Abstract
Response surface methodology was investigated to determine the operational parameters on the degradation of Congo red dye (CR) and chemical oxygen demand (COD) in two electrochemical systems evaluated individually on effluent pretreated by an up-flow anaerobic sludge blanket (UASB) reactor. The UASB reactor was fed with 100 mg L−1 of CR and was operated for 12 weeks at different hydraulic residence times (HRTs) of 12 h, 10 h, and 8 h. Once stabilized at an HRT of 8 h, the effluent was collected, homogenized, and independently treated by electrooxidation (EO) and electrocoagulation (EC) cells. On both electrochemical systems, two electrode pairs were used; solid for EC (Fe and stainless-steel) and mesh electrodes for EO (Ti/PbO2 and Ti), and the effect of intensity (A), recirculation flow rate (mL min−1), and experimental time (min) was optimized on response variables. The maximum efficiencies of sequential systems for COD degradation and CR decolorization were 92.78% and 98.43% by EC and ≥99.84% and ≥99.71% by EO, respectively. Results indicate that the coupled systems can be used in textile industry wastewater treatment for the removal of dyes and the decolorized by-products.
1. Introduction
The textile industry generates significant environmental pollution due to the excessive amount and diversity of colorants and color auxiliaries used during dyeing processes. In recent years, clothing demand and the use of azo-type dyes, which represent around 60–70% of the total dye used industrially, have increased [1]. The presence of small amounts of dyes in aquatic systems reduces light penetration, which inhibits photosynthesis, affecting gas solubility and, consequently, trophic chain [2,3]. Azo dyes are synthetic, highly toxic, and present resistance to degradation by oxidizing agents, photodegradation, and biological systems [4,5]. In addition, the textile industry consumes high amounts of water, and in places with scarce drinking water, textile effluents are generally recycled to reduce requirements, which increases the risk of exposure to these compounds [6]. Congo red (CR) is a synthetic benzidine-based anionic diazo dye widely used by the textile, paper, and plastic industries due to its high fixation in cotton. It is used in measurement as a natural dye and as an indicator. CR is difficult to biodegrade, and it is metabolized to benzidine, a human carcinogen that is an irritant to eyes and skin and can cause allergic reactions [7,8].
Biological, chemical, electrochemical, and physical systems have been evaluated in different studies [9]. Adsorption methods have been the most studied, using several materials [2,3,7,8,10,11,12,13]. However, high doses of adsorbents may be required [9,14,15], regeneration of the adsorbent is difficult and expensive, additional treatment methods for the mineralization of dyes are necessary, and is not applicable to a wide variety of dyes [16,17]. The application of aerobic systems does not allow the effective degradation of azo dyes by their electrophilic nature [18]. However, under anaerobic conditions, azo dyes are susceptible to duffer reductive biotransformation using up-flow anaerobic sludge blanket (UASB) reactors [19,20,21] and other systems [22]. The UASB reactors have the economic advantage of not requiring oxygen; they generate low biomass and can withstand high loads of organic matter compared to other systems [23]. Under anaerobic conditions, azo dyes are reduced to aromatic amines [20,21,23,24], which presents the need to use a posterior system for the complete oxidation/elimination of these toxic compounds.
For the complete removal of azo dyes, biological sequential anaerobic-aerobic treatment has been used. However, aromatic amines have substituents with nitro and sulfonic groups; these are highly recalcitrant for aerobic bacteria, which does not allow the efficient mineralization of these compounds in a biological aerobic post-treatment [22]. Nonetheless, the coupled system by electrochemical-biological methods, with the theoretical principle to convert the dye molecules to readily biodegradable compounds by electrochemical treatment [25,26], cannot be used with high loads of organic matter because of the competition with the dyes. Furthermore, the application of the electrochemical process may promote the mineralization of azo dyes to more toxic compounds, which limits efficient removal in the biological system.
The aromatic amines and color remnant from UASB reactors and the other anaerobic systems can be removed using electrochemical systems, in which electrooxidation contributes to the mineralization of contaminants and allows entrapment by electrocoagulation [27]. During the electrooxidation process, OH− radicals (highly oxidizing agents) are generated on the anode surface and are responsible for carrying out more than 90% of the oxidation (direct), until CO2 and H2O [28,29]. The material of the anode is an essential factor in the generation of OH− radicals. Diamond doped boron (BDD) is the anode with the highest reported efficiency. However, it has the disadvantage of having a very elevated cost. PbO2 coated anodes have a removal rate close to BDD, are of low cost, and can be used for the efficient oxidation of azo dyes and aromatics amines. On the other hand, in the electrocoagulation process by applying electrical current, adsorbents (Fe(OH)3, Fe(OH)2+, Fe(OH)2+ and Fe2(OH)24+ [30]) are generated on the Fe anode. For this type of dye, Fe has a higher affinity. The adsorbents generated will trap amines and other contaminants that could not be eliminated in the preliminary stage. Some studies of UASB reactors coupled to electrochemical systems have achieved decolorization of color above 92% and of COD ~67–90% on different wastewater effluents [31,32,33]. The objective of this work was to decolorize the CR dye by a sequential system using a conventional UASB reactor as a biological treatment to remove the organic matter and promote the reduction of the dye, coupled with two electrochemical treatments (electrooxidation and electrocoagulation), operated individually, as post treatment.
