Abstract
This study aimed at investigating the potential impacts of perfluorooctanoic acid (PFOA) exposure on the partial denitrification (PD) system. Our results indicated that nitrite accumulation rates were significantly decreased to 67.94 ± 1.25%–69.52 ± 3.13% after long-term PFOA exposure (0.5–20 mg/L), while the nitrate transformation ratio was slightly impacted. The PFOA removal efficiency gradually decreased from 67.42 ± 3.39% to 6.56 ± 5.25% with an increasing PFOA dosage, indicating that the main PFOA removal pathway was biosorption. The average EPS contents increased by two folds, which suggested that exposure to PFOA significantly stimulated EPS secretion. Excitation emission matrix analysis revealed that PFOA exposure promoted the secretion of tryptophan protein-like, humic acid-like, and aromatic protein II-like substances, which may act as a protective barrier against PFOA toxicity. Moreover, significant changes in characteristic peaks after PFOA exposure were shown as indicated by Fourier transform infrared spectroscopy. High-throughput sequencing suggested that PFOA significantly decreased bacterial richness and increased evenness, indicating that toxicity effects of PFOA were more pronounced for abundant species (e.g., Thauera) than rare species. Thauera was the most dominant genus responsible for nitrite accumulation, whose abundance significantly decreased from 35.99 ± 2.67% to 18.60 ± 2.18% after PFOA exposure. In comparison, the abundances of common denitrifiers, such as Denitratisoma, Bdellovibrio, and OLB8, significantly increased, suggesting that these genera were potential PFOA-resistant bacteria. This study presents new insights into the effect of PFOA on a PD system.
1. Introduction
The partial denitrification (PD) process is considered a promising way to provide a reliable NO2−-N supply for anammox bacteria because of its ability to reduce NO3−-N to NO2−-N as an end product [1]. The PD process is characterized with stable NO2−-N accumulation, lower carbon source demand, and fewer greenhouse gas emissions [2,3]. Therefore, the synergy of the PD and anammox processes is of great importance as an energy-efficient and environmentally sustainable alternative approach improving the nitrogen removal performance in mainstream municipal wastewater treatment [4,5].
The reliable and high accumulations of NO2−-N in the PD system are prerequisites for the practical application of the synergy system of PD and anammox [6]. Recent advances in the stability of the PD process have demonstrated that the accumulation of NO2−-N was subject to environmental factors. For instance, under a low temperature of 20 °C, the biofilms showed a significantly higher NO2−-N accumulation of 74.7% than that of 59.9% under a high temperature of 30 °C [7]. Moreover, Gao et al. [8] found that triclosan inhibited the NO2−-N accumulation as the triclosan dosage increased from 1 mg/L to 5 mg/L. It was found that the NO2−-N accumulation rate was influenced by varying C/N ratios, which was 17.9% at a C/N ratio of 3 and was 47.04% at 5 [9]. However, the accumulation of NO2−-N was observed to reach as high as 82.18% at a C/N ratio of 2.5 [10]. Furthermore, Qian et al. [11] investigated the influence of pH on NO2−-N accumulation and demonstrated that a NO2−-N accumulation rate under a pH of 9.0 was significantly higher than those under a pH of 7.0 and 5.0. Taken together, these efforts suggested that NO2−-N accumulation can be largely affected by external environmental factors. In addition to the above environmental factors, real wastewater commonly contains various pollutants, such as antibiotics [12], heavy metals [13], and persistent organic pollutants [14], which would potentially influence the NO2−-N accumulation of the PD process.
Perfluorinated alkyl substances (PFASs) are widely used in the manufacturing industry, such as plastic polymers, textile fibers, and the cosmetics industry, due to their relatively high lipophobicity, thermal stability, and hydrophobicity. Specifically, perfluorooctanoic acid (PFOA) is one of the representative PFASs. The extensive use of PFOA-containing products and PFOA precursors unavoidably led to their release into the aquatic environment. After discharged into aquatic environments, PFOA can be adsorbed to a wide range of environmental colloids and particles through electrostatic, hydrophobic, and ion exchange interactions [15,16]. Due to their extreme persistence and high bioaccumulation potential, PFOA can lead to acute and chronic impacts on individual, population, and community levels of living organisms via damaging the cell membrane, altering metabolism, triggering oxidative stress, and inhibiting growth [17,18]. Therefore, PFOA has been frequently observed in various ubiquitous environments, such as rivers, lakes, and groundwater [19], and even in human bodies [20] because of its high resistance to degradation and inappropriate management [21].
In recent years, numerous studies have concentrated on the impact of PFOA on the performance of activated sludge. For instance, a previous study showed that PFOA at 1.0 mg/L could suppress nitrate reduction and nitrite reduction by 13.1% and 5.8%, respectively [22]. However, Chen et al. [23] found that PFOA at 20 mg/L significantly improved the total nitrogen removal rate from 78.7 ± 6.89% to 86.8 ± 6.39% by improving nar and nir activities. In comparison, as PFOA concentration increased from 5 to 50 mg/L, the ammonia removal rate decreased from 93.90 ± 3.64% to 77.81 ± 6.86% [24]. Moreover, the COD removal performance inhibited by PFOA was observed previously [25]. In addition, the changes in microbial community compositions were also demonstrated [26]. For instance, the relative abundances of genera Thauera and Azoarcus decreased from 1.39% and 1.18% to 1.07% and 0.93%, respectively, after PFOA treatment [22]. Collectively, previous studies suggested that the PFOA addition would improve or inhibit the reactor performance and impact the microbial community of activated sludge. While considerable studies have been devoted to evaluating the impacts of PFOA on conventional activated sludge processes, the influence of PFOA on the PD process is insufficiently studied. As mentioned above, PFOA can result in bacterial metabolic disorder, cell damage, oxidative stress, and growth inhibition, and it is hypothesized that long-term PFOA exposure would inhibit nitrite accumulation in the PD system. Additionally, the microbial responses to PFOA stress at population and community levels have been rarely assessed.
Therefore, this study aimed to investigate the responses of the PD process to long-term PFOA stress. The reactor performances were monitored at regular intervals, with a focus on the concentrations of NO2−-N, NO3−-N, and PFOA. In addition, the composition and structure of EPS were termly analyzed, as well as the abundance and diversity of microbial communities at the phylum and gene levels. The findings of this investigation were expected to contribute to a comprehensive understanding on the influences of PFOA on the PD process.
