Use of δ13C and δ15N as Indicators to Evaluate the Influence of Sewage on Organic Matter in the Zhangjiang Mangrove–Estuary Ecosystem, Southeastern China
Abstract
1. Introduction
2. Materials and Methods
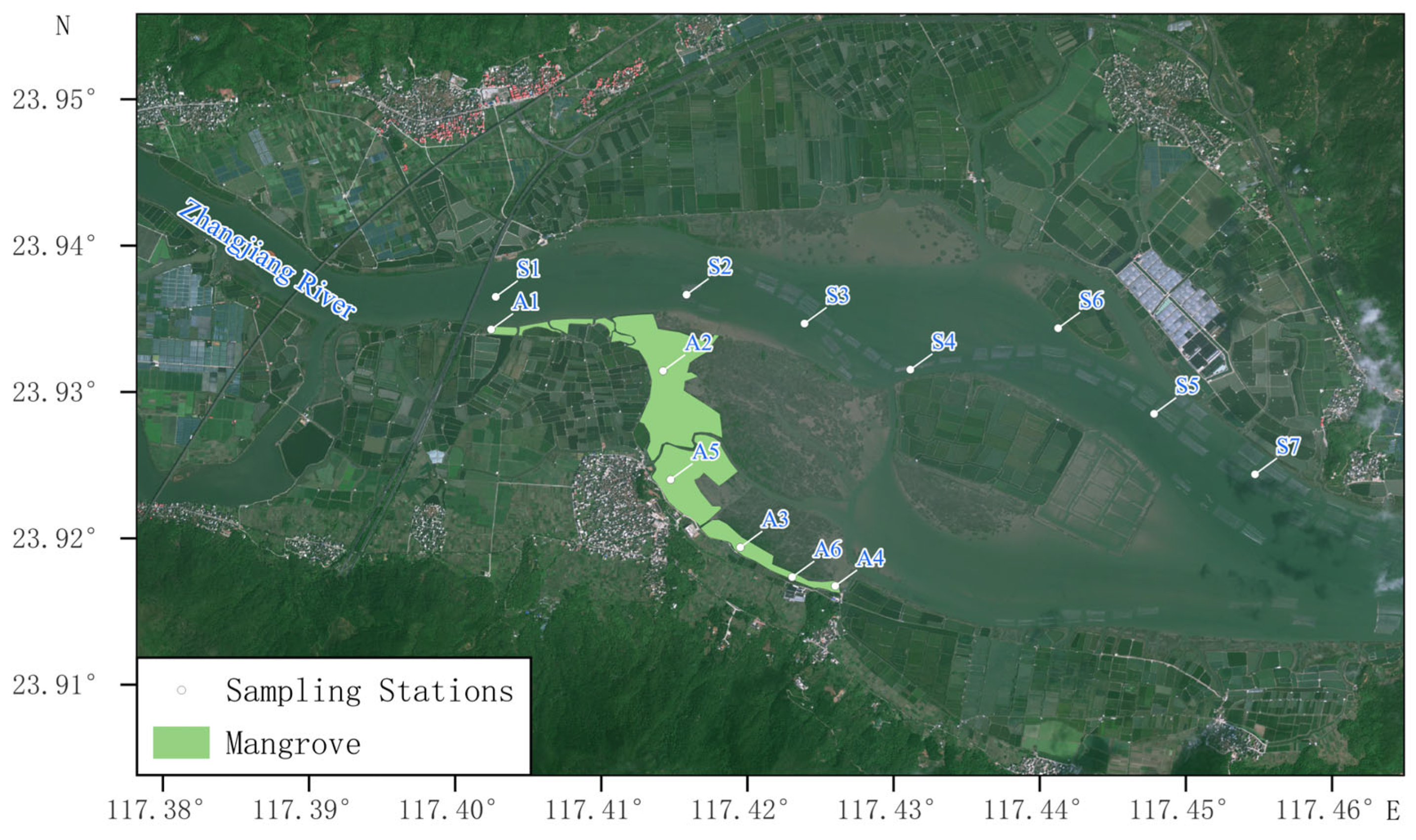
2.1. Sample Collection
2.2. Dissolved Inorganic Nutrients
2.3. Organic Carbon and Nitrogen
2.4. Primary Production
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Nutrients Concentrations
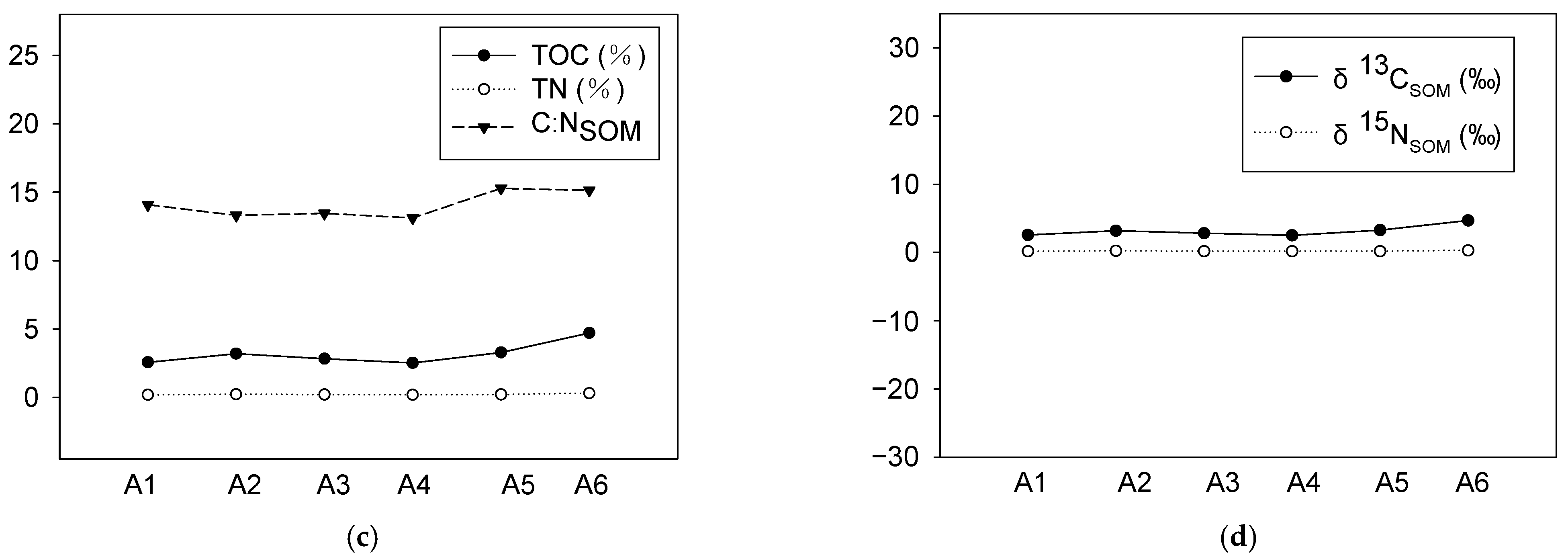
3.3. The Contents and the Isotopic Characters of Organic Matter
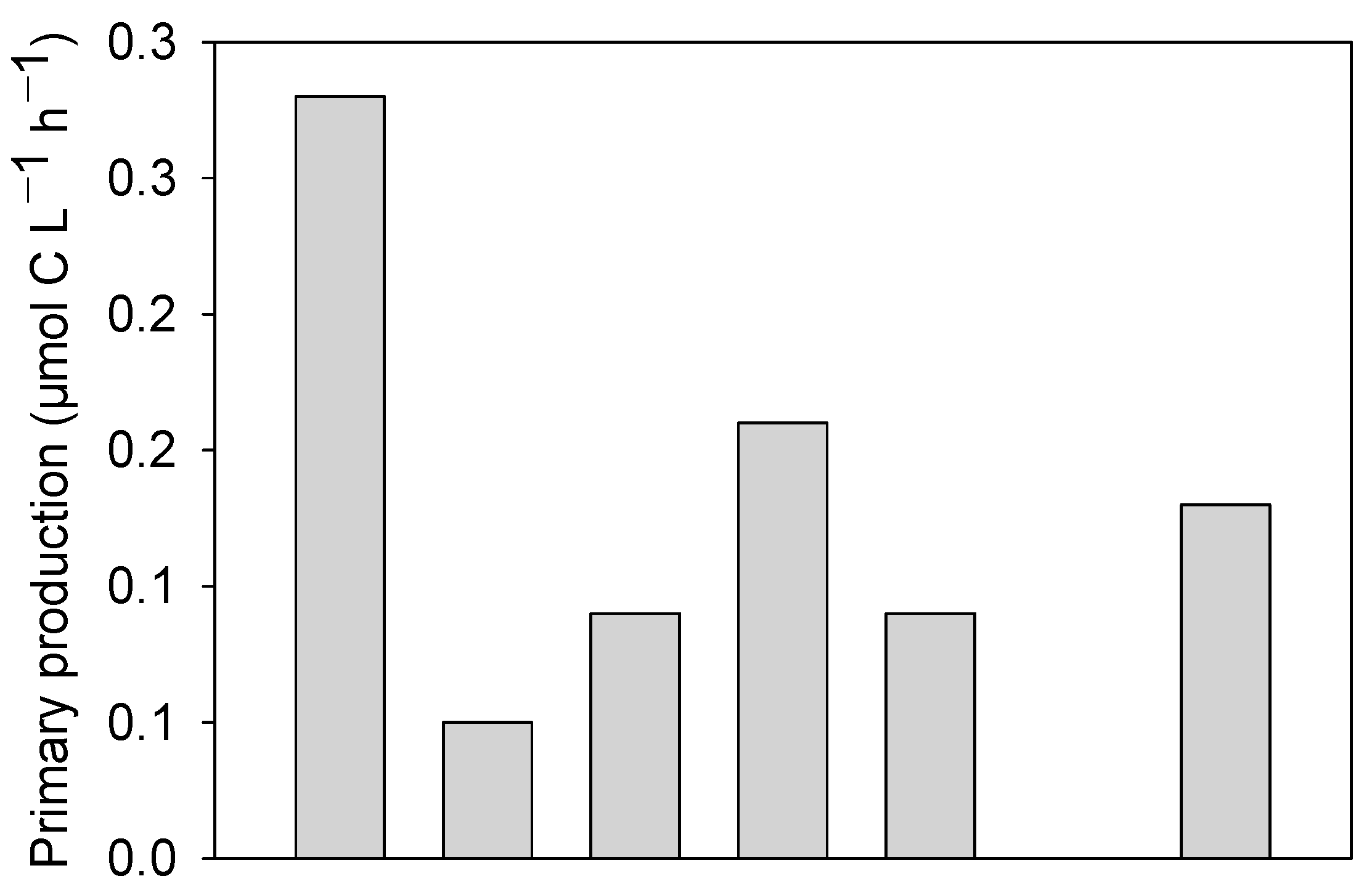
3.4. Primary Production
4. Discussion
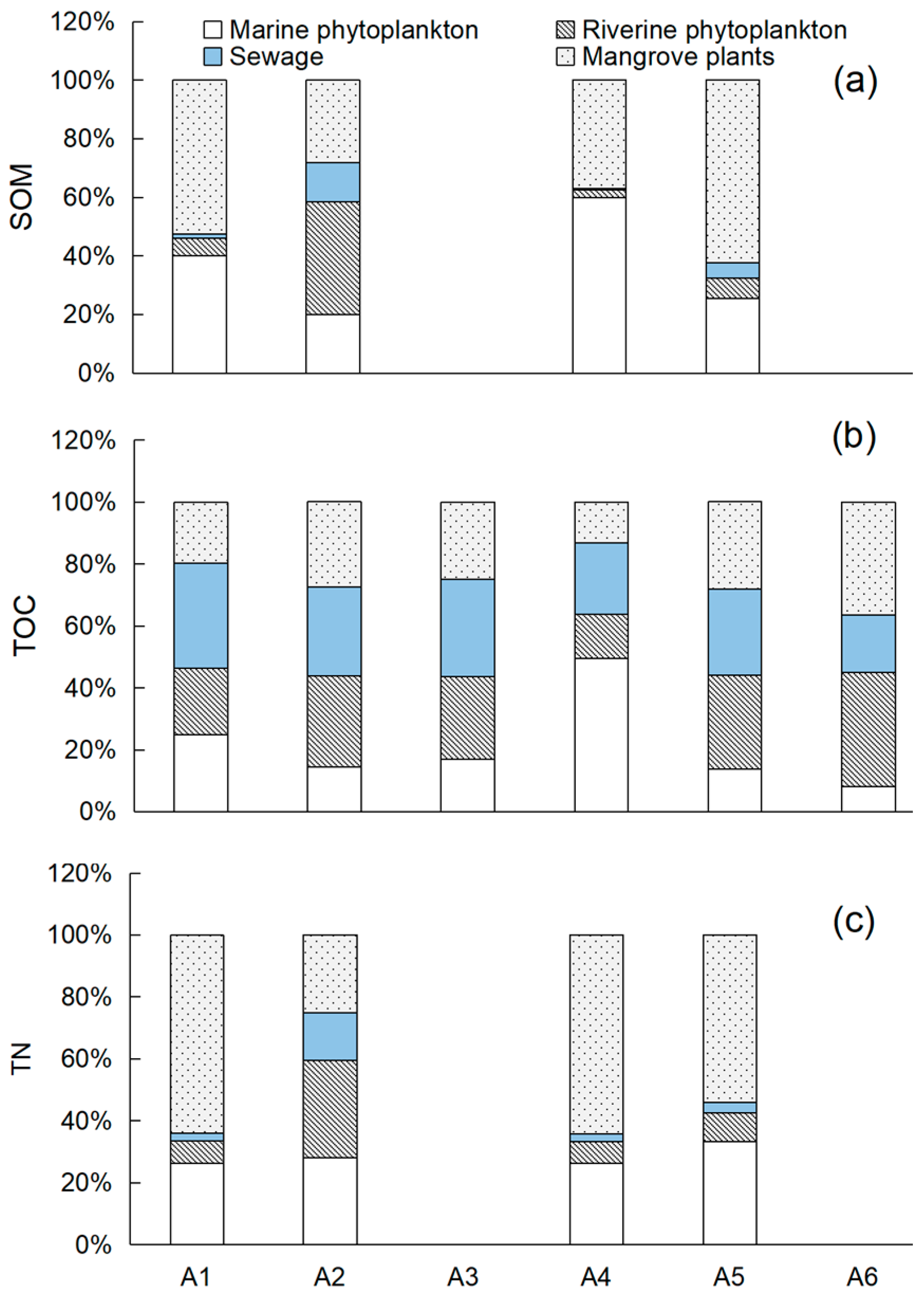
4.1. Contribution of Sewage to Organic Matter
4.2. Potential Effects of Sewage on Organic Matters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Bianchi, T.S. The Role of Terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, J.L.; Bi, X.Y.; Lin, G.H.; Feng, C.C.; Li, Z.J.; Qi, F.; Zheng, T.L.; Xie, L.Q. Trace metals in sediments and benthic animals from aquaculture ponds near a mangrove wetland in Southern China. Mar. Pollut. Bull. 2017, 117, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Peng, R.; Yang, Y.; He, L.; Wang, W.Q.; Zheng, T.L.; Lin, G.H. Mariculture pond influence on mangrove areas in South China: Significantly larger nitrogen and phosphorus loadings from sediment wash-out than from tidal water exchange. Aquaculture 2014, 426–427, 204–212. [Google Scholar] [CrossRef]
- Trott, L.A.; Alongi, D.M. The impact of shrimp pond effluent on water quality and phytoplankton biomass in a tropical mangrove estuary. Mar. Pollut. Bull. 2000, 40, 947–951. [Google Scholar] [CrossRef]
- Howarth, L.M.; Filgueira, R.; Jiang, D.; Koepke, H.; Frame, M.K.; Buchwald, C.; Finnis, S.; Chopin, T.; Grant, J. Using acroalgal bioindicators to map nutrient plums from fish farms and ohter source at a bay-wide scale. Aquac. Env. Interac. 2019, 11, 671–684. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, 7, e43542. [Google Scholar] [CrossRef]
