Abstract
Endotoxins, lipopolysaccharides released from the outer membrane of Gram-negative bacteria, pose a significant risk in healthcare environments, particularly in Central Sterile Supply Departments (CSSDs), where the delivery of sterile pyrogen-free medical devices is critical for patient safety. Traditional methods for controlling endotoxins in water systems, such as ultraviolet (UV) disinfection, have proven ineffective at reducing endotoxin concentrations to comply with regulatory standards (<0.25 EU/mL). This limitation presents a significant challenge, especially in the context of reverse osmosis (RO) permeate used in CSSDs, where water typically has very low conductivity. Despite the established importance of endotoxin removal, a gap in the literature exists regarding effective chemical-free methods that can meet the stringent endotoxin limits in such low-conductivity environments. This study addresses this gap by evaluating the effectiveness of the eBooster™ electrochemical technology—featuring proprietary electrode materials and a reactor design optimized for potable water—for endotoxin removal from water, specifically under the low-conductivity conditions typical of RO permeate. Laboratory experiments using the B250 reactor achieved >90% endotoxin reduction (from 1.2 EU/mL to <0.1 EU/mL) at flow rates ≤5 L/min and current densities of 0.45–2.7 mA/cm2. Additional real-world testing at three hospitals showed that the eBooster™ unit, when installed in the RO tank recirculation loop, consistently reduced endotoxin levels from 0.76 EU/mL (with UV) to <0.05 EU/mL over 24 months of operation, while heterotrophic plate counts dropped from 190 to <1 CFU/100 mL. Statistical analysis confirmed the reproducibility and flow-rate dependence of the removal efficiency. Limitations observed included reduced efficacy at higher flow rates, the need for sufficient residence time, and a temporary performance decline after two years due to a power fault, which was promptly corrected. Compared to earlier approaches, eBooster™ demonstrated superior performance in low-conductivity environments without added chemicals or significant maintenance. These findings highlight the strength and novelty of eBooster™ as a reliable, chemical-free, and maintenance-friendly alternative to traditional UV disinfection systems, offering a promising solution for critical water treatment applications in healthcare environments.
1. Introduction
Endotoxins are lipopolysaccharides (LPSs) found in the outer membrane of Gram-negative bacteria, many of which are common waterborne pathogens (e.g., Campylobacter, E. coli, Legionellas, Pseudomonas, Salmonella, and Vibrio cholerae). Chemically, LPS molecules consist of three main components: lipid A (the toxic portion), core sugars (which stabilize the structure), and the O-antigen (a variable sugar chain that helps the bacteria evade the immune response) [1]. Large amounts of LPSs are released when bacteria undergo lysis (cell rupture), while smaller quantities are shed during normal bacterial growth.
Unlike exotoxins—which are actively secreted by bacteria—endotoxins are highly stable and cannot be destroyed by standard autoclaving at 121 °C. Inactivation requires extreme dry heat (above 160 °C) or harsh chemicals, such as ethylene oxide gas [2]. While endotoxins are less toxic than exotoxins, they can still provoke severe systemic reactions, including septic shock, fever, and organ failure when present in high doses [3]. Even minute amounts (as little as 10−12–10−9 g) entering the bloodstream can trigger significant biological reactions.
In CSSDs, endotoxins present a major challenge, as the goal is to deliver sterile pyrogen-free medical devices. The key concerns in this context include the following:
- Pyrogenic reactions in patients [4]
- Fever and inflammation: even when instruments are microbiologically sterile, residual endotoxins can provoke pyrogenic reactions if introduced into the bloodstream or tissues.
- Risk of septic shock: contaminated surgical instruments, such as implants or catheters, may trigger systemic inflammatory response syndrome (SIRS).
- Difficulty in complete removal
- Beyond their heat resistance, LPSs strongly adhere to surfaces like glass, metal, and plastics, making them difficult to eliminate using standard cleaning procedures [5].
- False sterility assurance
- A device may pass microbiological sterility tests (i.e., show no bacterial growth) but still harbor harmful levels of endotoxins [6].
- Detecting such contamination requires the Limulus Amebocyte Lysate (LAL) test [7], adding complexity to quality assurance protocols.
Common sources of endotoxin contamination in CSSDs include biofilms in water lines, where endotoxins can accumulate in washer-disinfectors and ultrapure water systems [8]. Improper rinsing also plays a significant role: if cleaning agents are not thoroughly removed, residual detergents may bind endotoxins, preventing their effective elimination [9]. Additionally, inadequate storage practices can lead to airborne contamination, exposing sterilized items to endotoxin-laden particulates [10].
From a regulatory and compliance perspective, strict endotoxin limits are enforced for high-risk medical products such as implants, dialysis equipment, and injectable medication. For instance, intrathecal drugs must not exceed 0.25 endotoxin units (EU) per milliliter, as mandated by FDA and ISO standards [11]. Failure to comply with these limits can result in serious consequences, including product recalls, litigation, and, most critically, patient harm.
To mitigate these risks, CSSDs implement several control measures. One key intervention is the use of depyrogenation ovens, which destroy endotoxins by heating glassware at 250 °C for over 30 min or at 180 °C for more than 3 h. Additionally, water used in the cleaning process is often treated through ultrafiltration and RO systems to prevent endotoxin buildup in washers. Pre-sterilized disposable items are also used to minimize risks. Validated cleaning protocols ensure the thorough removal of organic debris, a potential source of endotoxins [9,12]. For high-risk medical devices, such as spinal needles and heart valves, LAL testing is mandatory to detect any residual endotoxin contamination [13].
