Abstract
Northwestern Jiangxi Province is rich in metasilicic acid (as H2SiO3) mineral water resources. Investigating their hydrogeochemical characteristics and formation mechanism is crucial for the rational utilization of water resources and the sustainable development of the local mineral water industry. Taking the Taoping water source area in northwestern Jiangxi as a case study, 11 sets of groundwater and surface water samples were systematically collected. By comprehensively applying mathematical statistics, ionic ratios, and isotopic analyses, the hydrogeochemical characteristics and formation processes of metasilicic acid-type mineral water were examined. The results indicate that: (1) The mineral waters in the area are weakly alkaline and belong to the metasilicic acid type, with concentrations ranging from 22.0 to 67.0 mg/L, of which 75% exceed 30 mg/L. (2) The primary hydrochemical types are HCO3−–Ca·Na, HCO3−–Ca·Mg, and HCO3−–Ca. Analysis of stable isotopes (δ18O and δ2H) and tritium (3H) indicates that metasilicic acid mineral water is primarily recharged by atmospheric precipitation, with an apparent groundwater age of approximately 60 years. (3) The enrichment of metasilicic acid primarily results from the weathering and leaching of silicate minerals, coupled with cation exchange. K+ and Na+ are mainly derived from silicate minerals such as feldspars and halite, whereas Ca2+ and Mg2+ originate primarily from carbonate minerals like calcite and dolomite. During recharge, atmospheric precipitation infiltrates the aquifer, dissolving aluminosilicate and siliceous minerals in the surrounding rocks, thereby releasing metasilicic acid into the groundwater and ultimately forming the metasilicic acid-type mineral water.
1. Introduction
As a type of natural mineral water, metasilicic acid mineral water is known for its health benefits, and is therefore highly favored by consumers [1,2]. In recent years, extensive research on its formation mechanisms has been conducted both in China and abroad, focusing on aspects such as water–rock interactions [3,4], hydrochemistry [5,6], isotopic analysis [7,8], and hydrogeochemical modeling [9,10,11]. Tingting et al. [10] investigated mineral waters in the Xishan region and found that the formation of metasilicic acid-type mineral waters was primarily controlled by the development of fractures. Using reverse hydrogeochemical modeling, they demonstrated that water–rock interactions are primarily represented by the dissolution of sodium and calcium feldspar, and pyroxene, which leads to the generation of metasilicic acid. Similarly, Zhu et al. [12] studied mineral waters in the Changbai Mountain basaltic region and found that groundwater was primarily recharged via atmospheric precipitation. In this area, groundwater not only interacts with host rocks, but also experiences other hydrogeochemical processes, such as rainfall infiltration, evaporation, and concentration. The metasilicic acid in groundwater primarily originates from the dissolution of feldspar and pyroxene, with its concentration gradually increasing along the runoff path. Jin et al. [8] used hydrogen–oxygen isotopic analysis and tritium (TU) dating to determine that metasilicic acid-type mineral waters in Zhangbei County are primarily recharged by local summer precipitation and glacial meltwater from nearby mountains, with groundwater ages ranging from approximately 15 to 60 yr. Fang et al. [11] analyzed the formation mechanism of mineral waters in Fushun County using PHREEQC simulations of water–rock interactions along three flow paths. His study systematically examined the genesis of local metasilicic acid-type mineral waters from qualitative, quantitative, temporal, and spatial perspectives. Similarly, Houyen et al. [13] employed rock geochemistry and isotopic tracing to identify factors influencing the spatial variation in silicic acid in groundwater and explored the genesis of silicic acid mineral water from the combined perspective of rock weathering and hydrogeochemical processes. Finally, Wang et al. [14] analyzed the hydrochemical characteristics of groundwater in the mountainous region of southern Jiangxi. He explored lithological controls and the primary mechanisms shaping these characteristics and concluded that groundwater types in this area are primarily governed by rock weathering, with major ionic sources derived primarily from the weathering and dissolution of silicate rocks, supplemented by the dissolution of carbonate minerals.
Jiangxi Province has abundant mineral water resources; surveys have identified 175 mineral water sources. Among these, metasilicic acid mineral waters were the most prevalent, accounting for 134 sources [15]. Lei et al. [16] applied multiple analytical approaches to investigate the distribution, hydrochemical characteristics, genesis, and material sources of potential metasilicic acid mineral waters in Xingguo County and evaluated their health benefits based on sensory and health-related indices. Panxi et al. [17] proposed rational development strategies for strontium-rich and metasilicic acid mineral waters in northern Pingxiang City. Fang analyzed the formation characteristics of mineral waters in Luxi County by examining the local geological setting and hydrogeological conditions. Similarly, Sun et al. [18] explored the exposure patterns of mineral waters in the mountainous regions of southern Jiangxi and concluded that fault structures provide both the hydrodynamic conditions necessary for mineral water formation and spatial pathways favorable for its migration and storage.
