Abstract
The involvement of the brain motor system in idiopathic scoliosis remains unclear. In this paper, we question whether the functional connectivity (FC) of the central motor circuitry is abnormal in adolescent idiopathic scoliosis (AIS) and whether it can be modified by flexion of the lower extremities. Functional magnetic resonance imaging (fMRI) in 18 patients with a right thoracic idiopathic curve greater than 30° (mean angle 49.4°, mean age 15.3 years, 4 males) and 22 healthy controls (mean age 18.2 years, 4 males) was explored using a 3T MR scanner. We measured their resting-state fMRI: (a) with extended lower extremities; (b) with semiflexion of the left lower extremity and extended right lower extremity, with hip abduction. Decreased FC between the secondary motor area (SMA) and postcentral cortex, pallidum and cuneus, postcentral gyrus and cerebellum, putamen and temporal lateral neocortex was observed in AIS. This pathological connectivity was reversed by lower extremity semiflexion. The FC between cortical and subcortical motor structures is significantly decreased in AIS. The decreased FC of the SMA, basal ganglia, cuneus (a hub structure), and cerebellum indicates the functional impairment of structures involved in regulating muscular tone. FC impairment in patients with AIS appears to be a reaction to the pathological condition. This pathological pattern flexibly reacts to changes in the positioning of the lower extremities, showing that the functional impairment of brain motor circuitry in AIS is reversible. We suggest that the reactivity of cerebral activity leading to brain activity normalization could be used for a rehabilitation program for patients with AIS.
1. Introduction
Adolescent idiopathic scoliosis (AIS) is a three-dimensional spinal deformity, with the main component in the frontal plane. It occurs in approximately 3% of the population, predominantly in girls [1]. The etiology of AIS is considered multifactorial, but it remains largely unknown. Several factors may contribute to AIS, including genetic, hormonal, metabolic, skeletal, biomechanical, and environmental factors [2,3]. There is growing support for a central nervous system (CNS) impairment that may be underlying or contributing to AIS [4,5].
The study follows the hypothesis that idiopathic scoliosis can be caused by an imbalance between the brain regions controlling muscle tone [6].
We explored whether the central motor circuit connectivity is impaired in AIS, and if so, whether this impairment is constant or modifiable. Brain connectivity was explored using functional magnetic resonance imaging (fMRI). We questioned whether the brain functional connectivity (FC) of the cortical and subcortical motor structures is abnormal in AIS and whether it can be modified by flexion of the lower extremities.
2. Materials and Methods
2.1. Subjects
The study evaluated 40 participants: 18 patients with idiopathic scoliosis (SC; mean age 15.3 years, ranging from 11 to 20; 4 males, 2 left-handed participants) and 22 healthy controls (HC; mean age 18.2 years, ranging from 12 to 20 years; 4 males, 2 left-handed participants). See Table A1 and Table A2 (Appendix A) for details. The inclusion criterion for SC was a right thoracic idiopathic curve greater than 30° using the Cobb method. The average value of the Cobb angle was 49.4° (range 35°–68°); the apex of the curve was from T7 to T10. Twelve patients were not treated by a brace; another three used a brace during the night, and one used a brace during the day, while two patients used a brace for 21–22 h per day. All curves were Lenke type 1 (main thoracic). All patients were treated by physiotherapy. Patients were examined at the Orthopedics Department of the University Hospital Brno, including an X-ray examination. The patients did not use any medication. All the controls were students. They were examined by the first author, an experienced spine surgeon, in a standing position and by a flexion test. No deformities or prominence were present. Physical activity was without limitation (except in the scoliosis group, for whom we did not recommend falls and jumping). No neural diseases were found in the participants. The study was approved by the Multicentric Ethical Committee of University Hospital, Brno on 27 July 2016, and all the participants (and their parents, if the participants were children) signed informed consent forms. All methods were carried out in accordance with the relevant guidelines and regulations.
