Abstract
Since the realization in the 1930s that elevated fluoride concentrations in drinking water can have detrimental effects on human health, new methods have been progressively developed in order to reduce fluoride to acceptable levels. In the developing world the necessity for filtration media that are both low-cost and sourced from locally available materials has resulted in the widespread use of bone char. Since the early 1990s metallic iron (Fe0) has received widespread use as both an adsorbent and a reducing agent for the removal of a wide range of contaminant species from water. The ion-selectivity of Fe0 is dictated by the positively charged surface of iron (hydr)oxides at circumneutral pH. This suggests that Fe0 could potentially be applied as suitable filter media for the negatively charged fluoride ion. This communication seeks to demonstrate from a theoretical basis and using empirical data from the literature the suitability of Fe0 filters for fluoride removal. The work concludes that Fe0-bearing materials, such as steel wool, hold good promise as low-cost, readily available and highly effective decentralized fluoride treatment materials.
1. Introduction
The realization that elevated fluoride levels in drinking water can cause tooth enamel degradation (via dental fluorosis) dates back to 1931 [1]. This discovery subsequently obliged two U.S. cities (Bauxite, Arkansas and Oakley, Idaho) to discard abundant water supplies in favor of those containing lower fluoride concentrations [2]. However, for some localities it became apparent that it is extremely technically difficult to find alternative water sources that contain low fluorine levels [2,3,4]. To date, countries including China, Estonia, Ethiopia, Ghana, India, Kenya, Rwanda, South Africa, Tanzania, and Thailand lack regions which have fluoride below acceptable concentrations for drinking [5,6,7,8,9,10,11,12,13]. Accordingly, removing excess fluoride from drinking water is a scientific challenge with worldwide significance [14,15,16,17].
Boruff [18] was among the first researchers who developed a methodology for the removal of excess fluoride from drinking water [2,3,4,19]. Among other materials, aluminum oxides and hydroxides received widespread attention [13]. As an example, activated alumina (AA) is synthesized by calcination (~500 °C) of aluminum ore (bauxite) to render it porous and highly adsorptive. The fundamental interfacial properties of AA are the protonation of surface hydroxyl groups resulting in the development of surface charge [13,20]. At pH values <6.0 the surface of AA is positively charged and the adsorption capacity for fluoride is high. In the neutral pH range the affinity of the AA surface for fluoride is much lower. This means that, as far as safe drinking water provision is concerned, AA is used below its capacity. Attempts to optimize the AA efficiency for safe drinking water provision include the development of oxide mixtures (Fe2O3, MgO, MnO2, TiO2) [21,22]. These operations appear to have been successful, but the mechanisms are still unknown [13].
In his pioneering work, Boruff [18] tested aluminum sulfate, sodium aluminate, zeolite, activated alumina and bauxite as Al-based materials. He also investigated the suitability of common adsorbents including silica gel, sodium silicate and ferric salts. Despite 80 years of intensive research since then, investigations into natural adsorbing materials that can be directly used without modifications (no chemical, physical or thermal treatment) and that are readily available and affordable in the developing world are lacking [14,17,23,24,25]. In the absence of such natural materials, an alternative is to test and use any manufactured material that is abundantly available and potentially affordable. Because manufactured materials of concern were not designed for water treatment, pre-treatment (e.g., degreasing) is typically required.
This work aims to establish from a theoretic basis and by using empirical data published in the literature the potential use of Fe0 for the removal of fluoride (F−) from drinking water. Fe0 filters were introduced around 25 years ago for groundwater remediation [26,27,28,29,30,31,32,33,34,35,36,37] and have received interest for both wastewater treatment [38] and safe drinking water provision [39,40]. However, Fe0 has received very little attention as a filter medium for fluoride removal. In fact, only three peer-reviewed articles on fluoride removal by Fe0 could be found in the literature: Fakhri and Adami [41] and Jahin [42] used nanoscale Fe0 in batch systems to remove excessive fluoride from water and Jeong et al. [43] treated industrial wastewater in Fe0/sand columns operating with 600 V (external power supply).
Herein an overview of contemporary technologies for fluoride removal from water is first given before presenting Fe0 as a new potential technology for decentralized defluoridation in the developing world.
2. Existing Technologies for F− Removal and Their Application in the Developing World
2.1. Water Quality Monitoring
The suitability of a water source for any use (including drinking) should be confirmed by laboratory analysis in order to determine its biological, chemical and physical composition. Accordingly, the first step to enable (universal) access to drinking water is to equip (and accredit) analytical laboratories worldwide and make them accessible. Based on analytical results there are three options: (i) do nothing because the water source is good for human consumption; (ii) mix contaminated water with clean water (dilution) in order to meet the guideline requirements; and (iii) treat the contaminated water.
In the developed world, district and/or province councils permanently monitor the biological and chemical properties of drinking water and make the results available to citizens. Citizens who are dependent on a private water supply have the possibility to make water analyses in accredited laboratories. Moreover, private suppliers have the obligation to monitor the quality of the supplied water [44]. In the developing world, analytical laboratories are scarce and, where available, water analysis is not affordable for the majority of the population.
This presentation underlines this discrepancy. In the era of modern analytical instrumentation, this discrepancy is not acceptable. Accordingly, a mandatory pre-requisite to universal access to safe drinking water is to equip water laboratories in the developing world [40].
2.2. Water Treatment Technologies for Fluoride (F−) Removal
Conventional F− treatment methods, which are typically applied in centralized treatment plants, include reverse osmosis, electrodialysis, coagulation-flocculation-filtration and adsorption with activated alumina [9,13,25]. In contrast, the most popular alternative (often decentralized) F− treatment methods are: (i) adsorption with local materials (clays and soils, brick chips); (ii) adsorption with activated alumina and bone char; and (iii) the Nalgonda technique [5,8,24,25,45,46]. Water filtration with biomaterials and bone char will be presented in the next section as common in the developing world (Table 1).

Table 1.
Availability of common adsorbents for aqueous fluoride removal.
| Material | Comments | Availability |
|---|---|---|
| Metal oxides and hydroxides | Exhibit relatively low efficiency without physico-chemical modification | low |
| Biosorbents | Exhibit relatively low efficiency without physico-chemical modification | very high |
| Geomaterials | Exhibit relatively low efficiency without physico-chemical modification | high |
| Carbonaceous Materials | Typically require expensive physico-chemical activation | low |
| Bone char | Produced by carbonizing animal bones | high |
| Industrial by-Products | Typically require expensive physico-chemical activation | low |
| Metallic iron (Fe0) | Under natural conditions a ubiquitous (hydr)oxide layer is also present | high |
2.3. Biomaterial-Based Filters for F− Removal
There is growing interest in the application of plant biomass for aqueous F− removal [47,48,49,50,51,52]. The main advantage of plant biomass is that it is readily available and can operate at circumneutral pH. The F− removal mechanism by this class of material is not well established. It is currently understood that the driving force for F− removal relies on its affinity for several species present in the biomass structure (e.g., Ca2+, Mg2+, OH− and –NH2 groups) [48,49,53,54]. Several plant materials such as bark from Moringa olifera and Emblica officinalis, as well as the roots from Vetiveria zizanoides and the leaves from Cyanodon tactylon sisal, have been determined as effective defluoridating agents ([52] and refs. cited therein). Literature reports that tea leaves could quantitatively remove fluoride from a aqueous 20 mg/L solution [55], Tamarind (Tamarindus indica) fruit cover powder treated with HCl could remove up to 57.1% of fluoride from natural water containing 3.5 mg/L F− at the pH of 7.6 [47]. A comparative study using a synthetic solution containing fluoride at 2.0 mg/L showed that biomass from vetiver (Vetiveria zizanoides) roots, tamarind seed (Tamarindus indica), clove (Eugenia carryophyllata), neem (Azardirachta indica), acacia (Acacia catechu willd), nutmeg (Myristica fragrans) and coffee husk (Coffea arabica) could remove up to 80%, 75%, 70%, 52%, 47%, 45% and 38% of fluoride ions, respectively, at neutral pH [49]. A more recent comparative study reported the F− removal efficiencies of maize leaf, goose grass, banana false stem, untreated sisal fiber and aloe vera leaf and sisal pith biomass to be 4.1%, 4.6%, 7.1%, 26.6%, 29.4% and 47.3%, respectively.
2.4. Bone Char-Based Filters for F− Removal
Bone char (BC) is generally considered the most efficient and affordable material for F− removal in decentralized safe drinking water production at present [25,56,57,58,59]. The four main reasons for this are: (i) BC is relatively inexpensive; (ii) BC is made from locally abundantly available spent bone; (iii) BC can be manufactured in sufficient amounts and quality for local needs, and (iv) BC can be recycled via a simple thermal treatment process at 200 °C.
BC predominantly consists of hydoxyapatite [Ca10(PO4)6(OH)2] [2,59,60,61]. BC therefore retains fluoride by chemisorption and ion exchange between dissolved F− ions and PO43−. Electrostatic attraction of dissolved F− is also known to occur in parallel with F− adsorbed via physisorption. The dominating mechanism of fluoride removal has therefore not been fully elucidated [59,62,63]; however, it is certain that the kinetics of hydoxyapatite dissolution limit the extent of ion exchange.
BC has been used as a versatile adsorbent for a wide variety of other pollutants, including arsenate, dyes and heavy metals [59]. Therefore, BC has the potential to be used universally for decentralized safe drinking water provision in chemical-free systems. However, some attempts to use locally produced BC for aqueous F− removal have not been successful [8]. Two major reasons for this are: (i) a societal aversion exists for the handling of fresh bone; and (ii) BC-filtered water produces an unpleasant taste [11,59]. Accordingly, alternatives to BC are still sought. Fe0 materials in packed bed are a potential candidate.
3. Fe0 Filters
Fe0-based filters have been demonstrated as capable of removing/inactivating different types of biological and chemical pollutants from water [64,65,66,67,68,69,70,71,72]. The performance of individual filters depends on the Fe0 type (intrinsic reactivity); Fe0 particle size (mm, μm or nm scale); filter design (e.g., depth of the reactive layer, dimensions of the filter), type and concentration of complimentary material (e.g., Fe0/sand ratio); water composition (e.g., pH and presence of competing species); and operating conditions (e.g., water flow, temperature). Thus, there is diversity among operational factors that can impact the efficiency of a Fe0 filter. This high degree of diversity for significant factors suggests that only a well-designed systematic approach could identify conditions for optimal operation [40,73,74,75,76,77].
3.1. Historical Background
During the past two decades, great efforts have been made to develop Fe0 filters for use in decentralized water supply systems in developing countries, including Argentina, Bangladesh, Cambodia, Cameroon, Chile, India, Nepal and Vietnam [39,40,64,66,72,78,79,80,81,82,83,84,85,86]. Several Fe0 filters exist and were also developed/optimized by scientists from many institutions worldwide, including the Massachusetts Institute of Technology [78,79], George Mason University [81] and the Swiss Federal Institute of Aquatic Science and Technology [86,87].
