Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater
Highlights
- PFOA/S sorption depends on pH, ionic strength, and co-existing ions in solution.
- PFOS consistently showed higher adsorption than PFOA on all adsorbents.
- PFOA/S adsorption occurs in the following order: AER> single-walled CNTs> AC.
- Sorption capacities for PFOA/S on all adsorbents decreased above pH 5.
- 1-2 year of in situ treatment adequately reduces PFOA/S concentrations in groundwater.
Abstract
1. Introduction
2. Per- and Polyfluoroalkyl Substances (PFAS) Physicochemical Characteristics and Occurrence in the Environment
3. Adsorbents for Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) Removal
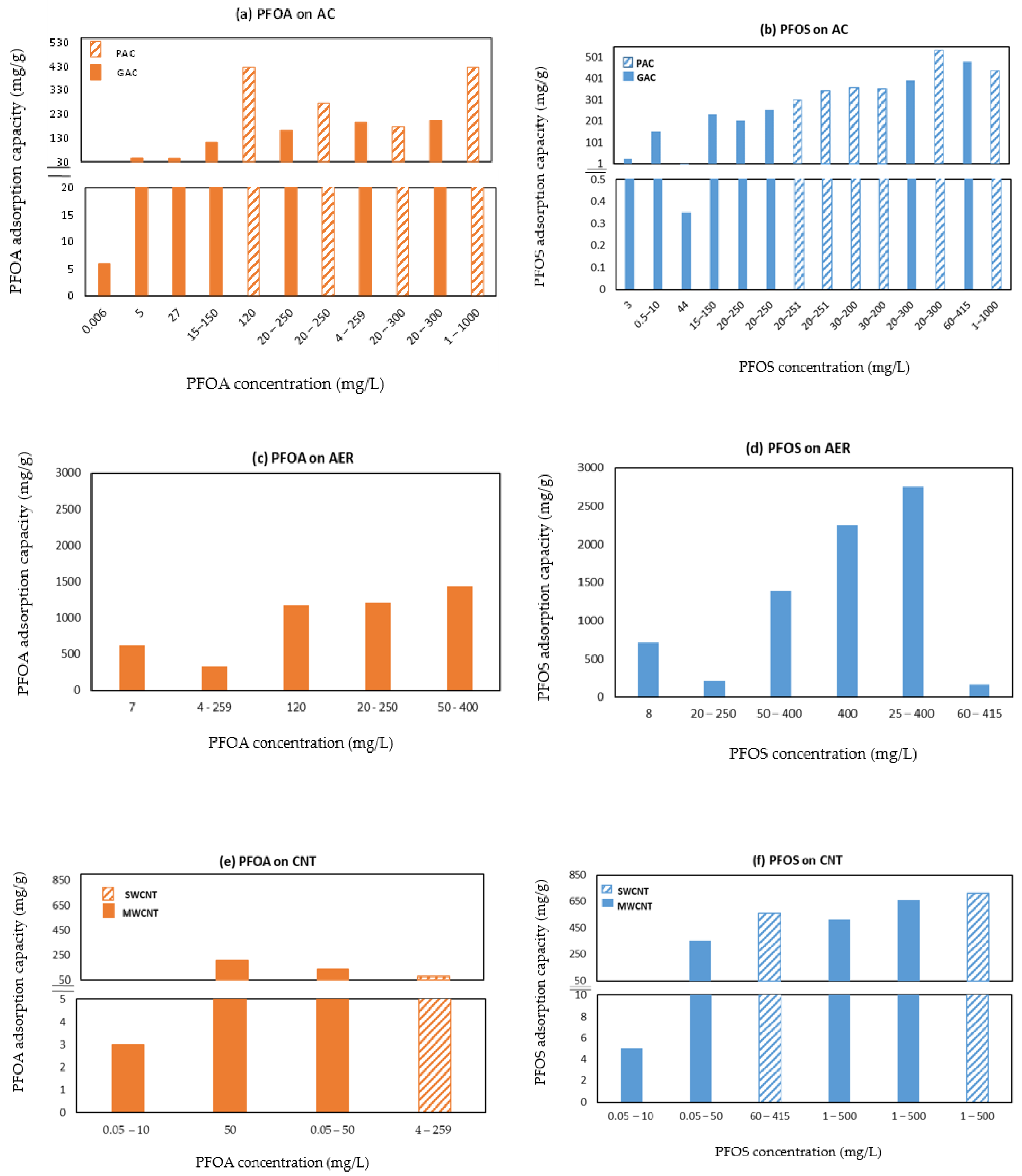
3.1. Activated Carbon (AC)
3.2. Anion Exchange Resins (AERs)
3.3. Carbon Nanotubes
4. Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) Adsorption Mechanism
4.1. Electrostatic Interactions
4.2. Hydrophobic Interactions
4.3. Anion and Ligand Exchange
4.4. Hydrogen Bonding
4.5. Charge-Assisted Hydrogen Bonding (CAHB)
5. Adsorption as a Function of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) Head Group
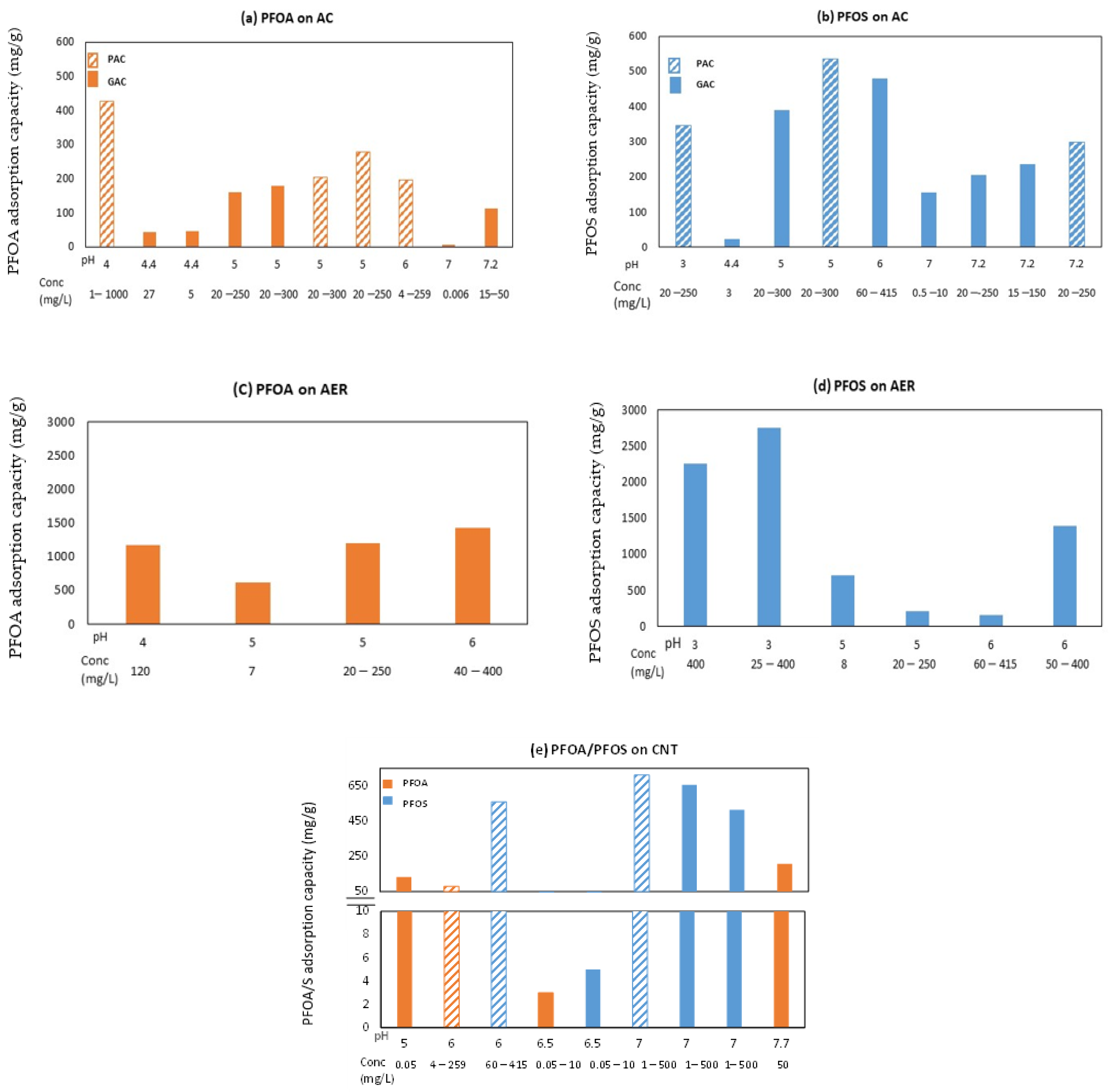
6. Combined Impact of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) Head Group and Solution pH on Adsorption
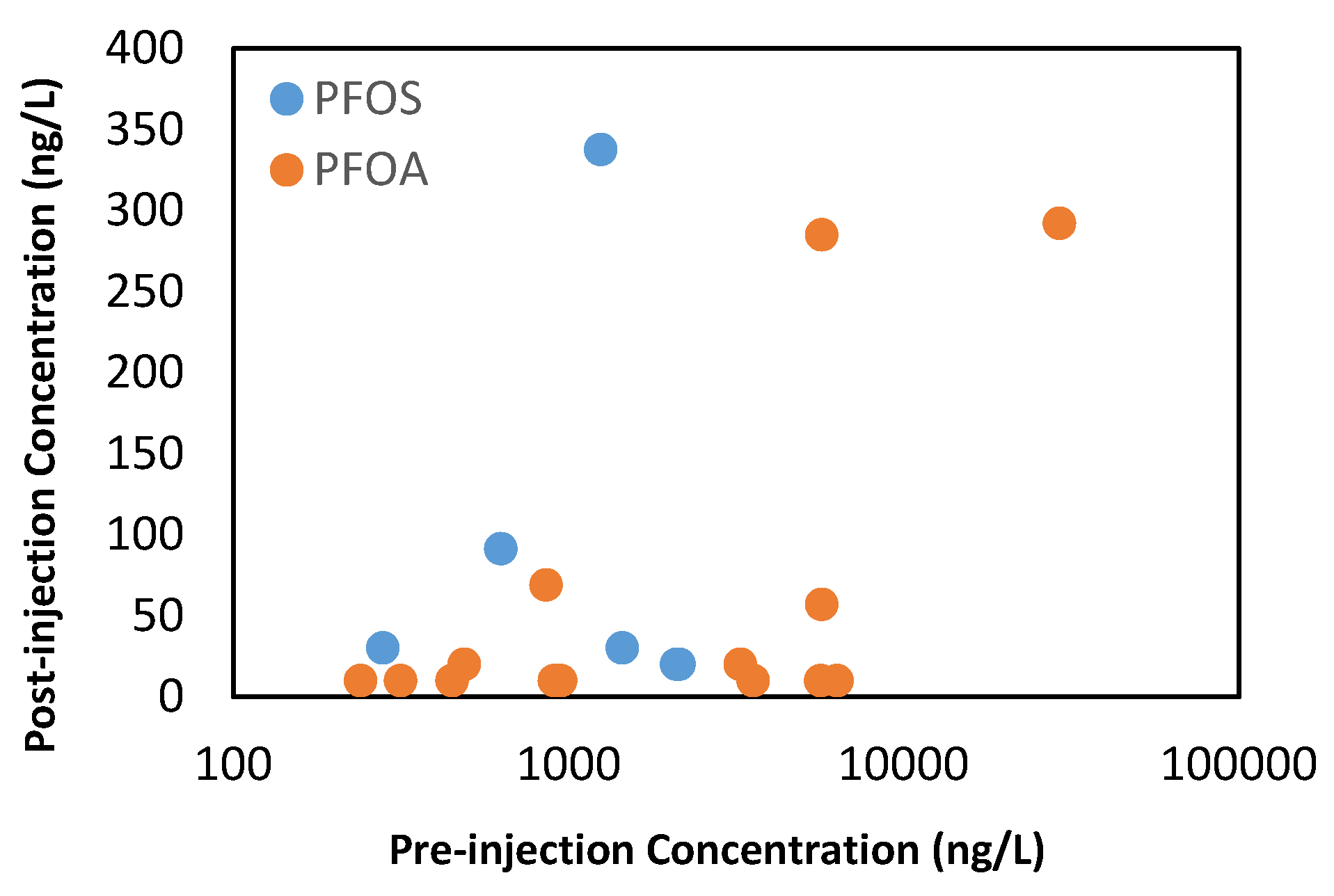
7. In Situ Treatment of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S)-Impacted Groundwater
7.1. In Situ Treatment of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S)-Impacted Groundwater Using Ion Exchange Resins
7.2. Adsorption of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) at the Air–Water Interface in the Vadose Zone
| PFOA/S | mbgs | Aquifer Properties | Time After Injection | C0 (ng/L) | C (ng/L)/Removal % | fcac (%) | ŋe | Vep (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| PFOA | 0.9–1.7 | iH 0.06 m/m, mean KH 2.6 m/day, Darcy Flux 0.8 m/day | 3, 6, 9, 12, 18 (18 months) | 490–3260 | <MDL (20) | 0.02 | N/A | N/A | [175] |
| PFOS | 280–1450 | <MDL (30) | |||||||
| PFOA | 5.0–6.2 | KH 1.1 × 10−6 to 8.6 × 10−4 m/s | 18 months | 6340 | <MDL (10) 96.5% | N/A | 0.002 | 33 | [171] |
| PFOA | 5.3–6.2 | 3560 | |||||||
| PFOA | 5.5, 7.0 | Averaged iH 0.008 m/m, KH 4.7 × 10−5 m/s–4.7 × 10−4 m/s, v ∼6 m/year | 92, 184, 278, 366, 549 days (18 months) | 450 | <MDL | 0.08 | 0.2 | 40 | [173] |
| PFOA | 9.5–12 | Averaged iv 0.018, iH 0.003, K 3.5 × 10−3–3.9 × 10−2 cm/s | 182, 273, and 366 (12 months) | 910 | <MDL | 0.76 | 0.2 | 50 | [174] |
| PFOS | 2105 | ||||||||
| PFOA | 3.2 | KH 4.8 × 10−6 to 6.3 × 10−4 m/s, v 9 m/year | 122, 248, 362, 547, and 724 (24 months) | 950 | <MDL (10 ng/L) | 0.07 | N/A | 30 | [188] |
| PFOS | 2140 | <MDL (20 ng/L) | |||||||
| PFOA | N/A | N/A | 41, 88, 116, 152, 178, 230 days | 316 | <MDL (0.2) | 0.26 | 0.15 | 70 | [177] |
| PFOS | 14.2 | <MDL (0.3) | |||||||
| PFOA | N/A | N/A | 90, 124, 145, 176, 208, 232, 329 days | 5660 | <MDL (5) | 0.25 | 0.23 | 70 | |