2.2. MRI Acquisition
All MRI measurements were taken at the Multimodal and Functional Imaging Laboratory (MAFIL), CEITEC, Masaryk University, using a Siemens Magnetom Prisma 3T scanner. The first part of the MRI protocol was measured with a 64-channel head/neck coil and with subjects lying in the standard position (on their backs, face up). This part consisted of T1-weighted MPRAGE anatomical images covering the whole brain, with isotropic voxels of 1 × 1 × 1 mm. The second part was measured with an open Tx/Rx Cp head coil and with subjects lying in a prone position, face down. We measured: (A) a resting-state fMRI in the prone position with extended lower extremities for 6 min; (B) a resting-state fMRI in the prone position with semiflexion of the left lower extremity and with an extended right lower extremity and slight right hip abduction (see Figure 1) for 6 min. A prone position during the examination was selected due to the relaxation position of the trunk and lower extremities—the position is also part of crawling. The complete position of the upper extremities (shoulder elevation of the extended left extremity and semiflexion of the right one) was not possible during the MRI examination because of the gantry diameter. Both of these fMRI parts were acquired with the following parameters: 150 scans (volumes), repetition time (TR) 2.1 s, TE 35 ms, voxel size 3 × 3 × 4 mm (slice thickness of 4 mm), 30 slices in a transversal orientation. The first author (an experienced spine surgeon) was present during the MRI examination and checked the positioning of the participants.
Figure 1.
Schematic picture of the position of the measured subject in the scanner, with semiflexion.
2.3. fMRI Data Processing
The processing of MRI data was performed with the SPM12 toolbox running under MATLAB R2015b. Functional scans were realigned to correct for the displacement caused by movement, transformed into the MNI space, resampled to a voxel size of 3 × 3 × 3 mm, and spatially smoothed by a Gaussian filter, with FWHM (full width at half maximum) = 5 mm. The fMRI data were also co-registered to the anatomical images. Before FC analysis, movement signals were regressed out of the fMRI data, using the translations and rotations obtained during the SPM realignment procedure. Moreover, representative signals (as the first principal component) from the white matter and cerebral-spinal fluid compartments were regressed out of the fMRI data to eliminate the effect of physiological artifacts and scanner instabilities. The data were filtered with a high-pass filter with a cut-off of 128 s.
The FC was calculated as a seed correlation analysis, with seeds placed in the primary motor area (Brodmann area—BA4), supplementary motor area (SMA), putamen, and pallidum—in all cases, both left and right regions were evaluated independently (i.e., 8 seeds were evaluated in this study).
Interactions between the factors of the group and lower extremity position on FC were finally evaluated using a full factorial second-level model, as implemented in SPM12, with two factors. One factor was the effect of the group (patients vs. healthy controls); the other factor was the effect of the lower extremity positions (extended vs. semiflexion). Two covariates (age and sex) were included in the statistical model. The age covariate was particularly necessary to obtain valid between-group comparisons because of the significant differences in mean age. The results were assessed with cluster-level inference. The initial threshold to create clusters was set at p < 0.001 and uncorrected. The cluster-level significance was set at p < 0.05 FWE and corrected. The simple difference between the FC in patients and in controls (with lower extremities extended) was assessed using a two-sample Student’s t-test with the same level of significance.
3. Results
No pathology was found on the brain MRI images.
First, we analyzed the differences between HC and SC in the position with extended lower extremities, to find the main differences in FC caused by idiopathic scoliosis (see Table 1 for details).

Table 1.
The effect of grouping (healthy controls (HC) vs. SC) in a prone position with lower extremities extended, based on two-sample t-tests. Age, sex, and the dominant hand were used as covariates in this statistical model. The results were thresholded at the cluster level of p < 0.05 FWE, corrected, with the initial threshold p < 0.001 uncorrected and used to form clusters.
We found more decreased FC between the left BA4 and right SMA, left parietal lobe, left temporal lobe, and right precuneus in SC than in HC. A decreased FC was also found between the right BA4 and left precuneus, right BA4 and right SMA, left pallidum and left rolandic operculum, left pallidum and right cerebellum posterior lobe, left putamen and right insula, and right putamen and right insula. Conversely, we found some connections with an increased FC in SC compared to HC. These are between the left SMA and right cerebellum anterior lobe, right SMA and right cerebellum anterior lobe, left pallidum and left precuneus, right pallidum and right precuneus, right pallidum and right parietal lobe, right pallidum and left parietal lobe, and right pallidum and left frontal lobe. Therefore, complex changes in FC were observed between HC and SC.
