Abstract
The extensive use of antibiotics in animal husbandry leads to the release of unmetabolised residues and the dissemination of antimicrobial resistance genes (ARGs) in manure, posing environmental and public health challenges. Conventional treatment technologies, including hydrolysis, photodegradation, and phytoremediation, are often constrained by incomplete mineralisation, high cost, and environmental variability. Biocatalytic and microbially mediated processes are increasingly recognised as sustainable alternatives. Enzymes, which in clinical contexts confer resistance, can, in environmental matrices, catalyse the dismantling of antibiotic scaffolds, attenuating bioactivity and promoting detoxification. Catalytic classes such as hydrolases, transferases, and oxidoreductases mediate diverse transformations, including hydrolytic cleavage, functional group transfer, and oxidative modification. Microbial consortia and bioaugmentation further enhance biodegradation, while biochar and other amendments reduce ARG persistence. Advances in multi-omics, enzyme engineering, and immobilisation have expanded catalytic repertoires, improved stability, and enabled integration with composting, anaerobic digestion, and hybrid bioprocesses. Nonetheless, incomplete degradation, recalcitrant intermediates, and horizontal gene transfer remain challenges. Importantly, since degradation products may leach into soils and aquatic systems, optimising these processes is critical to prevent residues from entering the water cycle. This review synthesises advances in microbial and enzymatic degradation strategies, highlighting opportunities for sustainable manure management while mitigating water pollution risks.
Keywords:
manure; microbiome; enzymes; antibiotics; biodegradation; antimicrobial resistance; water pollution 1. Introduction
The global intensification of livestock production has been accompanied by widespread and often prophylactic antibiotic use, both for therapeutic purposes and as growth-promoting agents. Approximately 70% of all antibiotics produced worldwide are administered to farm animals, with sheep and pigs receiving the highest per-kilogram doses, whereas poultry and cattle generally receive lower amounts due to shorter production cycles and reduced veterinary intervention for outdoor husbandry, respectively [1]. This extensive application results in the accumulation of unmetabolised antibiotic residues in manure, which subsequently enter agricultural soils and aquatic ecosystems, creating persistent environmental reservoirs of antimicrobial resistance (AMR) and mobile resistance determinants [2].
Environmental contamination with antibiotics constitutes a complex and multifaceted public health challenge. Residues excreted by livestock persist in soil, water, and sediments, exerting selective pressure on microbial communities, promoting the emergence of antibiotic-resistant bacteria (ARB) and antimicrobial resistance genes (ARGs), and facilitating horizontal gene transfer [3,4]. Beyond their immediate impact on resistance, these residues and their transformation products pose long-term risks of leaching into groundwater and surface waters, thereby contributing to water pollution and human exposure [1]. These compounds are often chemically stable under environmental conditions, and their presence has been linked to increased human exposure via food, water, and direct contact, underscoring the urgent need for regulatory oversight and effective mitigation strategies [2,3].
Livestock manure is a major vector for the dissemination of antibiotic residues and ARGs. Soils amended with manure frequently contain high concentrations of antibiotics, ARB, and mobile genetic elements, which enhance horizontal gene transfer across diverse bacterial taxa and may transfer resistance to clinically relevant pathogens [5,6]. Residual antibiotics such as doxycycline, oxytetracycline, and ciprofloxacin have been shown to disrupt microbial community structure, reduce enzymatic activity essential for organic matter decomposition, and impair nutrient cycling, while repeated applications of manure from antibiotic-treated livestock sustain ARGs over multiple years [7,8,9].
Microbial and enzymatic degradation constitute a key natural attenuation pathway for mitigating antibiotic persistence and the dissemination of ARGs. Microorganisms catalyse the breakdown of antibiotics and contribute to the reduction in resistance determinants, thereby alleviating selective pressures that drive AMR emergence [10,11]. Aerobic composting and anaerobic digestion (AD) have demonstrated significant reductions in antibiotic concentrations and ARG abundance through high-temperature inactivation of resistant microbes, suppression of horizontal gene transfer, and optimisation of physicochemical conditions [12,13]. Additionally, enzymatic degradation mediated by ligninolytic fungi and oxidative enzymes, including laccases and peroxidases, further enhances the decomposition of recalcitrant antibiotic compounds, contributing to resistance attenuation [10,14,15].
Biotechnological interventions that harness microbial metabolism and enzymatic activity offer sustainable and scalable strategies to mitigate antibiotic residues and ARGs in livestock manure. Thermophilic AD generally outperforms mesophilic regimes in reducing ARGs, although efficacy is influenced by antibiotic class, manure composition, and operational conditions [12,13]. Emerging approaches, such as hydrodynamic cavitation combined with natural oils, have demonstrated >99% removal of both Gram-negative and Gram-positive resistant bacteria, representing environmentally sustainable and economically viable alternatives to conventional chemical treatments [16].
Given the rising global demand for animal-derived foods and the associated risk of environmental antibiotic dissemination, particularly in regions of intensive livestock farming, understanding the mechanistic basis of microbial and enzyme-mediated antibiotic degradation is imperative [10,14]. Although these strategies primarily aim to control pollutants at the source, their ultimate significance lies in preventing antibiotic residues and degradation products from entering and polluting aquatic environments. This review provides a comprehensive synthesis of biotechnological strategies for microbial and enzyme-based degradation of antibiotics in livestock manure, focusing on mechanistic pathways, effectiveness in mitigating antimicrobial resistance, and identification of knowledge gaps to optimise sustainable interventions that support environmental safety, public health, and circular bioeconomy objectives.
2. Occurrence and Fate of Antibiotics in Livestock Manure
The widespread use of antibiotics in livestock production has become a major environmental concern. In 2017, more than 93,000 tonnes were administered worldwide for disease prevention, treatment, and growth promotion, and global usage is projected to exceed 104,000 tonnes by 2030 [17]. However, only 10–40% of these compounds are metabolised by animals, while the remainder is excreted largely unchanged in urine and faeces [18]. Residues from major antibiotic classes, including β-lactams, tetracyclines, sulphonamides, and macrolides, are frequently detected in livestock manure, as confirmed by recent surveys across pig and poultry farms in 5 European countries [19]. Due to their persistence and resistance to natural degradation, these residues accumulate in manure and act as a pathway for dissemination into soils, water bodies, and food crops. This dissemination is of particular concern because both parent compounds and degradation byproducts can leach into groundwater and surface waters, contributing to pollution of aquatic environments [18]. Antibiotics used in livestock are excreted in large quantities, accumulate in manure, and disseminate into soils, water, and crops, as illustrated in Figure 1.
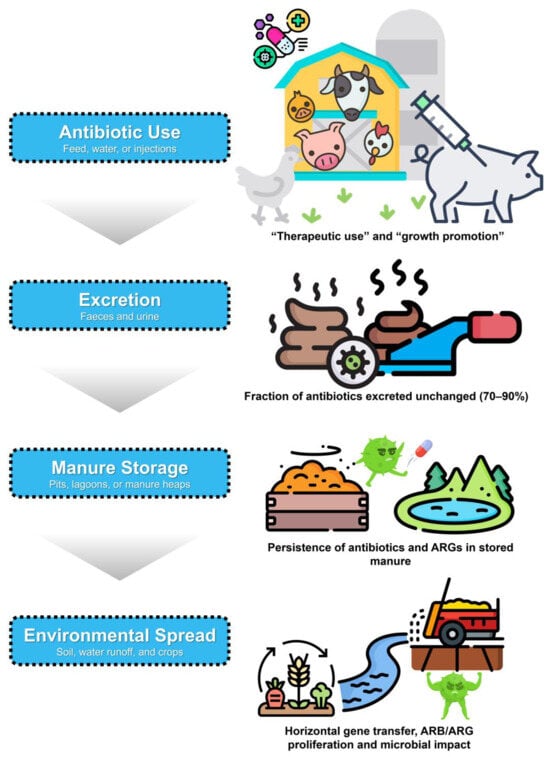
Figure 1.
Pathways of antibiotic entry and persistence in livestock manure. Antibiotics given to animals for therapy or growth promotion are partly metabolised, but much is excreted into manure together with resistant bacteria and genes. Manure storage and field application enable residues and resistance elements to enter soil, water, and crops, driving antimicrobial resistance. Microbial and enzymatic processes such as composting and anaerobic digestion can mitigate these risks and reduce environmental dissemination.
2.1. Classes of Antibiotics Frequently Detected
Among the diverse antibiotics used in livestock production, tetracyclines, sulphonamides, macrolides, and fluoroquinolones are the most frequently reported in manure. Tetracyclines (e.g., oxytetracycline, chlortetracycline, doxycycline) are extensively applied in poultry, swine, and cattle owing to their broad-spectrum antimicrobial activity and relatively low cost. Their strong binding affinity to divalent cations and soil particles promotes persistence in manure, where they can undergo epimerization and other transformations into stable metabolites that retain partial biological activity [20]. Sulphonamides (e.g., sulfamethoxazole, sulfadiazine, sulfamethazine) are likewise prevalent; their high aqueous solubility enhances their environmental mobility, thereby facilitating leaching and runoff into soils and water bodies when manure is used as fertiliser [21]. Macrolides, such as erythromycin and tylosin, are commonly administered to prevent and treat respiratory infections in livestock, and their residues are consistently detected in manure. These compounds are relatively stable and may accumulate in amended soils, contributing to long-term selective pressure on microbial communities [22]. Fluoroquinolones (e.g., ciprofloxacin, enrofloxacin), though applied in smaller amounts compared to tetracyclines, are structurally complex and halogenated, rendering them highly resistant to biodegradation and particularly concerning for the emergence of multidrug-resistant bacteria [23]. Overall, the recurrent detection of these antibiotic classes in animal waste highlights their environmental persistence, their role in disseminating antimicrobial residues beyond farm boundaries, and the challenges associated with their degradation and mitigation (Table 1).

Table 1.
Examples of antibiotic residue occurrence in livestock manure.
2.2. Physicochemical Properties Influencing Microbial Degradation
The persistence and transformation of antibiotics in livestock manure are strongly influenced by their intrinsic chemical structures and physicochemical properties. Functional groups such as lactone esters, β-lactam amides, sulphonamides, and polycyclic aromatic systems dictate susceptibility to hydrolysis, photolysis, oxidation, and enzymatic attack. These features not only determine the environmental stability of antibiotic residues but also shape the microbial and enzymatic pathways available for their degradation. Importantly, these pathways influence whether residues are fully detoxified or transformed into stable intermediates that may still enter the water cycle. Below, key antibiotic classes are discussed in relation to their structural chemistry and degradation behaviour [14,15].
2.2.1. Macrolides
Macrolides are large polycyclic lactones (14–16-membered rings) bearing multiple hydroxyl and amine substituents, often decorated with sugar residues. Their rigid scaffolds lack extended conjugation, conferring high resistance to direct photolysis under natural sunlight. Only high-energy UV or reactive oxygen species (ROS) induce peripheral modifications without disrupting the lactone core [30]. In contrast, microbial esterases specifically hydrolyse the lactone bond; for instance, EstX cleaves tylosin and tilmicosin to yield inactive ring-opened products [31]. Thus, while macrolides are chemically recalcitrant, they remain vulnerable to dedicated lactonases.
2.2.2. Fluoroquinolones
Fluoroquinolones possess a rigid bicyclic quinolone-4-one core with a C3 carboxylate, C4 carbonyl, a C6 fluorine, and a heterocyclic substituent at C7. This conjugated aromatic system strongly absorbs UV light, making fluoroquinolones more photoreactive than macrolides. Photolysis and advanced oxidation processes cleave the piperazine substituent, remove fluorine, and decarboxylate the C3 position, while hydroxyl radicals introduce aromatic hydroxylations [32]. Despite these transformations, the fluorinated core remains chemically stable, slowing complete breakdown. Microbial degradation proceeds slowly and primarily targets the side chains rather than the nucleus [33].
2.2.3. Aminoglycosides
Aminoglycosides are highly polar and polycationic, built on an aminocyclitol core glycosidically linked to multiple amino sugars. Their lack of conjugated chromophores renders them photochemically inert in aqueous systems. Instead, they undergo indirect photodegradation when sorbed onto natural organic matter, which facilitates ROS attack [34]. Microbial inactivation relies on cleavage of glycosidic bonds or sugar moieties; enzymes such as the FAD-dependent dehydrogenase AquKGD oxidise and detach the amino sugar groups, yielding inactive derivatives [35]. This dependence on specialised enzymatic pathways explains the persistence of aminoglycosides in untreated environments.
2.2.4. Tetracyclines
Tetracyclines consist of a linear four-ring scaffold with multiple functional groups, including a dimethylamino at C4 and keto-enols at C1/C2 and C11/C12. These features make tetracyclines amphoteric and strong metal chelators. Their conjugated system absorbs UV light, allowing photodegradation through radical-mediated ring cleavage and epimerization at the C4 dimethylamino group [36]. Hydrolysis can also lead to dehydration and isomerisation, producing inactive forms such as 4-epitetracycline and anhydrotetracycline. While tetracyclines degrade more readily than macrolides, their metal-chelating ability and soil sorption contribute to environmental persistence [37].
2.2.5. β-Lactams
β-Lactams (penicillins and cephalosporins) are defined by a strained four-membered β-lactam amide ring fused to a thiazolidine or dihydrothiazine ring. The extreme ring strain renders the β-lactam bond highly labile to hydrolysis. Both abiotic reactions (acid/base catalysis) and microbial β-lactamases cleave the amide bond to form inactive penicilloic acids [38]. In contrast to macrolides or quinolones, β-lactams are photochemically inert due to their lack of chromophores, but hydrolytic cleavage ensures rapid environmental breakdown. Thus, β-lactams are among the least persistent antibiotic classes.
2.2.6. Sulphonamides
Sulphonamides consist of an arylamine or heterocyclic moiety linked to an N-aryl sulphonamide group. The S–N bond is polarised and susceptible to cleavage under UV irradiation or oxidative conditions [39]. Direct photolysis and radical-mediated pathways often yield aromatic amines and sulphonic fragments. Biodegradation is typically slow unless mediated by specialised bacterial enzymes that acetylate or cleave the sulphonamide linkage. Consequently, sulphonamides are moderately stable, less recalcitrant than macrolides or fluoroquinolones, but more persistent than β-lactams.
The fate of antibiotics in manure depends on both their chemical stability and the presence of microbial enzymes targeting structural weak points. Rigid aromatic or macrocyclic scaffolds, such as those in macrolides and fluoroquinolones, confer high persistence, while strained or polarised bonds, as in β-lactams and sulphonamides, make compounds more susceptible to hydrolysis or cleavage. These differences explain why some antibiotics persist in the environment, whereas others degrade rapidly. Table 2 summarises the physicochemical properties influencing microbial degradation of major antibiotic classes.

Table 2.
Physicochemical properties influencing microbial degradation of major antibiotic classes.
2.3. Challenges in the Degradation of Antibiotics Using Traditional Manure Treatments
Veterinary antibiotics are often excreted largely unmetabolized, with 50–80% of doses passing into manure [41]. When this manure is applied to land, residues from multiple drug classes pose environmental and health risks. Conventional treatment approaches, such as composting, anaerobic digestion, aerobic storage, and chemical oxidation, can reduce antibiotic levels but face persistent challenges in achieving complete degradation across all compounds. Even when partial breakdown occurs, transformation products can persist and migrate into soils and waters, highlighting the need to optimise treatments not only for efficiency but also for preventing downstream water contamination.
2.3.1. Composting
Composting is effective at lowering concentrations of many antibiotics, with reported reductions between 47 and 91% [42,43]. However, complete elimination is rarely achieved. Tetracyclines and fluoroquinolones are especially persistent, with half-lives exceeding 100 days in manure due to strong sorption to solids and inherent chemical stability [44,45]. Another concern is the rebound of antibiotic resistance genes during the maturation phase, as residual antibiotics or extracellular DNA sustain selective pressure once temperatures fall [46]. Composting efficiency also depends heavily on maintaining high thermophilic conditions, and any lapse in process control can leave significant residues in the final product.
2.3.2. Anaerobic Digestion
Anaerobic digestion (AD) stabilises manure and produces biogas, but its capacity for antibiotic degradation is limited. Compounds such as tetracyclines and fluoroquinolones resist anaerobic breakdown and persist with long half-lives [44]. In many cases, antibiotics are not destroyed but instead partition into the solid fraction of digestate, where concentrations can even increase compared to raw manure [47]. This persistence is problematic because surviving residues maintain selective pressure, and studies have shown that AD can enrich certain antibiotic resistance genes under these conditions [48]. These outcomes highlight the mismatch between the design of AD for energy recovery and its limited efficacy in contaminant removal.
2.3.3. Aerobic Storage and Treatment
Simpler manure handling methods, such as stockpiling or lagoon storage, are even less effective at antibiotic removal. For example, studies report that residues of macrolides and fluoroquinolones often remain stable for months under such conditions, showing minimal natural attenuation [12]. While advanced aerobic treatments such as biofilm reactors can achieve high removal rates, including near-complete degradation of several drug classes [49], these systems face major barriers to full-scale use. Energy demands for aeration, risks of clogging, and long hydraulic retention times limit practicality on farms, making them difficult to implement outside of controlled trials.
