Abstract
Most water systems that support ecosystems and feed humans are depleted or stressed. Aquifer characteristics, topography, subsurface activities, climate, and geochemical processes regulate groundwater availability, a reliable source of fresh water. Globally, agriculture, industries, and the domestic sector are the three major sectors that consume vast quantities of freshwater resources. Further anthropogenic activities, such as soil leaching, acid rain, fertilizer, pesticides, mining, and other industrial activities, resulted in the release of organic and inorganic pollutants that affected global water resources. In India, groundwater is used in huge quantities, resulting in groundwater depletion of 1 to 2 m a year. Low-income countries face many issues related to water pollution, and the availability of safe water is minimal. In 2019, deaths due to unsafe sanitation accounted for 2.2% of the total global deaths, amounting to 1.2 million people’s deaths. India recorded 6.6% of deaths due to unsafe sanitation in 2019. India and China accounted for around 90.41% and 60.4% of the groundwater utilization for agricultural purposes, respectively. In 2020, China and India utilized vast quantities of nutrients (nitrate and phosphate) for crop growth to enhance crop yield, resulting in the highest nitrate and phosphate concentrations in groundwater. Remediating contaminants from different sources requires knowledge of their concentration, behavior, cycling, and degradation pathways. According to safety guidelines, limiting and optimizing crop organic and inorganic fertilizer, pesticide waste disposal, and empty container disposal can reduce groundwater contamination. The present study summarized groundwater utilization in various sectors, potential sources of groundwater contamination impacts on human health and the environment, preventive measures, and mitigation methods to overcome groundwater pollution.
1. Introduction
The increase in population, rapid urbanization, and industrial revolution resulted in the utilization of an enormous amount of fresh water [1]. The world’s freshwater source is not readily available for the human population since only 3% of the total water available is fresh water, and the remaining 97% is salt water [2]. Out of the 3% of the total fresh water available, 69% is in the form of ice and glaciers, 30% in the form of groundwater, and 1% in the form of surface water. A total of 87% of the total surface water is available in lakes, 11% in swamps, and 2% in rivers. So, approximately only 1% of the fresh water is available for the human population to meet its daily demands. The major drawback of this available fresh water is that it is not evenly distributed worldwide [3,4]. Most available fresh water is accumulated in Brazil, Canada, China, Colombia, Indonesia, Russia, and the United States. This results in most countries being in water scarcity and stress categories [5]. Globally, one-fifth of the total population needs to get the required amount of groundwater due to water stress or scarcity, resulting in a decrease in the economic growth of those developing countries [6].
Groundwater is the primary source for drinking, agricultural and industrial development. Around one-third of the total human population globally depends on groundwater for drinking purposes [7,8]. Groundwater is the only source in many arid and semi-arid regions where the rainfall is limited [9]. The leading sustainable development of nations is to provide a safe and renewable groundwater supply to the population [10]. The groundwater quality and quantity are degraded due to climate change, industrial, domestic, and agricultural activities [11,12]. Many organic and inorganic contaminants, namely nanoparticles, microplastics, and pesticides, are considered a severe threat to humans and the socioeconomic development of society [13,14,15]. Chemical contamination is the primary source of groundwater contamination, resulting in many researchers studying and understanding the process of groundwater contamination in the last three decades [16,17]. Critical Zone (CZ) studies the freshwater zones available, from green vegetation canopy to the bottom of the aquifers. Many researchers investigated the evolution and circulation of the groundwater to understand the CZ structures, processes, and functions [18,19].
Generally, in the olden days, groundwater contamination was assumed to be due only to geogenic origin, the dissolution of naturally available minerals in the earth’s crust to the groundwater in the aquifer. However, due to the vast expansion of urbanization and industrial activities, anthropogenic activities negatively impacted the groundwater [20,21]. Most countries where economic development is happening rapidly are affected by groundwater pollutants, and most of the countries are located in Africa and Asia [22,23]. Groundwater contamination is the addition of undesirable compounds in the water due to artificial activities, which may be chemicals, viruses, bacteria, heavy metals, dyes, and other substances [24]. Groundwater contamination differs from surface water contamination since the colour is invisible, and groundwater recovery is difficult to achieve [25,26]. Since groundwater contamination will result in chronic disease, detecting its negative impact on human health is complex. Since groundwater is located in subsurface earth strata, once it is contaminated, groundwater remediation is complex [8]. The natural recovery process will take more than 100 years to degrade the contaminants in the subsurface even though the contaminant’s source is reduced [27,28].
Synthetic or naturally occurring chemicals, as well as any microorganisms that are not regularly monitored in the environment but have the potential to do so and have known or suspected adverse effects on human health or the environment, are considered to be emerging contaminants (ECs) [29]. ECs are known to be contaminants of emerging environmental concerns due to their persistence in the environment and ability to alter the physiology of target receptors. Pharmaceuticals and personal care products (PPCPs), plasticizers, surfactants, fire retardants, nanomaterials, and pesticides are some of the well-known EC classes [30]. Ten Latin American and Caribbean (LAC) nations have confirmed the existence of 159 PhACs, primarily anti-inflammatory and analgesics. However, exceptionally high levels of ethinylestradiol (6.8 μg/L) and carbamazepine (830 μg/L) were discovered in Brazil and Ecuador, respectively [31]. Table 1 summarizes some of the most common ECs and their permissible values recommended by the World Health Organization (WHO) in water. Because of their detrimental effects on endocrine systems, several ECs have been classified as endocrine disruptive compounds (EDCs). Contaminants in aquatic environment resources are a significant environmental, public health and safety concern. The water distribution system is now seriously threatened by these contaminations. Emerging contaminants (ECs) include medications, X-ray media, pesticides, endocrine disruptors, and personal hygiene products. These contaminants have been found in surface water, wastewater, and groundwater sources across the globe in recent years. The degradation and removal of contaminants using various treatment techniques, including physical, chemical, and biological has been the subject of numerous previous or ongoing investigations. Nevertheless, there needs to be more experimental data available to make accurate predictions about the removal and mechanistic degradation fate of ECs in a variety of real-world systems. The fate of ECs and their transport into groundwater systems are critical in understanding groundwater pollution. Many researchers studied the fate of many ECs and their transport into soil and groundwater systems, namely formaldehyde [32,33], titanium dioxide [34], cotransport of biocolloids [35], cotransport of human adenoviruses [36], cotransport of clay colloids [37,38], transport of bio colloids [39], and cotransport of Pseudomonas putida and kaolinite particles [40].

Table 1.
Emerging contaminants (ECs) in the groundwater sources [41].
2. Global Scenario
Globally, freshwater utilization has increased six-fold from 1900 to 2014, twice that of the global population growth rate [42,43]. The World Meteorological Organization (WMO) estimated that 40% of the population lives in water-stressed regions. By 2050, the increase in population will be around 3 billion, resulting in water scarcity, a matter of life on earth [44]. In 2014, the world freshwater utilization was around 3.99 trillion m3, a 494% increase in utilization compared to 1900. The increasing population and industrial development is the main reason for freshwater utilization [45]. Globally, freshwater utilization was grouped into three categories based on the following regions: BRCIS (Brazil, Russia, China, India, and South Africa), OECD nations, and ROW (rest of the world) nations. India and China are the top two thickly populated countries, which may result in the overutilization of freshwater resources [45].
Similarly, OECD countries utilized approximately 20–25%, and ROW countries utilized around 30–33%. While 8.4% of the world’s population resides in Latin America and the Caribbean (LAC), the regions’ renewable water resources account for 35.1% of the world’s total. The availability of water per person in the LAC region is abundant compared to the rest of the World. On the other hand, rapid urbanization, poor governance, and inadequate infrastructure in some areas are to blame for water scarcity and an unequal social and geographical distribution of water resources. Furthermore, LAC has combined to become a global agricultural-product exporter, accounting for about 70% of freshwater withdrawal [46]. In LAC, the primary drivers of water consumption are agriculture and urbanization. Agricultural demands account for roughly 68% of freshwater availability, while industrial uses comprise 11% [46]. The available data show that developed countries utilize a low amount of fresh water compared to developing and poor countries [45].
India, China, and the United States are the top three countries where the freshwater utilization is maximum and accounts for about 647.5, 591.8 and 444.3 trillion m3 of water, respectively. India, China, and the United States account for 16.3, 14.9, and 11.21% of global freshwater utilization, resulting in approximately 42.5% of global freshwater resources [45]. Turkmenistan, Iraq, and Chile have the highest per capita freshwater utilization rates, with an average usage of 5753, 2646, and 2152 m3, respectively. In contrast, the per capita freshwater consumption in China, India, and the US is approximately 602.3, 425, and 1543 m3, respectively [45]. Water scarcity and security mainly depend on natural renewable resources, and naturally renewable resources depend on population and renewable flows through rainfall [47]. Similarly, the rainfall intensity decreased due to some seasonal variation, which will also decrease the rate of renewable resources. Generally, if the withdrawal of fresh water is more than the renewable capacity of the resource, it will result in water stress and scarcity in a county [48]. The renewable resource of India, China, and the United States was estimated at approximately 1045, 1998, and 8582 m3 per capita in 2019 [45]. However, due to the increase in population, this renewable rate decreased to 67, 53, and 44% for India, China, and the United States, respectively.
The increase in water demand, water scarcity, and security are the major concerns in the present century due to the increase in population and other developmental activities. Water stress indicates how fast the county utilizes groundwater rather than its renewable capacity [47,49]. South Asia, North Africa, and the Middle East are in the water stress region. Kuwait, United Arab Emirates, Saudi Arabia, Libya, and Qatar are the most water-stressed countries, and these countries’ availability of renewable water resources is more than 100% [45]. Those countries where this ratio is less than 50% are less water-stressed. India, China, and the United States have a water stress percentage of 66.49, 43.2, and 28.16%, respectively [45].
