Abstract
A series of chiral enantiomerically pure aziridines containing a phosphine moiety were synthesized and successfully applied as organocatalysts in asymmetric intramolecular Rauhut–Currier reactions of p-quinone derivatives. The desired chiral phenols were achieved in high chemical yields and with satisfactory values of enantiomeric excess (up to 98% ee, in some cases). The stereochemical course of the title reaction may be controlled by the use of an appropriate enantiomer of the catalyst. The individual enantiomers of the organocatalyst led to the formation of specific enantiomers of the chiral product.
1. Introduction
Asymmetric organocatalysis [1] is still a significant challenge for modern organic chemists, as well as an enabling technology for various aspects of medicinal chemistry [2]. Among the many approaches used in asymmetric organocatalysis [2], the most widely applied are chiral amine catalysis [3], bifunctional hydrogen bond catalysis [4], chiral phosphoric acid catalysis [5], N-heterocyclic carbene catalysis [6], and phase-transfer catalysis [7]. Moreover, recent literature reports describe interesting and not so obvious methods such as organomulticatalysis [8], enantioselective radical reactions [9], organocatalytic reactions employing prochiral carbocationic intermediates [10], asymmetric organocatalysis in continuous flow [11], and the use of carbon dioxide in asymmetric synthesis [12].
The asymmetric Rauhut–Currier (RC) reaction (vinylogous Morita–Baylis–Hillman reaction) constitutes a coupling of an activated alkene to a second Michael acceptor [13]. The process can take place in an intramolecular manner, thereby yielding useful derivatives of decalin [14], allenes [15], and acrylates [16]. In turn, an intermolecular RC reaction may lead to the creation of the useful β-perfluoroalkyl building blocks [17], 3,3-disubstituted oxindoles [18], spirocyclic oxindoles [19,20], and azepino [1,2-α] indoles [21].
Among the wide range of catalysts promoting an RC reaction, mention should be made of DMAP and its derivatives [22], N-heterocyclic carbenes [23], thiourea-phosphines [24], bisphosphines [25], and, especially, phosphines [26,27,28,29,30].
However, among the extremely wide range of organophosphorus catalysts used in asymmetric synthesis, phosphorus-containing aziridine derivatives have received only a few reports [31,32,33,34,35].
Taking into account all the aforementioned information and based on the experience of our group in working with chiral aziridine catalysts [36,37,38,39], we decided to continue our research on the use of organophosphorus derivatives of chiral aziridines in asymmetric synthesis [40,41,42,43,44]. Thus, in order to test the possibility of extending the applicability of our chiral aziridine phosphines, we decided to study their catalytic activity in an asymmetric intramolecular Rauhut–Currier reaction.
2. Results and Discussion
2.1. Synthesis of Chiral Aziridine-Phosphines 1–8
The optically pure aziridine-phosphines 1–8 (Figure 1) were synthesized according to the previously published protocol [42]. The synthetic pathway comprises the formation of the corresponding phosphine oxides [40] and then their reduction using the triethoxysilane-titanium (IV) isopropoxide system [42].
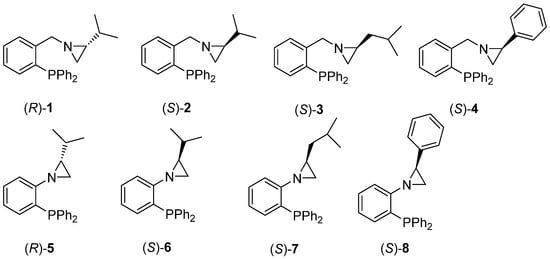
Figure 1.
Chiral aziridine-phosphines 1–8.
2.2. Asymmetric Intramolecular Rauhut–Currier Reaction in the Presence of Aziridines 1–8
Having the enantiomerically pure aziridine-phosphines 1–8 in hand, we decided to check their catalytic activity in the asymmetric intramolecular Rauhut–Currier reaction of p-quinone derivative 9 obtained from salicylaldehyde. (Z)-2-((tert-Butyl)-5-isopropyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl)phenyl acrylate 9 was stirred in dichloromethane at room temperature in the presence of 10 mol% of chiral catalysts 1–8 over 48 h (Scheme 1) [26].