The basic pattern of FC, assessed with seed correlation analysis for both groups, lower extremities in an extended position, and all seeds, is presented in Figure 2. There is a lesser extent of voxels that are significantly correlated with seeds in the SC group than in the HC group. The locations with statistical significance are summarized in Table 1. It is clear that the connectivity maps look symmetrical for all seeds and both groups. Functional connectivity from the SMA and BA4 seeds revealed motor networks with sensory-motor regions. The pallidum and putamen seeds reveal connectivity in the basal ganglia.
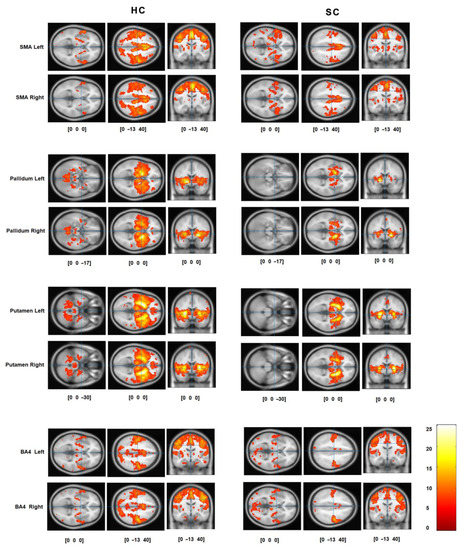
Figure 2.
The mean functional connectivity for healthy controls (column titled HC) and AIS patients (SC) was assessed with one sample t-test on individual seed correlation maps. The rows correspond to specific seeds (left and right SMA, left and right pallidum, left and right putamen, left and right BA4). The resulting maps were thresholded at p < 0.001, FWE corrected.
Subsequently, we focused on the change in connectivity patterns between HC and SC, caused by changes to lower extremity positions (extended vs. flexed). We found significant results for several seed regions. The significant results for the right pallidum as a seed region are presented in Figure 3. There are three significant clusters: one in the cerebellum posterior lobe, one in the precuneus, and one in the postcentral gyrus. The boxplots show the functional connectivity with the seed (right pallidum) for both groups and conditions. There are two different patterns. While the cerebellum and postcentral gyrus change the between-group effect from HC > SC during extension to SC > HC during flexion, the precuneus shows the opposite pattern, i.e., changing from SC > HC during extension to HC > SC during flexion. Figure 4 represents significant results for the left putamen as a seed region. There are two significant clusters (in the middle temporal gyrus and inferior temporal lobe), both showing a similar pattern of connectivity changes between the lower extremity conditions—from HC > SC during extension to SC > HC during flexion. The last significant result is presented in Figure 5 for the right SMA as a seed region. There is a significant change in connectivity between the extended and flexed positions in the postcentral gyrus. In contrast to the previous seeds, there is no flipping of the effect between the two groups, only a significant decrease in the difference (the large effect of HC > SC during extension became much smaller during flexion but still maintained the same direction of HC > SC). As a general result for all the presented seeds, the flexed position caused a decrease in FC differences between HC and SC.
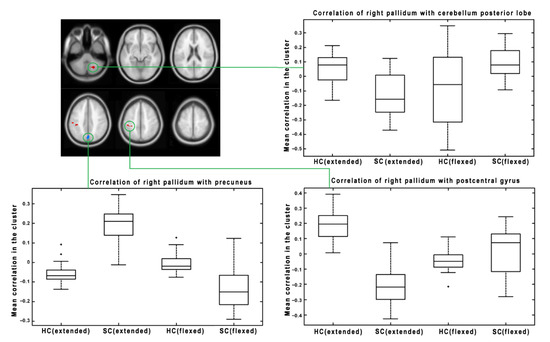
Figure 3.
The results of interactions between groups and lower extremity position factors for the right pallidum as a seed region. The results were thresholded at a cluster level of p < 0.05 FWE corrected, with p < 0.001 uncorrected as the initial threshold to form clusters. The mean functional connectivity of the individual conditions (HC and SC with extended lower extremities, HC and SC with flexed lower extremities) is visualized as a box plot for each significant cluster. The boxplots visualize the median (line within the box); 25th and 75th percentiles (lower/upper box boundaries); data range without outliers (whiskers). Outliers are visualized as individual points.
