α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications
Abstract
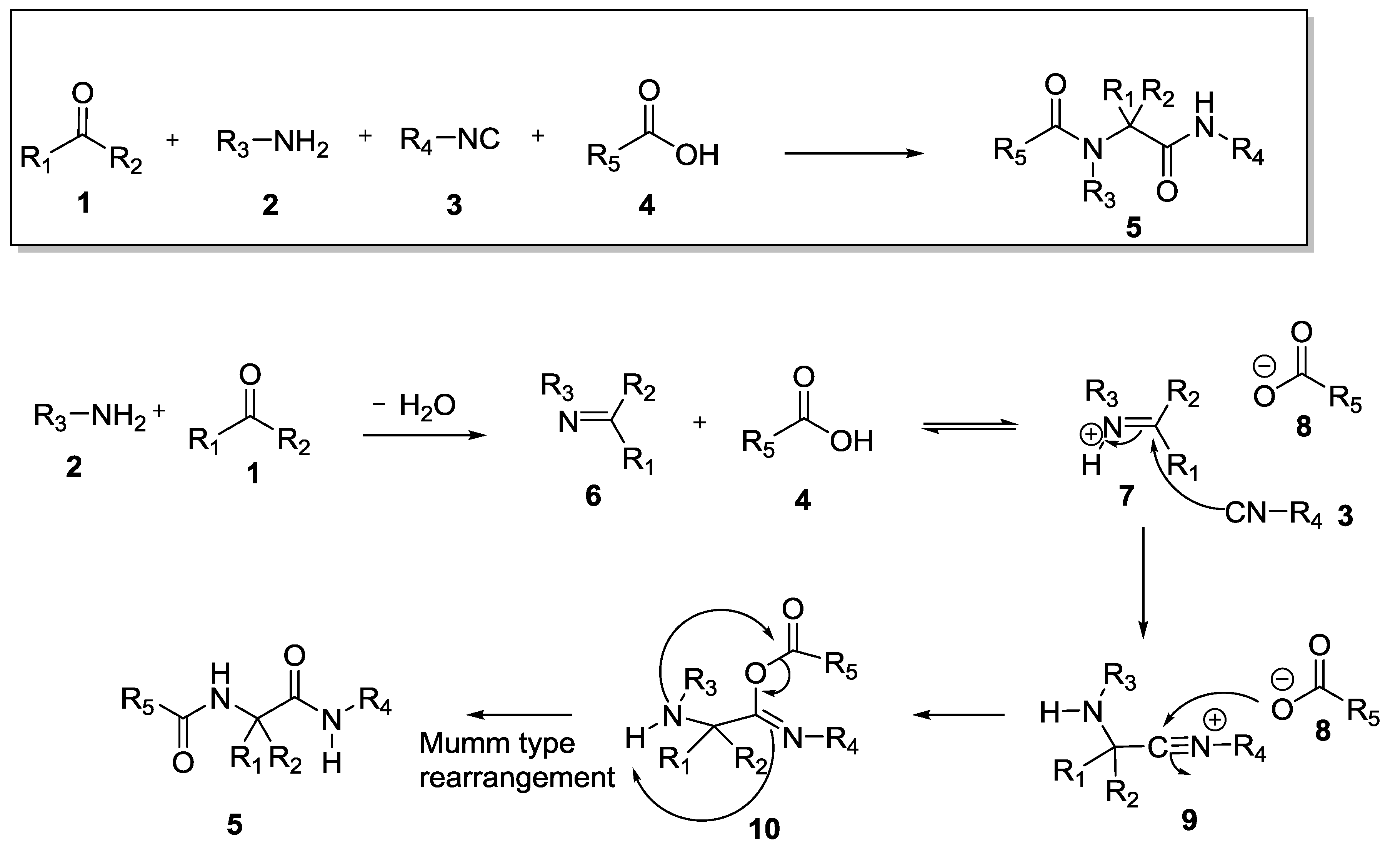
1. Introduction
2. Linear Compounds
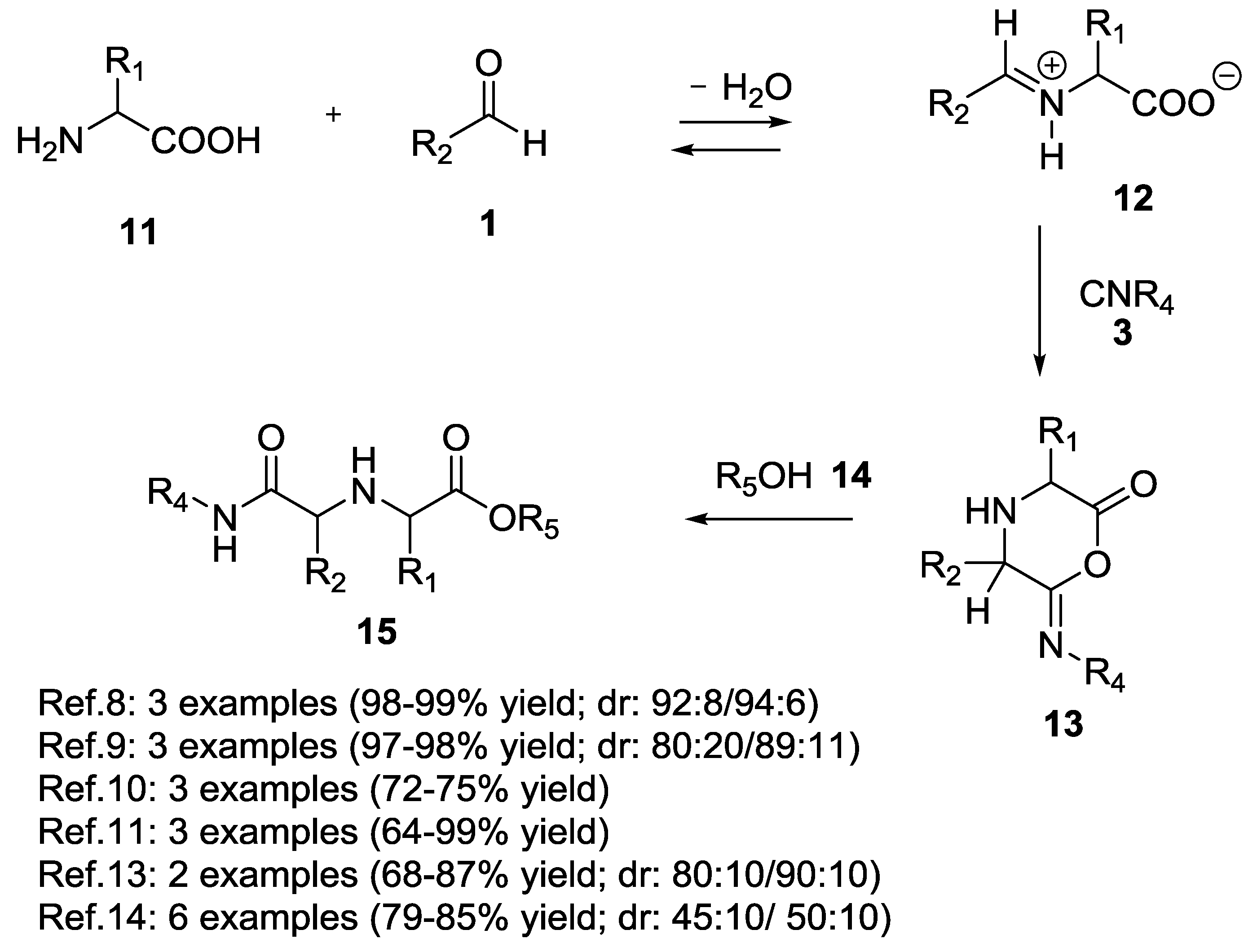
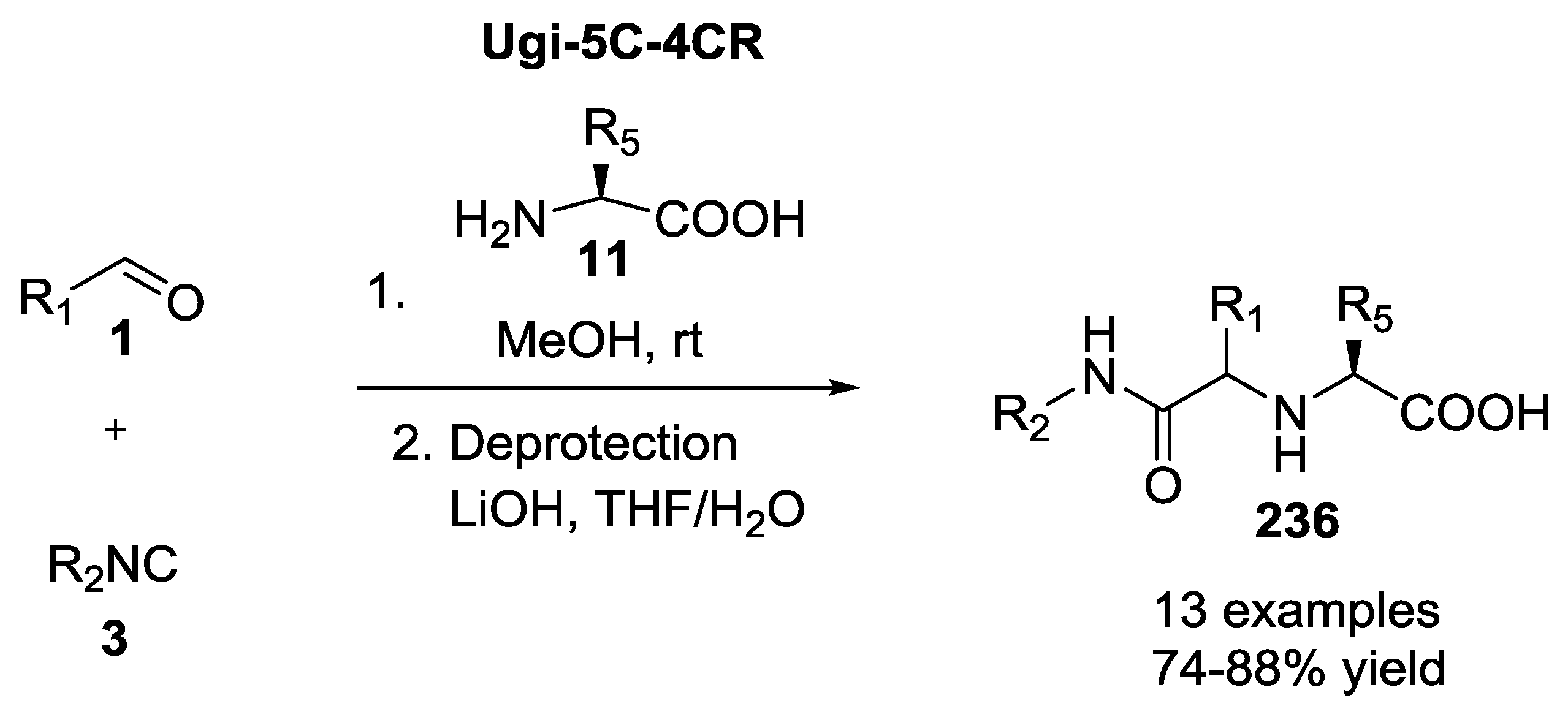
2.1. Ugi 5C-4CR Using α-Amino Acids, Aldehydes, Chloroaldehydes and Ketones
2.2. Tandem Ugi-asserini Reactions Involving α-Amino Acids
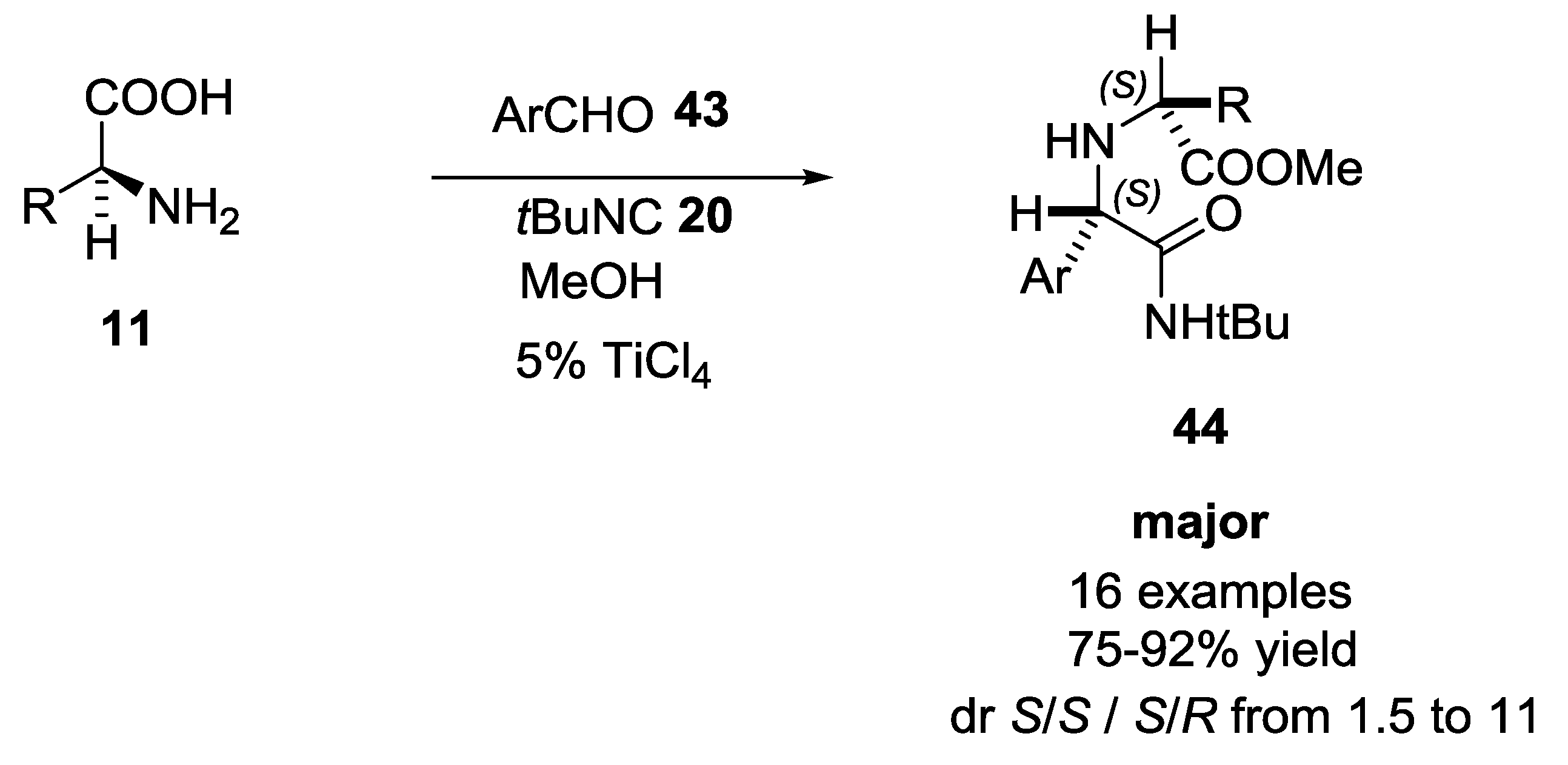
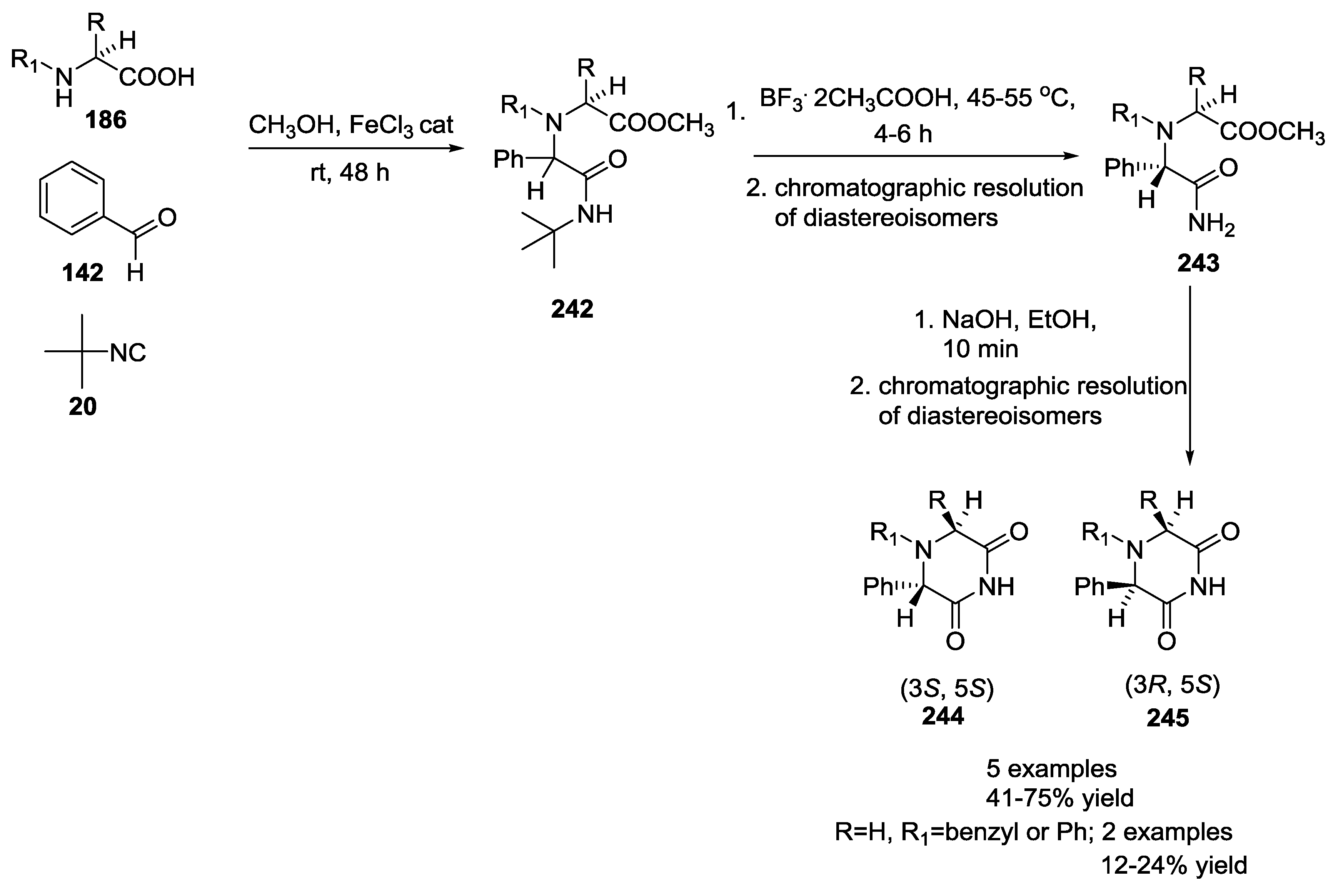
2.3. α-Amino Acids as Chiral Auxiliaries in the U-5C-4CR Reaction
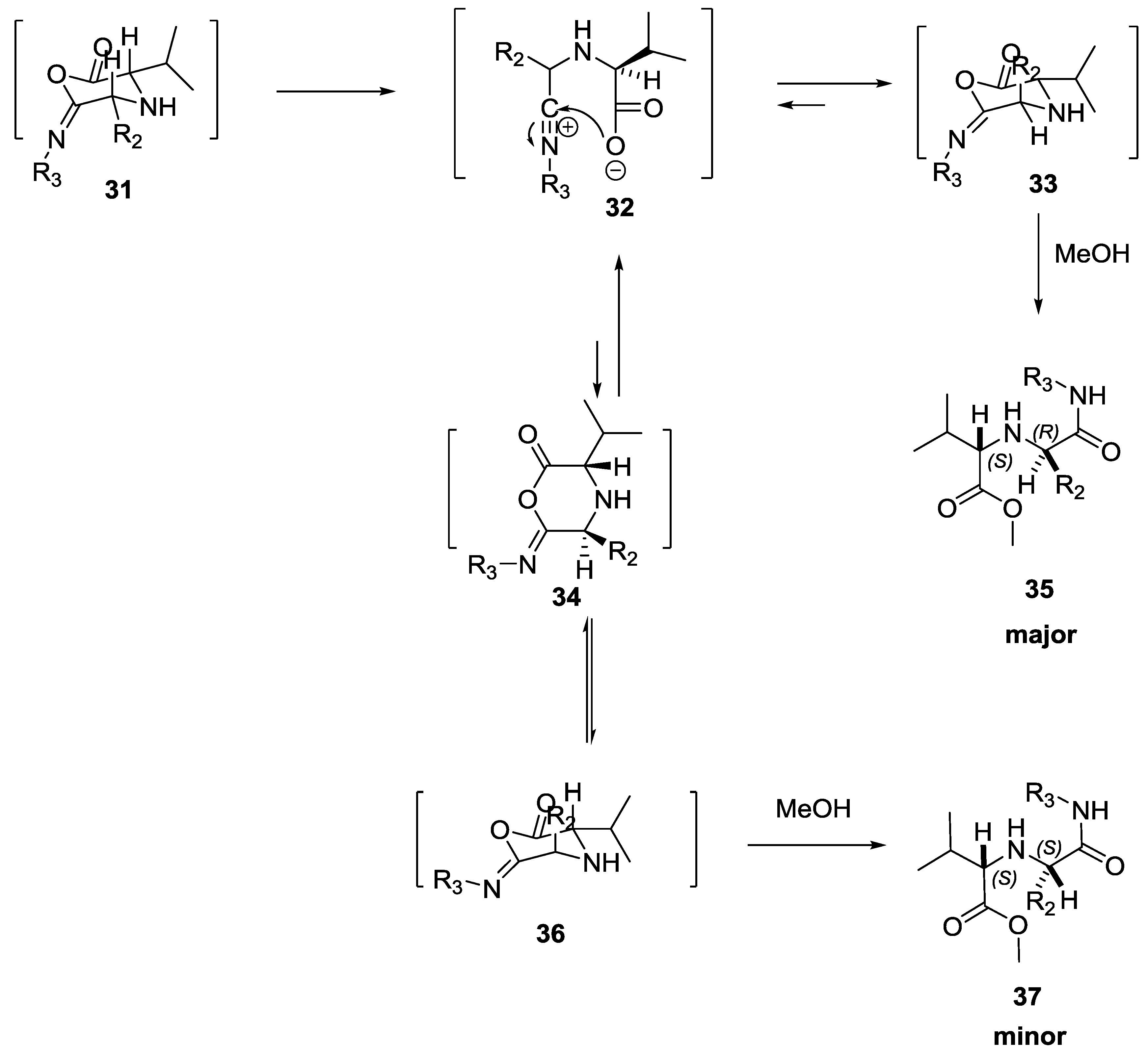
2.4. Diastereoselectivity in the U-5C-4CR Reaction
2.5. Use of TiCl4 as Lewis Acid in the U-5C-4CR Reaction
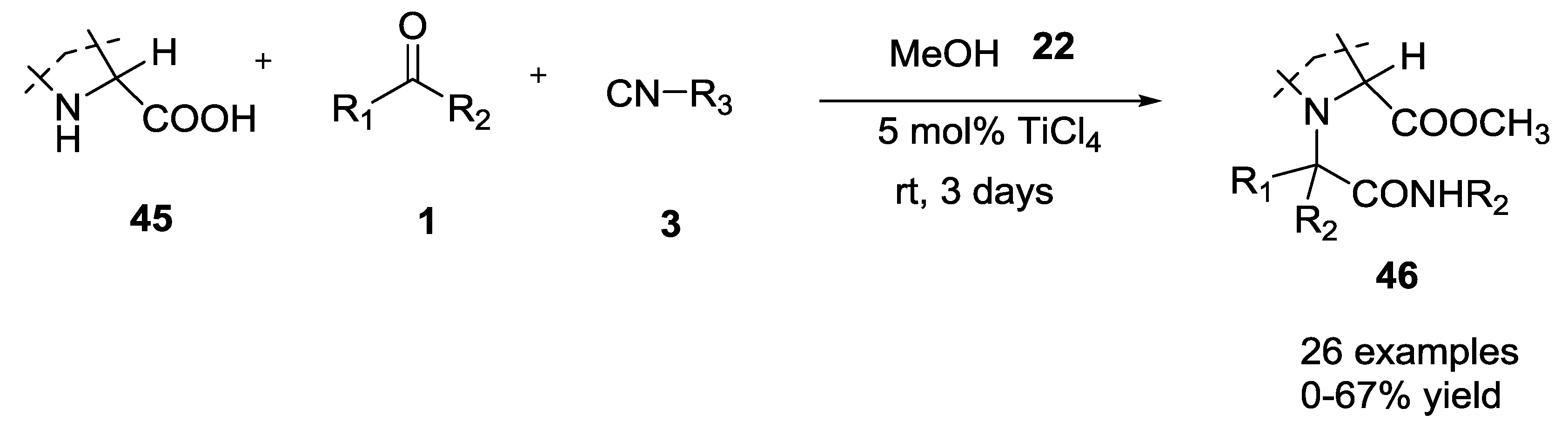
2.6. Ketones and Secondary α-Amino Acids in the U-5C-4CR Reaction
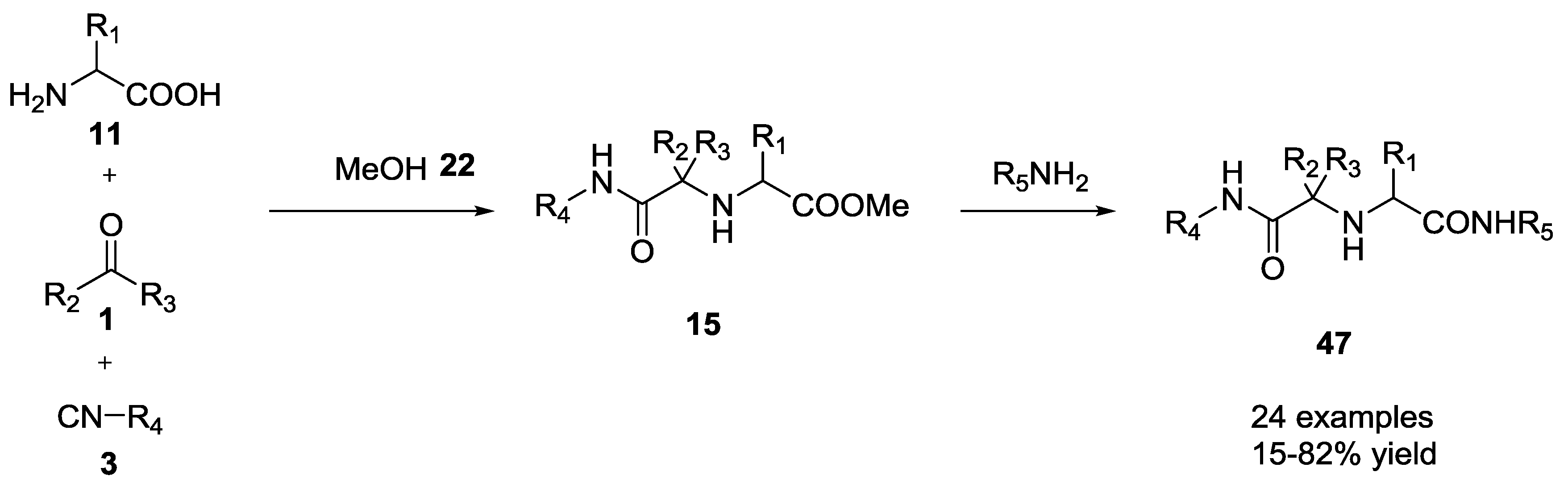
2.7. Direct Conversion of Ugi Ester Scaffold to Amide
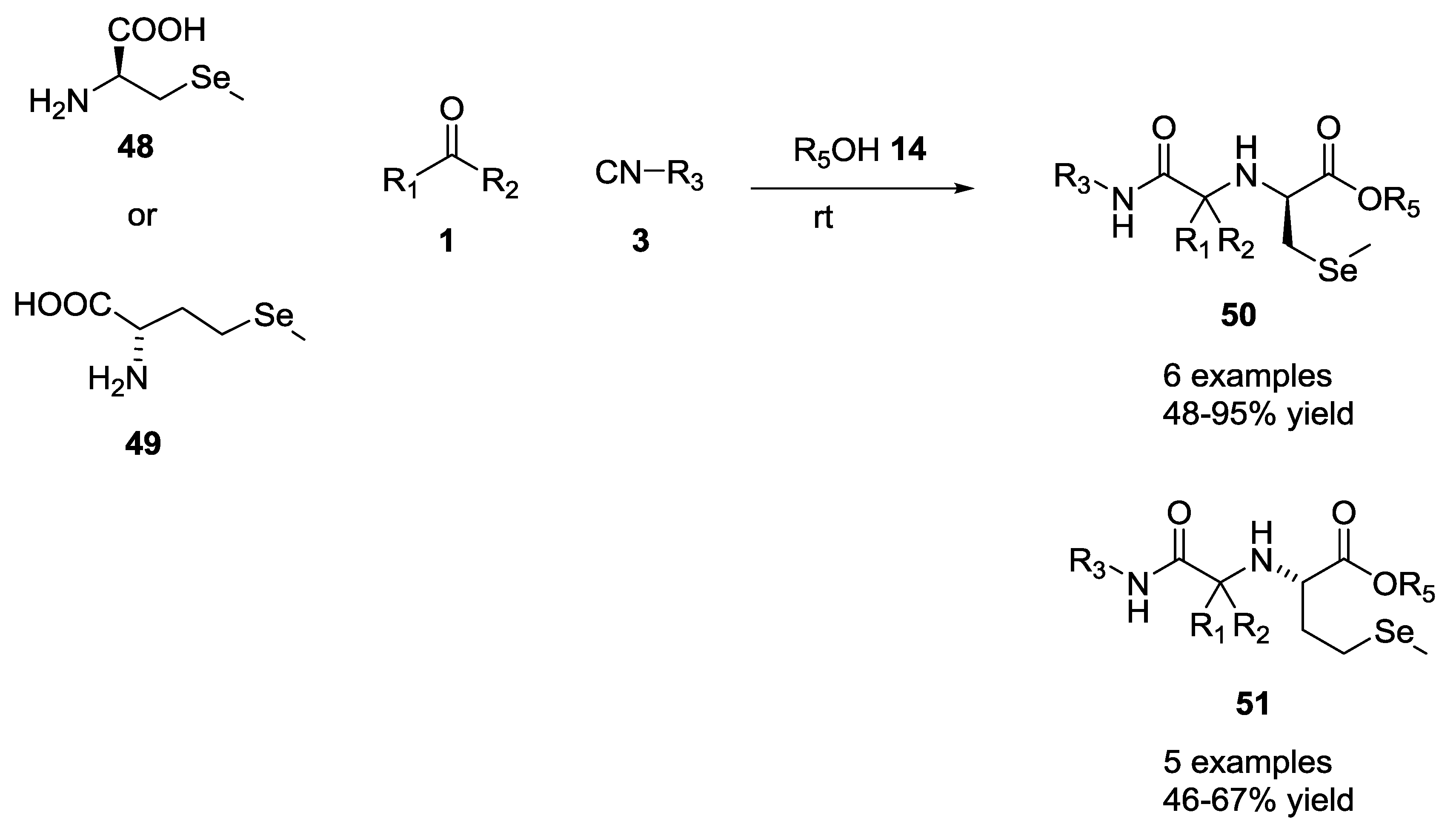
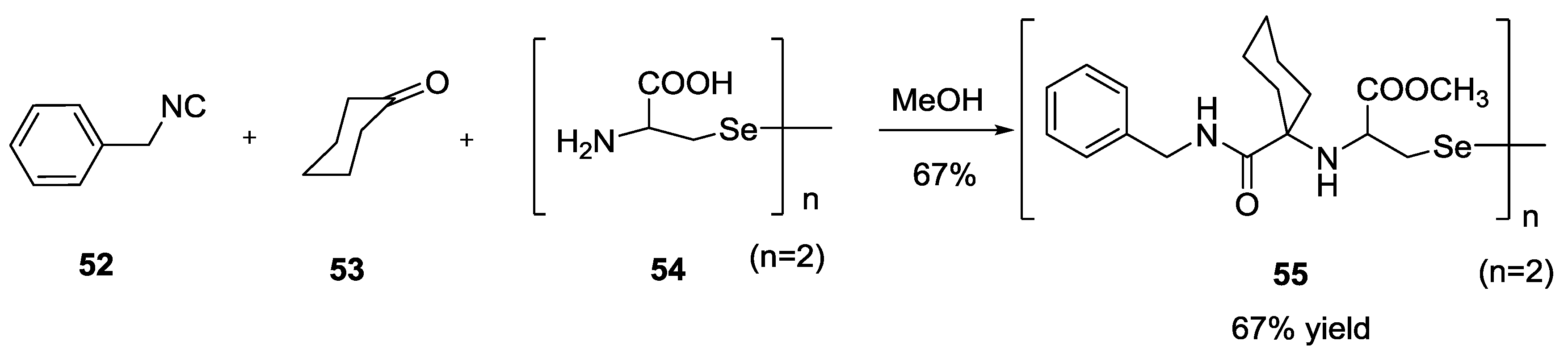
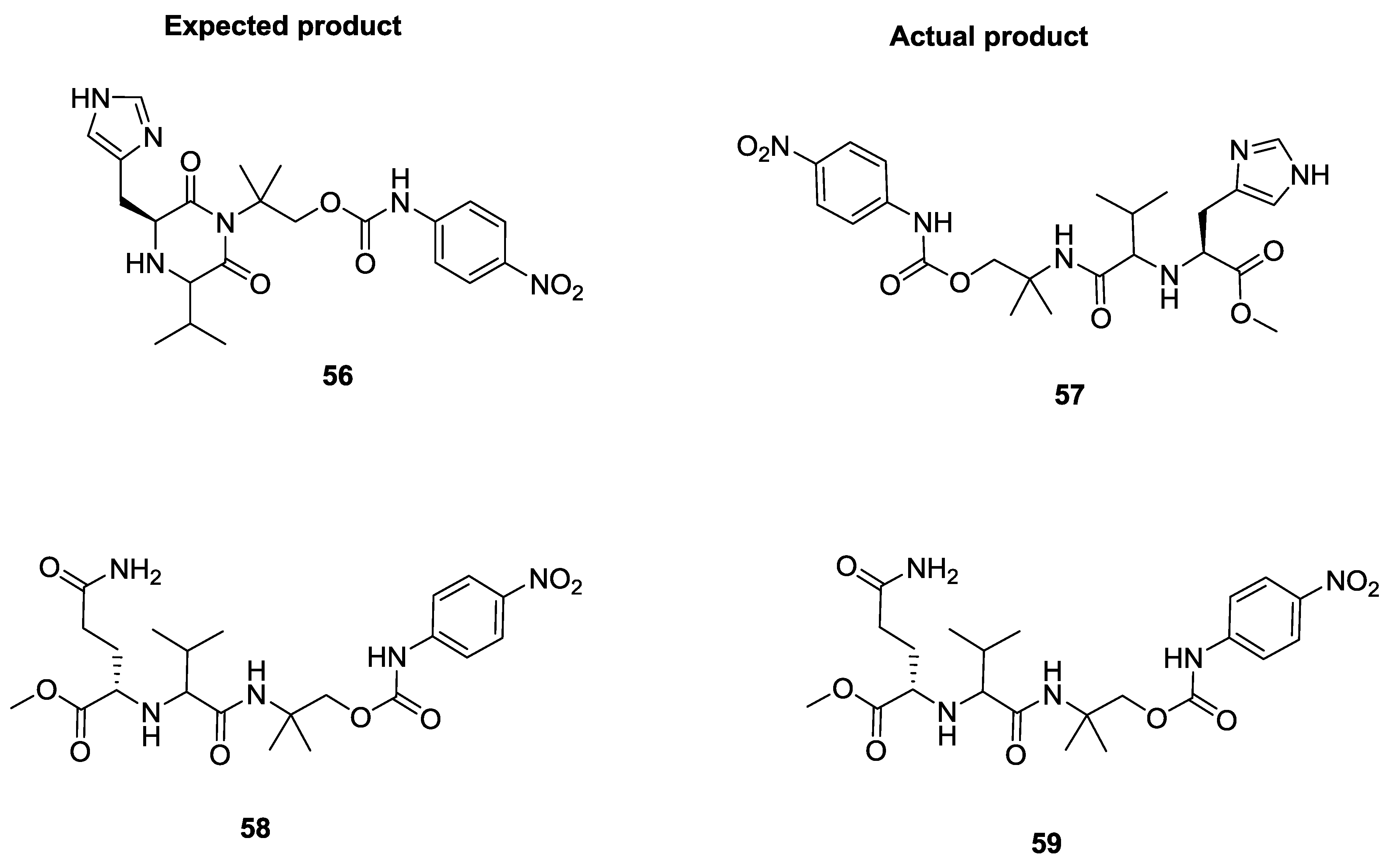
2.8. Selenium α-Amino Acids in the U-5C-4CR Reaction
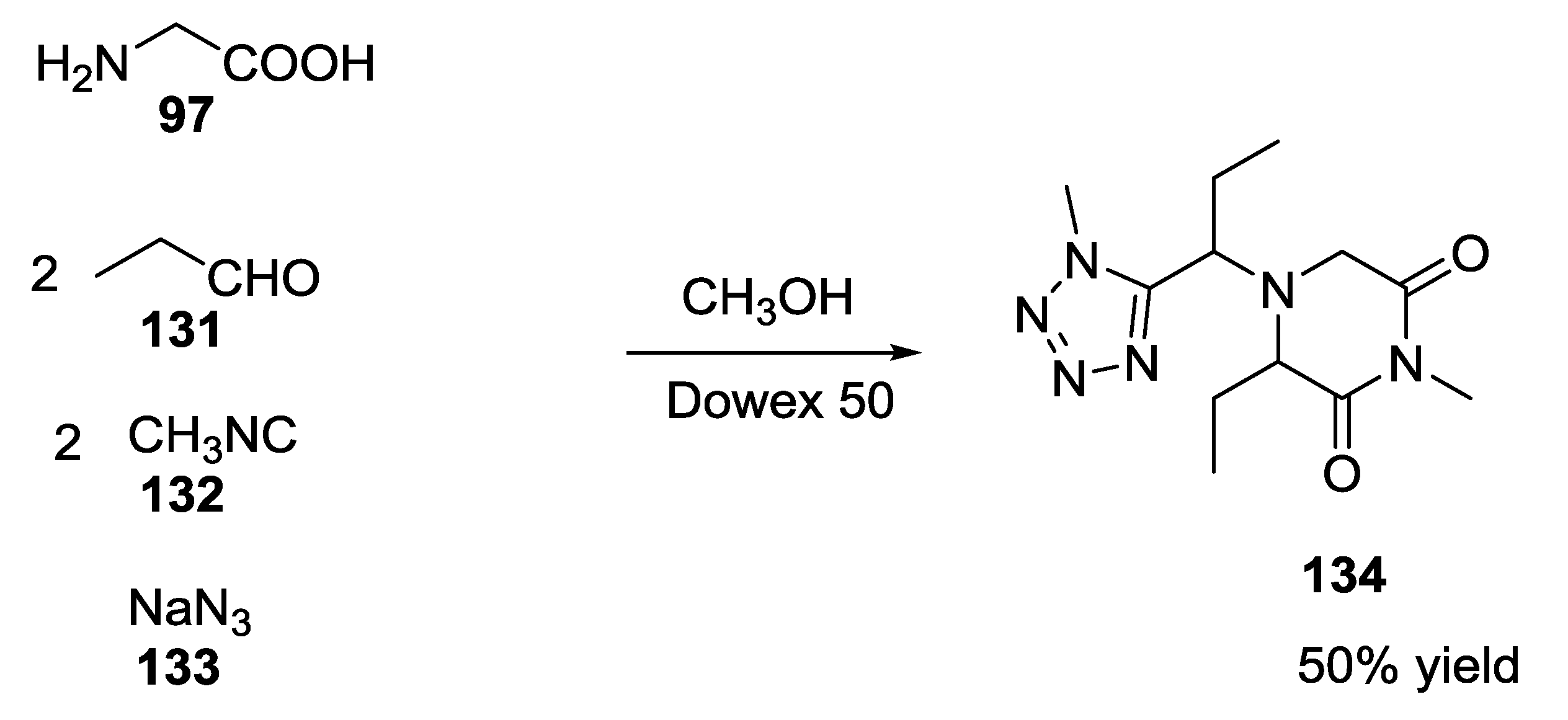
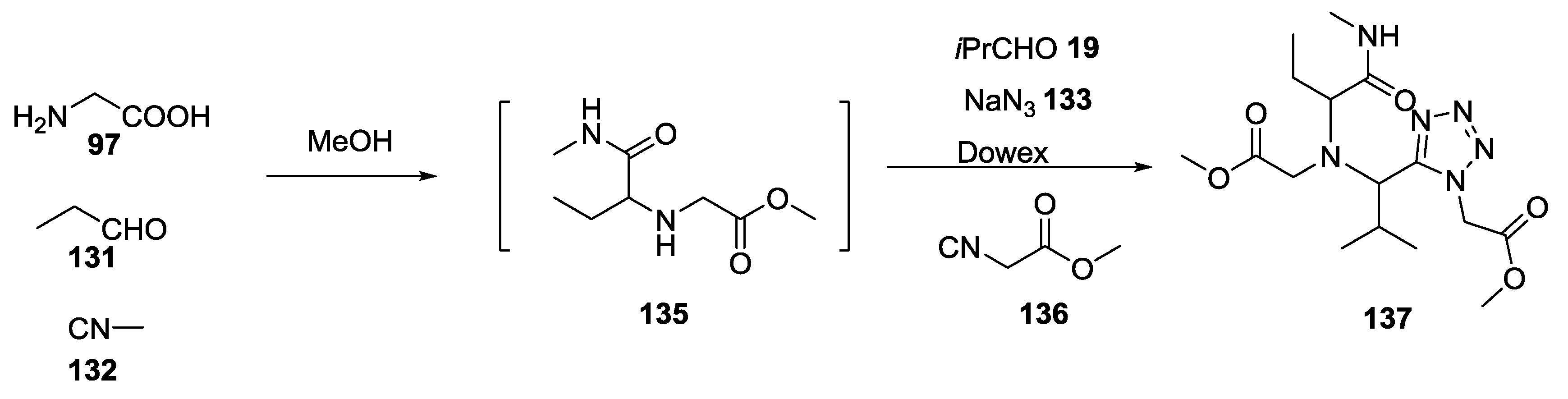
2.9. Isocyanocarbamates in the U-5C-4CR
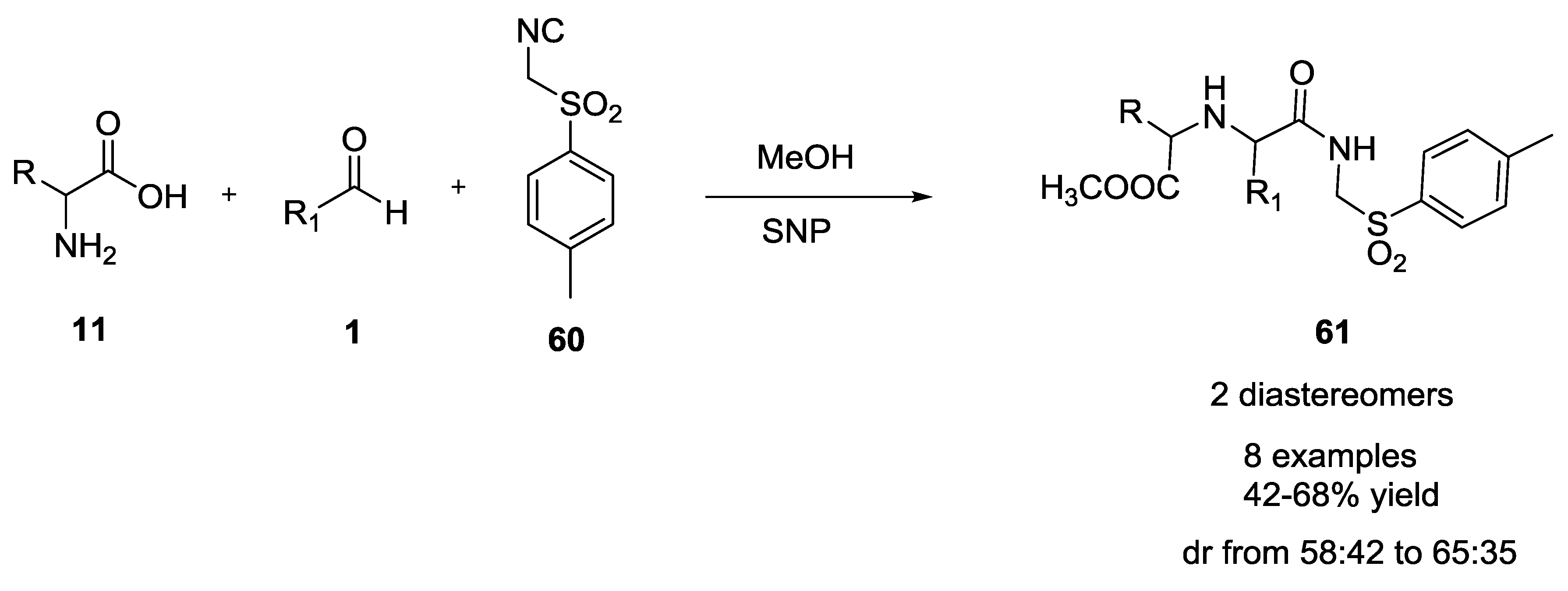
2.10. Silica Nanoparticles as Green Catalyst for U-5C-4CR
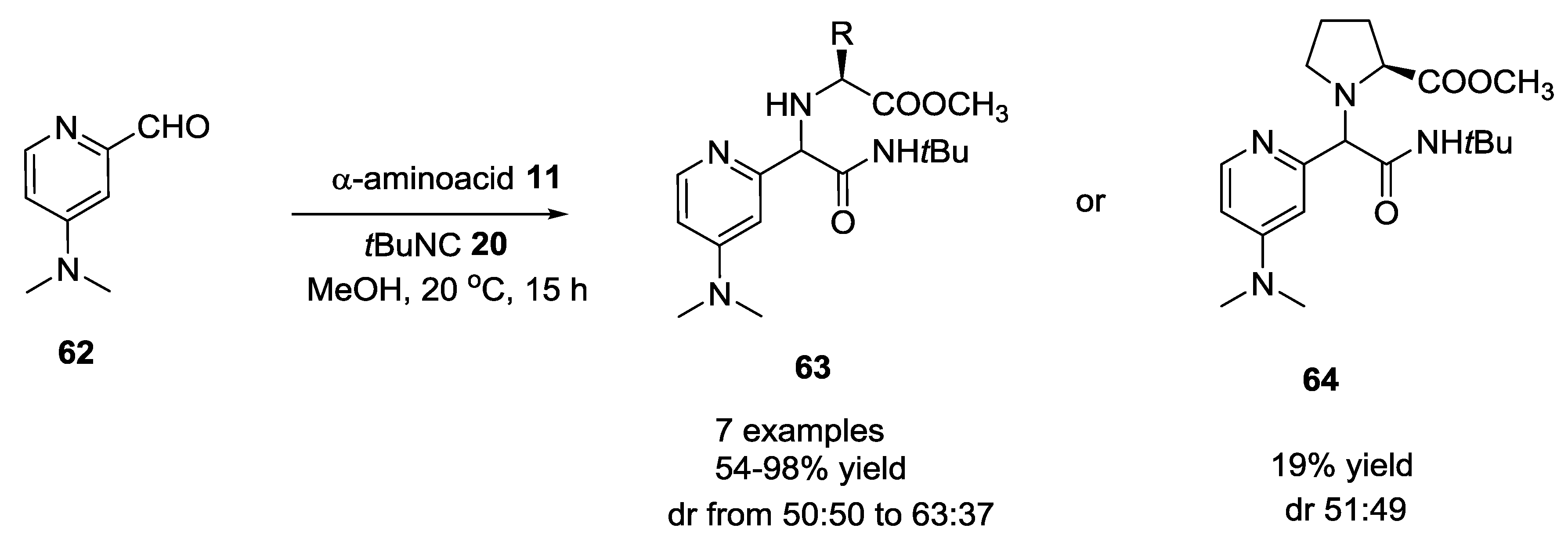
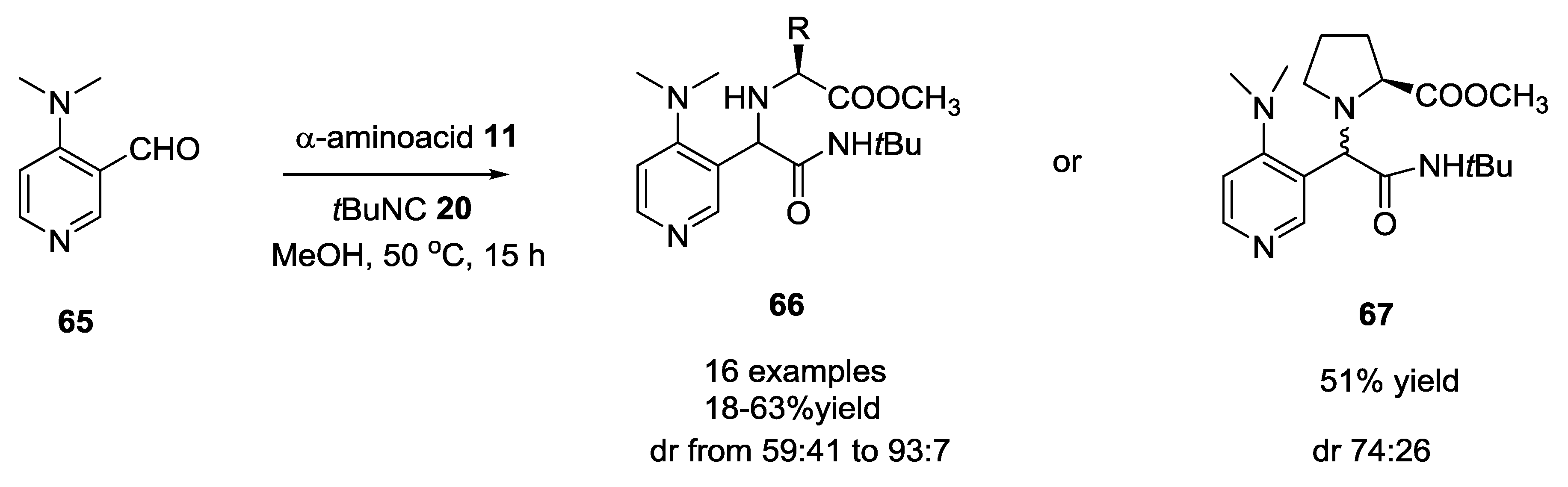
2.11. Use of DMAP-Based Aldehydes in U-5C-4CR
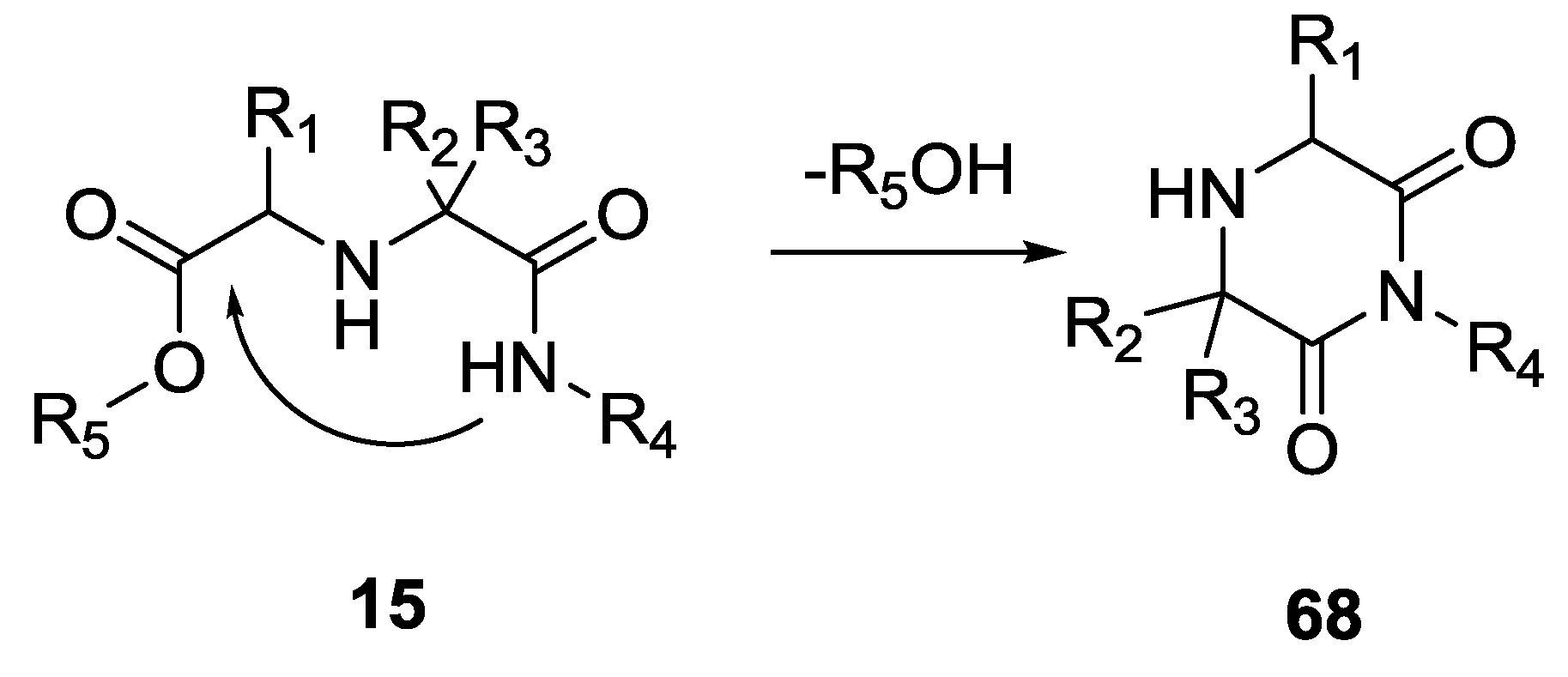
3. Cyclic Compounds
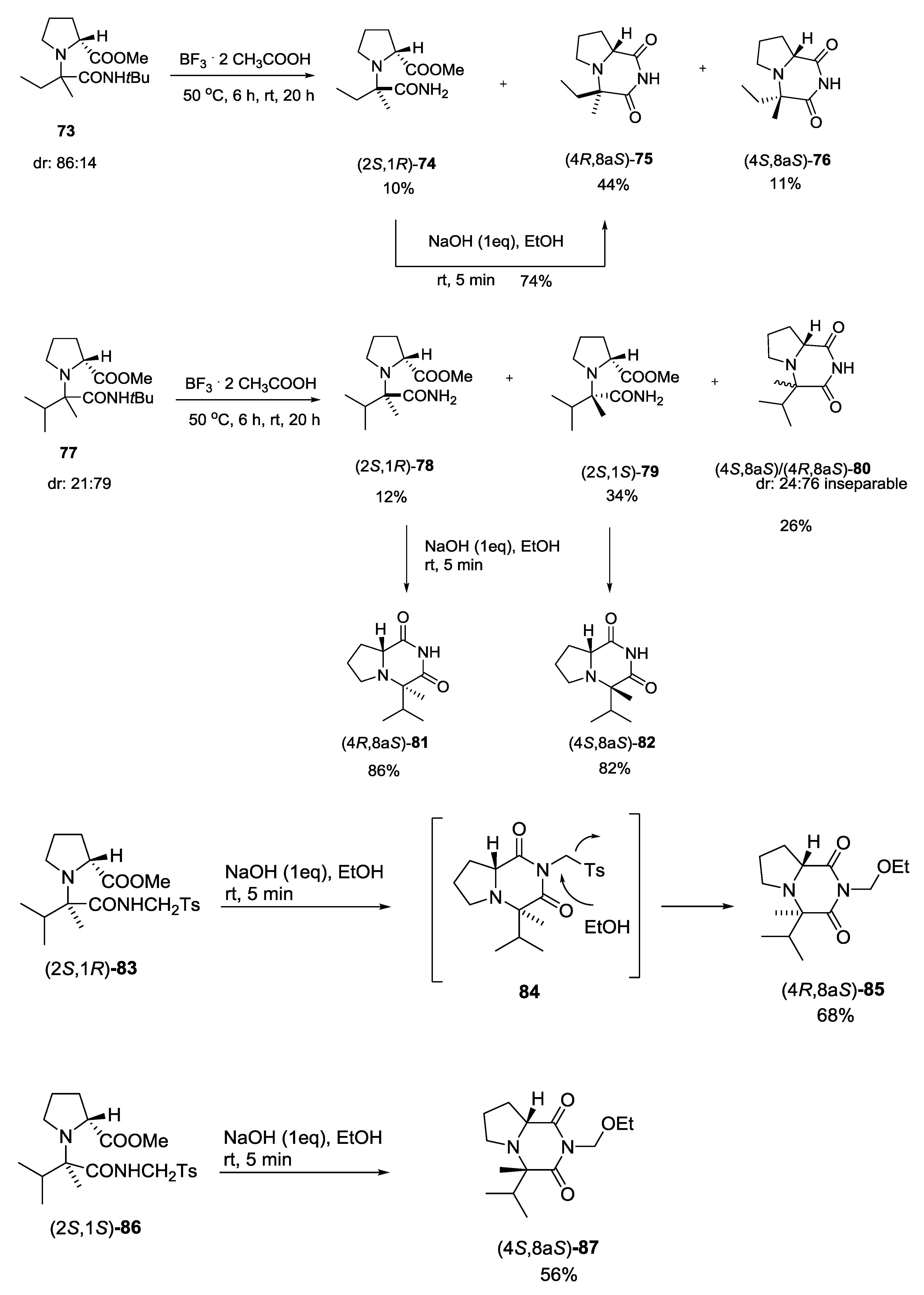
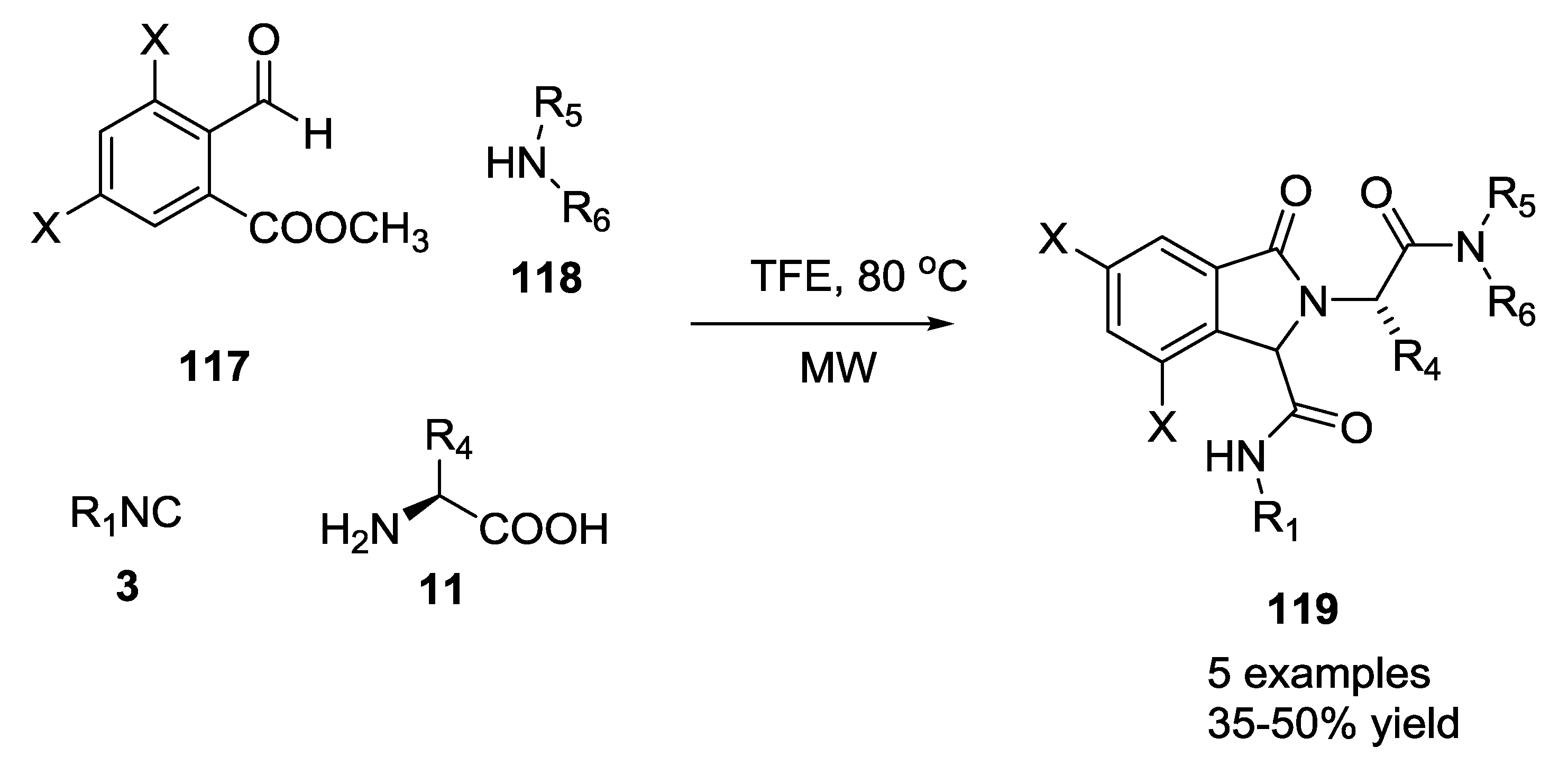
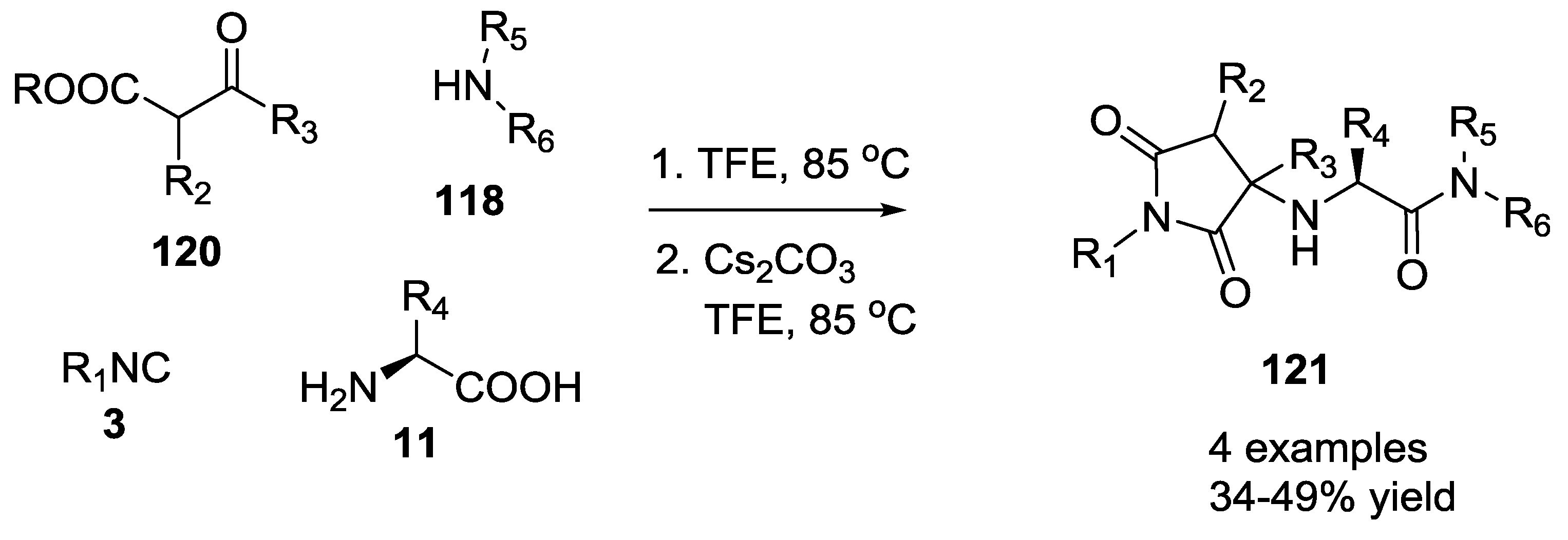
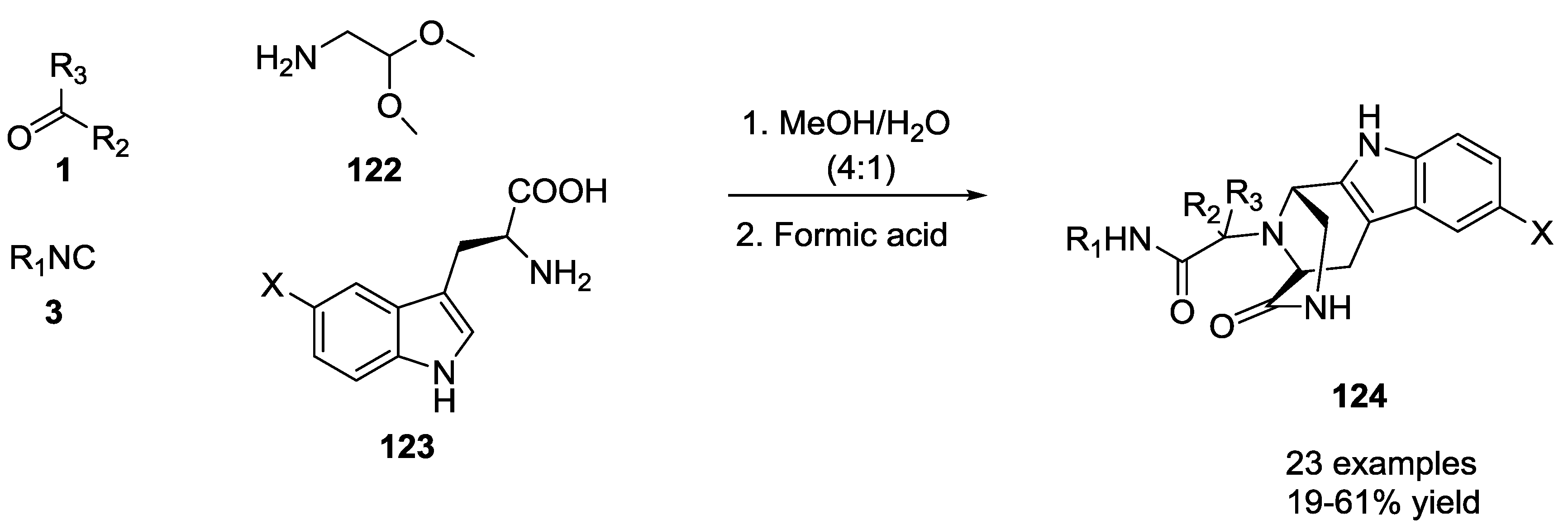
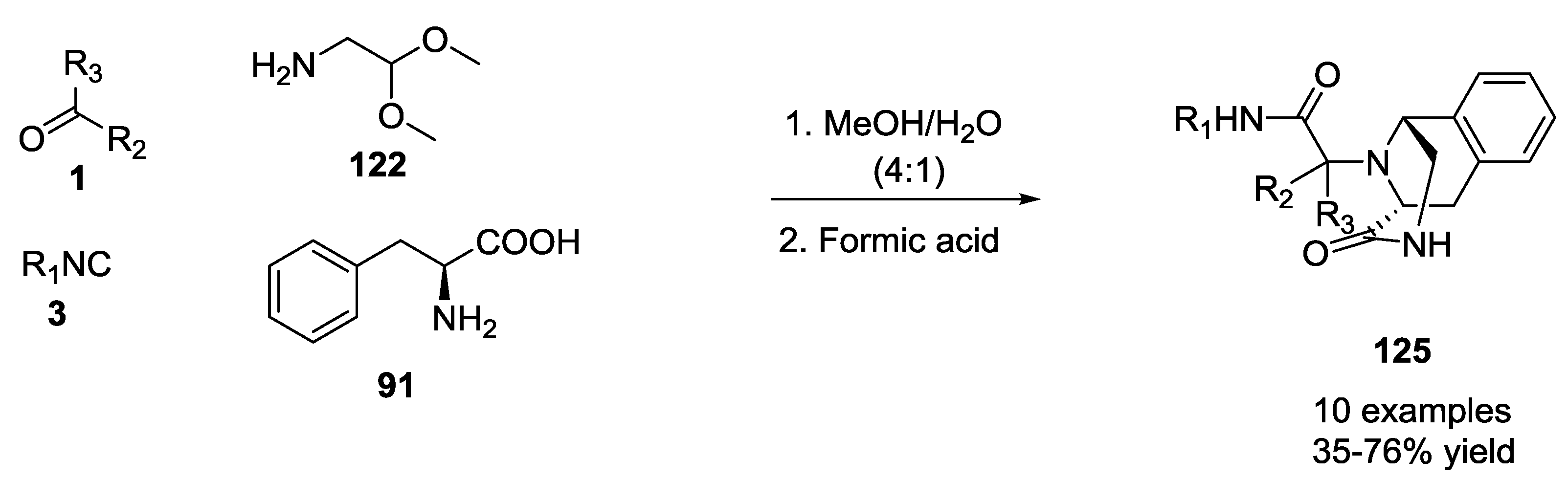
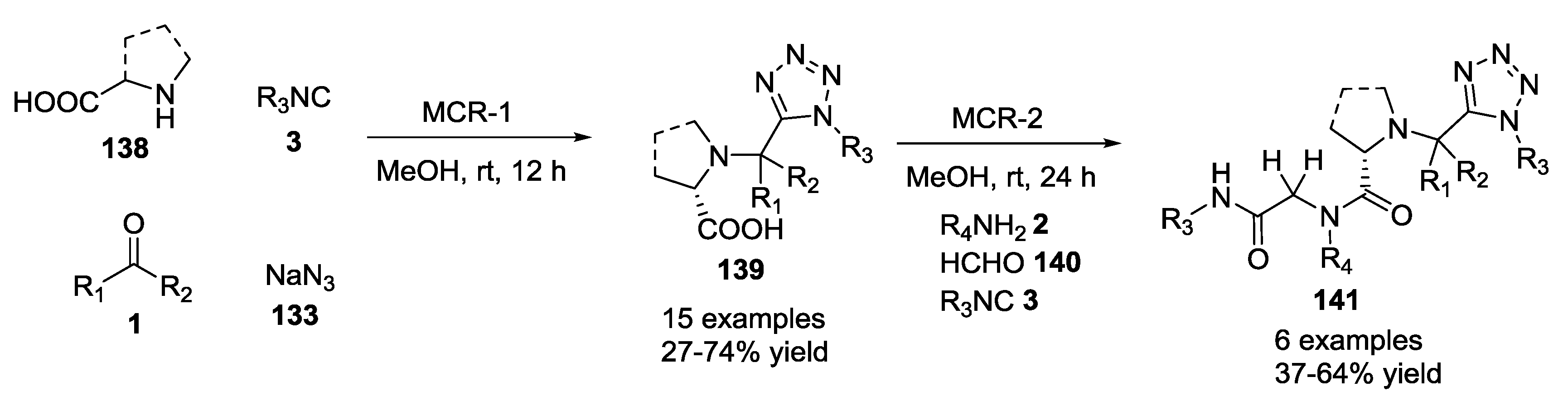
3.1. Ugi-5C-4CRs and One-Pot Post-Condensation Modifications
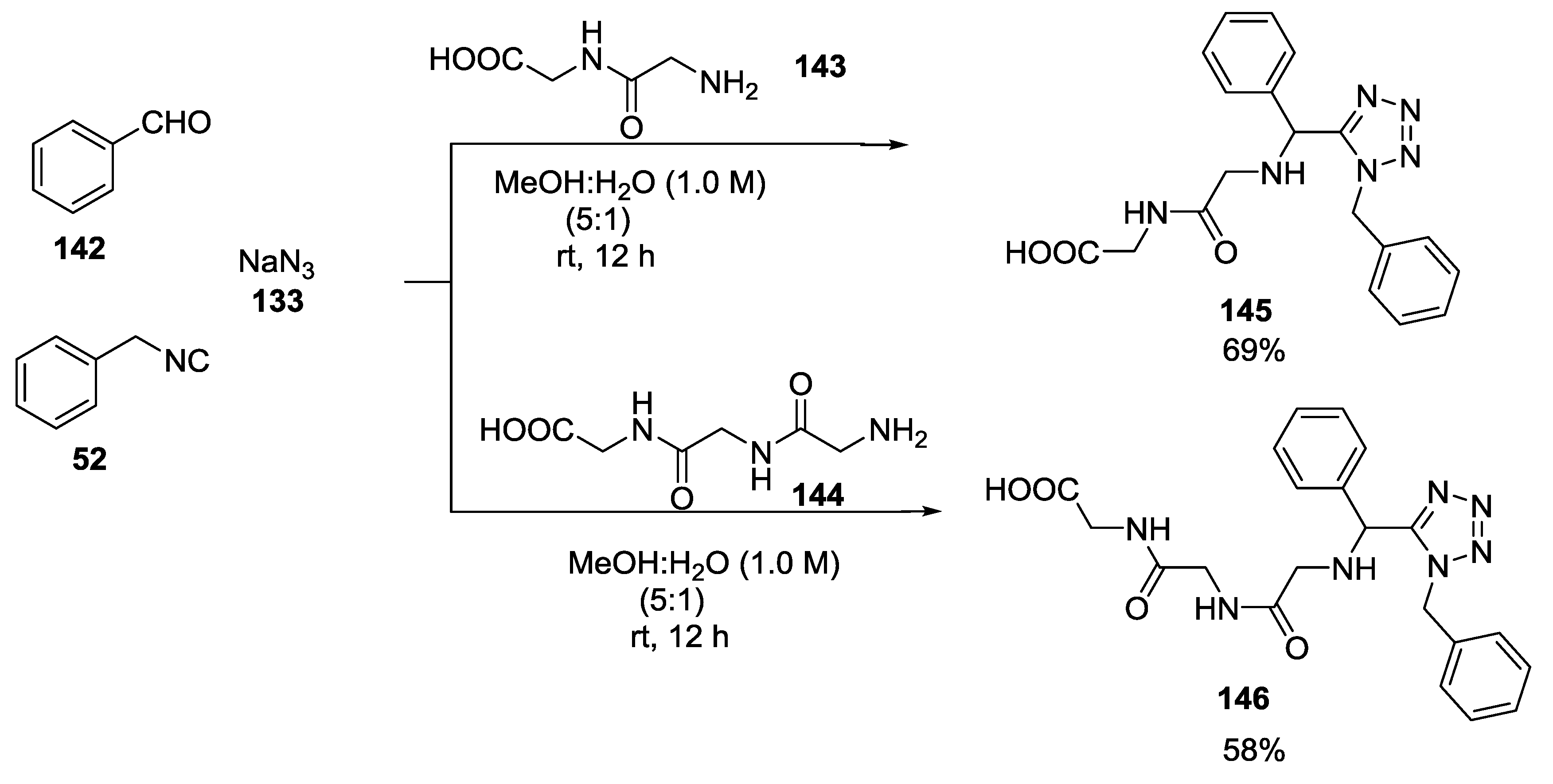
3.2. Exploiting α-Amino Acids in the Combination of Tandem MCRs
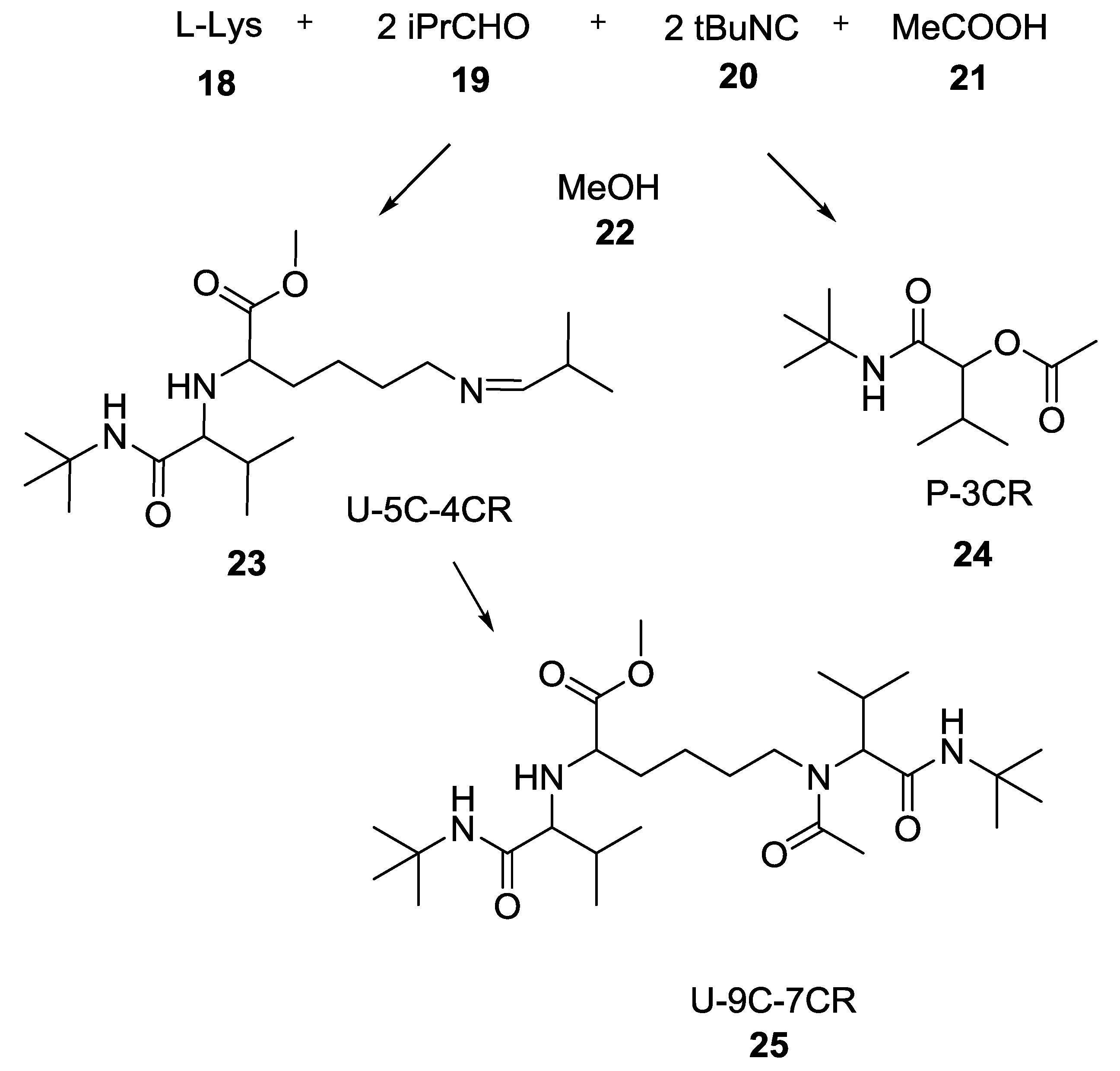
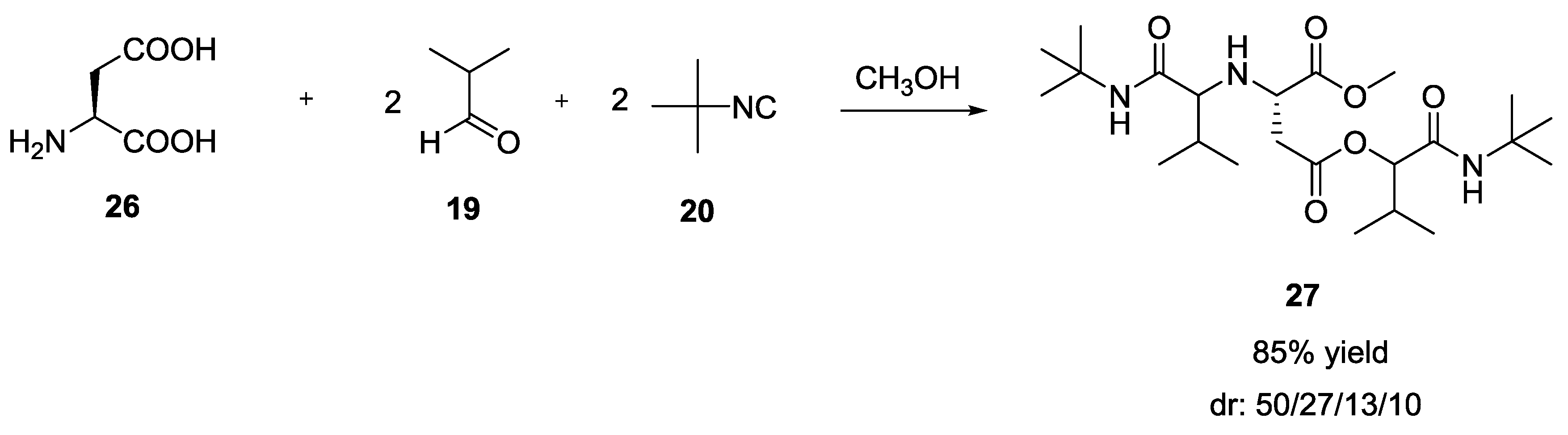
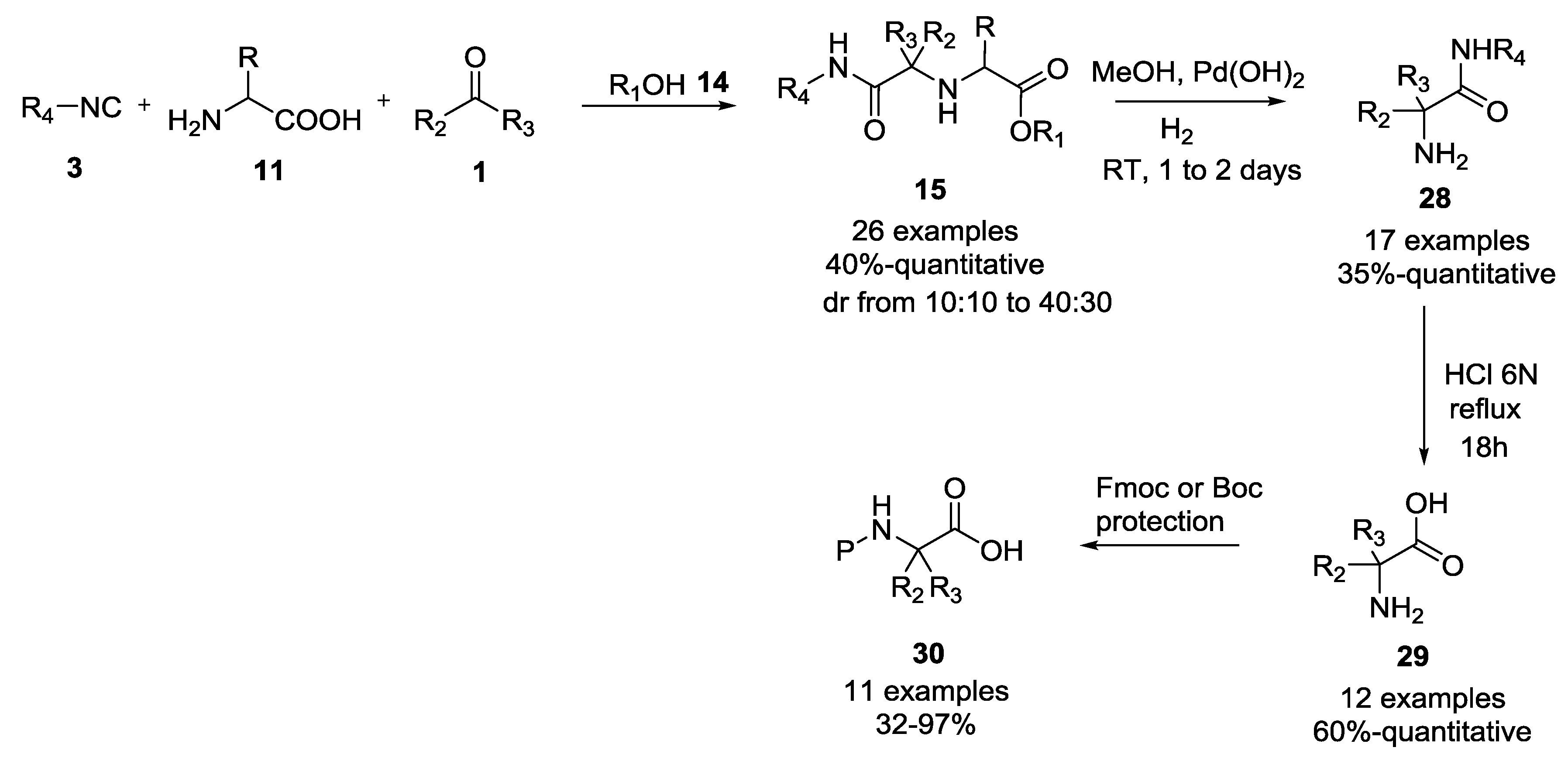
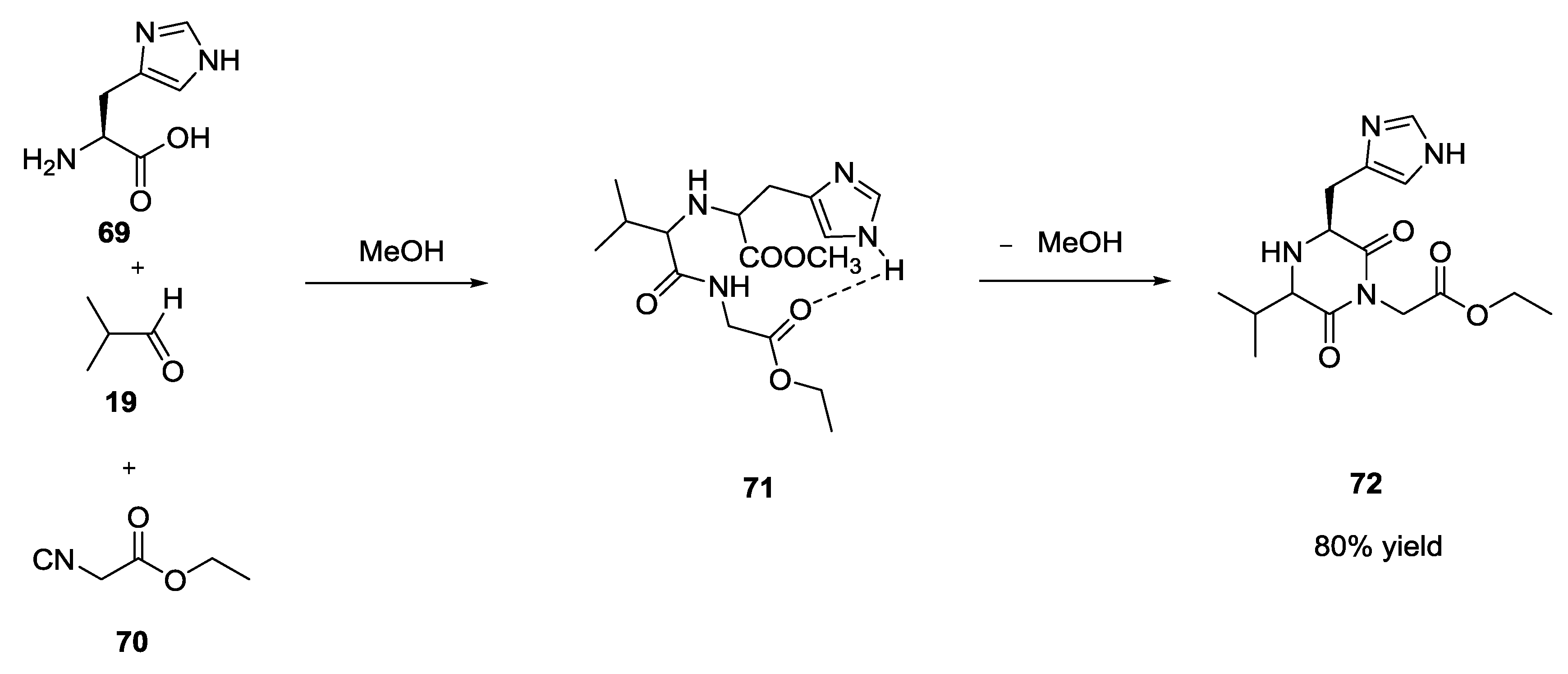
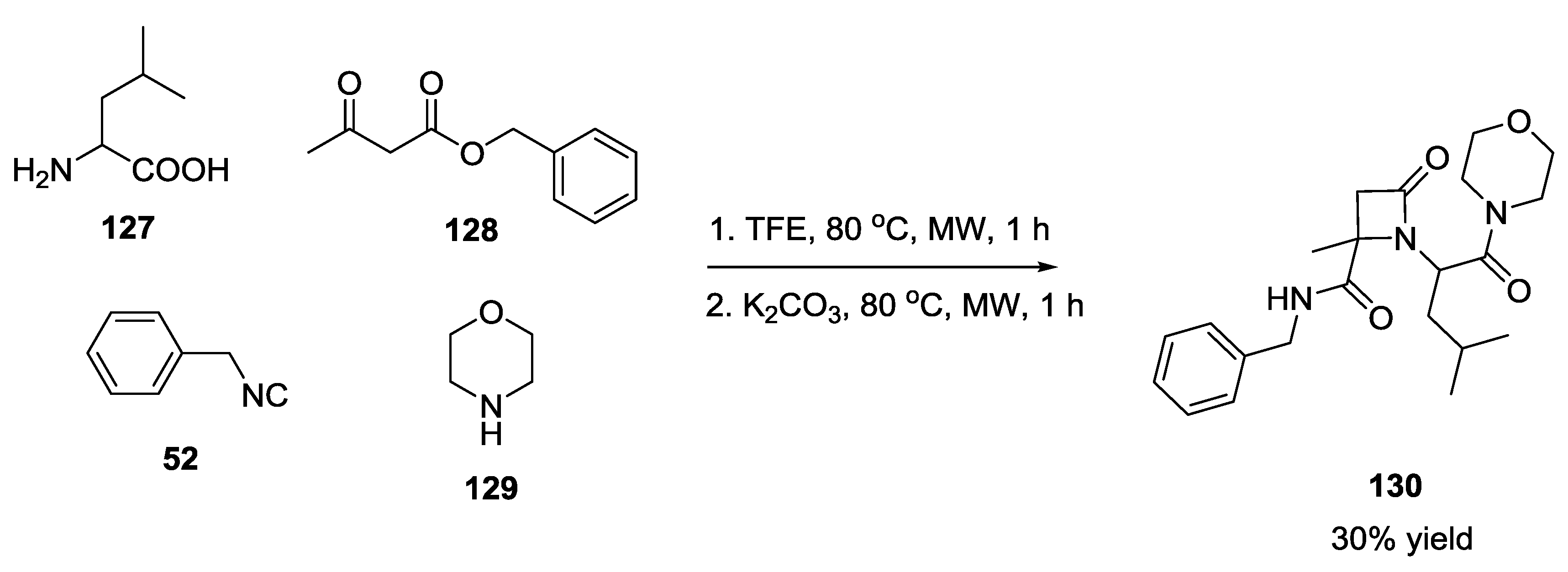
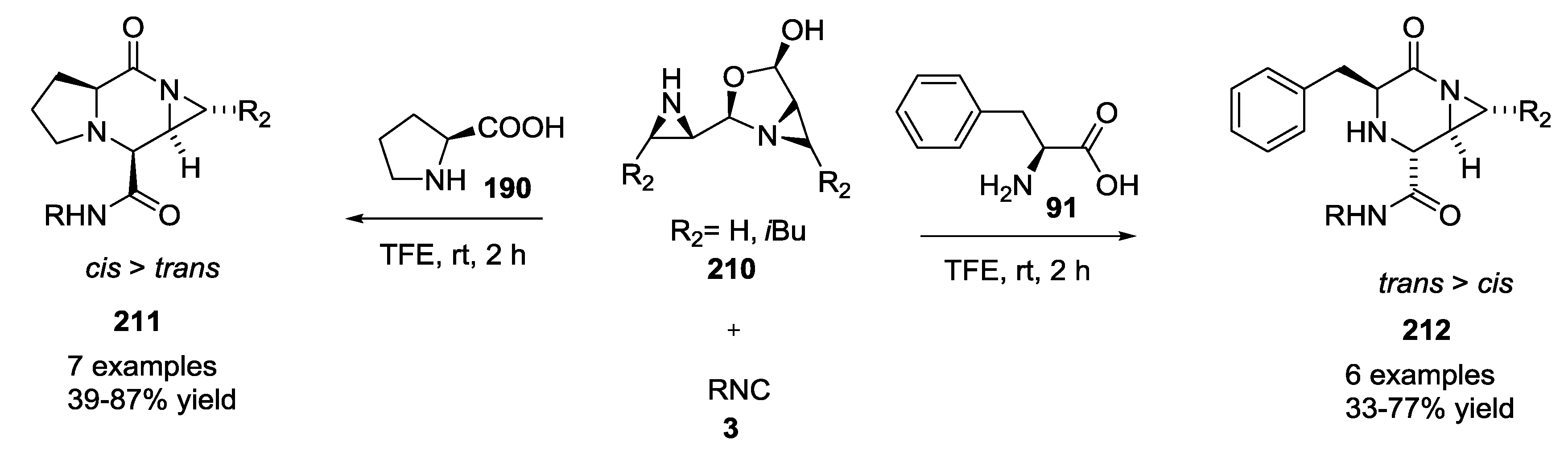
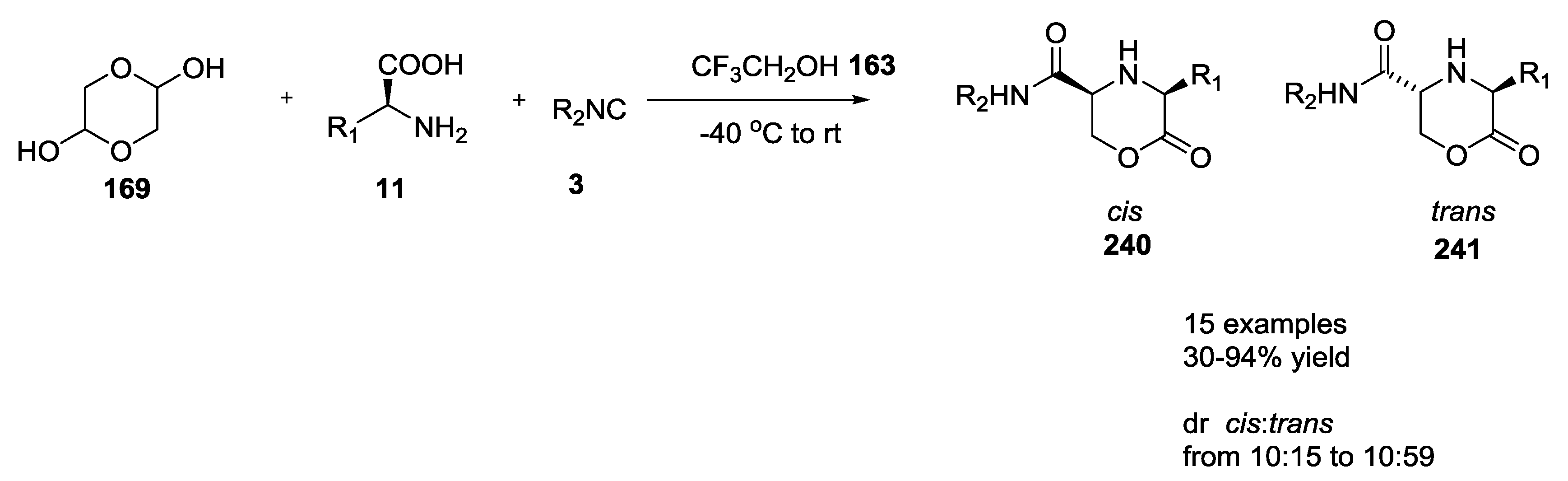
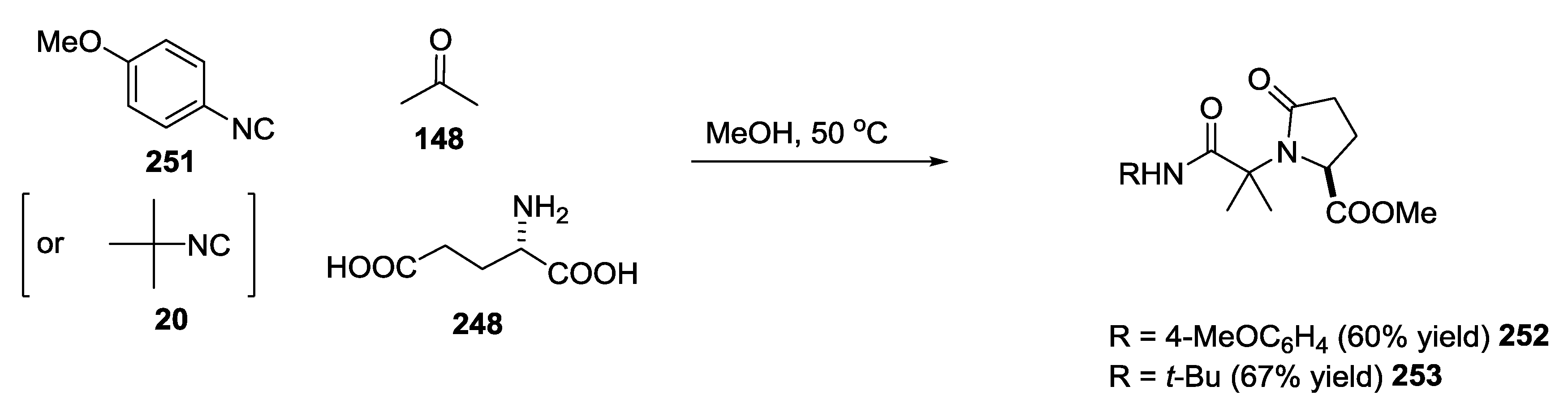
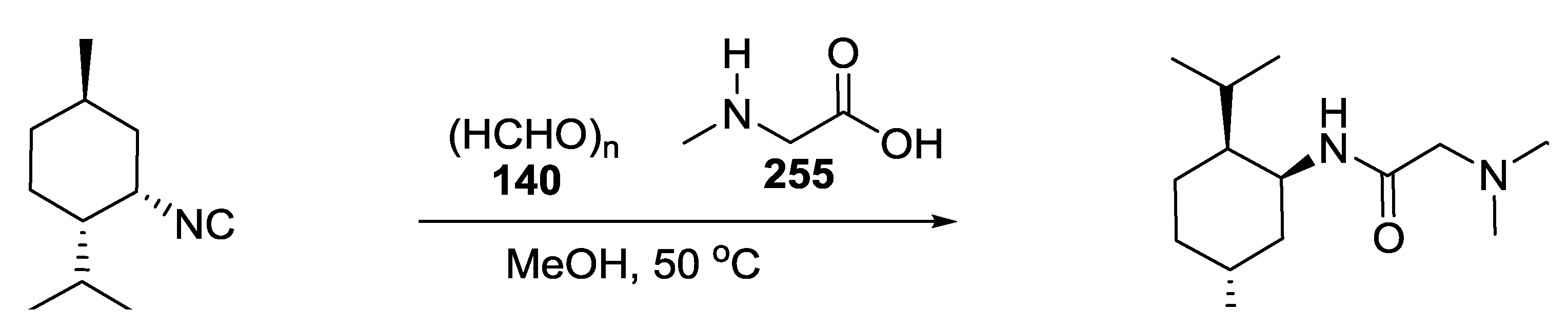
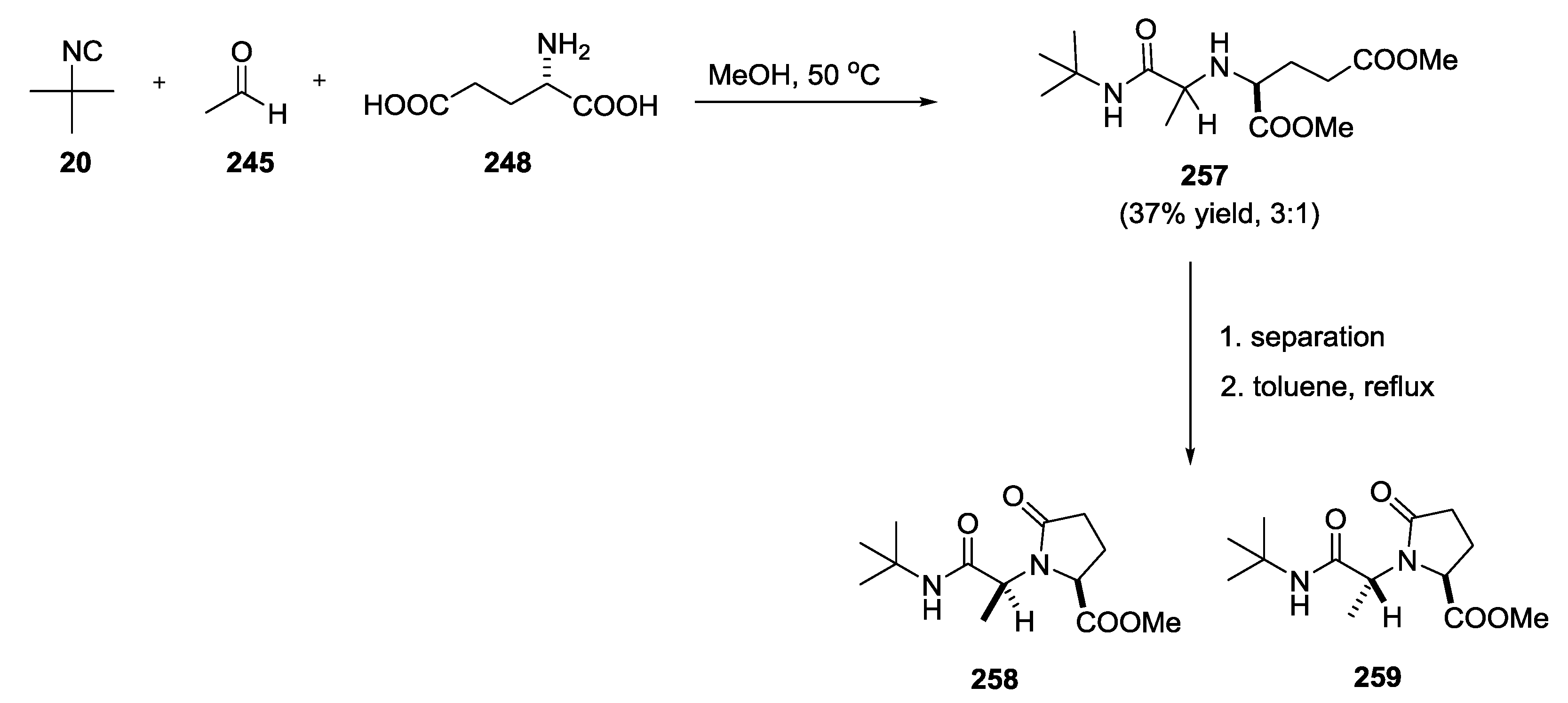
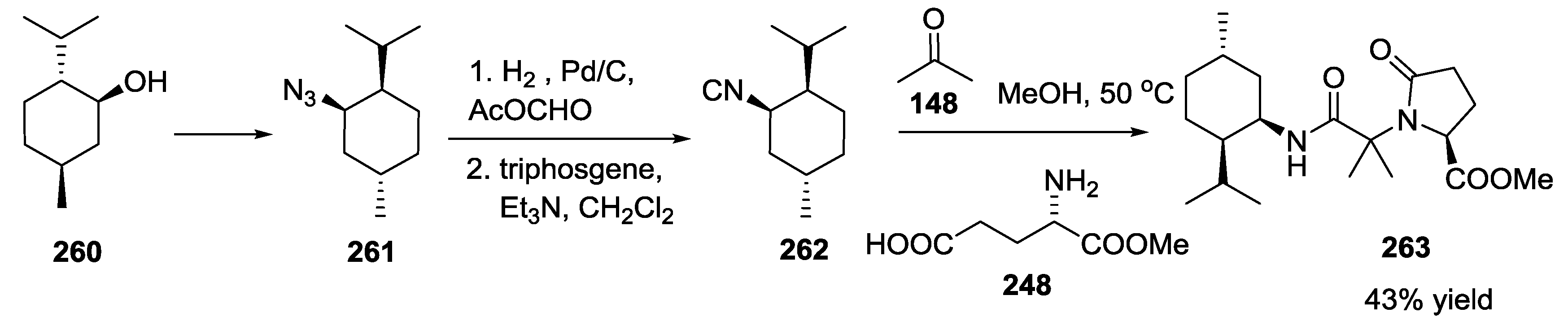
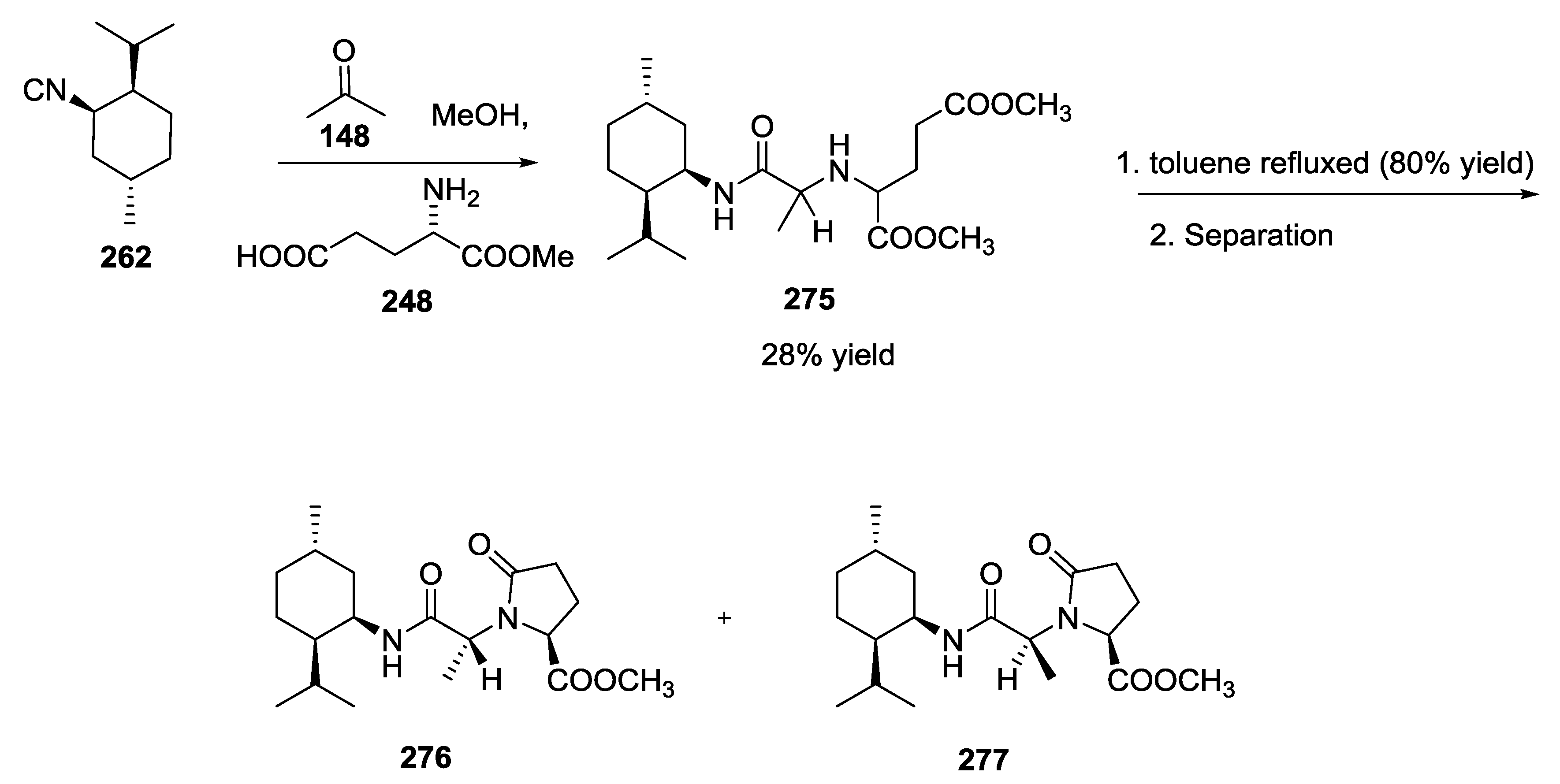
3.3. Use of tri-Functional α-Amino Acids in Ugi-5C-4CRs
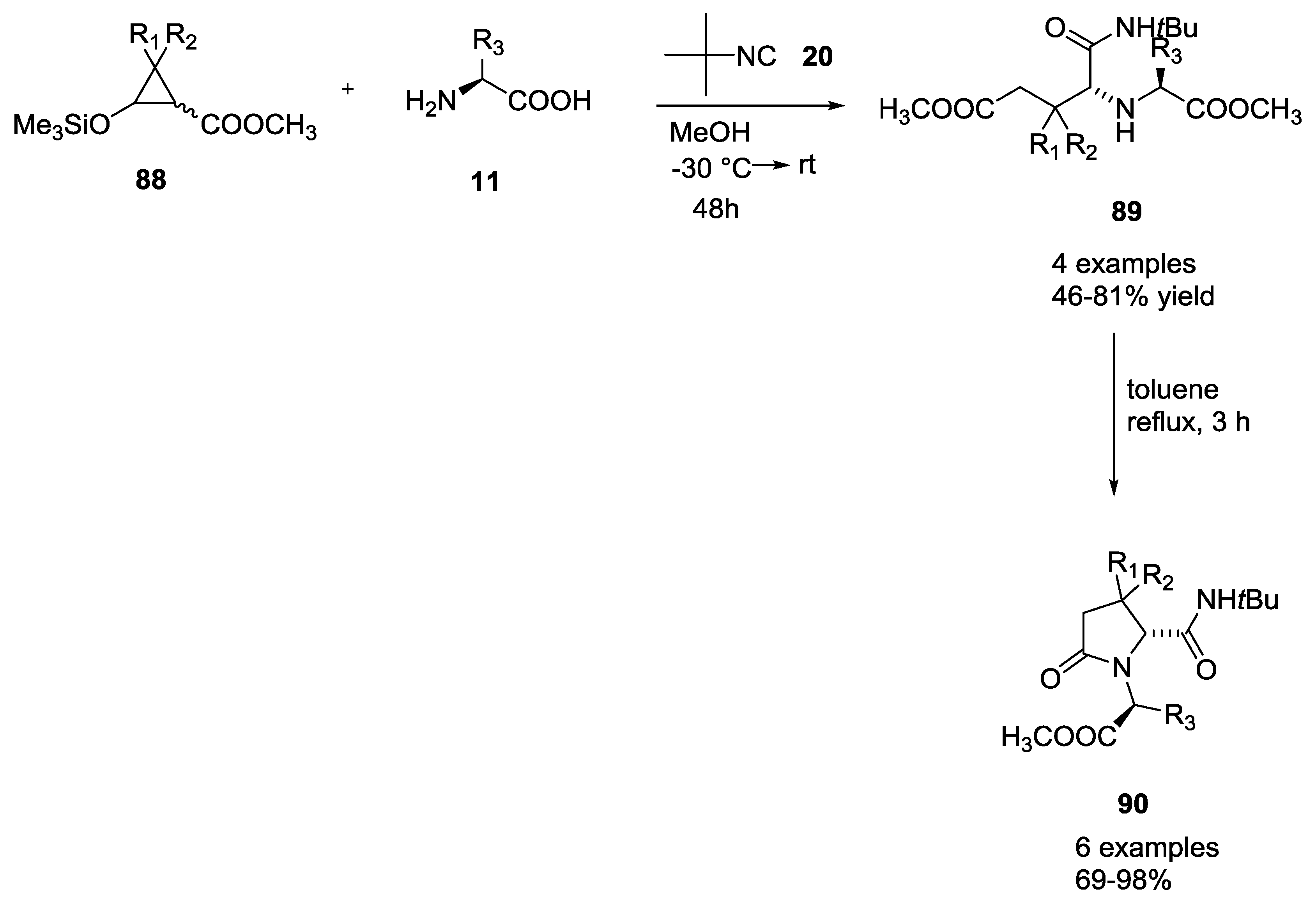
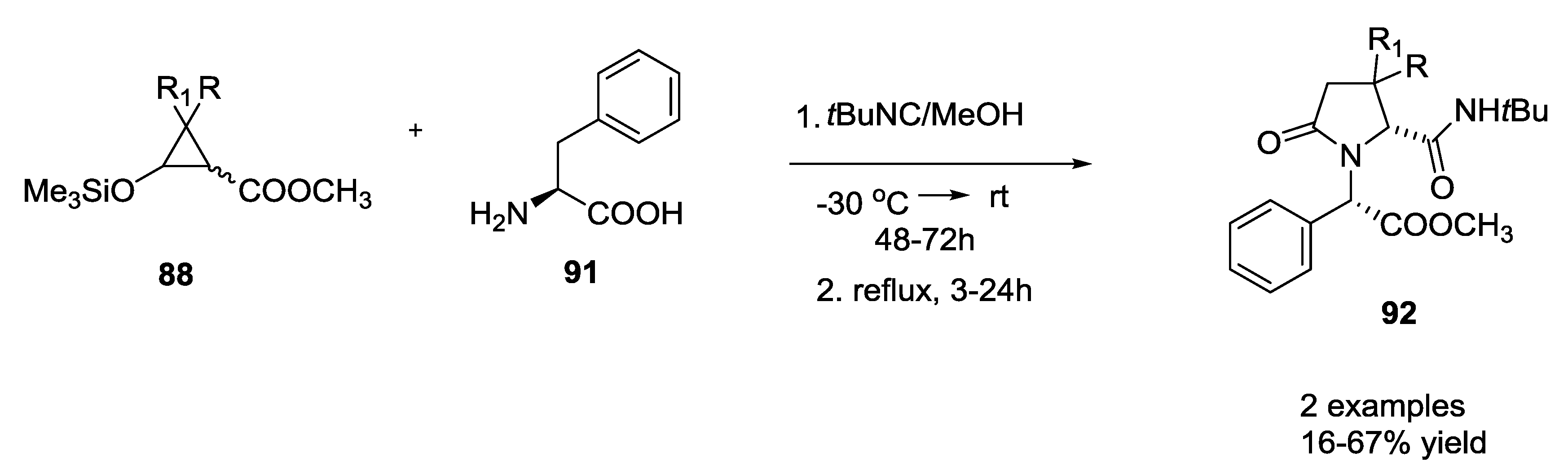
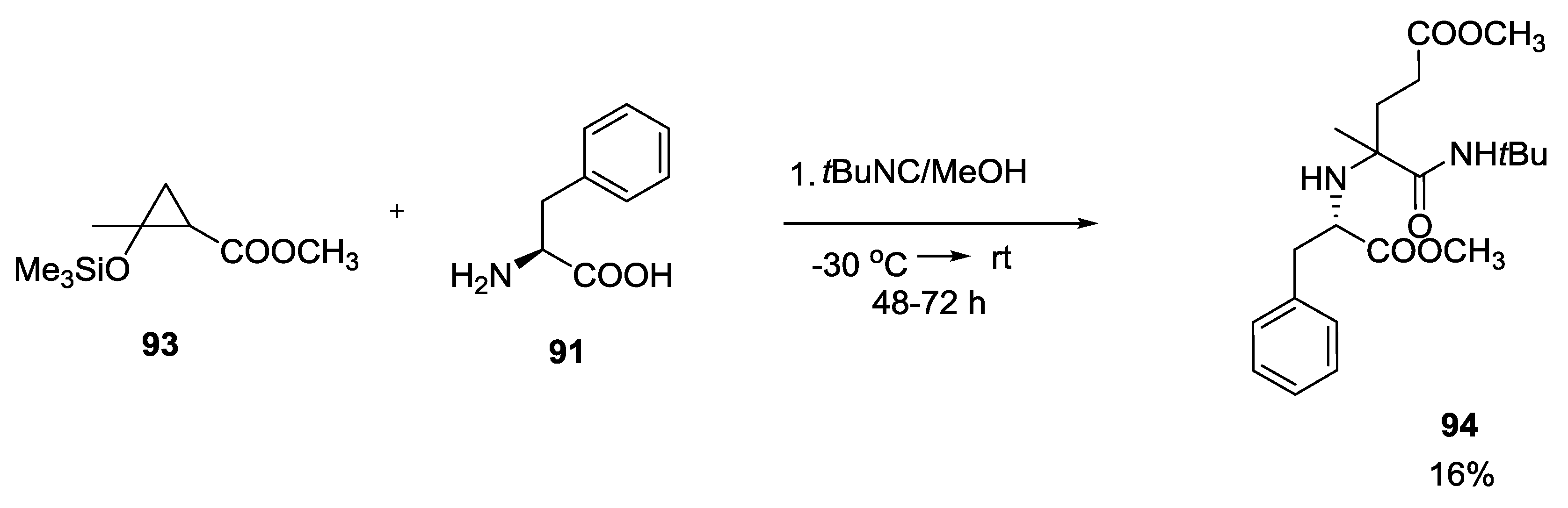
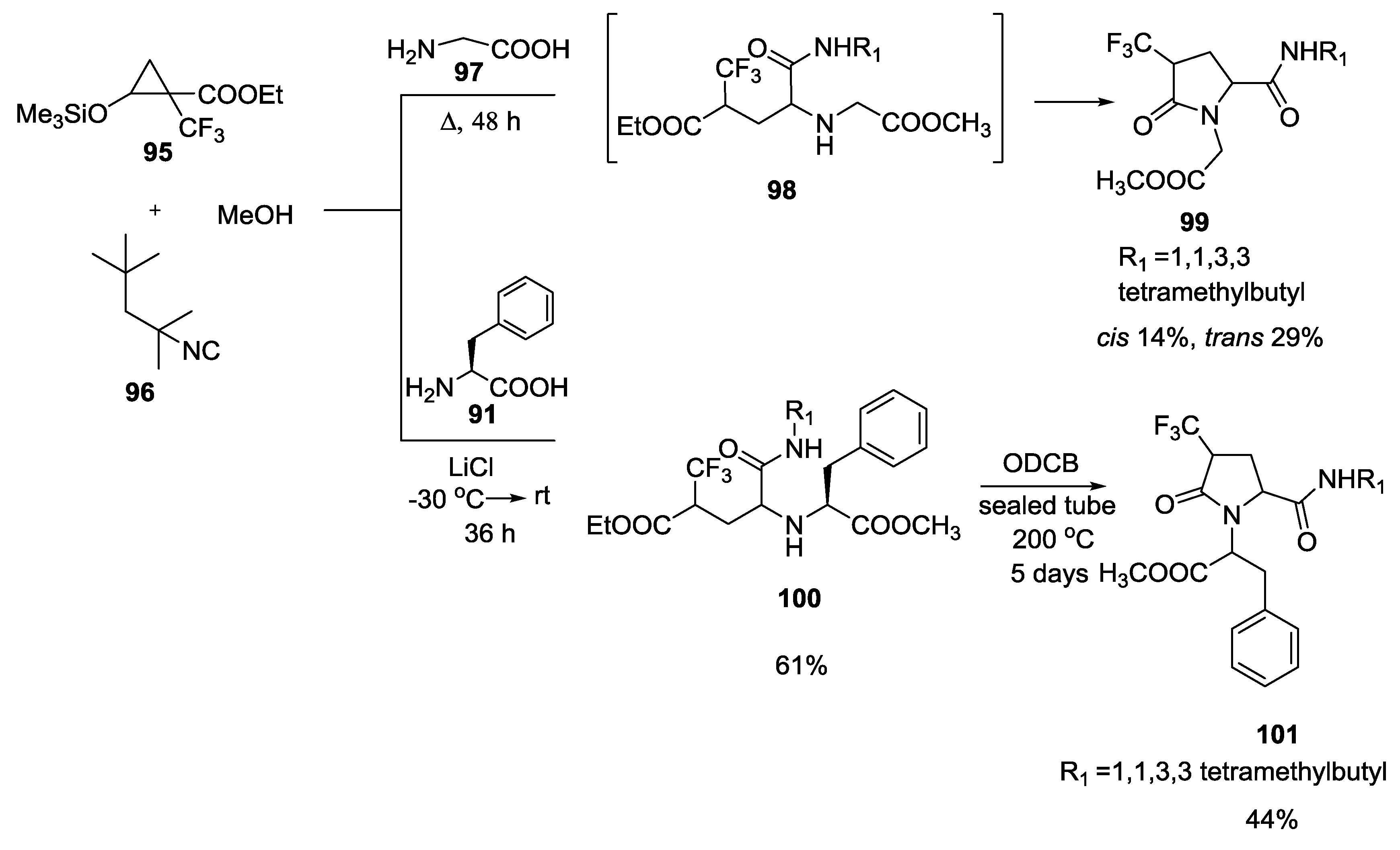