Several methods have been explored for removing endotoxins from water, including adsorption [14,15], retentive filtration [16], electrochemical techniques [17,18], UV treatment [19,20], and chemical oxidation processes [20,21]. Chlorination, alone or in combination with UV exposure [22], has also been considered. However, these approaches are generally unsuitable for CSSD applications due to the risk of introducing undesirable chemical byproducts, such as chlorites and chlorates, into the water used for cleaning or rinsing medical devices. Ozonation appears promising since ozone decomposes into oxygen, leaving no residue; however, convincing results remain lacking. Ren et al. [21] dosed 2 mg/L of ozone on solutions containing around 3000 EU/mL. While they observed significant reductions in the inflammation-inducing capacity of the treated solution, reductions were incomplete. Notably, the ozone dose was much higher than the LPS concentration (2 mg/L compared to 235 µg/L), and such a high ozone dosage could jeopardize material integrity in a real-world application [23].
UV radiation can reduce endotoxin levels when applied at doses much higher than those used for standard microbial disinfection [19]. However, its effectiveness is limited: while it may partially degrade endotoxins, residual fragments can remain biologically active or immunogenic. UV is generally more effective at killing bacteria (thus preventing further endotoxin release) than at breaking down existing endotoxins. As a result, UV is often used as a pretreatment step, followed by methods like ultrafiltration or adsorbent resins to achieve acceptable endotoxin levels.
Absorbent materials, such as the commercial resin ‘ToxinEraser’, have demonstrated substantial LPS removal efficiencies (though not exceeding 90%) [14,15]. However, their regeneration requires endotoxin-free water, a detail that is frequently overlooked in the literature.
Electrochemical methods, including oxidation [18] and electrodeionization [17], offer promising strategies for endotoxin removal. Electrochemical oxidation relies on reactive oxygen species—such as hydroxyl radicals—generated at the anode to degrade the lipopolysaccharide structure of endotoxins. For instance, Wang et al. [18] demonstrated LPS degradation using a Ti-based electrode in a batch reactor, achieving significant reductions under high LPS concentrations (20 mg/L) and extended treatment times (60 min) in a 20 mM Na2SO4 electrolyte. While effective, this approach is impractical for continuous-flow systems like those in CSSDs, where low-conductivity RO permeate (typically <20 μS/cm) and stringent endotoxin limits (e.g., ≤0.25 EU/mL) demand rapid chemical-free treatment without added electrolytes. Electrodeionization, by combining ion-exchange resins with an electric field, facilitates the continuous removal of charged endotoxin fragments without the need for chemical regenerants. However, its primary function is ion removal, and its capacity to reduce viable bacteria and endotoxins diminishes over time due to the saturation of adsorption sites [17]. These methods offer a chemical-free alternative, although their effectiveness depends on factors such as endotoxin concentration, electrode material, and system design.
Ecas4 Australia Pty Ltd. (Mile End South, SA, Australia) has recently introduced the eBooster™ technology, designed to enhance drinking water treatment while reducing operating costs and improving risk management in healthcare settings [24]. This system uses a patented reactor and electrodes specifically designed for drinking water treatment. By applying an electric field to the water, it directly and indirectly oxidizes microorganisms through the production of oxidizing species. Additionally, the system generates active chlorine by oxidizing naturally occurring chlorides in the water.
Water supplied to CSSDs typically undergoes a multi-step treatment process, including filtration through a cartridge filter (to remove particles like those found in tap water), softening (to exchange divalent cations such as Ca2+ and Mg2+ for monovalent Na+ cations), activated carbon filtration (to remove free and combined chlorine, protecting downstream equipment), reverse osmosis (to eliminate chemical contaminants not removed by the carbon filter), UV disinfection (to prevent bacterial regrowth in the RO permeate storage tank), and ultrafiltration (to target bacteria that UV treatment could not eliminate and remove endotoxins generated by UV disinfection) [17]. In this context, eBooster™ can replace UV disinfection, being installed within the recirculation loop of the RO permeate storage tank. This loop prevents water stagnation, which can lead to microbial growth, biofilm formation, and degradation of permeate quality. With eBooster™, the recirculated water undergoes continuous electrochemical treatment, enhancing disinfection and reducing bioburden. This configuration is ideal for facilities that require high-purity water with low microbial counts, such as in healthcare, food and beverage, and pharmaceutical applications.
This contribution presents laboratory data and field data supporting the hypothesis that eBooster™ technology can significantly enhance endotoxin removal from water, even under very low conductivity conditions.
2. Materials and Methods
2.1. eBooster™ Device
The eBooster™ electrochemical units are available in three different sizes [24]. The B250 model (Figure 1), the smallest version, is designed for localized applications, such as residential settings and plumbing systems, and supports flow rates of up to 25 L/min. This unit incorporates 12 planar DSA-type electrodes arranged in an interdigitated pattern, providing a total anode surface area of 255 cm2. The proprietary electrode coating is specifically designed for treating water intended for human consumption [25] while also being durable enough to withstand periodic polarity reversal (see, for example, [26]).

Figure 1.
Ecas4 Australia’s eBooster™ model B250. Specifications: Dimensions: 80 × 80 × 160 mm; Weight: 600 g; Maximum working pressure: 20 bar (2000 kPa); Maximum working temperature: 50 °C; and Materials: uPVC (food grade) and titanium.
Unlike the larger models, the B250 has not yet been deployed in standard applications. It has only been tested in controlled environments, including a couple of university laboratories [24] and a few hospitals, which have provided valuable opportunities to assess its potential. As a result, a standardized control panel for this model has not yet been established. For treating RO water, which typically has conductivity values below 20 µS/cm, the control panel must include a power supply capable of delivering currents in the range of a few amperes at voltages approaching 48 V. Similar to the control panels used for larger models, the B250’s panel typically includes a flow switch (to disable current when no flow is detected), polarity reversal contactors, and a timer to adjust the reversal frequency.