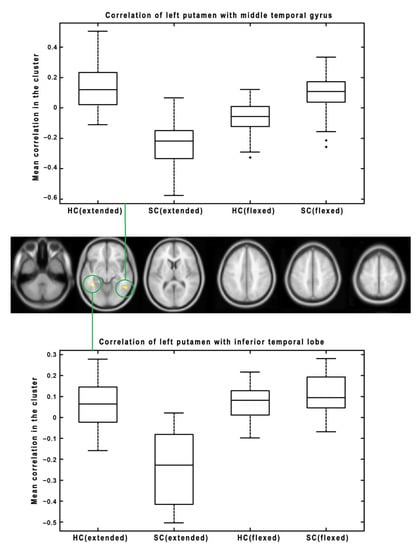
Figure 4.
The results of interactions between the groups and lower extremity position factors for the left putamen as a seed region. The results were thresholded at a cluster level of p < 0.05 FWE corrected, with p < 0.001 uncorrected as the initial threshold to form clusters. The mean functional connectivity of individual conditions (HC and SC with extended lower extremities, HC and SC with flexed lower extremities) is visualized as a box plot for each significant cluster. The boxplots visualize: the median (line within the box); 25th and 75th percentiles (lower/upper box boundaries); data range without outliers (whiskers). Outliers are visualized as individual points.
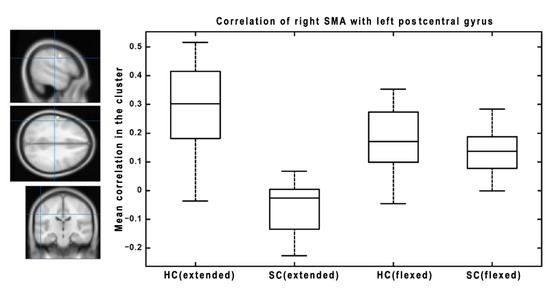
Figure 5.
The results of interactions between groups and lower extremity position factors for the right SMA as a seed region. The results were thresholded at a cluster level of p < 0.05 FWE corrected, with p < 0.001 uncorrected as the initial threshold to form clusters. The mean functional connectivity of individual conditions (HC and SC with extended lower extremities, HC and SC with flexed lower extremities) is visualized as a box plot for each significant cluster. The boxplots visualize: the median (line within the box); 25th and 75th percentiles (lower/upper box boundaries); data range without outliers (whiskers). Outliers are visualized as individual points.
4. Discussion
In this study, the FC of brain motor circuitry in SC compared to HC was studied. We focused on the FC of the motor cortical regions—the primary sensorimotor cortex, SMA, and basal ganglia (putamen and pallidum). The investigated structures form the cortico-basal ganglia-thalamocortical circuitry regulating motor activity. First, we found significant differences in the FC of the motor cortical and subcortical structures between SC and HC when in a static position with extended lower extremities (see Figure 2 and Table 1). Our study revealed abnormal FC in SC between the SMA and the postcentral cortex, pallidum and cuneus, postcentral gyrus and cerebellum, and the putamen and temporal lateral neocortex. This impaired connectivity of the SMA, basal ganglia, cuneus (a hub structure), and cerebellum indicates the functional impairment of structures involved in regulating muscle tone and movement. Second, this pathological connectivity was reversed by changing the lower extremity positioning (see Figure 3, Figure 4 and Figure 5). With a semiflexion of the left lower extremity and a slight hip abduction of the right lower extremity, the differences between SC and HC decreased and reversed (from HC > SC to SC > HC or vice versa, see Figure 3 and Figure 4 for these patterns) or practically disappeared (as visible in Figure 5). The tested positions represent the first phase of crawling or a relaxed position of the lower extremities [7].
It is probable that the observed impairment of FC reflects an impaired sensorimotor integration in AIS. Abnormal cortical dynamics of sensorimotor information processing in the sensorimotor cortex in AIS were reported in previous EEG studies [8,9]. Boček et al. [8] found lower intracortical inhibition, higher motor cortex excitability, and preserved spinal inhibitory circuits. These changes could be caused by impaired sensorimotor integration, predominantly at the cortical level. Our results demonstrate that this impairment is present not only on the cortical level but also involves the cortico-subcortical motor circuitry.
Greater interhemispheric asymmetry and increased activation in the contralateral SMA in AIS, compared to HC, were described in an fMRI study by Domenech et al. in 2011 [4]. An abnormal pattern of motor circuit activation with movement execution (opening and closing the hand) supported the hypothesis that a sensorimotor integration disorder underlies the pathogenesis of AIS. It was suggested that the abnormal function of motor circuits (abnormal sensorimotor integration) can lead to scoliosis via asymmetrical paraspinal muscle activation.