2.3.4. Photo-Fenton Oxidation
Chemical treatments such as photo-Fenton oxidation are capable of rapid and extensive degradation of antibiotics under laboratory conditions. For instance, nearly complete removal has been reported for sulphonamides and tetracyclines in optimised systems [41]. Yet these results do not readily translate to real manure handling. The high turbidity and organic matter content of manure interfere with light penetration and quench hydroxyl radicals, reducing treatment efficiency [50]. In addition, the process requires substantial chemical inputs, raising both cost and sustainability concerns. Even when degradation is achieved, transformation byproducts can remain biologically active, raising questions about overall risk reduction. Together, these factors make photo-Fenton systems technically complex and economically unrealistic for routine manure management.
2.4. Microbial Resistance Pressure and Horizontal Gene Transfer Potential
Manure and slurry enriched with antibiotic residues constitutes a critical hotspot for the emergence and dissemination of antimicrobial resistance. Even at sub-inhibitory concentrations, antibiotics exert strong selective pressure on microbial communities, enriching resistant bacteria and resistance determinants. Multiple studies have shown that both land application and controlled incubations of antibiotic-containing manure increase the abundance and diversity of antibiotic resistance genes (ARGs) in soil microbiota [51]. For example, a survey of soils amended with swine, poultry, or cattle manure—with or without supplementation of the macrolide tylosin—detected 185 unique ARGs, with macrolide–lincosamide–streptogramin B (MLSB) resistance genes dominating. ARG richness and gene copy numbers were significantly higher in manure-amended soils than in unamended controls, with tylosin-spiked manure producing the strongest proliferation. Importantly, elevated ARG levels persisted over time; one study found that soils still contained significantly more ARGs 130 days after manure application than baseline levels. These results underscore that antibiotic-laden manure exerts prolonged resistance pressure, enriching bacteria resistant to tetracyclines, macrolides, aminoglycosides, β-lactams, fluoroquinolones, sulphonamides, and other drug classes commonly present in livestock waste [52].
Beyond enriching resistant bacteria, the manure environment enhances horizontal gene transfer (HGT), thereby amplifying the potential for ARG dissemination. Manure-amended soils are enriched in mobile genetic elements (MGEs)—plasmids, integrons, transposons, and integrative conjugative elements—which facilitate gene exchange across diverse taxa [53]. Soil microcosm experiments have demonstrated manure-to-soil transfer of plasmid-encoded resistance. In one controlled study, an Escherichia coli donor introduced with manure carried a broad-host-range IncP1 plasmid encoding multiple resistance determinants. Within four days of application, transconjugants harbouring this plasmid were recovered from indigenous soil bacteria, albeit at low frequencies [54]. These transfer events, though transient, illustrate that manure-derived plasmids can bridge microbial communities and invade soil populations, even under natural, low-antibiotic conditions.
Plasmid-mediated transfer is a dominant HGT route in manure ecosystems. Long-read metagenomic sequencing of dairy cow manure revealed numerous multidrug resistance plasmids. While many efflux genes were chromosomally encoded, class-specific ARGs, including those conferring resistance to fluoroquinolones, aminoglycosides, MLS antibiotics, and β-lactams, were frequently plasmid-borne [52]. Enterobacteriaceae were identified as predominant hosts of these plasmids, many of which carried multiple linked determinants, including extended-spectrum β-lactamases in combination with aminoglycoside, tetracycline, sulphonamide, MLS, and even heavy-metal resistance genes. This physical linkage facilitates co-selection: exposure to a single antibiotic or heavy metal can maintain plasmids encoding multiple resistances. A cattle manure study provided further evidence, showing enrichment of low-GC plasmids carrying the tetracycline efflux gene tet(Y) at the manure–soil interface, with concomitant increases in tet(Y) copies in the soil resistome [53]. These plasmids, likely broad-host-range types, exemplify how plasmid dynamics drive ARG dissemination between manure and soil bacteria.
Other mobile genetic elements further enhance ARG mobility. Integrons, particularly class 1 integrons, are abundant in manure and can capture and express gene cassettes encoding resistance. Field studies have shown that repeated manure fertilisation significantly increases the abundance of the class 1 integron integrase gene (intI1) in soils, correlating with increased ARG diversity and abundance [51]. A recent study of E. coli from pig manure confirmed integron-mediated mobilisation, reporting widespread class 1 integrons with selective carriage of certain resistance determinants. Notably, β-lactamase genes showed positive correlations with intI1 abundance, while other ARGs were negatively associated, suggesting selective gene capture by integrons, with other resistances mobilised by plasmids or transposons [55]. Integrative conjugative elements (ICEs) and transposons also contribute substantially. In one case, a manure-derived Vagococcus isolate harboured an ICE encoding both MLS and tetracycline resistances flanked by multiple transposases, highlighting the modularity and transfer potential of such elements. Increases in transposase gene counts often accompany ARG enrichment in manure-amended soils, suggesting that transposons and insertion sequences actively mobilise ARGs into new genomic contexts.
Taken together, these findings demonstrate that manure not only acts as a reservoir of ARGs but also as a dynamic platform promoting their horizontal dissemination through diverse mobile elements. The combined selective pressure of residual antibiotics and the abundance of MGEs ensures that antibiotic use in intensive livestock farming has far-reaching consequences for resistance spread in agroecosystems. Thus, while source-level control of antibiotic residues in manure is critical, its broader significance lies in preventing both active residues and resistance determinants from entering and polluting water environments. At the same time, the microbial communities present in manure—though implicated in resistance, also possess the potential to degrade antibiotics, linking resistance dynamics to biodegradation processes discussed in the next section.
3. Microbial Communities Involved in Antibiotic Degradation
The degradation of antibiotics in manure is largely governed by indigenous microbiota originating from the animal gut and the surrounding environment. These communities possess diverse metabolic functions, enabling both direct enzymatic breakdown and co-metabolic transformations that yield fewer active metabolites, epimerised derivatives, or complete mineralisation products. Degradation efficiency differs across antibiotic classes, with β-lactams rapidly hydrolysed by microbial β-lactamases, while structurally complex fluoroquinolones and macrolides often persist due to their aromatic or macrocyclic scaffolds [44,52]. Metagenomic surveys reveal that manure microbiomes not only exhibit broad taxonomic diversity but also encode oxidoreductases, hydrolases, and transferases involved in antibiotic transformation [56]. However, these same communities can also serve as reservoirs of antibiotic resistance genes (ARGs), creating a paradox where biodegradation and resistance dissemination occur simultaneously. Moreover, while microbial degradation reduces the immediate load of active antibiotics, incomplete transformation can generate stable byproducts that leach into soils and waters, posing risks of downstream contamination. Thus, understanding microbial community dynamics is essential not only for predicting antibiotic fate in manure but also for safeguarding water environments from both residues and their degradation products.
3.1. Indigenous Manure Microbiota: Taxonomic and Functional Diversity
Livestock manure and slurry harbour complex microbial communities originating from the animal gut and the farm environment. Across cattle, swine, and poultry, a few major bacterial phyla consistently dominate these microbiomes—notably Firmicutes, Bacteroidetes, and Proteobacteria [57,58]. However, the relative abundance of these groups and the specific taxa present can vary with the host species and manure handling. High-throughput sequencing studies over the last decade have illuminated these community profiles, revealing both shared features and distinct patterns for cattle, swine, and poultry manure.
3.1.1. Cattle Manure Microbiome
Fresh cattle manure is typically enriched in Firmicutes, reflecting the fibre-degrading bacterial populations of the ruminant gut. In one metagenomic survey, Firmicutes constituted roughly one-third (~37%) of the bacterial community in dairy cow manure, followed by Bacteroidetes at ~22% [57]. Smaller fractions of Proteobacteria (~12%) and other phyla occur as well. This aligns with the prevalence of cellulolytic anaerobes from the rumen: for example, cattle manure is rich in Clostridia and Bacteroidia lineages that break down plant fibres. Dominant genera often include rumen-derived taxa such as Ruminococcus, Fibrobacter, and Prevotella. These microbes carry out crucial fermentative processes, producing short-chain fatty acids and supporting downstream methanogens in manure storage [58].
3.1.2. Swine Manure Microbiome
Swine manure contains a diverse microbiome reflecting the omnivorous diet and gut physiology of pigs. Studies consistently report Firmicutes and Bacteroidetes as co-dominant phyla in pig manure, alongside notable levels of Spirochaetes and Proteobacteria [59]. For example, a 16S rRNA gene survey of pig livestock slurry found the community to be mainly composed of Firmicutes and Bacteroidetes, with Spirochaetes and Proteobacteria also among dominant taxa. The presence of Treponema is a recurring feature of swine faecal microbiota and carries over into livestock slurry. Obligate anaerobes from the porcine colon, such as lactic acid bacteria, clostridia, and Bacteroidales, typically flourish in stored pig manure.
Metagenomic analyses further revealed that pig livestock slurry harbours not only commensal bacteria but also a reservoir of pathogens and mobile genetic elements. For instance, a recent high-depth metagenomic study of U.S. swine farm slurry recovered over 1700 viral genomes and detected pathogenic bacteria, including Listeria monocytogenes, Bordetella, and Enterococcus species [60]. The same study identified Entamoeba and Blastocystis among eukaryotic microbes in pig slurry. This indicates that swine manure can contain a broad spectrum of microbial life, from benign fermenters to potential pathogens. Nonetheless, the core bacterial community remains dominated by anaerobic fermenters that originate in the pig gut and thrive in the nutrient-rich, anoxic slurry environment [59].
3.1.3. Poultry Manure Microbiome
Poultry manure exhibits a microbial profile influenced by both the birds’ gastrointestinal microbiome and the pen environment. Firmicutes frequently emerge as the most dominant phylum in chicken faecal microbiomes. In a recent global survey of commercial poultry farms, Firmicutes accounted for ~59% of the faecal bacterial community on average, with Lactobacillus being the single most abundant genus [58]. Co-dominant groups in poultry manure include Actinobacteria and Proteobacteria. Actinobacterial taxa are common in litter, likely derived from bird skin and the high-oxygen, dry bedding environment, whereas Proteobacteria include enteric organisms like Escherichia coli and other Gram-negatives.
Indeed, sequencing of broiler litter has identified core genera such as Staphylococcus, Brevibacterium, and Lactobacillus present consistently. Many of these microbes are known for thriving on protein-rich skin secretions or feed remnants in the litter. Meanwhile, the gut-derived bacteria in poultry manure contribute to nutrient cycling once manure is applied to soil. High-throughput sequencing confirms that chicken manure supports a mixed community of aerobic and anaerobic bacteria: Lactobacillus and Enterococcus from the intestines, Bacillus and Staphylococcus from the environment, Escherichia/Shigella from faeces, and so on. These communities tend to be species-rich; for instance, one study found five phyla: Proteobacteria, Actinobacteria, Bacteroidetes, Firmicutes, and Planctomycetes, all significantly represented in chicken manure treatments [56]. Overall, poultry manure microbiomes are heterogeneous, combining intestinal fermentative bacteria with environmental aerobes, and their composition can shift with litter management, bird age, and housing conditions.
3.2. Identified Antibiotic-Degrading Microbial Genera
A wide range of microorganisms have been implicated in the biotransformation of antibiotics in manure, spanning bacteria, fungi, and archaea. These taxa differ not only in their ecological niches within the manure matrix but also in their enzymatic capacities to target specific antibiotic structures. While bacteria often dominate due to their abundance and metabolic versatility, fungi contribute oxidative enzymes such as laccases and peroxidases that broaden degradation potential, and archaea, though less frequently studied, participate indirectly through co-metabolic processes and community interactions. Collectively, these organisms underpin the biological attenuation of antibiotics in manure, yet their activity is strongly shaped by environmental factors such as oxygen availability, substrate load, and manure management practices. Importantly, their role extends beyond on-site detoxification: by controlling antibiotics at the source, microbial degraders reduce the likelihood that residues or metabolites migrate into groundwater or surface waters, where they could contribute to aquatic pollution.
3.2.1. Bacteria
Bacteria represent the most extensively studied group of microorganisms capable of antibiotic degradation in manure and associated environments. Their degradation capacities span all major antibiotic classes, utilising a broad repertoire of enzymatic strategies including hydrolysis, oxidation, hydroxylation, dealkylation, and conjugation.
Within the macrolide class, Streptomyces rochei has been identified as an actinomycete capable of degrading clarithromycin under aerobic, mesophilic conditions, removing nearly half of the antibiotic in three days [61]. The genus Paracoccus also demonstrates macrolide degradation potential, with P. versutus strain W7 achieving nearly 60% erythromycin removal via lactone ring hydrolysis mediated by erythromycin esterase EreA [62]. Similarly, Rhodococcus gordoniae rjjtx-2 dismantles erythromycin through multi-step hydrolysis, producing several metabolites with the complete dismantling of the lactone ring [63]. Other notable degraders include Acinetobacter pittii TR1, which oxidatively transforms roxithromycin during heterotrophic nitrification–denitrification [64], and Delftia lacustris RJJ-61, which encodes and expresses erythromycin esterase homologs for lactone hydrolysis, enabling complete detoxification of erythromycin residues [65].
For fluoroquinolones, anaerobic Paraclostridium sp. S2 exhibits a unique ability to biotransform ciprofloxacin under sulphate-reducing conditions, initiating degradation through piperazine ring cleavage and subsequent defluorination [66]. Thermophilic Thermus thermophilus strain C419 thrives at 70 °C and degrades ciprofloxacin along with several other fluoroquinolones by hydroxylating and fragmenting the quinolone nucleus [67]. Additionally, Microbacterium sp. 4N2-2 co-metabolises norfloxacin through hydroxylation, dealkylation, and conjugation, yielding four inactive metabolites over two weeks [68].
Among aminoglycoside degraders, Bacillus velezensis MAE16 can metabolise neomycin through glycosidase-mediated sugar cleavage and amine acetylation, producing detoxified intermediates such as 2-deoxystreptamine [69]. Brevundimonas diminuta BZC3 encodes the plasmid-borne aminoglycoside acetyltransferase gene aac(3)-IIa, enabling regioselective acetylation of gentamicin into inactive derivatives [70]. Classic work with Pseudomonas aeruginosa also demonstrated its ability to degrade streptomycin via oxidative dissimilation pathways, mediated by N-adenylyltransferases and oxidases, leading to ring cleavage and detoxification [71].
Tetracycline degradation is widely represented among enteric and environmental bacteria. Enterobacter hormaechei MEH2305 utilises both intracellular and extracellular pathways to degrade tetracycline, oxytetracycline, and doxycycline, converting them into inactive intermediates [72]. In addition, gut-derived Bacteroides fragilis carries the tetX gene, encoding a flavin-dependent monooxygenase that hydroxylates tetracyclines at carbon-11a, producing unstable intermediates that spontaneously disintegrate [73]. Spore-forming Lysinibacillus sp. strain 3 + I has been reported to degrade oxytetracycline through extracellular laccase-like oxidases, achieving near-complete mineralisation under aerobic conditions [74]. Other genera, such as Pandoraea, also contribute, with strain TJ3 co-metabolising oxytetracycline via hydrolytic dechlorination and amide cleavage [75].
In the β-lactam group, Bacillus pseudomycoides AH1 utilises a secreted β-lactamase to hydrolyse penicillin G into penicilloic acid and phenylacetic acid, achieving near-complete degradation in three days [72]. Alcaligenes faecalis, although less efficient, harbours penicillin acylases capable of hydrolysing penicillin and amoxicillin, with reported removal of up to 70% of amoxicillin under aerobic conditions [76].
Sulphonamide degraders include Achromobacter xylosoxidans strain JL9, which mineralises sulfamethoxazole via hydroxylation and sulphonamide bond cleavage, funnelling degradation products into central metabolism [77]. These findings underscore the taxonomic and mechanistic diversity of bacterial genera contributing to antibiotic degradation in manure environments. Representative bacterial strains capable of degrading major antibiotic classes in manure and related environments are summarised in Table 3.

Table 3.
Bacterial strains involved in antibiotic degradation.
3.2.2. Fungi
Fungi contribute significantly to antibiotic degradation owing to their robust extracellular enzyme systems, particularly ligninolytic enzymes such as laccases, lignin peroxidases, and manganese peroxidases, as well as intracellular cytochrome P450s. These enzymes enable fungi to oxidise, hydroxylate, demethylate, and cleave complex antibiotic structures, often transforming or mineralising them into less active compounds. Macrolide degradation has been demonstrated by Peniophora incarnata, which can utilise erythromycin as its sole carbon source [86]. White-rot fungi, such as Pleurotus ostreatus and Trametes versicolor, secrete enzyme preparations that rapidly degrade erythromycin and azithromycin, primarily under conditions that promote oxidative enzyme production [87,88]. Fluoroquinolone degradation by fungi is particularly efficient, with Bjerkandera adusta and Phanerochaete spadiceum fully degrading a broad range of compounds, while Trametes and Pleurotus spp. also display strong activity [89]. Mechanisms include hydroxylation, N-oxidation, and dealkylation, with B. adusta shown to degrade enrofloxacin via piperazine de-ethylation specifically [88]. Although aminoglycoside degradation is less frequently reported, T. versicolor laccase preparations achieved partial removal of neomycin [90], suggesting fungal oxidoreductases may play a role in modifying aminoglycoside structures. Tetracycline degradation is widely reported in fungi, with T. versicolor and B. adusta capable of >90% removal within hours to days. Mechanisms involve hydroxylation, ring opening, and peroxidase-mediated oxidative cleavage, leading to detoxification and even mineralisation [91]. Fungi also efficiently degrade β-lactams, as shown by Coriolopsis gallica, which eliminates ampicillin via laccase-mediated processes [92]. Penicillium and Aspergillus spp., too, produce β-lactamases or penicillin acylases capable of hydrolysing β-lactam antibiotics. Finally, sulphonamides are highly sensitive to fungal laccases, with T. versicolor and B. adusta removing up to 98% of sulfadiazine and sulfamethoxazole in hours [87]. Fungal mechanisms involve oxidative cleavage of the sulphonamide bond and polymerisation of breakdown products into humic-like substances.
