Validation and Application of the Diffusive Gradients in Thin-Films Technique for In Situ Measurement of β-Blocker Drugs in Waters and Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Gel Preparation and DGT Assemblies
2.3. Adsorption Properties of DGT Binding Gels
2.4. Diffusion Coefficient Measurements
2.5. Time and Diffusive Layer Thickness Dependence
2.6. DGT Performance Tests under Different Conditions
2.7. In Situ Application of DGT in Rivers
2.8. Deployment of DGT Probe at the Sediment–Water Interface
2.9. Chemical Analysis and DGT Calculation
3. Results and Discussion
3.1. Possible Adsorption on DGT Mouldings, Diffusive Gels and Filter Membranes
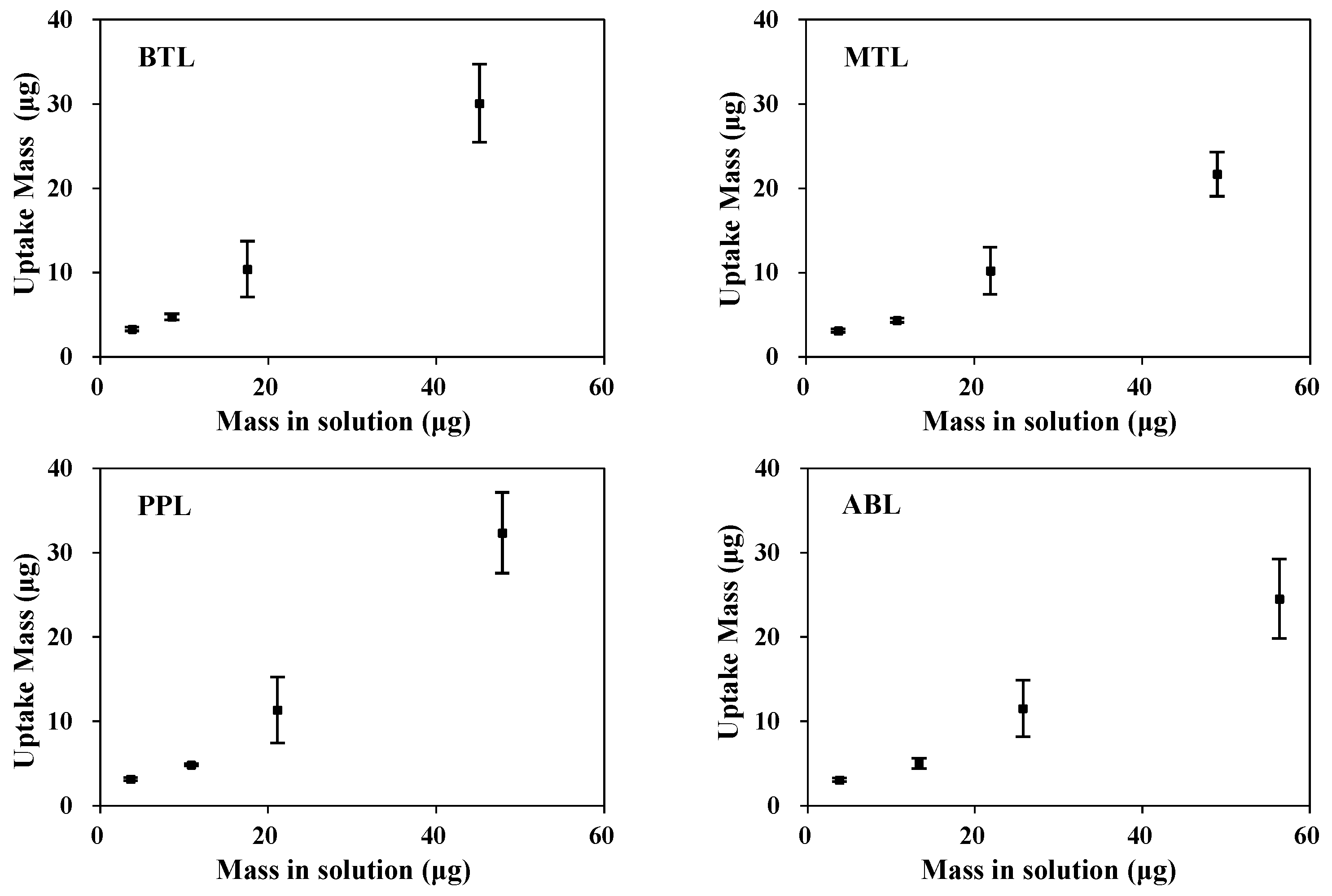
3.2. Uptake Kinetics and Effective Capacity of DGT Devices
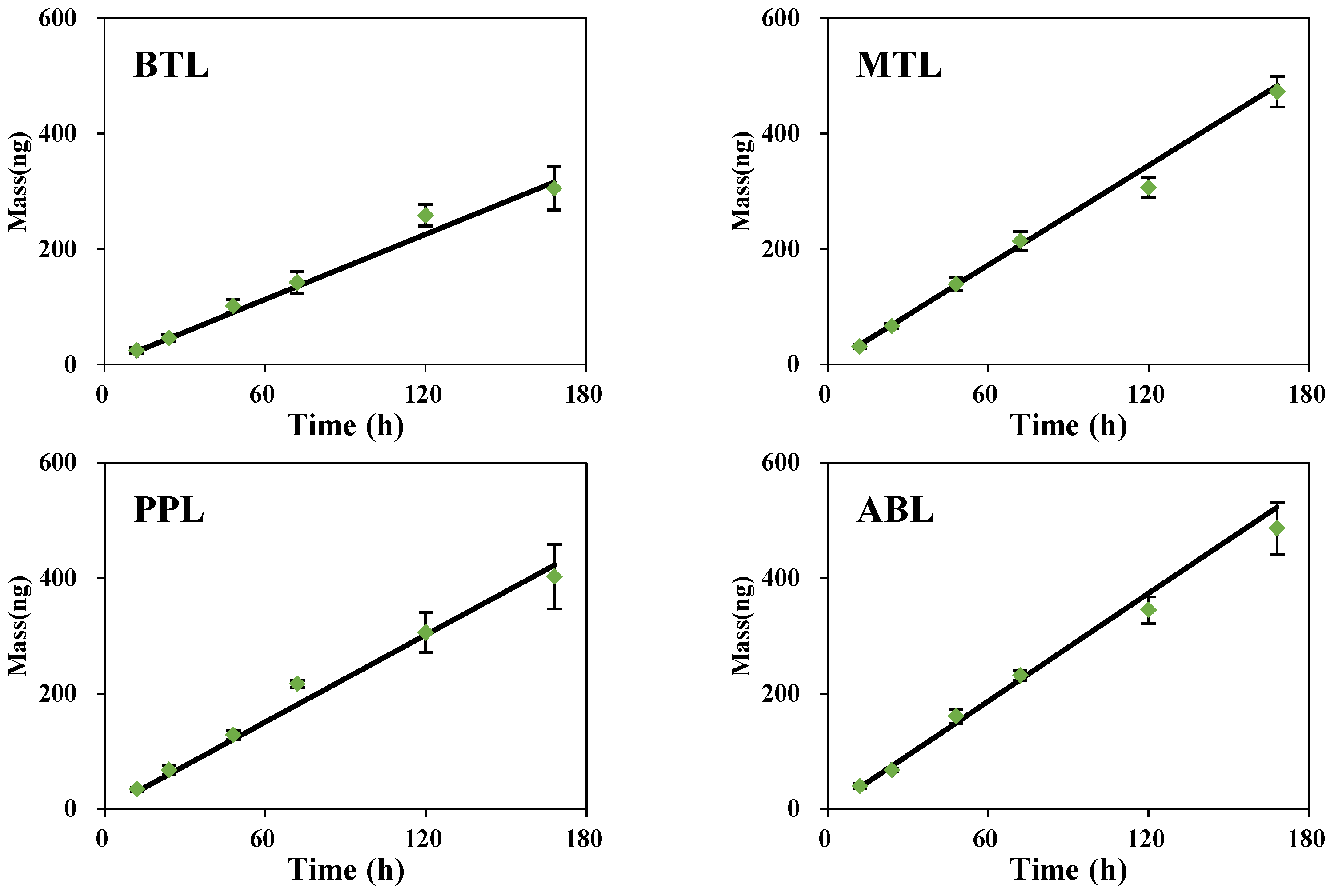
3.3. Diffusion Coefficient Measurements
3.4. Time and Diffusive Layer Thickness Dependence
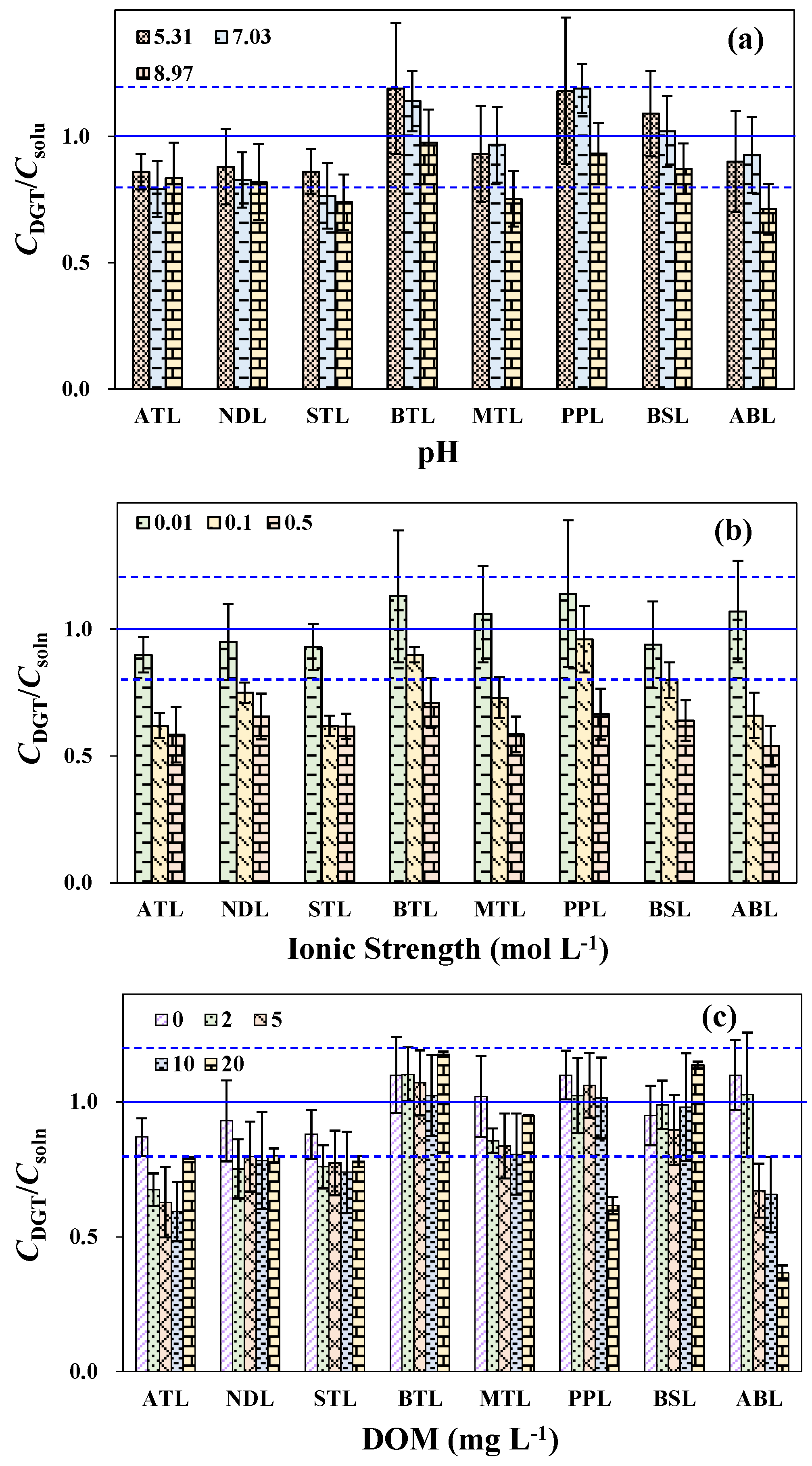
3.5. DGT Performances under Different Conditions
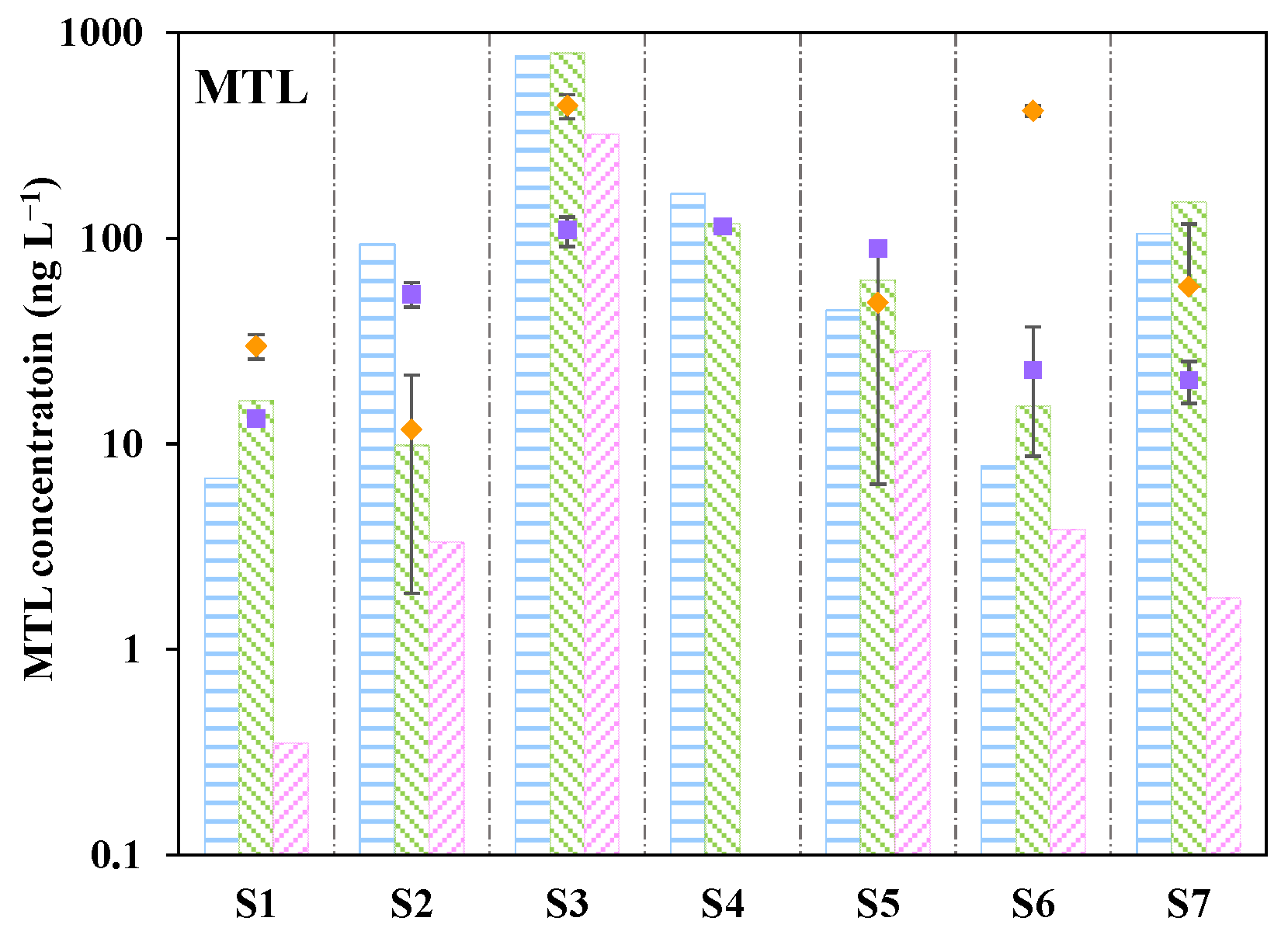
3.6. In Situ Application of DGT in River Waters
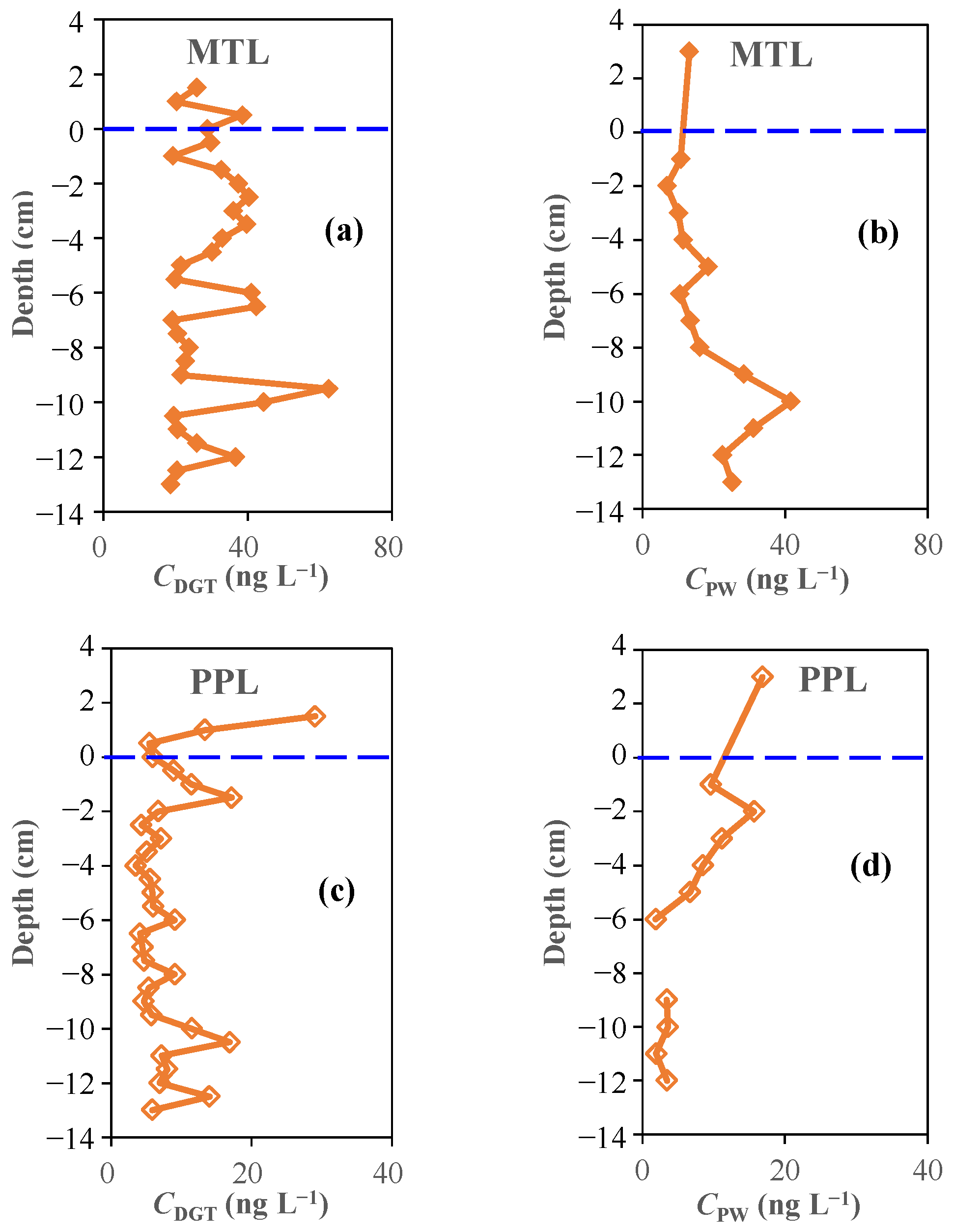
3.7. In Situ Profiling of β-Blockers at the Sediment–Water Interface Using DGT Probes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The combination of beta-blockers and ACE inhibitors across the spectrum of cardiovascular diseases. Cardiovasc. Drugs Ther. 2023, 37, 757–770. [Google Scholar] [CrossRef]