- Reis, C.R.G.; Reed, S.C.; Oliveira, R.S.; Nardoto, G.B. Isotopic evidence that nitrogen enrichment intensifies nitrogen losses to the atmosphere from subtropical mangroves. Ecosystems 2019, 22, 1126–1144. [Google Scholar] [CrossRef]
- Chen, G.C.; Chen, B.; Yu, D.; Tam, N.; Ye, Y.; Chen, S.Y. Soil greenhouse gas emissions reduce the contribution of mangrove plants to the atmospheric cooling effect. Environ. Res. Lett. 2016, 11, 124019. [Google Scholar] [CrossRef]
- Wang, F.F.; Xiao, K.; Santos, I.R.; Lu, Z.Y.; Tamborski, J.; Wang, Y.; Yan, R.F.; Chen, N.W. Porewater exchange drives nutrient cycling and export in a mangrove-salt marsh ecotone. J. Hydrol. 2022, 606, 127401. [Google Scholar] [CrossRef]
- Gu, X.X.; Qiao, P.Y.; Krauss, K.K.; Lovelock, C.E.; Adams, J.B.; Chapman, S.K.; Jennerjahn, T.C.; Lin, Q.L.; Chen, L.Z. Changes in mangrove blue carbon under elevated atmospheric CO2. Ecosyst. Health Sust. 2023, 9, 0033. [Google Scholar] [CrossRef]
- Mckinnon, A.D.; Trott, L.A.; Alongi, D.M.; Davidson, A. water column production and nutrient characteristics in mangrove creeks receiving shrimp farm effluent. Aquac. Res. 2002, 33, 55–73. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Guallar, C.; Flos, J. Linking phytoplankton primary production and chromophoric dissolved organic matter in the sea. Prog. Oceanogr. 2019, 176, 102116. [Google Scholar] [CrossRef]
- Castillo, J.A.A.; Apan, A.A.; Maraseni, T.N.; Salmo, S.G. Soil greenhouse gas fluxes in tropical mangrove forests and in land uses on deforested mangrove lands. Catena 2017, 159, 60–69. [Google Scholar] [CrossRef]
- Ho, D.T.; Ferrón, S.; Engel, V.C.; Larsen, L.G.; Barr, J.G. Air-water gas exchange and CO2 flux in a mangrove-dominated estuary. Geophys. Res. Lett. 2014, 41, 108–113. [Google Scholar] [CrossRef]
- Liu, K.K.; Kao, S.J.; Wen, L.S.; Chen, K.L. Carbon and nitrogen isotopic compositions of particulate organic matter and biogeochemical processes in the eutrophic Danshuei esturary in Northern Taiwan. Sci. Total Environ. 2007, 382, 103–120. [Google Scholar] [CrossRef]