| PFOS | 670 | <MDL (2.5) | |||||||
| PFOA | N/A | N/A | 47, 321, 386, 482, 542 days | 240 | <MDL (0.01 μg/L) | 0.26 | 0.25 | 80 | |
| PFOS | 280 | ||||||||
| PFOS | N/A | N/A | 27, 58, 100, 132, 170, 358, 595 days | 60 | <MDL (0.01 μg/L) | 0.06 | 0.25 | 46 | |
| PFOS | N/A | N/A | 104, 227, 619 days | 500 | 82–99% | 0.32 | 0.33 | 70 | |
| PFOA | N/A | N/A | 19, 47, 110, 146, 200, 291, 370 days | 858 | >92% | 0.14 | 0.15 | 80 | |
| PFOS | 3540 | ||||||||
| PFOA | N/A | N/A | Data collection every month for 3 months post application. | 29,200 | 95–99% | 0.21 | 0.23 | 70 | |
| PFOS | 153,000 | ||||||||
| PFOA | N/A | N/A | 30, 60, 120, 210, 365, 730 days | 5700 | 95–99% | 0.11 | 0.25 | 39 | |
| PFOS | 8 | ||||||||
| PFOS | 3 | K 10−3 to 10−5 m/s | 1, 7, 11, 24, 60, 95, 113 days | 1250–319,000 | 73% | N/A | 0.37 | N/A | [191] |
| PFOA | 0.4 | Aquifer cell (64 cm length × 40.5 cm height × 1.4 cm internal thickness), flow cell packed under water-saturated conditions with 40–50 mesh Ottawa sand, pore-water velocity 1.52 m/day | Every 3 h, 70 h | 50,000 | 630–1360 Overall, 85.5% | N/A | N/A | N/A | [189] |
| PFOS | 230 Overall, 99.2% | ||||||||
| PFOA | 3 | Soil chamber (L = 15 cm, d = 3.6 cm), length to diameter ratio > 4 to simulate field conditions and minimize transverse dispersity. Soil column (L = 2 cm, d = 3.6 cm), flow rate 288 mL/day | 150 days | 510 | 99.7% | 0.031 | N/A | 400 PV | [192] |
| PFOS | 270 |
7.3. Air-Water Interfacial Adsorption of Perfluorooctanoic Acid and Perfluorooctanesulfonic Acid (PFOA/S) as a Function of Surface/Interfacial Tension
7.3.1. Effect of Solution Concentration on Air–Water Interfacial Adsorption Coefficients (Kaw)
7.3.2. Effect of Ionic Strength of the Solution on Air–Water Interfacial Adsorption Coefficients (Kaw)
8. Future Research Directions
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jarvis, A.L.; Justice, J.R.; Elias, M.C.; Schnitker, B.; Gallagher, K. Perfluorooctane sulfonate in US ambient surface waters: A review of occurrence in aquatic environments and comparison to global concentrations. Environ. Toxicol. Chem. 2021, 40, 2425–2442. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.-T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef]
- Buser, A.; Morf, L. Substance flow analysis for Switzerland: Perfluorinated surfactants perfluorooctanesulfonate (PFOS) and perfluorooctanoic acid (PFOA). Environ. Stud. 2009, 144, 22. [Google Scholar]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Han, L.; Wu, W.; Chen, M. Human health risk-based soil generic assessment criteria of representative perfluoroalkyl acids (PFAAs) under the agricultural land use in typical Chinese regions. Environ. Pollut. 2023, 335, 122368. [Google Scholar] [CrossRef]
- USEPA. Per- and Polyfluoroalkyl Substances (PFAS), Final PFAS National Primary Drinking Water Regulation. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 29 April 2024).