In summary, previous research on underground mineral water in Jiangxi Province has primarily addressed topography, stratigraphy, lithology, geological structures, groundwater circulation, hydrochemistry, and distribution patterns. However, detailed studies on the hydrogeochemical processes governing mineral water formation remain limited. To address this gap, this study focuses on the Taoping water source area, employing hydrogeochemical and isotopic analyses to investigate the characteristics and formation processes of metasilicic acid mineral water. The novelty of this work is twofold. First, the geologically unique study area (distinct from the more commonly studied basalt and karst terrains in China) provides new insights into the hydrogeochemistry of metasilicic acid enrichment in this setting. Second, the research integrates multiple techniques—including mathematical statistics, ionic ratios, and stable (δ18O, δ2H) and radioactive (3H) isotope analyses—to enable a systematic analysis of the sources, recharge mechanisms, and residence times of water components. This study aims to advance the understanding of metasilicic acid mineral water genesis, thereby providing a theoretical basis for the sustainable development and utilization of these resources in Xiushui County and offering a reference for similar geological settings elsewhere.
2. Research Area Overview
2.1. Geographical Location
The study area was located at the Taoping mineral water source in Shankou Town, Xiushui County, Jiujiang City, with geographical coordinates of 114°32′47.8″ E and 29°01′34.60″ N (Figure 1). This water source lies between the Mufu and Jiuling mountain ranges and features a landform that is elevated along the periphery and lowered toward the center. The terrain is primarily mountainous and hilly, with elevations ranging from approximately 111 to 130 m. The region experiences a typical mid-latitude subtropical humid monsoon climate, with an average annual temperature of approximately 17 °C and mean annual precipitation of approximately 1600 mm. Numerous rivers traverse the region, forming a dense drainage network, and providing abundant surface water resources.
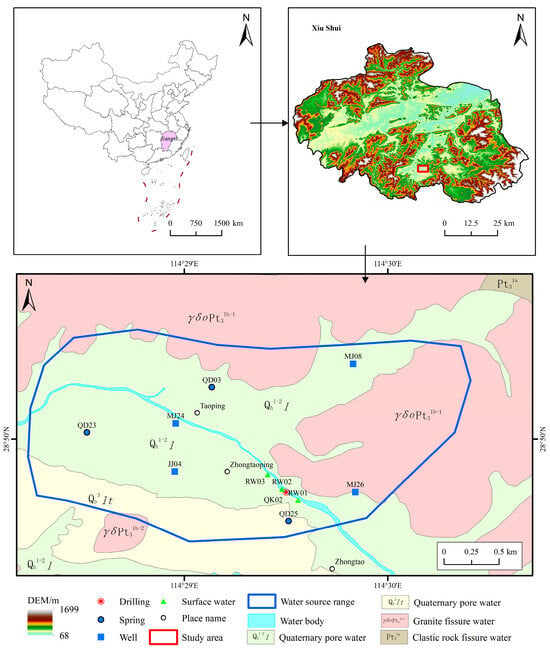
Figure 1.
Study area and sample distribution.
2.2. Geological Conditions
The study area is located on the northern margin of the Yangtze Geologic Province. Influenced by Caledonian and Yanshanian magmatic activity, the region has undergone multiple igneous rock intrusion episodes, leading to complex geological structures and well-developed strata. Except for the absence of Devonian, Carboniferous, and Jurassic formations, strata ranging from the Qingbaikou Formation to Quaternary deposits are exposed. The lithology primarily comprises Silurian granites and shallow metamorphosed Nanhua Group rocks. Two major faults, Fd1 and Fd2, are prominent within the study area. Fd1, located north of Taoping Village, contains a silicified fracture zone, in which an upwelling spring emerges along the hanging wall of the fault. Fd2, situated in Zhongtao Village, has a silicified zone trending northeasterly–southwesterly with a dip angle of approximately 47° and a visible width of 3–4 m. Fractures are densely developed in the vicinity of this fault, providing favorable pathways for groundwater movement.
2.3. Hydrogeological Conditions
Based on the groundwater occurrence conditions, the groundwater in the study area primarily comprised bedrock fracture water and Quaternary loose rock pore water. Bedrock fracture water primarily occurs within the Precambrian shallow-metamorphic and Paleozoic clastic rock fractures and is primarily recharged by atmospheric precipitation. Although it has favorable runoff conditions, the spring discharge is generally low. In river valleys, the Quaternary loose-rock pore water aquifer is characterized by a stable lithology, with an average thickness ranging from 3.1 to 4.91 m. The groundwater distribution in this aquifer is relatively uniform, with permeability coefficients between 13.6 and 110.08 m/d. The groundwater depth typically varies between 3 and 5 m, showing annual fluctuations of 0.5–1.5 m, indicating abundant groundwater resources.