Reviewing the history of Fe0 filter development, Noubactep et al. [76] demonstrated that with the exception of the SONO arsenic filters (Sono is the name of a city) [39,81], the volumetric expansive nature of iron corrosion [88,89] was not properly considered. Incorporating the volumetric expansion of Fe0 can be achieved via three ways: (i) decreasing the proportion of Fe0 in the filter [77,90]; (ii) using complimentary porous materials [39,81] and/or (iii) limiting the dissolved O2 concentration in the contaminated water [40,90,91]. Another limitation of the early Fe0 filters was that they were designed for Fe0 to serve either (i) as an electron donor for contaminant reduction [92]; (ii) as an iron oxide generator for species removal by adsorption and co-precipitation [93] or (iii) as an iron oxide generator to sustain sand filtration depending on the species to be removed [94,95,96,97,98,99,100,101].
3.2. The State-of-the Art
A central rationale for the applicability of Fe0 materials for water treatment is due to their ability to produce solid iron (hydr)oxide corrosion products which are highly effective aqueous contaminant-scavenging agents [102,103,104] for negatively charged substances [46,96,105,106], such as F− [40].
Another important feature of the Fe0/H2O system is that both Fe0 oxidative dissolution (Equation (1)) and the precipitation of Fe oxide-hydroxides (Equation (2)) occur in the presence of contaminants. This makes interactions between dissolved species, Fe0, Fe oxide-hydroxides and intermediate species very complex [107,108]. Intermediate species include FeII and H/H2 [109], which are reducing agents, with increased reducing power when already adsorbed onto (nascent) oxides [110,111]. The complexity of processes in Fe0/H2O systems has led to the misconception that Fe0 is a lone reducing agent [26,29,33,36,112,113]. This may explain why Fe0 has not been widely tested for F− removal, i.e., the standard electrode potentials for fluoride (E0 = 2.87 V, Equation (3)) and Fe0 (E0 = 0.44 V, Equation (4)) indicate that Fe0 in theory is unable to reduce F− (i.e., F2 would oxidize Fe0).
Fe0 + 2 H+ ⇒ Fe2+ + H2
Fe2+ + 2 OH− ⇒ Fe(OH)2
2 F− ⇒ F2 + 2 e−
Fe0 ⇒ Fe2+ + 2 e−
There are two other important features of Fe0 corrosion pathways that are essential for the process of contaminant removal: (i) the volume of each oxide (Voxide) is larger than the volume of the parent metallic iron (Viron) [88] and (ii) during the precipitation of oxides, contaminants can become enmeshed (i.e., physically sequestered) [107,108]. The volumetric expansive nature of iron corrosion (Voxide > Viron) implies that, irrespective of any adsorptive affinity between oxides and pollutants, decontamination by size exclusion is improved with the filter service life. On the other hand, “pure physical sequestration” can also occur independent of the Fe (hydr)oxide adsorptive affinity [114]. Therefore, Fe0/H2O systems are generally considered to be more suitable for negatively charged species but are often effective for the removal of other species as well [105,106,115].
Aqueous contaminants are typically removed in Fe0 filters by adsorption, co-precipitation and size-exclusion, making Fe0 filters a reactive filtration as opposed to adsorptive filtration in iron-oxide-coated sand filters. Here, iron oxides are progressively generated in the system [102,116,117,118,119], making the efficiency of a Fe0 filter highly dependent on its physical design (e.g., Fe0 reactivity, filter bed length, flow velocity). As a sinuous and tortuous material with, therefore, significant intrinsic porosity (available voids which can be used to accommodate the expansive Fe0 corrosion products), steel wool (SW) can be considered a suitable candidate for use as a filter material [73,74,75,90,120,121].
3.3. Designing Fe0 Filters for Fluoride Removal
3.3.1. Physico-Chemical Processes in Fe0 Filters
Several processes take place during Fe0 filter operation: (i) in-situ generation of Fe corrosion products; (ii) adsorption of dissolved species onto strained solids and all available surfaces, including Fe0 and its (hydr)oxides; and (iii) biologically induced processes such as biofouling [35,66,69,94,95,109]. Depending on the Fe0 type (intrinsic reactivity), the size and depth of the filter, the water flow velocity and the chemical and physical properties of the “raw water”, well-designed Fe0 filters can free water from all possible pollutants, namely: (i) chemical contaminants (organic and inorganic, including tastes and odors); (ii) biological contaminants (pathogens, including bacteria and viruses) and (iii) physical contaminants including suspended solids [40,76,82,83,122].
3.3.2. Biological Mediated Processes in Fe0 Filters
A Fe0 filter can be adapted to become a “self-sustaining” filter, such as a slow sand filter (SSF). For over 200 years, SSFs have been used to control microbiological water contamination ([123] and refs. cited therein). There is currently a renewed interest in SSF application as systems operating without chemicals or electricity. The processes involved within biofilms of SSF are still not fully understood. Relevant processes include adsorption, bio-oxidation, predation and scavenging. These processes have been hypothesized but have never been comprehensively verified [123,124]. Such a gap in knowledge pertaining to the optimal efficiency of SSFs hampers advances in their design.
Because Fe0 filters operate under similar conditions like SSFs, microbiological communities will develop within them and potentially impact the process of iron corrosion as well as the transformation of corrosion products. As an example, crystalline FeIII oxides can be microbiologically reduced to FeII oxides via colloidal hydroxides [125,126]. Thus, beside abiotic processes, biotic processes also contribute to decontamination in Fe0 filters and should also be considered.
3.3.3. Optimizing the Efficiency Fe0 Filters
The efficiency of Fe0 filters can be considerably increased when suspended solids are removed in pre-filters (e.g., filtration on coarse and fine sand). This operation increases the service life of Fe0 filters. The service life of Fe0 filters is also considerably lengthened if the formation of more voluminous corrosion products is avoided by working under anoxic conditions [77,89,90,127]. Low oxygen levels are known to be achieved in biofilms of SSFs. In fact, dissolved oxygen is used for respiration and, hence, the development of an aerobic microbial community building the biofilm [123]. In other words, an ideal Fe0 filter utilizes both the O2-removing capacity of a SSF and the well-documented aqueous contaminant-scavenging ability of the Fe0/H2O system [77,122,127]. A suitable design may consider the periodic replacement of the SSF units (e.g., after every six months) in order to utilize the full contaminant-scavenging ability of the Fe0 (Figure 1).
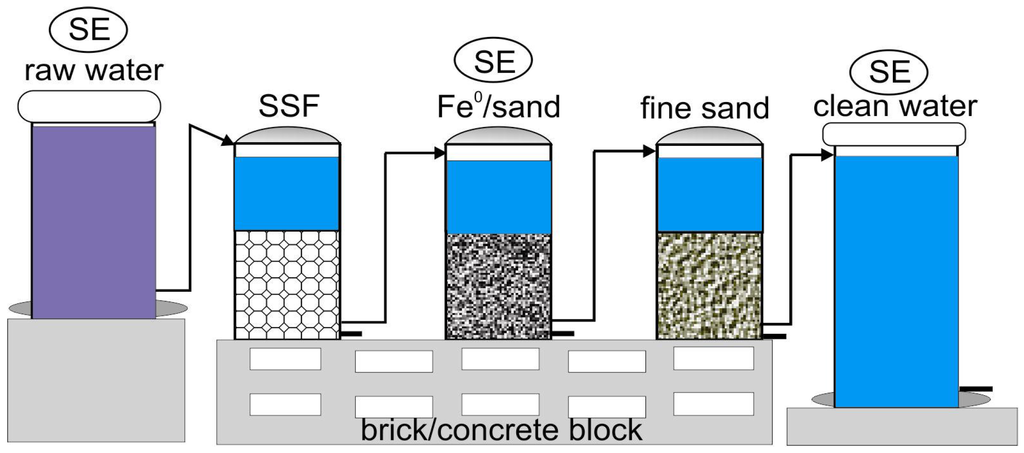
Figure 1.
Side view of a sequential Fe0-sand water filter. To optimize the efficiency of the filtration unit, the effluent should be clarified and deaerated before being introduced, using, for example, a slow sand filter (SSF). Water from the Fe0 filter subsequently flows through a sand layer. “SE” specifies the positions where solar energy is expected to optimize operation and monitoring of Fe0 filter. SE may have four key functions: (i) pumping the “raw water” to a storage tank; (ii) regulating the flow regime out of the storage tank; (iii) controlling the temperature of the Fe0-based units; and (iv) pasteurizing the stagnant treated water. Figure is reproduced from Salama et al. [130].
In water treatment scenarios where SSFs are too slow to produce low O2 concentrations (typically <2.0 mg/L), other O2 scavengers can be used, including Fe0 itself [29,90,91]. When Fe0 is used as a dissolved O2 scavenger, the user should remember that any preferential flow created in the sacrificial Fe0 unit will likely impact the efficiency of the treatment unit(s). In this case, equalizing units (gravel or anthracite columns) should be used [128,129]. Ideally, materials contained in the equalizing units should have good adsorptive affinity for positively charged species which therefore have less affinity to adsorb to iron oxides [105,106]. For the present study, however, equalization is the major goal as fluoride ions are negatively charged and, thus, possess good adsorptive affinity for the surface of Fe oxide-hydroxides which cover the Fe0 surface.
3.3.4. Fe0 Filters for Fluoride Removal
Section 3.3.1 discussed the parameters that determine the efficiency of Fe0 filters. From the material perspective, three relevant parameters are named: Fe0 type, Fe0 mass and Fe0 particle size. The depth of the reactive zone (e.g., Fe0/sand mixture, Figure 2) in the filter is an additional important parameter. The water flow velocity is a parameter that can easily be fixed, for example using the same velocity employed for the SSF.
For aqueous F− removal in Fe0/H2O systems, the pH value is not typically very important as only natural waters with pH >4.5 are concerned [131]. In this pH range, the solubility of iron is minimal, fluoride is negatively charged and the surface of Fe oxide-hydroxide positively charged. The concentration of dissolved anionic species (e.g., Cl−, HCO3−, NO3−, PO43−, SO42−), added, for example, as sodium salts, are often a more important factor than simply the pH value (Figure 3); however, the speciation of these ions (namely HCO3−, PO43− and SO42−) is pH-dependent. However, as F− removal is the main subject, the pH-dependent speciation of co-existing ions is of secondary importance (for a conceptual investigation).
