Abstract
Arsenic contamination in water remains a critical global challenge, particularly in rural and resource-limited regions. Clays have been widely studied as cost-effective and efficient adsorbents for arsenic removal. This systematic review provides a comprehensive analysis of the application of clays in arsenic adsorption, focusing on clay types, operational units, and study methodologies. The review classifies the adsorption mechanisms, highlights key factors influencing adsorption performance—such as pH, ionic strength, and surface modifications—and examines the effectiveness of various modifications. Furthermore, the study categorizes adsorption research into kinetic, iso-thermal, thermodynamic, and efficiency studies, providing insights into the state of the art and the experimental conditions that govern arsenic removal. It also discusses the scalability and practical application of clay-based adsorption technologies, emphasizing gaps in field validation, regeneration studies, and large-scale implementation. The findings highlight the potential of natural and modified clays in arsenic remediation, while underscoring the need for further research to optimize adsorption conditions and enhance sustainability in water treatment systems.
1. Introduction
Arsenic water contamination represents a serious public health problem worldwide due to the severe health damage it causes. In countries like the United States, Argentina, China, and Chile, tap water contains between 50 and 90 μg/L of arsenic [,]. The level recommended by the World Health Organization (WHO) for arsenic concentration in drinking water is a maximum of 10 μg/L of As []. Arsenic contamination of groundwater and surface water is a global public health problem that primarily affects populations in rural areas of developing countries, where the harmful effects of consuming contaminated water have been widely documented.
Arsenic is commonly found in groundwater, forming arsenite (As(III)) and arsenate (As(V)) species. The As(III) species is more difficult to remove from water than As(V) and is more harmful to health due to its higher cellular uptake. Some arsenic compounds can dissolve in water, accumulating in marine species such as fish and shellfish. It has been reported that in humans, exposure to inorganic arsenic due to the ingestion of small amounts of arsenic in water and other foods contaminated by water over long periods leads to severe health consequences, as continuous consumption of arsenic can cause chronic poisoning. Among the early health effects observed in children and adults are vascular diseases (premature heart attack), respiratory diseases (bronchiectasis), and skin lesions. Chronic effects, recorded 20 years after peak exposure, have been associated with a variety of health issues known as chronic endemic regional hydroarsenicism (CERHA), which includes various types of cancer (skin, lung, bladder, liver, kidney, and prostate); neurological, gastrointestinal, hematological, and perinatal pathologies; and other clinical immunological manifestations and vascular effects, including myocardial infarction, hypertension, diabetes, miscarriage, low birth weight, hyperkeratosis, hyperpigmentation, and neurotoxicity, among others [,,,]. For example, there is decades-old evidence in the literature of arsenic’s impact on human health. Smith et al. [] demonstrated that prenatal exposure to arsenic from drinking water in Antofagasta, Chile, resulted in increased mortality from bronchiectasis and chronic obstructive pulmonary disease in individuals aged 30 to 49. Additionally, they observed a 46-fold increase in bronchiectasis mortality for those born to mothers exposed to nearly 900 μg/L of drinking water but only 12-fold for those born to mothers exposed to around 100 μg/L.
Although water treatment technologies exist for arsenic removal, many have significant limitations in terms of cost and accessibility, particularly in rural communities and low-resource areas. Some conventionally applied techniques for removing arsenic species include biological processes [], oxidation [], coagulation–flocculation [], adsorption [], and membrane techniques []. These technologies offer efficiency rates of 90–95% for arsenic removal in water; however, some are complex and costly (investment and operation), with efficiency depending on the type of chemicals used and the dosage. The active material is not regenerated, and disposal is a problem. Clays have emerged as promising materials in this context due to their abundance, low cost, and unique adsorptive properties, making them ideal for low-cost water treatment applications.
Clays are natural materials composed mainly of hydrated silicate minerals abundant in the environment. One of their most remarkable features is their high adsorption capacity, attributed to their large surface area and porous structure. Clay particles consist of silicate layers arranged in sheets that can attract and retain various ions and molecules. These structural properties, with their widespread availability and low extraction cost, make clays particularly attractive for pollutant removal in water treatment.
Several types of clay (such as bentonite, kaolinite, and montmorillonite) differ in composition and adsorptive capacity. These variations affect their affinity and effectiveness in adsorbing arsenic, whether in arsenate (As(V)) or arsenite (As(III)) form. For in-stance, montmorillonite, known for its high cation exchange capacity, has been widely studied due to its potential to retain arsenic ions in aqueous conditions. The ability of each clay type to remove arsenic depends on factors such as its crystalline structure, surface charge, and the pH of the water to be treated.
The adsorption process of arsenic onto clays involves several mechanisms, such as electrostatic attraction, hydrogen bonding, and specific surface interactions. Electrostatic adsorption plays a key role when the clay surface is positively charged, facilitating the attraction of arsenate ions. Additionally, clay can modify its active sites under certain pH and temperature conditions to enhance its adsorptive capacity. Some studies have indicated that adding additives such as transition metals or chemical modifications can further enhance arsenic adsorption, optimizing the use of clays as adsorbents in water treatment processes.
Although numerous studies have explored using clay for arsenic removal in water, the findings are varied and sometimes contradictory. A systematic literature review provides a global and comparative overview that allows for synthesizing trends, identifying knowledge gaps, and establishing recommendations for future research. In this sense, the main objective of this work is to provide a comprehensive understanding of the state of the art in the application of clays for arsenic removal, focusing on the types of clays utilized, the operational units employed in arsenic removal, and the types of studies conducted.
2. Background
This section provides an overview of the main topics related to clays and possible clustering and classification used in this study.
2.1. Clay Classification
In conducting a systematic literature review on the use of clays in adsorption applications, it is essential to classify clays by their mineralogical groups to highlight the distinct structural and chemical properties that influence their adsorption capacities (Table 1). This classification allows for a more nuanced analysis of how each clay type interacts with contaminants like arsenic, providing insights into the mechanisms at play and enabling a clearer comparison of their adsorption efficiencies. Clays are highly heterogeneous materials with significant variation in structure, surface area, charge, and chemical composition across different groups, which directly impacts their adsorption capabilities. Therefore, categorizing them systematically enhances the consistency and interpretability of the findings in this review.

Table 1.
Classification and properties of clay types used in adsorption applications.
The chosen classification is based on well-established mineralogical groups, each exhibiting unique characteristics relevant to adsorption. It is important to clarify that bentonite is not a mineral name but rather a commercial or rock name that commonly refers to clays predominantly composed of minerals such as montmorillonite, saponite, and others. Therefore, for mineralogical classification, bentonite should be considered a type of rock composed primarily of smectite group minerals. A supplemental note has been added here for clarification. Smectites—including montmorillonite, saponite, and the rock type bentonite—are widely studied due to their high cation exchange capacity (CEC) and swelling properties, which facilitate strong adsorption [,]. The kaolinite group (kaolinite and halloysite), while having a lower CEC, is valued for its stability in acidic conditions and its frequent use in low-cost water purification applications. The illite group, containing illite and glauconite, features non-swelling properties and relatively high CEC, making these clays useful in specific adsorption contexts. Fibrous clays, such as sepiolite and palygorskite, possess unique fibrous structures and channels that enhance adsorption through increased surface area and pore accessibility. Finally, other clay types like vermiculite, chlorite, and pyrophyllite bring additional diversity in composition and structure, offering specialized adsorption behaviors suitable for contaminants or environmental conditions.
Each one of these classes could also be functionalized and modified. Independently of this tendency, when classified, they were grouped based on the described classification and were not further clustered by functionalized, modified, or natural state.
2.2. Operational Unit Classification
In conducting a systematic literature review on the operational units used for arsenic adsorption, we classified these systems based on their distinct operational mechanisms and application contexts. Each method offers unique approaches to arsenic removal, affecting adsorption efficiency, scalability, and suitability for various environmental and industrial settings. By organizing the literature into seven primary types of operational units—batch reactor, fixed-bed, ion exchange, chemical precipitation, membrane filtration, electrochemical treatment, and coagulation and flocculation—this review enables a focused and comparative analysis of the effectiveness and limitations inherent to each method.
The batch reactor method serves as a foundational system, particularly in laboratory studies where initial adsorption properties, kinetics, and isotherms are evaluated. Its controlled environment and simplicity make it ideal for parameter optimization and early-stage experimentation. The fixed-bed system, in contrast, offers a continuous flow configuration suited to large-scale or industrial applications, where adsorbents are used in columns to treat higher volumes of water. Fixed-bed systems are widely studied for their adaptability, scalability, and ability to handle various arsenic concentrations with minimal operational adjustments, making them a core component of full-scale adsorption systems.
Ion exchange processes encompass techniques such as cation and anion exchange, ion sorption, and ion capture. This category is defined by the exchange mechanism that selectively binds arsenic ions, providing high removal efficiency, especially for specific arsenic species. Chemical precipitation is another critical category, where arsenic is removed through chemical reactions that precipitate arsenic compounds, often complementing other methods in high-arsenic-concentration scenarios and allowing for easier separation of arsenic-laden solids.
Membrane filtration stands out as a method based on physical separation, utilizing selective adsorption or particle size exclusion to capture arsenic ions. It is particularly advantageous in applications where fine filtration and minimal chemical addition are preferred. Electrochemical treatment methods leverage electric fields to enhance arsenic adsorption or transform arsenic species, which can be adjusted through electrode materials and current densities. This flexibility makes electrochemical approaches suitable for complex aqueous environments or waste streams containing diverse contaminants.
Finally, coagulation and flocculation are commonly used treatment methods for arsenic removal, especially in large-scale water treatment. By promoting the aggregation and settling of arsenic-laden particles, this method offers a cost-effective solution for treating high-volume water systems and is often integrated with other methods to increase overall efficiency. Table 2 gives a broad description of the different units previously mentioned.

Table 2.
Classification and descriptions of operational units for arsenic adsorption processes.
While various operational units for arsenic adsorption have been described, it is important to critically assess their limitations and practical applicability. Batch reactor systems, although widely used in laboratory studies, present challenges in scalability due to their static nature and limited ability to simulate dynamic field conditions. In particular, batch systems often fail to account for factors such as reaeration and continuous contaminant flux, which are crucial for real-world water treatment scenarios. Their use may overestimate adsorption capacities compared to continuous systems, where conditions are less controlled.
Real-time and continuous treatment systems, such as fixed-bed reactors, face their own challenges. These include operational difficulties like maintaining consistent flow rates, preventing clogging or channeling, and ensuring long-term stability of the adsorbent material. Additionally, the effectiveness of these systems can vary significantly under fluctuating influent compositions or environmental conditions. Addressing these limitations is essential to bridge the gap between laboratory-scale findings and practical field-scale applications.
The results obtained in the following sections can be used to see the research gaps that exist in both operational unit process and clay-type analyses.
2.3. Type of Analyses
When conducting a systematic literature review on adsorption studies, classifying re-search by study type is essential to provide a clear and organized understanding of each aspect of the adsorption process. This classification allows us to appreciate the multi-faceted nature of adsorption research, where each study type—whether focused on kinetics, adsorption mechanisms, efficiency, or other parameters—addresses a unique aspect of understanding and optimizing the adsorption of contaminants like arsenic. This structured approach reveals not only the depth of each study area but also how they interrelate to form a comprehensive view of adsorption technology.
Kinetic Studies: Kinetic studies analyze the rate of arsenic adsorption and the time needed to reach equilibrium, using models such as pseudo-first-order, Boyd, Weber–Morris, and pseudo-second-order kinetics. By understanding the temporal dynamics of adsorption, researchers can optimize the process for scenarios requiring rapid contaminant removal, which is crucial in applications where time efficiency is key. Furthermore, if experimental data fit the pseudo-second-order model, it may suggest that the adsorption process is dominated by chemisorption. Nevertheless, further studies are required for confirmation.