2. Materials and Methods
2.1. Experimental Unit and Operation Conditions
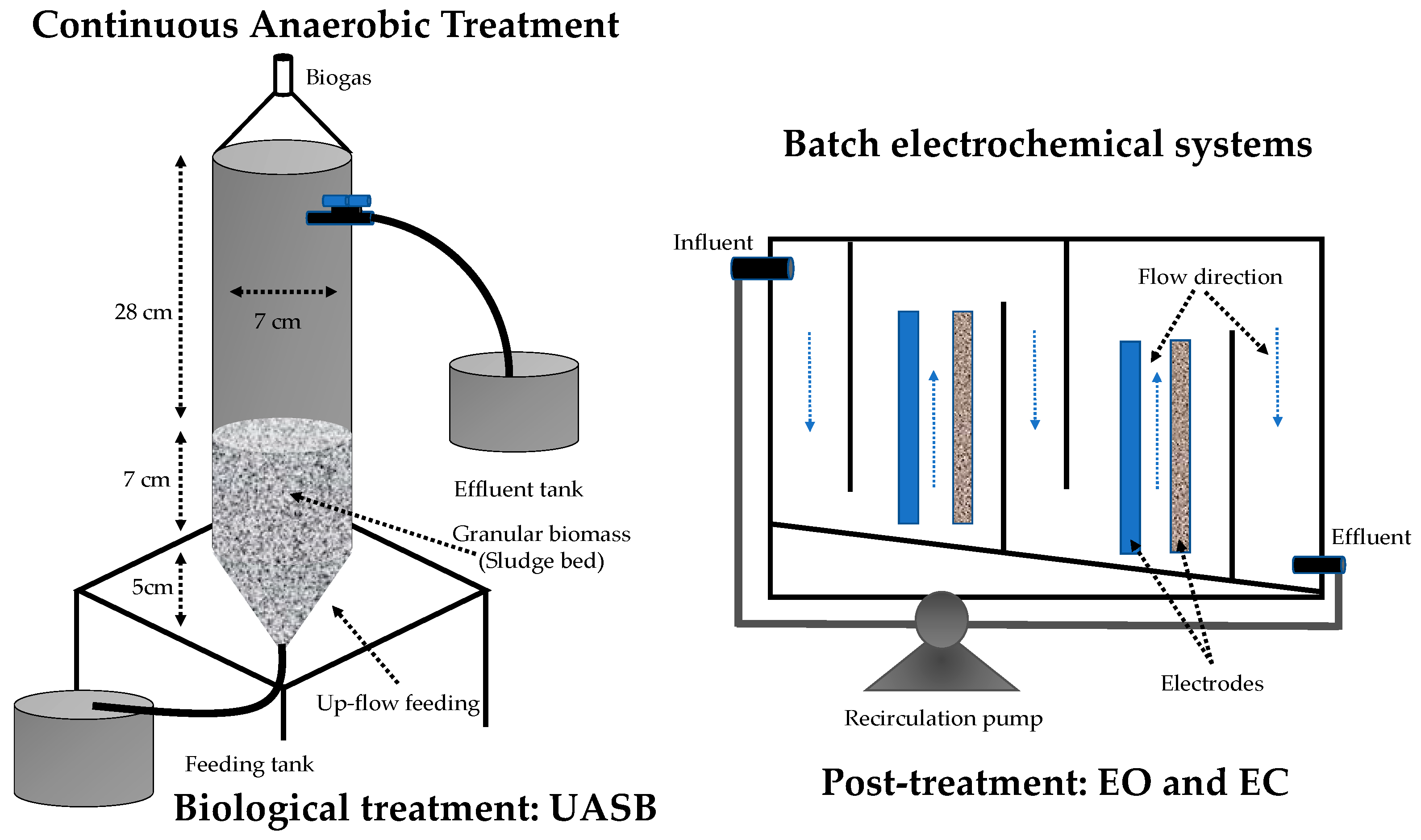
Biological treatment was conducted in a 900 mL cylindrical UASB reactor made of Plexiglas material (Figure 1). The bioreactor was inoculated with 340 g of anaerobic sludge, equivalent to 23.3 g volatile suspended solids (VSS) L−1. The sludge was collected from an industrial UASB reactor installed in a brewery. The bioreactor was fed with a solution composed of 153.85 mg L−1 of CR (65% Sigma Aldrich) and 1 g L−1 of sodium acetate (99% purity Faga Lab) as a carbon and energy source. In addition, the solution was prepared according to the basal medium described by Alvarez and Cervantes [34], with the following composition (g L−1, ≥99.0% Sigma Aldrich): NaHCO3, 1.68; NH4Cl, 0.3; KH2PO4, 0.2; MgCl2·6H2O, 0.03; CaCl2, 0.1; and 1 mL L−1 of trace elements solution. Trace elements solution contained (mg L−1): FeCl2·4H2O, 2000; H3BO3, 50; ZnCl2, 50; CuCl2·2H2O, 38; MnCl2·4H2O, 500; (NH4)6Mo7O24·4H2O, 50; AlCl3·6H2O, 90; CoCl2·6H2O, 2000; NiCl2·6H2O, 92; Na2SeO·5H2O, 162; EDTA, 1000; and 1 mL L−1 of HCl, 36% (≥99.0% Sigma Aldrich). The solution was adjusted to pH 7. Three hydraulic residence times (HRTs) of 12 h, 10 h, and 8 h were evaluated during 12 weeks of the total operation. Once the anaerobic system had stabilized at an HRT of 8 h, its effluent was periodically collected in containers of 1.5 L and was frozen at 4 °C in the dark. For subsequent treatment in electrochemical systems, a frozen container was taken for each experiment, and it was gradually thawed in the dark, homogenized, and evaluated under the parameters established in the experimental design.
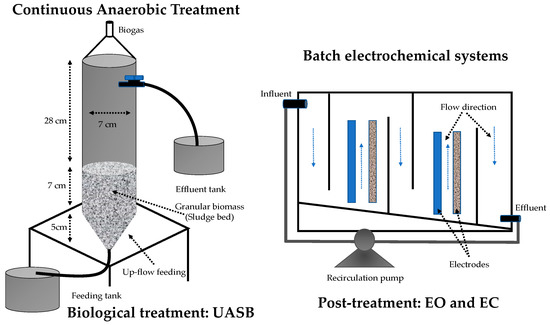
Figure 1.
Scheme of systems used for CR elimination. The left anaerobic reactor operated continuously and in the proper flow direction in the electrochemical cells operated in batch.
The electrochemical systems (electrocoagulation (EC) of 0.9 L and electrooxidation (EO) of 1.12 L) were operated with dynamic flow interaction. That is, the liquid followed a flow direction from top to bottom and from bottom to top in 5 different sections, parallel to the electrodes and separated by walls of acrylic (Figure 1). This operation method allowed for increasing the homogenization in the system. On the other hand, two pairs of electrodes were used. For EC, anode and cathode solids of Fe and stainless steel, with an electrochemically active surface area of 110 cm2, were used. In EO, mesh electrodes were used, Ti/PbO2 anodes and Ti cathodes, with an electrochemically active surface area of 366 cm2 and 320 cm2, respectively. The power was supplied with Single-Phase BK Precision® equipment. The color and chemical oxygen demand (COD) in the influents and effluents of the biological and ECH systems was monitored to establish the treatment efficiency.
2.2. Analytical Details
Samples were collected from the influent and the effluent of the UASB reactor during 12 weeks of operation. In addition, in the electrochemical systems, samples from the influent and effluent of each experiment, according to the experimental matrix conditions (Table 1), were collected and preserved for posterior analysis. Dye concentration was evaluated in a UV spectrophotometer (Thermo Fisher Scientific, UV GENESYSTM 10S, Waltham, MA, USA) at 495 nm. The chemical oxygen demand (COD) was determined using the colorimetric method [35]. CR and COD removal were calculated using Equations (1) and (2), respectively.
where CR0 is the initial concentration of CR (mg L−1), CRf is the final concentration of CR (mg L−1), CR removal is Congo red decolorization (%), COD0 is the initial concentration of COD (mg L−1), CODf is the final concentration of COD (mg L−1), and COD removal is the chemical oxygen demand removal (%).

Table 1.
Experimental matrix and values of independent factors.