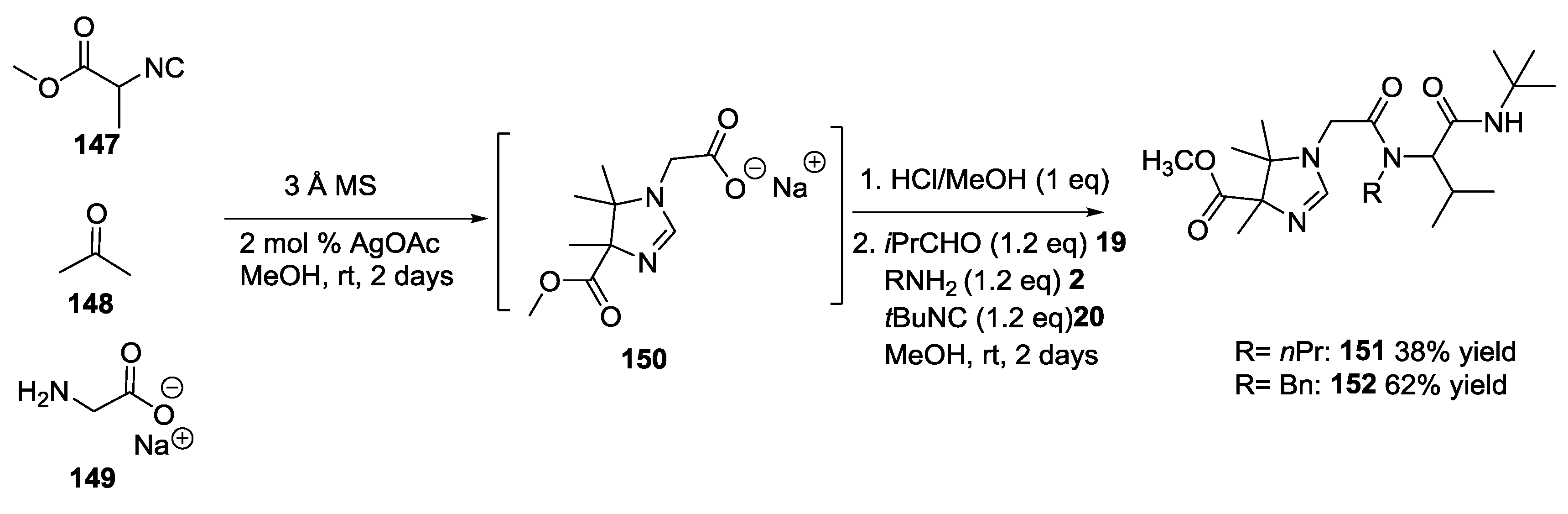
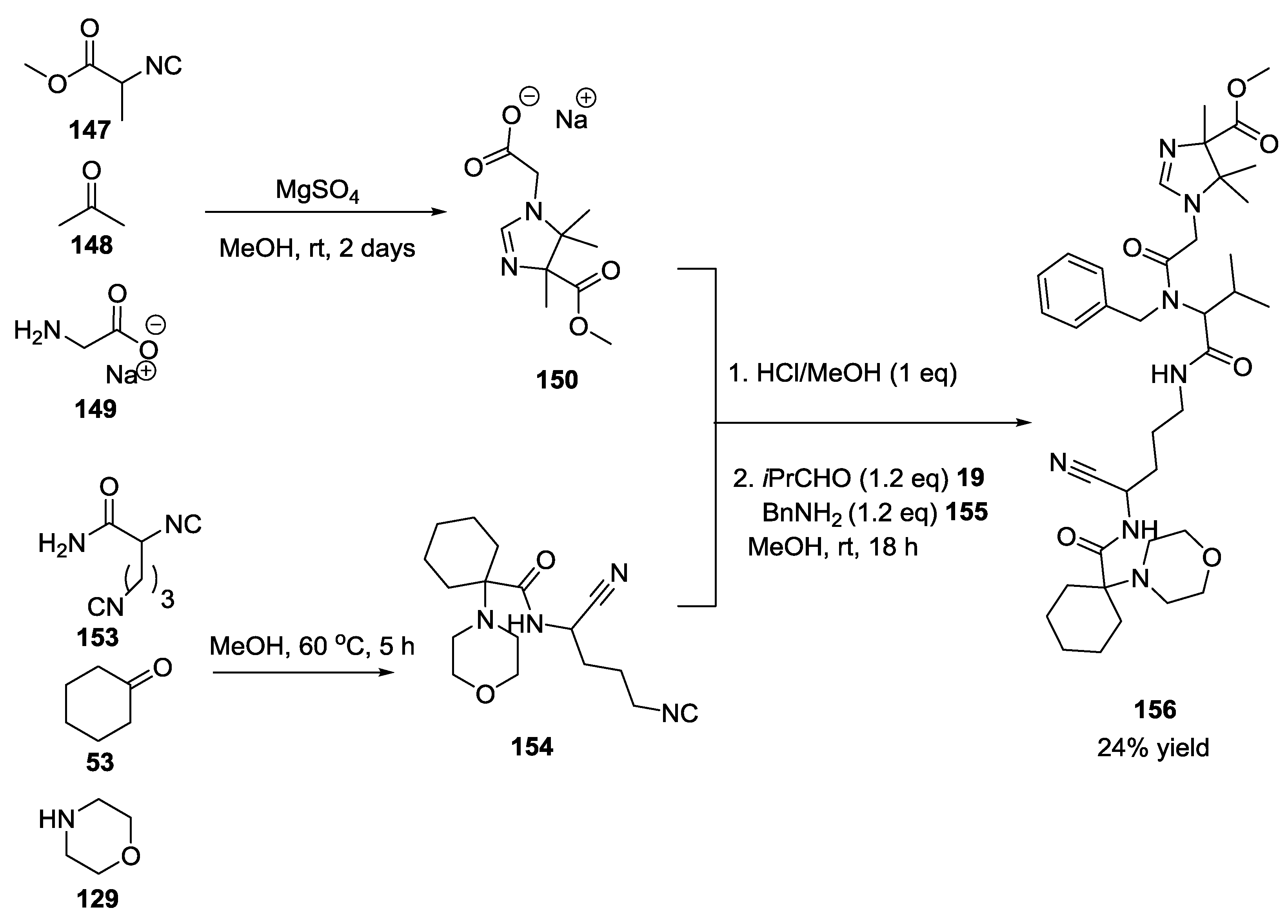
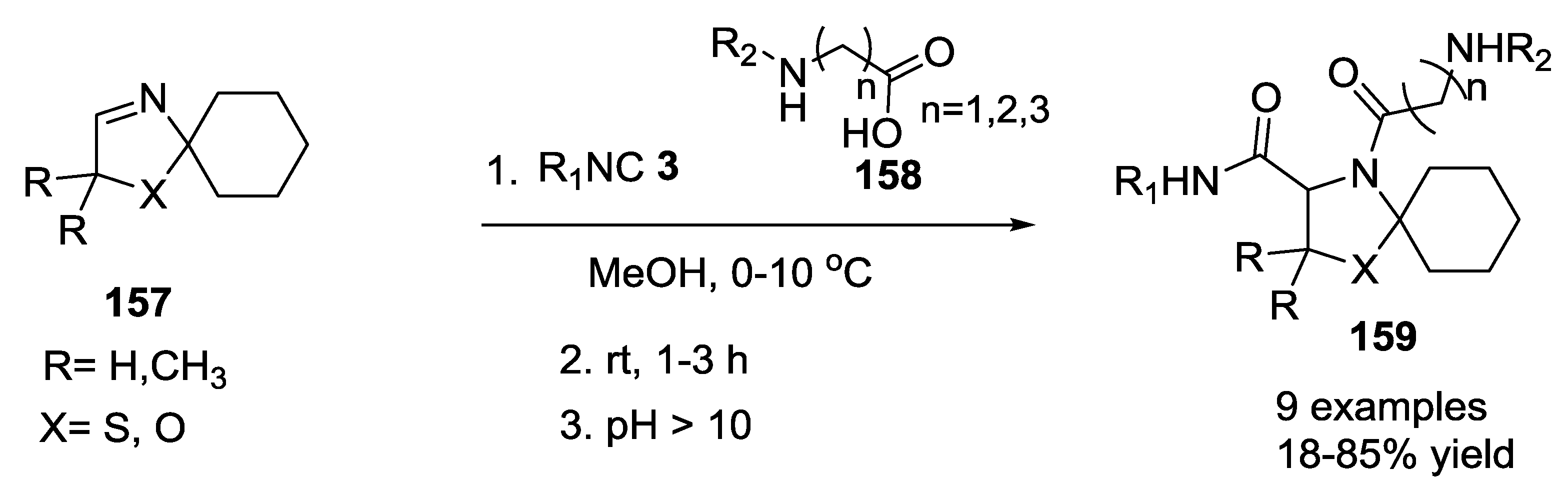
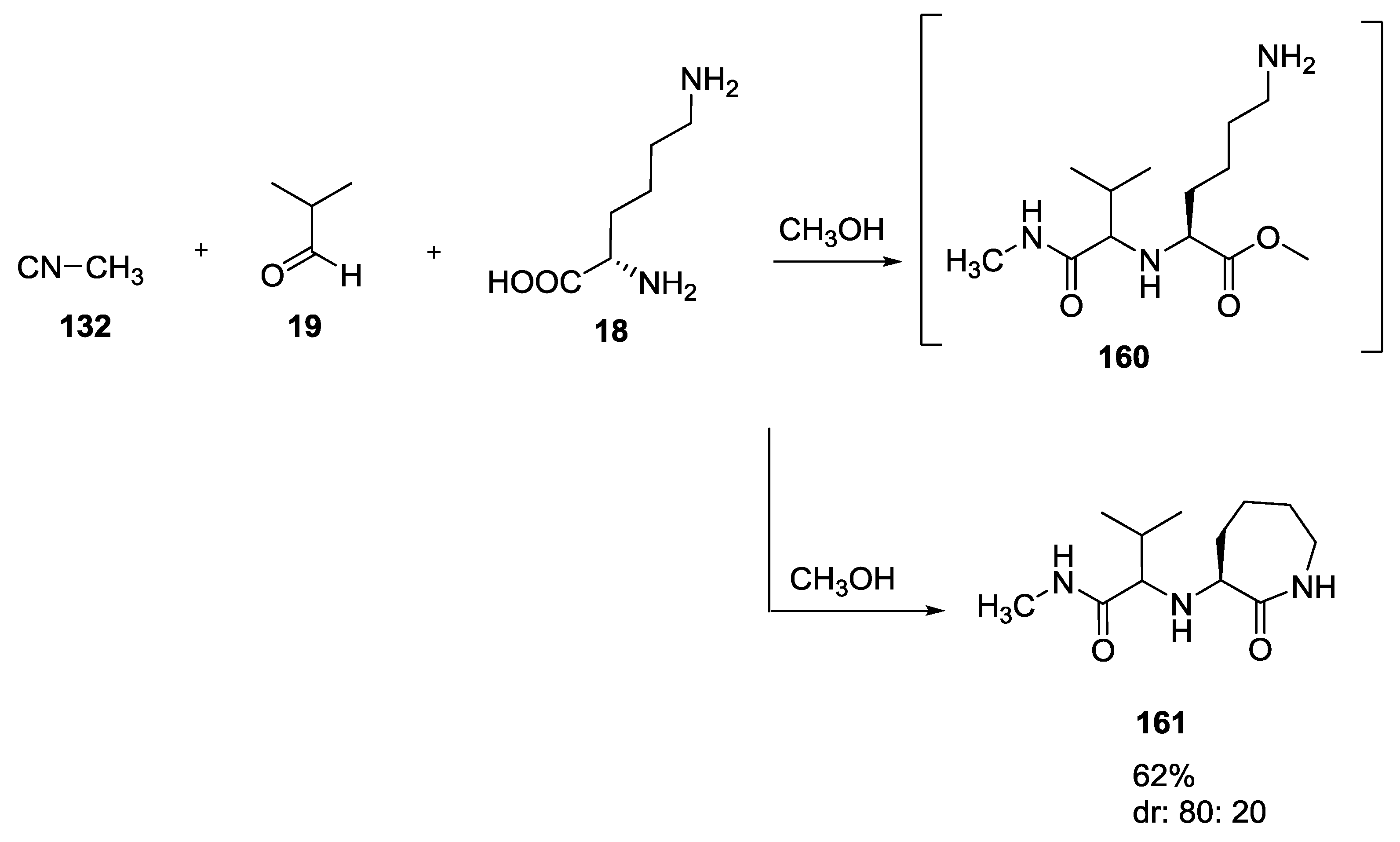
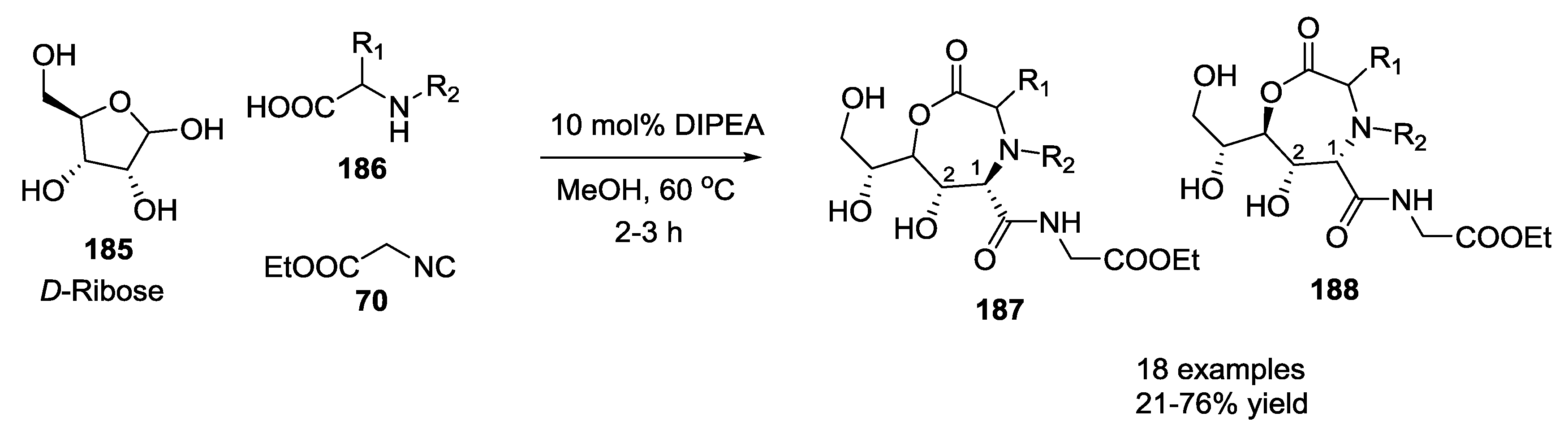
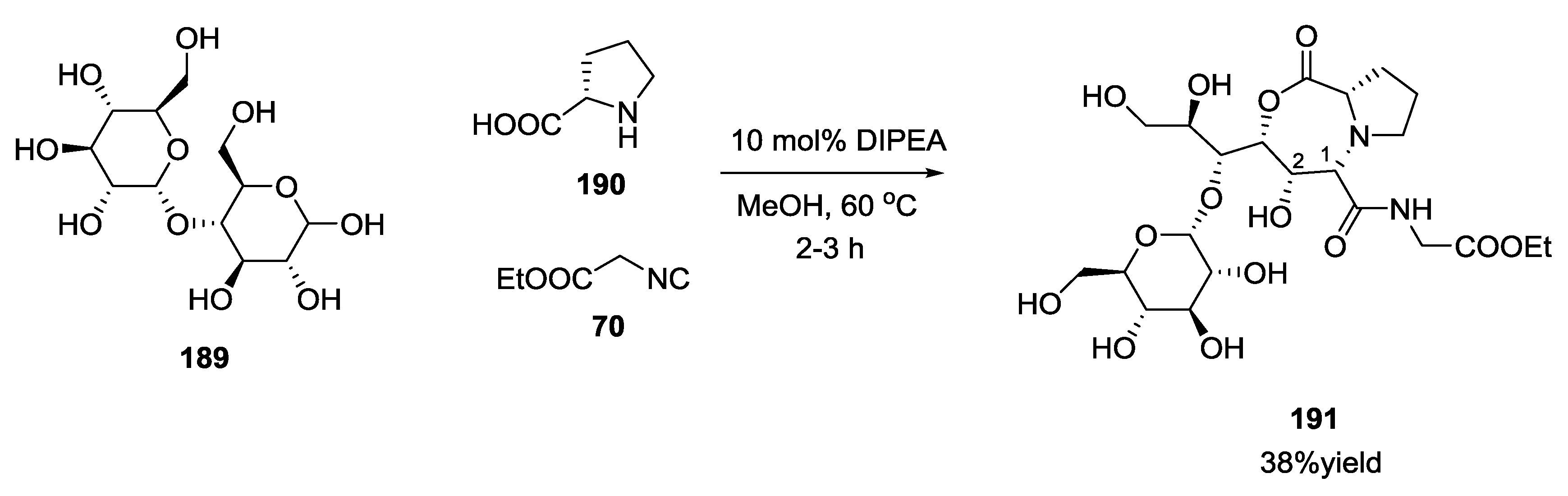
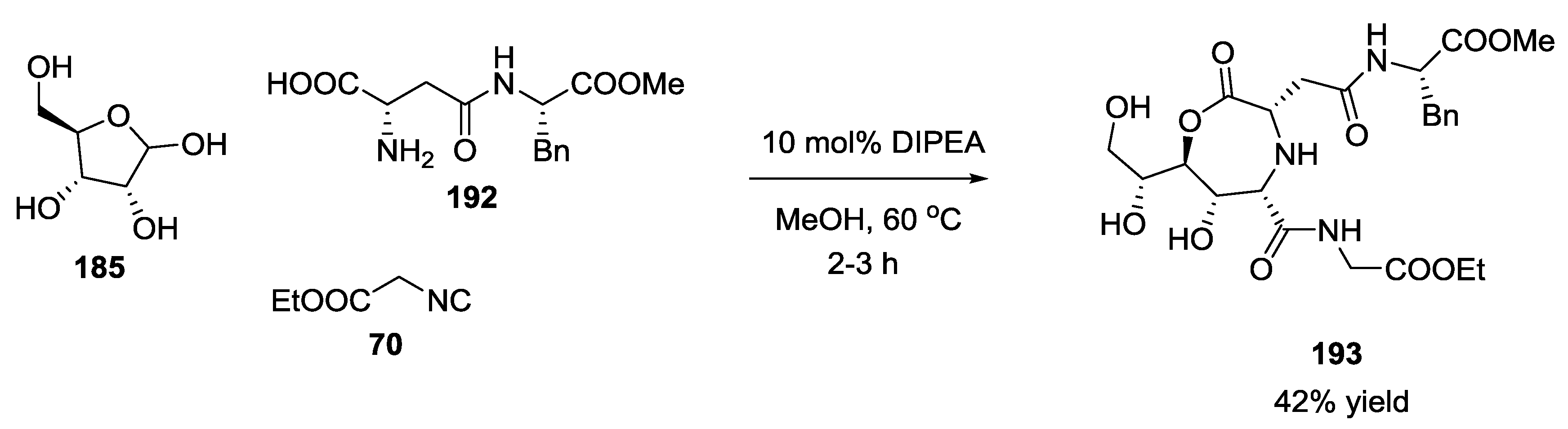
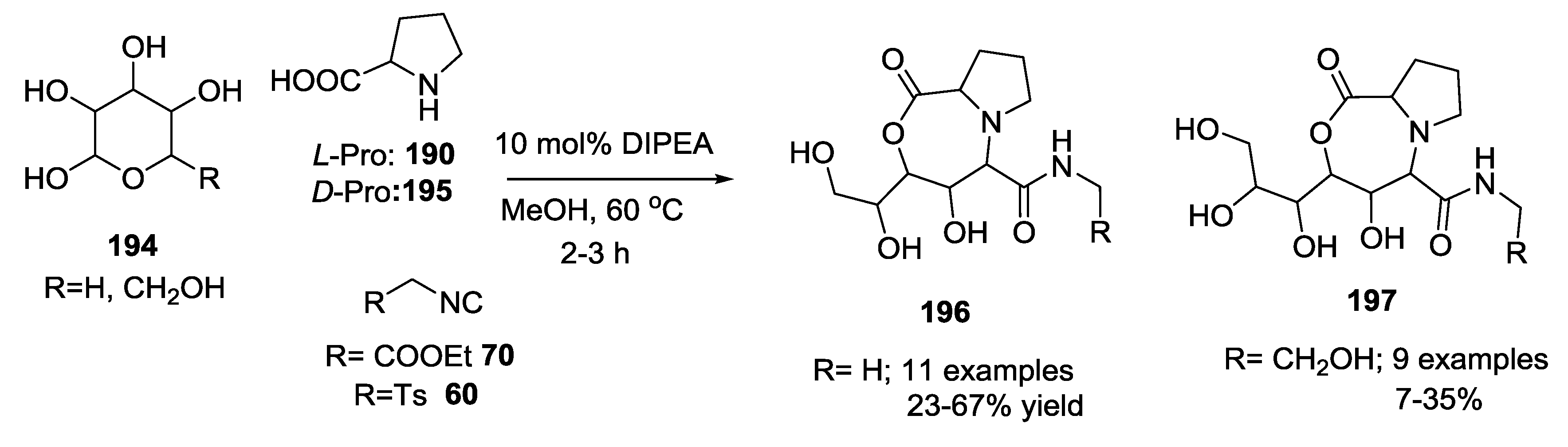
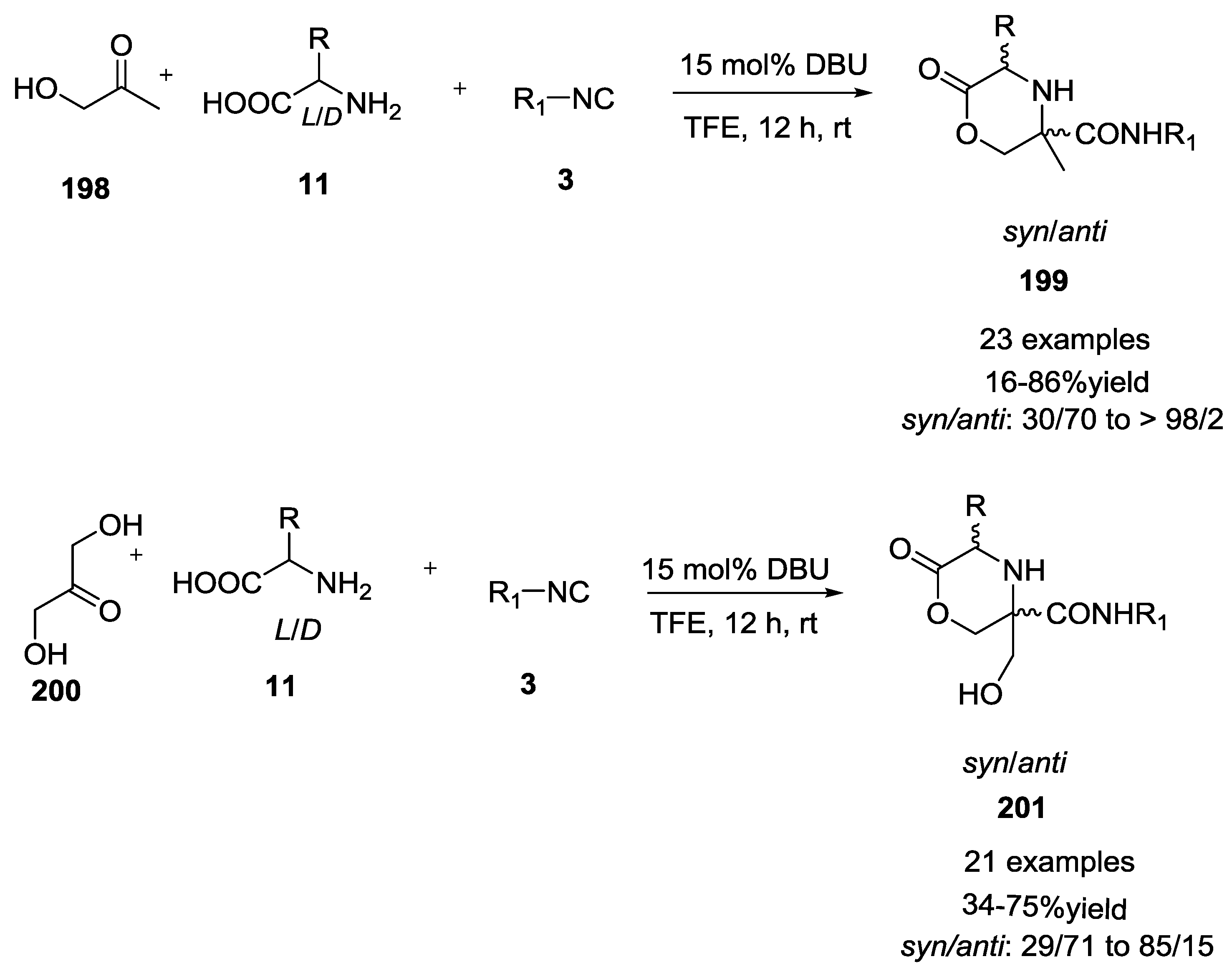
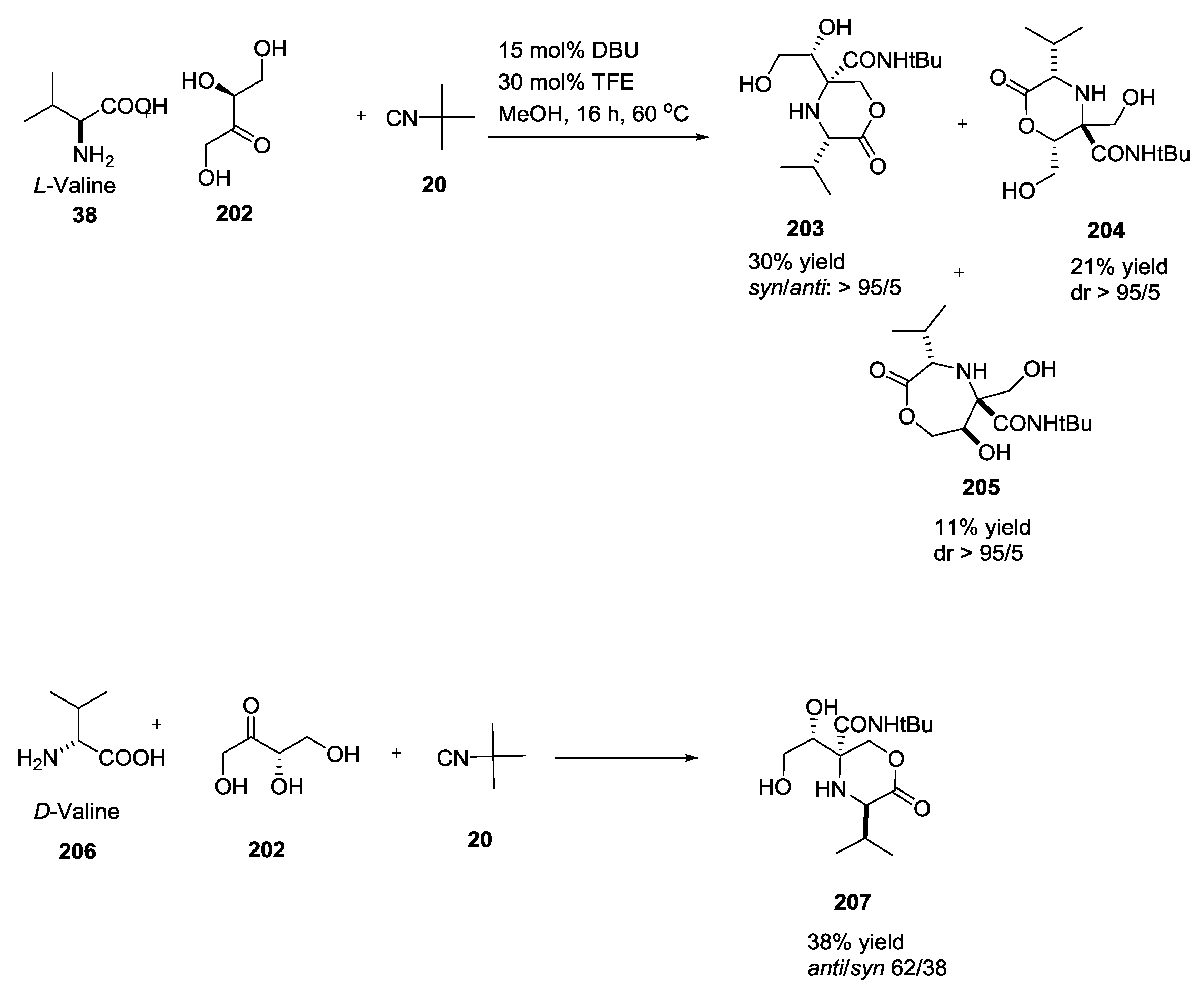
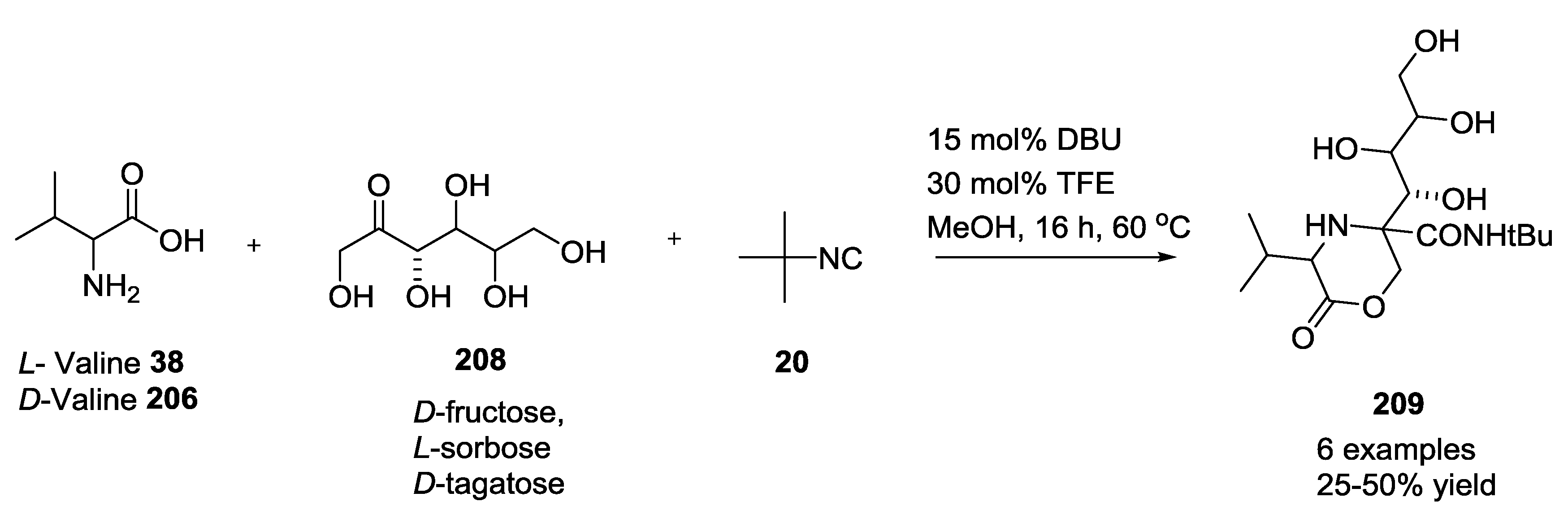
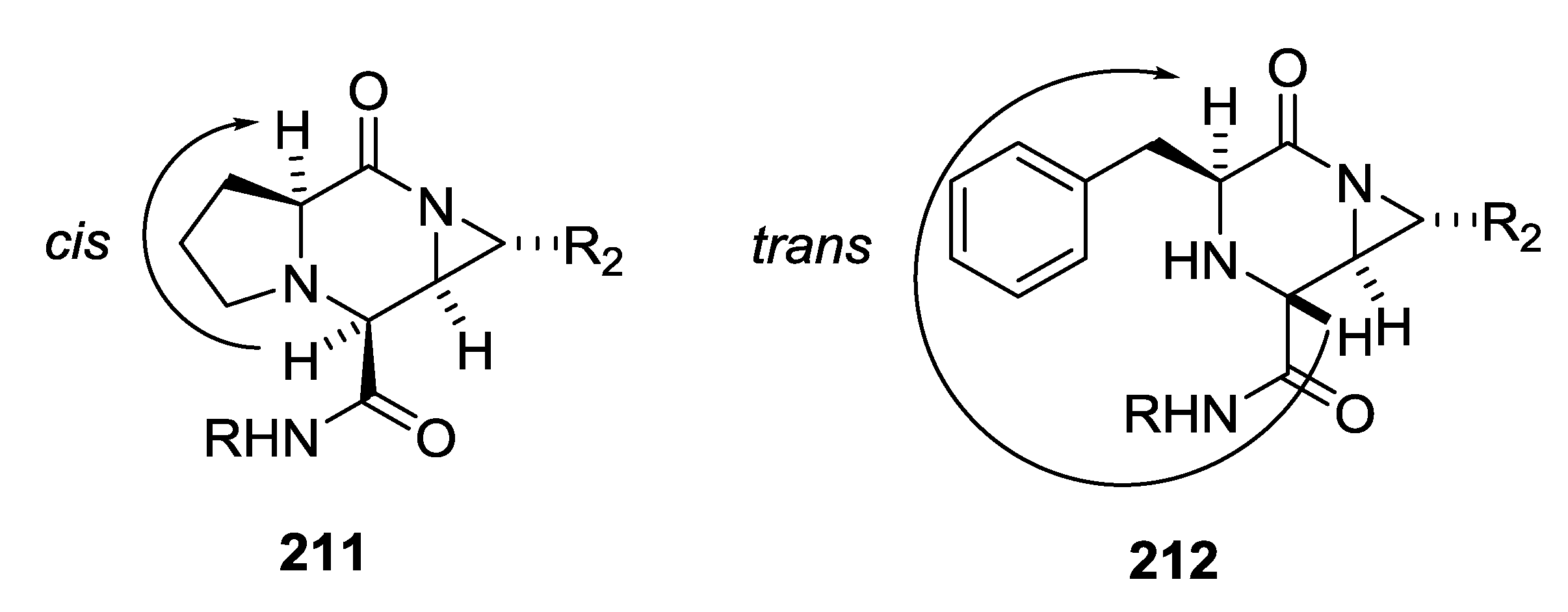
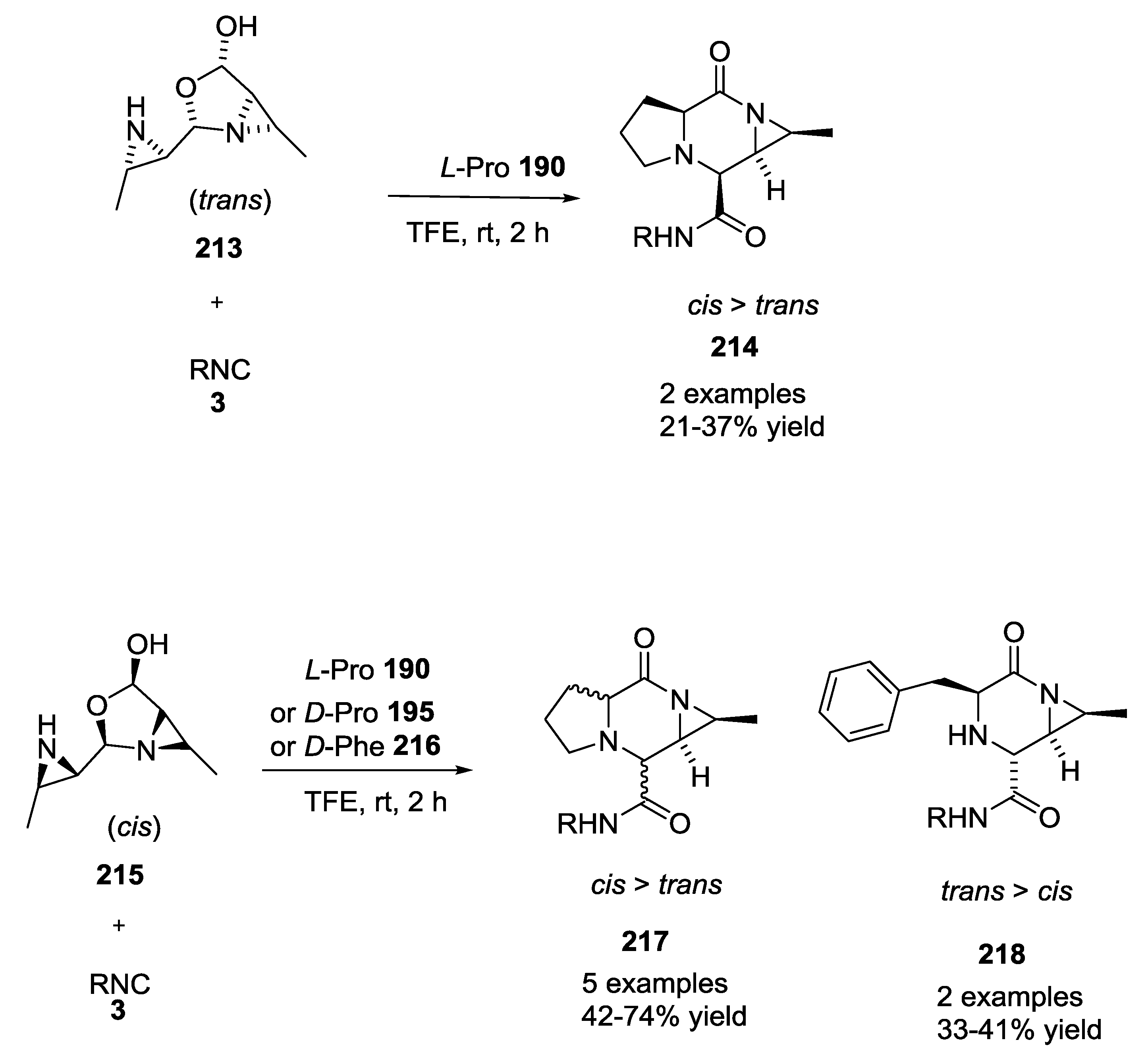
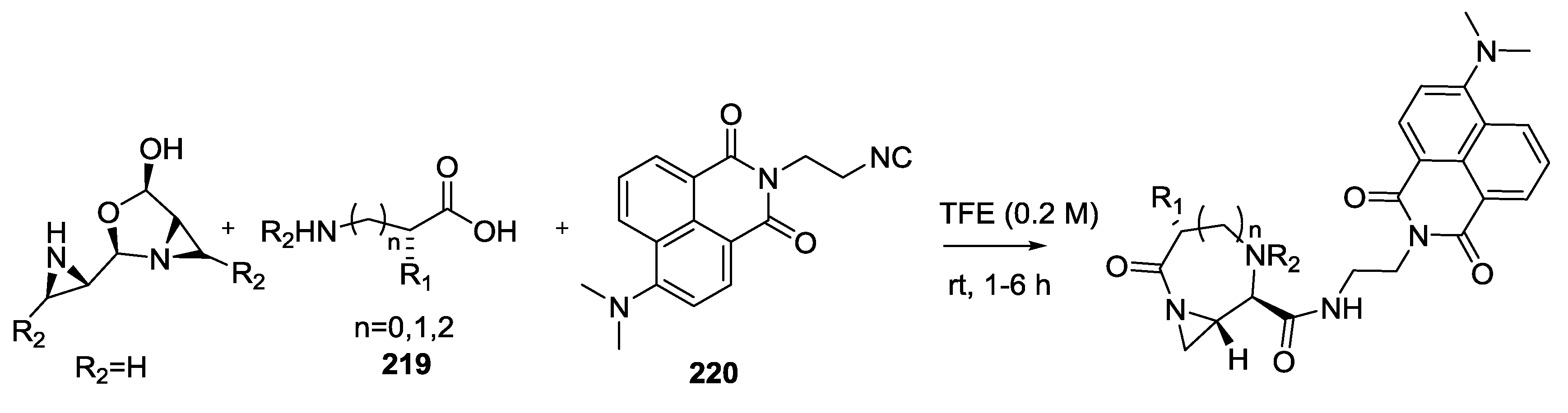
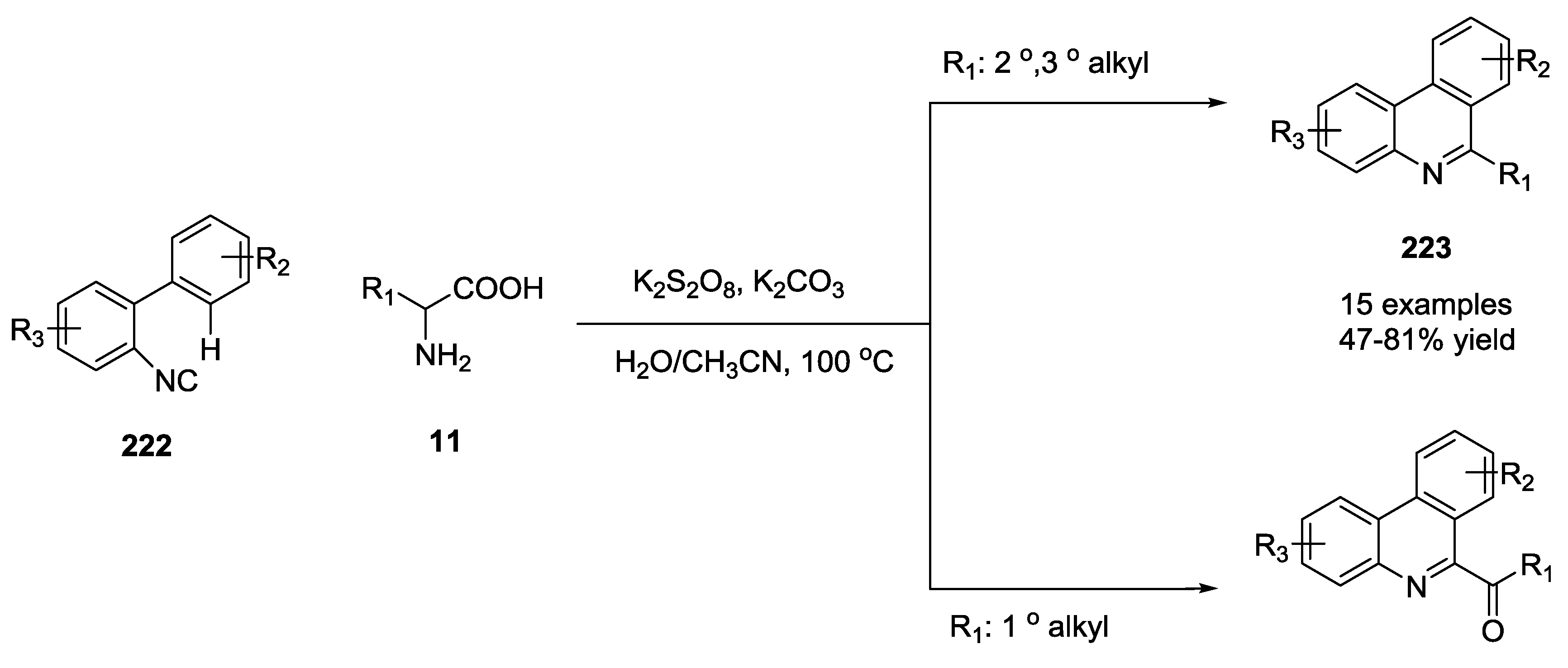
3.4. Combination of Other Bifunctional Starting Materials with α-Amino Acids
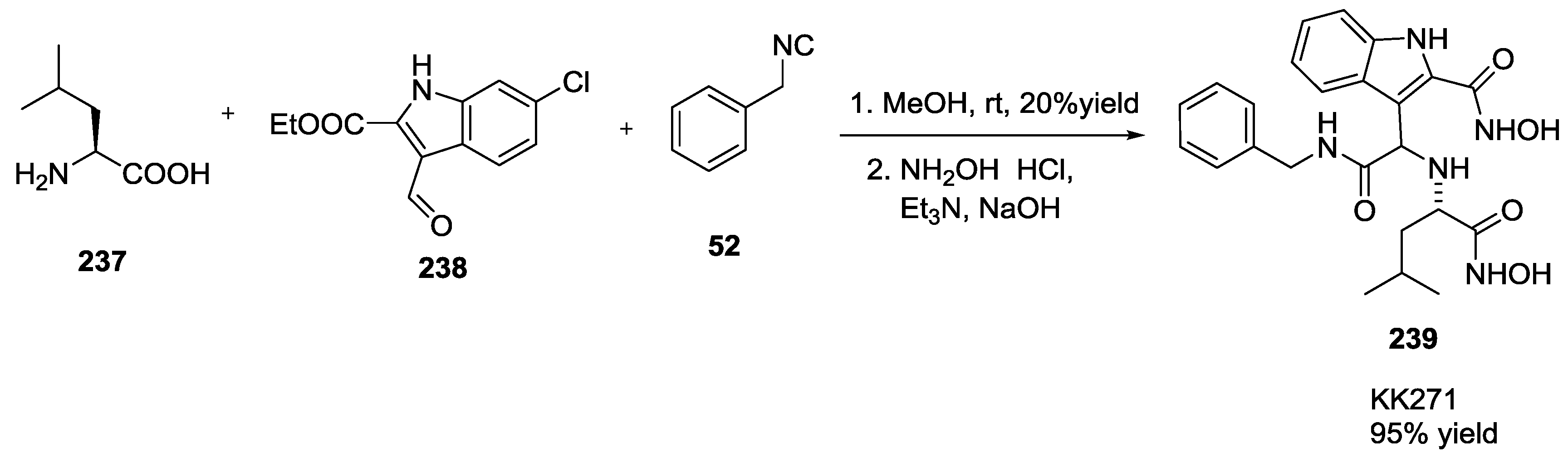
4. Ugi Compounds in Medicinal Chemistry
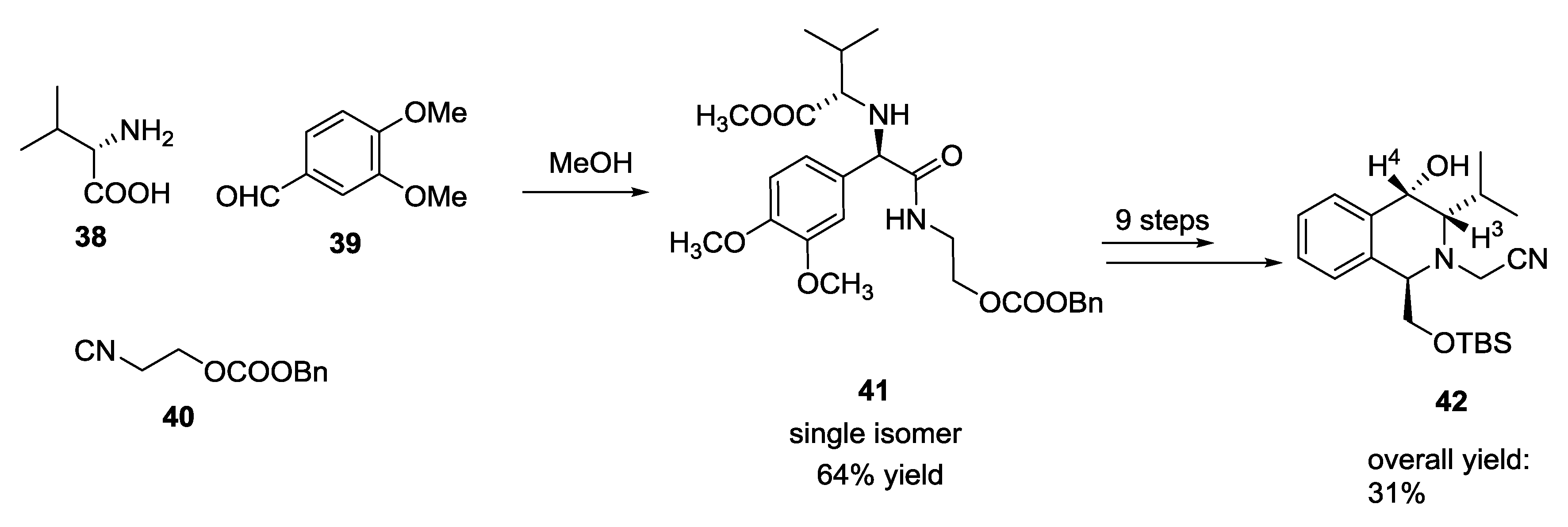
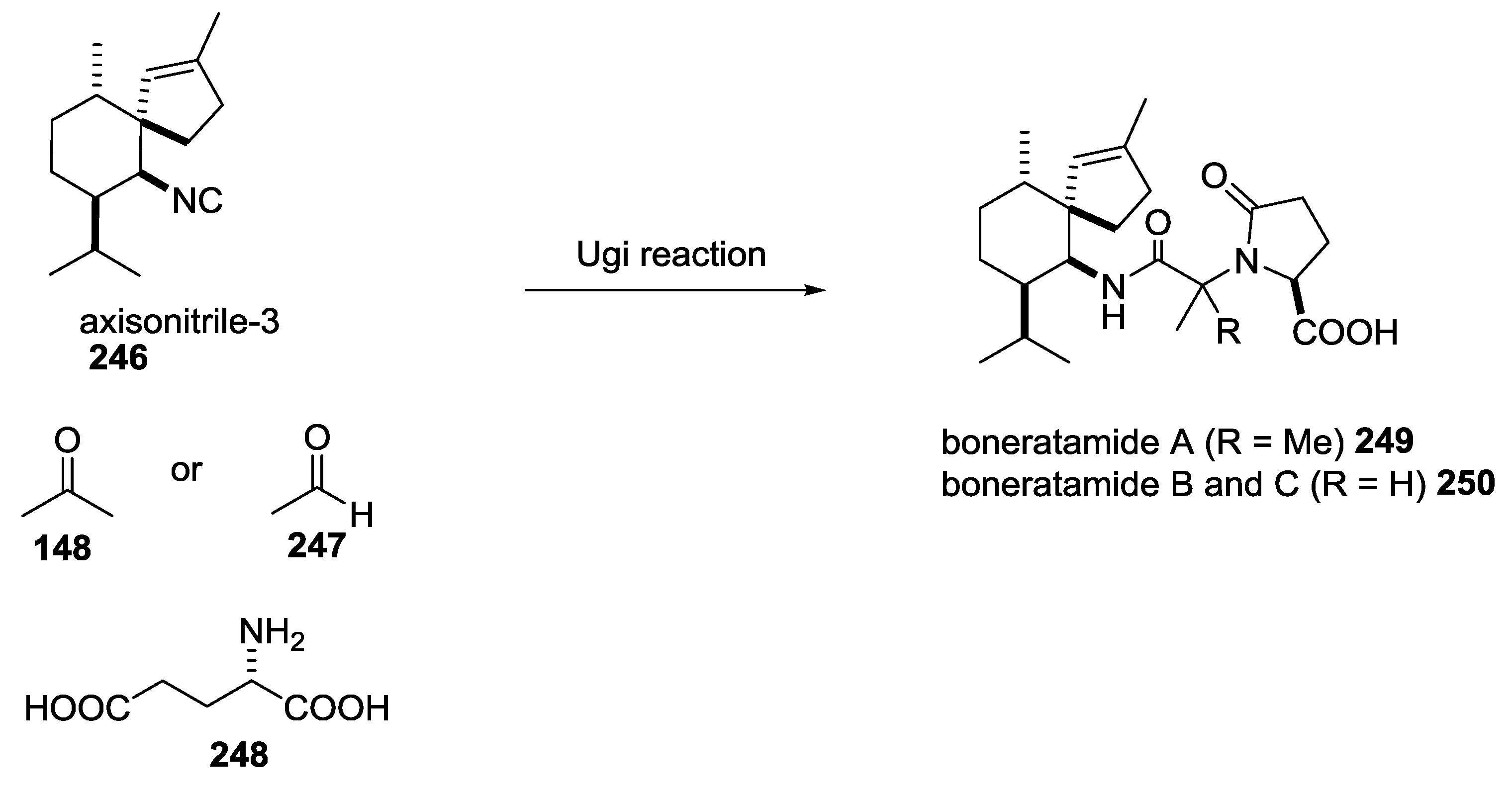
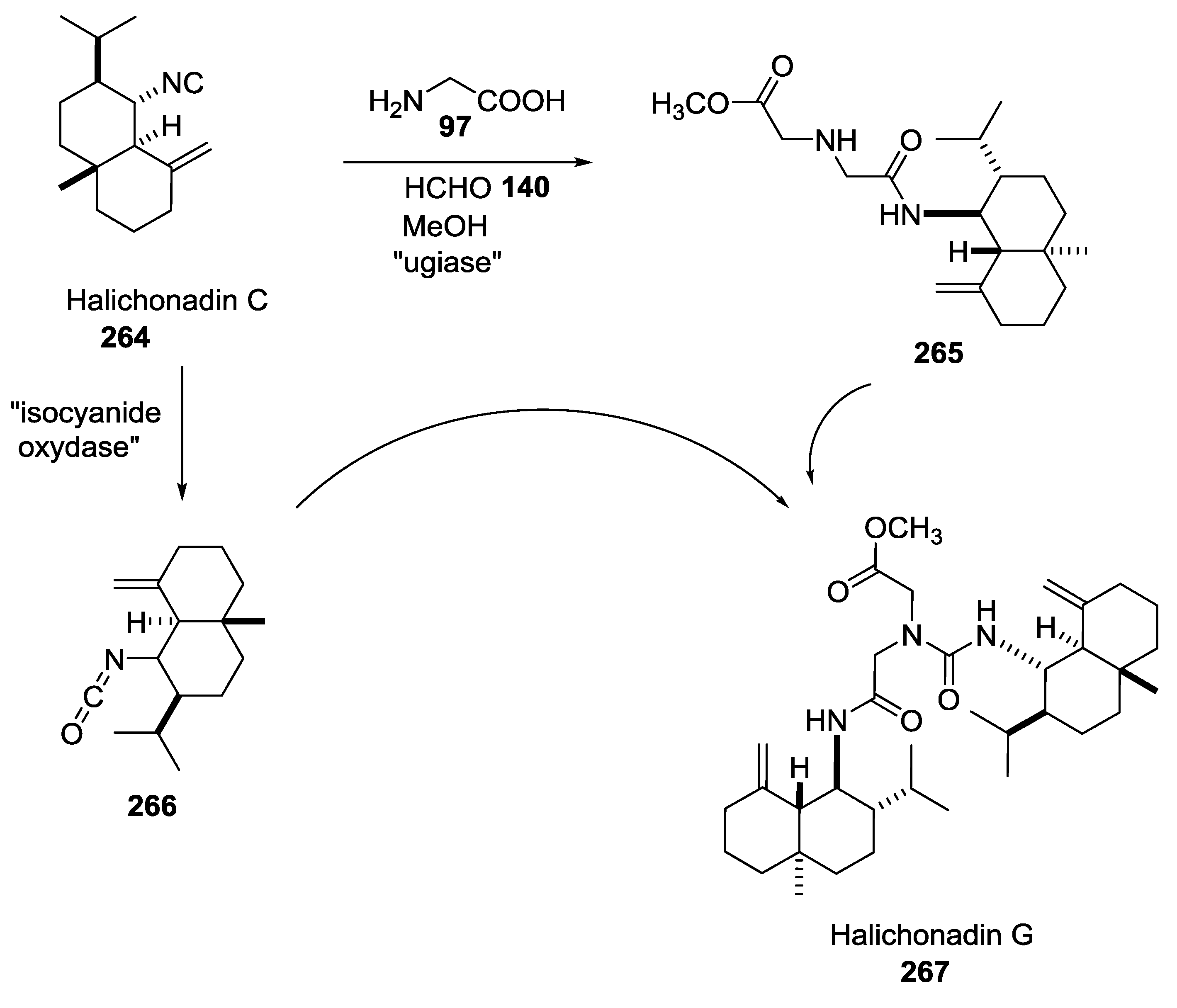
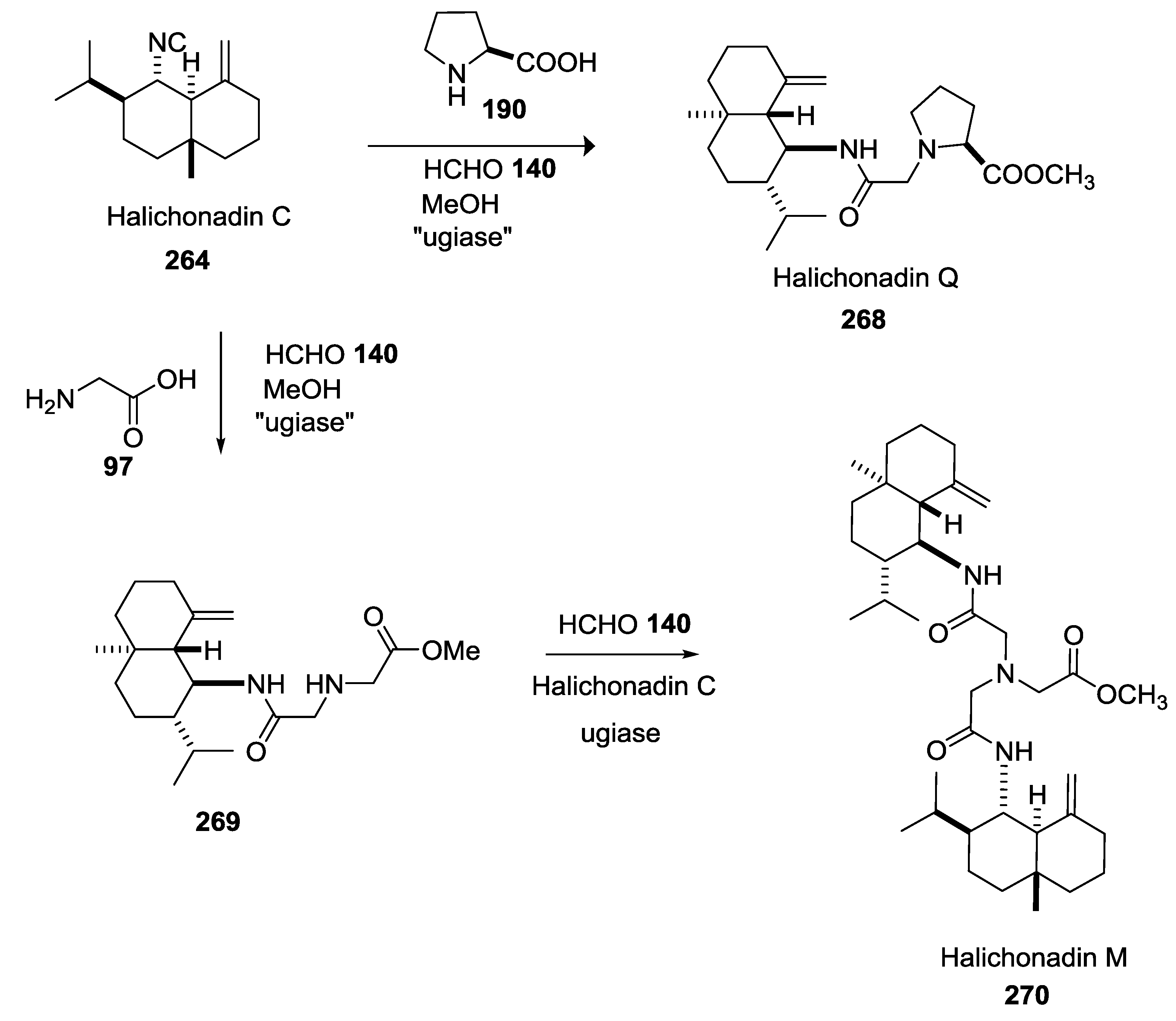
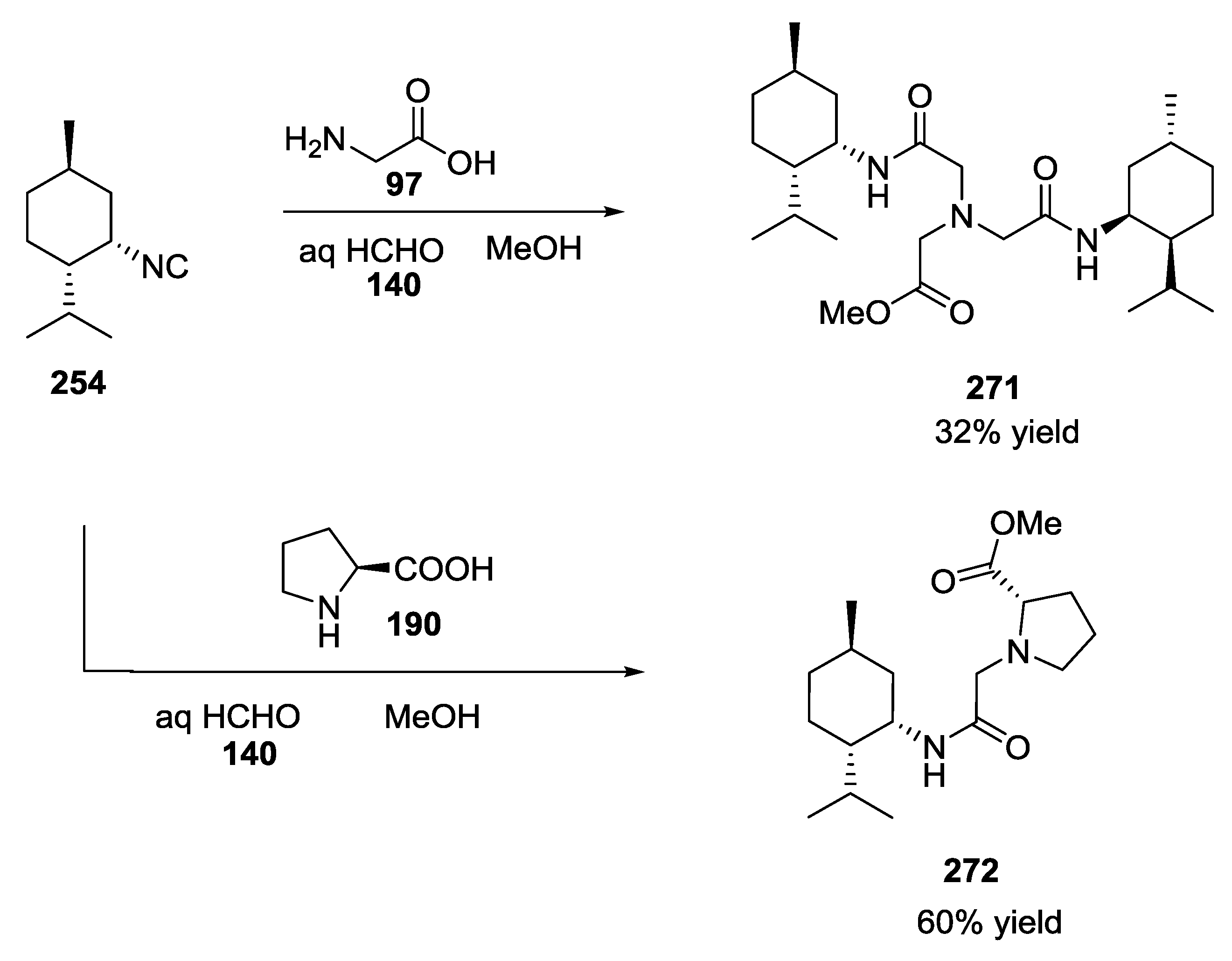
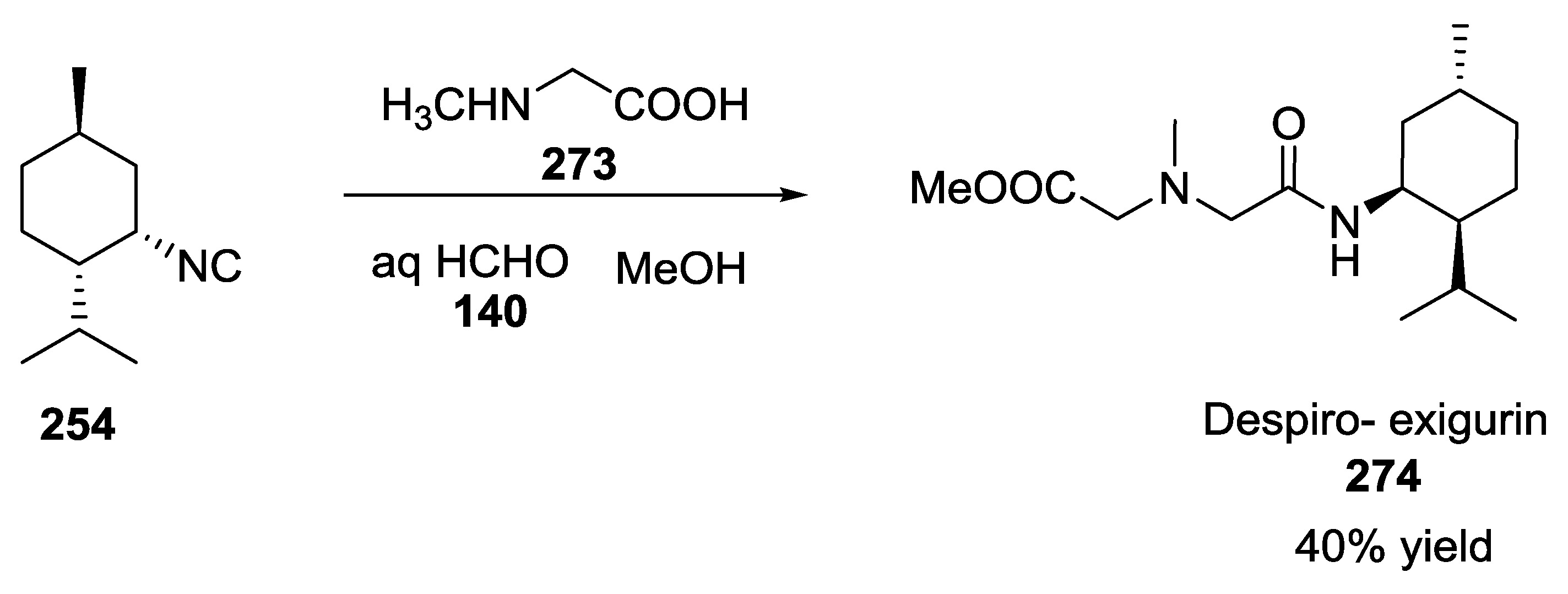
5. UGI-5C-4CR in the Synthesis of Natural Products
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Ugi, I.; Werner, B.; Dömling, A. The chemistry of isocyanides, their multicomponent reactions and their libraries. Molecules 2003, 8, 53–66. [Google Scholar] [CrossRef]
- Dömling, A. Recent advances in isocyanide-based multicomponent chemistry. Curr. Opin. Chem. Biol. 2002, 6, 306–313. [Google Scholar] [CrossRef]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Giustiniano, M.; Novellino, E.; Tron, G.C. Nitrile N-oxides and nitrile imines as new fuels for the discovery of novel isocyanide-based multicomponent reactions. Synthesis 2016, 48, 2721–2731. [Google Scholar] [CrossRef]
- Mercalli, V.; Massarotti, A.; Varese, M.; Giustiniano, M.; Meneghetti, F.; Novellino, E.; Tron, G.C. Multicomponent reaction of Z-chlorooximes, isocyanides, and hydroxylamines as hypernucleophilic traps. A one-pot route to aminodioximes and their transformation into 5-amino-1,2,4-oxadiazoles by mitsunobu–beckmann rearrangement. J. Org. Chem. 2015, 80, 9652–9661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Liu, J.; Xie, Z.; Luan, S.; Xiao, C.; Tao, Y.; Wang, X. Ugi reaction of natural amino acids: A general route toward facile synthesis of polypeptoids for bioapplications. ACS Macro Lett. 2016, 5, 1049–1054. [Google Scholar] [CrossRef]
- Demharter, A.; Hörl, W.; Herdtweck, E.; Ugi, I. Synthesis of chiral 1,1′-iminodicarboxylic acid derivatives from α-amino acids, aldehydes, isocyanides, and alcohols by the diastereoselective five-center–four-component reaction. Angew. Chem. Int. Ed. 1996, 35, 173–175. [Google Scholar] [CrossRef]