2.2. Laboratory Testing
All electrochemical treatments were performed using the B250 unit (Figure 1). The unit was electrically connected to a 360 W Series 2260B programmable DC power supply (Keithley/Tektronix, São Paulo, Brazil), which is capable of delivering up to 36 A at 30 V. The system was hydraulically connected to a 100 L HDPE reservoir through PVC piping and a variable-speed MasterFlex B/T peristaltic pump (Cole-Parmer, IL, USA), capable of delivering up to 42 L/min.
Two types of water were used for the experiments: tap water filtered through a carbon filter, and tap water purified by a Millipore Milli-Q® lab water system (Darmstadt, Germany) (resistivity ≥ 18.2 MΩ∙cm). To simulate endotoxin contamination, the water was spiked with 1 µg/L of endotoxins (equivalent to 12 EU/mL), and dilutions were made by factors of 3, 10, or 50 to create final endotoxin concentrations of 4.0, 1.2, and 0.25 EU/mL, respectively.
The water was circulated through the electrochemical cell at various flow rates of 2.5, 5.0, 7.8, 9.8, and 14.5 L/min. Notably, the conductivity difference between Milli-Q water and filtered tap water influenced the current density when applying a voltage of approximately 24 V. This resulted in 2.7 mA/cm2 for filtered tap water and 0.45 mA/cm2 for Milli-Q water.
All electrochemical treatments were conducted under constant current (galvanostatic mode), with the water passing only once through the B250 reactor before being collected in a second reservoir.
2.3. Analytical Methods
All endotoxin tests were conducted at least in triplicate using sterilized pyrogen-free glassware and water. The endotoxin quantification was performed utilizing the PierceTM Chromogenic Endotoxin Quant Kit (Thermo Scientific, Zürich, Switzerland), which uses Limulus Amebocyte Lysates (LALs) for detecting endotoxins [27]. The assay works by triggering an enzymatic cascade that releases p-nitroaniline (PNA), leading to a yellow coloration proportional to the endotoxin concentration. Absorbance was measured photometrically at 405 nm, and the endotoxin concentration was determined using a standard curve.
2.3.1. Preparation of Standard and Samples
A lyophilized E. coli endotoxin standard (Millipore SAS, Molsheim, France) was reconstituted in endotoxin-free water to prepare a 10 EU/mL endotoxin standard solution. The solution was vortexed at 1500 rpm for 15 min to ensure complete dissolution, followed by serial dilutions to prepare high (0.25–10 EU/mL) and low (0.01–0.25 EU/mL) standard ranges. Water samples were diluted with endotoxin-free water to match the selected standard range.
2.3.2. Test Procedure
Briefly, 50 µL of each standard or sample was added to each well in triplicate. LAL was reconstituted just before use with 1.7 mL of endotoxin-free water. Then, 50 µL of the LAL solution was added to each well, and the well plate was incubated at 37 °C for 14 min (high range) or 30 min (low range). A chromogenic substrate was reconstituted in 3.4 mL of endotoxin-free water and pre-warmed to 37 °C for 5 min before being added. After incubation with the lysate, 100 µL of the pre-warmed substrate was added to each well and incubated for an additional 6 min. The reaction was terminated by adding 50 µL of 25% acetic acid, and optical density (OD) was immediately measured at 405 nm using a MultiskanTM FC Microplate Photometer (Thermo Scientific, Zürich, Switzerland).
2.3.3. Quality Control Measures
Prior to testing, all consumables (e.g., pipette tips, tubes, well plates, and reservoirs) were confirmed to be pyrogen-free. All reagents were equilibrated to room temperature before use to ensure consistency and accuracy of results.
2.4. Real-World Scenario Testing: Field Analysis
In addition to laboratory testing, real-world scenario testing was conducted in collaboration with Aquastream Water Solutions (Toowoomba, Queensland). Three hospitals with CSSDs were selected as part of a pilot study. The selected hospitals were located in different regions of Australia, and the testing aimed to evaluate the performance of the B250 reactor in a practical setting.
2.4.1. Water Sample Analysis by NATA-Accredited Laboratories
Water samples collected from these CSSDs were sent to laboratories accredited by the National Association of Testing Authorities (NATA) for analysis. Two methods were used for testing:
- APHA 9215 D (membrane filter method)—Used to determine the total heterotrophic plate counts (HPC).
- European Pharmacopoeia 2.6.14 Method C (turbidimetric kinetic method)—Used to measure bacterial endotoxins.
The reporting limits for these parameters were as follows:
Total heterotrophic plate counts: 1 CFU/100 mL
Bacterial endotoxins: 0.05 EU/mL
The limit of reporting (LOR) is defined as the limit of detection (LOD) multiplied by a safety factor, accounting for daily variations in instrument sensitivity. The relationship between the values is given by LOD < LOR ≤ LOQ, where LOQ is defined as three times the LOD, representing the minimum concentration that can be measured with an accuracy of ±30%.
2.4.2. Field Installation of B250 Reactors
In early May 2022, three B250 reactors were installed in the recirculation loop of the RO permeate storage tanks at three hospital locations, replacing the existing UV disinfection system. The hospitals involved were as follows:
- North Lakes Day Hospital—Located in North Lakes, within the City of Moreton Bay, Queensland. According to the 2021 census, North Lakes had a population of 23,030 [28].
- Somerset Private Hospital—Situated in Penrith, approximately 50 km west of Sydney’s central business district. The City of Penrith had a population of 217,664 as per the 2021 census [29].
- Toowoomba Surgicentre—Based in Toowoomba, located 132 km west of Brisbane, Queensland’s capital. The city’s urban population was 142,163 as recorded in the 2021 census [30].
However, only one of the hospitals provided supporting analytical reports, which will be discussed in greater detail later.
Each installation had a RO permeate tank volume of approximately 300 L with a typical recirculation rate of 7 L/min. In all cases, the systems were equipped with control panels that included a 5 A/48 V power supply, capable of delivering approximately 0.4 A (equivalent to ~1.5 mA/cm2 at ~42 V) to the electrochemical unit.
