Abstract
Springs are unique ecosystems found in lowland areas. In urban environments, these springs often have niches that are heavily transformed by human activity. In this study, we identified and compared the taxonomic diversity of diatom communities across various microhabitats—epilithon, epipelon, epipsammon, epibryon, and epixylon—within altered lowland urban springs. Our results revealed differences in diatom communities among the microhabitats, with the highest species richness observed in the epibryon. Notably, the presence of extremely rare species such as Amphora eximia, Caloneis aerophila, and Stauroneis muriella suggest that, even under urban conditions, springs continue to serve a refugial function for diatom diversity. These findings underscore the important role of urban springs in maintaining diatom diversity despite high anthropogenic pressure. We also assessed the ecological status of the springs using the Polish Multimetric Diatom Index (IO), which incorporates indicators of trophy, saprobity, and the abundance of reference species. All studied springs were classified as having very good ecological status.
1. Introduction
Springs are natural outflows of groundwater to the surface, forming a connection between groundwater and surface water, which makes them unique ecosystems from a hydrological perspective [1,2,3]. Their stable, low-temperature conditions support steno-thermic organisms [4]. Among primary producers in aquatic ecosystems, diatoms are one of the most important groups. The majority of diatom species are highly sensitive to eutrophication and organic pollution. The development of benthic diatom assemblages is influenced by several factors, including the quality of the outflowing water, the type of substrate, and the degree of anthropogenic transformation of spring niches and their immediate surroundings [2,5]. Such habitat alternations are especially evident in urbanized areas [6,7,8]. The biodiversity of springs is further characterized by the presence of diatom species that are rarely found in other aquatic environments, including some that are newly described or previously unknown [4,9,10,11].
Springs located in mountains and upland areas have received the most attention from diatomologists to date [2,4,12,13,14,15,16,17]. In contrast, the lowland springs of central Europe remain poorly studied in terms of the organisms inhabiting them [16]. In northeastern Poland, only two publications have so far presented data on diatom assemblages from lowland springs in the Białystok and Knyszyn Forest [18,19], despite a long history of research focused on the physical and chemical parameters of spring waters [1,20,21]. A pilot study revealed remarkably high diatom diversity in these springs, particularly within the genus Diploneis with two species, D. burgitensis and D. parapetersenii recorded for the first time in Poland [19]. These findings confirmed the important role of springs as refugia for diatom development—especially for rare and poorly known species—and encouraged us to expand our research to include additional urban springs (Jaroszówka Małe, Dojlidy Górne) as well as previously unstudied microhabitats such as epibryon and epixilon. Moreover, these ecosystems offer significant research potential for assessing the vulnerability of individual species [4,19].
Diatoms are widely used as indicators for assessing the ecological condition of aquatic ecosystems [22,23,24]. Based on the composition of diatom communities, it is possible to track temporal changes in pH, microplastic pollution, organic matter content, and the trophic status of water bodies [23,24,25,26].
The aim of this study was to identify and compare the taxonomic diversity of diatom communities across different microhabitats in four forest springs located in Białystok and to determine whether these springs retain their refugial character under urban conditions. In addition, the ecological status of the springs was assessed using the Multimetric Diatom Index (IO), which takes into account indicators of trophy, saprobity, and the abundance of reference species.
2. Materials and Methods
2.1. Study Area
This study focused on four springs located within the administrative boundaries of Białystok, northeastern Poland (Figure 1). Natural outflow springs are rare in urban areas; therefore, only four suitable sites were identified for research. All springs are situated on the outskirts of the city, within forested areas (Figure 1), and fall within the catchments of the Jaroszówka, Supraśl, and Biała rivers (Table 1). Each spring is classified as reocrenic, with groundwater emerging through permeable sediments composed of sand, gravel, and, in most cases, peat. Springs 2, 3, and 4 exhibit descensional flow, where groundwater percolates downward through geological layers and emerges due to gravitational pressure. In contrast, Spring No. 1 is characterized by ascendant groundwater flow, where groundwater rises to the surface under hydrostatic pressure from a confined aquifer.
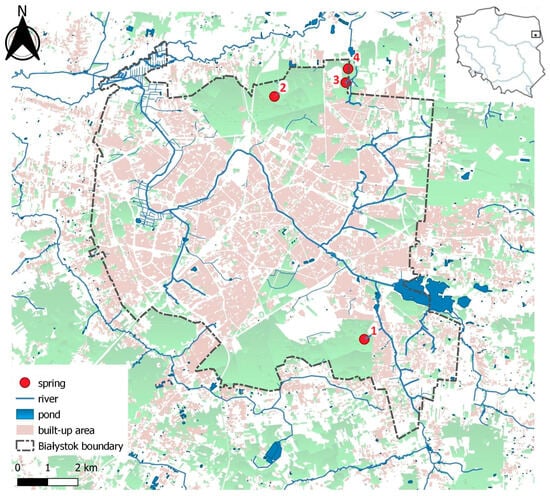
Figure 1.
The distribution of study sites in northeastern Poland. Springs in the city of Białystok are indicated with 1–4. Coordinates presented along the edges are in ETRS89/Poland CS92.

Table 1.
Morphological and hydrological characteristics of springs in Białystok: Dojlidy Górne spring (DG); Pietrasze spring (P); Jaroszówka Duże spring (JD); Jaroszówka Małe spring (JM).
Spring No. 1 (Dojlidy Górne) (Figure 2(1)) is located in the Biała River catchment, in the southern part of Białystok. It lies near a residential area composed of single-family houses but remains within a forested environment. This is a low-yield spring, with a discharge of 0.3 L s−1, situated at an elevation of 151 m a.s.l. Its surface area is approximately 150 m2. The aeration zone consists of sand, gravel, and peat.

Figure 2.
Four forest rheocrenes in Białystok: (1) Dojlidy Górne spring; (2) Pietrasze spring; (3) Jaroszówka Duże spring; (4) Jaroszówka Małe spring.
Spring No. 2 (Pietrasze) (Figure 2(2)) is located in the Supraśl catchment, within the largest forested area among the studied sites. The spring is situated in a deeply incised valley with slopes inclined at approximately 65°, and it is surrounded by recently established artificial plantings of yew (Taxus baccata L.). The aeration zone is composed of sand, gravel, and peat. This spring lies at an elevation of 140 m a.s.l., with an average discharge of 4.9 L s−1 and the largest surface area among all sites, covering approximately 700 m2. Its stable flow and extensive surface area suggest a well-functioning groundwater recharge system operating under relatively undisturbed environmental conditions.
Spring No. 3 (Jaroszówka Duże) (Figure 2(3)) is located in the Jaroszówka catchment, in a semi-forested area near Jaroszówka Małe. It has the highest discharge among the studied springs, averaging 5.2 L s−1, and is situated at an elevation of 150 m a.s.l., with a surface area of approximately 250 m2. The aeration zone consists of sand and gravel. The spring is surrounded by mixed forest, and the site is shaded and largely devoid of vegetation due to limited light availability. Historically, the site functioned as an illegal landfill. Although the waste has since been buried under a thin layer of soil, the area remains unrestored, with immobile debris still present beneath forest litter [18].