2.4. Groundwater Recharge and Drainage Conditions
Groundwater recharge primarily originates from atmospheric precipitation and lateral inflow from surface rivers, resulting in shallow groundwater levels. In areas where bedrock fissure water is distributed, groundwater levels are relatively high and some groundwater is discharged locally to form springs. In mountainous regions with large elevation differences and steep terrain, groundwater typically exhibits steep hydraulic gradients and short flow paths, which contribute to high water quality. Groundwater discharge primarily occurs near recharge areas, forming downslope springs at mountain bases. Water-rich zones commonly develop in densely fractured contact zones and low-lying regions. The bedrock fracture water is predominantly confined, with atmospheric precipitation infiltrating weathered fractures and structural joints to recharge the aquifer. Groundwater level dynamics largely correspond to precipitation patterns and exhibit distinct seasonal variations.
3. Materials and Methods
In May 2023 (without isotopic testing) and March 2025 (with isotopic testing), 11 sets of surface water and groundwater samples were collected for water quality analysis. All fieldwork strictly followed the Technical Specifications for Groundwater Environmental Monitoring (2021). Sampling points were distributed across major rivers, wells, and springs within the water source area, yielding 11 samples for stable hydrogen and oxygen isotopic analyses and one sample for TU isotopic analysis. The spatial distribution of the sampling points is shown in Figure 1. Each sampling location was precisely recorded using Garmin™ GPS devices (Garmin Ltd., Olathe, KS, USA) to document its coordinates. Prior to sampling, large-diameter wells and boreholes were thoroughly cleaned. The sampling bottles were rinsed three times with water and completely filled without air bubbles to prevent contamination. All collected samples were tightly sealed and transported to the laboratory within the prescribed time limit. Field parameters, including water temperature, pH, dissolved oxygen, and electrical conductivity, were measured on-site using a portable water quality analyzer (Model: SX731) (Shenzhen Sanxin Technology Co., Ltd., Shengzhen, China), whereas other indicators were analyzed in the laboratory. Water chemistry analyses were conducted at the Testing Center of the Jiangxi Provincial Survey and Design Institute Co., Ltd. Cations were determined by flame atomic absorption spectroscopy, CO32− and HCO3− were analyzed by titration, and other anions were determined using ionic chromatography (Thermo Fisher Scientific Inc., Waltham, MA, USA). Isotopic analyses were performed at the Third Institute of Oceanography, Ministry of Natural Resources, where D and 18O were measured using optical cavity decay spectroscopy (LGR, los gatos research, San Jose, CA, USA), with precision levels of <±1.5‰ and <±0.5‰, respectively. TU was measured using a Quantulus 1220 ultralow background liquid scintillation (erkinElmer, Inc., Waltham, MA, USA) counter with a detection limit of 0.1 Bq/L. The sampling and test results are listed in Table 1.

Table 1.
Hydrochemical composition of the studied samples.
4. Analysis and Discussion
4.1. Distribution and Hydrochemical Characteristics of Metasilicic Acid-Type Mineral Waters
4.1.1. Ionic Characteristics of Metasilicic Acid and Key Water Chemistry
Water temperatures at all sampling points were <25 °C. According to the Drinking Natural Mineral Water standard [19] (GB 8537-2018), the boundary value for metasilicic acid content in mineral waters is ≥25.0 mg/L (when the content ranges between 25.0 and 30.0 mg/L, the water temperature should exceed 25 °C). Statistical analysis of the water chemistry data revealed that the silicic acid concentrations in groundwater within the study area ranged from 22.0 to 67.0 mg/L (Table 1), with six sampling points (75% of all samples) showing concentrations > 30 mg/L. All samples meeting the silicic acid threshold were distributed within the igneous rock fissure aquifer and clastic rock pore-fissure aquifer groups. This indicates that silicic acid mineral water is widely present in local groundwater systems.
