Abstract
Springs provide ideal monitoring points for groundwater chemistry, which are important for managing groundwater resources. The chemistry of these spring waters aggregate geochemical reactions along the flow path. In this paper, part two of a two-part investigation, 104 perennial springs in the classic karst landscape of the Mitchell Plateau, Indiana, USA were sampled at base flow. Many of these springs are historically important for domestic, agricultural, commercial, and recreational use. Multifactor analysis of field measurements, principal ions, and stable isotopes revealed five primary clusters of springs emerging from the Mitchell Aquifer. Two clusters represented earth-alkaline-type karst groundwater that were discriminated by temperature and inorganic carbon concentration. Two other clusters comprised mineralized alkaline-earth-type groundwater with excess alkalis and elevated sulfate. The fifth cluster appeared to be groundwater that included meteoric and mineralized sources. Using the longitudinal data over two years from part one of this investigation, two mixing lines were used to describe the data set. The mixing lines pointed to sources of sulfur in mineralized springs from deep brines and from evaporite dissolution. Collectively, these regional data allow for a better delineation of water types and differentiation between the Upper and Lower Mitchell Aquifer.
1. Introduction
Groundwater springs play a vital role in the development of societies and have captured the minds of explorers, artists, and scientists. In karst regions, springs are sometimes the sole water source in an otherwise water-scarce landscape [1] and the access to and quality of that karst groundwater has helped guide the vectors of wildlife migration, human settlement, and water utilization. In fact, the classic sand column water filtration experiments of Henry Darcy relied on groundwater piped through an aqueduct from the Rosoir Spring in the karst massif northwest of the city of Dijon, France [2].
The water chemistry of karst springs amalgamates the complex biogeochemical processes occurring in their contributing basin [3]. Where contributing conduit systems to a karst spring remain unmapped, the chemical signature manifests as an output from an otherwise “black box” of information, where input signals are modulated [4]. Various methodologies can deconvolve the transformations along the flow path [5,6]. For example, the hydrodynamics are guided primarily by hydrograph analysis [7]. The geochemistry, however, involves more complex interrelationships between contributing end members of water and the reaction kinetics along the flow path between those waters and the intervening media [8,9].
This paper is the second of a two-part investigation. In the first part [10], two years of groundwater geochemistry data from two karst basins in the Mitchell Plateau of south-central Indiana, USA revealed that cave development, and therefore carbonate aquifer evolution, is polygenetic, embracing aspects of both carbonic and sulfuric acid speleogenesis. In this second paper, we leverage a regional data set of water chemistry from 104 groundwater springs in this same classic karst terrain to build upon these conclusions, reveal the nature of the end-member sources of water that contribute to spring chemistry, and highlight the importance of the geologic framework to the nature of water quality.
2. Prior Work
European colonists in North America followed rivers westward and encountered vast lowland plateaus west of the Appalachian Mountains. Here, wildlife trackways, or “traces”, connected these rivers to groundwater springs. Most of these springs were of fresh groundwater emerging from karst aquifers embedded in Paleozoic carbonates. A smaller subset, termed “licks”, were seeps of mineral-rich groundwater that provided essential salts. Both the fresh and mineral springs became economically important resources as homesteads and towns were established during the 18th and 19th centuries. Freshwater springs became municipal water supplies. Mineral springs hosted important hotels and spas; waters were bottled and widely exported as medicinal supplements [11], reaching a peak around the end of the 18th century.
Concurrent with that peak in mineral spring utilization, Oscar Meinzer traveled the nation for the U.S. Geological Survey and recorded the hydrochemical characteristics of the largest known springs in the United States at the time [12]. This followed other, more regional studies, such as those by Willis Blatchley, who detailed the discharge and chemistry of both fresh and mineral springs in Indiana [13]. Blatchley focused on the Mitchell Plateau and Crawford Upland physiographic regions of south-central Indiana [14] where Mississippian carbonates outcrop south of the glacial limit (Figure 1). These “Indiana Uplands” remain relatively rural to this day, a consequence of, in part, the more challenging landscape and the overall lack of available surface water or productive wells to serve industry and large-scale agriculture.
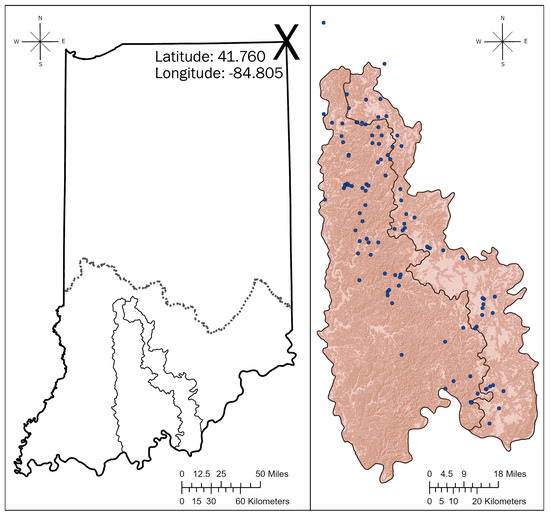
Figure 1.
(Left panel) is a map of Indiana, USA. The latitude and longitude of the northeast corner of the state (the large X) is given in a WGS 1984 datum. The southern limit of the Wisconsin episode of Quaternary glaciation is shown as a dotted line. The two irregular polygons shown south of the glacial limit are the Mitchell Plateau and Crawford Uplands physiographic regions to the right and left, respectively. The (right panel) is a map of the Mitchell Plateau and Crawford Uplands physiographic regions, with spring locations from this study as blue dots overlain on the topography.
Earlier, in 1837, the state geological survey was established in Indiana and David Dale Owen was appointed as the first state geologist. Among his first reports to the state legislature were discussions of the cave-rich landscape from Bloomington in the north to the Ohio River in the south, including notes on the Lost River that disappeared underground, only to rise again after 37 km of dry riverbed [15].
The work of Blatchley on spring chemistry and Owen on geology served as a foundation for more than a century of investigation of karst in Indiana, including pivotal studies on karst landscapes [16]; cave surveys [17]; cave geomorphology [18]; dye tracing and hydraulics [19]; and geochemistry [20,21,22,23]. Over the past few decades, the focus of research has shifted toward the effects of highway infrastructure [24], land use [23], and flooding [25,26]. A review of the state of Indiana karst science was published as part of a tribute to the careers of Art Palmer and Dick Powell at the Geological Society of America annual meeting held in 2018 in Indianapolis, Indiana [27].
Since that meeting, the Indiana Geological and Water Survey (IGWS), with funding from the Center for Rural Engagement, also at Indiana University, undertook a pair of studies in the karst aquifers of south-central Indiana. The first [28] involved two years of biweekly water sampling and monitoring in the Bluespring Caverns and Lost River karst basins during 2019 and 2020—the topic of this paper’s companion piece [10]. The results of that study extend the work of Palmer [18], who made hydraulic arguments to interpret speleogenesis, and Krothe and Libra [21], who made geochemical arguments to interpret hydrogeology, by using geochemical arguments to interpret speleogenesis. In short, using dissolved ions and δ34S, Burgess found that the bulk of measured sulfate load in terminal springs from adjacent karst basins has disparate origins. At Orangeville Rise in the Lost River karst basin, the sulfate is sourced to evaporites in the lower Blue River group carbonates (Figure 2). In the adjacent Bluespring Caverns, significant sulfate comes from oxidizing sulfides entrained in petroleum-associated brines upwelling into the cave through fractures [10].