Spring No. 4 (Jaroszówka Małe) (Figure 2(4)) is also located in the Jaroszówka catchment, on the northeastern outskirts of Białystok, within a partially forested area. It is a low-discharge spring (0.3 L s−1) situated at an elevation of 150.5 m a.s.l., with a surface area of approximately 125 m2. The aeration zone is composed of sand, gravel, and peat. Due to the removal of the surrounding tree stand, the site receives high levels of sunlight, which promotes the development of lush vegetation. However, the spring is highly sensitive to drought conditions and frequently dries out, making water collection impossible during such periods. In the case of both Jaroszówka Duże and Jaroszówka Małe, potential anthropogenic pressure is increased by the proximity of a relatively busy road and a nearby aggregate plant.
2.2. Sampling and Measurements
Diatom samples and water for physical and chemical analyses were collected in May and November 2019 (Table 2). In the field, water temperature, pH, electrolytic conductivity (EC), oxygen concentration, and oxygen saturation were measured in situ using an HQ40D Multi Meter (Hach-Lange GmbH, Berlin, Germany). Diatom material was collected from five microhabitats present at the following sites: epilithon (from stones), epipelon (from mud), epipsammon (from sand), epibryon (from bryophytes), and epixylon (from wood) (Table 2). Sampling and subsequent laboratory analyses followed procedures described by Kolada et al. [26]. The relative abundance of each taxon and the species richness of diatom assemblages were estimated from a minimum of 400 diatom valves per sample. Diatom identification was based on the morphological characteristics of valves, examined under an Olympus BX53 light microscope with Nomarski phase contrast. Taxonomic identifications primarily followed Cantonati et al. [27], Bąk et al. [28], and more recent publications. Genus and species names adhere to the most current taxonomic literature. For example, Staurosira sp. closely resembles S. chavauxii [29], although this species remains a nomen nudum as it has not yet been formally described. Staurosirella neopinnata is the correct name for the taxon commonly identifed as S. pinnata [30]. Based on original materials, S. pinnata is now considered conspecific with S. construens Ehrenberg. Navicula saugerresii (=Sellaphora saugerresii) is generally placed within the “minima–minutissima–seminulum” complex (e.g., Krammer and Lange–Bertalot 1986 [31]). The morphological concept of “Navicula minima”, and consequently, Eolimna, remains uncertain, because illustrations often depict a diatom that corresponds to the common understanding of “Sellaphora seminulum”, which is a distinct species [26]. In our study, we use Sellaphora saugerresii instead of the commonly (and incorrectly) applied name S. seminulum. Sellaphora seminulum is treated here as a valid species, with S. joubaudii (= Navicula seminulum var. radiosa and Navicula joubaudii) considered a taxonomic synonym [32]. Finally, the diatom identified as Placoneis clementis is reported as Placoneis cf. clementis because P. clementis is a fossil species [33].

Table 2.
Sampling date with the type of microhabitats of diatom communities and water parameters; n.d. no determined; Dojlidy Górne spring (DG); Pietrasze spring (P); Jaroszówka Duże spring (JD); Jaroszówka Małe spring (JM).
The Shannon–Wiener Diversity Index and Jaccard Similarity Index were calculated following the methodologies outlined by Kawecka and Eloranta [34] and Kolada et al. [26]. The ecological status of the waters was assessed based on the presence and abundance of diatom indicator species. The conservation status and potential threats to diatom taxa were evaluated using the German Red List of diatoms [35]. For overall assessment, Multimetric Diatom Index (IO) was applied [26].
3. Results
The springs showed similar water temperature values (Table 2). In May, temperatures ranged narrowly between 10.2 °C and 10.7 °C. In November, a lower temperature of 8.1 °C was recorded at the Dojlidy Górne spring. The highest EC value (870 µS cm−1) was recorded at Jaroszówka Duże spring, and the lowest (363 µS cm−1) in Pietrasze spring. In May, pH values across all springs remained within a narrow range of 6.04–6.19. However, in November, the pH at the Dojlidy Górne spring increased significantly to 8.78. The highest oxygen concentration and saturation (9.72 mg L−1, 88.2%) were recorded in May in Pietrasze spring. In contrast, the lowest oxygen levels were observed at Dojlidy Górne spring, with 6.04 mg L−1 (70.2%) in May and 6.01 mg L−1 (52%) in November.
A total of 118 benthic diatom taxa, belonging to 33 genera, were identified across all studied samples (Table 3).

Table 3.
Species of diatoms found in springs: Dojlidy Górne (DG), Pietrasze (P), Jaroszówka Duże (JD), Jaroszówka Małe (JM); microhabitats (MHs): PS—epipsammon, P—epipelon, L—epilithon, B—epibryon, X—epixylon, all—species present in all microhabitats; C—commonness according to Red List in Germany [35]: NT—Not Threatened, NI—Near-Threatened; TU—Threat of Unknown Extent, DD—Data-Deficient, TD—Threatened; ER—Extremely Rare, ND—no data; collection date: *—November 2019.
The 20 genera that contributed more than 2.0% to the total relative abundance are illustrated in Figure 3, Figure 4 and Figure 5.
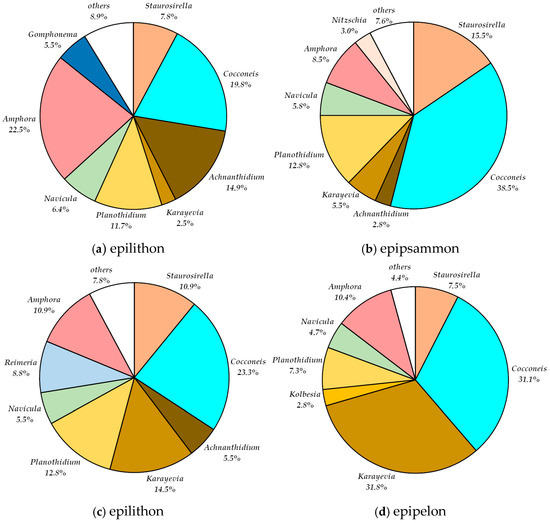
Figure 3.
Percentage share of genera in the relative abundance in the Dojlidy Górne spring in (a) epilithon, (b) epipsammon in May 2019 and (c) epilithon, (d) epipelon in November 2019.
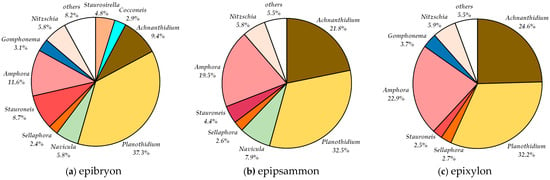
Figure 4.
Percentage share of genera in the relative abundance in the Pietrasze spring in May 2019 in (a) epibryon, (b) epipsammon, and (c) epixylon.