2. Materials and Methods
2.1. Reactor Setup and Operation
The experiments were carried out in a 4 L sequencing batch reactor (SBR) with domesticated PD sludge (the domestication process is shown in Figure S1). The major components of synthetic wastewater were as follows: sodium acetate (120 mg/L COD), KNO3 (50 mg/L NO3−-N), KH2PO3 (0.05 mg/L), CaCl2 (0.4 mg/L), and MgSO4·7H2O (0.2 mg/L). The SBR was performed at room temperature (about 25 °C), and the influent pH was maintained at 8.5 ± 0.3 and adjusted with NaHCO3. The reactor was operated for 12 cycles per day with a 50% drainage ratio per cycle, corresponding to a hydraulic retention time (HRT) of 24 h. Sequential feeding (8 min), reaction (30 min), settling (52 min), discharging (9 min) and an idle period (21 min) were involved in a cycle (2 h). PFOA (>99% purity) was purchased from Shanghai Aladdin, China. The environmentally relevant levels of PFOA were 0.1–1 mg/L [22]. To investigate the effect of a high concentration of PFOA exposure on the PD process, 0.1–20 mg/L PFOA was set in our study according to previous studies [27]. The SBR was operated for nearly 180 days, and the whole period was divided into seven phases depending on the PFOA concentrations, i.e., phase I (days 0–25, 0 mg-PFOA/L), phase II (days 26–50, 0.1 mg-PFOA/L), phase III (days 51–75, 0.5 mg-PFOA/L), phase IV (days 76–100, 2 mg-PFOA/L), phase V (days 101–125, 5 mg-PFOA/L), phase VI (days 126–150, 10 mg-PFOA/L), and phase VII (days 151–175, 20 mg-PFOA/L). During the operation period, no sludge was discharged from the SBR except for biomass sampling for analysis of EPS and microbial community.
2.2. Chemical and Physical Analysis
Influent and effluent samples were collected every day. A 0.45 µm filter was employed to filter the samples before analysis. NO2−-N and NO3−-N concentrations were measured after filtration through the membrane (0.45 μm) according to the Standard Methods [28]. The nitrite accumulation rate (NAR) and nitrate transformation ratio (NTR) were calculated by the following Equations (1) and (2).
NAR (%) = (NO2−-Nt − NO2−-Ninitial)/(NO3−-Ninitial − NO3−-Nt) × 100%
NTR (%) = (NO3−-Ninitial − NO3−-Nt)/NO3−-Ninitial × 100%
NO2−-Nt and NO3−-Nt represent the NO2−-N and NO3−-N concentrations of the SBR reactor at sampling time. NO2−-Ninitial and NO3−-Ninitial represent the NO2−-N and NO3−-N concentrations of the SBR reactor at initial time.
The concentrations of PFOA in effluent were determined by using liquid chromatography tandem mass spectrometry (LC-MS). The detection method was based on the previous studies [24]. The column used was an Agilent ZORBAX Plus C18 liquid chromatography column. The mobile phase A was acetonitrile, and the mobile phase B was 5 mmol/L of ammonium acetate. The LC-MS scan time was 6 min at a flow rate of 0.2 mL/min, and the ratio of mobile phase A to B was 58:42 from 0 to 2.5 min, 5:95 from 2.5 to 4 min, and 5:95 from 4 to 6 min. The ratio of mobile phase A to B returned to 58:42 at 4–6 min.
2.3. Extraction and Analysis of Bacterial Extracellular Polymeric Substances
In this experiment, bacterial extracellular polymeric substances (EPS) were extracted using a heat extraction method [29]. The detailed procedures were as follows: 20 mL mixed sludge suspensions were collected in the 50 mL centrifuge tube and rinsed with PBS (20 mmol/L, pH = 7) three times. The mixed sludge suspensions were centrifuged at 3000 rpm for 15 min. Removing the supernatant, the sediments were re-suspended with 0.05% NaCl to the original volumes and placed in a water bath (60 °C, 120 rpm/min) for 30 min. Then, the mixed sludge suspensions were centrifuged at 12,000 rpm for 15 min, and the supernatants were filtered through a 0.45 μm filter membrane. The supernatants collected were termed as the EPS of sludge samples. The polysaccharide (PS) of EPS was measured using the phenol–sulfuric acid assay [30], whereas the protein (PN) was determined by the modified Lowry method [31].
2.4. Excitation–Emission Matrix (EEM) and Fourier Transform Infrared (FTIR) Spectroscopy Analysis
Excitation–emission matrix (EEM) was used to analyze the organic components in EPS from partial denitrification sludge. The excitation wavelength’s setting parameters of the EEM spectrometer were 220 nm to 450 nm in increments of 5 nm, while the emission wavelengths were set from 250 nm to 550 nm in 1 nm increments [32]. The EEM spectrometer was scanned at 2000 nm/min, and ultra-pure water was used as a control group. Moreover, the EPS surface functional groups were characterized by infrared spectroscopy [6]. The EPS extracts were freeze-dried to powder and then homogenously mixed with potassium bromide powder. In addition, the secondary structures of the protein on the amide I region at 1700–1600 cm−1 were determined using PeakFit v4.12 software.
2.5. Sludge Morphology Analysis
Firstly, an appropriate amount of sludge was rinsed with PBS (20 mmol/L, pH = 7) three times. The cells were first fixed by adding glutaraldehyde. After 3 h, the samples were rinsed well with PBS (20 mmol/L, pH = 7) three times. Subsequently, samples were dehydrated using 50%, 60%, 70%, 80%, 90%, and 100% ethanol solutions. Then, the biomass samples were treated with a mixture of anhydrous ethanol and isoamyl acetate (1:1). Finally, samples were lyophilized and gold-plated. The sludge morphology could be observed using an emission scanning electron microscope (FE-SEM).
2.6. Microbial Community Analysis
16S rRNA high-throughput sequencing was used to investigate the changes in the microbial community structure under PFOA stress. At the end of each phase, biomass samples were collected. In total, 20 PD sludge samples were collected in this study, including 8 samples from phase I and 2 samples each from phases II to phase VII. DNA was extracted using an E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). Concentrations and quality of the extracted DNA were measured by microspectrophotometry (NanoDrop ND-1000, NanoDrop Technologies, Willmington, DE, USA). A reverse primer 806R (5′-GGAC-TACHVGGGTWTCTAAT-3′) and a forward primer 338F (5′-ACTCCTACGG-GAGGCAGCA-3′) were used for the amplification of the V3-V4 region of the polymerase chain reaction (PCR) of the bacterial 16S rRNA genes [33]. PCR reactions were conducted in triplicate in a 20 μL mixture, which contains 4 μL FastPfu Buffer, 0.4 μL FastPfu Polymerase, 2 μL 2.5 mM dNTPs, 1.6 μL 5 μM primers, and 10 ng template DNA. The resulted products of PCR were further purified by AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Purified products were pooled in equimolar and paired-end sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The operational taxonomic units (OTUs) were clustered with Uparse version 7.0 based on different degrees of similarity (≥97% in this experiment). After sequencing was completed, data analysis was conducted on the Majorbio online Platform (www.majorbio.com, accessed on 18 May 2023). The sequencing raw data for the present study have been uploaded in the Sequence Read Archive (SRA) with the accession number PRJNA977209.
