Abstract
In Diels–Alder reactions, 2H-pyran-2-ones as dienes can yield a large variety of cycloadducts with up to four contiguous carbon stereogenic centers. Some of the potentially most useful, however difficult to prepare due to their low thermal stability, are the primary CO2-containing oxabicyclo[2.2.2]octenes, which could be formed as eight distinctive isomers (two sets of regioisomers, each of these composed of four different stereoisomers). A high-pressure synthesis of such products was recently described in a few cases where vinyl-moiety-containing dienophiles were used as synthetic equivalents of acetylene. However, structures of the primary products have been so far only rarely investigated in detail. Herein, we present seven novel single-crystal X-ray diffraction structures of such cycloadducts of both stereoisomeric forms, i.e., endo and exo. Additionally, we present a single-crystal structure of a rare case of a cyclohexadiene system stable at room temperature, obtained as a secondary product upon the retro-hetero-Diels–Alder elimination of CO2 under thermal conditions (microwave irradiation), during this elimination the symmetry is increased and out of eight initially possible isomers of the reactant, this number in the product is decreased to four. In oxabicyclo[2.2.2]octene compounds, centrosymmetric hydrogen bonding was found to be the predominant motif and diverse supramolecular patterns were observed due to rich variety of C–H⋯O and C–H⋯π interactions.
1. Introduction
The Diels–Alder reaction, mechanistically classified as a [4+2] cycloaddition belonging to the larger group of pericyclic reactions, is one of the crucial synthetic tools for the construction of novel C–C bonds [1,2], gaining importance in all its varieties, including (organo)catalytic and enantioselective versions [3,4]. Cycloadditions are highly versatile reactions as a broad range of starting dienes and dienophiles (alkenes or alkynes) can be used. Additionally, they are also often highly stereoselective, providing up to four contiguous carbon stereogenic centers and opening access to other stereogenic centers as well, such as chiral phosphorus [5], thus being of paramount importance when a decrease in symmetry (i.e., increase in asymmetry) is desired or necessary as often is the case for the construction of scaffolds of many natural and related compounds. Consequently, cycloadditions provide a viable alternative to the other widespread options of preparing enantiomerically pure compounds, i.e., optical resolution [6] or application of asymmetric (auto)catalysis [7], both of these offering the possibility of enantiomeric excess amplification. There are many empirical rules, partially stemming from the solid theoretical background set by Woodward and Hoffmann [8,9,10], which govern regio-, enantio- and diastereoselectivity of cycloadditions. Generally accepted Alder endo rule predicts the formation of the endo product as the predominant one. As a consequence of all this, the Diels–Alder reaction is currently in the spotlight of various investigations where amplification of chirality is achieved by dynamic crystallization, an example demonstrated with maleimides and 2-methylfuran (Scheme 1) [11]. Another recent application of cycloaddition reactions, albeit under photochemical conditions, is for the preparation of photochromic organic crystals [12], offering promising properties.
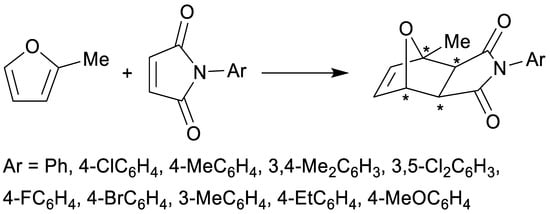
Scheme 1.
Highly enantioselective Diels–Alder reactions with 2-methylfuran as diene and N-substituted maleimides [11].
Furthermore, 2H-pyran-2-ones are rewarding diene systems capable of many [4+2] cycloaddition reactions [13,14,15] with a variety of suitable dienophiles, including maleic anhydride, N-substituted maleimides, vinyl ethers and their derivatives. The latter type of dienophiles has attracted our attention, as vinyl-group containing compounds can act as reactive partners in [4+2] cycloadditions [16]. Additionally, due to their highly asymmetrical electron density (especially in vinyl ethers and their nitrogen analogs) they can furthermore act as highly regio- and stereoselective partners. For example, in the case of cycloaddition between a substituted 3-acylamino-2H-pyran-2-one and ethyl vinyl ether [17] (or any other suitable alkene dienophile), in the first step a carbon-dioxide bridge system (i.e., 7-oxabicyclo[2.2.2]octene) is formed, such systems (including those formed via other reaction pathways) are known from the literature, but their solid-state structures were described just in a few cases, for some recent examples see ref. [18,19,20,21,22,23,24,25,26,27,28]. However, 7-oxabicyclo[2.2.2]octenes produced as described above can be formed as two possible regioisomers and each of these can exist as a mixture of two diastereoisomers (endo or exo), in turn each of the four distinctive possibilities is actually composed of a pair of enantiomers. Therefore, in a single synthetic step, eight isomeric products (i.e., two regioisomeric compounds each of them as four different stereoisomers) can be formed. However, the reaction can proceed further, via a retro-hetero-Diels–Alder reaction a molecule of carbon dioxide can be eliminated thus producing a cyclohexadiene system, the number of possible isomers is thus reduced to two regioisomers (each of them as a pair of enantiomers). These intermediates can react further via two different pathways. The first option is that an elimination (dehydrogenation in general or elimination of an alcohol in the case of vinyl ethers as dienophiles) takes place yielding the final benzene derivative (only two different regioisomers are possible, stereoisomery obviously not being possible anymore). The second option is that the cyclohexadiene system acts as a new diene and another cycloaddition step takes place (if the dienophile is still available), thus yielding bicyclo[2.2.2]octene systems [29]. Each of their regioisomers can possibly consist of the following stereoisomers: a pair of enantiomers (termed exo,endo and endo,exo) or one of two different meso compounds (exo,exo and endo,endo). So far all of these possibilities have been achieved [30,31] and various reaction conditions were developed, enabling the preferential formation of the desired products or even of the exact isomers.
Accordingly, many recent investigations have demonstrated that bicyclo[2.2.2]octene adducts are obtained under kinetically controlled reaction conditions, i.e., after shorter reaction times at lower temperatures, in general [32,33]. On the other hand, aromatization of the cyclohexadiene systems can be achieved under thermodynamic conditions and/or with the application of suitable dehydrogenation catalysts (such as active charcoal Darco) or bases (such as DABCO [1,4-diazabicyclo[2.2.2]octane]) when an elimination of a small stable molecule is the objective [34]. To stop the reactions at the first stage, i.e., before the elimination of CO2, various strategies have been devised, however all of them having in common mild reaction conditions, preferably taking place at room temperature and at increased pressure (up to 18 kbar) [35]. High pressure namely accelerates the cycloaddition step (due to the highly negative activation volume of this step), concomitantly suppressing the elimination of the CO2 (retro-hetero-Diels–Alder reaction) [36]. Additionally, application of high pressure in organic synthesis has proven to be important, as recently demonstrated by the total synthesis of (–)-aritasone [37], not least because it can lead to changes in regio- and stereoselectivity [38,39].
Our previous investigations [16,17] have shown that starting from substituted 2H-pyran-2-ones 1 and vinyl-moiety-containing dienophiles 2 the cycloadditions can be selectively directed towards each of the possible products: CO2-containing 7-oxabicyclo[2.2.2]octenes 3, cyclohexadienes 4 or final benzene rings 5 (Scheme 2). Furthermore, 7-oxabicyclo[2.2.2]octenes 3 could be prepared only at room temperature under high pressure (13–15 kbar) conditions in dichloromethane (or neat), without the addition of a base. On the other hand, cyclohexadienes 4 were prepared with thermal activation (microwave irradiation in acetonitrile at 120 °C, without a base), whereas the final elimination step to the end products 5 was successfully promoted by the addition of a suitable base, DABCO giving the best results (under microwave irradiation at 120 °C). When the whole reaction sequence (i.e., 1→5) is considered, vinyl ether has acted as a masked acetylene (as its synthetic equivalent, analogously as was previously shown for vinyl acetate (Scheme 3) [40] and vinyl borane (Scheme 4) [41] in other types of reactions), opening further synthetic possibilities as the gaseous acetylene without any electron-perturbing groups generally reacts in Diels–Alder reactions only sluggishly, at best.