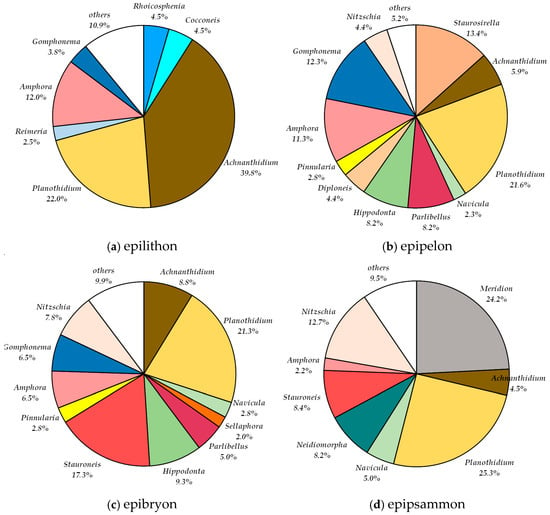
Figure 5.
Percentage share of genera in the relative abundance in May 2019 in (a) epilithon, (b) epipelon, (c) epibryon of Jaroszówka Duże, and (d) epipsammon of Jaroszówka Małe.
3.1. Dojlidy Górne Spring
The highest taxonomic diversity was observed at this site, with 68 taxa belonging to 23 genera. Samples were collected twice—in May and November 2019 (Table 2). The epilithon microhabitat exhibited the highest species richness, with 58 taxa identified. Lower numbers of taxa were recorded in the epipsammon (37 taxa) and in epipelon (26 taxa) microhabitats (Table 3).
During the spring sampling period, 19 diatom genera were recorded in the epilithon microhabitat. The most abundant genera were Amphora (22.5%), Cocconeis (19%), Achnanthidium (14.9%), and Planothidium (11.7%) (Figure 3a). Cocconeis was notable for its greatest richness, with six species identified (Table 3). The most dominant species were Amphora pediculus (20.2%), Cocconeis pseudothumensis (15.6%) and Achnanthidium minutissimum var. minutissimum (14.9%).
In autumn, the relative abundance of the genus Cocconeis in the epilithic diatom assemblages increased from 19.8% to 23.3%, making it the most dominant genus (Figure 3a,c). A slight increase was also observed for Planothidium, rising from 11.7% to 12.8%, which maintained its position among the four co-dominant genera alongside Karayevia (14.5%), Staurosirella (10.9%), and Amphora (10.9%) (Figure 3c). Some genera showed seasonal exclusivity: Hippodonta, Pinnularia, and Encyonema were recorded only in autumn while Placoneis, Caloneis, Cymbopleura, and Gomphonema were present only in spring (Table 3, Figure 3a,c). The genus Navicula was represented by the highest number of species (six) (Table 3). As in spring, Cocconeis pseudothumensis remained among the most abundant species (9.3%), followed by Planothidium frequentissimum (8.5%), Cocconeis neodiminuta, and Karayevia clevei (each at 8.1%), Staurosirella neopinnata (5.7%), and Achananthidium minnutissimum var. minutissimum (5.5%).
In the epipsammon, 37 diatom taxa representing 17 genera were identified. Although Navicula was the most species-rich genus (five species), it accounted for only a small proportion of the total abundance (5.7%) (Table 3). The most dominant genera were Cocconeis (38.5%), Staurosirella (15.5%) and Planothidium (12.8%) (Figure 3b). Within this microhabitat, Cocconeis pseudothumensis was by far the most abundant species, comprising 37.5% of the assemblage. Other notably abundant taxa included Staurosirella neopinnata (8.8%), Planothidium frequentissimum (7.5%), Amphora pediculus (7%) and Staurosirella martyi (6.8%).
In autumn, 26 diatom taxa were identified in the epipelon, representing 12 genera. Among them, the genus Cocconeis was the most taxonomically diverse, with five species (Table 3). The dominant genera were Karayevia (31.8%) and Cocconeis (31.1%) (Figure 3d). The most abundant species in this microhabitat was Cocconeis pseudothumensis, comprising 21.2% of the assemblage. Other frequently occurring species included Karayevia kolbei (17%) and K. clevei (14.9%). Notably, Cocconeis pseudothumensis was highly abundant across all analyzed microhabitats. Additionally, single valves of Staurosira sp., morphologically similar to S. chavauxii, were observed exclusively in this epipelon sample (Table 3).
3.2. Pietrasze Spring
Across the three analyzed microhabitats, a total of 63 diatom species belonging to 20 genera were identified (Table 3). The highest species richness was observed in the epibryon, with 42 species recorded, whereas the lowest diversity was found in the epixylon, which hosted 26 species.
In the epibryon microhabitat, 42 diatom taxa belonging to 20 genera were recorded. The genera Cocconeis, Navicula, and Stauroneis exhibited the highest species richness, each represented by five species (Table 3). The genus Planothidium dominated in terms of relative abundance, accounting for 37.3% of the total assemblage (Figure 4a). Other notable genera included Amphora (11.6%), Achnanthidium (9.4%), and Stauroneis (8.7%). Among all identified species, Planothidium frequentissimum was the most abundant (25.7%), followed by Planothidium lanceolatum (11.6%), Achnanthidium minutissimum var. minutissimum (9.4%), and Amphora pediculus (7.8%).
In the epipsammon microhabitat, 39 diatom taxa belonging to 15 genera were recorded. The genus Nitzschia was the most species-rich, with 12 species identified (Table 3). The most abundant genera were Planothidium (32.5%), Achnanthidium (21.8%), and Amphora (19.5%) (Figure 4b). Among the species, Planothidium frequentissimum showed the highest abundance (24.4%), followed closely by Achnanthidium minutissimum var. minutissimum (21.8%). Other abundant species included Amphora pediculus (15.8%), Planothidium lanceolatum (8.1%), and Navicula striolata (7.2%).
In the epixylon microhabitat, 26 diatom taxa from 12 genera were identified, with the genus Nitzschia being the most species-rich, comprising 4 species (Figure 4c). The genus Planothidium had the highest relative abundance, accounting for 32.2% of the total diatom valves (Figure 4c). Other genera with significant shares included Achnanthidium (24.6%) and Amphora (22.9%). Among species, Planothidium frequentissimum and Achnanthidium minutissimum var. minutissimum had very similar abundances, representing 26% and 24.6% of the total diatom valves, respectively. Amphora pediculus was less abundant at 17%. Notaly, Stauroneis muriella was recorded only in the epixylon habitat. Additionally, single valves of Cocconeis pseudothumensis were found in both epibryon and epixylon microhabitats.
3.3. Jaroszówka Duże Spring
In the epilithon microhabitat, 33 diatom taxa from 19 genera were identified (Table 3). The genus Navicula was the most taxonomically diverse, represented by 4 species. Achnanthidium was the most abundant genus, comprising 39.8% of the total diatom valves (Figure 5a). Planothidium and Amphora were also well represented, accounting for 22% and 12%, respectively. Among the species, Achnanthidium minutissimum var minutissimum had the highest relative abundance (39.8%), followed by Planothidium frequentissimum (18.5%) and Amphora pediculus (8%) (Figure 5a).