Adsorption Mechanism Studies: Adsorption mechanism studies delve into the molecular interactions between arsenic and adsorbents, distinguishing between physical adsorption (physisorption) and chemical bonding (chemisorption). These studies are fundamental to understanding the exact nature of arsenic binding, which informs the design of adsorbents tailored to specific contaminant interactions.
Efficiency Studies: Efficiency studies systematically vary operational conditions (such as pH, adsorbent dose, and temperature) to determine the optimal settings for maxi-mum arsenic removal. These studies are pivotal in scaling up adsorption processes, ensuring that high removal efficiency is achieved under different conditions and guiding industrial applications. When evaluating adsorption performance, two common metrics are normally used: percentage removal and adsorption capacity. These provide a standard way to compare how effective different adsorbents are under specific conditions. Percentage removal (%) measures how much of the contaminant is removed from the solution:
where is the initial concentration, and is the equilibrium concentration. Adsorption capacity indicates how much contaminant is adsorbed per unit mass of adsorbent:
where V is the volume of solution, and m is the mass of the adsorbent.
Isothermal Studies: Isothermal studies examine the equilibrium relationship between the arsenic concentration in the solution and the adsorbed amount, often using models like Langmuir (assumes monolayer adsorption), Temkin (considers the adsorbate–adsorbate interactions), Dubinin–Radushkevich (helps differentiate between physical and chemical adsorption), and Freundlich (adsorption onto heterogeneous surfaces) isotherms. The maximum absorption capacity qmax value from the Langmuir isotherm analyses results are commonly used for comparing the potential effectiveness of different materials. By assessing adsorption capacity and surface characteristics, these studies help determine whether a material is suited to monolayer or multi-layer adsorption, guiding material selection.
Material Characterization: Material characterization defines the physical and chemical properties of the adsorbent through techniques like X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX/EDS), Fourier transform infrared spectroscopy (FTIR), and Brunauer–Emmett–Taller (BET) surface area analysis. Characterization is vital to understanding the attributes that affect adsorption performance, such as surface area, pore structure, and composition, thereby informing the development of more efficient adsorbents.
Regeneration and Reuse: Potential regeneration studies assess the adsorbent’s ability to be reused after arsenic saturation, evaluating performance over multiple cycles. This area of study is important for sustainable development, as it provides insights into the economic viability and environmental impact of adsorption processes by highlighting materials that offer long-term reusability.
Thermodynamic Studies: Thermodynamic studies investigate the energy changes in adsorption, examining parameters like Gibbs free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°) to determine whether the process is spontaneous or requires external energy. Such studies are essential for understanding the feasibility and driving forces of arsenic adsorption under different conditions. More specifically, a negative ΔG° means the process is spontaneous, positive ΔH° indicates an endothermic process, and positive ΔS° implies increased randomness, which can be linked to the release of water molecules or ions during adsorption.
Simulation Studies: Simulation studies offer a means of modeling and predicting adsorption behavior under various environmental and operational conditions. These computational approaches complement experimental findings, providing deeper insights into the mechanisms and performance of adsorption systems. Molecular-level simulations, such as molecular dynamics and Monte Carlo methods, are used to examine the interaction between components, identifying preferred binding sites, adsorption energies, and diffusion dynamics. Density functional theory (DFT) enables the calculation of electronic structures and the energetic binding interactions, which are valuable for evaluating chemisorption mechanisms. At a larger scale, computational fluid dynamics (CFD) are employed to model flow behavior, pressure drops, and adsorption performance in continuous systems. Additionally, empirical modeling techniques such as response surface methodology (RSM) and artificial neural networks (ANNs) are used for process optimization and performance prediction by correlating experimental variables—such as pH, contact time, and adsorbent dose—with adsorption efficiency.
Real Scenario Validation: Validation in real-world settings is essential for proving the practical applicability of adsorbents under field conditions, such as in groundwater or industrial wastewater. These studies test performance amidst complex variables like competing ions and fluctuating pH, demonstrating that adsorbents are effective beyond controlled lab environments.
3. Research Methodology
Figure 1 shows a schematic in which the research methodology is described. The processes, in the top row of the Figure, are linked to their primary outcomes, in the bottom row of the figure. These include reviewing scope, all papers, bibliometric analysis, relevant papers, and classification scheme. Although the bibliometric analysis is not the core of the methodology, it is added to gain a better understanding of the topic trends. These are developed in the subsequent subsections according to the figure.
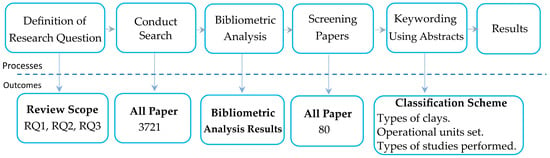
Figure 1.
Systematic mapping approach.
3.1. Definition of Research Question
The goal of the present study is defined as follows: to provide an understanding of the state of the art of the use of clays for arsenic removal. To achieve such a goal, the following research questions are posed (together with the main drive from the question):
- RQ1—What are the main types of clays used for arsenic removal?
- RQ2—What are the primary operational units used for arsenic adsorption by clay?
- RQ3—What are the main types of studies performed and the achievements obtained for arsenic adsorption using clay?
3.2. Search Methodology
To assess the state of the art, a systematic approach was conducted. The process starts with the definition of the key pillars that drive the topic and the use of targeted keywords to conduct literature searches. These keywords were used as markers, enabling efficient search across various platforms. Keywords and pillars were combined to address the research questions. Specifically, keyword selection was approached by establishing specific groups to help with the definition of the components of the keywords, taking a bottom-up approach.
The methodology applied to construct queries and define keywords follows the PICOC methodology—population, intervention, comparison, outcomes, and context [,,]. Each of these topics is defined next:
- Population: This refers to the specific group of individuals or subjects of interest to the study. In the context of this work, the population is the family and different types of clays that constitute the population of analysis (i.e., Section 2.1), as well as the group of manuscripts from which the research questions are formulated.
- Intervention: The intervention refers to the approach or technique applied in the empirical study. This study involves software methodologies, tools, technologies, and procedures. Different operational units that can be applied to specific procedures in the adsorption of arsenic are considered. These analyses were previously referred to in Section 2.2.
- Comparison: The comparison component involves differentiating methods, processes, or strategies to compare individuals. To do so, we refer to the differentiation of studies performed in different manuscripts (i.e., Section 2.3).
- Outcomes: The outcomes of the adsorption process using different technologies with different clays under different operational conditions should lead to different efficiency and clay uptake conditions.
- Context: The context provides a comprehensive view of whether the study was conducted in academia or industry, the industrial segment, and the subject’s incentives.
Thus, the context imposes keywords to constrain the search—for example, the use of the terms “heavy metal”, “arsenic”, and “As” define a branch of representation for the same component.
Based on the combination of components and context defined based on the PICOC approach, the keywords for queries were predefined based on classifications defined in Section 2.1, Section 2.2 and Section 2.3. The specific queries using the keywords mentioned above are shown in Table 3. An explanation of the differentiation based on the search engine is presented in the following section.

Table 3.
Queries to the databases IEEE, Scopus, ACS, and Google Scholar.
3.3. Bibliometric Analysis
Table 4 presents the findings before the screening phase, structured on publications per year. The last two columns of the table show the total number of publications for the analyzed period, labelled as “total,” and the relative percentage of queries that contained terms related to arsenic adsorption (i.e., as obtained by eliminating “heavy metal” and “arsenic” from all respective queries)—labelled as “Perc.” Importantly, for Scopus and GS, the results are those obtained only in titles, given the broad number and generalization of the topics when considering regular fields (i.e., abstract, articles, and keywords).

Table 4.
Searches in the IEEE, Scopus, ACS, and Google Scholar (GS) databases between the years 2014 and 2024.
As observed in the table, the ACS and Scopus engines showed a steady increase in publication activity. This consistent upward trend suggests a growing tendency of the subject covered here. Conversely, the IEEE and GS databases exhibited low and inconsistent search volumes, but with a similar trend of increase, especially between 2019 and 2024. The highest percentage (7.57%) was obtained for the ACS, possibly for the IEEE, due to it being more narrowly focused and based on the chemistry considerations involved in the adsorption of arsenic; on the other hand, Scopus covers a wider range of subjects, including technical and non-technical content, and thus, specific fields in the search process were needed.
3.4. Screening Papers
A systematic analysis was conducted on the collected dataset with a primary focus on assessing the relevance of the titles to the key facets identified previously. In cases where uncertainty arose, a more detailed analysis was performed based on the information provided in the abstracts. This evaluation process followed a structured pipeline approach, as illustrated in Figure 2. The main objective of this rigorous screening was to ensure that each manuscript included at least topics connected to clay and arsenic removal (absorption, desorption, precipitation, and others, as defined by the keyword approach) from fluids. Manuscripts that were not directly connected to clay’s absorption of heavy metals were further analyzed to check whether arsenic was considered in them.
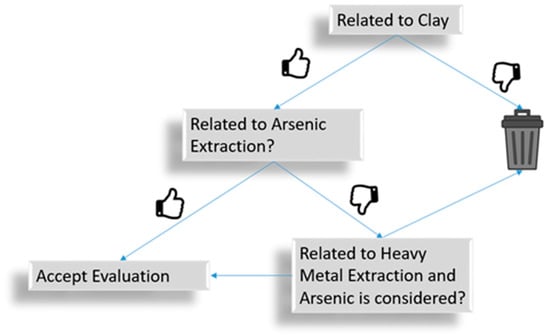
Figure 2.
Screening process followed to select the papers based on the keywords.
The screening process does not necessarily define whether the manuscript describes an important contribution to answer the settled research questions, and thus, only those that could be linked to the facets were included in the analysis.
3.5. Keywording Using Abstracts
The predefined set of keywords served as the basis for conducting the mapping and analysis processes in the study [,,]. However, during the screening process, and together with the keywords associated with the types of clays, operational units set, and types of studies performed, different keywords were also considered for inclusion for analysis.
4. Results and Discussions
This section shares the findings from our systematic literature review, which was carefully conducted through a rigorous screening process. We provide a detailed look at the analysis, including the sources we used to gather research questions and findings. To deepen our understanding of the information, we also included bibliometric analyses.
For each research question, we added a brief discussion to connect the insights directly with the figures shown. This discussion sets the stage for the next section, where we dive deeper into the findings, explore future research directions, and highlight any gaps we identified.
The next sections use grid charts to show how often certain topics appeared in the reviewed studies. The points where rows and columns meet show which topics were studied together. The numbers in the top row and last column show how many times each topic came up overall.
Only studies that focused on the specific topics being analyzed were included in the charts. More general studies that did not cover these topics were left out. Also, since one study can include more than one topic, the total number shown in the top right corner of each chart represents the number of topics discussed—not the number of studies.
Furthermore, results that showed 0 results were not included in the figures. For clays, these results include “glauconite”, “vermiculite”, “chlorite”, and “pyrophyllite”; for the operational processes, these results include “chemical precipitation”, “coagulation”, and “flocculation”.
The limited use of minerals like glauconite, vermiculite, chlorite, and pyrophyllite in arsenic adsorption could be attributed to several factors related to their structure, chemical properties, availability, and adsorption efficiency. Here are the key reasons:
Arsenic adsorption efficiency is highly dependent on the mineral’s surface area and pore structure. Minerals like glauconite, vermiculite, and chlorite typically have lower specific surface areas compared to other clay minerals, such as montmorillonite and activated carbon materials, which offer larger surface areas conducive to higher arsenic uptake. Minerals like illite, kaolinite, and palygorskite, often preferred in arsenic adsorption studies, have specific structural characteristics or modification potentials that maximize arsenic adsorption through enhanced surface interactions. Furthermore, the surface charge of a mineral influences its ability to attract or repel arsenic ions, which are generally negatively charged in common environmental forms. The net surface charge and composition of minerals like glauconite, chlorite, and pyrophyllite may not be as favorable for arsenic ion adsorption compared to minerals with higher positive charges on their surfaces, such as iron- and aluminum-based oxides or certain treated/modified clays. Minerals like illite and palygorskite, in contrast, can be modified to optimize surface charge and enhance adsorption sites for arsenic. Additionally, the adsorption of arsenic on mineral surfaces should rely on complexation with surface functional groups, such as hydroxyls in aluminum or iron oxide surfaces, which facilitate strong inner-sphere or outer-sphere binding.