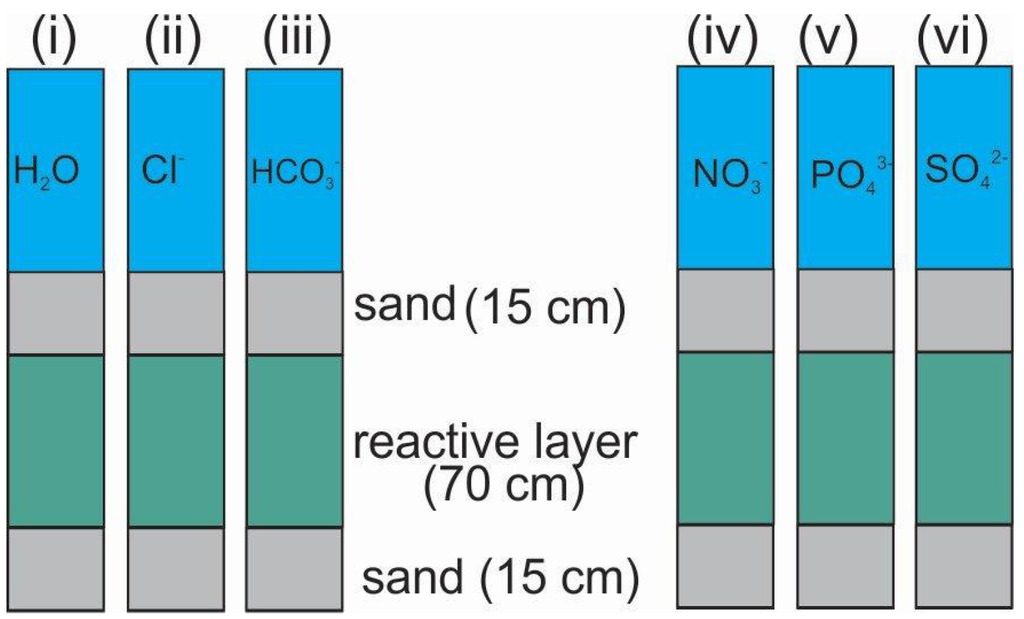
Figure 2.
Filtration unit modules used to investigate of the impact of different anions on the performance of a Fe0 packed bed for F− removal. Layered arrangement of iron filings and sand used for the experiments is also shown; however, by modifying this design other parameters (e.g., Fe0 type, sand grading/sorting) can also be investigated.
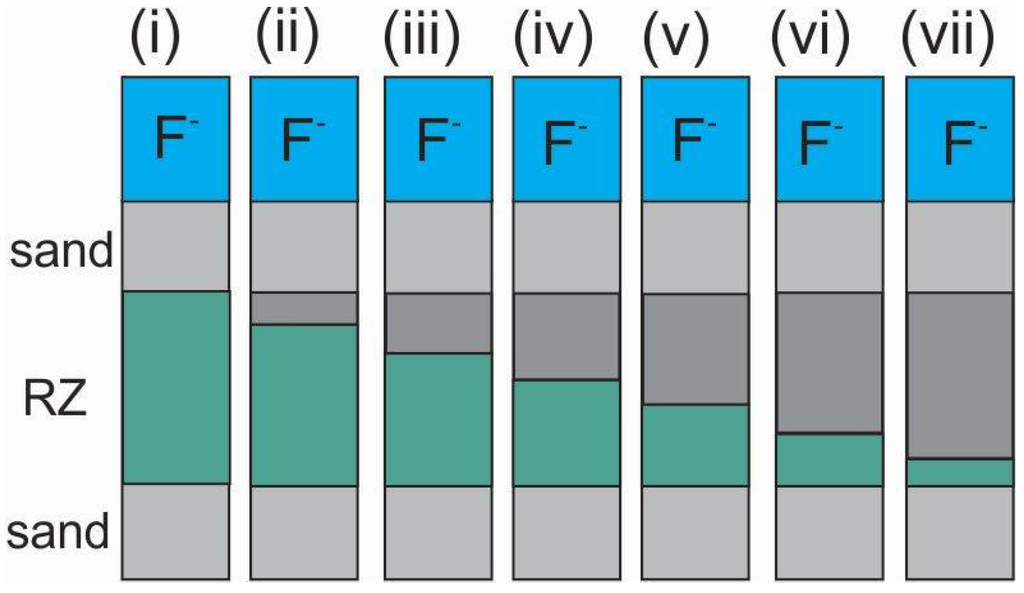
Figure 3.
Filtration unit modules used for the investigation of the effects of the thickness of the reactive zone (RZ) on the performance of a Fe0 packed bed for F− removal. Layered arrangement of iron filings and sand used for the experiments is roughly shown. Sand in RZ is not necessarily the same at the inlet/outlet.
In order to investigate the efficacy of a water filtration technology, a systematic approach must be adopted which comprises fixing certain parameters and testing all others in a pre-defined sequence. Some examples are the filter container (10 cm inner diameter; 100 cm height) and the depth of the reactive zone (Hrz = 70 cm). The other 30 cm could be made up of two items: coarse gravel, 5 cm; river sand, 5 cm; and fine gravel (drainage layer) (Figure 2 and Figure 3), 5 cm (one at the inlet and one at the outlet). Ideally, 25% of the reactive zone (70 cm)—volumetric ratio—should be made up of Fe0 [40]. Accordingly, comparatively testing the efficiency of three Fe0 materials for F− removal should consist in performing parallel experiments with three systems (ideally each in triplicate) differing solely by the Fe0 type in the reactive zone (modified Figure 2; each column contains another Fe0 material and the influent is the same). Ideally, the breakthrough of F− and Fe is monitored as a function of time, as well as the pH value and any changes in the hydraulic conductivity [40,115]. It is understood that the dissolved O2 level and common water quality parameters will be routinely monitored and documented. Relevant parameters include electric conductivity, temperature, Ca2+, Na+, K+, Mg2+, Mn2+, Cl−, HCO3−, NO3−, PO43−, SO42−.
Based on the results of Fe0 efficiency characterization, a material with acceptable corrosion kinetics could be selected to test the impact of all other operational parameters. For example, testing the impact of the five named anions (Cl−, HCO3−, NO3−, PO43−, SO42−) should consist of testing six systems in parallel, the sixth system being fed by the operational solvent (e.g., tap water) (Figure 2). Ideally, relevant anion concentrations for specific sites should be tested. Alternatively, experiments with equivalent amounts of the anions uncovering the range of natural concentrations could be performed. Such principle experiments are necessary to gain a general overview of the impact of all relevant influencing parameters and, thus, ease site-specific fine-tuning experiments.
The last types of experiments will consist in determining the depth of the reactive zone of the filter bed needed for efficient water treatment for a certain period (e.g., six months) (Figure 3). This is to determine the frequency for the replacement of Fe0 units. This investigation implies the determination of the numbers of beds having a reactive zone of 70 cm that will be needed to efficiently treat water for six months.
The next section will discuss methods to test commercially available SW [117,118,119,132,133] as a suitable fluoride filtration technology. If this operation is successful, such filtration systems will be able to be manufactured in the developing world using commonly available tools [39,78,79,81,85,134,135].
4. Steel Wool for Universal Fluoride Removal
4.1. Availability and Suitability of Steel Wool
Metal wools are globally abundant. They are used in sanitation and maintenance for cleaning and polishing many types of surfaces including granite, marble, metal and wood. The three most common types of metal wools are steel (Figure 4), bronze and copper. SW is often made of various raw materials including low alloyed steel and stainless steel. For the purpose of water treatment, a rule of thumb could be: “use only the SW that readily rusts in water” (i.e., no stainless steel wool).

Figure 4.
Photographs of six steel wool specimens found on the market in Douala/Cameroon (August 2015).
The thickness of SW fibers (μm range) suggests that they can deplete within a year or some within two years upon aqueous corrosion [136,137]. This makes SW a practical material to start characterization of key aspects of the long-term Fe0 efficiency (sustained reactivity), both at lab and field scales. Long-term parallel experiments with SW (e.g., diameters 30 to 300 μm—ref. [136]) and thicker Fe0 materials (e.g., shopped iron wires of different diameters >300 μm) are recommended in this context.
While SW is an extremely widely used and cheap material, some commercial forms of SW contain oil and grease from the manufacturing process that should first be washed (cleaned) before use [70,138,139]. In addition, a mild acid treatment may be necessary in order to remove any surface contamination including FeIII-bearing (hydr)oxide phases.
4.2. Current Use of Steel Wool for Water Treatment
Tseng et al. [138] used SW as generator of iron oxides for the “accumulation” of 60Co (pre-concentration) in environmental monitoring of a nuclear power plant. This demonstrates that this material is a potential suitable candidate to be used for water treatment in Fe0 filters. Since then, James et al. [102] tested SW as an amendment material for peat and sand beds in order to: (i) increase the performance for phosphate removal and (ii) lengthen the service life of such beds. The evidence that SW amended peat/sand beds are sustainable systems for phosphate removal was subsequently described some 15 years later by Erickson and colleagues [117,118,119].
With the introduction of the Fe0 technology, several investigators have tested various SW materials for water treatment (Table 2). Applications included in-situ production of (i) H2 for denitrification (Biswas and Bose [140]—Nr. 11); (ii) Fenton reagents for oxidative degradation (Teixeira et al. [141]—Nr. 1); (iii) iron oxides for As removal (Campos [133]—Nr. 12) and (iv) iron oxides for pathogen removal (Bradley et al. [96]—Nr. 6). However, investigations were performed on a pragmatic basis (Table 3 and Table 4) and have not considered pioneering works (Nr. 15 and Nr. 16, Table 2). The used experimental conditions were not properly documented (Table 2) and were so diverse (Table 3 and Table 4) that available results have solely a qualitative value (Section 4.3). Additionally, precedent works testing SW for decontamination in the framework of the “Fe0 remediation technology” have not been considered as a rule. Table 5 shows clearly that from the six most recent studies using SW, just one of seven possible previous peer-reviewed articles is referenced in two of six articles. This evidences the fact that SW is just qualitatively used as a Fe0-based material as it this been the case in classroom demonstrations of the oxygen content of air (approximately one-fifth of the dry air) for several decades [142,143,144,145]. For example, Birk et al. [142] allowed acid-washed steel wool to rust in an air-filled container inverted in water; the decrease of air volume is measured. In this context, the experimental vessels can be heated and all other possible tools can be used to accelerate Fe0 oxidation [144]. The intrinsic reactivity of used SW is not addressed—just its capability to reduce atmospheric O2 is of interest.

Table 2.