In epipelon microhabitat, 35 diatom taxa belonging to 17 genera were identified. The genera Amphora and Planothidium were the most species-rich, each represented by four species. Planothidium also had the highest relative abundance among the genera, accounting for 21.6% of the total diatom community (Figure 5b). Other genera with notable but lower shares included Staurosirella (13.4%), Gomphonema (12.3%), and Amphora (11.3%). At the species level, Planothidium frequentissimum was the most abundant, comprising 16.5% of the assemblage.
In the epibryon microhabitat, 43 diatom taxa representing 22 genera were identified. The genera Navicula and Nitzschia were the most species-rich, each represented by five species. In terms of relative abundance, Planothidium (21.3%) and Stauroneis (17.3%) were the most dominant genera (Figure 5c). At the species level, the most abundant taxa were Planothidium frequentissimum (14%), Staurosirella neopinnata (10.5%), Hippodonta costulata (9.3%), and Achnanthidium minutissimum var. minutissimum (8.8%).
3.4. Jaroszówka Małe Spring
In the epipsammon microhabitat, 21 diatom genera were recorded. The genera Planothidium (25.3%), Meridion (24.2%) and Nitzschia (12.7%) exhibited the highest relative abundances (Figure 5d). Nitzschia was the most species-rich genus, represented by seven 7 species. The most abundant species were Meridion circulare var. circulare (24.2%), Planothidium frequentissimum (19.3%), and Nitzschia homburgiensis (10%).
3.5. Species Distributions Across Microhabitats
A total of 14 diatom species were found inhabiting all studied microhabitats: Cocconeis neodiminuta, C. placentula var. placentula, C. pseudothumensis, Achnanthidium minutissimum var. minutissimum, Planothidium frequentissimum, P. lanceolatum, Navicula antonii, N. striolata, Hippodonta costulata, Stauroneis smithii, Amphora copulata, A. pediculus, Gomphonema micropus, and Nitzschia frustulum var. frustulum (Table 3). Among them, according to German Red List [35] 11 species are classified as Not Threatened. Two species (Cocconeis pseudothumensis and Navicula striolata) are considered to be Threat of Unknown Extent, and one species (Cocconeis placentula var. placentula) is categorized as Data-Deficient due to insufficient information for proper assessment (Table 3). The highest taxonomic diversity was recorded in the epipsammon, where 21 unique species were identified (Table 3). These included Diatoma moniliformis ssp. ovalis, Eunotia tenella, Navicula lanceolata, Placoneis elginensis, Neidiomorpha binodis, Neidium ampliatum, Frustulia vulgaris, Caloneis alpestris, Pinnularia perirrorata, Cymbopleura subaequalis, Nitzschia amphibia, N. capitellata, N. frustulum, N. homburgiensis, N. intermedia, N. microcephala, N. perminuta, N. pura, N. pusilla, and N. recta, Surirella angusta. Of these, 16 were classified as Not Threatened. The remaining five belonged to other categories: Eunotia tenella, Caloneis alpestris, and Nitzschia homburgiensis (Threat of Unknown Extent), Neidiomorpha binodis (Near-Threatened), and Cymbopleura subaequalis (Threatened) (Table 3). The epilithon microhabitat was inhabited by 11 unique species: Staurosirella leptostauron var. dubia, Cocconeis lineata, C. placentula var. klinoraphis, Psammothidium helveticum, Placoneis cf clementis, Sellaphora saugerresii, Caloneis fontinalis, Encyonema reichardtii, Gomphonema innocens, G. parvulum, and Nitzschia frustulum var. inconspicua. This microhabitat exhibited the lowest variation in rarity classification—10 of these species were considered Not Threatened, and one (Placoneis cf clementis) was classified as Data-Deficient. In the epibryon, the following species were found exclusively: Navicula tenelloides, Humidophila perpusilla, Neidium bisulcatum, Stauroneis anceps, S. thermicola, Diploneis fontanella, D. fontium, and Pinnularia obscura (Table 3). In contrast to the epilithon, this habitat showed the highest variation in rarity. Only three of the above species were classified as Not Threatened, while others were categorized as Threat of Unknown Extent (Diploneis fontanella, D. fontium), Near-Threatened (Stauroneis anceps), and Threatened (Neidium bisulcatum) groups. In the epipelon, only four rare species were identified: Sellaphora pupula sensu lato, Parlibellus protractoides, Neidiomorpha binodeformis, and Halamphora normanii. Except for Sellaphora pupula, which classified as Data-Deficient, all others are considered Not Threatened. The epixylon microhabitat had the lowest species-richness, with only three rare taxa: Gomphonema angustum (Threat of Unknown Extent), Nitzschia palea var. debilis, and N. palea var. palea (both Not Threatened). The presence, rarity status, and microhabitat preference are summarized in Table 3.
3.6. Comparison of Diatom Communities
To assess and compare species diversity across the studied sites, the Shannon–Wiener diversity index was used. The highest value of the index (1.896), indicating the greatest species diversity, was recorded in the epibryon of the Jaroszówka Duże spring. In contrast, the lowest value (0.974) was observed in the epixylon of the Pietrasze spring in May 2019 (Figure 6).
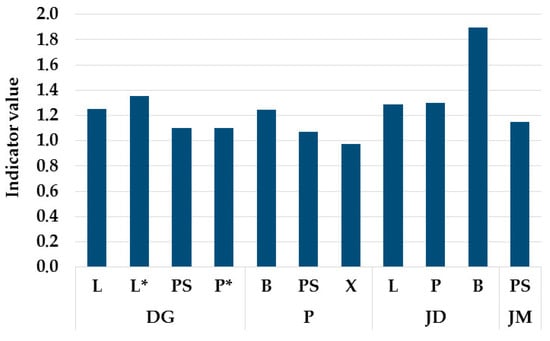
Figure 6.
The value of the Shannon–Wiener Index for individual locations: Dojlidy Górne (DG), Pietrasze (P), Jaroszówka Duże (JD), Jaroszówka Małe (JM); PS—epipsammon, P—epipelon, L—epilithon, B—epibryon, X—epixylon, *—November 2019.
To compare the structure of diatom communities across the study sites, the Jaccard Index was employed. Comparisons were made between microhabitats within individual springs as well as among identical microhabitats across different springs (Table 4). The highest value of the index (0.483), indicating the greatest similarity in diatom species composition, was observed in the epilithon assemblages from May and November in the Dojlidy Górne spring. In contrast, the lowest species similarity was found in the epipsammon assemblages between Dojlidy Górne and Pietrasze (0.267), and between Pietrasze and Jaroszówka Małe (0.270). All index values are presented in Table 4.

Table 4.
Species similarity of diatoms within one spring based on the Jaccard Index; Dojlidy Górne (DG), Pietrasze (P), Jaroszówka Duże (JD), Jaroszówka Małe (JM); PS—epipsammon, P—epipelon, L—epilithon, B—epibryon, X—epixylon, *—November 2019.