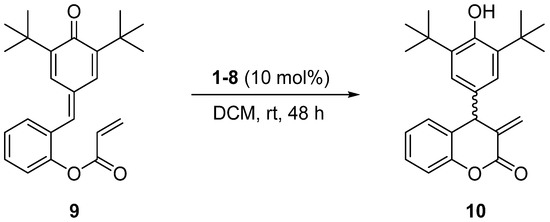
Scheme 1.
Asymmetric intramolecular Rauhut–Currier reaction of p-quinone derivative 9 catalyzed by chiral aziridine-phosphines 1–8.
All the results of the above asymmetric transformations comprising chemical yields, enantiomeric excess (ee), and absolute configurations of chiral products 10 are collected in Table 1.

Table 1.
Asymmetric Rauhut–Currier reaction promoted by aziridine-phosphines 1–8.
Inspection of the data collected in Table 1 reveals some findings. As anticipated, based on our previous studies [40,41,42,43,44], organocatalysts 1–4 bearing a methylene group connecting a phenyl ring of phosphine with a three-membered system of aziridine showed rather moderate activity in the title reaction, affording product 10 in moderate-to-high (51–81%) chemical yields, and with low-to-moderate enantiomeric excess values (30–66% of ee) (Table 1, entries 1–4). In turn, the application of the catalyst 5 and catalyst 6 (without the above methylene linker and with an isopropyl chain in an aziridine subunit) gave much better results in terms of yield and, especially, enantiomeric excess (Table 1, entries 5–6). Their analogs 7 and 8 with isobutyl and phenyl substituents were also more active in comparison with the methylene-linked systems of 1–4, but in these cases, the differences were not so pronounced (Table 1, entries 7–8). Moreover, the application of the enantiomeric forms of catalysts 1–2 (Table 1, entries 1–2) and 5–6 (Table 1, entries 5–6) resulted in the creation of the opposite enantiomers of product 10, which is in line with our previous findings [40,41,42,43,44]. In these cases, the differences in the enantiomeric excesses of products having the opposite absolute configurations seem somewhat surprising (52%, 66%, and 91%, and 99% of ee, respectively). Such discrepancies may sometimes result from the different chemical and optical purities of both enantiomeric catalysts. Finally, we also tried to lower the catalyst loading to 5 mol%; however, this resulted in a reduction in both the chemical yield and the enantiomeric excess.
2.3. Organocatalytic Asymmetric Rauhut–Currier Reaction in the Presence of the Phosphine 6–Scope of the Substrates
The aziridine phosphine 6 showed the best catalytic activity in the asymmetric intramolecular Rauhut–Currier reaction of substrate 9. Hence, we decided to extend the scope of its applicability in the further asymmetric Rauhut–Currier intramolecular cyclization reactions using the other or more p-quinone derivatives 11–15 bearing various substituents in an aromatic ring (Scheme 2). All the results are summarized in Table 2.
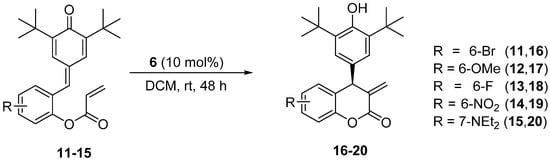
Scheme 2.
Asymmetric Rauhut–Currier reaction catalyzed by aziridine-phosphine 6.

Table 2.
Asymmetric Rauhut–Currier reaction promoted by aziridine-phosphine 6.
Careful analysis of the results collected in Table 2 reveals that (S)-configured chiral aziridine-phosphine 6 constitutes an efficient catalyst which is prone to successfully catalyzing the asymmetric organocatalytic intramolecular Rauhut–Currier reactions of variously substituted p-quinone derivatives. Much to our disappointment, the RC reaction employing 7-NEt2-substituted derivative 15 completely failed (Table 2, entry 5). Attempts to repeat it with higher catalyst loading and at elevated temperatures also resulted in isolation of the starting material. Moreover, TLC analysis of reaction mixture and 1H NMR of the recovered starting material 15 revealed its partial decomposition.