Summary of trade names, iron and carbon contents of 14 peer-reviewed articles using steel wool for contaminant removal. It is seen that only four articles have specified the composition of used steel wools while seven have not specified the trade name of their used specimens. “Nr” is the number referencing individual articles in Table 3, Table 4 and Table 5.
| Nr | Anno | Ref. | Trade Name | Element (%) | |
|---|---|---|---|---|---|
| Fe | C | ||||
| 1 | 2015 | [141] | Limpano (Brazil) | 99.8 | 0.1 |
| 2 | 2014 | [146] | n.s. | n.s. | n.s. |
| 3 | 2013 | [147] | n.s. | n.s. | n.s. |
| 4 | 2013 | [137] | n.s. | n.s. | n.s. |
| 5 | 2012 | [139] | n.s | 98.6 | n.s |
| 6 | 2011 | [96] | Peerless Metal Powders & Abrasive | n.s. | n.s. |
| 7 | 2010 | [148] | Bombril (Brazil) | n.s. | n.s. |
| 8 | 2009 | [149] | Mapavirulana (Argentina) | 98.5 | 0.1 |
| 9 | 2008 | [150] | n.s. | 98.5 | n.s. |
| 10 | 2007 | [118] | Global Material Technologies (USA) | n.s. | n.s. |
| 11 | 2005 | [140] | Rohit Industries (India) | n.s. | n.s. |
| 12 | 2002 | [133] | n.s. | n.s. | n.s. |
| 13 | 1998 | [132] | Rhodes American Company | n.s. | n.s. |
| 14 | 1997 | [136] | n.s. | n.s. | n.s. |
| 15 | 1992 | [102] | Rhodes American Company | n.s. | n.s. |
| 16 | 1984 | [138] | Rhodes Company (USA) | n.s. | n.s. |
In summary, 30 years ago Tseng et al. [138] endeavored to use SW as a source of iron oxides for aqueous contaminant removal. Since 1997, testing/using SW has become a common tool for the removal of individual aqueous species (Table 2, Table 3 and Table 4). Knowledge of this process is relatively poorly reported in the scientific literature at present, i.e., a systematic approach to investigate the operating mode of SW for water treatment is currently lacking. Considering the shortcomings of previous works, Section 4.4 introduces a concept for testing SW-based Fe0 filters for fluoride removal at the household level. The relevance of data/parameters summarized in Table 2 through Table 5 is discussed in the next section.

Table 3.
Summary of some experimental conditions used for the batch experiments in 13 of the peer-reviewed articles using steel wool for contaminant removal. The numbers (Nr.) are related to relevant references as specified in Table 2. Polychlorinated biphenyls are abbreviated to: “PCBs”. X stands for the used contaminant and [X] for its concentration.
| Nr. | X | [X] (mg/L) | Fe0 (g/L) | pH Value | Duration | Volume (mL) | Mixing | Speed (rpm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Phenol | 200 | 1 to 7 | 5–9 | 300 min | 500 | stirred | n.s. |
| 3 | Cr | 100 | 0.0083 | 3 | 2 h | 50 | n.s. | - |
| 4 | As(V) | 0.3 | n.s. | 3–12 | 480 min | 100 | shaken | 35–40 |
| 5 | PCBs | <12,800 | 500 | n.s. | 4 h | 2 | stirred | n.s. |
| 7 | Cr(VI) | 9.44 | 0.015 | n.s. | 3 h | 30 | n.s. | n.s. |
| 8 | As | 1 | 2 to 3 | 7.1 | 150 min | 500 | stirred | 300 |
| 9 | As | 1–1.3 | 1.3 | <8 | 24 h | 1000 | quiescient | - |
| 10 | PO43− | 0.5 | n.s. | 5.7 | 24 h | 150 | shaken | - |
| 11 | NO3− | 42 | 2.5–25 | n.s. | 60 d | 200 | n.s. | n.s. |
| 12 | As | 0.5 to 10 | n.s. | 6.1 to 6.8 | 15 min | 50 | n.s. | n.s. |
| 13 | NO3− | 50 | 40 | 5 to 11 | 12 d | 250 | shaken | 100 |
| 15 | PO43− | 4 | 7.41 | 5 | 60 min | 25 | agitated | - |
| 16 | 60Co | 200–1000 | 10 | 6.84 | 30 s | 20 | n.s. | n.s. |

Table 4.
Summary of some experimental conditions used for the column experiments in nine of the peer-reviewed articles using steel wool for contaminant removal. The numbers (Nr.) are related to relevant references as specified in Table 2. Point forming unit is abbreviated to: “PFU”. X stands for the used contaminant and [X] for its concentration. ID is the internal diameter of the column, L its length and RZ the thickness of the reactive zone (Fe0-bearing zone).
| Nr. | X | [X] (mg/L) | Fe0 (g) | Dimensions (cm) | Porosity (%) | pH | Duration | Flow (mL/h) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | L | RZ | ||||||||
| 2 | Cr | <100 | 10 | 3 | 30 | n.s. | n.s. | 3–7 | 120 min | 20 |
| 4 | As (V) | 0.3 | 47 | 4 | 0.1 | n.s. | n.s. | n.s. | 90 h | <600 |
| 5 | Virus | 1010 PFU | 260 | 2.5 | 8.9 | n.s. | n.s. | 6.2 | 170 d | <38.4 |
| 9 | PO43− | <0.8 | <5.5 | 5.08 | 84 | n.s. | n.s. | n.s. | <2 d | n.s. |
| 10 | NO3− | 40 | <1.5 | 4 | n.s. | 12 | 50 | n.s. | n.s. | n.s. |
| 13 | NO3− | 50 | 8 | 2.5 | 26.5 | n.s. | 90 | 6.7 | 131 d | 3 and 10 |
| 14 | Cr(VI) | 500 | 1 | 3.5 | 20 | n.s. | n.s. | n.s. | n.s. | 15,000 |
| 15 | PO43− | 50 | 0.3 | n.s. | <2.9 | n.s. | n.s. | n.s. | 22 d | 4.2 |
| 16 | 60Co | <180 | 40 | 5 | n.s. | 7 | n.s. | 6.84 | <24 h | 72,000 |

Table 5.
Extent to which recent articles which have investigated using steel wool for water treatment were considered by academic journal papers since 2010 (Nr. 1 through 6, Table 2). It is evident that existing articles are typically independent work as just one ancient article was cited by two of the six articles since 2010. Additionally, none of the pioneering work (Nr. 15 and 16) was considered by all articles of the Fe0 remediation technology area.
| Nr. | Recent Articles | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| 1 | no | no | no | no | no | no | no |
| 2 | no | no | no | no | no | no | yes |
| 3 | no | no | no | no | no | no | yes |
| 4 | no | no | no | no | no | no | no |
| 5 | no | no | no | no | no | no | no |
| 6 | no | no | no | no | no | no | no |
4.3. Lessons from the Peer-Reviewed Articles on “SW for Water Treatment”
Table 2 through Table 5 include peer-reviewed articles only. Admittedly, some relevant peer-reviewed articles have been overseen by the used search procedures [151,152,153,154,155,156,157,158]. Academic thesis [117], technical reports [159,160] and patents [161] do exist as well but are not considered herein because they have also not specified key issues addressed in this review. This section will corroborate the argumentation from Section 4.2 by giving a short comment on each table.
Table 2 shows that six of 14 articles have not specified the trade name of the materials they used. Giving the trade name is not necessarily a must but giving the elemental composition of used materials should be mandatory. Only four articles have done this. Additionally, only three articles have specified the specific surface area (not shown) of the material they tested. Accordingly, it is fair to state that efforts to define used steel wools were not satisfactorily.
Table 3 summarizes the experimental conditions for the 13 articles testing SW in batch experiments. It is seen that seven different contaminants (As, Co, Cr, NO3−, PCB, phenol and PO43−) were tested. The initial concentrations varied from 0.3 to 12,800 mg/L. The SW mass loading, where specified, varied from 0.01 to 500 g/L. The initial pH value, if specified, varied from 3.0 to 12.0 (for the purpose herein only pH > 5.5 is relevant). The experimental duration varied from 30 s to 60 days. The volume of the reaction vessels varied from 20 to 1000 mL and several mixing speeds and intensities were tested. The suitability of these operational parameters to achieve the given objectives of the individual studies has been discussed already and will not be repeated here [35,162]. It is sufficient here to recognize that results obtained under such diverse experimental conditions are highly qualitative. Additionally, batch experiments are of little usefulness when it comes to designing steady-state flow systems.
Table 4 summarizes the experimental conditions for the nine articles testing SW in column experiments. Six different contaminants (As, Co, Cr, NO3−, PO43− and a virus) were tested. The initial concentrations varied from 0.3 to 500 mg/L. The SW mass varied from 1.0 to 260 g/L. The dimensions of the columns and the height of the reactive zone (SW-containing layer) were not always specified. The initial pH value, where specified, varied from 3.0 to 7.0. The experimental duration, where specified, varied from 2 h to 170 days. The flow velocity, where specified, varied from 4 mL/h to 15 L/h (72 L/h is not considered as it was used for pre-concentration). Again, the large variability of tested experimental designs degrades available column results to qualitative results, even for columns testing the same contaminant such as Nr. 2 and Nr. 14 (testing SW for Cr removal). This situation is not ideal as lab-scale column experiments have the potential to be used as bench-scale tests for household filters. For example, testing the approach of Biswas and Bose [140], given the mass of SW in 125 cm3 of sand, one can rapidly select SW materials for further tests and optimize the operational parameters for efficient Fe0-SW filters. The question to answer in this primary research is twofold: (i) which SW samples are suitable for water treatment and (ii) which mass of suitable material per 125 cm3 should be used? Adopting such an approach within and across research groups will certainly accelerate knowledge acquisition for efficient SW-based Fe0 filters.
Table 5 compares the extent at which post-2010 peer-reviewed articles have referenced previous articles (1992 < year ≤ 2010) dealing with SW for water treatment. The preponderance of “no” in Table 5 (40/42) clearly demonstrates that available works are independent efforts. The scientific community cannot afford this approach anymore. In essence, presenting new results without putting them in a historical context is not acceptable and even not fair for readers wishing to learn from a given research field.
4.4. Steel Wool-Based Fe0 Filters for Water Treatment at the Household Level
A suitable example design of a stand-alone SW filtration system is described herein. SW was chosen as a suitable Fe0 material because of its wide commercial availability at relatively low cost and its high surface area with little resistance to fluid flow [102,118,149]. Due to the high levels of variability of both steel wool (SW) samples and contaminated water sources, only a systematic sequence of experiments can determine whether a SW specimen is suitable for water defluoridation.
Laboratory-based studies should be conducted to investigate the effect of bed design variables on the extent of F− removal, mostly in gravity-driven systems. Design parameters to be investigated include: (i) SW type, form and quantity; (ii) supporting filter media (e.g., anthracite, pumice, sand) and their particle sizes (e.g., 0.5 mm, 1.0 mm, 2.0 mm); (iii) dimension of the filter (e.g., ID = 10 mm and L = 50 mm), (iv) depth of the reactive zone (e.g., 15 mm, 30 mm or 40 mm) (Figure 3), and (v) packing configurations of the reactive zone (layered or mixed).
