Abstract
Fish–mussel polyculture is a promising strategy for sustainable aquaculture. This study investigated hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × P. vachelli ♂) and Chinese olive mussel (Solenaia oleivora) polyculture on water quality, bacterial community structure, and fish growth performance over a six-month production cycle. At harvest, polyculture fish had an 11.65% higher weight gain rate than those in monoculture. Polyculture reduced TN to 1.89–1.95 mg/L (vs. monoculture 2.74–3.44 mg/L) in July–October and kept TP at 0.29–0.73 mg/L (vs. monoculture 0.37–1.45 mg/L). The microbial α-diversity analysis revealed that the community richness and diversity of monoculture were reduced in July, whereas polyculture experienced decreased richness in October and diminished diversity in both July and October. Dominant bacterial phyla included Proteobacteria, Actinobacteriota, Bacteroidota, and Firmicutes, with Proteobacteria showing higher relative abundance in polyculture. Genus-level analysis revealed distinct successional patterns driven by season and cultivation mode. Notably, polyculture systems can effectively suppress potential pathogens. Redundancy analysis indicated that environmental factors played crucial roles in shaping the microbial community structure. More importantly, it provides scientific basis for optimizing freshwater polyculture models and offers practical technical support for promoting ecologically sustainable aquaculture through improved nutrient cycling and microbiome modulation.
1. Introduction
Aquaculture plays a crucial role in global food security, providing nearly half of the world’s fish supply for human consumption [1,2]. However, intensive monoculture practices often lead to environmental degradation, including water pollution, eutrophication, and disease outbreaks; this threatens the sustainability of aquaculture systems [3]. To address these challenges, integrated multi-trophic aquaculture and polyculture systems have been proposed as eco-friendly alternatives, where different species with complementary ecological functions are co-cultured to enhance resource utilization and minimize waste discharge [4,5]. Polyculture, a farming practice that involves raising multiple species in the same environment, has gained increasing attention in recent years due to its potential to enhance productivity and ecological sustainability [6]. Among various polyculture systems, fish–mussel polyculture stands out as a promising approach, particularly in aquatic ecosystems due to its potential to improve water quality and increase overall productivity [7]. Additionally, the integration of fish and mussels also affects water bacterial community structure, which is crucial for the overall health and productivity of the aquatic system [8,9].
Hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × P. vachelli ♂), a commercially important benthic fish in China, is known for its high market value and adaptability to intensive farming conditions [10,11]. However, its intensive cultivation often leads to organic waste accumulation due to uneaten feed and excretion, which can deteriorate water quality and promote harmful microbial blooms [12]. Conversely, Chinese olive mussel (Solenaia oleivora), an endemic rare freshwater mussel in China, acts as an efficient biofilter, removing suspended particles, phytoplankton, and organic detritus from the water column [13,14]. The ecological roles of these two species suggest that their combined culture could create a synergistic relationship, where S. oleivora mitigates the waste produced by the hybrid yellow catfish, while the fish’s bioturbation activity may enhance nutrient recycling for mussel organisms.
Water quality is a critical determinant of aquaculture success, as it directly influences fish health, growth performance, and disease susceptibility [15]. To evaluate aquaculture water quality, key parameters such as dissolved oxygen (DO), ammonia-nitrogen (NH3-N), nitrite (NO2−-N), and total phosphorus (TP) are commonly monitored, and excessive levels of these substances often induce physiological stress in cultured species and even lead to mortality [16]. Beyond water quality parameters, microbial communities in aquaculture systems also play a fundamental ecological role: they mediate nutrient cycling, drive the decomposition of organic matter, and help to control the proliferation of pathogenic microorganisms [17]. Previous studies have confirmed that bivalves can reshape the structure of bacterial communities by altering the availability of nutrients in the water, regulating dissolved oxygen levels, and modifying the composition of organic matter [18]. Additionally, some studies have demonstrated the ecological benefits of fish-bivalve polyculture systems [9,19,20]. However, studies on freshwater fish–mussel polyculture systems, particularly involving hybrid yellow catfish and S. oleivora, remain scarce. Understanding their combined effects on nutrient cycling and microbial ecology is essential for sustainable pond management.
The present study aims to investigate the effects of the hybrid yellow catfish and S. oleivora polyculture on two key aspects: (1) key water quality parameters, including nitrogen and phosphorus dynamics, and (2) the structure and diversity of water bacterial communities. We hypothesize that (1) the presence of S. oleivora will improve overall water quality compared to hybrid yellow catfish monoculture; and (2) the polyculture will lead to distinct shifts in bacterial community composition, favoring beneficial microbes involved in nutrient cycling and organic matter degradation. Elucidating these mechanisms will contribute to the development of sustainable aquaculture strategies that enhance productivity while minimizing environmental impacts, and also provides insights into the broader application of fish-bivalve polyculture in freshwater systems, supporting the transition toward more ecologically balanced aquaculture practices.