3.7. Ecological Status of the Springs
The ecological status of the spring waters was assessed using the Polish Multimetric Diatom Index (IO), which integrates three components: the trophic index (TI), saprobic index (SI), and the abundance of reference species (GR). The highest value (2.975), indicating the least favorable trophic conditions, was obtained for the Dojlidy Górne spring in the autumn epipelon. The lowest TI value (2.481), indicating more favorable conditions, was observed in the epilithon of the Jaroszówka Duże spring. The highest SI value (1.859), reflecting greater organic pollution, was found in the epipsammon of the Pietrasze spring during the spring survey, while the lowest SI (1.247) was noted in the epipsammon of the Dojlidy Górne spring in the same period. The highest GR values, reflecting the greatest abundance of reference species, were very similar and recorded in the Dojlidy Górne spring (0.834, epilithon) and in the Pietrasze spring (0.833, epixylon). The lowest GR value (0.569) occurred in the epipelon of the Dojlidy Górne spring in autumn. The highest values of the Multimetric Diatom Index (IO), indicating the best ecological status, were recorded in the Dojlidy Górne spring during spring in the epipsammon (0.675) and epilithon microhabitats (0.672). The lowest IO value (0.564) was noted in the epibryon of the Jaroszówka Duże spring. Despite these variations, all studied sites were classified as having a very good ecological status (Table 5).

Table 5.
Values of diatom indicators and assessment of the ecological status of waters in the studied springs; Dojlidy Górne (DG), Pietrasze (P), Jaroszówka Duże (JD), Jaroszówka Małe (JM); PS—epipsammon, P—epipelon, L—epilithon, B—epibryon, X—epixylon, *—November 2019; TI—trophic index, SI—saprobic index, GR—abundance of reference species, IO—Multimetric Diatom Index.
4. Discussion
4.1. Diatom Communities in Lowland Areas
Diatoms inhabit nearly all aquatic environments, including springs [24,29,36]. However, studies focusing on benthic diatom communities in lowland springs are scarce [18,19,37], as most springs are mainly located in the highlands and mountains [2,38,39]. Moreover, taxonomic studies of urban springs are limited [7,8,18]. Urban development often leads to significant transformation of spring habitats, in some cases resulting in the complete loss of water discharge [6]. In contrast, the presence of relatively unaltered spring habitats within semi-natural forest areas of Białystok (North Podlasie Lowland) provided a unique opportunity to investigate benthic diatom communities. The forested locations of these springs offer a degree of protection not found in more heavily transformed urban environments. This has contributed to the highest overall number of diatom species, especially in the Dojlidy Górne and Pietrasze springs. Currently, Dojlidy Górne and Pietrasze appear to be the best naturally preserved springs. Pietrasze is effectively isolated by a large forest and situated far from roads, while Dojlidy Górne—despite being close to single-family housing—is shielded by a dense forest barrier and lacks direct access paths, which limits human disturbance. In contrast, the Jaroszówka Duże spring, more exposed to anthropogenic pressure, exhibited the highest EC values (870 µS cm−1) (Table 2) recorded both in this and previous studies [18], as well as elevated sulfate (av. 133.5 mg L−1) and chloride (av. 88.6 mg L−1) concentrations [3,18], indicating degraded water quality compared to other studied springs. Interestingly, despite greater human influence, Jaroszówka Duże and Jaroszówka Małe showed similar Shannon–Wiener Index values (Table 4) in epipelon, epipsammon, and epilithon microhabitats when compared to the better-protected Dojlidy Górne and Pietrasze. This suggests that favorable conditions for diatom development persist across all studied sites. The highest index value was obtained in the epibryon Jaroszówka Duże, driven by a marked increase in species richness compared to epilithon and epipelon, particularly due to aerophytic taxa such as Humidophila perpusilla and Caloneis aerophila. Similar findings of higher diversity in epibryon versus epilithon have been reported in alpine springs [40]. Aerophytic diatoms are capable of withstanding temporary water shortages and are commonly found in the epibryon [41]. Higher water quality is further reflected by the presence of species sensitive to organic pollution, such as Cocconeis pseudothumensis. This species was dominant or co-dominant in all habitats of the Dojlidy Górne spring and was also detected in the epibryon and epixylon of the Pietrasze spring.
The majority of the identified diatoms, particularly dominant species such as Amphora pediculus, as well as less abundant taxa Nitzschia frustulum, Navicula antonii, N. gregaria, and N. lanceolata, are characterized as eurytopic taxa [7]. Many of the observed species are widespread, such as Gomphonema micropus, Reimeria sinuata, Stauroneis smithii [27]. However, our study also revealed the presence of rarely recorded diatoms—Caloneis aerophila, Cocconeis pseudothumensis, Navicula striolata, Nitzschia homburgiensis, Placoneis elginensis, P. paraelginensis, Sellaphora pseudopupula, and Stauroneis muriella [27], as well as Staurosira sp. (=S. chavauxii). Springs are known to harbor rare, endangered, poorly studied diatoms that are new to the country or science [4,7,9,11,19]. These findings highlight the critical ecological role of spring niches as irreplaceable refugia for diatom survival and hotspots for maintaining aquatic biodiversity [4,19].
Diatom Red Lists are tools important for the assessment and preservation of biodiversity and habitats [42]. They help evaluate the threat levels faced by native species. In our study, we began with the Red List of Plants and Fungi in Poland (2006) [43], which revealed the presence of various diatom groups in the forested urban springs under investigation. Navicula striolata and Sellaphora pseudopupulla, which were recorded in all urban springs, and Pinnularia viridiformis from Dojlidy Górne, Jaroszówka Duże, and Jaroszówka Małe springs are recognized as Declining—critically endangered species. According to Red List of Plants and Fungi in Poland (2006) [43] such diatoms as Caloneis fontinalis, Diploneis fontinum, Nitzschia pura are classified as rare in Europe [7,9,19]. However, under the current classification [35], Diploneis fontinum is listed as Threated of Unknown Extent, while Caloneis fontinalis, Nitzschia pura are now considered Not Threatened. Next, we applied the latest diatom threat list [35]. According to the German Red List [35], diatom taxa identified in our study were classified as follows: 1—Extremely Rare: Amphora eximia, Caloneis aerophila, and Stauroneis muriella; 2—Threat of Unknown Extent: Caloneis alpestris, Cocconeis pseudothumensis, Diploneis fontanella, D. krammeri, Eunotia tenella, Navicula oblonga, N. striolata, Nitzschia homburgiensis, Pinnularia viridiformis, and Sellaphora pseudopupula; 3—Threatened: Cymbopleura subaequalis; 4—Near-Threatened: Diploneis elliptica, Halamphora normanii, Neidium ampliatum, Stauroneis anceps, and S. separanda; 5—Data-Deficient: Cocconeis placentula var. placentula, Diploneis separanda, Placoneis elginensis, P. paraelginensis, and Stauroneis leguminopsis (Table 3). The remaining 80% of the species were classified as Not Threatened. The most threatened diatoms (Extremely Rare), such as Amphora eximia, Caloneis aerophila, and Stauroneis muriella may indicate the preservation of the refugial character of the springs despite their small populations. Several other notable rare or vulnerable diatoms were recorded: Stauroneis thermicola (Pietrasze spring), Cocconeis disculus (Dojlidy Górne, Pietrasze), C. placentula var. klinographis (Pietrasze), Parlibellus protractoides (Jaroszówka Duże) and among vulnerable: Sellaphora bacillum (Pietrasze, Jaroszówka Duże, Jaroszówka Małe), Cocconeis pseudothumensis (Dojlidy Górne, Pietrasze) (Table 3). Many of the diatom species we recorded remain poorly understood in terms of environmental preferences (e.g., Diploneis separanda) [19,27], underscoring the need for continued taxonomic research, with simultaneous analysis of water quality.