- Yang, B.; Cao, L.; Liu, S.M.; Zhang, G.S. Biogeochemistry of bulk organic matter and biogenic elements in surface sediments of the Yangtze River estuary and Adjacent Sea. Mar. Pollut. Bull. 2015, 96, 471–484. [Google Scholar] [CrossRef]
- Hu, J.F.; Peng, P.A.; Jia, G.D.; Mai, B.X.; Zhang, G. Distribution and sources of organic carbon, nitrogen and their isotopes in sediments of the subtropical Pearl River estuary and the adjacent shelf, Southern China. Mar. Chem. 2006, 98, 274–285. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, D.J.; Lu, H.W.X. Isotope variability of particulate organic matter at the PN section in the East China Sea. Biogeochemistry 2003, 65, 31–49. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Sharp, J.H.; Fogel, M.L. Stable carbon and nitrogen isotope biogeochemistry in the Delaware Estuary. Limnol. Oceanogr. 1988, 33, 1102–1115. [Google Scholar] [CrossRef]
- Granger, J.; Sigman, D.M.; Rohde, M.M.; Maldonado, M.T.; Tortell, P.D. N and O isotope effects during nitrate assimilation by unicellular prokaryotic and eukaryotic plankton cultures. Geochim. Cosmochim. Acta 2010, 74, 1030–1040. [Google Scholar] [CrossRef]
- Andrews, J.E.; Greenaway, A.M.; Dennis, P.F. Combined carbon isotope and C/N ratios as indicators of source and fate of organic matter in a poorly flushed, tropical estuary: Hunts bay, Kingston harbour, Jamaica. Estuar. Coast. Shelf Sci. 1998, 46, 743–756. [Google Scholar] [CrossRef]
- Sweeney, R.E.; Kalil, E.K.; Kaplan, I.R. Characterization of domestic and industrial sewage in Southern California coastal sediments using nitrogen, carbon, sulphur and uranium tracers. Mar. Environ. Res. 1980, 3, 225–243. [Google Scholar] [CrossRef]
- Wada, E.; Minagawa, M.; Mizutani, H.; Tsuji, T.; Karasawa, K. Biogeochemical studies on the transport of organic matter along the Otsuchi river watershed, Japan. Estuar. Coast. Shelf Sci. 1987, 25, 321–336. [Google Scholar] [CrossRef]
- Hama, T.; Miyazaki, T.; Ogawa, Y.; Iwakuma, T.; Takahashi, M.; Otsuki, A.; Ichimura, S. Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 1983, 73, 31–36. [Google Scholar] [CrossRef]