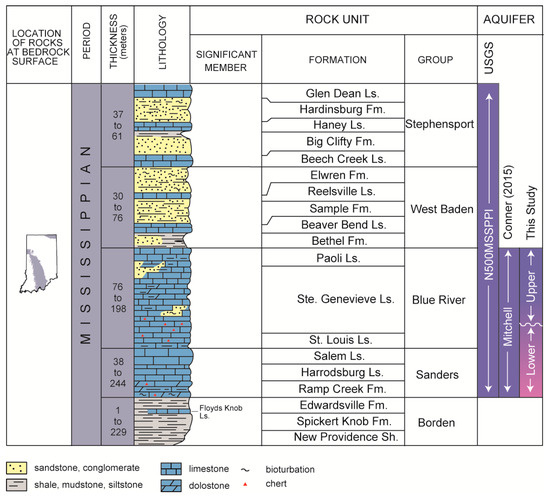
Figure 2.
Stratigraphic column showing Mississippian stratigraphic units in Southern Indiana and principal bedrock aquifer systems after [29]. Aquifer designations are identified in the left column and include the USGS principal bedrock aquifer system [30], the Mitchell Aquifer [31], and the subdivisions of the Mitchell Aquifer as presented in this study.
The purpose of the second study, which is the focus of this paper, was to inventory and characterize perennial springs throughout the Indiana Uplands. During the fall of 2019 and 2020, 104 springs (Figure 1), including Orangeville Rise and Bluespring Caverns, were sampled in base flow conditions to better understand the relationships among geology, land use, spring type, and resultant water quality and quantity. This manuscript uses a subset of those data to contextualize how this snapshot of spring water chemistry aligns with time-series data sets from Burgess et al. [10] and displays geochemical phenomena that are regional, if not broader, in scope.
3. Regional Groundwater Aquifers
Indiana is centered in a temperate midlatitude climate where plentiful rainfall drains into tributaries of the Wabash and Ohio Rivers. The physiography of Indiana was shaped by periodic glacial events that drove cycles of erosion and sedimentation. Glaciers cut, filled, and formed terraces in major outwash channels and deeply incised modern river valleys in the nonglaciated bedrock uplands. Climate change is altering regional conditions; the duration and intensity of rain and drought have increased and average annual precipitation in the region has increased by 14 cm since 1895 [32]. These changes pose significant challenges to residents through well-documented increases in flooding hazards [26,33] and exports of nutrients and topsoil [34].
The groundwater environment of Indiana is equally complex and guided by poorly understood surface–subsurface interactions. The success of agricultural and manufacturing industries in Indiana has been, in part, enabled by accessible groundwater in the glacial and alluvial aquifers that dominate the central and northern tiers of the state. Large-scale farms irrigate crops from high-capacity wells that intersect glacial sand and gravel sediments with high transmissivity [35]. These farms often use tile drains to route shallow groundwater to natural or constructed waterways. The intense management of the landscape drives a drawdown of the water table, among other lasting impacts on the critical zone [36].
The nonglaciated uplands include the outcrop for, principal recharge to, and active circulation zone of regional sedimentary bedrock aquifers. The bedrock units that host these aquifers parallel the fringe of and dip toward the Illinois structural basin (Figure 1). These bedrock aquifers are less heterogeneous, and on average less permeable, than glacial or alluvial aquifers. As a result, water availability is either “abundant or absent” [37], which limits economic development in Southern Indiana. Importantly, bedrock aquifers in Indiana are largely uncharacterized. At the level of the U.S. Geological Survey (USGS), regional aquifer assessment reports (RASA, 1978–1992) divided the bedrock aquifers according to systems [30]. Currently, in Indiana, the only two bedrock aquifers identified at the national level are the Silurian-Devonian (N400SLRDVN) and Mississippian aquifers (N500MSSPPI, Figure 2). At the level of the Indiana Department of Natural Resources (IDNR), bedrock aquifers are associated with stratigraphic groups that are mapped at the 1:250k scale by the IGWS. These boundaries have not been delineated with respect to water quality or productivity because of a lack of detailed mapping, sampling, and monitoring. Despite known karst terrain in both the Silurian-Devonian and Mississippian bedrock aquifer systems in Indiana [27], limited consideration is given to the influence of sinkholes, caves, and springs on groundwater basin boundaries, except where specific tracing has linked locations of dye injection to sites of dye recovery [38].
The Mitchell Aquifer
The lack of detailed aquifer mapping hampers an understanding of Indiana’s groundwater resources. For example, the Mississippian bedrock aquifer is a carbonate system with internal geochemical variation. Gradients of dissolved sulfur are one window into that variation [10]. More broadly, that variation includes shallow circulating groundwater [27] in the zone of outcrop that separates groundwater linked to meteoric flushing from deeper, saline, and nonpotable groundwater; [31] introduces this pairing as the Mitchell Aquifer (Figure 2). Furthermore, away from the influence of incised rivers, anecdotal evidence points toward meteoric waters circulating in the upper Blue River Group and more mineralized waters present in the Sanders Group and the lower Blue River Group. It appears that the confining qualities of the Lost River Chert in the lower Blue River Group influence the demarcation between water quality in the region of outcrop (Figure 2). We introduce that demarcation as an Upper Mitchell Aquifer of relatively fresh water that is shallower and focused toward updip settings and a Lower Mitchell Aquifer that is deeper, somewhat-to-highly mineralized, and focused toward downdip settings (Figure 2). We posit, a. priori, that the boundary between these paired aquifers is irregular sensu analogous work on the mineralized groundwater surface in Kentucky [39]. Following work in Southwest Indiana [40], the boundary will include salinity anomalies and salinity reversals or inversions related to meteoric flushing or upwelling brines identified elsewhere in the Illinois Basin [41]. The nature of those brines was germane to the focused study of the companion paper [10] and are a central theme of this paper. Monitoring springs emerging from karst basins can help partition the Mississippian bedrock aquifer system into these two functional units.
4. Methods
We selected perennial springs for this study based on historical records indicating geological, environmental, and/or commercial importance. Permission for access and sampling was obtained by the appropriate entity, including private landowners, not-for-profit organizations, state parks and preserves (IDNR), the Hoosier National Forest (U.S. Department of Agriculture), and the Crane Naval Warfare Center (U.S. Department of Defense). The springs were sampled during base flow conditions in the dry seasons (August through November) of 2020 and 2021. In total, 104 springs were sampled, 33 in 2019 and 71 in 2020, with 102 of the springs in the Mitchell Plateau and Crawford Uplands physiographic regions (Figure 1). The two northernmost springs, Porter Cave and Boone-Hutcheson Cave, discharged from small karst basins with a thin mantle of glacial sediments, but also hosted in Mississippian carbonates.
Inventory forms were completed for each spring, and discharge was estimated where possible. Field measurements of water temperature (T), specific conductance (SpC), dissolved oxygen (DO), and pH were collected using a calibrated YSI ProDSS handheld water quality sonde. Water samples were collected for laboratory measurements using a peristaltic pump and a stainless-steel tripod filtration unit housing 0.45-micron cellulose nitrate filters. The sample tubing and filtration system was flushed with deionized water between sampling sites and conditioned for the next sample by running the spring water through the filtration unit containing a new filter. Aliquots were collected in HDPE bottles. Aliquots for metals were preserved with concentrated nitric acid to a pH of <2. All samples were transported to the lab on ice and kept in cold storage until analysis.
