Abstract
Controlling the surface traps in metal halide perovskites (MHPs) is essential for device performance, stability, and commercialization. Here, a facile approach is introduced to passivate the methylammonium lead iodide (MAPbI3) perovskite single crystal (PSC) surface defects by benzylamine (BA) ligand treatment, and the natural crystallographic (100) facets surface of PSC is chosen as the research platform to provide a deeper understanding of the passivation process. The confocal photoluminescence (PL) results show that the pristine three-dimensional (3D) MAPbI3 PSC surface with a symmetric emission spectrum is normally converted to a pure two-dimensional (2D) BA2PbI4, and also forms a quasi-2D Ruddlesden–Popper perovskite (RPP) BA2MAn−1PbnI3n+1 (n = 2, 3, 4, … ∞) after BA exchange with cation defects. The blue shift in the PL peak, as well as the extended exciton lifetimes of time-resolved photoluminescence (TRPL), indicate the realization of surface defect passivation. Additionally, changes in surface morphology are also investigated. The reaction starts with the formation of small, layered crystallites over the surface; as time elapses, the layered crystallites spread and merge in contact with each other, eventually resulting in smooth features. Our findings present a simple approach for MAPbI3 PSC surface defect passivation, which aims to advance MHP optimization processes toward practical perovskite device applications.
1. Introduction
Organic–inorganic halide perovskites (OIHPs) ABX3 are solution-processable semiconductors that have great potential for applications, including photovoltaics [1,2,3], detectors [4,5], light-emitting diodes (LED) [6,7], lasers [8], and memory devices [9]. However, stability issues in perovskite polycrystalline film devices seem to block further advancements toward commercialization, because organic–inorganic hybrid perovskites have been found to be highly sensitive to ambient conditions. Polycrystalline perovskite-based devices contain a diversity of defects within the perovskite materials and the corresponding interfaces, affecting device performance, stability, and commercialization [10,11]. Fortunately, perovskite single crystals (PSCs) exhibit superior characteristics, such as higher stability than the polycrystalline films, and, thus, hold promise for high-efficiency and stable photovoltaics [12,13]. However, the PSC surface presents a major performance bottleneck, because the trap-state density at the surface is much higher than that in the crystal body. The uneven surface leads to the formation of unbalanced boundaries in the single-crystal process; moreover, the halogen ion and metal ions are unsaturated in the surface. The surface is critical for single crystals and the surface trap-state density is much higher compared with the bulk density, resulting in an increase in the trap-assisted recombination of excited charge carriers and limiting carrier diffusion lengths in the surface region [14,15]. As charge recombination and extraction occur at the surface areas of PSC planar devices, the application of appropriate surface modification is essential for device performance.
Fortunately, the surface passivation of OIHPs is an effective solution. Tremendous efforts in improving passivation strategies to annihilate surface defects, optimize carrier extraction, inhibit carrier recombination, and advance device stability have been developed in recent years [16]. Most recently, the formation of a thin, two-dimensional (2D) perovskite layer on the surface of three-dimensional (3D) perovskite films by introducing larger organic molecules has become a popular strategy for surface passivation [17,18,19,20,21].
Generally, the typical chemical formula of 2D perovskites is (A′)m(A)n−1BnX3n+1 (n = 1, 2, 3, 4, … ∞). A′ is a monovalent (m = 2) or divalent (m = 1) organic spacer cation and large in size, such as phenylethylamine (PEA+), butylamine, or benzylamine (BA+); A represents smaller cations, such as methylammonium (MA+ = CH3NH3+) or formamidinium CH2(NH2)2+ (FA+); B stands for a divalent metal ion; X represents a halide ion, such as iodine (I-), bromine ion (Br-), or chloride ion (Cl-); n stands for the number of 3D inorganic layers separated by the organic layers. The n value may range from one to positive infinity. The n = 1 structure is completely 2D perovskite, while the n = ∞ structure is pure 3D perovskite, and when n is between one and ∞ we have quasi-2D (Q-2D) Ruddlesden–Popper perovskite (RPP). In contrast with the 3D counterparts, lower dimensionality perovskites possess better thermal and structural stability, while their photovoltaic performance is poorer because of the corresponding wider bandgap of about 2.5 eV, and the charge transportation across inorganic layers is inhibited by the organic spacer. The fixed 2D/3D structure formed by surface passivation factually combines the stability of 2D layered perovskite with the excellent electron/hole carrier capabilities of 3D perovskite, exhibiting more efficient performance and improved stability [18].
Clarifying the mechanism of surface trap-state passivation in hybrid perovskites is of crucial significance to the improvement in electrical properties and the design of high-performance devices. To date, most passivation research has been focused on the surface of perovskite polycrystalline films. However, it is difficult to distinguish the influences of various crystal planes in polycrystalline materials, and perovskite single crystals show obvious orientation and anisotropy properties [22,23,24,25]. As a result, single crystals are better platforms for the investigation of the materials’ intrinsic properties. Nevertheless, there are rare reports of detailed discussions of the passivation processes regarding the natural crystallographic surface of the PSCs. In this work, we introduced a facile approach to the methylammonium lead iodide (MAPbI3) perovskite single crystal surface passivation. Firstly, we applied the inverse temperature crystallization (ITC) method to grow high quality PSCs of MAPbI3. We then chose larger organic molecules of BA as the passivators, and the natural crystallographic (100) facets surface of the as-prepared single crystal as the research platform. We found that BA was capable of reacting with MAPbI3 single crystal surface defective regions and reducing defect density, leading to the in situ formation of 2D perovskites on the 3D surface. Meanwhile, the perovskite superstructure also formed as the mixture of multiple perovskite phases with n = 1, 2, 3, 4, and ≈∞, denoted as 2D/Q-2D/3D. Therefore, the possible chemical reaction mechanisms were proposed.