- Gao, Y. Studies on Distribution Patterns of and Controlling Factors for Soil Carbon Pools of Selected Mangrove Wetlands in China. Ph.D. Thesis, Tsinghua University, Beijing, China, 2019. (In Chinese with English Abstract). [Google Scholar]
- Bai, Y. Investigating the Distribution and Sources of Organic Matter in Surface Sediment of Mangrove Wetland in Zhangjiang Estuary (China) Using Elemental, Isotopic and Fatty Acid Biomarkers. Master’s Thesis, Xiamen University, Fujian, China, 2009. (In Chinese with English Abstract). [Google Scholar]
- Wu, G. Organic Carbon Sources and Microbial Carbon Assimilation in Mangrove Ecosystems. Ph.D. Thesis, Xiamen University, Fujian, China, 2018. (In Chinese with English Abstract). [Google Scholar]
- Machiwa, J.F. Spatial zonation of benthic macrofauna and possible effects of sewage discharge on it in Futian mangrove swamp, Shenzhen. Tanz. J. Sci. 2000, 26, 15–28. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Wu, Y.; Jennerjahn, T.C.; Ittekkot, V.; He, Q. Distribution of organic matter in the Changjiang (Yangtze river) estuary and their stable carbon and nitrogen isotopic ratios: Implications for source discrimination and sedimentary dynamics. Mar. Chem. 2007, 106, 111–126. [Google Scholar] [CrossRef]
- Mou, X.Y. The Isotopic Composition of Carbon and Nitrogen in Suspended Particulate Organic Matter in the Daya Bay and the Jiaozhou Bay. Master’s Thesis, Xiamen University, Fujian, China, 2018. (In Chinese with English Abstract). [Google Scholar]
- Sigleo, A.C.; Macko, S.A. Carbon and Nitrogen Isotopes in Suspended Particles and Colloids, Chesapeake and San Francisco Estuaries, U.S.A. Estuar. Coast. Shelf Sci. 2002, 54, 701–711. [Google Scholar] [CrossRef]
- Tucker, J.; Sheats, N.; Giblin, A.E.; Hopkinson, C.; Montoya, J.P. Using stable isotopes to trace sewage derived material through Boston harbor and Massachusetts bay. Mar. Environ. Res. 1999, 48, 353–375. [Google Scholar] [CrossRef]
- Ostrom, N.E.; Macko, S.A.; Deibel, D.; Thompson, R.J. Seasonal variation in the stable carbon and nitrogen isotope biogeochemistry of a coastal cold ocean environment. Geochim. Cosmochim. Acta 1997, 61, 2929–2942. [Google Scholar] [CrossRef]
