Abstract
The study of the temporal evolution of chloride ions in groundwater is important for identifying whether their sources are due to anthropogenic pollution or natural factors. Groundwater in the northern part of Dalat Banner, Ordos City, has high chloride ion content and exhibits strong temporal variability. To identify the source of chloride ions and reveal their evolution mechanisms, the fast Fourier transform (FFT) was used to determine the trend and cycle of chloride ion evolution, and the groundwater dynamics field combined with multivariate statistical analysis was used to identify the source of chloride ion pollution. Calculations show that the background value of chloride ions in groundwater in the study area is 195.17 mg/L, reaching a maximum of 459 mg/L under the influence of rainfall. The fluctuation of chloride ion concentration is mainly related to the total rainfall in the study area over 165 days, and a single rainfall of more than 15 mm affects the concentration of chloride ions in groundwater. The results of this study show that the background values of chloride ions are mainly influenced by the groundwater dynamical field, and the temporal volatility is mainly influenced by atmospheric rainfall.
1. Introduction
Chloride ions are naturally abundant in the environmental background and play a crucial role in the recharge and discharge dynamics of groundwater. They are also highly sensitive to external disturbances, which can lead to frequent exceedances in groundwater concentrations. Consequently, chloride ions exhibit significant spatial and temporal variability within groundwater systems. Currently, numerical simulation [1,2], isotope [3,4,5] tracer [6,7], multivariate statistics [8,9,10,11,12], and other methods are utilized both domestically and internationally for the traceability analysis of groundwater pollutants, achieving good results. Among these methods, Cl ions, as conventional ions, have been analyzed for traceability since the 1960s. Scholars commonly use hydrological modeling, water chemistry methods, isotopes, artificial sweeteners, and other methods for tracing chloride ions [13,14,15,16]. Turson–Aishan conducted a study of chloride ion concentration in the Wei-Ku region and found that the coefficient of variation of salt content in all the layers of soil in the study area exceeded 100%, with strong spatial variability [17]; Fu Tengfei carried out a study on the spatial variability of chloride ion concentration along the south coast of Laizhou Bay, and the coefficient of variation of chloride ion concentration on the spatial scale under the effect of a large amount of groundwater pumping and rainfall drenching in the rainy season was up to 146% [18]. Liu Fei conducted a study on chloride ion concentration in Minqin Oasis and found that the coefficient of variation of chloride ion concentration was 103.83% in arable land and 102% in grassland, both of which were highly variable, depending on the land use and cropping system [19]. There are a large number of studies on the variability of Cl ions on spatial scales, but fewer studies on the mechanism of their evolution on long time series scales, especially the response mode of chloride ions to climate change, are not clearly understood.
Building on this context, we focus on the Ordos Plateau’s unique meteorological and hydrological characteristics to explore the source and evolution of chloride ions. Dalat Banner, located in the northern part of Ordos City, was selected as a representative study area. Utilizing long-term groundwater monitoring wells and integrating the local hydrogeological structure and hydrodynamic field, we aim to identify the sources of chloride ions in groundwater. Additionally, we seek to determine the rainfall intensity threshold that influences chloride concentrations. This study also examines the spatial and temporal characteristics of the atmospheric precipitation and chloride distribution based on a clear understanding of the local chloride background levels. By first establishing the background levels of chloride ions in the region, we can explore the spatiotemporal evolution of the atmospheric rainfall and chloride distribution. This allows us to identify the sources of chloride ions in the regional groundwater and to determine the thresholds of rainfall intensity that impact chloride concentrations. These findings will provide essential support for the control and management of the groundwater environment in the Ordos area.
2. Materials and Methods
2.1. Study Area
The Ordos Plateau is located in the core area of the northern agricultural and pastoral intertwined belt, with strong agricultural irrigation and due to a strong evaporation effect, which leads to the rapid evaporation of irrigation water, chloride, sodium, calcium, and other ions are very easy to be enriched in the shallow soil. Meanwhile, the Ordos Plateau is rainy and hot at the same time, and rainfall is mainly concentrated between June and September [20,21], with more extreme rainfall weather, and the frequency of heavy rainfall (24 h rainfall > 50 mm) is at least one time per year in the last 40 years, and it reached 14 times in 2016 [22]. Under intense rainfall, chloride ions reenter groundwater through vertical infiltration [23,24], resulting in a strong temporal variability of chloride ions in regional groundwater.
The study area is located in the northeastern part of Dalat Banner in the northern part of Ordos, covering an area of 178 km2. The geomorphology consists of alluvial plains with east–west distribution in the form of bands. The micro-geomorphic units mostly belong to hilly gentle slopes and river valley terraces, and the land surface is covered by wind-deposited sands forming flat sand land. The general trend of the topography is high in the west and low in the east, with a gentle transition and a steplike decrease in the north–south direction. Groundwater in the region is mainly deposited in the diving aquifer of the Upper Pleistocene to Holocene (Q3–4), and the lithology of the aquifer is dominated by fine and powdery sand, sand and clay, locally coarse sand, gravelly sand, and gravelly sand, with a loose structure and high porosity; the thickness of the aquifer in the alluvial floodplain area varies, with the southern part of the area slightly thinned, generally between 30 and 50 m, the central and northern parts are thicker, generally between 50 and 100 m, and the northern part of the Yellow River alluvial plain is generally between 10 and 40 m. In the northern part of the Yellow River alluvial plain, the thickness of the shallow groundwater aquifer is smaller, generally between 10 and 40 m, due to the deposit of silt loam and powdery clay strips in a cascading distribution and greater thickness.
The long-term groundwater monitoring well CQ01 is located in the northeastern part of the study area, which was completed in 2017 and has a depth of 50 m. The buried depth of the groundwater table ranges from 10 to 14 m, and the buried depth of the submersible aquifer ranges from 12 to 50 m. The well is located in the northeastern part of the study area.