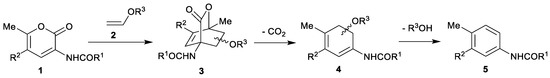
Scheme 2.
General reaction pathway of a Diels–Alder reaction between 2H-pyran-2-one derivatives 1 and vinyl ethers 2 acting as dienophiles.
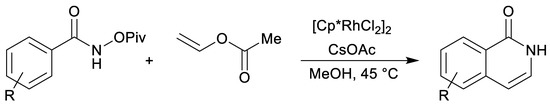
Scheme 3.
Vinyl acetate as a cheap and easy-to-handle synthetic equivalent of explosive and gaseous acetylene [40].
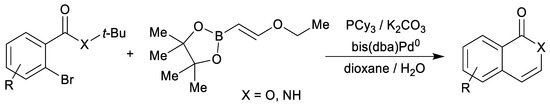
Scheme 4.
Vinyl borane as a two-carbon acetylene equivalent applied for the synthesis of isoquinolones (X = NH) and isocoumarins (X = O) [41].
The products thus obtained present some interesting structural characteristics, where the number of isomers possible in each step is decreasing towards the final product 5, this being consequence of the increase in symmetry as one proceeds from the primary intermediates (i.e., 7-oxabicyclo[2.2.2]octenes 3) via the cyclohexadiene intermediates 4 towards the final benzene products 5. Previously, we have revealed just a few X-ray structures of such cases therefore we would now like to report the results of additional single-crystal X-ray characterizations of the products 3 and 4 to determine their structural features including supramolecular aggregation in the solid state form that might influence their properties.
Examination of the number of possible isomers for each of the products obtained when a vinyl ether 2 acts as a dienophile in a Diels–Alder reaction with a substituted 2H-pyran-2-one (Figure 1) shows that 7-oxabicyclo[2.2.2]octenes 3, possessing three stereogenic centers, could theoretically exist as eight different stereoisomers. Due to the steric constraints of the bridged system, both bridgehead stereocenters (i.e., C-1 and C-4) are correlated leading to only four different stereoisomers, distinguished on the base of the configuration of alkoxy groups at the C-8 we get an enantiomeric pair labelled exo and another one termed endo (meaning that endo-3 and exo-3 each exist as a pair of enantiomers). Analogous is the situation with the regioisomeric product 3’, where alkoxy group is at the position C-7 and can also provide two pairs of enantiomers. Therefore, for all primary adducts 3 and 3’ together, there could be eight different isomers. Upon the elimination of the CO2, two stereogenic centers (previously C-1 and C-4) are lost, therefore just a pair of enantiomers for each regioisomeric product (i.e., 4 and 4’) is possible, whereas the final aromatic product 5 can exist only as a single isomer. The situation is analogous when instead of the vinyl ethers 2, their nitrogen derivatives, such as 1-vinyl-2-pyrrolidone (6a) or N-vinylcaprolactam (6b), are applied and these cases will therefore not be elaborated separately.
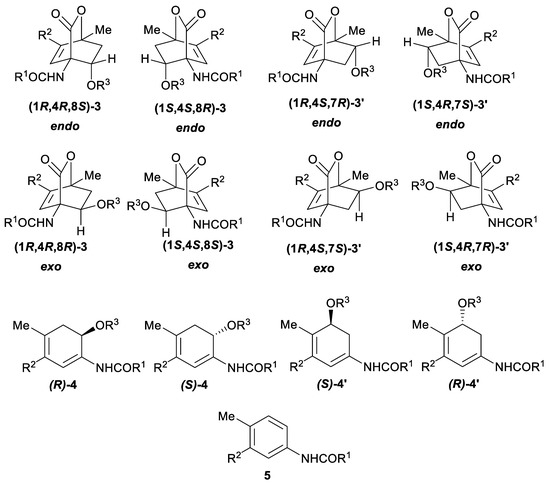
Figure 1.
Decreasing number of stereoisomers upon the increase in symmetry of the products (absolute configurations correspond to the following substituents: R1 = Ph, R2 = COMe, R3 = Et).
Our interest in the above described transformations was initially based on the application of ethyl vinyl ether (and its analogs) as a synthetic equivalent of acetylene for the production of substituted benzene derivatives 5. This being an interesting application, as it is well-known that acetylene is not a very suitable dienophile in Diels–Alder reactions. Therefore, when we disclosed the results of these syntheses in our previous publications [16,17], the structures of primary cycloadducts (i.e., 3) and those formed after the elimination of carbon dioxide (i.e., 4) in the solid state were not of primary importance. Nevertheless, we have investigated [17] the crystal structures of a pair of exo- and endo-isomers of N-[6-acetyl-1-methyl-3-oxo-8-(2-oxoazepan-1-yl)-2-oxabicyclo[2.2.2]oct-5-en-4-yl]benzamide obtained by the cycloaddition between the appropriate 2H-pyran-2-one 1a (R1 = Ph, R2 = Me) and N-vinylcaprolactam (6b) as the dienophile. X-ray diffraction analysis confirmed that the cycloaddition yielded a mixture of both stereoisomers that we were able to separate and based on the NMR data for these two compounds, the structures of other cycloadducts were established as well. However, we were intrigued by the possibility of further investigating the structures of some of the other cycloadducts of the type 3 and 4 in the solid state, as nowadays novel studies regularly underpin the importance of various intermolecular interactions [42,43,44,45], not just hydrogen bonds. Therefore, we prepared suitable crystals of six primary cycloadducts of the type 3 and one cyclohexadiene product 4 and embarked on thorough X-ray crystallographic investigation, including their crystal packing and supramolecular architectures. It turned out that the predominant motif in studied cycloadducts is the intermolecular hydrogen bonding between amide NH group and CO2 bridge of the adjacent molecule with the domination of centrosymmetric hydrogen-bonded dimers. Furthermore, diverse supramolecular patterns were observed due to rich variety of C–H⋯O and C–H⋯π interactions. These supramolecular interactions represent interesting examples of how chiral organic molecules can pack when crystallized as a racemate accommodating a high number of centrosymmetric hydrogen bonds, thus observing symmetry in the crystalline form, albeit the crystals are formed by the aggregation of asymmetric molecules.
2. Materials and Methods
2.1. Synthesis
Compounds 3a–e and 4a were prepared according the published procedure [17]. Briefly, adducts 3 were obtained by a high-pressure method conducted in Teflon ampules immersed in a piston-cylinder type of pressure apparatus (U101, Unipress Equipment, Warszawa, Poland) filled with white spirit (also known as mineral spirits) and pressurized at approximately 14 kbar (at room temperature). Reaction mixtures of the appropriate 2H-pyran-2-ones 1 (1 equiv.) and dienophiles: ethyl vinyl ether (2a) (20 equiv.), cyclohexyl vinyl ether (2b) (20 equiv.) or 1-vinyl-2-pyrrolidone (6a) (27 equiv.), were diluted with dichloromethane (to completely fill the 3.8 mL ampule) and pressurized for 336–408 h, thereafter the apparatus was disassembled, volatile components were removed in vacuo, crude products 3 precipitated with the addition of isopropyl ether and consequently recrystallized from suitable solvents: methanol for endo-3a, ethanol for endo-3b–d, dichloromethane for exo-3e, isopropyl ether for exo-3f. Isomer endo-3e was obtained from the mother liquor after exo-3e has precipitated. The crystals obtained in all cases were appropriate for X-ray diffraction analyses.
Compound 4a was prepared by microwave-assisted process in a focused monomode apparatus (Discover by CEM Corporation, Matthews, NC) by irradiation of a mixture of the appropriate 2H-pyran-2-one 1f (1 equiv.) and ethyl vinyl ether (2a) (20 equiv.) in acetonitrile (2 mL per mmol of 1) at 120 °C (180 W) for 2 h, as described previously [17]. After the reaction, product 4a precipitated upon cooling the mixture in an ice-water bath, filtration and washing with a cold (approximately 0 °C) mixture of ethanol and water (1:1) provided crude cyclohexadiene 4a, which was carefully recrystallized from ethanol (temperature during the crystallization should not exceed approximately 50–60 °C, as otherwise elimination of ethanol takes place causing partial aromatization of the product) to provide crystals suitable for X-ray diffraction analysis.