While chemical precipitation, coagulation, and flocculation are widely used in water treatment processes, they have limitations when specifically applied to arsenic adsorption. Arsenic could have low concentrations, making its removal challenging through precipitation, coagulation, and flocculation. These processes are more effective for high-concentration contaminants and may not be efficient enough for arsenic, especially when it exists in trace amounts. At lower concentrations, the formation of precipitates or flocs may be inadequate, resulting in incomplete arsenic removal. Furthermore, the effectiveness of chemical precipitation and coagulation is highly dependent on the pH of the solution. For instance, arsenate (As(V)) precipitates well with iron and aluminum salts in slightly acidic to neutral pH conditions. However, arsenite (As(III)), which is less reactive, requires oxidation to arsenate for effective precipitation, which adds a step and complicates the treatment process. Maintaining an optimal pH in large-scale water treatment can be costly and complex, reducing the efficiency of these methods for arsenic removal.
4.1. RQ1—What Are the Main Type of Clays Used for Arsenic Removal?
Figure 3 shows the different types of clays used in function of the different operational units used during the adsorption of arsenic. Montmorillonite, for example, is referenced 21 times, making it one of the most widely used clays, followed by bentonite, with 18 references, and nontronite, with 2. Kaolinite appears with a frequency of 17 references, suggesting its comparative effectiveness or availability in experimental studies. Other clays, like illite and sepiolite, are less frequently used, with six and eight references, respectively, indicating limited or niche applicability.
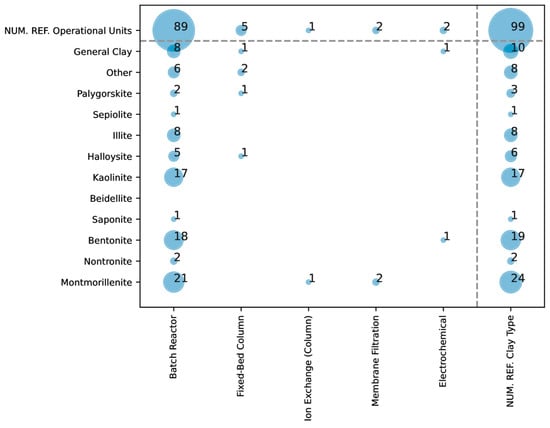
Figure 3.
Overlap in the literature between the type of clays used for arsenic removal and the type of operational unit used.
Montmorillonite: After performing the analysis in the provided manuscripts, we defined the research on montmorillonite for arsenic adsorption into five key areas of application: functional modification, adsorption kinetics and mechanisms, environmental influence, comparative efficiency, and regeneration potential.
Functional Modification: across the studies, various chemical modifications to montmorillonite have been explored to improve its arsenic adsorption capacity, highlighting its versatility and adaptability. For example, aluminum-modified montmorillonite demonstrated notable efficiency in the simultaneous adsorption of arsenic (As) and fluoride (F) in multielement groundwater samples, achieving removal efficiencies of up to 21 mg/g for arsenic, underscoring its potential in groundwater treatment applications [,]. Other modifications, such as carboxymethyl cellulose (CMC) grafting, enhanced the material’s interaction with arsenic ions, with removal efficiencies reaching up to 85% under optimized conditions [].
TiO2-pillared montmorillonite embedded with CeOx/MnOy nanoparticles achieved a high adsorption capacity of 46.58 mg/g for arsenic (III), facilitating the oxidation of As(III) to the less toxic As(V) form, making it especially valuable for treating contaminated industrial wastewater []. Similarly, the inclusion of hexadecyl trimethyl ammonium chloride (HDTMA-Cl) and cetyltrimethylammonium bromide (CTMAB) resulted in increased surface hydrophobicity, enhancing arsenic removal capabilities under diverse environmental conditions [,]. Furthermore, embedding organoclay into nanocomposite membranes provided a reusable material that integrates adsorption with filtration for long-term arsenic remediation [].
Adsorption Kinetics and Mechanisms: The kinetics and mechanisms of arsenic adsorption on montmorillonite and its modified forms indicate a consistent trend of chemisorption, with adsorption following a pseudo-second-order kinetic model, suggesting strong chemical bonding between arsenic ions and the modified clay surface. For example, studies on iron-modified montmorillonite showed rapid arsenic removal, with up to 99% of As(V) eliminated within the first 30 min at an initial concentration of 3 mg/L []. Additionally, thermodynamic analyses revealed that adsorption is often endothermic, as seen in studies involving CMC montmorillonite, where performance increased with temperature []. Hydroxy iron-modified montmorillonite displayed notably fast adsorption kinetics, achieving over 55% arsenic removal within just 30 s, which was further confirmed to fit a pseudo-second-order model []. The adoption of pseudo-second-order kinetics across various studies confirms that modified montmorillonite is suitable for applications requiring fast and stable arsenic capture, with modifications like FeOOH effectively enhancing sorption for As(V) [].
Environmental Influence: The adsorption efficiency of montmorillonite for arsenic removal is significantly influenced by solution pH and ionic strength, factors that impact adsorption capacity and material stability. Across the manuscripts, slightly acidic-to-neutral pH conditions (5–6) were generally found to optimize arsenic adsorption, with certain modifications (e.g., iron-modified montmorillonite) showing stability across broader pH ranges []. Natural and Moroccan clays demonstrated that higher ionic strengths can further improve arsenic removal by enhancing complex stability, which is advantageous in high-salinity environments like industrial effluents []. In specific instances, the introduction of competing ions, such as sulfate, nitrate, and chloride, revealed that Fe-modified montmorillonite retained robust adsorption capabilities even in saline solutions, showing the versatility of montmorillonite in challenging conditions []. The influence of environmental factors, including the presence of competitive ions, necessitates pH adjustments in practical applications to maximize arsenic uptake, especially in heterogeneous groundwater systems [].
Comparative Efficiency: Montmorillonite, especially in its modified forms, consistently outperformed other natural clays and conventional adsorbents, like activated carbon, in arsenic and heavy metal removal. Fe-modified smectites, for instance, displayed superior adsorption properties, outperforming traditional adsorbents across various pH levels and ionic conditions []. In comparative studies, aluminum and iron oxide-modified smectites showcased enhanced adsorption for arsenic, cadmium, and other heavy metals, making them viable alternatives to carbon-based materials, except in select cases like mercury, where carbon was marginally more effective []. Acid-processed montmorillonite also demonstrated a high adsorption capacity in high-salinity solutions, positioning it as a low-cost option for water treatment facilities that address multiple contaminants []. Additionally, Fe–Mn composites in montmorillonite further elevated its arsenic removal efficiency, making modified montmorillonite a highly competitive option for industrial and municipal wastewater treatment [].
Regeneration Potential: The ability to regenerate and reuse montmorillonite-based adsorbents presents a sustainable advantage, particularly for large-scale applications. For instance, TiO2–Ce/Mn-modified montmorillonite retained nearly 80% of its arsenic adsorption efficiency across multiple cycles, indicating robustness and reusability []. Studies on polysulfone/organoclay nanocomposite membranes demonstrated that the materials could undergo multiple adsorption regeneration cycles while maintaining efficacy, as shown in the retention of nearly 80% efficiency after five cycles []. This regenerative capability is further emphasized in hydroxy iron-modified montmorillonite, which maintained its adsorption capacity across cycles, reinforcing its suitability for long-term use in industrial and groundwater treatment systems []. As indicated in [], montmorillonite’s stability and reusability reduce operational costs and environmental impacts, underlining its role in sustainable and scalable water purification.
Nontronite: After performing the analysis in the provided manuscripts, we have defined the findings into three key areas of application: redox-driven arsenic adsorption, pH-dependent adsorption behavior, and structural impact on sorption efficiency.
Redox-driven arsenic adsorption plays a crucial role in the effectiveness of nontronite as a sorbent material, particularly in the redox transformation of arsenic. As explored in Ref. [], introducing Fe(II) into predominantly Fe(III)-based nontronite structures activates the mineral’s surface, enabling it to catalyze the oxidation of As(III) to As(V).
This redox activity occurs under both oxic and anoxic conditions, albeit more prominently in oxygen-rich environments. The mechanism involves electron transfer from As(III) to Fe(III) within the octahedral sheets of the mineral, showcasing that only certain structural Fe(III) sites are accessible for such transformations. Both synthetic and natural nontronites demonstrate this behavior, yet synthetic nontronite can be tuned for higher reactivity by adjusting Fe(II) content, making it a strong candidate for remediation in environments where arsenic oxidation is necessary.
pH-dependent adsorption behavior has a marked influence on arsenic sorption capacities, as observed in Ref. []. The study notes that As(V) and As(III) adsorption on nontronite is significantly influenced by pH variations. Specifically, As(V) adsorption is favored at lower pH levels, where positively charged nontronite surfaces create favorable conditions for attracting and binding anionic As(V) species. However, above pH 5.0, As(V) sorption decreases due to decreased electrostatic attraction, aligning with findings in prior studies on clay mineral surface charges. On the other hand, As(III) adsorption peaks at a neutral pH of approximately 7.0, where the mineral surface properties better facilitate the uptake of these neutral species. This pH-sensitive sorption underscores the adaptability of nontronite to a range of aqueous environments, allowing it to function effectively in both acidic and neutral water systems, which is essential for broader water treatment applications.
Structural impact on sorption efficiency further defines nontronite’s suitability for arsenic adsorption. With a comparison of clay minerals such as montmorillonite, kaolinite, and nontronite, it is evident that Fe-rich nontronites like NAu-2 demonstrate higher arsenic uptake efficiency due to their larger surface area and iron content []. For instance, NAu-2, which possesses a high Fe concentration and significant surface area, surpasses other clay minerals in its ability to remove arsenic, particularly under optimal pH and redox conditions. The presence of iron within the nontronite structure not only enhances its reactivity but also increases its affinity for arsenic species, further amplified in Fe(II)-enriched scenarios. This structural advantage, coupled with nontronite’s versatile surface characteristics, highlights its robustness as a viable material for arsenic adsorption across diverse aqueous environments, particularly in contaminated groundwater systems where arsenic levels are a persistent concern.
Bentonite: After performing the analysis across the provided manuscripts, we refined the collaborations into four key areas of application: modified bentonite adsorbents, composite bentonite structures, natural and acid-activated bentonite, and novel functionalized bentonite.
Modified bentonite adsorbents are often chemically or thermally treated to enhance their arsenic adsorption capabilities. For example, Mutar and Saleh’s research on nano-bentonite derived from Iraqi clay demonstrated that optimized factors like pH and dosage using central composite design could achieve high arsenic removal efficiency, showcasing bentonite’s adaptability as a low-cost adsorbent []. Similarly, Dehghani et al. investigated chitosan-modified bentonite (MBC), achieving a significant As(V) adsorption capacity of 122.23 mg/g. They identified that adsorption efficiency improved under optimized parameters, demonstrating modified bentonite’s effectiveness for arsenic removal []. Studies by Dousova et al. further illustrate that acid treatment of Fe-rich clays, like bentonite, enhances anion-active sites, achieving adsorption capacities of up to 39.2 mmol/g for As(V) and Sb(V), supporting modified bentonite’s efficacy in arsenic remediation through chemical treatment [].