2. Materials and Methods
2.1. Material
All reagents in our work were used as received without any further purification. Methylamine iodide (CH3NH3I, >98%) and benzylamine (99%) were both purchased from TCI Co., Ltd. (Tokyo, Japan). Lead iodide (PbI2, ≥98%) was purchased from Innochem Co., Ltd. (Beijing, China). Gamma-butyrolactone (GBL) was from Adamas Reagent Co., Ltd. (Basel, Switzerland). Chlorobenzene (99%) was purchased from Alfa Aesar Co., Ltd. (Tewksbury, MA, USA).
2.2. Growth of MAPbI3 Single Crystal
CH3NH3PbI3 single crystals were grown from a solution method using a modified version of the inverse temperature crystallization (ITC) approach, previously reported by Saidaminov et al. [26]. Here, methylamine iodide and lead iodide were dissolved in GBL by a molar ratio of 1:1, and with a [CH3NH3PbI3] concentration of 1.23 M at room temperature inside vessels. The solutions were filtered with PTFE filters (a 0.22 μm pore size) and then transferred into vessels. The vessels were then sealed and heated in an oil bath at 60 °C until no visible undissolved salts could be observed with the naked eye and the yellow solution became transparent. Then, the oil bath temperature was gradually raised to 90 °C and maintained at 90 °C for a certain number of days. Small crystals were observed after several hours and extracted after 2~3 days of crystallization. Such a slow growth rate guaranteed the crystalline quality and size. After using cleanroom wipers to dry the excess precursor on the surface of the PSC under ambient conditions, all crystals were stored in a dry box.
2.3. Process for BA Treatment
A ligand solution of 5 vol. % BA in anhydrous chlorobenzene was prepared inside a glove box filled with nitrogen. MAPbI3 perovskite crystals without polishing were immersed in the BA ligand solution for five typical processing times (30 s, 60 s, 30 min, 1 h, and 3 h), after which they were rinsed in chlorobenzene and naturally dried. Chlorobenzene was chosen, because it is extensively applied as an antisolvent for perovskite materials and is an ideal solvent for BA. The five types of processing times were selected because they were representative samples of the passivation effect, crystal surface flatness, and rapidity, all of which could help to clarify the principle.
2.4. Characterization
To avoid moisture effects on the PSC surfaces, all characterizations were carried out immediately after crystal growth or powder preparations. Powder X-ray diffraction (PXRD) spectra were obtained on an X-ray diffractometer (Rigaku SmartLab SE, Tokyo, Japan) with a Cu-Kα radiation source (λ = 1.5406 Å) at a scan rate of 5 °min−1. The powders were created by grinding a large MAPbI3 single crystal as prepared. The surface morphology of PSCs was investigated with a scanning electron microscope (SEM, Hitachi SU8010, Tokyo, Japan). Steady-state photoluminescence measurements (PL) and time-resolved photoluminescence (TRPL) spectra of bulk crystal were obtained with a laser Raman spectrometer (Acton SP2500, Princeton Instruments), a 488 nm pulsed diode laser (PicoQuant PDL 800-D) with a repetition rate of 0.25 MHz as the excitation source under ambient, and an industrial digital camera (Sony E3ISPM12000KPA), using a home-built confocal microscope.
3. Results
3.1. X-ray Diffraction Characterization of MAPbI3 Crystal
A pristine MAPbI3 single crystal synthesized by the ITC method is shown in Figure 1. A black and shiny crystal of MAPbI3 was formed, which exhibited facets consistent with rhombohexagonal dodecahedrons.
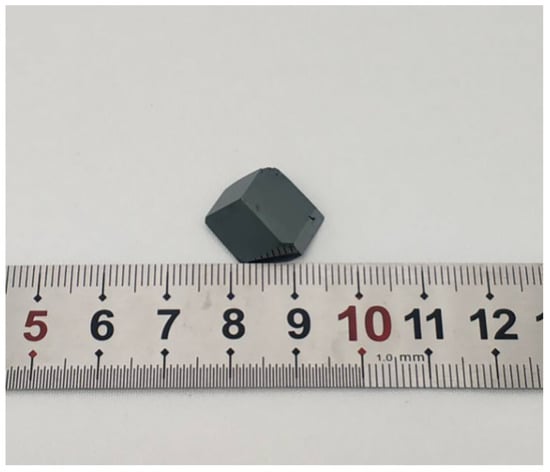
Figure 1.
Photograph of the MAPbI3 single crystal grown using the inverse temperature crystallization method.
The PXRD pattern of the MAPbI3 single crystal powder is presented in Figure 2a. It shows that the main peaks were at 2θ = 14.1°, 28.4°, and 31.9°, which could be assigned to the (110), (220), and (310) lattice planes of the lower-symmetrical tetragonal structure, respectively, (space group I4/mcm, a = b = 8.8725 Å and c = 12.547 Å) at room temperature, according to the results reported in the literature for powder samples [27,28,29,30]. The clear split of the (220) and (004) peaks further affirmed that the as-synthesized MAPbI3 single crystal adopted a tetragonal phase with a high crystalline quality [27,29]. The crystals can be conceived as a methylammonium ion (MA+) contained in an (inorganic) framework of a corner-sharing PbI63− octahedral, as shown in Figure 2b [31,32].