The equipment setup for each hospital is displayed in Figure 2, with images showing the control panel and electrochemical device. For example, Figure 2b shows the control panel (still unoptimized and, thus, significantly larger than the electrochemical device), while Figure 2c illustrates the electrochemical device, which is significantly smaller than the UV disinfection system it replaced.
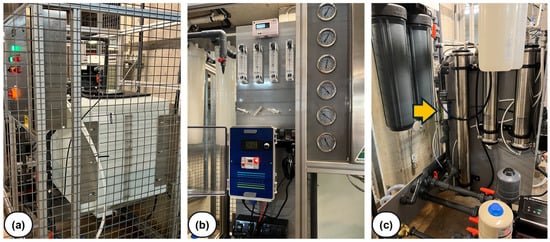
Figure 2.
Photos taken at one of the hospitals where the eBooster™ system is being tested. (a) RO permeate tank inside the restricted area of the plant; (b) control panel of the eBooster™ system; and (c) model B250 device (indicated by the arrow) installed in a vertical position within the recirculation loop (note: the nearby UV disinfection system was disconnected).
3. Results
3.1. Laboratory Testing
The initial tests, conducted without applying any current to the eBooster™ unit, aimed to assess whether passive water flow through the reactor and associated tubing could impact endotoxin concentrations, given that LPSs are known to adhere strongly to the surfaces of various materials [5].
The results in Figure 3 show that endotoxin concentration remained relatively stable around the baseline value of 4.0 EU/mL under passive conditions, with no significant changes observed across different water types (filtered water and Milli-Q water).
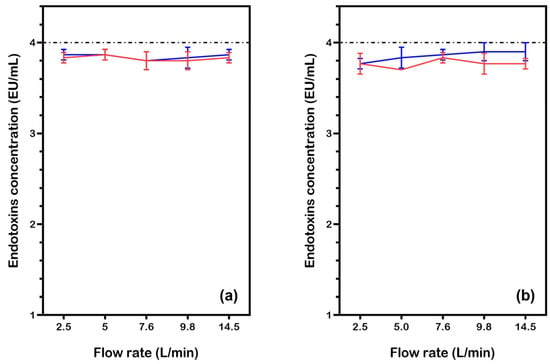
Figure 3.
Endotoxin concentration in water samples (initial LPS: 4.0 EU/mL) after passing through the eBooster™ unit without current, at various flow rates. Results are shown for two test conditions: (a) filtered water and (b) Milli-Q water. Data points and lines are color-coded for different tests (Test1: red, Test2: blue). Each point is the average of three measurements, with the corresponding standard deviation.
A two-sample t-test comparing the data in Figure 3a (filtered water) revealed no statistically significant differences between the two tests at any flow rate (all p > 0.05), confirming the reproducibility of the measurements. However, one-sample t-tests against the theoretical baseline value of 4 showed that both tests consistently produced values significantly lower than expected (all p < 0.001). The mean deviation ranged from −0.17 to −0.10 across flow rates, indicating a systematic bias.
Statistical analysis of tests performed with Milli-Q water (Figure 3b) also revealed no significant differences across flow rates (two-sample t-tests, all p > 0.05), further confirming reproducibility. However, both tests yielded mean values significantly lower than the baseline of 4 (one-sample t-tests, all p < 0.05), suggesting a systematic experimental bias. These results imply that while the two test sets are consistent within themselves, their deviations from the expected value require further investigation into potential methodological or instrumental errors. One possible contributing factor to this bias could be the aggregation of endotoxins over time, particularly at higher concentrations, which may reduce their effective quantification and alter the assay’s sensitivity. This possibility warrants further exploration to better understand its impact on the results.
Overall, the results in Figure 3 confirm that, under passive conditions (i.e., without current), the eBooster™ unit and its associated tubing do not significantly remove or retain endotoxins. This suggests that any reductions observed in subsequent experiments with applied current can be attributed to electrochemical activity rather than adsorption or physical loss within the system.
When current was applied to the eBooster™ unit, significant reductions in endotoxin concentrations were observed, especially at lower flow rates (2.5 to 5.0 L/min). As shown in Figure 4a, endotoxin levels in filtered water decreased from the baseline of 4.0 EU/mL to around 2.0 EU/mL at these lower flow rates. However, at higher flow rates (7.6–14.5 L/min), endotoxin concentrations returned closer to baseline, suggesting that shorter residence times reduce the effectiveness of electrochemical treatment. Statistical analysis confirmed that the applied current significantly lowered endotoxin levels below baseline (μ = 4) at flow rates ≤ 9.8 L/min (one-sample t-tests, all p ≤ 0.003). Both Test 1 and Test 2 showed excellent agreement (two-sample t-tests, all p ≥ 0.104), with treatment efficacy strongly dependent on flow rate: endotoxin levels were 50–60% lower than baseline at 2.5–5.0 L/min (means = 1.73–2.07), but the reduction diminished to 3–17% at higher flow rates. At 14.5 L/min, Test 2 (mean = 3.87, p = 0.080) and Test 1 (mean = 3.87, p = 0.002) diverged slightly in statistical significance, although both values approached baseline levels.
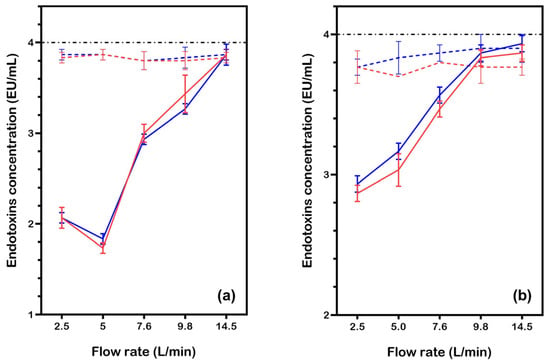
Figure 4.
