Application Potential of Sulfur-Based Autotrophic Denitrification in Low Carbon Wastewater Treatment: Efficiency, Cost and Greenhouse Gas Emission Reduction
Abstract
1. Introduction
2. Metabolic Mechanisms and Efficiency of SAD
2.1. Electron Donor Metabolic Mechanisms and Key Microbial Differences of SAD
2.2. Efficiency of SAD Compared to Conventional HD Processes
2.3. Efficiency of SAD Compared to Other Autotrophic Denitrification Processes
2.4. Denitrification Performance and Kinetics
3. Costs and Strategies to Improve the Economic Efficiency of SAD
3.1. Comparison with the Cost of the Traditional HD Process
| Process/System Type | Electron Donor/Carbon Source | Reported Operating Cost (USD/m3) | Notes | Unit Denitrification Cost (USD/m3) | Refs. |
|---|---|---|---|---|---|
| SAD filter | Elemental sulfur | 0.016 | The cost based on the consumables of HD was 2.23 times higher than SAD | 0.16 | [42] |
| HD filter | Acetic acid byproduct | 0.037 | 0.37 | ||
| SAD (lab) | Elemental sulfur | 0.41 | Real wastewater tested | 4.1 | [16] |
| Pyrite-assisted SAD (PSAD) biofilter | Pyrite | 0.34 | Lower cost due to pyrite price & reduced chemicals | 3.4 | |
| HD biofilter | CH3COONa | 2.04 | Typical in municipal WWTPs | 20.4 | |
| SAD filter | Elemental sulfur | 0.009 | 0.09 | [68] | |
| HD filter | Sodium acetate | 0.057 | 0.57 | ||
| SAD filter | Autotrophic denitrifying filter media | 0.029 | SAD filters save approximately 37.9% in operating costs compared to HD filters | 0.29 | [69] |
3.2. Strategies to Improve the Economic Efficiency of SAD
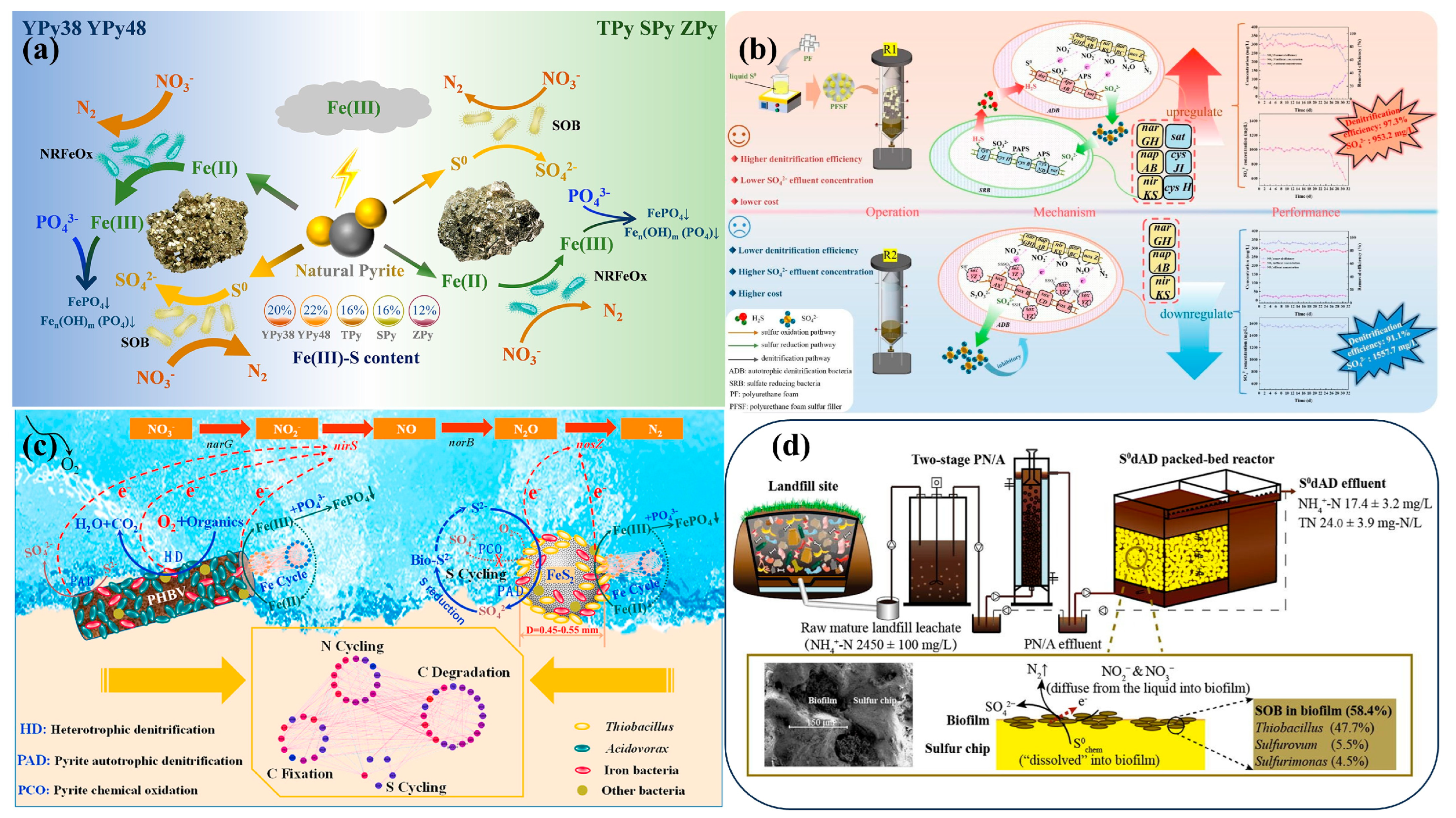
3.3. Integrated Testing and Optimization Strategies for Process Enhancement and Scale-Up of SAD
4. GHG Emission Reduction Effects of SAD
4.1. GHG Reduction Effects
| Process Type | Electron Donor | N2O Emission (% of Nitrate Load) | Carbon Emission (g/m3) | Refs. |
|---|---|---|---|---|
| HD | Methanol | - | 35.48 | [86] |
| SAD | S0 | - | −14.19 | |
| S2O32− | - | −12.31 | ||
| FeS2 | - | −40.16 | ||
| HD | Methanol | 3.01 | - | [87,88,89] |
| Acetate | 2.3–19.91 | - | ||
| SAD | Sulfur | 0.01−0.8 | - |
4.2. Environmental Benefits Assessment of SAD
4.3. Sustainability Discussions from a Life Cycle Perspective
5. Conclusions and Prospects
- (1)
- Future research should further explore the metabolic mechanism and community synergy of sulfur-oxidizing functional microorganisms (such as Thiobacillus and Thiomonas) and optimize the bacterial community structure through microbial ecological engineering methods to improve system stability and nitrogen removal efficiency.