3.2.3. Archaea
Although less studied than bacteria and fungi, archaea play distinct roles in antibiotic degradation, particularly in manure and wastewater environments where they form consortia with bacteria. Ammonia-oxidising archaea (AOA) such as those within Thaumarchaeota, cometabolise antibiotics like cephalexin through nonspecific oxidation catalysed by ammonia monooxygenase during nitrification [93]. This process introduces hydroxylations that destabilise antibiotic molecules, contributing to attenuation under aerobic, ammonia-rich conditions.
In anaerobic digestion, methanogenic archaea facilitate the degradation of antibiotics indirectly by consuming bacterial fermentation products, thereby driving syntrophic breakdown processes forward. Their involvement is essential in sulphonamide degradation under methanogenic or sulphate-reducing conditions, such as in mangrove sediments, where archaeal activity ensures complete removal of sulfamethoxazole [94].
Extremophilic haloarchaea also exhibit potential for antibiotic degradation, as they metabolise complex aromatic compounds in hypersaline environments [95]. Their enzymes, including haloalkane dehalogenases and monooxygenases, may be capable of attacking the aromatic structures present in quinolones or chloramphenicol.
Genomic evidence further supports archaeal capacity for antibiotic degradation, as multiple archaeal lineages encode β-lactamase homologs, including methanogens and haloarchaea [96]. These enzymes, structurally conserved with bacterial counterparts, may hydrolyse β-lactam antibiotics, provide resistance or enable direct degradation.
Notably, the intrinsic tolerance of Archaea to antibiotics allows them to persist in antibiotic-rich environments where bacterial activity may be suppressed [97]. This resilience ensures that archaeal metabolism continues to contribute to overall degradation dynamics in manure and wastewater systems.
Among the three microbial groups, bacteria remain the most versatile and rapid degraders under mesophilic and neutral pH conditions, benefiting from diverse catabolic pathways and adaptability to manure environments [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,78,79,80,81,82,83,84,85]. Fungi, particularly white-rot and ligninolytic species such as Trametes versicolor and Bjerkandera adusta, often outperform bacteria in degrading recalcitrant and polycyclic antibiotics (e.g., fluoroquinolones, tetracyclines) through extracellular oxidoreductases, though their activity typically requires aerobic, moderately acidic, and lignocellulose-rich conditions [87,88,89,90,91,92]. Archaea, by contrast, contribute indirectly or through co-metabolic oxidation in syntrophic or extremophilic systems, with lower degradation rates but high stability under anaerobic or high-salinity conditions [93,94,95,96,97]. For practical applications, bacterial consortia and fungal enzyme systems show the greatest promise for scale-up, bacteria for integration into composting and anaerobic digestion, and fungi or fungal enzymes for post-treatment or biofilter applications [98,99,100]. Optimising environmental parameters and co-culturing bacteria–fungi consortia may thus offer the most robust and scalable approach to antibiotic attenuation in manure management systems [101,102,103].
3.3. Factors Affecting Microbial Degradation
The microbial degradation of antibiotics in manure and livestock slurry depends on environmental conditions and the traits of the microbial community. Key drivers include temperature, pH, oxygen availability, and moisture/solids, which together determine degradation kinetics and pathways.
3.3.1. Temperature
Elevated temperatures generally accelerate antibiotic breakdown by enhancing microbial metabolism and abiotic reactions. For example, in swine manure, sulphonamide removal increased with temperature, reaching maximum reduction at 60 °C [98]. Thermophilic anaerobic digestion also promotes faster decay of tetracyclines [99], though excessive heat can reduce community diversity and hinder ARG removal [100]. Thus, thermophilic but not extreme conditions are often optimal.
3.3.2. pH
Antibiotic stability and microbial activity are highly dependent on pH. Acidification of swine manure enhanced sulphonamide degradation (75–86% vs. 26–61% at neutral pH) and suppressed resistant bacteria [101]. In contrast, alkaline conditions accelerated breakdown of β-lactams such as cephapirin, which disappeared within one day at pH 9–12 [102]. Hence, acidic conditions favour sulphonamide degradation, while alkaline environments promote β-lactam hydrolysis.
3.3.3. Oxygen Availability
Aerobic conditions generally enable faster and more complete antibiotic removal compared to anaerobic digestion. Composting reduces both residues and ARGs more effectively than storage lagoons or digesters [99,103]. Nonetheless, optimised anaerobic digestion can achieve complete removal of certain compounds, e.g., tylosin under mesophilic conditions [104], though outcomes remain variable and compound specific [105].
3.3.4. Moisture and Solids
Manure structure and amendments affect aeration and microbial access. Bulking agents (e.g., sawdust) improved antibiotic removal by 15–33% during swine manure composting [105], while impacts were weaker in poultry manure due to antibiotic sorption. Excess organic carbon may either compete with or promote co-metabolic degradation [8].
Optimal degradation occurs under moderately thermophilic conditions, tailored pH (acidic for sulphonamides, alkaline for β-lactams), aerobic treatment, and balanced solids/moisture. Environmental tuning combined with management strategies (e.g., bulking, co-substrates) can significantly enhance antibiotic attenuation and reduce ARG persistence in livestock slurry. Ultimately, these optimisations are vital not only for improving treatment efficiency but also for achieving the broader goal of preventing antibiotics and their breakdown products from entering and polluting water ecosystems. Building on these process-level improvements, the next section focuses on the microbial enzymes that directly catalyse antibiotic inactivation and biodegradation, forming the mechanistic basis for these observed reductions.
4. Microbial Enzymes for Antibiotic Inactivation and Biodegradation
Microbial enzymes can neutralise antibiotics either by structurally modifying them or by breaking down their chemical scaffolds. Figure 2 highlights representative enzymatic inactivation mechanisms across macrolides, tetracyclines, fluoroquinolones, aminoglycosides, β-lactams, and sulphonamides. For example, β-lactamases, which hydrolyse the β-lactam ring, and aminoglycoside-modifying enzymes (AMEs), which acetylate, phosphorylate, or adenylate aminoglycosides to block ribosome binding [106]. Beyond resistance, these and other enzymes, such as hydrolases, laccases, and oxidoreductases, play an important role in biodegradation, transforming antibiotic pollutants into less toxic compounds [14]. This enzymatic activity is significant because it not only lowers the risk of resistance but also reduces the likelihood that active residues persist and migrate into aquatic systems. However, incomplete enzymatic breakdown may generate stable metabolites that leach into water bodies, posing potential risks to ecosystems and human health. Thus, while microbial enzymes are aimed at controlling pollutants at the source, their broader role lies in preventing antibiotics and their byproducts from entering and polluting the water environment.
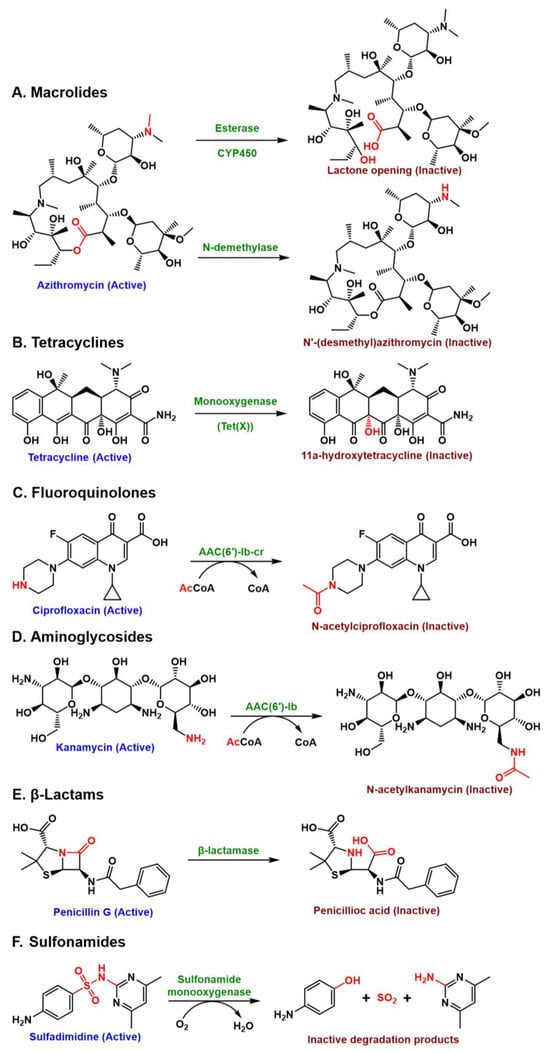
Figure 2.
Common enzymatic mechanisms that inactivate antibiotics across different classes. (A) Macrolides—azithromycin is inactivated via esterase-mediated lactone ring hydrolysis or N-demethylation of the desosamine sugar, both disrupting ribosome binding; (B) Tetracyclines—tetracycline is hydroxylated by Tet(X) at C11a, producing unstable, inactive derivatives; (C) Fluoroquinolones—ciprofloxacin is acetylated by AAC(6′)-Ib-cr, reducing binding to DNA gyrase and topoisomerase IV; (D) Aminoglycosides—kanamycin is acetylated, preventing 30S ribosomal subunit interaction; (E) β-Lactams—penicillin G is hydrolyzed by β-lactamases, opening the β-lactam ring and abolishing PBP binding; (F) Sulphonamides—sulfadimidine is oxidised by monooxygenases to inactive products that no longer inhibit dihydropteroate synthase.
4.1. Hydrolases
Hydrolases inactivate and biodegrade antibiotics by cleaving critical ester and amide bonds, disrupting their biological activity. Key examples include β-lactamases, which hydrolyse the β-lactam ring in penicillins and cephalosporins, and esterases, which break the macrolactone ring in macrolides. These enzymatic activities not only contribute to bacterial resistance but also enable the degradation of antibiotic residues in wastewater and the environment [18,69]. By dismantling antibiotic structures before they reach waterways, hydrolases help mitigate pharmaceutical pollution and protect aquatic ecosystems from chronic exposure.
4.1.1. β-Lactamases
β-Lactamases are a diverse group of enzymes that confer resistance to β-lactam antibiotics by hydrolysing their characteristic four-membered β-lactam ring, thereby neutralising their bactericidal activity [107,108]. These enzymes are produced by a wide range of microorganisms, including environmental and clinical isolates, and play a central role in both intra- and interspecies interactions, as they can protect neighbouring bacteria or even eukaryotic cells from β-lactam cytotoxicity [109]. Mechanistic studies have revealed that serine β-lactamases (e.g., KPC-2, OXA-48) and metallo-β-lactamases (e.g., NDM-1, VIM-1) catalyse the hydrolysis of the β-lactam ring, often generating enamine or imine intermediates from the pyrroline ring of carbapenems, with rapid tautomerization ensuring efficient turnover [108]. Beyond clinical resistance, β-lactamases contribute to the environmental persistence of antibiotics, as demonstrated in studies where Bacillus subtilis 1556WTNC produced β-lactamase to biodegrade penicillins and cephalosporins in treated sewage effluents under environmentally relevant pH and temperature conditions [110].
The widespread production of β-lactam antibiotics through microbial fermentation generates significant solid wastes containing residual drugs and antibiotic resistance genes (ARGs), which are classified as emerging contaminants due to their ecological and health risks [111,112]. Advanced treatment technologies such as ionising radiation, ozonation, and thermal processing have been employed to degrade cephalosporin residues and inactivate ARGs, achieving variable efficiencies depending on matrix composition and operational conditions [111]. Interestingly, biomimetic catalysts, including Zn-based metal–organic frameworks, have been developed to mimic β-lactamase activity, selectively hydrolysing β-lactam rings and providing insight into enzyme-substrate interactions via SCXRD and DFT calculations [113]. These findings underscore the dual relevance of β-lactamases in both clinical antibiotic resistance and environmental antibiotic degradation, highlighting the importance of understanding their molecular mechanisms, ecological impact, and potential biotechnological applications.
4.1.2. Esterases
Microbial esterases are a versatile class of hydrolases that play critical roles in antibiotic resistance and bioremediation by catalysing the hydrolysis of ester bonds in diverse compounds, including macrolide and cephalosporin antibiotics [114,115]. In pathogenic bacteria, macrolide esterases such as the Ere and Est families cleave the macrolactone ring, rendering antibiotics like erythromycin, tylosin, and leucomycin inactive, while preserving enzyme specificity through catalytic triads characteristic of the alpha/beta hydrolase superfamily [116,117]. Esterases like EstX are globally distributed, carried on mobile genetic elements such as integrons and transposons, and contribute significantly to the spread of resistance among clinically relevant species, including Escherichia coli, Salmonella enterica, and Klebsiella pneumoniae [117]. Beyond clinical resistance, esterases facilitate environmental biodegradation by converting cephalosporin C into deacetylcephalosporin C and breaking down erythromycin in aqueous systems [115,118]. Immobilised esterase reactors have demonstrated sustained catalytic activity, achieving continuous degradation of erythromycin at rates exceeding 15 mg h−1 for extended periods, highlighting their potential for wastewater treatment and removal of recalcitrant antibiotic pollutants [118]. Comprehensive understanding of microbial esterase specificity and mechanisms is therefore essential for designing novel antibiotics, mitigating resistance, and developing environmentally sustainable biodegradation strategies.
4.1.3. Amidases
Microbial amidases are hydrolase enzymes that catalyse the cleavage of amide bonds, exhibiting chemo-, regio-, and enantioselective specificity useful in biocatalysis and environmental remediation [119]. Signature amidases possess conserved Ser-Ser-Lys residues, while nitrilase superfamily amidases utilise Glu-Lys-Cys catalytic triads, enabling broad substrate activity. They degrade toxic amides in polymers, insecticides, and antibiotics, including amphenicols, through hydrolysis to inactive products, reducing antimicrobial activity [120]. Notably, substrate-induced inactivation of Escherichia coli AmiD amidase demonstrates a novel strategy to inhibit this enzyme class, revealing kinetic biphasic behaviour upon substrate binding [121]. Penicillin G amidase exemplifies industrial application, producing 6-aminopenicillanic acid for semisynthetic β-lactams. Advances in protein engineering and heterologous expression enhance amidase stability, efficiency, and environmental utility, highlighting their biotechnological and ecological relevance.
4.2. Transferases
Transferases are enzymes that catalyse the transfer of functional groups from one molecule to another. Aminoglycoside acetyltransferases (AAC) modify amino groups, aminoglycoside phosphotransferases (APH) add phosphate to hydroxyl groups, and aminoglycoside nucleotidyltransferases (ANT/adenylyltransferases) adenylate hydroxyl groups. Chloramphenicol acetyltransferases (CAT) acetylate hydroxyl groups, while variants like AAC(6′)-Ib-cr can also acetylate fluoroquinolone piperazinyl groups [122].
4.2.1. Acetyltransferases
Acetyltransferases are versatile enzymes that transfer acetyl groups from donors such as acetyl-CoA to specific substrates, profoundly influencing bacterial physiology, antibiotic resistance, and pathogenesis. Among these, aminoglycoside acetyltransferases (AACs) are key mediators of resistance, catalysing the acetylation of aminoglycoside antibiotics, thereby preventing their binding to the 30S ribosomal subunit [122,123]. Examples include AAC(2′), which confers low-level aminoglycoside resistance in mycobacteria, and Eis, a multifunctional enzyme involved in virulence and modulation of pro-inflammatory cytokines [122]. AAC(6′)-Ib and the recently identified AAC(6′)-Va confer high-level resistance in Gram-negative bacteria, including Acinetobacter baumannii and Aeromonas hydrophila, highlighting their clinical relevance [124,125].
Notably, Microbacterium sp. strain 4N2-2 was shown to inactivate the fluoroquinolone norfloxacin via N-acetylation, catalysed by glutamine synthetase (GS), producing N-acetylnorfloxacin and conferring low-level antibiotic resistance. Structural analyses suggested that norfloxacin binds near the ADP site and acetyl-CoA at a cleft in GS, highlighting environmental bacteria as potential mediators of fluoroquinolone persistence through enzymatic modification [126].
Acetyltransferases are not restricted to antibiotic modification. Chloramphenicol acetyltransferases (CATs) mediate resistance through enzymatic inactivation of chloramphenicol, often carried on mobile genetic elements that facilitate horizontal gene transfer [127]. Furthermore, protein lysine acetyltransferases (bKATs) regulate central metabolic enzymes and stress responses, including persister cell formation, pH tolerance, and adaptation to nutrient limitations, thereby contributing to bacterial fitness and pathogenesis [128].
The emergence of inhibitory strategies targeting AACs, such as metal ion adjuvants or small-molecule inhibitors, illustrates the therapeutic potential of acetyltransferase modulation to restore antibiotic efficacy [123]. Overall, bacterial acetyltransferases serve dual roles as mediators of antibiotic resistance and regulators of physiology, representing promising targets for novel antimicrobial development. Continued characterisation of these enzymes, including unannotated bKATs, is critical for understanding bacterial adaptation and combating multidrug-resistant infections.
