UV-Light-Driven Photocatalytic Dye Degradation and Antibacterial Potentials of Biosynthesized SiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extract
2.3. Biosynthesis of SiO2 Nanoparticles
2.4. Characterization of SiO2 Nanoparticles
2.5. Photocatalytic Activity
2.6. Antibacterial Activity
3. Result and Discussions
3.1. Biosynthesized SiO2 Nanoparticles Reaction Mechanism
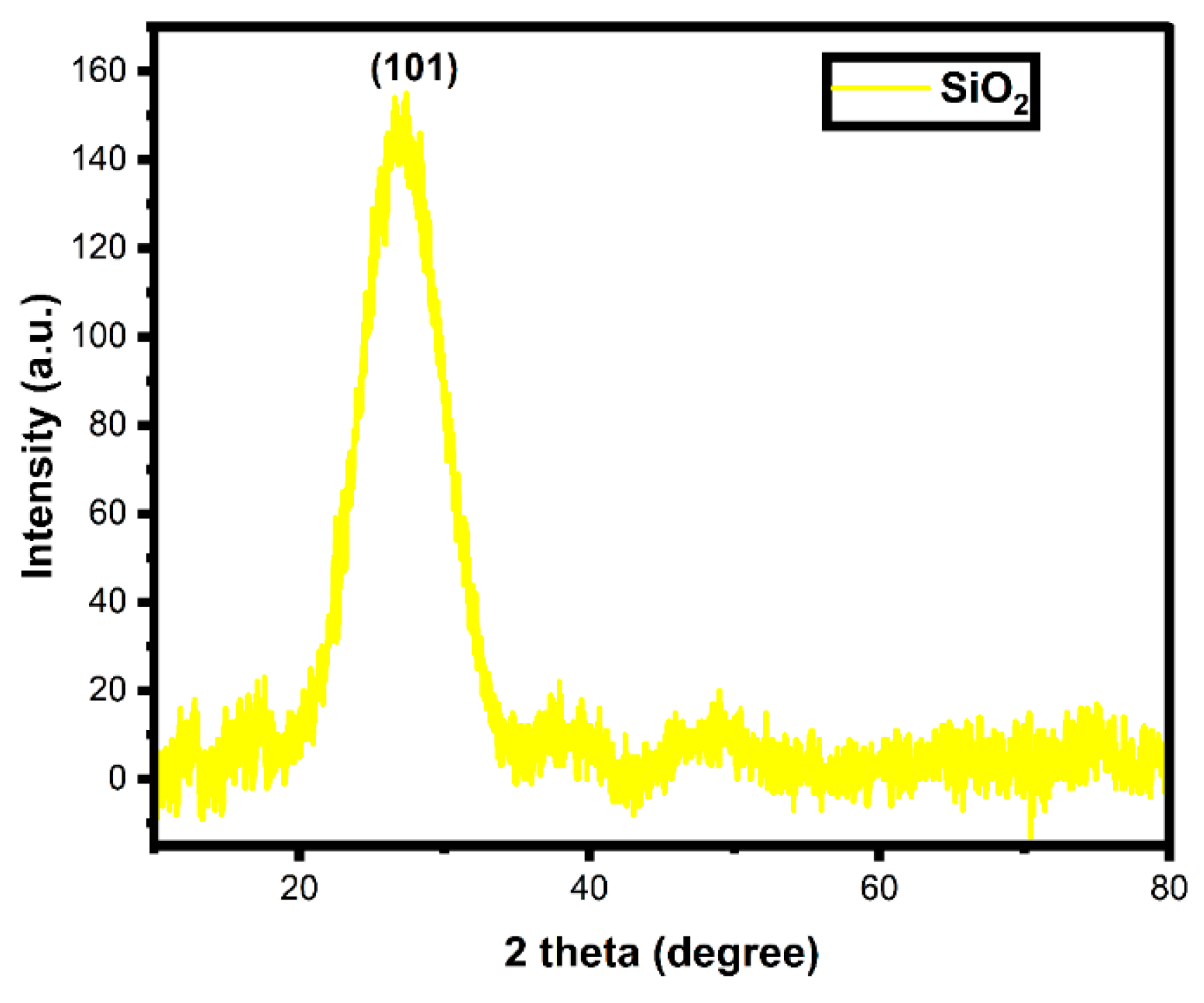
3.2. X-ray Diffraction Analysis
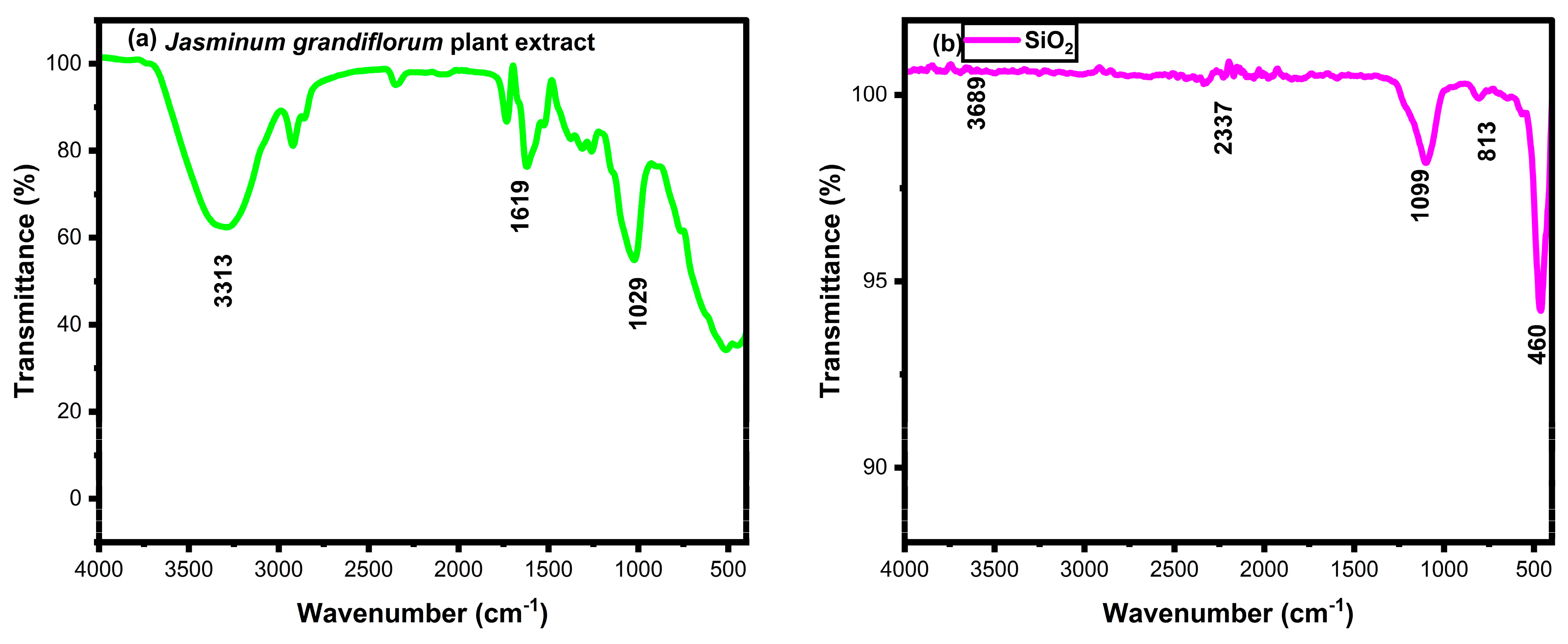
3.3. FTIR Analysis
3.4. UV-Vis DRS Analysis
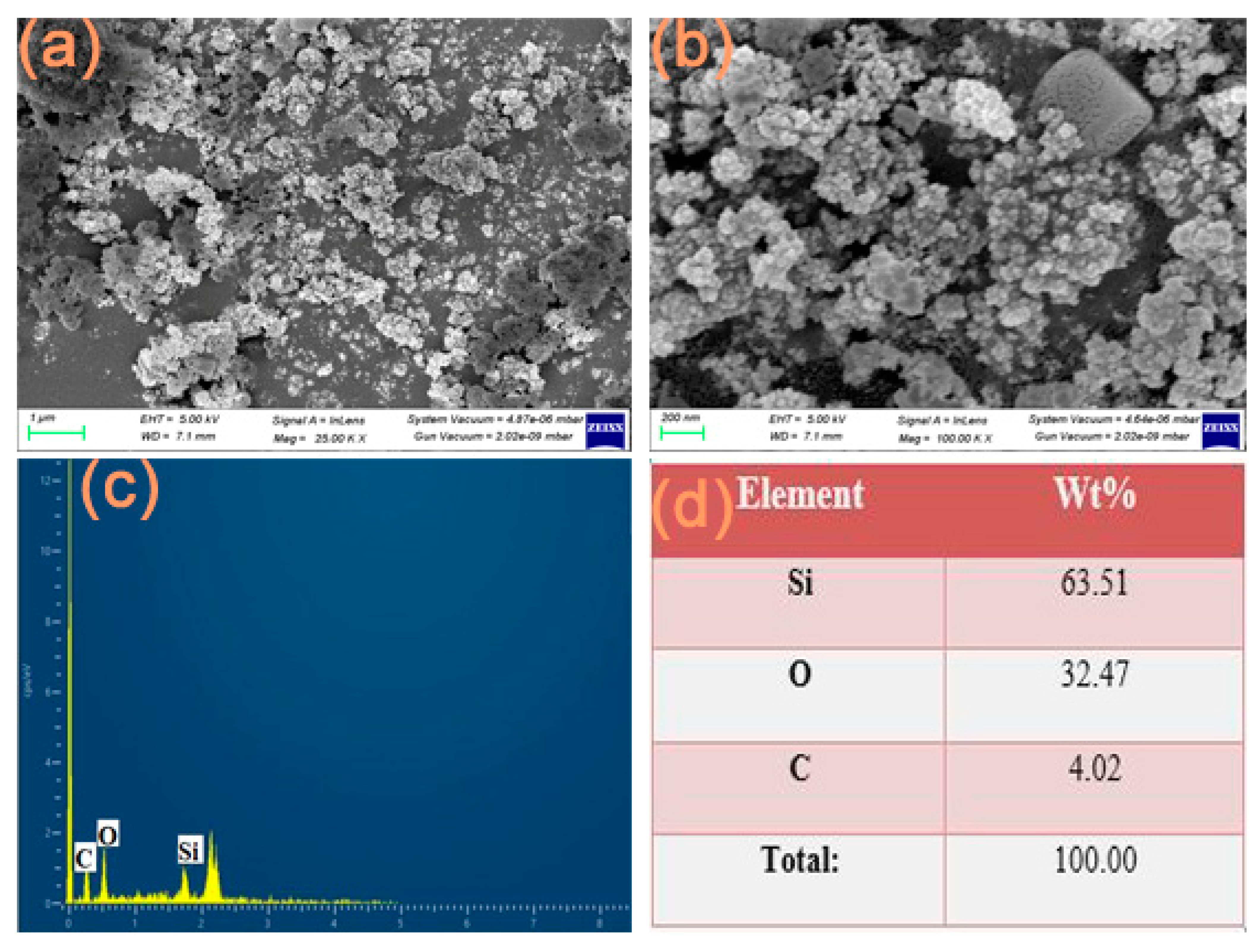
3.5. FESEM with EDX Analysis
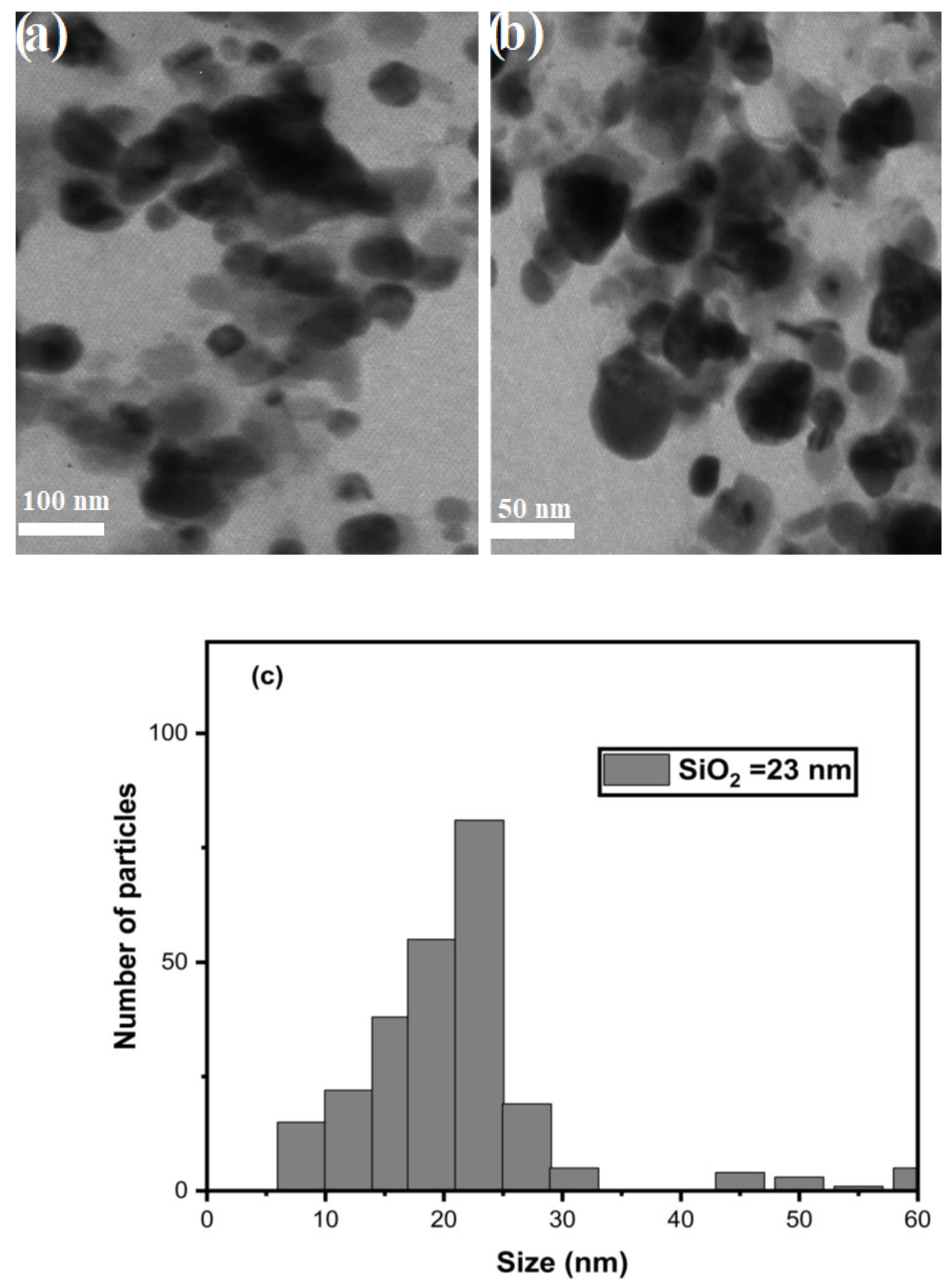
3.6. TEM Analysis
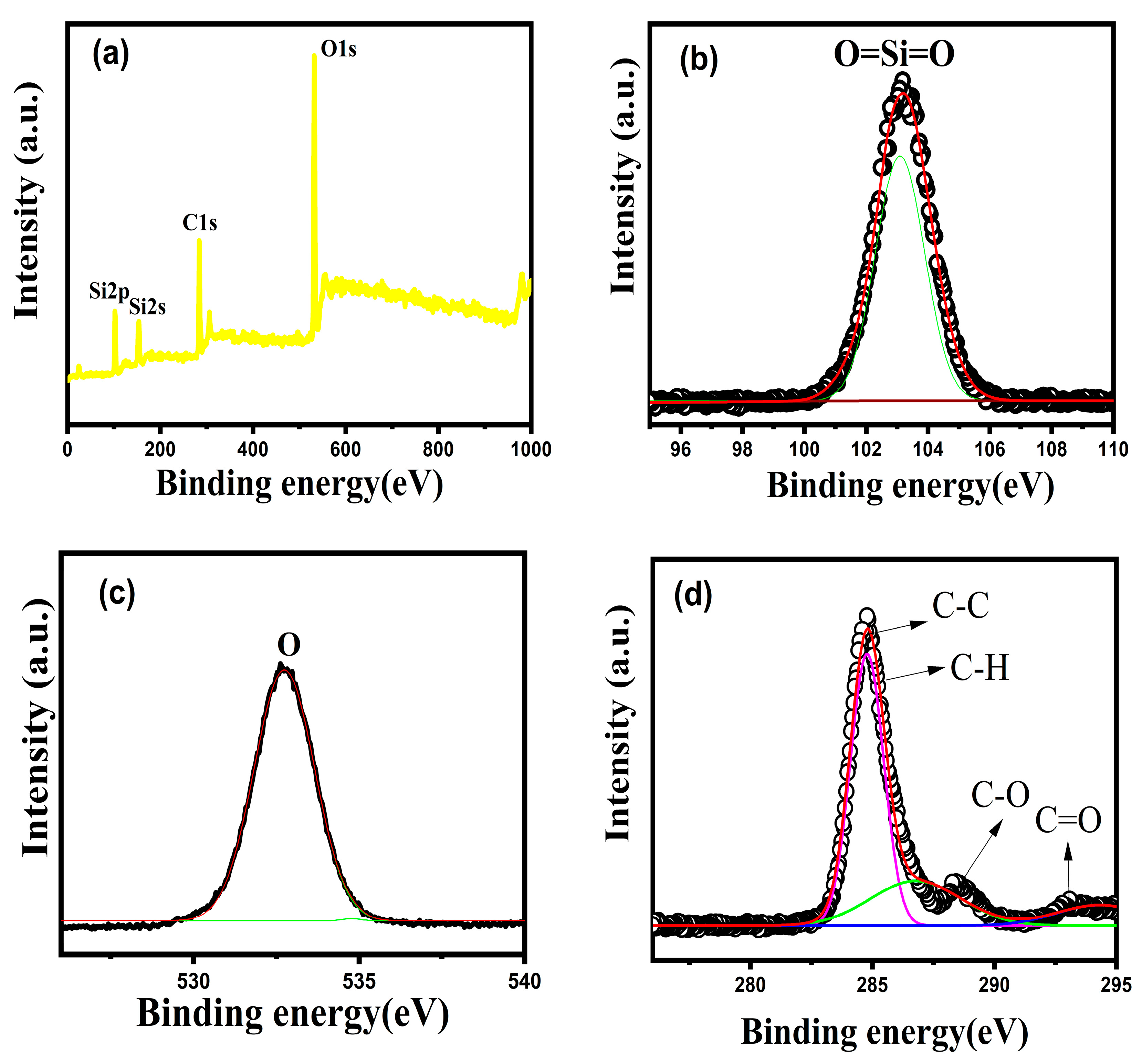
3.7. XPS Analysis
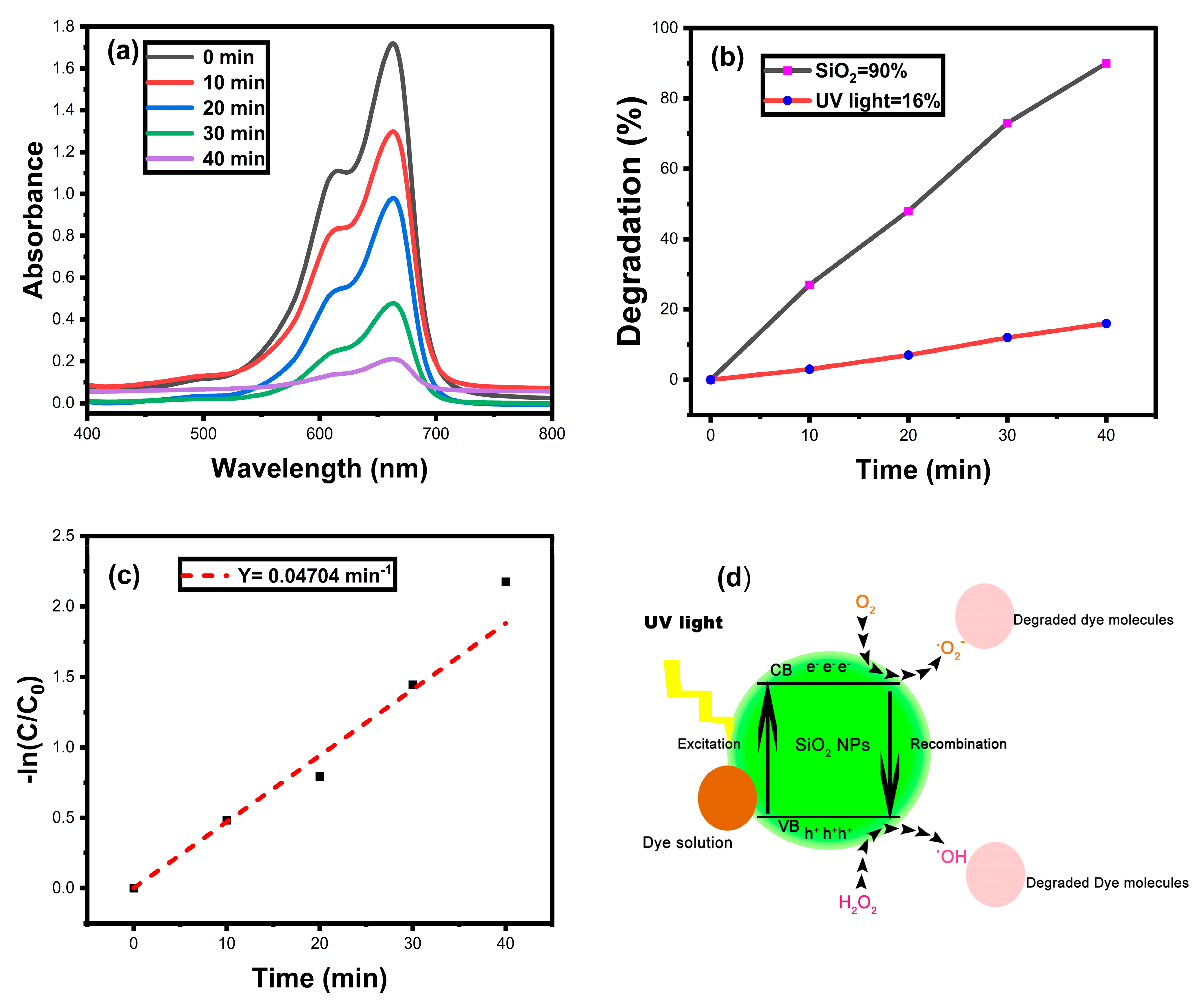
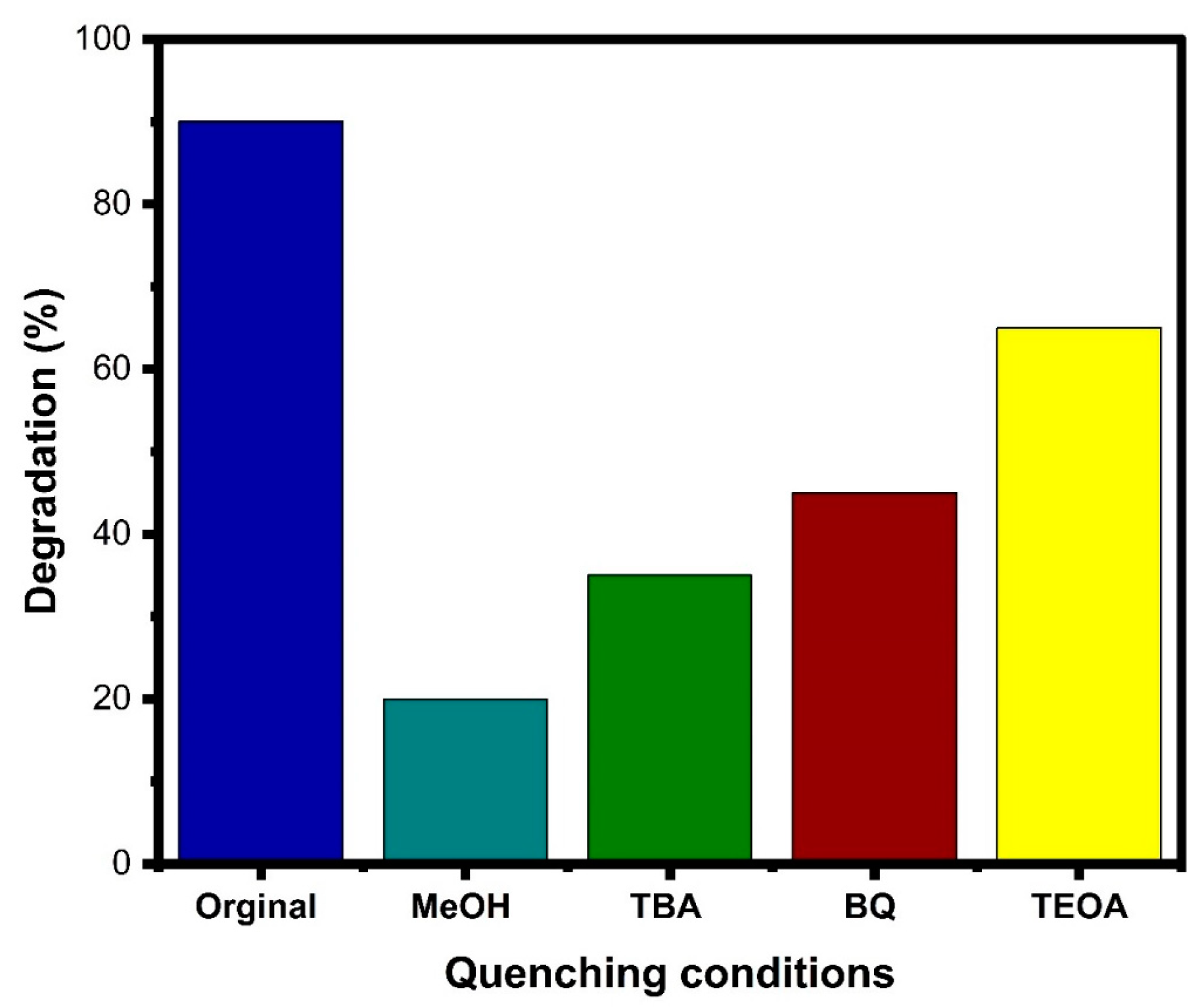
3.8. Photocatalytic Dye Degradation
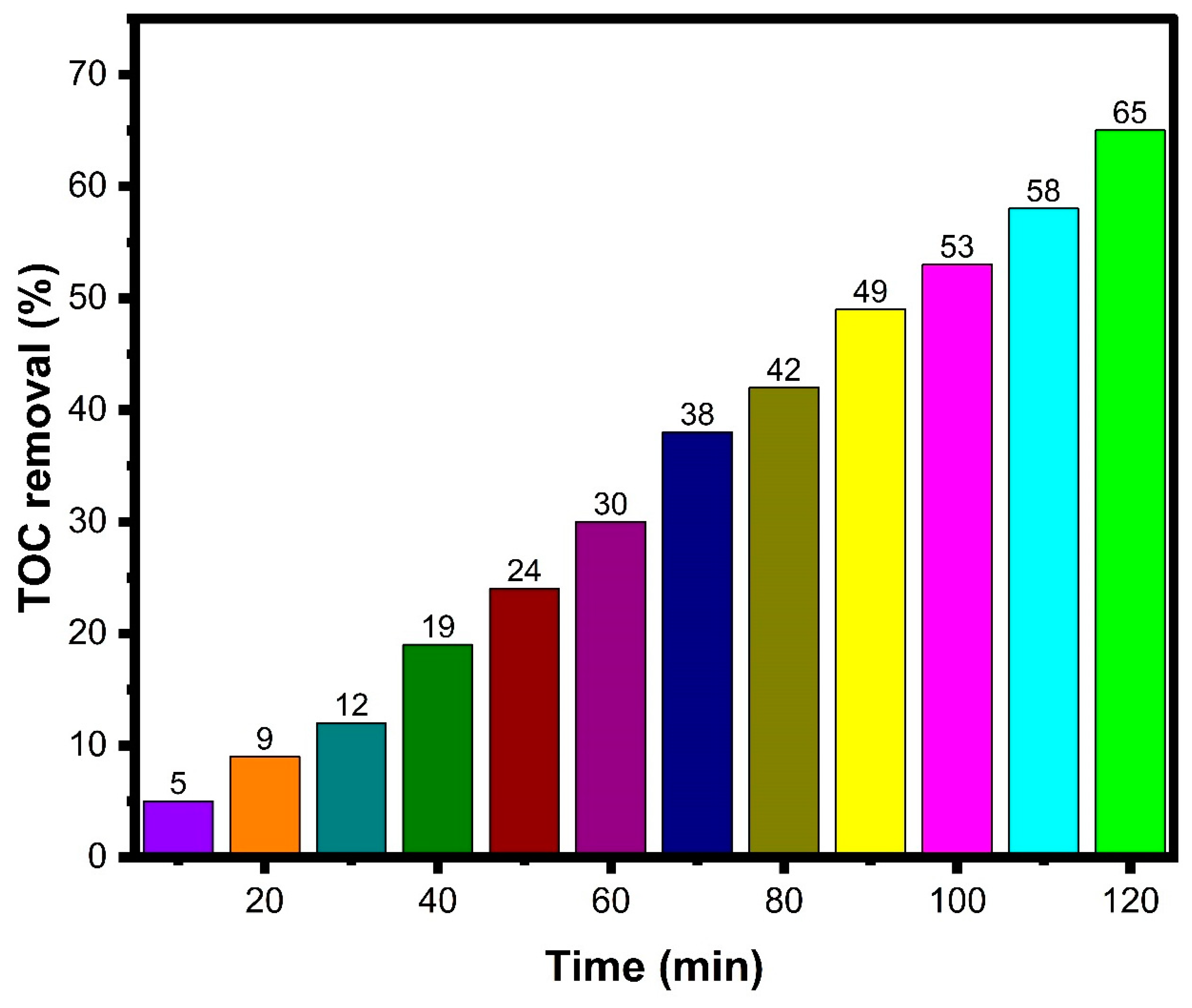
Reusability Analysis
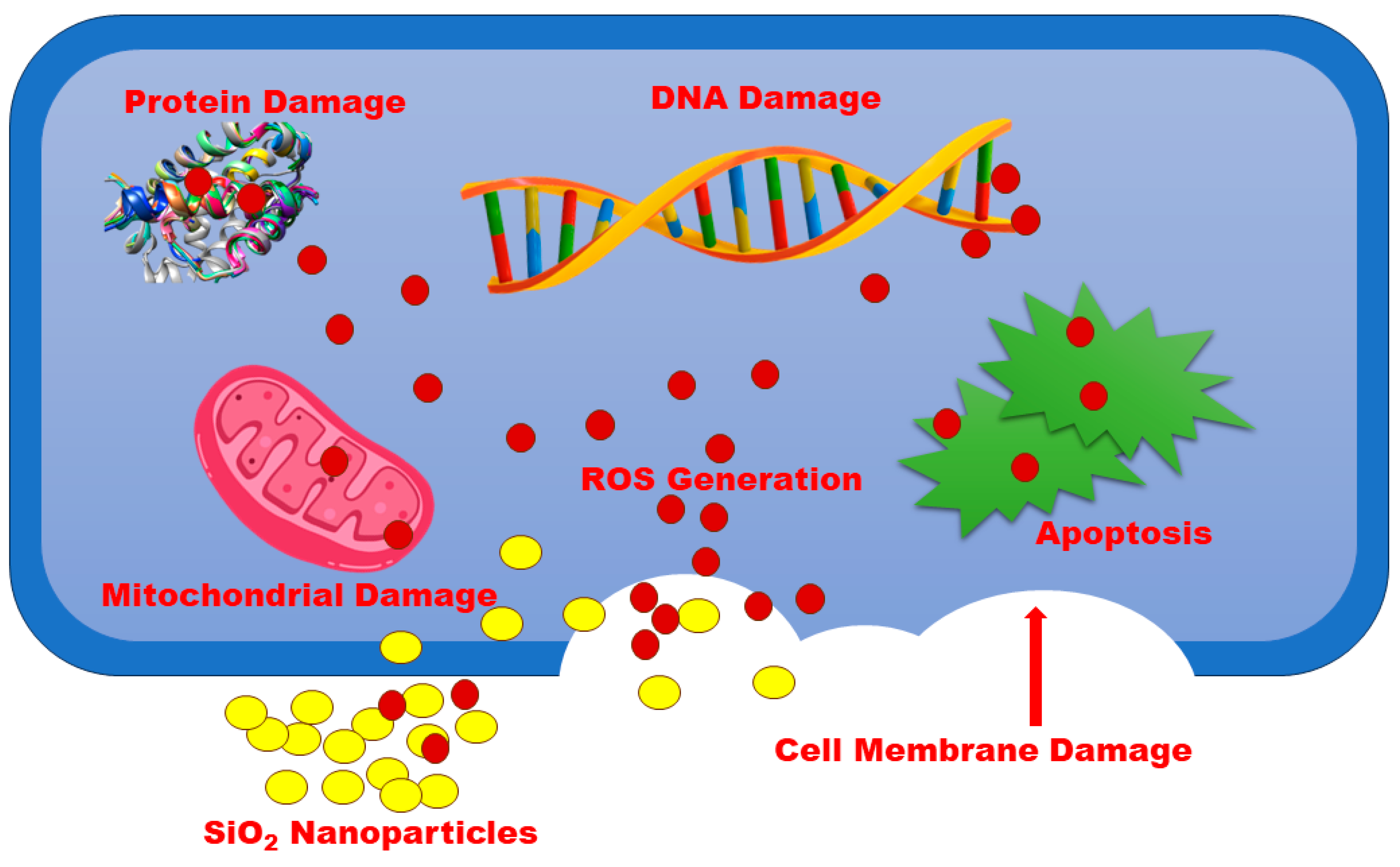
3.9. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: A review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Chaudhari, R.Y.; Nemade, M.S. Azadirachta indica leaves mediated green synthesis of metal oxide nanoparticles: A review. Talanta Open 2022, 5, 100083. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Toprak, M.S.; Fadeel, B. Toxicity of metal and metal oxide nanoparticles. In Handbook on the Toxicology of Metals; Academic Press: London, UK, 2022; pp. 87–126. [Google Scholar]
- Roy, A.; Murthy, H.A.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic synthesis of metal/metal oxide nanoparticles for degradation of dyes. J. Renew. Mater. 2022, 10, 1911–1930. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, J.; Zhang, Y.; Li, K.; Ye, X.; Fang, M.; Tan, X.; Kong, M.; Wang, X. Selective and efficient removal of radioactive ions from water with well-dispersed metal oxide nanoparticles@N-doped carbon. Sep. Purif. Technol. 2022, 285, 120366. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, J.; Kaur, A.; Singh, N. Antimycotic activity of biogenically synthesised metal and metal oxide nanoparticles against plant pathogenic fungus Fusarium moniliforme (F. fujikuroi). Indian J. Exp. Biol. (IJEB) 2022, 58, 263–270. [Google Scholar]
- Kundu, C.K.; Song, L.; Hu, Y. Chitosan-metal oxide nanoparticle hybrids in developing bi-functional polyamide 66 textiles with enhanced flame retardancy and wettability. Appl. Surf. Sci. Adv. 2022, 7, 100202. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M.; Martinez, F.; Kahrizi, D.; Khan, H.; Rose Alencar De Menezes, I.; Coutinho, H.D.M.; Costa, J.G.M. The efficiency of metal, metal oxide, and metalloid nanoparticles against cancer cells and bacterial pathogens: Different mechanisms of action. Cell. Mol. Biomed. Rep. 2022, 2, 10–21. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: A review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Hammad, E.N.; Salem, S.S.; Mohamed, A.A.; El-Dougdoug, W. Environmental impacts of ecofriendly iron oxide nanoparticles on dyes removal and antibacterial activity. Appl. Biochem. Biotechnol. 2022, 194, 6053–6067. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, P.; Zachariah, M.R. Direct Imaging and Simulation of the Interface Reaction of Metal/Metal Oxide Nanoparticle Laminates. J. Phys. Chem. C 2022, 126, 8684–8691. [Google Scholar] [CrossRef]