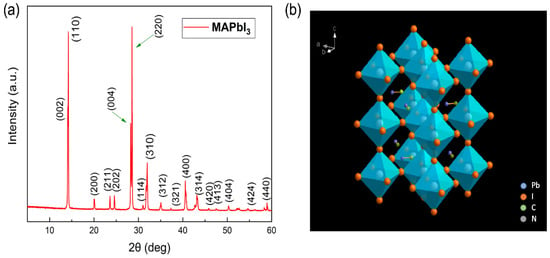
Figure 2.
(a) Powder X-ray diffraction pattern of the MAPbI3 sample; (b) the crystal structure of MAPbI3 in its tetragonal symmetry at room temperature. (The PbI6 octahedra are blue and the iodine atoms are orange.)
3.2. PL Analysis
The pristine MAPbI3 single crystal without polishing was then submerged in the BA ligand solution for a period of time (as shown in Figure 3). The BA ligand solution was initially clear and transparent, and there was no significant change after 60 s of immersion. After 3 h of the soaking process, the BA solution turned pale yellow, as shown in Figure 3c.

Figure 3.
Photos of the single crystalline perovskite with (a) 0 s, (b) 60 s, and (c) 3 h BA treatment.
Figure 4 shows the PXRD patterns of the MAPbI3 samples after the BA treatment for 30 s (purple line), 60 s (blue line), 30 min (green line), 1 h (orange line), and 3 h (pink line). There was no discernible difference in PXRD before and after passivation, which could be attributed to the surface passivation layer’s low component content, which could not be detected by an XRD measurement.
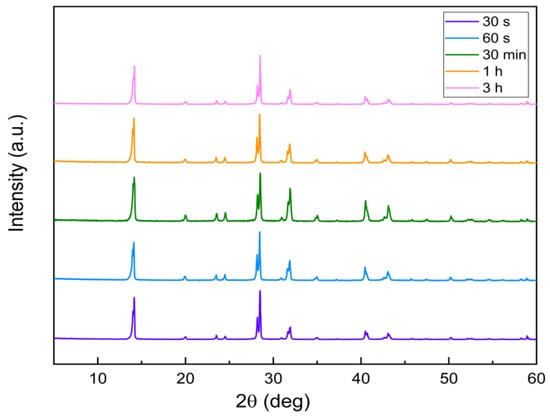
Figure 4.
Powder X-ray diffraction patterns of the MAPbI3 samples after BA treatment.
Figure 5 demonstrates the PL spectra of the PSC surface before and after BA treatment, and the corresponding optical microscopy images. The blue dot in the optical microscopy images (as shown in Figure 5b–e) is the position of the sample surface irradiated by the focused laser light source during PL measurement. The pristine PSC sample (purple line) exhibited a symmetric emission spectrum at the excitation of 775 nm (1.60 eV). After the BA ligand treatment, several different types of capping layer samples coexisted on the different positions of the treated 3D MAPbI3 single crystal surface. As shown for the 2D-type samples (orange line) in Figure 5a, the sample mainly exhibited a single fluorescence peak around 533 nm (2.33 eV). This emission feature suggested the existence of a pure 2D perovskite (BA)2PbI4, which exhibited a long, asymmetric tail extending toward a longer wavelength (lower energy side), possibly due to the self-trapped exciton [33] or the disordered structure [34]. This result implied that the BA treatment assisted the MAPbI3 conversion into (BA)2PbI4. The 2D/3D-type mixed-phase layer (green line) was also found in another position on the BA-treated surface, and showed the 2D (n = 1) and 3D PL peaks obviously separated from each other, which was ascribed to the excitonic band-edge emissions of 2D and 3D perovskites and coincided well with the previous findings [35]. In addition, multiple Q-2D perovskite phases simultaneously existed because multiple PL peaks for 2D/Q-2D/3D could be found (Figure 5a blue line), although nominally prepared as n = 1 because it is well-known that n = 1 nanoplatelets (pure 2D perovskite materials) possess larger formation energy than Q-2D. More importantly, the blue shift of 19 nm for the 3D perovskite PL peak suggested the occurrence of defect passivation on the perovskite surface [36].
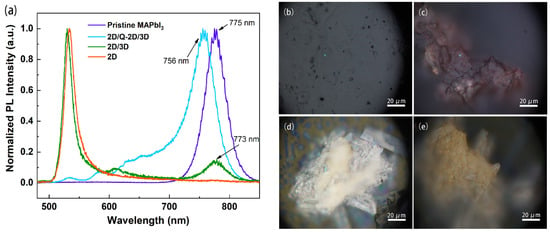
Figure 5.
(a) PL spectra of the crystal surface after BA treatment (blue, green, and orange lines) for 60 s and an untreated crystal (purple line); optical microcopy images showing the corresponding PL testing position: (b) pristine MAPbI3, and after 60 s BA treatment in the different positions (c) 2D/3D, (d) 2D/Q-2D/3D, and (e) 2D of the same treated single crystal.
The superstructure (Figure 6a, red line) similar to the 2D/Q-2D/3D sample in Figure 5a was occasionally found, mainly located at the edge of the 60 s BA-treated PSC surface with the laser focused on spot A.
