Abstract
Developmental noise is a variety that is not related to the usually distinguished sources of phenotypic diversity, i.e., differences in the genotype and in the environment. This variation arises in the process of the realization of genetic information and reflects the imperfection of ontogenetic processes. The most common measure of it is the value of fluctuating asymmetry as slight deviations from the symmetry. Developmental noise proves to be one of the main sources of intrapopulation phenotypic diversity. The magnitude of this variability is an ontogenetic response to environmental or genetic stress, and its assessment, in fact, provides a unique opportunity to estimate the developing system condition. The level of developmental noise, characterizing an organism’s condition, acts as another population parameter that allows to approach the evaluation of the community condition. Initial deviations in the system condition can be detected even against the background of optimal estimates of abundance, biodiversity, and ecosystem functioning.
1. Introduction
The study of the manifestations of fluctuating asymmetry, which essentially differ from other forms of asymmetry associated with significant and genetically determined deviations from symmetry, led to the conclusion that they are rather the result of imperfections in the realization of genetically predetermined symmetry and represent a special range of phenomena—ontogenetic noise [1,2,3,4,5]. The assessment of the significance of the phenomena for characterizing the nature of the observed phenotypic diversity and the condition of the developing system is the subject of this study. It is aimed to evaluate the applicability of the approach based on the study of the developmental noise level to characterize the state of biological systems both at the level of the organism and, more broadly, at the level of the population and community, especially in the highlight of the main requirements for such an assessment and in comparison with other possible approaches to realize the task. The assessment of the state of biological systems seems to be increasingly relevant due to the intensification of various forms of anthropogenic impact, including climate change. There is also a need to develop approaches to assess the health of biological systems and the favorable environment, especially in terms of ensuring human health and sustainable development [6,7,8,9,10].
2. Developmental Noise Assessment
Developmental noise is understood as the variability that is not associated with the usually considered causes of the emergence of phenotypic diversity and differences in the genotype and in the environment (developmental conditions). In fact, this is ontogenetic variability that occurs in the process of the realization of genetic information. This form of variability, apparently, is ubiquitous and takes place in the development of any morphological structure, but its isolation against the background of total phenotypic diversity is difficult due to the fact that it involves the assessment of phenotypic differences that arise on the basis of the same genotype under similar developmental conditions. This is possible when studying isogenic lines and clones, assessing deviations in the realization of a predetermined pattern of the morphological structure, due to the imperfection of ontogenetic processes. The most common way to assess the developmental noise level is the evaluation of the magnitude of fluctuating asymmetry (differences in the values of the trait on different sides of the body) [11,12,13,14,15,16,17].
As evidenced by laboratory experiments and natural population studies, the level of developmental noise increases under environmental and genetic stress, acting as a characteristic of developmental stability. Moreover, such changes take place before significant changes in the viability of the organism occur (Figure 1) [4,18,19,20].
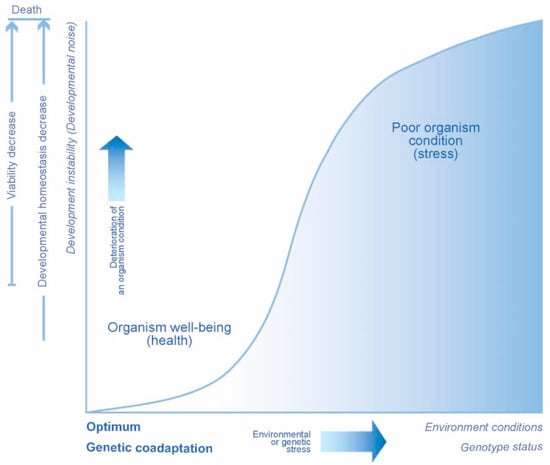
Figure 1.
Developmental noise assessment: scheme of possible changes.
An increase in the noise level is usually observed with a change in the fitness parameters, such as the breeding success index, indicating a deterioration of developmental stability [18,21,22,23,24,25].