- Kumar, P.; Tomar, V.; Kumar, D.; Joshi, R.K.; Nemiwal, M. Magnetically active iron oxide nanoparticles for catalysis of organic transformations: A review. Tetrahedron 2022, 106–107, 132641. [Google Scholar] [CrossRef]
- Mabrouk, M.; Ibrahim Fouad, G.; El-Sayed, S.A.; Rizk, M.Z.; Beherei, H.H. Hepatotoxic and neurotoxic potential of iron oxide nanoparticles in Wistar rats: A biochemical and ultrastructural study. Biol. Trace Elem. Res. 2022, 200, 3638–3665. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.; Ahmed, W.; Al Ayoubi, S.; Nisa, S.; Bibi, Y.; Sabir, M.; Khan, M.M.; Ahmed, W.; Qayyum, A. Fungicidal synergistic effect of biogenically synthesized zinc oxide and copper oxide nanoparticles against Alternaria citri causing citrus black rot disease. Saudi J. Biol. Sci. 2022, 29, 88–95. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; El-Belely, E.F.; Awad, M.A.; Hassan, S.E.D.; Al-Faifi, Z.E.; Hamza, M.F. Enhanced antimicrobial, cytotoxicity, larvicidal, and repellence activities of brown algae, Cystoseira crinita-mediated green synthesis of magnesium oxide nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, Q.; Zhang, M. A review of non-point source water pollution modeling for the urban–rural transitional areas of China: Research status and prospect. Sci. Total Environ. 2022, 826, 154146. [Google Scholar] [CrossRef] [PubMed]
- Mumbi, A.W.; Watanabe, T. Cost Estimations of Water Pollution for the Adoption of Suitable Water Treatment Technology. Sustainability 2022, 14, 649. [Google Scholar] [CrossRef]
- Haghnazar, H.; Cunningham, J.A.; Kumar, V.; Aghayani, E.; Mehraein, M. COVID-19 and urban rivers: Effects of lockdown period on surface water pollution and quality-a case study of the Zarjoub River, north of Iran. Environ. Sci. Pollut. Res. 2022, 29, 27382–27398. [Google Scholar] [CrossRef]
- Huang, Y.; Mi, F.; Wang, J.; Yang, X.; Yu, T. Water pollution incidents and their influencing factors in China during the past 20 years. Environ. Monit. Assess. 2022, 194, 182. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Zughaibi, T.A.; Javed, M. Water quality index, Labeo rohita, and Eichhornia crassipes: Suitable bio-indicators of river water pollution. Saudi J. Biol. Sci. 2022, 29, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Jiao, G.; Wang, J.; Li, J.; Li, M.; Jiang, H. Spatial Pattern of Technological Innovation in the Yangtze River Delta Region and Its Impact on Water Pollution. Int. J. Environ. Res. Public Health 2022, 19, 7437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Granger, S.J.; Semenov, M.A.; Upadhayay, H.R.; Collins, A.L. Diffuse water pollution during recent extreme wet-weather in the UK: Environmental damage costs and insight into the future? J. Clean. Prod. 2022, 338, 130633. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.R.; Barik, M.; Prasad, K.V.; Kumar, A.; Verma, A.K.; Sahoo, S.K.; Jha, V.; Sahoo, N.K. Hydro-geochemical analysis based on entropy and geostatistics model for delineation of anthropogenic ground water pollution for health risks assessment of Dhenkanal district, India. Ecotoxicology 2022, 31, 549–564. [Google Scholar] [CrossRef]