2.2. X-ray Single Crystal Analysis
Single-crystal X-ray diffraction data were collected at room temperature on a Nonius Kappa CCD diffractometer with the graphite monochromated Mo-Kα radiation (λ = 0.71073 Å). DENZO program was used to process the data [46]. Structures were solved by direct methods implemented in SHELXS-97 and SHELXL-97 was used for the refinement using a full-matrix least-squares procedure based on F2 [47]. All non-hydrogen atoms were refined anisotropically. All hydrogen atoms were readily located in difference Fourier maps and were subsequently treated as riding atoms in geometrically idealized positions with Uiso(H) = kUeq(C,N), where k = 1.5 for NH and methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all the other H atoms. In endo-3b atoms C9–C10 and C24 in each ethoxy group were disordered over two positions with the refined ratios 0.805(19):0.195(19) and 0.444(11):0.556(11), respectively. Atoms C9A, C9B, C10A and C10B were refined with restrained Uij components. Resolution in endo-3b is lower than expected; however, the data were of sufficient quality to determine the connectivity. All compounds crystallize as racemates. The crystallographic data are listed in Table 1. The crystallographic data have been deposited at Cambridge Crystallographic Data Centre (CCDC 2034987–2034994) and can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Appropriate cif-files with single-crystal XRD structures are provided as Supplementary Materials also.

Table 1.
Experimental data for the X-ray diffraction studies.
3. Results
3.1. Chemistry
As presented in Table 2, crystals suitable for X-ray diffraction were prepared for the following oxabicyclo[2.2.2]octene products [17]: (i) set of three endo adducts 3a–c obtained with the cycloaddition of ethyl vinyl ether (2a) on the appropriate 2H-pyran-2-ones 1a–c; (ii) for endo-3d obtained by the cycloaddition of cyclohexyl vinyl ether (2b) on 2H-pyran-2-one 1d; (iii) for the pair of endo and exo adducts 3e produced by the cycloaddition of 1-vinyl-2-pyrrolidone (6a) on the 2H-pyran-2-one 1e; (iv) for the exo-3f obtained by the cycloaddition between 6a and 2H-pyran-2-one 1d. Additionally, a single crystal of cyclohexadiene product 4a was also prepared; however, in contrast to the high-pressure conditions appropriate for the preparation of 3, here thermal activation with microwaves was necessary. Microwave irradiation as a way to facilitate organic reactions has during the last decades found some surprisingly widespread applications, as recently vividly described by Kappe [48], including acceleration of Diels–Alder reactions [2,49], Kabachnik–Fields reaction [50] and many others.

Table 2.
Prepared products [17].
It is worth noting that product endo-3e was isolated by precipitation upon slow evaporation of the volatile components from the mother liquor (which remained after the precipitation of exo-3e and its removal by vacuum filtration) containing an appreciable amount of the starting dienophile 6a (which was initially applied in a large excess, also as a co-solvent, in molar ratio 1e:6a = 1:27). Consequently, endo isomer was found to crystallize as a solvate endo-3e∙6a.
3.2. X-Ray Single Crystal Determination
Compounds endo-3a and endo-3c crystallize in the orthorhombic space group Pcab, compound endo-3d in the monoclinic space group C2/c and compound exo-3e in the monoclinic space group P21/n while compounds endo-3b, endo-3e and exo-3f crystallize in the triclinic space group P–1. Compound 4a crystallizes in the orthorhombic space group Pcab. In all crystal structures asymmetric units are composed of one molecule except in endo-3b where two crystallographically independent molecules are present in the asymmetric unit and in endo-3e∙6a where the asymmetric unit is composed of a molecule endo-3e and a molecule of 1-vinyl-2-pyrrolidone (6a) as a solvate. Crystal structures are presented in Figure 2.
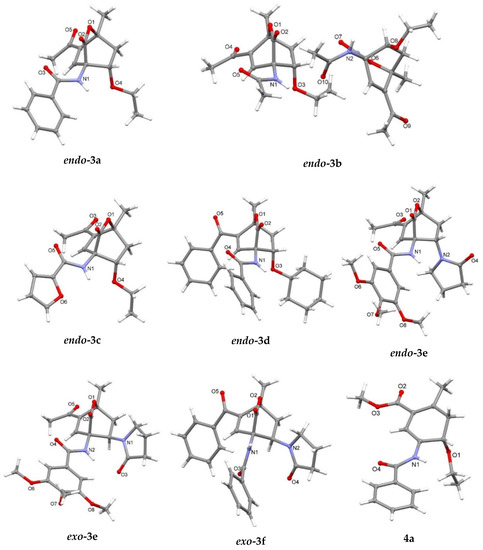
Figure 2.
Crystal structures of 7-oxabicyclo[2.2.2]octenes 3a–f and cyclohexadiene 4a. In endo-3b two crystallographic molecules are present in the asymmetric unit, disorder is omitted for clarity. In endo-3e, solvate molecule 6a is omitted for clarity.
Compounds 3 possess rigid 7-oxabicyclo[2.2.2]octene skeleton and thus structures variate primarily due to different substituents at the position 6 (acetyl, benzoyl), 8 (cyclohexyloxy, ethoxy, 2-oxopyrrolidin-1-yl) and 4 (benzoylamino, acetylamino, trimethoxybenzoylamino, furan-2-carboxylamino) while position 1 is always occupied by a methyl group. Different substituents present at positions 4, 6 and 8 enable diverse interactions in the crystal structure. All compounds possess one classical hydrogen bond donor, namely amide NH group. On the other hand, there are several potential hydrogen bond acceptors, such as oxygen atoms of side arms at the positions 4, 6 and 8 as well as both oxygen atoms in the CO2 bridge.
In compound endo-3a, hydrogen-bonded centrosymmetric dimer is formed via N1–H1⋯O2i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the CO2 bridge of the symmetry related molecule as a hydrogen bond acceptor with the graph-set motif R22(10) [51] (Figure 3a, Table 3). This interaction is further supported by centrosymmetric C4–H4b⋯π interaction between the bicyclo[2.2.2]octene scaffold and the phenyl ring of the benzoylamino substituent with d(H4b⋯Cg4) = 2.83 Å and <(C4–H4b ⋯Cg4) = 155°, where Cg4 is C14–C19 ring centroid. Dimeric structure is further connected into a layer along the ab plane via C9–H9c⋯π interaction between acetyl moiety and phenyl ring of the benzoylamino substituent of the adjacent hydrogen-bonded dimer (Figure 3b–d). There are no significant π⋯π interactions.
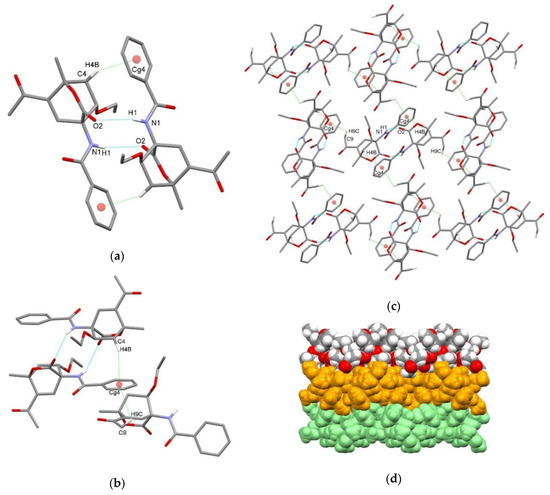
Figure 3.
Supramolecular aggregation of endo-3a. (a) Hydrogen-bonded centrosymmetric dimer formed through N–H⋯O and C–H⋯π interactions; (b) hydrogen-bonded dimers are further connected with adjacent dimers through C–H⋯π interactions forming (c) layer; (d) packing of layers (arbitrary colors). Hydrogen atoms not involved in the motifs shown have been omitted for clarity.

Table 3.