- (2)
- To address issues such as low mass transfer efficiency and sulfur media clogging, there is an urgent need to develop composite sulfur carriers with high specific surface area, controlled-release properties, and excellent mass transfer performance. This will enhance electron transfer processes and effectively suppress sulfate accumulation.
- (3)
- To resolve alkalinity imbalance and microbial community instability, promote deep integration of SAD with low-carbon-consumption processes like anaerobic ammonium oxidation (ANAMMOX), establishing SAD–ANAMMOX synergistic systems to achieve self-balancing alkalinity and microbial ecological regulation, thereby enhancing system resilience to disturbances.
- (4)
- Pilot-scale and large-scale engineering studies should be intensified to establish intelligent control strategies based on real-time water quality and operational characteristics. This will drive the SAD process toward modularization, standardization, and intelligent upgrades, providing reliable support for its widespread application.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, X.; Zhang, C.; Zhang, L.; Zhang, R.; Chen, G.; Hu, W. Thoughts on the development direction of sludge treatment and resource recovery under the background of carbon neutrality. Water Wastewater Eng. 2021, 47, 1–5. [Google Scholar] [CrossRef]
- Song, X.; Li, P.; Zhang, B.; Yu, K.; Zhang, D.; He, Y. Realization approaches for constructing energy self-sufficient wastewater treatment plants: A review. Carbon Neutrality 2025, 4, 21. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Yang, W.; Wei, Y.; Li, W. Denitrification Performance and Microbiological Mechanisms Using Polyglycolic Acid as a Carbon Source. Water 2024, 16, 1277. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, V.; Cheng, L.; Hussain, A.; Ormeci, B. Nitrogen removal from wastewater: A comprehensive review of biological nitrogen removal processes, critical operation parameters and bioreactor design. J. Environ. Chem. Eng. 2022, 10, 107387. [Google Scholar] [CrossRef]
- Huang, X.; Guida, S.; Jefferson, B.; Soares, A. Economic evaluation of ion-exchange processes for nutrient removal and recovery from municipal wastewater. NPJ Clean Water 2020, 3, 7. [Google Scholar] [CrossRef]
- Choi, B.; Kim, T.-I.; Kim, H.H.; Kim, C.-M.; Park, S.; Lee, S. Enhancing energy and nitrogen removal efficiency through automatic split injection and innovative aeration device: A study of low C/N ratio environments. Water Res. 2024, 266, 122389. [Google Scholar] [CrossRef] [PubMed]
- Brozinčević, A.; Grgas, D.; Štefanac, T.; Habuda-Stanić, M.; Zelić, B.; Landeka Dragičević, T. Cost Reduction in the Process of Biological Denitrification by Choosing Traditional or Alternative Carbon Sources. Energies 2024, 17, 3660. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Y. A review on effect mechanism of sulfur/iron-based electron donors on denitrification in constructed wetlands. J. Environ. Chem. Eng. 2025, 13, 118684. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Li, T. Research progress on the microbial technology of nitrogen transformation from low carbon wastewater. Ind. Water Treat. 2022, 42, 22–32. [Google Scholar] [CrossRef]
- Carranza Muñoz, A.; Malovanyy, A.; Singh, A.; Baresel, C.; Karlsson, J.; Stark-Fujii, K.; Schnürer, A. Replacing methanol with internally produced VFA-based carbon source for denitrification at the Henriksdal WWTP. Water Sci. Technol. 2025, 92, 139–152. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Rind, S.; Rani, K. Systematic review: External carbon source for biological denitrification for wastewater. Biotechnol. Bioeng. 2022, 120, 642–658. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Wang, H.; Xue, G.; Zhou, M.; Ran, X.; Wang, Y. Sulfur autotrophic denitrification as an efficient nitrogen removals method for wastewater treatment towards lower organic requirement: A review. Water Res. 2023, 245, 120569. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Xing, L.; Hu, G.; Li, Z.; Liu, B.; Han, Y.; Liu, Y.; Li, G. Research progress on advanced treatment of municipal sewage by sulfur autotrophic denitrification. Appl. Chem. Ind. 2024, 53, 699–702, 707. [Google Scholar] [CrossRef]
- Hao, X.; Wei, H.; Yu, W.; Zhu, Y. Analysis of the pros and cons for sulfur autotrophic denitrification technology. Acta Sci. Circumstantiae 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wang, C.; Xu, T.; Liu, W.; Chen, Q.; Liu, X.; Zhang, T.; Gao, X.; Zhao, C. Pyrite and Sulfur Autotrophic Denitrification for Simultaneous Nitrogen and Phosphorus Removal and Greenhouse Gas Emission Reduction Potential. Environ. Eng. Sci. 2024, 41, 347–357. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Tang, S.; Fan, M.; Yang, H.; Ma, Z.; Guan, Y.; Li, R. Re-analysis of the pros and cons for sulfur autotrophic denitrification technology. Environ. Chem. 2025, 45, 1–18. Available online: https://www.sciengine.com/EC/doi/10.7524/j.issn.0254-6108.2025010306 (accessed on 30 September 2025). [CrossRef]