Endotoxin concentration in water samples (initial LPS: 4.0 EU/mL) after treatment with the eBooster™ unit under current, at various flow rates. Results are shown for two test conditions: (a) filtered water (conductivity: 350 μS/cm, 2.7 mA/cm2 at 24 V) and (b) Milli-Q water (conductivity: 8–10 μS/cm, 0.45 mA/cm2 at 25 V). Data points and lines are color-coded for different tests (Test1: red, Test2: blue). Each point is the average of three measurements, with the corresponding standard deviation. The dashed lines indicate data without current (from Figure 3).
In Milli-Q water (Figure 4b), a modest reduction in endotoxin levels was observed at lower flow rates (2.5 to 5.0 L/min), with values dropping from the baseline (~3.7–3.8 EU/mL) to around 2.8–3.2 EU/mL. This suggests that electrochemical treatment remains somewhat effective, even with the low current (0.45 mA/cm2) resulting from the very low conductivity of Milli-Q water. However, as the flow rate increased beyond 5.0 L/min, endotoxin concentrations rose back toward baseline levels. Statistical analysis showed excellent reproducibility between Test 1 and Test 2 across all flow rates (two-sample t-tests, all p > 0.05). Measurements at lower flow rates (2.5–7.6 L/min) remained significantly below baseline (μ = 4; p < 0.001), but treatment efficacy diminished at higher flow rates. At 9.8 L/min, Test 1 (mean = 3.87, p = 0.063) showed no statistically significant difference from baseline, while Test 2 (mean = 3.83, p = 0.016) remained marginally significant. By 14.5 L/min, both tests approached baseline levels (p = 0.052–0.028), indicating a flow-rate-dependent decrease in efficacy.
These results demonstrate that under low-conductivity conditions, the effectiveness of the eBooster™ unit is greatly reduced at low current densities. To achieve substantial endotoxin reduction, longer contact times at lower flow rates are required.
Further tests using water with lower initial endotoxin concentrations were conducted to better simulate real-world conditions. Figure 5 presents the results of three separate tests, with four samples collected at each flow rate. The experiments used Milli-Q water with endotoxin concentrations of either 1.2 EU/mL (Figure 5a,b) or 0.25 EU/mL (Figure 5c). Tests were performed both without current (Figure 5a) and with current applied at a density of 0.45 mA/cm2 (Figure 5b,c), across the standard flow rate range.
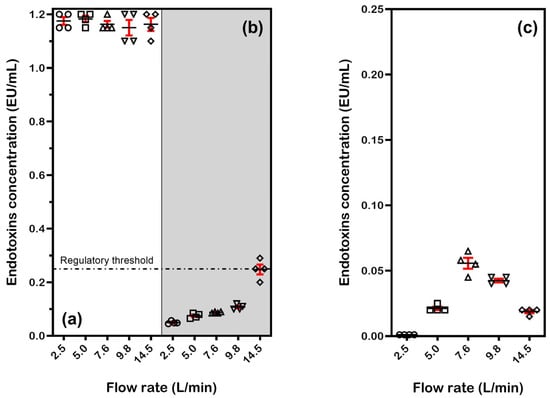
Figure 5.
Endotoxin concentration in Milli-Q water samples collected after passing through the eBooster™ unit at various flow rates: (a) water containing 1.2 EU/mL without current; (b) water containing 1.2 EU/mL treated at 0.45 mA/cm2 (under 27 V); and (c) water containing 0.25 EU/mL treated at 0.45 mA/cm2 (under 27 V). For each flow rate, individual data points, along with the mean and standard deviation, are reported.
When no current was applied (Figure 5a), endotoxin concentration remained stable at approximately 1.2 EU/mL across all flow rates, consistent with previous results and confirming that the system does not significantly remove endotoxins under passive conditions. One-sample t-tests (α = 0.05) showed no statistically significant deviation from the baseline value of 1.2 EU/mL (all p > 0.05), confirming system stability. No flow-rate-dependent trends in deviation magnitude were observed (R2 = 0.12 for mean vs. flow rate).
In contrast, when current was applied (Figure 5b), a substantial reduction in LPS concentration was observed at lower flow rates. At 2.5 and 5.0 L/min, the average concentration dropped below 0.1 EU/mL, achieving over 90% endotoxin removal. This performance surpasses that of commercially available systems, such as the ‘ToxinEraser’, previously discussed in [14]. As the flow rate increased, the process became less effective, with endotoxin levels rising progressively and approaching 0.3 EU/mL at 14.5 L/min (75% reduction). This indicates that residence time is critical for effective electrochemical treatment, although this limitation could be addressed by adding a recycle loop for multiple treatment cycles.
Statistical analysis confirmed significant reductions from the baseline (μ = 1.2) at all flow rates (one-sample t-tests, all p < 0.001), supporting the expected effect of applied current. The magnitude of reduction showed a strong flow-rate dependence, decreasing from 95.9–96.3% at lower flows (2.5–5.0 L/min) to 75.8–80.8% at intermediate flows (7.6–9.8 L/min), with partial recovery (76.0–80.4% reduction) at the highest flow rate (14.5 L/min). Overall, the system demonstrates robust current-dependent effects that are modulated by flow rate, with >90% reduction at flows ≤9.8 L/min and a substantial 79.3% reduction even at 14.5 L/min.