2. Materials and Methods
2.1. Pond Culture and Experimental Design
The cultivation experiment was conducted in the Fishery Institute of Anhui Academy of Agricultural Sciences, Hefei, Anhui Province, China (31°53′10″ N, 117°14′45″ E), which spanned from April to October 2024. The total duration of the experiment was six months, covering key growth phases of the cultured species. The study employed a comparative experimental design consisting of two treatment groups: (1) Fish monoculture, where only hybrid yellow catfish (P. fulvidraco ♀ × P. vachelli ♂) was reared, and (2) fish–mussel polyculture, where hybrid yellow catfish and S. oleivora were co-cultured. Each treatment group was replicated in three earthen ponds (approximately 0.1 ha each) to ensure statistical robustness. Prior to seedling stocking, the aquaculture ponds were subjectd to thorough rectification. Pond water was completely drained, followed by removal of debris and excessive silt, maintaining the silt thickness at the pond bottom within 15 cm. Subsequently, quicklime was dissolved in water and immediately applied across the entire pond for disinfection purposes. After exposure to sunlight for 10–15 days, water was reintroduced into the ponds. All experimental ponds shared a unified water source (underground well water) to ensure consistency in water quality parameters. During the water injection process, a sieve silk was used at the water inlet to filter out impurities.
S. oleivora was stocked in early April, during which water transparency was controlled at 10–30 cm. In contrast, hybrid yellow catfish were stocked in early May, when the water temperature stabilized above 18 °C. For hybrid yellow catfish, 11 fingerlings/m2 were stocked, with an initial individual weight of 25 g. S. oleivora individuals were suspended in cages at a density of 0.3 cage/m2, with each cage holding 200 juveniles (a stocking density of 65 juveniles/m2). The initial individual weight of S. oleivora seedlings was 8–10 g per individual (Figure 1). Feeding primarily relied on a specialized feed formulated for hybrid yellow catfish. To further control environmental variability, a unified feeding regime was strictly implemented for all experimental ponds. After the fingerlings were domesticated to feed in groups, the feeding amount was generally controlled at 3% of the fish body weight, with the requirement of being consumed within 1 h. Feeding was conducted twice a day, from 7:00 to 8:00 in the morning and from 17:00 to 18:00 in the afternoon, with the afternoon feeding amount accounted for 60% of the total daily feed intake.
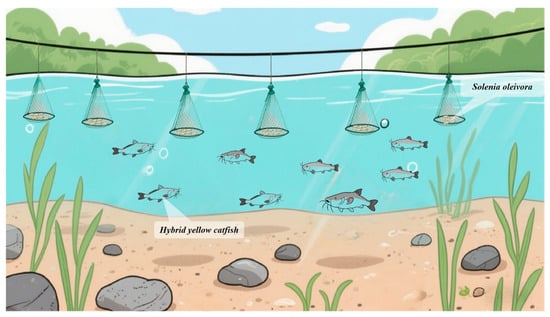
Figure 1.
Schematic diagram of fish–mussel polyculture. The cages are connected in series with ropes, spaced 40 cm to 50 cm apart, and suspended at a position 60 cm to 70 cm below the water surface.
2.2. Sample Collection
Samples were collected at three critical time-points: initial stage (May, after two weeks of acclimation), peak growing season (July, peak growth stage) and final sampling before harvest (October, harvest stage). For each aquaculture mode, water samples were collected from the corresponding ponds. Specifically, the monoculture system yielded 18 samples across three sampling months: May (labeled MW1-1–MW1-6), July (JW1-1–JW1-6), and October (OW1-1–OW1-6). The polyculture system was sampled in the same three months, providing another 18 samples (May: MW2-1–MW2-6; July: JW2-1–JW2-6; October: OW2-1–OW2-6). In total, 36 water samples were obtained from the two aquaculture systems. Water samples were collected from the surface, middle, and bottom of the pond; samples were mixed, filtered through a 0.22-μm filter, and placed in a sterile 5 mL tube. All samples were immediately stored at −80 °C before DNA extraction.
The on-site water quality monitoring was also conducted. Key physicochemical parameters including temperature (°C), dissolved oxygen (DO) (mg/L), pH, and conductivity (μS/cm) were measured on-site using a YSI ProDSS multiparameter water quality meter (YSI Incorporated, Yellow Springs, OH, USA) at three fixed sampling points in each pond. All YSI measurements were conducted between 10:00 and 11:00 AM to minimize diurnal variation effects. The probe was calibrated before each sampling event following manufacturer protocols. Water samples were collected from each pond at a depth of 50 cm using a water sampler (1 L per pond). The following water quality parameters were measured: ammonia-nitrogen (NH3-N), nitrite (NO2−-N), total nitrogen (TN), dissolved total nitrogen (DTN), total phosphorus (TP), dissolved total phosphorus (DTP), orthophosphate (PO43−-P) and permanganate index (CODMn). All water samples were preserved on ice and processed within 6 h.
2.3. DNA Extraction, PCR Amplification, and 16S rRNA Sequencing
Microbial community genomic DNA was extracted from all samples using the MagPure Soil DNA LQ Kit (Magen, Guangzhou, Guangdong, China; D6356-02) according to the manufacturer’s instructions. The DNA concentration and purity were determined using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo FisherScientific, Waltham, MA, USA). The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with universal primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′) using a polymerase chain reaction (PCR) thermocycler (Bio-rad, Hercules, CA, USA; 580BR10905). The PCRs were performed in triplicate using 25 μL mixtures containing 0.5 U of TaKaRa Ex Taq DNA polymerase, 1× PCR buffer, forward and reverse primers (0.4 μM each), and 10 ng of DNA template. The PCR amplification of the 16S rRNA gene was performed as follows: initial denaturation at 94 °C for 5 min; followed by 26 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 20 s; and a single extension at 72 °C for 5 min.
