. Behi et al. [92], the utilization of heat pipes demonstrated superior performance in the thermal management of 18,650 batteries compared to the other two cooling methods: natural and forced air convection, as well as the implementation of the cell distance effect. The study focused on a 24-cell battery module and employed a mathematical modeling and thermal equations to assess the effectiveness of different cooling techniques. The results revealed that the use of Cu sheets heat pipes led to a significant reduction in module temperature, specifically by 42.7% compared to natural air cooling methods.
In the study done by Behi et al. [93], the simultaneous use of heat pipes and air coolers was investigated and compared to the natural convection method and heat pipes used without air coolers. The findings from [92] demonstrated that heat pipes, specifically those incorporating copper sheets, exhibited the best performance. Building upon this, the researchers in the current study sought to further enhance performance by integrating air coolers with the heat pipes, surpassing the previous method. Research 6 utilized a cooling-assisted heat pipe approach and employed the same mathematical and thermal modeling techniques as [92]. Simulations conducted with experimental data indicated that the addition of air coolers resulted in a temperature drop 1.8 times greater compared to heat pipes used without air coolers. This study highlighted the significant role that coolers, when combined with heat pipes, can play in facilitating heat loss.
In the study done by T. Deng et al. [94], a novel cooling design was introduced to enhance temperature dissipation in lithium-ion batteries. The proposed approach involved the utilization of cooling plates with symmetrical and reverting bifurcation designs to facilitate efficient heat exchange. The researchers examined thermodynamic parameters and heat loss while implementing these cooling plates, which were positioned in a sandwich-like manner around the battery cells. Numerical simulations revealed that this innovative design led to a significant 17.19% increase in the heat transfer coefficient. Furthermore, the branched cooling plates also influenced the friction factor, resulting in a substantial change of 85.53%.
In the study done by M. A. Bamdezh et al. [95], phase change materials (PCM) were incorporated alongside air cooling (TMS). To reduce the weight of the battery, the researchers applied a phase change material with an aluminum shell to the surrounding area of each cell within the cooler. This approach not only reduced the weight of the batteries but also improved their cost-effectiveness. The simulation results demonstrated a maximum thermal gradient of 1.5 degrees Celsius within the battery module, indicating a uniform thermal distribution. Moreover, the researchers observed that while increasing the thickness of the PCM layer could lower the average cell temperature, it could also result in an increased maximum temperature difference.
One solution to enhance heat dissipation is the utilization of phase change materials (PCM) and coolants. Due to their inherently low thermal conductivity, researchers have explored the use of additives to increase the thermal conductivity of PCMs. The extent of the increase in thermal conductivity is dependent on the latent heat capacity of the materials. However, instead of focusing on the composition of PCMs, S. Arora et al. [96] examined the positioning of cells within batteries. As outlined in [10], reversing the cell layout can facilitate convective flow and compensate for the low conductivity effect of these materials. In contrast, previous designs where battery cells were arranged vertically resulted in a greater temperature gradient compared to the reverse design.
C. Lin et al. [97] employed graphite sheets to enhance the thermal conductivity of phase change materials (PCM) in LiFePO4 battery modules. The researchers then conducted discharge tests both with and without PCM. The experimental results demonstrated the significant impact of PCM cooling on temperature reduction, as the maximum temperature gradient at the end of the discharge process remained below 5 degrees Celsius. Subsequently, a 3D numerical model was utilized to simulate the temperature profile throughout the discharge duration.
In the study done by S. Basu et al. [98], the amount of heat loss and the impact on battery capacity reduction were investigated using Newman’s pseudo-two-dimensional (P2D) module. Through numerical calculations, the researchers discovered that when the temperature exceeds 45 °C, the battery’s lifespan decreases significantly. At a temperature of 55 °C, the battery capacity experiences a substantial drop of 70%. To address these issues, phase change material (PCM) was employed to achieve a uniform thermal distribution. However, since PCM alone was insufficient for effective heat dissipation, liquid cooling was also implemented in this study, resulting in a notable improvement in the performance of the battery’s heat control system.
The presence of an air conditioner is crucial for the efficient operation of a battery module. In the study done by J. Cen et al. [99], several experimental tests were conducted to enhance the performance of the refrigerant. One of the strategies involved assessing the gas valve opening. This approach not only increased the heat loss of the coolant but also reduced the maximum temperature gradient within the battery to 2 degrees Celsius. Additionally, the researchers explored the sensitivity of the thermostat and implemented the proportional-integral-derivative (PID) control algorithm as another strategy for thermal management. These approaches yielded promising results in maintaining optimal temperature conditions for a module comprising 64 lithium-ion batteries.
According to [100], the researchers discovered that the thickness of the phase-change composite material significantly affects thermal loss. When the thickness was set to 6 mm, a notable increase of approximately 1.5 times in heat loss was observed in the absence of cooling, highlighting the significant impact of this parameter. However, the effectiveness diminishes when using thicker plates or increasing the thickness beyond 6 mm. These findings were obtained through experimental research conducted on LiFePO4 prismatic cells with a capacity of 20 Ah. The cells were connected in series, and temperature measurements were monitored using 18 thermocouples.
Based on the numerical analysis conducted by X. Zhang et al. [101], it was determined that the utilization of internal air circulation through an active air cooling system is over 70% more energy-efficient compared to an external air circulation system. The primary objective of this study was to comprehensively examine the energy consumption of a battery module comprising 32 lithium-ion batteries, specifically focusing on the impact of air coolers. As a result, the findings indicate that energy consumption is reduced as the rate of decrease in temperature from the maximum temperature increase increases by 1 degree Celsius.
Biemolt and colleagues [102] conducted an analysis of recent advancements in alternative battery systems employing sodium, magnesium, zinc, and aluminum. In each case, they organized these metals based on the overarching cathode material and placed emphasis on the energy storage mechanism. Specifically, sodium-ion batteries closely resemble today’s lithium-ion batteries in terms of technology and chemistry. This resemblance facilitates a smoother short-term transition, but it presents a long-term challenge due to their limited specific capacity. The lower reactivity of magnesium makes pure magnesium metal anodes safer compared to alkali alternatives, although they still face deactivation issues over time. Combining magnesium with different metals can effectively address this problem. Zinc boasts the lowest theoretical specific capacity, yet its metal anodes exhibit remarkable stability and can be utilized without alterations. This results in capacities similar to those of other materials and is suitable for immediate use in applications where weight is not a critical factor. Aluminum appears to be the most promising alternative in theory, primarily due to its high specific capacity resulting from a three-electron redox reaction. Nevertheless, a significant challenge persists in finding the right balance between stability and specific capacity [102].