3. Results and Discussion
3.1. Performance of the Partial Denitrification Process under PFOA Stress
The performances of the reactor at seven different PFOA concentrations are shown in Figure 1 and Table S1. The average effluent NO3−-N and NO2−-N concentrations were 5.68 ± 1.30 mg/L and 30.76 ± 1.43 mg/L in phase I, respectively. Accordingly, the average NAR and NTR in the PD reactor were 71.28 ± 1.41% and 88.35 ± 2.70%, respectively. In phase I, when 0.1 mg/L PFOA was added, the average NAR slightly decreased to 71.19 ± 2.55%. However, as PFOA concentration increased during phase II-VII, NAR continuously declined to 67.94 ± 1.25%–69.52 ± 3.13%, which suggested a severe inhibition on nitrite accumulation under PFOA stress. The average effluent NO3−-N concentrations declined from 5.68 ± 1.30 mg/L (phase I) to 3.84 ± 1 mg/L (phase III), leading to an increase in the average NTR from phase I (88.35 ± 2.70%) to phase III (92.33 ± 1.95%). This indicated that low PFOA concentrations (0.1–0.5 mg/L) could promote nitrate reduction, which was in agreement with a previous study that showed 0.5 mg/L of PFOA could enhance denitrification [23] (Table S2). In comparison, Yang et al. [22] found that nitrate and nitrite reduction were inhibited by 13.1% and 5.8%, respectively, under 1 mg/L PFOA concentration. Intriguingly, high PFOA concentrations (2–20 mg/L) showed a slight effect on NTR (Table S1). Similarly, Yu et al. [34] and Cao et al. [27] demonstrated that <20 mg/L PFOA showed minor influence on both nitrification and denitrification, which indicated a safe PFOA exposure concentration for a nitrogen removal system. Overall, our results showed that nitrite accumulation was significantly influenced by PFOS, while nitrate reduction was slightly impacted. Generally, the structure and cell surface integrity affect the performance of an activated sludge system [35]. A recent study demonstrated that PFOA induced the production of reactive oxygen species in activated sludge, which can result in the inactivation of bacterial cells [36]. However, in this study, considerable deterioration in the reactor performance did not occur, suggesting a minor impact of PFOA on the basic characteristics of PD sludge. This was supported by SEM results that the structure and cell surface integrity of PD sludge flocs were merely affected after PFOA exposure (Figure S2). A similar finding was also found in a previous study [23], which was likely attributed to the bacterial self-protection of EPS, as discussed in Section 3.2.
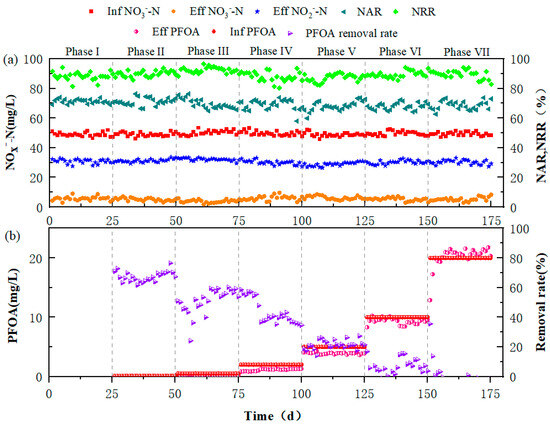
Figure 1.
Operational performance of reactor during the whole experiment: (a) Variations in NO2−-N, NO3−-N, NAR, and NTR in the PD system. (b) Changes in influent and effluent PFOA concentrations. The corresponding PFOA concentrations of Phase I, II, III, IV, V, VI, and VII were 0, 0.1, 0.5, 2, 5, 10, and 20 mg/L, respectively.
As shown in Figure 1b, the removal rate of PFOA gradually decreased from 67.42 ± 3.39% (phase II) to 6.56 ± 5.25% (phase VI) with an increasing PFOA concentration (0.1–10 mg/L). This was in line with a previous report [37], which showed that a high removal efficiency of PFOA was achieved initially due to the adsorption of the sludge, while the PFOA removal efficiency gradually decreased with extended experimentation. Additionally, our results indicated that the main PFOA removal pathway was biosorption, which was also supported by previous studies demonstrating that PFOA removal in wastewater treatment systems mainly depend on the adsorption of activated sludge [22,27]. In phase VII with 20 mg/L PFOA addition, the effluent PFOA concentrations were higher than those of the influent, which was probably due to the desorption of PFOA from the sludge. A similar phenomenon was also observed previously, which was mainly due to strong desorption hysteresis effects of proteins [38].
3.2. Contents and Characteristics of EPS in Partial Denitrification Sludge
EPS is an important component of activated sludge, which can protect the bacteria from toxic substances [23] and provide active adsorption sites for contaminants [39]. The variations of PS and PN contents in EPS are shown in Figure 2. Apparently, the results reveals that the higher concentration of PFOA, the more EPS was secreted by the PD sludge. Specifically, the average PS and PN contents increased from 15.97 mg/g VSS to 35.24 ± 1.51 mg/g VSS and from 43.36 to 93.01 ± 4.07 mg/g VSS, respectively. This suggested that long-term exposure to PFOA significantly stimulated EPS secretion by PD bacteria, which was in accordance with previous results that a significant increase in EPS contents were found under PFOA stress [40]. The increase in EPS content was mainly attributed to the bacterial self-protection and/or stress response to resist the PFOA toxicity [25]. A previous study has demonstrated that functional groups in EPS, such as alcoholic amide and hydroxyl groups, could provide multiple active binding sites for adsorption of PFOA [41]. Also of note, the increase in PN concentration was particularly significant as compared with the PS concentration, suggesting that PN in EPS was more sensitive to PFOA toxicity than PS and that the PN played a major role in resisting the PFOA toxicity.
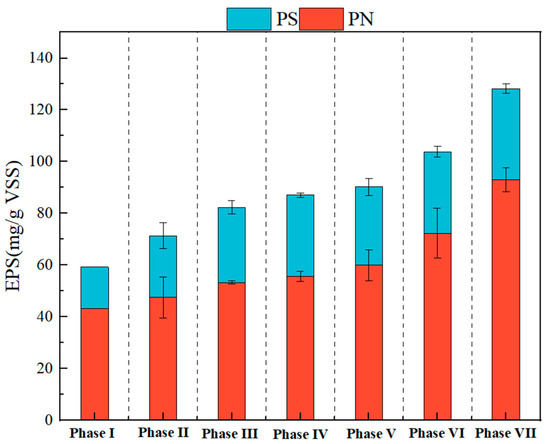
Figure 2.