In the lab, the samples were analyzed for alkalinity and ion chemistry. Quality assurance and quality control were conducted in accordance with accepted protocols including instrument calibrations before fieldwork and normalizing laboratory measurements to established standards. In the IGWS laboratory, end-point titrations for carbonate ([CO32−]) and bicarbonate ([HCO3−]) alkalinity were conducted using a Radiometer autotitrator with a H2SO4 titrant. The IGWS laboratory measured nutrient concentrations using a Hach DR2700 spectrophotometer with methods and detection limits in Table 1. The Indiana State Department of Health measured aliquots for cations, anions, and trace metals using inductively coupled plasma–optical emission spectroscopy (ICP-OES), ICP mass spectrometry (ICP-MS), and ion chromatography using methods and detection limits for drinking water (Table 1).

Table 1.
Methods, detection limits, and error estimates for water analyses in this study.
5. Results
Inventory data, field measurements, and geochemical results are available on a public-facing web service [42]. Charge balances for individual samples are available on this web service—maximum errors were 23.4% and −12.5% with an average of 4.6% and 96 samples having <10% error. Multifactor cluster analysis using the XLSTAT macro in Microsoft Excel and principal component analysis (PCA) using XLSTAT revealed five independent groupings of data for a subset of all parameters, Clusters 1–5, differentiated according to the first two eigenvalues, Factor 1 and Factor 2 (Table 2, Figure 3). Factor 1 incorporates significant positive loadings of SpC, [SO42−], [F−], [Cl−], [Ca2+], [Mg2+], [K+], [Na+], and [Sr2+], and negative loadings of DO and computed Eh. Factor 2 incorporates loadings of T and alkalinity.

Table 2.
PCA factor loadings.
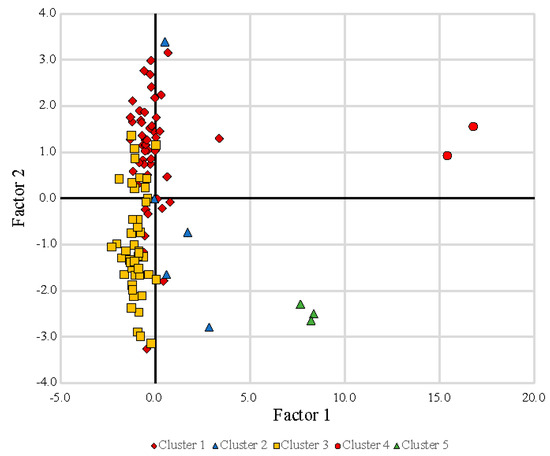
Figure 3.
Scatterplot showing factor loadings from principal component analysis of spring geochemistry data (Table 1) with sites subdivided into clusters identified using XLSTAT in Microsoft Excel.
Clusters 1 (49 springs) and 3 (45 springs) divided with considerable overlap into the Mitchell Plateau and Crawford Uplands, respectively (Figure 4); they divided mostly on the basis of T and [HCO3−]. On Piper (Figure 5) and Durov (Figure 6) diagrams, neither Clusters 1 or 3 appear as independent populations; they are functionally alkaline earth waters dominant in Ca2+, Mg2+, and HCO3− with an SpC of <1000 μS/cm and pH values that range between 6.8 and 8.4.
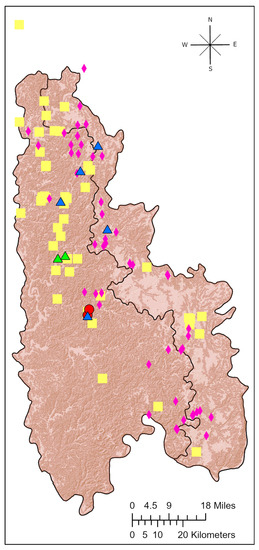
Figure 4.
Map of the Mitchell Plateau (right) and Crawford Uplands (left) physiographic regions with spring locations from this study shown according to their cluster and overlain on the topography. Cluster 1 springs are magenta diamonds. Cluster 2 springs are blue triangles. Cluster 3 springs are yellow squares. Cluster 4 springs are red circles. Cluster 5 springs are green triangles.
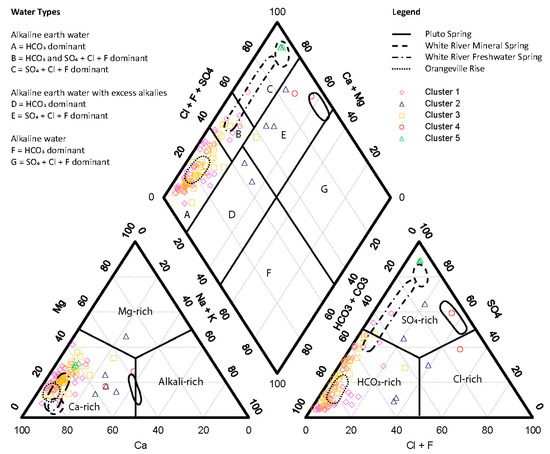
Figure 5.
Piper diagram of spring data divided into clusters with previously identified areas of data shown in [21]. The axes of the ternary diagrams are the proportional percentage of each component to the cations (lower left) and anions (lower right) in solution. Piper plot constructed using the USGS groundwater software package, Version 1.30 [43] with fields modified from [44].
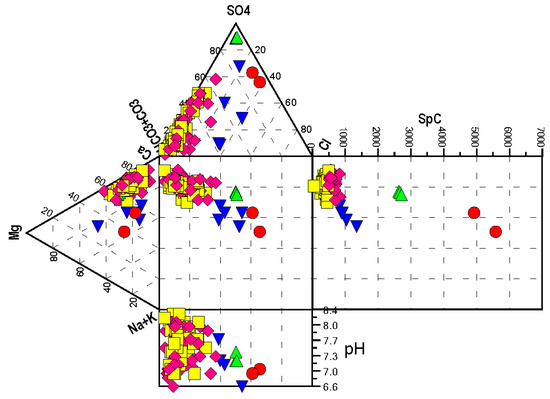
Figure 6.
Durov diagram of spring data divided into clusters. Cluster 1 springs are magenta diamonds. Cluster 2 springs are blue triangles. Cluster 3 springs are yellow squares. Cluster 4 springs are red circles. Cluster 5 springs are green triangles. The axes of the ternary diagrams are the proportional percentage of each component to the cations (left) and anions (top) in solution. The field-measured pH of the spring water is shown at the bottom. The field-measured specific conductance (SpC) of the spring water is shown at (right) in mS/cm.
Clusters 4 (two springs) and 5 (three springs), are distinctly different from Clusters 1 and 3 and are the mineral-rich springs of the inventory in the Crawford Uplands. They all share enriched [SO42−] on the Piper and Durov diagrams (Figure 5 and Figure 6), aligning well with earlier study [21]. These two clusters have strong PCA loading on factor 1; however, they have opposing PCA loading on factor 2 (Figure 3). Cluster 4 consists of the mineral springs at French Lick. They are alkaline earth waters with excess alkalines having a high SpC (>4500 μS/cm) and pH values below 7. Cluster 5 consists of mineral springs north of French Lick along the White River. They are alkaline earth waters with SpC clustered at 2600 μS/cm and pH values between 7.1 and 7.4. Cluster 2, comprising five springs in both the Mitchell Plateau and the Crawford Uplands, are alkaline earth waters with excess alkalines (Figure 5) and combine the characteristics of both fresh and mineral springs with somewhat-elevated SpC (Figure 6).