Evaluating various metals for battery applications, particularly with regard to performance factors such as electrolyte compatibility, capacity, capacity retention, cycle durability, and voltage characteristics, holds immense significance within battery research. Such comparisons aid in the identification of optimal materials for specific purposes and contribute to the advancement of battery technology. Below are key aspects to take into account when assessing metals for batteries:
Electrolyte Compatibility: It is essential to determine how well a metal interacts with a particular electrolyte since different metals may necessitate specific electrolyte types. Compatibility issues can result in corrosion, reduced capacity, and safety concerns.
Capacity: Battery capacity measures its energy storage capability. Metals with higher capacity can store more energy, making them advantageous for applications requiring sustained power.
Capacity Retention: As batteries age, their capacity tends to decrease. Assessing a metal’s ability to maintain its capacity over numerous charge-discharge cycles is critical for long-term battery performance.
Cycle Durability: The number of charge-discharge cycles a battery can endure before experiencing significant capacity loss is a crucial factor. Metals capable of withstanding a high number of cycles are preferred for applications involving frequent recharges.
Voltage and Potential: A metal’s voltage and potential in a battery system influence its energy density and power output. High-voltage metals may be preferred for specific applications requiring elevated energy density.
Cost: Material cost is a significant consideration for practical battery applications. Some metals may be costly or challenging to procure, making them less viable for widespread use.
Safety: Certain metals may pose safety risks due to their reactivity with electrolytes or susceptibility to dendrite formation, which can lead to short circuits and thermal issues. Safety considerations are paramount, particularly for portable devices and electric vehicles.
Environmental Impact: The environmental implications of metal usage in batteries, including extraction, processing, and disposal, should be considered. Sustainable and eco-friendly materials are gaining importance.
Specific Application Requirements: Diverse applications have specific demands. For instance, lithium-ion batteries are prevalent in portable electronics due to their high energy density, while lead-acid batteries are favored in automotive applications for their reliability.
Research and Development: Ongoing research and development efforts may lead to enhancements in the performance of certain metals or the discovery of new materials. Staying informed about the latest developments is crucial.
When assessing metals for battery applications, thorough testing and analysis are vital to determining their suitability for a given purpose. This often involves laboratory experiments, including electrochemical characterization, to gather data on capacity, capacity retention, cycle life, and safety considerations. Furthermore, real-world testing in relevant environments or applications can offer valuable insights into metal performance in practical scenarios.
10. Mechanism of Battery Electrode Materials
The deterioration of electrode materials over time can lead to a reduction in capacity and an increase in resistance within the entire lithium-ion battery, significantly impacting its overall performance. Moreover, describing aging processes accurately is challenging due to the interplay of multiple factors. Lin et al. [103] provided an explanation for the decline in capacity and power associated with aging mechanisms specific to electrodes during cycling and various storage conditions, focusing on metallic oxide-based cathodes and carbon-based anodes. For the cathodes in lithium-ion batteries, the primary aging mechanism involves the mechanical stress and strain induced by the insertion and extraction of lithium ions, which predominantly results in structural degradation. Another critical aging factor is the dissolution of metals from the cathode and their subsequent deposition onto the anode. Regarding the anodes, the key aging mechanisms include the loss of recyclable lithium ions due to the formation and growth of a solid electrolyte interphase (SEI) layer, along with mechanical fatigue caused by diffusion-induced stress on the carbon anode particles. Additionally, the aging of electrodes is heavily influenced by their electrochemical behavior during cycling and storage conditions and is the outcome of both structural and morphological changes, as well as side reactions exacerbated by the decomposition products and protic impurities present in the electrolyte [103].
The operation of battery electrode materials is an intricate process characterized by a range of physical and chemical reactions that transpire during a battery’s charging and discharging cycles. Batteries, as electrochemical devices, store and release electrical energy through reversible chemical reactions that occur within the electrode materials. The following delve into this mechanism in-depth:
Electrode Materials: Typically, batteries consist of two types of electrode materials: the anode (negative electrode) and the cathode (positive electrode). Each electrode plays a distinct role in how the battery functions.
Anode: During discharge, the anode is where oxidation (the loss of electrons) occurs. Common anode materials include graphite (found in lithium-ion batteries), lithium metal (utilized in certain advanced batteries), and various other materials suited for different battery types.
Cathode: On the other hand, during discharge, the cathode is where reduction (the gain of electrons) takes place. The choice of cathode materials varies based on the type of battery, such as lithium cobalt oxide (LiCoO2) for lithium-ion batteries, manganese dioxide (MnO2) for alkaline batteries, and others for different applications.
Charging and Discharging:
Discharge: When a battery is in use (discharging), electrons flow from the anode to the cathode through an external circuit, generating an electric current. Simultaneously, ions (typically lithium ions in lithium-ion batteries) move from the anode to the cathode through the electrolyte, ensuring electrical charge balance.
Charge: During the charging process, an external voltage is applied to the battery, causing the opposite reactions to occur. Electrons are pushed back from the cathode to the anode, and ions move from the cathode to the anode.
Ion Movement: The electrolyte, typically a lithium salt dissolved in a solvent for lithium-ion batteries, facilitates the movement of ions between the anode and cathode while preventing the flow of electrons, which could lead to a short circuit.
Chemical Reactions:
Anode Reaction: During discharge at the anode, lithium ions intercalate (insert) into the anode material (e.g., graphite), resulting in a negative charge on the anode. This process is accompanied by oxidation reactions that generate electrons.
Cathode Reaction: During discharge at the cathode, the cathode material (e.g., LiCoO2) undergoes a reduction reaction, where lithium ions from the electrolyte and electrons from the external circuit combine to form lithium compounds.
Reversibility: An important characteristic of battery electrode materials is their ability to undergo reversible chemical reactions. In well-designed batteries, these reactions can be reversed, allowing for multiple charge and discharge cycles without significant degradation.
Capacity and Voltage: The capacity of a battery depends on the amount of active material in the electrodes and the number of charge/discharge cycles it can endure. Voltage is determined by the electrochemical potential difference between the anode and cathode materials.
Aging and Degradation: Over time, repeated charge and discharge cycles can lead to electrode degradation. This degradation may result from various factors, including the formation of a solid-electrolyte interphase (SEI), side reactions, and structural changes in the electrode materials. Battery management systems are employed to mitigate these effects and extend the battery’s lifespan.