- Cordoba, A.; Saldias, C.; Urzúa, M.; Montalti, M.; Guernelli, M.; Focarete, M.L.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12, 756. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Shi, B.; Fan, W.; Li, Z.; Chen, Y. Facile fabrication of superhydrophobic paper with durability, chemical stability and self-cleaning by roll coating with modified nano-TiO2. Appl. Nanosci. 2020, 10, 4063–4073. [Google Scholar] [CrossRef]
- Akbari, R.; Godeau, G.; Mohammadizadeh, M.; Guittard, F.; Darmanin, T. The influence of bath temperature on the one-step electrodeposition of non-wetting copper oxide coatings. Appl. Surf. Sci. 2020, 503, 144094. [Google Scholar] [CrossRef]
- Cooney, R.; Wan, A.H.; O’Donncha, F.; Clifford, E. Designing environmentally efficient aquafeeds through the use of multicriteria decision support tools. Curr. Opin. Environ. Sci. Health 2021, 23, 100276. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, W.; Huang, J.; Wang, Y.; Guo, H. ZnO Promoted Persulfate Activation in Discharge Plasma System for Ofloxacin Degradation. Catalysts 2023, 13, 847. [Google Scholar] [CrossRef]
- Lopes, O.F.; Paris, E.C.; Ribeiro, C. Synthesis of Nb2O5 nanoparticles through the oxidant peroxide method applied to organic pollutant photodegradation: A mechanistic study. Appl. Catal. B Environ. 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Batista, L.M.B.; Dos Santos, A.J.; da Silva, D.R.; de Melo Alves, A.P.; Garcia-Segura, S.; Martínez-Huitle, C.A. Solar photocatalytic application of NbO2OH as alternative photocatalyst for water treatment. Sci. Total Environ. 2017, 596, 79–86. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Batista, L.M.B.; Martínez-Huitle, C.A.; Alves, A.P.D.M.; Garcia-Segura, S. Niobium oxide catalysts as emerging material for textile wastewater reuse: Photocatalytic decolorization of azo dyes. Catalysts 2019, 9, 1070. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Pang, H.; Xiong, Y.; Ma, S.; Duan, Y.; Hou, Y.; Mao, C. Lightweight PPy aerogel adopted with Co and SiO2 nanoparticles for enhanced electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 97, 213–222. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface functionalization of bamboo leave mediated synthesized SiO2 nanoparticles: Study of adsorption mechanism, isotherms and enhanced adsorption capacity for removal of Cr (VI) from aqueous solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Alqahtani, M.J.; Mostafa, S.A.; Hussein, I.A.; Elhawary, S.; Mokhtar, F.A.; Albogami, S.; Tomczyk, M.; Batiha, G.E.-S.; Negm, W.A. Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation. Metabolites 2022, 12, 792. [Google Scholar] [CrossRef]
- Mansour, K.A.; El-Neketi, M.; Lahloub, M.F.; Elbermawi, A. Nanoemulsions of Jasminum humile L. and Jasminum grandiflorum L. essential oils: An approach to enhance their cytotoxic and antiviral effects. Molecules 2022, 27, 3639. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Wen, S.; Li, Q.; Chen, R.; Lai, X.; Zhang, Z.; Zhou, Z.; Xie, Y.; Zheng, X.; et al. Extract of Jasminum grandiflorum L. alleviates CCl4-induced liver injury by decreasing inflammation, oxidative stress and hepatic CYP2E1 expression in mice. Biomed. Pharmacother. 2022, 152, 113255. [Google Scholar] [CrossRef]