4.2. Microhabitat Type Versus Species Communities
Previous studies of diatom communities in the springs of Bialystok focused exclusively on two sites: Jaroszówka Duże and Pietrasze. In Jaroszówka Duże, epilithic diatom communities were examined only once [18], and only during summer. A second study in both niches focused only on the diversity of the genus Diploneis in the psammon and/or epipelon [19]. Our research is the first to document diatom communities in the Jaroszówka Małe and Dojlidy springs. Additionally, it expands the scope of earlier studies by including new microhabitats in Jaroszówka Duże (psammon and epibryon), and by presenting—for the first time—a complete species list from psammon, epipelon, and epixylon in Pietrasze. The greater diversity of substrates within Jaroszówka Duże clearly contributes to an increase in the number of recorded diatom taxa. This is particularly evident in the Dojlidy Górne and Pietrasze springs. While some species were restricted to a single microhabitat, only 14 out of 118 taxa were found in all examined microhabitats. The diversity of microhabitats in our springs contributed to a high species richness, comparable to findings from other regions in Poland [4,28] and globally [2,40,42]. A similar relationship between microhabitat type and diatom community composition was previously reported by Cantonati et al. (2023) [2] in German mountain springs, where out of 127 taxa, 27 were exclusive to the epilithon and 38 to the epibryon. With a similar total number of taxa in our (118 taxa) and Alpine springs, the numbers of taxa exclusive to the epilithon or epibryon in the mountains were several times higher than in our study. Interestingly, none of the listed species appeared in our springs with the same types of habitats. This difference is likely due to climatic conditions and geographical location. Moreover, diatom communities are influenced by other key environmental factors that contribute to spring classification, including spring type, spring efficiency, and water quality [39,40,44].
Our results showed that species richness in the epilithon of Dojlidy Górne (40–46 taxa/400 valves) and Jaroszówka Duże (31 taxa/400 valves) was lower than that reported from carbonate lowland springs in Flanders (av. 50 taxa/500 valves) [37], but higher than in many upland and mountain springs elsewhere in Europe (14–37/300–450 valves) (for Denys, Oosterlynck 2015) [37].
Only in the case of the Jaroszówka Duże spring was it possible to compare epilithon communities between our study and previous research [18]. Our results documented more than twice the number of taxa (31) compared to an earlier study (14). Only eight taxa were shared between the two sampling periods: Achnanthidium minutissimum var. minutissimum, Amphora pediculus, Meridion circulare var. circulare, Planothidium frequentissimum, P. lanceolatum, Rhoicosphenia abbreviata, Staurosirella neopinnata, and Navicula gregaria. In addition, our study confirmed the presence of four species of the genus Diploneis previously reported by Grabowska et al. 2023 [19] in the Jaroszówka Duże and Pietrasze springs. Specifically, D. fontinum and D. elliptica were recorded in Jaroszówka Duże, while D. fontanella and D. krammeri were observed in the Pietrasze spring.
4.3. Environmental Impact, Ecological Status
The use of bioindicators—organisms sensitive to changes in environmental conditions—is a common approach to evaluating the ecological status of aquatic ecosystems [39,45]. According to the Water Framework Directive, benthic diatoms, along with phytoplankton, macrophytes, macrozoobenthos, and fish, are integral components in the assessment of surface water quality [26]. A notable example is Cocconeis pseudothumensis, which was identified in all microhabitats of the Dojlidy Górne spring and in the epibryon and epixylon of the Pietrasze spring. This species, in terms of saprobic index, does not tolerate pollution [27,28]. Its presence in these two springs suggests that they are currently the most effectively protected from anthropogenic pressure among the studied sites. This conclusion is further supported by the highest values of the Polish Multimetric Diatom Index (IO), which integrates three components: trophic index, saprobic index, and abundance of reference species. The most favorable assessments were found for the Dojlidy Górne and Pietrasze springs, which are subject to relatively low anthropogenic impact. In contrast, the Jaroszówka Duże and Jaroszówka Małe springs, more exposed to external influences, received lower IO values. Across all springs, the epipelon communities received the weakest evaluation. Nevertheless, for all diatom assemblage types studied, the final classification of ecological status was the same—defined as very good. Our findings demonstrate that a reliable assessment of the ecological status of lowland springs is also possible using diatom communities other than epilithic ones. Epilithon communities are recommended for water quality assessment, but such assemblages are virtually absent in several aquatic lowland systems [22,45].
Extreme climate change poses a serious threat to ecosystems worldwide. The impact of increasing drought events has been significant for aquatic ecosystems, where lower water levels can lead to increased concentrations of nutrients and pollutants, increased water temperature, and decreased flow rates [46]. Springs are not exempt from these pressures, especially those with small capacities (of the order of 1–5 L/s), such as the springs in Bialystok. This vulnerability is especially evident in the case of the Jaroszówka Małe spring, where prolonged periods of low water availability throughout much of the year present a risk of habitat loss and the potential disappearance of this niche. Anthropogenic pressures also represent a significant threat to aquatic ecosystems. Unsuitable land use, urbanization, industrialization, and agriculture, contribute to the introduction of pollutants into water bodies. Climate and anthropogenic changes have contributed to conservation efforts [46].
5. Conclusions
Our research has demonstrated that semi-natural, forested urban springs are characterized by high diatom species richness, encompassing taxa with diverse habitat preferences and varying degrees of threat. Among the 118 identified benthic diatom taxa, the majority (approximately 80%) are classified as Not Threatened, which may reflect a notable decline in the biodiversity of communities in the urban environment. Nevertheless, even in springs exposed to greater anthropogenic pressure, three extremely rare species were recorded: Amphora eximia, Stauroneis muriella, and Caloneis aerophila. These findings indicate that extremely endangered diatoms occurred in all studied springs and in all microhabitats.
Based on the highest values of the Shannon–Wiener Index, the greatest species diversity was observed in the epibryon communities, while the lowest was recorded in the epipelon. The low similarity of diatom communities within the same substrate types was found between springs indicating effective isolation of niches and preservation of their unique character. The ecological status of the waters was assessed as very good at all the studied sites, regardless of the type of substrate inhabited by diatoms and the varying potential anthropogenic pressure.