4.2.2. Phosphotransferases
Aminoglycoside O-phosphotransferases (APHs) catalyse ATP-dependent phosphorylation of specific hydroxyl groups on aminoglycosides, rendering the antibiotics inactive [129]. Structurally, APHs resemble eukaryotic protein kinases despite low sequence homology, and some exhibit dual activity as serine protein kinases [123,130]. APHs are classified into seven subfamilies, including APH(3′), APH(2″), and APH(6′), and are often chromosomal or plasmid-borne, facilitating horizontal gene transfer. The recently identified aph(3′)-Ie from Citrobacter gillenii mediates high-efficiency phosphorylation of multiple aminoglycosides, including ribostamycin and kanamycin, highlighting ongoing diversification of APH-mediated resistance [131].
4.2.3. Nucleotidyltransferases (Adenylyltransferases)
Aminoglycoside nucleotidyltransferases (ANTs), also termed adenylyltransferases, mediate ATP-dependent transfer of an adenylyl group to hydroxyl or amino moieties on aminoglycosides, thereby reducing antibiotic affinity for the ribosome and inactivating the drug [132,133]. ANT enzymes exhibit substrate specificity, such as ANT(6) targeting streptomycin’s streptidine moiety or ANT(2″)-Ia modifying gentamicin, tobramycin, and kanamycin. Structural studies reveal a two-domain fold similar to DNA polymerases, with Mg2+ ions and catalytic residues coordinating the nucleotidyl transfer reaction [134]. Other clinically relevant ANTs include ANT(4′) and LinB, which catalyse adenylylation of diverse aminoglycosides and lincosamides, respectively, highlighting ANTs as widespread mediators of bacterial resistance and promising targets for inhibitor development [135].
4.3. Oxidoreductases/Monooxygenases
Oxidoreductases, including monooxygenases, modify antibiotics and metabolites via redox reactions. Tet(X) hydroxylates tetracyclines, and sulphonamide monooxygenases oxidise sulphonamides and O- and N-demethylases remove methyl groups. Laccases, peroxidases, cytochrome P450s, and dioxygenases carry out diverse oxidative transformations that support bacterial survival and resistance.
4.3.1. Demethylases
Bacterial N-demethylases play a critical role in antibiotic inactivation and environmental degradation by removing methyl groups from drugs or related compounds. For instance, the PdmAB system in Sphingobium sp. mediates mono-N-demethylation of N, N-dimethyl-substituted phenylurea herbicides, functioning as a Rieske non-heme iron oxygenase with low substrate specificity and broad environmental distribution [136]. Similarly, O-demethylases in Desulfitobacterium hafniense cleave methylated ethers, transferring methyl groups to corrinoid proteins and tetrahydrofolate, enabling biodegradation under anaerobic conditions [137]. Notably, the N-demethylation of macrolides, such as erythromycin, azithromycin, clarithromycin, and clindamycin, is significantly depressed in murine Toxoplasma gondii infection, indicating that host–pathogen interactions can influence demethylation activity [138]. These enzymatic demethylation processes illustrate how microbial N-demethylases contribute to both antibiotic resistance and the detoxification of xenobiotics in natural and clinical environments.
4.3.2. Monooxygenases
Monoxygenases play a central role in bacterial antibiotic inactivation and degradation, acting through oxidative modifications that reduce drug efficacy or facilitate complete mineralisation. Ammonia monooxygenase (AMO), present in ammonia-oxidising archaea (AOA) and bacteria, exemplifies this versatility: while primarily oxidising ammonia, it can cometabolically transform antibiotics. In nitrifying sludge, AMO enabled >99% removal of cephalexin in the presence of ammonium, with amoA upregulation and no toxic intermediates [97], highlighting its potential for antibiotic attenuation in manure and wastewater systems.
Flavin-dependent monooxygenases, such as the tetracycline-inactivating Tet(X) family, catalyse NADPH-dependent hydroxylation of tetracycline antibiotics, conferring broad-spectrum resistance across clinical, environmental, and commensal bacteria [139,140]. Tet(X) enzymes have been successfully expressed in Pichia pastoris, demonstrating robust degradation of tetracyclines in environmental matrices, highlighting their potential for bioremediation while mitigating gene transfer risks [141]. Similarly, sulphonamide-degrading bacteria utilise two-component flavin monooxygenase systems, such as SulX and SulR in Microbacterium sp., to initiate ipso-hydroxylation of sulphonamides, releasing 4-aminophenol and enabling further mineralisation; co-expression in E. coli reduces susceptibility to sulfamethoxazole, illustrating resistance potential [142,143]. Cytochrome P450 enzymes further expand the oxidative repertoire, catalysing the biodegradation of persistent xenobiotics, including fluorinated pharmaceuticals, pyrethroids, and PFAS, with bacterial and fungal CYPomes showing significant sequence diversity and environmental adaptation [144,145,146,147,148]. Collectively, these monooxygenases—flavin-dependent, P450, and related oxidoreductases mediate antibiotic inactivation and environmental degradation, underscoring their dual role in resistance evolution and bioremediation. Understanding their structure, cofactor requirements, and substrate specificity is essential for developing inhibitors or harnessing these enzymes for sustainable detoxification strategies while mitigating the spread of antimicrobial resistance.
4.3.3. Laccases
Laccases, particularly from Trametes versicolor, emerge as effective biocatalysts for antibiotic inactivation and environmental remediation. Laccases can degrade sulphonamides, macrolides, and tetracyclines, and some fungi produce their own redox mediators to enhance breakdown. For instance, a fungal laccase consortium removed 93.4% of chlortetracycline from contaminated straw and soil in situ, demonstrating effective antibiotic degradation without external chemical mediators [149]. When immobilised on supports such as gelatin beads, laccases exhibit enhanced stability, reusability, and catalytic efficiency, achieving up to 72% tetracycline degradation in fluidised bed reactors, while reducing acute toxicity in water [150]. This highlights their role not only in residue removal but also in protecting water quality, as enzymatic treatment reduces ecotoxicological risks associated with antibiotic leakage into rivers, lakes, and groundwater.
The addition of mediators like syringaldehyde further expands their substrate range, enabling the removal of diverse antibiotics, although this can induce temporary toxicity [151]. Laccase-based enzymatic treatments offer a sustainable and mild alternative for mitigating antibiotic pollution in wastewater, supporting both resistance control and ecosystem protection [152].
4.3.4. Peroxidases
Peroxidases, including lignin peroxidase (LiP) and manganese peroxidase (MnP), have demonstrated significant potential for antibiotic inactivation and environmental remediation [153,154]. LiP from Phanerochaete chrysosporium efficiently degraded tetracycline and oxytetracycline in vitro, achieving ~95% removal within minutes under optimised conditions [155]. Similarly, MnP-producing Bacillus velezensis Al-Dhabi 140 effectively removed tetracycline in fibrous-bed reactors, reducing antibacterial activity over time [156]. Extremophilic catalase-peroxidases from Bacillus ligniniphilus L1 also showed enhanced degradation of multiple antibiotics compared to commercial enzymes, with high stability under variable pH and temperature [157]. Peroxidase-mediated antibiotic degradation thus represents a sustainable and efficient strategy for mitigating pharmaceutical pollution in wastewater.
4.3.5. Unspecific Peroxygenases
Unspecific peroxygenases (UPOs) from fungi are versatile oxidative enzymes capable of degrading and inactivating diverse antibiotics under mild conditions. For example, Agrocybe aegerita UPO and Caldariomyces chloroperoxidase have been shown to mineralise or transform multiple pharmaceutical classes, often achieving 50–90% removal in optimised lab reactions [158,159]. Their broad substrate specificity and mild reaction requirements make UPOs promising tools for eco-friendly antibiotic bioremediation. By contributing to the breakdown of antibiotics before they enter the hydrological cycle, UPOs and related enzymes support the ultimate goal of preventing water pollution and safeguarding environmental and public health.
The microbial enzymes involved in antibiotic inactivation and biodegradation are summarized in Table 4.

Table 4.
Microbial enzymes involved in antibiotic inactivation and biodegradation.
5. Strategies to Enhance Microbial Degradation
Antibiotic residues in livestock manure pose a significant environmental and public health risk due to the potential spread of antibiotic-resistant bacteria and resistance genes (ARGs) [160]. Traditional disposal and physicochemical treatment methods, such as hydrolysis, photodegradation, and phytoremediation, are often limited by incomplete degradation, high cost, or environmental constraints. In contrast, biological degradation is increasingly recognised as a sustainable, efficient, and environmentally friendly approach for mitigating antibiotic pollution in manure [161]. In addition to reducing antibiotic residues at the source, microbial degradation plays a crucial role in preventing these contaminants, or their potentially harmful transformation products, from entering the water cycle and contributing to aquatic pollution. Thus, the ultimate aim is not only source control but also safeguarding water environments and ecosystems from long-term contamination [18,69]. This approach not only reduces antibiotic concentrations but also helps maintain microbial diversity, protects ecosystem balance, and limits the propagation of ARGs [162]. Current research emphasises strategies to enhance biodegradation efficiency, including bioaugmentation, biostimulation, enzyme and microbial engineering, and synergistic treatments.
5.1. Bioaugmentation with Specialised Degraders
Bioaugmentation involves introducing targeted microorganisms capable of efficiently degrading specific antibiotics. This strategy is widely applied in composting, where aerobic microbial activity can accelerate antibiotic removal while simultaneously sanitising manure. For instance, Sphingobacterium changzhouense TC931, isolated from farm manure, achieved tetracycline removal rates of 58% on day 2 and 87% on day 3, primarily through biosorption via extracellular polymeric substances and enzymatic biodegradation [163]. Similarly, complex microbial inoculants combining bacteria and fungi have been shown to regulate composting temperature, enhance key enzymatic activities (cellulase, urease, polyphenol oxidase), and significantly reduce residual antibiotics and ARGs [164]. Strains such as Bacillus licheniformis and Aspergillus niger have also been applied successfully to reduce both antibiotic residues and the relative abundance of ARGs during manure composting [165]. These studies demonstrate that carefully selected and applied degraders can markedly accelerate antibiotic removal while maintaining ecological stability.
5.2. Biostimulation Using Nutrient Amendments or Carbon Sources
Biostimulation enhances the activity of native or added microorganisms through nutrient amendments, carbon sources, or other stimulatory agents. Biochar, a stable carbon-rich material with high surface area, is frequently used in manure composting to adsorb antibiotics, regulate microbial communities, and suppress ARG proliferation. For example, biochar addition to chicken manure compost achieved up to 98% reduction in ARG abundance, while in pig manure compost, total ARGs decreased by 17% [166,167]. Co-composting pig manure with crop residues and biochar has been shown to accelerate antibiotic removal and inhibit the horizontal transfer of ARGs [168]. Vermicomposting, i.e., utilising earthworms in combination with microbial activity, similarly enhanced antibiotic degradation; experiments with sheep manure demonstrated tetracycline removal rates of 80% when earthworms were combined with lactic acid bacteria [169]. These results highlight the importance of manipulating environmental conditions to optimise microbial activity for efficient antibiotic degradation.
5.3. Enzyme and Microbial Engineering
The excessive use of antibiotics in livestock production leads to the accumulation of unmetabolized residues in manure, which contributes to environmental contamination and the spread of antibiotic resistance genes (ARGs). Conventional manure treatments often fail to completely eliminate these residues, highlighting the need for advanced enzyme- and microbial-based strategies. Enzymatic degradation has shown significant promise in breaking down recalcitrant antibiotics into less active or non-toxic products. For example, unspecific peroxygenases (UPOs) from Agrocybe aegerita and vanadium chloroperoxidases (VCPOs) from Curvularia inaequalis removed more than 90% of sulfamethoxazole in continuous reactors, reducing both antimicrobial activity and phytotoxicity [159]. Similarly, β-lactamase OtLac from Ochrobactrum tritici efficiently degraded penicillin V under physiological conditions, suggesting its potential for manure treatment [170]. Beyond single-enzyme systems, biofilm-dispersing enzymes such as glycoside hydrolases and proteases can disrupt microbial biofilms that shelter resistant bacteria, thus enhancing antibiotic removal [171].
Microbial engineering offers complementary solutions by constructing strains and consortia with enhanced catabolic activity. Synthetic microbial consortia, combining bacteria and fungi, can perform stepwise degradation of antibiotics, increasing resilience in complex manure environments [172]. Advances in genetic and enzyme engineering now allow the creation of recombinant microorganisms expressing degradative enzymes. For instance, Saccharomyces boulardii EMYA-01 engineered with esterase EreA degraded over 70% of erythromycin residues in faecal suspensions within 24 h, while maintaining microbial diversity [173]. Such approaches expand substrate specificity, improve enzyme stability, and enable simultaneous targeting of multiple antibiotic classes.
However, the deliberate release of genetically modified microorganisms (GMMs) into open agricultural environments raises biosafety concerns, particularly regarding the unintended spread of recombinant DNA and ARGs, since many degradation and resistance mechanisms overlap [174,175,176]. Therefore, the application of engineered strains should be confined to controlled or contained systems, such as closed bioreactors, composting units, or isolated storage facilities, where gene transfer risks can be effectively managed. At the farm level, manure could first be collected and treated in dedicated containment areas before land application, thereby preventing dissemination of engineered organisms and residual ARGs into soil and water systems. Figure 3 illustrates the stepwise process of bioremediation of livestock manure using an engineered microbial consortium (ex situ approach).
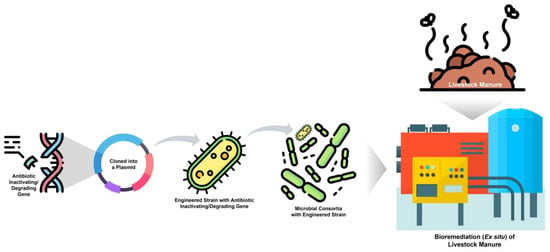
Figure 3.
Bioremediation of livestock manure using an engineered microbial consortium (ex situ approach). The figure illustrates the stepwise process of developing and applying engineered microbial systems for controlled manure bioremediation. An antibiotic-inactivating or -degrading gene is first identified and cloned into a plasmid vector, which is then introduced into a suitable host bacterium to create an engineered strain with antibiotic-degrading capability. This engineered strain is combined with other beneficial microbes to form a microbial consortium. The consortium is applied to livestock manure under contained or ex situ conditions, such as bioreactors or isolated treatment units, to enhance antibiotic degradation while minimising environmental release of genetically modified organisms and antibiotic resistance genes.
Future research should prioritise safe containment strategies, including immobilised enzyme systems, cell-free biocatalysts, or encapsulated microbial formulations that enable efficient degradation without environmental release. In this way, enzyme and microbial engineering can remain powerful tools for sustainable livestock waste management while ensuring biosafety and regulatory compliance. Ultimately, scaling up these technologies will require integrated designs that combine enzymatic biocatalysis with physical containment and real-time monitoring to balance degradation performance with ecological safety.
5.4. Synergistic and Integrated Treatments
Integrating biological degradation with complementary physicochemical or ecological methods further improves efficiency and robustness. Composting and anaerobic digestion, when combined with specialised degraders or engineered strains, can accelerate antibiotic removal and ARG reduction. Synergistic approaches may also include co-treatment with oxidation, coagulation, or photocatalytic processes to further enhance biodegradation [175,176]. Utilising microbial consortia, carefully selected mixtures of bacteria and fungi can exploit synergistic metabolic pathways to target complex antibiotic mixtures that single strains may degrade inefficiently.
Hybrid biological–chemical systems have shown particularly strong potential. For instance, an electro-Fenton process combined with peracetic acid (EF–PAA) achieved complete norfloxacin degradation, 98% mineralisation within 120 min, and total ARG elimination within 20 min, with low energy demand (1.18 kWh/m3) and confirmed ecological safety [177]. Similarly, dielectric barrier discharge (DBD) plasma coupled with sodium persulfate (PS) degraded 89% of levofloxacin versus 59% by plasma alone, with enhanced kinetics driven by ·OH, SO4−·, and O3 radicals [178].
Microbial consortia of bacteria and fungi enable synergistic degradation of complex antibiotic mixtures, expanding enzymatic diversity, improving stability under variable conditions, and reducing ARGs. Integrated treatments can deliver rapid and safe removal of antibiotics from manure, and when combined with advanced oxidation or ecological processes, they address both chemical residues and resistance risks, offering a sustainable solution for livestock waste management.
6. Omics Approaches to Uncover Microbial Degradation Potential
Omics approaches, including metagenomics, metatranscriptomics, metaproteomics, and metabolomics, provide powerful tools to explore the microbial potential for antibiotic degradation in manure. By analysing the complete DNA, RNA, proteins, and metabolites of microbial communities, these techniques reveal which microbes and enzymes are involved, the metabolic pathways they use, and how manure treatment strategies influence antibiotic breakdown [179]. Beyond manure management, understanding these degradation pathways is critical for preventing antibiotic residues and their potentially harmful transformation products from entering the water cycle, thereby reducing environmental and aquatic pollution. This holistic insight not only identifies key degraders and their functional genes but also informs bioremediation strategies, uncovers novel degradation pathways, and supports risk assessment of antibiotic residues and resistance genes in treated manure. Figure 4 shows how multi-omics approaches, including metagenomics, metatranscriptomics, proteomics, isotope labelling, metabolomics, and systems biology, can be combined to reveal microbial-mediated antibiotic degradation pathways, active genes and enzymes, metabolite transformations, and community interactions in livestock manure.
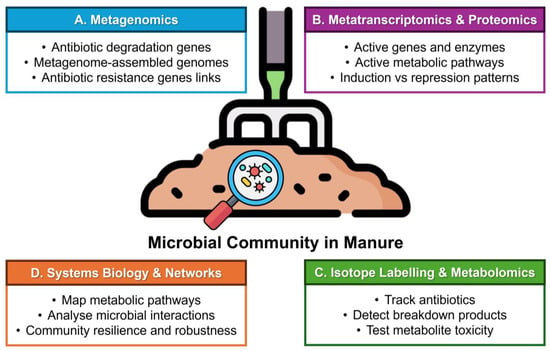
Figure 4.