Safety Considerations: A comprehensive understanding of the battery electrode materials’ mechanisms is essential for ensuring battery safety. Overcharging, overheating, or mechanical damage can lead to short circuits, thermal runaway, and potentially fires or explosions, especially in lithium-ion batteries.
The operation of battery electrode materials involves intricate chemical reactions, ion transportation through the electrolyte, and electron flow in the external circuit. This process enables batteries to efficiently store and release electrical energy, making them indispensable in a wide array of applications, ranging from portable electronics to electric vehicles and grid energy storage.
11. Safety Concerns about Inorganic Electrolytes-Based Solid-State Batteries
In contrast to conventional lithium-ion setups, solid-state batteries have the potential to attain enhanced safety and energy density. Despite significant advancements, particularly in solid-state electrolytes, there are enduring fundamental challenges that solid-state systems face in the realms of chemistry and mechanics. Wang et al. [104] outlined the underlying problems in solid-state batteries, with particular emphasis on three pivotal aspects: (i) the fundamental principles governing the development of high-ionic conductors; (ii) the structural transformations occurring at chemically unstable interfaces between the electrolyte and electrodes; and (iii) the impacts of solid-state battery manufacturing, encompassing electrode and electrolyte design [104].
Solid-state batteries with inorganic electrolytes hold great potential for the future of energy storage, especially in the realm of lithium-ion batteries (Li-ion). They bring several advantages over conventional liquid electrolytes, primarily in terms of safety, as they are non-flammable and do not leak. Nonetheless, as you pointed out, safety concerns tied to heat generation and thermal runaway are still significant aspects of solid-state battery development. Here are some critical points to consider regarding safety issues concerning inorganic electrolyte-based solid-state batteries:
Heat Generation: Solid-state batteries can still generate heat during their operation, particularly when charging or discharging rapidly. This heat may cause thermal stress within the battery components, potentially impacting their performance and lifespan.
Thermal Runaway: While solid-state electrolytes are not flammable, they are not immune to thermal runaway. Under extreme conditions or material defects, solid-state batteries can experience thermal runaway, which is a self-sustaining reaction that generates heat and could result in catastrophic failure.
Material Selection: The selection of materials for solid-state batteries is crucial to addressing safety concerns. Researchers are actively working on developing solid electrolytes that are not only safe but also exhibit excellent ionic conductivity and mechanical stability. Materials like ceramics and polymers are being explored for their potential to enhance safety.
Design and Engineering: Battery design plays a pivotal role in minimizing safety risks. This involves strategies like incorporating protective layers, implementing thermal management systems, and designing cells to withstand mechanical stresses. Proper engineering and manufacturing techniques are vital for ensuring the safety and reliability of solid-state batteries.
Testing and Certification: Establishing comprehensive testing and certification standards is essential to ensuring that solid-state batteries meet safety requirements. Industry and regulatory bodies are collaborating to establish protocols for testing and certifying the safety of these batteries.
Monitoring and Control: Advanced battery management systems (BMS) are crucial for monitoring temperature, voltage, and current within solid-state batteries. These systems can detect anomalies early, helping prevent potential safety issues by controlling the operating conditions.
In summary, while inorganic electrolyte-based solid-state batteries offer superior safety compared to traditional Li-ion batteries, concerns about heat generation and thermal runaway remain valid. Ongoing research and development efforts are aimed at addressing these concerns through material innovation, improved battery design, and the implementation of stringent testing and safety standards. The ultimate objective is to harness the benefits of solid-state batteries while ensuring their safety for widespread use across various applications.
12. Fast Charging and Safety Problems
High-speed charging (known as extreme fast charging, or XFC) plays a crucial role in advancing electric transportation. While prior research primarily concentrated on enhancing the movement of lithium ions within electrodes and electrolytes, the challenges associated with the efficient transfer of charge at the interface between the electrode and electrolyte have not received much attention. In a study conducted by Xing Yao et al. [105], it was revealed that the speed at which lithium ions transfer across the interface between the cathode and electrolyte is the limiting factor during XFC. However, to prevent the undesirable occurrence of lithium plating, it is necessary to simultaneously lower the energy barrier for charge transfer at both the cathode and anode. This reduction is achieved through modifications in the electrolyte composition [105].
Identifying the step that limits the rate of the process makes it challenging to completely prevent lithium (Li) plating on graphite anodes during rapid charging. To tackle this problem, Yue et al. [106] introduced a strategy for controlling Li plating and its morphology. Specifically, they achieved reversible Li plating on the graphite anode by utilizing a localized high-concentration electrolyte (LHCE). This approach effectively regulated Li plating with a high degree of reversibility during high-rate cycling. The researchers also conducted a detailed examination of the evolution of the solid electrolyte interphase (SEI) both before and after Li plating, aiming to gain insights into the interaction between lithiation behavior and electrochemical interface polarization [106].
Accelerating the charging speed and reducing the charging duration for lithium-ion batteries are essential steps in making electric vehicles more mainstream. Nevertheless, the challenge of preventing lithium plating on the graphite anode during rapid charging remains formidable. Despite significant advancements in lithium detection techniques, there is still a lack of understanding about the fundamental processes involved in lithium plating and its chemical/electrochemical responses during repeated charging cycles. In a study by Qian et al. [107], a comprehensive electrochemical approach was employed to investigate the behavior of graphite electrodes under fast-charging conditions. The research delved into a detailed analysis of how the phase, composition, and morphology of the graphite electrode changed when subjected to rapid charging. By implementing a resting period, they examined the subsequent reactions of the plated lithium, which tends to become irreversible or “dead” lithium. Furthermore, the researchers developed an improved graphite electrode with a thin silver (Ag) coating, acting as a reservoir for lithium. This plated lithium can be effectively “absorbed” by the Ag layer, forming a solid solution of lithium and silver (Li-Ag). This Li-Ag solid solution helps suppress the formation of irreversible lithium and provides structural stability. Consequently, this promotes the continued lithiation of the graphite electrode and enhances its reversibility during charging cycles [107].
Lithium-ion batteries (LIBs), as the most advanced energy storage devices, have garnered significant attention and seen rapid development in recent decades. However, there is an increasing urgency to enhance their fast-charging capabilities for time-saving and convenience, which is often hindered by the limitations of the graphite anode. Recent research has uncovered that the fast-charging performance of graphite anodes is largely influenced by the characteristics of the electrolyte used. In light of this, Zhang et al. [108] have provided a summary of investigations into improving the fast-charging performance of graphite anodes through modifications to the electrolyte. These investigations focus on two key aspects: the structures of the solid electrolyte interphase (SEI) and the arrangements of solvated lithium ions. Finally, the article outlines the challenges and potential avenues for further research aimed at advancing the fast-charging capabilities of graphite anodes [108].
