Abstract
Escherichia coli is an important indicator microorganism of fecal contamination in water. However, routine government monitoring often fails to capture the actual state of pollution, because E. coli concentrations in urban rivers are highly variable. This study presents a case study on factors influencing E. coli concentrations in an urban river draining a fully sewered area. An approximately 70-fold higher concentration compared with the average dry-weather concentration (1.9 CFU/mL) was observed under wet-weather conditions, probably due to the effects of combined sewer overflows. A very short survival of E. coli (less than one day) was expected in the unfiltered overlying water, due to the contributions of bacteriophages, protozoan predation, and bacterial competition, whereas a longer survival was expected in the sediment. Such a short survival may be a characteristic of the target watershed, where treated wastewater accounted for approximately 75% of the total flow. The highly variable antimicrobial resistance among E. coli populations under dry-weather conditions was possibly caused by the regrowth of a limited number of E. coli individuals in the sediment. Rising temperatures due to global warming are expected to decrease the concentration of E. coli in the target watershed, where E. coli populations are strongly suppressed by predation and competition.
1. Introduction
Waterborne diseases have declined significantly over recent decades owing to socioeconomic development and public health measures, but global warming may reverse some of these positive trends [1]. In sub-Saharan Africa and South Asia, global warming is expected to increase deaths from shigellosis, cryptosporidiosis, and typhoid fever, while in some regions, a reduction in deaths attributable to viral infections is anticipated [2]. The dependence of pathogen survival on temperature is an important research topic for discussing the future direction of public health countermeasures [3].
Escherichia coli has long been regarded as a representative indicator of fecal contamination in water, although its suitability as a biological marker for a broad range of potential pathogens in wastewater has been questioned [4]. One reason for its use as an indicator is its ubiquitous presence in wastewater. A combined sewer system is designed to collect both rainwater and wastewater in the same pipes. A major drawback of such systems is the discharge of untreated wastewater into water bodies during heavy rainfall events, leading to environmental pollution through combined sewer overflows (CSOs). Monitoring E. coli using culture-based methods is common practice for assessing the impact of CSOs on public health in receiving waters [5], although Bacteroides spp., Enterococcus, and coliphages are also considered candidate indicator microorganisms for fecal contamination by CSOs [6,7]. In addition to culture-based methods, environmental DNA methods have been applied to evaluate the impact of CSOs [8]. However, concentrations estimated by culture-based methods are often higher than those obtained by molecular methods [3].
Another reason for the use of E. coli as an indicator microorganism is its poor reproducibility in water environments, since conditions outside the gastrointestinal tracts of animals are generally unfavorable for E. coli growth [9]. Regrowth of E. coli in drinking water distribution systems has been shown to be unlikely, except in certain cases of biofilm formation [10]. However, several recent studies have reported that specific strains of E. coli can survive for extended periods and potentially reproduce in extraintestinal environments [11,12,13], where a limited amount of growth substrates remains available [9,14]. The multiplication of E. coli is often suppressed by the co-presence of indigenous microorganisms, which is partly explained by competition for growth substrates in the environment, as these substrates can be utilized by both E. coli and indigenous bacteria [9].
Survival of E. coli in the water environment is influenced by abiotic factors such as light intensity and temperature as well as biotic factors such as predation and competition [3,15]. The increase in decay rates is often associated with an increase in salinity and sunlight intensity [16] and an increase in pH and dissolved oxygen [13]. Extended survival at lower temperature has been often observed [13,14,17]; a study reported higher survival periods (1 log reduction in 13 weeks) of E. coli O157 at 8 °C, while shorter survival periods of time (2 weeks in lake water and 11 weeks for autoclaved water) were reported at 25 °C [18]. In another study, different survivals depending on the species were reported [19]; E. coli and Salmonella typhimurium survived throughout the 28 experimental days at 4 °C in sediments, while Vibrio and Salmonella survived longer (28 days) than E. coli (14 days) and Shigella dysenteriae (4 days) at 30 °C [19].
It has been noted that predatory protozoan populations play an important role in the decay process of E. coli. Removal of indigenous microbiota generally prolongs the survival of E. coli [15,20]. In addition, bacteriophage infection has been considered a major factor contributing to the reduction in E. coli, as filtered river water shortened its survival compared with autoclaved river water [5]. The contribution of bacteriophages and protozoa at higher temperatures has also been observed for other species like Ralstonia solanacearum [21]. Longer survival is expected in sediments, where higher levels of growth substrates, lower UV intensity, and fewer predatory protozoa are present [7,22,23]. A case study reported that E. coli can survive for up to 14 days in the water column and up to 21 days in bed and bank sediments in an urban estuary [24]. Another case study showed that the time required for a 99% reduction in E. coli in sediments (27–37 d) was reported to be approximately six times that in the overlying water (5 d) [23].