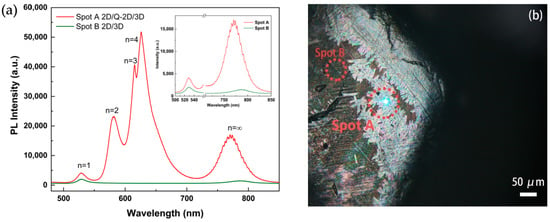
Figure 6.
(a) PL spectra comparison of a 2D/Q-2D/3D spot (red line) and a 2D/3D spot (green line); inset: intercepted 2D and 3D peaks for comparison; (b) optical microscopy image of a 60 s BA-treated PSC edge.
As shown in Figure 6a, the PL spectra exhibited three strong emission peaks at 575, 608, and 645 nm, which were consistent with (BA)2(MA)n−1PbnI3n+1 perovskites with n = 2, 3, and 4, respectively (red line, spot A) [37]. Interestingly, the peaks’ intensity was particularly high, the peaks’ half-width of different n value phases was narrow, and the peaks were obviously separated. Zooming in, as shown in the inset of Figure 6a to see more clearly, the 2D/Q-2D/3D perovskite actually had a much stronger PL intensity than the 2D/3D perovskite. The formed 2D/Q-2D/3D perovskite crystallites capping layer was found to have a high coverage rate along the crystal edge and/or a vertical plate-like appearance on the surface of the 3D perovskite, as shown in Figure 6b. The dense small-n perovskite capping layer contained hydrophobic bulky ligands that could make it difficult for water molecules to penetrate through the surface and protected the perovskite below [38]. The 2D/3D perovskite could also be found, as shown in Figure 6a (green line), with the laser focused on lateral spot B. Therefore, through the BA treatment, the PL results showed that various 2D perovskite phases coexisted on the surface of the same BA-treated single crystal, and defects on the surface were passivated.
3.3. TRPL Analysis
To further investigate the charge-transfer and recombination aims to prove that BA can passivate the surface defects at the single crystal surface, a TRPL measurement was conducted. Figure 7 shows the TRPL results of three typical PSC samples, corresponding to the pristine MAPbI3 PSC (purple dots), the 2D/3D position of the PSC after the 60 s BA treatment (green dots), and the 2D/Q-2D/3D position of the PSC after the 1 h BA treatment (orange dots).
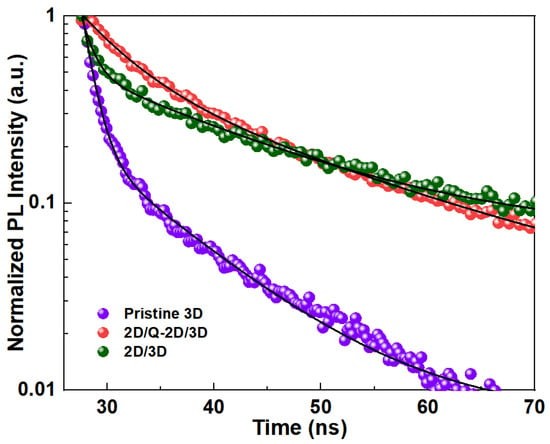
Figure 7.
PL decay for pristine MAPbI3 single crystal, 60 s (2D/3D), and 1 h (2D/Q-2D/3D) BA-treated samples (the solid lines are the fitting data).
Two time components were extracted to calculate the carrier recombination lifetime of the as-synthesized PSC samples by fitting the TRPL curve according to a bi-exponential function: I(t) = A1 exp(−t/τ1) + A2 exp(−t/τ2). Here, I(t) is the normalized PL intensity at time t; A1 and A2 are fitting parameters that represent the relative exponent weight; and τ1 and τ2 are the lifetimes of the exponents that reflect the time constant of the fast and slow decay process, respectively. Previous studies showed that the fast decay (τ1) component is attributed to the bimolecular recombination of photogenerated free carriers, and the slow decay (τ2) component is mainly associated with trap-assisted recombination [39]. All the fitted TRPL parameters for the pristine 3D and BA-treated perovskite samples are listed in Table 1. As expected, the PSCs treated by the BA ligand solution showed slightly longer PL lifetimes (τ1 = 4.57 ns, τ2 = 19.92 ns for 2D/Q-2D/3D and τ1 = 1.09 ns, τ2 = 15.4 ns for 2D/3D) than those of the pristine 3D PSCs (τ1 = 1.06 ns, τ2 = 9.03 ns), as shown in Table 1. Here, the BA passivation mainly influenced the slow decay process, consisting of the proposed mechanism according to the PL results that BA eliminated the surface charge traps.

Table 1.
Comparison of PL decay lifetime in pristine MAPbI3, 2D/Q-2D/3D, and 2D/3D samples in Figure 6.
3.4. Surface Morphology and Stability Analysis
In order to further observe the passivation process, the typical surface morphology changes in MAPbI3 single crystals, treated by BA at different times, were compared by SEM, as shown in Figure 8. The morphology of pristine MAPbI3 is shown in Figure 8a, in which there are some visible pinholes (scattered dark dots) and a few small particles on the surface, which might have resulted from the partial decomposition of the PSC surface because of contamination through the attachment of the nonstoichiometric precursors when removing the PSCs out of the super-saturated precursor solution [28]. After exposure to BA, the morphology of the perovskite surface was obviously affected, as shown in Figure 8b–f. The reaction started with the formation of small, layered crystallites over the surface (Figure 8b,e). As time elapsed, these layered crystallites tended to spread and merge in contact with each other (Figure 8c). After deliberately prolonging the BA treatment time to 1 h for a sufficient reaction of the whole surface, the typical morphology of the ultimate surface showed a newly formed, more uniform, and smoother crystal surface (Figure 8d). Instead, the surface became rough after the 3 h BA treatment because there were many needle-like aggregates on the single crystal surface (Figure 8f), and the solution simultaneously became pale yellow, as shown in Figure 3c. We supposed that lead iodide was formed.