- Koju, R.; Cheng, Y.; Gao, S.; Joshi, T.P.; Hu, C.; Yang, M.; Qu, J. High-performance and short-process sulphur autotrophic denitrification from low-C/N wastewater using novel suspended bio-S0 filters. J. Clean. Prod. 2025, 495, 145083. [Google Scholar] [CrossRef]
- Wang, N.; Bai, S.; Zhang, Y.-n.; Xu, D.; Zhang, Q.; Wang, M.; Zhong, Y. Sulfur autotrophic denitrification as a sustainable nitrogen removal technology to achieve carbon neutrality: Recent advances and optimization strategies. J. Water Process Eng. 2025, 70, 107154. [Google Scholar] [CrossRef]
- Wang, H.-C.; Liu, Y.; Yang, Y.-M.; Fang, Y.-K.; Luo, S.; Cheng, H.-Y.; Wang, A.-J. Element sulfur-based autotrophic denitrification constructed wetland as an efficient approach for nitrogen removal from low C/N wastewater. Water Res. 2022, 226, 119258. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Chen, N.; Peng, T.; Dong, S.; Feng, C. Denitrification performance and mechanism of biofilter constructed with sulfur autotrophic denitrification composite filler in engineering application. Bioresour. Technol. 2021, 340, 125699. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Zhou, Y.; Wang, B.; Wang, S.; Li, J. Response characteristics of the microbial community, metabolic pathways, and anti-resistance genes under high nitrate and sulfamethoxazole stress in a fluidized sulfur autotrophic denitrification process. Bioresour. Technol. 2025, 425, 132310. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Z.; Liang, H.; Du, Z.; Zhang, Y.; Lu, H.; Peng, Y. Metagenomic insights into nitrite accumulation in sulfur-based denitrification systems utilizing different electron donors: Functional microbial communities and metabolic mechanisms. Water Res. 2025, 270, 122805. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.H.; Wang, Q.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Efficient bio-refractory industrial wastewater treatment with mitigated membrane fouling in a membrane bioreactor strengthened by the micro-scale ZVI@GAC galvanic-cells-initiated radical generation and coagulation processes. Water Res. 2022, 209, 117943. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Li, D.S.; Cui, Y.W.; Xing, W.; Deng, S.H. Micro-electrolysis/retinervus luffae-based simultaneous autotrophic and heterotrophic denitrification for low C/N wastewater treatment. Environ. Sci. Pollut. Res. 2017, 24, 16651–16658. [Google Scholar] [CrossRef]
- Cui, P.; Wan, N.; Li, C.; Zou, L.; Ma, M.; Du, J.; Jiang, Y. Comparative analysis of sulfur-driven autotrophic denitrification for pilot-scale application: Pollutant removal performance and metagenomic function. Bioresour. Technol. 2024, 413, 131433. [Google Scholar] [CrossRef]
- Guo, G.; Li, Z.; Chen, L.; Ling, Q.; Zan, F.; Isawi, H.; Hao, T.; Ma, J.; Wang, Z.; Chen, G.; et al. Advances in elemental sulfur-driven bioprocesses for wastewater treatment: From metabolic study to application. Water Res. 2022, 213, 118143. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in Research Into and Applications of Heterotrophic Nitrifying and Aerobic Denitrifying Microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Shao, L.; Wang, D.; Chen, G.; Zhao, X.; Fan, L. Advance in the sulfur-based electron donor autotrophic denitrification for nitrate nitrogen removal from wastewater. World J. Microbiol. Biotechnol. 2023, 40, 7. [Google Scholar] [CrossRef]
- Li, R.; Lu, M.-Y.; Guo, R.-B.; Duan, H.; Ni, B.-J.; Fu, S.-F. Life cycle assessment of hydrogenotrophic denitrification in membrane aerated biofilm reactors for sustainable wastewater treatment. Water Res. 2024, 267, 122529. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y.; Zhang, J.; Dai, X.; Li, H.; Zhang, X.; Wu, Z.; Zheng, J. Model Evaluation of the Microbial Metabolic Processes in a Hydrogen-Based Membrane Biofilm Reactor for Simultaneous Bromate and Nitrate Reduction. Membranes 2022, 12, 774. [Google Scholar] [CrossRef]