Finally, in order to prove the importance of the phosphine moiety for the course of the title reaction, a model intramolecular Rauhut–Currier reaction of derivative 9 was performed in the presence of aziridine-phosphine oxide 21 (Scheme 3). As anticipated, based on our previous studies on the asymmetric Morita–Baylis–Hillman reaction [44], not even traces of the product 10 formation were observed. This phenomenon confirms the decisive importance of the phosphine moiety for the course of the asymmetric Rauhut–Currier reaction (i.e., the vinyl Morita–Baylis–Hillman reaction), and it also indicates the validity of the previously proposed transition state model [44].
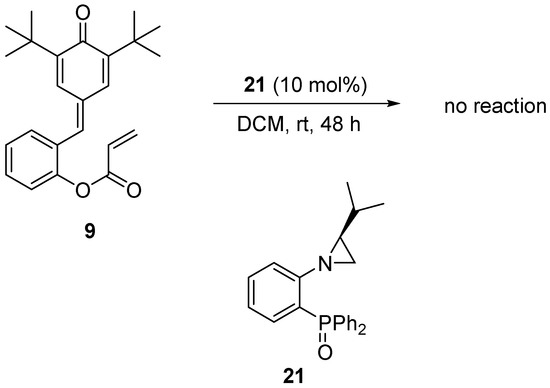
Scheme 3.
Attempt at the asymmetric Rauhut–Currier reaction in the presence of phosphine oxide 21.
3. Materials and Methods
3.1. Materials
Dichloromethane (DCM) (anhydrous, 99.8%) was purchased from Merck (Merck KgaA, Darmstadt, Germany) and used without purification. Ethyl acetate and n-hexane were distilled before use. NMR spectra were recorded on a Bruker (Bruker, Billerica, MA, USA) apparatus at 600 and 150 MHz with the use of CDCl3 as a solvent and TMS as an internal standard. The data are reported as s = singlet, d = doublet, t = triplet, dd = doublet of doublets, dt = doublet of triplets, and m = multiplet. Optical rotations were measured on an Anton Paar MCP500 polarimeter with a sodium lamp at room temperature. Thin layer chromatography (TLC) was carried out using Merck 60 F254 silica gel plates (Merck KgaA, Darmstadt, Germany). Column chromatography was performed on Merck 60 silica gel. Enantiomeric excess was determined using chiral HPLC on a Chiralcel OD-H column (Daicel Corporation, Osaka, Japan). The Knauer HPLC chromatograph (Knauer, Wissenchaftliche Geräte GmbH, Berlin, Germany) was used for HPLC analysis. Aziridine-phosphines 1–8 and phosphine oxide 21 were synthesized according to previous protocols [40,42].
3.2. Methods
3.2.1. Substrates
Substrates 9, 11–13, and 15 were prepared according to literature [26]. Their spectroscopic data are in full agreement with the aforementioned published data [26]. In turn, 6-NO2-subsituted starting material 14 was synthesized on the basis of the work of Enders et al. [45]. As no description of the spectroscopic data of substrate 14 has been found in the literature, we present it below:
- 2-((3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)methyl)-4-nitrophenyl acrylate 14Yellow solid, mp 112.4–113.9 °C; 1 H NMR (600 MHz, CDCl3) δ = 1.30 (s, 9H), 1.35 (s, 9H), 6.16 (dd, J = 0.5, 10.5 Hz, 1H), 6.38 (dd, J = 10.5, 17.3 Hz, 1H), 6.70 (dd, J = 0.5, 17.3 Hz, 1H), 7.00 (s, 2H), 7.30 (d, J = 2.0 Hz, 1H), 7.48 (d, J = 9.0 Hz, 1H), 8.33 (dd, J = 2.7, 8.9 Hz, 1H), 8.42 (d, J = 2.6 Hz, 1H); 13 C NMR (150 MHz, CDCl3) δ = 29.4, 29.5, 35.6, 123.9, 124.9, 126.7, 127.0, 130.0, 132.1, 133.9, 134.7, 134.9, 145.3, 149.1, 153.5, 163.1, 186.4. Anal. calcd for C24H27NO5: C, 70.40, H, 6.60, N, 3.30; found C, 70.22, H, 6.44, N, 3.20.