The PCR products of the same sample were mixed using Tris–HCl elution buffer and were detected using 2% agarose gel electrophoresis. After extraction and purification using the AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA, USA) according to the manufacturer’s instructions, the sample was quantified using a Quantus Fluorometer (Promega, Madison, WI, USA). Purified amplicons were pooled in equimolar concentrations, and paired-end sequencing (2 × 250) was performed on an Illumina MiSeq platform (Illumina Inc., San Diego, CA, USA) according to the standard protocols by OE biotech Co., Ltd. (Shanghai, China).
2.4. Bacterial Community Profiling and Analysis
Raw sequencing data were in FASTQ format. Paired-end reads were then preprocessed using cutadapt software (version 5.2) to detect and cut off the adapter. After trimming, paired-end reads were filtered for low quality sequences, denoised, merged and checked for chimera reads using DADA2 with the default parameters of Quantitative Insights into Microbial Ecology (QIIME) 2.0 [21,22]. Finally, the software output the representative reads and the ASV abundance table. The representative read of each ASV was selected using QIIME2 package. All representative reads were annotated and blasted against Silva database Version 138 using q2-feature-classifier with the default parameters.
To investigate the differences in community richness and diversity, alpha diversity indexes, including the Chao index and abundance-based coverage estimator (ACE; community richness indexes) and the Simpson and Shannon diversity indexes, were calculated. The index was calculated for each treatment, and the Wilcoxon rank-sum test was used to compare the water bacterial diversity between any two groups. Significance was defined at an adjusted p-value less than 0.05. Principal coordinates analysis of the Bray–Curtis distances was performed to calculate the overall community similarity between the two aquaculture modes. Microbial community bar plots were used to determine taxonomic composition. Linear discriminant analysis effect size (LEfSe) [23] was performed using the free online Oebiotech Cloud Platform (www.cloud.oebiotech.com, accessed on 15 November 2024) to elucidate the abundance of microbial communities within the different aquaculture modes.
2.5. Bacterial Community-Water Quality Parameter Relationships
To clarify associations between bacterial community composition (at both phylum and genus levels) and water quality parameters, redundancy analysis [22] was performed. The environmental variables involved were T, NH3-N, NO2−-N, TN, TP, PO43−-P, and CODMn. All RDA calculations and ordination plot visualizations were carried out using the company’s cloud-based bioinformatics platform, following its standardized analytical workflows (www.cloud.oebiotech.com, accessed on 25 November 2024). This multivariate approach quantifies the contribution of each water quality parameter to variations in bacterial community assemblages across different aquaculture modes and sampling stages. The significance of the RDA model and the individual effects of environmental variables were validated using 999 permutations, with statistical significance set at p < 0.05 to ensure the reliability of observed correlations.
3. Results
3.1. The Influence of Polyculture Mode on the Weight Gain Rate
The growth performance of fish under polyculture and monoculture systems was evaluated based on body weight and weight gain rate (Table 1). At the initial stage, no significant differences were detected between the two culture modes (p > 0.05). At the harvest stage, however, fish in the polyculture system achieved a significantly higher body weight than those in the monoculture system (p < 0.05), accompanied by a greater weight gain rate. These results indicate that the polyculture environment enhanced growth efficiency.

Table 1.
Growth Performance of Fish in Polyculture and Monoculture Systems.
3.2. Water Quality Dynamics
Water quality indices of the monoculture and polyculture systems across May, July, and October, as shown in Table 2, revealed similar seasonal trends in temperature (peak in July) and pH (increase to stabilization). No significant difference in dissolved oxygen was observed between systems. Total nitrogen increased significantly over time in monoculture but decreased significantly in polyculture by July and October. Ammonia nitrogen was significantly higher in monoculture than polyculture in October. Monoculture exhibited consistently and significantly higher levels of total phosphorus, dissolved total phosphorus, and phosphate (May–July) across all sampling periods. Polyculture also showed significantly lower chemical oxygen demand in July. These findings demonstrate that polyculture offers advantages in nutrient management, particularly in reducing nitrogen accumulation seasonally and maintaining lower phosphorus levels, likely due to enhanced biological interactions and waste assimilation.

Table 2.
Water quality indices of the two culture patterns over three times.
3.3. Overview of 16S rRNA Sequencing Analysis
A total of 2,774,506 high-quality sequences were retained after rigorous quality control, with per-sample read counts ranging from 74,454 to 79,510, demonstrating exceptional uniformity in sequencing depth across the 36 samples. Denoising via DADA2 resolved 25,263 biologically valid amplicon sequence variants (ASVs), defining the total microbial diversity pool. Substantial heterogeneity in α-diversity was observed at the sample level, where ASV richness (ASV_counts) spanned a 2.92-fold gradient from 283 (JW1 to 1) to 827 (MW2-3).