There are many factors that influence the concentration of E. coli in aquatic environments, and key factors such as its origin and the role of predatory protozoan populations vary by location. The effect of global warming on E. coli survival will also be an important consideration in future public health discussions. This article presents a case study of E. coli concentrations and survival in an urban river draining a fully sewered area in Japan, with attention to several influencing factors.
2. Materials and Methods
2.1. Sample Origin
Surface water and sediment samples were collected near the Kamenoko Bridge on the Tsurumi River in Kanagawa Prefecture, Japan (a photograph of the sampling location is shown in Figure S1). Approximately 99% of the population in the target watershed was served by a public sewer system in 2022 according to the Yokohama City Office. Treated wastewater from four wastewater treatment plants—Naruse (0.87 m3/s), Tsurumigawa (0.35 m3/s), Aso (0.41 m3/s), and Tsuduki (2.11 m3/s)—accounted for 75% of the total flow at the sampling location in dry-weather conditions. The locations of the sampling point and wastewater treatment plants are mapped in Figure 1. The annual average water quality measurements at the sampling location, according to the Ministry of Land, Infrastructure, Transport and Tourism (MLIT), Japan [25], in dry-weather conditions in 2024 were BOD 3.2 mg/L, suspended solids (SS) 4.4 mg/L, total nitrogen (TN) 7.1 mg/L, total phosphorous (TP) 0.37 mg/L, NH4-N 0.69 mg/L, and total organic carbon (TOC) 4.4 mg/L. Although all of these four wastewater treatment plants were designed for a separate sewer system, the sampling location was affected by CSOs from another sewer network on rainy days. Water and sediment samples, collected in sterilized glass bottles in the morning, were immediately transported to the laboratory for analysis later the same day. E. coli concentrations were measured approximately once a month from 2022 to 2024. Both dry-weather and wet-weather days were selected as sampling dates to investigate the residual effects of CSOs on the concentration and antimicrobial resistance profiles of E. coli. The exact sampling dates and the amount of precipitation (rainfall) within the preceding 72 h are shown in Table S1.
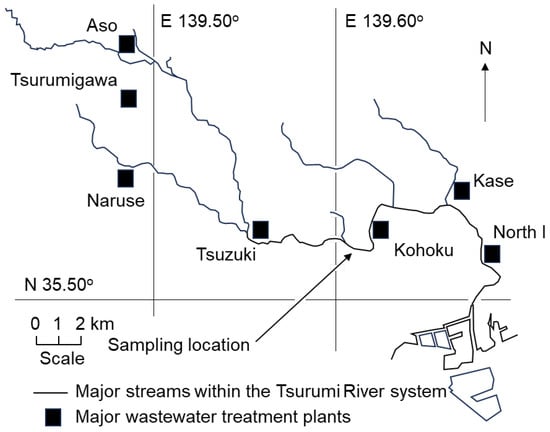
Figure 1.
A map of sampling location and wastewater treatment plants.
2.2. Decay Rate of Native E. coli Contained in the Sample
To examine the decay rates of E. coli inherent in the river water at the sampling location (Kamenoko bridge), changes in E. coli concentrations were monitored for one week in the samples kept in incubators set at 4 °C, 20 °C and 37 °C under dark, static conditions in the indoor laboratory. Original samples were collected on 7 June 2022 and 28 June 2022, and the same tests were conducted. The SS concentrations in the water samples for the test of decay rates ranged from 2 to 7 mg/L. To evaluate the persistence of the target microorganisms in sediment, the sediment samples were prepared in 50 mL glass bottles by mixing 30 g (wet weight) of sediments with overlying water (to a final volume of 50 mL). The changes in the number of E. coli in the supernatant of the sediment-containing samples were monitored for one week under the same conditions as those used for the measurement of the water samples. In the analysis of E. coli numbers in the water samples and in the sediment-containing water samples, the supernatants were obtained by allowing the sample to settle for 10 min after vigorously shaking the sampling bottles for two minutes.