Hydrogen bonds for 3a–f and 4a [Å and °].
In compound endo-3b, hydrogen-bonded centrosymmetric tetramer is present, formed via a combination of N1–H29⋯O10 and N2–H30⋯O2i interactions with the graph-set motif R44(18). In this tetramer, molecule A forms a hydrogen bond through the amide NH group acting as a hydrogen bond donor with the carbonyl oxygen atom of the amide group of molecule B acting as a hydrogen bond acceptor. Furthermore, molecule B forms a hydrogen bond through amide NH group acting as a hydrogen bond donor with the carbonyl oxygen atom of the CO2 bridge of symmetry related molecule A acting as a hydrogen bond acceptor (Figure 4a, Table 3). Tetramer is further supported by C1–H1⋯O7 interaction between the bicyclo[2.2.2]octene scaffold of molecule A and the carbonyl oxygen atom of the CO2 bridge of molecule B, as well as C28–H28A⋯O2 interaction between methyl group of acetylamino substituent of molecule B and the carbonyl oxygen atom of the CO2 bridge of molecule A. Tetramers are further connected into layers along the ab plane via C12–H12A⋯O9ii and C16–H16B⋯O7iii interactions (Figure 4b). There are no significant C–H⋯π and π⋯π interactions.
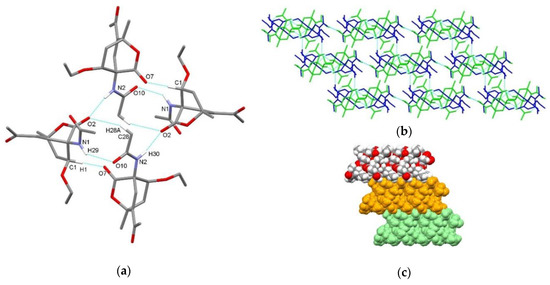
Figure 4.
Supramolecular aggregation of endo-3b. (a) Hydrogen-bonded centrosymmetric tetramer formed through N–H⋯O and C–H⋯O interactions; (b) layer formation connecting tetramers through C–H⋯O interactions (color code: symmetry equivalents); (c) packing of layers (arbitrary colors). Hydrogen atoms not involved in the motifs shown and disorder have been omitted for clarity.
In compound endo-3c, hydrogen-bonded centrosymmetric dimer is formed via N1–H1⋯O2i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the CO2 bridge of the adjacent molecule as a hydrogen bond acceptor with the graph-set motif R22(10) (Figure 5a, Table 3). Dimers are further connected into 3D structure due to C–H⋯O and C=O⋯π interactions. C16–H16⋯O5ii interactions enable the formation of layers along the ac plane between furyl ring and carbonyl oxygen atom of the furan-2-carboxylamino substituent of the adjacent hydrogen-bonded dimer (Figure 5b), while C9=O3⋯π interactions between the acetyl moiety and the furyl ring of the adjacent hydrogen-bonded dimer enable the formation of layers along the ab plane with d(O3⋯Cg1) = 3.5759(19) Å and <(C9=O3⋯Cg1) = 74.16(11)°, where Cg1 is O6/C14–C17 ring centroid (Figure 5c). There are no significant π⋯π interactions.
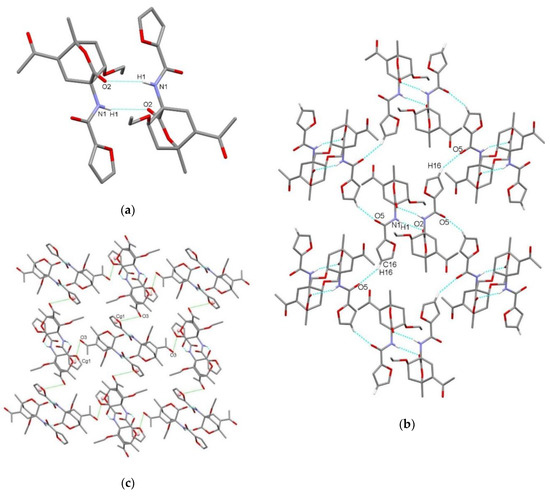
Figure 5.
Supramolecular aggregation of endo-3c. (a) Hydrogen-bonded centrosymmetric dimer formed through N–H⋯O interaction; (b) layer formation along ac plane through the C–H⋯O interactions; (c) layer formation along ab plane through the C=O⋯π interactions. Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
In compound endo-3d, hydrogen-bonded centrosymmetric dimer is formed via N1–H1⋯O2i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the CO2 bridge of the adjacent molecule as a hydrogen bond acceptor with the graph-set motif R22(10) (Figure 6a, Table 3). This interaction is further supported by centrosymmetric C4–H4b⋯π interaction between the bicyclo[2.2.2]octene scaffold and the phenyl ring of the benzoylamino substituent with d(H4b⋯Cg6) = 2.92Å and <(C4–H4b ⋯Cg6) = 149°, where Cg6 is C23–C28 ring centroid. Dimers are further connected into layers along the (–101) plane through the combination of C11–H11⋯O4ii and C18–H18a⋯O4iii interactions connecting phenyl ring of benzoylamino moiety and cyclohexyl substituent, both as hydrogen bond donors, with the carbonyl oxygen atom of the amide group of two different adjacent molecules (Figure 6b), thus carbonyl O4 atom acts as an acceptor of two hydrogen bonds from two different adjacent molecules. Three-dimensional supramolecular structure is achieved through the C27–H27⋯O1iv interactions along c axis between the phenyl ring of benzoylamino moiety and the ring oxygen atom of the CO2 bridge (Figure 6c). There are no significant π⋯π interactions.
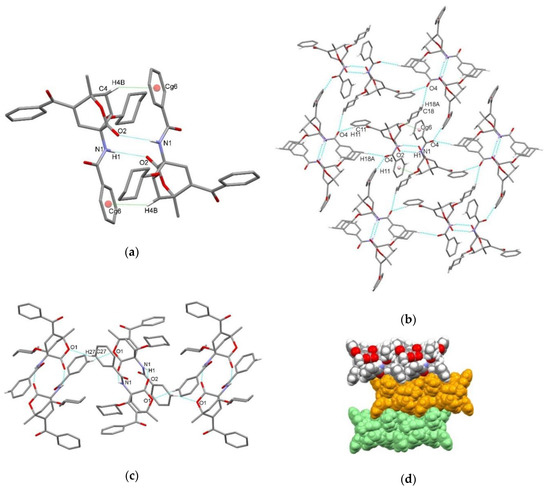
Figure 6.
Supramolecular aggregation of endo-3d. (a) Hydrogen-bonded centrosymmetric dimer formed through N–H⋯O and C–H⋯π interactions; (b) hydrogen-bonded dimers connected into layer through C–H⋯O interactions (C–H⋯π interactions within a dimer are presented only for the central one); (c) C–H⋯O interactions between dimers along c axis; (d) packing of layers (arbitrary colors). Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
In compound endo-3e∙6a, hydrogen-bonded centrosymmetric dimer is formed via N1–H1⋯O4i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the 2-oxopyrrolidin-1-yl group of the adjacent molecule as a hydrogen bond acceptor with the graph-set motif R22(14) (Figure 7a, Table 3). Dimers are further connected into chains along a axis through the combination of centrosymmetric C3–H3⋯O8ii and C9–H9c⋯O8ii interactions connecting acetyl moiety and bicyclo[2.2.2]octene scaffold, both as hydrogen bond donors, with the methoxy oxygen atom of the trimethoxybenzoylamino group of the adjacent molecule, thus methoxy O8 atom acts as an acceptor of two hydrogen bonds from one adjacent molecule (Figure 7a). Interactions between hydrogen-bonded chains of 3e and solvate molecules 6a enable the formation of layers through the C27–H27a⋯O1 and C27–H27b⋯O5iii interactions composed of centrosymmetric hydrogen-bonded tetramer with ABAB pattern with the graph-set motif R44(18) (Figure 7b). There are no significant C–H⋯π and π⋯π interactions.