2.2. Sample Collection and Measurements
2.2.1. Groundwater Sample Collection and Testing
To investigate the spatial and temporal evolution characteristics of groundwater chloride ions in the region, a total of 18 sets of groundwater chemistry samples were collected from January 2021 to November 2023 at the long-term monitoring well CQ01 at a frequency of one set every 2 months. To determine the background value of chloride ion concentration, seven sets of samples were collected in the area in May 2023 to test 39 indicators such as Cl and Na, while 23 points of groundwater level measurements were carried out in the study area to characterize the groundwater flow field in the study area.
Before the sample collection work, sampling is conducted using a low-flow submersible pump to wash the sampling point; after the pumping volume is greater than 3–5 times the volume of the well, it is regarded as the completion of the well washing and formally starts the sampling work. Sampling was carried out in polyethylene plastic bottles with a capacity of 1 L, which were moistened and washed for collection.
After completing the sampling, the samples were restored in an insulated box with built-in frozen blue ice and sent to the laboratory to test the content of each ion within 48 h. The chemical reagents for the experiments were prepared at the Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences. And the chemical reagents required for soil sample testing are also included. The main testing instruments include plasma emission spectrometer (iCAP6300, Thermo Fisher, Los Angeles, CA, USA), gas chromatography-mass spectrometry (GC-MSQP2010plus, Yidian, Shanghai, China) and atomic absorption spectrophotometer (TAS-986, Yidian, Shanghai, China).
2.2.2. Soil Sample Collection and Testing
In 2023, soil samples were collected from within the air-packed zone (0–14 m) at the long-term monitoring well CQ01 and its upstream and downstream ZK06 and ZK07, respectively, at 2 m intervals, with seven sets of soil samples collected at each point, for a cumulative total of 21 sets of soil samples.
After completing the sample collection, the chloride ion content in the soil was determined according to the industry standard “Determination of Chloride Ion Content in Soil” (NY/T1378-2007) [25].
2.2.3. Rainfall Data
In this study, daily precipitation data were obtained from the Dongsheng meteorological station (Station ID: 53543, Station Name: DONGSHENG, CH). The station is at coordinates 109.983° E, 39.833° N, at an elevation of 1461 m above sea level.
2.3. Research Methods
2.3.1. Autocorrelation Function (ACF)—Periodic Analysis
The autocorrelation function is used to characterize the degree of correlation between a set of time-series samples and itself after a certain time lag. The autocorrelation function can eliminate the random error of the data itself in the calculation. The autocorrelation function can be calculated to obtain the periodic pattern of the groundwater chloride ion concentration within the study area over time. The autocorrelation coefficient calculation formula is shown in Equation (1):
where is the autocorrelation coefficient with lag ; is the observed value of the time series; is the mean value of the time series; is the length of the time series; and is the number of lag periods.
2.3.2. Fast Fourier Transform (FFT)—Periodicity Analysis
The Fast Fourier Transform converts time-series chloride concentration test data into the frequency domain. In turn, its periodicity is analyzed in the frequency domain. The fast Fourier transform is calculated as shown in Equation (2):
where is the complex-valued spectral component with frequency ; is the observed value of the time series; is the length of the time series; and is the sampling frequency.
Since the sampling frequency is one sample every two months, the period of the chloride concentration (months).
2.3.3. Multivariate Statistical Analysis
The groundwater in the study area flows from southwest to northeast, and CQ01 is located downstream of the study area (Figure 1). During the groundwater flow, the content of chloride ions gradually increases with the groundwater flow due to water–rock interaction. On top of that, under the effect of agricultural irrigation and strong evaporation, the content of Cl ions in the air pocket is also high. Under the effect of atmospheric rainfall, the Cl ions in the air envelope enter the submerged aquifer again, and due to the fluctuation of atmospheric rainfall, the Cl ions in the groundwater in the agricultural irrigation area have a strong spatial variability on the time scale.
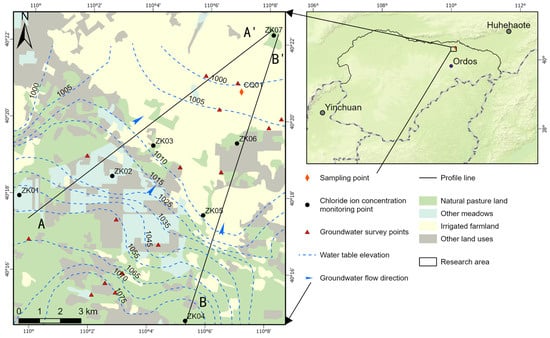
Figure 1.
Location of the study area.
In the rainfall leaching of chloride ions in the air pocket, the larger the rainfall amount and the longer the time, the content of chloride ions entering the groundwater from the soil gradually increases, and with the decrease of the content of chloride ions in the soil, the content of chloride ions entering the groundwater gradually decreases, and finally tends to be a constant amount, and its evolution of the time scale in the groundwater is close to a normal distribution.
The more fully chlorine ions are leached, the less chlorine ions are retained in the soil. Therefore, when the content of chloride ions in the soil is high, the leaching effect of rainfall on chloride ions is better. Along with the gradual increase of rainfall, the leaching effect of rainfall on chloride ions gradually decreases and finally tends to a constant amount.
In order to explore the response mode of groundwater chloride ions to atmospheric rainfall in the study area, daily rainfall data from meteorological stations in the region were collected since January 2020, and a Gaussian function was applied to fit the changes in rainfall and chloride ions concentration in the monitoring wells of CQ01; the regression Equation (3) is:
where Chloride ion concentration is , rainfall is ; , and are constants obtained from the fitting calculation; represents the theoretical maximum value of chloride ion concentration, i.e., the concentration of chloride ions in groundwater after all chloride ions are leached out of the soil, and represents the amount of rainfall that achieves the maximum effect of chlorine ion leaching. When the value of is 0, the calculated value of is the background value of the chloride concentration in the study area when there is no interference from rainfall and other anthropogenic factors.
3. Results and Discussion
3.1. Characteristics of Rainfall and Cl Concentration Evolution with Time in the Study Area
Figure 2 illustrates the variation in chloride ion concentrations in groundwater at the sampling sites from 2021 to 2023. The average chloride ion concentrations in the monitoring wells from 2021 to 2023 were 261 mg/L, 272.83 mg/L, and 300.41 mg/L, respectively, showing an increasing trend each year. Figure 3 illustrates the variation in rainfall in the study area from 2021 to 2023. The total rainfall from 2021 to 2023 was 296.93 mm, 542.29 mm, and 425.45 mm, respectively, with the total rainfall in 2022 being higher than in 2021 and 2023.