Piper diagrams were used to visually assess the types of water chemistry and relative distribution of major ions, thereby clarifying the geochemical evolution patterns. As shown in Table 1, groundwater samples exhibited pH values between 6.45 and 6.85, whereas surface water samples ranged from 6.95 to 6.99. Overall, both water types were predominantly neutral and exhibited good chemical stability. The total dissolved solids (TDS) in both groundwater and surface water were <100 mg/L, consistent with low-mineralization freshwater characteristics. Regarding ionic composition, the dominant anion in both water types was HCO3−, whereas the dominant cations were primarily Ca2+, followed by Na+ and Mg2+, indicating a similarity in ionic sources. Figure 2 shows that anions cluster near the HCO3− axis and cations near the Ca2+–Mg2+ axis. This pattern suggests that HCO3− overwhelmingly dominates over Cl− and SO42−, whereas Ca2+ and Mg2+ are the principal cations. Combined with the low TDS levels (<100 mg/L), the Shukarev classification indicates that the silicic acid groundwater in the study area primarily belongs to the HCO3−–Ca·Na, HCO3−–Ca·Mg, and HCO3−–Ca types.
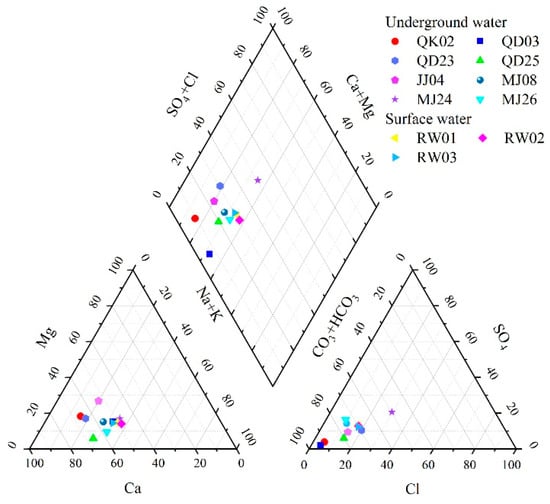
Figure 2.
The piper three-line graph of groundwater geochemistry in the study area.
4.1.2. Pearson Correlation Analysis
Through mathematical and statistical analyses of the hydrochemical components, the relationships between the parameters and their sources can be identified more effectively [9]. The Pearson correlation coefficient is used to measure the linear relationship between two variables, ranging from −1 to +1. A value of −1 indicates a perfect negative correlation, +1 a perfect positive correlation, and 0 no correlation. The greater the absolute value, the stronger the relationship. The formula used is as follows:
To explore the hydrochemical component sources and correlation strengths in metasilicic acid-type mineral waters, Pearson correlation analysis was performed using SPSS software (SPSS 27.) for the measured parameters: H2SiO3, K+, Na+, Ca2+, Mg2+, Cl−, HCO3−, SO42−, TDS, and pH. Table 2 shows that TDS exhibits significantly positive correlations with HCO3− (r = 0.922) and Mg2+ (r = 0.849), indicating that these ions are major groundwater constituents and likely share common sources, significantly influencing TDS levels. Groundwater components primarily originate from atmospheric precipitation recharge, weathering, and the dissolution of rock-forming minerals. The metasilicic acid content shows positive correlations with pH, Na+, and Ca2+, with correlation coefficients of 0.485, 0.201, and 0.129, respectively.

Table 2.
Correlation coefficient matrix of groundwater chemical components in the study area.
4.2. Analysis of Hydrochemical Component Sources
4.2.1. Gibbs and Three-Component Diagrams
To explain the natural factors controlling the chemical composition of water bodies, Gibbs [20] proposed using the scatter distribution of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) ratios plotted against the TDS. Gibbs diagrams provide an intuitive method for analyzing the composition, origin, and interrelationships among water samples and are crucial for understanding the hydrogeochemical genesis of groundwater [21]. In the Gibbs diagram, the vertical axis represents the TDS (logarithmic scale), whereas the horizontal axis depicts either the cation ratio Na+/(Na+ + Ca2+) or the anion ratio Cl−/(Cl− + HCO3−). These diagrams help identify whether the water chemistry is controlled by precipitation, rock weathering, or evaporation–concentration processes [22,23].
Plotting the hydrochemical data of the study area (Figure 3) shows that all the silicate mineral water samples fall within the rock weathering-dominated zone. This indicates that the major ions were primarily controlled by rock weathering, although some samples showed transitional trends toward precipitation effects. At sampling points within the loose rock pore aquifers, the molar concentration ratios Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) were both <0.5, suggesting dominance of alkaline earth cations and bicarbonate ions—typical of groundwater formed through mineral leaching. The rocks contributing to these processes are primarily carbonate and silicate. To further elucidate the sources of major ions, ratios such as Ca2+/Na+, Mg2+/Na+, and HCO3−/Na+ were employed [24]. Figure 4 shows that both the surface and groundwater samples plot between the silicate and carbonate rock-controlled end members, indicating that the hydrochemical evolution of the water in this area is jointly governed by the weathering and dissolution of silicate and carbonate rocks.