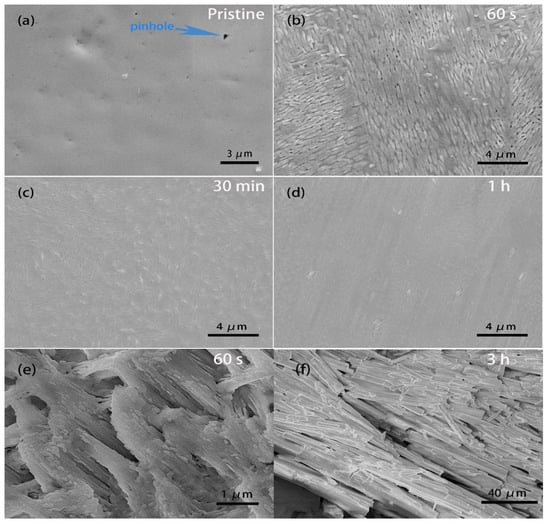
Figure 8.
Typical scanning electron microscope images of the single crystal of MAPbI3: (a) pristine, and after (b) 60 s, (c) 30 min, (d) 1 h, (e) 60 s, and (f) 3 h BA treatment.
The surface wettability is critical for the moisture stability of high-quality MAPbI3 SC devices. To examine the influence of BA treatment on the surface wettability, we observed the water contact angle change of SCs with BA treatment. As shown in Figure 9a, the contact angle of the pristine SC surface was 84.5°. The contact angle of the pristine PSC was larger than that of the conventional polycrystalline perovskite films because the single crystal had a compact structure, no grain boundary, and high crystallinity, resulting in an enhanced moisture stability compared with polycrystalline films. As shown in Figure 9b, the contact angle of the 1 h BA-treated SC surface was 98.0°, which was much larger than that of the pristine SC. This increase in the contact angle could be attributed to the improved morphology quality of the perovskite SC surface, as demonstrated by the SEM characterization, which prevented H2O intake. The 2D perovskites had better moisture resistance than 3D perovskite materials due to their enhanced hydrophobicity and high formation energy [40,41], which could also improve the moisture resistance of the PSC. Thus, the BA treatment can increase the hydrophobicity of SC, which is expected to benefit the stability and formation of high-quality PSC devices.
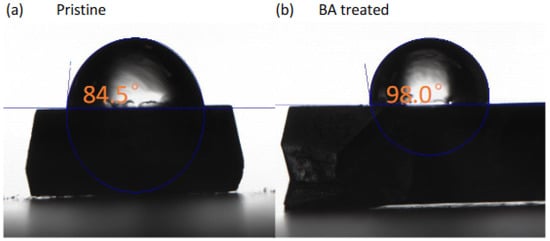
Figure 9.
Water contact angle measurements of the PSCs (a) before and (b) after BA treatment.
4. Discussion
Based on all the results presented herein, the possible chemical equation mechanism was proposed as Equations (1) and (2):
where MA+ and BA+ denote the protonated ion, that is, the ammonium forms of methylamine and BA, respectively. The basic mechanisms that we speculated as being involved in the surface passivation of MAPbI3 PSC by BA are depicted schematically in Figure 10. Previous reports had shown that the basic N atom with an electron lone pair in alkylamine molecules intercalated into the PbI6-octahedra framework in MAPbI3 perovskite [42,43]. Therefore, a partial proton transfer process occurred at the top perovskite lattice. Because BA+ possesses a large ionic radius, which is incompatible with a 3D perovskite structure, large alkylammonium cation organic molecules (BA) acted as a spacer between the lead halide octahedral planes, slicing the 3D perovskite structure. Following this, perovskites based on this cation tended to rebuild the perovskite structure by rapid nucleation and growth into many small crystallites with different corner-sharing octahedral slab thicknesses. The driving force for this rebuild process might have been the optimized stability of the 2D perovskite materials containing aligned BA groups through the vdW interactions [44,45]. Thus, the BA molecules gradually diffused into the crystalline structure through the surface, reacting with MAPbI3. Ultimately, the formation of 2D and Q-2D perovskites healed the surface defects due to such layered lower dimensional perovskite crystals, which would spread and merge when in contact with each other, resulting in a smooth surface and CH3NH2 molecules being released from the liquid at the same time.
2BA+(n+1)MA+PbI3 → (BA+)2MAn−1PbnI3n+1 + PbI2 + 2MA↑
3BA+(n+2)MA+PbI3 → (BA+)2MAn−1PbnI3n+1 + 2PbI2 + 3MA↑ + BAI
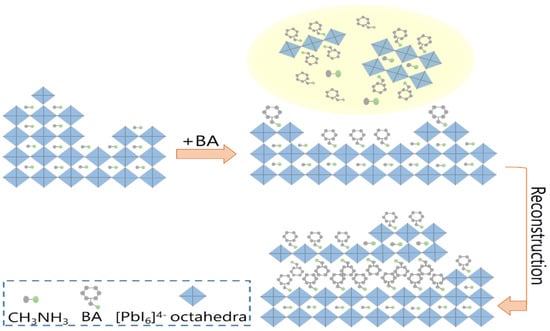
Figure 10.