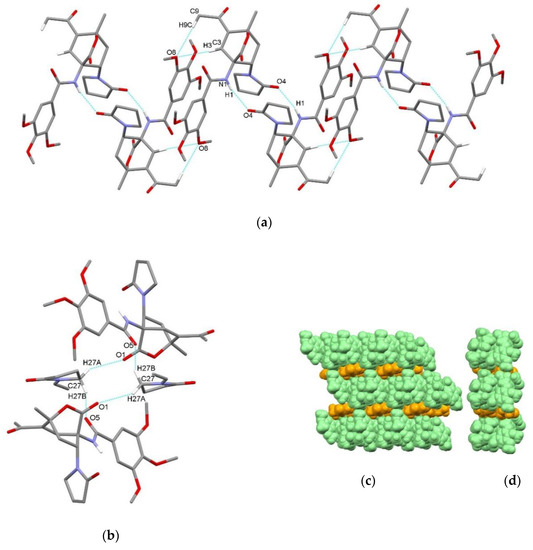
Figure 7.
Supramolecular aggregation of endo-3e∙6a. (a) Hydrogen-bonded centrosymmetric dimers formed through N–H⋯O interactions are further connected through centrosymmetric C–H⋯O interactions; (b) centrosymmetric hydrogen-bonded tetramer composed of two endo-3e molecules and two solvate molecule connected through C–H⋯O interactions; packing of chains of endo-3e (green) and 1-vinyl-2-pyrrolidone (6a) solvate molecules (orange) (c) front and (d) side view. Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
In compound exo-3e, amide NH group is not involved in intermolecular hydrogen bonding; however, it forms intramolecular N2–H2⋯O3 interaction with the carbonyl oxygen atom of the 2-oxopyrrolidin-1-yl substituent. However, centrosymmetric dimer is still formed, in this case via C10–H10c⋯O7i interaction between acetyl substituent and methoxy group of trimethoxybenzoylamino substituent and via C22–H22a⋯O3i interaction between methoxy group and the 2-oxopyrrolidin-1-yl oxygen atom with the graph-set motifs R22(16) and R22(26) (Figure 8a, Table 3). Furthermore, dimers connect into a chain along c axis via centrosymmetric C23–H23c⋯O6ii interaction between methoxy groups of two adjacent molecules (Figure 8a). There are no significant C–H⋯π and π⋯π interactions.
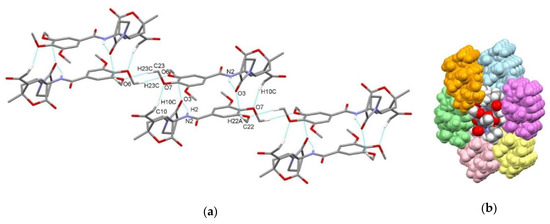
Figure 8.
Supramolecular aggregation of exo-3e. (a) Hydrogen-bonded centrosymmetric dimers formed through C–H⋯O interactions extended into chain through additional centrosymmetric C–H⋯O interactions; (b) packing of chains (color code: arbitrary colors). Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
In compound exo-3f, hydrogen-bonded centrosymmetric dimer is formed via N1–H1⋯O1i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the CO2 bridge of the adjacent molecule as a hydrogen bond acceptor with the graph-set motif R22(10). Dimers are further connected into chain along c axis through centrosymmetric C19–H19b⋯O4ii interactions connecting methyl substituent at the bicyclo[2.2.2]octene skeleton with the carbonyl oxygen atom of the 2-oxopyrrolidin-1-yl substituent of the adjacent molecule with the graph-set motif R22(16) (Figure 9a, Table 3). Chains are further connected into a layer along the (–110) plane through C12–H12⋯O5iii interactions between the phenyl ring of benzoylamino group and the carbonyl oxygen atom of the benzoyl unit (Figure 9b). There are no significant C–H⋯π and π⋯π interactions.
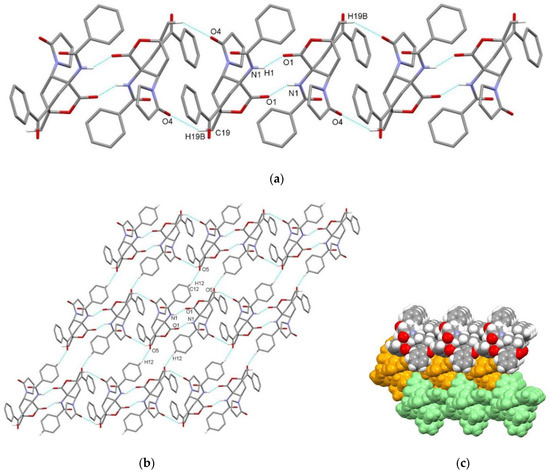
Figure 9.
Supramolecular aggregation of exo-3f. (a) Hydrogen-bonded chain formed through centrosymmetric N–H⋯O and C–H⋯O interactions; (b) chains connected into a layer through C–H⋯O interactions; (c) packing of layers (color code: arbitrary colors). Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
Compound 4a represents a very rare case of a cyclohexadiene system stable at room temperature, albeit containing groups that are prone to elimination (in this case of a molecule of ethanol), which would furnish a benzene derivative, thus achieving aromatic stabilization. Another case from the literature was described by Afarinkia et al. [52]. In compound 4a, hydrogen-bonded chain along b axis is formed via N1–H1⋯O2i interaction between the amide NH group acting as a hydrogen bond donor and the carbonyl oxygen atom of the ester group of the adjacent molecule as a hydrogen bond acceptor supported by C6–H6⋯O2i interaction forming graph-set motif R12(6) (Figure 10a, Table 3). Chains are further connected into a zig-zag layer along bc plain through centrosymmetric C18–H18⋯O4ii interactions between benzoylamino substituents of adjacent molecules with graph-set motif R22(10) (Figure 10b). There are no significant C–H⋯π and π⋯π interactions.
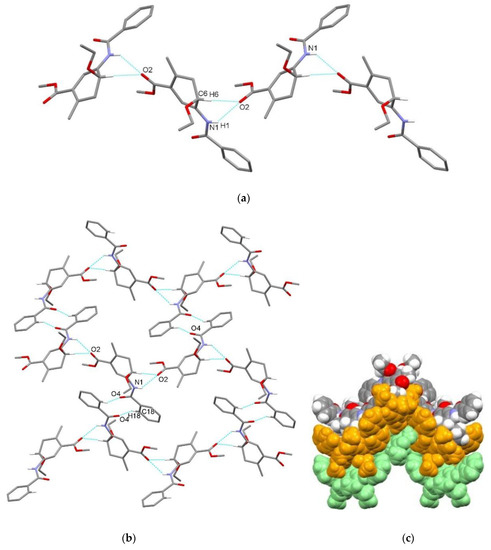
Figure 10.
Supramolecular aggregation of 4a. (a) Hydrogen-bonded chain formed through N–H⋯O and C–H⋯O interactions; (b) chains connected into a layer through centrosymmetric C–H⋯O interactions; (c) packing of zig-zag layers (color code: arbitrary colors). Hydrogen atoms not involved in the motifs shown have been omitted for clarity.
4. Discussion
We have demonstrated that the cycloadditions between 2H-pyran-2-one derivatives 1a–e and various vinyl-moiety-containing dienophiles 2 and 6 in the first step yield oxabicyclo[2.2.2]octene products of the type 3. However, the reaction can be successfully stopped at this step only if it is taking place under mild conditions, temperatures above approximately 50–60 °C cause immediate and irreversible elimination of carbon dioxide via retro-hetero-Diels–Alder reaction and thus cyclohexadiene systems 4 are formed. However, the cycloadditions are sluggish, at best, at ambient conditions, therefore we found that the only possibility to obtain products 3 was to avoid thermal activation of the reaction and instead use activation with high pressure [35,36,39], which is known to accelerate processes having negative activation volumes (among such processes are generally all reactions where two molecules give just one; however, the solvation effects have to be considered as well). Empirically it was established that the majority of Diels–Alder reactions indeed possess negative activation volumes [53,54] and are therefore prone to be accelerated under high pressure conditions. As cycloadditions are equilibrium processes, we applied large excess of the dienophile 2 to push the position of the equilibrium towards the products 3; however, due to the practical reasons of solubility (and to decrease the viscosity), some dichloromethane as a solvent was also necessary.