Variation in the content of protein (PN) and polysaccharide (PS) at different phases. The corresponding PFOA concentrations of Phase I, II, III, IV, V, VI, and VII were 0, 0.1, 0.5, 2, 5, 10, and 20 mg/L, respectively.
In addition, we performed EEM analysis to investigate the fluorescence properties of EPS at different PFOA concentrations (Figure 3). Three fluorescence peaks, i.e., peak A (Ex/Em of 290/350 nm), peak B (Ex/Em of 360/450 nm), and peak C (Ex/Em of 230/300 nm), were identified in the samples, which were relevant to tryptophan protein-like, humic acid-like, and aromatic protein II-like substances, respectively [32]. As shown in Table S3 and Figure 3, the intensities of all three peaks considerably increased after a PFOA addition. Specifically, the intensities of peak A, peak B, and peak C considerably increased from 35,017 a.u. to 204,070 a.u., from 7729 a.u. to 25,219 a.u., and from 7733 a.u. to 155,109 a.u., respectively. This result indicated a substantial increase in tryptophan protein-like, humic acid-like, and aromatic protein II-like substances, confirming the increase in PN content in EPS. This was also consistent in a previous study showing that the production of tryptophan protein-like, humic acid-like [27,42], and aromatic protein II-like [43] substances were promoted under PFOA stress. Moreover, under long-term PFOA stress, all three peaks eventually showed a blue shift. For instance, peak C blue-shifted from Ex/Em of 230/331 to Ex/Em of 225/306, indicating the breakdown of large molecules to small molecules [44]. In conclusion, the intensity of the fluorescence peak and position of EPS significantly changed after PFOA exposure, which may be served as a protective barrier against the toxicity of PFOA.
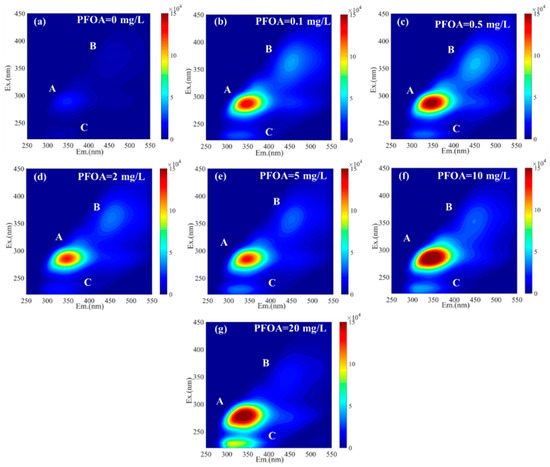
Figure 3.
EEM fluorescence spectra of EPS under different PFOA concentrations: (a) PFOA = 0 mg/L (Phase I); (b) PFOA = 0.1 mg/L (Phase II); (c) PFOA = 0.5 mg/L (Phase III); (d) PFOA = 2 mg/L (Phase IV); (e) PFOA = 5 mg/L (Phase V); (f) PFOA = 10 mg/L (Phase VI); and (g) PFOA = 20 mg/L (Phase VII).
To further assess the changes in EPS functional groups, FTIR analysis was performed. The region of the infrared spectrum related to the typical characteristic peaks (400–1800 cm−1) is shown in Figure 4a. Under long-term PFOA stress, the characteristic peaks of the infrared spectra significantly changed as compared with phase I without PFOA. Under PFOA stress, the characteristic peak at 1637 cm−1 and 1404 cm−1 were enhanced, which were related to the C=O stretching of the amide I region, and C-H symmetric deformations and asymmetric bending vibration of the methyl groups of the amide II region, respectively [45]. Moreover, a weak characteristic peak appeared at 1244 cm−1 after PFOA exposure, which belongs to a protein-related region [6]. This was consistent with the changes in the concentration and EEM fluorescence spectra of PN. Furthermore, the enhancement of the characteristic peak at 1080 cm−1 was likely related to the variations in the content and characteristic of PS, which was linked to the C-H and C-O-C stretching of PS [46]. In addition, a new characteristic peak appeared at 871 cm−1, which was attributed to the ring vibrations of nucleotides and aromatic amino acids. It suggested that long-term exposure to PFOA may have had effects on the synthesis of aromatic compounds, which supported the result of EEM.
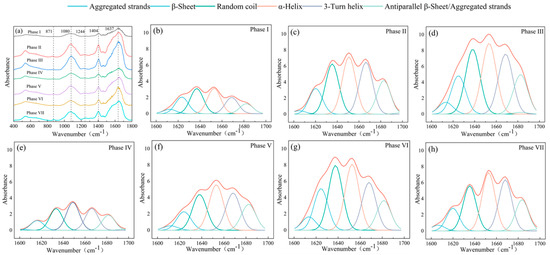
Figure 4.
FTIR analysis of EPS in different phases: (a) FTIR spectra of EPS extracted from PD sludge at different PFOA stresses. The curve—fitted amide I region (1600–1700 cm−1) for PN at different PFOA stresses: (b) phase I (0 mg/L); (c) phase II (0.1 mg/L); (d) phase III (0.5 mg/L); (e) phase IV (2 mg/L); (f) phase V (5 mg/L); (g) phase VI (10 mg/L); and (h) phase VII (20 mg/L).
In order to understand the subtle differences in the amide I region (1600–1700 cm−1) of PN in EPS among different PFOA concentrations, a second derivative analysis and a curve-fitting procedure were performed in the present study. As shown in Table S4 and Figure 4b–h, the protein secondary structure had a considerable change after the PFOA addition. For instance, the relative percentage of β-Sheet, Aggregated strands, and α-Helix generally showed decreasing trends with an increasing PFOA concentration, while Antiparallel β-structures and Random coil increased after PFOA exposure. Additionally, the α-Helix/(β-Sheet + Random coil) value was 1.01 for phase I, which was obviously lower than those of other phases except for phase IV. This indicated that the PN in EPS of phase I had a looser structure [47]. The above changes in protein secondary structures suggested that PFOA exposure deteriorated the bacterial flocculation and aggregation of sludge cells, which was probably due to the shifts in microbial communities and, consequently, the characteristics of secreted PN. EPS is known to determine the surface hydrophilicity/hydrophobicity of activated sludge. Overall, the compositions and characteristics of the secreted EPS were changes under PFOA stress, thus affecting the compressibility and settling ability of the sludge.
Taken together, PFOA exposure altered the compositions and characteristics of the secreted EPS, which was responsible for alleviating the toxicity of PFOA. For instance, aromatic protein can bind with C-F tail of PFOA via hydrophobic interaction, while amide groups could form an electrostatic attraction with carboxyl head of PFOA [41]. Additionally, humic acid-like substances possess aromatic groups, which were also involved in the adsorption of PFOA through hydrophobic interaction.