Composite bentonite structures represent an innovative approach, combining bentonite with other materials to form stable, multi-functional adsorbents. Masindi et al.’s magnesite–bentonite clay composite illustrates how bentonite composites offer high arsenic adsorption rates, optimized through both physical adsorption and chemical binding. This composite proved effective even in industrial effluents, validating its large-scale application potential []. Saleh et al.’s study on bentonite/chitosan/TiO2 composites uses UV-induced photooxidation to convert As(III) to As(V) before adsorption, highlighting recyclability and cost-effectiveness as key benefits for bentonite-based composites []. Hokkanen et al.’s hydroxyapatite–bentonite–nanocrystalline cellulose (CHA–BENT–NCC) composite also achieved rapid As(III) removal rates, achieving 95% efficiency within 5 min, underscoring the potential for composites that combine bentonite with nanoscale materials []. Baigorria et al. presented an innovative polyvinyl alcohol/alginate hydrogel with bentonite, creating porous, ecofriendly beads for arsenic removal, which highlighted the versatility of composite materials in arsenic adsorption applications [].
Natural and acid-activated bentonite provides a cost-effective solution suitable for sustainable, accessible water treatment, particularly in regions with limited resources. Studies by Fazlali et al. reveal that acid-activated exfoliated bentonite significantly improves adsorption efficiency for toxic ions like arsenic by increasing surface area and pore size []. Uddin’s review corroborates this, noting the benefits of natural and minimally processed bentonite for heavy metal removal, emphasizing environmental sustainability []. Asere et al. expanded on the feasibility of natural bentonite as a low-cost adsorbent in developing countries, underscoring its cost-efficiency and potential for localized arsenic remediation []. Jha’s work on chemically activated bentonite supports the enhanced adsorption capacities for metal ions, validating the practical benefits of natural and acid-activated bentonite in diverse settings [].
Novel functionalized bentonite combines bentonite with advanced organic or polymeric modifiers to enhance selectivity and adsorption strength. Mukhopadhyaya et al. developed surfactant-modified smectite, achieving 66.9% arsenic removal via ligand exchange and electrostatic attraction, demonstrating that organic modifications increase bentonite’s adsorption potential []. Hua’s co-modification of bentonite with manganese oxides and PDMDAAC shows a unique synergy between components, achieving high arsenic uptake through tailored interactions, proving that it is highly effective for low-concentration arsenic adsorption in varied water chemistries []. Similarly, Pawar et al.’s study on iron oxide-modified clay-activated carbon composites demonstrates that multi-component functionalization provides new adsorption pathways, increasing arsenic retention and applicability in industrial settings [].
Kaolinite Group: After performing the analysis on the provided manuscripts, we refined and focused the classification exclusively on the applications of kaolinite and halloysite clays for arsenic adsorption into four key areas: modified clays for enhanced adsorption, influence of pH and competing anions, high-temperature applications for gaseous arsenic, and natural kaolinite and halloysite for arsenic adsorption.
Modified Clays for Enhanced Adsorption: Various studies highlight the effectiveness of modifying kaolinite and halloysite clays to improve arsenic adsorption. For instance, ZnO–halloysite nanocomposites have been synthesized and optimized using the Box–Behnken methodology, achieving over 90% arsenic (As(III)) removal through controlled pH, contact time, and adsorbent dosage, underscoring the potential of metal oxide–halloysite composites in aqueous arsenic remediation []. Additionally, magnetite (Fe3O4) nanoparticle-loaded halloysite nanotubes demonstrated high efficiency in arsenate (As(V)) removal, reaching over 99% removal efficiency in groundwater applications. These Fe3O4–halloysite composites benefit from magnetic properties that facilitate easy separation and reuse, making them an ecofriendly solution for large-scale treatment []. Acid treatment of Fe-rich kaolinite and halloysite further underscores the benefits of modification. For example, kaolinite modified with HCl or oxalic acid has shown increased adsorption for both As(V) and antimonate ions due to the creation of additional active sites on acid-leached surfaces []. Intercalating kaolinite with organic and inorganic surfactants also produces hybrid adsorbents with high adsorption across pH 4–8, emphasizing how structural modifications enhance adsorption and align with chemisorptive mechanisms []. Iron-impregnated kaolinite further demonstrates this by promoting arsenic binding via newly introduced ferrihydrite phases, which create iron-rich surfaces effective for arsenic remediation in aqueous systems [].
Influence of pH and competing anions: pH and competing ions significantly influence arsenic adsorption onto kaolinite and halloysite. Studies on phosphate-bound kaolinite and Fe-exchanged smectite indicate that optimal adsorption generally occurs in acidic to near-neutral pH ranges, particularly around pH 5, as lower pH conditions enhance arsenic binding to the clay surface. Competing anions, such as phosphate and sulfate, are known to interfere, with phosphate showing a particularly strong inhibitory effect. This competitive effect implies that in environments with high anion concentrations, Fe-modified kaolinite or halloysite clays are preferable, as they can maintain higher arsenic uptake under diverse ionic conditions []. Leonardite char, although less selective than kaolinite or halloysite in the presence of certain anions, performs effectively for arsenic removal when supplemented with iron, aligning with selective adsorption behavior seen in Fe-modified kaolinite []. These findings emphasize that controlling pH and considering coexisting ions is crucial for optimizing arsenic adsorption, particularly in systems where multiple ions interact with arsenic [].
High-Temperature Applications for Gaseous Arsenic: Kaolinite and halloysite clays demonstrate unique adaptability in high-temperature applications, such as coal combustion settings where gaseous arsenic capture is required. CuCl2-modified halloysite nanotubes exhibit robust arsenic adsorption from flue gas, maintaining high efficiency even at 600 °C, with minimal interference from other flue gas constituents like NOx and SOx. This modification stabilizes arsenic by converting As(III) to As(V) within the nanotube structure, underscoring the potential of CuCl2–halloysite composites in industrial arsenic scrubbing applications []. Additionally, kaolinite in sodium vapor-rich environments enables effective arsenic oxidation, achieving adsorption capacities of up to 878 μg/g through the formation of As–O–Al and Na–O–As–O–Al complexes. This highlights kaolinite’s suitability for high-temperature arsenic capture applications, particularly in environments with elevated temperatures and oxidative potential [].
Natural Kaolinite and Halloysite for Arsenic Adsorption: Unmodified kaolinite and halloysite also exhibit arsenic adsorption properties, although with lower capacities compared to their modified counterparts. Studies on Moroccan kaolinite reveal effective arsenic adsorption in acidic conditions, with R-clay showing a maximum adsorption capacity of 1.076 mg/g following the Langmuir isotherm model, indicating uniform adsorption sites on the clay surface []. Iron-rich kaolinite naturally contains significant Fe and Al oxides, which enhance its ability to bond with arsenic species, making it a suitable option for direct adsorption applications in areas where unmodified materials are preferred []. Nontronite and montmorillonite studies confirm the effectiveness of Fe-rich natural kaolinite at low pH, supporting the Freundlich model and reflecting the heterogeneous adsorption potential of these clays [,]. Expanded clay aggregates produced from unmodified halloysite and kaolinite have also shown multifunctional properties, including adsorption of arsenic and other heavy metals, making them practical for rural and low-cost water purification needs [].
In summary, the classifications illustrate the broad applicability of kaolinite and halloysite in arsenic remediation, with structural modifications, environmental parameters such as pH, and operational conditions like temperature all playing essential roles in optimizing these materials for various applications across different settings.
Illite Group: After performing the analysis across the manuscripts [,,,,,,,,,], we defined the applications of illite and glauconite in arsenic remediation into four key areas: natural adsorption mechanisms, the role of iron modification in adsorption enhancement, environmental stability under redox conditions, and practical considerations for remediation effectiveness and feasibility.
Natural Adsorption Mechanisms: In examining illite’s unmodified adsorption characteristics, studies illustrate its capacity to adsorb arsenic (As) through inner- and outer-sphere mechanisms, facilitated by illite’s unique surface chemistry. The adsorption mechanism primarily involves inner-sphere complexation at edge sites where arsenic binds directly with aluminum and oxygen atoms, forming As–O–Al structures. This bonding alters illite’s surface charge, shifting its isoelectric point (IEP) to a more negative value, enhancing its attraction to arsenic under specific pH conditions []. Illite’s adsorption of arsenic follows a surface complexation model (SCM), where As(III) and As(V) create monodentate and bidentate complexes. Experimental adsorption capacities indicate illite’s affinity for As(V) (0.667 μmol/g) over As(III) (0.251 μmol/g), with adsorption kinetics showing rapid initial binding that stabilizes over time []. For glauconite, its natural iron content supports arsenic adsorption via electrostatic attraction and Fe-mediated complexation, particularly in acidic environments typical of contaminated waters, which enhances its role in natural arsenic remediation [].
Role of Iron Modification in Adsorption Enhancement: Iron-modified illite and glauconite show substantial increases in adsorption capacity, with Fe-oxide modifications introducing more active sites for arsenic binding. Modified illite, for instance, demonstrated enhanced adsorption through FeOOH coatings, adding ferrihydrite phases that interact with As(V) through electron-donating properties, significantly increasing its adsorptive capacity in low-to-neutral pH waters, as common in arsenic-contaminated groundwater []. Similarly, Fe-modified glauconite’s added iron sites improved arsenic binding efficiency, especially for As(V), by creating stronger electrostatic attractions and complexation with iron. Studies confirm that iron coatings increase adsorption rates by creating accessible binding sites, enabling glauconite to retain arsenic effectively even in high-contamination regions []. This demonstrates that surface modification with iron compounds improves arsenic uptake on both minerals, with iron acting as a powerful mediator for bonding, especially in Fe-enriched environments.
Environmental Stability Under Redox Conditions: Illite’s stability under fluctuating redox conditions positions it well for applications in acid mine drainage (AMD) remediation, where environmental conditions alternate between oxidative and reductive states. Illite maintains arsenic adsorption even with changing redox conditions, providing stable containment of arsenic over time, as demonstrated in studies involving acid mine drainage scenarios []. The study highlights illite’s ability to immobilize arsenic through Fe(III) nanoparticle precipitation under oxic conditions and retain it even as Fe reduces in anoxic settings, making illite especially effective in AMD sites where arsenic mobility poses severe risks. Glauconite, with its natural iron content, also shows resilience under redox cycling, although its adsorption performance improves significantly with additional Fe-oxide modification, as this modification supports stable binding of arsenic ions in varying environmental conditions []. This redox stability is essential for both minerals, especially in long-term arsenic containment in ecologically sensitive settings.
Practical Considerations for Remediation Effectiveness and Feasibility: Finally, practical considerations for applying illite and glauconite in large-scale arsenic remediation focus on cost, modification ease, and multi-environment effectiveness. Due to their natural abundance, both minerals present cost-effective alternatives to synthetic adsorbents, particularly in arsenic remediation efforts within resource-limited regions. Illite has shown effectiveness in groundwater decontamination, with modified glauconite performing well in regions with extreme arsenic contamination [,]. Iron modification methods, such as ferrihydrite or FeOOH coatings, offer scalable solutions that do not require complex processing, making these minerals practical for widespread environmental applications. Both minerals also display robust arsenic adsorption across varying pH levels, underscoring their versatility and reinforcing their role as low-cost, high-efficiency adsorbents for broad remediation use [].
Fibrous Clays: After performing the analysis across the provided manuscripts, we defined the collaborations into four key areas of application: thermal activation of palygorskite, hybridization with layered double hydroxides (LDHs), iron modification of clay structures, and advanced composite design through biochar integration and computational modeling.