- Furlow, B. US EPA Sets Historic New Restrictions on Toxic PFAS in Drinking Water. Available online: https://www.sciencedirect.com/science/article/pii/S1470204524002158 (accessed on 20 May 2023).
- Grunfeld, D.A.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Zhang, W.L.; Liang, Y.N. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution—A review. Sci. Total Environ. 2019, 694, 133606. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mamadiev, M.; Wang, Z. Sorption of perfluorooctane sulfonate and perfluorooctanoate on polyacrylonitrile fiber-derived activated carbon fibers: In comparison with activated carbon. RSC Adv. 2017, 7, 927–938. [Google Scholar] [CrossRef]
- Goss, K.-U. The pKa Values of PFOA and Other Highly Fluorinated Carboxylic Acids. Environ. Sci. Technol. 2008, 42, 456–458. [Google Scholar] [CrossRef]
- Brooke, D.; Footitt, A.; Nwaogu, T. Environmental Risk Evaluation Report: Perfluorooctanesulphonate (PFOS); RPA Europe: Milano, Italy, 2004. [Google Scholar]
- Zhang, Y.; Zhou, Y.; Dong, R.; Song, N.; Hong, M.; Li, J.; Yu, J.; Kong, D. Emerging and legacy per- and polyfluoroalkyl substances (PFAS) in fluorochemical wastewater along full-scale treatment processes: Source, fate, and ecological risk. J. Hazard. Mater. 2024, 465, 133270. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Wang, B.; Lu, G.; Liu, J.; Wu, D.; Yan, Z. Toxicity comparison of perfluorooctanoic acid (PFOA), hexafluoropropylene oxide dimer acid (HFPO-DA), and hexafluoropropylene oxide trimer acid (HFPO-TA) in zebrafish gut. Aquat. Toxicol. 2023, 262, 106655. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.; Park, H.; Kim, M.-K.; Eom, S.; Choe, Y.; Choe, J.K.; Zoh, K.-D. Kinetics and proposed mechanisms of hexafluoropropylene oxide dimer acid (GenX) degradation via vacuum-UV (VUV) photolysis and VUV/sulfite processes. J. Hazard. Mater. 2024, 463, 132864. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Hebert, G.N.; Strauss, S.H.; Field, J.A. Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J. Environ. Monit. 2003, 5, 341–345. [Google Scholar] [CrossRef]
- Munoz, G.; Labadie, P.; Botta, F.; Lestremau, F.; Lopez, B.; Geneste, E.; Pardon, P.; Dévier, M.-H.; Budzinski, H. Occurrence survey and spatial distribution of perfluoroalkyl and polyfluoroalkyl surfactants in groundwater, surface water, and sediments from tropical environments. Sci. Total Environ. 2017, 607, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef]
- Higgins, C.P.; Luthy, R.G. Sorption of Perfluorinated Surfactants on Sediments†. Environ. Sci. Technol. 2006, 40, 7251. [Google Scholar] [CrossRef]
- Yamabe, T.; Moroi, Y.; Abe, Y.; Takahasi, T. Micelle formation and surface adsorption of N-(1,1-dihydroperfluoroalkyl)-N,N,N-trimethylammonium chloride. Langmuir 2000, 16, 9754–9758. [Google Scholar] [CrossRef]
- Kubo, K.; Moroi, Y.; Nomura, K.; Abe, Y.; Takahashi, T. Study on molecular aggregates of N-(1,1-dihydroperfluoroalkyl)-N,N,N-trimethylammonium chloride. Langmuir 2002, 18, 8770–8776. [Google Scholar] [CrossRef]
- Blanco, E.; González-Pérez, A.; Ruso, J.M.; Pedrido, R.; Prieto, G.; Sarmiento, F. A comparative study of the physicochemical properties of perfluorinated and hydrogenated amphiphiles. J. Colloid Interface Sci. 2005, 288, 247–260. [Google Scholar] [CrossRef]
- Riess, J.G. Highly fluorinated amphiphilic molecules and self-assemblies with biomedical potential. Curr. Opin. Colloid Interface Sci. 2009, 14, 294–304. [Google Scholar] [CrossRef]
- Umeh, A.C.; Naidu, R.; Shilpi, S.; Boateng, E.B.; Rahman, A.; Cousins, I.T.; Chadalavada, S.; Lamb, D.; Bowman, M. Sorption of PFOS in 114 well-characterized tropical and temperate soils: Application of multivariate and artificial neural network analyses. Environ. Sci. Technol. 2021, 55, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.H.; Bräunig, J.; Thompson, K.; Thompson, J.; Kabiri, S.; Navarro, D.A.; Kookana, R.S.; Grimison, C.; Barnes, C.M.; Higgins, C.P. Influences of chemical properties, soil properties, and solution pH on soil–water partitioning coefficients of per-and polyfluoroalkyl substances (PFASs). Environ. Sci. Technol. 2020, 54, 15883–15892. [Google Scholar] [CrossRef] [PubMed]
- Campos-Pereira, H.; Makselon, J.; Kleja, D.B.; Prater, I.; Kögel-Knabner, I.; Ahrens, L.; Gustafsson, J.P. Binding of per-and polyfluoroalkyl substances (PFASs) by organic soil materials with different structural composition–Charge-and concentration-dependent sorption behavior. Chemosphere 2022, 297, 134167. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Palau, J.; Vidal, M.; Rigol, A. Examining sorption of perfluoroalkyl substances (PFAS) in biochars and other carbon-rich materials. Chemosphere 2022, 302, 134733. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, J. Sorption and desorption of anionic, cationic and zwitterionic polyfluoroalkyl substances by soil organic matter and pyrogenic carbonaceous materials. Chem. Eng. J. 2018, 346, 682–691. [Google Scholar] [CrossRef]
- Campos-Pereira, H.; Kleja, D.B.; Sjöstedt, C.; Ahrens, L.; Klysubun, W.; Gustafsson, J.P. The adsorption of per-and polyfluoroalkyl substances (PFASs) onto ferrihydrite is governed by surface charge. Environ. Sci. Technol. 2020, 54, 15722–15730. [Google Scholar] [CrossRef]
- Oliver, D.P.; Li, Y.; Orr, R.; Nelson, P.; Barnes, M.; McLaughlin, M.J.; Kookana, R.S. The role of surface charge and pH changes in tropical soils on sorption behaviour of per-and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2019, 673, 197–206. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fu, Q.S.; Gao, D.; Criddle, C.S.; Leckie, J.O. Effect of solution chemistry on the adsorption of perfluorooctane sulfonate onto mineral surfaces. Water Res. 2010, 44, 2654–2662. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, X.; Penn, L.; Gulliver, J.S.; Simcik, M.F. Effects of monovalent cations on the competitive adsorption of perfluoroalkyl acids by kaolinite: Experimental studies and modeling. Environ. Sci. Technol. 2011, 45, 10028–10035. [Google Scholar] [CrossRef]
- Jeon, J.; Kannan, K.; Lim, B.J.; An, K.G.; Kim, S.D. Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. J. Environ. Monit. 2011, 13, 1803–1810. [Google Scholar] [CrossRef]
- Luft, C.M.; Schutt, T.C.; Shukla, M.K. Properties and mechanisms for PFAS adsorption to aqueous clay and humic soil components. Environ. Sci. Technol. 2022, 56, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.; Roccaro, P. Removal of poly-and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- McCleaf, P.; Englund, S.; Östlund, A.; Lindegren, K.; Wiberg, K.; Ahrens, L. Removal efficiency of multiple poly-and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Res. 2017, 120, 77–87. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Wan, H.; Mills, R.; Qu, K.; Hower, J.C.; Mottaleb, M.A.; Bhattacharyya, D.; Xu, Z. Rapid removal of PFOA and PFOS via modified industrial solid waste: Mechanisms and influences of water matrices. Chem. Eng. J. 2022, 433, 133271. [Google Scholar] [CrossRef]
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef]
- Umeh, A.C.; Hassan, M.; Egbuatu, M.; Zeng, Z.; Al Amin, M.; Samarasinghe, C.; Naidu, R. Multicomponent PFAS sorption and desorption in common commercial adsorbents: Kinetics, isotherm, adsorbent dose, pH, and index ion and ionic strength effects. Sci. Total Environ. 2023, 904, 166568. [Google Scholar] [CrossRef]
- Amen, R.; Ibrahim, A.; Shafqat, W.; Hassan, E.B. A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges. Sustainability 2023, 15, 16173. [Google Scholar] [CrossRef]
- Hu, X.C.; Andrews, D.Q.; Lindstrom, A.B.; Bruton, T.A.; Schaider, L.A.; Grandjean, P.; Lohmann, R.; Carignan, C.C.; Blum, A.; Balan, S.A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3, 344–350. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Bentel, M.J.; Yu, Y.; Xu, L.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management. Environ. Sci. Technol. 2019, 53, 3718–3728. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Politzer, P.; Jin, P.; Murray, J.S. Atomic polarizability, volume and ionization energy. J. Chem. Phys. 2002, 117, 8197–8202. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Park, H.; Cheng, J.; Mader, B.T.; Hoffmann, M.R. Enhancement of Perfluorooctanoate and Perfluorooctanesulfonate Activity at Acoustic Cavitation Bubble Interfaces. J. Phys. Chem. C 2008, 112, 16850–16857. [Google Scholar] [CrossRef]
- Wesseler, E.P.; Iltis, R.; Clark, L.C., Jr. The solubility of oxygen in highly fluorinated liquids. J. Fluor. Chem. 1977, 9, 137–146. [Google Scholar] [CrossRef]
- Hao, F.; Guo, W.; Wang, A.; Leng, Y.; Li, H. Intensification of sonochemical degradation of ammonium perfluorooctanoate by persulfate oxidant. Ultrason. Sonochem. 2014, 21, 554–558. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Long-Chain Perfluorinated Chemicals. Action Plan. Available online: https://www.epa.gov/sites/default/files/2016-01/documents/pfcs_action_plan1230_09.pdf (accessed on 20 May 2023).