3.3. Effects of PFOA Stress on the Microbial Community Structures
The microbial communities in PD sludge samples at different phases were assessed using 16S rRNA high-throughput sequencing. As shown in the Venn diagram (Figure S3), all sludge samples shared 291 OTUs and the unique OTUs in phase I, II, III, IV, V, VI, and VII were 96, 39, 33, 19, 20, 15, and 37, respectively. This suggested that exposure to PFOA significantly decreased bacterial diversity, which coincided with alpha diversity analysis. As shown in Table S5, significant (p < 0.01) decreases in the species richness indices, including sobs, chao 1, and ace, were found after PFOA addition. A previous study also demonstrated that after 90 days of PFOA exposure, bacterial richness of activated sludge declined under both anaerobic and aerobic conditions [48]. In contrast, the bio-diversities in the soil microbial community increased with both PFOA and PFOS treatment [49]. Additionally, an increase in evenness was observed as revealed by the Shannon index, which indicated that toxicity effects of PFOA were more pronounced for abundant species (e.g., Thauera as discussed below) than rare species.
The variations and distances among samples in all the phases were visualized using PCoA based on the OTU level. As shown in Figure 5, clear differences among samples from different phases were found, which fell into seven groups based on phases. Noticeably, undeniable distances among samples of phase I (PFOA = 0 mg/L), samples of phase II-V (PFOA = 0.1–5 mg/L), and samples of phase VI-VII (PFOA = 10–20 mg/L) were observed. The above results suggested that PFOA played a crucial role in shaping PD sludge microbial compositions, and the effect of PFOA on community compositions was concentration-dependent. This was in agreement with previous studies that showed PFOA induced significant changes in microbial communities after long-term exposure [27,34].
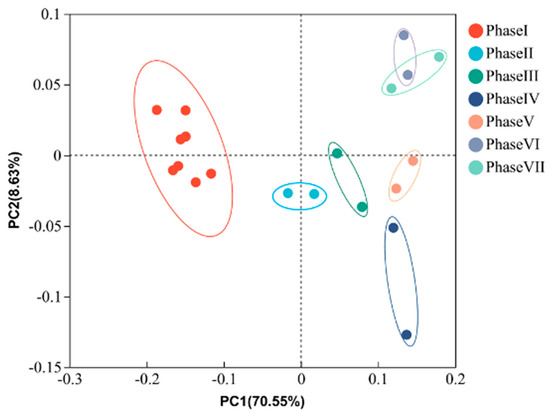
Figure 5.
The similarity of community structures in PCoA ordination. Each point in the plot represents a sample, and different colors indicate different phases. The corresponding PFOA concentrations of phase I, II, III, IV, V, VI, and VII were 0, 0.1, 0.5, 2, 5, 10, and 20 mg/L, respectively.
The microbial community structure at the phylum level under different PFOA concentrations is shown in Figure 6a. Proteobacteria (average relative abundance at 42.16 ± 8.09%) were the predominant phylum throughout the experiment, followed by phylum Bacteroidetes (23.33 ± 7.29%) and Patescibacteria (13.34 ± 3.72%). Other less abundant phylum included Planctomycetota (4.71 ± 0.93%), Chloroflexi (2.91 ± 0.80%), and Elusimicrobiota (2.52 ± 3.73%). Phylum Proteobacteria are usually considered as the dominant bacteria in denitrification reactors, which play an important role in the denitrification process [24,37]. In this study, the relative abundance of Proteobacteria gradually declined from 50.48 ± 2.99% in phase I to 39.24 ± 1.00% in phase VII, suggesting that exposure to PFOA restrained the abundance of Proteobacteria [50]. In contrast, the abundance of phylum Bacteroidetes gradually increased from 15.96 ± 1.11% in phase I to 34.55 ± 3.95% in phase VII with the increasing PFOA concentration, which indicated their tolerance towards PFOA stress. Notably, the abundance of a little studied phylum Elusimicrobiota substantially increased after exposure to PFOA. Phylum Elusimicrobiota have recently been demonstrated to possess the ability to utilize various polysaccharides [51]; thus, their increasing abundance was likely due to the increase in PS content induced by a PFOA addition. Additionally, Zhao et al. [52] found that Elusimicrobia have the functional potentials in synthesis of various essential amino acids. As such, the enrichment of Elusimicrobia under PFOA stress might take responsibility, at least in part, for the significant increase in PN content.
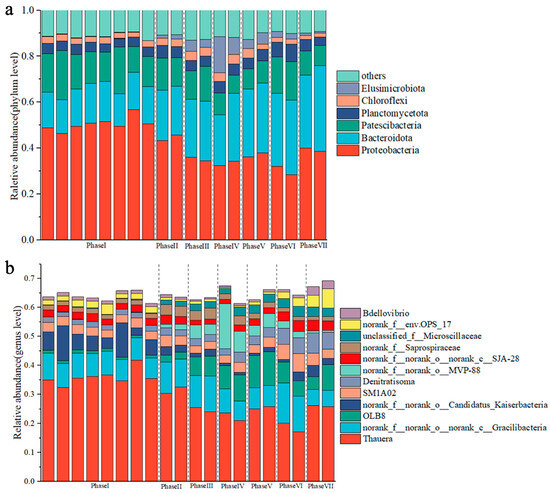
Figure 6.
Variations of microbial compositions at phylum level (a) and genus level (b) under different PFOA concentrations. The corresponding PFOA concentrations of phase I, II, III, IV, V, VI, and VII were 0, 0.1, 0.5, 2, 5, 10, and 20 mg/L, respectively.