3. Freshwater Utilization
Freshwater withdrawals are directly related to the population, and like climate change, groundwater is also a significant concern in the present decades. Based on the present groundwater withdrawal, future withdrawals of the groundwater can be anticipated [50,51]. Agriculture, industries, and the domestic sector are the three major sectors that consume vast quantities of freshwater resources. Globally, population increases and associated daily activities resulted in increased freshwater consumption and the implementation of water conservation methods [48]. The government and many non-governmental organizations framed policies and methodologies to reduce water consumption and save for future generations.
3.1. Agricultural
Water is an essential source for agricultural activities, either in the form of rainwater or groundwater. The cultivation of food crops, biofuels, non-food crop production, and livestock are some of the primary reasons for the increased freshwater utilization [52]. The population is directly related to food demand, resulting in increased groundwater utilization for the last few decades. For example, in 2010, India consumed a freshwater resource of around 700 billion m3 for agricultural activities, which is twice the consumption of 1975 [45]. In 2015, India ranked first in freshwater consumption, with a withdrawal of 688 million m3, followed by China, the second largest freshwater-consuming country, with 385 billion m3, and the United States consumed around 175 billion m3 [45]. It is estimated that around 70% of the total fresh water utilized is for agricultural sectors [53]. However, freshwater withdrawal for agricultural purposes varies significantly from county to country. In 2019, Somalia, Afghanistan, and Nepal consumed around 99.48, 98.17, and 98.14%, respectively, for agricultural purposes. India, China, and the United States accounted for around 90.41, 60.4, and 39.66%, respectively. It is also estimated that low-income countries withdraw more than 90%, middle-income countries consume between 80 and 90%, and high-income countries consume less than 41% for agricultural purposes. Germany and Belgium consumed less than 2% of the fresh water for agriculture. Furthermore, in 2020, the total agricultural land irrigated with groundwater was estimated, and Bangladesh stands first, with 82% of the total available land irrigated using groundwater, followed by Suriname (71.43%) and Pakistan (52.66%). In India, 39.96% of the total land is irrigated using groundwater, which is 7% higher compared to 2001. The United Kingdom, Austria, and Belgium irrigate less than 1% of the available agricultural land.
3.2. Industries
Approximately 20% of the available fresh water is globally used for industrial activities [54]. Next to agricultural sectors, industries depend on fresh water for industrial applications, namely cooling towers, washing, tanning, dyeing, dilution, steam generation, and manufacturing processes [55]. The energy sector, which utilizes coal, lignite, and other fossil fuels, and nuclear power plants consume huge quantities of fresh water for power generation [56]. In 2015, the United States, China, and Russia consumed approximately 248, 138, and 39 billion m3 per year for industrial development. India consumed around 39 billion m3 of fresh water for industrial development. Counties across America, Europe, and East Asia and some countries of the Pacific region consume more than 1 billion m3 of water for industrial activities. In contrast, South Asian and Sub-Saharan African countries consume less than 0.5 billion m3 of fresh water.
Freshwater withdrawal is very high in high-income countries and less in low-income countries. Since industries play a significant role in the economic development of a country, high-income countries consume more fresh water [57]. In 2019, Estonia, Slovenia, and Belgium consumed approximately 91, 82, and 81% of the total fresh water available within the country [45]. Since India is an agricultural country, only 2.23% of the available freshwater resources were consumed for industrial activities. China and the United States consumed around 22.3% and 47.2% of freshwater resources [45]. The industrial utilization of fresh water results in water pollution, further affecting the fresh water. Due to industrial activities, the pollutant concentration in fresh water is increased and affects human health and the environment [58].
3.3. Domestic
Around 10–12% of the available fresh water is globally used for municipal purposes [45], and the domestic use of fresh water includes cooking, drinking, bathing, and washing. Domestic utilization of fresh water is relatively less when compared to agricultural and industrial developments [2]. China tops first in municipal freshwater utilization, with an average consumption of 70 billion m3 in 2015. The United States consumed 62 billion m3, and India consumed 56 billion m3 [45]. Despite the lower population in the United States, freshwater consumption is high due to the highest per capita water demand. Freshwater utilization in many countries is less than 30% for municipal purposes. India, China, and the United States consumed around 7.55, 13.55, and 13.14% of the available freshwater resources. On average, low-income countries consume 6.96%, lower-middle-income countries consume 8.2%, middle-income countries consume 10.77%, upper-middle-income countries consume 14.02%, and higher-income countries consume 16.26% of the freshwater resources. South Asian countries consume only 6.8% of the available fresh water, whereas European Union countries and West Bank and Gaza countries consume 23.45 and 46.23%, respectively [45]. Figure 1 illustrates the overall freshwater consumption of India, China, and the United States.

Figure 1.
Freshwater consumption in the year 2019.
4. Potential Sources of Contamination
Generally, groundwater contamination may be due to natural and anthropogenic activities. Natural processes such as climate change, natural disasters, soil matrix, rock–water interactions, and geological factors will contaminate the freshwater sources [12]. Anthropogenic activities, namely industrial waste discharge, solid and liquid waste, improper disposal of hazardous and biomedical waste, poor management of E-waste, mining activities, utilization of pesticides in agricultural practices, fertilizers, municipal waste, rapid urbanization, and land use, will result in groundwater contamination [12]. Figure 2 illustrates the different sources of groundwater pollution.

Figure 2.
Potential sources of groundwater pollution.
4.1. Natural Process
Natural disasters, namely volcanic eruptions, tsunamis, floods, drought, hurricanes, cyclones, and earthquakes, release vast quantities of natural waste and affect the fresh water’s quality [59]. Worldwide, from 2000 to 2022, 9142 natural disasters occurred, with an average of 415 natural disasters per year [60]. In 2022, the USA recorded the highest number of natural disasters, at 26, followed by 20 in Indonesia and 14 in Colombia. These natural disasters will affect the quality of the fresh water and cause pollution by mixing sewage water, municipal waste, and other contaminants. Next to natural disasters, geogenic contaminants are the primary concern, as they result in the degradation of the groundwater [12]. Geogenic contaminants occur naturally, resulting in elevated concentrations of certain chemical compounds that damage human health [61]. The most common geogenic pollutants are arsenic (As); chloride (Cl); fluoride (F); iodine (I); uranium (U); sodium (Na); sulfate (SO4); and trace elements such as chromium (Cr), iron (Fe), manganese (Mn), selenium (Se), etc. [62]. Some of the primary reasons for the geogenic contaminant in the aquifer are the weathering of rocks, rock–water interaction, environmental conditions like climate change, aquifer redox conditions and congestion of groundwater flow [63]. Anthropogenic activities also may be one of the reasons for the geochemical changes in the subsurface of the earth [64].
Fluoride is considered one of the significant critical geogenic contaminants since it affects groundwater worldwide. Igneous and sedimentary rocks are considered significant sources of fluoride minerals. They are present in these rocks as fluorite and apatite; these minerals have less solubility in the water [65]. Fluoride based pesticides result in fluoride leaching into the soil, getting mixed with clay minerals, and reaching groundwater [66]. Asian and African countries are mostly affected due to fluoride contamination, and fluorosis is the most common problem that people are facing in these countries. Many countries recorded a fluoride concentration of 5 to 9 mg/L, indicating severe groundwater pollution since the WHO recommended level of fluoride is 1 mg/L [67]. Iron is the most commonly used ore in the industrial process; during mining and due to the weathering of iron mineral rocks, iron will become soluble in groundwater and affect the quality [68]. Iron is also released from the animal and plant metabolism process. Similarly, arsenic concentration in the groundwater is increased due to the weathering of sedimentary and volcanic rocks, fossil fuels, and geothermal areas [69]. Rocks containing nickel ore dissolve and leach into the groundwater when they come into contact with the water flowing downstream [70].
4.2. Agricultural Practices
The agricultural practices resulted in emerging pollutants, namely nutrients (nitrate and phosphate), fertilizers, pesticides, and insecticides. Nitrate is considered one of the essential nutrients for the growth of the crop and to increase the yield of the crop [71]. Nitrates present in the nitrogen fertilizer, when applied to the crop, will result in water, soil, and air pollution [72,73]. The crops will only partially utilize the total applied fertilizers, resulting in soil deposition. During heavy rainfall, nitrate in the soil dissolves with rainwater, reaches surface water through runoff, and percolates into the soil and reaches aquifers. This will result in the contamination of both surface and groundwater. In 2020, China, India, and the United States were the top three countries that utilized vast quantities of nutrients for crop growth. China utilized 45 million tons, India utilized 32.54 million tons, and the United States utilized 20.96 million tons. To meet the population’s demand, the utilization of fertilizers has been increasing vigorously over the last two decades [74]. Several parts of Indian county reported the highest concentration of nitrate in the groundwater, between 40 and 1000 mg/L, and the threshold level of nitrate in the groundwater should not exceed 50 mg/L [75].
Followed by nitrate, phosphorus is the second major nutrient required for crops for proliferation and growth. Phosphorus is available in orthophosphate and dihydrogen phosphate; orthophosphate is the most preferred source of phosphorus for plants’ growth [76]. The plants do not utilize this soluble orthophosphate, causing it to eventually reach water bodies, resulting in an eutrophication process [77]. Phosphates reach the water bodies through industry effluent discharges, domestic wastewater, agricultural runoff, soil erosion, weathering of rocks, and livestock [78]. Pesticides, namely insecticides, herbicides, fungicides, and nematicides, are used in considerable quantities in agricultural practices to control weeds, kill insects, and control plant diseases [66]. Dichlorodiphenyltrichloroethane (DDT) is a well-known pesticide; it is most commonly used and has a half-life period of 15 years. It is less soluble [79] and reaches groundwater quickly, resulting in severe groundwater contamination.
4.3. Hazardous Waste Disposal
Urban areas experience severe stress in water bodies due to water pollution caused by anthropogenic activities. Sewer networks play a significant role in urban infrastructure development. Sewer networks protect the urban water environment, prevent human health threats, and eliminate waterborne diseases [80,81]. Due to the corrosive nature and complex nature of wastewater, sewer network leakage occurs and contaminates groundwater. Leachate generated from the landfill also results in soil pollution, followed by wastewater that will interact with the rocks and result in the leaching of rock minerals to the confined and unconfined aquifers.