- Mcclelland, J.W.; Valiela, I. Linking nitrogen in estuarine producers to land derived sources. Limnol. Oceanogr. 1998, 43, 577–585. [Google Scholar] [CrossRef]
- Lehmann, M.F.; Bernasconi, S.M.; Barbieri, A.; McKenzie, J.A. Preservation of organic Matter and alteration of its carbon and nitrogen isotope composition during simulated and in Situ early sedimentary diagenesis. Geochim. Cosmochim. Acta 2002, 66, 3573–3584. [Google Scholar] [CrossRef]
- Wang, X.C.; Ma, H.Q.; Li, R.H.; Song, Z.S.; Wu, J.P. Seasonal fluxes and source variation of organic carbon transported by two major Chinese rivers: The Yellow river and Changjiang (Yangtze) river. Glob. Biogeochem. Cycles 2012, 26, GB2025.1–10. [Google Scholar] [CrossRef]
- Yu, F.L.; Zong, Y.Q.; Lloyd, J.M.; Huang, G.Q.; Leng, M.J.; Kendrick, C.; Lamb, A.L.; Yim, W.W. Bulk organic δ13C and C/N as indicators for sediment sources in the Pearl river delta and estuary, Southern China. Estuar. Coast. Shelf Sci. 2010, 87, 618–630. [Google Scholar] [CrossRef]
- Cai, D.L.; Tan, F.C.; Edmond, J.M. Sources and transport of particulate organic carbon in the Amazon river and estuary. Estuar. Coast. Shelf Sci. 1988, 26, 1–14. [Google Scholar] [CrossRef]
- Mortillaro, J.M.; Abril, G.; Moreira-Turcq, P.; Sobrinho, R.L.; Perez, M.; Meziane, T. Fatty acid and stable isotope (δ13C, δ15N) signatures of particulate organic matter in the lower Amazon river: Seasonal contrasts and connectivity between Floodplain lakes and the mainstem. Org. Geochem. 2011, 42, 1159–1168. [Google Scholar] [CrossRef]
- Hamilton, S.K.; Lewis, W.M., Jr. Stable carbon and nitrogen isotopes in algae and detritus from the Orinoco river floodplain, Venezuela. Geochim. Cosmochim. Acta 1992, 56, 4237–4246. [Google Scholar] [CrossRef]
- Kuramoto, T.; Minagawa, M. Stable Carbon and nitrogen isotopic characterization of organic matter in a mangrove ecosystem on the Southwestern coast of Thailand. J. Oceanogr. 2001, 57, 421–431. [Google Scholar] [CrossRef]