- Yıldırım, S.; Erkmen, C.; Uslu, B. Novel trends in analytical methods for β-blockers: An overview of applications in the last decade. Crit. Rev. Anal. Chem. 2022, 52, 131–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong, Y.; Xue, Y.; Liu, Z.; Tang, M.; Huang, L.-Z.; Shao, S.; Fang, Z. Degradation of the β-blocker propranolol by sulfite activation using FeS. Chem. Eng. J. 2020, 385, 123884. [Google Scholar] [CrossRef]
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and enantiomer profiles of β-blockers in wastewater and a receiving water body and adjacent soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; He, K.; Zhang, Y.; Li, P.; Lin, Y.; Yue, J. Predicting environmental risks of pharmaceutical residues by wastewater surveillance: An analysis based on pharmaceutical sales and their excretion data. Sci. Total Environ. 2024, 916, 170204. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, D.; Nałęcz-Jawecki, G.; Drzewicz, P.; Skowronek, A.; Mianowicz, K.; Strzelecka, A.; Giebułtowicz, J. The assessment of environmental risk related to the occurrence of pharmaceuticals in bottom sediments of the Odra River estuary (SW Baltic Sea). Sci. Total Environ. 2022, 828, 154446. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Rezania, S.; Nazari, M. Pharmaceuticals and personal care products in aquatic environments and their removal by algae-based systems. Chemosphere 2022, 288, 132580. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, J.W.; Hartman, K.J. The Potential Impacts of Statins and Beta-Blockers on West Virginia Ichthyofauna. Water 2023, 15, 3536. [Google Scholar] [CrossRef]