The changes in the microbial community at a genus level in each phase are shown in Figure 6b. The results demonstrated that the genus Thauera was the most dominant genus throughout the experiment with an average relative abundance of 29.27 ± 6.67%, followed by Gracilibacteria (8.70 ± 2.27%), OLB8 (4.59 ± 3.79%), Candidatus_Kaiserbacteria (3.75 ± 3.28%), SM1A02 (3.43 ± 0.91%), and Denitratisoma (3.00 ± 1.62%). The genus Thauera was susceptible to the variable PFOA concentration. Specifically, the relative abundance of Thauera considerably decreased from 35.99 ± 2.67% in phase I to 18.60 ± 2.18% in phase VI but rebounded to 26.00 ± 0.33% in phase VII. The Spearman correlation analysis (Figure S4) indicated that genus Thauera was significantly and positively correlated with NAR (r = 0.85, p < 0.05), which was in good agreement with a previous study showing that Thauera is incomplete denitrifying bacteria that are responsible for NO2−-N accumulation [53]. Moreover, the abundance of genera Denitratisoma (r = 0.95, p < 0.001), Bdellovibrio (r = 0.86, p < 0.05), and env.OPS_17 (r = 0.92, p < 0.001) showed a positive correlation with PFOA concentration. Similarly, the abundance of OLB8 sharply increased from 0.96 ± 0.11% in phase I to11.37 ± 0.24% in Phase V. Previous studies have demonstrated that Denitratisoma [54], Bdellovibrio [55], env.OPS_17 [56], and OLB8 [57] are involved in denitrification processes. This suggested that these denitrifying genera were potential PFOA-resistant members. Additionally, Kondrotaite et al. [58] showed that the genus OLB8 possesses the metabolic capabilities for the degradation of polysaccharides and proteins. Thus, the significant increase in PS and PN contents stimulated by PFOA stress enabled the proliferation of OLB8. In this context, although NAR significantly decreased under PFOA stress, a significant increase in EPS content provided a protective barrier for bacteria against PFOA toxicity. On the other hand, PFOA exposure unexpectedly improved the availability of substrate (i.e., PS and PN) for OLB8 and induced the proliferation of other PFOA-tolerant denitrifying bacteria (e.g., Denitratisoma), which enabled relatively high NTR of the PD process throughout the whole period. Therefore, the breakdown of PD sludge did not occur even under a long-term high concentration of PFOA exposure.
4. Conclusions
In summary, the results found that the NAR continuously decreased to 67.94 ± 1.25%–69.52 ± 3.13% as the PFOA increased to 20 mg/L, while NTR was slightly influenced by PFOA. The removal efficiency of PFOA gradually decreased from 67.42 ± 3.39% to 6.56 ± 5.25%, indicating that the main PFOA removal pathway was biosorption. After PFOA exposure, PS and PN content increased significantly, which suggested that long-term exposure to PFOA significantly stimulated EPS secretion. EEM analysis showed that PFOA stress promoted the production of tryptophan protein-like, humic acid-like, and aromatic protein II-like substances. Additionally, FTIR analysis indicated substantial changes in functional groups, especially those associated with proteins. High-throughput sequencing suggested that PFOA exposure induced considerable changes in community structures. Although the abundance of the PD-related genus Thauera significantly decreased, the abundance of other denitrification-related genera Denitratisoma, Bdellovibrio, env.OPS_17, and OLB8 increased under PFOA stress.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15162977/s1, Table S1: Summary of the reactor performances throughout the whole experimental period; Table S2: The effects of PFOA on nitrogen removal performances; Table S3: EEM spectrum information of EPS under different PFOA stresses; Table S4: Relative percentage of protein secondary structure in amide I region at different PFOA concentrations; Table S5: Species diversity indices of sludge samples at different PFOA concentrations; Figure S1: Changes in the performance of partial denitrification during PD domestication process; Figure S2: SEM image of the sludge before and after PFOA stress; Figure S3: Venn diagram showing the unique and shared OTUs in different phases; Figure S4: Spearman’s rank correlations between performance parameters and the major genera.
Author Contributions
Methodology, H.Z.; Formal analysis, H.Z.; Writing—original draft, S.Z.; Writing—review & editing, N.R.M.; Visualization, Y.F.; Supervision, S.L. and L.Z.; Funding acquisition, S.Z., S.L. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 52200065), Science and Technology Program of Guangzhou (202102010396), Youth Innovative Talents Project of Department of Education of Guangdong Province (2022KQNCX076), Science and Technology Projects of Shaoguan (220609104530715), and Shaoguan University Research Project (SZ2021KJ09).
Data Availability Statement
The data sets supporting the results of this article are available upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Du, R.; Cao, S.; Zhang, H.; Li, X.; Peng, Y. Flexible Nitrite Supply Alternative for Mainstream Anammox: Advances in Enhancing Process Stability. Environ. Sci. Technol. 2020, 54, 6353–6364. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Y.; Li, X.; Du, R. Feasibility of partial-denitrification/anammox for pharmaceutical wastewater treatment in a hybrid biofilm reactor. Water Res. 2022, 208, 117856. [Google Scholar] [CrossRef]
- Izadi, P.; Sinha, P.; Andalib, M.; Samberger, C.; Lehman, G.; Messologitis, K.; Jacangelo, J. Coupling fundamental mechanisms and operational controls in mainstream partial denitrification for partial denitrification anammox applications: A review. J. Clean. Prod. 2023, 400, 136741. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Hassan, G.K.; Lu, X.; Piechota, G.; Mannina, G.; Bott, C.B.; Mąkinia, J. An advanced synergy of partial denitrification-anammox for optimizing nitrogen removal from wastewater: A review. Bioresour. Technol. 2023, 381, 129168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Maddela, N.R.; Li, H.; An, Y.; Li, S. Exposure to Cr(VI) affects partial denitrification process-nitrite accumulation, EPS characteristic and microbial community assembly. J. Environ. Chem. Eng. 2023, 11, 109001. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, Y.; Zhao, Y.; Zhao, Q.; Li, X.; Zhang, Q.; Sui, J.; Wang, C.; Li, J. Excellent anammox performance driven by stable partial denitrification when encountering seasonal decreasing temperature. Bioresour. Technol. 2022, 364, 128041. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F.; Liu, X.H.; Fan, X.Y.; Dai, H.H. Effects of triclosan on performance, microbial community and antibiotic resistance genes during partial denitrification in a sequencing moving bed biofilm reactor. Bioresour. Technol. 2019, 281, 326–334. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Zhu, W.; Xie, T.; Li, L. Effect of COD/NO(3) (-)-N ratio on nitrite accumulation and microbial behavior in glucose-driven partial denitrification system. Heliyon 2023, 9, e14920. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, J.; Liu, Q.; Fan, Y.; Zhu, C.; Liu, Y.; He, C.; Wu, J. Nitrite accumulation and microbial behavior by seeding denitrifying phosphorus removal sludge for partial denitrification (PD): The effect of COD/NO(3)(-) ratio. Bioresour. Technol. 2021, 323, 124524. [Google Scholar] [CrossRef]