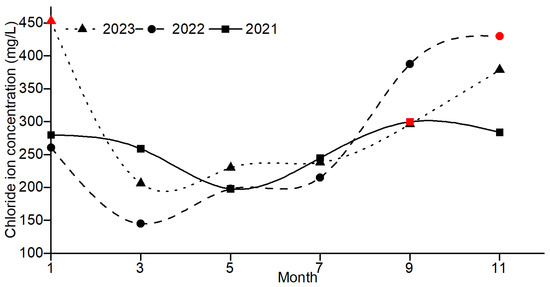
Figure 2.
Changes in chloride ion concentrations by year. Note: The red symbols are the maximum chloride ion concentrations during the year.
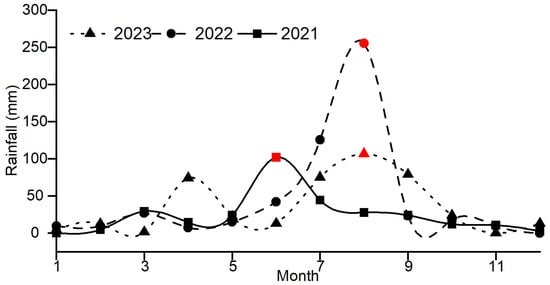
Figure 3.
Monthly rainfall changes from 2021 to 2023. Note: The red symbols are the maximum rainfall during the year.
3.2. Background Values of Cl Ions in the Study Area
As shown in Figure 1, the evolution of groundwater chloride ion concentrations at the seven monitoring points along the groundwater flow path is shown in the table below:
Of the eight sampling points mentioned above, all were located within the grassy area except for CQ01, where chloride concentrations were calculated by fitting the chloride concentrations at each point of the AA’ and BB’ profiles to the distance from the first sampling point, respectively.
Table 1 shows that the Cl− ion concentration has a significant tendency to increase with groundwater flow. At the same time, the increase of Cl− ion concentration is smaller in the region with faster groundwater flow and larger hydraulic gradient. The increase of Cl− ion concentration is larger in the region with a smaller hydraulic gradient and slower groundwater flow.

Table 1.
Concentration of chloride ions at monitoring points.
The scatter plot of the distance–chloride ion concentration at each sampling point was graphed according to Table 1, and a fitted line was plotted. The scatter plot of the distance–chloride ion concentration of profile AA’ is shown in Figure 4; the fitting equation of the change of chloride ion concentration with distance is , which has a coefficient of determination , indicating a good fit. The background value of the corresponding chloride ion concentration at the long-term monitoring point is 158.78 mg/L.
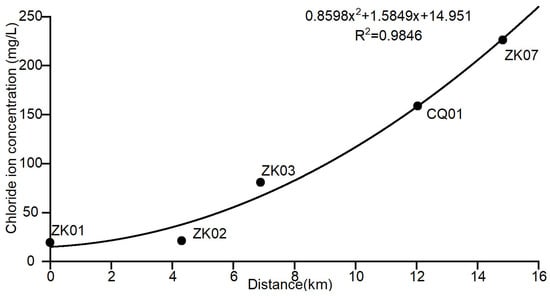
Figure 4.
Scatter plot of the distance–chloride concentration for profile AA’.
The scatter plot of the distance–chloride ion concentration for profile BB’ is shown in Figure 5; the fitting equation for the variation of chloride ion concentration with distance is , which has a coefficient of determination of , indicating a good fit, and the background value of the corresponding chloride ion concentration at the long-term monitoring point is 158.95 mg/L.
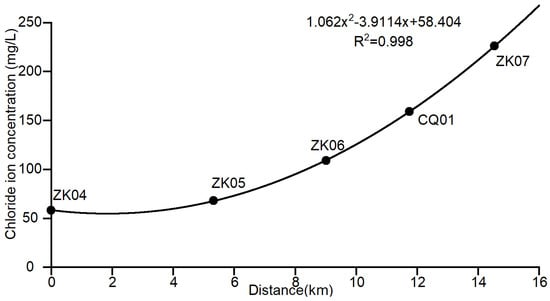
Figure 5.
Scatter plot of the distance–chloride concentration profile for BB’.
Combining the distance–chloride ion concentration fitting of the monitoring points in profile AA’ and profile BB’, and taking the average value of the calculation results of the two groundwater flow directions, it can be preliminarily concluded that under the influence of water–rock interaction, the natural background value of the chloride ion concentration in groundwater of CQ01 is approximately 158.865 mg/L.
3.3. Chloride Ion Content in Soil
Using the chloride ion concentration profile, it was calculated that the chloride ion concentration at point CQ01 should be around 158.865 when it is not disturbed by human factors. However, the average values of chloride ion concentration at point CQ01 measured from 2021 to 2023 were 261 mg/L, 272.83 mg/L, and 300.41 mg/L, respectively. Therefore, there are other sources of chloride ions at CQ01.
Soil samples were taken from the sampling points CQ01, ZK06, and ZK07 to test the soluble chlorine content in the soil layers at various burial depths. The results are shown in Table 2. It can be observed that the soluble chlorine content in the soil at sampling point CQ01, which is located in an irrigated farmland area, is higher than that at ZK06 and ZK07, which are located in a grassland area. This indicates that chloride ions in the groundwater are significantly retained in the soil due to irrigation.

Table 2.
Soluble chlorine content in soil layers.