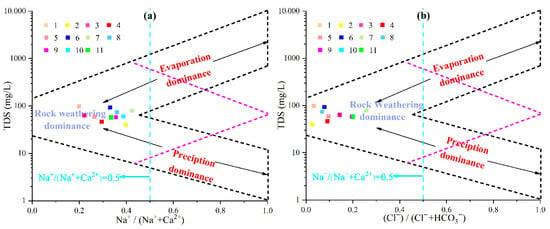
Figure 3.
Gibbs diagram of groundwater chemistry in the study area. (a) represents the relationship of mass concentration ratios Na/(Na + Ca) and TDS of groundwater. (b) represents the relationship of mass concentration ratios Cl/(Cl + HCO3) and TDS of groundwater.
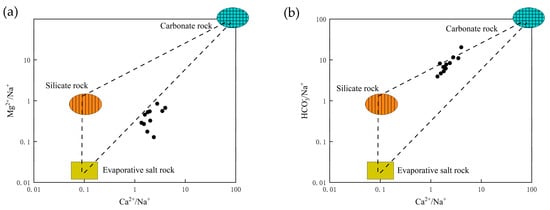
Figure 4.
Three-terminal diagram of groundwater sources in the study area. (a) represents the relationship between the molar concentration ratio of Ca to Na and that of Mg to Na. (b) represents the relationship between the molar concentration ratio of Ca to Na and that of HCO3 to Na.
4.2.2. Chlor-Alkali Index (CAI)
The CAI, also known as the Scheele index, is used to analyze the strength and direction of the cation exchange. The formula used is as follows:
During flow, groundwater interacts continuously with the surrounding rock through cation exchange processes, which exert a significant influence on its hydrochemical composition. Cation exchange refers to the reversible replacement of metal cations in groundwater with cations adsorbed on mineral surfaces within an aquifer matrix under specific geochemical conditions. This process is a key hydrogeochemical mechanism controlling cation concentrations in groundwater [13]. Previous studies have commonly used ion ratio methods to determine whether cation exchange occurs in groundwater [25,26]. When the molar concentration ratios of groundwater ions (Mg2+ + Ca2+ − SO42− − HCO3−) and (Na+ − Cl−) are approximately 1, it indicates that cation exchange occurs during groundwater circulation within the study area. The CAI was used to evaluate the direction and intensity of the exchange reactions. Positive CAI values represent forward cation exchange, in which Na+ and K+ in the groundwater replace Mg2+ and Ca2+ in the aquifer minerals. Conversely, negative CAI values indicate a reverse exchange, where Mg2+ and Ca2+ in the groundwater are replaced by Na+ and K+ from the rock matrix [27].
As shown in Figure 5a, the negative molar concentration ratios of (Mg2+ + Ca2+ − SO42− − HCO3−) to (Na+ − Cl−) suggest the occurrence of cation exchange in the groundwater. This is further supported by Figure 5b, where most groundwater samples exhibit negative CAI-I and CAI-II values, indicating reverse cation exchange—i.e., Na+ and K+ from the surrounding rocks replace Mg2+ and Ca2+ in the groundwater. This exchange process explains the dominance of Mg2+ and Ca2+ ions in the study area’s groundwater.
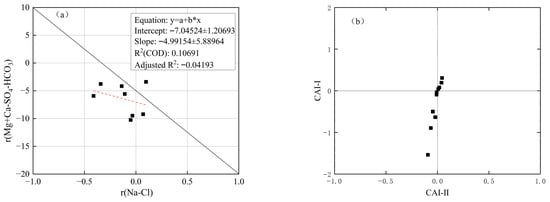
Figure 5.
Analysis diagram of alternating cation adsorption. (a) (Mg2+ + Ca2+ − SO42− − HCO3−) and (Na+ − Cl−) molar concentration correlation. (b) CAI (CAI-I and CAI-II) relationship diagram.
4.3. Analysis of Supply Sources and Ages
Stable hydrogen and oxygen isotopic analysis is essential for identifying groundwater recharge sources and elucidating transformation processes and evolutionary mechanisms within the hydrological cycle [28,29]. The distribution and enrichment levels of stable isotopes in groundwater are typically expressed using δ values. A negative δ value indicates isotopic depletion in groundwater, whereas a positive δ value indicates isotopic enrichment. The calculation formulae for δ2H and δ18O are as follows:
In 1981, the International Atomic Energy Agency modified this formula, resulting in the Global Mean Wetline (GMWL) formula:
In 1983, Zheng Shuhui derived the China Mainland Weather Line (CMWL) formula:
Owing to variations in climatic and environmental conditions among different regions, studies have generally employed the local meteoric water line (LMWL) of the study area for isotopic analysis. Considering no local precipitation isotopic data were available for this region, the regional meteoric water line established by Bao Zhicheng [30] was adopted.