- Ulvi, A.; Aydın, S.; Aydın, M.E. Fate of selected pharmaceuticals in hospital and municipal wastewater effluent: Occurrence, removal, and environmental risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 75609–75625. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Jiang, L.; Wang, Y.F.; Ji, X.W.; Zhang, D.L.; Xu, G.Z.; Wu, D.S.; Li, A.M.; Xie, X.C. Validation and application of diffusive gradient in thin-film (DGT) equipped novel cyclodextrin polymer gels for monitoring endocrine disrupting chemicals (EDCs) and environmental risk assessment in the Taihu lake basin. Environ. Res. 2022, 212, 113391. [Google Scholar] [CrossRef]
- Gui, W.; Tian, C.; Sun, Q.; Li, S.; Zhang, W.; Tang, J.; Zhu, G. Simultaneous determination of organotin pesticides by HPLC-ICP-MS and their sorption, desorption, and transformation in freshwater sediments. Water Res. 2016, 95, 185–194. [Google Scholar] [CrossRef]
- Zhou, C.; Gaulier, C.; Luo, M.; Guo, W.; Baeyens, W.; Gao, Y. Fine scale measurements in Belgian coastal sediments reveal different mobilization mechanisms for cationic trace metals and oxyanions. Environ. Int. 2020, 145, 106140. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, Y.; Li, Y.; Yang, D.; Zhang, H.; Jones, K.C.; Gu, C.; Luo, J. Development and applications of novel DGT passive samplers for measuring 12 per-and polyfluoroalkyl substances in natural waters and wastewaters. Environ. Sci. Technol. 