Multi-omics approaches to uncover antibiotic degradation potential in livestock manure. The schematic illustrates how different omics strategies can be applied to investigate microbial communities responsible for antibiotic biodegradation in manure. (A) Metagenomics identifies antibiotic-degrading genes, reconstructs metagenome-assembled genomes (MAGs), and links these to antibiotic resistance genes (ARGs). (B) Metatranscriptomics and Proteomics reveal actively expressed genes, enzymes, and metabolic pathways, while distinguishing induction versus repression patterns under antibiotic stress. (C) Isotope Labelling and Metabolomics employ 13C/15N-labelled antibiotics to trace substrate utilisation, detect degradation products, and assess metabolite toxicity. (D) Systems Biology and Networks integrate multi-omics data to map metabolic pathways, analyse microbial interactions, and predict community resilience and robustness under environmental pressures. Together, these approaches provide a holistic understanding of microbial-mediated antibiotic degradation in livestock manure.
6.1. Metagenomics
Metagenomics has emerged as a transformative approach for identifying antibiotic-degrading and antibiotic resistance genes (ARGs) in complex environmental matrices, including livestock manure, which acts as both a reservoir and a dissemination pathway for ARGs [58]. Traditional culture-based methods fail to capture most of the microbial diversity in manure, as most environmental microbes are unculturable. Culture-independent metagenomic strategies, including shotgun sequencing and functional metagenomic screening, overcome these limitations by analysing the total microbial DNA, enabling the discovery of novel resistance determinants and their potential mobilisation pathways [180]. By identifying microbes and genes capable of antibiotic degradation, metagenomics can help assess how effectively manure treatment prevents residual antibiotics or their transformation products from leaching into surface and groundwater.
Functional metagenomic screens of soil and waste-derived DNA libraries have revealed extensive variability in resistance proteins, with multiple variants of aminoglycoside acetyltransferases, rifampin ADP-ribosyltransferases, dihydrofolate reductases, and efflux transporters exhibiting comparable resistance despite substantial sequence divergence [180]. Such studies highlight the functional redundancy and plasticity of ARGs in environmental microbial communities, including those associated with manure. Shotgun metagenomic analyses have further demonstrated the prevalence of ARGs across diverse classes, including β-lactams, tetracyclines, sulphonamides, and fluoroquinolones, often linked to mobile genetic elements (MGEs) that facilitate horizontal gene transfer between microbes [181,182].
Manure metagenomes frequently reveal co-occurrence of contaminant-degrading bacteria with ARGs, particularly in environments enriched for both anthropogenic inputs and xenobiotics [183]. Such co-selection suggests that microbial populations adapted to degrade environmental contaminants may also serve as reservoirs for ARGs, amplifying potential health risks. Metagenomic approaches thus enable not only the detection of ARGs but also the identification of their microbial hosts, resistance mechanisms, and contextual genomic elements, providing a comprehensive framework for assessing manure-associated antimicrobial resistance [60]. Furthermore, linking antibiotic-degrading microbes to potential ARG hosts allows risk assessment of whether degradation products might still enter water systems and exert ecological or toxicological effects. Ultimately, these insights inform the development of management and treatment strategies aimed at reducing the environmental dissemination of ARGs while enhancing biodegradation of residual antibiotics in livestock waste.
6.2. Metatranscriptomics and Metaproteomics
Metatranscriptomics and metaproteomics have become essential tools for characterising functional microbial activity in livestock manure under treatment conditions, enabling the identification of metabolically active microorganisms and their expressed enzymes. While metagenomics provides insights into the genetic potential of microbial communities, metatranscriptomics assesses gene expression patterns, revealing which microbes are actively engaged in processes such as hydrolysis, fermentation, and methanogenesis. Proteomics complements this by identifying the enzymes that are produced, thereby linking microbial identity with function [184,185].
For instance, in continuous anaerobic digestion of cattle manure, metatranscriptomic analyses demonstrated that bioaugmentation enhanced the expression of cellulolytic bacteria including Clostridium, Cellvibrio, Cellulomonas, and Fibrobacter, which correlated with elevated cellulase activity (Rb: 0.917–1.081 vs. Rc: 0.551–0.677) and improved hydrolysis of lignocellulosic substrates [186]. Simultaneously, uncultured Bathyarchaeia exhibited increased transcription of methyl-coenzyme M reductase, accelerating methanogenesis and highlighting their dual role as acetogens and methanogens. Such findings underscore the capacity of metatranscriptomics to uncover functional responses of indigenous microbial communities to treatment interventions.
Metaproteomic analyses further reveal the expressed enzyme repertoire, including cellulases, hemicellulases, and key metabolic enzymes, allowing reconstruction of active metabolic pathways in manure treatment systems 184,185]. Combining sample-specific metagenomic databases with public reference datasets enhances peptide identification and functional annotation, ensuring comprehensive interpretation of complex microbial communities in manure. Together, metatranscriptomics and proteomics provide a dynamic picture of microbial activity, revealing how treatment strategies such as bioaugmentation, co-digestion, or process optimisation influence functional gene expression and enzyme production [185]. These approaches enable the identification of key microbial taxa and enzymes that drive organic matter degradation, nutrient cycling, and biogas production, offering critical insights for designing targeted interventions to enhance the efficiency and sustainability of livestock manure management.
6.3. Isotope Labelling and Metabolomics
Stable isotope labelling combined with metabolomics also provides critical information on the fate of antibiotic transformation products that may enter water systems. Detecting active metabolites and mapping degradation pathways enables the prediction of which compounds might persist in effluents or groundwater, helping to mitigate aquatic contamination. Traditional monitoring of antibiotics in complex matrices such as manure, soil, and sewage sludge often suffers from matrix interference; however, the use of isotope-labelled internal standards in LC–MS/MS substantially improves selectivity, recovery, and accuracy across diverse antibiotic classes [187]. For example, Huang et al. optimised ultrasonic-assisted extraction with hydrophilic–lipophilic balance cartridges and applied isotope-labelled internal standards for tetracyclines, sulphonamides, fluoroquinolones, and macrolides, achieving linearity (R2 > 0.997), high recoveries (60–140%), and detection limits down to sub-µg kg−1 levels across soil, manure, and sludge. Such advances provide a robust foundation for routine multi-residue surveillance of veterinary antibiotics in livestock environments [188].
Beyond quantification, isotope-assisted non-target metabolomics allows identification of transformation products and degradation pathways. For example, Qiu et al. applied stable isotope-guided screening to florfenicol in soil, revealing 74 candidate metabolites and confirming 12 transformation products, including hydrolysis, dechlorination, defluorination, dehydration, and sulphone reduction products [189]. Notably, several novel metabolites were also detected in field soils amended with manure, some of which retained antimicrobial activity, highlighting their environmental risk. Monitoring such metabolites is crucial for assessing whether partially degraded antibiotics can enter water bodies and impact aquatic microbiota. Similarly, compound-specific isotope analysis (CSIA) has been employed to trace biotic and abiotic transformations of sulphonamides. Birkigt et al. demonstrated carbon isotope fractionation of sulfamethoxazole (SMX) during biodegradation by Microbacterium sp. BR1 compared to photolysis, showing that isotope signatures (δ13C) can discriminate microbial hydroxylation from abiotic cleavage [190].
Integrating stable isotope labelling with metabolomics thus provides dual benefits: quantitative determination of antibiotic residues and qualitative elucidation of metabolic pathways. These approaches enable differentiation of degradative versus non-degradative processes, discovery of novel metabolites, and improved risk assessment of antibiotic persistence and transformation products in livestock manure. Future developments in multi-element isotope analysis (C, N, Cl, S) and non-target metabolomics will further refine our understanding of antibiotic degradation and support sustainable manure management strategies.
6.4. Systems Biology and Microbial Interaction Networks
Systems biology approaches provide an integrated framework to unravel how antibiotics shape microbial communities and their capacity to degrade contaminants in livestock manure. Antibiotic residues not only persist during storage but also restructure microbial networks, suppress key degraders, and alter nutrient cycling processes. For instance, Fang et al. demonstrated that oxytetracycline (OTC) and ciprofloxacin (CIP) reduced carbon, nitrogen, and phosphorus release by up to 87%, coinciding with reduced Gram-negative bacterial abundance and extracellular enzyme activities. Co-occurrence network analysis revealed that antibiotics disrupted interactions among Gram-negative, Gram-positive bacteria, and actinomycetes, weakening the functional connectivity required for efficient manure decomposition [8]. Incorporating a water pollution perspective, systems biology allows tracking how antibiotic degradation or incomplete degradation affects the potential for runoff or leaching of residual compounds into aquatic environments [18].
Network-based analyses also shed light on the spread of antibiotic resistance genes (ARGs). ARGs persist through vertical transmission and propagate via horizontal gene transfer, forming self-sustaining networks across diverse microbial taxa [191,192]. Manure application introduces these resistomes into agricultural soils, where co-selection with metals such as Cu and Zn further promotes ARG maintenance [193]. Such disruptions not only constrain microbial decomposition but also pose risks to soil fertility, food safety, and human health.
Anaerobic digestion (AD) represents a model system to apply systems biology for mitigating antibiotics. Multi-omic and network studies show that thermophilic AD more effectively reduces ARG abundance than mesophilic processes [12]. By integrating community structure, enzyme activity, and metabolite data, systems-level analyses can identify keystone taxa and functional modules responsible for antibiotic degradation. Such analyses can also assess whether transformation products are fully mineralised or remain in liquid effluents, helping to prevent water contamination [18]. These insights are critical to optimise manure treatment strategies, support nutrient recycling, and reduce the dissemination of resistance in agroecosystems.
7. Challenges and Research Directions
Microbial and enzymatic strategies for antibiotic degradation in livestock manure represent promising avenues for mitigating environmental contamination, yet their effective implementation is constrained by multiple interconnected challenges. These limitations span chemical, biological, environmental, and regulatory dimensions, and addressing them requires coordinated, multidisciplinary advances.
A primary obstacle arises from the structural and compositional diversity of antibiotics commonly applied in livestock production. Residues often include tetracyclines, sulphonamides, fluoroquinolones, and macrolides, resulting in heterogeneous mixtures that complicate universal degradation strategies [194]. Enzymes and microbes frequently achieve only partial degradation, leading to the accumulation of transformation intermediates that can persist or even exhibit increased toxicity [47,172]. Incomplete degradation poses an additional environmental concern: transformation products may leach into surface or groundwater, contributing to aquatic contamination and ecosystem disruption. The ecotoxicological implications of these products remain poorly characterised, and their role in shaping antimicrobial resistance (AMR) dynamics is not fully understood [195]. Advanced analytical platforms such as high-resolution mass spectrometry and non-target metabolomics are needed to characterise these products and guide enzyme engineering toward complete mineralisation [196,197,198]. To address this, multi-omics approaches (metagenomics, metatranscriptomics, metaproteomics) combined with stable isotope probing and flux modelling can identify active taxa and pathways driving anaerobic degradation [179,184]. Integrating these approaches can also assess whether degradation pathways prevent the release of active metabolites into the water cycle, helping safeguard aquatic environments.
Enzyme- and microbe-related limitations further restrict progress. Oxidative enzymes such as laccases, peroxidases, and monooxygenases often require unstable cofactors and are inhibited by competing organic matter, humic substances, or pH fluctuations typical of manure matrices [14,15]. Exogenous microbial degraders also face challenges in establishing themselves due to competition with native microbiota or predation by bacteriophages [181]. Compounding this, antibiotic residues exert selective pressure that favours resistant strains and promotes horizontal gene transfer (HGT) of resistance determinants through plasmids, transposons, and integrons [5,54,167,191]. Incomplete or inefficient degradation in situ could thus result in residual antibiotics and ARGs entering waterways, underlining the importance of designing treatments that minimise aquatic dissemination [18].
Environmental heterogeneity represents another significant barrier. Manure systems exhibit wide variations in pH, temperature, moisture, and nutrient load, leading to inconsistent biodegradation outcomes [98,99,100,101,102,103,104,105]. Anaerobic conditions, which dominate in manure storage and anaerobic digestion, are particularly problematic since many antibiotic-degrading enzymes and microbial pathways are oxygen-dependent [103,104,105]. Anaerobic degradation mechanisms remain poorly characterised, with studies showing limited removal efficiencies and inhibition of processes such as methanogenesis [103,104]. Poorly understood anaerobic pathways could allow residual antibiotics or transformation products to persist and potentially contaminate water bodies after manure application [88]. Mapping the microbial consortia, electron transfer mechanisms, and metabolic pathways involved in such environments is an urgent research priority.
In parallel, novel and emerging mitigation strategies such as constructed wetlands (CWs) and bio-based amendments show strong potential to complement microbial approaches. Constructed wetlands have been recognised as cost-effective, low-energy systems capable of removing antibiotics and emerging contaminants through coupled plant–microbe interactions, redox gradients, and adsorption processes [199]. Hybrid and newly engineered CWs demonstrate high scalability, low operational cost, and integration potential with existing livestock waste treatment infrastructures. Similarly, chitosan-derived flocculants represent a sustainable amendment for antibiotic and heavy-metal adsorption due to their biocompatibility, strong binding capacity, and non-ecotoxic nature [200]. These bio-based materials, along with advanced physicochemical treatments, such as photo-Fenton, electro-Fenton, and catalytic oxidation, offer complementary means to accelerate antibiotic attenuation when combined with biological systems.
Operational, methodological, and socio-economic constraints also impede translation into practice. Most studies remain confined to laboratory conditions, with a notable lack of pilot- or field-scale validation. The absence of standardised protocols for evaluating antibiotic degradation, ARG suppression, and by-product toxicity hinders cross-study comparability and slows regulatory approval [201]. Without assessing waterborne risks of degradation products, scale-up and regulatory acceptance of microbial or enzymatic treatments may be limited [154]. Cost, scalability, and farmer adoption remain key barriers: implementation in real farms requires affordable technologies, technical training, and infrastructure support. Constructed wetlands and bio-based amendments, being low-input systems, may offer near-term feasibility for small- and medium-scale farms, while advanced catalytic or enzyme-based reactors may remain suited for centralised or industrial facilities. Biosafety concerns associated with genetically engineered degraders further complicate regulatory acceptance and raise ecological risk considerations [202].
Regulatory frameworks also shape feasibility. The recent European veterinary regulations on antibiotic use emphasise responsible prescription, residue monitoring, and surveillance of antimicrobial consumption [203]. Integrating manure treatment standards within these frameworks could accelerate the adoption of safe bioremediation technologies. Ultimately, bridging laboratory advances with field implementation will require multi-stakeholder collaboration, incentive policies, and stepwise scaling from contained pilot systems to controlled ex situ treatment facilities. Given current technological maturity, such translation could realistically occur within the next 5–10 years under supportive regulatory and economic conditions.
In summary, tackling these multifaceted challenges will require integrated solutions that couple enzyme engineering, microbial consortia design, and systems-level optimisation with robust analytical methods, standardised assays, and pilot-scale demonstrations. Ensuring that degradation outcomes prevent residual antibiotics and transformation products from entering the water cycle should be a central objective, alongside ARG suppression and ecotoxicological safety [151]. Future research should also focus on techno-economic feasibility, life-cycle assessment, and policy frameworks that ensure scalability and farmer adoption of sustainable manure management solutions [192]. Table 5 summarises these challenges, their implications, and potential research and mitigation strategies.

Table 5.
Key challenges, implications, and research needs in microbial antibiotic degradation.
8. Conclusions and Future Perspectives
Microbial enzymes play a dual role in antibiotic dynamics, acting both as mediators of resistance in pathogens and as potent biocatalysts for environmental detoxification. Hydrolases, including β-lactamases, esterases, and amidases, together with transferases, oxidoreductases, and fungal oxidative enzymes, exhibit the molecular versatility necessary to degrade a wide spectrum of antibiotic classes. Notable examples include β-lactamase OtLac, which efficiently degraded penicillin V; engineered Saccharomyces boulardii expressing erythromycin esterase EreA, achieving over 70% removal of erythromycin in faecal suspensions within 24 h; and unspecific peroxygenases and chloroperoxidases, which eliminated more than 90% of sulphonamides under reactor conditions. Such enzymatic degradation is crucial not only for reducing antibiotic residues in manure but also for preventing their release into surface and groundwater, thereby mitigating potential water pollution.
Complementary strategies such as bioaugmentation with targeted degraders like S. changzhouense, achieving 87% tetracycline removal in 3 days, and biostimulation via biochar, resulting in 98% ARG reduction in chicken manure compost, demonstrate practical avenues to enhance antibiotic degradation in complex matrices. By achieving efficient degradation at the source, these strategies reduce the risk of both antibiotics and their transformation products entering the water cycle.
Hybrid approaches combining enzymatic or microbial degradation with chemical oxidation, such as electro-Fenton treatment with peracetic acid, achieving complete norfloxacin removal and ARG elimination within 20 min, further illustrate the feasibility of rapid, safe, and scalable interventions. Omics-driven approaches, including metagenomics, metatranscriptomics, and isotope-assisted metabolomics, provide mechanistic insights by identifying active degraders, key enzymes, and intermediate metabolites, some of which may persist and retain antimicrobial activity if released, highlighting the need for careful monitoring to prevent water contamination.