Thermal activation of palygorskite has emerged as a highly effective method for enhancing arsenic adsorption on clay materials. Studies have demonstrated that thermal treatment of palygorskite significantly improves its adsorption capacity due to structural transformations that increase surface area and pore volume. Specifically, thermally activated palygorskite showed distinct adsorption behaviors for arsenic, lead, and zinc, with arsenic achieving the highest binding affinity. This method capitalizes on the material’s SiO and MgO sites, which exhibit stable adsorption energies when arsenic is present. The theoretical analysis confirms that the MgO surface in particular supports the highest arsenic affinity, aligning with observed results that arsenic consistently outperformed other metals in terms of adsorption stability []. The implication of this trend is that thermally activated palygorskite could be highly suited for environments with predominant arsenic contamination, where selective adsorption is a priority.
Hybridization with layered double hydroxides (LDHs) has proven a valuable strategy for achieving multifunctional adsorbent properties. By integrating LDHs with natural clays such as sepiolite, bentonite, and halloysite, researchers have developed bifunctional materials that effectively adsorb both cations and anions. The “in situ” assembly process, wherein LDHs are grown directly on the clay surface, was especially effective, yielding uniform materials with enhanced adsorption characteristics. These hybrids exhibit high anion and cation exchange capacities, which facilitate arsenic adsorption alongside other pollutants, like cadmium. The structural compatibility between clay and LDH components fosters a synergistic effect, where the combined material performs better than its individual constituents. The “in situ” hybrid approach therefore represents a promising technique for creating versatile adsorbents capable of handling complex contaminant mixtures in natural water systems [].
Iron modification of clay structures represents a critical advance in the development of low-cost, high-efficiency adsorbents for arsenic removal, especially in rural and underserved areas. Iron impregnation onto clays, such as attapulgite, leverages the affinity of iron for arsenic species, effectively boosting adsorption capacity through the addition of reactive sites and increased surface area. Iron-modified clays, particularly those formed through calcination, retain mechanical stability and perform well across a wide pH range, making them adaptable to varied environmental conditions. These properties make iron-modified clays a suitable candidate for application in both batch and column modes of water treatment, as demonstrated in studies where modified attapulgite achieved arsenic adsorption efficiencies surpassing those of traditional low-cost adsorbents. The durability and regeneration potential of iron-impregnated clays further emphasize their value in sustainable arsenic remediation efforts [].
Advanced composite design through biochar integration and computational modeling highlights an innovative approach to enhancing adsorption properties through the fusion of biochar and clay structures. Biochar, known for its high porosity and functional surface groups, complements the adsorption capabilities of clays like attapulgite when combined as a composite material. These biochar–clay composites, developed through slow pyrolysis and further treated with agents like zinc chloride, have demonstrated exceptional ability to reduce bioavailable arsenic and cadmium in sediment applications. Computational methods, such as computer-aided molecular design (CAMD) and adsorbate solid solution (ASS) theory, offer additional enhancements by optimizing functional group interactions for targeted adsorption. The CAMD approach allows for the fine-tuning of adsorption capacity by predicting molecular interactions between arsenic and specific clay components, such as beidellite and sepiolite, resulting in adsorbents with significantly higher capacities than those of conventional clays. These advanced composites are positioned as an optimal solution for in situ treatment of sediments, particularly in regions where cost-effective and high-capacity adsorbents are essential [,].
General Clay and Other Types: After performing the analysis in the provided manuscripts, we defined the collaborations into four key areas of application: natural clay minerals, modified clay composites, multi-functional adsorbents, and point-of-use (POU) applications for arsenic removal.
Natural clay minerals represent the baseline adsorbents in arsenic removal studies, utilizing the inherent properties of unmodified clay minerals to retain arsenic through basic adsorption mechanisms. For instance, studies involving phyllosilicate clay minerals highlight how arsenic adsorption can be enhanced with the presence of Ca/Mg ions, which prevent desorption in specific groundwater recharge conditions []. Similarly, Shanghai silty clay has been examined for its baseline adsorption efficiency towards As(V) in a high-surface-area batch process, revealing that although effective, unmodified clays are generally limited in their adsorption capacity relative to modified variants [].
Modified clay composites demonstrate a clear advancement in arsenic adsorption efficiency through targeted chemical modifications. Studies with iron oxide or manganese oxide modifications, such as those performed on laterite clay, reveal significant improvements in adsorption capacities and oxidative capabilities, which facilitate the conversion of more toxic As(III) to less toxic As(V) []. The introduction of aluminum oxide nanoparticles on treated laterite has similarly shown enhanced arsenic removal, with adsorption capacities reaching up to 48.5 mg/g, indicating a promising avenue for arsenic and fluoride co-removal []. Such modifications not only increase the clay’s adsorptive surface area but also introduce new reactive sites that enhance its ability to bind arsenic more effectively compared to unmodified clay [,].
Multi-functional adsorbents incorporate additional elements to address arsenic and other heavy metals simultaneously, reflecting a trend towards broader, multi-pollutant applications. Research on composite materials that combine clay with calcium carbonate or metal oxides has shown an increased capacity for adsorbing multiple contaminants. For example, in high-calcium environments, clays enhanced with CaCO3 show stronger adsorption behavior due to increased ionic interactions, beneficially altering the pH to immobilize arsenic and other metal contaminants []. Additionally, kinetic and thermodynamic studies on clay containing tailings with electrochemical modifications have been used to optimize arsenic extraction processes, indicating that multifunctional adsorbents could serve as a foundation for further industrial-scale applications [].
Point-of-use applications are particularly significant in regions where centralized treatment is lacking. Iron-coated ceramics, as well as sand–clay mixtures, have been developed for household arsenic filtration, showing practical effectiveness and ease of production, making them accessible to low-resource settings. Studies on iron-coated ceramic clay pitchers demonstrate arsenic removal efficiencies of up to 94–98%, making them viable for direct application in contaminated areas []. Additionally, lanthanum-coated ceramics have been identified as promising materials for both As(V) and As(III) removal, particularly suitable for household use due to their high stability and ability to sustain filtration over extended periods [].
4.2. RQ2—What Are the Primary Operational Units Used for Arsenic Adsorption by Clay?
In the field of arsenic adsorption, batch reactors are prominently featured in experimental studies, with a substantial number of research papers employing this system for initial adsorption analyses. In fact, as observed in Figure 3, about 90 references to the use of batch reactors exist within the screened manuscripts. The popularity of batch reactors in these studies could be defined by their simplicity and flexibility, making them an ideal choice for fundamental investigations into adsorption behavior at lower technology readiness levels (TRLs). Batch reactors offer a straightforward, controlled environment that allows researchers to systematically explore adsorption characteristics, enabling precise control over experimental parameters such as pH, temperature, and adsorbent concentration. This controlled setting is essential for conducting kinetic and equilibrium studies, which are critical in understanding adsorption mechanisms and developing isotherm models (e.g., Langmuir, Freundlich), both foundational for subsequent scaling. Independent of this, we identified the arsenic adsorption applications in four other operational units: fixed-bed columns, ion exchange, membrane filtration, and electrochemical methods.
Fixed-bed columns represent a versatile and cost-effective solution for arsenic removal, particularly in applications requiring simple, scalable setups that allow for long contact times between the contaminant and adsorbent materials. Several studies demonstrated the efficacy of fixed-bed columns with modified adsorbents, such as iron and lanthanum-coated porous materials. For instance, porous attapulgite impregnated with hydrated iron oxide effectively captured arsenite (As(III)) and arsenate (As(V)) in fixed-bed configurations, achieving strong adsorption capacities with high regeneration potential across several cycles using NaOH as a regenerant []. This method demonstrates particular strength in managing groundwater contaminants due to its adaptability across various pH levels and resilience to interference from ions like SO2 and HCO. Likewise, lanthanum-coated ceramic materials employed in column setups effectively removed both As(III) and As(V) and could serve as a promising low-cost option for decentralized, point-of-use water treatment, especially in low-resource settings where infrastructure for extensive water treatment is limited []. Additionally, montmorillonite clays, modified to enhance adsorption characteristics, showed significant potential in fixed-bed systems due to their high affinity for As(III) under acidic conditions. This natural, inexpensive adsorbent can be adapted for rural areas, making it a feasible option for large-scale water purification systems [].
Ion exchange methods emphasize the use of ionic adjustments to stabilize arsenic, particularly in aquifer and groundwater applications where natural clay minerals serve as the primary medium for adsorption. One notable application of ion exchange is within managed aquifer recharge (MAR) processes, where adding calcium (Ca+2) and magnesium (Mg+2) ions to recharge water significantly enhances arsenate adsorption onto aquifer sediments. By adjusting recharge water chemistry, arsenic mobilization from aquifer sediments can be minimized, allowing for a controlled and sustained reduction in arsenic levels in groundwater []. Another example includes the use of mixed-metal oxyhydroxides, such as those derived from laterite and ferromanganese slag, which leverage ion exchange properties inherent to Mn and Fe oxyhydroxides to oxidize and adsorb arsenic in contaminated water []. These materials function both as adsorbents and as oxidative agents, creating a dual-action mechanism that can effectively lower arsenic levels, particularly in groundwater.
Membrane filtration systems represent a sophisticated approach to arsenic removal, utilizing advanced materials to enhance both adsorptive capacity and physical stability. Membrane filtration solutions that incorporate adsorptive properties, such as organoclay-embedded polysulfone (PSf) nanocomposite membranes, have demonstrated high arsenate removal efficiencies. These membranes combine high mechanical strength, surface hydrophilicity, and reusability, making them suitable for continuous arsenic adsorption in water treatment applications []. Polysulfone membranes with embedded organoclay offer durability and high flux, which are important characteristics for systems that require repeated regeneration. Additionally, lanthanum-coated ceramic granules and disks were fabricated into membranelike setups to facilitate water flow while retaining effective arsenic removal capabilities. Such configurations offer a promising solution for household or small-scale use in areas where filtration options are limited and low-maintenance systems are preferred []. Furthermore, the development of polysulfone/organoclay nanocomposite membranes for arsenic removal has been discussed []. These membranes demonstrated effective arsenate removal in contaminated water. The organoclay addition increased the membrane’s surface hydrophilicity, roughness, and mechanical strength, which are key characteristics for adsorptive efficiency and reusability in filtration applications. Moreover, the membrane’s performance showed promise across multiple cycles, indicating its potential for sustainable use in water treatment systems requiring frequent regeneration [].
Electrochemical methods offer a targeted approach to the removal of arsenic from contaminated soils, particularly in environmental cleanup applications involving mining tailings or heavily polluted sites []. Electrochemical extraction, as studied in one manuscript, used a kinetic modeling approach to optimize conditions for arsenic, iron, and manganese removal from soils containing high levels of clay. The Elovich kinetic model was found to best describe arsenic extraction behavior, allowing the researchers to refine the process by adjusting factors such as current intensity, extractant concentration, and the liquid-to-solid ratio. This electrochemical method presents an attractive solution for soil remediation, particularly in large-scale applications where arsenic levels exceed those typically found in drinking-water sources. By directly targeting arsenic within the soil matrix, this approach mitigates environmental contamination and offers a scalable method for high-volume, high-yield extraction in contaminated areas.
4.3. RQ3—What Are the Main Types of Studies Performed and the Achievements Obtained for Arsenic Adsorption Using Clay?
Figure 4 and Figure 5 show the results when considering the different types of studies performed. The data reveal that certain study types are predominant in arsenic adsorption research, while others are less commonly explored. Kinetic studies, efficiency assessments, isothermal analyses, material characterization, and adsorption mechanism investigations are among the most frequently conducted. These study types are essential for understanding the adsorption process’s dynamics, efficacy, and interactions at a molecular level, which are critical for optimizing materials and processes in a controlled environment.