We have to stress that the crystal structures of the products 3 presented herein are the only 7-oxabicyclo[2.2.2]octenes possessing a substituted amino group at one of the bridgehead atoms described so far, in addition to one publication (describing three structures) by Haufe et al., [22] and our own previous report [17]. It is interesting to note that in all cases where oxygen-based dienophiles 2 (i.e., 2a,b) were used, the only stereoisomer of the products 3 obtained was of the endo type; however, with the nitrogen-based dienophile (i.e., 6a) in one case (i.e., formation of 3f) only exo stereoisomer was obtained, whereas in the other case (i.e., formation of 3e) a mixture of both endo and exo stereoisomers was produced. Obviously, all of the products were obtained as racemic mixtures of both possible enantiomers. As there is no clear correlation between the steric demands of the dienophiles (2 and 6 in combination with 1) and the outcome of these cycloadditions, it is appropriate to propose that they are governed not only by the steric hindrance between the groups of the reacting molecules, but also by their electronic properties (i.e., change of stereoselectivity when oxygen dienophiles 2 are applied instead of their nitrogen analogs 6). However, the regioselectivity of all these cycloadditions was complete, in all cases furnishing only products 3, where the group formerly bound at position 3 in the starting 2H-pyran-2-ones 1 is in the product adjacent to the group stemming from the dienophiles 2 or 6, meaning that exclusively products 3 and no 3’ are formed (see Figure 2).
The rigid 7-oxabicyclo[2.2.2]octene skeleton in compounds 3 leads to structural variations only due to rotations of side arms or to exo/endo orientation of substituents at position 8 as can be seen from the superposition of molecular structures (Figure 11a). At position 6, acetyl substituents incline in respect to the 7-oxabicyclo[2.2.2]octene skeleton with torsion angle Φ1 (see Figure 11b) in the range −179.14(15)° (for endo-3a) and –137.4(2)° (for endo-3e), while benzoyl substituents in endo-3d and exo-3f incline by 138.6(3)° and 138.9(2)°, respectively (Figure 11a). Inclination of amide group at position 4 shows much smaller differences with torsion angle Φ2 being in the range 65.0(4)–67.3(4)° for all structures except for exo-3f where torsion angle is much larger (172.01(17)°).
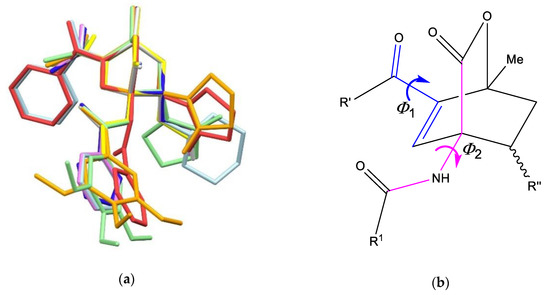
Figure 11.
(a) Superposition of compounds 3. Hydrogen atoms and disorder in endo-3b have been omitted for clarity. Color code: endo-3a (violet), endo-3b molecule A (yellow), endo-3b molecule B (light grey), endo-3c (blue), endo-3d (light blue), endo-3e (light green), exo-3e (orange), exo-3f (red); (b) torsion angles for substituents at the positions 6 and 4.
In all compounds 3, amide NH is the only classical hydrogen bond donor and is involved in intermolecular hydrogen bonding except in compound exo-3e, which forms intramolecular interaction with the 2-oxopyrrolidin-1-yl substituent. When participating in intermolecular interactions diverse patterns can be expected for the amide group. For example, chains could be formed between amide units acting both as hydrogen bond donors and acceptors (see Figure 12a,b). However, among the compounds investigated in this series predominates the hydrogen bond formation between amide NH group and CO2 bridge of the adjacent 7-oxabicyclo[2.2.2]octene molecule as observed in five out of seven cases (endo-3a, endo-3b, endo-3c, endo-3d and exo-3f). In most cases, centrosymmetric hydrogen-bonded dimers are formed (Figure 12c), while in endo-3b a centrosymmetric hydrogen-bonded tetramer is formed with a combination of the interaction between amide NH group and CO2 bridge of the adjacent molecule and the interaction of the amide NH group with the amide carbonyl group. Such interactions in endo-3b are most probably possible due to the presence of two crystallographically independent molecules and/or due to the presence of sterically less demanding acetylamino group (in comparison with benzoylamino) at position 4. On the other hand, two different patterns were found in 3e (Figure 12d,e), in exo-3e, NH group is involved in intramolecular interaction with the 2-oxopyrrolidin-1-yl substituent. Such intramolecular interaction could be expected in all 2-oxopyrrolidin-1-yl-containing compounds 3 (endo-3e∙6a, exo-3e, exo-3f) since carbonyl group can be positioned suitably for the interaction with the NH group due to the free rotation of the C–Npyrrolidone bond. However, this is not the case in endo-3e∙6a and also not in the case of exo-3f even though the 2-oxopyrrolidin-1-yl substituent is also in exo position as is in exo-3e. While in exo-3e, NH group forms intramolecular interaction with the 2-oxopyrrolidin-1-yl substituent, the situation is different in endo-3e∙6a where NH group forms intermolecular interactions with the 2-oxopyrrolidin-1-yl substituent of the adjacent molecule. These findings imply that the exo/endo orientation of the 2-oxopyrrolidin-1-yl substituent is not the governing factor for the formation of hydrogen bonding with the NH group. It is worth noting that the carbonyl oxygen atom of CO2 bridge is not involved as a hydrogen bond acceptor only in exo-3e, whereas in all of the other structures it is involved either in NH⋯O or CH⋯O interactions. Another interesting peculiarity of structures 3 is that ring oxygen atom of the CO2 bridge is a hydrogen bond acceptor only in the structure of endo-3d.
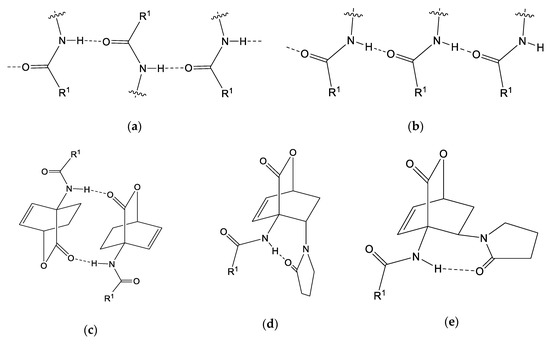
Figure 12.
Possible hydrogen-bonded motifs formed by amide NH group. (a,b) chain formation through the amide groups as hydrogen bond donors and acceptors; (c) centrosymmetric hydrogen-bonded dimers with NH and CO2 groups as hydrogen bond donor and acceptor, respectively; (d) possible intramolecular hydrogen bonding with 2-oxopyrrolidin-1-yl in endo position; (e) possible intramolecular hydrogen bonding with 2-oxopyrrolidin-1-yl in exo position.
Complete regioselectivity of the first cycloaddition step is also reflected in the formation of 4a as the only regioisomer, again of the type having the group stemming from the dienophile 2, and the group being in the starting 2H-pyran-2-one 1f at the position 3, in 1,2-position. Therefore, cyclohexadiene product obtained was only of the type 4 (and no 4’ was detected, see Figure 1). Understandably, 4a was formed as a racemic mixture of both possible enantiomers. Amide NH in 4a is the only classical hydrogen bond donor and enables, in combination with centrosymmetric C–H⋯O interactions, the formation of hydrogen bonded layers.