The observed reduction in endotoxins at a very low initial concentration (0.25 EU/mL, Figure 5c) confirms that the eBoosterTM system can achieve near-complete endotoxin removal even with a single pass. All measurements showed statistically significant reductions from baseline (one-sample t-tests, all p < 0.001), with strong flow-rate dependence. The current effect was most pronounced at low flows (99.6% reduction at 2.5 L/min) and gradually decreased with increasing flow rate (91.2–94.0% reduction at 14.5 L/min). The decreased effectiveness at intermediate flow rates (7.6–9.8 L/min) suggests that mass transfer limitations and local hydrodynamics may influence treatment efficiency. Key observations include the following:
- Low-flow regime (<5.0 L/min): in laminar flow, the fluid moves smoothly in parallel layers, keeping the boundary layer stable. This allows for efficient reactions due to good contact between the reactants and the surface.
- Critical transition (5.0 → 7.6 L/min): As the flow shifts from laminar to transitional (not yet fully turbulent), fluid movement becomes irregular, reducing boundary layer contact time. Even though the current is kept constant, the reaction rate becomes more dependent on the kinetics of the process, slowing down if reactants cannot reach the surface fast enough (loss of efficiency).
- High-flow regime: in turbulent flow conditions, enhanced mixing helps renew the electrochemical double layers, compensating for shorter residence time and maintaining reaction efficiency.
To explore the system’s broader performance, a heatmap was generated to visualize the relationship between endotoxin removal efficiency (%), flow rate, and current density. Efficiencies were simulated using Equations (1) and (2), where j is the current density (mA/cm2), and Q is the flow rate (L/min).
Equations (1) and (2) reflect laboratory findings, showing efficiencies up to 95% at low flow rates (e.g., 2.5 L/min) and high current density (2.7 mA/cm2), with efficiency decreasing at higher flow rates due to reduced residence time and lower current density. The ‘min’ function (a built-in Python function that returns the smallest value among its arguments) caps efficiency at 95%, while the ‘decay’ term (1 − 0.05 × (Q − 2.5)) models the efficiency reduction observed in Figure 4 and Figure 5.
The heatmap shown in Figure 6 highlights the eBooster™ system’s performance under optimized conditions, supporting its potential use in CSSDs for producing pyrogen-free water.
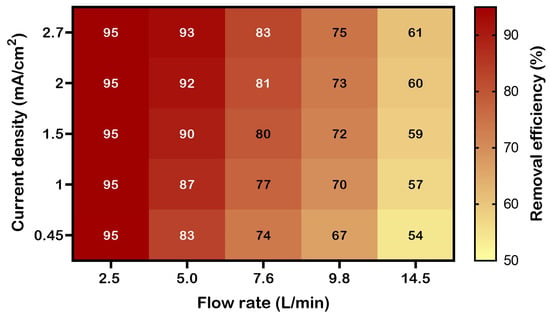
Figure 6.
Heatmap showing simulated endotoxin removal efficiency (%) as a function of flow rate and current density during treatment with the eBooster™ B250 unit. Higher efficiencies (exceeding 90%) are observed at lower flow rates (e.g., 2.5–5.0 L/min) and higher current densities (e.g., 2.7 mA/cm2), reflecting increased contact time and electrochemical energy input.
3.2. Real-World Scenario Testing
Water supplied to CSSDs typically comes from the municipal network and undergoes multi-stage treatment. Disinfection is usually carried out using UV light to prevent bacterial regrowth, followed by ultrafiltration to remove any residual bacteria and endotoxins produced by the UV treatment. Real-world testing of the eBooster™ system in hospital environments revealed that, despite challenges with variable water quality and system performance over time, the technology successfully maintained the required water quality standards for CSSD operations.
Table 1 presents typical analytical results from a NATA-accredited laboratory for water samples collected at one of the participating hospitals. These results confirm that the treated water met the required chemical quality parameters for CSSD use, with low levels of conductivity, hardness, and chemical contaminants.

Table 1.
Typical analytical results from NATA-accredited laboratories based on water samples collected at one hospital. Certificate of Analysis (CoA) date: 15 July 2024.
The microbiological testing results, extracted from multiple CoAs and summarized in Table 2, show that despite the presence of a UV disinfection system, endotoxin levels initially exceeded acceptable limits. After installing the eBoosterTM system, a significant reduction in both HPC and endotoxin levels was observed. The system performed stably for nearly two years, with only a brief disruption due to a technical issue. After 24 months of optimal operation, a slight increase in endotoxin levels was detected, which was traced to a malfunction in the power supply. Following the replacement of the power supply, the system’s effectiveness was quickly restored, bringing water quality back within acceptable limits.

Table 2.
Microbiological results from NATA-accredited laboratories, based on water samples collected at one hospital. According to AS 5369:2023, the acceptable limit for LPSs is ≤0.25 EU/mL, and HPC must be below 100 CFU/100 mL (ISO 15883-1:2024 [33]).
As anticipated, test reports were provided by only one hospital, following an investigation into a slight increase in endotoxin and HPC levels after nearly two years of optimal operation (the hospital’s name, known to the authors, has been withheld for privacy reasons). The other two hospitals reported no such issues, and Aquastream Water Solutions confirmed that all systems remain in operation.
It is important to note that testing in real-world conditions, especially in hospital environments where patient health is critical and the margin for error is minimal, presents unique challenges. Therefore, it is understandable that participating facilities may prefer to keep detailed performance data confidential.
Despite these limitations, the findings demonstrate the eBoosterTM system’s ability to maintain high microbiological and chemical water quality standards for CSSD operations. The consistent reduction in microbial load and endotoxins, along with the prompt recovery of performance after resolving a technical fault, highlights the reliability and robustness of the technology. Overall, the data support eBooster™ as a viable and efficient alternative to traditional UV disinfection for critical water treatment applications. As expected from the nature of the water being treated, as well as from experiences conducted elsewhere [24], no problems of electrode scaling, limescale formation, or performance degradation over time were encountered at any installation sites.