Several researchers conducted an analysis of leachate and concluded it is composed of organic and inorganic pollutants, ammonia, metal ions, fluoride and some of the heavy metals [82]. Leachate with high heavy metals concentration seriously threatens the aquatic ecosystem [83]. In addition to industrial waste, biomedical waste from hospitals and biowaste from research centers result in severe issues for human health and the environment. In the hospital, many chemicals are used to diagnose disease and are thrown into the dust bins [84]. This waste, when disposed of in a landfill, causes serious issues. For example, ethidium bromide is a chemical used in the hospital for visualizing nucleic acid and is thrown out in the dust bins. These chemicals and many radioactive wastes are generated from the hospital during cancer diagnosis and X-ray studies [85]. This chemical waste has emerged in the past two decades due to advancements in medical diagnosis. It needs to be recycled appropriately; source reduction will be the one possible remediation [86].
Heavy metals are metallic compounds, and some of the most common heavy metals detected in water are arsenic, lead, chromium, cadmium, nickel, and antimony. These heavy metals enter the human body through air, water, and soil. These heavy metals enter the human body through ingestion (drinking water) and dermal activities [87]. Metal smelting, chemical manufacturing, battery manufacturing, secondary metal processing, and paint production are the significant sources of lead contamination in groundwater [88]. The pH, minerals in water, and dissolved salts in the water decide the amount of lead solubility. Chromium is not available in elemental composition; it is available in its natural form. Electroplating industries and chromium-containing material disposal will contaminate chromium [89]. Cadmium is present in nickel–cadmium batteries, and the improper disposal of these batteries will release an increased concentration in groundwater sources [90]. Similarly, fossil fuel burning, the mining of metals, fertilizer application, and the disposal of municipal and industrial waste are some of the significant sources of nickel contamination in the groundwater [91].
4.4. Municipal Solid Waste
Globally, due to rapid industrialization, urbanization, and modernization, solid waste generation has increased. The municipal authorities collect the solid waste generated from the urban areas, and it is then recycled and disposed of adequately using some treatment techniques. In rural areas, waste collection is not strictly regulated, so it is disposed of without proper guidelines. In the long run, these practices will result in several environmental issues and harm human health. Plastic waste is becoming a huge problem, and the utilization of plastic is increasing due to its advantages, like strength, low cost, and adaptability [92,93]. However, plastics are non-biodegradable, resulting in several issues in treatment and disposal, and plastics that are less than 5 mm are called microplastics, one of the emerging pollutants degrading groundwater quality [94,95]. Other pollutants released from households are detergents, chemicals used in floor washing, E-waste, food waste, medicines, and other organic and inorganic waste. PCPs include anti-dandruff shampoo, toothpaste with fluoride, skin moisturizers, deodorants, face and body cleansers, hair removal creams, and other cosmetics that contain medications and chemicals that, when combined with discarded container settings, can significantly contribute to water contamination [96].
The landfill is the most preferred disposal method for solid waste. All the solid and hazardous waste collected from the urban waste will be pretreated, resource recovery will happen, and, finally, the remaining waste will be disposed of in a landfill. The biodegradable, industrial, and biomedical waste, namely drugs and medicines, plastics, and batteries, will mix with this municipal waste [97]. The hydrolysis process will result in the breakdown of the compound using water and result in the generation of a considerable quantity of liquid waste. This liquid waste will leach through the soil and enter into aquifers. The groundwater is affected by these landfill sites, and communities close to the dumping site will experience severe groundwater contamination [98].
4.5. Extraction of Natural Gas
Natural gas is also a fossil fuel, but burning natural gas has less greenhouse gas emissions when compared to other conventional fossil fuels, like coal, lignite, and crude oil. Methane gas is the primary component of natural gas, and during extraction and transportation through pipelines, there is a greater chance of leakage. Natural gas extraction is an unconventional method that utilizes some chemicals for the borehole and fracturing of wells and some chemicals for the refining and processing of gas, which will affect the nearby community by creating water pollution [99]. The extraction of methane gas will result in the release of radioactive materials, gases, and minerals that will mix with the groundwater due to leaking [100]. Natural gas extraction will also utilize a large quantity of water, creating an additional pollution load and depletion of groundwater. During extraction, gas from the well will leak, and sometimes the well will fail, and these natural gases will get mixed with the groundwater and cause pollution [101]. Groundwater pollution due to natural gas has been reported in many places throughout the world.
4.6. Mining and Quarrying
Earth is abundantly filled with many minerals and water, the two essential components of the day-to-day activities of human being, and mining minerals is a primary source for many industrial activities [102]. However, these mining activities have several environmental impacts, and groundwater has a strong interrelationship with mining activities. Mining activities will result in the degradation of both surface and groundwater. Some of the impacts of mining activities on surface water are mine flooding, water logging, chemical pollution, effluent discharge, and other anthropogenic activities [103]. Similarly, mining activities also affect the groundwater and have several disadvantages, namely reduction in the groundwater table, saline water intrusion, and aquifer contamination that may be permanent or temporary. Some significant impacts of mining are acid attacks, heavy metal contamination, chemical pollution due to processing, and erosion and sedimentation [102]. During mining activities, the sulfur that is present in the minerals, when exposed to the atmosphere, moisture content, and iron-oxidizing bacteria, results in an acid attack on the nearby areas, and groundwater will become more acidic. Furthermore, these acids will interact with the mined minerals and result in the leaching of some heavy metals, namely arsenic, nickel, cadmium, and other trace compounds, resulting in severe water pollution [104]. In mining areas, some of the chemical, namely cyanide or sulfuric acid, and ferric hydroxide precipitation are used to process the ores, resulting in chemical pollution to the nearby water bodies.
4.7. Climate Change
Groundwater is the only source in the coastal regions that meets the demands of domestic, agricultural, and industrial activities. The surface water in the coastal regions is saline, mainly due to the mixing of seawater with the surface water [105]. The natural backwater flow of the sea will convert the fresh water available in the rivers and estuaries to salt water. Approximately 40% of the population lives in developed coastal regions worldwide [106]. The increase in population and density resulted in increased utilization of fresh water from the ground [1]. In coastal regions, the average population density is approximately 80 per sq. km, and it is twice the population density of the world [105]. In coastal regions, the evaporation rate is high and will result in non-availability of surface water for the community. Climate change is another critical natural phenomenon which has affected the coastal region vigorously in the past two decades. Due to climate change, the seawater level has increased due to ice melting and seawater expansion due to warming [107]. An increase in seawater levels and groundwater depletion will result in saltwater intrusion into the aquifers of the coastal regions. Saltwater intrusion will affect the groundwater quality and result in many health impacts on human beings. The impact and extent of the saltwater intrusion vary from place to place. Many researchers studied the impact of saltwater intrusion in groundwater aquifers [108,109,110]. Seawater intrusion and groundwater degradation are predominately found in Asian countries.
India is one of the fastest-growing countries, and the country’s economic development depends on industries and agricultural practices; groundwater is utilized in huge quantities to meet the demands [111]. India accounts for around 7500 km of coastal state surrounded by the Indian Ocean, the Bay of Bengal, and the Arabian Sea. Many districts of South India are already severely affected due to seawater intrusion, resulting in 21,000 sq. km of soil being affected and becoming saline soil [105]. Climate change will also result in natural disasters, namely floods, drought, heatwaves, hurricanes, and changes in weather patterns. The earth’s average temperature is increasing continuously, and the present situation is that, by the end of 2100, the earth will experience an average temperature of 1.5 to 2.5 °C [112]. Climate change has affected the weather pattern, and in the present decade, many countries have experienced an unexpectedly high rainfall intensity, which resulted in floods [113].
The Intergovernmental Panel on Climate Change (IPCC) was formed in 1988; the primary goal of the IPCC is to mandate the nations to adopt specific mitigation strategies to reduce the effects of climate change. About 1700 scientists in 1992 gave the first warnings about climate change and its detrimental effects on the ozone layer, biodiversity, sea level rise, human health, and other environmental damages. The Conference of Parties (COP) was established under the United Nations Framework Convention on Climate Change (UNFCCC) in 1992 to frame the global response to climate change. Its first meeting was held in Berlin in 1995, and subsequent meetings have been held annually since then. The average earth temperature will rise by 3 to 4 °C by 2100 compared to preindustrial times (1850), according to the IPCC’s fifth assessment report on climate change, released in November 2014. With 196 participating countries, the COP held its 21st meeting in 2015, with the primary objective of lowering the planet’s temperature by 2 °C, ideally by at least 1.5 °C. In August of 2021, the IPCC published its sixth assessment report on climate change, and according to the report, the average earth temperature will rise by 3.9 °C by the end of 2100. In October 2021, Scotland hosted the 26th Conference of Parties (COP), preceded by the 6th IPCC meeting, and the primary goal of the 26th COP meeting is to implement the Paris Agreement’s goal of a 1.5 °C global temperature reduction.
5. Consequences of Groundwater Contamination
Groundwater pollution poses several impacts on human health, affects the country’s economic growth, creates an imbalance in the aquatic ecosystem and freshwater scarcity, and affects green vegetation on the earth [58]. Figure 3 illustrates the impact of groundwater pollution on different sectors.
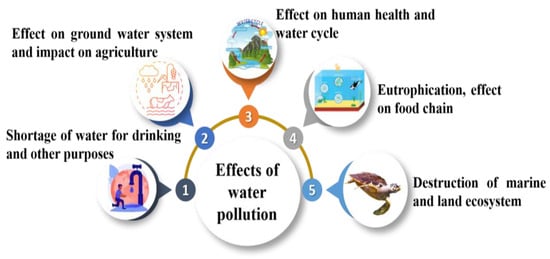
Figure 3.
Effects of groundwater contamination on different sectors.