General rules of thumb for filter design can often be used as a guideline. For example: (i) the size of filter media particles significantly impacts the hydraulic conductivity (permeability) and thus the overall contaminant removal performance; (ii) filters with smaller particles had better straining efficiency, but subsequently shorter lifespan; (iii) deeper filters have a longer lifespan; (iv) two layers of distinct-sized media in the filter bed improved performance over the single-layered systems; (v) mixed systems showed improved performance as compared with single-layered systems; and (vi) low water flow velocities are better for filter performance [40,77,163,164]. It is certain that simple modifications of SW-based water filters can significantly affect their performance and/or reduce maintenance intervals.
4.5. Proposed Design Procedure
The idea of using Fe0 filtration for universal safe drinking water provision [73] has now progressed to the point where point-of-use systems should be piloted [40]. Although no real world prototype has been presented yet, examples given below target at designing typical SW-based systems for individual households. This option may be improved in the future and tools presented in related articles [40,68,69,76,122] would assist in scaling up for larger population sizes.
4.5.1. Required Water Production Rate
It is assumed that an average household (model household) in a rural settlement in the developing world has 10 inhabitants. The World Health Organization (WHO) recommends 7.5 liters (L) of water per person per day, meaning that the model household needs a minimum of 75 L of water per day. Considering a surplus water use of 25 L per day, a suitable filtration system would therefore be required to produce approximately 100 L of water per day. Furthermore, the system could be designed such that 50 L of water is filtered in the morning and the remaining 50 L in the evening.
4.5.2. Media and Water Properties
Locally available anthracite, gravel, pumice or sand should be (crushed and) sieved and the most suitable particle size (e.g., 0.5 ≤ d (mm) ≤ 1.5) used [163]. Fe0 material herein is operationally SW that is either used as received or chopped into pieces of suitable sizes. SW should be characterized for its chemical composition, density, and chemical reactivity after available tests ([165] and referenced cited therein). The “raw water” quality has to be analytically determined and after the filter effluent, must contain F− at less than 1.5 mg/L (WHO guideline, [166]).
The major challenge of this research is to experimentally determine a suitable media volume (or mass) required to efficiently treat 100 L water/day at a minimal filtration rate of 5 L/h (50 L in 10 h). The maximal filtration rate should also be specified as well as the suitable bed depth, and the mass of SW in a predetermined volume of the reactive zone. Once this goal is achieved the service life of such a filter should be specified and ways and means should be sought to lengthen it to at least six months (if applicable). Relevant tools include using several beds in a series [40,167].
The first exploratory research could start with a reactive zone of 500 mL made up of graded sand (e.g., 0.5 ≤ d (mm) ≤ 1.5) and 10% (w/w) chopped SW. Depending on the results, the SW proportion as well as the depth of the reactive zone can be varied. It is understood that for each SW sample the experiment has to be started at point zero until some correlations are established between chemical composition, density and efficiency at removing F−. It is also understood that the reactive zone is confined by two layers of granular material (Figure 2) with incidence on the water quality and the water flow velocity. It has already been discussed that the layering of materials at the outlet should be selected to avoid a high iron concentration in treated water [40,76].
Other steps in the design of SW filters are common to other filtration systems and include the determination of (i) the orifice diameter; (ii) the total height of the filter (heights of support layers + media + reactive zone + supernatant). The total height of a SW unit dictates the container to be used or manufactured. In essence, in case ideal containers are not affordable, filters could be designed with available ones following a similar approach as described herein. Mathematical equations are also available to modify filter design accordingly [75,76,122,163].
4.5.3. Filter Design
A modular SW-based filter for F− removal for a 10-person-large household is sought. It is assumed that the available water source contains 10 mg/L F−. The filter should be gravity-fed and requires only medium replacement once or twice per year for maintenance. The filtered water should not contain more than 1.5 mg/L F−. The reasoning herein is based on a filter container with the following dimensions: 10 cm inner diameter (D) and 100 cm height (L). Of the total length, a depth of 70 cm is reserved for the reactive zone (RZ or Hrz) (Figure 2). The volume of the reactive zone is thus: π × (D2/4) × H = 3.14 × (10)2/4 × 70 = 5500 cm3 or 5.5 L. The total volume of the filter is 7.9 L, and 2.6 L (corresponding to 30 cm) is left for sand and gravel.
The research question is which mass of a steel wool should be mixed with sand to build the 5.5 L reactive zone? The mass (or volume) of sand and SW vary but their total volume is fixed. An additional consideration is that the diameter of the sand particles (d) should not exceed 1.0 cm (d/D ≤ 0.1). Typically sand particles used in such filters have a d-value lower than 5.0 mm ([40] and refs. cited therein), meaning that filters with D = 5.0 cm can be envisaged as well. The case of a 100% SW reactive zone used as received or with properly pretreated material is envisaged, but will not be discussed herein. It is sufficient to consider that, because of their mechanical properties, the pure SW-Hrz may need to be supported.
For spherical particles, it has been established that the most sustainable Fe0:sand volumetric ratio is 1:3 (1 volume Fe0 and 3 volumes of sand) [76,77,122,168,169]. This rule cannot be applied to fibrous SW. Therefore, fixing the Hrz volume and measuring the SW mass to be balanced with sand to fill it is a good starting point. In this effort, both the resulting mass of sand and SW are to be documented, such that in the middle term, when enough data is available, statistical analysis can be performed to check whether the rules of thumb can be elaborated for the construction of SW Fe0 filters. It is understood that each tested SW is characterized for its elemental composition, its intrinsic reactivity (e.g., kEDTA value, see Btatkeu-K et al. [165]), and the results are made available to the scientific community, at least as supporting information (appended to any publication, including technical documents).
A good starting point is to test the suitability of available SW materials with a design close to the one presented by Biswas and Bose [140] (based on SW mass in 125 cm3 of sand) and to use testing methods summarized in Btatkeu-K et al. [165] (e.g., kEDTA). Selected materials will then be tested in the modular SW filter while varying the mass of SW in the 5.5 L reactive zone to optimize the filter efficiency. The experiments should be performed for at least one year regardless of whether a breakthrough is observed or not. A specific objective will be to characterize the long-term behavior of SW (through F−/Fe breakthrough and permeability loss). The results for such experiments would demonstrate whether the initial modular system is satisfactorily designed or over-designed. In the case that the system is over-designed, there are at least two ways to improve it: (i) using a smaller filter (e.g., D = 5.0 cm) or (ii) reducing the height of the reactive zone.
Summarizing the first step on the way to popular Fe0 filters for F− removal, it can be described as follows:
- (i)
- Select a SW material for its intrinsic reactivity using available tools [165];
- (ii)
- Characterize the selected SW material for its elemental composition;
- (iii)
- Test the suitability of selected SW materials at a small scale with a design comparable to the one described by Biswas and Bose [140];
- (iv)
- Test relevant SW materials with the modular filter described in this section (or a well-described modification).
The results of the modular test will assist in establishing a base F− treatment facility at bench scale. The system could be composed of several tanks in a series of which just some are filled with specially prepared SW fibers mixed with sand. A design figure is appended (Figure 2). Once the optimal SW/sand mixture is found, it can be contained in a fabric bag that is well-packed to facilitate insertion into and removal from the tank.
4.5.4. Beyond Steel Wool Filters
The present study is a continuation of a vast effort to fully exploit the ability of metallic iron (Fe0) for in-situ generation of adsorbing (and reducing) agents for contaminant removal and, thus, water treatment. In-situ generation of adsorbing agents circumvents many limitations associated with the use of synthetic and natural adsorbents such as ferrihydrites and other iron oxides/hydroxides [160,170]. Relevant limitations include mass transfer concerns and the occupation of reactive sites by co-contaminants. In a properly designed Fe0 filter, a relevant contaminant (e.g., F−) and co-contaminants (e.g., PO43−, SO42−) will be independently removed from the aqueous phase. In other words, the main question with Fe0 filters is how long and with which reaction kinetics will they produce sufficient corrosion products for efficient contaminant removal?
Previous work mostly based on the premise that Fe0 is a reducing agent has not adequately addressed this question. In addition, linear relationships used to describe reaction kinetics are not relevant for iron corrosion processes under environmental conditions [89]. Recent investigations have recalled the stochastic nature of environmental aqueous iron corrosion [128,129]. This means that relevant data, obtained under well-defined conditions, are needed in order to derive appropriate kinetic parameters for long-term efficient Fe0 filters (Equation (5), Section 6.3). The achievement of such data is essential as small-size Fe0 materials are allowed to corrode under water saturation for the first time. This assertion is valid despite five centuries’ experience in using iron shavings and scrap iron for the recovery of metals in the mining industry (cementation) [171,172].
To fill this gap, the research envisaged herein should be advantageously extended to larger-size Fe0 materials. For example, a series of Fe0 coils/wires and Fe0 nails with various diameters and similar intrinsic reactivity could be tested in long-term experiments to gain insights into the size-dependent corrosion of Fe0 materials during water treatment. The first advantage of this would be to determine a material that could be used in lieu of steel wool for a longer operation time without maintenance (e.g., 18 or 24 months).
5. Economic Considerations
The costs for a SW-based water filtration system include: the material cost (including SW and the filter container), construction cost, operation cost, monitoring cost and decommission cost [40]. Currently, every SW material might be regarded as unique to its site (local market). Thus, the overall cost of resulting Fe0 filters will be site-specific. Additionally, the manufacture of filter containers using local material should be encouraged.
The most reliable cost estimation for Fe0 filters has been presented by Gottinger et al. [82,83] after testing such filters at pilot scale for As and U removal. A comparison to adsorptive filtration reveals that Fe0/sand is economical with respect to media and treatment cost, with a satisfactory service life. Used Fe0 filings can be obtained for less than $1.50/kg and the cost of manufacturing this filter is comparable to the slow sand filter. Their calculations yielded a total treatment cost of less than $0.01/L for the modular treatment train, including filter installation, media, operation and maintenance [83].