- Mansour, K.A.; Elbermawi, A.; Al-Karmalawy, A.A.; Lahloub, M.F.; El-Neketi, M. Cytotoxic effects of extracts obtained from plants of the Oleaceae family: Bio-guided isolation and molecular docking of new secoiridoids from Jasminum humile. Pharm. Biol. 2022, 60, 1374–1383. [Google Scholar] [CrossRef]
- Sharmila, M.; Mani, R.J.; Parvathiraja, C.; Kader, S.M.A.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Photocatalytic Dye Degradation and Bio-Insights of Honey-Produced α-Fe2O3 Nanoparticles. Water 2022, 14, 2301. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schweiger, T.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Chemical composition, olfactory evaluation and antimicrobial activities of Jasminum grandiflorum L. absolute from India. Nat. Prod. Commun. 2007, 2, 1934578X0700200411. [Google Scholar] [CrossRef]
- Zhao, G.; Yin, Z.; Dong, J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J. Ethnopharmacol. 2009, 125, 265–268. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Depciuch, J.; Fryc, B.; Kus-Liskiewicz, M. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using clove eugenol. Appl. Organomet. Chem. 2018, 32, e4276. [Google Scholar] [CrossRef]
- Amritphale, S.S.; Mishra, D.; Mudgal, M.; Chouhan, R.K.; Chandra, N. A novel green approach for making hybrid inorganic-organic geopolymeric cementitious material utilizing fly ash and rice husk. J. Environ. Chem. Eng. 2016, 4, 3856–3865. [Google Scholar] [CrossRef]
- Da Silva, V.J.; de Almeida, E.P.; Gonçalves, W.P.; da Nóbrega, R.B.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; de Lima Santana, L.N. Mineralogical and dielectric properties of mullite and cordierite ceramics produced using wastes. Ceram. Int. 2019, 45, 4692–4699. [Google Scholar] [CrossRef]
- Gonçalves, W.P.; Silva, V.J.; Menezes, R.R.; Neves, G.A.; Lira, H.L.; Santana, L.N. Microstructural, physical and mechanical behavior of pastes containing clays and alumina waste. Appl. Clay Sci. 2017, 137, 259–265. [Google Scholar] [CrossRef]
- Basak, M.; Rahman, M.L.; Ahmed, M.F.; Biswas, B.; Sharmin, N. The use of X-ray diffraction peak profile analysis to determine the structural parameters of cobalt ferrite nanoparticles using Debye-Scherrer, Williamson-Hall, Halder-Wagner and Size-strain plot: Different precipitating agent approach. J. Alloys Compd. 2022, 895, 162694. [Google Scholar] [CrossRef]
- Selvanayaki, R.; Rameshbabu, M.; Muthupandi, S.; Razia, M.; Florence, S.S.; Ravichandran, K.; Prabha, K. Structural, optical and electrical conductivity studies of pure and Fe doped ZincOxide (ZnO) nanoparticles. Mater. Today Proc. 2022, 49, 2628–2631. [Google Scholar] [CrossRef]
- Dong, R.; Wang, L.; Zhu, J.; Liu, L.; Qian, Y. A novel SiO2–GO/acrylic resin nanocomposite: Fabrication, characterization and properties. Appl. Phys. A 2019, 125, 551. [Google Scholar] [CrossRef]