Figure 6 shows the variation of chloride ion concentration with the depth of the vadose zone. It can be seen that the chlorine content in the soil layers of the study area is higher in the surface soil from 0 to 2 m, decreases with increasing depth, rises again at about 6 m, reaches a maximum value at about 10 m, and stabilizes at 12 m and downwards. This variation is in accordance with the conclusions reached in this study. The study area is located in an irrigated farmland area, and when groundwater is utilized for irrigation, water is lost, and salts accumulate at the surface. As a result, soluble chlorine is slightly higher in the surface soil. The process of rainfall leaching soluble chlorine to the subsurface could not leach all the chlorine ions into the subsurface. Therefore, the soluble chlorine content gradually decreases with increasing depth until it reaches a depth of 4~6 m. Along with many times of groundwater irrigation containing chloride ions and rainfall leaching without chloride ions, 71% of the chloride ions in the soil would be transported downward with the leaching solution [23], so the depth of the groundwater table at the sampling site fluctuated at about 10~14 m, and a large number of chloride ions were leached to the shallowest point of the fluctuation of the groundwater table and gradually leached into the groundwater. Therefore, the concentration of chloride ions in the soil reaches the maximum value at a depth of 8~10 m and tends to stabilize at a deeper depth.
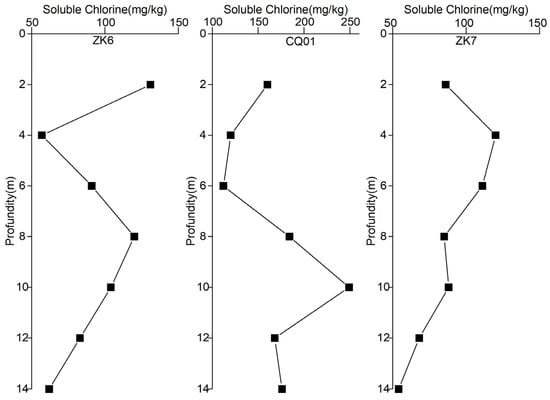
Figure 6.
Distribution of soluble chlorine content by sampling point.
The difference in soluble chlorine content in the soil between the CQ01 sampling site and the ZK06 and ZK07 sampling sites indicates that irrigation has had a more pronounced effect on the chlorine ion content in the soil. Due to rainfall and other factors, this effect is applied to the groundwater so that the concentration of chloride ions in the groundwater is increased. Therefore, the correlation between groundwater chloride concentration and rainfall at CQ01 was investigated.
3.4. Analysis of the Correlation between Cl Ions and Rainfall
3.4.1. Cyclic Analysis of Chloride Ion Concentration Variations
The autocorrelation function (ACF) was used to analyze the changes in groundwater chloride ion concentration from January 2021 to November 2023, and the results of the analysis are shown in Figure 7, which shows that the autocorrelation coefficient is −0.5643 when the lag value is 3, and the autocorrelation coefficient is located outside the confidence interval; the results are significant. The results show that the concentration of chloride ions in groundwater in the study area has significant autocorrelation with a lag period of 6 months (one group of samples is collected every 2 months, the lag period is 3 × 2 = 6). That is to say, the concentration of groundwater chloride ions in the study area shows a regular change trend with a cycle of 6 months. This trend of changes is opposite in every two adjacent cycles.
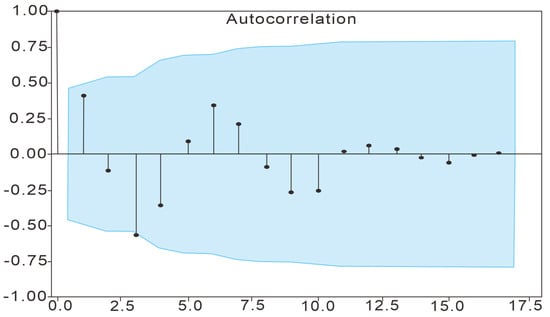
Figure 7.
ACF analysis results. Note: The blue region represents the confidence interval with a confidence level of 95%.
The trend of groundwater chloride ion concentration in the study area was further analyzed using a Fast Fourier Transform (FFT); as shown in Figure 8, when the spike frequency is 0, the spike amplitude is 5000, which is much higher than the amplitude of other spike frequencies. Figure 8 shows that the baseline level (background value) accounts for the major part of the overall concentration of chloride ions in groundwater in the study area; the spike amplitude is 965.60095 when the spike frequency is 0.08333, which is the maximum value except for the spike frequency of 0. Since the sampling frequency is one sample every two months, the variation period of chloride ion concentration in the study area is months.
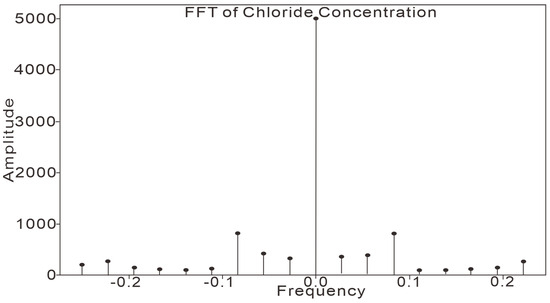
Figure 8.
FFT analysis results.
As can be seen from the FFT calculations, the groundwater chloride ion concentration in the study area is mainly dominated by a baseline level that remains stable, indicating that the groundwater chloride ion concentration in the study area is mainly determined by water–rock interactions [26,27], etc., and is a characterization of the background value. The period of the changing part is 24 months, and considering the significantly larger rainfall in 2022, it is presumed that the changing part of the groundwater chloride ion concentration is mainly influenced by rainfall.
From the results of the ACF calculation, it can be seen that the trend of groundwater chloride ion concentration in the study area is opposite to that of January to June and July to December every year, and the groundwater chloride ion concentration shows a decreasing trend from January to June and an increasing trend from July to December. The rainy season in Erdos City is mainly from June to September, and after the beginning of the rainy season, the trend of groundwater chloride ion concentration changed significantly from decreasing to increasing and maintained the increasing trend after the end of the rainy season until January of the next year. The correlation between rainfall and chloride ion concentration was further analyzed.
3.4.2. Correlation Analysis between Chloride Ion Concentration and Rainfall Amount
Figure 3 shows that the rainfall is slightly higher from March to April every year. Meanwhile, from June to September, rainfall averages 71.1% of the year. Among them, 2022 was relatively rainy and had the highest rainfall for the year. This variation coincides with the variation of chloride ion concentration to some extent, and the effect of rainfall on chloride ion concentration continues for some time.