2021, 55, 9548–9556. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, T.Y.; Wan, Q.; Chai, B.B.; Lei, X.H.; He, L.X.; Chen, B. Microbial pathways in the coupling of iron, sulfur, and phosphorus cycles at the sediment-water interface of a river system: An in situ study involving the DGT technique. Sci. Total Environ. 2023, 863, 160855. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.-E.L.; Chen, W.; Chen, J.; Cai, X.; Jones, K.C.; Zhang, H. Development of a passive sampling technique for measuring pesticides in waters and soils. J. Agric. Food Chem. 2019, 67, 6397. [Google Scholar] [CrossRef]
- Xie, H.; Chen, J.; Chen, Q.; Chen, C.-E.L.; Du, J.; Tan, F.; Zhou, C. Development and evaluation of diffusive gradients in thin films technique for measuring antibiotics in seawater. Sci. Total Environ. 2018, 618, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Chen, C.; Sweetman, A.; Zhang, H.; Jones, K. DGT passive sampling for quantitative in situ measurements of compounds from household and personal care products in waters. Environ. Sci. Technol. 2017, 51, 13274–13281. [Google Scholar] [CrossRef]
- Fang, W.; Williams, P.N.; Fang, X.; Amoah-Antwi, C.; Yin, D.X.; Li, G.; Ma, L.Q.; Luo, J. Field-Scale Heterogeneity and Geochemical Regulation of Arsenic, Iron, Lead, and Sulfur Bioavailability in Paddy Soil. Environ. Sci. Technol. 2018, 52, 12098–12107. [Google Scholar] [CrossRef]