6. Discussion
The geochemistry of regional aquifers is guided by water–rock interactions that follow geospatial patterns. Analogous carbonate aquifers in the U.S. include the Madison Aquifer of the northern Great Plains [45], the Edwards Aquifer of Texas [46], and the Floridan aquifer of the southeast coastal plain [47]. Each of these includes meteoric and mineralized components in a karst-rich setting. In the Madison Aquifer, montane recharge from outcrops in the Black Hills and Rocky Mountains injects a plume of fresh water that becomes longitudinally mineralized toward sedimentary basins, such as the Williston Basin [48]. In the Edwards Aquifer, rivers and streams in the Texas Hill Country provide recharge along the Balcones Escarpment into down-stepping fault blocks where a wedge of largely fresh water overlies or is upgradient of a mineralized zone at depth or where the Edwards is confined [49]. In the Floridan aquifer, an upper lens of freshwater resides atop a mineralized lower component [47]. The size and scope of this freshwater lens have changed repeatedly during Quaternary sea-level eustacy [50].
In each example, the composition and position of the mineralized-meteoric water interface directs carbonate diagenesis. In the Madison Aquifer, Late-Paleozoic dissolution of anhydrite and Cenozoic water table fluctuations influenced the development of secondary porosity in the Black Hills, forming world-renowned caves [51]. In the Edwards Aquifer, secondary porosity at multiple scales [52] developed along recharge pathways and where sulfur redox occurs at the mineralized water interface [53]. In the Floridan aquifer, sea level rise and fall, and thus the water table, helped guide the evolution of multigenerational, stratiform, cavernous porosity [54] that provides pathways for up-coning sulfur rich waters in coast-proximal settings [55]. We suggest an equally complicated diagenetic history of carbonate evolution in south-central Indiana that has regional implications for the Interior Lowland Plateaus of the North American midcontinent, and, by extension, similar geologic settings globally.
6.1. Observed Geochemical Processes
The clusters of sampled springs in this study show five independent populations of data. Principal component analysis (Figure 3) illustrates how Clusters 1 and 3 (the largest populations) separate along factor 2, in other words, T and [HCO3−]. However, these two clusters functionally overlap on both Piper (Figure 5) and Durov (Figure 6) diagrams, and are both earth-alkaline-type waters. Thus, Clusters 1 and 3 most likely represent the same water source, meteoric recharge, but are separated by the time available for water–rock interactions. Lower T and higher [HCO3−] suggest longer equilibration times and more carbonate dissolution in the shallow subsurface that supports an interpretation of a correlation to length of subsurface flow path. The apparent meteoric water–carbonate dissolution reactions follow the carbonic acid speleogenesis (CAS) model for karst aquifer evolution [56], or as presented as a chemical equation,
CO2(aq) + H2O + CaCO3(s) → Ca2+(aq) + 2HCO3−(aq).
Meteoric springs generally follow the simple dissolution line on the Durov diagram (Figure 6). The springs in Cluster 1 include longer flow paths with waters closer to calcite saturation ([HCO3−] = 4.9 +/− 1.0 mmol) and warmer temperatures (Tav = 14.1 +/− 1.8 °C). Warmer temperatures seemingly conflict with the interpretation of longer flow paths, but could be explained by significant recharge contributions during the warm season from soil storage in the overlying sinkhole plain. In contrast, springs in Cluster 3 seem to have shorter flow paths with cooler temperatures (Tav = 13.0 +/− 1.2 °C) and lower alkalinity ([HCO3−] = 3.4 +/− 0.9 mmol). The cooler temperatures likely reflect limited epikarst recharge and a dominance of matrix flow.
Groundwater chemistry at most karst springs is more complex than this straightforward CAS model because water–rock interactions include minerals other than calcite and dolomite. In this study, Clusters 4 and 5 are clearly separate populations from both the shallow aquifer water and from each other (Figure 3). As these two clusters share common elevated [SO42−], it stands to reason that these groundwaters have interacted with a sulfate-rich source. One potential source is sedimentary gypsum and anhydrite interbedded in the stratigraphy of carbonate aquifers, such as in the case of the Lower Blue River Group in this study [57]. During groundwater interactions with these evaporites, highly soluble sulfate minerals are released into solution, increasing [SO42−], while sometimes suppressing [HCO3−] through the common-ion effect [58]. Presented in chemical equation form,
where waters at saturation with calcium and bicarbonate dissolve anhydrite and result in calcite precipitation.
Ca2+(aq) + 2HCO3−(aq) + CaSO4(s) → Ca2+(aq)+ HCO3−(aq) + SO42−(aq) + CaCO3(s)↓,
At Orangeville Rise, a terminal rise of the Lost River Karst Basin, time-series data [10] illustrate that significant [SO42−] is isotopically akin to, and represents contributions from, the anhydrite of the lower Blue River Group. This supports previous interpretations [22]. On a Durov diagram (Figure 6), the Orangeville Rise data do not follow a simple dissolution or mixing line. Rather, they trend toward the data of Cluster 5, the mineral springs along the White River (Figure 6). Given the increase in [SO42−] from anhydrite without a proportional increase in [Ca2+], this horizontal trend could be related to the common-ion effect driving contemporaneous calcite precipitation as anhydrite is dissolved. Hydrogen sulfide is stable in solution at Trinity Springs in Cluster 5 on an Eh-pH diagram due to bacterial sulfate reduction in a low-oxygen setting (Figure 7).
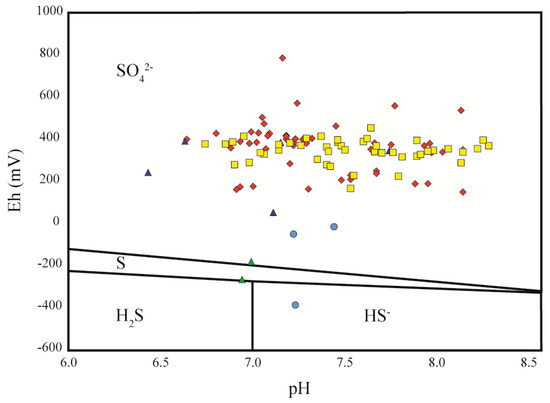
Figure 7.
Scatterplot showing Eh and pH of clustered spring data from this study. Cluster 1 springs are magenta diamonds. Cluster 2 springs are blue triangles. Cluster 3 springs are yellow squares. Cluster 4 springs are red circles. Cluster 5 springs are green triangles. Stability fields for sulfur species are also shown for this Eh-pH range.
It is also not uncommon for groundwater in carbonate aquifers to interact with hydrocarbons and associated brines with elevated [Na+], [K+], [Cl−], and [H2S−] [59]. Incorporating these brines in shallow karst groundwater can drive additional carbonate dissolution through the production of sulfuric acid—the sulfuric acid speleogenesis (SAS) model for karst aquifer evolution [60]. In this process, sulfide oxidizes and dissolves calcite, generates additional acidity, and precipitates gypsum. In equation form,
H2S−(aq) + 202(aq) + 2H2O + 2CaCO3(s) → Ca2+(aq)+ 2HCO3−(aq) + (CaSO4 • 2H2O)(s)↓.
Regional evidence of this influence was already established in the Cumberland Plateau of Southern Kentucky [61]. In Cluster 4, the mineral springs at French Lick, significant sulfide is present and the oxidation of this sulfide contributes to high [SO42−] and to lower pH. Elemental sulfur was precipitating at one of the two sampled springs (New Sprudel), an observation matching the Eh-pH diagram (Figure 7). SpC values in these samples are very elevated because of high concentrations of total dissolved solids, including significant contributions of Na+ and Cl− (Figure 5).
In the Burgess study [28], time-series data from Bluespring Caverns, adjacent to the East Fork White River, include elevated [SO42−] during times of low flow. In situ observations in the cave and δ34S of the dissolved sulfate identify discovered seeps of petroleum-rich brine as a primary source—upwards of 61% at peak contribution. The methods used to compute this contribution are detailed in the companion paper [10]. Elevated [Na+], [K+], and [Cl−] above baseline at Bluespring Caverns connect the seeps to deeper brines as at Cluster 4 (Figure 6), and the data from Cluster 2 lie between samples from Cluster 1 and Cluster 4 (Figure 6); they appear to follow a simple mixing line.