As shown in Figure 9, the trends of chloride ion concentration and rainfall over time are the same, but there is a certain lag between the chloride ion concentration and rainfall changes on the time scale, which indicates that the effect of rainfall on chloride ions is not occurring in real time, so the relationship between the chloride ion concentration and its relationship with the sum of the daily rainfall in the period prior to the time point of the test was further investigated.
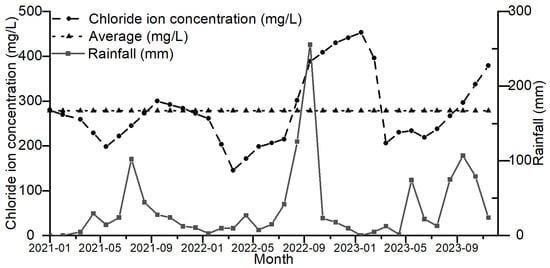
Figure 9.
Temporal Trends in Chloride Ion Concentration and Rainfall.
3.4.3. Result of the Correlation Analysis
1. Rainfall period analysis
The cumulative rainfall was calculated from 10 to 200 days before the sampling date, and the trends of different rainfall periods and chloride ion concentration were observed, as shown in Figure 10, along with the increase in the cumulative time of rainfall, the trends of rainfall and chloride ion concentration were gradually the same. This indicates that rainfall over a period of time is an important factor influencing the concentration of chloride ions in the groundwater in the study area.
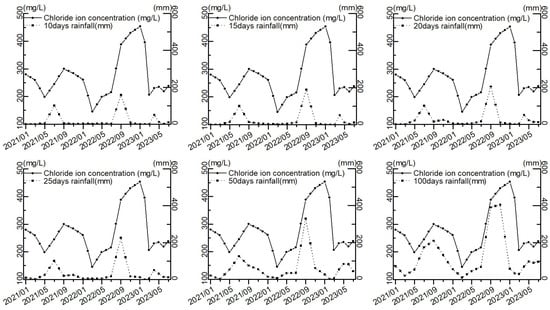
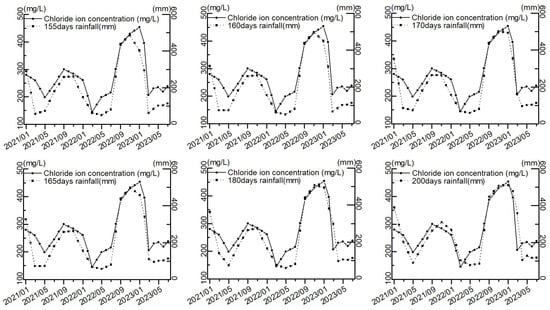
Figure 10.
Trends in rainfall and chloride ion concentration at different times.
In order to find the cumulative rainfall period with the highest correlation to the chloride ion concentration, the Pearson correlation coefficient method was used to find the correlation coefficients between the cumulative rainfall and the chloride ion concentration in groundwater for the first 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 155, 160, 165, 170, 180, 190, and 200 days, as shown in Figure 11. The correlation coefficients between the cumulative rainfall and groundwater chloride concentration for the 180, 190, and 200 days and the correlation coefficients with chloride concentration for each time are shown in Figure 11.
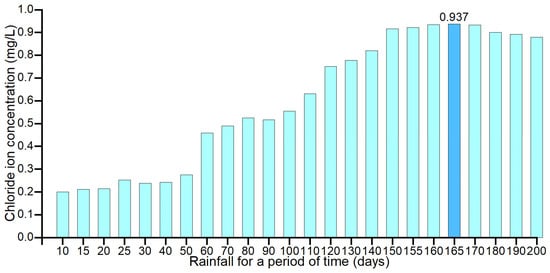
Figure 11.
Histogram of the correlation coefficients for each time of rainfall statistics.
From the correlation coefficients between the rainfall and chloride ion concentration in groundwater for each period with sliding properties, it can be seen that the rainfall for 165 days in the study area is an important factor in determining the concentration of chloride ions in groundwater. It shows that the fluctuation of chloride ions in groundwater in the study area is mainly caused by rainfall leaching chloride ions from surface soil and carrying them to groundwater.
2. Analysis of the rainfall intensity
The results of this study were further analyzed by screening the number of days with single-day rainfall greater than 10 mm, 20 mm, 30 mm, 40 mm, and 50 mm within 165 days, respectively, and the number of days with single-day rainfall for each day is shown in Figure 12 and Table 3, which show that rainfall with higher intensity occurred during the 165 days prior to the collection of the samples with a higher concentration of chloride ions. At the sampling time nodes with chloride ion concentrations of 388 mg/L, 430 mg/L, 453 mg/L, and 396 mg/L, there was a single day of rainfall greater than 50 mm during the 165 days prior to sampling. This indicates a certain correlation between rainfall intensity and the concentration of chloride ions in the groundwater during the 165 days and that there is a rainfall threshold value above which rainfall on a single day will have a more obvious effect on the concentration of chloride ions in the groundwater. A single day’s rainfall above this threshold has a more pronounced effect on groundwater chloride concentrations.
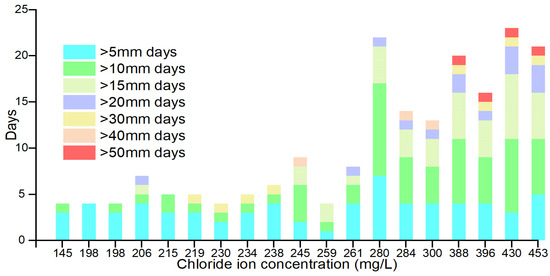
Figure 12.
Histogram of the number of days of occurrence of each rainfall intensity.

Table 3.
Number of days for each rainfall intensity.
In order to clarify the threshold value of the impact of a single rainfall on the chloride ion concentration in groundwater, the sum of rainfall intensities greater than 10 mm, 20 mm, 30 mm, 40 mm, and 50 mm in a single day within 165 days was calculated, as shown in Table 4. The trend of each rainfall intensity and chloride ion concentration is shown in Figure 4.

Table 4.
Cumulative rainfall by rainfall intensity.