- Ugi, I.; Demharter, A.; Hörl, W.; Schmid, T. Ugi reactions with trifunctional α-amino acids, aldehydes, isocyanides and alcohols. Tetrahedron 1996, 52, 11657–11664. [Google Scholar] [CrossRef]
- Silva, E.H.B.; Emery, F.S.; Ponte, G.D.; Donate, P.M. Synthesis of some functionalized peptomers via Ugi four-component reaction. Synth. Commun. 2015, 45, 1761–1767. [Google Scholar] [CrossRef]
- Vazquez, M.P.; Morshed, M.M.; Hickey, J.L.; Poupart, M.-A.; Yang, G.; Gilard, J.; Kafal, A.P.; Roughton, A.L. Fragment Synthesis of Cyclic Peptides. Publication No. WO2017079821A1, 18 May 2017. [Google Scholar]
- Mollica, A.; Pelliccia, S.; Famiglini, V.; Stefanucci, A.; Macedonio, G.; Chiavaroli, A.; Orlando, G.; Brunetti, L.; Ferrante, C.; Pieretti, S.; et al. Exploring the first Rimonabant analog-opioid peptide hybrid compound, as bivalent ligand for CB1 and opioid receptors. J. Enzyme Inhib. Med. Chem. 2017, 32, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Sollis, S.L. Short and novel stereospecific synthesis of trisubstituted 2,5-diketopiperazines. J. Org. Chem. 2005, 70, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Méndez, Y.; Pérez-Labrada, K.; González-Bacerio, J.; Valdés, G.; De Los Chávez, M.Á.; Osuna, J.; Charli, J.-L.; Pascual, I.; Rivera, D.G. Combinatorial multicomponent access to natural-products-inspired peptidomimetics: Discovery of selective inhibitors of microbial metallo-aminopeptidases. ChemMedChem 2014, 9, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Ugi, I.; Hörl, W.; Hanusch-Kompa, C.; Schmid, T.; Herdtweck, E. MCR 6: Chiral 2,6-piperazinediones via Ugi reactions with a-amino acids, carbonyl compounds, isocyanides and alcohols. Heterocycles 1998, 47, 965–975. [Google Scholar] [CrossRef]
- Ugi, I.K.; Ebert, B.; Hörl, W. Formation of 1,1′-iminodicarboxylic acid derivatives, 2,6-diketo-piperazine and dibenzodiazocine-2,6-dione by variations of multicomponent reactions. Chemosphere 2001, 43, 75–81. [Google Scholar] [CrossRef]
- Mjalli, A.M.M. A method for the Synthesis of Compounds of Formula 1 and Derivatives Thereof. Publication No. WO2000043352A1, 27 July 2000. [Google Scholar]
- Sung, K.; Chen, F.-L.; Chung, M.-J. Application of MCR: Facile one-pot diastereoselective syntheses of novel chiral α,α-iminodiacetic acid analogues. Mol. Divers. 2003, 6, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, L.; Chen, R.; Ni, D.; Xia, L. An approach to the synthesis of enantiopure tetrahydroisoquinoline via a key asymmetric Ugi reaction. Synlett 2013, 24, 241–245. [Google Scholar] [CrossRef][Green Version]