6.2. Contributing End Members
In reality, shallow carbonate aquifer systems may combine all three described processes, CAS, SAS, and the common-ion effect, and the chemistry of karst springs can be recast as mixing models that combine these three end members. Graphically, these end members can be presented in various ways that can highlight or diminish the differences. For example, the Piper and Durov diagrams will show a progressive evolution of water types, but may not allow for simple algebraic solutions to end-member proportions.
If we compartmentalize these into a Cartesian system of expected chemical profiles [62], with key cations on the x-axis and key anions on the y-axis, systems that are solely guided by CAS will proportionally have more [Ca2+], [Mg2+], and [HCO3−], than [Na+], [K+], [Cl−], and [SO42−] and thus plot in the +x, +y quadrant. Alternatively, SAS-dominated systems will plot in the −x, −y quadrant because [Na+], [K+], [Cl−], and [SO42−] outpace the production of [Ca2+], [Mg2+], and [HCO3−]. Finally, in evaporite-dominated systems, [Ca2+], [Mg2+] are more important than [Na+], [K+], and [SO42−] is released from dissolution in significant quantities, and will plot in the +x, −y quadrant. Given that karst springs in temperate landscapes have ample active circulation in the critical zone, the plotted envelope of expected groundwater chemistry will be parabolic in shape and weighed toward the CAS end member.
In the Chadha plot (Figure 8), the data from this study do not present a clean parabolic pattern; however, the discrete sample data clearly define the following: (1) karst springs with chemistry evolved principally from epigenetic interactions with meteoric water and carbonate bedrock (94 sites); (2) mineral spring end members with solute loads derived from hypogenetic interactions with hydrocarbon brines (2 sites); (3) mineral spring end members with solute loads derived from evaporites (3 sites); and (4) karst springs with water chemistry evolved from the proportional mixing of epigenetic recharge and the two end-member types of mineralized water (5 sites). Notably, compared to other published applications of the Chadha diagram, the data collected from Southern Indiana springs plot relatively close to each other. This illustrates how the differences in water chemistry in these carbonate aquifers are the result of subtle mixing and geochemical evolution driven by intersecting biological and geological processes in the hydrosphere.
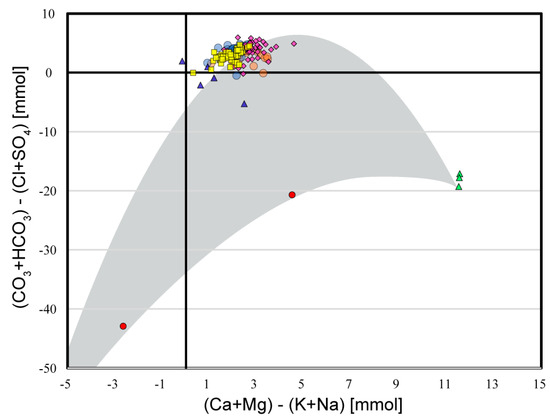
Figure 8.
Chadha diagram of spring chemistry data and time-series data from [10]. Cluster 1 springs are magenta diamonds. Cluster 2 springs are blue triangles. Cluster 3 springs are yellow squares. Cluster 4 springs are red circles. Cluster 5 springs are green triangles. Bluespring Cavern data are shown in light blue circles. Orangeville Rise data are shown as light orange circles. The parabolic curve shown in gray is illustrative of the envelope of expected data in a system of mixing waters comprising three end members: (1) shallow, meteoric groundwater (Clusters 1 and 3), (2) this meteoric groundwater equilibrated with sedimentary sulfate (Cluster 5), and (3) deep, mineralized groundwater (off the lower right of this graph).
The distribution of these spring types is, in part, stratigraphically guided. Springs with largely epigenetic waters emerge from springsheds that are either (1) smaller with localized flow systems, or (2) from flow systems in stratigraphic zones devoid of evaporites or petroleum seeps. In contrast, springs that incorporate hypogenetic waters are often from (1) larger springsheds or (2) deeper flow systems. Moreover, the geographic distribution of spring chemistry, while not precise, is separated into:
- Larger springsheds in the Mitchell Plateau where Middle-Mississippian carbonates are at the surface on a mature karst landscape of autogenic recharge with a well-developed epikarst, and where long-term meteoric flushing has removed most of the evaporites and basin brine;
- Smaller springsheds in the Mitchell Plateau and Crawford Uplands in a less mature karst landscape that lack a well-developed epikarst and include significant autogenic recharge;
- Springs in the Crawford Uplands of rising hypogenic waters where the carbonates are at or below grade and covered by noncarbonate caprock, and where meteoric flushing of evaporites and basin brines is incomplete.
6.3. Groundwater Chemistry in the Mitchell Aquifer System
Given the data of this study, considering related data sets from previous studies [21], and building upon earlier work [31], the Mitchell Aquifer includes two subaquifers that span the carbonates of the Sanders and Blue River Groups—the Upper and Lower Mitchell Aquifers (Figure 2). The boundary between these has stratigraphic influences, but is, in practice, a geochemical division partly, but not completely, aligned with geography. The upper and lower stratigraphic bounds of this aquifer are the contact between the Bethel Formation of the West Baden Group and the Paoli Limestone of the Blue River Group, and the lowermost permeable carbonates in the Sanders Group (spatially variable position in the Harrodsburg-Ramp Creek Limestone), respectively (Figure 2).
The Upper Mitchell Aquifer, defined by the springs in Clusters 1 and 3 and comprising most of the sampled springs (94), is largely alkaline earth water rich in Ca2+, Mg2+, and HCO3−, and relatively poor in Na+, K+, Cl−, and SO42− (Figure 5). The SpC of these waters (<1000 μS/cm) reflects the interaction between meteoric recharge and carbonate bedrock. The smaller springsheds with considerable allogenic recharge in the Crawford Uplands to the west and bordering the Norman Uplands in the east have lower SpC than the larger springsheds with largely autogenic recharge in the Mitchell Plateau (Figure 4). In essence, the chemistry of the Upper Mitchell Aquifer is of a typical carbonate groundwater and is characteristic of karst springs emerging from Paleozoic limestone and dolostone throughout the U.S. Midcontinent.
In contrast, only five sampled springs (Clusters 4 and 5) are representative of mineralized water rising to the surface from the Lower Mitchell Aquifer. At these springs, SpC values > 2500 μS S/cm, pH is lower, and SO42− is significantly elevated (Figure 5 and Figure 6). Two divergent geochemical pathways are visible in those samples. The cluster from mineral springs along the White River clearly has a connection to interactions with evaporites in the Lower Blue River Group. In contrast, the mineral springs at French Lick are dominated by interactions with basin brines. Transitional springs, mixing waters from both the Upper and Lower Mitchell Aquifers, are represented by the five springs in Cluster 2. These transitional springs illustrate the complexity and need for future investigation into the spatial and temporal position of the mineralized water boundary that separates the upper and lower aquifers. This includes mixing zone thickness, areas of suppression and up-coning, and the response to changes in recharge and pumping.
6.4. Implications for Speleogenesis and Carbon Flux
While these data are a geographic snapshot over a regional scale during base flow in the dry season, the guiding trends are defined by time-series chemistry data collected in the terminal conduits of adjacent springsheds [28] and point to a larger phenomenon. Bluespring Caverns includes elevated solute loading guided in part by input from identified petroleum seeps [10]. Orangeville Rise, in contrast, has a greater proportion of calcium contributed by interactions with gypsum and anhydrite. At Bluespring Caverns, contributions of excess sulfide from these petroleum seeps enhance the dissolution of carbonate through SAS. At Orangeville Rise, the excess sulfate from evaporite dissolution may drive carbonate out of solution by the common-ion effect.