2.3. Decay Rate of E. coli Strains Under Competitive and Non-Competitive Conditions
The decay rates of three E. coli strains (NBRC 13168, NBRC 13965, and NBRC 15034) in the water environment of the target location were examined under competitive and non-competitive conditions. NBRC13168 is known as a propagating strain for phages T1, T2, T3, T4, T5, T6 and T7. NBRC13965 is a male strain of W3110 and a propagating strain for phages f1, f2, Qβ, M12, f1, and MS2. A colony of the strain was picked up from the agar plate and incubated in liquid broth (the constituents were glucose 1.5 g/L, peptone 10 g/L, yeast extract 5 g/L, MgSO4-7H2O 0.2 g/L, MnSO4-4H2O 0.05 g/L, NaCl 5 g/L, pH at 7.2) for four hours. The solution with approximately 109–1010 CFU/mL was diluted 100 times with phosphorous buffer solution to minimize the organic constituents before 1000-fold dilution with river water taken from the sampling location. The decay rates of the strains were examined in three types of river water collected on 12 December 2023: (1) untreated river water, (2) autoclaved river water, and (3) filtered river water (Sterivex PVDF, 0.22 μm). The autoclaved river water simulated a biologically stable environment, while the filtered river water simulated bacteria-free river water with the presence of coliphages. Changes in the number of E. coli during incubation at 37 °C under aerobic conditions with shaking were monitored for 5 days in the same manner as the measurement of the decay rate for native E. coli.
2.4. Enumeration of E. coli Numbers
An appropriate volume (0.1–10 mL) of each water sample was filtered through a nitrocellulose membrane (47 mm diameter, 0.45 μm pore size; Millipore, Billerica, MA, USA) to collect the bacteria present in the sample. Membranes with different sample volumes were prepared to obtain countable numbers of colonies on agar plates. Each membrane filter was then placed on an agar plate prepared with the selective chromogenic medium, CHROMagar ECC (CHROMagar, Paris, France), which allows for simultaneous detection and differentiation of E. coli and other Enterobacterales based on colony coloration after incubation at 37 °C for 24 h. The number of blue colonies on the plates was counted as the number of E. coli. From each sample, 8 to 40 colonies (the exact number of colonies for each sample is shown in Table S1) were randomly selected and streaked onto fresh CHROMagar ECC plates to obtain isolates after 24 h of incubation at 37 °C.
2.5. Disk-Diffusion Test for Antibiotic Susceptibility
The resistant profile to several antimicrobials (ampicillin (ABPC), cefazolin (CEZ), cefotaxime (CTX), meropenem (MPM), gentamicin (GM), sulfamethoxazole/trimethoprim (ST), tetracycline (TC) and levofloxacin (LVX)) was determined to characterize the obtained E. coli isolates, as described in previous research [26]. The disk-diffusion test based on the Kirby–Bauer method was conducted to identify the susceptibility of isolates on the Mueller–Hinton agar medium (Eiken Chemical Co. Ltd., Tokyo, Japan). The susceptibility was interpreted as susceptible (S), intermediate (I), or resistant (R) based on the comparisons of the inhibition-zone diameters around the diffusion disks with the criteria (CLSI M-100-28) [27]. The quality check strain used in this test was E. coli NRBC 15034, which is available in Japan as the identical strain of ATCC 25922, recommended by CLSI [27]. The quality control ranges for ATCC 25922 (Table 4A-1 in the CLSI document [27]) were referred to. In this study, CTX resistance was evaluated with the newer cutoff (4 mg/L) to broaden the definition of resistant bacteria [28].
3. Results
3.1. Numbers of E. coli at the Sampling Point
Figure 2 shows the E. coli concentration in the samples taken from the target river. The results obtained in this study (Figure 2a) include the results obtained in both dry- and wet-weather conditions. The closed plots represent results obtained with more than 10 mm of precipitation within the preceding 72 h, while the open plots represent results not influenced by rain events (less than 10 mm of precipitation within the preceding 72 h). Most of the observed concentrations exceeding 10 CFU/mL were recorded on wet-weather days, whereas most samples collected on dry-weather days contained less than 10 CFU/mL of E. coli. The pronounced impact of rainfall on E. coli concentrations in receiving waters has been documented in many studies [6,7,8]. For example, during rainfall events in a river in Tokyo, extremely high concentrations of E. coli—more than 100 times higher than those observed on dry-weather days—have been reported [5]. According to the monthly monitoring conducted by the Ministry of Land, Infrastructure, Transport and Tourism (MLIT), Japan [25], as shown in Figure 2b based on the downloaded numbers in Table S2, the average E. coli count at the same location was 1.9 CFU/mL (n = 26, 2022–2024), with a maximum of 9.8 CFU/mL. The more stable values observed by the MLIT compared to our observations were due to sampling times, because sampling by the MILT was planned to avoid the effect of wet-weather conditions. The results obtained in this study with dry-weather condition were consistent with the monitoring by the MLIT, while high values above 10 CFU/mL were concentrated with closed plots in Figure 2a, measured in wet-weather conditions. The high concentration of E. coli (33 CFU/mL on 8 August 2023, and 11 CFU/mL on 15 October 2024) without recorded precipitation was an exception, probably due to localized heavy rain that was not recorded by the Meteorological Agency’s monitoring station in Yokohama, Japan. The highest value (145 CFU/mL, approximately 70-fold higher concentration compared to the average value on dry-weather days) was observed on 13 May 2024.