- Godet, T.; Bonvin, Y.; Vincent, G.; Merle, D.; Thozet, A.; Ciufolini, M.A. Titanium catalysis in the Ugi reaction of α amino acids with aromatic aldehydes. Org. Lett. 2004, 6, 3281–3284. [Google Scholar] [CrossRef]
- Dawidowski, M.; Sobczak, S.; Wilczek, M.; Kulesza, A.; Turło, J. Expanding the substrate scope of Ugi five-center, four-component reaction U-5C-4CR): Ketones as coupling partners for secondary amino acids. Mol. Divers. 2014, 18, 61–77. [Google Scholar] [CrossRef]
- Wang, W.; Dömling, A. Efficient synthesis of arrays of amino acid derived Ugi products with subsequent amidation. J. Comb. Chem. 2009, 11, 403–409. [Google Scholar] [CrossRef]
- Liu, H.; Dömling, A. One-pot synthesis of highly functionalized seleno amino acid derivatives. Chem. Biol. Drug Des. 2009, 74, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Totaro, K.A.; Okandeji, B.O.; Sello, J.K. Use of a multicomponent reaction for chemoselective derivatization of multiple classes of metabolites. ChemBioChem 2012, 13, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Edjlali, L.; Vessally, E.; Jafari, Z.; Esrafili, M.D. A novel multicomponent reaction between amino acids, aromatic aldehydes and p-toluenesulfonylmethyl isocyanide: An efficient and green one-pot synthesis using nanosilica reaction between amino acids, aromatic aldehydes and ptoluenesulfonylmethyl isocyanide: An efficient and green one-pot synthesis using nanosilica. Green Chem. Lett. Rev. 2016, 9, 13–19. [Google Scholar]
- Mandai, H.; Irie, S.; Mitsudo, K.; Suga, S. Studies on the synthesis of DMAP derivatives by diastereoselective Ugi reactions. Molecules 2011, 16, 8815–8832. [Google Scholar] [CrossRef]
- Mandai, H.; Irie, S.; Akehi, M.; Yuri, K.; Yoden, M.; Mitsudo, K.; Suga, S. Kinetic Resolution of Secondary alcohols by chiral DMAP derivatives prepared by the Ugi Multicomponent Reaction. Heterocycles 2013, 87, 329–340. [Google Scholar] [CrossRef]
- Zimmer, R.; Ziemer, A.; Gruner, M.; Brüdgam, I.; Hartl, H.; Reissig, H.-U. Siloxycyclopropanes in Ugi four-component reaction: A new method for the synthesis of highly substituted pyrrolidinone derivatives. Synthesis 2001, 11, 1649–1658. [Google Scholar] [CrossRef]
- Gladow, D.; Senf, D.; Wiecko, J.; Lentz, D.; Zimmer, R.; Reissig, H.-U. New trifluoromethyl-substituted heterocycles by multicomponent reactions of siloxycyclopropanes. Chem. Heterocycl. Compd. 2017, 53, 416–421. [Google Scholar] [CrossRef]
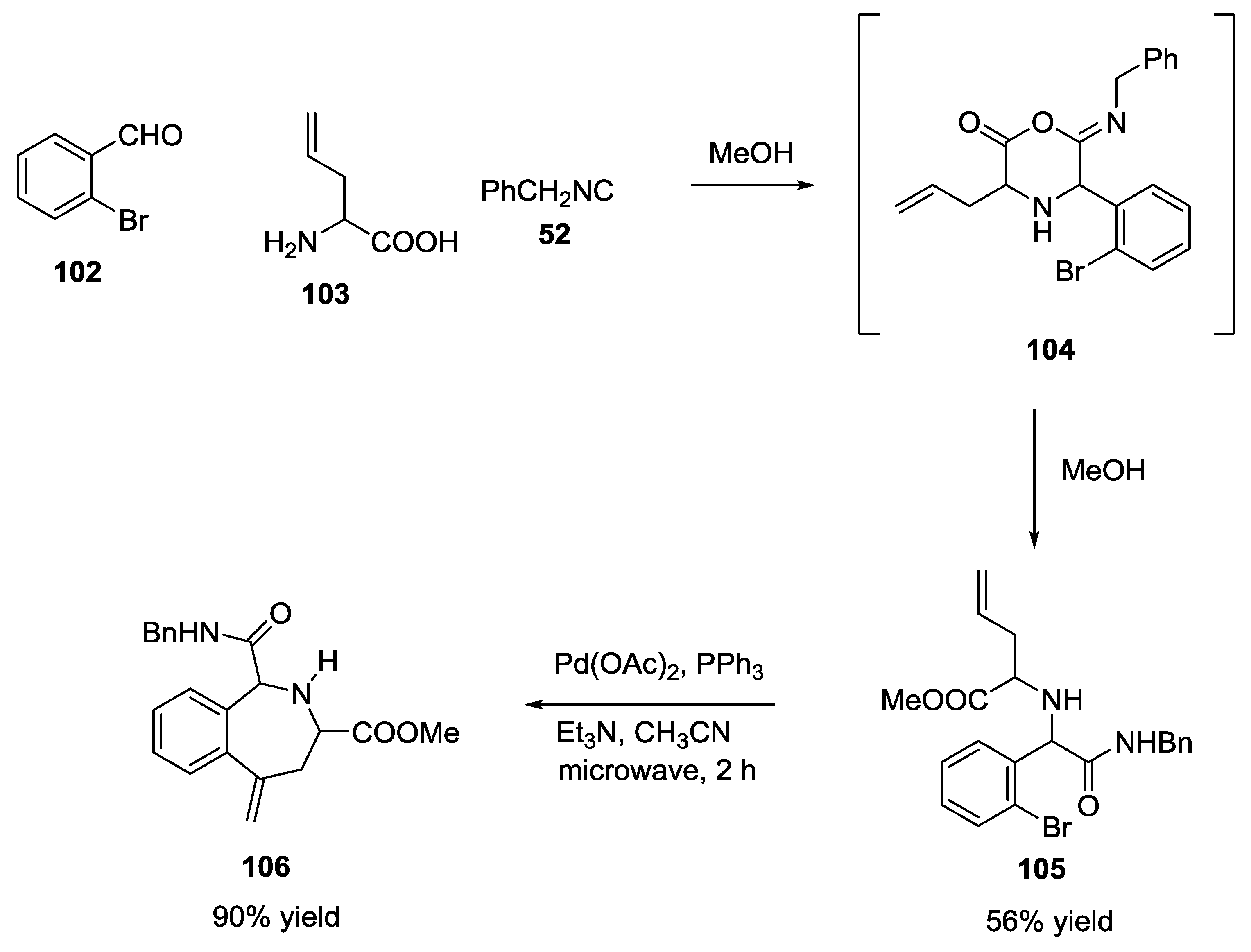