3. Assessment of the Developing System Condition
The developmental noise turns out to be not only a source of phenotypic diversity but also a characteristic of the developing system condition. In genetically homogeneous, highly homozygous lines and clones (as well as in the cases of genetic coadaptation disruption), a higher level of phenotypic variability is observed than in ordinary heterogeneous populations. The main reason for this is the growth of developmental noise. This indicates that the main factor for determining the magnitude of this form of variability is not genetic or environmental diversity but the state of the developing system. This form of variability, along with genetic variability, acts as one of the main sources of the observed intrapopulation phenotypic diversity [4,19,26]. Under normal conditions, the level of developmental noise does not show consistent changes even for correlated morphological traits, while at the same time, under stressful exposure, the noise level shows consistent changes even for uncorrelated traits. The variability of the pholidosis characters (number of scales) in reptiles and craniological characters (number of foramina) in mammals can be used as examples. The consistency of changes in the level of developmental noise with other genetic and physiological parameters allows us to speak about the characteristic of an organism’s condition (Figure 2 and Figure 3). At the same time, an increase in the noise level, marking a change in an organism’s condition, is not the cause of its death [4,27,28,29].

Figure 2.
Developmental noise assessment: what does it mean?
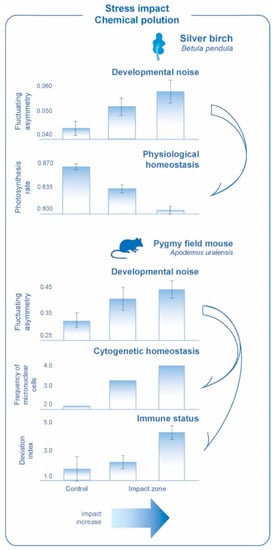
Figure 3.
Correlated response of the level of developmental noise with other parameters for different species under stress impact and chemical pollution.
Developmental noise was assessed by the value of the integrated index: the average scaled difference between the sides per character (for five leaf measurements) for plants and the average frequency of asymmetric manifestation per character (for 10 craniological characters, number of foramina) for mammals. The efficiency of photosynthesis was evaluated by the ratio of the variable fluorescence to the maximum. Cytogenetic homeostasis was assessed by the frequency of cells with micronuclei. Immune status was assessed by the value of the integrated index of deviation from the conditional norm for some parameters of the immune system [29].
4. Applicability of the Approach
The level of developmental noise, characterizing an organism’s condition, acts as another population parameter [21,30]. The approach can also be used for biological systems of a higher rank. In this case, the characteristic of the state of the system is carried out according to the noise level estimates of the constituent elements. Consistency in the change in indicators of the noise level in populations of different species allows us to approach the characterization of the community condition (Figure 4).

Figure 4.
Biological system condition: a possible approach for assessing.
Initial deviations in the state of the system can be detected even against the background of optimal estimates of the indicators of abundance, biodiversity, and ecosystem functioning. Such assessments of the state of a biological system can also serve to characterize the habitat quality (Figure 5) [4,27,31,32,33].

Figure 5.
Biological system condition: a scheme for assessing.
Among the features of the approach, which determine the possibilities of its practical use, the following should be noted. The generality of the assessment is associated with a characteristic of developmental stability, the possibility of characterizing a conditional norm at a minimum level of noise, and the degree of deviation from it according to the increase in the level of developmental variability. The sensitivity of the assessment is due to the fact that an increase in the level of noise up to a certain limit is within the backlash allowed by natural selection, which makes it possible to detect even initial changes in the system condition. This makes it possible to characterize such an assessment as an early warning system. An increase in the level of universality, which is due to the fact that developmental noise is represented in the phenotypic variability of any morphological structure of living beings, is a non-specific response to various types of adverse impact. These features allow us to talk about the prospects of using the approach in accordance with the requirements to characterize the state of a biological system from an organism to a community (Figure 6) [27,34,35].

Figure 6.
Developmental noise assessment: specific features.
In the field of environmental toxicology [36,37,38,39], the assessment of developmental noise can act as a biomarker of the state of biological systems under the whole complex of natural and anthropogenic influences. In fact, this approach assesses the well-being of living beings [40,41], opening up the possibility for such a characteristic, not only for animals but also for plants [4,42]. In the field of environmental epidemiology and conservation medicine [43], the approach characterizes a favorable environment for living beings, including humans.
Thus, the level of developmental noise seems to be a promising indicator of biological system conditions. Among the main advantages of the approach is the existence of relevant criteria for the assessment and justification of the significance of the evaluation obtained for characterizing the well-being or health of the system and the degree of its possible deviation from the conditional norm.