- Zou, Y.-T.; Fang, Z.; Li, Y.; Wang, R.; Zhang, H.; Jones, K.C.; Cui, X.-Y.; Shi, X.-Y.; Yin, D.; Li, C. Novel Method for in Situ Monitoring of Organophosphorus Flame Retardants in Waters. Anal. Chem. 2018, 90, 10016–10023. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Hou, S.; Lv, J.; Zhang, Y.; Wu, F.; Hua, Z.; Meng, W.; Zhang, H.; Xu, J. Investigation and application of a new passive sampling technique for in situ monitoring of illicit drugs in waste waters and rivers. Environ. Sci. Technol. 2017, 51, 9101–9108. [Google Scholar] [CrossRef]
- Challis, J.K.; Hanson, M.L.; Wong, C.S. Development and calibration of an organic-diffusive gradients in thin films aquatic passive sampler for a diverse suite of polar organic contaminants. Anal. Chem. 2016, 88, 10583–10591. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Luo, J.; Jones, K.C.; Zhang, H. Use of the Dynamic Technique DGT to Determine the Labile Pool Size and Kinetic Resupply of Pesticides in Soils and Sediments. Environ. Sci. Technol. 2021, 55, 9591–9600. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Wang, R.; Li, C.; Tang, J.; Chen, T.; Ying, G.-G.; Chen, C.-E. Diffusive gradients in thin films (DGT) probe for effectively sampling of per-and polyfluoroalkyl substances in waters and sediments. J. Environ. Sci. 2022, 121, 90–97. [Google Scholar] [CrossRef]