6. Discussion
6.1. The Major Challenge
Dental fluorosis and other health problems caused by elevated fluoride in drinking water have been known for years now [14,17,18,45,56]. Efficient F− removal by chemical methods alone (e.g., precipitation) is not possible because the typical F− concentration in natural waters is generally too low (typically less than 30 mg·L−1) to result in super-saturation and subsequent precipitation ([24] and refs. cited therein). Accordingly, only physical methods (adsorption, size exclusion) or a combination of physical and chemical methods can enable defluoridation within WHO standards (≤1.5 mg·L−1). Additionally, to be relevant for remote locations, any method should be easily used by a layman while being affordable and efficient for the long term [6,24,39,40,73,79]. Such a method has yet not been universally agreed upon, with the main two reasons being (i) the active surface of any filtration media is often very quickly passivated by adsorbed solutes, rendering the theoretical removal capacity extremely difficult to predict/quantify (hereafter Problem 1) and (ii) the long-term kinetics of the removal process (e.g., dissolution of calcite or apatite) are difficult to quantify (hereafter Problem 2). To maintain compliance with the WHO standards the fluoride content of the treated water needs to be monitored during the entire service life of the filter. Problem 1 (reduced adsorption capacity) is common to all conventional adsorbent materials which have a maximum surface typically available at the start of the operation and then depreciating as a function of time. For cases where adsorbents are generated in situ (e.g., Fe0/H2O system), the situation may be different because continuously generated adsorbents separately remove fluoride ions and concurrent species (Cl−, HCO3−, NO3−, PO43−, SO42−). However, a system can be customized to selectively remove F− and other species. The main challenge is to couple the kinetics of Fe0 corrosion to the other relevant variables (water quality, water flow velocity, temperature). In another phase, Problem 2 (the long-term kinetics of iron corrosion) is the major challenge of designing efficient Fe0 filters.
6.2. Steel Wool-Based Fe0 Filters as Starting Point
The present work has introduced a concept for a systematic investigation of defluoridation using Fe0 materials, starting with locally available steel wool. Fe0 filtration technologies also provide the additional benefit of co-removing numerous other undesirable species when present even in trace quantities. In order to progress most efficiently, research with a standard Fe0 reference material (e.g., Connelly-GPM, iPutec GmbH or Peerless Metals) is required with results always published in open-access journals. All other operative conditions can be changed (other Fe0 materials including steel wool, column dimensions, Fe0 particle size), in particular the water chemistry, as soon as natural waters are used. To optimize result comparability, sorbent materials (Fe0 and additives) should be characterized extensively. Relevant parameters for granular material include: grain size, height of the reactive zone, point of zero charge, relative mass of Fe0 and additives (or volumetric proportion), specific surface area, sphericity [76,163]. Moreover, testing common pollutants in addition to fluoride in synthetic solutions (e.g., AsIII/AsV, F−I and UVI) while using comparable experimental designs will accelerate the identification of strengths and weaknesses of the Fe0 filtration technology. It is hoped that such an approach will soon be adopted worldwide to enable the exploitation of the potential of this still innovative technology. This includes the identification of its limitations on a scientific basis.
6.3. Filter Design Considerations
The real merit of long-term laboratory and field experiments based on principles exposed herein will be the estimation of long-term kinetics of iron corrosion for practical Fe0 materials (including steel wool and iron wire) under relevant field conditions. In fact, first-order models have been typically used to model contaminant removal in Fe0/H2O systems [33,173]. Admittedly, this approach was acceptable because Fe0 was considered a reducing agent for the pollutants of concern. Now that the reducing transformation concept has been proven as false [35,174], alternative approaches are needed. Results from corrosion science have shown that the function describing the instantaneous rate of iron corrosion is expressed as follows [175]:
where q0 is a constant, v0 is the short-term and vP the long-term average rate of Fe0 corrosion. The instantaneous rate drops exponentially from v0 to vP, v0 > vP. Consequently, vP ≤ v(t) ≤ v0. The form of Equation (5) allows relating the instantaneous rate v(t) to the average rate (vP); “v0” is associated with laboratory measurements while “vP” can be directly associated with the field measurements. Accordingly, results of experiments performed following the concept presented herein will enable the determination of −q0 and, thus, vp. Then v0 can be obtained from short-term column experiments using, for example, diluted EDTA solutions [176]. These parameters will enable a better design of Fe0 filters. As expected, these results will be available for the whole Fe0 research community [73].
v(t) = vp + (v0 − vp) × exp(−q0 × t)
6.4. Steel Wool Filters for All
It has been demonstrated herein that while steel wool filters can become reactively exhausted (corroded to end member hydroxides) within relatively short timescales such as two to three months, due to their cheap cost and high availability worldwide they would be easy to replace often in the developing world. Because steel wool is simply the starting material for a hand-built and locally sourced water filter, there is a real potential to foster local industry during filter construction, which includes: (i) designing and manufacturing plastic or glass filter containers; (ii) purchasing and conditioning Fe0 materials (including steel wool) and additives; and (iii) collecting and recycling spent (corroded) filters [122,127,169,176]. Testing metal foams should be also considered in this effort [177]. In summary, the demand for decentralized water treatment can be exploited to start a new area of self-reliance in the developing world.
7. Concluding Remarks
Recent investigations have determined Fe0-bearing packed bed filters as a promising technology for decentralized and small-scale clean drinking water provision. Fe0-bearing filters, however, have not yet been tested for this purpose. However, (i) taking into account the various drawbacks and challenges identified in the Fe0 filter research and (ii) using a novel methodical approach for filter design, great progress in Fe0 filter application can be expected in the near future.
Steel wool (SW) is suggested herein as an ideal candidate material for use as a Fe0-bearing filter material for the removal of F− from drinking water. It is suggested that the use of SW as filtration media could be particularly beneficial in the developing world because of the material’s low cost and widespread abundance. A systematic methodology for the empirical testing of the suitability of SW as a filter for F− is reported, including methodology to determine the impact of hydrological changes in filter design to incorporate SW corrosion processes (volumetric expansion) and methods to determine the influence of different concentrations of anions (e.g., added as sodium salts), cations (e.g., added in the nitrate form) and humic substances in the permeate fluid (contaminated water). Given the fibrous nature of SW, the addition of a constant SW mass (e.g., 25 g) to a defined volume of sand (e.g., 250 cm3) would enable the characterization of the effects of shape, size and grading of sands on the efficiency of combined SW-sand filtration systems.
There is still overwhelming evidence that governments, charities and non-governmental organizations cannot effectively provide safe drinking water to everyone worldwide. For example, the United Nations (UN) millennium goal of halving the proportion of people without safe drinking water in 1990 by 2015 was achieved by only a very close margin, and in some parts of the world there is evidence to suggest that clean drinking water provision is worse than in the past. Some 780 million people still do not have access to safe drinking water. The concept presented herein is directly aimed to empower people for self-reliance in drinking water provision. An effective Fe0 water filter can be both constructed and managed by local communities in remote locations even in politically unstable and economically deprived conditions because construction and maintenance will be cheap and easy to implement. The scientific community is invited to catalyze the development of such self-reliant, community-led water filtration systems in the future, which, if achieved, will significantly benefit the health of millions of people worldwide. Evidence presented herein suggests that Fe0 filters can provide a key part in this revolution, particularly for fluoride removal, and can be scaled up or down to meet the requirements of each community.
Acknowledgments
Gerhard Max Hundertmark from the Geosciences Center (University of Göttingen) is acknowledged for technical support. Mohammad Azizur Rahman (ISU, Leibniz University, Hannover/Germany) is thanked for his valuable advice. The manuscript was improved by the insightful comments of anonymous reviewers from Water. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Author Contributions
The text of this article was written by Chicgoua Noubactep, Richard Crane and Karoli Njau with contributions from Arnaud Igor Ndé-Tchoupé and Hezron T. Mwakabona. Arnaud Igor Ndé-Tchoupé conducted background research on “steel wool for water treatment” while Hezron T. Mwakabona summarized previous work on all other adsorbents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Churchill, H.V. Occurrence of fluorides in some waters of the United States. Ind. Eng. Chem. 1931, 23, 996–998. [Google Scholar] [CrossRef]
- Davey, W.B. The Use of Bone and Other Phosphates for the Removal of Fluorine from Drinking Water. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 1939. [Google Scholar]
- Mckee, R.H.; Johnston, W.S. Removal of flourides from drinking water. Ind. Eng. Chem. 1934, 26, 849–851. [Google Scholar] [CrossRef]
- Fink, G.J.; Lindsay, F.K. Activated alumina for removing fluorides from drinking water. Ind. Eng. Chem. 1936, 28, 947–948. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Ocampo-Perez, R.; Garcia de la Cruz, R.F.; Aragon-Piña, A.; Martinez-Rosales, J.M.; Guerrero-Coronado, R.M.; Fuentes-Rubio, L. Adsorption of fluoride from water solution on bone char. Ind. Eng. Chem. Res. 2007, 46, 9205–9212. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, I.A. Fluorosis varied treatment options. J. Conserv. Dent. 2010, 13, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Smittakorn, S.; Jirawongboonrod, N.; Mongkolnchai-Arunya, S.; Durnford, D. Homemade bone charcoal adsorbent for defluoridation of groundwater in Thailand. J. Water Health 2010, 8, 826–836. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in drinking water and defluoridation of water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sharma, R.; Singh, V.K.; Steele, P.; Pittman, C.U., Jr. Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: Equilibrium uptake and sorption dynamics modeling. Ind. Eng. Chem. Res. 2012, 51, 900–914. [Google Scholar] [CrossRef]
- Thole, B. Chapter 4: Ground Water Contamination with Fluoride and Potential Fluoride Removal Technologies for East and Southern Africa. Available online: http://www.intechopen.com/books/perspectives-in-water-pollution/ground-water-contamination-with-fluoride-and-potential-fluoride-removal-technologies-for-east-and-so (accessed on 20 November 2015).