- Bristow, L.A.; Jickells, T.D.; Weston, K.; Marca, A.D.; Parker, E.R.; Andrews, J.E. Tracing estuarine organic matter sources into the Southern North sea using C and N isotopic signatures. Biogeochemistry 2013, 113, 9–22. [Google Scholar] [CrossRef]
- Benner, R.; Fogel, M.L.; Sprague, E.K.; Hodson, R.E. Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 1987, 329, 708–710. [Google Scholar] [CrossRef]
- Boschker, H.T.; Moerdijk, T.C.; Breugel, P.V.; Houtekamer, M.; Middelburg, J.J. A versatile method for stable carbon isotope analysis of carbohydrates by high performance liquid chromatography and isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Mccallister, S.L.; Bauer, J.E.; Ducklow, H.W.; Canuel, E.A. Sources of estuarine dissolved and particulate organic matter: A multi-tracer approach. Org. Geochem. 2006, 37, 454–468. [Google Scholar] [CrossRef]
- Qian, Y.; Ii, M.C.K.; Svalberg, J.; Walker, J. Suspended particulate organic matter (SPOM) in gulf of Mexico estuaries: Compound specific isotope analysis and plant pigment compositions. Org. Geochem. 1996, 24, 875–888. [Google Scholar] [CrossRef]
- Thornton, S.F.; McManus, J. Application of organic carbon and nitrogen stable isotope and C/N ratios as source indicators of organic matter provenance in estuarine systems evidence from the Tay estuary, Scotland. Estuar. Coast. Shelf. Sci. 1994, 38, 219–233. [Google Scholar] [CrossRef]
- Debruyn, A.M.H.; Rasmussen, J.B. Quantifying assimilation of sewage derived organic matter by riverine benthos. Ecol. Appl. 2002, 12, 511–520. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef]
- Green, P.A.; Vörösmarty, C.J.; Meybeck, M.; Galloway, J.N.; Peterson, B.J.; Boyer, E.W. Preindustrial and contemporary fluxes of nitrogen through rivers: A global assessment based on typology. Biogeochemistry 2004, 68, 71–105. [Google Scholar] [CrossRef]
- Neumann, B.; Vafeidis, A.T.; Zimmermann, J.; Nicholls, R.J. Future coastal population growth and exposure to sea level rise and coastal flooding: A global assessment. PLoS ONE 2015, 10, e0118571. [Google Scholar] [CrossRef]