- You, N.; Deng, S.H.; He, H.Y.; Hu, J.Y. Ferromanganese oxide-functionalized TiO2 for rapid catalytic ozonation of PPCPs through a coordinated oxidation process with adjusted composition and strengthened generation of reactive oxygen species. Water Res. 2024, 258, 121813. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.B.; Yao, H.; Li, X.Y.; Deng, S.H.; Zhao, S.L.; Zhang, W. Enhanced Degradation of Sulfamethoxazole (SMX) in Toilet Wastewater by Photo-Fenton Reactive Membrane Filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Yang, S.; He, Z.; Li, Y.; Wang, Y.; Li, J. Iron-dependent autotrophic denitrification as a novel microbial driven and iron-mediated denitrification process: A critical review. Environ. Res. 2025, 273, 120808. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Meng, J.; Hu, Y.; Lee, P.-H.; Zhan, X. A review in Fe(0)/Fe(II) mediated autotrophic denitrification for low C/N wastewater treatment. Water Res. 2025, 282, 123925. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, H.; Lee, P.-H.; Zhussupbekova, A.; Shvets, I.V.; Du, B.; Terada, A.; Zhan, X. Nitrate removal in iron sulfide-driven autotrophic denitrification biofilter: Biochemical and chemical transformation pathways and its underlying microbial mechanism. Sci. Total Environ. 2023, 901, 165908. [Google Scholar] [CrossRef]
- Li, H.; Sun, R.; Zhang, X.; Lin, H.; Xie, Y.; Han, Y.; Pan, Y.; Wang, D.; Dong, K. Characteristics of denitrification and microbial community in respect to various H2 pressures and distances to the gas supply end in H2-based MBfR. Front. Microbiol. 2022, 13, 1023402. [Google Scholar] [CrossRef]
- Chen, Z.; Lou, M.; Fang, P.; Xiao, D.; Zhu, W.; Chen, H.; Qian, W. Impact of different sulfur sources on the structure and function of sulfur autotrophic denitrification bacteria. Sci. Rep. 2023, 13, 19404. [Google Scholar] [CrossRef]
- Xu, B.; Yang, X.; Li, Y.; Yang, K.; Xiong, Y.; Yuan, N. Pyrite-Based Autotrophic Denitrifying Microorganisms Derived from Paddy Soils: Effects of Organic Co-Substrate Addition. Int. J. Environ. Res. Public Health 2022, 19, 11763. [Google Scholar] [CrossRef]
- Xu, J.; Lu, Z.; Xu, Y.; Liang, C.; Peng, L. Improved Performance of Sulfur-Driven Autotrophic Denitrification Process by Regulating Sulfur-Based Electron Donors. Water 2024, 16, 730. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Huang, Y.; Zhu, L.; Yang, P.-b.; Zhu, Q. Characteristics of a Combined Heterotrophic and Sulfur Autotrophic Denitrification Technology for Removal of High Nitrate in Water. Huanjing Kexue 2016, 37, 2646–2651. [Google Scholar] [CrossRef]
- Wang, S.-S.; Cheng, H.-Y.; Zhang, H.; Su, S.-G.; Sun, Y.-L.; Wang, H.-C.; Han, J.-L.; Wang, A.-J.; Guadie, A. Sulfur autotrophic denitrification filter and heterotrophic denitrification filter: Comparison on denitrification performance, hydrodynamic characteristics and operating cost. Environ. Res. 2021, 197, 111029. [Google Scholar] [CrossRef]
- Klaus, S.; Campolong, C.; Rosenthal, A.; Sabba, F.; Baideme, M.; Wells, G.; De Clippeleir, H.; Chandran, K.; Bott, C. Comparison of carbon sources in a partial denitrification/anammox MBBR using glycerol, acetate, and methanol. Environ. Sci. Water Res. Technol. 2023, 9, 1041–1052. [Google Scholar] [CrossRef]
- Moghaddam, R.; Barkle, G.; Rivas, A.; Torres-Rojas, D.; Schipper, L. Constant carbon dosing of a pilot-scale denitrifying bioreactor to improve nitrate removal from agricultural tile drainage. Ecol. Eng. 2023, 187, 106851. [Google Scholar] [CrossRef]
- Farmer, M.; Sabba, F.; Wells, G. Nitrous oxide production, truncated denitrification pathways, and omics-informed process modeling in a denitrifying phosphorus removal bioprocess. Water Res. X 2025, 29, 100391. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Yan, G.; Dong, W.; Chu, Z.; Wang, H.; Chang, Y.; Ling, Y.; Zhang, Y. Initial carbon release characteristics, mechanisms and denitrification performance of a novel slow release carbon source. J. Environ. Sci. 2022, 118, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Li, Z.R.; Zhang, X.N.; Dong, H.; Qian, Z.M.; Yi, S.; Zhuang, W.Q.; Cheng, H.Y.; Wang, A.J. Design and operation insights concerning a pilot-scale S0-driven autotrophic denitrification packed-bed process. Chem. Eng. J. 2023, 470, 144396. [Google Scholar] [CrossRef]
- Li, W.; Nan, G.; Shang, J. Effect of sulfur autotrophic denitrification constructed wetland on nitrogen removal in tailwater of WWTP. Water Purif. Technol. 2023, 42, 94–100. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, C.; Li, N.; Zou, P.; Gao, X.; Cao, Q. Nitrogen removal performances and metabolic mechanisms of denitrification systems using different volatile fatty acids as external carbon sources. Chem. Eng. J. 2023, 474, 145998. [Google Scholar] [CrossRef]