3.2.2. Asymmetric Organocatalytic Rauhut–Currier Reaction–General Procedure
A solution of chiral catalyst (10 mol%, 0.02 mmol) and substrate (0.2 mmol) in dichloromethane (2 mL) was stirred at room temperature until complete consumption of the starting material took place (usually 48 h, reactions monitored by TLC). The solvent was evaporated in vacuo and the residue was purified by column chromatography on silica gel (hexane: ethyl acetate 4:1). The NMR spectra of products 10 and 16–18 were consistent with the literature data [26]. For product 19, full spectroscopic analysis has been included. Copies of the NMR spectra of RC products and their HPLC tracks are collected in the Supplementary Materials.
- (S)-4-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methylenechroman-2-one 10
- 1H NMR (600 MHz, CDCl3) δ = 1.39 (s, 18H), 4.88 (s, 1H), 5.19 (s, 1H), 5.76 (s, 1H), 6.91 (s, 2H), 7-15-7.17 (m, 3H), 7.31-7.34 (m, 1H).
- (S)-4-(3,5-di-tert-butyl-4-hydroxyphenyl)-6-bromo-3-methylenechroman-2-one 16
- 1H NMR (600 MHz, CDCl3) δ = 1.41 (s, 18H), 4.84 (s, 1H), 5.22 (s, 1H), 5.75 (s, 1H), 6.44 (s, 1H), 6.89 (s, 1H), 7.04 (d, J = 8.6 Hz, 1H), 7.42 (dd, J = 2.3, 8.7 Hz, 1H).
- (S)-4-(3,5-di-tert-butyl-4-hydroxyphenyl)-6-methoxy-3-methylenechroman-2-one 17
- 1H NMR (600 MHz, CDCl3) δ = 1.40 (s, 1H), 3.84 (s, 3H), 4.82 (s, 1H), 5.18 (s, 1H), 5.73 (s, 1H), 6.41 (s, 1H), 6.69-6.71 (m, 2H), 6.91 (s, 2H), 7.04 (d, J = 9.1 Hz, 1H).
- (S)-4-(3,5-di-tert-butyl-4-hydroxyphenyl)-6-fluoro-3-methylenechroman-2-one 18
- 1H NMR (600 MHz, CDCl3) δ = 1.41 (s, 18H), 4.84 (s, 1H), 5.23 (s, 1H), 5.74 (s, 1H), 6.45 (s, 1H), 6.84 (dd, J = 3.0 Hz, 8.4 Hz, 1H), 6.91 (s, 2H), 7.01 (dt, J = 3.1 Hz, 8.3 Hz, 1H), 7.12 (dd, J = 4.6 Hz, 8.9 Hz, 1H).
- (S)-4-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-methylene-6-nitrochroman-2-one 19
- White solid, mp 171.3–172.2 °C; [α]d = 38.0 (c 0.5, CHCl3); 1H NMR (600 MHz, CDCl3) δ = 1.41 (s, 18H), 4.97 (s, 1H), 5.27 (s, 1H), 5.82 (s, 1H), 6.54 (s, 1H), 6.91 (s, 2H), 8.08 (d, J = 2.6 Hz, 1H), 8.22 (dd, J = 2.6, 9.0 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ = 30.1, 34.5, 48.1, 118.2, 124.1, 124.5, 124.6, 127.1, 129.7, 130.8, 135.4, 136.9, 144.4, 153.6, 161.7. Anal. calcd for C24H27NO5: C, 70.40, H, 6.60, N, 3.30; found C, 70.20, H, 6.44, N, 3.11.