Other fMRI studies showed an impaired FC of the postcentral [5] and temporal [10] cortices in AIS. Wang et al. [10] found an increased central role of the temporal and occipital cortex and a decreased central role of the limbic cortex in AIS patients, compared to healthy controls. These findings show a larger impairment of brain FC in AIS.
Many authors using MRI have described structural changes in the brain. Xue et al. [5] found damaged white matter of the corpus callosum and corresponding interhemispheric connections in the somatosensory and optical cortex (Brodmann areas 1, 2, 3, 17, and 18). This altered white matter microstructure within the brain of patients with AIS could be related to abnormal brain maturation during adolescence and could possibly explain the previously documented somatosensory function impairment. Using diffusion tensor imaging, Joly et al. [11] found lower fraction anisotropy of the corpus callosum in AIS patients, showing a different evolution of white matter. Hitier et al. [12] described an asymmetry of the vestibular apparatus that influences the vestibulospinal tracts, hypothalamus, and cerebellum participating in balance maintenance. Liu et al. [13] found neuroanatomic asymmetry (volume changes) in those regions controlling motor function and coordination. Shi et al. [14] found a significantly increased cerebellum volume in AIS patients; they explained it as an increased effort to compensate for body balance due to an asymmetrical spine. Asymmetries in the different regions of the CNS were found by Domenech [4] and Joly [11].
In the HC in our study, the connections are more extensive/larger and are between more regions. A steady state in the prone position, with extended lower extremities, leads to larger connections between more regions in HC; more neurons and more regions are involved, with the use of more tracts. This could be caused by a greater number of neurons, which is a congenital/inherited factor, and by the connection of more regions during the development of motor functions, due to a greater number of sensory, motor, and proprioceptive impulses, which are connected with greater intensive motor activity. In SC, the connections are smaller, between fewer regions, and different from those in the HC. This finding could be caused by a thinner brain cortex in SC, or by a formation of different connections during motor development and may be due to motor activities. A thinner brain cortex could be congenital/inherited, with fewer neurons, or by the halting of cell division by external or internal factors during brain development, predominantly in the intrauterine period. CNS development after birth, with fewer sensory, motor, and proprioceptive impulses and with fewer motor activities, will cause the connection of fewer regions in comparison to HC. Using a 1.5 T apparatus, Wang et al. [10] found an increased role of the temporal and occipital cortex, along with decreased interhemispheric connectivity and increased connectivity in some cortical regions. We evaluated the brain using a 3.0 T apparatus in the subcortical regions and the cerebellum. The positions of the patients were different in the two studies, including the prone position, and we evaluated the lower extremities when extended and then in the semiflexion (relaxed position). The greater brain connectivity in HC can be evaluated as being usual and, in SC, as compensatory, due to hardware or software disorders of the body’s “central computer”—the CNS. The question remains if this disorder is primary or secondary.
Our results indicate that this pathological brain activation is not a fixed reaction to a fixed axial posture anomaly; instead, it flexibly reacts to changed lower extremity positions. This leads to the suggestion that the pathological FC in patients with AIS is a reaction to a pathological condition. It is probable that the brain’s motor circuitry plays an important role in AIS. Conversely, it also shows that the functional impairment of brain motor circuitry in AIS is reversible. It is possible that the reactivity of cerebral activity, leading to brain activity normalization, could be used in rehabilitation programs for patients with AIS. We can recommend dynamic crawling and static relaxation positions in the early and subsequent periods of motor development. Hypothetically, stimulation of the cortical motor structures using invasive or non-invasive methods could be tested. However, a larger and longitudinal study would be needed for further progress.
The main limitation of our study is the small number of participants. We were still able, however, to demonstrate significant differences between SC and HC.
5. Conclusions
The FC examined by fMRI in SC and HC is different when positioned with extended extremities. In HC, the FC is significantly greater and appears between many regions; it can be evaluated in the same way as the usual connectivity in a standard network. In SC, fewer and different regions are involved; this can be evaluated in the same way as compensatory connectivity. The cause of these differences could be a form of compensation for a hardware or software disorder of the body’s “central computer”, the CNS.