- Gracias, V.; Moore, J.D.; Djuric, S.W. Sequential Ugi/Heck cyclization strategies for the facile construction of highly functionalized N-heterocyclic scaffolds. Tetrahedron Lett. 2004, 45, 417–420. [Google Scholar] [CrossRef]
- Famiglini, V.; Coluccia, A.; Brancale, A.; Pelliccia, S.; La Regina, G.; Silvestri, R. Arylsulfone-based HIV-1 non-nucleoside reverse transcriptase inhibitors. Future Med. Chem. 2013, 5, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
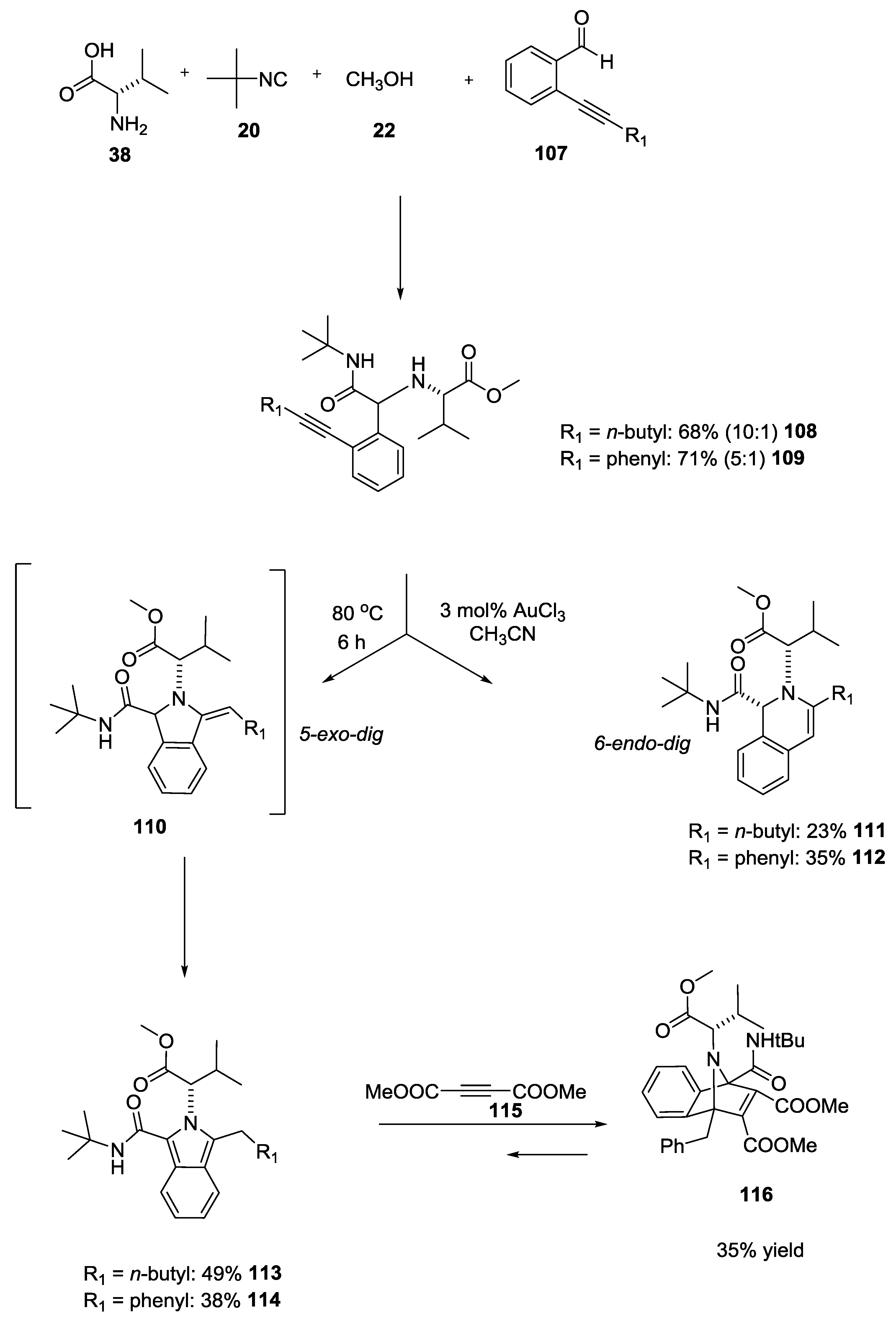
- Kadzimirsz, D.; Hildebrandt, D.; Merz, K.; Dyker, G. Isoindoles and dihydroisoquinolines by gold-catalyzed intramolecular hydroamination of alkynes. Chem. Commun. 2006, 6, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Khoury, K.; Sinha, M.K.; Nagashima, T.; Herdtweck, E.; Dömling, A. Efficient assembly of iminodicarboxamides by a “truly” four-component reaction. Angew. Chem. Int. Ed. 2012, 51, 10280–10283. [Google Scholar] [CrossRef]
- Sinha, M.K.; Khoury, K.; Herdtweck, E.; Dömling, A. Various cyclization scaffolds by a truly Ugi 4-CR. Org. Biomol. Chem. 2013, 11, 4792–4796. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Khoury, K.; Herdtweck, E.; Dömling, A. Tricycles by a new Ugi variation and pictet–spengler reaction in one pot. Chem. Eur. J. 2013, 19, 8048–8052. [Google Scholar] [CrossRef] [PubMed]
- Madhavachary, R.; Wang, Q.; Dömling, A. With unprotected amino acids to tetrazolo Peptidomimetics. Chem. Commun. 2017, 53, 8549–8552. [Google Scholar] [CrossRef]
- Elders, N.; Van der Born, D.; Hendrickx, L.J.D.; Timmer, B.J.J.; Krause, A.; Janssen, E.; de Kanter, F.J.J.; Ruijter, E.; Orru, R.V.A. The efficient one-pot reaction of up to eight components by the union of multicomponent reactions. Angew. Chem. Int. Ed. 2009, 48, 5856–5859. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, J.; Martens, J. Vereinfachte Peptidsynthese mit Schutzgruppenfreien Aminosäure- Hydrochloriden nach dem Prinzip der Vierkomponenten- Kondensation. Synthesis 1992, 9, 837–838. [Google Scholar] [CrossRef]
- Hatam, M.; Tehranfar, D.; Martens, J. Single-Step Synthesis of Racemic Di- and Tripeptides derived from unnatural β-hydroxy and β-mercapto α-amino acids by the Ugi Reaction. Synthesis 1994, 6, 619–623. [Google Scholar] [CrossRef]
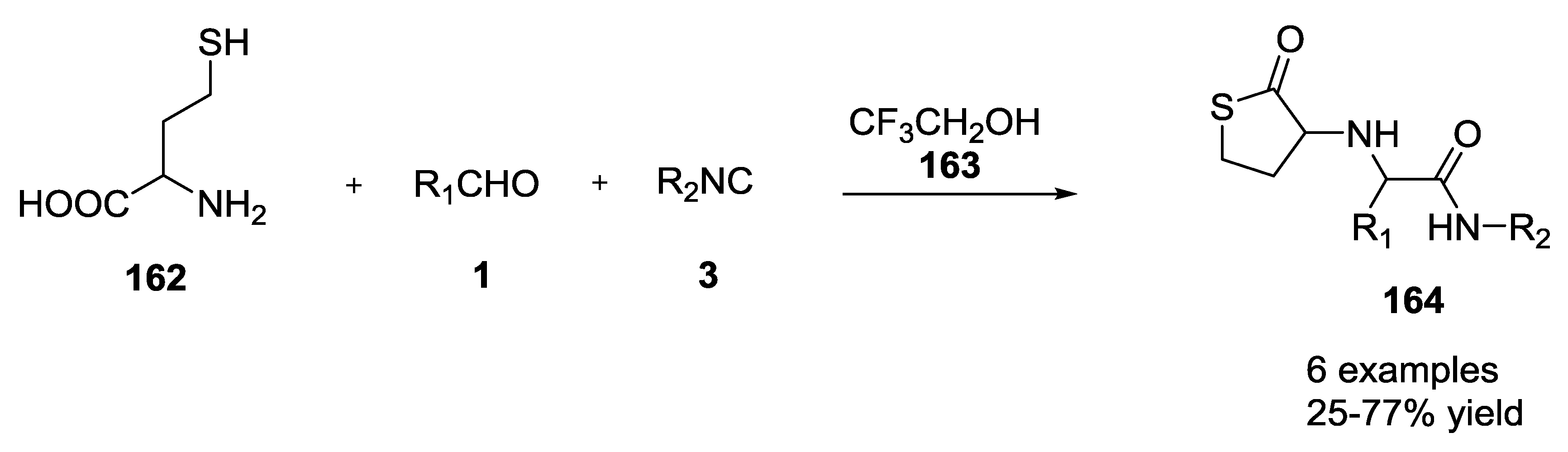
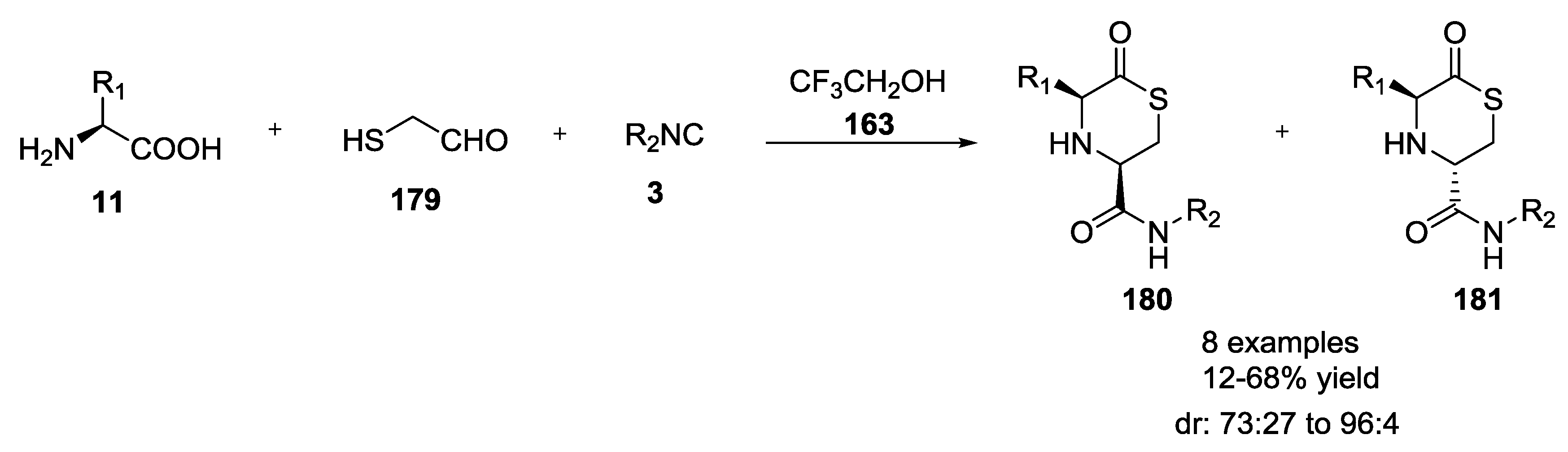
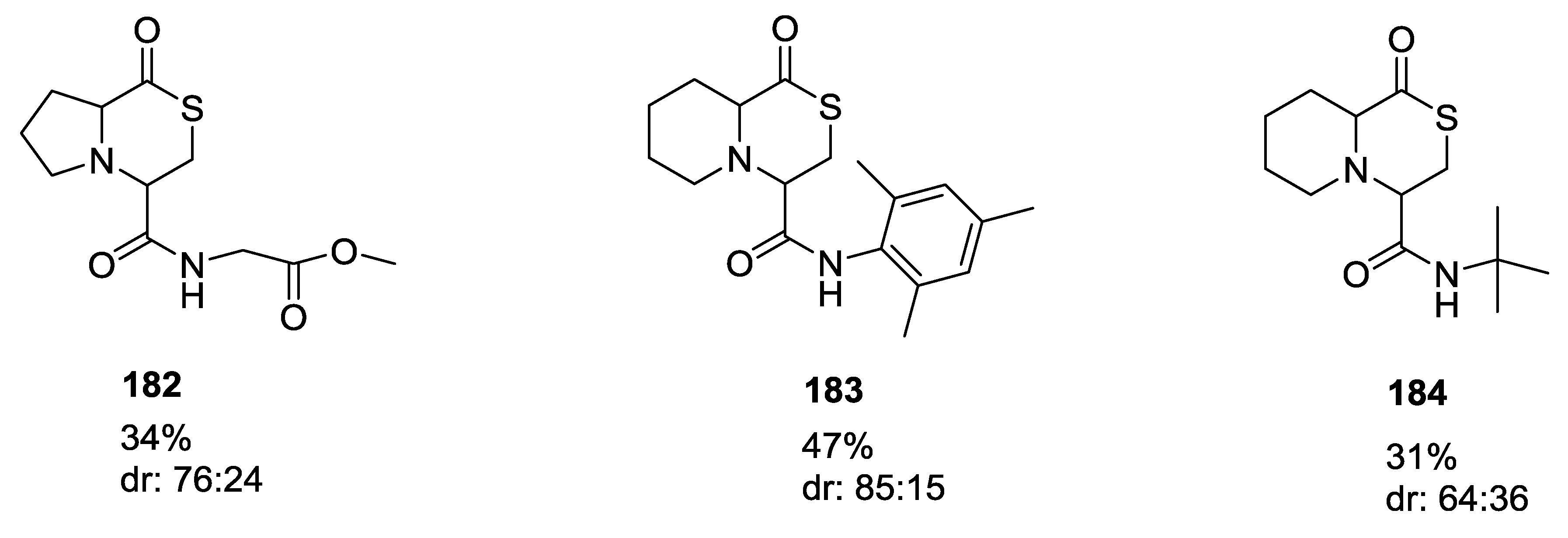
- Beck, B.; Srivastava, S.; Dömling, A. New End-on Thiolactone Scaffold by an isocyanide-based Multicomponent Reaction. Heterocycles 2007, 73, 177–182. [Google Scholar]
- Beck, B.; Srivastava, S.; Khoury, K.; Herdtweck, E.; Dömling, A. One-pot multicomponent synthesis of two novel thiolactone scaffolds. Mol. Divers. 2010, 14, 479–491. [Google Scholar] [CrossRef]