- Rayne, S.; Forest, K. Perfluoroalkyl sulfonic and carboxylic acids: A critical review of physicochemical properties, levels and patterns in waters and wastewaters, and treatment methods. J. Environ. Sci. Health Part A 2009, 44, 1145. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L. Polyfluoroalkyl compounds in the aquatic environment: A review of their occurrence and fate. J. Environ. Monit. 2011, 13, 20–31. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Per-and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid Interface Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Cousins, I.T.; Vestergren, R.; Wang, Z.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef]
- Kwok, K.Y.; Taniyasu, S.; Yeung, L.W.; Murphy, M.B.; Lam, P.K.; Horii, Y.; Kannan, K.; Petrick, G.; Sinha, R.K.; Yamashita, N. Flux of perfluorinated chemicals through wet deposition in Japan, the United States, and several other countries. Environ. Sci. Technol. 2010, 44, 7043–7049. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Gerwinski, W.; Theobald, N.; Ebinghaus, R. Sources of polyfluoroalkyl compounds in the North Sea, Baltic Sea and Norwegian Sea: Evidence from their spatial distribution in surface water. Mar. Pollut. Bull. 2010, 60, 255–260. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, Z.; Möller, A.; Sturm, R.; Tang, J.; Zhang, G.; Ebinghaus, R. Distribution and long-range transport of polyfluoroalkyl substances in the Arctic, Atlantic Ocean and Antarctic coast. Environ. Pollut. 2012, 170, 71–77. [Google Scholar] [CrossRef]
- Xiao, F.; Halbach, T.R.; Simcik, M.F.; Gulliver, J.S. Input characterization of perfluoroalkyl substances in wastewater treatment plants: Source discrimination by exploratory data analysis. Water Res. 2012, 46, 3101–3109. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Zhang, H.; Sheng, N.; Dai, J. Elevated concentrations of perfluorohexanesulfonate and other per-and polyfluoroalkyl substances in Baiyangdian Lake (China): Source characterization and exposure assessment. Environ. Pollut. 2018, 241, 684–691. [Google Scholar] [CrossRef]
- Möller, A.; Ahrens, L.; Surm, R.; Westerveld, J.; van der Wielen, F.; Ebinghaus, R.; de Voogt, P. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ. Pollut. 2010, 158, 3243–3250. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wiberg, K.; Ribeli, E.; Josefsson, S.; Futter, M.; Gustavsson, J.; Ahrens, L. Spatial distribution and source tracing of per-and polyfluoroalkyl substances (PFASs) in surface water in Northern Europe. Environ. Pollut. 2017, 220, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, R.; Zhang, C.; Han, J.; Wang, X.; Han, G.; He, X. Occurrence, spatial and temporal distributions of perfluoroalkyl substances in wastewater, seawater and sediment from Bohai Sea, China. Environ. Pollut. 2016, 219, 389–398. [Google Scholar] [CrossRef]
- Guelfo, J.L.; Adamson, D.T. Evaluation of a national data set for insights into sources, composition, and concentrations of per-and polyfluoroalkyl substances (PFASs) in US drinking water. Environ. Pollut. 2018, 236, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Hwang, Y.; Andersen, H.R.; Stasinakis, A.S.; Thomaidis, N.S.; Aloupi, M. Reductive degradation of perfluorinated compounds in water using Mg-aminoclay coated nanoscale zero valent iron. Chem. Eng. J. 2015, 262, 133–139. [Google Scholar] [CrossRef]
- NSW Health. PFOS and PFOA. Available online: https://www.health.nsw.gov.au/environment/factsheets/Pages/pfos.aspx (accessed on 27 November 2023).
- Health Canada. Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/water-talk-per-polyfluoroalkyl-substances-drinking-water.html (accessed on 23 January 2024).
- Union, O.J.o.t.E. European Parliament & Council of the European Union. EUR-Lex—52020SC0249—EN—EUR-Lex. Brussels, 14.10.2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52020SC0249 (accessed on 6 April 2025).
- Moody, C.A.; Field, J.A. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol. 2000, 34, 3864. [Google Scholar] [CrossRef]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from US military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678. [Google Scholar] [CrossRef]
- Jogsten, I.E.; Perelló, G.; Llebaria, X.; Bigas, E.; Martí-Cid, R.; Kärrman, A.; Domingo, J.L. Exposure to perfluorinated compounds in Catalonia, Spain, through consumption of various raw and cooked foodstuffs, including packaged food. Food Chem. Toxicol. 2009, 47, 1577–1583. [Google Scholar] [CrossRef]
- Mulabagal, V.; Liu, L.; Qi, J.; Wilson, C.; Hayworth, J.S. A rapid UHPLC-MS/MS method for simultaneous quantitation of 23 perfluoroalkyl substances (PFAS) in estuarine water. Talanta 2018, 190, 95–102. [Google Scholar] [CrossRef]
- Jogsten, I.E.; Nadal, M.; van Bavel, B.; Lindström, G.; Domingo, J.L. Per-and polyfluorinated compounds (PFCs) in house dust and indoor air in Catalonia, Spain: Implications for human exposure. Environ. Int. 2012, 39, 172–180. [Google Scholar] [CrossRef]
- Domingo, J.L. Health risks of dietary exposure to perfluorinated compounds. Environ. Int. 2012, 40, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per-and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Zango, Z.U.; Garba, A.; Haruna, A.; Imam, S.S.; Katsina, A.U.; Ali, A.F.; Abidin, A.Z.; Zango, M.U.; Garba, Z.N.; Hosseini-Bandegharaei, A. A systematic review on applications of biochar and activated carbon derived from biomass as adsorbents for sustainable remediation of antibiotics from pharmaceutical wastewater. J. Water Process Eng. 2024, 67, 106186. [Google Scholar] [CrossRef]
- Adetokun, A.A.; Uba, S.; Garba, Z.N. Optimization of adsorption of metal ions from a ternary aqueous solution with activated carbon from Acacia senegal (L.) Willd pods using Central Composite Design. J. King Saud Univ.-Sci. 2019, 31, 1452–1462. [Google Scholar] [CrossRef]
- Garba, Z.N.; Imam, M.; Adamu, H.; Agbaji, E.B. Optimization of adsorption conditions for Acid Chrome Blue K removal from aqueous solution using sugar-based activated carbon: Equilibrium isotherms and kinetics modeling. Sustain. Chem. One World 2024, 1, 100001. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y.; Hamid, H.; Jeronimo, M. Sludge-based activated carbon and its application in the removal of perfluoroalkyl substances: A feasible approach towards a circular economy. Chemosphere 2022, 294, 133707. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Akenga, T.A.; Olatunji, O.S. Removal of PFOA and PFOS from aqueous solutions using activated carbon produced from Vitis vinifera leaf litter. Environ. Sci. Pollut. Res. 2017, 24, 13107–13120. [Google Scholar] [CrossRef]
- Omo-Okoro, P.N.; Curtis, C.J.; Karásková, P.; Melymuk, L.; Oyewo, O.A.; Okonkwo, J.O. Kinetics, isotherm, and thermodynamic studies of the adsorption mechanism of PFOS and PFOA using inactivated and chemically activated maize tassel. Water Air Soil Pollut. 2020, 231, 485. [Google Scholar] [CrossRef]
- Levchuk, I.; Màrquez, J.J.R.; Sillanpää, M. Removal of natural organic matter (NOM) from water by ion exchange—A review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Beeftink, M.; Hofs, B.; Kramer, O.; Odegard, I.; van Der Wal, A. Carbon footprint of drinking water softening as determined by life cycle assessment. J. Clean. Prod. 2021, 278, 123925. [Google Scholar] [CrossRef]