Despite these advances, challenges remain. Enzyme inhibition by complex manure matrices, poorly characterised anaerobic degradation pathways, incomplete transformation leading to toxic intermediates, and biosafety concerns surrounding engineered degraders limit practical implementation. Standardised protocols and pilot-scale studies are urgently needed to enable cross-comparison, regulatory approval, and large-scale application. Future research should also assess the fate of degradation products to ensure that water bodies remain uncontaminated.
Future research should focus on discovering novel antibiotic-degrading enzymes through functional metagenomics and integrated multi-omics, designing engineered microbial consortia and multi-enzyme reactors for broad-spectrum degradation, and integrating hybrid biological and chemical systems into sustainable manure management practices. Concurrent biosafety and ecotoxicological assessments are essential to ensure safe environmental deployment. Aligning enzymatic and microbial strategies with the goal of preventing the release of active residues and transformation products into water bodies is crucial for environmental protection.
Author Contributions
Conceptualisation, supervision, project administration, funding acquisition, M.F.K., O.S., L.M. and D.B.; visualisation, M.F.K.; methodology, validation, formal analysis, data curation, writing—original draft preparation, Z.G., J.T., M.F.K. and G.C.; writing—review and editing, Z.G., J.T., M.F.K., G.C., O.S., L.M. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
Z.G. and J.T. acknowledge PhD funding from the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS). M.F.K. acknowledges financial support from the UCD Internal Awards (Award number: 82930-NP).
Data Availability Statement
This review does not include original experimental data. All information used is drawn from previously published studies, as cited in the references.
Acknowledgments
All authors acknowledge University College Dublin (UCD) and the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS), for providing excellent research facilities and for their support with laboratory resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ritchie, H.; Spooner, F. Large Amounts of Antibiotics Are Used in Livestock, But Several Countries Have Shown This Doesn’t Have to Be the Case. Our World Data, 9 December 2024. Available online: https://ourworldindata.org/antibiotics-livestock (accessed on 13 September 2025).
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231. [Google Scholar] [CrossRef] [PubMed]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazard. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Abraham, A.; Mtewa, A.G.; Chiutula, C.; Mvula, R.L.S.; Maluwa, A.; Eregno, F.E.; Njalam’mano, J. Prevalence of antibiotic-resistant bacteria in manure, soil, and vegetables in urban Blantyre, Malawi, from a farm-to-fork perspective. Int. J. Environ. Res. Public Health 2025, 22, 1273. [Google Scholar] [CrossRef]
- Wen, X.; Chen, M.; Ma, B.; Xu, J.; Zhu, T.; Zou, Y.; Liao, X.; Wang, Y.; Worrich, A.; Wu, Y. Removal of antibiotic resistance genes during swine manure composting is strongly impaired by high levels of doxycycline residues. Waste Manag. 2024, 177, 76–85. [Google Scholar] [CrossRef]
- Fang, L.; Lakshmanan, P.; Su, X.; Shi, Y.; Chen, Z.; Zhang, Y.; Sun, W.; Wu, J.; Xiao, R.; Chen, X. Impact of residual antibiotics on microbial decomposition of livestock manures in Eutric Regosol: Implications for sustainable nutrient recycling and soil carbon sequestration. J. Environ. Sci. 2025, 147, 498–511. [Google Scholar] [CrossRef]
- Shawver, S.; Wepking, C.; Ishii, S.; Strickland, M.S.; Badgley, B.D. Application of manure from cattle administered antibiotics has sustained multi-year impacts on soil resistome and microbial community structure. Soil Biol. Biochem. 2021, 157, 108252. [Google Scholar] [CrossRef]
- Khmaissa, M.; Zouari-Mechichi, H.; Sciara, G.; Record, E.; Mechichi, T. Pollution from livestock farming antibiotics: An emerging environmental and human health concern. J. Hazard. Mater. Adv. 2024, 13, 100410. [Google Scholar] [CrossRef]
- Zhao, E.; Li, Y.; Zhang, J.; Geng, B. A review on the degradation of antibiotic resistance genes during composting of livestock manure. Toxics 2025, 13, 667. [Google Scholar] [CrossRef]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The antibiotics degradation and its mechanisms during the livestock manure anaerobic digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef] [PubMed]
- Massé, D.I.; Saady, N.M.C.; Gilbert, Y. Potential of biological processes to eliminate antibiotics in livestock manure: An overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F. Fungi for sustainable pharmaceutical remediation: Enzymatic innovations, challenges, and applications—A review. Processes 2025, 13, 1034. [Google Scholar] [CrossRef]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Balapure, K.; Ranade, V.V. Destroying antimicrobial-resistant bacteria (AMR) and difficult opportunistic pathogens using cavitation and natural oils/plant extracts. Ultrason. Sonochem. 2020, 69, 105272. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Zhi, S.; Zhou, J.; Yang, F.; Tian, L.; Zhang, K. Systematic analysis of occurrence and variation tendency about 58 typical veterinary antibiotics during animal wastewater disposal processes in Tianjin, China. Ecotoxicol. Environ. Saf. 2018, 165, 376–385. [Google Scholar] [CrossRef]
- Lacroix, M.Z.; Ramon-Portugal, F.; Huesca, A.; Angastiniotis, K.; Simitopoulou, M.; Kefalas, G.; Ferrari, P.; Levallois, P.; Fourichon, C.; Wolthuis-Fillerup, M. Residues of veterinary antibiotics in manures from pig and chicken farms in a context of antimicrobial use reduction by implementation of health and welfare plans. Environ. Res. 2023, 238, 117242. [Google Scholar] [CrossRef]
- Patyra, E.; Osiński, Z.; Kwiatek, K. Residues of veterinary antibiotics in solid natural and organic fertilisers—Method development and sample analysis. Environ. Sci. Pollut. Res. 2024, 31, 1–15. [Google Scholar]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Wang, J.; Wang, J.; Zhu, L.; Ge, W. Macrolide- and quinolone-resistant bacteria and resistance genes as indicators of antibiotic resistance gene contamination in farmland soil with manure application. Ecol. Indic. 2019, 106, 105456. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y.; Li, F.; Li, X.; Liu, H.; Fazilani, S.A.; Guo, W.; Xu, G.; Zhang, X. The prevalence and mechanism of fluoroquinolone resistance in Escherichia coli isolated from swine farms in China. BMC Vet. Res. 2020, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Rasschaert, G.; Elst, D.; Colson, L. Antibiotic residues and antibiotic-resistant bacteria in pig slurry used to fertilise agricultural fields. Antibiotics 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, H.; Li, S.; Li, C.; Ma, Y. The presence of tetracyclines and sulfonamides in swine feeds and faeces: Dependence on the antibiotic type and swine growth stages. Environ. Sci. Pollut. Res. 2020, 27, 43093–43102. [Google Scholar] [CrossRef]
- Joseph, M.W.; Moturi, W.N.; Ogendi, G.M. Assessment of veterinary antibiotic use and occurrence of veterinary antibiotics in livestock manure from farms in Rongai Sub-County, Kenya. Curr. World Environ. 2024, 19, 778. [Google Scholar] [CrossRef]
- Topi, D.; Spahiu, J. Presence of veterinary antibiotics in livestock manure in two Southeastern Europe countries, Albania and Kosovo. Environ. Sci. Pollut. Res. 2020, 27, 44552–44560. [Google Scholar] [CrossRef]
- Du, T.; Feng, L.; Zhen, X. Microbial community structures and antibiotic biodegradation characteristics during anaerobic digestion of chicken manure containing residual enrofloxacin. J. Environ. Sci. Health B 2022, 57, 102–113. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Figueira, R.F.; Tornisielo, V.L.; Regitano, J.B. Occurrence and sorption of fluoroquinolones in poultry litters and soils from São Paulo State, Brazil. Sci. Total Environ. 2012, 432, 344–349. [Google Scholar] [CrossRef]
- Ge, L.; Cui, N.; Halsall, C.; Yang, Y.; Cao, S.; Zhang, P. Exploring the aqueous photodegradation of three ionisable macrolide antibiotics: Kinetics, intermediates and photoinduced toxicity. J. Water Process Eng. 2024, 62, 105383. [Google Scholar] [CrossRef]
- Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From toxins to therapeutics. Toxins 2021, 13, 347. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Z.; Pu, Q.; Li, X.; Cui, Y.; Yang, H.; Li, Y. Identification and mechanistic analysis of toxic degradation products in the advanced oxidation pathways of fluoroquinolone antibiotics. Toxics 2024, 12, 203. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chang, J.; Fu, B.; Wang, H. Insight into advanced oxidation processes for the degradation of fluoroquinolone antibiotics: Removal, mechanism, and influencing factors. Sci. Total Environ. 2023, 857, 159172. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Chen, L.; Han, Y.; Shen, Y.; Chen, B.; Wang, M. Deglycosylation inactivation initiated by a novel periplasmic dehydrogenase complex provides a novel strategy for eliminating the recalcitrant antibiotic kanamycin. Environ. Sci. Technol. 2023, 57, 4298–4307. [Google Scholar] [CrossRef] [PubMed]
- Moharrami, E.; Keshipour, S. Photocatalytic degradation of tetracycline antibiotic using nitrogen-doped reduced graphene oxide-supported titania/platinum nanoparticles. NPJ Mater. Degrad. 2025, 9, 57. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Kato, G.; Mitome, H.; Takeda, S.; Hidaka, N.; Tanaka, M.; Akira, K. Study on the chemical stability of β-lactam antibiotics in concomitant simple suspensions with magnesium oxide. J. Pharm. Health Care Sci. 2024, 10, 73. [Google Scholar] [CrossRef]
- Pu, H.; Chen, J.; Luo, J.; Yi, J. Photolysis mechanism of sulfonamide moiety in five-membered sulfonamides: A DFT study. Chemosphere 2018, 197, 569–575. [Google Scholar] [CrossRef]
- Topp, E.; Renaud, J.; Sumarah, M.; Sabourin, L. Reduced persistence of the macrolide antibiotics erythromycin, clarithromycin and azithromycin in agricultural soil following several years of exposure in the field. Sci. Total Environ. 2016, 562, 136–144. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Waigi, M.G.; Gudda, F.O.; Cheng, P.; Ling, W. Simultaneous removal of estrogens and antibiotics from livestock manure using Fenton oxidation technique. Catalysts 2019, 9, 644. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilised soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef]
- Yang, B.; Meng, L.; Xue, N. Removal of five fluoroquinolone antibiotics during broiler manure composting. Environ. Technol. 2018, 39, 373–381. [Google Scholar] [CrossRef]
- Kasumba, J.; Appala, K.; Agga, G.E.; Loughrin, J.H.; Conte, E.D. Anaerobic digestion of livestock and poultry manures spiked with tetracycline antibiotics. J. Environ. Sci. Health B 2020, 55, 135–147. [Google Scholar] [CrossRef]
- Massini, G.; Barra Caracciolo, A.; Rauseo, J.; Spataro, F.; Scordo, G.; Patrolecco, L.; Garbini, G.L.; Visca, A.; Grenni, P.; Rolando, L. Anaerobic digestion of cattle manure contaminated with an antibiotic mixture: A nature-based solution for environmental management. Land 2025, 14, 353. [Google Scholar] [CrossRef]
- Yang, X.; Sun, P.; Liu, B.; Ahmed, I.; Xie, Z.; Zhang, B. Effect of extending high-temperature duration on ARG rebound in a co-composting process for organic wastes. Sustainability 2024, 16, 5284. [Google Scholar] [CrossRef]
- Wallace, J.S.; Garner, E.; Pruden, A.; Aga, D.S. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ. Pollut. 2018, 236, 764–772. [Google Scholar] [CrossRef]
- Black, Z.; Balta, I.; Black, L.; Naughton, P.J.; Dooley, J.S.; Corcionivoschi, N. The fate of foodborne pathogens in manure-treated soil. Front. Microbiol. 2021, 12, 781357. [Google Scholar] [CrossRef] [PubMed]
- Larsson, Y.; Nikolausz, M.; Møller, H.B.; Bester, K. Removal of antibiotic and disinfectant compounds from digested pig manure by an aerobic hybrid biofilm process. Sci. Total Environ. 2025, 982, 179600. [Google Scholar] [CrossRef]
- Lima, K.V.; Nogueira, R.F.P.; Sousa, É.M.; Simões, M.M.; Lima, D.L.; Calisto, V. Magnetic activated carbon for improving the removal of antibiotics by heterogeneous solar photo-Fenton at circumneutral pH. Water Res. 2025, 248, 123679. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Hu, H.-W.; Gou, M.; Wang, J.-T.; Chen, D.; He, J.-Z. Temporal succession of soil antibiotic resistance genes following application of swine, cattle and poultry manures spiked with or without antibiotics. Environ. Pollut. 2017, 231, 1621–1632. [Google Scholar] [CrossRef]
- Qian, X.; Gunturu, S.; Sun, W.; Cole, J.R.; Norby, B.; Gu, J.; Tiedje, J.M. Long-read sequencing revealed co-occurrence, host range, and potential mobility of antibiotic resistome in cow faeces. Proc. Natl. Acad. Sci. USA 2021, 118, e2024464118. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valera, E.; Kyselková, M.; Ahmed, E.; Sladecek, F.X.J.; Goberna, M.; Elhottová, D. Native soil microorganisms hinder the soil enrichment with antibiotic resistance genes following manure applications. Sci. Rep. 2019, 9, 6760. [Google Scholar] [CrossRef] [PubMed]
- Macedo, G.; Olesen, A.K.; Maccario, L.; Hernandez Leal, L.; van der Maas, P.; Heederik, D.; Mevius, D.; Sørensen, S.J.; Schmitt, H. Horizontal gene transfer of an IncP1 plasmid to soil bacterial community introduced by Escherichia coli through manure amendment in soil microcosms. Environ. Sci. Technol. 2022, 56, 11398–11408. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, A.; Buscaroli, E.; Modesto, M.; Luise, D.; Blasioli, S.; Scarafile, D.; Di Vito, M.; Bugli, F.; Trevisi, P.; Braschi, I. The swine waste resistome: Spreading and transfer of antibiotic resistance genes in Escherichia coli strains and the associated microbial communities. Ecotoxicol. Environ. Saf. 2024, 283, 116774. [Google Scholar] [CrossRef]
- Minkina, T.; Sushkova, S.; Delegan, Y.; Bren, A.; Mazanko, M.; Kocharovskaya, Y.; Filonov, A.; Rajput, V.D.; Mandzhieva, S.; Rudoy, D. Effect of chicken manure on soil microbial community diversity in poultry keeping areas. Environ. Geochem. Health 2023, 45, 9303–9319. [Google Scholar] [CrossRef]
- Günel, G.; İnce, O.; Uzun, Ö.; Erdem, E.I.; İnce, B. Enhancing biomethane production from cattle manure by integrating rumen bacteria: A microbial analysis with next-generation sequencing and quantitative PCR. Biomass Convers. Biorefin. 2025, 1–15. [Google Scholar] [CrossRef]
- Shi, J.; Su, H.; He, S.; Dai, S.; Mao, H.; Wu, D. Pan-Genomic Insights into Rumen Microbiome-Mediated Short-Chain Fatty Acid Production and Regulation in Ruminants. Microorganisms 2025, 13, 1175. [Google Scholar] [CrossRef]
- Chen, T.; Xie, G.; Mi, J.; Wen, X.; Cao, Z.; Ma, B.; Zou, Y.; Zhang, N.; Wang, Y.; Liao, X. Recovery of the structure and function of the pig manure bacterial community after enrofloxacin exposure. Microbiol. Spectr. 2022, 10, e02004-21. [Google Scholar] [CrossRef]
- Ramesh, A.; Bailey, E.S.; Ahyong, V.; Langelier, C.; Phelps, M.; Neff, N.; Sit, R.; Tato, C.; DeRisi, J.L.; Greer, A.G. Metagenomic characterisation of swine slurry in a North American swine farm operation. Sci. Rep. 2021, 11, 16994. [Google Scholar] [CrossRef]
- Ercoli, L.; Rossetto, R.; Di Giorgi, S.; Raffaelli, A.; Nuti, M.; Pellegrino, E. Effective bioremediation of clarithromycin and diclofenac in wastewater by microbes and Arundo donax L. Environ. Sci. Pollut. Res. 2023, 30, 77193–77209. [Google Scholar] [CrossRef]
- Ren, J.; Qi, X.; Zhang, J.; Niu, D.; Shen, Y.; Yu, C.; Zhi, J.; Wang, C.; Jiang, X.; Zhang, W. Biodegradation efficiency and mechanism of erythromycin degradation by Paracoccus versutus W7. J. Environ. Manag. 2023, 332, 117372. [Google Scholar] [CrossRef]