Comparing the three precipitation lines (Figure 6), it can be seen that the slope (8.4) and intercept (15.6) of the regional meteoric water line in the study area are higher than those of the global and national standard meteoric water lines. This characteristic reflects the unique water vapor circulation mechanism during the formation and deposition of precipitation in the study area. On the one hand, the study area is situated in a mountain valley between the Mufu Mountains and the Jiuling Mountains, where local water vapor circulation is weak. Precipitation is primarily replenished by long-distance marine water vapor transport, with no significant isotopic fractionation occurring during water vapor migration. On the other hand, the region has high vegetation coverage and low surface evaporation intensity, meaning that precipitation is less affected by secondary evaporation during deposition. Therefore, the isotopic composition of precipitation can better preserve the original isotopic signature of atmospheric precipitation. This observation is consistent with the findings of Ge et al. [31] and Zhou et al. [24] in the southern red bed regions, which further confirms the typical isotopic characteristics of precipitation in the humid mountainous areas of southern China.
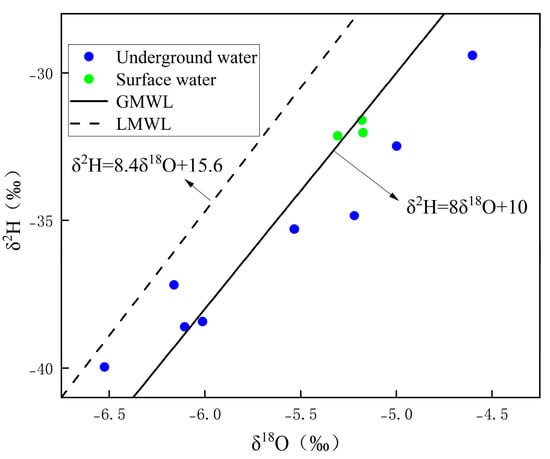
Figure 6.
The relationship between groundwater and surface water (δ2H–δ18O) in the study area.
TU isotopic dating is a key technique used for short-term groundwater age estimation in modern and recent hydrological studies. The principle is based on the radioactive decay of Tritium 3H, which dates over a time range of several decades to centuries. This method is particularly useful for assessing hydrological characteristics such as groundwater age, residence time, and renewal capacity [32,33]. In this study, deep groundwater samples were collected from borehole QK02 for TU isotopic analysis. The measured TU content was 1.44 TU (Table 3). Considering the relatively simple aquifer structure and slow groundwater flow velocity in the study area, a total mixing model was applied to estimate the groundwater age. Based on the interpolation model proposed by previous researchers [8], the estimated residence time of the groundwater in the study area was approximately 60 yr. This indicates that the deep groundwater within the metasilicic acid mineral water source area is characterized by a long residence time, extended recharge distance, and significant water–rock interactions.

Table 3.
Hydrogen and oxygen isotopic analysis at water sample points in the study area.
5. Conclusions
This investigation elucidates the hydrogeochemical characteristics and formation mechanisms of silica-rich mineral water in the Taoping water source area, northwestern Jiangxi. The main findings are:
- (1)
- The groundwater in this magmatic rock terrain is characterized by low salinity (TDS < 100 mg/L) and metasilicic acid-enriched characteristics, qualifying as high-quality metasilicic acid mineral water. The strata contain abundant carbonates and dolomites, resulting in high contents of Ca2+ and Mg2+ ions. The dominant hydrochemical facies are HCO3−–Ca·Na, HCO3−–Ca·Mg, and HCO3−–Ca types.
- (2)
- Ionic ratio analysis indicates that the enrichment of silicic acid in groundwater is mainly controlled by the weathering and leaching of silicate minerals, with cation exchange adsorption serving as a secondary contributing process.
- (3)
- Stable isotopic (18O and δ2H) analyses show that both surface water and groundwater share a common recharge source—atmospheric precipitation. However, surface water showed greater isotopic enrichment owing to enhanced evaporation during its exposure to the atmosphere. Tritium (3H) dating results suggest that the groundwater in the study area has a residence time of approximately 60 years, reflecting long-term water–rock interactions and significant hydrogeochemical evolution.