2.3. Experimental Design for Electrochemical Systems
CR and COD degradation on the electrochemical systems was performed using response surface methodology (RSM). Intensity (X1, A), recirculation flow rate (X2, mL min−1), and experimental time (X3, min) were the independent variables evaluated. Five levels and a total of 20 treatments comprised eight runs for factorial design, six replicates at the center point, and six runs for high and low extreme levels (Table 2). CR (Y1) decolorization and COD (Y2) removal in % were the two investigated responses variables. Design Expert® 7 (version 7.0.0) was used to generate the model and to perform the analysis variance (ANOVA). The experimental matrix and values of each independent factor are represented in Table 1. The following second-order equation gives the predicted response in all experimental fields [28,36] (Equation (3)).
where Y is the experimental response; bo is the average of the experimental response; Coefficients bi, bii, and bij are the linear, quadratic, and interaction effects between i and j factors for the response Y, respectively.

Table 2.
Experimental levels and factorial 22 and central composite matrix for EO and EC.
3. Results and Discussion
3.1. Biological Treatment: Upflow Anaerobic Sludge Blanket
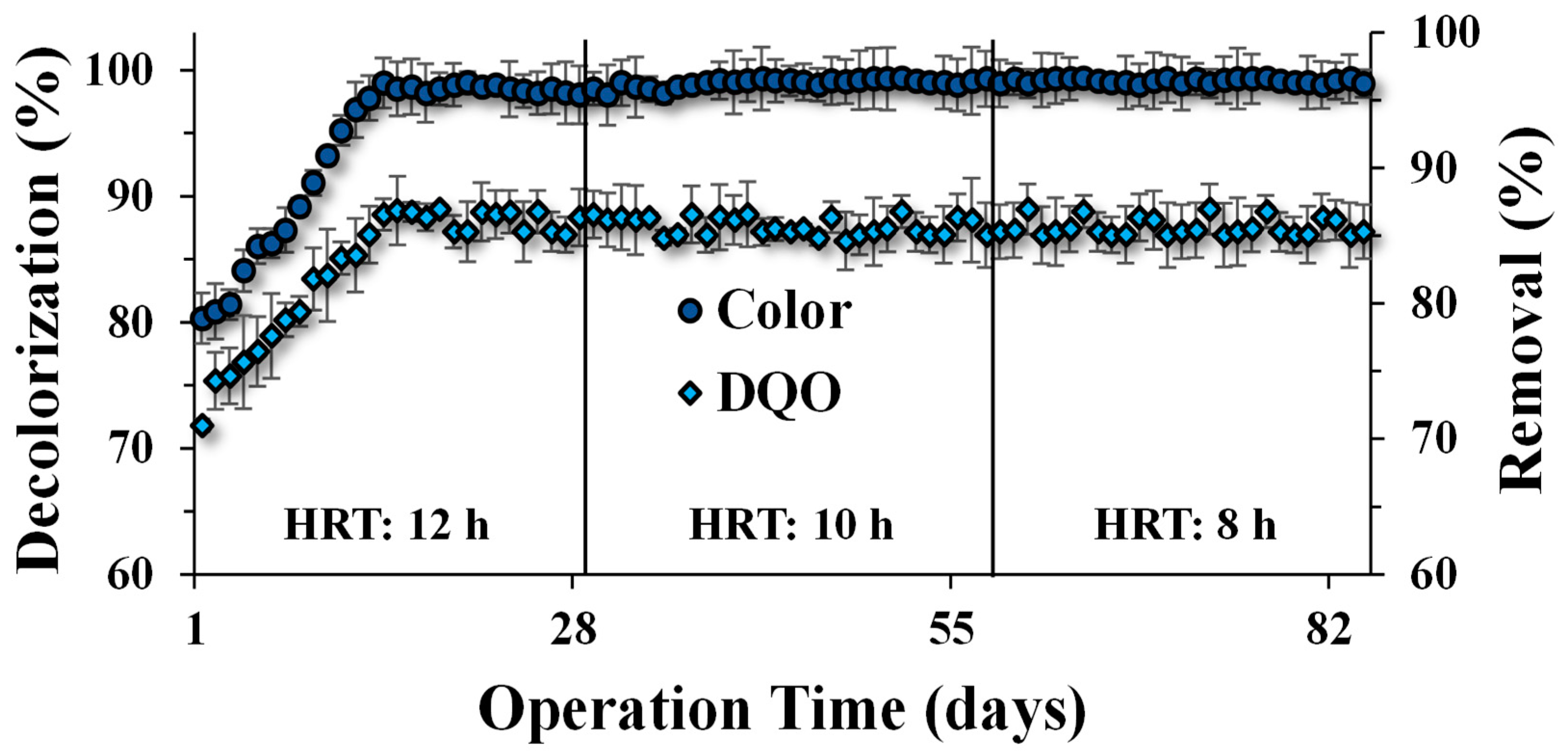
Biological treatment for CR decolorization and COD removal was evaluated over 84 d (12 weeks) at different HRTs. The initial decolorization efficiency for CR was 80% and removal for COD 72%, which gradually increased until reaching ≥96.6% and ≥84.2%, respectively. The concentrations at the effluent of the UASB reactor were 3.4 mg L−1 of CR (from 100 mg L−1 of initial dye) and 158 mg L−1 of COD (from 1000 mg L−1 of initial COD), after three weeks of operation with an HRT of 12 h (Figure 2). Similar removal efficiency values were observed in the following stages despite the change in the TRH to 10 h and 8 h, suggesting that the anaerobic sludge was already acclimated to the presence of the azo compound [37]. Under anaerobic conditions, azo compounds are susceptible to suffering reductive biotransformation by the cleavage of azo linkages, producing generally colorless but potentially dangerous aromatic amines. The reduction mechanism of azo dyes is mainly attributable to the capacity of anaerobic microorganisms to produce redox-active molecules (e.g., favines and hydroquinones) capable of transferring reducing equivalents to promote the reduction [6]. The reduction mechanism for CR suggests that three products were obtained: benzidine and two aromatic amines (with the same structure). The reduction is a two-stage process, in which the rate of benzidine production is slower than the decolorization rate, which indicates that one azo bond is broken first, promoting the decolorization, followed by a slower breaking of the second azo bond for benzidine production [38]. According to the results, CR was easily reduced by the anaerobic sludge according to the extent indicated (Figure 2). Previous studies indicate that tri-azo structures, as presented in the CR structure, are more recalcitrant compared to mono- and di-azo structures [39]. Nonetheless, the reduction of different azo dyes may occur differently, according to the toxicity extent, recalcitrance, and resistance to removal, regardless of the number of azo bonds [40]. In addition, the production of aromatic amines by the reduction in anaerobic systems treating azo compounds may result in highly toxic effluent, which makes necessary a subsequent treatment to eliminate them. Some processes, such as oxidation or adsorption systems can be applied [24,37,41].