- Ji, X.; Challis, J.K.; Brinkmann, M. A critical review of diffusive gradients in thin films technique for measuring organic pollutants: Potential limitations, application to solid phases, and combination with bioassays. Chemosphere 2022, 287, 132352. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. In situ speciation measurements. Using diffusive gradients in thin films (DGT) to determine inorganically and organically complexed metals. Pure Appl. Chem. 2001, 73, 9–15. [Google Scholar] [CrossRef]
- Guan, D.X.; Li, Y.Q.; Yu, N.Y.; Yu, G.H.; Wei, S.; Zhang, H.; Davison, W.; Cui, X.Y.; Ma, L.Q.; Luo, J. In situ measurement of perfluoroalkyl substances in aquatic systems using diffusive gradients in thin-films technique. Water Res. 2018, 144, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Geng, J.J.; Xie, X.C.; Wang, X.R.; Ren, H.Q.; Gao, S.X. Determination of phosphite in a eutrophic freshwater lake by suppressed conductivity ion chromatography. Environ. Sci. Technol. 2012, 46, 10667–10674. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, K.; Li, Y.; Zhang, H.; Jones, K.C.; Liu, X.; Liu, S.; Ma, L.Q.; Luo, J. Development and application of the diffusive gradients in thin-films technique for measuring psychiatric pharmaceuticals in natural waters. Environ. Sci. Technol. 2019, 53, 11223–11231. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Rong, Q.; Zhang, H.; Jones, K.C.; Li, Y.; Luo, J. Evaluation and Application of a Novel Diffusive Gradients in Thin-Films Technique for In Situ Monitoring of Glucocorticoids in Natural Waters. Environ. Sci. Technol. 2022, 56, 15499–15507. [Google Scholar] [CrossRef]