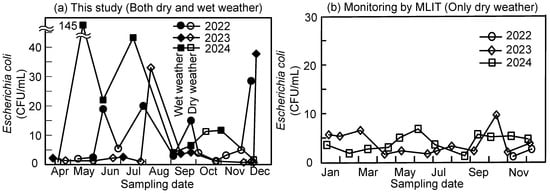
Figure 2.
E. coli concentrations at the sampling point (Kamenoko bridge). (a) This study; closed plots indicate data points where more than 10 mm of rainfall occurred in the 72 h preceding each sampling occasion. (b) Monthly spot monitoring by MLIT; all results were obtained for dry-weather days.
3.2. Susceptibility of the Isolates to Antimicrobials
Table 1, based on the numbers of isolates shown in Table S3, presents the results of antimicrobial susceptibility tests of the E. coli isolates obtained in this study, in comparison with clinical and livestock isolates. The resistance profiles of the isolates obtained in this study showed greater similarity to those of clinical isolates, but poor similarity to those of farm-animal-origin isolates. Higher prevalences (18.7–41.2%) of resistance to β-lactams (ABPC, CEZ, and CTX) were observed, comparable to those of clinical isolates [29]. In contrast, the resistance ratios to CEZ and CTX in the environmental isolates were much higher than those observed in isolates from livestock animals [30], although resistance to ABPC was ubiquitous regardless of the origin of the isolates. The voluntary withdrawal of ceftiofur in 2012 led to a dramatic decrease in CTX resistance in E. coli from healthy broilers at farms in Japan [31]. Since then, CTX resistance has been considered a useful indicator for distinguishing human-origin E. coli from farm-animal-origin E. coli [32]. On the other hand, the resistance ratios to TC and ST among livestock isolates were much higher than those of the environmental isolates in this study, likely reflecting the antimicrobials commonly used in livestock production.

Table 1.
The resistant ratios to antimicrobials in E. coli. A comparison of environmental isolates obtained in this study to the clinical and livestock isolates by national surveillance.
CTX resistance, which reflects the source of E. coli, was plotted against the E. coli concentration of individual samples (n = 29) in Figure 3. Although an increase in E. coli concentration due to CSOs during wet-weather conditions was expected to cause a corresponding increase in CTX resistance, the experimental results did not show a positive correlation (r = −0.15). On 13 May 2024, during intense rainfall, only 2 out of 16 isolates (12.5%) were resistant to CTX, even though the E. coli concentration reached 145 CFU/mL, the highest value observed in this study. In contrast, on 24 July 2024, when the E. coli concentration was only 1.1 CFU/mL, all 36 examined isolates were resistant to CTX. This observation suggests that a high E. coli concentration does not necessarily correspond to a high proportion of CTX-resistant isolates.
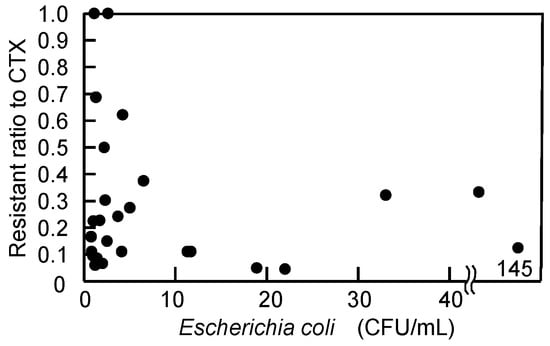
Figure 3.
Effect of number of E. coli in the samples on the resistant ratio of isolates to cefotaxime. A negative insignificant correlation (r = −0.15, n = 29) was found.