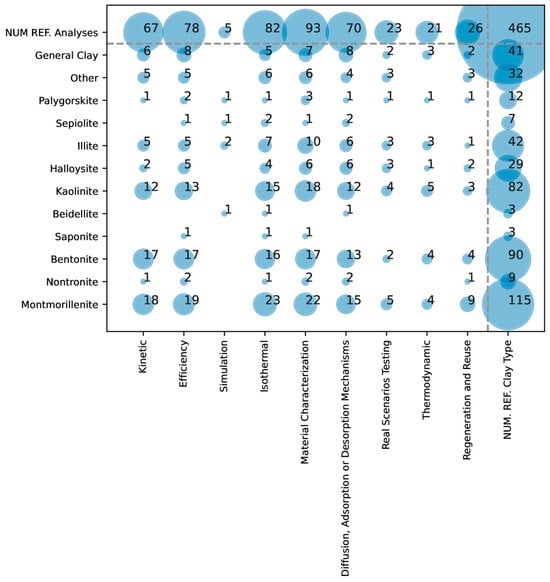
Figure 4.
Overlap in the literature between the types of clays and the types of studies performed in arsenic removal.
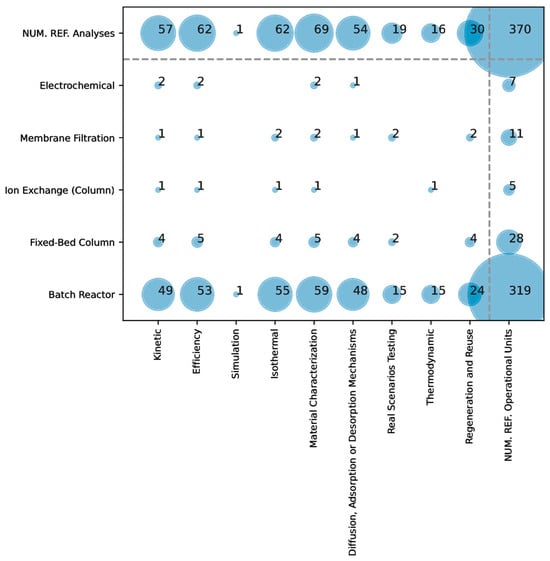
Figure 5.
Overlap in literature between the types of operational units and types of analyses performed.
Conversely, simulation studies, real scenario testing, thermodynamic analyses, and regeneration/reuse studies are less common. The lower frequency of these studies suggests that researchers focus more on fundamental laboratory-based analyses than on applied, large-scale, or real-world testing (i.e., lower TRLs). Real scenario testing and regeneration studies are likely constrained by the need for additional resources, longer timelines, and complexities inherent to field conditions. Additionally, thermodynamic studies, which involve intricate calculations of energy changes, might be less prioritized in initial experimental setups focused on basic adsorption efficiencies and kinetics.
When analyzing the less frequent types of studies, the tendencies observed were the following:
Simulation: After performing the analysis in the provided manuscripts, we defined the contributions into three key areas of application: mechanistic modeling and surface complexation models, first principles and density functional theory (DFT) studies, and innovative adsorbent design using computational techniques.
Mechanistic Modeling and Surface Complexation Models: One key area involves understanding arsenic adsorption through mechanistic modeling, with a focus on surface complexation models (SCMs) that predict adsorption behaviors on mineral surfaces. For instance, in Ref. [], the study of arsenic (III/V) adsorption onto illite uses SCMs to decode the adsorption mechanisms in detail, considering specific interactions such as cation bridging on basal surfaces and complexation through monodentate and bidentate configurations. This process-based SCM accurately models arsenic adsorption patterns by accounting for electrostatic forces and inner-sphere complexation mechanisms that influence adsorption on edge and basal surfaces. Such studies emphasize the critical role of surface characteristics, demonstrating that adsorption on minerals like illite can be highly specific, with clear trends based on molecular configurations and charge interactions. These mechanistic insights contribute to the development of predictive models that are valuable for both environmental management and industrial applications where precise arsenic adsorption control is required.
First-Principle and Density Functional Theory (DFT) Studies: A second area of focus is the use of first-principles and DFT-based approaches to understand adsorption mechanisms at an atomic level. In Refs. [,], DFT calculations were utilized to assess the adsorption of heavy metals, including arsenic, on mineral surfaces such as thermally activated palygorskite and illite. Specifically, Ref. [] provides an in-depth analysis of arsenic adsorption on the illite (001) surface, highlighting its adsorption energy and the electron transfer that stabilizes arsenic on the mineral surface. These studies show that arsenic exhibits a stronger adsorption energy compared to other heavy metals, particularly on oxygen-rich sites, where chemisorption is preferred over physisorption. The preference for stable adsorption sites with high electron density, especially on oxygen-rich surfaces, suggests a notable trend where arsenic exhibits stronger retention characteristics on certain mineral substrates. This atomic-level understanding, facilitated by DFT, underscores the importance of specific surface structures in influencing adsorption behavior, providing a theoretical basis for selecting and optimizing mineral surfaces for effective arsenic adsorption.
Innovative adsorbent design using computational techniques: The third classification lies in the development of adsorbents with enhanced arsenic removal capabilities through computational techniques. The research in Ref. [] demonstrates the use of computer-aided molecular design (CAMD) and adsorbate solid solution (ASS) theory to create optimized clay-based adsorbents such as beidellite, zeolite, and sepiolite. These computational approaches enable the design of adsorbents that possess an adsorption capacity one order of magnitude higher than that of traditional materials, primarily by adjusting functional groups and structural parameters for ideal arsenic retention. By leveraging computational optimization algorithms, these studies identify adsorbent configurations that are both cost-effective and highly efficient, addressing a significant need in global arsenic remediation efforts. Such advancements in adsorbent design through CAMD and ASS represent a promising avenue for large-scale applications, particularly in regions with severe arsenic contamination in groundwater.
Real Scenario Testing: Modified natural clays for adsorption enhancement show significant promise in practical arsenic remediation through targeted modifications that enhance adsorption efficiency. For example, leonardite char, a coal mining byproduct, demonstrated high adsorption for As(III) and As(V) at optimal pH levels, fitting both Langmuir and Freundlich isotherms []. Similarly, acid–base and thermally treated laterite clays were applied with notable success, being further stabilized in clay brick formations to manage spent adsorbents in field applications []. Aluminum-modified montmorillonite has also proven effective in arsenic and fluoride removal in groundwater, and halloysite nanoclay demonstrated an 82.4% As(III) adsorption efficiency in polluted water [,]. These studies underscore the adaptability of modified clays, allowing for multi-contaminant targeting, high adsorption capacities, and multiple adsorption cycles without efficiency loss []. Together, these findings reinforce the practicality and sustainability of enhanced natural clays in real-world groundwater treatment.
Nano-engineered composite materials for selectivity and regeneration emerge as powerful solutions, especially in scenarios where high selectivity and reusability are essential. Composite materials such as polysulfone–organoclay nanocomposites provided an efficient method for As(V) removal from contaminated water, combining high adsorption capacity with structural stability, making them suitable for repeated adsorption cycles []. Additionally, a composite using Fe3O4-immobilized halloysite nanotubes demonstrated magnetically separable and easily regenerable properties, ideal for field applications []. Another approach involved biochar functionalized with attapulgite clay, significantly improving the stability and capacity for metal adsorption, thus immobilizing arsenic and cadmium in river sediment []. These nano-engineered materials exemplify durability, reusability, and cost-effectiveness, addressing long-term water purification and supporting low-cost, scalable solutions.
Geochemical interactions and environmental contexts in arsenic mobilization emphasize the need to understand environmental influences on arsenic behavior, which is critical for informed remediation strategies. Studies on managed aquifer recharge (MAR) demonstrate that cations such as Ca+2 and Mg+2 limit arsenic desorption, thus minimizing contamination risks during recharge events []. Furthermore, arsenic mobilization in paddy soils shows strong dependence on redox cycling, with reduction leading to arsenic release and reoxidation facilitating its sequestration, highlighting implications for irrigation practices in arsenic-prone regions []. Biochar–clay composites also effectively reduce arsenic bioavailability in sediment, illustrating a potential in situ approach for limiting arsenic mobility in natural settings []. The stabilization of spent adsorbents in clay bricks further addresses environmental concerns, reducing the risk of long-term pollutant re-release [].
Thermodynamic Studies: After performing the analysis in the provided manuscripts, we defined the collaborations into three key areas of application: clay and modified clay adsorbents, iron- and magnetite-based nanocomposites, and multi-metal and competitive adsorption systems.
Clay and modified clay adsorbents are widely studied due to their availability, cost-effectiveness, and versatility in adsorptive properties. Several studies, such as those in Refs. [,], focus on using unmodified and modified clay minerals like kaolin, montmorillonite, and bentonite to enhance arsenic adsorption. Modification techniques include intercalation with surfactants or metal oxides, which improves surface area and stability. Thermodynamic analyses in these studies often reveal spontaneous adsorption processes, indicated by negative Gibbs free energy (ΔG) values. The adsorption mechanisms align well with the Langmuir isotherm, suggesting monolayer adsorption on a homogeneous surface, particularly with modified clay minerals. Additionally, exothermic adsorption processes (negative ΔH) imply that lower temperatures favor arsenic uptake, making these adsorbents suitable for practical applications in groundwater treatment.
Iron- and magnetite-based nanocomposites represent a modern approach to arsenic adsorption, particularly in their ability to provide high surface area and magnetic properties for easy separation post-treatment. Studies using magnetite-loaded halloysite nanotubes, as described in [], demonstrate that these nanocomposites exhibit endothermic adsorption behavior, suggesting increased efficiency at higher temperatures. Thermodynamic evaluations show that adsorption is often chemisorptive, as chemical bonding occurs between arsenic ions and the composite surface, as confirmed through techniques like X-ray photoelectron spectroscopy (XPS) and Fourier-transform infrared spectroscopy (FTIR). The reusability and stability of these nanocomposites underscore their economic feasibility, enhancing their appeal for long-term arsenic removal applications.
Multi-metal and competitive adsorption systems examine arsenic adsorption in solutions containing various co-existing ions, simulating complex groundwater environments. For example, in Ref. [], the adsorption of arsenic alongside ions like fluoride shows that competitive ion interactions can influence adsorption capacity. Fluoride, in particular, is observed to enhance arsenic uptake due to its effect on surface interaction dynamics. Thermodynamically, these studies indicate an entropy-driven process (positive ΔS), suggesting that the randomness at the solid–liquid interface increases with arsenic adsorption. These findings highlight the complexities of adsorption behavior in multicomponent systems, where ionic interactions and competitive effects can impact the overall thermodynamic profile, including the spontaneity and enthalpy of the process.
These thermodynamic studies collectively indicate that arsenic adsorption is generally spontaneous across adsorbent types, with differences in enthalpy (ΔH) reflecting material-specific behaviors. Clay-based adsorbents and their modified forms are exothermic and tend to favor arsenic removal at ambient or lower temperatures, whereas magnetite-based nanocomposites show endothermic behavior, benefiting from higher temperatures. Furthermore, studies on competitive adsorption reveal that co-existing ions can alter adsorption dynamics, presenting both challenges and insights for optimizing arsenic removal in natural water systems. These findings support the development of efficient and economically viable arsenic adsorbents, aligning with global needs for safe and sustainable water treatment solutions.
5. Conclusions
This study provides a systematic review of the role of clays in arsenic adsorption, highlighting their potential as cost-effective and efficient adsorbents for water treatment. Through an extensive analysis of existing literature, the findings confirm that montmorillonite, bentonite, and kaolinite stand out as the most studied and effective clay minerals for arsenic removal. The review categorizes research based on adsorption kinetics, thermodynamics, and isotherms, offering insights into the mechanisms that govern arsenic adsorption on natural and modified clays.