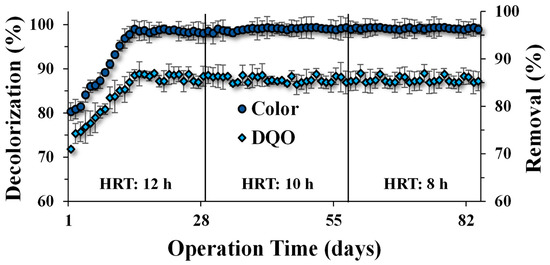
Figure 2.
COD removal and CR decolorization (%) in the UASB reactor, after 12 weeks (84 days) of operation at different hydraulic residence times: 12 h, 10 h, and 8 h.
3.2. Post-Treatment Optimization: Electrochemical Systems
3.2.1. Congo Red and Chemical Oxygen Demand Degradation in Electrooxidation Process
Three variables were evaluated: intensity (X1, I), recirculation flow rate (X2, RFR), and experimental time (X3, ET) on congo red CR decolorization and chemical oxygen demand (COD) removal by the electrooxidation (EO) process using a response surface with a total of 20 experiments and five levels. Table 2 shows the experimental results obtained. The highest removal efficiencies were found at the higher I levels tested (2.34 A), at 20 mL min−1 RFR and 100 min ET, achieving values of 91.25% for CR and 99% for COD. The lowest removal efficiencies were obtained for I = 1 A and 0.65 A, RFR = 10 mL min−1 and 20 mL min−1, and ET = 60 min and 100 min for CR (10.8%) and COD (26.4 7%), respectively. Second-order polynomial equations are given by Equations (3) and (4) for CR and COD removal, respectively:
Coefficients were calculated by the difference of half the arithmetic average of the highest and lowest level of results obtained. Positive coefficients have a positive effect on the response, and negative have the opposite effect [28,36]. b0 = 50.52 and 50.50 in Equations (4) and (5) are the average of the responses obtained in all experiments for CR and COD removal. b1 = +18.03 and +13.54 indicate that CR and COD degradation increase 36.06% (2 × 18.03) and 27.08% (2 × 13.54) when I increases from 1 A to 2 A. b2 = +1.47 and +1.43 means that CR decolorization and COD removal increase 2.94% (2 × 1.47) and 2.86% (2 × 1.43) when RFR increases from 10 mL min−1 to 30 mL min−1. b3 = +21.57 and +16.47 show that CR and COD degradation increase 43.14% (2 × 21.57) and 32.94% (2 × 16.47) when ET increases from 60 min to 140 min.
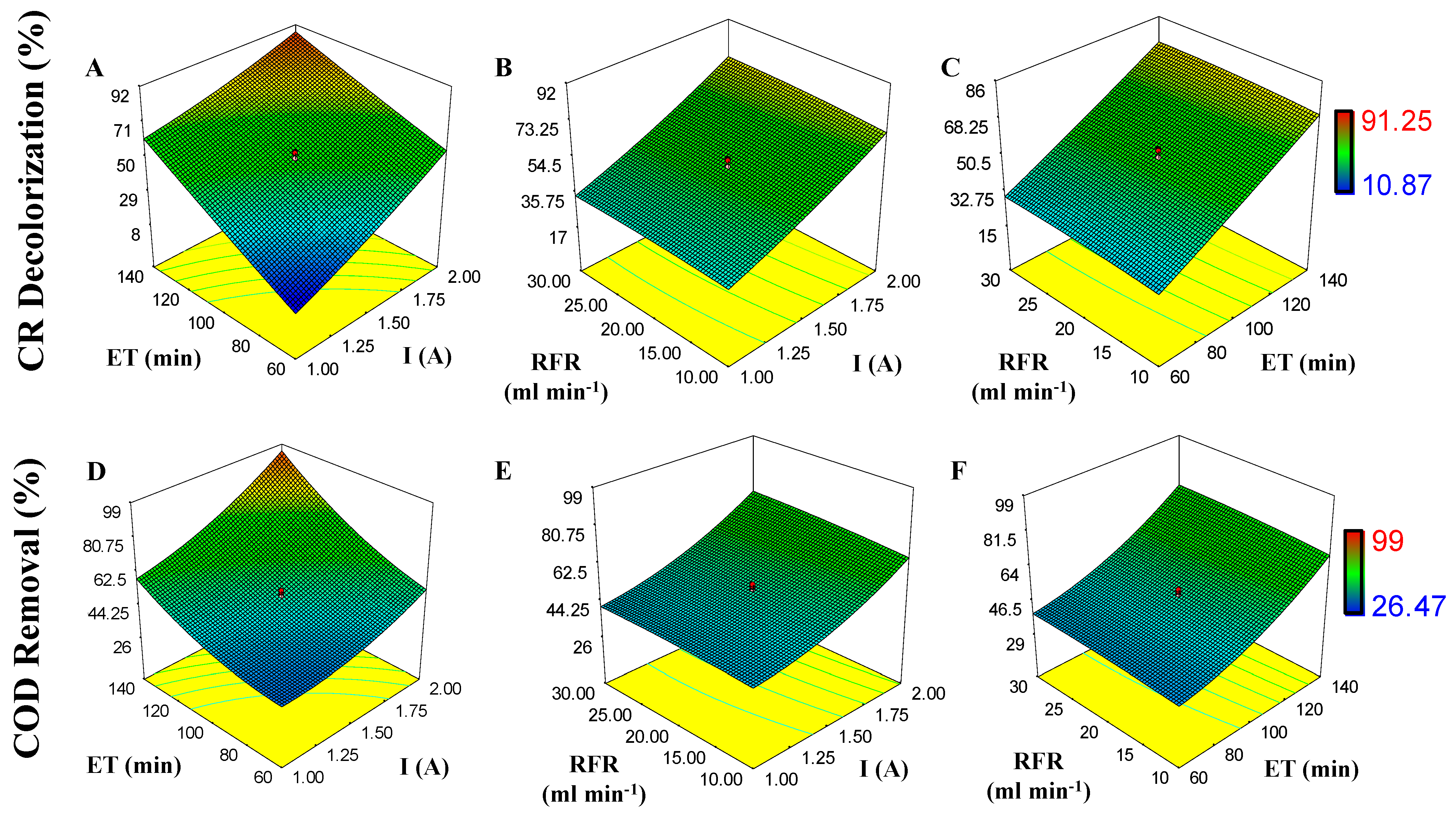
The effect of I, RFR, and ET on CR and COD removal is shown in response surface plots (Figure 3). As observed, the decolorization of CR and removal of COD can reach 91.25% and 99% if response variables (X1, X2, and X3) are increased. For CR R and COD R, the p-value model is <0.0001 and 0.0008; this indicates that the model is significant (Table 3). For CR decolorization, the p-value for X1 and X3 is <0.0001 (significant, p ˂ 0.05 with an F-value of 192.48 and 275.59) and for X2 is 0.2783 (not significant). Similar results are seen for COD removal; p-value for X1 and X3 is 0.0003 and <0.0001 (significant, p ˂ 0.05, with an F-value of 30.26 and 44.76) and for X2 is 0.5746 (not significant). Low dispersion is observed on data in Table 3, with a coefficient correlation value of R2 = 0.9735 (for CR decolorization) and R2 = 0.8961 (for COD removal), which means that only 2.65% and 10.39% of variation do not belong to the models.