3.3. Decay Rate of Native E. coli in Overlying Water and Sediment-Containing Water
Figure 4 shows the survival of native E. coli in overlying water and sediment-containing water under different temperatures. The results of the repeated experiments showed similar trends despite differences in the initial concentrations, as presented in Tables S4 and S5. The increase in temperature resulted in the increase in decay rate. At 20 °C, E. coli counts in water decreased to 10% of the initial count after 3-day incubation. By increasing the incubation temperature from 20 °C to 37 °C, a higher decay rate was observed, while more than one week was required for a 90% reduction in E. coli numbers at 4 °C. Figure 4b shows higher survival of E. coli in sediment-containing water compared with that in overlying water. Following the rapid decrease in E. coli counts in the initial three days, a slower reduction continued. Only a 20% decrease was observed at 4 °C in the sediment sample for seven days, while a 90% decrease was observed at 20 and 37 °C for seven days. The results imply that E. coli can survive in sediments for a longer time than in overlying water, especially in low-temperature conditions.
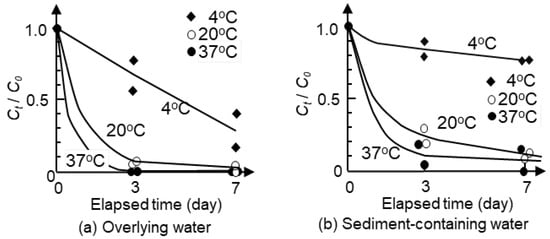
Figure 4.
Decay rates of native E. coli at different temperatures in overlying water and water-containing samples. Plots are the results of repeated (twice) experiments. Smoothed curves were drawn to connect the data points.
3.4. Decay Rate of E. coli Strains Under Competitive and Non-Competitive Conditions
All three E. coli strains (NBRC 13168, NBRC 13965 and NBRC 15034) showed similar trends regardless of the host ranges of the strains to coliphages, as shown in Figure 5; E. coli added to non-sterile river water decreased below the detection limit within 2 days, while a considerable increase in E. coli concentration was observed in autoclaved river water. This result implied that autoclave treatment of river water degraded refractory organic compounds into assimilable compounds which can be utilized by E. coli strains [33]. A retarded decrease compared to the non-sterile condition was observed in the case of E. coli in the filtered river water, as described by previous studies [15,20]. However, even with filtration, the E. coli concentration was below the detection limit after 2 days of incubation, possibly due to the effect of residual coliphages in the filtered sample [17]. These results suggest the combined contribution of predation by protists, infection by coliphages, or competition with other bacteria to the decrease in E. coli concentration in the environment.
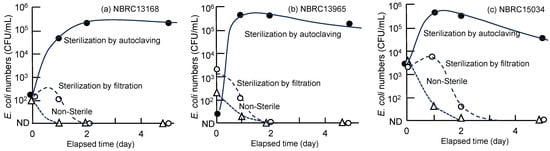
Figure 5.
Decay rate of three E. coli strains in competitive and non-competitive environments at 37 °C.
4. Discussion
4.1. Monitoring During Wet-Weather Conditions
The concentration level shown in Figure 2a is quite different from that of government monitoring data in Figure 2b. Government monitoring is usually carried out to understand baseline environmental conditions unaffected by wet weather, although sanitary indicator levels, such as E. coli in water quality measurements, are known to be significantly influenced by CSOs [5,6,7,8,34]. Several simplified methods have been proposed for the frequent monitoring of highly variable E. coli concentrations in river water [12,35], although these simplified methods are not actually employed in governmental monitoring. Usual baseline monitoring carried out in dry-weather conditions may not be sufficient to understand the health risk of the target watershed. In addition, the microorganisms in sediments originating from wet-weather deposition (CSO) will raise the dry-weather baseline level through resuspension of sediments, in which E. coli can survive for a long time in spite of the rapid decrease expected in the water phase [7,22,23]. Our monitoring results, showing large fluctuations in the proportion of CTX-resistant isolates under low E. coli concentration conditions, suggest that the E. coli detected in samples on dry-weather days may have originated from the regrowth of a few individuals in the environment. Regrowth of E. coli in aquatic environments is plausible, as indicated by recent studies [11,12,13], which reported the utilization of residual growth substrates in river water [9,14]. Such regrowth may occur in sediments rather than in the overlying water, reflecting differences in suppression pressure. Sediments can serve as sources of E. coli on dry-weather days.
4.2. Effect of Temperature on Survival of Pathogens
Figure 4 compares the results at 4, 20, and 37 °C, showing longer survival at lower temperatures, and this observed trend was consistent with previous studies [13,14,17,18,19]. The temperature dependence of E. coli decay rates can be partly explained by differences in the biological activity not only of E. coli itself but also of its predators and coliphages. Higher temperatures are favorable for predation by protozoa and infection by viruses. The lower survival under high-temperature conditions can also be applicable to the survival in sediments, as shown in Figure 4b, although E. coli can survive for a longer time in sediments than in overlying water. The higher survival in sediments was consistent with previous studies [7,22,23].