4. Conclusions
Chiral, optically pure aziridine-phosphines were proven to be effective catalysts of asymmetric organocatalytic intramolecular Rauhut–Currier reactions of the corresponding p-quinone derivatives. The appropriate chiral phenols were obtained efficiently in terms of chemical yield and enantiomeric excess. Moreover, the use of both the enantiomeric forms of the chiral catalysts led to the formation of the products bearing opposite absolute configurations. Thus, access to the appropriate enantiomers of the reaction products can be provided by the use of catalysts of the appropriate absolute configuration regarding the various interactions of the substrate with chiral aziridine-phosphine [44]. The formation of the second enantiomeric product is due to the fact that the use of a phosphine catalyst of the opposite configuration generates a mirror image transition state.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym14081631/s1: NMR spectra of substrate 14 and products of the RC reactions and HPLC chromatograms of the Rauhut–Currier reaction products.
Author Contributions
Conceptualization and methodology, S.L. and M.R.; software, A.B.-S. and M.R.; investigation, A.B.-S.; writing–original draft preparation, M.R.; writing–review and editing, S.L. and M.R.; supervision, M.R. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre (NCN) (Grant No. 2016/21/B/ST5/00421 for M.R.). The scientific grant InterChemMed No. POWR.03.02.00-00-00-I029/16 for A.B. is also acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiang, S.-H.; Tan, B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020, 11, 3786. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric organocatalysis: An enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- France, S.; Guerin, D.J.; Miller, S.J.; Lectka, T. Nucleophilic Chiral Amines as Catalysts in Asymmetric Synthesis. Chem. Rev. 2003, 103, 2985–3012. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional Amine-Squaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Woldegiorgis, A.G.; Lin, X. Recent advances in the asymmetric phosphoric acid-catalyzed synthesis of axially chiral compounds. Beilstein J. Org. Chem. 2021, 17, 2729–2764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Jin, Z.; Chi, Y.-R. N-Heterocyclic Carbene Organocatalysis: Activation Modes and Typical Reactive Intermediates. Chin. J. Chem. 2020, 38, 1167–1202. [Google Scholar] [CrossRef]
- Maruoka, K. Design of high-performance chiral phase-transfer catalysts with privileged structures. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 1–16. [Google Scholar] [CrossRef]
- Xiao, X.; Shao, B.-X.; Lu, Y.-J.; Cao, Q.-Q.; Xia, C.-N.; Chen, F.-E. Recent Advances in Asymmetric Organomulticatalysis. Adv. Synth. Catal. 2021, 363, 352–387. [Google Scholar] [CrossRef]
- Mondal, S.; Dumur, F.; Gigmes, D.; Sibi, M.P.; Bertrand, M.P.; Nechab, M. Enantioselective Radical Reactions Using Chiral Catalysts. Chem. Rev. 2022, 122, 5842–5976. [Google Scholar] [CrossRef]
- Lei, C.-W.; Mu, B.-S.; Zhou, F.; Yu, J.-S.; Zhou, Y.; Zhou, J. Organocatalytic enantioselective reactions involving prochiral carbocationic intermediates. Chem. Commun. 2021, 57, 9178–9191. [Google Scholar] [CrossRef]
- Atodiresei, I.; Vila, C.; Rueping, M. Asymmetric Organocatalysis in Continuous Flow: Opportunities for Impacting Industrial Catalysis. ACS Catal. 2015, 5, 1972–1985. [Google Scholar] [CrossRef]
- Ran, C.-K.; Chen, X.-W.; Gui, Y.-Y.; Liu, J.; Song, L.; Ren, K.; Yu, D.-G. Recent advances in asymmetric synthesis with CO2. Sci. China Chem. 2020, 63, 1336–1351. [Google Scholar] [CrossRef]