- Hu, J.; Tian, J.; Deng, X.; Liu, X.; Zhou, F.; Yu, J.; Chi, R.; Xiao, C. Heterotrophic nitrification processes driven by glucose and sodium acetate: New insights into microbial communities, functional genes and nitrogen metabolism from metagenomics and metabolomics. Bioresour. Technol. 2024, 408, 131226. [Google Scholar] [CrossRef]
- Farmer, M.; Sabba, F.; Wells, G. Nitrous oxide production, mechanisms, and modeling from a denitrifying phosphorus removal bioreactor. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, B.; Kang, F.; Wang, Y.; Yuan, Z.; Lyu, Z.; Zhu, T.; Zhang, Z. Denitrification Performance in Packed-Bed Reactors Using Novel Carbon-Sulfur-Based Composite Filters for Treatment of Synthetic Wastewater and Anaerobic Ammonia Oxidation Effluent. Front. Microbiol. 2022, 13, 934441. [Google Scholar] [CrossRef]
- Barkhordari, D.; Mathew, J.; Haroun, B.; Rehmann, L.; Murthy, S.; Santoro, D. Wastewater Denitrification with Solid-Phase Carbon: A Sustainable Alternative to Conventional Electron Donors. Nitrogen 2025, 6, 22. [Google Scholar] [CrossRef]
- Li, Y.; Han, Q.; Li, B. Engineering-scale application of sulfur-driven autotrophic denitrification wetland for advanced treatment of municipal tailwater. Bioresour. Technol. 2023, 379, 129035. [Google Scholar] [CrossRef]
- Han, Y.; Yang, P.; Feng, Y.; Wang, N.; Yuan, X.; An, J.; Liu, J.; Li, N.; He, W. Liquid-gas phase transition enables microbial electrolysis and H2-based membrane biofilm hybrid system to degrade organic pollution and achieve effective hydrogenotrophic denitrification of groundwater. Chemosphere 2023, 331, 138819. [Google Scholar] [CrossRef] [PubMed]
- Thant, K.J.W.; Anh-Vu, N.; Yun-Je, K.; Masumi, K.; Visvanathan, C. Performance of pilot-scale membrane aerated biofilm reactors integrated with anoxic nano-biotechnological reactor for domestic wastewater treatment. Chemosphere 2023, 319, 137927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, L.; Cui, C.; Lindeboom, R.E.F. High nitrite accumulation in hydrogenotrophic denitrification at low temperature: Transcriptional regulation and microbial community succession. Water Res. 2024, 263, 122144. [Google Scholar] [CrossRef]
- Ntagia, E.; Lens, P. Pyrite-based denitrification combined with electrochemical disinfection to remove nitrate and microbial contamination from groundwater. NPJ Clean Water 2023, 6, 59. [Google Scholar] [CrossRef]
- Hu, Z.F.; Yao, H.; Deng, S.H.; Zhang, C.; Peng, S.; Zhang, Z.G.; Li, D.S. Iron [Fe(0)]-carbon micro-electrolysis enhances simultaneous nitrogen and phosphorus removal in vertical flow constructed wetlands for advanced treatment of reclaimed water. J. Environ. Manag. 2023, 335, 117528. [Google Scholar] [CrossRef]
- Hu, Z.F.; Li, D.S.; Deng, S.H.; Liu, Y.H.; Ma, C.Y.; Zhang, C. Combination with catalyzed Fe(0)-carbon microelectrolysis and activated carbon adsorption for advanced reclaimed water treatment: Simultaneous nitrate and biorefractory organics removal. Environ. Sci. Pollut. Res. 2019, 26, 5693–5703. [Google Scholar] [CrossRef]
- Dong, K.; Feng, X.; Yao, Y.; Zhu, Z.; Lin, H.; Zhang, X.; Wang, D.; Li, H. Nitrogen Removal From Nitrate-Containing Wastewaters in Hydrogen-Based Membrane Biofilm Reactors via Hydrogen Autotrophic Denitrification: Biofilm Structure, Microbial Community and Optimization Strategies. Front. Microbiol. 2022, 13, 924084. [Google Scholar] [CrossRef]
- Huiliñir, C.; Acosta, L.; Yánez Sevilla, D.; Montalvo, S.; Esposito, G.; Retamal-Morales, G.; Levican, G.; Guerrero, L. Elemental sulfur-based autotrophic denitrification in stoichiometric S0/N ratio: Calibration and validation of a kinetic model. Bioresour. Technol. 2020, 307, 123229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Yang, X.; Ren, Z.; Huang, K.; Qian, F.; Li, J. Long-Term Operation of a Pilot-Scale Sulfur-Based Autotrophic Denitrification System for Deep Nitrogen Removal. Water 2023, 15, 428. [Google Scholar] [CrossRef]
- Yao, S.; Liu, L.; Zhang, S.; Tang, X. Nitrate Removal from Groundwater by Heterotrophic and Electro-Autotrophic Denitrification. Water 2022, 14, 1759. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 2022, 817, 153061. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron donors for autotrophic denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Carboni, M.F.; Florentino, A.P.; Costa, R.B.; Zhan, X.; Lens, P.N.L. Enrichment of Autotrophic Denitrifiers From Anaerobic Sludge Using Sulfurous Electron Donors. Front. Microbiol. 2021, 12, 678323. [Google Scholar] [CrossRef]
- Liu, B.; Guo, Y. Application of Sulfur Autotrophic Denitrification Technology in Advanced Treatment of Municipal Sewage. China Water Wastewater 2022, 38, 91–95. [Google Scholar] [CrossRef]