- Indermitte, E.; Saava, A.; Karro, E. Reducing exposure to high fluoride drinking water in Estonia—A Countrywide study. Int. J. Environ. Res. Public Health 2014, 11, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, E.; Zewge, F.; Johnson, C.A.; Chandravanshi, B.S. Aluminium hydro(oxide)-based (AO) adsorbent for defluoridation of drinking water: Optimisation, performance comparison, and field testing. Water SA 2015, 41, 121–128. [Google Scholar] [CrossRef]
- Onyango, M.S.; Leswifi, T.Y.; Ochieng, A.; Kuchar, D.; Otieno, F.O.; Matsuda, H. Breakthrough analysis for water defluoridation using surface-tailored zeolite in a fixed bed column. Ind. Eng. Chem. Res. 2009, 48, 931–937. [Google Scholar] [CrossRef]
- Jing, C.; Cui, J.; Huang, Y.; Li, A. Fabrication, characterization, and application of a composite adsorbent for simultaneous removal of arsenic and fluoride. ACS Appl. Mater. Interfaces 2012, 4, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, S.; Sharma, S.; Kulshrestha, V. Electrodeionization: An efficient way for removal of fluoride from tap water using an aluminum form of phosphomethylated resin. Ind. Eng. Chem. Res. 2015, 54, 4664–4671. [Google Scholar] [CrossRef]
- Shen, J.; Mkongo, G.; Abbt-Braun, G.; Ceppi, S.L.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: Fluoride removal in a rural community in northern Tanzania. Sep. Purif. Technol. 2015, 149, 349–361. [Google Scholar] [CrossRef]
- Boruff, C.S. Removal of fluorides from drinking waters. Ind. Eng. Chem. 1934, 26, 69–71. [Google Scholar] [CrossRef]
- Adler, H.; Klein, G.; Lindsay, F.K. Removal of fluorides from potable water by tricalcium phosphate. Ind. Eng. Chem. 1938, 30, 163–165. [Google Scholar] [CrossRef]
- Goldberg, S.; Davis, J.A.; Hem, J.D. The surface chemistry of aluminium oxides and hydroxides. In The Environmental Chemistry of Aluminium, 2nd ed.; Sposito, G., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 271–328. [Google Scholar]
- Tripathy, S.S.; Raichur, A.M. Abatement of fluoride from water using manganese dioxide coated activated alumina. J. Hazard. Mater. 2008, 153, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Saha, S.K.; Ghosh, U.C. Adsorption of fluoride from aqueous solution by a synthetic iron(III)-aluminium(III) mixed oxide. Ind. Eng. Chem. Res. 2007, 46, 5346–5356. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.K.; Dutta, R.K. Fluoride removal from water using crushed limestone. Indian J. Chem. Technol. 2010, 17, 120–125. [Google Scholar]
- Murutu, C.; Onyango, M.S.; Ochieng, A.; Otieno, F.A.O. Fluoride removal performance of phosphoric acid treated lime: Breakthrough analysis and point-of-use system performance. Water SA 2012, 38, 279–285. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Desalination of water using ZVI (Fe0). Water 2015, 7, 3671–3831. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based Metallic Systems: An Excellent Choice for Sustainable Water Treatment. Freib. Online Geosci. 2015, 38, 80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Nkundimana, E.; Noubactep, C.; Uwamariya, V. Metallic iron for water treatment and environmental remediation: A handout to young researchers. Fresenius Environ. Bull. 2015, 24, 1–14. [Google Scholar]
- Chiu, P.C. Applications of zero-valent iron (ZVI) and nanoscale ZVI to municipal and decentralized drinking water systems—A review. In Novel Solutions to Water Pollution; Ahuja, S., Hristovski, K., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1123, pp. 237–249. [Google Scholar]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Response surface methodology for adsorption of fluoride ion using nanoparticle of zero valent iron from aqueous solution. J. Chem. Eng. Process. Technol. 2013, 4. [Google Scholar] [CrossRef]
- Jahin, H.S. Fluoride removal from water using nanoscale zero-valent iron (NZVI). Int. Water Technol. J. 2014, 4, 173–182. [Google Scholar]
- Jeong, J.-Y.; Song, Y.-H.; Kim, J.-H.; Park, J.-Y. Simultaneous removal of nitrate, phosphate, and fluoride using a ZVI-packed bed electrolytic cell. Desal. Water Treat. 2014, 52, 737–743. [Google Scholar] [CrossRef]
- Kalt, P.; Birzer, C.; Evans, H.; Liew, A.; Padovan, M.; Watchman, M. A solar disinfection water treatment for remote communities. Procedia Eng. 2014, 78, 250–258. [Google Scholar] [CrossRef]
- Wambu, E.W.; Onindo, C.O.; Ambusso, W.; Muthakia, G.K. Removal of fluoride from aqueous solutions by adsorption using a siliceous mineral of a Kenyan origin. Clean Soil Air Water 2013, 41, 340–348. [Google Scholar] [CrossRef]
- Habuda-Stani, M.; Ravanèi, M.E.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Kumar, N.P.; Kumar, N.S.; Krishnaiah, A. Defluoridation of water using Tamarind (Tamarindus indica) fruit cover: Kinetics and equilibrium studies. J. Chil. Chem. Soc. 2012, 57, 1224–1131. [Google Scholar] [CrossRef]
- Pandey, P.K.; Pandey, M.; Sharma, R. Defluoridation of water by biomass: Tinospora cordifolia. J. Environ. Prot. 2012, 3, 610–616. [Google Scholar] [CrossRef]
- Harikumar, P.S.P.; Jaseela, C.; Megha, T. Defluoridation of water using biosorbents. Nat. Sci. 2012, 4, 245–251. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.L.; Gupta, A.; Dadashzadeh, M. Removal of fluoride from aqueous solution and ground water by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol. Eng. 2013, 52, 211–218. [Google Scholar] [CrossRef]
- Balouch, A.; Kolachi, M.; Talpur, F.N.; Khan, H.; Bhanger, M.I. Sorption kinetics isotherm and thermodynamic modelling of defluoridation of groundwater using natural adsorbents. Am. J. Anal. Chem. 2013, 4, 221–228. [Google Scholar] [CrossRef]
- Mwakabona, H.T.; Machunda, R.L.; Njau, K.N. Defluoridation of water by sisal leaf biomass: The influence of stereochemistry of the active compounds. Am. J. Chem. Eng. 2014, 2, 42–47. [Google Scholar] [CrossRef]
- Vardhan, C.M.V.; Karthkeyan, J. Removal of fluoride from water using low-cost materials. Int. Water Technol. J. 2011, 1, 120–131. [Google Scholar]
- Bhatnagar, A.; Kumar, E.; Sillanpaa, M. Fluoride removal from water by adsorption: A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Malde, M.K.; Greiner-Simonsen, R.; Julshamn, K.; Bjorvatn, K. Tealeaves may release or absorb fluoride, depending on the fluoride content of water. Sci. Total Environ. 2006, 366, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Mjengera, H.; Mkongo, G. Appropriate defluoridation technology for use in fluorotic areas in Tanzania. Phys. Chem. Earth 2003, 28, 1097–1104. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Rivera-Utrilla, J.; Medellin-Castillo, N.A.; Sanchez-Polo, M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem. Eng. J. 2010, 158, 458–467. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernández-Montoya, V.; Moreno-Virgena, M.R.; Tovar-Gómez, R.; Montes-Morán, M.A. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrol. 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Awwad, N.S.; Aboterika, A.H.A. Removal of mercury from wastewater using camel bone charcoal. J. Hazard. Mater. 2008, 154, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Gwala, P.; Andey, S.; Nagarnaik, P.; Ghosh, S.P.; Pal, P.; Deshmukh, P.; Labhasetwar, P. Design and development of sustainable remediation process for mitigation of fluoride contamination in ground water and field application for domestic use. Sci. Total Environ. 2014, 488–489, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Gómez, R.; Moreno-Virgen, M.R.; Dena-Aguilar, J.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Modeling of fixed-bed adsorption of fluoride on bone char using a hybrid neural network approach. Chem. Eng. J. 2013, 228, 1098–1109. [Google Scholar] [CrossRef]
- Kariuki, S.M.; Ngari, M.S.; Mavura, W.J.; Ollengo, M.S.; Ongoma, P.O. Effect of essential mineral ions from aqueous media on adsorption of fluoride by bone char. J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 9–17. [Google Scholar]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for groundwater of bangladesh. J. Environ. Sci. Health A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Delowar, H.K.M.; Uddin, I.; Abou el Hassan, W.H.; Perveen, M.F.; Irshad, M.; Islam, A.F.M.S.; Yoshida, I. A comparative study of household groundwater arsenic removal technologies and their water quality parameters. J. Appl. Sci. 2006, 6, 2193–2200. [Google Scholar]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for safe drinking water worldwide. Chem. Eng. J. 2010, 165, 740–749. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water production. Freib. Online Geosci. 2011, 27, 38. [Google Scholar]
- Deng, Y.; Englehardt, J.D.; Abdul-Aziz, S.; Bataille, T.; Cueto, J.; de Leon, O.; Wright, M.E.; Gardinali, P.; Narayanan, A.; Polar, J.; et al. Ambient iron-mediated aeration (IMA) for water reuse. Water Res. 2013, 47, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.S.; Chaudhari, S.K. Arsenic removal from simulated groundwater using household filter columns containing iron filings and sand. J. Water Proc. Eng. 2015, 6, 151–157. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Enhancing sustainability of household water filters by mixing metallic iron with porous materials. Chem. Eng. J. 2010, 162, 635–642. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Designing laboratory metallic iron columns for better result comparability. J. Hazard. Mater. 2011, 189, 809–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.-B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ arsenic filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended biosand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Gottinger, A.M. Chemical-free Arsenic Removal from Potable Water with a ZVI-amended Biofilter. Master’s Thesis, University of Regina, Regina, SK, Canada, 2010; p. 90. [Google Scholar]
- Gottinger, A.M.; McMartin, D.W.; Wild, D.J.; Moldovan, B. Integration of zero valent iron sand beds into biological treatment systems for uranium removal from drinking water wells in rural Canada. Can. J. Civ. Eng. 2013, 40, 945–950. [Google Scholar] [CrossRef]
- Kowalski, K.P.; Søgaard, E.G. Implementation of zero-valent iron (ZVI) into drinking water supply—Role of the ZVI and biological processes. Chemosphere 2014, 117, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.K.K.; Fenner, R.A. Designing programme implementation strategies to increase the adoption and use of biosand water filters in rural India. Water Altern. 2014, 7, 320–341. [Google Scholar]
- Wenk, C.B.; Kaegi, R.; Hug, S.J. Factors affecting arsenic and uranium removal with zero-valent iron: Laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ. Chem. 2014, 11, 547–557. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Metals 1923, 29, 529–591. [Google Scholar]
- Cox, G.L.; Roetheli, B.E. Effect of oxygen concentration on corrosion rates of steel and composition of corrosion products formed in oxygenated water. Ind. Eng. Chem. 1931, 23, 1012–1016. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabro, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Lackovic, J.A.; Nikolaidis, N.P.; Dobbs, G.M. Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 2000, 17, 29–39. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited: The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.B.; Popescu, I.C.; Crane, R.A.; Noubactep, C. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J. Hazard. Mater. 2011, 186, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Crane, R. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Noubactep, C. Elemental metals for environmental remediation: Lessons from hydrometallurgy. Fresenius Environ. Bull. 2012, 215, 1192–1196. [Google Scholar]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. Freib. Online Geosci. 2015, 42, 80. [Google Scholar]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3057–3062. [Google Scholar] [CrossRef]
- London, M.R.; Wahman, D.G.; Katz, L.E.; Speitel, G.E., Jr. Zero-valent iron/biotic treatment system for perchlorate-contaminated water: Lab-scale performance, modeling, and full-scale implications. J. Environ. Eng. 2013, 139, 1361–1367. [Google Scholar] [CrossRef]
- White, A.F.; Peterson, M.L. Reduction of aqueous transition metal species on the surfaces of Fe(II)-containing oxides. Geochim. Cosmochim. Acta 1996, 60, 3799–3814. [Google Scholar] [CrossRef]
- Charlet, L.; Liger, E.; Gerasimo, P. Decontamination of TCE- and U-rich waters by granular iron: Role of sorbed Fe (II). J. Environ. Eng. 1998, 124, 25–30. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.C.; Coughlin, J.U.; Hunter, B.D. Uranium co-precipitation with iron oxide minerals. Geochim. Cosmochim. Acta 2002, 66, 3533–3547. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the efficiency of the MB discoloration method for the characterization of Fe0/H2O systems using accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior. J. Electrochem. Soc. 2000, 147, 4087–4092. [Google Scholar] [CrossRef]
- Erickson, A.J. Enhanced sand Filtration for Storm Water Phosphorus Removal. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2005. [Google Scholar]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. ASCE 2007, 133, 485–497. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Masse, J.P.; Salvo, L.; Rodney, D.; Bréchet, Y.; Bouaziz, O. Influence of relative density on the architecture and mechanical behaviour of a steel metallic wool. Scr. Mater. 2006, 54, 1379–1383. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Togue-Kamga, F.; Schöner, A.; Woafo, P. Extending service life of household water filters by mixing metallic iron with sand. Clean Soil Air Water 2010, 38, 951–959. [Google Scholar] [CrossRef]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu-K, B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Haig, S.-J. Characterising the Functional Ecology of Slow sand Filters through Environmental Genomics. Ph.D. Thesis, School of Engineering, University of Glasgow, Glasgow, UK, 2014. [Google Scholar]
- Campos, L.C.; Su, M.F.J.; Graham, N.J.D.; Smith, S.R. Biomass development in slow sand filters. Water Res. 2002, 36, 4543–4551. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T.; Braul, L. Evaluation of treatment systems for the removal of arsenic from groundwater. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2005, 9, 152–157. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Biological filtration for removal of arsenic from drinking water. J. Environ. Manag. 2009, 90, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Flaws in the design of Fe0-based filtration systems? Chemosphere 2014, 117, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 60. [Google Scholar]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Salama, H.; Rahman, M.A.; Noubactep, C. Solar energy sustains universal self-reliance in water supply. In Presented at the First International Conference on Solar Energy Solutions for Electricity and Water Supply in Rural Areas, Cairo, Egyt, 7–10 October 2015.