Author Contributions
Conceptualization, D.L.; Methodology, D.L.; Software, X.W.; Formal analysis, D.L. and X.B.; Investigation, S.Y., T.L. and F.D.; Writing—original draft, X.W. and F.D.; Writing—review and editing, D.L.; Supervision, X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Nanchang Key Laboratory of Hydrogeology and High Quality Groundwater Resources Exploitation and Utilization, the grant number is 20243C11.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Dian Liu, Ximin Bai, Shengpin Yu, Tian Li, and Fei Deng was employed by the company Jiangxi Institute of Survey & Design Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, J.; Malhi, K.K.; Li, X.; Xu, X.; Kang, J.; Zhao, B.; Xu, Y.; Li, X.; Li, J. Metasilicate-based alkaline mineral water improves the growth performance of weaned piglets by maintaining gut-liver axis homeostasis through microbiota-mediated secondary bile acid pathway. Anim. Nutr. 2025, 20, 95–109. [Google Scholar] [CrossRef]
- Chen, J.; Xu, X.; Kang, J.; Zhao, B.; Xu, Y.; Li, J. Metasilicate-based alkaline mineral water confers diarrhea resistance in maternally separated piglets via the microbiota-gut interaction. Pharmacol. Res. 2023, 187, 106580. [Google Scholar] [CrossRef]
- Yang, X.; Jia, C.; Zhu, H.; Liu, Z.; Liu, Z. Characteristics and genesis of high-quality metasilicate mineral water in Liaocheng City, Shandong Province. Environ. Monit. Assess. 2024, 196, 1155. [Google Scholar] [CrossRef]
- Xu, M.; Hu, C.; Zhu, L.; Song, G.; Peng, W.; Yang, S.; Song, J. Spatial Distribution Characteristics and Genetic Mechanism of the Metasilicate-Rich Groundwater in Ji’nan Rock Mass Area, Shandong Province, China. Water 2023, 15, 713. [Google Scholar] [CrossRef]
- Kong, F.; Xue, Y.; Qiu, D.; Su, M.; Gong, H.; Wang, X. Assessment of the Mineralization Processes of Potable Natural Mineral Water Using the Chemical Thermodynamics Analysis. Pol. J. Environ. Stud. 2021, 31, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, Y.; Ma, Y.; Li, J.; Yu, Y.; Sun, W. Study on hydrochemical characteristics and formation process of Antu mineral water in Changbai Mountain, China. Water 2022, 14, 2770. [Google Scholar] [CrossRef]
- Li, Y.; Bian, J.; Li, J.; Ma, Y.; Auguiano, J.H.H. Hydrochemistry and stable isotope indication of natural mineral water in Changbai Mountain, China. J. Hydrol. Reg. Stud. 2022, 40, 101047. [Google Scholar] [CrossRef]
- He, J.; Ma, X.; Deng, Q.; Li, W.; Ma, X.; Zheng, Y.; Liu, Z. Hydrochemical characteristics and formation mechanism of metasilicate mineral water in a Cenozoic basaltic aquifer in Zhangbei County, Hebei Province. Geol. China 2021, 50, 1887–1902. [Google Scholar]
- Liu, Y.; Li, M.; Zhang, Y.; Wu, X.; Zhang, C. Analysis of the hydrogeochemical characteristics and origins of groundwater in the changbai mountain region via inverse hydrogeochemical modeling and unsupervised machine learning. Water 2024, 16, 1853. [Google Scholar] [CrossRef]
- Shan, T.; Xu, S.; Fan, Z.; Ruan, W. Characteristics and formation mechanism of metasilicate mineral water in Xishan mountain of Kunming. J. Kunming Univ. Sci. Technol. (Nat. Sci.) 2019, 44, 39–47. [Google Scholar]
- Fang, Z.; Bian, J.; Sun, X.; Tian, X. Mineral water formation mechanism and process modeling in Fusong County. Sci. Technol. Eng. 2017, 17, 39–44. [Google Scholar]
- Zhu, Z.; Xiao, C.; Liang, X.; Yang, W.; Jia, L. Hydrochemical characteristics and formation mechanism of metasilicic acid type natural mineral water in basalt area of Antu County. Water Resour. Hydropower Eng. 2021, 52, 146–156. [Google Scholar]
- Sun, H.; Sun, X.; Wei, X.; Chen, Z.; Liu, W.; Huang, X.; Li, X.; Yin, Z.; Liu, W. Formation mechanism of metasilicate mineral water in Chengde, Hebei Province: Evidence from rock weathering and water-rock interaction. Geol. China 2022, 49, 1088–1113. [Google Scholar]
- Wang, X.; Wang, Y.; Gao, G.; Li, M.; An, H.; Mao, K.; Wang, Y.; You, Y.; Gong, L. Hydrochemical Characteristics and Genesis of Groundwater in Taoshan Granite Body, Yushan Uplift Area, South Jiangxi, China. Water 2025, 17, 974. [Google Scholar] [CrossRef]
- Deng, J.; Xu, F.; Bai, X.; Ye, H. Study on the Distribution Patterns and Formation Mechanism of Natural Drinking Mineral Water in Jiangxi Province. Jiangxi Sci. 2024, 42, 1190–1197. [Google Scholar]
- Gong, L.; Wang, X.; Song, M.; Hu, Q.; Liao, S.; Chen, H. Hydrochemical characteristics and water quality health function evaluation of potential metasilicate mineral water in Xingguo County, Jiangxi Province. Rock Miner. Anal. 2021, 40, 894–906. [Google Scholar]
- Wang, P.; Cao, Y.; Feng, N.; Zhao, Y.; Wang, Z.; Zhong, B. Discovery and development suggestion of strontium-rich and metasilicate mineral water in the northern Pingxiang City, Jiangxi Province. Geol. China 2022, 49, 677–678. [Google Scholar]
- Sun, Z.; Gao, Z.; Wang, X.; Lin, H.; Song, M. Exploration of mineral water outcropping pattern in the mountainous area of South Jiangxi. Acta Geosci. Sin. 2018, 39, 565–572. [Google Scholar]
- GB 8537-2018; National Food Safety Standard Drinking Natural Mineral Water. Standards Press of China: Beijing, China, 2018.