3.4. Bacterial Community Shifts
To assess variations in microbial community characteristics, we calculated several α-diversity indices, including community richness estimators (Chao1 and ACE indices) and diversity measures (Simpson and Shannon indices). In the monoculture system, we observed significant reductions in the ACE index (community richness), as well as in both Simpson and Shannon diversity indices during July. The polyculture system showed a distinct temporal pattern: the ACE index significantly declined in October, while the Simpson index decreased in both July and October. Similarly, the Shannon diversity index exhibited a significant reduction in October under polyculture conditions. In the early stage of farming (May), there were no significant differences in ACE and Chao1 between the two farming modes, while Shannon and Simpson indices were significantly lower in the polyculture system compared to the monoculture system. During the peak farming period (July), the polyculture system showed significantly higher ACE and Chao1 indices than the monoculture system, while the Simpson index was significantly lower, and Shannon did not exhibit significant changes. By the late farming stage (October), the polyculture system demonstrated significantly lower ACE, Chao1, Shannon, and Simpson indices compared to the monoculture system (Figure 2).
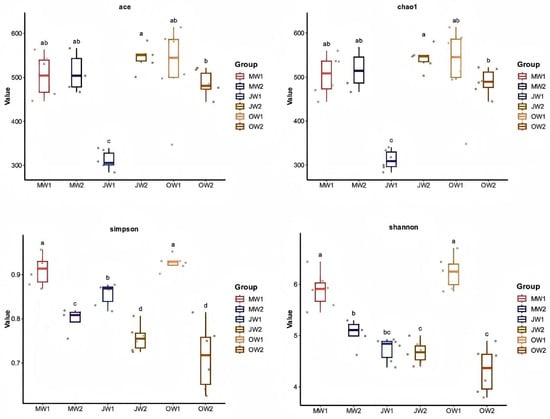
Figure 2.
Comparative analysis of microbial α-diversity (ACE index, Chao1 index, Simpson index, Shannon index) across different culture systems and sampling months. Groups are defined as: MW1 (monoculture, May), MW2 (polyculture, May); JW1 (monoculture, July), JW2 (polyculture, July); OW1 (monoculture, October), OW2 (polyculture, October). Different lowercase letters above bars indicate statistically significant differences among groups based on post hoc tests (p < 0.05), while shared letters denote no significant difference.
Principal coordinates analysis (PCoA) based on Binary–Jaccard distances visualized differences in pond water bacterial community composition both across months within the same culture mode and between different culture modes within the same month. The results revealed distinct clustering patterns, indicating that seasonal variation significantly influences bacterial communities within a given culture mode, and that the aquaculture practice itself substantially shapes the water microbiota composition (Figure 3).
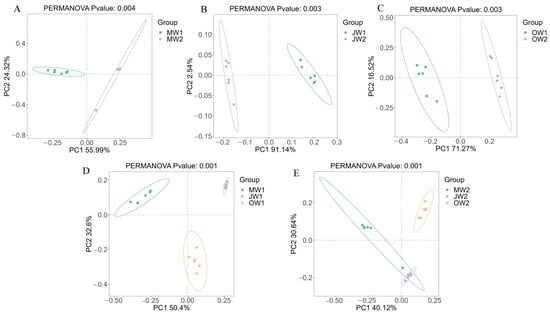
Figure 3.
Principal Coordinates Analysis (PCoA) of bacterial communities. Bray–Curtis PCoA ordination showing significant separation: Panels (A–C) represent bacterial community differences between two aquaculture modes at each of the three sampling time-points, respectively; Panels (D,E) represent community differences across the three time-points within the same aquaculture mode.
3.5. Taxonomic Composition of the Microbial Community
Proteobacteria, Actinobacteria, Bacteroidota and Firmicutes were the dominant phyla in the three times of the two aquaculture modes, while the relative abundance of bacterial phyla varied across the three sampling times and between the two aquaculture modes (Figure 4A). Proteobacteria emerged as the dominant phylum across all sampling periods, consistently exhibiting higher relative abundance in polyculture systems compared to monocultures. In May, Proteobacteria accounted for 53.13% (monoculture) versus 60.64% (polyculture), followed by July (44.97% vs. 61.72%) and October (52.19% vs. 78.74%). Actinobacteriota ranked second in abundance with notably reduced proportions in polyculture systems in October (30.19% monoculture vs. 8.39% polyculture). Bacteroidota, Firmicutes, Cyanobacteria, and Bdellovibrionota collectively comprised <20% of communities across all samples.
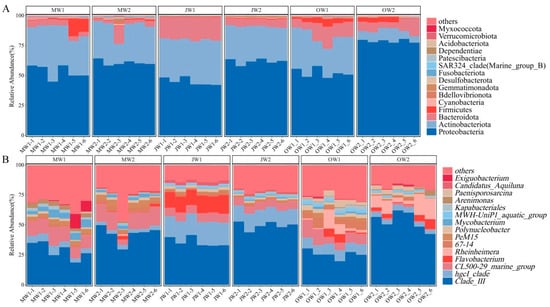
Figure 4.
Relative abundance of bacterial community composition at the phylum (A) and genus (B) level in the surrounding water for the two feeding modes in three times.