Fast charging poses a risk of hazardous lithium (Li) plating on graphite anodes, but the challenge of pinpointing the exact rate-limiting step has made it difficult to completely eliminate this problem. Therefore, the conventional approach to preventing Li plating needs to be reconsidered. In a study by Xu et al. [109], they developed an elastic solid electrolyte interphase (SEI) with a consistent flow of Li ions on the graphite anode. This was achieved by introducing a synergistic additive consisting of triglyme (G3) and LiNO3 (referred to as GLN) into a commercial carbonate electrolyte. The goal was to enable dendrite-free and highly reversible Li plating even under high charging rates [109].
Swift charging is undeniably a significant focus of advancement within battery technology, particularly in light of the growing popularity of electric vehicles (EVs) and consumers’ desire for shorter charging durations. Nonetheless, rapid charging presents a range of issues, encompassing safety worries, thermal instability, and the risk of thermal runaway. Below, advanced strategies and approaches aimed at addressing these challenges are discussed:
Enhanced Battery Chemistry: Scientists are actively researching battery chemistries that can endure rapid charging without experiencing degradation or overheating. This involves the utilization of advanced materials such as silicon anodes, solid-state electrolytes, and more stable cathode materials.
Thermal Control Systems: Effective thermal management systems are essential for rapid charging. This includes the development of efficient cooling mechanisms capable of dissipating heat generated during fast charging to avert thermal runaway. Techniques like liquid cooling and phase-change materials are under investigation.
Battery Pack Redesign: Modifying the battery pack design to enhance heat dissipation and ensure even temperature distribution is critical. This might entail alterations to cell layout, the incorporation of cooling channels, or the adoption of modular pack configurations.
Smart Charging Algorithms: Intelligent charging algorithms can optimize the charging process to minimize heat generation. These algorithms can adapt the charging rate based on factors such as battery temperature, charge status, and historical charging data.
Higher Voltage Charging: Elevating the voltage at which batteries are charged can reduce charging duration, but it necessitates robust insulation and safety measures to prevent electrical issues such as arcing.
Pulse Charging: Instead of providing a constant current, pulse charging involves intermittent bursts of energy. This approach can reduce heat generation and enhance safety during fast charging.
Fast-Charging Standards: Industry standards are continuously evolving to support higher charging speeds while ensuring safety and compatibility.
Battery Thermal Modeling: Employing advanced thermal modeling techniques enables real-time monitoring of battery temperature, allowing for dynamic adjustments to charging rates to avert overheating.
Battery Preconditioning: Preconditioning the battery through heating or cooling before charging can help maintain a stable temperature during rapid charging.
Battery Management Systems (BMS): Advanced BMS can monitor the health of individual cells and balance charge across cells to prevent thermal runaway. They can also communicate with charging infrastructure to regulate charging rates as needed.
Materials and Coatings: Implementing advanced materials and coatings can enhance the thermal stability of battery components, reducing the risk of thermal runaway.
Fast-Charging Networks: Expanding fast-charging networks and infrastructure can reduce the demand for ultra-fast charging, thereby mitigating safety concerns associated with extremely rapid charging.
It is important to emphasize that fast charging is a multifaceted challenge that requires collaboration among battery manufacturers, automakers, infrastructure providers, and researchers to develop safe and efficient solutions. While progress is underway, safety remains a paramount consideration in the advancement of fast-charging technology.
13. Discussion
The discussion provided offers a comprehensive overview of the key points discussed in the paper regarding heat generation, temperature effects, and thermal management strategies for lithium-ion batteries. Here is a breakdown of the main points covered in this section:
Heat Generation and Temperature Behavior:
- Charge and Discharge Process: The charging and discharging of lithium-ion batteries involve various charge transport and chemical reactions, which lead to the generation of heat. The balance between reversible and irreversible heat components is crucial for understanding temperature behavior.
- Effects of Elevated Temperatures: Elevated temperatures within batteries can trigger detrimental side reactions, accelerate degradation processes, and potentially lead to thermal runaway incidents. Understanding and managing temperature is critical for maintaining battery performance and safety.
- Heat Generation Mechanisms: Heat generation in lithium-ion batteries includes both reversible and irreversible components. Reversible heat is linked to entropy changes, while irreversible heat encompasses overpotential heating, ohmic heating, and reaction heating.
Temperature’s Impact on Battery Performance:
- Temperature and Performance: Temperature significantly influences the performance of lithium-ion batteries. Different temperature conditions can lead to distinct negative effects, impacting the battery’s capacity, power output, and overall efficiency.
- Temperature Measurement and Management: Accurate temperature measurement within batteries is essential for effective battery management. Understanding temperature’s influence on battery behavior helps design efficient thermal management systems to maintain optimal operating conditions.
Comprehensive Review and Insights:
- Review of Investigations: The paper provides a comprehensive review and discussion of experimental and modeling investigations into the thermal behavior of different lithium-ion technologies. This review enhances the understanding of heat generation mechanisms, aiding in the design and optimization of thermal management systems.
- Heat Source Terms: Recent studies are emphasizing the inclusion of additional heat source terms, such as enthalpy heating and heat of mixing. These terms account for factors like lithium-ion diffusion and ion concentration gradients, providing a more complete assessment of heat generation.
- Comparing Heat Generation Mechanisms: Comparisons between reversible reaction contributions and joule losses help researchers assess the significance of different heat generation mechanisms during battery charge and discharge. This understanding is vital for optimizing thermal management strategies.
Incorporating Shell Casing into Modeling:
- Importance of Shell Casing: While existing literature often focuses on modeling the internal components of batteries, neglecting the properties of the shell casing is a limitation. Incorporating the shell casing into battery modeling is crucial due to its strength and fracture resistance under mechanical loads.
In conclusion, the discussion section summarizes the critical aspects related to heat generation, temperature effects, and thermal management in lithium-ion batteries. It highlights the importance of accurately estimating heat generation, understanding its influence on battery performance, and incorporating all relevant components into modeling efforts. This knowledge is invaluable for optimizing the design of thermal management systems and ensuring the safety, reliability, and longevity of lithium-ion batteries in various applications.