- Baas-Becking, L.G.M.; Kaplan, I.R.; Moore, D. Limits of the natural environments in terms of pH and oxidation-reduction potentials. J. Geol. 1960, 68, 243–284. [Google Scholar] [CrossRef]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe(0)-supported autotrophic denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Campos, V. The effect of carbon steel-wool in removal of arsenic from drinking water. Environ. Geol. 2002, 42, 81–82. [Google Scholar] [CrossRef]
- Campbell, A.D.; Bolton, M. The Vhembe filter: A product for rural South Africa. Image Text J. Des. 2010, 16, 4–20. [Google Scholar]
- Barstow, C.K.; Ngabo, F.; Rosa, G.; Majorin, F.; Boisson, S.; Clasen, T.; Thomas, E.A. Designing and piloting a program to provide water filters and improved cookstoves in Rwanda. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Özer, A.; Altundogan, H.S.; Erdem, M.; Tümen, F. A study on the Cr(VI) removal from aqueous solutions by steel wool. Environ. Pollut. 1997, 97, 107–112. [Google Scholar] [CrossRef]
- Rao, T.S.; Murthy, D.S. Removal of arsenic (V) from water by adsorption onto low-cost and waste materials. Int. J. Res. Eng. Technol. 2013, 2, 206–212. [Google Scholar]
- Tseng, C.L.; Yang, M.H.; Lin, C.C. Rapid determination of cobalt-60 in sea water with steel wool adsorption. J. Radioanal. Nucl. Chem. Lett. 1984, 85, 253–260. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liao, W.; Yak, H.-K. Complete reduction of polychlorinated biphenyls by acid-etched steel wool in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2012, 51, 6625–6630. [Google Scholar] [CrossRef]
- Biswas, S.; Bose, P. Zero-valent iron-assisted autotrophic denitrification. J. Environ. Eng. 2005, 131, 1212–1220. [Google Scholar] [CrossRef]
- Teixeira, L.A.C.; de Abreu Vieira Junior, N.; Yokoyama, L.; da Fonseca, F.V. Degradation of phenol in mine waters using hydrogen peroxide and commercial steel wool. Int. J. Miner. Process. 2015, 138, 15–19. [Google Scholar] [CrossRef]
- Birk, J.P.; McGrath, L.; Gunter, S.K. A general chemistry experiment for the determination of the oxygen content of air. J. Chem. Educ. 1981, 58, 804–805. [Google Scholar] [CrossRef]
- Martins, G.F. Percent oxygen in air. J. Chem. Educ. 1987, 64. [Google Scholar] [CrossRef]
- Najdoski, M.; Petrusevski, V.M.; Alexander, M.D. A novel experiment for fast and simple determination of the oxygen content in the air. J. Chem. Educ. 2000, 77, 1447–1448. [Google Scholar] [CrossRef]
- Vera, F.; Rivera, R.; Núñez, C. A simple experiment to measure the content of oxygen in the air using heated steel wool. J. Chem. Educ. 2011, 88, 1341–1342. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Mitra, P.; Banerjee, P.; Sarkar, D. Reduction of hexavalent chromium present in wastewater by steel wool in a continuous flow system. APCBEE Procedia 2014, 10, 59–63. [Google Scholar] [CrossRef]
- Mitra, P.; Banerjee, P.; Sarkar, D.; Chakrabarti, S. Commercial steel wool for reduction of hexavalent chromium in wastewater: Batch kinetic studies and rate model. Int. J. Environ. Sci. Technol. 2013, 11, 449–460. [Google Scholar] [CrossRef]
- Gromboni, C.F.; Donati, G.L.; Matos, W.O.; Neves, E.F.A.; Nogueira, A.R.A.; Nobrega, J.A. Evaluation of metabisulfite and a commercial steel wool for removing chromium(VI) from wastewater. Environ. Chem. Lett. 2010, 8, 73–77. [Google Scholar] [CrossRef]
- Triszcz, J.M.; Porta, A.; Einschlag, F.S.G. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. Chem. Eng. J. 2009, 150, 431–439. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Contreras, D.; Yáñez, J.; Mansilla, H.D. In field arsenic removal from natural water by zero-valent iron assisted by solar radiation. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Mason, M.G.; Picken, H.D.; Weathers, L.J.; Parkin, G.F. Bioaugmentation of Fe(0) for the remediation of chlorinated aliphatic hydrocarbons. Environ. Eng. Sci. 2000, 17, 169–181. [Google Scholar] [CrossRef]
- Karschunke, K.; Jekel, M. Arsenic removal by iron hydroxides, produced by enhanced corrosion of iron. Water Sci. Technol. Water Supply 2002, 2, 237–245. [Google Scholar]
- Wang, Y.-H.; Lin, S.-H.; Juang, R.-S. Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents. J. Hazard. Mater. 2003, 102, 291–302. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wang, K.-S.; Hu, P.-I.; Lui, I.-C. Rapid recovery of dilute copper from a simulated Cu–SDS solution with low-cost steel wool cathode reactor. J. Hazard. Mater. 2009, 163, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction, and recirculation. Sustainability 2010, 2, 2988–3073. [Google Scholar] [CrossRef]
- Wei, M.-C.; Wang, K.-S.; Huang, C.-L.; Chiang, C.-W.; Chiang, T.-J.; Lee, S.-S.; Chang, S.-H. Improvement of textile dye removal by electrocoagulation with low-cost steel wool cathode reactor. Chem. Eng. J. 2012, 192, 37–44. [Google Scholar] [CrossRef]
- Yamashita, T.; Yamamoto-Ikemoto, R. Nitrogen and phosphorus removal from wastewater treatment plant effluent via bacterial sulfate reduction in an anoxic bioreactor packed with wood and iron. Int. J. Environ. Res. Public Health 2014, 11, 9835–9853. [Google Scholar] [CrossRef] [PubMed]
- Callery, O.; Brennan, R.B.; Healy, M.G. Use of amendments in a peat soil to reduce phosphorus losses from forestry operations. Ecol. Eng. 2015, 85, 193–200. [Google Scholar] [CrossRef]
- Golder Associates Inc. (Golder) Literature Review of Treatment Technologies to Remove Selenium from Mining Influenced Water. Available online: http://namc.org/docs/00057713.PDF (accessed on 26 November 2015).
- CH2M Hill Inc. (CH2M) Review of Available Technologies for the Removal of Selenium from Water. Final Report Prepared for North American Metals Council. 2010, p. 233. Available online: http://namc.org/docs/00062756.PDF (accessed on 20 November 2015).
- Kane, T.P.; Lovett, R.J.; Bouse, S.A. Element Removal Process. U.S. Patent 8,101,087, 24 January 2002. [Google Scholar]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kubare, M.; Haarhoff, J. Rational design of domestic biosand filters. J. Water SRT Aqua 2010, 59, 1–15. [Google Scholar] [CrossRef]
- Kandra, H.S.; Deletic, A.; McCarthy, D. Assessment of impact of filter design variables on clogging in stormwater filters. Water Resour. Manag. 2014, 28, 1873–1885. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Noubactep, C. Affordable safe drinking water for victims of natural disasters. In Natural Disasters and Sustainable Development; Kätsch, C., Meliczek, H., Eds.; Cuvillier Verlag: Göttingen, Germany, 2014; pp. 57–75. [Google Scholar]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2015. [Google Scholar] [CrossRef] [PubMed]
- Twidwell, L.; McCloskey, J.; Joyce, H.; Dahlgren, E.; Hadden, A. Removal of selenium oxyanions from mine waters utilizing elemental iron and galvanically coupled metals. In Innovations in Natural Resource Processing—Proceedings of the Jan. D. Miller Symposium; Young, C.A., Kellar, J.J., Free, M.J., Drelich, J., King, P.P., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2005. [Google Scholar]
- Nicol, M.J.; Schalch, E.; Balestra, P.; Hegedus, H. A modern study of the kinetics and mechanism of the cementation of gold. J. S. Afr. Inst. Min. Metall. 1979, 79, 191–198. [Google Scholar]
- Noubactep, C. Elemental metals for environmental remediation: Learning from cementation process. J. Hazard. Mater. 2010, 181, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Alamilla, J.L.; Espinosa-Medina, M.A.; Sosa, E. Modelling steel corrosion damage in soil environment. Corros. Sci. 2009, 51, 2628–2638. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the reactivity of metallic iron in Fe0/EDTA/H2O systems with column experiments. Chem. Eng. J. 2010, 162, 656–661. [Google Scholar] [CrossRef]
- Stergioudi, F.; Kaprara, E.; Simeonidis, K.; Sagris, D.; Mitrakas, M.; Vourlias, G.; Michailidis, N. Copper foams in water treatment technology: Removal of hexavalent chromium. Mater. Des. 2015, 87, 287–294. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).