The geochemical pathways of groundwater in this study are yet one more node of a larger revelation about speleogenesis and carbon flux in midcontinent settings. First, the CAS model of speleogenesis only captures the geochemical pathway of apparent dominance in modern groundwater. Caves of the Mitchell Plateau, as with elsewhere in the Interior Lowlands Plateaus of North America, are clearly throughways of epigenetic waters—both by cave morphology and by bulk water chemistry. However, this simple view overlooks indications of the active role of sulfur. For example, using analogous time-series data and geochemical modeling from Southern Kentucky, USA, Ref. [61] computed that upwards of 28% of dissolved inorganic carbon in a Cumberland Plateau karst spring was derived from reactions with entrained basin brines. In another nearby example, Ref. [63] noted morphological indicators in caves suggestive of hypogenetic origins. The companion paper [10] reports on secondary gypsum in Bluespring Caverns in proximity to observed petroleum seeps depleted in 34S and suggest that the early speleogenesis may owe more to SAS than CAS in incipient fractures. These are just a few examples in a growing recognition of hypogenetic processes to carbonate diagenesis in the carbonate critical zone [64,65] and the broader role of exogenous acids on the rates of global carbon flux regardless of lithology [66].
The other global implication of these regional observations is that modeling carbon flux must not rely simply on the carbonate equilibrium reactions to ascertain the origin and fate of dissolved inorganic carbon in karst groundwater. Ref. [61] illustrated this discrepancy clearly in the Cumberland Plateau study and scaled that result, with noted imprecision, to a global carbon flux. From that study and this one, it is clear that (1) interactions with reduced sulfur-rich basin bines can enhance the magnitude of carbonate weathering and carbon flux without a concurrent increase in atmospheric CO2 removal, and (2) interactions of karst groundwater with evaporites have the potential to mineralize carbon in the aquifer matrix and therefore reduce carbon flux. We posit that these are not just localized instances, but part of regional, and perhaps global, divergence from the commonly accepted paradigm of carbonate aquifer evolution.
7. Conclusions
This manuscript and the companion paper are one illustration of a more holistic view of groundwater geochemistry and the evolution of carbonate aquifers. Specifically, longitudinal data from key sites in the Mitchell Plateau of Indiana, USA inform a regional perspective and synthesis on the underlying process, evolution of secondary permeability, and the delineation of aquifer characteristics and groundwater quality. In the companion paper, two years’ worth of detailed measurements reveal two pathways leading to enriched sulfur in groundwater. One pathway, from the Lost River karst basin, is from the dissolution of evaporites in the stratigraphy, and the other, in the Bluespring Caverns karst basin, is sourced to rising basin bines. This second pathway sets the stage for enhanced carbonate dissolution from sulfuric acid, particularly in early-stage speleogenesis. In contrast, the first pathway may sequester dissolved carbon through the common-ion effect. Both are germane to better calibrations of groundwater carbon flux and demonstrate that cave development may indeed be a polygenetic geochemical process.
This paper applies these same geochemical concepts in a regional analysis of aquifer chemistry through discrete observations of groundwater springs. Some springs are dominated by interactions between meteoric recharge and the carbonate bedrock, with variations in chemistry guided by flow path length, basin size, and recharge characteristics. Other springs represent mineralized groundwater that are separately sourced to interactions with evaporites and basin brines. Yet other springs are a mixture between these end members. In addition to speleogenesis and carbon flux discussions, these groundwater types are an indication of two separate carbonate aquifers that are geochemically distinct, yet demonstrate clear signs of mixing—an Upper and Lower Mitchell Aquifer.
Author Contributions
Conceptualization, T.D.B. and L.J.F.; methodology, S.A.B., L.J.F. and T.D.B.; validation, S.A.B. and T.D.B.; formal analysis, S.A.B. and T.D.B.; investigation, T.D.B., S.A.B. and L.J.F.; resources, L.J.F. and T.D.B.; data curation, T.D.B.; writing—original draft preparation, L.J.F., S.A.B. and T.D.B.; writing—review and editing, L.J.F., S.A.B. and T.D.B.; visualization, L.J.F., S.A.B. and T.D.B.; supervision, L.J.F. and T.D.B.; project administration, L.J.F. and T.D.B.; funding acquisition, L.J.F. and T.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Center for Rural Engagement at Indiana University. The APC was funded in part by the Indiana University Open Access Article Publishing Fund.
Data Availability Statement
All primary data products produced as part of this investigation are included as part of an online spring database available through the Indiana Geological and Water Survey at Indiana University and are at the following website: https://igws.indiana.edu/springs (accessed on 25 September 2023).
Acknowledgments
Anion and cation analyses were performed by staff at the Indiana State Department of Health. Fieldwork was made possible by cooperation with the Hoosier National Forest, Indiana State Parks, Crane Naval Warfare Center, the Indiana Karst Conservancy, the owners and staff of Bluespring Caverns Park, and many private landowners. We are grateful for the many eyes of colleagues and reviewers that have considered and edited this work and are appreciative of the positive impact that those comments have had on the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parise, M.; Sammarco, M. The historical use of water resources in karst. Environ. Earth Sci. 2015, 74, 143–152. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon: Exposition et Application des Principes à Suivre et des Formules à Employer dans les Questions de Distribution d’eau; Victor Dalmont: Nantes, France, 1856; Volume 1. [Google Scholar]
- White, W.B. Springwater geochemistry. In Groundwater Hydrology of Springs; Kresic, N., Stevanovic, Z., Eds.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 231–268. [Google Scholar]
- Halihan, T.; Wicks, C.M.; Engeln, J.F. Physical response of a karst drainage basin to flood pulses: Example of the Devil’s Icebox cave system (Missouri, USA). J. Hydrol. 1998, 204, 24–36. [Google Scholar] [CrossRef]
- Eisenlohr, L.; Kiraly, L.; Bouzelboudjen, M.; Rossier, Y. Numerical simulation as a tool for checking the interpretation of karst spring hydrographs. J. Hydrol. 1997, 193, 306–315. [Google Scholar] [CrossRef]
- Labat, D.; Ababou, R.; Mangin, A. Rainfall–runoff relations for karstic springs. Part I: Convolution and spectral analyses. J. Hydrol. 2000, 38, 123–148. [Google Scholar] [CrossRef]
- Dreiss, S.J. Regional scale transport in a karst aquifer: 1. Component separation of spring flow hydrographs. Water Resour. Res. 1989, 25, 117–125. [Google Scholar] [CrossRef]