14. Conclusions
The conclusion effectively summarizes the key points discussed throughout the article and provides a concise overview of the significance of thermal analysis for lithium-ion battery systems. Here is a breakdown of the main points covered in this conclusion:
Evaluation of Existing Approaches:
- Basis for Effective Techniques: The review establishes a foundation by evaluating different models, algorithms, and methods used for analyzing the thermal properties of lithium-ion battery systems. This serves as a starting point for the development of more advanced and effective techniques for modeling and diagnosing battery thermal characteristics, ultimately enhancing safety.
Importance of Heat Generation Measurement:
- Heat Generation Rate and Safety: Accurate measurement of heat generation rates within lithium-ion cells is critical for ensuring safety and optimizing battery performance. The temperature distribution within the cell affects performance, cycle life, and overall safety. Heat generation and dissipation rates play a vital role in these dynamics.
Challenges and Model Development:
- Challenges in Experimental Measurements: The article points out the limited literature available on experimental measurements of heat generation rates, especially at high discharge rates. Developing dedicated thermal model systems is necessary to address the uneven temperature distribution in pouch cells.
- Importance of Suitable Thermal Models: To achieve more uniform temperature distribution and efficient thermal management, suitable thermal models specific to Li-ion batteries are required. These models are versatile tools that can be applied even with limited information about cell assembly and chemistry.
Practical Applications and Industry Impact:
- Utilization of Findings: The insights gained from thermal analysis techniques can be used by battery manufacturers to enhance battery designs and by researchers to advance the field. The non-uniform temperature distribution within battery packs highlights the need for optimized thermal management systems.
- Guiding Thermal Management Design: The uneven heat dissipation across battery packs emphasizes the importance of designing advanced thermal management systems. These systems can address specific needs such as mitigating capacity degradation, enhancing performance, and improving energy extraction.
Future Directions:
- Research and Further Exploration: The conclusion emphasizes the need for ongoing research to address various aspects of thermal issues in Li-ion batteries. This includes developing comprehensive thermal management techniques that incorporate features like cold storage, rapid heating, uniform cooling, and rapid cooling during critical events.
In conclusion, the article effectively summarizes the importance of accurate thermal analysis for lithium-ion battery systems. It highlights the need for further research to develop effective techniques for modeling and managing thermal characteristics, ultimately leading to improved safety, performance, and efficiency in battery applications.
Author Contributions
S.S.M. proposed the idea of the paper; S.S.M. wrote the paper; C.Z. provided suggestions on the content and structure of the paper; C.Z. and M.M. reviewed the draft manuscripts. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Helmholtz Association, grant number FE.5341.0118.0012, in the program Materials and Technologies for the Energy Transition (MTET) and the APC was funded by the KIT-Publication Fund of the Karlsruhe Institute of Technology. We want to express our gratitude for the funding.
Acknowledgments
This work contributes to the research performed at CELEST (Center of Electrochemical Energy Storage Ulm-Karlsruhe).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries; Springer: New York, NY, USA, 2009; Volume 1. [Google Scholar]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Rivas, M.H.; Van Mierlo, J. Key issues of lithium-ion batteries–from resource depletion to environmental performance indicators. J. Clean. Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, T.H.; Hu, S.J.; Cai, W.W.; Abell, J.A. Joining technologies for automotive lithium-ion battery manufacturing: A review. In Proceedings of the International Manufacturing Science and Engineering Conference, Erie, PA, USA, 12–15 October 2010; Volume 49460, pp. 541–549. [Google Scholar]
- Warner, J.T. The Handbook of Lithium-Ion Battery Pack Design: Chemistry, Components, Types and Terminology; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pistoia, G. (Ed.) Lithium-Ion Batteries: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Chen, D.; Jiang, J.; Kim, G.-H.; Yang, C.; Pesaran, A. Comparison of different cooling methods for lithium ion battery cells. Appl. Therm. Eng. 2016, 94, 846–854. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Applying Different Configurations for the Thermal Management of a Lithium Titanate Oxide Battery Pack. Electrochem 2021, 2, 50–63. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Thermal analysis of an indirect liquid cooling with different geometries for a lithium-ion battery. ECS Trans. 2019, 95, 105. [Google Scholar] [CrossRef]
- Madani, S.S. Numerical Simulation of an Air Cooling System for Lithium-Ion Batteries. ECS Trans. 2020, 99, 357. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Simulation of thermal behaviour of a lithium titanate oxide battery. Energies 2019, 12, 679. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Design and Simulation of Internal Flowing Twisted Conduits for Cooling of Lithium-Ion Batteries through Thermal Characterization. Batteries 2020, 6, 31. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Thermal analysis of cold plate with different configurations for thermal management of a lithium-ion battery. Batteries 2020, 6, 17. [Google Scholar] [CrossRef]
- Madani, S.S.; Schaltz, E.; Kær, S.K. Heat loss measurement of lithium titanate oxide batteries under fast charging conditions by employing isothermal calorimeter. Batteries 2018, 4, 59. [Google Scholar] [CrossRef]