- Bogart, K.H.A.; Dalleska, N.F.; Bogart, G.R.; Fisher, E.R. Plasma enhanced chemical vapor deposition of SiO2 using novel alkoxysilane precursors. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 476–480. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef]
- Sankareswaran, M.; Vanitha, M.; Periakaruppan, R.; Anbukumaran, A. Phyllanthus emblica mediated silica nanomaterials: Biosynthesis, structural and stability analysis. Silicon 2022, 14, 10123–10127. [Google Scholar] [CrossRef]
- Ulfa, M.; Prasetyoko, D.; Trisunaryanti, W.; Bahruji, H.; Fadila, Z.A.; Sholeha, N.A. The effect of gelatin as pore expander in green synthesis mesoporous silica for methylene blue adsorption. Sci. Rep. 2022, 12, 15271. [Google Scholar] [CrossRef]
- Salimi, E.; Nigje, A.K. Investigating the antibacterial activity of carboxymethyl cellulose films treated with novel Ag@ GO decorated SiO2 nanohybrids. Carbohydr. Polym. 2022, 298, 120077. [Google Scholar] [CrossRef]
- Madani, M.; Mansourian, M.; Almadari, S.; Mirzaee, O.; Tafreshi, M.J. Enhanced photosensitivity of heterostructure SiO2/Bi2WO6/GO composite nanoparticles. Phys. B Condens. Matter 2022, 645, 414241. [Google Scholar] [CrossRef]
- Nikpassand, M.; Fekri, L.Z.; Varma, R.S.; Hassanzadi, L.; Pashaki, F.S. Green synthesis of novel 5-amino-bispyrazole-4-carbonitriles using a recyclable Fe3O4@SiO2@vanillin@thioglycolic acid nano-catalyst. RSC Adv. 2022, 12, 834–844. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Montoro Bustos, A.R.; Pereira Reyes, R.; Paniagua, S.A.; Vega-Baudrit, J.R. Novel pathway for the sonochemical synthesis of silver nanoparticles with near-spherical shape and high stability in aqueous media. Sci. Rep. 2022, 12, 882. [Google Scholar] [CrossRef]
- Barnawi, N.; Allehyani, S.; Seoudi, R. Biosynthesis and characterization of gold nanoparticles and its application in eliminating nickel from water. J. Mater. Res. Technol. 2022, 17, 537–545. [Google Scholar] [CrossRef]
- Borzęcka, W.; Pereira, P.M.; Fernandes, R.; Trindade, T.; Torres, T.; Tomé, J.P. Spherical and rod shaped mesoporous silica nanoparticles for cancer-targeted and photosensitizer delivery in photodynamic therapy. J. Mater. Chem. B 2022, 10, 3248–3259. [Google Scholar] [CrossRef]
- Rybin, M.V.; Sinelnik, A.D.; Tajik, M.; Milichko, V.A.; Ubyivovk, E.V.; Yakovlev, S.A.; Pevtsov, A.B.; Yavsin, D.A.; Zuev, D.A.; Makarov, S.V. Optically Reconfigurable Spherical Ge-Sb-Te Nanoparticles with Reversible Switching. Laser Photonics Rev. 2022, 16, 2100253. [Google Scholar] [CrossRef]
- Arif, M.; Kumam, P.; Kumam, W.; Mostafa, Z. Heat transfer analysis of radiator using different shaped nanoparticles water-based ternary hybrid nanofluid with applications: A fractional model. Case Stud. Therm. Eng. 2022, 31, 101837. [Google Scholar] [CrossRef]
- Zonarsaghar, A.; Mousavi-Kamazani, M.; Zinatloo-Ajabshir, S. Sonochemical synthesis of CeVO4 nanoparticles for electrochemical hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 5403–5417. [Google Scholar] [CrossRef]
- Behzadnia, H.; Jin, H.; Najafian, M.; Hatami, M. Investigation of super-critical water-based nanofluid with different nanoparticles (shapes and types) used in the rectangular corrugated tube of reactors. Alex. Eng. J. 2022, 61, 2330–2347. [Google Scholar] [CrossRef]