- Ren, J.; Ni, S.; Shen, Y.; Niu, D.; Sun, R.; Wang, C.; Deng, L.; Zhang, Q.; Tang, Y.; Jiang, X. Characterization of the erythromycin degradation pathway and related enzyme in Rhodococcus gordoniae rjjtx-2. J. Clean. Prod. 2022, 379, 134758. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Song, G.; Ma, X.; Jiang, H.; Lu, J. Simultaneous degradation of roxithromycin and nitrogen removal by Acinetobacter pittii TR1: Performances, pathways, and mechanisms. J. Environ. Manag. 2025, 374, 123890. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Z.; Deng, L.; Niu, D.; Fan, B.; Huhe, T.; Li, Z.; Zhang, J.; Li, C. Biodegradation of erythromycin by Delftia lacustris RJJ-61 and characterization of its erythromycin esterase. J. Basic Microbiol. 2021, 61, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Oberoi, A.S.; He, Z.; Khanal, S.K.; Lu, H. Ciprofloxacin-degrading Paraclostridium sp. isolated from sulfate-reducing bacteria-enriched sludge: Optimization and mechanism. Water Res. 2021, 191, 116808. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-J.; Li, J.; Li, C.-X.; Tang, X.-D.; Yu, G.-W.; Wang, Y. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge. J. Hazard. Mater. 2018, 343, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Heinze, T.M.; Kim, B.-S.; Schnackenberg, L.K.; Woodling, K.A.; Sutherland, J.B. Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant. Appl. Environ. Microbiol. 2011, 77, 6100–6108. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, W.; Yan, X.; Ding, Q.; Liu, J.; Cheng, B.; Duan, T. Efficient degradation of neomycin by Bacillus velezensis and Cupriavidus basilensis isolated from mangrove soil and pharmaceutical wastewater. Front. Microbiol. 2025, 16, 1544888. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, H.; Li, Z.; Feng, Y.; Cheng, D.; Xue, J. Biodegradation of gentamicin by bacterial consortia AMQD4 in synthetic medium and raw gentamicin sewage. Sci. Rep. 2017, 7, 11004. [Google Scholar] [CrossRef]
- Klein, D.; Pramer, D. Bacterial dissimilation of streptomycin. J. Bacteriol. 1961, 82, 505–510. [Google Scholar] [CrossRef]
- Farag, A.M.; Ghonam, H.E.; El-Borai, A.M. Eco-friendly biotransformation of penicillin G by free and immobilized marine halophilic Bacillus pseudomycoides AH1. Ann. Microbiol. 2024, 74, 30. [Google Scholar] [CrossRef]
- Volkers, G.; Palm, G.J.; Weiss, M.S.; Wright, G.D.; Hinrichs, W. Structural basis for a new tetracycline resistance mechanism relying on the TetX monooxygenase. FEBS Lett. 2011, 585, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Sudha, S.; Parthasarathi, N.; Prabha, D.; Velmurugan, P.; Sivakumar, S.; Anitha, V.; Shrestha, A.; Chinnathambi, A.; Alharb, S.A.; Lakshmanaperumalsamy, P. Oxytetracycline degrading potential of Lysinibacillus sp. strain 3+I isolated from poultry manure. Evid. Based Complement. Altern. Med. 2022, 2022, 2750009. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Chen, Z.; Shen, J.; Jiang, F.; Wang, X.; Kang, J. Isolation of oxytetracycline-degrading bacteria and its application in improving the removal performance of aerobic granular sludge. J. Environ. Manag. 2020, 272, 111115. [Google Scholar] [CrossRef]
- Xue, W.; Li, F.; Zhou, Q. Degradation mechanisms of sulfamethoxazole and its induction of bacterial community changes and antibiotic resistance genes in a microbial fuel cell. Bioresour. Technol. 2019, 289, 121632. [Google Scholar] [CrossRef]
- Song, J.; Hao, G.; Liu, L.; Zhang, H.; Zhao, D.; Li, X.; Yang, Z.; Xu, J.; Ruan, Z.; Mu, Y. Biodegradation and metabolic pathway of sulfamethoxazole by Sphingobacterium mizutaii. Sci. Rep. 2021, 11, 23130. [Google Scholar] [CrossRef]
- Ahmad, F.; Zhu, D.; Sun, J. Environmental fate of tetracycline antibiotics: Degradation pathway mechanisms, challenges, and perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Mohisn, A.; Zhang, G.; Wang, Z.; Wu, S. Biodegradation of penicillin G sodium by Sphingobacterium sp. SQW1: Performance, degradation mechanism, and key enzymes. J. Hazard. Mater. 2024, 468, 133485. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Peng, M.-W.; Qing, T.; Feng, B.; Zhang, P. Amoxicillin degradation and high-value extracellular polymer recovery by algal-bacterial symbiosis systems. J. Hazard. Mater. 2023, 460, 132344. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Liu, Y.; Wan, Z.; Guo, X.; Lu, S.; Qiu, D. Sulfamethoxazole degradation by Pseudomonas silesiensis F6a isolated from bioelectrochemical technology-integrated constructed wetlands. Ecotoxicol. Environ. Saf. 2022, 240, 113698. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Biodegradation and metabolic pathway of sulfamethoxazole by a novel strain Acinetobacter sp. Appl. Microbiol. Biotechnol. 2018, 102, 425–432. [Google Scholar] [CrossRef]
- Plaisance, A.; Wayment, D.; Raje, H.; Boopathy, R. Biodegradation of sulfamethoxazole by a bacterium isolated from the Hurricane overtop sediments. Bioresour. Technol. Rep. 2024, 27, 101926. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Lv, M.; Wang, X.; Chen, L. Sulfamethoxazole degradation by Aeromonas caviae and co-metabolism by the mixed bacteria. Chemosphere 2023, 317, 137882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, H.; Jiang, Y.; Lv, M.; Wang, X.; Chen, L. Biotransformation mechanism of Vibrio diabolicus to sulfamethoxazole at transcriptional level. J. Hazard. Mater. 2021, 411, 125023. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Mao, L.; Yin, D.; Niu, D.; Taoli, H.; Wang, C.; Liu, Q.; Ren, J. Immobilization of Peniophora incarnata F1 in PVA-SA-biochar matrix and its degradation performance and mechanism for erythromycin degradation. J. Environ. Manag. 2025, 375, 124297. [Google Scholar] [CrossRef]
- Hultberg, M.; Golovko, O. Use of sawdust for production of ligninolytic enzymes by white-rot fungi and pharmaceutical removal. Bioproc. Biosyst. Eng. 2024, 47, 475–482. [Google Scholar] [CrossRef]
- Tan, Z.; Beltrán-Flores, E.; Ramos-Meza, G.D.; Alonso, L.L.; Sarrà, M. Eliminating antibiotics by white-rot fungi Trametes versicolor from manure solids and synthetic wastewater. Environ. Pollut. 2025, 378, 126504. [Google Scholar] [CrossRef]
- Akrout, I.; Staita, K.; Zouari-Mechichi, H.; Ghariani, B.; Khmaissa, M.; Navarro, D.; Doan, A.; Albert, Q.; Faulds, C.; Sciara, G. Valorizing fungal diversity for the degradation of fluoroquinolones. Heliyon 2024, 10, e25649. [Google Scholar] [CrossRef]
- Stenholm, Å.; Hedeland, M.; Pettersson, C.E. Neomycin removal using the white rot fungus Trametes versicolor. J. Environ. Sci. Health Part A 2022, 57, 436–447. [Google Scholar] [CrossRef]
- Tan, H.; Kong, D.; Ma, Q.; Li, Q.; Zhou, Y.; Jiang, X.; Wang, Z.; Parales, R.E.; Ruan, Z. Biodegradation of tetracycline antibiotics by the yeast strain Cutaneotrichosporon dermatis M503. Microorganisms 2022, 10, 565. [Google Scholar] [CrossRef]
- Ghariani, B.; Alessa, A.H.; Ben Atitallah, I.; Louati, I.; Alsaigh, A.A.; Mechichi, T.; Zouari-Mechichi, H. Fungal bioremediation of the β-lactam antibiotic ampicillin under laccase-induced conditions. Antibiotics 2024, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Liu, M.; Yang, Z.; Wu, D.; Chen, F.; Wang, J.; Zhu, Z.; Wang, L. The synergistic mechanism of β-lactam antibiotic removal between ammonia-oxidizing microorganisms and heterotrophs. Environ. Res. 2023, 216, 114419. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Tsai, L.-L.; Chang, B.-V. Anaerobic degradation of sulfamethoxazole in mangrove sediments. Sci. Total Environ. 2018, 643, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Espinosa, R.M. Halophilic archaea as tools for bioremediation technologies. Appl. Microbiol. Biotechnol. 2024, 108, 401. [Google Scholar] [CrossRef]
- Khelaifia, S.; Drancourt, M. Susceptibility of archaea to antimicrobial agents: Applications to clinical microbiology. Clin. Microbiol. Infect. 2012, 18, 841–848. [Google Scholar] [CrossRef]
- Wang, B.; Ni, B.-J.; Yuan, Z.; Guo, J. Cometabolic biodegradation of cephalexin by enriched nitrifying sludge: Process characteristics, gene expression and product biotoxicity. Sci. Total Environ. 2019, 672, 275–282. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, J.; Chen, H.; Wang, J.; Sun, W.; Zhang, X.; Yang, Y.; Wang, Q.; Ma, J. Effect of temperature on sulfonamide antibiotics degradation, and on antibiotic resistance determinants and hosts in animal manures. Sci. Total Environ. 2017, 607, 725–732. [Google Scholar] [CrossRef]
- Proskynitopoulou, V.; Vourros, A.; Garagounis, I.; Toursidis, P.D.; Lorentzou, S.; Kougias, P.; Zouboulis, A.; Panopoulos, K.D. Treatment of anaerobically digested pig manure by applying membrane processes for nutrient recovery and antibiotics removal. Environ. Sci. Pollut. Res. 2024, 1–13. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.; Tian, S.; Liu, C.; Liu, L.; Wei, L.; Fan, H.; Zhang, T.; Wang, L.; Zhu, G. Higher temperatures do not always achieve better antibiotic resistance gene removal in anaerobic digestion of swine manure. Appl. Environ. Microbiol. 2019, 85, e02878-18. [Google Scholar] [CrossRef]
- Lin, H.; Sun, W.; Yu, Q.; Ma, J. Acidic conditions enhance the removal of sulfonamide antibiotics and antibiotic resistance determinants in swine manure. Environ. Pollut. 2020, 263, 114439. [Google Scholar] [CrossRef]
- Li, M.M.; Ray, P.; Knowlton, K.F.; Pruden, A.; Xia, K.; Teets, C.; Du, P. Fate of pirlimycin and antibiotic resistance genes in dairy manure slurries in response to temperature and pH adjustment. Sci. Total Environ. 2020, 710, 136310. [Google Scholar] [CrossRef]
- Riaz, L.; Wang, Q.; Yang, Q.; Li, X.; Yuan, W. Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. Sci. Total Environ. 2020, 718, 137414. [Google Scholar] [CrossRef]
- Hosseini Taleghani, A.; Lim, T.-T.; Lin, C.-H.; Ericsson, A.C.; Vo, P.H. Degradation of veterinary antibiotics in swine manure via anaerobic digestion. Bioengineering 2020, 7, 123. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Ma, J.; Sun, W.; Yang, Y.; Zhang, X. Compost-bulking agents reduce the reservoir of antibiotics and antibiotic resistance genes in manures by modifying bacterial microbiota. Sci. Total Environ. 2019, 649, 396–404. [Google Scholar] [CrossRef]
- De Pascale, G.; Wright, G.D. Antibiotic resistance by enzyme inactivation: From mechanisms to solutions. ChemBioChem 2010, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Lohans, C.T.; Freeman, E.I.; Groesen, E.V.; Tooke, C.L.; Hinchliffe, P.; Spencer, J.; Brem, J.; Schofield, C.J. Mechanistic insights into β-lactamase-catalysed carbapenem degradation through product characterisation. Sci. Rep. 2019, 9, 13608. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Ahmad, S.; Irudayaraj, J. Dynamic effect of β-lactam antibiotic inactivation due to the inter- and intra-species interaction of drug-resistant microbes. ACS Biomater. Sci. Eng. 2024, 10, 1461–1472. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Ismail, N. Biodegradation of pharmaceutical wastes in treated sewage effluents by Bacillus subtilis 1556WTNC. Environ. Process. 2014, 1, 459–481. [Google Scholar] [CrossRef]
- Chu, L.; Chen, D.; Wang, J.; Yang, Z.; Yang, Q.; Shen, Y. Degradation of antibiotics and inactivation of antibiotic resistance genes (ARGs) in cephalosporin C fermentation residues using ionizing radiation, ozonation and thermal treatment. J. Hazard. Mater. 2020, 382, 121058. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Escamilla, P.; Bartella, L.; Sanz-Navarro, S.; Percoco, R.M.; Di Donna, L.; Prejanò, M.; Marino, T.; Ferrando-Soria, J.; Armentano, D.; Leyva-Pérez, A.; et al. Degradation of penicillinic antibiotics and β-lactamase enzymatic catalysis in a biomimetic Zn-based metal–organic framework. Chem.–Eur. J. 2023, 29, e202301325. [Google Scholar] [CrossRef]
- Larsen, E.M.; Johnson, R.J. Microbial esterases and ester prodrugs: An unlikely marriage for combating antibiotic resistance. Drug Dev. Res. 2019, 80, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Walton, R.B.; Newkirk, J.F.; Miller, I.M. Microbial degradation of cephalosporin C. Nature 1963, 199, 909–910. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and functional insights into esterase-mediated macrolide resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Lin, J.; Lv, H.; Wang, T.; Tao, H.; Zhong, Y.; Zhou, Y.; Tang, Y.; Xie, F.; Zhuang, G.; Xu, C.; et al. The global distribution of the macrolide esterase EstX from the alpha/beta hydrolase superfamily. Commun. Biol. 2024, 7, 781. [Google Scholar] [CrossRef]
- De Cazes, M.; Belleville, M.P.; Petit, E.; Salomo, M.; Bayer, S.; Czaja, R.; De Gunzburg, J.; Sanchez-Marcano, J. Erythromycin degradation by esterase (EreB) in enzymatic membrane reactors. Biochem. Eng. J. 2016, 114, 70–78. [Google Scholar] [CrossRef]
- Singh, R.; Shahul, R.; Kumar, V.; Yadav, A.K.; Mehta, P.K. Microbial amidases: Characterization, advances and biotechnological applications. Biotechnol. Notes 2024, 6, 44–58. [Google Scholar] [CrossRef]
- Qian, Y.; Lai, L.; Cheng, M.; Fang, H.; Fan, D.; Zylstra, G.J.; Huang, X. Identification, characterization, and distribution of novel amidase gene aphA in sphingomonads conferring resistance to amphenicol antibiotics. Appl. Environ. Microbiol. 2024, 90, e01512-24. [Google Scholar] [CrossRef]
- Pennartz, A.; Généreux, C.; Parquet, C.; Mengin-Lecreulx, D.; Joris, B. Substrate-induced inactivation of the Escherichia coli AmiD N-acetylmuramoyl-L-alanine amidase highlights a new strategy to inhibit this class of enzyme. Antimicrob. Agents Chemother. 2009, 53, 2991–2997. [Google Scholar] [CrossRef]
- Sanz-García, F.; Anoz-Carbonell, E.; Pérez-Herrán, E.; Martín, C.; Lucía, A.; Rodrigues, L.; Aínsa, J.A. Mycobacterial aminoglycoside acetyltransferases: A little of drug resistance, and a lot of other roles. Front. Microbiol. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Reeves, C.M.; Magallon, J.; Rocha, K.; Tran, T.; Phan, K.; Vu, P.; Yi, Y.; Oakley-Havens, C.L.; Cedano, J.; Jimenez, V.; et al. Aminoglycoside 6′-N-acetyltransferase type Ib [AAC (6′)-Ib]-mediated aminoglycoside resistance: Phenotypic conversion to susceptibility by silver ions. Antibiotics 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, L.; Sha, Y.; Chen, Q.; Lin, N.; Zhao, J.; Zhang, Y.; Ji, Y.; Jiang, W.; Zhang, X.; et al. Identification and characterization of a novel 6′-N-aminoglycoside acetyltransferase AAC (6′)-Va from a clinical isolate of Aeromonas hydrophila. Front. Microbiol. 2023, 14, 1229593. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sony, S.A.; Chowdhury, M.B.; Ullah, M.M.; Paul, S.; Hossain, T. Retention of antibiotic activity against resistant bacteria harbouring aminoglycoside-N-acetyltransferase enzyme by adjuvants: A combination of in-silico and in-vitro study. Sci. Rep. 2020, 10, 19381. [Google Scholar] [CrossRef]
- Kim, D.W.; Feng, J.; Chen, H.; Kweon, O.; Gao, Y.; Yu, L.R.; Burrowes, V.J.; Sutherland, J.B. Identification of the enzyme responsible for N-acetylation of norfloxacin by Microbacterium sp. strain 4N2-2. Appl. Environ. Microbiol. 2013, 79, 314–321. [Google Scholar] [CrossRef]
- Moffa, M.; Brook, I. Tetracyclines, glycylcyclines, and chloramphenicol. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Philadelphia, PA, USA, 2015; pp. 322–338. [Google Scholar]
- Dash, A.; Modak, R. Protein acetyltransferases mediate bacterial adaptation to a diverse environment. J. Bacteriol. 2021, 203, e00128-21. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; A Syed, S.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Duman, M.; Saticioglu, I.B.; Buyukekiz, A.G.; Balta, F.; Altun, S. Molecular characterization and antimicrobial resistance profile of atypical Citrobacter gillenii and Citrobacter sp. isolated from diseased rainbow trout (Oncorhynchus mykiss). J. Global Antimicrob. Resist. 2017, 10, 136–142. [Google Scholar] [CrossRef]
- Lin, N.; Sha, Y.; Zhang, G.; Song, C.; Zhang, Y.; Zhao, J.; Huang, D.; Lu, J.; Bao, Q.; Pan, W. APH (3′)-Ie, an aminoglycoside-modifying enzyme discovered in a rabbit-derived Citrobacter gillenii isolate. Front. Cell. Infect. Microbiol. 2024, 14, 1435123. [Google Scholar] [CrossRef]
- Latorre, M.; Revuelta, J.; García-Junceda, E.; Bastida, A. 6-O-Nucleotidyltransferase: An aminoglycoside-modifying enzyme specific for streptomycin/streptidine. MedChemComm 2016, 7, 177–183. [Google Scholar] [CrossRef]