Genus-level analysis revealed distinct successional patterns driven by seasonal variation and cultivation practices (Figure 4B). Clade III (SAR11 clade) consistently dominated microbial communities, exhibiting significantly higher relative abundance in polyculture systems across all sampling periods. Temporal shifts occurred among taxa: hgcI-clade (Actinobacteria) peaked in July monocultures (18.71%), while Flavobacterium (Bacteroidota) showed exclusive July dominance in monoculture (13.95%). In contrast, its relative abundance in polyculture systems was merely 0.06% during this period. Conversely, Rheinheimera (Gammaproteobacteria) expanded >200-fold in October polycultures (10.44% vs. 0.04% in July), and Paenisporosarcina (Firmicutes) emerged solely in October systems (2.31–3.51%). Additionally, some microorganisms exhibited distinct temporal dynamics throughout the study period. Specifically, the genus Polynucleobacter maintained a relatively stable occupancy (1.47–2.92% across samples), whereas the CL500-29 marine group progressively declined from May (12.03–13.08%) to October (0.96–1.28%), and in July, especially in October, its content in the monoculture system is higher than that in the polyculture system.
Heatmap analysis revealed significant variation at the species level in microbial community composition across sample groups (Figure 5). In monoculture systems, Flavobacterium, Vibrio, and Aeromonas exhibited high relative abundances during July and October, emerging as dominant taxa, suggesting that monoculture systems may elevate pathogenic risks. Candidatus Nanopelagicus, Sporosarcina sp., and Pararheinheimera chironomi exhibited seasonal specificity, being significantly enriched in the ponds of different culture systems during October.
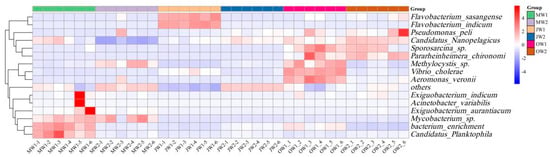
Figure 5.
Species-level heatmap of microbial community composition.
The LEfSe between MW1 and MW2 samples revealed that Clade III was a family-level biomarker and Proteobacteria was a phylum level biomarker in MW2 samples. The Xanthomonadaceae, Mycobacteriaceae and Moraxellaceae were family-level biomarkers and hgcI-clade, Arenimonas, Mycobacterium, Acinetobacter were genus-level biomarkers in MW1 samples. According to the LEfSe between JW1 and JW2 samples, Clade III was a family-level biomarker and Proteobacteria was a phylum-level biomarker in JW2. The hgcI-clade and Flavobacterium were genus-level biomarkers in JW1 samples. The LEfSe between OW1 and OW2 samples indicated that Clade III was a family-level biomarker and Proteobacteria was a phylum level biomarker in OW2. CL500-29 marine group, hgcI-clade, Kapabacteriales, 67-14 and PeM15 were genus-level biomarkers in OW1 samples (Figure 6).
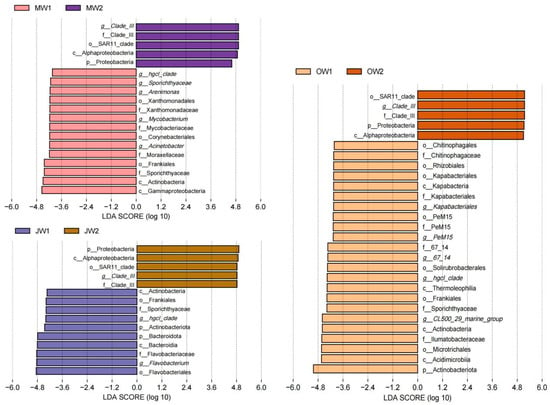
Figure 6.
Linear discriminant analysis effect size (LEfSe) of water microbiomes in May, July and October between the two culture modes.
3.6. Correlations Between Water Quality Indicators and Dominant Bacteria
Redundancy analysis was performed to explore the relationships between environmental factors and the microbial community. For the phylum-level analysis (Figure 7A), the RDA model revealed that the measured environmental factors collectively explained 72.5% of the variation in the microbial community (R2 = 0.725, adjusted R2 = 0.679, model p = 0.001). Of this explained variance, the first two RDA axes captured the majority, with RDA1 accounting for 82.3% and RDA2 for 14.1%, resulting in a cumulative contribution of 96.4% to the environmental constraint. The relatively long arrows of factors like NO2−-N and microbial groups such as Actinobacteria suggest that they play important roles in shaping the microbial community structure, and the similar directions of the NO2−-N arrow and the Actinobacteria arrow. In contrast, factors like DO and TP with shorter arrows have relatively weaker impacts. When arrows point in opposite directions (with a large included angle), it suggests a negative correlation, as seen between the NO2−-N arrow and the Proteobacteria arrow. For the genus-level analysis (Figure 7B), the RDA model explained 82.1% of the microbial community variation (R2 = 0.821, adjusted R2 = 0.792, model p = 0.001). The horizontal axis (RDA1) accounted for 54.8% of the variance, and the vertical axis (RDA2) accounted for 20.6%, with a cumulative interpretation of about 75.4% of the correlation information. Factors like NO2−-N and CODMn had relatively long arrows, indicating their significant roles in influencing the microbial genus-level community structure. The NO2−-N arrow had a similar direction to arrows of microbial genera like CL500-29 marine group, PeM15, 67-14 and Mycobacterium. On the other hand, the arrow of NH3-N and TN had an opposite direction to arrows of genera such as Rheinheimera and Clade III.
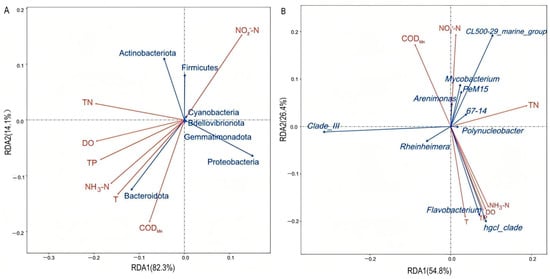
Figure 7.