5. Conclusions
We have carried out a detailed structural elucidation of cycloadducts formed between 2H-pyran-2-one derivatives 1 and various vinyl-moiety-containing dienophiles (ethyl vinyl ether, cyclohexyl vinyl ether and 1-vinyl-2-pyrrolidone) with oxabicyclo[2.2.2]octene skeleton (products of the type 3) obtained only under high pressure. In all cases where oxygen-based dienophiles 2 were used (ethyl vinyl ether and cyclohexyl vinyl ether), the only stereoisomer of the products 3 obtained was of the endo type; however, with the nitrogen-based dienophile (1-vinyl-2-pyrrolidone) in one case (i.e., formation of 3f) only exo stereoisomer was obtained, whereas in the other cases (i.e., formation of 3e) a mixture of both endo and exo stereoisomers was produced. All of the products 3 were obtained as racemic mixtures of both possible enantiomers. However, the regioselectivity of all these cycloadditions was complete, in all cases furnishing only products 3, where the group formerly bound at position 3 in the starting 2H-pyran-2-ones 1 is in the product adjacent to the group stemming from the dienophiles 2 or 6, meaning that exclusively products 3 and no 3’ are formed.
In all 7-oxabicyclo[2.2.2]octene compounds 3, amide NH is the only classical hydrogen bond donor and is involved in intermolecular hydrogen bonding except in compound exo-3e where intramolecular interaction is formed. The predominant motif is the intermolecular hydrogen bonding between amide NH group and CO2 bridge of the adjacent 7-oxabicyclo[2.2.2]octene molecule as observed in five out of seven cases (endo-3a, endo-3b, endo-3c, endo-3d and exo-3f) with the domination of centrosymmetric hydrogen-bonded dimers. Diverse supramolecular patterns were observed due to rich variety of C–H⋯O and C–H⋯π interactions while no significant π⋯π interactions were found.
Finally, complete regioselectivity of the first cycloaddition step is also reflected in the formation of 4a as the only regioisomer (albeit as a racemic mixture of both enantiomers). This compound represents a very rare case of a cyclohexadiene system stable at room temperature, even though it contains groups that are prone to elimination (here a molecule of ethanol could be eliminated), which would provide a benzene derivative, thus reaching aromatic stabilization. Additionally, in this case, supramolecular aggregation has been achieved through N–H⋯O and C–H⋯O interactions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-8994/12/10/1714/s1, cif-files with single-crystal XRD structures of 3a–f and 4a.
Author Contributions
Conceptualization, K.K., M.K., F.P.; methodology, K.K., M.K., F.P.; syntheses, A.J. and K.K.; formal analysis, A.J., K.K.; X-ray diffraction, F.P.; writing—original draft preparation, K.K. and F.P.; writing—review and editing, K.K., F.P.; visualization, K.K., F.P.; funding acquisition, M.K., F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science and Sport of the Republic of Slovenia and the Slovenian Research Agency, grant numbers P1-0230-0103 and P1-0230-0175.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dyan, O.T.; Borodin, G.I.; Zaikin, P.A. The Diels–Alder reaction for the synthesis of polycyclic aromatic compounds. Eur. J. Org. Chem. 2019, 7271–7306. [Google Scholar] [CrossRef]
- Mancini, P.M.E.; Ormachea, C.M.; Kneeteman, M.N. Polar Diels–Alder reactions under microwave irradiation employing different heterocyclic compounds as electrophiles. Mini-Rev. Org. Chem. 2019, 16, 527–543. [Google Scholar] [CrossRef]
- Harada, S.; Nishida, A. Catalytic and enantioselective Diels–Alder reactions of siloxydienes. Asian J. Org. Chem. 2019, 8, 732–745. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Asymmetric organocatalytic reactions of α,β-unsaturated cyclic ketones. Symmetry 2011, 3, 84–125. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Koprowski, M.; Drzazga, Z.; Parcheta, R.; Łastawiecka, E.; Demchuk, O.M.; Justyniak, I. Efficient oxidative resolution of 1-phenylphosphol-2-ene and Diels–Alder synthesis of enantiopure bicyclic and tricyclic P-stereogenic C-P heterocycles. Symmetry 2020, 12, 346. [Google Scholar] [CrossRef]
- Rádai, Z.; Bagi, P.; Czugler, M.; Karaghiosoff, K.; Keglevich, G. Optical resolution of dimethyl α-hydroxy-arylmethylphosphonates via diastereomer complex formation using calcium hydrogen O,O′-dibenzoyl-(2R,3R)-tartrate; X-ray analysis of the complexes and products. Symmetry 2020, 12, 758. [Google Scholar] [CrossRef]
- Soai, K.; Kawasaki, T.; Matsumoto, A. Role of asymmetric autocatalysis in the elucidation of origins of homochirality of organic compounds. Symmetry 2019, 11, 694. [Google Scholar] [CrossRef]
- Hoffmann, R.; Woodward, R.B. The conservation of orbital symmetry. Acc. Chem. Res. 1968, 1, 17–22. [Google Scholar] [CrossRef]
- Woodward, R.B.; Hoffmann, R. Selection rules for concerted cycloaddition reactions. J. Am. Chem. Soc. 1965, 87, 2046–2048. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic aspects of Diels–Alder reactions: A critical survey. Angew. Chem. Int. Ed. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Uemura, N.; Toyoda, S.; Shimizu, W.; Yoshida, Y.; Mino, T.; Sakamoto, M. Absolute asymmetric synthesis involving chiral symmetry breaking in Diels–Alder reaction. Symmetry 2020, 12, 910. [Google Scholar] [CrossRef]
- Kitagawa, D.; Bardeen, C.J.; Kobatake, S. Symmetry breaking and photomechanical behavior of photochromic organic crystals. Symmetry 2020, 12, 1478. [Google Scholar] [CrossRef]
- Ram, V.J.; Srivastava, P. Pyran-2-ones as a versatile synthon for the synthesis of diverse arenes and heteroarenes. Curr. Org. Chem. 2001, 5, 571–599. [Google Scholar] [CrossRef]
- Afarinkia, K.; Vinader, V.; Nelson, T.D.; Posner, G.H. Diels–Alder cycloadditions of 2-pyrones and 2-pyridones. Tetrahedron 1992, 48, 9111–9171. [Google Scholar] [CrossRef]
- Štefane, B.; Perdih, A.; Pevec, A.; Šolmajer, T.; Kočevar, M. The participation of 2H-pyran-2-ones in [4 + 2] cycloadditions: An experimental and computational study. Eur. J. Org. Chem. 2010, 5870–5883. [Google Scholar] [CrossRef]
- Kranjc, K.; Kočevar, M. Ethyl vinyl ether as a synthetic equivalent of acetylene in a DABCO-catalyzed microwave-assisted Diels–Alder–elimination reaction sequence starting from 2H-pyran-2-ones. Synlett 2008, 2613–2616. [Google Scholar] [CrossRef]
- Juranovič, A.; Kranjc, K.; Perdih, F.; Polanc, S.; Kočevar, M. Comparison of the reaction pathways and intermediate products of a microwave-assisted and high-pressure-promoted cycloaddition of vinyl-moiety-containing dienophiles on 2H-pyran-2-ones. Tetrahedron 2011, 67, 3490–3500. [Google Scholar] [CrossRef]
- Cole, C.J.F.; Fuentes, L.; Snyder, S.A. Asymmetric pyrone Diels–Alder reactions enabled by dienamine catalysis. Chem. Sci. 2020, 11, 2175–2180. [Google Scholar] [CrossRef]
- Geist, E.; Berneaud-Kötz, H.; Baikstis, T.; Dräger, G.; Kirschning, A. Toward chromanes by de novo construction of the benzene ring. Org. Lett. 2019, 21, 8930–8933. [Google Scholar] [CrossRef]