5.1. Human Health
Low-income countries face many issues related to water pollution, and the availability of safe water is minimal. In 2019, the deaths that occurred due to unsafe sanitation amounted to 2.2% of the total global deaths, and it accounts for 1.2 million people’s deaths. India recorded 6.6% of deaths being due to unsafe sanitation in 2019. The highest death rate, 10.9%, due to unsafe sanitation, was observed in Chad, and in India, for every 1 Lakh people, 57 died in 2019 [114]. The nitrate concentration in the fresh water is increasing due to fertilizer application. As per the Environmental Protection Agency (EPA), a nitrate concentration greater than 10 mg/L is considered harmful to humans. Nitrate is easily soluble in water and forms N-nitroso, which causes cancer in human being and quickly enters through drinking water [115,116]. An excess nitrate concentration will result in blue baby syndrome in newborn babies and gastrointestinal cancer in adults and children [117].
According to the EPA, 5 mg/L is the permissible phosphate limit in drinking water. Continuous consumption of phosphate-contaminated water for a more extended period will result in kidney disease and osteoporosis. Phosphate content also affects natural water bodies by enhancing the eutrophication process. Algae and cyanobacteria consume this excess phosphate and release toxins into the water bodies, further affecting human health. Phosphate also affects the micro and macronutrients and leads to hormonal imbalance in the human body [118]. Pesticide application in agricultural practices also results in congenital disabilities, cancer, and nervous system damage. Pesticides also result in diarrhoea, vomiting, skin irritation, nausea, and abdominal pain [119]. Excess fluoride concentration in the drinking water results in fluorosis and creates skeletal, dental, and non-skeletal problems. Fluorosis also results in muscle damage, gastrointestinal system issues, and a combined impact on several organs.
A high chloride concentration in the drinking water will result in elevated blood pressure and human cardiovascular disease [120]. It also affects plants, like crop burning and defoliation issues. Iron is essential for human health, and as per the World Health Organization (WHO), the permissible limit of iron in groundwater is 0.3 mg/L, and excess ion concentration in drinking water results in hemochromatosis conditions that affect organs and organ systems. At extreme levels, it results in liver failure, heart disease, and diabetes [121]. As per the WHO, the permissible level of manganese in the groundwater is 0.4 mg/L, and as per the Bureau of Indian Standards (BIS), it is 0.1 mg/L. The excess manganese intake will result in neurological conditions similar to Parkinson’s disease. An excess manganese concentration will also result in DNA replication and mutation in mammalian cells [122].
Heavy metals are hazardous to human health and are reused in the human body through drinking water. Due to industrialization, the weathering of rocks and mining of minerals result in increased levels of heavy metals in groundwater [123,124]. Because of the high levels of arsenic in groundwater, people might experience several health issues, including heart failure, myocardial depolarization, gastrointestinal symptoms, abdominal colic, and respiratory distress due to mucous membrane irritation, which can lead to rhinitis or laryngitis, bronchitis, etc. Skin conditions brought on by long-term arsenic use include generalized hyperkeratosis; warts or corns on the palms and soles; and hypopigmentation in the face, neck, and back [125]. As per the WHO, 10 μg/L is the permissible lead level in drinking water, and lead is a poisonous metal that harms humans when consumed in excess. Excess lead consumption will result in mental illness, heart disease, and kidney failure [126]. As per the WHO, the permissible limit of chromium in drinking water is 0.05 mg/L. Chromium results in various health problems, such as nosebleeds and irritations, skin rashes, allergic responses, ulcers, kidney and liver damage, weaker immune systems, and genetic material changes, and chromium overdose results in death [66]. Cadmium in drinking water should be less than 0.005 mg/L, as per the WHO’s standards, and excess cadmium results in detrimental health effects in people, including lung cancer, prostate cancer, and renal impairment. Itai-itai disease’s chronic cadmium poisoning was initially identified in Japan in the early 20th century [127]. Nickel in drinking water should be less than 0.1 mg/L, resulting in allergic reactions in humans. Humans working in nickel processing plants will develop nasal sinus issues, lung cancer, and chronic bronchitis.
5.2. Economic Growth
The water and the economic growth of a country are interlinked. Since water is required for health, industrial activities, and economic growth, pollution increases costs for additional treatment. Pollutants are the by-products of each process, and when mixed with water, this creates water pollution, and water pollution will result in economic loss to society. The economic impacts can be seen in health care sectors, water treatment costs, tourism, real estate, fisheries, aquaculture, industries, and other sectors that depend on water for regular operations. Algal blooms and nitrates in drinking water sources can significantly raise treatment costs. For instance, in Minnesota, nitrate-removal systems raised supply costs from 5 to 10 cents per 1000 gallons to more than USD 4 per 1000 gallons. In addition, cleaning up polluted water sources might cost billions of dollars. Spending money on water source protection results in cost savings for water treatment [128] The EPA estimates that each year, water pollution accounts for loss of USD 1 billion revenue in the tourism industry [128]. The cause of this is nutrient contamination and the resulting algae blooms. An urgent health issue was caused in August 2018 by a red algae bloom off the southwest coast of Florida [129]. Toxic vapors released by decomposing algae increased hospital admissions by 54% [130], and between 2004 and 2007, the state spent USD 11,114 and USD 250,000 on red-tide cleanup projects [131]. Many researchers formulated a model for studying the economic loss due to water pollution. It is estimated that if water is moderately polluted, it results in an economic loss of 1.4%, and when water is heavily polluted, it results in an economic loss of 2% [132].
5.3. Imbalance in Aquatic Ecosystem
Due to the industrial revolution and increase in population, aquatic ecosystems have become a sink for pollutants. Pollutants from agricultural practices, industrial processing, and domestic applications will reach the aquatic ecosystem. This will result in the degrading of the water quality and several diseases affecting human beings and aquatic species [133]. Naturally, all water bodies have a natural self-purification process, but when the concentration of the contaminant increases vigorously, the self-purification process is reduced and results in water pollution. Chemicals reaching the aquatic environment include heavy metals, volatile organic compounds, dyes, detergents, microfibers, plastics, microplastic, and several other chemicals [134]. Globally, 80% of the sewage and millions of tons of industrial waste are disposed into the water bodies. An aquatic ecosystem comprises freshwater ecosystems and marine ecosystems. The marine ecosystem comprises 70% of the earth’s surface and comprises oceans, coral reefs, estuaries, and coastal ecosystems [135]. At the same time, the freshwater ecosystem comprises only 1% of the earth’s surface and consists of a wetland, lentic, and lotic ecosystem.
Anthropogenic activities, including deforestation, road and bridge construction, and other industrial activities, result in water pollution. Agriculture is the main reason for aquatic ecosystem damage. European Union countries experience 36% of the aquatic ecosystem being affected by water pollution. In the USA, agricultural activity is the leading source of pollution in rivers and streams, followed by wetlands and lakes. In China, agricultural practices account for water pollution in surface and groundwater. Excess application of nutrients, namely nitrate, phosphate, and organic manure, to the soil will be utilized less than 100% [135]. During heavy rainfall, these pollutants will reach the water bodies and increase the nutrient concentration in the water bodies, resulting in eutrophication. Due to the increased nutrient content, algal blooms and other aquatic plants will be grown in the ecosystem. These plants will severely damage the other aquatic species in the ecosystem. Around 420 coastal areas are globally experiencing eutrophication, and 169 are hypoxic. Over the century, harmful algal blooms (HABs) have resulted in water quality degradation, the killing of many microorganisms and aquatic species, and public health risks [135,136]. Cyanobacteria are one of the main HABS in the freshwater ecosystem. Cyanobacteria results in poisoning cattle, animals, and humans and also creates an off-flavor in domestic water supplies.
Water bodies act as a sink for sewage disposal, and sewage consists of domestic, solid, and industrial waste. Almost 58% of the liquid waste from urban areas and 81% from industries are discharged into waste bodies. These activities resulted in extensive damage to the aquatic ecosystem by killing several aquatic species and affecting the structure of aquatic biota [137]. Sewage mixing into the aquatic environment will result in the depletion of dissolved oxygen, and its level in the water will become less than 5 mg/L. Most industrial waste consists of heavy metals, dyes, and toxic substances, which will not be degraded easily, resulting in a long-term impact on the aquatic ecosystem and the food chain of human beings [138].
5.4. Freshwater Scarcity
Groundwater pollution will result in water scarcity, and the available fresh water for public use will be limited. Water scarcity is uncommon in many parts of the world, and poor countries are affected very much due to water scarcity [48]. Water scarcity is mainly caused by several factors, varying from country to country, including population growth, climate change, natural disasters, war and conflict, wastewater treatment facilities, and a lack of regulatory bodies. Around 1.1 million people are struggling due to a lack of water, and 2.7 people are struggling for fresh water for one month in a year [139]. Water pollution is widespread worldwide, and the primary sources come from agricultural practices, industries, and domestic applications. Freshwater scarcity further affects the lives of humans, livestock, and agricultural activities, and industrial development is also affected. Due to groundwater pollution and water scarcity, many waterborne diseases will affect low-income and poor countries [58]. Water scarcity will also result in the migration of humans and animals, leaving the larger land area unsuitable for cultivation due to lack of water.
5.5. Vegetation
Groundwater is considered a critical source of water for plants during drought due to the non-availability of surface water. Groundwater plays a major role in vegetation, and it has several impacts on vegetation, namely composition, diversity, richness, distribution, structure, and function [140]. Groundwater affects vegetation function in several ways: transpiration, productivity, survival, nutrient cycle, respiration, and habitat changes in groundwater depth result in alteration in the vegetation composition. Several studies confirmed that groundwater contributes to the diversity and richness of green vegetation [141]. Groundwater depth and groundwater chemistry are important to species diversity since the pH, carbonate, bicarbonate, calcium, and magnesium salt concentrations vary according to the groundwater depth. Maximum diversity was observed in the herbs with the concentration of calcium and magnesium at a shallower groundwater level [142]. Severe diversity was observed in trees when the groundwater’s salinity and total dissolved solids were less with deeper groundwater levels. Due to alterations in the groundwater level, vegetation stress and mortality are increased, and shifts in the vegetation distribution and native species are mostly affected. A lack of groundwater availability and anthropogenic activity will increase vegetation stress and result in a progressive alteration in the vegetation distribution of a locality [143]. Deeper groundwater does not directly impact vegetation distribution; oxygen level and water stress play a significant role in vegetation distribution. In water-limited conditions, due to water stress, the vegetation structure is affected [144].