- Caltran, I.; Heijman, S.; Shorney-Darby, H.; Rietveld, L. Impact of removal of natural organic matter from surface water by ion exchange: A case study of pilots in Belgium, United Kingdom and the Netherlands. Sep. Purif. Technol. 2020, 247, 116974. [Google Scholar] [CrossRef]
- Dong, H.; Lin, T.; SenGupta, A.K. Field validation of multifunctional ion exchange process for reverse osmosis pretreatment and phosphate recovery during impaired water reuse. J. Water Process Eng. 2020, 36, 101347. [Google Scholar] [CrossRef]
- Zhang, J.; Amini, A.; O’Neal, J.A.; Boyer, T.H.; Zhang, Q. Development and validation of a novel modeling framework integrating ion exchange and resin regeneration for water treatment. Water Res. 2015, 84, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Deng, S.; Du, Z.; Liu, K.; Yu, G. Adsorptive removal of emerging polyfluoroalky substances F-53B and PFOS by anion-exchange resin: A comparative study. J. Hazard. Mater. 2017, 323, 550–557. [Google Scholar] [CrossRef]
- Bolto, B.; Dixon, D.; Eldridge, R.; King, S. Removal of THM precursors by coagulation or ion exchange. Water Res. 2002, 36, 5066–5073. [Google Scholar] [CrossRef]
- Hsu, S.; Singer, P.C. Removal of bromide and natural organic matter by anion exchange. Water Res. 2010, 44, 2133–2140. [Google Scholar] [CrossRef]
- Hu, Y.; Foster, J.; Boyer, T.H. Selectivity of bicarbonate-form anion exchange for drinking water contaminants: Influence of resin properties. Sep. Purif. Technol. 2016, 163, 128–139. [Google Scholar] [CrossRef]
- Rokicki, C.A.; Boyer, T.H. Bicarbonate-form anion exchange: Affinity, regeneration, and stoichiometry. Water Res. 2011, 45, 1329–1337. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Zhou, Q.; Shuang, C.; Zhou, W.; Wang, M. Effect of pore size distribution on tetracycline adsorption using magnetic hypercrosslinked resins. Microporous Mesoporous Mater. 2014, 184, 105–111. [Google Scholar] [CrossRef]
- Carter, K.E.; Farrell, J. Removal of Perfluorooctane and Perfluorobutane Sulfonate from Water via Carbon Adsorption and Ion Exchange. Sep. Sci. Technol. 2010, 45, 762–767. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, Q.; Nie, Y.; Wei, H.; Wang, B.; Huang, J.; Yu, G.; Xing, B. Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ. Pollut. 2012, 168, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xia, X.; Wang, X.; Qiao, J.; Chen, H. A comparative study on sorption of perfluorooctane sulfonate (PFOS) by chars, ash and carbon nanotubes. Chemosphere 2011, 83, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, D.; Li, C.; Ji, R.; Tian, X. Metal nanoparticles by doping carbon nanotubes improved the sorption of perfluorooctanoic acid. J. Hazard. Mater. 2018, 351, 206–214. [Google Scholar] [CrossRef]
- Wu, C.; Klemes, M.J.; Trang, B.; Dichtel, W.R.; Helbling, D.E. Exploring the factors that influence the adsorption of anionic PFAS on conventional and emerging adsorbents in aquatic matrices. Water Res. 2020, 182, 115950. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Glubt, S.V. The influence of surfactant and solution composition on PFAS adsorption at fluid-fluid interfaces. Water Res. 2019, 161, 17–26. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, C.; Ji, R.; Tian, X. Improved sorption of perfluorooctanoic acid on carbon nanotubes hybridized by metal oxide nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 15507–15517. [Google Scholar] [CrossRef]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Quan, X.; Chen, S.; Zhang, Y.; Yu, H. Adsorption of ionizable organic contaminants on multi-walled carbon nanotubes with different oxygen contents. J. Hazard. Mater. 2011, 186, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Speltini, A.; Maiocchi, M.; Cucca, L.; Merli, D.; Profumo, A. Solid-phase extraction of PFOA and PFOS from surface waters on functionalized multiwalled carbon nanotubes followed by UPLC–ESI-MS. Anal. Bioanal. Chem. 2014, 406, 3657–3665. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.; Shih, K. Adsorption behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on boehmite. Chemosphere 2012, 89, 1009–1014. [Google Scholar] [CrossRef]
- Li, F.; Fang, X.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Adsorption of perfluorinated acids onto soils: Kinetics, isotherms, and influences of soil properties. Sci. Total Environ. 2019, 649, 504–514. [Google Scholar] [CrossRef]
- Feng, G.; Zhou, B.; Yuan, R.; Luo, S.; Gai, N.; Chen, H. Influence of soil composition and environmental factors on the adsorption of per- and polyfluoroalkyl substances: A review. Sci. Total Environ. 2024, 925, 171785. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Pan, G.; Zhang, M. Assembling structures and dynamics properties of perfluorooctane sulfonate (PFOS) at water–titanium oxide interfaces. J. Colloid Interface Sci. 2013, 405, 189–194. [Google Scholar] [CrossRef]
- Xiao, F.; Davidsavor, K.J.; Park, S.; Nakayama, M.; Phillips, B.R. Batch and column study: Sorption of perfluorinated surfactants from water and cosolvent systems by Amberlite XAD resins. J. Colloid Interface Sci. 2012, 368, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Song, X.; Wang, Q.; Hu, Z. Sorption kinetics, isotherms and mechanisms of PFOS on soils with different physicochemical properties. Ecotoxicol. Environ. Saf. 2017, 142, 40–50. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Quan, X.; Zhang, Y. Enhanced Adsorption of PFOA and PFOS on Multiwalled Carbon Nanotubes under Electrochemical Assistance. Environ. Sci. Technol. 2011, 45, 8498–8505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Deng, S.; Zhang, Q.; Fan, Q.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated sludge. Chemosphere 2010, 81, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environ. Sci. Eur. 2023, 35, 12. [Google Scholar] [CrossRef]
- Liang, X. Facile preparation of magnetic separable powdered-activated-carbon/Ni adsorbent and its application in removal of perfluorooctane sulfonate (PFOS) from aqueous solution. J. Environ. Sci. Health A 2011, 46, 1482–1490. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Recent progress in adsorptive removal of per- and poly-fluoroalkyl substances (PFAS) from water/wastewater. Crit. Rev. Environ. Sci. Technol. 2022, 52, 90–129. [Google Scholar] [CrossRef]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The overlooked short-and ultrashort-chain poly-and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef]
- Kassar, C.; Graham, C.; Boyer, T.H. Removal of perfluoroalkyl acids and common drinking water contaminants by weak-base anion exchange resins: Impacts of solution pH and resin properties. Water Res. X 2022, 17, 100159. [Google Scholar] [CrossRef] [PubMed]
- Du, Z. Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins. J. Hazard. Mater. 2015, 286, 136–143. [Google Scholar] [CrossRef]
- Lampert, D.J.; Frisch, M.A.; Speitel, G.E., Jr. Removal of perfluorooctanoic acid and perfluorooctane sulfonate from wastewater by ion exchange. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2007, 11, 60–68. [Google Scholar] [CrossRef]