- Sakata, T.; Ogawa, S.; Inoue, K.; Shimizu, Y.; Tanahashi, Y. Effects of post-deposition annealing on chemical composition of SiNx film in SiO2/SiNx/SiO2/Si stacked structure. Surf. Interface Anal. 2022, 54, 661–666. [Google Scholar] [CrossRef]
- Arellano-Galindo, L.G.; Reynosa-Martínez, A.C.; Gaitán-Arévalo, J.R.; Valerio-Rodríguez, M.F.; Vargas-Gutiérrez, G.; López-Honorato, E. Superhydrophobic to superhydrophilic wettability transition of functionalized SiO2 nanoparticles. Ceram. Int. 2022, 48, 21631–21637. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhang, Y.; Yu, H.; Qu, Y.; Yu, J. Inorganic Metal-Oxide Photocatalyst for H2O2 Production. Small 2022, 18, 2104561. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Khan, A.A.P.; Singh, A.; Nadda, A.K.; Hussain, C.M.; Van Le, Q.; Rizevsky, S.; Nguyen, V.H.; Raizada, P. Photocatalytic transition-metal-oxides-based p–n heterojunction materials: Synthesis, sustainable energy and environmental applications, and perspectives. J. Nanostruct. Chem. 2023, 13, 129–166. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd (ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Wang, J.; Rao, C.; Lu, L.; Zhang, S.; Muddassir, M.; Liu, J. Efficient photocatalytic degradation of methyl violet using two new 3D MOFs directed by different carboxylate spacers. CrystEngComm 2021, 23, 741–747. [Google Scholar] [CrossRef]
- Li, Q.; Wei, G.; Duan, G.; Zhang, L.; Li, Z.; Wei, Z.; Zhou, Q.; Pei, R. Photocatalysis activation of peroxydisulfate over oxygen vacancies-rich mixed metal oxide derived from red mud-based layered double hydroxide for ciprofloxacin degradation. Sep. Purif. Technol. 2022, 289, 120733. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Sasaki, K. Fabrication of visible-light-active ZnCr mixed metal oxide/fly ash for photocatalytic activity toward pharmaceutical waste ciprofloxacin. J. Ind. Eng. Chem. 2022, 108, 263–273. [Google Scholar] [CrossRef]
- Govindhan, P.; Pragathiswaran, C. Silver nanoparticle decorated on ZnO@SiO2 nanocomposite and application for photocatalytic dye degradation of methylene blue. Natl. Acad. Sci. Lett. 2019, 42, 323–326. [Google Scholar] [CrossRef]
- Govindhan, P.; Pragathiswaran, C. Synthesis and characterization of TiO2@SiO2–Ag nanocomposites towards photocatalytic degradation of rhodamine B and methylene blue. J. Mater. Sci. Mater. Electron. 2016, 27, 8778–8785. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Mansoor, Q.; Ahmed, I. Graphene/SiO2 nanocomposites: The enhancement of photocatalytic and biomedical activity of SiO2 nanoparticles by graphene. J. Appl. Phys. 2017, 121, 244901. [Google Scholar] [CrossRef]
- Kim, C.; Choi, M.; Jang, J. Nitrogen-doped SiO2/TiO2 core/shell nanoparticles as highly efficient visible light photocatalyst. Catal. Commun. 2010, 11, 378–382. [Google Scholar] [CrossRef]
- Arshad, M.; Qayyum, A.; Shar, G.A.; Soomro, G.A.; Nazir, A.; Munir, B.; Iqbal, M. Zn-doped SiO2 nanoparticles preparation and characterization under the effect of various solvents: Antibacterial, antifungal and photocatlytic performance evaluation. J. Photochem. Photobiol. B Biol. 2018, 185, 176–183. [Google Scholar] [CrossRef]