Key findings indicate that modifications such as acid activation, metal oxide impregnation, and composite material integration significantly enhance arsenic adsorption efficiency. The study further emphasizes the importance of operational conditions—including pH, ionic strength, and temperature—on the adsorption capacity of clays. The effectiveness of various experimental setups, particularly batch reactors and fixed-bed systems, was also analyzed to determine their applicability in real-world water treatment scenarios.
While montmorillonite and bentonite are justifiably emphasized due to their strong performance, the review underrepresents other promising clays such as illite, sepiolite, and palygorskite. These materials offer unique structural and chemical properties that may enhance arsenic adsorption but remain insufficiently studied. This research gap could be addressed by exploring the performance and modification potential of these underutilized clays, helping to identify alternative materials.
Despite the promising potential of clay-based adsorbents, several challenges remain. The limited scalability of laboratory studies, the lack of field-scale validation, and uncertainties regarding the long-term stability and regeneration of spent adsorbents highlight areas requiring further research. Future work should focus on optimizing modified clay adsorbents for real-world applications, improving reusability, and integrating cost-effective large-scale implementation strategies.
By consolidating current knowledge, this review serves as a foundation for advancing clay-based arsenic remediation technologies, bridging the gap between experimental research and practical applications. Continued efforts in this area will be essential in ensuring safe and sustainable water treatment solutions, particularly for communities affected by arsenic contamination.
Author Contributions
Writing—review and editing, L.R.-B. and E.V.; writing—original draft preparation and formal analysis, G.G.C. and J.C.; methodology, J.Z.; validation and funding acquisition, H.V.-G. and C.S.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agencia Nacional de Investigación y Desarrollo, Chile (ANID/Subdirección de Investigación Aplicada/Fondef IDeA I+D/ID23I10021). Further, the authors gratefully acknowledge the partial funding from Agencia Nacional de Investigación y Desarrollo, Chile (ANID, InES I+D 240002 Project and ANID, ING 2030 Etapa 2, Project ING222010005).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors want to express their particular thanks to the Faculty of Engineering and Business, Universidad de Las Américas; Faculty of Engineering, Universidad Autónoma de Chile; and Faculty of Engineering, Universidad del Desarrollo, for providing financial support for the publication of this paper.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shaji, E.; Santosh, M.; Sarath, K.; Prakash, P.; Deepchand, V.; Divya, B. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- van Halem, D.; Bakker, S.A.; Amy, G.L.; van Dijk, J.C. Arsenic in drinking water: A worldwide water quality concern for water supply companies. Drink. Water Eng. Sci. 2009, 2, 29–34. [Google Scholar] [CrossRef]
- WHO. Sanitary Inspection Package: A Supporting Tool for the Guidelines for Drinking-Water Quality: Small Water Supplies; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Fauser, P.; Czub, M.J.; Bełdowski, J.; Niemikoski, H.; Vanninen, P.; Popiel, S.; Nawała, J.; Dziedzic, D.; Sanderson, H. Chemical warfare agents and their risk assessment in Daphnia magna and fish in the Baltic Sea—15 years of measurements. J. Hazard. Mater. Adv. 2023, 12, 100386. [Google Scholar] [CrossRef]
- Hasan, N.T.; Han, D.; Xu, X.; Sansom, G.; Roh, T. Relationship between low-level arsenic exposure in drinking water and kidney cancer risk in Texas. Environ. Pollut. 2024, 363, 125097. [Google Scholar] [CrossRef]
- Smith, A.H.; Marshall, G.; Yuan, Y.; Ferreccio, C.; Liaw, J.; von Ehrenstein, O.; Steinmaus, C.; Bates, M.N.; Selvin, S. Increased mortality from lung cancer and bronchiectasis in young adultsafter exposure to arsenic in utero and in early childhood. Environ. Health Perspect. 2006, 114, 1293–1296. [Google Scholar] [CrossRef]
- Maity, J.P.; Chen, C.-Y.; Bhattacharya, P.; Sharma, R.K.; Ahmad, A.; Patnaik, S.; Bundschuh, J. Advanced application of nano-technological and biological processes as well as mitigation options for arsenic removal. J. Hazard. Mater. 2021, 405, 123885. [Google Scholar] [CrossRef]
- Sorlini, S.; Miino, M.C.; Lazarova, Z.; Collivignarelli, M.C. Electrochemical Treatment of Arsenic in Drinking Water: Effect of Initial As3+ Concentration, pH, and Conductivity on the Kinetics of Oxidation. Clean Technol. 2023, 5, 203–214. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Tzollas, N.M.; Tolkou, A.K.; Mitrakas, M.; Ernst, M.; Zouboulis, A.I. Use of novel composite coagulants for arsenic removal from waters—Experimental insight for the application of polyferric sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated carbons for arsenic removal from natural waters and wastewaters: A review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- Usman, M.; Katsoyiannis, I.; Rodrigues, J.H.; Ernst, M. Arsenate removal from drinking water using by-products from conventional iron oxyhydroxides production as adsorbents coupled with submerged microfiltration unit. Environ. Sci. Pollut. Res. 2021, 28, 59063–59075. [Google Scholar] [CrossRef] [PubMed]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Petersen, K.; Vakkalanka, S.; Kuzniarz, L. Guidelines for conducting systematic mapping studies in software engineering: An update. Inf. Softw. Technol. 2015, 64, 1–18. [Google Scholar] [CrossRef]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Kitchenham, B. Guidelines for Performing Systematic Literature Reviews in Software Engineering; Technical Report, Version 2.3, EBSE Technical Report; Keele University: Keele, UK; University of Durham: Durham, UK, 2007. [Google Scholar]
- Petersen, K.; Feldt, R.; Mujtaba, S.; Mattsson, M. Systematic mapping studies in software engineering. In Proceedings of the International Conference on Evaluation and Assessment in Software Engineering (EASE 2008), Bari, Italy, 26–27 June 2008. [Google Scholar]
- Acharya, A.; Jeppu, G.; Girish, C.R.; Prabhu, B.; Murty, V.R.; Martis, A.S.; Ramesh, S. Adsorption of arsenic and fluoride: Modeling of single and competitive adsorption systems. Heliyon 2024, 10, e31967. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Melotta, M.; Fernández, M.; Iriel, A. Arsenic and fluoride adsorption from multielement solutions onto aluminium modified montmorillonite. Groundw. Sustain. Dev. 2024, 26, 101205. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Yan, J.; Wang, Y.; Yu, B.; Zhang, X.; Ye, S. TiO2 pillared montmorillonite in-situ growth of CeOx/MnOy nanoparticles for effective arsenic (III) adsorption in wastewater. Environ. Sci. Pollut. Res. 2020, 27, 17986–17996. [Google Scholar] [CrossRef]
- Bandpei, A.M.; Mohseni, S.M.; Sheikhmohammadi, A.; Sardar, M.; Sarkhosh, M.; Almasian, M.; Avazpour, M.; Mosallanejad, Z.; Atafar, Z.; Nazari, S.; et al. Optimization of arsenite removal by adsorption onto organically modified montmorillonite clay: Experimental & theoretical approaches. Korean J. Chem. Eng. 2016, 34, 376–383. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Z.; Luo, H.; Hu, B.; Dang, Z.; Yang, C.; Li, L. Adsorption of arsenic on modified montmorillonite. Appl. Clay Sci. 2014, 97–98, 17–23. [Google Scholar] [CrossRef]
- Shokri, E.; Yegani, R.; Pourabbas, B.; Kazemian, N. Preparation and characterization of polysulfone/organoclay adsorptive nanocomposite membrane for arsenic removal from contaminated water. Appl. Clay Sci. 2016, 132–133, 611–620. [Google Scholar] [CrossRef]
- Iriel, A.; Marco-Brown, J.L.; Diljkan, M.; Trinelli, M.A.; Afonso, M.d.S.; Cirelli, A.F. Arsenic Adsorption on Iron-Modified Montmorillonite: Kinetic Equilibrium and Surface Complexes. Environ. Eng. Sci. 2020, 37, 22–32. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Wang, J.; Du, Y.; Wang, Z.; Guo, Q.; Pan, Z.; Ma, X.; Planer-Friedrich, B.; Luo, Y.; et al. Mobilization of Colloid- and Nanoparticle-Bound Arsenic in Contaminated Paddy Soils during Reduction and Reoxidation. Environ. Sci. Technol. 2023, 57, 9843–9853. [Google Scholar] [CrossRef] [PubMed]
- Almasri, D.A.; Rhadfi, T.; Atieh, M.A.; McKay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Ozola, R.; Krauklis, A.; Leitietis, M.; Burlakovs, J.; Vircava, I.; Ansone-Bertina, L.; Bhatnagar, A.; Klavins, M. FeOOH-modified clay sorbents for arsenic removal from aqueous solutions. Environ. Technol. Innov. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Bentahar, Y.; Hurel, C.; Draoui, K.; Khairoun, S.; Marmier, N. Adsorptive properties of Moroccan clays for the removal of arsenic(V) from aqueous solution. Appl. Clay Sci. 2016, 119, 385–392. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Jung, W.; Halajnia, A.; Lakzian, A.; Kabra, A.N.; Jeon, B.-H. Removal of arsenate and arsenite from aqueous solution by adsorption on clay minerals. Geosystem Eng. 2015, 18, 302–311. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Barman, A.; Datta, S.C.; Manjaiah, K.M. Arsenic Adsorption on Modified Clay Minerals in Contaminated Soil and Water: Impact of pH and Competitive Anions. CLEAN—Soil Air Water 2021, 49, 2000259. [Google Scholar] [CrossRef]
- Franco, F.; Benítez-Guerrero, M.; Gonzalez-Triviño, I.; Pérez-Recuerda, R.; Assiego, C.; Cifuentes-Melchor, J.; Pascual-Cosp, J. Low-cost aluminum and iron oxides supported on dioctahedral and trioctahedral smectites: A comparative study of the effectiveness on the heavy metal adsorption from water. Appl. Clay Sci. 2016, 119, 321–332. [Google Scholar] [CrossRef]
- Wang, M.; Bera, G.; Mitra, K.; Wade, T.L.; Knap, A.H.; Phillips, T.D. Tight sorption of arsenic, cadmium, mercury, and lead by edible activated carbon and acid-processed montmorillonite clay. Environ. Sci. Pollut. Res. 2021, 28, 6758–6770. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Adeleye, A.S.; Farjadfard, S.; Esvandi, Z.; Arfaeinia, H.; Sorial, G.A.; Ramavandi, B.; Sahebi, S. Efficient arsenic(V) removal from contaminated water using natural clay and clay composite adsorbents. Environ. Sci. Pollut. Res. 2019, 26, 29748–29762. [Google Scholar] [CrossRef] [PubMed]
- Kanel, S.R.; Das, T.K.; Varma, R.S.; Kurwadkar, S.; Chakraborty, S.; Joshi, T.P.; Bezbaruah, A.N.; Nadagouda, M.N. Arsenic Contamination in Groundwater: Geochemical Basis of Treatment Technologies. ACS Environ. Au 2023, 3, 135–152. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Kruichak, J.N.; Artyushkova, K.; Newville, M.G.; Sun, C. Redox Transformations of As and Se at the Surfaces of Natural and Synthetic Ferric Nontronites: Role of Structural and Adsorbed Fe(II). Environ. Sci. Technol. 2017, 51, 11105–11114. [Google Scholar] [CrossRef]