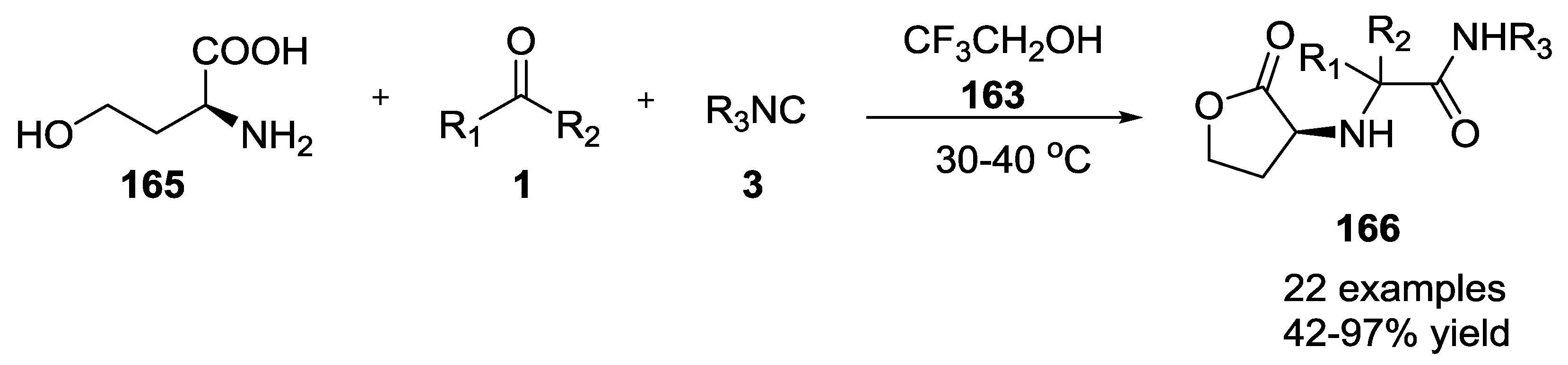
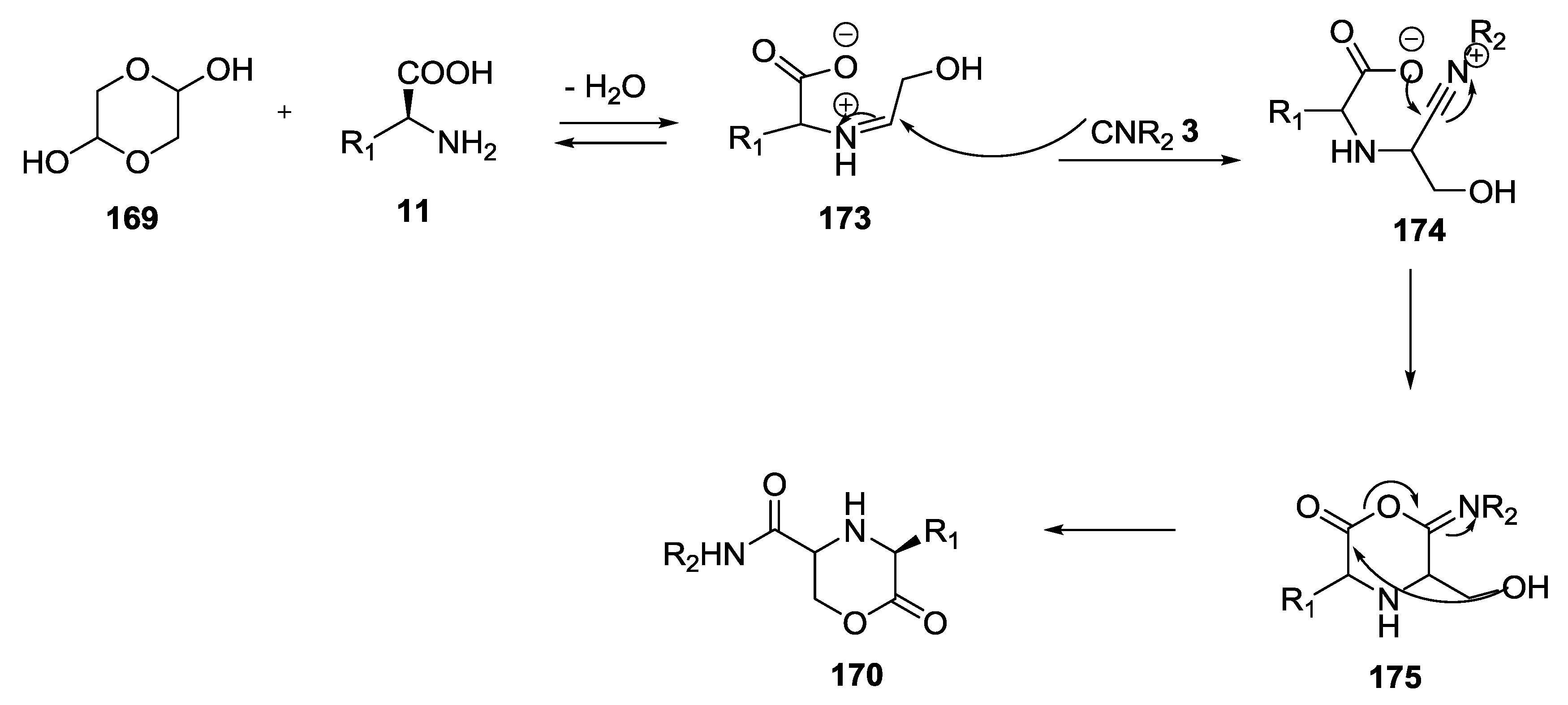
- Park, S.J.; Keum, G.; Kang, S.B.; Koh, H.Y.; Kim, Y.; Lee, D.H. A facile synthesis of N-carbamoylmethyl- α -aminobutyrolactones by the Ugi multicomponent condensation reaction. Tetrahedron Lett. 1998, 39, 7109–7112. [Google Scholar] [CrossRef]
- Kim, Y.B.; Choi, E.H.; Keum, G.; Kang, S.B.; Lee, D.H.; Koh, H.Y.; Kim, Y. An efficient synthesis of morpholin-2-one derivatives using glycolaldehyde dimer by the ugi multicomponent reaction. Org. Lett. 2001, 3, 4149–4152. [Google Scholar] [CrossRef] [PubMed]
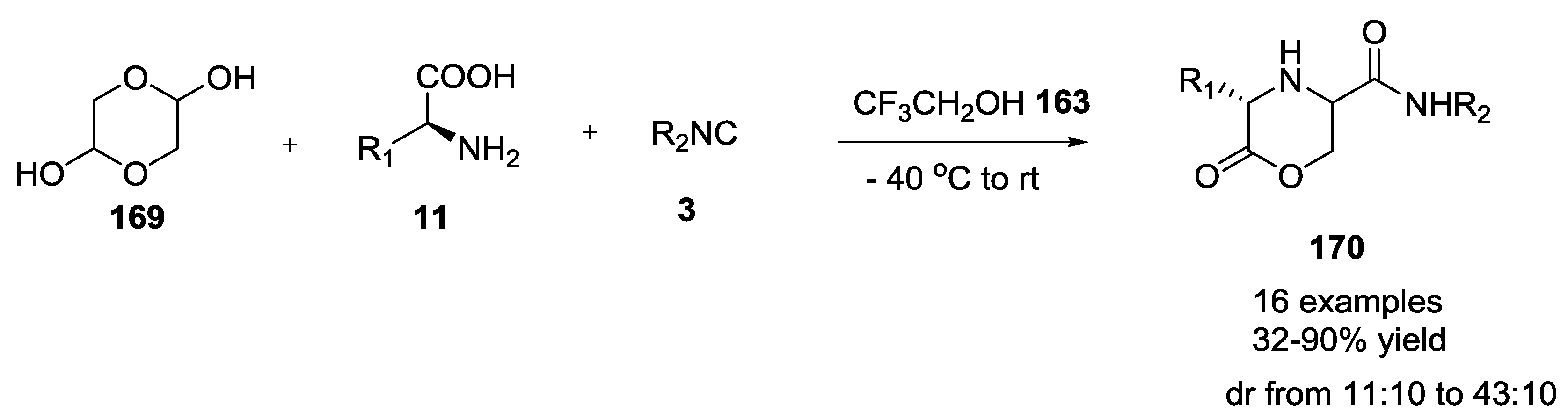
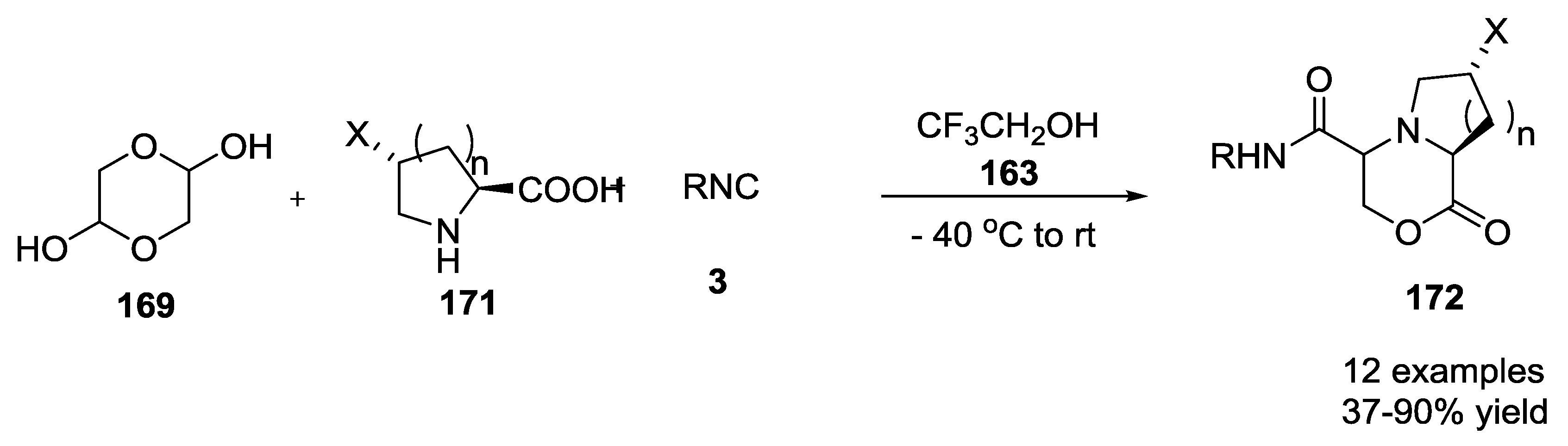
- Torre, A.F.D.L.; Rivera, D.G.; Concepción, O.; Echemendia, R.; Correa, A.G.; Paixão, M.W. Multicomponent synthesis of cyclic depsipeptide mimics by Ugi reaction including cyclic hemiacetals derived from asymmetric organocatalysis. J. Org. Chem. 2016, 81, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Beck, B.; Herdtweck, E.; Khoury, K.; Dömling, A. A novel Δ-thiolactone scaffold by a versatile intramolecular multicomponent reaction. Heterocycles 2009, 77, 731–738. [Google Scholar]
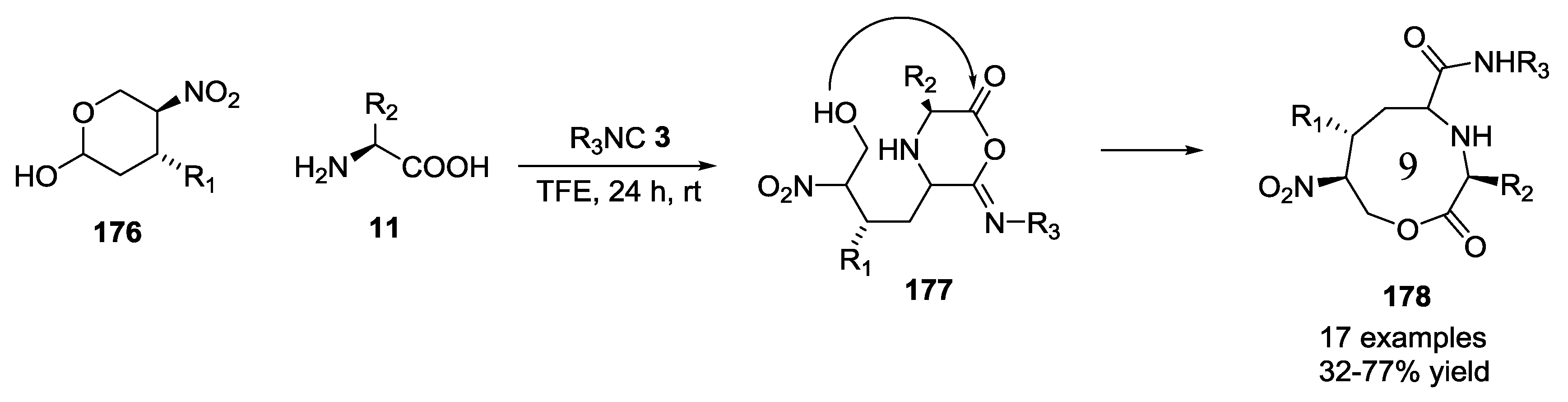
- Voigt, B.; Linke, M.; Mahrwald, R. Multicomponent cascade reactions of unprotected carbohydrates and amino acids. Org. Lett. 2015, 17, 2606–2609. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Trung, M.N.; Mahrwald, R. Multicomponent cascade reactions of unprotected ketoses and amino acids—Access to a defined configured quaternary stereogenic center. J. Org. Chem. 2015, 80, 10849–10865. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.; Hili, R. Cyclic Amino Acid Molecules and Methods of Preparing the Same. Publication No. WO2010105363A1, 23 September 2010. [Google Scholar]
- Heine, N.B.; Kaldas, S.J.; Belding, L.; Shmatova, O.; Dudding, T.; Nenajdenko, V.G.; Studer, A.; Yudin, A.K. Synthesis of chiral piperazinones using amphoteric aziridine aldehyde dimers and functionalized isocyanides. J. Org. Chem. 2016, 81, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, S.; Adachi, S.; Rotstein, B.H.; Hickey, J.L.; Scully, C.C.G.; Denis, J.D.S.; Courtemanche, R.; Yu, J.C.Y.; Chung, B.K.W.; Yudin, A.K. Stereocontrolled disruption of the Ugi reaction toward the production of chiral piperazinones: Substrate scope and process development. J. Org. Chem. 2014, 79, 9948–9957. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Rai, V.; Ryan Hili, R.; Yudin, A.K. Synthesis of peptide macrocycles using unprotected amino aldehydes. Nat. Protoc. 2010, 5, 1813–1822. [Google Scholar] [CrossRef]