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Ji, Y.; Wang, Y.; Zhang, L. The Hydrogeochemical Characteristics and Formation Mechanisms of the High-Salinity Groundwater in Yuheng Mining Area of the Jurassic Coalfield, Northern Shaanxi, China. Water 2025, 17, 1459. [Google Scholar] [CrossRef]
- Gao, S.; Li, C.; Liu, Y.; Sun, B.; Zhao, Z.; Lv, M.; Gang, S. Hydrogeochemical Characteristics and Evolution Processes of Karst Groundwater Affected by Multiple Influencing Factors in a Karst Spring Basin, Eastern China. Water 2023, 15, 3899. [Google Scholar] [CrossRef]
- Yan, M.; Wang, L.; Wang, Q.; Liu, Z. Hydrochemical Characteristics and Origin Analysis of Groundwater in Nanling County, Anhui Province. Water 2024, 16, 1579. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Q.; Wang, Y.; Luo, F.; Liang, J.; Xiong, J. Recharge sources and hydrochemical evolution mechanism of surface water and groundwater in typical karst mining area. Huan Jing Ke Xue 2024, 45, 5264–5276. [Google Scholar]
- Yadav, P.; Sreekesh, S.; Nandimandalam, J.R. Groundwater Quality and Its Suitability in the Semi-Arid River Basin in India: An Analysis of Hydrogeochemical Processes Using Multivariate Statistics. Environ. Model. Assess. 2025, 30, 625–646. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Wang, Y.; Zhang, L.; Li, X.; Geng, H.; Lu, Y. Chemical characteristics and evolution of groundwater in northeastern margin of the Tibetan Plateau, China. Environ. Geochem. Health 2025, 47, 11. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, H.; Wu, Y.; Cao, F.; Jia, F.; Qu, P. The evolution of hydrogeochemical characteristics of a typical piedmont karst groundwater system in a coal-mining area, Northern China. Environ. Earth Sci. 2019, 78, 557. [Google Scholar] [CrossRef]
- Oerter, E.J.H. Hydrogen and Oxygen Stable Isotope Compositions of Kaolinite Hydroxyl Water and their Paleoenvironmental Significance. Geochim. Cosmochim. Acta 2025, 398, 1–10. [Google Scholar] [CrossRef]
- Zuecco, G.; Marchina, C.; Censini, M.; Todini-Zicavo, D.; Cassiani, G.; Borga, M. A simple experiment to trace stemflow infiltration: Advantages and challenges of using stable isotopes of hydrogen and oxygen and electrical resistivity tomography. Vadose Zone J. 2025, 24, e20397. [Google Scholar] [CrossRef]
- Bao, Z.; Zha, X.; Gao, X.; Zhao, Y.; Zhao, A.; Xu, Z.; Chen, J.; Jiang, J. Hydrogeochemincal characteristics and genesis for jiujiang NO. 2 well in Jiangxi Province. China Earthq. Eng. J. 2022, 44, 920–928. [Google Scholar]
- Ge, Q.; Shao, Z.; Liang, X.; Li, X.; Wang, J.; Wu, P.; Chen, Y.; Li, J.; Zhang, Q. Study on the Hydrochemical Characteristics and Formation Mechanism of Shallow Groundwater in Karst Area, Meijiao Town, Ganzhou. Environ. Chem. 2024, 43, 1608–1620. [Google Scholar]
- Zhao, Z.; Sun, Y.; Chen, Q.; Li, T.; Liu, F.; Yan, T.; Zheng, W. Research Progress in Tritium Processing Technologies: A Review. Separations 2025, 12, 33. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Barešić, J. Application of Stable Isotopes and Tritium in Hydrology. Water 2021, 13, 430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.