- Chen, C.P.; Gao, Y.H.; Lin, P. Seasonal change of phytoplankton community in waters of mangrove in the estuarine of the Zhangjiang river, Fujiang Province, China. Mar. Sci. 2007, 31, 25–31. [Google Scholar] [CrossRef]
- Altabet, M.A.; Francois, R. Nitrogen isotope biogeochemistry of the Antarctic Polar Frontal Zone at 170°W. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 4247–4273. [Google Scholar] [CrossRef]
- DiFiore, P.J.; Sigman, D.M.; Karsh, K.L.; Trull, T.W.; Dunbar, R.B.; Robinson, R.S. Poleward decrease in the isotope effect of nitrate assimilation across the Southern Ocean. Geophys. Res. Lett. 2010, 37, L17601. [Google Scholar] [CrossRef]




| Station | T (°C) | S | DO (mg L−1) | Phycoerythrin (μg L−1) | Chl a (μg L−1) |
|---|---|---|---|---|---|
| S1 | 17.7 | 20.0 | 5.81 | 4.94 | 4.03 |
| S2 | 17.6 | 20.3 | 6.04 | 3.27 | 3.40 |
| S3 | 17.5 | 19.1 | 6.43 | 3.85 | 3.31 |
| S4 | 17.5 | 20.4 | 6.08 | 2.19 | 2.48 |
| S5 | 17.6 | 20.5 | 6.30 | 1.71 | 2.51 |
| S6 | 18.0 | 20.3 | 7.79 | 1.75 | 1.76 |
| S7 | 17.6 | 22.3 | 6.51 | 0.699 | 1.96 |
| Station | NO3− | NO2− | NH4+ | PO43− | SiO32− |
|---|---|---|---|---|---|
| S1 | 73.8 | 0.118 | 621.7 | 3.34 | 98.6 |
| S2 | 61.0 | 0.112 | 1067.0 | 2.91 | 89.9 |
| S3 | 65.4 | 0.112 | 1427 | 3.73 | 101.5 |
| S4 | 61.3 | 0.109 | 1899 | 3.03 | 92.4 |
| S5 | 55.9 | 0.107 | 895.3 | 3.07 | 89.5 |
| S6 | 51.8 | 0.0986 | 605.6 | 2.99 | 77.4 |
| S7 | 51.0 | 0.0993 | 836.3 | 3.22 | 80.3 |
| Research Areas | δ13C (‰) | δ15N (‰) | References |
|---|---|---|---|
| Yunxiao mangrove sediments | −28.6–−23.2 | 6.3–10.7 | This study |
| Yunxiao mangrove sediments | −25.5–−21.6 | 4.7–7.8 | [27,28] |
| Gaoqiao mangrove sediments | −26.68 ± 0.38 | - | |
| Dongzhai harbor mangrove sediments | −24.87 ± 1.76 | - | |
| Wenchang mangrove sediments | −26.29 ± 1.02 | - | |
| Jiulong River estuary mangrove sediments | −28.2–−23.0 | 2.4–11.2 | [29] |
| Futian mangrove sediments | −28.0 ± 0.3 | - | [30] |
| Research Areas | δ13C (‰) | δ15N (‰) | References |
|---|---|---|---|
| Zhangjiang esturay | −27.1~−24.1 | 1.9 ~ 4.1 | This study |
| Danshuei estuary | −25.5~−19.0 | −16.4~3.8 | [17] |