Author Contributions
The article was prepared at all stages, from beginning to end, including the reaction to the reviewers’ comments and suggestions, in collaboration with all authors V.M.Z. and I.E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by the Government program of basic research at the Koltzov Institute of Developmental Biology of the Russian Academy of Sciences in 2022 № 0088-2021-0019.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the members of the laboratory of postnatal ontogenesis of the Koltzov Institute of Developmental Biology of the Russian Academy of Sciences for their fruitful cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waddington, C.H. The Strategy of the Genes; George Allen & Unwin: London, UK, 1957; p. 262. [Google Scholar]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Willmore, K.E.; Hallgrímsson, B. Within Individual Variation: Developmental Noise Versus Developmental Stability in Variation; Hallgrímsson, B., Hall, B.K., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 191–218. [Google Scholar]
- Zakharov, V.M.; Shadrina, E.G.; Trofimov, I.E. Fluctuating Asymmetry, Developmental Noise and Developmental Stability: Future Prospects for the Population Developmental Biology Approach. Symmetry 2020, 12, 1376. [Google Scholar] [CrossRef]
- Roy, S.; Majumdar, S.M. Noise and Randomness in Living System; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Rapport, D.J.; Costanza, R.; McMichael, A.J. Assessing ecosystem health. Trends Ecol. Evol. 1998, 13, 397–402. [Google Scholar] [CrossRef]
- Wilcox, B.A.; Aguirre, A.A.; Horwitz, P. Connecting Ecology, Health, and Sustainability. In New Directionsin Conservation Medicine: Applied Cases of Ecological Health; Aguirre, A.A., Ostfeld, R., Daszak, P., Eds.; Oxford University Press: Oxford, MI, USA, 2012; pp. 17–32. [Google Scholar]
- Aguirre, A.A.; Basu, N.; Kahn, L.H.; Morin, X.K.; Echaubard, P.; Wilcox, B.A.; Beasley, V.R. Transdisciplinary and social-ecological health frameworks—Novel approaches to emerging parasitic and vector-borne diseases. Parasite Epidemiol. Control 2019, 4, e00084. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M. Animal welfare complementing or conflicting with other sustainability issues. Appl. Anim. Behav. Sci. 2019, 219, 104829. [Google Scholar] [CrossRef]
- Tarazona, A.M.; Ceballos, M.C.; Broom, D.M. Human relationships with domestic and other animals: One health, one welfare, one biology. Animals 2020, 10, 43. [Google Scholar] [CrossRef]
- Astauroff, B.L. Analyse der erblichen Störungsfälle der bilateralen Symmetrie. Z. Ver-Erbungslehre 1930, 55, 183–262. [Google Scholar] [CrossRef]
- Mather, K. Genetical control of stability in development. Heredity 1953, 7, 297–336. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, Analysis, Patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Zakharov, V.M. Future prospects for population phenogenetics. Sov. Sci. Rev. F. Physiol. Gen. Biol. 1989, 4, 1–79. [Google Scholar]
- Pertoldi, C.; Kristensen, T.N.; Loeschcke, V. A new method for estimating environmental variability for clonal organisms, and the use of fluctuating asymmetry as an indicator of developmental Instability. J. Theor. Biol. 2001, 210, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.C.; Graham, J.H.; Emlen, J.M.; Tracy, M.; Hough, R.A.; Alados, C.L.; Escós, J. Plant developmental instability: New measures, applications, and regulation. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 367–386. [Google Scholar]
- Sinclair, J.P.; Kashian, D.M.; Bradford, J.B.; Freeman, D.C. Variation in Fractal Symmetry of Annual Growth in Aspen as an Indicator of Developmental Stability in Trees. Symmetry 2015, 7, 354–364. [Google Scholar] [CrossRef]
- Moller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Zakharov, V.M.; Trofimov, I.E. Fluctuating asymmetry as an indicator of stress. Emerg Top Life Sci 2022, 6, 295–301. [Google Scholar] [CrossRef]
- Zakharov, V.M. Population phenogenetics: Analysis of developmental stability in natural populations. Acta Zool. Fenn. 1992, 191, 7–30. [Google Scholar]