- Rad, M.S.; Danilov, D.L.; Baghalha, M.; Kazemeini, M.; Notten, P.H.L. Adaptive thermal modeling of Li-ion batteries. Electrochim. Acta 2013, 102, 183–195. [Google Scholar]
- Zhu, C.; Li, X.; Song, L.; Xiang, L. Development of a theoretically based thermal model for lithium ion battery pack. J. Power Sources 2013, 223, 155–164. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Experimental and theoretical investigation of temperature distributions in a prismatic lithium-ion battery. Int. J. Therm. Sci. 2016, 99, 204–212. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. ETransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Baghdadi, I.; Briat, O.; Eddahech, A.; Vinassa, J.-M.; Gyan, P. Electro-thermal model of lithium-ion batteries for electrified vehicles applications. In Proceedings of the 2015 IEEE 24th International Symposium on Industrial Electronics (ISIE), Buzios, Brazil, 3–5 June 2015; pp. 1248–1252. [Google Scholar]
- Song, L.; Evans, J.W. Electrochemical-thermal model of lithium polymer batteries. J. Electrochem. Soc. 2000, 147, 2086. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Cao, K.; Li, S.; Su, Z.; Jin, G.; Fu, C. Core temperature estimation based on electro-thermal model of lithium-ion batteries. Int. J. Energy Res. 2020, 44, 5320–5333. [Google Scholar] [CrossRef]
- Choi, S.S.; Lim, H.S. Factors that affect cycle-life and possible degradation mechanisms of a Li-ion cell based on LiCoO2. J. Power Sources 2002, 111, 130–136. [Google Scholar] [CrossRef]
- Thomas, E.V.; Case, H.L.; Doughty, D.H.; Jungst, R.G.; Nagasubramanian, G.; Roth, E.P. Accelerated power degradation of Li-ion cells. J. Power Sources 2003, 124, 254–260. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S. Use of organic esters as cosolvents in electrolytes for lithium-ion batteries with improved low temperature performance. J. Electrochem. Soc. 2002, 149, A361. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Rao, L.; Newman, J. Heat-generation rate and general energy balance for insertion battery systems. J. Electrochem. Soc. 1997, 144, 2697. [Google Scholar] [CrossRef]
- Thomas, K.E.; Newman, J. Thermal modeling of porous insertion electrodes. J. Electrochem. Soc. 2003, 150, A176. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Effect of electrode configuration on the thermal behavior of a lithium-polymer battery. J. Power Sources 2008, 180, 909–916. [Google Scholar] [CrossRef]
- Kim, U.S.; Shin, C.B.; Kim, C.S. Modeling for the scale-up of a lithium-ion polymer battery. J. Power Sources 2009, 189, 841–846. [Google Scholar] [CrossRef]
- Smith, K.A.; Kim, G.H. Modeling of Non-Uniform Degradation in Large Format Lithium Ion Cells; InECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2009; p. 255. [Google Scholar]
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.L.; Bae, S.J. A study of the relationship between coulombic efficiency and capacity degradation of commercial lithium-ion batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A general energy balance for battery systems. J. Electrochem. Soc. 1985, 132, 5. [Google Scholar] [CrossRef]
- Sun, Y.S.; Bai, R.H.; Ma, J. Design and thermal analysis of a new topological cooling plate for prismatic lithium battery thermal management. Appl. Therm. Eng. 2023, 219, 119547. [Google Scholar] [CrossRef]
- Bazinski, S.J.; Wang, X. The Influence of Cell Temperature on the Entropic Coefficient of a Lithium Iron Phosphate (LFP) Pouch Cell. J. Electrochem. Soc. 2013, 161, A168–A175. [Google Scholar] [CrossRef]
- Kumaresan, K.; Sikha, G.; White, R.E. Thermal Model for a Li-Ion Cell. J. Electrochem. Soc. 2008, 155, A164. [Google Scholar] [CrossRef]
- Botte, G.G. Influence of Some Design Variables on the Thermal Behavior of a Lithium-Ion Cell. J. Electrochem. Soc. 1999, 146, 914. [Google Scholar] [CrossRef]
- Srinivasan, V.; Wang, C.Y. Analysis of Electrochemical and Thermal Behavior of Li-Ion Cells. J. Electrochem. Soc. 2003, 150, A98. [Google Scholar] [CrossRef]
- Pals, C.R.; Newman, J. Thermal Modeling of the Lithium/Polymer Battery—I. Discharge behaviour of a Single Cell. J. Electrochem. Soc. 1995, 142, 3274–3281. [Google Scholar] [CrossRef]
- Al Hallaj, S.; Maleki, H.; Hong, J.S.; Selman, J.R. Thermal modeling and design considerations of lithium-ion batteries. J. Power Sources 1999, 83, 1–8. [Google Scholar] [CrossRef]
- Smith, K.; Wang, C.Y. Power and thermal characterization of a lithium-ion battery pack for hybrid-electric vehicles. J. Power Sources 2006, 160, 662–673. [Google Scholar] [CrossRef]
- Verbrugge, M.W. Three dimensionai temperature and current distribution in a battery module. AIChE J. 1995, 41, 1550–1562. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.W. Heat Transfer Phenomena in Lithium/Polymer-Electrolyte Batteries for Electric Vehicle Application. J. Electrochem. Soc. 1993, 140, 1833–1838. [Google Scholar] [CrossRef]
- Gomadam, P.M.; Weidner, J.W.; Dougal, R.A.; White, R.E. Mathematical modeling of lithium-ion and nickel battery systems. J. Power Sources 2002, 110, 267–284. [Google Scholar] [CrossRef]
- Shen, X.Y.; Cai, T.N.; He, C.M.; Yang, Y.; Chen, M. Thermal analysis of modified Z-shaped air-cooled battery thermal management system for electric vehicles. J. Energy Storage 2023, 58, 106356. [Google Scholar] [CrossRef]
- Wu, L.X.; Liu, K.; Liu, J.H.; Pang, H. Evaluating the heat generation characteristics of cylindrical lithium-ion battery considering the discharge rates and N/P ratio. J. Energy Storage 2023, 64, 107182. [Google Scholar] [CrossRef]
- Chen, S.C.; Wan, C.C.; Wang, Y.Y. Thermal analysis of lithium-ion batteries. J. Power Sources 2005, 140, 111–124. [Google Scholar] [CrossRef]
- Zhu, N.N.; Wang, X.H.; Huang, Q.; Ding, C.; Wang, J. Assessment on fire risk of lithium-ion battery packs with different sizes and states of charge by cone calorimeter. J Therm Anal Calorim 2023, 148, 6119–6132. [Google Scholar] [CrossRef]
- Chen, S.-C.; Wang, Y.-Y.; Wan, C.-C. Thermal Analysis of Spirally Wound Lithium Batteries. J. Electrochem. Soc. 2006, 153, A637. [Google Scholar] [CrossRef]