Redundancy analysis diagrams illustrating the relationships between water quality indicators and dominant bacterial phyla (A) as well as genera (B).
4. Discussion
4.1. Water Quality Dynamics in Polyculture Systems
Water quality, which directly affects fish health and well-being, is the key factor to be considered in all aquaponic systems [15]. This study confirms that the polyculture system of hybrid yellow catfish (P. fulvidraco ♀ × P. vachelli ♂) and Chinese olive mussel (S. oleivora) significantly optimizes water quality compared to the catfish monoculture system, directly supports our first hypothesis. Specifically, the polyculture system effectively reduces nitrogen and phosphorus concentrations, particularly during the high-temperature season (July), and demonstrates clear advantages in nutrient regulation and organic pollution control. These findings align with results from other fish–mussel polyculture studies [20]. Although both systems exhibited consistent seasonal trends in temperature and pH, polyculture’s superior water quality performance underscores its ecological benefits.
In terms of nitrogen dynamics, the monoculture system showed a continuous upward trend in total nitrogen (TN) throughout the culture cycle, while the polyculture system exhibited a significant downward trend after July. This contrast indicates that S. oleivora effectively inhibits nitrogen accumulation—likely by filtering particulate organic nitrogen (e.g., uneaten feed and fish excreta) and converting it into biomass, thereby reducing the substrate for microbial nitrogen mineralization [9]. Ammonia nitrogen (NH3-N) showed more pronounced differences: in October (the harvest stage, when system effects are most stable), its concentration in the monoculture system was higher than the polyculture system (p < 0.05). This aligns with the findings that bivalve filtration reduced ammonia release by mitigating organic waste decomposition [24].
The polyculture system also showed advantages in organic pollution control: its permanganate index (CODMn) in July was significantly lower than the monoculture system (p < 0.05). This reflects reduced organic matter accumulation in the polyculture system, as mussels filter organic detritus and phytoplankton, reducing the substrate for microbial decomposition and lowering oxygen consumption associated with organic breakdown. Notably, although there was no significant difference in dissolved oxygen (DO) between the two systems (p > 0.05), the polyculture system maintained more stable DO levels during the high-temperature period (July), which may provide a more suitable aerobic environment for fish growth and microbial nutrient cycling.
4.2. Microbial Community Restructuring
The polyculture system significantly reshapes the structure and function of aquatic bacterial communities, supporting our second hypothesis that polyculture favors beneficial microbes involved in nutrient cycling and organic decomposition. Notably, temperature fluctuates seasonally and is well recognized as a key driver of bacterial growth and community dynamics, alongside other environmental factors like nutrient availability and dissolved oxygen. The present study revealed that during the peak farming period in July, polyculture systems exhibited higher ACE and Chao1 indices, indicating increased species richness and better preservation of rare taxa compared to monoculture, which was consistent with findings from pearl mussel-fish polyculture [19]. Additionally, a lower Simpson index indicated a more balanced distribution of biomass among species, suggesting that polyculture may disrupt the dominance of species typical in monocultures, thereby enhancing functional redundancy within the system [25].
Actinobacteria and Proteobacteria were the main microorganisms in denitrification process, while Firmicutes were the main microorganisms in nitrogen fixation process [26]. In the present study, Proteobacteria was the dominant phylum in both systems, but its relative abundance was consistently higher in the polyculture system. Phosphorus control is critical for mitigating eutrophication, Actinobacteria could effectively solubilize the inorganic phosphorus from calcium phosphate to intensify lake eutrophication, and many Actinobacteriota are heterotrophic decomposers that release nutrients during organic breakdown, their reduction in the polyculture system during October may be related to reduced nutrient accumulation—consistent with the lower nitrogen and phosphorus levels in this system [27].
At the genus level, Clade III (SAR11 clade) was a key indicator of community differences. The SAR11 group of bacteria plays an important role in the oceanic carbon cycle; its abundance increased in July as a result of a better adaptation toward more oligotrophic conditions [28,29]. It maintained significantly higher relative abundance in the polyculture system across all stages. This persistent dominance suggests that the polyculture environment may provide a more stable and compatible niche for SAR11. In our study, hgcI-clade was enriched in July and October. This result is consistent with findings from an integrated multi-trophic aquaculture system of P. fulvidraco, where enrichment was also observed in August and October but was lower in April and July [30]. Furthermore, hgcI-clade exhibited a strong negative correlation with NOx concentrations and nitrogen reduction genes, suggesting a putative role in nitrogen fixation. Consistent with its potential functional importance, this clade has been proposed as a novel keystone taxon and may represent a core component of the microbiome in oligotrophic aquatic ecosystems worldwide [31]. The percentage of CL500-29 marine group decreased progressively from May to October. In October, its relative abundance was higher in the monoculture system than in the polyculture system, and it showed a positive correlation with nitrate or nitrite concentrations. These findings are consistent with a previous study on the bacterioplankton community composition along the Pearl Estuary, China [32].