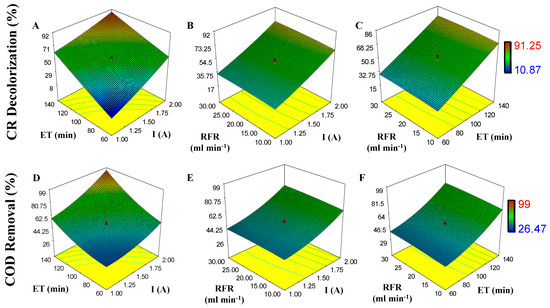
Figure 3.
Effect of independent variables (I, X1; RFR, X2, and ET, X3) on response variables (CR decolorization and COD removal) during electrooxidation process (EO): (A) ET and I on CR decolorization; (B) RFR and I on CR decolorization; (C) ET and RFR on CR decolorization; (D) ET and I on COD removal; (E) RFR and I on COD removal; (F) RFR and ET on COD removal.

Table 3.
ANOVA results for the response surface quadratic model for CR decolorization and COD removal in EO and EC processes.
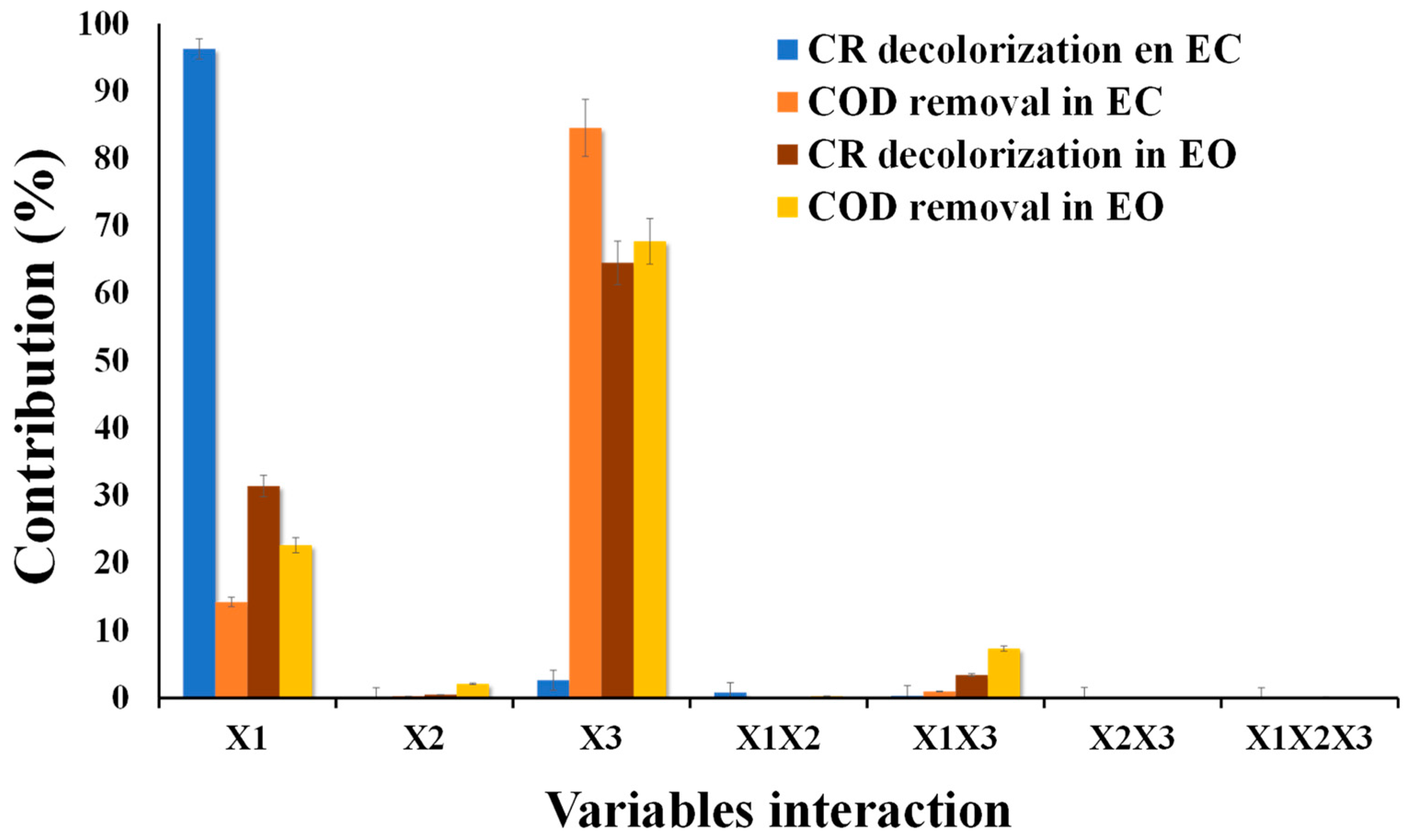
The contribution of independent variables to response variables is expressed as a percentage and is shown in Figure 4. The highest contribution to the response variable is 64.51% and 67.7% for X3 and 31.43% and 22.63% for X1 on CR decolorization and COD removal. The remainder is attributed to X2 and interaction between variables. This high contribution in X1 and X3 is because they control hydroxyl radical (OH−) production in the surface anode and the other oxidants indirectly generated, such as HClO, H2S2O8, and H2O2 [28,29,42,43,44].
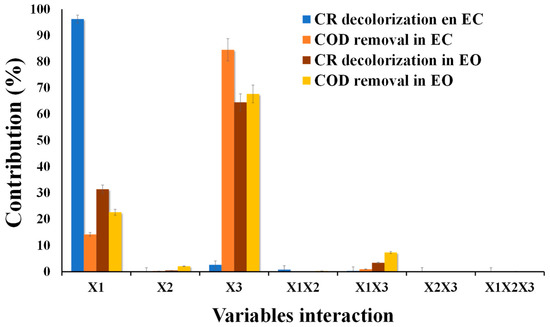
Figure 4.
Contribution percentage of each variable and the interaction (X1, X2, X3, X1X2, X1X3, X2X3) in both electrochemical systems.
To establish optimal conditions in response variables, energy consumption (I) and operating time (ET) were minimized, assigning an importance of 3 out of 5 for X1 and X3 (variables with the greatest effect in this study). Likewise, COD removal and CR decolorization were maximized, giving them an importance of 4 out of 5 and 5 out of 5, respectively. Optimization conditions obtained were I = 1.96 A, RFR = 23.51 mL min−1, and ET = 110.80 min to obtain a degradation of 74.12% and 73.74% of CR and COD. These conditions show that high removals can be obtained by reducing the operating conditions of the variables studied (X1, X2, and X3).
