The term “organic/inorganic hybrid materials” here refers to a metal complex consisting of an inorganic metal ion and an organic ligand, a metalloprotein, or a composite functional material in which an inorganic compound and an organic material are combined (Figure 1). From the level of intra-atomic electron or nuclear spins and optical transitions of metal ions, stereochemistry related to chirality or resolution and organic or biochemical reactions, molecular structure with spectroscopic selection rule, crystal structure dominated space group and showing magnetic or elastic properties, forming organic or biological supramolecular assemblies such as biomolecules, e.g., phospholipids, amino acids, proteins and DNA, and nanomaterials such as supramolecular liquid crystals, catalyst, or nanoparticles, polymers, etc. should be targeted. In addition, although incidental, it is considered that anisotropy and symmetry induced by external fields such as light irradiation or circular polarization can be included in this category.
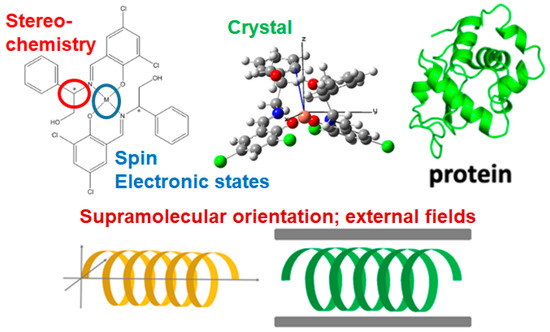
Figure 1.
Possible factors exhibiting “symmetry” (in particular chirality) in chemistry of organic/inorganic hybrid materials.
In any case, it has the characteristic that it is given properties and functions that cannot be possessed by each single component alone (These papers corresponding to each item in the above paragraph were actually published in Symmetry in 2020. But they are not quoted now, because they did not treat with hybrid materials!). Here, I would like to introduce some selected examples of metal complexes from recent researches in Symmetry, focusing on not only structural hierarchy but also "what types of or whose symmetry” is discussed. In short, there is an impression that research that skillfully utilizes symmetry in the chirality of organic/biological materials caused by asymmetric carbon, electron spin (magnetism/conductivity), and crystal structure / molecular structure is conspicuous.
Currently, in the field of metal complex chemistry, research on the synthesis of Metal-Organic Framework (MOF) and the use of void space is active. By synthesizing a two-dimensional thin film MOF incorporating chirality, it has been reported that the efficiency of optical resolution of racemic molecules and spectroscopic detection of chirality can be improved [1].
The development of optically active (chiral) porphyrin derivative ligands and the synthesis of copper and nickel complexes enabled the linear arrangement of magnetic ions within the molecule. Magnetic susceptibility and NMR measurements suggested antiferromagnetic interactions between paramagnetic metal ions [2].
The oxygen reduction efficiency of the metalloenzyme laccase-modified electrode depended on the chirality of the oligopeptide linker used to bind the enzyme to the surface (chirality selectivity of electron spins). At this time, the electron transfer between the cathode electrode and the enzyme was improved by using a copper complex having an amino acid derivative Schiff base ligand containing/not containing the azobenzene moiety as a mediator [3].
Antiferromagnetic interactions were confirmed by magnetic susceptibility and neutron diffraction measurements of the organic-inorganic hybrid of organic cations and manganese chloride. The crystal lattice was confirmed from the annihilation law, and the spin arrangement was clarified from the irreducible representation method. In this paper, magnetic structure analysis using magnetic space groups has been developed [4].
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Li, C.; Heinke, L. Thin Films of Homochiral Metal–Organic Frameworks for Chiroptical Spectroscopy and Enantiomer Separation. Symmetry 2020, 12, 686. [Google Scholar] [CrossRef]
- Setsune, J.-i.; Omae, S.; Tsujimura, Y.; Mochida, T.; Sakurai, T.; Ohta, H. Synthesis, Structure, and Magnetic Properties of Linear Trinuclear CuII and NiII Complexes of Porphyrin Analogues Embedded with Binaphthol Units. Symmetry 2020, 12, 1610. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Tassinari, F.; Haraguchi, T.; Banerjee-Gosh, K.; Akitsu, T.; Naaman, R. Electron Transfer via Helical Oligopeptide to Laccase Including Chiral Schiff Base Copper Mediators. Symmetry 2020, 12, 808. [Google Scholar] [CrossRef]
- Park, G.; Oh, I.-H.; Park, J.M.S.; Hahn, S.; Park, S.-H. Magnetic Structure of Inorganic–Organic Hybrid (C6H5CH2CH2NH3)2MnCl4 Using Magnetic Space Group Concept. Symmetry 2020, 12, 1980. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).