6. Prevention of Groundwater Pollution
Clean drinking water is good for the environment, personal hygiene, and health, and it promotes economic growth. Therefore, having efficient methods to stop groundwater pollution is essential. The cheapest and most practical alternative to remediation is the prevention of groundwater pollution [145]. Groundwater pollution can be undetected for years and severely damage human health when detected; therefore, prevention saves treatment costs [146]. The following factors should be considered in a successful preventative strategy: stormwater management, hazardous waste management, small- and medium-sized businesses, storage tanks and pipelines, monitoring wells, and the development of water policy and wastes need to be disposed of appropriately [147]. On-site septic systems must be used and maintained correctly according to the plan. Surface tanks should be preferred because they are easier to monitor. Control regarding overloading and storage locations must be exercised, and chemical use may be diminished or replaced. Stormwater pollution must be avoided to stop chemicals and garbage from entering aquifers during heavy rain and flooding.
Periodically conducting environmental audits is crucial, and this would result in the creation of a plan to prevent pollution. When an emergency response strategy is being created, high-risk regions must be routinely evaluated. Important aquifers and healthy fields would need to be protected in land use plans. Businesses and residents need to be educated and informed. Every household is required to collect hazardous waste. Monitoring is required for all storage tanks and pipelines, and it is essential to purchase materials of the proper caliber. Keeping track of their lifespan and replacing them as necessary is essential. Water wells that have not yet been used should be appropriately disposed of. Individual, small-, and medium-sized businesses that generate chemical waste must receive special attention. It is best to stop using insecticides and pesticides altogether or to use them as little as possible. The pipeline network and the area around storage tanks should have monitoring wells built regularly. To ensure the early discovery of leaks, these would be inspected regularly. Additionally, a body must be assigned the job of implementing the water policy, and the water policy should explicitly outline all prevention efforts. The public should be well informed, and awareness initiatives and penalties should be clearly stated. Figure 4 illustrates the different solutions to overcome groundwater pollution.
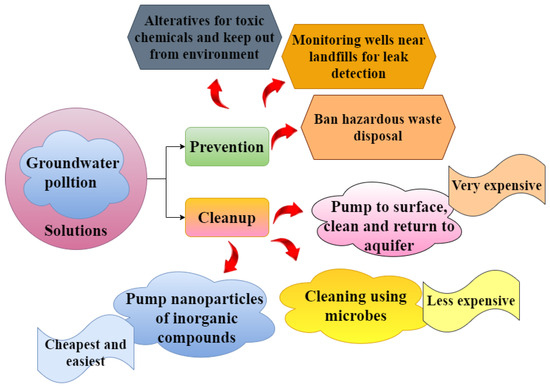
Figure 4.
Different solutions for groundwater contamination.
7. Remediation Technique for Groundwater Pollution
The technique adopted to clean up contaminated groundwater is known as groundwater remediation. Because each groundwater aquifer is different, the heterogeneity properties like hydraulic conductivity, pore structure, and microorganisms determine whether organic contaminants are mineralized by biotic or abiotic forces. The two methods discussed below share some processes but differ because in situ operations are significantly more compatible with bioremediation procedures. In situ bioremediation techniques have a definite economic and environmental advantage over physical–chemical ones if they can successfully be applied from the lab to the field [148].
Drilling a well into the contaminating plume is often one of the physical–chemical approaches of aquifer remediation. Techniques, namely reverse osmosis, air stripping, and activated charcoal, are available to remove contaminants from groundwater aquifers [149]. It is possible to eliminate emerging contaminants from pharmaceuticals by using membrane filtration, ultrasound, and single or combined biological methods [150]. Toxic organic substances must still be disposed of after contaminants have been removed from the aqueous phase, often achieved through incineration. Studies performed recently using modified clays have shown that they can absorb polluting chemicals from groundwater and degrade pollutants that have sorbed to the surface [151]. Biological degradation happens spontaneously in the subsoil. However, the breakdown rates in many soil conditions are sluggish for some refractory compounds, like organochlorine insecticides and some organochlorine solvents. As a result, the concentration of these compounds does not significantly decrease as they move away from their source of entrance [152]. Surface-based bioreactors have the benefit that bacterial growth may be precisely managed within these containments with the addition of nutrients (nitrogen, phosphorus, and carbon sources) to encourage a quick breakdown and high rates of conversion. This approach has some serious flaws. The capital and operating expenses could be more favorable due to the high pumping cost for removing contaminated groundwater from the subsurface [153]. This technique cannot remove pollutants embedded or adsorbed in the subsurface’s porous media. These leftovers are long-term sources of groundwater pollution release. Microorganisms taken from contaminated aquifers have been used in several laboratory experiments to test their capacity to break down a wide range of organic compounds [148].
Biotransformation was accelerated by alternately pumping pulses of oxygen and methane-containing groundwater into the subsurface’s contaminated zone. These field studies have shown that the right environmental factors can stimulate in situ microbial changes in the field, just as they can in the lab. Pollution can be eliminated in a variety of ways. These techniques can be roughly split into two categories. Both in situ and ex situ technologies exist [153]. Whatever the technique, cleaning up groundwater is costly. However, the price varies according to the scope, potential health effects, and accessible alternatives [154]. To stop further contamination, the aquifer might be sealed off. The water can subsequently be treated on the surface using physical, chemical, or biological technologies by draining the contaminated aquifer, which is recharged into the aquifer [155]. In situ technology involves the thermal, chemical, or biological treatment of groundwater within the aquifer. However, ex situ technology may use the following techniques: Adding steam to the water during the steam stripping process removes the pollutants in the pumped-out groundwater. The condensate can be used to recover the extracted steam, and further treatment options include incineration—the introduction of oxidizing and reducing chemicals during oxygen sparging. O3, H2O2, and hypochlorite are examples. These will chemically change the hazardous pollutants into less hazardous substances [148].
Without drawing water from the aquifer, in situ technology treats groundwater where it is. These could be produced via air sparging. Air sparging converts hydrocarbons from a dissolved form to a vapor phase by injecting contaminant-free air into the subsurface saturation zone. Bioremediation, in-well air stripping, chemical oxidation, thermal treatment, and phytoremediation are other in situ technology techniques [156]. Bioremediation involves injecting oxygen to speed up biodegradation [157]. It also incorporates the infusion of nutrients and degrading bacteria into the aquifer to promote biodegradation. In-well air stripping injects air into a well with two screens to force water out of the upper screen and up the well. Regarding chemical oxidation, reduction–oxidation processes change harmful pollutants into less dangerous compounds [148].
8. Conclusions
The maintenance of natural ecosystems, societal advancement, and human life and health all depend on groundwater resources. Pollution-related quality degradation of groundwater bodies is a significant global problem. There are many different and interconnected sources of groundwater pollution, including natural and anthropogenic sources. The primary sources of groundwater contamination are anthropogenic activities, namely excessive abstraction, inadequate wastewater treatment, industrial activities, disposal, and the use of fertilizers in agriculture. Seawater intrusion causes coastal groundwater bodies to become more salinized and unsuitable for drinking. Remediation is less appropriate than preventing groundwater pollution. While remediation methods include oxygen sparging, stream stripping, air stripping, thermal treatment, bioremediation, and chemical oxidation, prevention methods include monitoring hazardous materials, conducting periodic environmental audits, and health education. Additionally, most groundwater pollution is anthropocentric and can be avoided by providing extensive health education.