- Qian, W.; Ma, B.; Li, X.; Zhang, Q.; Peng, Y. Long-term effect of pH on denitrification: High pH benefits achieving partial-denitrification. Bioresour. Technol. 2019, 278, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Zhang, S.; Chen, S.; Zhang, L. Stress responses of partial denitrification system under long-term ciprofloxacin exposure in an anaerobic sequencing batch reactor. J. Environ. Chem. Eng. 2023, 11, 110141. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alshammari, R.H.; Hasan, I. In Situ Polyaniline Immobilized ZnO Nanorods for Efficient Adsorptive Detoxification of Cr (VI) from Aquatic System. Water 2023, 15, 1949. [Google Scholar] [CrossRef]
- Chinnadurai, K.; Prema, P.; Veeramanikandan, V.; Kumar, K.R.; Nguyen, V.-H.; Marraiki, N.; Zaghloul, N.S.S.; Balaji, P. Toxicity evaluation and oxidative stress response of fumaronitrile, a persistent organic pollutant (POP) of industrial waste water on tilapia fish (Oreochromis mossambicus). Environ. Res. 2022, 204, 112030. [Google Scholar] [CrossRef] [PubMed]
- Guelfo, J.L.; Korzeniowski, S.; Mills, M.A.; Anderson, J.; Anderson, R.H.; Arblaster, J.A.; Conder, J.M.; Cousins, I.T.; Dasu, K.; Henry, B.J.; et al. Environmental Sources, Chemistry, Fate, and Transport of Per- and Polyfluoroalkyl Substances: State of the Science, Key Knowledge Gaps, and Recommendations Presented at the August 2019 SETAC Focus Topic Meeting. Environ. Toxicol. Chem. 2021, 40, 3234–3260. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, K.; Majeed, I.; Bilal, M.; Rasheed, T.; Shakeel, A.; Iqbal, S. Phytochemistry and Diverse Pharmacology of Genus Mimosa: A Review. Biomolecules 2022, 12, 83. [Google Scholar] [CrossRef]
- Mao, W.; Li, M.; Xue, X.; Cao, W.; Wang, X.; Xu, F.; Jiang, W. Bioaccumulation and toxicity of perfluorooctanoic acid and perfluorooctane sulfonate in marine algae Chlorella sp. Sci. Total Environ. 2023, 870, 161882. [Google Scholar] [CrossRef]
- Riaz, T.; Munnwar, A.; Shahzadi, T.; Zaib, M.; Shahid, S.; Javed, M.; Iqbal, S.; Rizwan, K.; Waqas, M.; Khalid, B.; et al. Phyto-mediated synthesis of nickel oxide (NiO) nanoparticles using leaves’ extract of Syzygium cumini for antioxidant and dyes removal studies from wastewater. Inorg. Chem. Commun. 2022, 142, 109656. [Google Scholar] [CrossRef]
- Sims, J.L.; Stroski, K.M.; Kim, S.; Killeen, G.; Ehalt, R.; Simcik, M.F.; Brooks, B.W. Global occurrence and probabilistic environmental health hazard assessment of per- and polyfluoroalkyl substances (PFASs) in groundwater and surface waters. Sci. Total Environ. 2022, 816, 151535. [Google Scholar] [CrossRef]
- Chiavola, A.; Di Marcantonio, C.; Boni, M.R.; Biagioli, S.; Frugis, A.; Cecchini, G. Experimental investigation on the perfluorooctanoic and perfluorooctane sulfonic acids fate and behaviour in the activated sludge reactor. Process Saf. Environ. Prot. 2020, 134, 406–415. [Google Scholar] [CrossRef]
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, bioaccumulation, and distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in plants. iScience 2022, 25, 104061. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, N.; Yang, J.; Fu, Q.; Wang, Y.; Wang, D.; Tang, L.; Xia, J.; Liu, X.; Li, X.; et al. Interaction between perfluorooctanoic acid and aerobic granular sludge. Water Res. 2020, 169, 115249. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zou, M.; Zhou, Y.; Zeng, L.; Yang, X. Monitoring the nitrous oxide emissions and biological nutrient removal from wastewater treatment: Impact of perfluorooctanoic acid. J. Hazard. Mater. 2021, 402, 123469. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Su, C.; Fan, C.; Li, R.; Wang, Y.; Gao, S.; Chen, M. Long-term effect of perfluorooctanoic acid on the anammox system based on metagenomics: Performance, sludge characteristic and microbial community dynamic. Bioresour. Technol. 2022, 351, 127002. [Google Scholar] [CrossRef]
- Cao, L.; Liao, Y.; Su, C.; Tang, L.; Qi, Z.; Wei, L.; Wu, J.; Gao, S. Effects of PFOA on the physicochemical properties of anaerobic granular sludge: Performance evaluation, microbial community and metagenomic analysis. J. Environ. Manag. 2022, 313, 114936. [Google Scholar] [CrossRef]
- Chen, C.; Fang, Y.; Cui, X.; Zhou, D. Effects of trace PFOA on microbial community and metabolisms: Microbial selectivity, regulations and risks. Water Res. 2022, 226, 119273. [Google Scholar] [CrossRef]
- Cao, L.; Su, C.; Wu, J.; Wei, L.; Zhou, Y.; Tang, L.; Wang, Q.; Xian, Y. Impact of perfluorooctanoic acid on treatment wastewater by a tandem AnSBR-ASBR system: Performance, microbial community and metabolism pathway. Process Saf. Environ. Prot. 2022, 164, 373–383. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Hou, Z.; Dong, W.; Wang, H.; Zhao, Z.; Li, Z.; Liu, H.; Li, Y.; Zeng, Z.; Xie, J.; Zhang, L.; et al. Response of nitrite accumulation to elevated C/NO- 3-N ratio during partial denitrification process: Insights of extracellular polymeric substance, microbial community and metabolic function. Bioresour. Technol. 2023, 384, 129269. [Google Scholar] [CrossRef]
- Tan, C.H.; Koh, K.S.; Xie, C.; Tay, M.; Zhou, Y.; Williams, R.; Ng, W.J.; Rice, S.A.; Kjelleberg, S. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 2014, 8, 1186–1197. [Google Scholar] [CrossRef]
- Yu, G.-H.; He, P.-J.; Shao, L.-M.; Zhu, Y.-S. Extracellular proteins, polysaccharides and enzymes impact on sludge aerobic digestion after ultrasonic pretreatment. Water Res. 2008, 42, 1925–1934. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, K.; Xu, H.; Li, Y.; Xue, Q.; Xue, W.; Zhang, A.; Wen, X.; Xu, G.; Huang, X. Spectroscopic fingerprints profiling the polysaccharide/protein/humic architecture of stratified extracellular polymeric substances (EPS) in activated sludge. Water Res. 2023, 235, 119866. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, B.; Li, X.; Wang, S.; Wang, W.; Peng, Y. Enrichment of anammox biomass during mainstream wastewater treatment driven by achievement of partial denitrification through the addition of bio-carriers. J. Environ. Sci. 2024, 137, 181–194. [Google Scholar] [CrossRef]