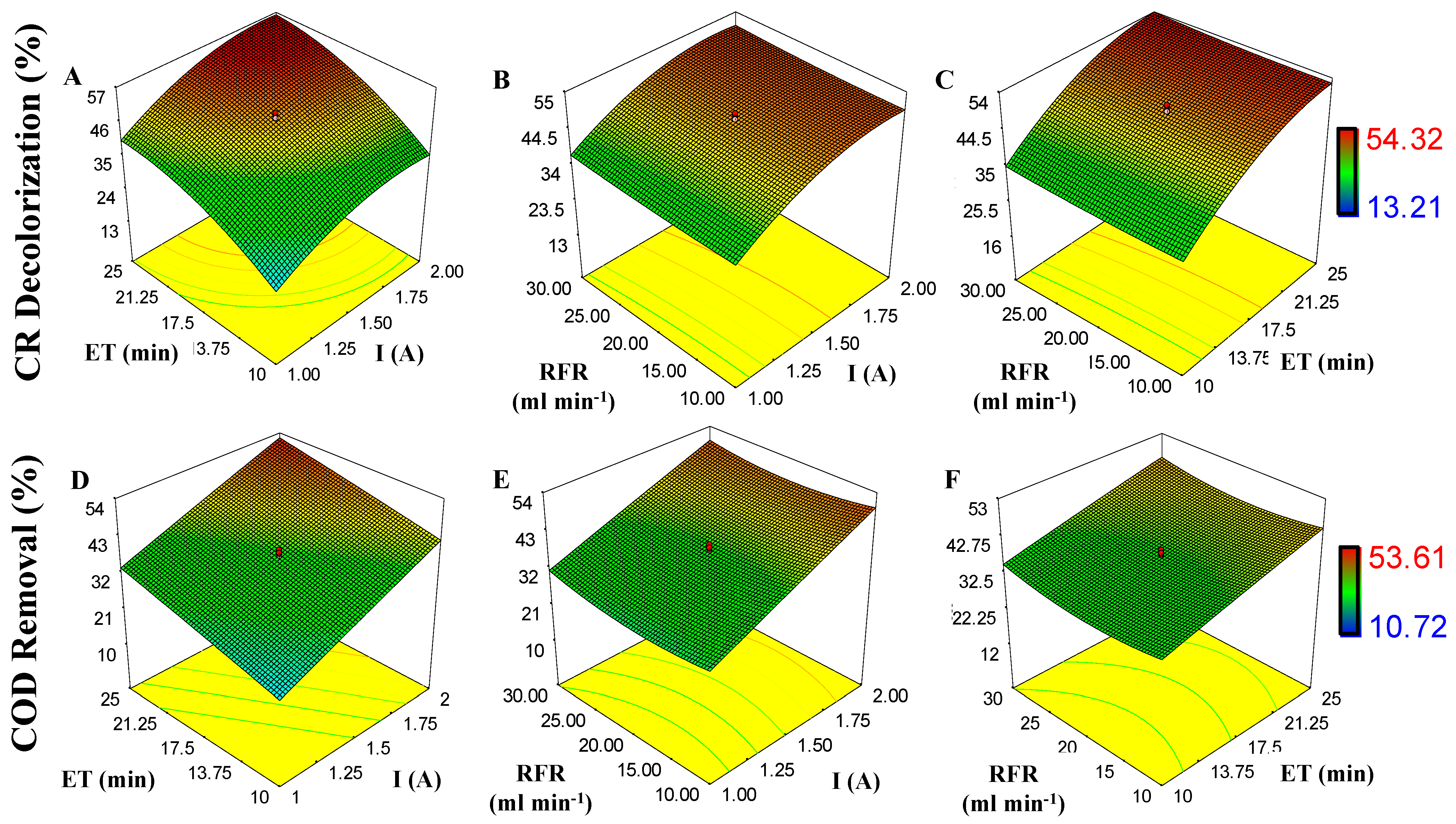
3.2.2. CR Decolorization and COD Removal in EC Process
For EC, the range for X3 was reduced to 10–25 min (Table 1); this is because the EC process is faster than EO [29]. Preliminary experiments were the basis for taking X3 values. Table 2 shows the experimental results obtained. Best degradations were found at the higher I levels tested (2.34 A), at 20 mL min−1 RFR and 17.5 min ET with a CR decolorization and COD removal of 53.61% and 54.32%. Second-order polynomial and lineal equations are given by Equations (6) and (7) for CR and COD degradation, respectively:
Coefficients were calculated by the difference of half the arithmetic average of the highest and lowest level of the results obtained. Positive coefficients have a positive effect on the response, and negative have the opposite effect [28,36]. b0 = 47.70 and 37.80 in Equations (6) and (7) are the averages of the responses obtained in all experiments for CR decolorization and COD removal.
The effects of I, RFR, and ET on CR decolorization and COD removal are shown in response surface plots (Figure 5). As observed, the degradation of CR and COD can reach 53.61% and 54.32% if response variables (X1, X2, and X3) are increased. For CR and COD degradation, the p-value model is 0.0011 and 0.0003; this indicates the model is significant (significant, p ˂ 0.05). For CR decolorization, the p-value for X1 and X3 is 0.0008 and for X2 is 0.6831 (not significant). Similar results are found for COD removal, where X2 is 0.7318. Low dispersion is observed on data in Table 3, with a coefficient correlation value of R2 = 0.8880 and R2 = 0.8961 for CR and COD degradation, respectively.
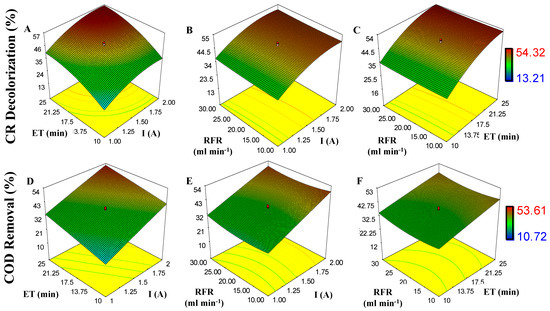
Figure 5.
Effect of independent variables (I, X1; RFR, X2, and ET, X3) on response variables (CR decolorization and COD removal) during electrocoagulation process (EC): (A) ET and I on CR decolorization; (B) RFR and I on CR decolorization; (C) ET and RFR on CR decolorization; (D) ET and I on COD removal; (E) RFR and I on COD removal; and (F) RFR and ET on COD removal.
The variables with the highest contribution in CR decolorization and COD removal were X1 with 96.25% and 14.21% and X3 with 2.61% and 84.54% (Figure 5). This is because, at high current intensity, the coagulant production increases, and pollutant entrapment takes place [45,46,47]. Likewise, using iron anodes, we can have coagulant species of Fe(OH)3, Fe(OH)2+, Fe(OH)2+, and Fe2(OH)24+ [30]. On the other hand, optimization was to minimize energy consumption (I) and operating time (ET), assigning an importance of 3 out of 5, and response variables (COD and CR removal) were given maximium importance (4 out of 5 and 5 out of 5, respectively). The optimization conditions obtained were I = 1.98 A, RFR = 10.10 mL min−1, and ET = 24.30 min, to obtain a degradation of 56.01% and 54.11% of CR and COD. These conditions show that high removals can be obtained by reducing the operating conditions of the variables studied (X1, X2, and X3).