| Yangtze river | −29.2~−25.8 | 2.4~7.0 | [31] |
| Daya bay | −25.7~−16.9 | −6.3~10.4 | [32] |
| Jiaozhou bay | −28.6~−21.2 | −3.1~13.2 | [33] |
| Potomac river | −27.3~−18.4 | 3.1~11.6 | |
| Chesapeake bay | −26.9~−17.5 | 5.0~9.5 | |
| San Francisco bay | −26.4~−26.0 | 0.7~1.0 | |
| Boston harbor | −26.9~−17.5 | 5.0~9.5 | [34] |
| Delaware estuary | −23.1~−18.7 | 5.5~12.2 | [21] |
| Conception bay | −27.2~−21.6 | 3.7~8.4 | [35] |
| Traverse bay | −30.7~−23.9 | 1.7~11.6 | [36] |
| Lugao bay | −29.9~−26.9 | 4.7~7.0 | [37] |
| Yellow river | −25.6~−23.1 | - | [38] |
| Pearl river | −25.6~−24.3 | - | [39] |
| Amazon river | −28.4~−17.5 | 3.6 ± 1.5 | [40,41] |
| Chl a (μg L−1) | NO3− (μmol L−1) | |
|---|---|---|
| POCriverine | r = 0.771 p < 0.01 n = 7 | r = 0.896 p < 0.01 n = 7 |
| δ13CPOM | r = −0.802 p < 0.05 n = 7 | r = −0.833 p < 0.05 n = 7 |
| δ15NPOM | r = −0.835 p < 0.05 n = 7 | r = −0.810 p < 0.05 n = 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yan, J.; Lu, Z.; Chu, T.; Li, J.; Chu, T. Use of δ13C and δ15N as Indicators to Evaluate the Influence of Sewage on Organic Matter in the Zhangjiang Mangrove–Estuary Ecosystem, Southeastern China. Water 2023, 15, 3660. https://doi.org/10.3390/w15203660
Li D, Yan J, Lu Z, Chu T, Li J, Chu T. Use of δ13C and δ15N as Indicators to Evaluate the Influence of Sewage on Organic Matter in the Zhangjiang Mangrove–Estuary Ecosystem, Southeastern China. Water. 2023; 15(20):3660. https://doi.org/10.3390/w15203660
Chicago/Turabian StyleLi, Danyang, Jinpei Yan, Zhiqiang Lu, Tianshu Chu, Jun Li, and Tajen Chu. 2023. "Use of δ13C and δ15N as Indicators to Evaluate the Influence of Sewage on Organic Matter in the Zhangjiang Mangrove–Estuary Ecosystem, Southeastern China" Water 15, no. 20: 3660. https://doi.org/10.3390/w15203660
APA StyleLi, D., Yan, J., Lu, Z., Chu, T., Li, J., & Chu, T. (2023). Use of δ13C and δ15N as Indicators to Evaluate the Influence of Sewage on Organic Matter in the Zhangjiang Mangrove–Estuary Ecosystem, Southeastern China. Water, 15(20), 3660. https://doi.org/10.3390/w15203660