Notably, heatmap analysis showed that the monoculture system had high relative abundances of Flavobacterium, Vibrio cholerae, and Aeromonas veronii in July and October—taxa associated with fish diseases—while these pathogens were significantly reduced in the polyculture system, which indicated that the polyculture system effectively suppressed potential pathogens. This may be because mussel filtration reduces the organic substrate available for pathogen growth and enriched beneficial bacteria compete with pathogens for nutrients or secrete antimicrobial metabolites, which is consistent with the P. fulvidraco integrated multi-trophic system and the lumpfish aquaculture system [30,33].
5. Conclusions
The introduction of S. oleivora enhanced the conversion of nitrogen and phosphorus, promoted microbial nutrient cycling, and improved water quality, thereby reducing the risk of opportunistic pathogen proliferation. This synergistic process facilitates the transformation of waste into resources, which highlights the ecological benefits of integrated multi-trophic aquaculture. By simultaneously enhancing the production environment and reducing environmental impacts, this system addresses two key challenges in the development of modern aquaculture [30,34]. Notably, the small experimental scale and short monitoring period in this study restrict result extrapolation and long-term trend capture. Future research should therefore focus on exploring the scalability of the polyculture model in large-scale ponds and replicating experiments under varied conditions to verify result stability. Nevertheless, these findings strongly support the adoption of fish–mussel polyculture as a sustainable alternative to traditional monoculture in freshwater aquaculture, particularly in regions prone to eutrophication.
Author Contributions
Conceptualization, H.W. and G.D.; methodology, H.W. and H.Z.; software, H.W. and Y.Z.; validation, Y.H., A.L. and Y.Z.; formal analysis, H.W.; investigation, F.H.; resources, H.W. and G.D.; data curation, H.W. and F.H.; writing—original draft preparation, H.W.; writing—review and editing, H.W. and H.Z.; visualization, Y.Z.; supervision, A.L.; project administration, G.D.; funding acquisition, H.W. and G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Anhui Academy of Agricultural Sciences Research Team Project (2025YL047), Anhui Key Laboratory of Aquaculture and Stock Enhancement project (2025YL083), Anhui Provincial Agricultural Germplasm Resource Center Bank Special Fund (2025), and Anhui Province Modern Agricultural Industrial Technology System Construction Special Fund ([2021] No. 711).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
We thank Funan County Jinghuai Special Aquatic Products Co., Ltd. for providing the experimental materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmed, N.; Thompson, S. The blue dimensions of aquaculture: A global synthesis. Sci. Total Environ. 2019, 652, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.O.; Akinsanola, A.B.; Okon, M.E.; Shitu, T.; Jagunna, I.I. Emerging challenges in aquaculture: Current perspectives and human health implications. Vet. World 2025, 18, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Turchini, G.M. Organic aquaculture productivity, environmental sustainability, and food security: Insights from organic agriculture. Food Secur. 2020, 12, 1253–1267. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hasanuzzaman, A.F.M.; Islam, S.S.; Sarower, M.G.; Mistry, S.K.; Arafat, S.T.; Huq, K.A. Integrated multi-trophic aquaculture (IMTA): Enhancing growth, production, immunological responses, and environmental management in aquaculture. Aquac. Int. 2025, 33, 336. [Google Scholar] [CrossRef]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When more is more: Taking advantage of species diversity to move towards sustainable aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef]
- Batır, E.; Aydın, İ.; Theodorou, J.A.; Rakaj, A. Mytilus galloprovincialis’s role in Integrated Multi-Trophic Aquaculture (IMTA): A comprehensive review. J. World Aquac. Soc. 2025, 56, e70013. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Qin, J.; Wang, Y. The effect of C/N ratio on bacterial community and water quality in a mussel-fish integrated system. Aquac. Res. 2018, 49, 1699–1708. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, H.; Chen, Y.; Ma, X.; Yu, G.; Hong, Y.; Hu, B. Fish-mussel-algae-bacteria model remedied eutrophication pollution: Application in Dongxiang district reservoir. Environ. Pollut. 2024, 342, 123011. [Google Scholar] [CrossRef]
- Ji, Z.; Lu, X.; Xue, M.; Fan, Y.; Tian, J.; Dong, L.; Zhu, C.; Wen, H.; Jiang, M. The probiotic effects of host-associated Bacillus velezensis in diets for hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × Pelteobagrus vachelli ♂). Anim. Nutr. 2023, 15, 114–125. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, H.; Tu, X.; Gu, Z. First Investigation of Trichodinid Species (Ciliophora: Trichodinidae) on Farmed Hybrid Yellow Catfish (Tachysurus fulvidraco × Tachysurus vachelli) in China. J. Fish Dis. 2025, 48, e14100. [Google Scholar] [CrossRef] [PubMed]