Global warming is generally considered to increase the risk of infectious diseases in humans [1,2]. As mentioned in the previous section, the highly fluctuating ratio of CTX-resistant isolates under dry-weather conditions in this study suggests probable regrowth of E. coli in aquatic environments, although the time-averaged resistance ratio may not be influenced by the regrowth, judging from a report in which the metabolic burden imposed by a plasmid was not a factor in the decrease in the survival [17]. Higher temperatures approaching 37 °C are favorable for such regrowth. However, changes in E. coli concentrations are determined by the balance between decay and growth. At least in the case of E. coli, global warming is thought to reduce overall concentrations in the environment by accelerating the decay rate, although highly variable antimicrobial resistances may be anticipated by the enhanced regrowth. Note that this discussion is based solely on the decay rates obtained in this study. Hydrological modeling incorporating bacterial source tracking [16,36] is required to distinguish between wildlife and human contributions in a more generalized discussion of temperature-dependent change.
4.3. Importance of Biological Factors in the Decay Rate
In this study, the observed half-life periods of E. coli at 37 °C and 20 °C (shown in Figure 4 and Figure 5) were less than one day. A comparable high decay rate was observed in a study using unfiltered Rhine River water in the Netherlands at 20 °C, indicating a high contribution of biological factors [15], whereas higher survivals were reported in several studies [18,19,23]. The shorter survival observed in this study may have resulted from the high biological activity of the samples affected by treated wastewater, whereas the reference studies used relatively clean water, such as reservoir water for drinking water supply. Although a higher salinity and stronger sunlight (UV) intensity have been shown to accelerate the decay rate [16], they may have only minor effects in the target sampling site, which is characterized by complete freshwater with a short hydraulic retention time, because E. coli populations at the sampling site are considered to be strongly suppressed by predation and competition.
One possible reason for the short survival of E. coli in overlying water is the fact that treated wastewater accounted for approximately 75% of the dry-weather total flow at the sampling site. The sewage effluent treated by the activated sludge process generally contains 100–102 PFU/mL of F-specific coliphage and 101–103 PFU/mL of F-specific coliphage, and it is relatively more resistant to chlorination than E. coli [37]. A high contribution of coliphage to the reduction in E. coli could also be seen from the rapid reduction in E. coli even in filtered samples in this study. Our study implies that lower survival of E. coli can be expected in water bodies affected by treated wastewater, especially at higher temperatures.
5. Conclusions
This study presents a case study on the factors influencing E. coli concentrations in an urban river draining a fully sewered area. An approximately 70-fold higher concentration than the average dry-weather level obtained from governmental routine monitoring (1.9 CFU/mL) was observed under wet-weather conditions, probably due to the effects of combined sewer overflows. A very short survival of E. coli (less than one day) was expected in the unfiltered overlying water, likely due to the effects of bacteriophages, protozoan predation, and bacterial competition, whereas a longer survival was expected in the sediment. A considerable fraction of E. coli in the overlying water on dry-weather days was probably supplied from the resuspension of the sediment. The highly variable antimicrobial resistance among E. coli populations under dry-weather conditions was possibly caused by the regrowth of a limited number of E. coli individuals in the sediment. Rising temperatures associated with global warming are expected to decrease E. coli concentrations in the target watershed, where E. coli populations are considered to be strongly suppressed by predation and competition. Some of the characteristics of the watershed may be attributed to the fact that treated wastewater accounted for approximately 75% of the dry-weather total flow.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17203026/s1, Figure S1: Photograph of the sampling site at the Tsurumi River; Table S1: Date of sampling, E. coli concentration, precipitation (rainfall), examined number of isolates for microbial susceptibility test; Table S2: Results of MLIT monitoring at Kamenoko bridge at the Tsurumi River; Table S3: Results on antimicrobial susceptibility test for isolates taken in 2024; Table S4: Results of decay rates of native E. coli at the sampling location in the samples taken on 7 June 2022; Table S5: Results of decay rates of native E. coli at the sampling location in the samples taken on 28 June 2022.
Author Contributions
Writing—original draft preparation and editing, T.U.; Writing—review, S.G.; Experimental supervision, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from the Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number: 24 K15344 under Grant-in-Aid for Scientific Research (C) program).