- Chen, Y. Three-Dimensional Thermal Modeling of Lithium-Polymer Batteries under Galvanostatic Discharge and Dynamic Power Profile. J. Electrochem. Soc. 1994, 141, 2947. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.W. Thermal analysis of lithium polymer electrolyte batteries by a two dimensional model-thermal behaviour and design optimization. Electrochim. Acta 1994, 39, 517–526. [Google Scholar] [CrossRef]
- Du, S.; Lai, Y.; Ai, L.; Ai, L.; Cheng, Y.; Tang, Y.; Jia, M. An investigation of irreversible heat generation in lithium ion batteries based on a thermo-electrochemical coupling method. Appl. Therm. Eng. 2017, 121, 501–510. [Google Scholar] [CrossRef]
- Drake, S.J.; Martin, M.; Wetz, D.; Ostanek, J.K.; Miller, S.; Heinzel, J.; Jain, A. Heat generation rate measurement in a Li-ion cell at large C-rates through temperature and heat flux measurements. J. Power Sources 2015, 285, 266–273. [Google Scholar] [CrossRef]
- Gümüşsu, E.; Ekici, Ö.; Köksal, M. 3-D CFD modeling and experimental testing of thermal behavior of a Li-Ion battery. Appl. Therm. Eng. 2017, 120, 484–495. [Google Scholar] [CrossRef]
- Xiao, M.; Choe, S.Y. Theoretical and experimental analysis of heat generations of a pouch type LiMn2O4/carbon high power Li-polymer battery. J. Power Sources 2013, 241, 46–55. [Google Scholar] [CrossRef]
- Abdul-Quadir, Y.; Laurila, T.; Karppinen, J.; Jalkanen, K.; Vuorilehto, K.; Skogström, L.; Paulasto-Kröckel, M. Heat generation in high power prismatic Li-ion battery cell with LiMnNiCoO2 cathode material. Int. J. Energy Res. 2014, 38, 1424–1437. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.M. Thermal characterization of a high-power lithium-ion battery: Potentiometric and calorimetric measurement of entropy changes. Energy 2013, 61, 432–439. [Google Scholar] [CrossRef]
- Nieto, N.; Díaz, L.; Gastelurrutia, J.; Alava, I.; Blanco, F.; Ramos, J.C.; Rivas, A. Thermal Modeling of Large Format Lithium-Ion Cells. J. Electrochem. Soc. 2013, 160, A212–A217. [Google Scholar] [CrossRef]
- Vertiz, G.; Oyarbide, M.; Macicior, H.; Miguel, O.; Cantero, I.; de Arroiabe, P.F.; Ulacia, I. Thermal characterization of large size lithium-ion pouch cell based on 1d electro-thermal model. J. Power Sources 2014, 272, 476–484. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Son, K.; Hwang, S.M.; Woo, S.G.; Paik, M.; Song, E.H.; Kim, Y.J. Thermal and chemical characterization of the solid-electrolyte interphase in Li-ion batteries using a novel separator sampling method. J. Power Sources 2019, 440, 227083. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium-ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Y.; Xiao, Z.; Wang, C.; Long, T. Review on Thermal Runaway of Lithium-Ion Batteries for Electric Vehicles. J. Electron. Mater. 2022, 51, 30–46. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Ramadass, P.; Zhang, Z. Analysis of internal short-circuit in a lithium-ion cell. J. Power Sources 2009, 194, 550–557. [Google Scholar] [CrossRef]
- Wei, G.; Huang, R.J.; Zhang, G.X.; Jiang, B.; Zhu, J.G.; Guo, Y.Y.; Han, G.S.; Wei, X.Z.; Dai, H.F. A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Appl. Energy 2023, 349, 121651. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. Abuse testing of lithium-ion batteries: Characterization of the overcharge reaction of LiCoO2/graphite cell. J. Electrochem. Soc. 2001, 148, A838–A844. [Google Scholar] [CrossRef]
- Zavalis, T.G.; Behm, M.; Lindbergh, G. Investigation of short-circuit scenarios in a lithium-ion battery cell. J. Electrochem. Soc. 2012, 159, A848–A859. [Google Scholar] [CrossRef]
- Maleki, H.; Howard, J.N. Internal short circuit in Li-ion cells. J. Power Sources 2009, 191, 568–574. [Google Scholar] [CrossRef]
- Yamauchi, T.; Mizushima, K.; Satoh, Y.; Yamada, S. Development of a simulator for both property and safety of a lithium secondary battery. J. Power Sources 2004, 136, 99–107. [Google Scholar] [CrossRef]
- Xia, Y.; Wierzbicki, T.; Sahraei, E.; Zhang, X. Damage of cells and battery packs due to ground impact. J. Power Sources 2014, 267, 78–97. [Google Scholar] [CrossRef]
- Zhang, C.; Santhanagopalan, S.; Sprague, M.A.; Pesaran, A.A. A representative sandwich model for simultaneously coupled mechanical-electric-thermal simulation of a lithium-ion cell under quasi-static indentation tests. J. Power Sources 2015, 298, 309–321. [Google Scholar] [CrossRef]
- Zhang, C.; Santhanagopalan, S.; Sprague, M.A.; Pesaran, A.A. Coupled mechanical-electrical thermal modeling for short-circuit prediction in a lithium-ion cell under mechanical abuse. J. Power Sources 2015, 290, 102–113. [Google Scholar] [CrossRef]
- Xia, Y.; Li, T.; Ren, F.; Gao, Y.; Wang, H. Failure analysis of pinch–torsion tests as a thermal runaway risk evaluation method of Li-ion cells. J. Power Sources 2014, 265, 356–362. [Google Scholar] [CrossRef]
- Sahraei, E.; Meier, J.; Wierzbicki, T. Characterizing and modeling mechanical properties and onset of short circuit for three types of lithium-ion pouch cells. J. Power Sources 2014, 247, 503–516. [Google Scholar] [CrossRef]
- Ali, M.Y.; Lai, W.-J.; Pan, J. Computational models for simulations of lithium-ion battery cells under constrained compression tests. J. Power Sources 2013, 242, 325–340. [Google Scholar] [CrossRef]
- Lai, W.-J.; Ali, M.Y.; Pan, J. Mechanical behavior of representative volume elements of lithium-ion battery cells under compressive loading conditions. J. Power Sources 2014, 245, 609–623. [Google Scholar] [CrossRef]
- Greve, L.; Fehrenbach, C. Mechanical testing and macro-mechanical finite element simulation of the deformation, fracture, and short circuit initiation of cylindrical lithium-ion battery cells. J. Power Sources 2012, 214, 377–385. [Google Scholar] [CrossRef]
- Sahraei, E.; Hill, R.; Wierzbicki, T. Calibration and finite element simulation of pouch lithium-ion batteries for mechanical integrity. J. Power Sources 2012, 201, 307–321. [Google Scholar] [CrossRef]
- Zhang, X.; Wierzbicki, T. Characterization of plasticity and fracture of shell casing of lithium-ion cylindrical battery. J. Power Sources 2015, 280, 47–56. [Google Scholar] [CrossRef]
- McKerracher, R.D.; Guzman-Guemez, J.; Wills, R.G.A.; Sharkh, S.M.; Kramer, D. Advances in prevention of thermal runaway in Lithium-Ion batteries. Adv. Energy Sustain. Res. 2021, 2, 2000059. [Google Scholar] [CrossRef]