4. Conclusions
Endotoxins, primarily lipopolysaccharides from Gram-negative bacteria, present a significant challenge in industries such as pharmaceuticals, healthcare, and food production. Due to their potent immunogenic properties and persistence in aqueous environments, they require stringent monitoring and effective control strategies.
Laboratory testing with the eBooster™ B250 electrochemical reactor demonstrated effective endotoxin reduction under optimized operating conditions, particularly at low flow rates and adequate current densities. Even in Milli-Q water with very low conductivity (8–10 μS/cm), the system achieved substantial reductions—over 95% in some cases—when treating samples containing 1.2 EU/mL of endotoxins. Residual endotoxin concentrations consistently fell well below the 0.25 EU/mL threshold, which is critical for many sensitive applications [11].
Field data collected from hospital CSSDs further validated the real-world effectiveness of the technology. After replacing conventional UV disinfection systems with the eBooster™, both microbial counts and endotoxin levels dropped significantly, remaining well below regulatory limits over extended periods. While one facility experienced a transient performance deviation due to a power supply fault (promptly resolved), no electrode fouling or system degradation was observed, contrasting with UV systems requiring annual lamp replacements.
Although our multi-site evaluation demonstrates promising operational longevity and maintenance advantages, we acknowledge that broader validation would strengthen generalizability. The elimination of consumable components and consistent performance observed suggest compelling economic and operational benefits versus conventional UV treatment, warranting future lifecycle cost analyses. These findings position eBooster™ as a viable and sustainable alternative for endotoxin control in critical water treatment applications.
Author Contributions
Conceptualization, S.F.; methodology, J.E.L.S., L.G.A.C., S.F. and C.A.M.-H.; software, S.F. and C.A.M.-H.; validation, J.E.L.S., L.G.A.C. and C.A.M.-H.; formal analysis, J.E.L.S., L.G.A.C., S.F. and C.A.M.-H.; investigation, J.E.L.S., L.G.A.C. and C.A.M.-H.; resources, S.F. and C.A.M.-H.; data curation, S.F. and C.A.M.-H.; writing—original draft preparation, S.F.; writing—review and editing, S.F. and C.A.M.-H.; visualization, S.F.; supervision, C.A.M.-H.; project administration, C.A.M.-H.; funding acquisition, C.A.M.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil): 408110/2022-8, 421313/2023-4.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors greatly acknowledge the support and assistance of Aquastream Water Solutions (www.aquastream.com.au).
Conflicts of Interest
Sergio Ferro is employed by the company Ecas4 Australia Pty Ltd., a supplier of in-line electrochlorination systems (www.ebooster.com.au). However, his contributions to this work and manuscript were made independently, with no guidance, requirement, or input from his employer. He did not receive any financial compensation for his work on this study or the manuscript. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFU | Colony-Forming Unit |
| CoA | Certificate of Analysis |
| CSSD | Central Sterile Supply Department |
| DSA | Dimensionally Stable Anode |
| EU | Endotoxin Unit |
| FDA | Food and Drug Administration |
| HDPE | High-Density Polyethylene |
| HPC | Heterotrophic Plate Count |
| ISO | International Organization for Standardization |
| LAL | Limulus Amebocyte Lysate |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| LPS | Lipopolysaccharide |
| mg/L | Milligrams per Liter |
| NATA | National Association of Testing Authorities |
| PNA | p-Nitroaniline |
| RO | Reverse Osmosis |
| UV | Ultraviolet (light) |
| µS/cm | Microsiemens per Centimeter |
References
- Ryan, J. Endotoxins and cell culture. Corning Life Sci. Tech. Bull. 2008, pp. 1–8. Available online: https://www.gongyingshi.com/item/doc/20141221/cc_endotoxins_tc_305_rev1.pdf (accessed on 1 May 2025).
- Miyamoto, T.; Okano, S.; Kasai, N. Inactivation of Escherichia coli endotoxin by soft hydrothermal processing. Appl. Environ. Microbiol. 2009, 75, 5058–5063. [Google Scholar] [CrossRef]
- Kellum, J.A.; Ronco, C. The role of endotoxin in septic shock. Crit. Care 2023, 27, 400. [Google Scholar] [CrossRef] [PubMed]
- Daufenbach, L.Z.; Alves, W.A.; de Azevedo, J.B.; Arduino, M.J.; Forster, T.S.; Carmo, E.H.; Hatch, D.L. Pyrogenic reactions and hemorrhage associated with intrinsic exposure to endotoxin-contaminated intravenous solutions. Infect. Control Hosp. Epidemiol. 2006, 27, 735–741. [Google Scholar] [CrossRef]
- Kimble, A.; Hauschild, J.; McDonnell, G. Affinity and inactivation of bacterial endotoxins for medical device materials. Biomed. Instrum. Technol. 2023, 57, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, M.; Hageman, D.J.; Tomaszewski, W.H.; Chandra, G.M.; Skousen, J.L.; Capadona, J.R. The effect of residual endotoxin contamination on the neuroinflammatory response to sterilized intracortical microelectrodes. J. Mater. Chem. B 2014, 2, 2517–2529. [Google Scholar] [CrossRef] [PubMed]
- McCartney, A.C. The Limulus Amoebocyte Lysate assay for bacterial endotoxins. Altern. Lab. Anim. 1985, 13, 180–192. [Google Scholar] [CrossRef]
- Kulakov, L.A.; McAlister, M.B.; Ogden, K.L.; Larkin, M.J.; O’Hanlon, J.F. Analysis of bacteria contaminating ultrapure water in industrial systems. Appl. Environ. Microbiol. 2002, 68, 1548–1555. [Google Scholar] [CrossRef]
- World Health Organization and Pan American Health Organization. Decontamination and Reprocessing of Medical Devices for Health Care Facilities. 2016. Available online: https://www3.paho.org/hq/dmdocuments/2017/who-Decontamination-and-reprocessing-of-medical-devices.pdf (accessed on 1 May 2025).