- Liang, S.; Yun, D.; Wang, Y.; Fu, M.; Xie, R.; Ashan, G.; Wang, F.; Li, S. Advanced Nitrogen Removal of Sulfur Autotrophic Denitrification Filter under Low Temperature in Winter: A Pilot-scale Study. China Water Wastewater 2025, 41, 14–21. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, G.; Li, R.; Xiao, L.; Zhan, X. Iron sulphides mediated autotrophic denitrification: An emerging bioprocess for nitrate pollution mitigation and sustainable wastewater treatment. Water Res. 2020, 179, 115914. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Dong, H.; Liu, L.; Zhou, C.; Du, Z.; Dang, Y.; Holmes, D.E. Magnetite-augmented sulfur-siderite autotrophic denitrification: Deep nitrogen removal at ultra-low HRT from lab to pilot scale. Water Res. 2025, 284, 124034. [Google Scholar] [CrossRef]
- Li, R.; Guan, M.; Wang, W. Simultaneous arsenite and nitrate removal from simulated groundwater based on pyrrhotite autotrophic denitrification. Water Res. 2021, 189, 116662. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, Q.; Li, Z.; Meng, G.; Liu, B.; Jiang, Y.; Li, S. Autotrophic denitrification by sulfur-based immobilized electron donor for enhanced nitrogen removal: Denitrification performance, microbial interspecific interaction and functional traits. Bioresour. Technol. 2024, 401, 130747. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, D.; Zhong, D.; Li, J.; Zhang, J.; Su, P.; Liu, X.; Dong, J.; Zhang, S.; Du, X. Study of nitrogen removal efficiency of the filled bed reactors using alkali-treated corncobs-sulfur (mixotrophic) for treating the effluent from simulated urban wastewater plants. Bioresour. Technol. 2022, 349, 126630. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Jin, D.; Wu, P. A critical review of sulfur autotrophic denitrification coupled with anammox. J. Environ. Manag. 2025, 383, 125417. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Liu, T.; Xu, T. Research status and prospect of sulfur autotrophic denitrification process. Ind. Water Treat. 2023, 43, 21–31. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, L.; Wu, W.; Wu, W. Introducing PHBV and controlling the pyrite sizes achieved the pyrite-based mixotrophic denitrification under natural aerobic conditions: Low sulfate production and functional microbe interaction. J. Clean. Prod. 2022, 366, 132986. [Google Scholar] [CrossRef]
- Zeng, C.; Su, Q.; Peng, L.; Sun, L.; Zhao, Q.; Diao, X.; Lu, H. Elemental sulfur-driven autotrophic denitrification for advanced nitrogen removal from mature landfill leachate after PN/A pretreatment. Chem. Eng. J. 2021, 410, 128256. [Google Scholar] [CrossRef]
- Strotmann, U.J.; Eismann, F.; Hauth, B.; Bias, W.R. An integrated test strategy for the assessment of anaerobic biodegradability of wastewaters. Chemosphere 1993, 26, 2241–2254. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, Z.; Wei, Q.; Han, I.; Jaffé, P.R. Performance of sulfur-based autotrophic denitrification and denitrifiers for wastewater treatment under acidic conditions. Bioresour. Technol. 2019, 294, 122176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Y.; Zuo, Y.; Huo, S.; Liang, C.; Hu, C. Integrated sulfur- and iron-based autotrophic denitrification process and microbial profiling in an anoxic fluidized-bed membrane bioreactor. Chemosphere 2019, 221, 375–382. [Google Scholar] [CrossRef]
- Deng, S.H.; Wang, C.Q.; Ngo, H.H.; Guo, W.S.; You, N.; Tang, H.; Yu, H.B.; Tang, L.; Han, J. Comparative review on microbial electrochemical technologies for resource recovery from wastewater towards circular economy and carbon neutrality. Bioresour. Technol. 2023, 376, 128906. [Google Scholar] [CrossRef]
- Zhu, T.; Ding, J.; Liu, Y.; Li, X.; Wang, Z.; Liu, Y. The effect of organic sources on the electron distribution and N2O emission in sulfur-driven autotrophic denitrification biofilters. Sci. Total Environ. 2023, 903, 166126. [Google Scholar] [CrossRef]
- Kim, B.; Baek, G.; Kim, C.; Lee, S.Y.; Yang, E.; Lee, S.; Kim, T.; Nam, J.-Y.; Lee, C.; Chae, K.-J.; et al. Progress and Prospects for Applications of Extracellular Electron Transport Mechanism in Environmental Biotechnology. ACS EST Eng. 2024, 4, 1520–1539. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, X.; Gao, G.; Ren, K.; Li, Z.; Xu, Z.; Wei, D.; Zhang, J. A new insight on simultaneous water purification and greenhouse gas reduction by constructing sulfur-siderite driven autotrophic denitrification pathways in constructed wetlands. Water Res. 2025, 274, 123130. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xi, Z.; Chen, Y.; Xu, W.; Sun, C.; Zhuang, W.; Zhang, T.; Li, D.; Pan, Y.; Li, Y.-Y.; et al. Biofiltration for low-carbon rural wastewater treatment: A review of advancements and opportunities towards carbon neutrality. J. Environ. Chem. Eng. 2024, 12, 114373. [Google Scholar] [CrossRef]