- Clarke, G.M. Relationships between developmental stability and fitness: Application for conservation biology. Conserv. Biol. 1995, 9, 18–24. [Google Scholar] [CrossRef]
- Clarke, G.M. Developmental Stability—Fitness Relationships. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 187–195. [Google Scholar]
- Parsons, P.A. Environments and evolution: Interactions between stress, resource inadequacy and energetic efficiency. Biol. Rev. 2005, 80, 589–610. [Google Scholar] [CrossRef]
- Zakharov, V.M.; Trofimov, I.E.; Sheftel, B.I. Fluctuating Asymmetry and Population Dynamics of the Common Shrew, Sorex araneus, in Central Siberia under Climate Change Conditions. Symmetry 2020, 12, 1960. [Google Scholar] [CrossRef]
- Leary, R.F.; Allendorf, F.W.; Knudsen, K.L. Genetic, environmental, and developmental causes of meristic variation in rainbow trout. Acta Zool. Fenn. 1992, 191, 79–95. [Google Scholar]
- Zakharov, V.M.; Krysanov, E.Y.; Pronin, A.V.; Trofimov, I.E. Study of developmental homeostasis in natural populations. Health of environment concept: Methodology and practice of estimation. Russ. J. Dev. Biol. 2017, 48, 355–368. [Google Scholar] [CrossRef]
- Krysanov, E.Y.; Ordzhonikidze, K.G.; Simanovsky, S.A. Cytogenetic indicators in estimation of environmental state. Russ. J. Dev. Biol. 2018, 49, 36–41. [Google Scholar] [CrossRef]
- Pronin, A.V.; Nikolaeva, T.N.; Deeva, A.V. Immunological approach to assessing the health of the environment. Russ. J. Dev. Biol. 2018, 49, 42–47. [Google Scholar] [CrossRef]
- Soule, M.E. Phenetics of Natural Populations. II. Asymmetry and Evolution in a Lizard. Am. Nat. 1967, 101, 141–160. [Google Scholar] [CrossRef]
- Zakharov, V.M. Linking Developmental Stability and Environmental stress: A Whole Organism Approach. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 402–414. [Google Scholar]
- Shadrina, E.G.; Vol’pert, Y.L. Developmental instability of the organism as a result of pessimization of environment under anthropogenic transformation of natural landscapes. Russ. J. Dev. Biol. 2014, 45, 117–126. [Google Scholar] [CrossRef]
- Shadrina, E.G.; Vol’pert, Y.L. Experience of applying plant and animal fluctuating asymmetry in assessment of environmental quality in terrestrial ecosystems: Results of 20-year studies of wildlife and anthropogenically transformed territories. Russ. J. Dev. Biol. 2018, 49, 23–35. [Google Scholar] [CrossRef]
- Zakharov, V.M.; Clarke, G.M. BIOTEST: A New Integrated Biological Approach for Assessing the Condition of Natural Environments; International Biotest Foundation: Moscow, Russia, 1993. [Google Scholar]
- Leung, B.; Knopper, L.; Mineau, P. A Critical Assesment of the Utility of Fluctuating Asymmetry as a Biomarker of Anthropogenic Stress. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 415–426. [Google Scholar]
- Peakall, D.B. Animal Biomarkers as Pollution Indicators; Chapman & Hall: London, UK, 1992; p. 292. [Google Scholar]
- Peakall, D.B.; Walker, C.H.; Migula, P. (Eds.) Biomarkers: A Pragmatic Basis for Remediation of Severe Pollution in Eastern Europe; Springer: Dodrecht, The Netherlands, 1999; p. 324. [Google Scholar]
- Kendall, R.J.; Lacher, T.E.; Cobb, G.P.; Cox, S.B. (Eds.) Wildlife Toxicology: Emerging Contaminant and Biodiversity Issues; CRC Press: Boca Raton, FL, USA, 2010; p. 322. [Google Scholar]
- Kendall, R.J. Wildlife Toxicology: Where We Have Been and Where We Are Going. J. Environ. Anal. Toxicol. 2016, 6, 348. [Google Scholar] [CrossRef]
- Broom, D.M.; Johnson, K.G. Stress and Animal Welfare; Chapman & Hall: London, UK, 1993; p. 211. [Google Scholar]
- Broom, D.M. Welfare in Relation to Feelings, Stress and Health. REDVET. Rev. Electrónica Vet. 2007, 1695, 7504. [Google Scholar]
- Erofeeva, E.A.; Yakimov, B.N. Change of Leaf Trait Asymmetry Type in Tilia cordata Mill. and Betula pendula Roth under Air Pollution. Symmetry 2020, 12, 727. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Ostfeld, R.S.; Tabor, G.M.; House, C.; Pearl, M.C. Conservation Medicine: Ecological Health in Practice; Oxford University Press: New York, NY, USA, 2002; p. 408. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).