- Bei, Y.; Deng, S.; Du, Z.; Wang, B.; Huang, J.; Yu, G. Adsorption of perfluorooctane sulfonate on carbon nanotubes: Influence of pH and competitive ions. Water Sci. Technol. 2014, 69, 1489–1495. [Google Scholar] [CrossRef]
- Yan, T.; Chen, H.; Jiang, F.; Wang, X. Adsorption of Perfluorooctane Sulfonate and Perfluorooctanoic Acid on Magnetic Mesoporous Carbon Nitride. J. Chem. Eng. Data 2014, 59, 508–515. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Wang, X.; Zhu, J.; Zhang, R.; He, P.; Fang, Y. The application of β-cyclodextrin derivative functionalized aligned carbon nanotubes for electrochemically DNA sensing via host–guest recognition. Anal. Chim. Acta 2011, 689, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Bei, Y.; Lu, X.; Du, Z.; Wang, B.; Wang, Y.; Huang, J.; Yu, G. Effect of co-existing organic compounds on adsorption of perfluorinated compounds onto carbon nanotubes. Front. Environ. Sci. Eng. 2015, 9, 784–792. [Google Scholar] [CrossRef]
- Wang, B.; Lee, L.S.; Wei, C.; Fu, H.; Zheng, S.; Xu, Z.; Zhu, D. Covalent triazine-based framework: A promising adsorbent for removal of perfluoroalkyl acids from aqueous solution. Environ. Pollut. 2016, 216, 884–892. [Google Scholar] [CrossRef]
- Wang, W.; Mi, X.; Zhou, Z.; Zhou, S.; Li, C.; Hu, X.; Qi, D.; Deng, S. Novel insights into the competitive adsorption behavior and mechanism of per- and polyfluoroalkyl substances on the anion-exchange resin. J. Colloid Interface Sci. 2019, 557, 655–663. [Google Scholar] [CrossRef]
- Pignatello, J.J. Soil organic matter as a nanoporous sorbent of organic pollutants. Adv. Colloid Interface Sci. 1998, 76, 445–467. [Google Scholar] [CrossRef]
- Xing, B.; Pignatello, J.J.; Gigliotti, B. Competitive Sorption between Atrazine and Other Organic Compounds in Soils and Model Sorbents. Environ. Sci. Technol. 1996, 30, 2432–2440. [Google Scholar] [CrossRef]
- Zhang, T.; Yue, X.; Yang, D.; Guo, Q.; Qiu, F.; Li, Z. Hybridization of Al2O3 microspheres and acrylic ester resins as a synergistic absorbent for selective oil and organic solvent absorption. Appl. Organomet. Chem. 2018, 32, e4244. [Google Scholar] [CrossRef]
- Guo, W.; Huo, S.; Feng, J.; Lu, X. Adsorption of perfluorooctane sulfonate (PFOS) on corn straw-derived biochar prepared at different pyrolytic temperatures. J. Taiwan Inst. Chem. Eng. 2017, 78, 265–271. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv. Colloid Interface Sci. 2004, 110, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Rattanaoudom, R.; Visvanathan, C.; Boontanon, S. Removal of concentrated PFOS and PFOA in synthetic industrial wastewater by powder activated carbon and hydrotalcite. J. Water Sustain. 2012, 2, 245–258. [Google Scholar]
- Saeidi, N.; Kopinke, F.-D.; Georgi, A. Understanding the effect of carbon surface chemistry on adsorption of perfluorinated alkyl substances. Chem. Eng. J. 2020, 381, 122689. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Niederer, C.; Goss, K.U. Predicting the partitioning behavior of various highly fluorinated compounds. Environ. Sci. Technol. 2006, 40, 7298–7304. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, J. Adsorption of perfluoroalkyl acids by carbonaceous adsorbents: Effect of carbon surface chemistry. Environ. Pollut. 2015, 202, 168–176. [Google Scholar] [CrossRef]
- Oen, A.M.P.; Beckingham, B.; Ghosh, U.; Kruså, M.E.; Luthy, R.G.; Hartnik, T.; Henriksen, T.; Cornelissen, G. Sorption of organic compounds to fresh and field-aged activated carbons in soils and sediments. Environ. Sci. Technol. 2012, 46, 810–817. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. Towards an unified hydrogen-bond theory. J. Mol. Struct. 2000, 552, 1–15. [Google Scholar] [CrossRef]
- Gilli, P.; Pretto, L.; Bertolasi, V.; Gilli, G. Predicting hydrogen-bond strengths from acid–base molecular properties. The pKa slide rule: Toward the solution of a long-lasting problem. Acc. Chem. Res. 2009, 42, 33. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, X.; Jiang, Z.; Zhang, H.; Yuan, S. Contribution of air-water interface in removing PFAS from drinking water: Adsorption, stability, interaction and machine learning studies. Water Res. 2023, 236, 119947. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- Vo, P.H.; Buckley, T.; Xu, X.; Nguyen, T.M.H.; Rudolph, V.; Shukla, P. Foam fractionation of per-and polyfluoroalkyl substances (PFASs) in landfill leachate using different cosurfactants. Chemosphere 2023, 310, 136869. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sengupta, A.K. Intraparticle diffusion during selective sorption of trace contaminants: The effect of gel versus macroporous morphology. Environ. Sci. Technol. 2000, 34, 5193–5200. [Google Scholar] [CrossRef]
- Gu, B.; Ku, Y.K.; Brown, G.M. Sorption and desorption of perchlorate and U(VI) by strong-base anion-exchange resins. Environ. Sci. Technol. 2005, 39, 901–907. [Google Scholar] [CrossRef]
- Boyer, T.H.; Singer, P.C. Stoichiometry of removal of natural organic matter by ion exchange. Environ. Sci. Technol. 2008, 42, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Volchek, K.; Brown, C.E.; Robinson, A.; Obal, T. Comparative study on adsorption of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) by different adsorbents in water. Water Sci. Technol. 2014, 70, 1983–1991. [Google Scholar] [CrossRef]
- Bolto, B.; Dixon, D.; Eldridge, R.; King, S.; Linge, K. Removal of natural organic matter by ion exchange. Water Res. 2002, 36, 5057–5065. [Google Scholar] [CrossRef] [PubMed]
- Maimaiti, A.; Deng, S.; Meng, P.; Wang, W.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chem. Eng. J. 2018, 348, 494–502. [Google Scholar] [CrossRef]
- Deng, S.; Yu, Q.; Huang, J.; Yu, G. Removal of perfluorooctane sulfonate from wastewater by anion exchange resins: Effects of resin properties and solution chemistry. Water Res. 2010, 44, 5188–5195. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Surridge, B.; Hoover, D.; Grace, R.; Gobas, F.A.P.C. Perfluoroalkyl contaminants in an arctic marine food web: Trophic magnification and wildlife exposure. Environ. Sci. Technol. 2009, 43, 4037–4043. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Syers, J.; Tillman, R. Surface charge and solute interactions in soils. Adv. Agron. 1999, 67, 87–140. [Google Scholar]
- Naidu, R.; Kookana, R.S.; Sumner, M.E.; Harter, R.D.; Tiller, K. Cadmium sorption and transport in variable charge soils: A review. J. Environ. Qual. 1997, 26, 602–617. [Google Scholar] [CrossRef]
- Wang, W.; Rhodes, G.; Zhang, W.; Yu, X.; Teppen, B.J.; Li, H. Implication of cation-bridging interaction contribution to sorption of perfluoroalkyl carboxylic acids by soils. Chemosphere 2022, 290, 133224. [Google Scholar] [CrossRef] [PubMed]
- Field, J.; Schwichtenberg, T.; Deeb, R.A.; Hawley, E.L.; Sayler, C.; Bogdan, D.; Shaefer, C.E., Jr.; DiGuiseppi, B.; Struse, A. Assessing the potential for bias in PFAS concentrations during groundwater and surface water sampling. Groundw. Monit. Remediat. 2019, 29, 31–48. [Google Scholar] [CrossRef]
- Simon, J.A.; Abrams, S.; Bradburne, T.; Bryant, D.; Burns, M.; Cassidy, D.; Cherry, J.; Chiang, S.Y.; Cox, D.; Crimi, M.; et al. PFAS Experts Symposium: Statements on regulatory policy, chemistry and analtyics, toxicology, transport/fate, and remediation for per- and polyfluoroalkyl substances (PFAS) contamination issues. Remediat.-J. Environ. Cleanup Costs Technol. Tech. 2019, 29, 31–48. [Google Scholar] [CrossRef]