The proposed graphic process for the 2D/Q-2D/3D stacking structures via BA treatment.
The surface passivation strategy of 3D perovskite materials has been used in photoelectronic devices [21,46,47], which improved stability, eliminated surface defects, smoothened surfaces, and suppressed ion migration. Furthermore, the 2D/3D structure formed during the passivation process belonged to the perovskite/perovskite heterostructures (PPHSs). PPHSs are a novel and arresting method used to construct type II heterostructures, to promote carrier separation and self-powered metal halide materials for perovskite solar cells, photodetectors, LEDs, and lasers applications in recent years [48,49,50,51]. It is expected that future work will further concentrate on the following aspects: (1) the fabrication of high-quality, multilayer single crystal heterostructure and corresponding devices; (2) building new carrier transmission modes in such PPHSs.
5. Conclusions
In summary, a facile approach was introduced herein to passivate the MAPbI3 PSC surface defects, and we used the natural crystallographic (100) facets surface of the PSCs as the research platform to investigate the surface passivation process. We showed that BA2MAn−1PbnI3n+1 (n = 1, 2, 3, 4, … ∞) formed after BA treatment, according to the multiple absorption peaks of PL spectra, including pure 2D and Q-2D perovskite phases with n at a judiciously chosen value. The blue shift in PL spectra peaks, as well as the extended exciton lifetimes of TRPL, indicated the realization of defect density reduction via BA passivation. Additionally, exposure to BA was accompanied by the breaking up of the defective 3D perovskite crystal regions, which reconstructed small crystallites during the BA molecule intercalation process. Upon prolonged exposure to the ligand solution, the small crystallites spread and merged in contact with each other, eventually giving rise to smoother features. These experimental findings revealed the feasibility of BA passivation on the defective surface regions, which is critical for advancing the material optimization process toward practical applications of perovskite devices.
Author Contributions
Conceptualization, M.C. and W.W.; methodology, W.W., K.J. and X.L.; characterization and investigation, W.W., Y.W. and B.C.; writing—review and editing, W.W. and M.C.; supervision, S.D.; funding acquisition, S.D., M.C., H.L. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2020YFB1506404), the 111 Project (B16016), the National Natural Science Foundation of China (no. 51961165106, 51572080, and 61904053), the Fundamental Research Funds for the Central Universities (no. 2019MS026, 2019MS027, and 2020MS080), HBUT Doctoral Research Initiation Fund Project (grant no. BSQD2019058), and the Project of Outstanding Young and Middle-aged Science and Technology Innovation Team of Colleges and Universities in Hubei Province under grant no. T201907.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Yunxia Zhang is gratefully acknowledged for her useful advice on the synthesis of MAPbI3 single crystals. The authors thank Ye Tao for help during the TRPL experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Bouich, A.; Mari-Guaita, J.; Bouich, A.; Pradas, I.G.; Mari, B. Towards manufacture stable lead perovskite APbI3 (A = Cs, MA, FA) based solar cells with low-cost techniques. Eng. Proc. 2021, 12, 81. [Google Scholar]
- Marí-Guaita, J.; Bouich, A.; Marí, B. Shedding Light on Phase Stability and Surface Engineering of Formamidinium Lead Iodide (FaPbI3) Thin Films for Solar Cells. Eng. Proc. 2021, 12, 2001. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [PubMed]
- Tie, S.; Zhao, W.; Xin, D.; Zhang, M.; Long, J.; Chen, Q.; Zheng, X.; Zhu, J.; Zhang, W. Robust Fabrication of Hybrid Lead-Free Perovskite Pellets for Stable X-ray Detectors with Low Detection Limit. Adv. Mater. 2020, 32, e2001981. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.B.; Sadhanala, A.; Pazos-Outon, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Yuan, M.; Na Quan, L.; Comin, R.; Walters, G.; Sabatini, R.; Voznyy, O.; Hoogland, S.; Zhao, Y.; Beauregard, E.M.; Kanjanaboos, P.; et al. Perovskite energy funnels for efficient light-emitting diodes. Nat. Nanotechnol. 2016, 11, 872–877. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X.-Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef]
- Yoo, E.J.; Lyu, M.; Yun, J.H.; Kang, C.J.; Choi, Y.J.; Wang, L. Resistive Switching Behavior in Organic-Inorganic Hybrid CH3NH3PbI3-xClx Perovskite for Resistive Random Access Memory Devices. Adv. Mater. 2015, 27, 6170–6175. [Google Scholar] [CrossRef]