Author Contributions
W.L. undertook field analyses and sampling, microscopic analyses, and writing of the manuscript; M.G. designed the study and undertook field analyses and sampling, microscopic analyses, and writing of the manuscript; A.Z.W., taxonomy consultations, writing and revising the manuscript; K.P. participated in field analyses and writing of the manuscript; A.W. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by a subsidy from the Biology Department of the University of Bialystok for the maintenance and development of research potential.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the anonymous reviewers for their valuable comments, which improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jekatierynczuk-Rudczyk, E.; Zieliński, P.; Puczko, K.; Micun, K.; Puczyłowska, E. The role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland). Water 2022, 14, 3202. [Google Scholar] [CrossRef]
- Cantonati, M.; Casoria, C.; Gerecke, R.; Bilous, O.P.; Maisto, G.; Segadelli, S.; Spitale, D.; Steinbauer, A.; Vogel, S.; Saber, A.A. Diatom Indicators of Fluctuating/ Intermittent Discharge from Springs in Two Bavarian Nature Conservation Areas. Diversity 2023, 15, 915. [Google Scholar] [CrossRef]
- Puczko, K.; Zieliński, P.; Jusik, S.; Kołakowska, A.; Jekatierynczuk-Rudczyk, E. Vascular plant and bryophyte species richness in response to water quality in lowland spring niches with different anthropogenic impacts. Environ. Monit. Assess. 2018, 190, 338. [Google Scholar] [CrossRef]
- Wojtal, A.Z. Species Composition and Distribution of Diatom Assemblages in Spring Waters in Southern Poland; Bibliotheca Diatomologica: Stuttgart, Germany, 2013; Volume 59, pp. 1–436. [Google Scholar]
- Szczepocka, E.; Żelazna-Wieczorek, J. Diatom biomonitoring—Scientific foundations, commonly discussed issues and frequently made errors. Oceanol. Hydrobiol. Stud. 2018, 47, 313–325. [Google Scholar] [CrossRef]
- Wang, X.; Chengxi, W.; Botao, W.; Shenghe, L.; Jinping, S. Protection of urban features during urbanization based on the roles of springs in Jinan. Chin. J. Pop. Res. Environ. 2017, 15, 93–102. [Google Scholar] [CrossRef]
- Wojtal, A.Z.; Okoń, D.; Różkowski, J. Diatom diversity (Bacillariophyta) in springs near Zawiercie. In XXVIII Jurassic Symposium Man and Nature of the Krakow-Wielun Upland. Conservation of Biodiversity and Landscape Identity; Selected issues. Post-conference materials; Zespół Parków Krajobrazowych Województwa Śląskiego: Będzin, Poland, 2017; pp. 67–73. (In Polish) [Google Scholar]
- Knysak, P.J. Human Impact on Crenic Ecosystems Based on the Diversity of Diatoms and Their Autecology. Ph.D. Thesis, University of Lodz, Łódź, Poland, 2019. [Google Scholar]
- Żelazna-Wieczorek, J. Diatom Flora in Springs of Łódź Hills (Central Poland). Biodiversity, Taxonomy, and Temporal Changes of Epipsammic Diatom Assemblages in Springs Affected by Human Impact; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2011; pp. 1–419. [Google Scholar]
- Delgado, C.; Ector, L.; Novais, M.H.; Blanco, S.; Hoffmann, L.; Padro, I. Epilithic diatoms of springs and spring–fed streams in Majorca Island (Spain) with the description of a new diatom species Cymbopleura margalefii sp. nov. Fottea 2013, 13, 87–104. [Google Scholar] [CrossRef]
- Cid-Rodríguez, M.; Cantonati, M.; Angeli, N.; Bilous, O.; Al-Harbi, M.; Lange-Bertalot, H.; Levkov, Z.; Piana, L.; Spitale, D.; Saber, A.A. The Diatom Genus Navicula in Spring Ecosystems with the Description of Navicula aquaesuavis sp. nov. Water 2024, 16, 2751. [Google Scholar] [CrossRef]
- Sabater, S.; Roca, J.R. Ecological and biogeographical aspects of diatom distribution in Pyrenean springs. Br. Phycol. J. 1992, 27, 203–213. [Google Scholar] [CrossRef]
- Solak, C.N.; Wojtal, A.Z. Diatoms in springs and streams of Türkmen Mountain (Sakarya river basin) common in Turkish inland waters. Pol. Bot. J. 2012, 57, 375–425. [Google Scholar]
- Hindáková, A.; Hindák, F. Cyanobacteria and diatoms of cold mineral springs in the National Natural Landmark of Mičiná (Central Slovakia). Bull. Slov. Bot. Spoločn. 2016, 38, 13–19. (In Slovak) [Google Scholar]
- Leira, M.; Meijide-Failde, R.; Torres, E. Diatom communities in thermo-mineral springs of Galicia (NW Spain). Diat. Res. 2017, 32, 29–42. [Google Scholar] [CrossRef]
- Lai, G.G.; Padedda, B.M.; Wetzel, C.E.; Cantonati, M.; Sechi, N.; Lugliè, A.; Ector, L. Diatom assemblages from different substrates of the Casteldoria thermo-mineral spring (Northern Sardinia, Italy). Bot. Lett. 2018, 166, 14–31. [Google Scholar] [CrossRef]
- Taxböck, L.; Karger, D.N.; Kessler, M.; Spitale, D.; Cantonati, M. Diatom Species Richness in Swiss Springs Increases with Habitat Complexity and Elevation. Water 2020, 12, 449. [Google Scholar] [CrossRef]
- Jekatierynczuk-Rudczyk, E.; Puczko, K.; Żukowska, J.; Sawicka, A. Biota communities influence on nutrients circulation in hyporheic zone—A case study in urban spring niches in Bialystok (NE Poland). Aquat. Sci. 2021, 83, 75. [Google Scholar] [CrossRef]
- Grabowska, M.; Wojtal, A.; Jekatierynczuk-Rudczyk, E.; Kryvosheia-Zakharova, O. Spatial patterns of Diploneis genus-level diversity in the Podlasie springs (NE Poland). Diversity 2023, 15, 897. [Google Scholar] [CrossRef]
- Jekatierynczuk-Rudczyk, E. Effects of drainage basin management on the chemical composition of waters in lowland springs. Acta Hydrobiol. 1999, 41, 97–105. [Google Scholar]
- Wojtal, A.Z.; Sobczyk, Ł. The influence of substrates and physicochemical factors on the composition of diatom assemblages in karst springs and their applicability in water-quality assessment. Hydrobiologia 2012, 695, 97–108. [Google Scholar] [CrossRef][Green Version]
- Potapova, M.; Charles, D.F. Choice of substrate in algae-based water-quality assessment. J. N. Am. Benthol. Soc. 2005, 24, 415–427. [Google Scholar] [CrossRef]