- Lin, C.; Burggräf, P.; Liu, L.; Adlon, T.; Mueller, K.; Beyer, M.; Xu, T.; Kammerer, V.; Hu, J.; Liu, S.; et al. Deep-Dive analysis of the latest Lithium-Ion battery safety testing standards and regulations in Germany and China. Renew. Sustain. Energy Rev. 2023, 173, 113077. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, H.; Xu, X. Thermal fault prognosis of lithium-ion batteries in real-world electric vehicles using self-attention mechanism networks. Appl. Therm. Eng. 2023, 226, 120304. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhou, Y.; Zhao, S.; Yuan, Y. Towards High-Safety Lithium-Ion Battery Diagnosis Methods. Batteries 2023, 9, 63. [Google Scholar] [CrossRef]
- Xia, Q.; Ren, Y.; Wang, Z.; Yang, D.; Yan, P.; Wu, Z.; Sun, B.; Feng, Q.; Qian, C. Safety risk assessment method for thermal abuse of lithium-ion battery pack based on multiphysics simulation and improved bisection method. Energy 2023, 264, 126228. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, Z.; Wang, B.; Gao, H.; Chen, S.; Zong, S.; Luo, K. A Li-ion battery thermal management system combining a heat pipe and thermoelectric cooler. Energies 2020, 13, 841. [Google Scholar] [CrossRef]
- Talluri, T.; Kim, T.H.; Shin, K.J. Analysis of a battery pack with a phase change material for the extreme temperature conditions of an electrical vehicle. Energies 2020, 13, 507. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, J.; Gu, J. The Effects of Cooling Structure and Li-Ion Battery Specification on the Cooling Performance of a Passive Thermal Management System. In Proceedings of the 2017 International Conference on Sensing, Diagnostics, Prognostics, and Control (SDPC), Shanghai, China, 16–18 August 2017; pp. 662–669. [Google Scholar]
- Jilte, R.D.; Kumar, R. Numerical investigation on the cooling performance of Li-ion battery thermal management system at high galvanostatic discharge. Eng. Sci. Technol. 2018, 21, 957–969. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Ghanbarpour, M.; Jaguemont, J.; Sokkeh, M.A.; Gandoman, F.H.; Berecibar, M.; Van Mierlo, J. A new concept of thermal management system in Li-ion battery using air cooling and heat pipe for electric vehicles. Appl. Therm. Eng. 2020, 174, 115280. [Google Scholar] [CrossRef]
- Behi, H.; Kalogiannis, T.; Suresh Patil, M.; Mierlo, J.V.; Berecibar, M. A new concept of air cooling and heat pipe for electric vehicles in fast discharging. Energies 2021, 14, 6477. [Google Scholar] [CrossRef]
- Deng, T.; Ran, Y.; Yin, Y.; Liu, P. Multi-objective optimization design of thermal management system for lithium-ion battery pack based on Non-dominated Sorting Genetic Algorithm II. Appl. Therm. Eng. 2020, 164, 114394. [Google Scholar] [CrossRef]
- Bamdezh, M.A.; Molaeimanesh, G.R. Impact of system structure on the performance of a hybrid thermal management system for a Li-ion battery module. J. Power Sources 2020, 457, 227993. [Google Scholar] [CrossRef]
- Arora, S.; Kapoor, A.; Shen, W. A novel thermal management system for improving discharge/charge performance of Li-ion battery packs under abuse. J. Power Sources 2018, 378, 759–775. [Google Scholar] [CrossRef]
- Lin, C.; Xu, S.; Chang, G.; Liu, J. Experiment and simulation of a LiFePO4 battery pack with a passive thermal management system using composite phase change material and graphite sheets. J. Power Sources 2015, 275, 742–749. [Google Scholar] [CrossRef]
- Basu, S.; Hariharan, K.S.; Kolake, S.M.; Song, T.; Sohn, D.K.; Yeo, T. Coupled electrochemical thermal modelling of a novel Li-ion battery pack thermal management system. Appl. Energy 2016, 181, 1–13. [Google Scholar] [CrossRef]
- Cen, J.; Jiang, F. Li-ion power battery temperature control by a battery thermal management and vehicle cabin air conditioning integrated system. Energy Sustain. Dev. 2020, 57, 141–148. [Google Scholar] [CrossRef]
- Malik, M.; Dincer, I.; Rosen, M.; Fowler, M. Experimental Investigation of a New Passive Thermal Management System for a Li-Ion Battery Pack Using Phase Change Composite Material. Electrochim. Acta 2017, 257, 345–355. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhang, Y.; Wang, F.; Wu, K. Experimental and Numerical Investigation of Thermal Energy Management with Reciprocating Cooling and Heating Systems for Li-Ion Battery Pack. J. Energy Eng. 2018, 144, 04018039. [Google Scholar] [CrossRef]
- Biemolt, J.; Jungbacker, P.; van Teijlingen, T.; Yan, N.; Rothenberg, G. Beyond lithium-based batteries. Materials 2020, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tang, A.; Mu, H.; Wang, W.; Wang, C. Aging mechanisms of electrode materials in lithium-ion batteries for electric vehicles. J. Chem. 2015, 2015, 104673. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, G.; Li, W.; Tao, Q.; Shi, C.; Jin, H.; Chen, G.; Wang, S. Fundamentals of electrolytes for solid-state batteries: Challenges and perspectives. Front. Mater. 2020, 7, 111. [Google Scholar] [CrossRef]
- Yao, Y.-X.; Chen, X.; Yao, N.; Gao, J.-H.; Xu, G.; Ding, J.-F.; Song, C.-L.; Cai, W.-L.; Yan, C.; Zhang, Q. Unlocking charge transfer limitations for extreme fast charging of Li-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202214828. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, J.; Dong, Y.; Chen, Y.; Shi, Z.; Xu, X.; Li, X.; Liang, Z. Reversible Li Plating on Graphite Anodes through Electrolyte Engineering for Fast-Charging Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302285. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, T.; Huang, D.; Liu, G.; Tong, W. Insights into the enhanced reversibility of graphite anode upon fast charging through Li reservoir. ACS Nano 2022, 16, 20197–20205. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Z.; Fang, J.; Li, K.; Zhang, M.; Li, Z.; Yang, L.; Pan, F. Electrolyte optimization for graphite anodes toward fast charging. J. Phys. Chem. C 2023, 127, 2755–2765. [Google Scholar] [CrossRef]
- Xu, X.; Yue, X.; Chen, Y.; Liang, Z. Li Plating Regulation on Fast-Charging Graphite Anodes by a Triglyme-LiNO3 Synergistic Electrolyte Additive. Angew. Chem. Int. Ed. 2023, 62, e202306963. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).