The low yield eliminations in EC are due to the short experimental time used (it was chosen according to the preliminary kinetics). Efficiencies can be increased by increasing the current intensity and the experimental time as these were the variables with the greatest influence and contribution to the response variables [30,47].
3.3. CR and COD Degradation by Coupled Biological-ECH Systems
Total degradations for the UASB reactor coupled to EO were 99.71% for CR decolorization and 99.84% for COD removal, obtaining final concentrations of 0.29 mg L−1 of CR and 1.58 mg L−1 of COD. For the UASB + EC, the maximum degradations for CR and COD were 98.43% and 92.78%. Previously, Aravind et al. [48] coupled the electrooxidation process to the photodegradation and biological system, achieving eliminations of 92% and 90% for color and COD degradation, respectively. Venkatesh et al. [26] used ozonation coupled to the UASB reactor for Reactive Black 5 and found a removal of 90% for COD and 94% for dye decolorization. Likewise, Buzzini et al. [31] used an inverse treatment coupling electrocoagulation to UASB, reaching 98% color and 67% COD degradation. Yetilmezsoy et al. [32] decolorized and removed the organic matter from poultry manure wastewater pretreated by a UASB and using EC as a post-treatment. They achieved degradations in the coupled system of 90% of COD and 92% of residual color. Likewise, Makwana and Ahammed [33] treated urban wastewater using a UASB coupled to an EC system, reaching removals of 71.3% for COD and 96.8% for color. The literature addresses the inverted process (electrochemical-biological); however, the by-products of these contaminants are more toxic than the original pollutant, and this limits the complete mineralization in the biological (secondary) treatment. In addition to the high degradation capacity of electrochemical systems as post-treatment, they have the advantage of occupying small spaces and do not require the addition of chemical agents [49]. In this study, anaerobic-electrochemical treatment is approached due to the azo dyes being reduced in the biological system forming the aromatic amines: benzidine and two aromatic amines (with the same structure). These amines are oxidized and desulphonated by the secondary treatment (electrooxidation) or by trapping in an adsorbent (electrocoagulation). During the electro-oxidation process, 8-Amino naphthalene diazine 3-sulfonic acid will oxidize and form metabolites (3-Hydroperoxy 8-nitrosonaphthol) [50], which will continue to oxidize until completely mineralized [51]. On EC, using iron anodes, coagulant species of Fe(OH)3, Fe(OH)2+, Fe(OH)2+, and Fe2(OH)24+ [30] are formed. These compounds trap in their matrix the amines and metabolites formed in the anaerobic system as well as the remaining color.
The advantage of using electrooxidation for organic pollutant degradation is that complete mineralization of these compounds can be achieved up until CO2 and H2O, directly by the OH− radicals generated in the anode and by other oxidants generated in the process (such as HClO, H2S2O8, H2O2, etc.) [28,29,51]. Likewise, methane generated in a biological treatment [40] can fully or partially supply the system demand. EO, compared to EC, has the disadvantage that the reaction process requires more time; however, in EC, the electrodes are sacrificial; that is, they are worn during each operation. On the other hand, the EC system requires less operation time, which makes it more economical. However, if an anaerobic biological system is used as a preliminary treatment (as in this study), a large part of the energy requirement can be supplied. EC has the disadvantage of generating a coagulant to trap the pollutants. It will require a subsequent treatment to remove them from the treated effluent [29]. The coupled system evaluated in this research allowed analysis of how these electrochemical systems behave when used as post-treatment of an anaerobic system in dye decolorization. In future works, it will be essential to evaluate the methane production (in specific conditions) to know the contribution that a preliminary anaerobic treatment would have in an electrochemical system. Likewise, it is crucial to evaluate the toxicity after applying EO and EC and the degree of mineralization after applying EO.
4. Conclusions
The coupled system evaluated in this study improved contaminant degradation. The biological treatment system (UASB) eliminated a large concentration of CR and COD and contributed to biogas production (anaerobic system), which can be used in electrochemical cell energy requirements. When an azo bond is broken during pretreatment, it generates new pollutants (aromatic amines), which can be degraded and mineralized post-treatment (electrochemical reactors). The maximum efficiencies of sequential systems for COD removal and CR decolorization were 92.78% and 98.43% by EC and ≥99.84% and ≥99.71% by EO. The best degradation achieved was in the UASB + EO coupled system, despite the fact that this system requires more experimental time compared to the EC. The most critical variables in both electrochemical systems were I, with a 96.25% contribution in color degradation, and ET, with 84.54% in DQO removal. Likewise, EC requires treatment of subsequent sedimentation to reduce the clots present. According to the results, this coupled system can be applied and scaled to wastewater treatment with high concentrations of colorants.