Author Contributions
G.R. (Gokulan Ravindiran): Conceptualization, Investigation, Resources, Data Curation, Writing—Original Draft, Visualization; S.R.: Conceptualization, Visualization, Data Curation, Writing—Original Draft; S.S.: Data Curation, Resources, Writing—Original Draft, Visualization; B.K.S.: Investigation, Writing—Review and Editing; G.R. (Gobinath Ravindran): Investigation, Supervision, Writing—Review and Editing; S.K.M.: Writing—Review and Editing; G.H.: Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No original data were generated during this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Okello, C.; Tomasello, B.; Greggio, N.; Wambiji, N.; Antonellini, M. Impact of Population Growth and Climate Change on the Freshwater Resources of Lamu Island, Kenya. Water 2015, 7, 1264–1290. [Google Scholar] [CrossRef]
- Cassardo, C.; Jones, J.A.A. Managing Water in a Changing World. Water 2011, 3, 618–628. [Google Scholar] [CrossRef]
- Kummu, M.; de Moel, H.; Ward, P.J.; Varis, O. How Close Do We Live to Water? A Global Analysis of Population to Freshwater Bodies. PLoS ONE 2011, 6, e20578. [Google Scholar] [CrossRef] [PubMed]
- Dinka, M.O.; Dinka, M.O. Safe Drinking Water: Concepts, Benefits, Principles and Standards. In Water Challenges of an Urbanizing World; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ismail, Z.; Go, Y.I. Fog-to-Water for Water Scarcity in Climate-Change Hazards Hotspots: Pilot Study in Southeast Asia. Glob. Chall. 2021, 5, 2000036. [Google Scholar] [CrossRef] [PubMed]
- Damkjaer, S.; Taylor, R. The Measurement of Water Scarcity: Defining a Meaningful Indicator. Ambio 2017, 46, 513. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Dhakate, R.; Kasarla, A.; Taloor, A.K. Appraisal of Groundwater Quality for Drinking and Irrigation Purposes in Central Telangana, India. Groundw. Sustain. Dev. 2020, 10, 100334. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lenin Sundar, M.; Ragunath, S.; Hemalatha, J.; Vivek, S.; Mohanraj, M.; Sampathkumar, V.; Mohammed Siraj Ansari, A.; Parthiban, V.; Manoj, S. Simulation of Ground Water Quality for Noyyal River Basin of Coimbatore City, Tamilnadu Using MODFLOW. Chemosphere 2022, 306, 135649. [Google Scholar] [CrossRef] [PubMed]
- Carrard, N.; Foster, T.; Willetts, J. Groundwater as a Source of Drinking Water in Southeast Asia and the Pacific: A Multi-Country Review of Current Reliance and Resource Concerns. Water 2019, 11, 1605. [Google Scholar] [CrossRef]
- Swain, S.; Taloor, A.K.; Dhal, L.; Sahoo, S.; Al-Ansari, N. Impact of Climate Change on Groundwater Hydrology: A Comprehensive Review and Current Status of the Indian Hydrogeology. Appl. Water Sci. 2022, 12, 120. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/Nanoplastic Toxicity in Plants: An Imminent Concern. Environ. Monit. Assess. 2022, 195, 27. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, S.; Marín-Benito, J.M.; Peña, A.; Antonio Rodríguez-Liébana, J.; Delgado-Moreno, L. Interactions of Microplastics with Pesticides in Soils and Their Ecotoxicological Implications. Agronomy 2023, 13, 701. [Google Scholar] [CrossRef]
- Subaramaniyam, U.; Allimuthu, R.S.; Vappu, S.; Ramalingam, D.; Balan, R.; Paital, B.; Panda, N.; Rath, P.K.; Ramalingam, N.; Sahoo, D.K. Effects of Microplastics, Pesticides and Nano-Materials on Fish Health, Oxidative Stress and Antioxidant Defense Mechanism. Front. Physiol. 2023, 14, 1217666. [Google Scholar] [CrossRef] [PubMed]
- Ullah, Z.; Rashid, A.; Ghani, J.; Nawab, J.; Zeng, X.C.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M.; et al. Groundwater Contamination through Potentially Harmful Metals and Its Implications in Groundwater Management. Front. Environ. Sci. 2022, 10, 1021596. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Du, S.; Wang, H.; Liu, S.; Du, S. The Investigation and Assessment on Groundwater Organic Pollution. Org. Pollut. Monit. Risk Treat. 2013, 4, 87–110. [Google Scholar] [CrossRef]
- Riebe, C.S.; Hahm, W.J.; Brantley, S.L. Controls on Deep Critical Zone Architecture: A Historical Review and Four Testable Hypotheses. Earth Surf. Process. Landforms 2017, 42, 128–156. [Google Scholar] [CrossRef]
- Wymore, A.S.; Ward, A.S.; Wohl, E.; Harvey, J.W. Viewing River Corridors through the Lens of Critical Zone Science. Front. Water 2023, 5, 1147561. [Google Scholar] [CrossRef]
- Triassi, M.; Cerino, P.; Montuori, P.; Pizzolante, A.; Trama, U.; Nicodemo, F.; D’Auria, J.L.; de Vita, S.; de Rosa, E.; Limone, A. Heavy Metals in Groundwater of Southern Italy: Occurrence and Potential Adverse Effects on the Environment and Human Health. Int. J. Environ. Res. Public Health 2023, 20, 1693. [Google Scholar] [CrossRef]
- Brindha, K.; Paul, R.; Walter, J.; Tan, M.L.; Singh, M.K. Trace Metals Contamination in Groundwater and Implications on Human Health: Comprehensive Assessment Using Hydrogeochemical and Geostatistical Methods. Environ. Geochem. Health 2020, 42, 3819. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Subramanian, K.; Sreevidya, V.; Venkatasubramani, R.; Sivakumar, V. DRASTIC Model Developed with Lineament Density to Map Groundwater Susceptibility: A Case Study in Part of Coimbatore District, Tamilnadu, India. Environ. Dev. Sustain. 2023, 25, 10411–10423. [Google Scholar] [CrossRef]
- Meenakshi Balasubramanian, S.; Bhaskar, A.S.; Sivakumar, V. Block Level Assessment of Groundwater Potential Zones Using Hydrogeological and Remote Sensing and GIS Data’s: A Scientific Approach to Prevent Water Scarcity Problems. Desalination Water Treat. 2023, 297, 227–239. [Google Scholar] [CrossRef]
- El-taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.S.; Radwan, A.G. A Review of Coagulation Explaining Its Definition, Mechanism, Coagulant Types, and Optimization Models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Sharma, I.; Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Alori, E.T.; Gabasawa, A.I.; Elenwo, C.E.; Agbeyegbe, O.O. Bioremediation Techniques as Affected by Limiting Factors in Soil Environment. Front. Soil Sci. 2022, 2, 937186. [Google Scholar] [CrossRef]
- Câmara, J.S.; Montesdeoca-Esponda, S.; Freitas, J.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Perestrelo, R. Emerging Contaminants in Seafront Zones. Environmental Impact and Analytical Approaches. Separations 2021, 8, 95. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Valdez-Carrillo, M.; Abrell, L.; Ramírez-Hernández, J.; Reyes-López, J.A.; Carreón-Diazconti, C. Pharmaceuticals as Emerging Contaminants in the Aquatic Environment of Latin America: A Review. Environ. Sci. Pollut. Res. 2020, 27, 44863–44891. [Google Scholar] [CrossRef]
- Stefanarou, A.S.; Chrysikopoulos, C.V. Interaction of Titanium Dioxide with Formaldehyde in the Presence of Quartz Sand under Static and Dynamic Conditions. Water 2021, 13, 1420. [Google Scholar] [CrossRef]
- Fountouli, T.V.; Chrysikopoulos, C.V. Effect of Clay Colloid Particles on Formaldehyde Transport in Unsaturated Porous Media. Water 2020, 12, 3541. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Fountouli, T.V. Cotransport of Titanium Dioxide Nanoparticles and Formaldehyde in Saturated and Unsaturated Columns Packed with Quartz Sand. Vadose Zone J. 2023, 22, e20175. [Google Scholar] [CrossRef]
- Georgopoulou, M.P.; Syngouna, V.I.; Chrysikopoulos, C.V. Influence of Graphene Oxide Nanoparticles on the Transport and Cotransport of Biocolloids in Saturated Porous Media. Colloids Surf. B Biointerfaces 2020, 189, 110841. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V.; Kokkinos, P.; Tselepi, M.A.; Vantarakis, A. Cotransport of Human Adenoviruses with Clay Colloids and TiO2 Nanoparticles in Saturated Porous Media: Effect of Flow Velocity. Sci. Total Environ. 2017, 598, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Cotransport of Clay Colloids and Viruses through Water-Saturated Vertically Oriented Columns Packed with Glass Beads: Gravity Effects. Sci. Total Environ. 2016, 545–546, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Cotransport of Clay Colloids and Viruses in Water Saturated Porous Media. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 416, 56–65. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Transport of Biocolloids in Water Saturated Columns Packed with Sand: Effect of Grain Size and Pore Water Velocity. J. Contam. Hydrol. 2011, 126, 301–314. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Chrysikopoulos, C.V. Cotransport of Pseudomonas Putida and Kaolinite Particles through Water-Saturated Columns Packed with Glass Beads. Water Resour. Res. 2011, 47, 2543. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Islam, S.M.F.; Karim, Z.; Islam, S.M.F.; Karim, Z. World’s Demand for Food and Water: The Consequences of Climate Change. In Desalination—Challenges and Opportunities; IntechOpen: London, UK, 2019; pp. 1–27. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The World’s Road to Water Scarcity: Shortage and Stress in the 20th Century and Pathways towards Sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future Global Urban Water Scarcity and Potential Solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Water Use and Stress. Our World Data 2017. Available online: https://ourworldindata.org/water-use-stress (accessed on 23 August 2023).
- Rodríguez, C.; García, B.; Pinto, C.; Sánchez, R.; Serrano, J.; Leiva, E. Water Context in Latin America and the Caribbean: Distribution, Regulations and Prospects for Water Reuse and Reclamation. Water 2022, 14, 3589. [Google Scholar] [CrossRef]
- Odhiambo, G.O. Water Scarcity in the Arabian Peninsula and Socio-Economic Implications. Appl. Water Sci. 2017, 7, 2479–2492. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water Supply and Water Scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Tariq, M.A.U.R.; Alotaibi, R.; Weththasinghe, K.K.; Rajabi, Z. A Detailed Perspective of Water Resource Management in a Dry and Water Scarce Country: The Case in Kuwait. Front. Environ. Sci. 2022, 10, 1073834. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Yaashikaa, P.R. Introduction—Water. In Water in Textiles and Fashion. Consumption, Footprint, and Life Cycle Assessment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Costa, D.; Zhang, H.; Levison, J. Impacts of Climate Change on Groundwater in the Great Lakes Basin: A Review. J. Great Lakes Res. 2021, 47, 1613–1625. [Google Scholar] [CrossRef]
- Schaible, G.D.; Aillery, M.P. Water Conservation in Irrigated Agriculture: Trends and Challenges in the Face of Emerging Demands; United States Department of Agriculture Economic Research Service: Washington, DC, USA, 2012. [Google Scholar]
- Gleick, P.H. The World’s Water; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Atimtay, A.T.; Sikdar, S.K. Security of Industrial Water Supply and Management; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Brook, B.W.; Alonso, A.; Meneley, D.A.; Misak, J.; Blees, T.; van Erp, J.B. Why Nuclear Energy Is Sustainable and Has to Be Part of the Energy Mix. Sustain. Mater. Technol. 2014, 1, 8–16. [Google Scholar] [CrossRef]
- Doungmanee, P. The Nexus of Agricultural Water Use and Economic Development Level. Kasetsart J. Soc. Sci. 2016, 37, 38–45. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Elumalai, V.; Ning, J.; Xu, F.; Mu, D. Groundwater Quality Assessment Using EWQI With Updated Water Quality Classification Criteria: A Case Study in and Around Zhouzhi County, Guanzhong Basin (China). Expo. Health 2022, 1–16. [Google Scholar] [CrossRef]
- Our World in Data. Natural Disasters. Available online: https://ourworldindata.org/natural-disasters (accessed on 12 August 2023).