- Rani, N.; Ahlawat, R.; Goswami, B. Annealing effect on bandgap energy and photocatalytic properties of CeO2–SiO2 nanocomposite prepared by sol-gel technique. Mater. Chem. Phys. 2020, 241, 122401. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, L.; Yang, R.; Zheng, J.; Li, X. Synthesis, characterization, and photocatalytic properties of Ag modified hollow SiO2/TiO2 hybrid microspheres. Appl. Catal. B Environ. 2011, 103, 181–189. [Google Scholar] [CrossRef]
- Sarani, M.; Joshaghani, A.B.; Najafidoust, A.; Asl, E.A.; Hakki, H.K.; Bananifard, H.; Sillanpaa, M. Sun-light driven photo degradation of organic dyes from wastewater on precipitation Ag2CrO4 over SiO2-aerogel and nano silica. Inorg. Chem. Commun. 2021, 133, 108877. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Shao, C.; Li, X.; Jiang, X.; Liu, Y. Fabrication of g-C3N4/SiO2-Au composite nanofibers with enhanced visible photocatalytic activity. Ceram. Int. 2017, 43, 15699–15707. [Google Scholar] [CrossRef]
- Hu, C.; Tang, Y.; Jimmy, C.Y.; Wong, P.K. Photocatalytic degradation of cationic blue X-GRL adsorbed on TiO2/SiO2 photocatalyst. Appl. Catal. B Environ. 2003, 40, 131–140. [Google Scholar] [CrossRef]
- Li, X.; Ye, J. Photocatalytic degradation of rhodamine B over Pb3Nb4O13/fumed SiO2 composite under visible light irradiation. J. Phys. Chem. C 2007, 111, 13109–13116. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Nd2O3-SiO2 nanocomposites: A simple sonochemical preparation, characterization and photocatalytic activity. Ultrason. Sonochem. 2018, 42, 171–182. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Synthesis of highly monodispersed teardrop-shaped core–shell SiO2/TiO2 nanoparticles and their photocatalytic activities. Appl. Surf. Sci. 2015, 351, 320–326. [Google Scholar] [CrossRef]
- Alavi, M. Bacteria and fungi as major bio-sources to fabricate silver nanoparticles with antibacterial activities. Expert Rev. Anti-Infect. Ther. 2022, 20, 897–906. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Nivetha, A.; Prabha, I. Surfactant-Enhanced Nano Spinel Oxide for Applications in Catalysis, Dye Degradation and Antibacterial Activity. ChemistrySelect 2022, 7, e202202389. [Google Scholar] [CrossRef]
- Al-Mushki, A.A.; Ahmed, A.A.A.; Abdulwahab, A.M.; Al-Asbahi, B.A.; Abduljalil, J.; Saad, F.A.; Al-Hada, N.M.; Qaid, S.M.; Ghaithan, H.M. Structural, optical, and antibacterial characteristics of mixed metal oxide CdO–NiO–Fe2O3 nanocomposites prepared using a self-combustion method at different polyvinyl alcohol concentrations. Appl. Phys. A 2022, 128, 279. [Google Scholar] [CrossRef]
- Prisrin, S.A.; Priyanga, M.; Ponvel, K.M.; Kaviarasan, K.; Kalidass, S. Plant Mediated Approach for the Fabrication of Nano CuO–NiO Mixed Oxides Using Aqueous Extract of Mimusops Elengi Leaf: Green Synthesis, Characterization and Antibacterial Activity Studies. J. Clust. Sci. 2022, 33, 765–772. [Google Scholar] [CrossRef]