- Ilin, A.; Forsh, P.A.; Martyshov, M.N.; Kazanskii, A.G.; Forsh, E.A.; Kashkarov, P.K. Humidity Sensing Properties of Organometallic Perovskite CH3NH3PbI3. ChemistrySelect 2020, 5, 6705–6708. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in perovskite-halides and their effects in solar cells. Nat. Energy 2016, 1, 16149. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, Y.X.; Zhu, X.J.; Feng, J.S.; Spanopoulos, I.; Ke, W.J.; He, Y.H.; Ren, X.D.; Yang, Z.; Xiao, F.W.; et al. Triple-Cation and Mixed-Halide Perovskite Single Crystal for High-Performance X-ray Imaging. Adv. Mater. 2021, 33, 2006010. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Turedi, B.; Alsalloum, A.Y.; Yang, C.; Zheng, X.; Gereige, I.; AlSaggaf, A.; Mohammed, O.F.; Bakr, O.M. Single-Crystal MAPbI3 Perovskite Solar Cells Exceeding 21% Power Conversion Efficiency. ACS Energy Lett. 2019, 4, 1258–1259. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Hutter, E.M.; Fang, Y.J.; Dong, Q.F.; Huang, J.S.; Savenije, T.J. Charge Carrier Lifetimes Exceeding 15 mu s in Methylammonium Lead Iodide Single Crystals. J. Phys. Chem. Lett. 2016, 7, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Yengel, E.; Yang, C.; Peng, W.; Alarousu, E.; Bakr, O.M.; Mohammed, O.F. The Surface of Hybrid Perovskite Crystals: A Boon or Bane. ACS Energy Lett. 2017, 2, 846–856. [Google Scholar] [CrossRef]
- Naghadeh, S.B.; Luo, B.; Abdelmageed, G.; Pu, Y.-C.; Zhang, C.; Zhang, J.Z. Photophysical Properties and Improved Stability of Organic–Inorganic Perovskite by Surface Passivation. J. Phys. Chem. C 2018, 122, 15799–15818. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, Y.; Fang, Y.; Chen, Z.; Yang, S.; Zheng, X.; Tang, S.; Liu, Y.; Zhao, J.; Huang, J. Enhanced Thermal Stability in Perovskite Solar Cells by Assembling 2D/3D Stacking Structures. J. Phys. Chem. Lett. 2018, 9, 654–658. [Google Scholar] [CrossRef]
- Wang, F.; Geng, W.; Zhou, Y.; Fang, H.-H.; Tong, C.-J.; Loi, M.A.; Liu, L.-M.; Zhao, N. Phenylalkylamine Passivation of Organolead Halide Perovskites Enabling High-Efficiency and Air-Stable Photovoltaic Cells. Adv. Mater. 2016, 28, 9986–9992. [Google Scholar] [CrossRef]
- Li, J.; Bu, T.; Lin, Z.; Mo, Y.; Chai, N.; Gao, X.; Ji, M.; Zhang, X.-L.; Cheng, Y.-B.; Huang, F. Efficient and stable perovskite solar cells via surface passivation of an ultrathin hydrophobic organic molecular layer. Chem. Eng. J. 2020, 405, 126712. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Allen, A.L.; Li, X.; Xia, H.; Peng, L.; Zhang, J.Z. Enhancing the Photoluminescence and Stability of Methylammonium Lead Halide Perovskite Nanocrystals with Phenylalanine. J. Phys. Chem. C 2021, 125, 2793–2801. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Zhang, W.; Li, F.; Wang, Z.; Wang, X.; Shao, Y.; Shao, J. Surface Passivation of MAPbBr3 Perovskite Single Crystals to Suppress Ion Migration and Enhance Photoelectronic Performance. ACS Appl. Mater. Interfaces 2022, 14, 10917–10926. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, M.; Wu, G.; Zhu, L.; Liu, X.; Lv, H.; Dai, S. Facet Control of the Lead-Free Methylammonium Bismuth Iodide Perovskite Single Crystals via Ligand-Mediated Strategy. Cryst. Growth Des. 2021, 21, 5840–5847. [Google Scholar] [CrossRef]
- Leblebici, S.Y.; Leppert, L.; Li, Y.; Reyes-Lillo, S.E.; Wickenburg, S.; Wong, E.; Lee, J.; Melli, M.; Ziegler, D.; Angell, D.K.; et al. Facet-dependent photovoltaic efficiency variations in single grains of hybrid halide perovskite. Nat. Energy 2016, 1, 16093. [Google Scholar] [CrossRef]
- Lv, Q.R.; He, W.H.; Lian, Z.P.; Ding, J.; Li, Q.; Yan, Q.F. Anisotropic moisture erosion of CH3NH3PbI3 single crystals. Crystengcomm 2017, 19, 901–904. [Google Scholar] [CrossRef]
- Kim, D.; Yun, J.-H.; Lyu, M.; Kim, J.; Lim, S.; Yun, J.S.; Wang, L.; Seidel, J. Probing Facet-Dependent Surface Defects in MAPbI3 Perovskite Single Crystals. J. Phys. Chem. C 2019, 123, 14144–14151. [Google Scholar] [CrossRef]
- Saidaminov, M.; Abdelhady, A.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; Maculan, G.; et al. High-quality bulk hybrid perovskite single crystals within minutes by inverse temperature crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Yang, Z.; Cui, D.; Ren, X.D.; Sun, J.K.; Liu, X.J.; Zhang, J.R.; Wei, Q.B.; Fan, H.B.; Yu, F.Y.; et al. Two-Inch-Sized Perovskite CH3NH3PbX3 (X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Dong, Q.F.; Fang, Y.J.; Shao, Y.C.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J.S. Electron-hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [Green Version]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, P.S.; Herron, N.; Guise, W.E.; Page, K.; Cheng, Y.Q.; Milas, I.; Crawford, M.K. Structures, Phase Transitions and Tricritical Behavior of the Hybrid Perovskite Methyl Ammonium Lead Iodide. Sci. Rep. 2016, 6, 35685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.Y.; Liu, Y.; Sun, Y.X.; Yuan, D.S.; Liu, X.L.; Lu, W.Q.; Liu, G.F.; Xia, H.B.; Tao, X.T. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. Crystengcomm 2015, 17, 665–670. [Google Scholar] [CrossRef]