Figure 13 shows that with the increase in rainfall intensity threshold, the rainfall and chloride concentration curves tend to approach and then deviate from each other. Pearson’s correlation coefficients between the rainfall and chloride ion concentration for each rainfall intensity were further investigated, and the correlation coefficients are shown in Figure 14 and Table 5, which show that the correlation coefficients between rainfall and chloride ion showed an increasing trend and then decreasing along with the increase of the rainfall in a single day. This indicates that when screening the single-day rainfall, the single-day rainfall that has no or very little correlation with the chloride ion concentration is first removed. The single-day rainfall with a high correlation with chloride ion concentration is removed later. Therefore, the single-day rainfall with the largest correlation coefficient with the chloride ion concentration is the threshold of single-day rainfall that affects the chloride ion concentration, which is 15 mm. This means that a single rainfall of more than 15 mm can effectively leach chloride ions from the surface soil and carry them to the groundwater.
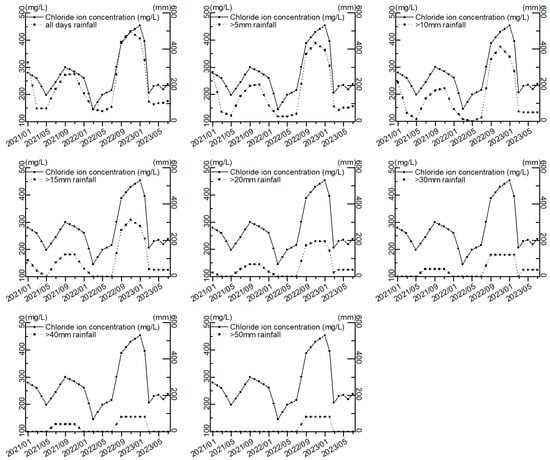
Figure 13.
Trends in rainfall and chloride ion concentration at various rainfall intensities.
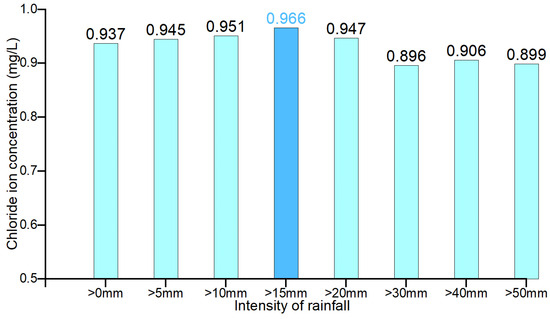
Figure 14.
Correlation coefficients between rainfall and chloride concentration at various rainfall intensities.

Table 5.
Correlation coefficients between rainfall intensity and chloride ion concentration for each rainfall intensity.
3.4.4. Result of the Regression Analysis
As shown in Figure 15, The Gaussian regression equation between groundwater chloride concentration and rainfall is .

Figure 15.
Chloride concentration—regression plot of single day > 15 mm rainfall in the first 165 days.
The sum of single rainfall events greater than 15 mm in the 165 days with the highest correlation with groundwater chloride concentration was used as the independent variable x, and groundwater chloride concentration was used as y.
The model efficiency coefficient of this regression equation, , was a good fit, and it can be seen from the regression equation that the higher the cumulative rainfall, the less chlorine ions are leached out per unit of rainfall. It is in line with the natural law. Meanwhile, when the rainfall was 400.2 mm, the chlorine ions retained in the soil were maximally leached into the groundwater; at this time, the groundwater chloride ion concentration was 459.1 mg/L. According to the Gaussian function to find the background value of chloride ion concentration in the study area, when the rainfall () takes the value of 0, the background value of chloride ion in the groundwater of the monitoring wells is 195.165 mg/L, which is higher than that of the background value (158.865 mg/L) carved out according to the local profile of the concentration of chloride ions.
The effect of water–rock interaction on chloride ion concentration in the study area was calculated as 158.865 mg/L using chloride ion concentration distance, which is different from the result obtained using chloride ion concentration–rainfall in the study area (195.165 mg/L), and this difference is attributed to the fact that the sampling site of the former is a pasture land that is not affected by anthropogenic activities while the latter is located in the irrigated farmland. As shown in Figure 6 and Table 2, under the influence of perennial irrigation, the soil was endowed with more chloride ions than the pastureland. Under irrigation and rainfall, more chloride ions enter the groundwater. This leads to some elevation of the background value of groundwater chloride ion concentration.
3.5. Error Verification of the Regression Equations
To further verify the accuracy of the test results, groundwater chemistry samples were collected from the sampling sites in September 2023 and November 2023 for the determination of chloride ion concentrations.
The results of the regression equation show that in September 2023, the concentration of chloride ion at the sampling point was 296.5 mg/L, the amount of rainfall greater than 15 mm on a single day in the previous 165 days was 126.81 mm, and the concentration of chloride ion calculated according to the regression equation was 307.99 mg/L. The error between the predicted value and the measured value was 3.9%, and the two were of the same level of water quality, which are both class IV; in 2023, the concentration of chloride ion at the sampling point was 379 mg/L in November, and the amount of rainfall greater than 15 mm on a single day in the previous 165 days was 202.95 mm. In November 2023, the chloride ion concentration at the sampling point was 379 mg/L, the rainfall amount greater than 15 mm on a single day in the previous 165 days was 202.95 mm, and the chloride ion concentration calculated according to the regression equation was 372.95 mg/L, and the error between the predicted value and the measured value was 1.6%, and the two were of the same water quality, both of which are classified as class V. The validation results showed that the Gaussian equation had a significant effect on the water quality of the sampling point. The validation results show that the Gaussian equation can accurately depict the relationship between the chloride ion concentration in the groundwater and rainfall in the study area (the sum of single-day rainfall greater than 15 mm in 165 days). For the chloride ion concentration > 380 mg/L, there were cases of rainfall higher than 50 mm in a single day in 165 days before sampling.
Sun Lei et al. found that the leaching of chloride ions from the soil reached the maximum leaching volume when the rainfall reached 440 mm [23], which is in line with the results obtained in this study (400.2 mm). Dustin W. Kincaid et al. found that contrary to the past belief that chloride ions are only stored for a short period in the soil, the soil can be a temporary reservoir of chloride ions, which can be stored in the soil over a longer time. Storage of chloride ions in a soil column 15 cm high, the leaching of chloride ions from the soil was only more effective when rainfall exceeded 150 mm and took 2 weeks, and exogenous chloride ions from the use of local snow removers in the winter still impacted the local groundwater in the summer of the following year [24], suggesting that it also took two seasons for the region to leach chloride ions into the groundwater gradually. This is consistent with the 165-day impact time obtained in this study.