- Mutar, R.F.; Saleh, M.A. Optimization of arsenic ions adsorption and removal from hospitals wastewater by nano-bentonite using central composite design. Mater. Today Proc. 2022, 60, 1248–1256. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Zarei, A.; Mesdaghinia, A.; Nabizadeh, R.; Alimohammadi, M.; Afsharnia, M. Response surface modeling, isotherm, thermodynamic and optimization study of arsenic (V) removal from aqueous solutions using modified bentonite-chitosan (MBC). Korean J. Chem. Eng. 2017, 34, 757–767. [Google Scholar] [CrossRef]
- Dousova, B.; Lhotka, M.; Filip, J.; Kolousek, D. Removal of arsenate and antimonate by acid-treated Fe-rich clays. J. Hazard. Mater. 2018, 357, 440–448. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, M.W.; Tutu, H.; De Beer, M. Application of magnesite–bentonite clay composite as an alternative technology for removal of arsenic from industrial effluents. Toxicol. Environ. Chem. 2014, 96, 1435–1451. [Google Scholar] [CrossRef]
- Saleh, S.; Mohammadnejad, S.; Khorgooei, H.; Otadi, M. Photooxidation/adsorption of arsenic (III) in aqueous solution over bentonite/ chitosan/TiO2 heterostructured catalyst. Chemosphere 2021, 280, 130583. [Google Scholar] [CrossRef]
- Hokkanen, S.; Doshi, B.; Srivastava, V.; Puro, L.; Koivula, R. Arsenic (III) removal from water by hydroxyapatite-bentonite clay-nanocrystalline cellulose. Environ. Prog. Sustain. Energy 2019, 38, 13147. [Google Scholar] [CrossRef]
- Baigorria, E.; Cano, L.A.; Sanchez, L.M.; Alvarez, V.A.; Ollier, R.P. Bentonite-composite polyvinyl alcohol/alginate hydrogel beads: Preparation, characterization and their use as arsenic removal devices. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100364. [Google Scholar] [CrossRef]
- Fazlali, F.; Mahjoub, A.R.; Aghayan, H. Adsorption of toxic heavy metals on organofunctionalized acid activated exfoliated bentonite clay for achieving wastewater treatment goals. Desalin. Water Treat 2019, 152, 338–350. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total. Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K. Chemical Science Review and Letters Adsorption of Cr(VI) and Arsenic onto Bentonite. Chem. Sci. Rev. Lett. 2014, 12, 3. [Google Scholar]
- Mukhopadhyay, R.; Manjaiah, K.; Datta, S.; Sarkar, B. Comparison of properties and aquatic arsenic removal potentials of organically modified smectite adsorbents. J. Hazard. Mater. 2019, 377, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Hua, J. Synthesis and characterization of bentonite based inorgano–organo-composites and their performances for removing arsenic from water. Appl. Clay Sci. 2015, 114, 239–246. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Kim, M.; Kim, J.-G.; Hong, S.-M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Khoddam, M.A.; Norouzbeigi, R.; Velayi, E.; Cavallaro, G. Statistical-based optimization and mechanism assessments of Arsenic (III) adsorption by ZnO-Halloysite nanocomposite. Sci. Rep. 2024, 14, 21629. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.K.; Biswas, B.; Rahman, M.M.; Xi, Y.; Paul, S.K.; Naidu, R. Magnetite Nanoparticles Loaded into Halloysite Nanotubes for Arsenic(V) Removal from Water. ACS Appl. Nano Mater. 2022, 5, 12063–12076. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Enhanced As(III) and As(V) Adsorption from Aqueous Solution by a Clay Based Hybrid Sorbent. Front. Chem. 2020, 7, 913. [Google Scholar] [CrossRef]
- Botto, I.L.; Tuti, S.; Gonzalez, M.J.; Gazzoli, D. Correlation between Iron Reducibility in Natural and Iron-Modified Clays and Its Adsorptive Capability for Arsenic Removal. Adv. Mater. Phys. Chem. 2016, 6, 129–139. [Google Scholar] [CrossRef]
- Chammui, Y.; Sooksamiti, P.; Naksata, W.; Thiansem, S.; Arqueropanyo, O.-A. Removal of arsenic from aqueous solution by adsorption on Leonardite. Chem. Eng. J. 2014, 240, 202–210. [Google Scholar] [CrossRef]
- Duan, X.-L.; Yuan, C.-G.; He, K.-Q.; Yu, J.-X.; Jiang, Y.-H.; Guo, Q.; Li, Y.; Yu, S.-J.; Liu, J.-F. Gaseous Arsenic Capture in Flue Gas by CuCl2-Modified Halloysite Nanotube Composites with High-Temperature NOx and SOx Resistance. Environ. Sci. Technol. 2022, 56, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Liu, H.; Zhang, X.; Huang, Y.; Li, H.; Huang, B.; Hu, H.; Yao, H. In-Furnace Control of Arsenic Vapor Emissions Using Kaolinite during Low-Rank Coal Combustion: Influence of Gaseous Sodium Compounds. Environ. Sci. Technol. 2019, 53, 12113–12120. [Google Scholar] [CrossRef]
- Rehman, A.; Rukh, S.; Al Ayoubi, S.; Khattak, S.A.; Mehmood, A.; Ali, L.; Khan, A.; Malik, K.M.; Qayyum, A.; Salam, H. Natural Clay Minerals as Potential Arsenic Sorbents from Contaminated Groundwater: Equilibrium and Kinetic Studies. Int. J. Environ. Res. Public Health 2022, 19, 16292. [Google Scholar] [CrossRef]
- Ihekweme, G.O.; Obianyo, I.I.; Anosike-Francis, E.N.; Anyakora, V.N.; Odusanya, O.S.; Onwualu, A.P. Expanded clay aggregates multi-functionality for water purification: Disinfection and adsorption studies. Cogent Eng. 2021, 8, 1883232. [Google Scholar] [CrossRef]
- Chi, Z.; Xie, X.; Pi, K.; Wu, Y.; Wang, Y. Spectroscopic and modeling approaches of arsenic (III/V) adsorption onto Illite. J. Hazard. Mater. 2024, 477, 135284. [Google Scholar] [CrossRef]
- Qi, C.; Xu, X.; Chen, Q.; Liu, H.; Min, X.; Fourie, A.; Chai, L. Ab initio calculation of the adsorption of As, Cd, Cr, and Hg heavy metal atoms onto the illite(001) surface: Implications for soil pollution and reclamation. Environ. Pollut. 2022, 312, 120072. [Google Scholar] [CrossRef]
- Lefticariu, L.; Sutton, S.R.; Lanzirotti, A.; Flynn, T.M. Enhanced Immobilization of Arsenic from Acid Mine Drainage by Detrital Clay Minerals. ACS Earth Space Chem. 2019, 3, 2525–2538. [Google Scholar] [CrossRef]
- Schaefer, M.V.; Abernathy, M.J.; Nguyen, D.; Cornell, T.; Ying, S.C. Firing Increases Arsenic Leaching from Ceramic Water Filters via Arsenic and Iron Phase Transformations. Environ. Sci. Technol. 2021, 55, 9826–9835. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, L.-M.; Gao, W.; Dong, Y.-X.; Zhao, Y.-J.; He, M.-C. Adsorption mechanisms of heavy metal atoms As, Pb, and Zn on thermally activated palygorskite Si–O/Mg–O (200) surfaces: A first-principles calculations. Micro Nanostruct. 2024, 193, 207917. [Google Scholar] [CrossRef]
- Liñán-González, A.E.; Aguilar-Aguilar, A.; Robledo-Cabrera, A.; Collins-Martínez, V.H.; Flores-Cano, J.V.; Ocampo-Perez, R.; Padilla-Ortega, E. Synthesis of bifunctional nanostructured adsorbents based on anionic/cationic clays: Effect of arrangement on simultaneous adsorption of cadmium and arsenate. Environ. Sci. Pollut. Res. 2024, 31, 40100–40116. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kong, M.; Gu, X.; Chen, H. Removal of arsenic from water by porous charred granulated attapulgite-supported hydrated iron oxide in bath and column modes. J. Clean. Prod. 2017, 166, 88–97. [Google Scholar] [CrossRef]
- Doshi, R.K.; Mukherjee, R.; Diwekar, U.M. Application of Adsorbate Solid Solution Theory To Design Novel Adsorbents for Arsenic Removal Using CAMD. ACS Sustain. Chem. Eng. 2018, 6, 2603–2611. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Tan, X.; Liu, Y.; Zhou, Y.; Hu, X.; Cai, X.; Xu, W.; Zhang, C.; Liu, S. Functionalized Biochar/Clay Composites for Reducing the Bioavailable Fraction of Arsenic and Cadmium in River Sediment. Environ. Toxicol. Chem. 2019, 38, 2337–2347. [Google Scholar] [CrossRef]
- Fakhreddine, S.; Dittmar, J.; Phipps, D.; Dadakis, J.; Fendorf, S. Geochemical Triggers of Arsenic Mobilization during Managed Aquifer Recharge. Environ. Sci. Technol. 2015, 49, 7802–7809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W. Evaluating the adsorption of Shanghai silty clay to Cd(II), Pb(II), As(V), and Cr(VI): Kinetic, equilibrium, and thermodynamic studies. Environ. Monit. Assess. 2021, 193, 1–23. [Google Scholar] [CrossRef]
- Jain, N.; Maiti, A. Arsenite Oxidation and Arsenic Adsorption Strategy Using Developed Material from Laterite and Ferromanganese Slag: Electron Paramagnetic Resonance Spectroscopy Analysis. Ind. Eng. Chem. Res. 2023, 62, 15600–15612. [Google Scholar] [CrossRef]
- Rathore, V.K.; Mondal, P. Stabilization of arsenic and fluoride bearing spent adsorbent in clay bricks: Preparation, characterization and leaching studies. J. Environ. Manag. 2017, 200, 160–169. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current trends of arsenic adsorption in continuous mode: Literature review and future perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Wu, X.; Cui, M.; Zhang, J.; Huang, X. Adsorption of heavy metals on soil collected from lixisol of typical karst areas in the presence of CaCO3 and soil clay and their competition behavior. Sustainability 2020, 12, 7315. [Google Scholar] [CrossRef]
- Rosa, M.; Egido, J.; Márquez, M. Empirical kinetic models for the electrochemical extraction of arsenic and heavy metals from clay containing tailings. Appl. Clay Sci. 2019, 182, 105254. [Google Scholar] [CrossRef]
- Saeed, R.; Qureshi, K.; Shaikh, M.S.; Bhatti, Z.A. Removal of Arsenic from synthetic-water using Iron Coated Sand Adsorbent and Modified Ceramic Clay House Hold Pitcher. In Proceedings of the 2021 4th International Conference on Energy Conservation and Efficiency, ICECE 2021, Lahore, Pakistan, 16–17 March 2021. [Google Scholar] [CrossRef]
- Yang, H.; Min, X.; Xu, S.; Wang, Y. Lanthanum(III)-Coated Ceramics as a Promising Material in Point-of-Use Water Treatment for Arsenite and Arsenate Removal. ACS Sustain. Chem. Eng. 2019, 7, 9220–9227. [Google Scholar] [CrossRef]
- Zehhaf, A.; Benyoucef, A.; Quijada, C.; Taleb, S.; Morallón, E. Algerian natural montmorillonites for arsenic(III) removal in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 595–602. [Google Scholar] [CrossRef]
- Aljohani, N.S.; Kavil, Y.N.; Al-Farawati, R.K.; Alelyani, S.S.; Orif, M.I.; Shaban, Y.A.; Al-Mhyawi, S.R.; Aljuhani, E.H.; Salam, M.A. The effective adsorption of arsenic from polluted water using modified Halloysite nanoclay. Arab. J. Chem. 2023, 16, 104652. [Google Scholar] [CrossRef]
- Mishra, T.; Mahato, D.K. A comparative study on enhanced arsenic(V) and arsenic(III) removal by iron oxide and manganese oxide pillared clays from ground water. J. Environ. Chem. Eng. 2016, 4, 1224–1230. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Performance evaluation of surfactant modified kaolin clay in As(III) and As(V) adsorption from groundwater: Adsorption kinetics, isotherms and thermodynamics. Heliyon 2019, 5, e02756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).