- Wang, J.-J.; Huang, B.-C.; Li, J.; Jin, R.-C. Advances and challenges of sulfur-driven autotrophic denitrification (SDAD) for nitrogen removal. Chin. Chem. Lett. 2020, 31, 2567–2574. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, X.; Xue, M.; Chen, J.; Li, S. Evaluation of nitrogen removal and N2O emission in a novel anammox coupled with sulfite-driven autotrophic denitrification system: Influence of pH. J. Clean. Prod. 2021, 321, 128984. [Google Scholar] [CrossRef]
- Cui, Y.-X.; Biswal, B.K.; Guo, G.; Deng, Y.-F.; Huang, H.; Chen, G.-H.; Wu, D. Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 2019, 103, 6023–6039. [Google Scholar] [CrossRef]
- Xue, M.; Nie, Y.; Cao, X.; Zhou, X. Deciphering the influence of S/N ratio in a sulfite-driven autotrophic denitrification reactor. Sci. Total Environ. 2022, 836, 155612. [Google Scholar] [CrossRef]
- Lv, Y.-T.; Zhang, X.; Zhu, C.; Lin, L.; Sun, T.; Wang, X.; Wang, L. Micro-analysis of nitrous oxide accumulation in denitrification under acidic conditions: The role of pH and free nitrous acid. J. Water Process Eng. 2022, 47, 102767. [Google Scholar] [CrossRef]
- Lin, X.; Li, B.; Tian, M.; Li, X.; Wang, J. Denitrification effect and strengthening mechanism of SAD/A system at low temperature by gel-immobilization technology. Sci. Total Environ. 2023, 900, 165599. [Google Scholar] [CrossRef]
- Yang, J.; Fang, F.; Chen, L.; Weng, J. Review of heterotrophic-sulfur autotrophic synergistic denitrification technology. Appl. Chem. Ind. 2024, 53, 1377–1382. [Google Scholar] [CrossRef]
- Tian, T.; Yu, H.-Q. Denitrification with non-organic electron donor for treating low C/N ratio wastewaters. Bioresour. Technol. 2020, 299, 122686. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, J. Sulfur-based mixotrophic denitrification: A promising approach for nitrogen removal from low C/N ratio wastewater. Sci. Total Environ. 2024, 957, 177419. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, M.; Huang, G.; Li, R. Foam FeSO4 modified limestone sulfur concrete for non-stink and high-rate nitrogen and phosphorus removal from wastewater. Water Res. 2025, 271, 122996. [Google Scholar] [CrossRef]
- Wang, F.; Liang, D.; He, W.; Liu, G.; Li, J.; Tian, Y.; Feng, Y. Insights into sulfur cycling contribution to nitrogen removal in sulfur autotrophic denitrification combined microbial electrochemical denitrification. Chem. Eng. J. 2024, 482, 148882. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, K.; Kong, L.; Liu, S. Research progress and prospect of low-carbon biological technology for nitrate removal in wastewater treatment. Front. Environ. Sci. Eng. 2024, 18, 80. [Google Scholar] [CrossRef]
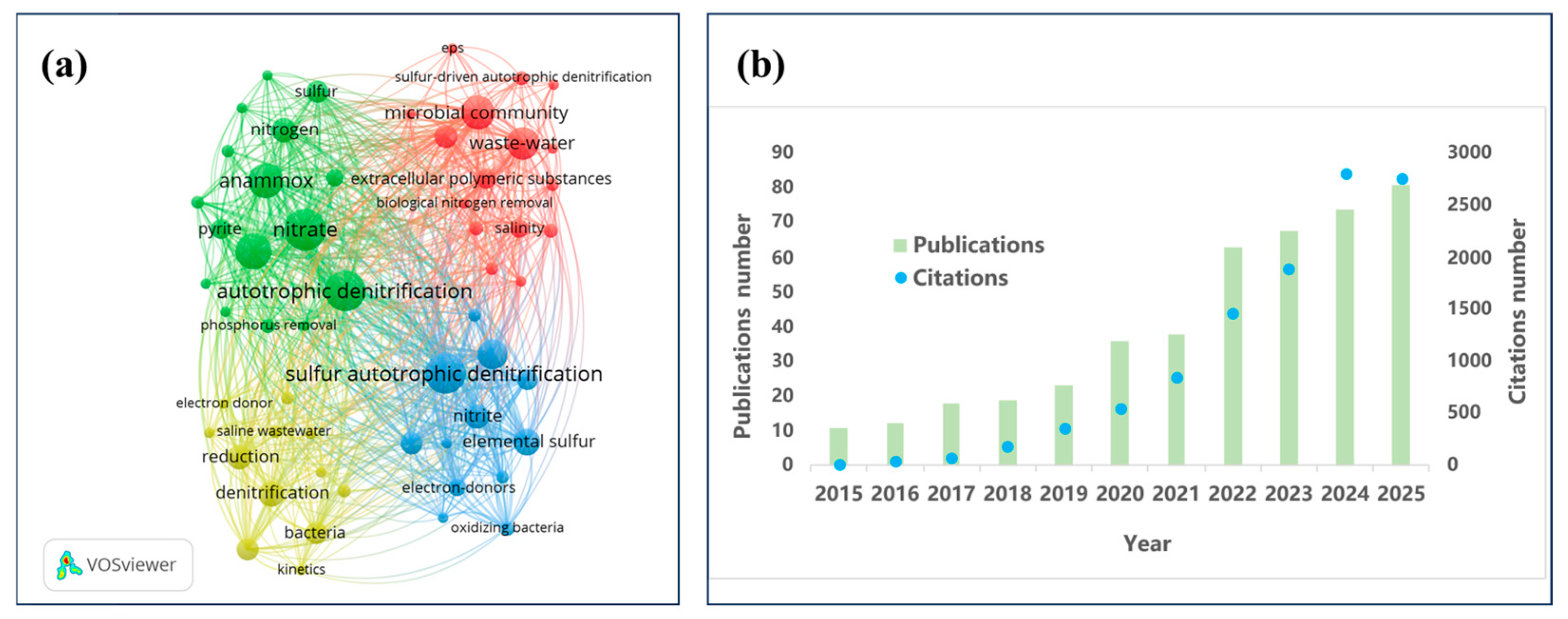
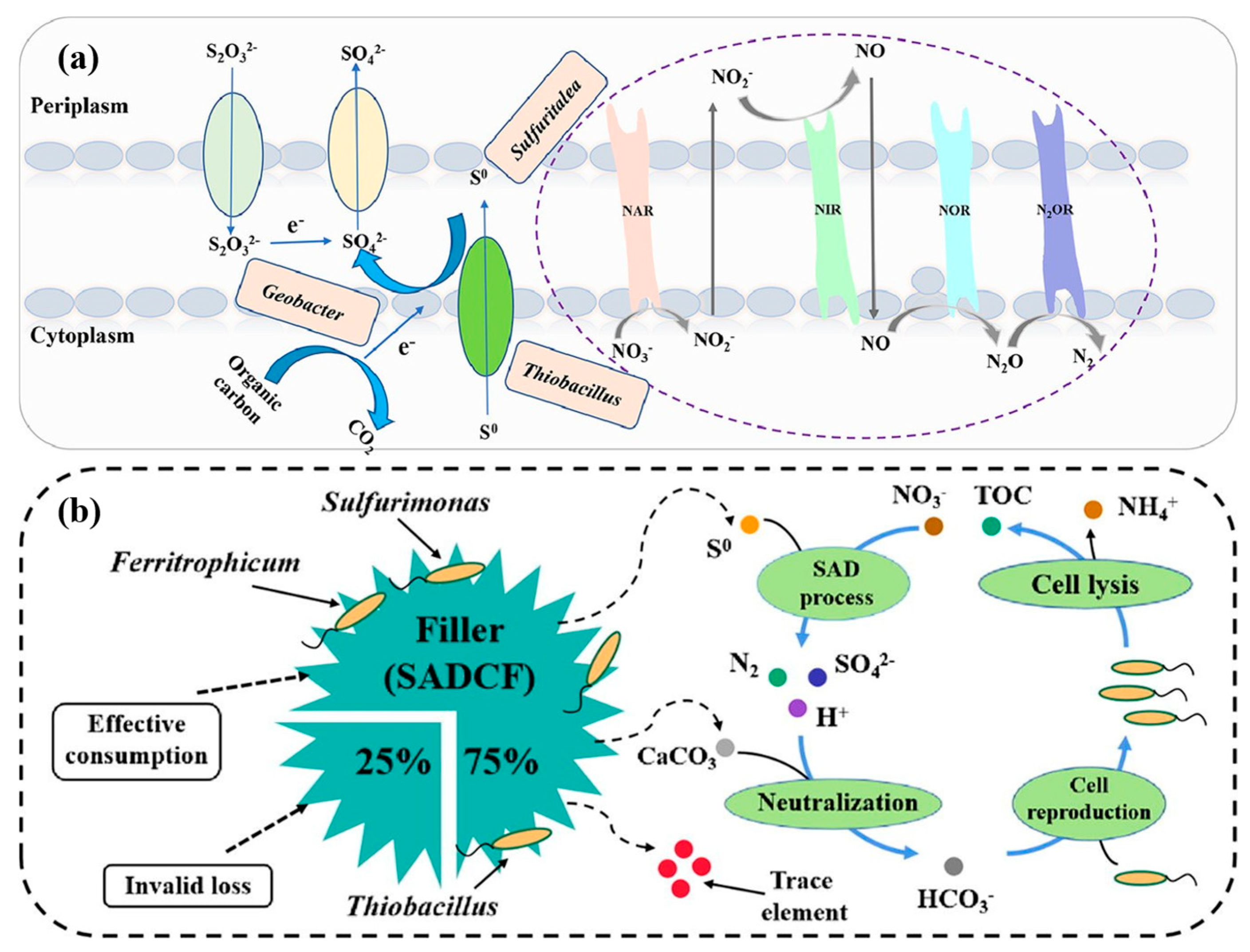
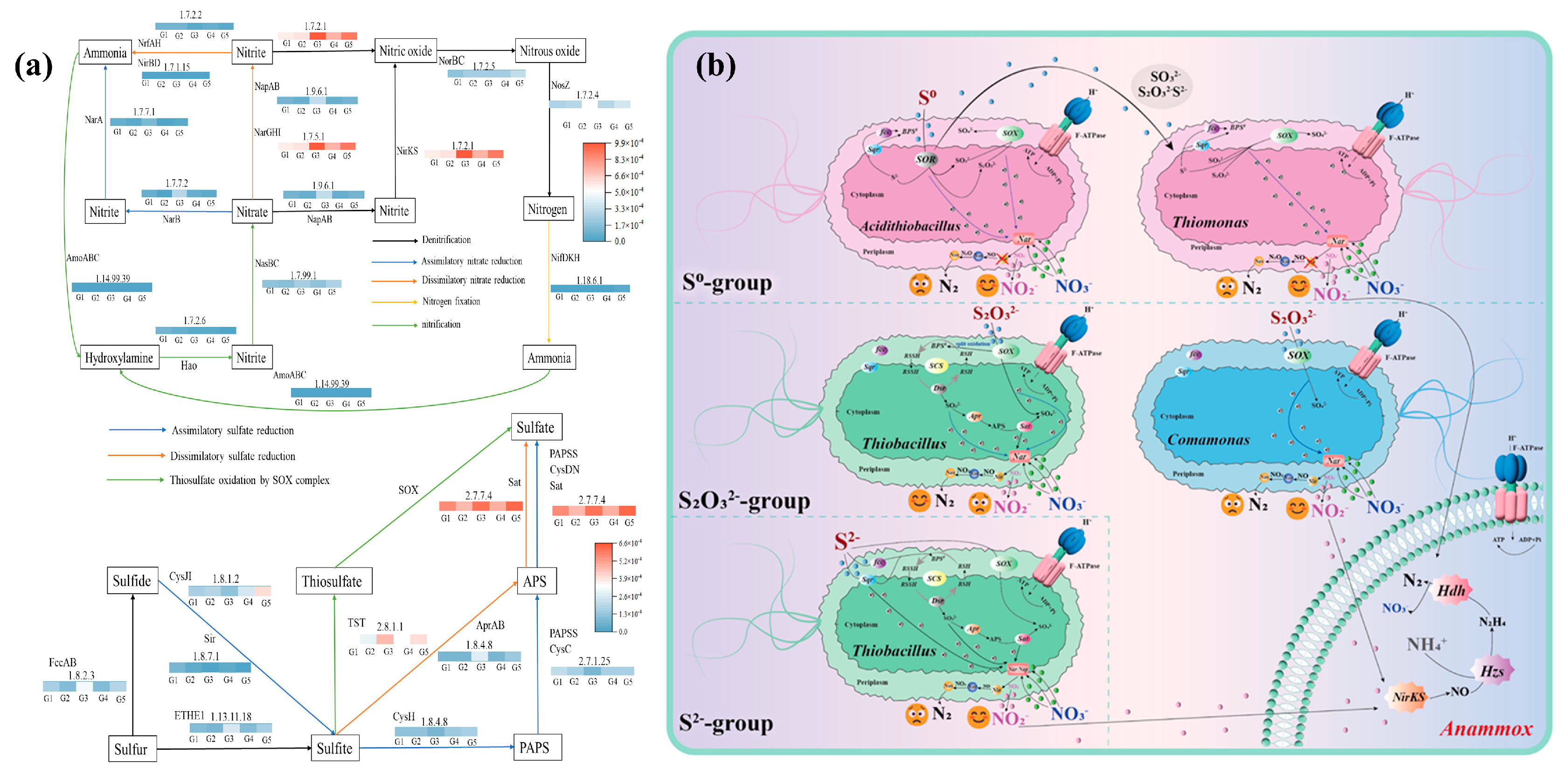
| Type | Representative Functional Genera | Dominant Phyla | Environmental Preferences/Sensitivities | Refs. |
|---|---|---|---|---|
| HD | Pseudomonas, Paracoccus, Acinetobacter, Bacillus | Proteobacteria, Firmicutes | Fast growth, requires sufficient organic carbon, highly sensitive to DO and temperature changes | [28] |
| IAD | Gallionella (Gallionellaceae), Acidovorax, Dechloromonas, Thiobacillus | Proteobacteria, Nitrospirae | Kinetics generally slower; strongly affected by mineral dissolution/passivation, pH, alkalinity; requires longer HRT | [34] |
| HAD | Hydrogenophaga, Paracoccus, Thauera, Pseudomonas | Proteobacteria, Bacteroidetes | Sensitive to gas–liquid mass transfer; temperature-sensitive; prone to NO2− accumulation at low temperature | [37] |
| SAD | Thiobacillus, Sulfurimonas, Sulfuricurvum, Sulfuricella | Proteobacteria | Tolerant to salinity and sulfate stress; diverse sulfur-oxidizing pathways; performs well under low C/N conditions | [38] |
| Type | Carbon Source | Applicable Scenarios | Representative Removal Efficiency | Representative Volumetric Rate/Load | Operational Considerations/By-Products | Refs. |
|---|---|---|---|---|---|---|
| HD | Methanol | Municipal plant post-denitrification, media bed/biological filter | Typically, 90–99% removal under proper dosing | Pilot reports show instantaneous rates from 0.5 up to 1.4 kg N·m−3·d−1 in optimized systems or startup peaks | Note the flammability risks and safety requirements; over-injection may cause COD levels in effluent to exceed standards and pose N2O risks | [11,44] |