The test of the regression equation and the corroboration of the results with the findings of others show that the results obtained in this study are accurate and reliable.
This paper focuses on the problem of chloride ions exceeding the standard in the typical area of Dalat Banner, Ordos City, and the main sources of chloride ions in the groundwater of the study area were determined by correlation analysis and regression analysis between the concentration of chloride ion in the groundwater of the study area and the local rainfall. The high concentration of chloride ions in groundwater in the study area is not related to human activities. This is mainly due to the high background value of chloride ion concentration caused by the geological background and the migration of chloride ions from rainfall–leaching surface soil into groundwater. Since the study area is an irrigated farmland, it is assumed that the chloride ions in the surface soil are mainly due to the loss of water due to absorption by vegetation and evaporation after pumping groundwater for irrigation and the retention of salts in the surface soil.
The study area is primarily irrigated farmland, where groundwater is extracted from aquifers and transferred to surface fields for irrigation. The region experiences high evaporation rates, which contribute to water loss and salt accumulation. This study does not consider variations in evaporation rates. However, evaporation plays a significant role in salt accumulation. This research uniquely considers the impact of rainfall when analyzing variations in chloride ion concentrations in groundwater. Yet, using only rainfall as a factor to fit chloride concentrations may not fully account for environmental changes. So, this study primarily focuses on cases where chloride concentration fluctuations are significant under stable environmental conditions over time.
4. Conclusions
Based on the measured water chemistry ion content, groundwater table burial depth, and precipitation data in the field, the background values of chloride ions in the study area were identified using FFT and multivariate statistical analyses. This study revealed the mechanisms of the cyclical changes of chloride ions and explored their contamination sources. The specific conclusions are listed below.
The chloride content in the monitoring well area is not only high but also exhibits significant temporal variability. This phenomenon can be attributed to two main reasons. First, the monitoring well is located in a groundwater discharge zone, where the interaction between groundwater and rock leads to elevated levels of chlorides as the water flows through. Consequently, the background chloride concentration in the groundwater surrounding the monitoring well is relatively high. Second, this well is situated in an irrigated agricultural area, where atmospheric precipitation causes vertical migration of chloride ions from the surface soil. Given that precipitation in Ordos City is unevenly distributed throughout the year, primarily occurring between June and September, the chloride concentration in the monitoring well also demonstrates a regular pattern of temporal and spatial variation.
The fluctuation of chloride ion concentration is primarily influenced by total precipitation over 165 days. When a single rainfall exceeds 15 mm, it effectively leaches surface soil salts from the irrigated agricultural zone into the groundwater. This leaching effect persists for 165 days following rainfall events. Rainfall in the study area mainly occurs from June to October, resulting in higher chloride ion concentrations measured from June to January and lower concentrations measured from January to June.
This temporal variability in chloride ion concentration should be taken into account by relevant authorities for improved monitoring and managing of groundwater environmental conditions in the study area.
Author Contributions
Conceptualization, L.S.; Data curation, L.S.; Formal analysis, L.S. and R.J.; Funding acquisition, B.Z., R.Z. and R.M.; Investigation, L.S. and B.Z.; Methodology, L.S.; Project administration, B.Z. and R.M.; Resources, B.Z., R.Z. and S.L.; Supervision, W.D.; Validation, R.J., W.D. and R.M.; Writing—original draft, L.S.; Writing—reviewing and editing, L.S. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the “Basic Research Funds of Chinese Academy of Geological Sciences (SK202327)”, “Geological Survey Projects Foundation of the Institute of Hydrogeology and Environmental Geology (DD20221773)”, and “Investigation of Groundwater Environment in Ordos (Phase II) (ESZC-G-F-220140)”.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The editor and reviewers are sincerely acknowledged for their instructive and detailed comments on the early versions of this manuscript. The authors appreciate all centers supplying open-source data sets used in the present research.
Conflicts of Interest
Ruiqing Zhou was employed by Ordos Environmental Protection Investment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relation-ships that could be construed as a potential conflict of interest. All authors have read and agreed to the published version of the manuscript.