- Stroski, K.M.; Challis, J.K.; Wong, C.S. The influence of pH on sampler uptake for an improved configuration of the organic-diffusive gradients in thin films passive sampler. Anal. Chim. Acta 2018, 1018, 45–53. [Google Scholar] [CrossRef]
- Guibal, R.; Buzier, R.; Charriau, A.; Lissalde, S.; Guibaud, G. Passive sampling of anionic pesticides using the Diffusive Gradients in Thin films technique (DGT). Anal. Chim. Acta 2017, 966, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pan, S.; Cheng, H.; Sweetman, A.J.; Zhang, H.; Jones, K.C. Diffusive gradients in thin-films (DGT) for in situ sampling of selected endocrine disrupting chemicals (EDCs) in waters. Water Res. 2018, 137, 211–219. [Google Scholar] [CrossRef]
- Jeong, Y.; Schäffer, A.; Smith, K. Equilibrium partitioning of organic compounds to OASIS HLB® as a function of compound concentration, pH, temperature and salinity. Chemosphere 2017, 174, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.; Morriss, A.; Paton, G. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Ma, R.; Wang, B.; Lu, S.; Zhang, Y.; Yin, L.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Characterization of pharmaceutically active compounds in Dongting Lake, China: Occurrence, chiral profiling and environmental risk. Sci. Total Environ. 