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Hug, S.J.; Winkel, L.H.E.; Voegelin, A.; Berg, M.; Johnson, A.C. Arsenic and Other Geogenic Contaminants in Groundwater—A Global Challenge. Chimia 2020, 74, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Ma, L.; Wu, J.; Abuduwaili, J.; Liu, W. Geochemical Responses to Anthropogenic and Natural Influences in Ebinur Lake Sediments of Arid Northwest China. PLoS ONE 2016, 11, e0155819. [Google Scholar] [CrossRef] [PubMed]
- Onipe, T.; Edokpayi, J.N.; Odiyo, J.O. Geochemical Characterization and Assessment of Fluoride Sources in Groundwater of Siloam Area, Limpopo Province, South Africa. Sci. Rep. 2021, 11, 14000. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Chand, S.; Chand, S.; Rout, P.R.; Naik, S.K. Emerging Groundwater Contaminants: A Comprehensive Review on Their Health Hazards and Remediation Technologies. Groundw. Sustain. Dev. 2023, 20, 100868. [Google Scholar] [CrossRef]
- Rehman, F.; Siddique, J.; Shahab, A.; Azeem, T.; Bangash, A.A.; Naseem, A.A.; Riaz, O.; ur Rehman, Q. Hydrochemical Appraisal of Fluoride Contamination in Groundwater and Human Health Risk Assessment at Isa Khel, Punjab, Pakistan. Environ. Technol. Innov. 2022, 27, 102445. [Google Scholar] [CrossRef]
- Priyanka, M.; Venkata, R.G.; Ratnakar, D. Groundwater Quality Appraisal and Its Hydrochemical Characterization in and around Iron Ore Mine, Chitradurga, Karnataka. Int. J. Hydrol. 2017, 1, 151–161. [Google Scholar] [CrossRef]
- Masuda, H. Arsenic Cycling in the Earth’s Crust and Hydrosphere: Interaction between Naturally Occurring Arsenic and Human Activities. Prog. Earth Planet. Sci. 2018, 5, 68. [Google Scholar] [CrossRef]
- Åhlgren, K.; Sjöberg, V.; Allard, B.; Bäckström, M. Groundwater Chemistry Affected by Trace Elements (As, Mo, Ni, U and V) from a Burning Alum Shale Waste Deposit, Kvarntorp, Sweden. Environ. Sci. Pollut. Res. 2021, 28, 30219–30241. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of Organic Farming for Achieving Sustainability in Agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Lee, E.; Rout, P.R.; Bae, J. The Applicability of Anaerobically Treated Domestic Wastewater as a Nutrient Medium in Hydroponic Lettuce Cultivation: Nitrogen Toxicity and Health Risk Assessment. Sci. Total Environ. 2021, 780, 146482. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, P.; Yang, N.; Yang, C.; Zhou, Y.; Li, J. Distribution, Sources and Main Controlling Factors of Nitrate in a Typical Intensive Agricultural Region, Northwestern China: Vertical Profile Perspectives. Environ. Res. 2023, 237, 116911. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.C.; Mandal, B.; Sarkar, D. Assessment of the Potential Hazards of Nitrate Contamination in Surface and Groundwater in a Heavily Fertilized and Intensively Cultivated District of India. Environ. Monit. Assess. 2008, 146, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ayyasamy, P.M.; Rajakumar, S.; Sathishkumar, M.; Swaminathan, K.; Shanthi, K.; Lakshmanaperumalsamy, P.; Lee, S. Nitrate Removal from Synthetic Medium and Groundwater with Aquatic Macrophytes. Desalination 2009, 242, 286–296. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Modeling Isotherms, Kinetics and Understanding the Mechanism of Phosphate Adsorption onto a Solid Waste: Ground Burnt Patties. J. Environ. Chem. Eng. 2014, 2, 1331–1342. [Google Scholar] [CrossRef]
- Rout, P.R.; Dash, R.R.; Bhunia, P. Nutrient Removal from Binary Aqueous Phase by Dolochar: Highlighting Optimization, Single and Binary Adsorption Isotherms and Nutrient Release. Process Saf. Environ. Prot. 2016, 100, 91–107. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Evaluation of Kinetic and Statistical Models for Predicting Breakthrough Curves of Phosphate Removal Using Dolochar-Packed Columns. J. Water Process Eng. 2017, 17, 168–180. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Sahoo, M.M.; Raut, P.R.; Mahanty, B.; Sahoo, N.K. Kinetic Modelling and Process Engineering of Phenolics Microbial and Enzymatic Biodegradation: A Current Outlook and Challenges. J. Water Process Eng. 2021, 44, 102421. [Google Scholar] [CrossRef]
- Wear, S.L.; Acuña, V.; McDonald, R.; Font, C. Sewage Pollution, Declining Ecosystem Health, and Cross-Sector Collaboration. Biol. Conserv. 2021, 255, 109010. [Google Scholar] [CrossRef]
- Guo, W.; Li, P.; Du, Q.; Zhou, Y.; Xu, D.; Zhang, Z. Hydrogeochemical Processes Regulating the Groundwater Geochemistry and Human Health Risk of Groundwater in the Rural Areas of the Wei River Basin, China. Expo. Health 2023, 1–16. [Google Scholar] [CrossRef]
- Hosseini Beinabaj, S.M.; Heydariyan, H.; Mohammad Aleii, H.; Hosseinzadeh, A. Concentration of Heavy Metals in Leachate, Soil, and Plants in Tehran’s Landfill: Investigation of the Effect of Landfill Age on the Intensity of Pollution. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef]
- Abd El-Salam, M.M.; Abu-Zuid, G.I. Impact of Landfill Leachate on the Groundwater Quality: A Case Study in Egypt. J. Adv. Res. 2015, 6, 579. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, K.K.; Barik, D. Health Hazards of Medical Waste and Its Disposal. Energy from Toxic Organic Waste for Heat and Power Generation; Woodhead Publishing: Sawston, UK, 2019; pp. 99–118. [Google Scholar] [CrossRef]
- Ravichandran, R.; Binukumar, J.; Sreeram, R.; Arunkumar, L. An Overview of Radioactive Waste Disposal Procedures of a Nuclear Medicine Department. J. Med. Phys. 2011, 36, 95. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cui, M.; Ye, Z.; Guo, Q. Environmental Challenges from the Increasing Medical Waste since SARS Outbreak. J. Clean. Prod. 2021, 291, 125246. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Dongre, R.S.; Dongre, R.S. Lead: Toxicological Profile, Pollution Aspects and Remedial Solutions. Lead Chem. 2020, 1–18. [Google Scholar] [CrossRef]
- IARC. Chromium and Chromium Compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1990. [Google Scholar]
- Sidhu, G.P.S.; Bali, A.S. Cd in the Environment: Uptake, Toxicity and Management. In Appraisal of Metal(loids) in the Ecosystem; Elsevier: Amsterdam, The Netherlands, 2022; pp. 283–300. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and Removal Characteristics of Microplastics in Different Processes of the Leachate Treatment System. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Mehra, K.; Gupta, A. Microplastics as Contaminants in Indian Environment: A Review. Environ. Sci. Pollut. Res. 2021, 28, 68025–68052. [Google Scholar] [CrossRef]
- Rout, P.R.; Mohanty, A.; Aastha; Sharma, A.; Miglani, M.; Liu, D.; Varjani, S. Micro- and Nanoplastics Removal Mechanisms in Wastewater Treatment Plants: A Review. J. Hazard. Mater. Adv. 2022, 6, 100070. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Peysson, W.; Vulliet, E. Determination of 136 Pharmaceuticals and Hormones in Sewage Sludge Using Quick, Easy, Cheap, Effective, Rugged and Safe Extraction Followed by Analysis with Liquid Chromatography-Time-of-Flight-Mass Spectrometry. J. Chromatogr. A 2013, 1290, 46–61. [Google Scholar] [CrossRef]
- Slack, R.J.; Gronow, J.R.; Voulvoulis, N. Household Hazardous Waste in Municipal Landfills: Contaminants in Leachate. Sci. Total Environ. 2005, 337, 119–137. [Google Scholar] [CrossRef]
- Buerge, I.J.; Buser, H.R.; Kahle, M.; Müller, M.D.; Poiger, T. Ubiquitous Occurrence of the Artificial Sweetener Acesulfame in the Aquatic Environment: An Ideal Chemical Marker of Domestic Wastewater in Groundwater. Environ. Sci. Technol. 2009, 43, 4381–4385. [Google Scholar] [CrossRef]
- Woda, J.; Wen, T.; Oakley, D.; Yoxtheimer, D.; Engelder, T.; Clara Castro, M.; Brantley, S.L. Detecting and Explaining Why Aquifers Occasionally Become Degraded near Hydraulically Fractured Shale Gas Wells. Proc. Natl. Acad. Sci. USA 2018, 115, 12349–12358. [Google Scholar] [CrossRef]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of Shale Gas Development on Regional Water Quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef]
- Colborn, T.; Kwiatkowski, C.; Schultz, K.; Bachran, M. Natural Gas Operations from a Public Health Perspective. Hum. Ecol. Risk Assess. Int. J. 2011, 17, 1039–1056. [Google Scholar] [CrossRef]
- Soni, A.K.; Soni, A.K. Mining of Minerals and Groundwater in India. In Groundwater-Resource Characterisation and Management Aspects; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Kausher, R.; Singh, R.; Sinha, A.K.; Sethy, S.N.; Kumar, S.; Pandey, S.; Ragab, A.E.; Mohamed, A. Assessing Impacts of Mining-Induced Land Use Changes on Groundwater and Surface Water Quality Using Isotopic and Hydrogeochemical Signatures. Sustainability 2023, 15, 11041. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Isa, Y.M. Bioremediation of Acid Mine Drainage—Review. Alexandria Eng. J. 2023, 65, 1047–1075. [Google Scholar] [CrossRef]
- Prusty, P.; Farooq, S.H. Seawater Intrusion in the Coastal Aquifers of India—A Review. HydroResearch 2020, 3, 61–74. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K.; Shankar, K.; Yang, Q.; Jayasena, H.C. Coastal Groundwater Dynamics, Environmental Issues and Sustainability: A Synthesis. Mar. Pollut. Bull. 2023, 191, 114973. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Abdalla, F. Ionic Ratios as Tracers to Assess Seawater Intrusion and to Identify Salinity Sources in Jazan Coastal Aquifer, Saudi Arabia. Arab. J. Geosci. 2016, 9, 40. [Google Scholar] [CrossRef]
- Suhartono, E.; Purwanto, P.; Suripin, S. Seawater Intrusion Modeling on Groundwater Confined Aquifer in Semarang. Procedia Environ. Sci. 2015, 23, 110–115. [Google Scholar] [CrossRef]
- Manivannan, V.; Elango, L. Seawater Intrusion and Submarine Groundwater Discharge along the Indian Coast. Environ. Sci. Pollut. Res. 2019, 26, 31592–31608. [Google Scholar] [CrossRef]
- Bierkens, M.F.P.; Wada, Y. Non-Renewable Groundwater Use and Groundwater Depletion: A Review. Environ. Res. Lett. 2019, 14, 063002. [Google Scholar] [CrossRef]
- Shivanna, K.R. Climate Change and Its Impact on Biodiversity and Human Welfare. Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 2022, 88, 160. [Google Scholar] [CrossRef]
- Ajani, A.; van der Geest, K. Climate Change in Rural Pakistan: Evidence and Experiences from a People-Centered Perspective. Sustain. Sci. 2021, 16, 1999–2011. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Clean Water and Sanitation. Our World Data 2021. Available online: https://ourworldindata.org/water-use-stress (accessed on 23 August 2023).