- Amirkolaie, A.K. Reduction in the environmental impact of waste discharged by fish farms through feed and feeding. Rev. Aquac. 2011, 3, 19–26. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.-R.; Guo, S.-S.; Guo, X.-Z.; Wei, K.-J.; Ge, T.-M. Isolation and characterization of fifteen polymorphic microsatellite loci in the threatened freshwater mussel Solenaia oleivora (Bivalvia: Unionidae). Biochem. Syst. Ecol. 2013, 47, 104–107. [Google Scholar] [CrossRef]
- Chen, P.; Li, D.; Chen, X.; Zhang, G.; Yang, S. Molecular identification and phylogenetic analysis of the mitogenome of Solenaia oleivora MG. Mitochondrial DNA Part B 2020, 5, 2796–2798. [Google Scholar] [CrossRef] [PubMed]
- Yavuzcan Yildiz, H.; Robaina, L.; Pirhonen, J.; Mente, E.; Domínguez, D.; Parisi, G. Fish Welfare in Aquaponic Systems: Its Relation to Water Quality with an Emphasis on Feed and Faeces—A Review. Water 2017, 9, 13. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Foysal, M.J.; Fotedar, R.; Gupta, S.K.; Siddik, M.A.B.; Tay, C.-Y. The Effect of Two Dietary Protein Sources on Water Quality and the Aquatic Microbial Communities in Marron (Cherax cainii) Culture. Microb. Ecol. 2021, 82, 299–308. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Waiho, K.; Fazhan, H.; Azwar, E.; Shu-Chien, A.C.; Hersi, M.A.; Kasan, N.A.; Foo, S.S.; Wong, K.Y.; Draman, A.S.; et al. Emerging paradigms in sustainable shellfish aquaculture: Microalgae and biofloc technologies for wastewater treatment. Aquaculture 2024, 587, 740835. [Google Scholar] [CrossRef]
- Lacoste, É.; McKindsey, C.W.; Archambault, P. Biodiversity–Ecosystem Functioning (BEF) approach to further understanding aquaculture–environment interactions with application to bivalve culture and benthic ecosystems. Rev. Aquac. 2020, 12, 2027–2041. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, J.; Zhang, C.; Qin, J.; Wang, Y. Bacterial composition, abundance and diversity in fish polyculture and mussel–fish integrated cultured ponds in China. Aquac. Res. 2017, 48, 3950–3961. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, J.; Dong, J.; Yang, H.; Yu, G.; Hong, Y. Association of algae diversity and Hyriopsis schlegelii growth in mixed fish-mussel aquaculture. Algal Res. 2022, 65, 102736. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, Y.; Wen, H.; Ma, X.; Xu, D. Integrated analysis of physiological, transcriptome, and metabolome analyses of the gills in Solenaia oleivora under ammonia exposure. Ecotoxicol. Environ. Saf. 2024, 271, 115949. [Google Scholar] [CrossRef]
- Stefani, F.; Schiavon, A.; Tirozzi, P.; Gomarasca, S.; Marziali, L. Functional response of fish communities in a multistressed freshwater world. Sci. Total Environ. 2020, 740, 139902. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Li, K.; Meng, Q.; Wang, S.; Wang, Y.; Zhu, P.; Niu, Q.; Yan, H.; Li, X.; et al. Red mud conserved compost nitrogen by enhancing nitrogen fixation and inhibiting denitrification revealed via metagenomic analysis. Bioresour. Technol. 2022, 346, 126654. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, R.; Lu, Q.; Huang, Q.; Chen, J. Response of bacterioplankton communities and their phosphorus metabolic functions to algal extinction and growth in a eutrophic plateau lake. Environ. Technol. Innov. 2025, 38, 104108. [Google Scholar] [CrossRef]
- Korlević, M.; Šupraha, L.; Ljubešić, Z.; Henderiks, J.; Ciglenečki, I.; Dautović, J.; Orlić, S. Bacterial diversity across a highly stratified ecosystem: A salt-wedge Mediterranean estuary. Syst. Appl. Microbiol. 2016, 39, 398–408. [Google Scholar] [CrossRef]
- Brindefalk, B.; Ettema, T.J.G.; Viklund, J.; Thollesson, M.; Andersson, S.G.E. A Phylometagenomic Exploration of Oceanic Alphaproteobacteria Reveals Mitochondrial Relatives Unrelated to the SAR11 Clade. PLoS ONE 2011, 6, e24457. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Liu, X.-G.; Lu, M.; Zhou, R.-F.; Sun, Z.-Y.; Xiao, S.-W. Characteristics of Bacterial Community in Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System. Water 2022, 14, 3192. [Google Scholar] [CrossRef]
- Ruprecht, J.E.; Birrer, S.C.; Dafforn, K.A.; Mitrovic, S.M.; Crane, S.L.; Johnston, E.L.; Wemheuer, F.; Navarro, A.; Harrison, A.J.; Turner, I.L.; et al. Wastewater effluents cause microbial community shifts and change trophic status. Water Res. 2021, 200, 117206. [Google Scholar] [CrossRef]
- Liu, J.; Fu, B.; Yang, H.; Zhao, M.; He, B.; Zhang, X.-H. Phylogenetic shifts of bacterioplankton community composition along the Pearl Estuary: The potential impact of hypoxia and nutrients. Front. Microbiol. 2015, 6, 64. [Google Scholar] [CrossRef]
- Dahle, S.W.; Bakke, I.; Birkeland, M.; Nordøy, K.; Dalum, A.S.; Attramadal, K.J.K. Production of lumpfish (Cyclopterus lumpus L.) in RAS with distinct water treatments: Effects on fish survival, growth, gill health and microbial communities in rearing water and biofilm. Aquaculture 2020, 522, 735097. [Google Scholar] [CrossRef]
- Wang, M.; Feng, W.; Wang, Y.; Li, B.; Wang, J.; Zhu, X.; Zhang, L. Water quality, plankton composition, and growth performance of juvenile yellow catfish (Pelteobagrus fulvidraco) in mono- and polyculture systems. Aquaculture 2022, 552, 738017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).