| Acetate/VFAs | Tertiary/post denitrification, SBRs, packed beds | Very high >90% NO3− removal; faster kinetics and higher specific denitrification rates than methanol | Higher specific denitrification rates reported vs. methanol; Volumetric rates depend on HRT/biomass | Fast kinetics, can be prepared on-site, requires fermentation equipment/raw materials, ingredient fluctuations, over-dosage may leave residual COD | [10,49] | |
| Glucose | Primarily used for laboratory mechanism research and certain pilot projects | Achieves high nitrate removal ≈85–95% (under appropriate C/N ratio and HRT) | Initial/instantaneous rates are high (easily assimilated by microorganisms), but long-term rates depend on community succession | High instantaneous rates require long-term monitoring. Nitrite/N2O issues are prone to occur in high-load/short HRT systems | [50,51] | |
| Starch/SRC | Decentralized/remote wastewater treatment stations, aquaculture water polishing | Long-term removal efficiency typically ranges from 70–95% | Instantaneous volumetric flow rates are generally lower than for soluble small-molecule carbon, exhibiting lower peaks and long-term stability | Release rates depend on material, temperature, and microbes; show initial lag; need replacement; risk clogging, local anaerobia, and short COD spikes | [52,53] | |
| SAD | - | Low C/N municipal secondary effluents for advanced nitrogen removal; saline/brackish groundwater | In most studies, NO3−-N removal >90%; Constructed wetlands and filters treating real/simulated tailwater reach 96% | Fixed bed/packed filter: peak 164 g NO3−-N·m−3·h−1 (≈3.9 kg N ·m−3·d−1); long-term maximum load ≈ 1158 mg·L−1·d−1(≈1.158 kg N·m−3·d−1); stable even at short HRT | Manage sulfate formation and alkalinity consumption; anti-clogging and backwashing to maintain hydraulics; suppress H2S accumulation | [12] |
| Type | Applicable Scenarios | Representative Removal Efficiency | Representative Volumetric Rate/Load | Achievable Effluent NO3−–N NO3−-N | Operational Considerations | Refs. |
|---|---|---|---|---|---|---|
| SAD | Low C/N municipal secondary effluents for advanced nitrogen removal; reclaimed water; saline wastewater | Typically 92 ± 5%, with reported ranges of 80–98% under various operational conditions. | Peak: 164 g NO3−-N·m−3·h−1 (≈3.9 kg N·m−3·d−1); maximum load: 1158 mg·L−1·d−1 (≈1.158 kg N·m−3·d−1) (fixed-bed/packed filter) | Typically, 2–10 mg·L−1; <5 mg·L−1 achievable in advanced processes (municipal effluents) | Sulfate generation and alkalinity consumption; risk of clogging, requires backwashing to maintain hydraulic performance | [12] |
| HAD | Groundwater/drinking water denitrification; post-treatment of reclaimed water | 90–99%; 97.8% in real groundwater | On the order of 135 g N·m−3·d−1 (≈0.135 kg N·m−3·d−1) under optimized MBfR conditions; strongly affected by H2 supply and mass transfer | 2.2 ± 1.0 mg·L−1 (real groundwater, continuous operation) | H+ production causing pH decrease; requires alkalinity or CO2 dosing; sensitive to temperature and mass transfer | [61] |
| IAD | Groundwater/drinking water (low C/N); mineral-driven systems | Typically, 85 ± 7%, with reported ranges of 70–95% under comparable hydraulic and nitrogen loading conditions; ≈94% at HRT of 24 h | Continuous-flow P-FBR: 168 mg NO3−·L−1·d−1 (≈0.168 N kg·m−3·d−1) at HRT of 24 h; efficiency drops significantly at 12 h, with NO2− accumulation | 10 mg NO3−·L−1 (as NO3−; at HRT of 24 h) | Limited by mineral dissolution/passivation; influenced by pH/alkalinity and co-release of metals/sulfate; sensitive to organic co-substrates | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, Q.; Tang, J.; Chen, Y.; Xu, Z.; Deng, S. Application Potential of Sulfur-Based Autotrophic Denitrification in Low Carbon Wastewater Treatment: Efficiency, Cost and Greenhouse Gas Emission Reduction. Water 2025, 17, 3281. https://doi.org/10.3390/w17223281
Zhang X, Ma Q, Tang J, Chen Y, Xu Z, Deng S. Application Potential of Sulfur-Based Autotrophic Denitrification in Low Carbon Wastewater Treatment: Efficiency, Cost and Greenhouse Gas Emission Reduction. Water. 2025; 17(22):3281. https://doi.org/10.3390/w17223281
Chicago/Turabian StyleZhang, Xiaolong, Qiqi Ma, Jing Tang, Ying Chen, Ziyu Xu, and Shihai Deng. 2025. "Application Potential of Sulfur-Based Autotrophic Denitrification in Low Carbon Wastewater Treatment: Efficiency, Cost and Greenhouse Gas Emission Reduction" Water 17, no. 22: 3281. https://doi.org/10.3390/w17223281
APA StyleZhang, X., Ma, Q., Tang, J., Chen, Y., Xu, Z., & Deng, S. (2025). Application Potential of Sulfur-Based Autotrophic Denitrification in Low Carbon Wastewater Treatment: Efficiency, Cost and Greenhouse Gas Emission Reduction. Water, 17(22), 3281. https://doi.org/10.3390/w17223281