- Jebrail, M.J.; Ng, A.H.C.; Rai, V.; Hili, R.; Yudin, A.K.; Wheeler, A.R. Synchronized synthesis of peptide-based macrocycles by digital microfluidics. Angew. Chem. 2010, 122, 8807–8811. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Mourtada, R.; Kelley, S.O.; Yudin, A.K. Solvatochromic reagents for multicomponent reactions and their utility in the development of cell-permeable macrocyclic peptide vectors. Chem. Eur. J. 2011, 17, 12257–12261. [Google Scholar] [CrossRef]
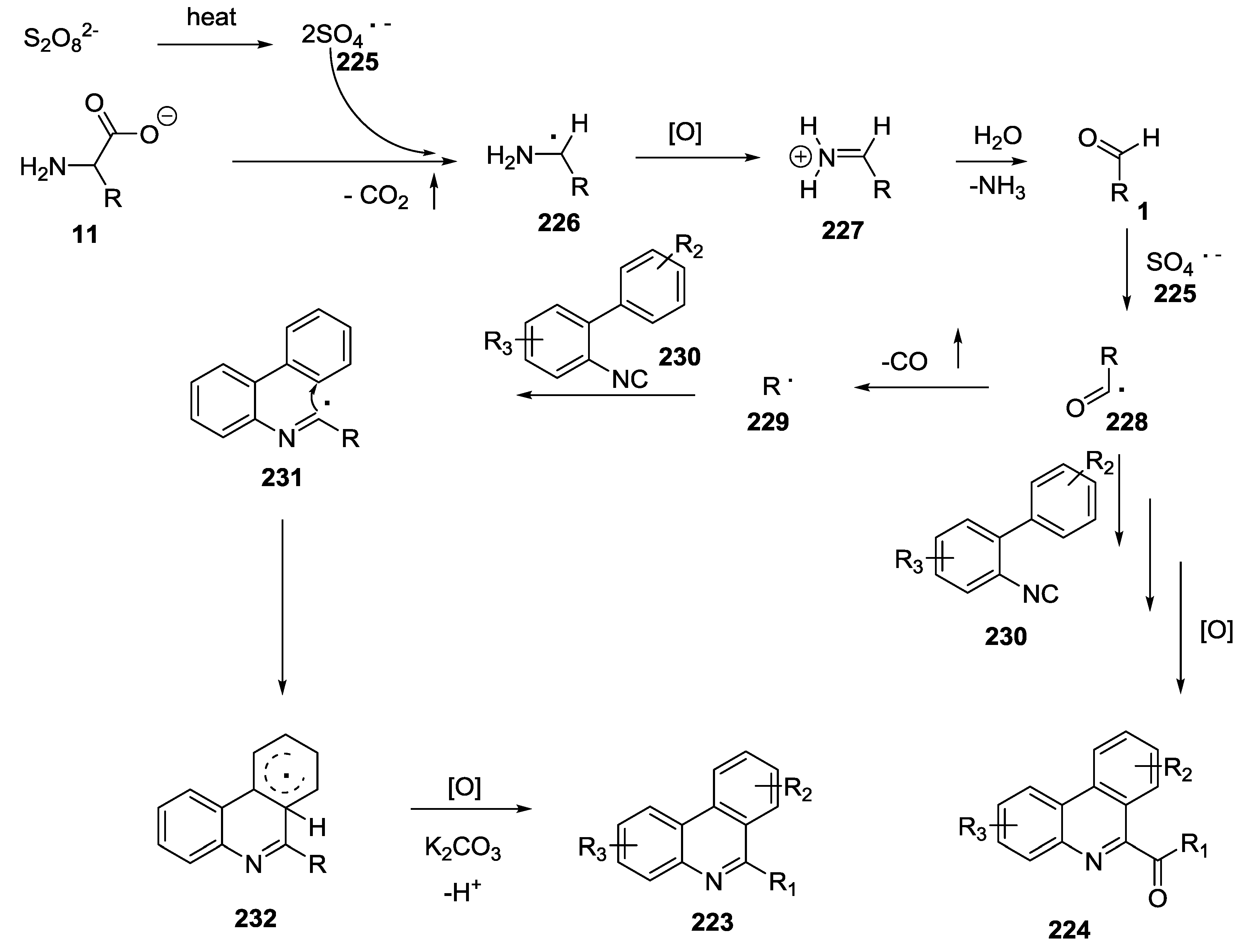
- Lu, S.-C.; Li, H.-S.; Gong, Y.-L.; Wang, X.-L.; Li, F.-R.; Li, F.; Duan, G.-Y.; Xu, S. Use of unprotected amino acids in metal-free tandem radical cyclization reactions: Divergent synthesis of 6-alkyl/acyl phenanthridines. RSC Adv. 2017, 7, 55891–55896. [Google Scholar] [CrossRef]
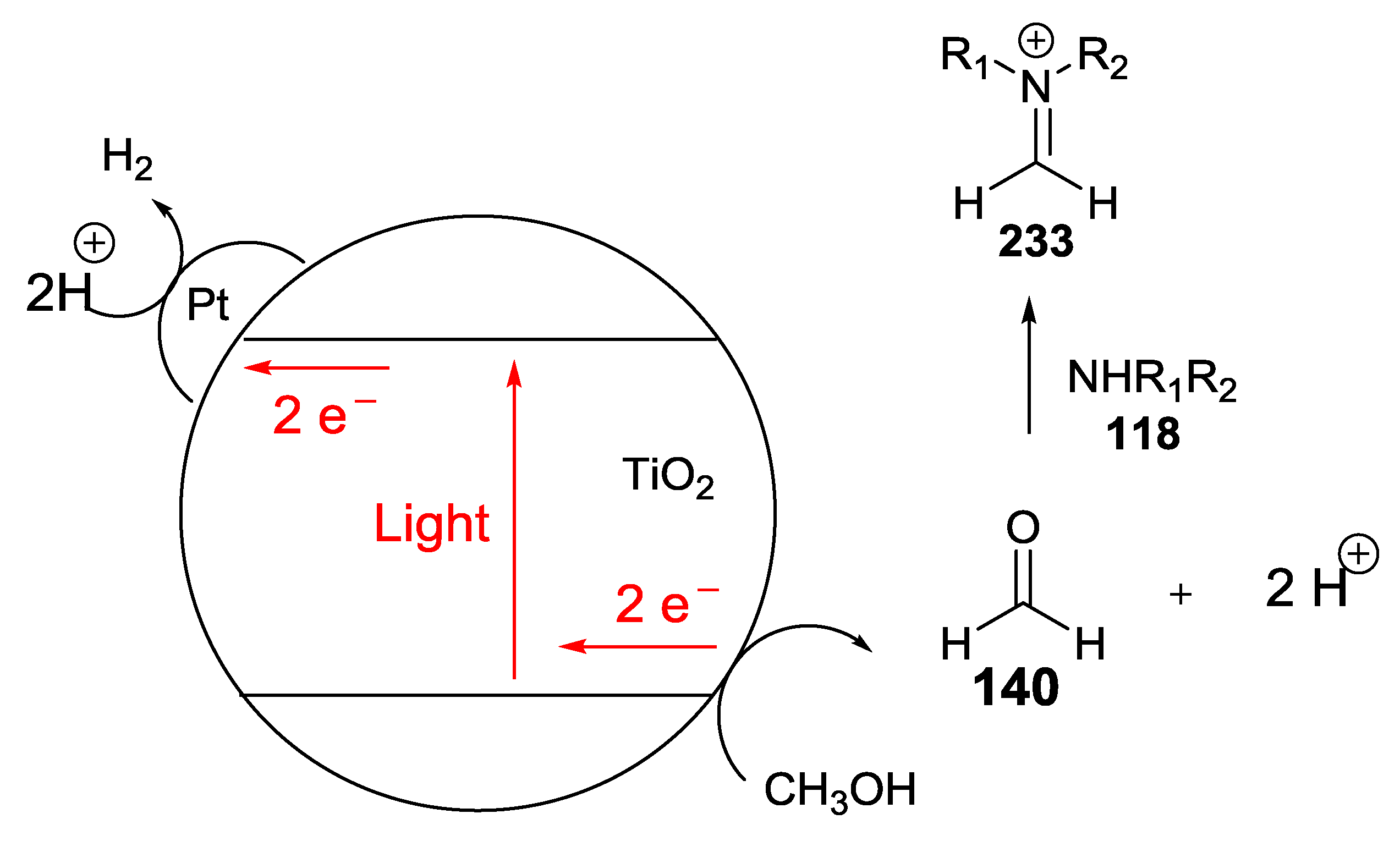
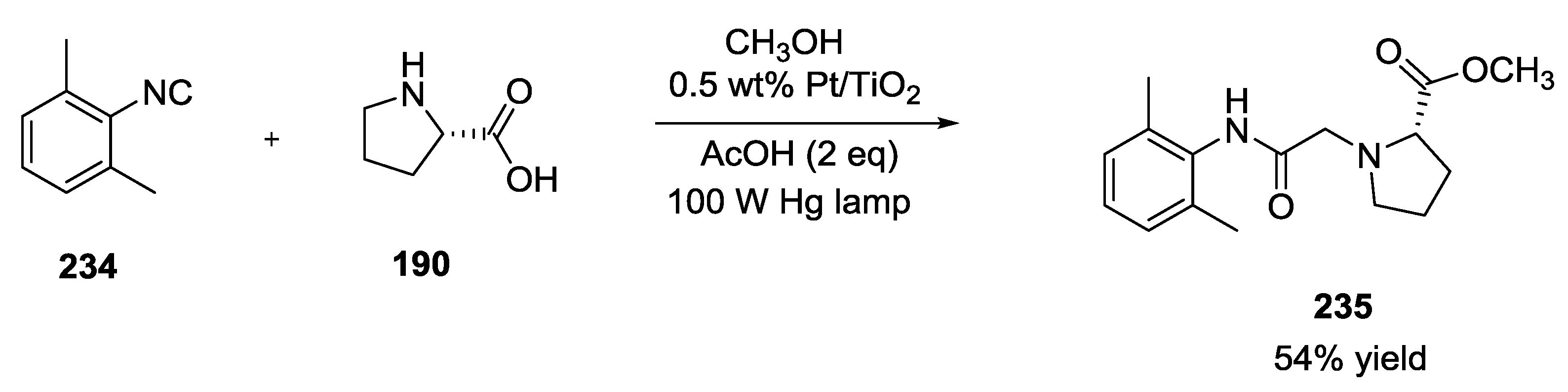
- Adolph, C.M.; Werth, J.; Selvaraj, R.; Wegener, E.C.; Uyeda, C. Dehydrogenative Transformations of Imines Using a Heterogeneous Photocatalyst. J. Org. Chem. 2017, 82, 5959–5965. [Google Scholar] [CrossRef] [PubMed]
- González-Bacerio, J.; Maluf, S.E.C.; Méndez, Y.; Pascual, I.; Florent, I.; Melo, P.M.; Budu, A.; Ferreira, J.C.; Moreno, E.; Carmona, A.K.; et al. KBE009: An antimalarial bestatin-like inhibitor of the Plasmodium falciparum M1 aminopeptidase discovered in an Ugi multicomponent reaction-derived peptidomimetic library. Bioorg. Med. Chem. 2017, 25, 4628–4636. [Google Scholar] [CrossRef] [PubMed]
- Bista, M.; Wolf, S.; Khoury, K.; Kowalska, K.; Huang, Y.; Wrona, E.; Arciniega, M.; Popowicz, G.M.; Holak, T.A.; Dömling, A. Transient protein states in designing inhibitors of the MDM2-p53 interaction. Structure 2013, 21, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Ku, I.W.; Cho, S.; Doddareddy, M.R.; Jang, M.S.; Keum, G.; Lee, J.-H.; Chung, B.Y.; Kim, Y.; Rhim, H.; Kang, S.B. Morpholin-2-one derivatives as novel selective T-type Ca2 channel blockers. Bioorg. Med. Chem. Lett. 2006, 16, 5244–5248. [Google Scholar] [CrossRef]
- Dawidowski, M.; Turło, J. Multicomponent synthesis and anticonvulsant activity of monocyclic 2,6-diketopiperazine derivatives. Med. Chem. Res. 2014, 23, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D.; Hatley, R.J.; Hickey, D.M.B.; Liddle, J.; Livermore, D.G.H.; Mason, A.M.; Miller, N.D.; Nerozzi, F.; Sollis, S.L.; Szardenings, A.K.; et al. Substituted Diketopiperazines as Oxytocin Antagonists. Publication No. WO2003053443A1, 3 July 2003. [Google Scholar]