- Wu, X.; Trinh, M.T.; Niesner, D.; Zhu, H.; Norman, Z.; Owen, J.S.; Yaffe, O.; Kudisch, B.J.; Zhu, X.-Y. Trap States in Lead Iodide Perovskites. J. Am. Chem. Soc. 2015, 137, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Zhang, X.W.; Deng, J.X.; Chu, Z.M.; Jiang, Q.; Meng, J.H.; Wang, P.Y.; Zhang, L.Q.; Yin, Z.G.; You, J.B. Efficient green light-emitting diodes based on quasi-two-dimensional composition and phase engineered perovskite with surface passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Stoumpos, C.C.; Kanatzidis, M.G. Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. J. Am. Chem. Soc. 2018, 141, 1171–1190. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, F.; Liu, H.; Li, X.; Xiao, Y.; Wang, S. Tuning the crystal growth of perovskite thin-films by adding the 2-pyridylthiourea additive for highly efficient and stable solar cells prepared in ambient air. J. Mater. Chem. A 2017, 5, 13448–13456. [Google Scholar] [CrossRef]
- Liu, J.; Leng, J.; Wu, K.; Zhang, J.; Jin, S. Observation of Internal Photoinduced Electron and Hole Separation in Hybrid Two-Dimentional Perovskite Films. J. Am. Chem. Soc. 2017, 139, 1432–1435. [Google Scholar] [CrossRef]
- Lu, Y.-B.; Guan, C.; Sun, H.; Cong, W.-Y.; Yang, H.; Zhang, P. Investigation on Enhanced Moisture Resistance of Two-Dimensional Layered Hybrid Organic–Inorganic Perovskites (C4H9NH3)2PbI4. J. Phys. Chem. C 2018, 122, 11862–11869. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Saidaminov, M.I.; Haque, A.; Alarousu, E.; Sarmah, S.P.; Murali, B.; Dursun, I.; Miao, X.-H.; Abdelhady, A.; Wu, T.; et al. Formamidinium Lead Halide Perovskite Crystals with Unprecedented Long Carrier Dynamics and Diffusion Length. ACS Energy Lett. 2016, 1, 32–37. [Google Scholar] [CrossRef]
- Soe, C.M.M.; Nie, W.; Stoumpos, C.; Tsai, H.; Blancon, J.-C.; Liu, F.; Even, J.; Marks, T.J.; Mohite, A.D.; Kanatzidis, M.G. Understanding Film Formation Morphology and Orientation in High Member 2D Ruddlesden–Popper Perovskites for High-Efficiency Solar Cells. Adv. Energy Mater. 2017, 8, 1700979. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Zakeeruddin, S.M.; Grätzel, M.; Fan, Z. Large-Grain Tin-Rich Perovskite Films for Efficient Solar Cells via Metal Alloying Technique. Adv. Mater. 2018, 30, 1705998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.M.; Wang, Z.W.; Zhou, Y.Y.; Pang, S.P.; Wang, D.; Xu, H.X.; Liu, Z.H.; Padture, N.P.; Cui, G.L. Methylamine-Gas-Induced Defect-Healing Behavior of CH3NH3PbI3 Thin Films for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 9705–9709. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.F.; Liang, W.Y. Raman spectroscopy of new lead iodide intercalation compounds. J. Phys. Condens. Matter 1993, 5, 6407–6418. [Google Scholar] [CrossRef]
- Bučko, T.; Hafner, J.; Lebègue, S.; Ángyán, J.G. Improved Description of the Structure of Molecular and Layered Crystals: Ab Initio DFT Calculations with van der Waals Corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef] [PubMed]
- Duim, H.; Fang, H.-H.; Adjokatse, S.; Brink, G.H.T.; Marques, M.A.L.; Kooi, B.J.; Blake, G.R.; Botti, S.; Loi, M.A. Mechanism of surface passivation of methylammonium lead tribromide single crystals by benzylamine. Appl. Phys. Rev. 2019, 6, 031401. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Younas, M.; Gondal, M.A.; Dastageer, M.A. Fabrication of perovskite solar cells using novel 2D/3D-blended perovskite single crystals. Int. J. Energy Res. 2020, 45, 5555–5566. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, Y.; Zhang, R.; Li, Y.; Yan, Q.; Lee, S.; Yu, Y.; Tsai, H.; Choi, W.; Wang, K.; et al. A fabrication process for flexible single-crystal perovskite devices. Nature 2020, 583, 790–795. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ji, C.M.; Liu, X.T.; Wang, S.S.; Li, L.N.; Peng, Y.; Yao, Y.P.; Hong, M.C.; Luo, J.H. Solution-Grown Large-Sized Single-Crystalline 2D/3D Perovskite Heterostructure for Self-Powered Photodetection. Adv. Opt. Mater. 2020, 8, 2000311. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Ni, Z.; Xu, S.; Zhao, J.; Xiao, X.; Huang, J. Heterojunction structures for reduced noise in large-area and sensitive perovskite X-ray detectors. Sci. Adv. 2021, 7, eabg6716. [Google Scholar] [CrossRef]
- He, Y.; Pan, W.; Guo, C.; Zhang, H.; Wei, H.; Yang, B. 3D/2D Perovskite Single Crystals Heterojunction for Suppressed Ions Migration in Hard X-ray Detection. Adv. Funct. Mater. 2021, 31, 2104880. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).