- Groves, C.G.; Howard, A.D. Early development of karst systems: 1. Preferential flow path enlargement under laminar flow. Water Resour. Res. 1994, 30, 2837–2846. [Google Scholar] [CrossRef]
- Ford, D.; Williams, P.D. Karst Hydrogeology and Geomorphology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Burgess, S.A.; Florea, L.F.; Branam, T. Divergent geochemical pathways of carbonate aquifer evolution in a classic karst terrain: (1) polygenetic cave development identified using longitudinal groundwater geochemistry. Water 2023, in press. [Google Scholar]
- Peale, A.C. Lists and Analyses of the Mineral Springs of the United States (a Preliminary Study); U.S. Geological Survey Report 32; Government Printing Office: Washington, DC, USA, 1886; p. 235. [Google Scholar]
- Meinzer, O.E. Large Springs in the United States; Water Supply Paper 557; U.S. Geological Survey: Reston, VA, USA; Government Printing Office: Washington, DC, USA, 1927; p. 94. [Google Scholar]
- Blatchley, W.S. The Mineral Waters of Indiana: Their Location, Origin and Character; W.B. Burford, Contractor for State Printing and Binding: Indianapolis, IN, USA, 1903. [Google Scholar]
- Gray, H.H. Physiographic Divisions of Indiana; Indiana Geological and Water Survey, Special Report 61; Indiana Geological Survey: Bloomington, IN, USA, 2000; p. 15. [Google Scholar]
- Owen, D.D. Report of a Geological Reconnaissance and Survey of the State of Indiana, Made in the Years 1837 and 1838; Osborn & Willets, Printers to the State: Indianapolis, IN, USA, 1859. [Google Scholar]
- Malott, C.A. The swallow-holes of Lost River, Orange County, Indiana. Proc. Natl. Acad. Sci. USA 1952, 61, 187–231. [Google Scholar]
- Powell, R. Caves of Indiana; Indiana Geological Survey Circular 8; Indiana Geological Survey: Bloomington, IN, USA, 1961; p. 127. [Google Scholar]
- Palmer, A.N. A Hydrologic Study of the Indiana Karst: An Evaluation of the Effects of Geologic Setting upon Ground-Water Flow and Water Supply in a Karst Region, with Special Reference to Northwestern Lawrence County, Indiana. Doctoral Dissertation, Indiana University, Bloomington, IN, USA, 1969. [Google Scholar]
- Bassett, J.L. Hydrology and Geochemistry of Karst Terrain, Upper Lost River Drainage Basin, Indiana: Bloomington. Master’s Thesis, Indiana University, Bloomington, IN, USA, 1974; p. 102. [Google Scholar]
- Bassett, J.L. Hydrology and geochemistry of the upper Lost River drainage basin, Indiana. Bull. Natl. Speleol. Soc. 1976, 38, 80–87. [Google Scholar]
- Krothe, N.C.; Libra, R.D. Sulphur isotopes and hydrochemical variations in spring waters of southern Indiana, U.S.A. J. Hydrol. 1983, 61, 267–283. [Google Scholar] [CrossRef]
- Lee, E.S.; Krothe, N.C. Delineating the karstic flow system in the upper Lost River drainage basin, south central Indiana: Using sulphate and δ34SSO4 as tracers. Appl. Geochem. 2003, 18, 145–153. [Google Scholar] [CrossRef]
- Hasenmueller, N.R.; Buehler, M.A.; Krothe, N.C.; Comer, J.B.; Branam, T.D.; Ennis, M.V.; Smith, R.T.; Zamani, D.D.; Hahn, L.; Rybarczyk, J.P. Water-quality characteristics and contaminants in the rural karst-dominated Spring Mill Lake watershed, southern Indiana. In Perspectives on Karst Geomorphology, Hydrology, and Geochemistry—A Tribute Volume to Derek C. Ford and William B. White; Harmon, R.S., Wicks, C., Eds.; GSA Special Paper 404; Geological Society of America: Boulder, CO, USA, 2016. [Google Scholar]
- Beck, B.; Pearson, F.M. Application of fluorescent dye tracing techniques for delineating sinkhole drainage routes, Highway 37 improvement project Lawrence County, Indiana. In Karst Geohazards Engineering and Environmental Problems in Karst Terrane; Beck, B.F., Ed.; Routledge: London, UK, 1995. [Google Scholar]
- Bayless, E.R.; Cinotto, P.J.; Ulery, R.L.; Taylor, C.J.; McCombs, G.K.; Kim, M.H.; Nelson, H.L. Surface-Water and Karst Groundwater Interactions and Streamflow-Response Simulations of the Karst-Influenced Upper Lost River Watershed, Orange County, Indiana; Scientific Investigations Report 2014-5028; U.S. Geological Survey: Washington, DC, USA, 2014; p. 39. [Google Scholar]
- Burgess, S.A.; Florea, L.J. Economic Exclusion and Forgotten Floodplains on Karst Terrain. Environ. Eng. Geosci. 2023, in press. [Google Scholar]
- Florea, L.J.; Hasenmueller, N.R.; Branam, T.D.; Frushour, S.S.; Powell, R.L. Karst geology and hydrogeology of the Mitchell Plateau of south-central Indiana. In Ancient Oceans, Orogenic Uplifts, and Glacial Ice: Geologic Crossroads in America’s Heartland; Florea, L., Ed.; Geological Society of America Special Paper 51; Geolgical Society of America: Boulder, CO, USA, 2018; p. 95. [Google Scholar]
- Burgess, S.A. Sulfur Systematics and Carbonate Diagenesis in the Mitchell Plateau, Indiana. Master’s Thesis, Indiana University, Bloomington, IN, USA, 2021; p. 164. [Google Scholar]
- Thompson, T.A.; Sowder, K.; Johnson, M.R. Generalized Stratigraphic Column of Indiana Bedrock (Poster). Available online: https://igws.indiana.edu/ignis/GeneralizedStratigraphicColumn.pdf (accessed on 31 July 2023).
- Sun, R.J.; Johnston, R.H. Regional Aquifer-System Analysis Program of the US Geological Survey, 1978–1992; Circular 1099; U.S. Geological Survey: Washington, DC, USA, 1994. [Google Scholar]
- Conner, G.A. Karst Spring Cutoffs, Cave Tiers, and Sinking Stream Basins Correlated to Fluvial Base Level Decline in South-Central Indiana. In 14th Sinkhole Conference; NCKRI Symposium 5; National Cave and Karst Research Institute: Carlsbad, CA, USA, 2015; pp. 53–61. [Google Scholar]
- Widhalm, M.; Hamlet, A.; Byun, K.; Robeson, S.; Baldwin, M.; Staten, P.; Chiu, C.; Coleman, J.; Hall, E.; Hoogewind, K.; et al. Indiana’s Past & Future Climate: A Report from the Indiana Climate Change Impacts Assessment; Purdue Climate Change Research Center, Purdue University: West Lafayette, IN, USA, 2018. [Google Scholar] [CrossRef]
- First Street Foundation. The First National Flood Risk Assessment: Defining America’s Growing Risk. Available online: https://assets.firststreet.org/uploads/2020/06/first_street_foundation__first_national_flood_risk_assessment.pdf (accessed on 24 July 2023).
- Wagner, L.E. The Impact of Storm Characteristics and Land Use on Nutrient Export in Two Glaciated Watersheds in Central Indiana, USA. Doctoral Dissertation, Indiana University, Bloomington, IN, USA, 2007. [Google Scholar]
- Arihood, L.D.; Basch, M.E. Geohydrology and Simulated Ground-Water Flow in an Irrigated Area of Northwestern Indiana; Water-Resources Investigations Report 92-4046; U.S. Geological Survey: Washington, DC, USA, 1994. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.; Le, P.V.; Papanicolaou, A.T.; Rhoads, B.L.; Anders, A.M.; Stumpf, A.; Belmont, P. Critical transition in critical zone of intensively managed landscapes. Anthropocene 2018, 22, 10–19. [Google Scholar] [CrossRef]
- Wittman, J. Water and Economic Development in Indiana–Modernizing the State’s Approach to a Critical Resource; Interra Geoscience and Engineering Solutions for the Indiana Chamber of Commerce: Indianapolis, IN, USA, 2014; p. 81. Available online: http://share.indianachamber.com/media/WaterStudyReport2014LoRes.pdf (accessed on 13 November 2017).