The brain FC of cortical and subcortical motor structures is abnormal in SC, and this dysfunction is modified by the semiflexion of the lower extremities in a relaxed position. The normalization of activation in the motor circuits induced by movement indicates that the central motor circuit impairment could be secondary or also causative to scoliosis, being influenced by brain plasticity. These findings could support the introduction of dynamic crawling and static relaxation positions in the early and subsequent stages of motor development.
Author Contributions
R.C.—study design and hypothesis, orthopedic examination of patients, discussion and interpretation, manuscript preparation; M.M.—design of acquisition protocol, data processing, and analysis, participation oi interpretation, and manuscript preparation; M.N.—data processing and analysis; M.R.—participation in discussion and interpretation; I.R.—discussion and interpretation and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the core facility of MAFIL in CEITEC MU, supported by the Czech BioImaging large RI project (LM2018129, funded by MEYS CR), for their support in obtaining the scientific data presented in this paper. The results of this research have been acquired within the CEITEC 2020 (LQ1601) project, with financial contributions made by the Ministry of Education, Youths, and Sports of the Czech Republic, and with special support from the National Program for Sustainability II funds.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Brno University Hospital (protocol code 01-270716/EK, date of approval 27 July 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, due to restrictions in informed consent, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Patient characteristics.
Table A1.
Patient characteristics.
| Subject ID | Age (Years) | Menarche (Years) | Handedness | Apex | X-ray | Thoracic Kyphosis T5-T12 | Lumbar Lordosis T12-S1 | Prom cm | Orthosis |
|---|---|---|---|---|---|---|---|---|---|
| SC_01 | 17 | 15 | right | T8-9 | 59 | 23 | −48 | 3 | 0 |
| SC_02 | 18 | 15 | right | T9 | 59 | 12 | −55 | 2.1 | 0 |
| SC_03 | 14 | 0 | right | T9 | 49 | 19 | −79 | 1.2 | 0 |
| SC_04 | 12 | 0 | right | T9-10 | 48 | 24 | −67 | 2.2 | night |
| SC_05 | 16 | 12 | right | T9 | 39 | 10 | −52 | 2.1 | 0 |
| SC_06 | 18 | 11 | right | T8 | 40 | 10 | −57 | 1.9 | 0 |
| SC_07 | 15 | 12 | right | T7-8 | 47 | 18 | −63 | 1.7 | 0 |
| SC_08 | 16 | 15 | right | T9-10 | 68 | 32 | −59 | 2.5 | 0 |
| SC_09 | 14 | 13 | right | T9 | 56 | 30 | −74 | 3 | 0 |
| SC_10 | 11 | 0 | left | T8-9 | 46 | 28 | −65 | 2.5 | 21/24 h |
| SC_11 | 20 | male | right | T8-9 | 40 | 17 | −48 | 1.8 | 0 |
| SC_12 | 15 | male | right | T8 | 50 | 50 | −56 | 3.5 | 0 |
| SC_13 | 13 | 0 | right | T8-9 | 60 | −12 | −46 | 2.7 | Day |
| SC_14 | 13 | 13 | right | T7 | 48 | 1 | −49 | 2.5 | 0 |
| SC_15 | 17 | male | right | T10 | 51 | 45 | −67 | 3 | Night |
| SC_16 | 18 | male | right | T8-9 | 49 | 16 | −54 | 3 | 0 |
| SC_17 | 16 | 11 | right | T8 | 35 | 20 | −59 | 2 | Night |
| SC_18 | 12 | 12 | left | T9 | 45 | 19 | −47 | 1.5 | 22/24 h |
Legend: Menarche is age (onset) of menarche in years; T = thoracic, S = sacrum; X-ray = X-ray of thoracic curve, thoracic kyphosis, and lumbar lordosis all given in Cobb angles; prom cm = thoracic prominence in cm.

Table A2.
Healthy controls characteristics.
Table A2.