- Abrams, S.; McGregor, R.; Burns, M.; Galasso, J.; Havranek, T.; Hesemann, J.; Longsworth, J.; McDonough, J.; Mora, R. PFAS Experts Symposium 2: Statements on available in situ remediation technologies. Remediat.-J. Environ. Cleanup Costs Technol. Tech. 2022, 32, 45–53. [Google Scholar] [CrossRef]
- Darlington, R.; Barth, E.; McKernan, J. The challenges of PFAS remediation. Mil. Eng. 2018, 110, 58. [Google Scholar]
- Nzeribe, N.; Crimi, M.; Thagard, S.M.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review Blossom. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Brusseau, M.L. Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Sci. Total Environ. 2018, 613–614, 176–185. [Google Scholar] [CrossRef]
- Psillakis, E.; Cheng, J.; Hoffmann, M.R.; Colussi, A.J. Enrichment factors of perfluoroalkyl oxoanions at the air/water interface. J. Phys. Chem. A 2009, 113, 8826–8829. [Google Scholar] [CrossRef]
- Carey, G.R.; McGregor, R.; Pham, A.L.T.; Sleep, B.; Hakimabadi, S.G. Evaluating the longevity of a PFAS in situ colloidal activated carbon remedy. Remediation 2019, 29, 17. [Google Scholar] [CrossRef]
- McGregor, R. The in situ treatment of PFAS within porewater at the air–water interface of a PFAS source zone. Remediat. J. 2023, 33, 265–278. [Google Scholar] [CrossRef]
- Ben-Hur, M.; Yolcu, G.; Uysal, H.; Lado, M.; Paz, A. Soil structure changes: Aggregate size and soil texture effects on hydraulic conductivity under different saline and sodic conditions. Soil Res. 2009, 47, 688–696. [Google Scholar] [CrossRef]
- McGregor, R. Six pilot-scale studies evaluating the in situ treatment of PFAS in groundwater. Remediat.-J. Environ. Cleanup Costs Technol. Tech. 2020, 30, 39–50. [Google Scholar] [CrossRef]
- McGregor, R.; Benevenuto, L. The effect of heterogeneity on the distribution and treatment of PFAS in a complex geologic environment. Front. Environ. Chem. 2021, 2, 729779. [Google Scholar] [CrossRef]
- McGregor, R. In Situ treatment of PFAS-impacted groundwater using colloidal activated Carbon. Remediat. J. 2018, 28, 33–41. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Yan, N.; Van Glubt, S.; Wang, Y.; Chen, W.; Lyu, Y.; Dungan, B.; Carroll, K.C.; Holguin, F.O. Comprehensive retention model for PFAS transport in subsurface systems. Water Res. 2019, 148, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Carey, G.R.; Hakimabadi, S.G.; Singh, M.; McGregor, R.; Woodfield, C.; Van Geel, P.J.; Pham, A.L.-T. Longevity of colloidal activated carbon for in situ PFAS remediation at AFFF-contaminated airport sites. Remediat. J. 2022, 33, 3–23. [Google Scholar] [CrossRef]
- Umeh, A.C.; Stegh, J.; Naidu, R. Toward In Situ Sequestration of Multicomponent PFAS Using Injectable Adsorbent Suspensions. ACS EST Water 2023, 3, 3858–3873. [Google Scholar] [CrossRef]
- Liu, C.; Chu, J.; Cápiro, N.L.; Fortner, J.D.; Pennell, K.D. In-situ sequestration of perfluoroalkyl substances using polymer-stabilized ion exchange resin. J. Hazard. Mater. 2022, 422, 126960. [Google Scholar] [CrossRef]
- Zaggia, A.; Conte, L.; Falletti, L.; Fant, M.; Chiorboli, A. Use of strong anion exchange resins for the removal of perfluoroalkylated substances from contaminated drinking water in batch and continuous pilot plants. Water Res. 2016, 91, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.J.; Valsaraj, K.T. Adsorption and Reaction of Trace Gas-Phase Organic Compounds on Atmospheric Water Film Surfaces: A Critical Review. Environ. Sci. Technol. 2010, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-M.; Vieira, V.M.; Ryan, P.B.; Detwiler, R.; Sanders, B.; Steenland, K.; Bartell, S.M. Environmental Fate and Transport Modeling for Perfluorooctanoic Acid Emitted from the Washington Works Facility in West Virginia. Environ. Sci. Technol. 2011, 45, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.K.; Barber, L.B.; Leblanc, D.R.; Sunderland, E.M.; Vecitis, C.D. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51, 4269–4279. [Google Scholar] [CrossRef]
- Silva, J.A.K.; Martin, W.A.; McCray, J.E. Air-water interfacial adsorption coefficients for PFAS when present as a multi-component mixture. J. Contam. Hydrol. 2021, 236, 103731. [Google Scholar] [CrossRef]
- Xiao, F.; Simcik, M.F.; Halbach, T.R.; Gulliver, J.S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a U.S. metropolitan area: Migration and implications for human exposure. Water Res. 2015, 72, 64–74. [Google Scholar] [CrossRef]
- Anderson, R.H.; Adamson, D.T.; Stroo, H.F. Partitioning of poly-and perfluoroalkyl substances from soil to groundwater within aqueous film-forming foam source zones. J. Contam. Hydrol. 2019, 220, 59–65. [Google Scholar] [CrossRef]
- Lyu, Y.; Brusseau, M.L.; Chen, W.; Yan, N.; Fu, X.; Lin, X. Adsorption of PFOA at the Air-Water Interface during Transport in Unsaturated Porous Media. Environ. Sci. Technol. 2018, 52, 7745–7753. [Google Scholar] [CrossRef]
- McGregor, R.; Zhao, Y. The in situ treatment of TCE and PFAS in groundwater within a silty sand aquifer. Remediat.-J. Environ. Cleanup Costs Technol. Tech. 2021, 31, 7–17. [Google Scholar] [CrossRef]
- Liu, C.; Hatton, J.; Arnold, W.A.; Simcik, M.F.; Pennell, K.D. In Situ Sequestration of Perfluoroalkyl Substances Using Polymer-Stabilized Powdered Activated Carbon. Environ. Sci. Technol. 2020, 54, 6929–6936. [Google Scholar] [CrossRef]
- Mahinroosta, R.; Senevirathna, L. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Høisæter, Å.; Arp, H.P.H.; Slinde, G.; Knutsen, H.; Hale, S.E.; Breedveld, G.D.; Hansen, M.C. Excavated vs novel in situ soil washing as a remediation strategy for sandy soils impacted with per- and polyfluoroalkyl substances from aqueous film forming foams. Sci. Total Environ. 2021, 794, 148763. [Google Scholar] [CrossRef] [PubMed]
- Niarchos, G.; Ahrens, L.; Kleja, D.B.; Fagerlund, F. Per- and polyfluoroalkyl substance (PFAS) retention by colloidal activated carbon (CAC) using dynamic column experiments. Environ. Pollut. 2022, 308, 119667. [Google Scholar] [CrossRef]
- Meng, P.; Deng, S.; Lu, X.; Du, Z.; Wang, B.; Huang, J.; Wang, Y.; Yu, G.; Xing, B. Role of air bubbles overlooked in the adsorption of perfluorooctanesulfonate on hydrophobic carbonaceous adsorbents. Environ. Sci. Technol. 2014, 48, 13785–13792. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, W.; Yu, G.; Deng, S. Contribution of Nanobubbles for PFAS Adsorption on Graphene and OH- and NH2-Functionalized Graphene: Comparing Simulations with Experimental Results. Environ. Sci. Technol. 2021, 55, 13254–13263. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.; Karanam, K.; Han, H.; Vo, H.N.P.; Shukla, P.; Firouzi, M.; Rudolph, V. Effect of different co-foaming agents on PFAS removal from the environment by foam fractionation. Water Res. 2023, 230, 119532. [Google Scholar] [CrossRef]
- Costanza-Robinson, M.; Brusseau, M. Air-Water Interfacial Areas in Unsaturated Porous Media: Evaluation of Interfacial Domains. In AGU Fall Meeting Abstracts; Wiley and American Geophysical Union; p. H52D–03. 2001. Available online: https://ui.adsabs.harvard.edu/abs/2001AGUFM.H52D..03C/abstract (accessed on 6 April 2025).