- Alam, S.M.K.; Li, P.; Fida, M. Groundwater Nitrate Pollution Due to Excessive Use of N-Fertilizers in Rural Areas of Bangladesh: Pollution Status, Health Risk, Source Contribution, and Future Impacts. Expo. Health 2023, 1–24. [Google Scholar] [CrossRef]
- Xu, D.; Li, P.; Chen, X.; Yang, S.; Zhang, P.; Guo, F. Major Ion Hydrogeochemistry and Health Risk of Groundwater Nitrate in Selected Rural Areas of the Guanzhong Basin, China. Hum. Ecol. Risk Assess. Int. J. 2023, 29, 701–727. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Khalid, S.; Murtaza, B.; Shaheen, I.; Ahmad, I.; Ullah, M.I.; Abbas, T.; Rehman, F.; Ashraf, M.R.; Khalid, S.; Abbas, S.; et al. Assessment and Public Perception of Drinking Water Quality and Safety in District Vehari, Punjab, Pakistan. J. Clean. Prod. 2018, 181, 224–234. [Google Scholar] [CrossRef]
- Melanda, V.S.; Galiciolli, M.E.A.; Lima, L.S.; Figueiredo, B.C.; Oliveira, C.S. Impact of Pesticides on Cancer and Congenital Malformation: A Systematic Review. Toxics 2022, 10, 676. [Google Scholar] [CrossRef]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride Occurrences, Health Problems, Detection, and Remediation Methods for Drinking Water: A Comprehensive Review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Khan, M.J.H.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Tanaka, H. Human Health Risk Assessment of Elevated and Variable Iron and Manganese Intake with Arsenic-Safe Groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef]
- Aminur Rahman, M.; Abul Hashem, M.; Sohel Rana, M.; Rashidul Islam, M. Manganese in Potable Water of Nine Districts, Bangladesh: Human Health Risk. Environ. Sci. Pollut. Res. 2021, 28, 45663–45675. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Jayasena, H.A.H.C. Perchlorate Contamination in Groundwater and Associated Health Risks from Fireworks Manufacturing Area (Sivakasi Region) of South India. Expo. Health 2022, 14, 359–373. [Google Scholar] [CrossRef]
- Sivakumar, V.; Chidambaram, S.M.; Velusamy, S.; Rathinavel, R.; Shanmugasundaram, D.K.; Sundararaj, P.; Shanmugamoorthy, M.; Thangavel, R.; Balu, K. An Integrated Approach for an Impact Assessment of the Tank Water and Groundwater Quality in Coimbatore Region of South India: Implication from Anthropogenic Activities. Environ. Monit. Assess. 2023, 195, 88. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, D.; Singh, S.K.; Rahman, M.M.; Dutta, R.N.; Mukherjee, S.C.; Pati, S.; Kar, P.B. Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int. J. Environ. Res. Public Health 2018, 15, 180. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cadmium Toxicity and Treatment: An Update. Casp. J. Intern. Med. 2017, 8, 135. [Google Scholar] [CrossRef]
- US EPA. The Effects: Economy. Available online: https://www.epa.gov/nutrientpollution/effects-economy (accessed on 16 August 2023).
- Red Tide Report (August 10, 2018). Available online: https://content.govdelivery.com/accounts/FLFFWCC/bulletins/20510d9 (accessed on 16 August 2023).
- Florida Disaster. Gov. Scott Directs Additional $4 Million for Counties Impacted by Red Tide. 18 September 2018. Available online: https://www.floridadisaster.org/news-media/news/20180918-gov.-scott-directs-additional-$4-million-for-counties-impacted-by-red-tide/ (accessed on 16 August 2023).
- Morgan, K.L.; Larkin, S.L.; Adams, C.M. Public Costs of Florida Red Tides: A Survey of Coastal Managers; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Russ, J.; Zaveri, E.; Desbureaux, S.; Damania, R.; Rodella, A.S. The Impact of Water Quality on GDP Growth: Evidence from around the World. Water Secur. 2022, 17, 100130. [Google Scholar] [CrossRef]
- Learn Science at Scitable. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Available online: https://www.nature.com/scitable/knowledge/library/eutrophication-causes-consequences-and-controls-in-aquatic-102364466/ (accessed on 16 August 2023).
- Saito, M.; Magara, Y.; Wisjnuprapto. Study on Self-Purification Capacity for Organic Pollutants in Stagnant Water. Water Sci. Technol. 2002, 46, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. ISBN 978-3-030-35691-0. [Google Scholar]
- Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K.; Yang, Q. Environmental Chemistry, Toxicity and Health Risk Assessment of Groundwater: Environmental Persistence and Management Strategies. Environ. Res. 2022, 214, 113884. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.U.; Qayoom, U. Implications of Sewage Discharge on Freshwater Ecosystems; China Agricultural University: Beijing, China, 2021. [Google Scholar]
- ScienceDirect Topics. Dissolved Oxygen—An Overview. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/dissolved-oxygen (accessed on 16 August 2023).
- WWF. Water Scarcity|Threats. Available online: https://www.worldwildlife.org/threats/water-scarcity (accessed on 15 August 2023).
- Glanville, K.; Sheldon, F.; Butler, D.; Capon, S. Effects and Significance of Groundwater for Vegetation: A Systematic Review. Sci. Total Environ. 2023, 875, 162577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, T.; Zhou, J.; Cai, W.; Gao, N.; Du, H.; Jiang, L.; Lai, L.; Zheng, Y. Groundwater Depth and Soil Properties Are Associated with Variation in Vegetation of a Desert Riparian Ecosystem in an Arid Area of China. Forests 2018, 9, 34. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; He, X.; He, S. Exploring the Response of Shallow Groundwater to Precipitation in the Northern Piedmont of the Qinling Mountains, China. Urban Clim. 2023, 47, 101379. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Zhang, D.; Chen, X. Environmental Groundwater Depth for Groundwater-Dependent Terrestrial Ecosystems in Arid/Semiarid Regions: A Review. Int. J. Environ. Res. Public Health 2019, 16, 763. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, D.; Chen, X. Vegetation Response to Groundwater Variation in Arid Environments: Visualization of Research Evolution, Synthesis of Response Types, and Estimation of Groundwater Threshold. Int. J. Environ. Res. Public Health 2019, 16, 1849. [Google Scholar] [CrossRef]
- Anandhi, A.; Karunanidhi, D.; Sankar, G.M.; Panda, S.; Kannan, N. A Framework for Sustainable Groundwater Management. Water 2022, 14, 3416. [Google Scholar] [CrossRef]
- Madsen, E.L. Determining in Situ Biodegradation. Environ. Sci. Technol. 1991, 25, 1662–1673. [Google Scholar] [CrossRef]
- ScienceDirect Topics. Wastewater Management—An Overview. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/wastewater-management (accessed on 16 August 2023).
- Talabi, A.O.; Kayode, T.J.; Talabi, A.O.; Kayode, T.J. Groundwater Pollution and Remediation. J. Water Resour. Prot. 2019, 11, 1–19. [Google Scholar] [CrossRef]
- Baier, J.H.; Lykins, B.W.; Fronk, C.A.; Kramer, S.J. Using Reverse Osmosis to Remove Agricultural Chemicals from Groundwater. J. Am. Water Works Assoc. 1987, 79, 55–60. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Badawi, A.K.; Emam, H.E.; Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Kataoka, R. Biodegradability and Biodegradation Pathways of Chlorinated Cyclodiene Insecticides by Soil Fungi. J. Pestic. Sci. 2018, 43, 314. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation Techniques–Classification Based on Site of Application: Principles, Advantages, Limitations and Prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Sankar, K.; Karunanidhi, D.; Kalaivanan, K.; Subramani, T.; Shanthi, D.; Balamurugan, P. Integrated Hydrogeophysical and GIS Based Demarcation of Groundwater Potential and Vulnerability Zones in a Hard Rock and Sedimentary Terrain of Southern India. Chemosphere 2023, 316, 137305. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, P.; Wang, Y.; Du, Q. Integration of Hydrochemistry and Stable Isotopes for Assessing Groundwater Recharge and Evaporation in Pre- and Post-Rainy Seasons in Hua County, China. Nat. Resour. Res. 2023, 32, 1959–1973. [Google Scholar] [CrossRef]
- Sharma, A.; Varandani, P.D.N.S. Ground Water Remediation Technologies. Int. J. Eng. Res. Technol. 2013, 2, 1552–1559. [Google Scholar] [CrossRef]
- Li, P.; Sabarathinam, C.; Elumalai, V. Groundwater Pollution and Its Remediation for Sustainable Water Management. Chemosphere 2023, 329, 138621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).