References
- Zheng, N.; Liu, J.; Xia, X.; Gu, S.; Wu, Y.; Li, X.; Jiang, S. Identification of Contaminant Source and Hydraulic Conductivity Field Based on an ILUES-SOM Surrogate Model. Stoch. Environ. Res. Risk Assess. 2023, 37, 2725–2738. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Pan, Z.; Wang, Z.; Dong, G. Simultaneous Identification of Groundwater Contaminant Source and Hydraulic Parameters Based on Multilayer Perceptron and Flying Foxes’ Optimization. Environ. Sci. Pollut. Res. 2023, 30, 78933–78947. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wu, Y.; Fan, J.; Ye, F.; Xie, C.; Fu, X.; Sun, Y. Identification of Nitrogen Pollution Sources and Transport Transformation Processes in Groundwater of Different Landforms Using C, H, N, and O Isotope Techniques: An Example from the Lower Weihe River. Environ. Sci. Pollut. Res. 2023, 30, 29442–29457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, D.; Sun, Q.; Gan, Y.; Bai, L.; Li, S.; Liu, D.; Dai, J. Combining Multi-Isotope Technology, Hydrochemical Information, and MixSIAR Model to Identify and Quantify Nitrate Sources of Groundwater and Surface Water in a Multi-Land Use Region. Environ. Sci. Pollut. Res. 2023, 30, 80070–80084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gui, H.; Jiang, C.; Zha, J.; Zheng, L. Hydrogeochemistry and Stable Hydrogen and Oxygen Isotope Characteristics of Deep Limestone Water in the Huainan Panxie Mining Area. Arab. J. Geosci. 2022, 15, 1133. [Google Scholar] [CrossRef]
- Irrgeher, J.; Prohaska, T. Application of Non-Traditional Stable Isotopes in Analytical Ecogeochemistry Assessed by MC ICP-MS—A Critical Review. Anal. Bioanal. Chem. 2015, 408, 369–385. [Google Scholar] [CrossRef]
- Wang, G.; Gao, H.; Long, B.; Wu, J. Progress of Nitrate Isotope-Coupled Multi-Tracer Traceability of Groundwater Nitrate Contamination. J. Appl. Ecol. 2024, 35, 970–984. [Google Scholar]
- Long, Z. Huangshui River Basin Water Environment Quality Comprehensive Evaluation and Typical Pollutants Traceability and Control Countermeasures Research. Ph.D. Thesis, Shandong University, Jinan, China, 2022. [Google Scholar]
- Lin, B.; Qi, F.; An, X.; Zhao, C.; Gao, Y.; Liu, Y.; Zhong, Y.; Qiu, B.; Wang, Z.; Hu, Q.; et al. Review: The Application of Source Analysis Methods in Tracing Urban Non-Point Source Pollution: Categorization, Hotspots, and Future Prospects. Environ. Sci. Pollut. Res. 2024, 31, 23482–23504. [Google Scholar] [CrossRef]
- Peng, L.; Jianyuan, C.; Ji, L.; Tiantian, W.; Jian, Y. Hydrogeochemical Characteristics and Solute Sources of Groundwater in the Yuhengbei Mining Area, Shaanxi Province, China. Environ. Earth Sci. 2022, 81, 516. [Google Scholar] [CrossRef]
- Chakraborty, P.; Wood, D.A.; Singh, S.; Hazra, B. Trace Element Contamination in Soils Surrounding the Open-Cast Coal Mines of Eastern Raniganj Basin, India. Environ. Geochem. Health 2023, 45, 7275–7302. [Google Scholar] [CrossRef]
- Han, X.; Tang, F.; Liu, A.L. Drinking Water Quality Evaluation in Supply Systems in Wuhan, China: Application of Entropy Weight Water Quality Index and Multivariate Statistical Analysis. Environ. Sci. Pollut. Res. 2024, 31, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Jean-Michel, M.; Alexandre, D. Origin of Cl− and High δ37Cl Values in Radiocarbon Dated-Fracture Groundwaters at the Tournemire URL (France). Procedia Earth Planet. Sci. 2013, 7, 574–577. [Google Scholar] [CrossRef]
- Moncada-Aguilar, A.M.; Ramírez-Hernández, J.; Quintero-Núñez, M.; Avendaño-Reyes, L. Origin of Salinity in Groundwater of Neighboring Villages of the Cerro Prieto Geothermal Field. Water Air Soil Pollut. 2010, 213, 389–400. [Google Scholar] [CrossRef]
- Khazaei, E.; Milne-Home, W. Applicability of Geochemical Techniques and Artificial Sweeteners in Discriminating the Anthropogenic Sources of Chloride in Shallow Groundwater North of Toronto, Canada. Environ. Monit. Assess. 2017, 189, 218. [Google Scholar] [CrossRef]
- Ma, R.; Li, L.; Zhang, B. Impact Assessment of Anthropogenic Activities on the Ecological Systems in the Xiongan New Area in the North China Plain. Integr. Environ. Assess. Manag. 2021, 12, 866–876. [Google Scholar] [CrossRef]
- Tursun, A. Study on Spatial and Temporal Changes of Salinized Soil and Groundwater Characteristics in Wei-Ku Oasis. Ph.D. Thesis, Xinjiang University, Ürümqi, China, 2012. [Google Scholar]
- Tengfei, F. Research Application of Spatial and Temporal Variability of Soil Salinization and Monitoring System in Typical Coastal Areas. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences (Institute of Oceanography), Qingdao, China, 2015. [Google Scholar]
- Fei, L. Soil Water-Salt Characteristics of Minqin Oasis and Its Relationship with Groundwater. Master’s Thesis, Lanzhou University, Lanzhou, China, 2019. [Google Scholar]
- Huang, G.; Sun, J.; Qi, J.; Zang, Y.; Chen, J.; Jing, J. Isotopic Composition of Ordos Groundwater in Relation to Climate Change. J. Earth Sci. 2007, 06, 550–554. [Google Scholar]
- Xuekun, Y. Characterization of Precipitation in Ordos City in the Recent 50 Years. Inn. Mong. Sci. Econ. 2017, 22, 66–67, 81. [Google Scholar]
- Zhang, L.; Zheng, Y.; Xu, J.; Xu, G.; Li, Y. Characteristics of Spatial and Temporal Distribution of Heavy Rainfall and Disaster Defense in Ordos City under the Background of Climate Warming. Inn. Mong. Meteorol. 2020, 4, 14–16. [Google Scholar]
- Sun, L.; Yuan, L.; Fu, Q.; Nie, X.; Yu, H.; Tang, J.; Dong, L. Effects of Rainfall on Exogenous Cl⁻ Leaching Efficiency and Its Spatial Distribution in Soils with Different Textures. J. Northeast Agric. Univ. 2022, 53, 27–38. [Google Scholar]
- Kincaid, D.W.; Findlay, S.E.G. Sources of Elevated Chloride in Local Streams: Groundwater and Soils as Potential Reservoirs. Water Air Soil Pollut. 2009, 203, 335–342. [Google Scholar] [CrossRef]
- NY/T 1378-2007; Determination of Chloride Ion Content in Soil. China Standards Press: Beijing, China, 2007.
- Jiang, W.; Sheng, Y.; Wang, G.; Shi, Z.; Liu, F.; Zhang, J.; Chen, D. Cl, Br, B, Li, and Noble Gases Isotopes to Study the Origin and Evolution of Deep Groundwater in Sedimentary Basins: A Review. Environ. Chem. Lett. 2022, 20, 1497–1528. [Google Scholar] [CrossRef]
- Sheng, Y.; Baars, O.; Guo, D.; Whitham, J.; Srivastava, S.; Dong, H. Mineral-Bound Trace Metals as Cofactors for Anaerobic Biological Nitrogen Fixation. Environ. Sci. Technol. 2023, 57, 7206–7216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).