- Spaan, S.; Heederik, D.J.; Thorne, P.S.; Wouters, I.M. Optimization of airborne endotoxin exposure assessment: Effects of filter type, transport conditions, extraction solutions, and storage of samples and extracts. Appl. Environ. Microbiol. 2007, 73, 6134–6143. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2006, 26, 6811–6817. [Google Scholar] [CrossRef]
- US Food & Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. 2015. Available online: https://www.fda.gov/media/80265/download (accessed on 1 May 2025).
- US Food & Drug Administration. Pyrogen and Endotoxins Testing: Questions and Answers. 2012. Available online: https://www.fda.gov/media/83477/download (accessed on 1 May 2025).
- Wan, Z.; Yu, Y.; Cheng, Q.; Tang, W.; Tan, W.; Liu, M. Rational design of hydrogel polymer nanoparticles based on a receptor recognition mechanism of endotoxin and its application in endotoxin removal. ACS Appl. Polym. Mater. 2024, 6, 14490–14504. [Google Scholar] [CrossRef]
- Jandosov, J.; Berillo, D.; Misra, A.; Alavijeh, M.; Chenchik, D.; Baimenov, A.; Bernardo, M.; Azat, S.; Mansurov, Z.; Silvestre-Albero, J.; et al. Biomass-derived nanoporous carbon honeycomb monoliths for environmental lipopolysaccharide adsorption from aqueous media. Int. J. Mol. Sci. 2025, 26, 952. [Google Scholar] [CrossRef]
- Catapano, G.; Morrone, G.; Hu, L.; Fragomeni, G.; Buscaroli, A. Endotoxin-retentive filters for the online preparation of ultrapure dialysis fluid and non-pyrogenic substitution fluid: A critical review and reference guide. Membranes 2025, 15, 51. [Google Scholar] [CrossRef]
- Ase, T.; Watabe, T.; Sato, T. Enhanced production of water for haemodialysis using electrodeionization. Sep. Sci. Technol. 2017, 52, 332–343. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Huang, J.; Xu, L.; Yin, L.; Ji, Y.; Wang, C.; Xu, Z.; Niu, J. Insights into electrochemical decomposition mechanism of lipopolysaccharide using TiO2 nanotubes arrays electrode. J. Hazard. Mater. 2020, 391, 122259. [Google Scholar] [CrossRef]
- Anderson, W.B.; Huck, P.M.; Dixon, D.G.; Mayfield, C.I. Endotoxin inactivation in water by using medium-pressure UV lamps. Appl. Environ. Microbiol. 2003, 69, 3002–3004. [Google Scholar] [CrossRef] [PubMed]
- Stange, C.; Sidhu, J.P.S.; Toze, S.; Tiehm, A. Comparative removal of antibiotic resistance genes during chlorination, ozonation, and UV treatment. Int. J. Hyg. Environ. Health 2019, 222, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kong, J.; Xue, J.; Shi, X.; Li, H.; Qiao, J.; Lu, Y. Effects of ozonation on the activity of endotoxin and its inhalation toxicity in reclaimed water. Water Res. 2019, 154, 153–161. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, J.; Qiao, J.; Lu, Y. Effects of chlorination and combined UV/Cl2 treatment on endotoxin activity and inhalation toxicity of lipopolysaccharide, gram-negative bacteria and reclaimed water. Water Res. 2019, 155, 124–130. [Google Scholar] [CrossRef]
- Sato, Y.; Suzuki, M.; Matsudaira, M. Dissolved ozone effect on corrosion of metals in water. Boshoku Gijutsu 1982, 31, 319–324. [Google Scholar] [CrossRef][Green Version]
- Ferro, S.; Vallelonga, D.; Romeo, D.; Mondello, B.; Gus, G.; Caruso, P.; Amorico, T. eBoosterTM: The first electrochemical disinfection system to reduce microbial contamination in drinking water networks without maintenance. Water 2025, 17, 1361. [Google Scholar] [CrossRef]
- Ferro, S. Challenges in designing electrochemical disinfection systems for reducing microbial contamination in drinking water distribution networks. Water 2025, 17, 754. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Zhang, X.; Yu, Y.; Chen, X. High-performance Ti/IrO2−RhOx−Ta2O5 electrodes for polarity reversal applications. Ind. Eng. Chem. Res. 2021, 60, 4310–4320. [Google Scholar] [CrossRef]
- Pierce™ Chromogenic Endotoxin Quant Kit—Thermo Fisher Scientific. Available online: https://www.thermofisher.com/order/catalog/product/A39552 (accessed on 21 May 2025).
- Australian Bureau of Statistics—North Lakes. Available online: https://www.abs.gov.au/census/find-census-data/quickstats/2021/314021579 (accessed on 18 May 2025).
- Australian Bureau of Statistics—Penrith. Available online: https://www.abs.gov.au/census/find-census-data/quickstats/2021/LGA16350 (accessed on 18 May 2025).
- Australian Bureau of Statistics—Toowoomba. Available online: https://www.abs.gov.au/census/find-census-data/quickstats/2021/3016 (accessed on 18 May 2025).
- AS 5369:2023; Reprocessing of Reusable Medical Devices and Other Devices in Health and Non-Health Related Facilities. Standards Australia Ltd: Sydney, NSW, Australia, 2023.
- ANSI/AAMI/ISO 23500-3:2019; Preparation and Quality Management of Fluids for Haemodialysis and Related Therapies—Part 3: Water for Haemodialysis and Related Therapies. Association for the Advancement of Medical Instrumentation: Arlington, VA, USA, 2020.
- ISO 15883-1:2024; Washer-Disinfectors Part 1: General Requirements, Terms and Definitions and Tests. ISO Copyright Office: Geneva, Switzerland, 2024.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).