- Earth Tech. Delineation of Sinkhole Drainage Routes Utilizing Fuorescent Dye Tracing Techniques along State Route 60 between Mitchell and U.S. Highway 50, Lawrence County, Indiana; Report to the Indiana Department of Transportation, Environmental Assessment Section; Indiana Department of Transportation: Indianapolis, IN, USA, 1995; p. 16. [Google Scholar]
- Hopkins, H.T. Fresh-Saline Water Interface Map of Kentucky; Kentucky Geological Survey. Series X, 1(500,000); Kentucky Geological Survey: Lexington, MA, USA, 1966. [Google Scholar]
- Mitchell, W.M. Assessment of the 3,000 ppm and 10,000 ppm Total Dissolved Solids Boundaries in the Mississippian and Pennsylvanian Aquifers of Southwestern Indiana. Indiana Geological and Water Survey, Open File Study 94-02; Indiana Geological Survey: Bloomington, IN, USA, 1993. [Google Scholar]
- Panno, S.V.; Askari, Z.; Kelly, W.R.; Parris, T.M.; Hackley, K.C. Recharge and groundwater flow within an Intracratonic basin, Midwestern United States. Groundwater 2018, 56, 32–45. [Google Scholar] [CrossRef]
- Indiana Geological and Water Survey (IGWS). Springs. 2023. Available online: https://igws.indiana.edu/springs (accessed on 19 July 2023).
- Furtak, H.; Langguth, H.R. Zur hydrochemischen Kennzeichnung von Grundwässern und Grund-wassertypen mittels Kennzahlen. In Memoires IAH-Congress; IAH-Congress: Hannover, Germany, 1967; pp. 86–96. [Google Scholar]
- Winston, R.B. GW_Chart version 1.30: U.S. Geological Survey Software Release, 26 June 2020. USGS Digit. Object Identifier Cat. 2020. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busby, J.F.; Lee, R.W.; Hanshaw, B.B. Geochemical modeling of the Madison aquifer in parts of Montana, Wyoming, and South Dakota. Wat. Resour. Res. 1990, 26, 1981–2014. [Google Scholar] [CrossRef]
- Oetting, G.C.; Banner, J.L.; Sharp, J.M., Jr. Regional controls on the geochemical evolution of saline groundwaters in the Edwards aquifer, central Texas. J. Hydrol. 1996, 181, 251–283. [Google Scholar] [CrossRef]
- Williams, L.J.; Kuniansky, E.L. Revised Hydrogeologic Framework of the Floridan Aquifer System in Florida and Parts of Georgia, Alabama, and South Carolina; Regional Aquifer Analysis Professional Paper 1807; U.S. Geological Survey: Washington, DC, USA, 2016; p. 140. [Google Scholar]
- Downey, J.S.; Busby, J.F.; Dinwiddie, G.A. Regional aquifers and petroleum in the Williston Basin region of the United States. Am. Assoc. Petrol. Geol. Bull. 1987, 69, 299. [Google Scholar]
- Pavlicek, D.; Small, T.A.; Rettman, P.L. Hydrogeologic Data from a Study of the Freshwater Zone/Salinewater Zone Interface in the Edwards Aquifer, San Antonio Region, Texas; Open File Report 87-389; U.S. Geological Survey: Washington, DC, USA, 1987; p. 108. [Google Scholar]
- Gulley, J.D.; Florea, L.J. Caves as paleo-water table indicators in the unconfined Upper Floridan aquifer. Fla. Sci. 2016, 79, 239–256. [Google Scholar]
- Palmer, A.N.; Palmer, M.V.; Paces, J.B.; Feinberg, J.; Gao, Y.; Alexander, E.C. Geologic history of the Black Hills caves, South Dakota. In Caves and Karst Across Time; Feinberg, J., Gao, Y., Alexander, E.C., Eds.; Geological Society of America Special Paper 516; Geological Society of America: Boulder, CO, USA, 2016; pp. 87–101. [Google Scholar]
- Halihan, T.; Sharp, J.M., Jr.; Mace, R.E. Interpreting flow using permeability at multiple scales. In Karst Modeling; Palmer, A.N., Palmer, M.V., Eds.; Karst Waters Institute Special Publication 5; Karst Waters Institute: Charles Town, WV, USA, 1999; pp. 82–96. [Google Scholar]
- Engel, A.S.; Randall, K.W. Experimental evidence for microbially mediated carbonate dissolution from the saline water zone of the Edwards Aquifer, Central Texas. Geomicrol. J. 2011, 28, 313–327. [Google Scholar] [CrossRef]
- Florea, L.J.; Vacher, H.L.; Donahue, B.; Naar, D. Quaternary cave levels in peninsular Florida. Quat. Sci. Rev. 2007, 26, 1344–1361. [Google Scholar] [CrossRef]
- Stewart, J.W.; Mills, L.R. Hydrogeology of the Sulphur Springs Area, Tampa, Florida; U.S. Geological Survey Water-Resources Investigations Report 83-4085; U.S. Geological Survey: Washington, DC, USA, 1984. [Google Scholar]
- Palmer, A.N. Sulfuric acid vs. epigenic carbonic acid in cave origin and morphology. In Proceedings of the Deep Karst: Origins, Resources, and Management of Hypogene Karst, Carlsbad, NM, USA, 11–14 April 2016; pp. 11–14. [Google Scholar]
- McGregor, D.J. Gypsum and Anhydrite Deposits in Southwestern Indiana; Indiana Geological and Water Survey Report of Progress 08; Indiana Geological Survey: Bloomington, IN, USA, 1954; p. 24. [Google Scholar]
- Klimchouk, A.B. Hypogene Speleogenesis. In Treatise on Geomorphology; Shroder, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 220–240. [Google Scholar] [CrossRef]
- Stueber, A.M.; Walter, L.M. Origin and chemical evolution of formation waters from Silurian-Devonian strata in the Illinois basin, USA. Geochim. Cosmochim. Acta 1991, 55, 309–325. [Google Scholar] [CrossRef]
- Hill, C.A. Sulfuric acid speleogenesis of Carlsbad Cavern and its relationship to hydrocarbons, Delaware Basin, New Mexico and Texas. Am. Assoc. Petrol. Geol. Bull. 1990, 74, 1685–1694. [Google Scholar]
- Florea, L.J. Carbon flux and landscape evolution in epigenic karst aquifers modeled from geochemical mass balance. Earth Surf. Proc. Land. 2015, 40, 1072–1087. [Google Scholar] [CrossRef]
- Chadha, D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrog. J. 1990, 7, 431–439. [Google Scholar] [CrossRef]
- Florea, L.J. Sulfur-Based Speleogenesis in the Cumberland Plateau, USA. In Hypogene Karst Regions and Caves of the World; Klimchouk, A., Palmer, A., Waele, J., Auler, A., Audra, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 683–690. [Google Scholar]
- Covington, M.D.; Martin, J.B.; Toran, L.E.; Macalady, J.L.; Sekhon, N.; Sullivan, P.L.; Garcia, A.A.; Heffernan, J.B.; Graham, W.D. Carbonates in the critical zone. Earth’s Future 2023, 11, e2022EF002765. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Qian, J.; Wang, Z.; Hou, X.; Fu, C.; Ma, J.; Li, J. Hydrogeochemical Characteristics and Evolution of Karst Groundwater in Heilongdong Spring Basin, Northern China. Water 2023, 15, 726. [Google Scholar] [CrossRef]
- Li, C.; Smith, P.; Bai, X.; Tan, Q.; Luo, G.; Li, Q.; Wang, J.; Wu, L.; Chen, F.; Deng, Y.; et al. Effects of carbonate minerals and exogenous acids on carbon flux from the chemical weathering of granite and basalt. Glob. Plan. Chang. 2023, 221, 104053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).