Data Availability Statement
The original data presented in this study are included in the article and in the Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the experimental support provided by R. Aono, T. Hashimoto, A. Otani and M. Waki (School of Bioscience and Biotechnology, Tokyo University of Technology).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef]
- Chua, P.L.C.; Huber, V.; Ng, C.F.S.; Seposo, X.T.; Madaniyazi, L.; Hales, S.; Woodward, A.; Hashizume, M. Global projections of temperature-attributable mortality due to enteric infections: A modelling study. Lancet Planet. Health 2021, 5, e436–e445. [Google Scholar] [CrossRef]
- Korajkic, A.; Wanjugi, P.; Brooks, L.; Cao, Y.; Harwood, V.J. Persistence and decay of fecal microbiota in aquatic habitats. Microbiol. Mol. Biol. Rev. 2019, 83, e00005-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liang, J.; Wang, Y.; Tao, Y.; Lu, Y.; Wang, A. A global perspective on microbial risk factors in effluents of wastewater treatment plants. J. Environ. Sci. 2024, 138, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Poopipattana, C.; Suzuki, M.; Furumai, H. Impact of long-duration CSO events under different tidal change conditions on distribution of microbial indicators and PPCPs in Sumida river estuary of Tokyo Bay, Japan. Environ. Sci. Pollut. Res. 2021, 28, 7212–7225. [Google Scholar] [CrossRef] [PubMed]
- Ekhlas, D.; Kurisu, F.; Kasuga, I.; Cernava, T.; Berg, G.; Liu, M.; Furumai, H. Identification of new eligible indicator organisms for combined sewer overflow via 16S rRNA gene amplicon sequencing in Kanda River, Tokyo. J. Environ. Manag. 2021, 284, 112059. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Martín-Díaz, J.; Vinas-Balada, E.; Calero-Caceres, W.; Lucena, F.; Blanch, A.R. Mobilisation of microbial indicators, microbial source tracking markers and pathogens after rainfall events. Water Res. 2017, 112, 248–253. [Google Scholar] [CrossRef]
- Zan, R.; Blackburn, A.; Plaimart, J.; Acharya, K.; Walsh, C.; Stirling, R.; Kilsby, C.G.; Werner, D. Environmental DNA clarifies impacts of combined sewer overflows on the bacteriology of an urban river and resulting risks to public health. Sci. Total Environ. 2023, 889, 164282. [Google Scholar] [CrossRef]
- Ishii, Y.; Kurisu, F.; Kasuga, I.; Furumai, H. Competition for growth substrates in river water between Escherichia coli and indigenous bacteria illustrated by high-resolution mass spectrometry. Lett. Appl. Microbiol. 2021, 72, 133–140. [Google Scholar] [CrossRef]
- Abberton, C.L.; Bereschenko, L.; van der Wielen, P.W.; Smith, C.J. Survival, biofilm formation, and growth potential of environmental and enteric Escherichia coli strains in drinking water microcosms. Appl. Environ. Microbiol. 2016, 82, 5320–5331. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Rumball, N.A.; Alm, E.W.; McLellan, S.L. Genetic Determinants of Escherichia coli survival in beach sand. Appl. Environ. Microbiol. 2023, 89, e0142322. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.E.; El-Liethy, M.A.; Abia, A.L.K.; Hemdan, B.A.; Shaheen, M.N. Survival of E. coli O157:H7, Salmonella typhimurium, HAdV2 and MNV-1 in river water under dark conditions and varying storage temperatures. Sci. Total Environ. 2019, 648, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sutton, N.B.; Wagner, T.V.; Rijnaarts, H.H.M.; van der Wielen, P.W.J.J. Influence of combined abiotic/biotic factors on decay of P. aeruginosa and E. coli in Rhine River water. Appl. Microbiol. Biotechnol. 2024, 108, 294. [Google Scholar] [CrossRef]
- Liang, L.; Goh, S.G.; Gin, K.Y.H. Decay kinetics of microbial source tracking (MST) markers and human adenovirus under the effects of sunlight and salinity. Sci. Total Environ. 2017, 574, 165–175. [Google Scholar] [CrossRef]
- Flint, K.P. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 1987, 63, 261–270. [Google Scholar] [CrossRef]
- Wang, G.; Doyle, M.P. Survival of enterohemorrhagic Escherichia coli 0157:H7 in water. J. Food Prot. 1998, 61, 662–667. [Google Scholar] [CrossRef]
- Abia, A.L.K.; Ubomba-Jaswa, E.; Momba, M.N.B. Competitive Survival of Escherichia coli, Vibrio cholerae, Salmonella typhimurium and Shigella dysenteriae in Riverbed Sediments. Microb. Ecol. 2016, 72, 881–889. [Google Scholar] [CrossRef]