- Yu, X.; Nishimura, F.; Hidaka, T. Impact of Long-Term Perfluorooctanoic Acid (PFOA) Exposure on Activated Sludge Process. Water Air Soil Pollut. 2018, 229, 134. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Zhu, X.; Zheng, X.; Feng, L. Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ. Sci. Technol. 2012, 46, 12452–12458. [Google Scholar] [CrossRef]
- Jiao, Y.; Zou, M.; Yang, X.; Tsang, Y.F.; Chen, H. Perfluorooctanoic acid triggers oxidative stress in anaerobic digestion of sewage sludge. J. Hazard. Mater. 2022, 424, 127418. [Google Scholar] [CrossRef]
- Tang, L.; Su, C.; Wang, Q.; Cao, L.; Xian, Y.; Wen, S.; Zhou, Y.; Gao, S. Use of iron-loaded biochar to alleviate anammox performance inhibition under PFOA stress conditions: Integrated analysis of sludge characteristics and metagenomics. Sci. Total Environ. 2023, 865, 161178. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Song, M.; Zhou, Y. Redistribution of perfluorooctanoic acid in sludge after thermal hydrolysis: Location of protein plays a major role. Water Res. 2023, 241, 120135. [Google Scholar] [CrossRef]
- Du, M.; Xu, D.; Trinh, X.; Liu, S.; Wang, M.; Zhang, Y.; Wu, J.; Zhou, Q.; Wu, Z. EPS solubilization treatment by applying the biosurfactant rhamnolipid to reduce clogging in constructed wetlands. Bioresour. Technol. 2016, 218, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, Y.; Wang, Y.; Kong, Z.; Cao, W.; Zhang, Y. Insight into the Impacts and Removal Pathways of Perfluorooctanoic Acid (PFOA) in Anaerobic Digestion. Water 2022, 14, 2255. [Google Scholar] [CrossRef]
- Yan, W.; Qian, T.; Zhang, L.; Wang, L.; Zhou, Y. Interaction of perfluorooctanoic acid with extracellular polymeric substances—Role of protein. J. Hazard. Mater. 2021, 401, 123381. [Google Scholar] [CrossRef]
- Huang, D.-Q.; Wang, Y.; Li, Z.-Y.; Huang, B.-C.; Yang, M.; Fan, N.-S.; Jin, R.-C. Metabolomics and molecular simulation reveal the responding mechanism of anammox consortia to perfluorooctanoic acid by regulating metabolic network. Chem. Eng. J. 2023, 460, 141712. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Li, B.; Peng, L.; Xu, Y.; Li, R.; Song, K. Effect and mechanism of perfluorooctanoic acid (PFOA) on anaerobic digestion sludge dewaterability. Chemosphere 2023, 335, 139142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, J.; Lv, M.; Yu, H.; Zhao, H.; Xu, X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere 2015, 121, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Deng, D.; Li, R.; Guo, C.; Ma, J.; Chen, M. Investigation of extracellular polymeric substances (EPS) in four types of sludge: Factors influencing EPS properties and sludge granulation. J. Water Process Eng. 2021, 40, 101924. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Korpol, B.R.; Chellam, S.; Gassman, P.L.; Engelhard, M.H.; Lea, A.S.; Rosso, K.M. Spectroscopic Characterization of Extracellular Polymeric Substances from Escherichia coli and Serratia marcescens: Suppression Using Sub-Inhibitory Concentrations of Bismuth Thiols. Biomacromolecules 2008, 9, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, S.; Zhang, Z. Role of extracellular polymeric substance in determining the high aggregation ability of anammox sludge. Water Res. 2015, 75, 51–62. [Google Scholar] [CrossRef]
- Huang, D.; Xu, R.; Sun, X.; Li, Y.; Xiao, E.; Xu, Z.; Wang, Q.; Gao, P.; Yang, Z.; Lin, H.; et al. Effects of perfluorooctanoic acid (PFOA) on activated sludge microbial community under aerobic and anaerobic conditions. Environ. Sci. Pollut. Res. 2022, 29, 63379–63392. [Google Scholar] [CrossRef]
- Xu, R.; Tao, W.; Lin, H.; Huang, D.; Su, P.; Gao, P.; Sun, X.; Yang, Z.; Sun, W. Effects of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonic Acid (PFOS) on Soil Microbial Community. Microb. Ecol. 2022, 83, 929–941. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Wang, Y.; Qian, X. The fate and behavior of perfluorooctanoic acid (PFOA) in constructed wetlands: Insights into potential removal and transformation pathway. Sci. Total Environ. 2023, 861, 160309. [Google Scholar] [CrossRef]
- Uzun, M.; Koziaeva, V.; Dziuba, M.; Alekseeva, L.; Krutkina, M.; Sukhacheva, M.; Baslerov, R.; Grouzdev, D. Recovery and genome reconstruction of novel magnetotactic Elusimicrobiota from bog soil. ISME J. 2023, 17, 204–214. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, L.; Zhang, T.; Zhuang, D.; Wu, Q.; Yu, J.; Tian, C.; Zhang, Z. Gut microbiome signatures of extreme environment adaption in Tibetan pig. NPJ Biofilms Microbiomes 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Qian, W.; Yuan, C.; Yuan, Z.; Peng, Y. Achieving Mainstream Nitrogen Removal through Coupling Anammox with Denitratation. Environ. Sci. Technol. 2017, 51, 8405–8413. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Fernandes, L.; Pereira, A.D.; Leal, C.D.; Davenport, R.; Werner, D.; Filho, C.R.M.; Bressani-Ribeiro, T.; de Lemos Chernicharo, C.A.; de Araujo, J.C. Effect of temperature on microbial diversity and nitrogen removal performance of an anammox reactor treating anaerobically pretreated municipal wastewater. Bioresour. Technol. 2018, 258, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Chen, P.; Sathishkumar, K.; Lu, Y.; Naraginti, S.; Wu, Y.; Wu, H. Biological mediated synthesis of reduced graphene oxide (rGO) as a potential electron shuttle for facilitated biological denitrification: Insight into the electron transfer process. J. Environ. Chem. Eng. 2022, 10, 108225. [Google Scholar] [CrossRef]
- Huang, X.; Yao, K.; Yu, J.; Dong, W.; Zhao, Z. Nitrogen removal performance and microbial characteristics during simultaneous chemical phosphorus removal process using Fe(3). Bioresour. Technol. 2022, 363, 127972. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Liao, H.; Li, X.; Shen, L.; Lin, H.; Sun, L.; Ou, R.; He, D. Hot-pressed membrane assemblies enhancing the biofilm formation and nitrogen removal in a membrane-aerated biofilm reactor. Sci. Total Environ. 2022, 833, 155003. [Google Scholar] [CrossRef]
- Kondrotaite, Z.; Valk, L.C.; Petriglieri, F.; Singleton, C.; Nierychlo, M.; Dueholm, M.K.D.; Nielsen, P.H. Diversity and Ecophysiology of the Genus OLB8 and Other Abundant Uncultured Saprospiraceae Genera in Global Wastewater Treatment Systems. Front. Microbiol. 2022, 13, 917553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).