- Aroyan, C.E.; Dermenci, A.; Miller, S.J. The Rauhut-Currier reaction: A history and its synthetic application. Tetrahedron 2009, 65, 4069–4084. [Google Scholar] [CrossRef]
- Morgan Ross, T.; Burke, S.J.; Malachowski, W.P. Enantioselective synthesis of decalin structures with all-carbon quaternary centers via one-pot sequential Cope/Rauhut-Currier reaction. Tetrahedron Lett. 2014, 55, 4616–4618. [Google Scholar] [CrossRef][Green Version]
- Yao, W.; Dou, X.; Wen, S.; Wu, J.; Vittal, J.J.; Lu, Y. Enantioselective desymmetrization of cyclohexadienones via an intramolecular Rauhut-Currier reaction of allenoates. Nat. Commun. 2016, 7, 13024. [Google Scholar] [CrossRef]
- Zhou, X.; Nie, H.; Liu, X.; Long, X.; Jiang, R.; Chen, W. Ferrocene-based bifunctional organocatalyst for highly enantioselective intramolecular Rauhut-Currier reaction. Catal. Commun. 2019, 121, 78–83. [Google Scholar] [CrossRef]
- Tao, M.; Zhou, W.; Zhang, J. Phosphine-Catalyzed Asymmetric Intermolecular Cross Rauhut-Currier Reaction of β-Perfluoroalkyl-Substituted Enones and Vinyl Ketones. Adv. Synth. Catal. 2017, 359, 3347–3353. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; Man, Y.; Gao, X.; Yang, L.; Ren, Y.; Li, N.; Tang, B.; Zhao, G. Asymmetric Intermolecular Rauhut-Currier Reaction for the Construction of 3,3-Disubstituted Oxindoles with Quaternary Stereogenic Centers. Adv. Synth. Catal. 2017, 359, 3934–3939. [Google Scholar] [CrossRef]
- He, Q.; Yang, Z.-H.; Yang, J.; Du, W.; Chen, Y.-C. Enantioselective Formal Arylation of (7-Aza)isatylidene Malononitriles with α’-Alkylidene-2-cyclohexenones. Adv. Synth. Catal. 2020, 362, 4438–4443. [Google Scholar] [CrossRef]
- Hu, F.-L.; Wei, Y.; Shi, M. Phosphine-Catalyzed Asymmetric Formal [4 + 2] Tandem Cyclization of Activated Dienes with Isatylidenemalononitriles: Enantioselective Synthesis of Multistereogenic Spirocyclic Oxindoles. Adv. Synth. Catal. 2014, 356, 736–742. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, L.; Maiti, R.; Mou, C.; Pan, L.; Chi, Y.R. Sulfinate and Carbene Co-catalyzed Rauhut-Currier Reaction for Enantioselective Access to Azepino [1,2-α]indoles. Angew. Chem. Int. Ed. 2019, 58, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Bania, N.; Mondal, B.; Ghosh, S.; Pan, S.C. DMAP Catalyzed Domino Rauhut-Currier Cyclization Reaction between Alkylidene Pyrazolones and Nitro-olefins: Access to Tetrahydropyrano[2,3-c]pyrazoles. J. Org. Chem. 2021, 86, 4304–4312. [Google Scholar] [CrossRef]
- Bae, S.; Zhang, C.; Gillard, R.M.; Lupton, D.W. Enantioselective N-Heterocyclic Carbene Catalyzed Bis(enoate) Rauhut-Currier Reaction. Angew. Chem. Int. Ed. 2019, 58, 13370–13374. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jin, Z.; Chan, W.-L.; Lu, Y. Enantioselective Construction of Bicyclic Pyran and Hydrindane Scaffolds via Intramolecular Rauhut-Currier Reactions Catalyzed by Thiourea-Phosphines. ACS Catal. 2018, 8, 8810–8815. [Google Scholar] [CrossRef]
- Zhou, W.; Su, X.; Tao, M.; Zhu, C.; Zhao, Q.; Zhang, J. Chiral Sulfinamide Bisphosphine Catalysts: Design, Synthesis, and Application in Highly Enantioselective Intermolecular Cross-Rauhut-Currier Reactions. Angew. Chem. Int. Ed. 2015, 54, 14853–14857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Gan, K.-J.; Liu, X.-X.; Deng, Y.-H.; Wang, F.-X.; Yu, K.-Y.; Zhang, J.; Fan, C.-A. Enantioselective Synthesis of Functionalized 4-Aryl Hydrocoumarins and 4-Aryl Hydroquinolin-2-ones via intramolecular Vinylogous Rauhut-Currier Reaction of para-Quinone Methides. Org. Lett. 2017, 19, 3207–3210. [Google Scholar] [CrossRef]