- Colosi, L.M.; Pinto, R.A.; Huang, Q.; Weber, W.J.J. Peroxidase-mediated degradation of perfluorooctanoic acid. Environ. Toxicol. Chem. 2009, 28, 264–271. [Google Scholar] [CrossRef]
- Adamson, A.W. Physical Chemistry of Surfaces; Interscience Publishers: New York, NY, USA, 1967. [Google Scholar]
- Schick, M.J. Nonionic Surfactants: Physical Chemistry; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Möbius, D.; Miller, R.; Fainerman, V.B. Surfactants: Chemistry, Interfacial Properties, Applications; Elsevier: Amsterdam, The Netherlands, 2001; Volume 13. [Google Scholar]
- Barnes, G.; Gentle, I. Interfacial Science: An Introduction; Oxford University Press: Cary, NC, USA, 2011. [Google Scholar]
- Berg, J.C. An Introduction to Interfaces & Colloids: The Bridge to Nanoscience; World Scientific: Singapore, 2010. [Google Scholar]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhong, H.; El Ouni, A.; Lin, D.; Wang, B.; Brusseau, M.L. The two-phase flow IPTT method for measurement of nonwetting-wetting liquid interfacial areas at higher nonwetting saturations in natural porous media. Water Resour. Res. 2016, 52, 5506–5515. [Google Scholar] [CrossRef]
- Silva, J.A.; Martin, W.A.; Johnson, J.L.; McCray, J.E. Evaluating air-water and NAPL-water interfacial adsorption and retention of perfluorocarboxylic acids within the vadose zone. J. Contam. Hydrol. 2019, 223, 103472. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Glubt, S.V. The influence of molecular structure on PFAS adsorption at air-water interfaces in electrolyte solutions. Chemosphere 2021, 281, 130829. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Prescher, D.; Hirte, R.; Geggel, K. Adsorption Properties of Surface Chemically Pure Sodium Perfluoro-n-alkanoates at the Air/Water Interface: Counterion Effects within Homologous Series of 1:1 Ionic Surfactants. Langmuir 2015, 31, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Wang, B.; Du, X.; Guo, B.; Brusseau, M.L. Air-water interfacial adsorption of C4-C10 perfluorocarboxylic acids during transport in unsaturated porous media. Sci. Total Environ. 2022, 831, 154905. [Google Scholar] [CrossRef]
- Brusseau, M.L. Examining the robustness and concentration dependency of PFAS air-water and NAPL-water interfacial adsorption coefficients. Water Res. 2021, 190, 116778. [Google Scholar] [CrossRef] [PubMed]
- Brusseau, M.L.; Guo, B.; Huang, D.; Yan, N.; Lyu, Y. Ideal versus nonideal transport of PFAS in unsaturated porous media. Water Res. 2021, 202, 117405. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lyu, X.; Gao, B.; Xu, H.; Wu, J.; Sun, Y. Effects of ionic strength and cation type on the transport of perfluorooctanoic acid (PFOA) in unsaturated sand porous media. J. Hazard. Mater. 2021, 403, 123688. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Lavorgna, G.M.; Lippincott, D.R.; Nguyen, D.; Christie, E.; Shea, S.; O’Hare, S.; Lemes, M.C.S.; Higgins, C.P.; Field, J. A field study to assess the role of air-water interfacial sorption on PFAS leaching in an AFFF source area. J. Contam. Hydrol. 2022, 248, 104001. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Culina, V.; Nguyen, D.; Field, J. Uptake of poly-and perfluoroalkyl substances at the air–water interface. Environ. Sci. Technol. 2019, 53, 12442–12448. [Google Scholar] [CrossRef]
- Kim, H.; Annable, M.D.; Rao, P.S.C. Influence of Air—Water Interfacial Adsorption and Gas-Phase Partitioning on the Transport of Organic Chemicals in Unsaturated Porous Media. Environ. Sci. Technol. 1998, 32, 1253–1259. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Liu, D.; Yao, X.; Wang, Y.; Lu, X.; Wang, B.; Huang, J.; Wang, Y.; Xing, B. Efficient adsorption of PFOS and F53B from chrome plating wastewater and their subsequent degradation in the regeneration process. Chem. Eng. J. 2016, 290, 405–413. [Google Scholar] [CrossRef]
| Compound | Abbreviation | Chemical Formula | Chemical Structure | Molecular Weight (g·mol−1) | Boiling Point (°C) | Log Kow | pKa |
|---|---|---|---|---|---|---|---|
| Perfluorooctanoic acid | PFOA | C7F15CO2H | 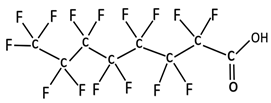 | 414.07 | 192 | 4.59 | −0.20 [9] −0.5 to −3.8 [10,11] |
| Perfluorooctanesulfonic acid | PFOS | C8F17SO3H | 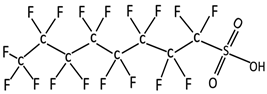 | 500.13 | 249 | 4.49 | −3.27 [9,10,12] |
| Perfluorononanoic acid | PFNA | C8F17CO2H | 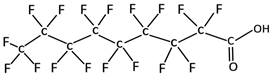 | 464.08 | N/A | 5.48 | −0.21 [13] |
| Perfluorohexanesulfonic acid | PFHxS | C6HF13O3S | 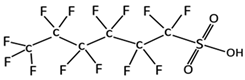 | 400.12 | 238.5 °C | 3.16 | 0.14 [9] |
| Hexafluoropropylene oxide dimer acid (GenX) | HFPO-DA | C6F11O3H | 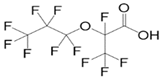 | 330.06 | N/A | N/A | 2.8 [14,15] |
| Toxicity Reference Value | PFOS | PFOA | Authority | References |
|---|---|---|---|---|
| Drinking water, MCL (μg/L) | 0.004 | 0.004 | US EPA | [6,7] |
| Latest restriction toxic PFAS in drinking water | 0 | 0 | ||
| Tolerable daily intake (μg/kg/d) | 0.02 | 0.16 | Commonwealth Department of Health, Australia | [73] |
| Drinking water quality guideline (μg/L) | 0.07 | 0.56 | ||
| Recreational water quality guideline (μg/L) | 0.7 | 5.6 | ||
| Sum of all PFAS | <30 ng/L | Health Canada | [74] | |
| Sum of all PFAS | <500 ng/L | European Union | [75] | |
| Sum of 20 selected PFAS | <100 ng/L | |||
| PFOA/S | PFOA/S Conc (mg/L) | Surface Tension/Interfacial Tension (mN/m) Background Solution a/b | Ionic Strength of Background Electrolyte | Surface Tension/Interfacial Tension (mN/m) in Electrolyte Solutions | Calculated Kaw (cm) | Reference |
|---|---|---|---|---|---|---|
| PFOA | 0.4 | N/A | N/A | N/A | 0.064 ± 0.048 | [212] |
| 10 | 0.06996 a | 0.023 M, NaHCO3, CaSO4·2H2O, MgSO4 and KCl | N/A | 0.0086 | [184] | |
| 1 | 0.06982 a | 0.01 M NaCl | 0.07022 b | 0.0027 | [176] | |
| 1 | 0.07076 b | 0.01 M NaCl | 0.07106 b | 0.002 ± 0.0005 | [187] | |
| 1 | 71.96 a | N/A | N/A | 0.00274 | [205] | |
| 1 | N/A | N/A | 71.77 b | 0.00285 | [187] | |
| 2.41 | 74.69 b | N/A | N/A | 0.0234 | [53] | |
| 0.01 | N/A | 0.01 M NaCl | 71.48 b | 0.0032 | [209] | |
| 10 | N/A | 0.01 M NaCl | 69.45 b | 0.0037 | [210] | |
| 0.0068 | N/A | 0.0015 M NaCl | 72.96 b | 0.00193 | [211] | |
| 0.0068 | N/A | 0.0015 M CaC2 | 72.90 b | 0.00529 | ||
| 0.0068 | N/A | 0.01 M NaCl | 72.80 b | 0.00536 | ||
| 0.0068 | N/A | 0.01 M CaCl2 | 72.86 b | 0.00662 | ||
| 0.0068 | N/A | 0.03 M NaCl | 72.73 b | 0.00771 | ||
| 0.0068 | N/A | 0.03 M CaCl2 | 72.71 b | 0.00830 | ||
| Na-PFOA | 1 | 73.39 b | deionized water | N/A | 0.00023 | [206] |
| 0.1 | 72.56 b | deionized water | N/A | 0.00023 | ||
| 0.1 | N/A | 0.01 M NaCl | 71.75 b | 0.0017 | ||
| 0.1 | N/A | 0.1 M NaCl | 71.12 b | 0.0087 | ||
| 0.1 | N/A | 0.01 M KCl | 72.38 b | 0.0015 | ||
| 0.1 | N/A | 0.01 M CaCl2 | 72.38 b | 0.017 | ||
| 0.1 | 72.29 a | N/A | N/A | 0.006 | ||
| PFOS | 0.01 | 72.68 a | 0.01 M NaCl | 72.15 b | N/A | |
| 1 | 0.07409 b | deionized water | N/A | 0.0076 | [168] | |
| 10 | 0.07271 b | deionized water | N/A | 0.0007 | ||
| 1 | 67.42 a | 0.01 M NaCl | 69.33 b | 0.02 | [176] | |
| 10 | 60.81 a | 0.023 M NaHCO3, CaSO4·2H2O, MgSO4 and KCl | N/A | 0.0129 | [184] | |
| 9.8 | N/A | 0.01 M NaCl | N/A | 0.18 ± 0.029 | [212] | |
| 1.99 | 74.69 b | N/A | N/A | 0.0755 | [53] | |
| 1 | N/A | 0.01 M NaCl | 70.76 b | 0.027 | [210] | |
| 0.01 | N/A | 0.001 M NaCl | 71.85 b | 0.033 | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Kelso, C.; Hai, F. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water 2025, 17, 1401. https://doi.org/10.3390/w17091401
Fatima M, Kelso C, Hai F. Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water. 2025; 17(9):1401. https://doi.org/10.3390/w17091401
Chicago/Turabian StyleFatima, Mehak, Celine Kelso, and Faisal Hai. 2025. "Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater" Water 17, no. 9: 1401. https://doi.org/10.3390/w17091401
APA StyleFatima, M., Kelso, C., & Hai, F. (2025). Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) Adsorption onto Different Adsorbents: A Critical Review of the Impact of Their Chemical Structure and Retention Mechanisms in Soil and Groundwater. Water, 17(9), 1401. https://doi.org/10.3390/w17091401