- Ichikawa, Y.; Saito, K.; Nishimori, A.; Kotsuki, H.; Nakano, K. A biomimetic approach to terpenes isolated from marine sponges: A Ugi coupling reaction in a hypothetical biosynthesis. Synlett 2013, 24, 757–761. [Google Scholar] [CrossRef][Green Version]
- Chianese, G.; Yu, H.-B.; Yang, F.; Sirignano, C.; Luciano, P.; Han, B.-N.; Khan, S.; Lin, H.-W.; Taglialatela-Scafati, O. PPAR modulating polyketides from a chinese plakortis simplex and clues on the origin of their chemodiversity. J. Org. Chem. 2016, 81, 5135–5143. [Google Scholar] [CrossRef]
- Saito, K.; Nishimori, A.; Mimura, R.; Nakano, K.; Kotsuki, H.; Masuda, T.; Ichikawa, Y. A biomimetic approach to the synthesis of the core structure of the marine sponge terpene halichonadin G. Eur. J. Org. Chem. 2013, 31, 7041–7043. [Google Scholar] [CrossRef]
- Mimura, R.; Kitamori, A.; Ikeda, A.; Masuda, T.; Nakano, K.; Kotsuki, H.; Ichikawa, Y. Biomimetic approaches employing the Ugi five-center four- component reaction for synthesis of the right-hand portion of halichonadin Q and the central part of halichonadin M. Synthesis 2015, 47, 3043–3048. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Saito, K.; Mimura, R.; Kitamori, A.; Matsukawa, A.; Ikeda, A.; Masuda, T.; Kotsuki, H.; Nakano, K. A Biomimetic Approach to the synthesis of terpene-amino acid conjugates. The Ugi reaction in the hypothetical biosynthesis of marine natural products. Heterocycles 2016, 92, 1040–1053. [Google Scholar] [CrossRef]
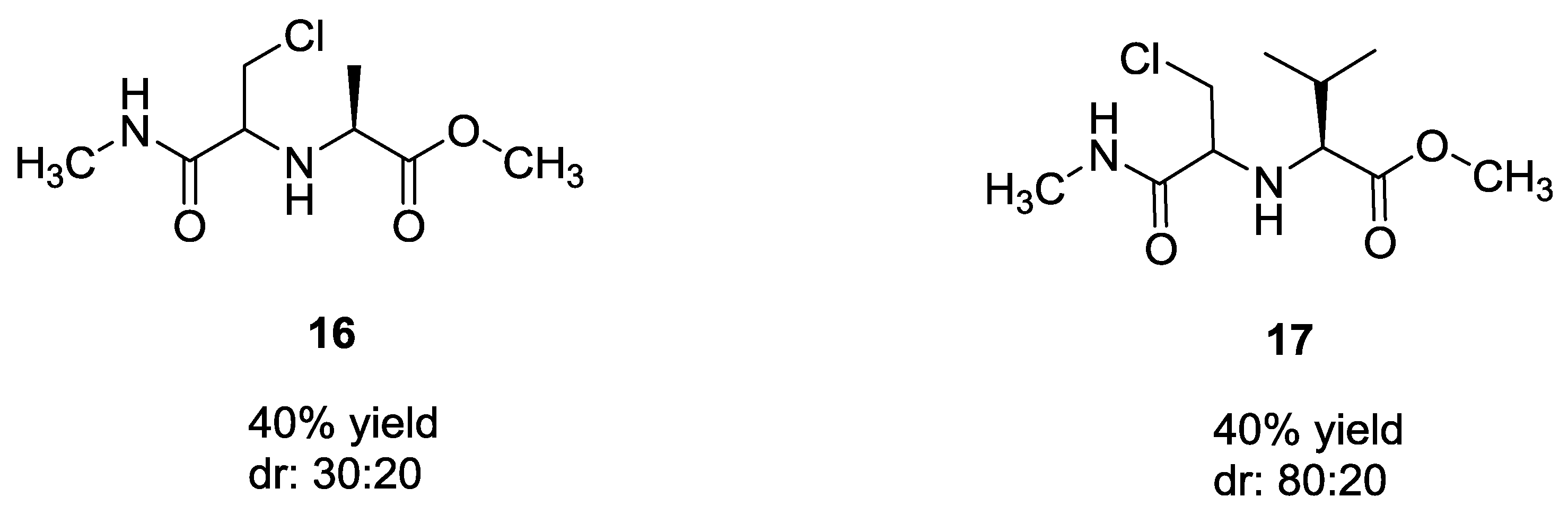
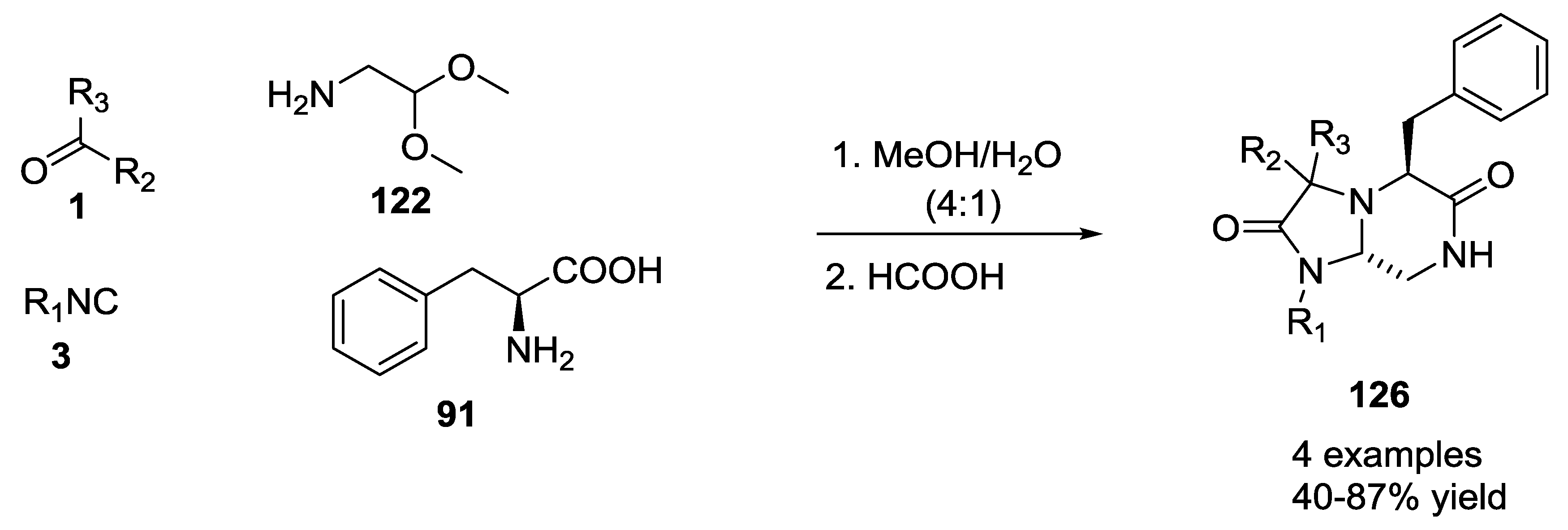

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelliccia, S.; Alfano, I.A.; Galli, U.; Novellino, E.; Giustiniano, M.; Tron, G.C. α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications. Symmetry 2019, 11, 798. https://doi.org/10.3390/sym11060798
Pelliccia S, Alfano IA, Galli U, Novellino E, Giustiniano M, Tron GC. α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications. Symmetry. 2019; 11(6):798. https://doi.org/10.3390/sym11060798
Chicago/Turabian StylePelliccia, Sveva, Ilenia Antonella Alfano, Ubaldina Galli, Ettore Novellino, Mariateresa Giustiniano, and Gian Cesare Tron. 2019. "α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications" Symmetry 11, no. 6: 798. https://doi.org/10.3390/sym11060798
APA StylePelliccia, S., Alfano, I. A., Galli, U., Novellino, E., Giustiniano, M., & Tron, G. C. (2019). α-Amino Acids as Synthons in the Ugi-5-Centers-4-Components Reaction: Chemistry and Applications. Symmetry, 11(6), 798. https://doi.org/10.3390/sym11060798