- Cox, G.; Stogios, P.J.; Savchenko, A.; Wright, G.D. Structural and molecular basis for resistance to aminoglycoside antibiotics by the adenylyltransferase ANT (2″)-Ia. mBio 2015, 6, e00128-15. [Google Scholar] [CrossRef]
- Martí, S.; Bastida, A.; Świderek, K. Theoretical studies on mechanism of inactivation of kanamycin A by 4′-O-Nucleotidyltransferase. Front. Chem. 2019, 6, 660. [Google Scholar] [CrossRef] [PubMed]
- Morar, M.; Bhullar, K.; Hughes, D.W.; Junop, M.; Wright, G.D. Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure 2009, 17, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhou, C.; Sørensen, S.R.; Zhang, J.; He, J.; Yu, P.; Yan, X.; Li, S. The novel bacterial N-demethylase PdmAB is responsible for the initial step of N,N-dimethyl-substituted phenylurea herbicide degradation. Appl. Environ. Microbiol. 2013, 79, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Studenik, S.; Vogel, M.; Diekert, G. Characterization of an O-demethylase of Desulfitobacterium hafniense DCB-2. J. Bacteriol. 2012, 194, 3317–3326. [Google Scholar] [CrossRef]
- Berg-Candolfi, M.; Candolfi, E. Depression of the N-demethylation of erythromycin, azithromycin, clarithromycin and clindamycin in murine Toxoplasma infection. Int. J. Parasitol. 1996, 26, 1321–1323. [Google Scholar] [CrossRef]
- Gasparrini, A.J.; Markley, J.L.; Kumar, H.; Wang, B.; Fang, L.; Irum, S.; Symister, C.T.; Wallace, M.; Burnham, C.A.D.; Andleeb, S.; et al. Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance. Commun. Biol. 2020, 3, 241. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-inactivating enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- He, Q.; Cui, C.Y.; Zhang, X.J.; Lin, Z.Y.; Jia, Q.L.; Li, C.; Ren, H.; Cai, D.T.; Zheng, Z.J.; Long, T.F.; et al. Reducing tetracycline antibiotics residues in aqueous environments using Tet(X) degrading enzymes expressed in Pichia pastoris. Sci. Total Environ. 2021, 799, 149360. [Google Scholar] [CrossRef]
- Ricken, B.; Kolvenbach, B.A.; Bergesch, C.; Benndorf, D.; Kroll, K.; Strnad, H.; Vlček, Č.; Adaixo, R.; Hammes, F.; Shahgaldian, P.; et al. FMNH2-dependent monooxygenases initiate catabolism of sulfonamides in Microbacterium sp. strain BR1 subsisting on sulfonamide antibiotics. Sci. Rep. 2017, 7, 15783. [Google Scholar] [CrossRef]
- Kim, D.W.; Thawng, C.N.; Lee, K.; Wellington, E.M.; Cha, C.J. A novel sulfonamide resistance mechanism by two-component flavin-dependent monooxygenase system in sulfonamide-degrading actinobacteria. Environ. Int. 2019, 127, 206–215. [Google Scholar] [CrossRef]
- Khan, M.F.; Liao, J.; Liu, Z.; Chugh, G. Bacterial cytochrome P450 involvement in the biodegradation of fluorinated pyrethroids. J. Xenobiotics 2025, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Cytochrome P450 5208A3 is a promiscuous xenobiotic biotransforming enzyme in Cunninghamella elegans. Enzyme Microb. Technol. 2022, 161, 110102. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Cunninghamella spp. produce mammalian-equivalent metabolites from fluorinated pyrethroid pesticides. AMB Express 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Fluorotelomer alcohols are efficiently biotransformed by Cunninghamella elegans. Environ. Sci. Pollut. Res. 2023, 30, 23613–23623. [Google Scholar] [CrossRef]
- Khan, M.F.; Paul Guin, J.; Thampi, R.K.; Sullivan, J.A.; Murphy, C.D. Enhanced removal of perfluorooctanoic acid with sequential photocatalysis and fungal treatment. Environ. Sci. Pollut. Res. 2023, 30, 91478–91486. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, L.; Lin, X. Laccase-based self-amplifying catalytic system enables efficient antibiotic degradation for sustainable environmental remediation. Adv. Sci. 2023, 10, 2300210. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Pochat-Bohatier, C.; Sanchez-Marcano, J.; Belleville, M.P. Enzymatic degradation of tetracycline by Trametes versicolor laccase in a fluidized bed reactor. Sci. Total Environ. 2024, 907, 168152. [Google Scholar] [CrossRef]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase–degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Environmental remediation by novel nanomaterials and fungi with high-degradation capacity of hazardous contaminants. In Bio and Nanoremediation of Hazardous Environmental Pollutants; Fernández Luqueño, F., López-Valdez, F., Medina Pérez, G., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 283–310. [Google Scholar]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar] [CrossRef]
- Son, H.; Seo, H.; Han, S.; Kim, S.M.; Pham, L.T.M.; Khan, M.F.; Sung, H.J.; Kang, S.H.; Kim, K.J.; Kim, Y.H. Extra disulfide and ionic salt bridge improves the thermostability of lignin peroxidase H8 under acidic condition. Enzyme Microb. Technol. 2021, 148, 109803. [Google Scholar] [CrossRef]
- Wen, X.; Jia, Y.; Li, J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium–a white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Arasu, M.V. Effective degradation of tetracycline by manganese peroxidase producing Bacillus velezensis strain Al-Dhabi 140 from Saudi Arabia using fibrous-bed reactor. Chemosphere 2021, 268, 128726. [Google Scholar] [CrossRef]
- Nawaz, M.Z.; Khalid, H.R.; Mirza, M.U.; Xu, L.; Haider, S.Z.; Al-Ghanim, K.A.; Barceló, D.; Zhu, D. Elucidating the bioremediation potential of laccase and peroxidase enzymes from Bacillus ligniniphilus L1 in antibiotic degradation: A computationally guided study. Bioresour. Technol. 2024, 413, 131520. [Google Scholar] [CrossRef]
- De Boer, S.R.; Schäffer, A.; Moreira, M.T. Towards oxidoreductase-based processes for the removal of antibiotics from wastewater. Rev. Environ. Sci. Biotechnol. 2023, 22, 899–932. [Google Scholar] [CrossRef]
- De Boer, S.; Sastre, D.; Castillo, A.; Mendez, S.B.; Hollmann, F.; Lores, M.; Schaeffer, A.; Moreira, M.T. Advancing the enzymatic removal of antibiotics with unspecific peroxygenase and vanadium chloroperoxidase. J. Environ. Chem. Eng. 2025, 13, 115795. [Google Scholar] [CrossRef]
- Zhao, C.; Xin, L.; Xu, X.; Qin, Y.; Wu, W. Dynamics of antibiotics and antibiotic resistance genes in four types of kitchen waste composting processes. J. Hazard. Mater. 2022, 424, 127526. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The effect review of various biological, physical and chemical methods on the removal of antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Goulas, A.; Livoreil, B.; Grall, N.; Benoit, P.; Couderc-Obert, C.; Dagot, C.; Patureau, D.; Petit, F.; Laouénan, C.; Andremont, A. What are the effective solutions to control the dissemination of antibiotic resistance in the environment? A systematic review protocol. Environ. Evid. 2018, 7, 3. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, J.; Liu, Y.; Chen, L.; Xu, Y.; Zou, Y.; Li, Y.; Gong, B. The survival and removal mechanism of Sphingobacterium changzhouense TC931 under tetracycline stress and its ecological safety after application. Bioresour. Technol. 2021, 333, 125067. [Google Scholar] [CrossRef]
- Li, Y.; Gu, J.; Zhang, S.-Q.; Shi, L.-X.; Sun, W.; Qian, X.; Duan, M.-L.; Yin, Y.-N.; Wang, X.-J. Effects of adding compound microbial inoculum on microbial community diversity and enzymatic activity during co-composting. Environ. Eng. Sci. 2018, 35, 270–278. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, L.; Cai, Q.; Xu, Y.; Xie, Z.; Liu, J.; Ning, X. Simultaneous reduction of antibiotics and antibiotic resistance genes in pig manure using a composting process with a novel microbial agent. Ecotoxicol. Environ. Saf. 2021, 208, 111724. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiong, J.; Yang, Z.; Han, L.; Huang, G. Exploring the impact of biochar on antibiotics and antibiotic resistance genes in pig manure aerobic composting through untargeted metabolomics and metagenomics. Bioresour. Technol. 2022, 352, 127118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qiu, X.; Wu, X.; Lu, S. Horizontal gene transfer is a key determinant of antibiotic resistance genes profiles during chicken manure composting with the addition of biochar and zeolite. J. Hazard. Mater. 2021, 408, 124883. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Liu, F.; Tian, Y.; Zhang, J.; Liu, H.; Duan, J.; Bi, W.; Qin, J.; Xu, S. Effect of biochar on antibiotics and antibiotic resistance genes variations during co-composting of pig manure and corn straw. Front. Bioeng. Biotechnol. 2022, 10, 960476. [Google Scholar] [CrossRef]
- Liu, P.; Sun, M.; Xia, S.; Ju, J.; Mao, W.; Zhao, H.; Hao, Y. Earthworms and lactic acid bacteria (LAB) cooperate to promote the biodegradation of tetracycline residues in livestock manure. Waste Manag. 2024, 186, 166–175. [Google Scholar] [CrossRef]
- Wang, P.; Shen, C.; Cong, Q.; Xu, K.; Lu, J. Enzyme-catalyzed biodegradation of penicillin fermentation residues by β-lactamase OtLac from Ochrobactrum tritici. Microb. Cell Fact. 2021, 20, 117. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. NPJ Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef]
- Zheng, C.C.; Gao, L.; Sun, H.; Zhao, X.Y.; Gao, Z.Q.; Liu, J.; Guo, W. Advancements in enzymatic reaction-mediated microbial transformation. Heliyon 2024, 10, 19. [Google Scholar] [CrossRef]
- He, Q.; Lin, Z.; Zhang, X.; Qin, M.; Huang, Y.; Liao, X.; Liu, Y.; Ren, H.; Sun, J. Designing a reengineered probiotic yeast to spontaneously degrade residual antibiotics in gut during antimicrobial therapy. J. Clean. Prod. 2024, 483, 144177. [Google Scholar] [CrossRef]
- Bhatia, V.; Dhir, A.; Ray, A.K. Integration of photocatalytic and biological processes for treatment of pharmaceutical effluent. J. Photochem. Photobiol. A Chem. 2018, 364, 322–327. [Google Scholar] [CrossRef]
- Katiyar, R.; Chen, C.W.; Singhania, R.R.; Tsai, M.L.; Saratale, G.D.; Pandey, A.; Dong, C.D.; Patel, A.K. Efficient remediation of antibiotic pollutants from the environment by innovative biochar: Current updates and prospects. Bioengineered 2022, 13, 14730–14748. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Mulla, S.I., Bharagava, R.N., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2022; Volume 30, pp. 93–118. [Google Scholar] [CrossRef]
- Feng, L.; Dong, W.; Li, B.; Li, Q.; Liu, Z.; Feng, R.; Giannakis, S. Highly synergistic degradation of norfloxacin and outstanding elimination of antibiotic-resistant elements by a combined electro-Fenton/peracetic acid process under mild conditions. J. Environ. Chem. Eng. 2025, 13, 116153. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Wang, Y. Synergistic degradation of levofloxacin through dielectric barrier discharge and sodium persulfate. J. Environ. Chem. Eng. 2023, 11, 111158. [Google Scholar] [CrossRef]
- Fanfoni, E.; Bellassi, P.; Fontana, A.; Sinisgalli, E.; Rocchetti, G.; Piccinini, S.; Morelli, L.; Cappa, F. Metagenomics and untargeted metabolomics reveal antibiotic resistance dynamics in an anaerobic digestion–composting system treating organic fraction of municipal solid waste. Environ. Microbiome 2025, 20, 106. [Google Scholar] [CrossRef]
- McGarvey, K.M.; Queitsch, K.; Fields, S. Wide variation in antibiotic resistance proteins identified by functional metagenomic screening of a soil DNA library. Appl. Environ. Microbiol. 2012, 78, 1708–1714. [Google Scholar] [CrossRef]
- Olsen, N.S.; Riber, L. Metagenomics as a transformative tool for antibiotic resistance surveillance: Highlighting the impact of mobile genetic elements with a focus on the complex role of phages. Antibiotics 2025, 14, 296. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Boulund, F.; Fick, J.; Kristiansson, E.; Larsson, D.J. Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Front. Microbiol. 2014, 5, 648. [Google Scholar] [CrossRef]
- Gao, F.Z.; Jia, W.L.; Li, B.; Zhang, M.; He, L.Y.; Bai, H.; Liu, Y.S.; Ying, G.G. Contaminant-degrading bacteria are super carriers of antibiotic resistance genes in municipal landfills: A metagenomics-based study. Environ. Int. 2025, 195, 109239. [Google Scholar] [CrossRef]
- Hassa, J.; Maus, I.; Off, S.; Pühler, A.; Scherer, P.; Klocke, M.; Schlüter, A. Metagenome, metatranscriptome, and metaproteome approaches unraveled compositions and functional relationships of microbial communities residing in biogas plants. Appl. Microbiol. Biotechnol. 2018, 102, 5045–5063. [Google Scholar] [CrossRef]
- Jouffret, V.; Miotello, G.; Culotta, K.; Ayrault, S.; Pible, O.; Armengaud, J. Increasing the power of interpretation for soil metaproteomics data. Microbiome 2021, 9, 195. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Zhang, Z. Implementing metatranscriptomics to unveil the mechanism of bioaugmentation adopted in a continuous anaerobic process treating cow manure. Bioresour. Technol. 2021, 330, 124962. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Richnow, H.H.; Imfeld, G. Novel stable isotope concepts to track antibiotics in wetland systems. J. Environ. Sci. 2024, 146, 298–303. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, M.; Li, W.; Wu, L.; Chen, Y.; Luo, Y.; Christie, P.; Zhang, H. Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal. Methods 2013, 5, 3721–3731. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, A.; Yu-ming, M.H.; Zhao, Y.; He, Y.; Xu, J.; Lu, Z. Elucidating degradation mechanisms of florfenicol in soil by stable-isotope assisted nontarget screening. J. Hazard. Mater. 2021, 403, 123974. [Google Scholar] [CrossRef]
- Birkigt, J.; Gilevska, T.; Ricken, B.; Richnow, H.H.; Vione, D.; Corvini, P.F.X.; Nijenhuis, I.; Cichocka, D. Carbon stable isotope fractionation of sulfamethoxazole during biodegradation by Microbacterium sp. strain BR1 and upon direct photolysis. Environ. Sci. Technol. 2015, 49, 6029–6036. [Google Scholar] [CrossRef]
- de Sousa, J.M.; Lourenço, M.; Gordo, I. Horizontal gene transfer among host-associated microbes. Cell Host Microbe 2023, 31, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shen, Z.; Zhou, Q.; Ren, J.; Wang, H.; Zhang, D.; Pan, X. Antibiotic resistance genes in the animal manure-amended soil-plant system: Occurrence, transmission, bacterial hosts, and human health risks. Ecotoxicol. Environ. Saf. 2025, 303, 118890. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; He, M.; Zhang, J.; Liu, J.; Su, J.; Dai, J. Effects of the coexistence of antibiotics and heavy metals on the fate of antibiotic resistance genes in chicken manure and surrounding soils. Ecotoxicol. Environ. Saf. 2023, 263, 115367. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, K.; Deng, C.; Yu, Z.; Shi, J.; Johnson, A.C. Persistence and migration of tetracycline, sulfonamide, fluoroquinolone, and macrolide antibiotics in streams using a simulated hydrodynamic system. Environ. Pollut. 2019, 252, 1532–1538. [Google Scholar] [CrossRef]
- Cavany, S.; Nanyonga, S.; Hauk, C.; Lim, C.; Tarning, J.; Sartorius, B.; Dolecek, C.; Caillet, C.; Newton, P.N.; Cooper, B.S. The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance. Nat. Commun. 2023, 14, 6153. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F. Recent progress and challenges in microbial defluorination and degradation for sustainable remediation of fluorinated xenobiotics. Processes 2025, 13, 2017. [Google Scholar] [CrossRef]
- Khan, M.F. Recent advances in microbial enzyme applications for sustainable textile processing and waste management. Sci 2025, 7, 46. [Google Scholar] [CrossRef]
- Khan, M.F. Enhancing stability of enzymes for industrial applications: Molecular insights and emerging approaches. World J. Microbiol. Biotechnol. 2025, 41, 362. [Google Scholar] [CrossRef]
- Monali, M.; Choudharya, M.; Ray, S. A review on constructed wetlands for environmental and emerging contaminants removal from wastewater. Environ. Dev. Sustain. 2024, 26, 30181–30220. [Google Scholar]
- Zubair, M.; Arshad, M.; Ullah, A. Chitosan-based materials for water and wastewater treatment. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Volume 3: Chitin and Chitosan based Polymer Materials for Various Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 773–809. [Google Scholar]
- Kümmerer, K.; Alexy, R.; Hüttig, J.; Schöll, A. Standardized tests fail to assess the effects of antibiotics on environmental bacteria. Water Res. 2004, 38, 2111–2116. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).