- Grant, P.S.; Brimble, M.A.; Furkert, D.P. Synthesis of the bicyclic lactone core of leonuketal, enabled by a telescoped Diels–Alder reaction sequence. Chem. Asian J. 2019, 14, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, I.S.; Tolmachova, N.A.; Dolovanyuk, V.G.; Gerus, I.I.; Daniliuc, C.-G.; Haufe, G. Synthesis of trifluoromethyl-containing polysubstituted aromatic compounds by Diels–Alder reaction of ethyl 3-benzamido-2-oxo-6-(trifluoromethyl)-2H-pyran-5-carboxylate. Eur. J. Org. Chem. 2015, 2482–2491. [Google Scholar] [CrossRef]
- Kondratov, I.S.; Tolmachova, N.A.; Dolovanyuk, V.G.; Gerus, I.I.; Bergander, K.; Daniliuc, C.-G.; Haufe, G. Diels–Alder reaction of ethyl 3-benzamido-2-oxo-6-(trifluoromethyl)-2H-pyran-5-carboxylate with alkoxyalkenes as an effective approach to trifluoromethyl-containing 3-aminobenzoic acid derivatives. Eur. J. Org. Chem. 2014, 2443–2450. [Google Scholar] [CrossRef]
- Burch, P.; Binaghi, M.; Scherer, M.; Wentzel, C.; Bossert, D.; Eberhardt, L.; Neuburger, M.; Scheiffele, P.; Gademann, K. Total synthesis of gelsemiol. Chem. Eur. J. 2013, 19, 2589–2591. [Google Scholar] [CrossRef] [PubMed]
- Bartelson, K.J.; Singh, R.P.; Foxman, B.M.; Deng, L. Catalytic asymmetric [4 + 2] additions with aliphatic nitroalkenes. Chem. Sci. 2011, 2, 1940–1944. [Google Scholar] [CrossRef]
- Afarinkia, K.; Abdullahi, M.H.; Scowen, I.J. A new, general method for the synthesis of carbasugar-sugar pseudodisaccharides. Org. Lett. 2009, 11, 5182–5184. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, Y.-Q.; Liu, Y.; Foxman, B.M.; Deng, L. Asymmetric Diels–Alder reactions of 2-pyrones with a bifunctional organic catalyst. J. Am. Chem. Soc. 2007, 129, 6364–6365. [Google Scholar] [CrossRef]
- Shimo, T.; Yasutake, M.; Shinmyozu, T.; Somekawa, K. Crystal structure of methyl endo-8-cyano-exo-8-methyl-3-oxo-2-oxabicyclo[2.2.2]oct-5-ene-6-carboxylate. Anal. Sci. 2003, 19, 471–472. [Google Scholar] [CrossRef]
- Zhang, H.; Appels, D.C.; Hockless, D.C.R.; Mander, L.N. A new approach to the total synthesis of the unusual diterpenoid tropone, harringtonolide. Tetrahedron Lett. 1998, 39, 6577–6580. [Google Scholar] [CrossRef]
- Hren, J.; Polanc, S.; Kočevar, M. The synthesis and transformations of fused bicyclo[2.2.2]octenes. Arkivoc 2008, i, 209–231. [Google Scholar] [CrossRef]
- Kranjc, K.; Kočevar, M. Green chemistry starting from 2H-pyran-2-one derivatives. Curr. Green Chem. 2014, 1, 202–215. [Google Scholar] [CrossRef]
- Kranjc, K.; Kočevar, M. Regio- and stereoselective syntheses and cycloadditions of substituted 2H-pyran-2-ones and their fused derivatives. Arkivoc 2013, i, 333–363. [Google Scholar] [CrossRef]
- Kranjc, K.; Polanc, S.; Kočevar, M. Diels–Alder reactions of fused pyran-2-ones with maleimides: Efficient syntheses of benz[e]isoindoles and related systems. Org. Lett. 2003, 5, 2833–2836. [Google Scholar] [CrossRef]
- Kranjc, K.; Perdih, F.; Kočevar, M. Effect of ring size on the exo/endo selectivity of a thermal double cycloaddition of fused pyran-2-ones. J. Org. Chem. 2009, 74, 6303–6306. [Google Scholar] [CrossRef] [PubMed]
- Krivec, M.; Gazvoda, M.; Kranjc, K.; Polanc, S.; Kočevar, M. A way to avoid using precious metals: The application of high-surface activated carbon for the synthesis of isoindoles via the Diels–Alder reaction of 2H-pyran-2-ones. J. Org. Chem. 2012, 77, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Gladysz, J.A.; Lee, S.J.; Tomasello, J.A.V.; Yu, Y.S. High-pressure cycloadditions of pyrones: Synthesis of highly functionalized six-membered rings by inhibition of carbon dioxide loss. J. Org. Chem. 1977, 42, 4171–4172. [Google Scholar] [CrossRef]
- Klärner, F.-G.; Breitkopf, V. The effect of pressure on retro Diels–Alder reactions. Eur. J. Org. Chem. 1999, 2757–2762. [Google Scholar] [CrossRef]
- Uroos, M.; Pitt, P.; Harwood, L.M.; Lewis, W.; Blake, A.J.; Hayes, C.J. Total synthesis of (–)-aritasone via the ultra-high pressure hetero-Diels–Alder dimerisation of (–)-pinocarvone. Org. Biomol. Chem. 2017, 15, 8523–8528. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Prasad, D.L.V.K.; Yadav, A.; Yadav, K. On the solvent- and temperature-driven stereoselectivity of the Diels–Alder cycloaddition reactions of furan with maleic anhydride and maleimide. J. Phys. Org. Chem. 2020, e4131. [Google Scholar] [CrossRef]
- Tsypysheva, I.P.; Borisevich, S.S.; Lobov, A.N.; Kovalskaya, A.V.; Shamukaev, V.V.; Safiullin, R.L.; Khursan, S.L. Inversion of diastereoselectivity under high pressure conditions: Diels–Alder reactions of 12-N-substituted derivatives of (–)-cytisine with N-phenylmaleimide. Tetrahedron Asymmetry 2015, 26, 732–737. [Google Scholar] [CrossRef]
- Webb, J.N.; Marsden, P.S.; Raw, A.S. Rhodium(III)-catalyzed C–H activation/annulation with vinyl esters as an acetylene equivalent. Org. Lett. 2014, 16, 4718. [Google Scholar] [CrossRef]
- Toure, M.; Jaime-Figueroa, S.; Burslem, G.M.; Crews, C.M. Expeditious synthesis of isoquinolones and isocoumarins with a vinyl borane as an acetylene equivalent. Eur. J. Org. Chem. 2016, 4171–4175. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Gavezzotti, A. Pillars of crystal engineering: Crystal energies and symmetry operators. CrystEngComm 2018, 20, 2511–2518. [Google Scholar] [CrossRef]
- Lengauer, H.; Makuc, D.; Šterk, D.; Perdih, F.; Pichler, A.; Trdan Lušin, T.; Plavec, J.; Časar, Z. Co-crystals, salts or mixtures of both? The case of tenofovir alafenamide fumarates. Pharmaceutics 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, D.S.; Il’in, M.V.; Suslonov, V.V.; Novikov, A.S. Symmetrical noncovalent interactions Br⋯Br observed in crystal structure of exotic primary peroxide. Symmetry 2020, 12, 637. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Kappe, C.O. My twenty years in microwave chemistry: From kitchen ovens to microwaves that aren’t microwaves. Chem. Rec. 2019, 19, 15–39. [Google Scholar] [CrossRef]
- Sarotti, A.M.; Pisano, P.L.; Pellegrinet, S.C. A facile microwave-assisted Diels–Alder reaction of vinylboronates. Org. Biomol. Chem. 2010, 8, 5069–5073. [Google Scholar] [CrossRef]
- Tajti, Á.; Szatmári, E.; Perdih, F.; Keglevich, G.; Bálint, E. Microwave-assisted Kabachnik–Fields reaction with amino alcohols as the amine component. Molecules 2019, 24, 1640. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Afarinkia, K.; Abdullahi, M.H.; Scrowen, I.J. A synthesis of carbasugar–sugar pseudodisaccharides via a cycloaddition–cycloreversion reaction of 2H-pyran-2-ones. Org. Lett. 2010, 12, 5564–5566. [Google Scholar] [CrossRef]
- Drljaca, A.; Hubbard, C.D.; van Eldik, R.; Asano, T.; Basilevsky, M.V.; le Noble, W.J. Activation and reaction volumes in solution. 3. Chem. Rev. 1998, 98, 2167–2289. [Google Scholar] [CrossRef] [PubMed]
- Van Eldik, R.; Asano, T.; le Noble, W.J. Activation and reaction volumes in solution. 2. Chem. Rev. 1989, 89, 549–688. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).