- Lim, C.H.; Flint, K.P. The effects of nutrients on the survival of Escherichia coli in lake water. J. Appl. Bacteriol. 1989, 66, 559–569. [Google Scholar] [CrossRef]
- Alvarez, B.; López, M.M.; Biosca, E.G. Influence of native microbiota on survival of Ralstonia solanacearum phylotype II in river water microcosms. Appl Environ Microbiol. 2007, 73, 7210–7217. [Google Scholar] [CrossRef]
- Liu, B.; Lee, C.W.; Bong, C.W.; Wang, A.J. Investigating Escherichia coli habitat transition from sediments to water in tropical urban lakes. PeerJ 2024, 12, e16556. [Google Scholar] [CrossRef]
- Kim, M.; Wuertz, S. Survival and persistence of host-associated Bacteroidales cells and DNA in comparison with Escherichia coli and Enterococcus in freshwater sediments as quantified by PMA-qPCR and qPCR. Water Res. 2015, 87, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Schang, C.; Lintern, A.; Cook, P.L.M.; Osborne, C.; McKinley, A.; Schmidt, J.; Coleman, R.; Rooney, G.; Henry, R.; Deletic, A.; et al. Presence and survival of culturable Campylobacter spp. and Escherichia coli in a temperate urban estuary. Sci. Total Environ. 2016, 569–570, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Land, Infrastructure, Transport and Tourism, Japan, Water Information System. Available online: http://www1.river.go.jp/ (accessed on 1 April 2025).
- Urase, T.; Sato, T. Quantitative Monitoring of Resistance in Escherichia coli to Clinically Important Antimicrobials in an Urban Watershed. J. Water Environ. Technol. 2016, 14, 341–349. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). M100 Performance Standard for Antimicrobial Susceptibility Testing, 28th ed.; CLSI: Malvern, PA, USA, 2018. [Google Scholar]
- Tsutsui, H.; Urase, T. Characterization of extended spectrum β-lactamase-producing Escherichia coli in the environment isolated with different concentrations of cefotaxime. J. Water Environ. Technol. 2019, 17, 262–272. [Google Scholar] [CrossRef]
- Antimicrobial Resistance (AMR) One Health Platform System. Available online: https://amr-onehealth-platform.ncgm.go.jp/ (accessed on 1 April 2025).
- National Veterinary Assay Laboratory; Ministry of Agriculture, Forestry and Fisheries. Countermeasures of Antimicrobial resistance (AMR). Available online: https://www.maff.go.jp/nval/yakuzai/yakuzai_AMR_2.html (accessed on 1 April 2025).
- Hiki, M.; Kawanishi, M.; Abo, H.; Kojima, A.; Koike, R.; Hamamoto, S.; Asai, T. Decreased resistance to broad-spectrum cephalosporin in Escherichia coli from healthy broilers at farms in Japan after voluntary withdrawal of ceftiofur. Foodborne Pathog. Dis. 2015, 12, 639–643. [Google Scholar] [CrossRef]
- Urase, T.; Okazaki, M.; Tsutsui, H. Prevalence of ESBL-producing Escherichia coli and carbapenem-resistant Enterobacteriaceae in treated wastewater: A comparison with nosocomial infection surveillance. J. Water Health 2020, 18, 899–910. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, L.; Gong, D.; Lu, J. Effects of sterilization treatments on the analysis of TOC in water samples. J. Environ. Sci. 2010, 22, 789–795. [Google Scholar] [CrossRef]
- Sylvestre, É.; Burnet, J.B.; Smeets, P.; Medema, G.; Prévost, M.; Dorner, S. Can routine monitoring of E. coli fully account for peak event concentrations at drinking water intakes in agricultural and urban rivers? Water Res. 2020, 170, 115369. [Google Scholar] [CrossRef]
- Satoh, H.; Katayose, Y.; Hirano, R. Simple enumeration of Escherichia coli concentrations in river water samples by measuring β-d-glucuronidase activities in a microplate reader. Water Sci. Technol. 2021, 83, 1399–1406. [Google Scholar] [CrossRef]
- Jeong, J.; Wagner, K.; Flores, J.; Cawthon, T.; Her, Y.; Osorio, J.; Yen, H. Linking watershed modeling and bacterial source tracking to better assess E. coli sources. Sci. Total Environ. 2019, 648, 164–175. [Google Scholar] [CrossRef]
- Kelmer, G.A.R.; Ramos, E.R.; Dias, E.H.O. Coliphages as viral indicators in municipal wastewater: A comparison between the ISO and the USEPA methods based on a systematic literature review. Water Res. 2023, 230, 119579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).