- Zgrudno, A.; Picińska-Fałtnowicz, J.; Błachuta, J.; Pasztaleniec, A. Phytobenthos in rivers and dam reservoirs. In Handbook for Monitoring Biological Elements and Classifying the Ecological Status of Surface Waters; Kolada, A., Ed.; Environmental Monitoring Library: Warsaw, Poland, 2020; pp. 45–74. [Google Scholar]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.B.; Selmeczy, G.; Lengyel, E.; Tapolczai, K. Ecosystem services provided by freshwater and marine diatoms. Hydrobiologia 2023, 850, 2707–2733. [Google Scholar] [CrossRef]
- Parikh, H.S.; Dave, G.; Tiwari, A. Microplastic pollution in aquatic ecosystems: Impacts on diatom communities. Environ. Monit. Assess. 2025, 197, 206. [Google Scholar] [CrossRef]
- Kolada, A.; Adamczyk, M.; Bielczyńska, A.; Bis, B.; Błachuta, J.; Błeńska, M.; Bociąg, K.; Brzeska-Roszczyk, P.; Ciecierska, H.; Dziemian, Ł.; et al. Manual for Monitoring of Biological Elements and Classification of Ecological Status of Surface Waters. Methods Update; Library of Environmental Monitoring: Warsaw, Poland, 2020. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H.; Hofmann, G. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Glashutten, Germany, 2017; pp. 1–942. [Google Scholar]
- Bąk, M.; Witkowski, A.; Żelazna-Wieczorek, J.; Wojtal, A.; Szczepocka, E.; Szulc, K.; Szulc, B. The Key to the Determination of Diatoms in Phytobenthos for the Assessment of the Ecological Status of Surface Waters in Poland; Library of Environmental Monitoring: Warsaw, Poland, 2012; pp. 1–452. (In Polish) [Google Scholar]
- Bey, M.-Y.; Ector, L. Atlas des Diatomées des Cours D’eau de la Région Rhône-Alpes, Tome 2: Araphidées, Brachyraphidées; Direction régionale de l’Environnement, de l’Aménagement et du Logement Rhône-Alpes: Lyon, France, 2013; pp. 1–168. [Google Scholar]
- Morales, E.A.; Wetzel, C.E.; Haworth, E.Y.; Ector, L. Ending a 175-year taxonomic uncertainty: Description of Staurosirella neopinnata sp. nov. (Bacillariophyta) to accommodate Fragilaria pinnata, a highly misconstrued taxon with a purported worldwide distribution. Phytotaxa 2013, 402, 75–87. [Google Scholar] [CrossRef]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Part 1: Naviculaceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Jena, Germany, 1986; p. 876. [Google Scholar]
- Wetzel, C.; Ector, L.; Van de Vijver, B.; Compère, P.; Mann, D. Morphology, typification and critical analysis of some ecologically important small naviculoid species (Bacillariophyta). Fottea 2015, 15, 203–234. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Wojtal, A.Z. Diversity in species complexes of Placoneis clementis (Grunow) Cox and Paraplaconeis placentula (Ehrenberg) Kulikovskiy, Lange-Bertalot & Metzeltin. Nova Hedwigia Beiheft 2014, 143, 403–420. [Google Scholar]
- Kawecka, B.; Eloranta, P.V. Outline of Algal Ecology of Freshwater and Terrestrial Environments; PWN Scientific Publishers: Warsaw, Poland, 1994. (In Polish) [Google Scholar]
- Hofmann, G.; Lange-Bertalot, H.; Werum, M.; Klee, R. Rote Liste und Gesamtartenliste der limnischen Kieselalgen (Bacillariophyta) Deutschlands. In Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands; Band 7: Pflanzen. Münster (Landwirtschaftsverlag); Metzing, D., Hofbauer, N., Ludwig, G., Matzke-Hajek, G., Eds.; Naturschutz und Biologische Vielfalt: Bonn, Germany, 2018; Volume 70, pp. 601–708. [Google Scholar]
- Araujo, C.R.; Wyatt, K.H.; Rober, A.R. For richer or poorer: Diatoms as indicators of biological condition across a gradient of boreal fen types. Hydrobiologia 2025, 852, 575–592. [Google Scholar] [CrossRef]
- Denys, L.; Oosterlynck, P. Diatom assemblages of non–living substrates in petrifying Cratoneurion springs from lower Belgium. Fottea 2015, 15, 123–138. [Google Scholar] [CrossRef]
- Michalczyk, Z.; Chmiel, S.; Głowacki, S.; Zielińska, B. Changes of springs’ yield of Lublin Upland and Roztocze Region in 1998-2008. J. Water Land Dev. 2008, 12, 113–125. [Google Scholar] [CrossRef]
- Rajchel, L.; Rajchel, J.; Wołowski, K. Microorganisms in selected sulphuric springs of the Polish Carpathians. Geol. Quart. 2002, 46, 189–198. [Google Scholar]
- Cantonati, M.; Angeli, N.; Bertuzzi, E.; Spitale, D.; Lange-Bertalot, H. Diatoms in springs of the Alps: Spring types, environmental determinants, and substratum. Freshw. Sci. 2012, 31, 499–524. [Google Scholar] [CrossRef]
- Żelazna-Wieczorek, J. Diatoms Bacillariophyta in springs and riverhead stream section in the upper part of the San river. Roczniki Bieszczadzkie 2025, 20, 220–229. (In Polish) [Google Scholar]
- Cantonati, M.; Hofmann, G.; Spitale, D.; Werum, M.; Lange-Bertalot, H. Diatom Red Lists: Important tools to assess and preserve biodiversity and habitats in the face of direct impacts and environmental change. Biodivers. Conserv. 2022, 31, 453–477. [Google Scholar] [CrossRef]
- Mirek, Z.; Zarzycki, K.; Wojewoda, W.; Szeląg, Z. Red List of Plants and Fungi in Poland; W. Szafer Institute of Botany. Polish Academy of Sciences: Kraków, Poland, 2006. (In Polish) [Google Scholar]
- Cantonati, M.; Bilous, O.; Spitale, D.; Angeli, N.; Segadelli, S.; Bernabè, D.; Lichtenwöhrer, K.; Gerecke, R.; Saber, A.A. Diatoms from the Spring Ecosystems Selected for the Long-Term Monitoring of Climate-Change Effects in the Berchtesgaden National Park (Germany). Water 2022, 14, 381. [Google Scholar] [CrossRef]
- Winter, J.G.; Duthie, H.C. Stream epilithic, epipelic and epiphytic diatoms: Habitat fidelity and use in biomonitoring. Aquat. Ecol. 2000, 34, 345–353. [Google Scholar] [CrossRef]
- Nicolosi Gelis, M.M.; Sathicq, M.B.; Paredes del Puerto, J.M.; Pazos, R.S.; Tarda, A.S.; Gómez, N. Impact of extreme drought on diatom traits and species composition in temperate lowland streams. Hydrobiologia 2025, 852, 629–644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).