- Qin, C.; Liu, Y.; Yu, Y.; Fu, Y.; Li, H.; Wang, W. α-Functionalization of 2-Vinylpyridines via a Chiral Phosphine Catalyzed Enantioselective Cross Rauhut-Currier Reaction. Org. Lett. 2018, 20, 1304–1307. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, B.; Du, W.; Chen, Y. Phosphine-Catalyzed Formal [6 + 2] Cycloadditions of α’-Methylene 2-Cyclopentenones. Chin. J. Org. Chem. 2019, 39, 2218–2225. [Google Scholar] [CrossRef]
- Liang, S.-Y.; Jiang, B.; Xiao, B.-X.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Phosphine Catalyzed Enantioselective Cascade Reaction Initiated by Intermolecular Cross Rauhut-Currier Reaction of Electron-Deficient ortho-Formyl Styrenes. ChemCatChem 2020, 12, 5374–5377. [Google Scholar] [CrossRef]
- Xiao, B.-X.; Jiang, B.; Song, X.; Du, W.; Chen, Y.-C. Phosphine-catalysed asymmetric dearomative formal [4 + 2] cycloadditions of 3-benzofuranyl vinyl ketones. Chem. Commun. 2019, 55, 3097–3100. [Google Scholar] [CrossRef]
- Eröksüz, S.; Dogan, Ö.; Garner, P.P. A new chiral phosphine oxide ligand for enantioselective 1,3-dipolar cycloaddition reactions of azomethine ylides. Tetrahedron Asymmetry 2010, 21, 2535–2541. [Google Scholar] [CrossRef]
- Dogan, Ö.; Bulut, A.; Ali Tecimer, M. Chiral phosphine oxide aziridinyl phosphonate as a Lewis base catalyst for enantioselective allylsilane addition to aldehydes. Tetrahedron Asymmetry 2015, 26, 966–969. [Google Scholar] [CrossRef]
- Dogan, Ö.; Isci, M.; Aygun, M. New phosphine oxide aziridinyl phosphonates as chiral Lewis bases for the Abramov-type phosphonylation of aldehydes. Tetrahedron Asymmetry 2013, 24, 562–567. [Google Scholar] [CrossRef]
- Dogan, Ö.; Tan, D. Enantioselective direct aldol reactions promoted by phosphine oxide aziridinyl phosphonate organocatalysts. Tetrahedron Asymmetry 2015, 26, 1348–1353. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Pieczonka, A.M. Optically Pure Aziridinyl Ligands as Useful Catalysts in the Stereocontrolled Synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Direct asymmetric aldol condensation catalyzed by aziridine semicarbazide zinc (II) complexes. Tetrahedron Lett. 2014, 55, 2373–2375. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Jarzyński, S.; Obijalska, E. Lactic acid derived aziridinyl alcohols as highly effective catalysts for asymmetric additions of an organozinc species to aldehydes. Tetrahedron Asymmetry 2013, 24, 1336–1340. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Jarzyński, S.; Pieczonka, A.M.; Leśniak, S.; Rachwalski, M. Highly enantioselective addition of arylzinc reagents to aldehydes promoted by chiral aziridine alcohols. Tetrahedron Asymmetry 2016, 27, 1238–1244. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich Reaction Promoted by Chiral Phosphinoyl-Aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Adamczyk, J.; Marciniak, L.; Pieczonka, A.M.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts 2021, 11, 968. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Highly Efficient Asymmetric Morita-Baylis-Hillman Reaction Promoted by Chiral Aziridine-Phosphines. Catalysts 2022, 12, 394. [Google Scholar] [CrossRef]
- Zhao, K.; Zhi, Y.; Shu, T.; Valkonen, A.; Rissanen, K.; Enders, D. Organocatalytic Domino Oxa-Michael/1,6-Addition Reactions: Asymmetric Synthesis of Chromans Bearing Oxindole Scaffolds. Angew. Chem. Int. Ed. 2016, 55, 12104–12108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).