2016, 557, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.C.; Schaffner, C.; Majewsky, M.; Klasmeier, J.; Fenner, K. Fate of β-blocker human pharmaceuticals in surface water: Comparison of measured and simulated concentrations in the Glatt Valley Watershed, Switzerland. Water Res. 2010, 44, 936–948. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545, 48–56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, M.; Fu, M.; Tan, D.; Zhang, P.; Zhou, Z.; Li, X. Validation and Application of the Diffusive Gradients in Thin-Films Technique for In Situ Measurement of β-Blocker Drugs in Waters and Sediments. Water 2024, 16, 1478. https://doi.org/10.3390/w16111478
Li Y, Wu M, Fu M, Tan D, Zhang P, Zhou Z, Li X. Validation and Application of the Diffusive Gradients in Thin-Films Technique for In Situ Measurement of β-Blocker Drugs in Waters and Sediments. Water. 2024; 16(11):1478. https://doi.org/10.3390/w16111478
Chicago/Turabian StyleLi, Yanying, Mingzhe Wu, Mengnan Fu, Dongqin Tan, Peng Zhang, Zhimin Zhou, and Xiaoyan Li. 2024. "Validation and Application of the Diffusive Gradients in Thin-Films Technique for In Situ Measurement of β-Blocker Drugs in Waters and Sediments" Water 16, no. 11: 1478. https://doi.org/10.3390/w16111478
APA StyleLi, Y., Wu, M., Fu, M., Tan, D., Zhang, P., Zhou, Z., & Li, X. (2024). Validation and Application of the Diffusive Gradients in Thin-Films Technique for In Situ Measurement of β-Blocker Drugs in Waters and Sediments. Water, 16(11), 1478. https://doi.org/10.3390/w16111478