- Matalkeh, M.; Nasrallah, G.K.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, K.M. Visible light photocatalytic activity of Ag/WO3 nanoparticles and its antibacterial activity under ambient light and in the dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Approved Standard M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, pp. 11–13. [Google Scholar]
- Dolgov, A.; Lopaev, D.; Lee, C.J.; Zoethout, E.; Medvedev, V.; Yakushev, O.; Bijkerk, F. Characterization of carbon contamination under ion and hot atom bombardment in a tin-plasma extreme ultraviolet light source. Appl. Surf. Sci. 2015, 353, 708–713. [Google Scholar] [CrossRef]



| S.no | Sample | Dye | Light Source | Dosage | Dye Volume | Degradation Percentage | Ref |
|---|---|---|---|---|---|---|---|
| 1. | ZnO@SiO2-Ag | MB | Visible light | 0.1 g | 100 mL | 81 | [76] |
| 2. | TiO2@SiO2-Ag | Rh-B and MB | Visible light | 100 mg | 100 mL | 92 and 85 | [77] |
| 3. | Core–shell SiO2/TiO2 | MB | Sun light | 10 mg | 100 mL | 97.7 | [88] |
| 4. | Nitrogen-doped SiO2/TiO2 | MB | Visible light | 0.010 g | 20 mL | 96 | [79] |
| 5. | Zn-doped-SiO2 | CV | Solar light | 0.5 mg | 100 mL | 85.5 | [80] |
| 6. | CeO2-SiO2 | Rhodamine 6G | Visible light | 5 mg | 5 mL | 98 | [81] |
| 7. | Ag-modified hollow SiO2/TiO2 | Rh-B | Visible light | 30 mg | 50 mL | 90 | [82] |
| 8. | Graphene/SiO2 | MO | UV light | 0.03g | 100 mL | 99 | [78] |
| 9. | Ag2CrO4 over SiO2-aerogel | ORGANIC DYE | Visible light | 0.2 g | 100 mL | 95.4 | [83] |
| 10. | g-C3N4/SiO2-Au | Rh-B | Visible light | 0.05 g | 50 mL | 99.8 | [84] |
| 11. | TiO2/SiO2 | cationic blue X-GRL | UV light | 0.08 g | 50 mL | 95 | [85] |
| 12. | Pb3Nb4O13/fumed SiO2 | Rhodamine B | Visible light | 0.3 g | 100 mL | 96 | [86] |
| 13. | Nd2O3-SiO2 | MV | UV light | 120 mg | 50 mL | 95 | [87] |
| 14. | SiO2 | MB | UV light | 10 mg | 100 mL | 90 | Present work |
| Control | Zone Diameter According to the Criteria Published CLSI [95] | ||
|---|---|---|---|
| Resistant (mm) | Intermediate (mm) | Susceptible (mm) | |
| Amikacin | ≤14 | 15–16 | ≥17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, P.; Gupta, J.K.; Mohammad Wabaidur, S.; Siddiqui, M.R.; Foon Lee, S.; Lai, W.-C. UV-Light-Driven Photocatalytic Dye Degradation and Antibacterial Potentials of Biosynthesized SiO2 Nanoparticles. Water 2023, 15, 2973. https://doi.org/10.3390/w15162973
Chelliah P, Gupta JK, Mohammad Wabaidur S, Siddiqui MR, Foon Lee S, Lai W-C. UV-Light-Driven Photocatalytic Dye Degradation and Antibacterial Potentials of Biosynthesized SiO2 Nanoparticles. Water. 2023; 15(16):2973. https://doi.org/10.3390/w15162973
Chicago/Turabian StyleChelliah, Parvathiraja, Jeetendra Kumar Gupta, Saikh Mohammad Wabaidur, Masoom Raza Siddiqui, Siaw Foon Lee, and Wen-Cheng Lai. 2023. "UV-Light-Driven Photocatalytic Dye Degradation and Antibacterial Potentials of Biosynthesized SiO2 Nanoparticles" Water 15, no. 16: 2973. https://doi.org/10.3390/w15162973
APA StyleChelliah, P., Gupta, J. K., Mohammad Wabaidur, S., Siddiqui, M. R., Foon Lee, S., & Lai, W.-C. (2023). UV-Light-Driven Photocatalytic Dye Degradation and Antibacterial Potentials of Biosynthesized SiO2 Nanoparticles. Water, 15(16), 2973. https://doi.org/10.3390/w15162973