Healthy controls characteristics.
| Subject ID | Age | Menarche | Handedness |
|---|---|---|---|
| HC_01 | 18 | 13 | right |
| HC_02 | 18 | 12 | right |
| HC_03 | 18 | male | right |
| HC_04 | 18 | male | right |
| HC_05 | 20 | 12 | right |
| HC_06 | 20 | male | left |
| HC_07 | 20 | 11 | right |
| HC_08 | 20 | male | right |
| HC_09 | 19 | 12 | right |
| HC_10 | 19 | 12 | right |
| HC_11 | 12 | 0 | right |
| HC_12 | 19 | 15 | right |
| HC_13 | 19 | 13,5 | right |
| HC_14 | 19 | 11 | right |
| HC_15 | 19 | 14 | right |
| HC_16 | 20 | 13 | right |
| HC_17 | 20 | 13 | left |
| HC_18 | 13 | 14 | right |
| HC_19 | 19 | 10 | right |
| HC_20 | 20 | 12 | right |
| HC_21 | 19 | 12 | right |
| HC_22 | 12 | 11 | right |
References
- Lenke, L.G.; Betz, R.R.; Clements, D.; Merola, A.; Haher, T.; Lowe, T.; Newton, P.; Bridwell, K.H.; Blanke, K. Curve prevalence of a new classification of operative adolescent idiopathic scoliosis: Does classification correlate with treatment? Spine 2002, 27, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Latalski, M.; Danielewicz-Bromberek, A.; Fatyga, M.; Latalska, M.; Kröber, M.; Zwolak, P. Current insights into the aetiology of adolescent idiopathic scoliosis. Arch. Orthop. Trauma Surg. 2017, 137, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, L.; Chu, W.C.W.; Burwell, R.G.; Cheng, J.C.Y.; Ahuja, A.T. Abnormal cerebral cortical thinning pattern in adolescent girls with idiopathic scoliosis. Neuroimage 2012, 59, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; García-Martí, G.; Martí-Bonmatí, L.; Barrios, C.; Tormos, J.M.; Pascual-Leone, A. Abnormal activation of the motor cortical network in idiopathic scoliosis demonstrated by functional MRI. Eur. Spine J. 2011, 20, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Shi, L.; Hui, S.; Wang, D.; Lam, T.; Ip, C.-B.; Ng, B.; Cheng, J.; Chu, W. Altered white matter microstructure in the corpus callosum and its cerebral interhemispheric tracts in adolescent idiopathic scoliosis: Diffusion tensor imaging analysis. Am. J. Neuroradiol. 2018, 39, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, R. Influence of Sensorymotor Cortical Area Damage on Spine Development: Spine Development after Pinealectomy and Sensorymotor Area Damage, 1st ed; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Vojta, V. Infant Movement Disorders Caused by Brain Damage, 1st ed; Grada-Avicenum: Prague, Czech Republic, 1993. [Google Scholar]
- Boček, V.; Krbec, M.; Vaško, P.; Brabec, K.; Pavlíková, M.; Štětkářová, I. Alteration of cortical but not spinal inhibitory circuits in idiopathic scoliosis. J. Spinal Cord Med. 2022, 45, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.; Pialasse, J.P.; Knoth, I.S.; Lippé, S.; Duclos, C.; Simoneau, M. Cortical dynamics of sensorimotor information processing associated with balance control in adolescents with and without idiopathic scoliosis. Clin. Neurophysiol. 2019, 130, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, L.; Liu, S.; Hui, S.; Wang, Y.; Cheng, J.; Chu, W.C.W. Altered topological organization of cortical network in adolescent girls with idiopathic scoliosis. PLoS ONE 2013, 8, e83767. [Google Scholar] [CrossRef] [PubMed]
- Joly, O.; Rousié, D.; Jissendi, P.; Rousié, M.; Frankó, E. A new approach to corpus callosum anomalies in idiopathic scoliosis using diffusion tensor magnetic resonance imaging. Eur. Spine J. 2014, 23, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Hitier, M.; Hamon, M.; Denise, P.; Lacoudre, J.; Thenint, M.-A.; Mallet, J.-F.; Moreau, S.; Quarck, G. Lateral Semicircular Canal Asymmetry in Idiopathic Scoliosis: An Early Link between Biomechanical, Hormonal and Neurosensory Theories? PLoS ONE 2015, 10, e0131120. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chu, W.C.; Young, G.; Li, K.; Yeung, B.H.; Guo, L.; Man, C.W.; Lam, W.W.; Wong, S.T.; Cheng, J. MR analysis of regional brain volume in adolescent idiopathic scoliosis: Neurological manifestation of a systemic disease. J. Magn. Reson. Imaging 2008, 27, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, D.; Hui, S.C.N.; Tong, M.C.F.; Cheng, J.C.Y.; Chu, W.C.W. Volumetric changes in cerebellar regions in adolescent idiopathic scoliosis compared with healthy controls. Spine J. 2013, 13, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).