Author Contributions
Conceptualization, P.G.-M., L.H.Á.-V., C.G.-G., L.A.L.-S., M.A.C.-R. and I.C.R.-S.; methodology, M.O.C.-G., R.G.U.-M., E.R.M.-E., M.A.C.-R., P.G.-M. and I.C.R.-S.; software, C.G.-G., L.H.Á.-V. and I.C.R.-S.; validation, L.H.Á.-V., C.G.-G. and I.C.R.-S.; formal analysis, P.G.-M., L.M.D.-T., C.G.-G., L.A.L.-S., L.H.Á.-V. and I.C.R.-S.; investigation, P.G.-M., M.A.C.-R., M.O.C.-G., C.G.-G., L.H.Á.-V. and I.C.R.-S.; resources, L.A.L.-S., C.G.-G., L.H.Á.-V., M.O.C.-G., P.G.-M., L.M.D.-T. and M.A.C.-R.; data curation, E.R.M.-E., L.A.L.-S., L.H.Á.-V., R.G.U.-M. and I.C.R.-S.; writing—original draft preparation, C.G.-G., L.H.Á.-V., P.G.-M., M.O.C.-G., M.A.C.-R. and I.C.R.-S.; writing—review and editing, P.G.-M., C.G.-G., L.H.Á.-V., M.O.C.-G., M.A.C.-R. and I.C.R.-S.; visualization, R.G.U.-M., L.H.Á.-V. and E.R.M.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvalho, J.R.S.; Amaral, F.M.; Florencio, L.; Kato, M.T.; Delforno, T.P.; Gavazza, S. Microaerated UASB reactor treating textile wastewater: The core microbiome and removal of azo dye Direct Black 22. Chemosphere 2020, 242, 125157. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Kampoosaen, C.; Kengnok, O. Adsorption characteristics of Congo red on carbonized leonardite. J. Clean. Prod. 2016, 134, 506–514. [Google Scholar] [CrossRef]
- Zhang, L.; Song, F.; Wang, S.; Wang, H.; Yang, W.; Li, Y. Efficient Removal of Hexavalent Chromium and Congo Red by Graphene Oxide/Silica Nanosheets with Multistage Pores. J. Chem. Eng. Data 2020, 65, 4354–4368. [Google Scholar] [CrossRef]
- Taher, T.; Rohendi, D.; Mohadi, R.; Lesbani, A. Congo red dye removal from aqueous solution by acid-activated bentonite from sarolangun: Kinetic, equilibrium, and thermodynamic studies. Arab J. Basic Appl. Sci. 2019, 26, 125–136. [Google Scholar] [CrossRef]
- Bedekar, P.A.; Saratale, R.G.; Saratale, G.D.; Govindwar, S.P. Development of low cost upflow column bioreactor for degradation and detoxification of Blue HERD and textile effluent by Lysinibacillus sp. RGS immobilized on Loofa. Int. Biodeterior. Biodegrad. 2014, 96, 112–120. [Google Scholar] [CrossRef]
- Cervantes, F.J.; Dos Santos, A.B. Reduction of azo dyes by anaerobic bacteria: Microbiological and biochemical aspects. Rev. Environ. Sci. Bio/Technol. 2011, 10, 125–137. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloys Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Islam, M.A.; Mozumder, M.S.I. Development of Treatment Technology for Dye Containing Industrial Wastewater. J. Sci. Res. 2010, 2, 567. [Google Scholar]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V.K. Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef]
- El Haddad, M.; Regti, A.; Slimani, R.; Lazar, S. Assessment of the biosorption kinetic and thermodynamic for the removal of safranin dye from aqueous solutions using calcined mussel shells. J. Ind. Eng. Chem. 2014, 20, 717–724. [Google Scholar] [CrossRef]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Rahman, N.U.; Ullah, I.; Alam, S.; Khan, M.S.; Shah, L.A.; Zekker, I.; Burlakovs, J.; Kallistova, A.; Pimenov, N.; Vincevica-Gaile, Z.; et al. Activated Ailanthus altissima Sawdust as Adsorbent for Removal of Acid Yellow 29 from Wastewater: Kinetics Approach. Water 2021, 13, 2136. [Google Scholar] [CrossRef]
- Umar, A.; Khan, M.S.; Alam, S.; Zekker, I.; Burlakovs, J.; dC Rubin, S.S.; Bhowmick, G.D.; Kallistova, A.; Pimenov, N.; Zahoor, M. Synthesis and Characterization of Pd-Ni Bimetallic Nanoparticles as Efficient Adsorbent for the Removal of Acid Orange 8 Present in Wastewater. Water 2021, 13, 1095. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Recent Advances in Biopolymer-Based Dye Removal Technologies. Molecules 2021, 26, 4697. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Role of Azoreductases in Bacterial Decolorization of Azo Dyes. Curr. Trends Biomed. Eng. Biosci. 2017, 9, 50–52. [Google Scholar] [CrossRef]
- Zaroual, Z.; Azzi, M.; Saib, N.; Chainet, E. Contribution to the study of electrocoagulation mechanism in basic textile effluent. J. Hazard. Mater. 2006, 131, 73–78. [Google Scholar] [CrossRef]
- Isik, M.; Sponza, D.T. Effects of alkalinity and co-substrate on the performance of an upflow anaerobic sludge blanket (UASB) reactor through decolorization of Congo Red azo dye. Bioresour. Technol. 2005, 96, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.H.; Arvizu, I.C.; Garcia-Reyes, R.B.; Martinez, C.M.; Olivo-Alanis, D.; Del Angel, Y.A. Quinone-functionalized activated carbon improves the reduction of congo red coupled to the removal of p-cresol in a UASB reactor. J. Hazard Mater. 2017, 338, 233–240. [Google Scholar] [CrossRef]
- Silva, S.Q.; Silva, D.C.; Lanna, M.C.; Baeta, B.E.; Aquino, S.F. Microbial dynamics during azo dye degradation in a UASB reactor supplied with yeast extract. Braz. J. Microbiol. 2014, 45, 1153–1160. [Google Scholar] [CrossRef]
- Dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Somasiri, W.; Li, X.F.; Ruan, W.Q.; Jian, C. Evaluation of the efficacy of upflow anaerobic sludge blanket reactor in removal of colour and reduction of COD in real textile wastewater. Bioresour. Technol. 2008, 99, 3692–3699. [Google Scholar] [CrossRef]
- Lu, X.; Liu, R. Treatment of Azo Dye-Containing Wastewater Using Integrated Processes. In Biodegradation of Azo Dyes; Atacag Erkurt, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–155. [Google Scholar]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B 2019, 200, 111655. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.; Venkatesh, K.; Quaff, A.R. Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J. Appl. Res. Technol. 2017, 15, 340–345. [Google Scholar] [CrossRef]
- Katsoni, A.; Mantzavinos, D.; Diamadopoulos, E. Coupling digestion in a pilot-scale UASB reactor and electrochemical oxidation over BDD anode to treat diluted cheese whey. Environ. Sci. Pollut. Res. 2014, 21, 12170–12181. [Google Scholar] [CrossRef] [PubMed]
- Romero-Soto, I.C.; Dia, O.; Leyva-Soto, L.A.; Drogui, P.; Buelna, G.; Diaz-Tenorio, L.M.; Ulloa-Mercado, R.G.; Gortares-Moroyoqui, P. Degradation of Chloramphenicol in Synthetic and Aquaculture Wastewater Using Electrooxidation. J. Environ. Qual. 2018, 47, 805–811. [Google Scholar] [CrossRef]
- Drogui, P.; Blais, J.A.; Mercier, G. Review of Electrochemical Technologies for Environmental Applications. Recent Pat. Eng. 2007, 1, 257–272. [Google Scholar] [CrossRef]
- Akhtar, A.; Aslam, Z.; Asghar, A.; Bello, M.M.; Raman, A.A.A. Electrocoagulation of Congo Red dye-containing wastewater: Optimization of operational parameters and process mechanism. J. Environ. Chem. Eng. 2020, 8, 104055. [Google Scholar] [CrossRef]
- Buzzini, A.P.; Patrizzi, L.J.; Motheo, A.J.; Pires, E.C. Preliminary evaluation of the electrochemical and chemical coagulation processes in the post-treatment of effluent from an upflow anaerobic sludge blanket (UASB) reactor. J. Environ. Manag. 2007, 85, 847–857. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Sakar, S. Improvement of COD and color removal from UASB treated poultry manure wastewater using Fenton’s oxidation. J. Hazard. Mater. 2008, 151, 547–558. [Google Scholar] [CrossRef]
- Makwana, A.R.; Ahammed, M.M. Electrocoagulation process for the post-treatment of anaerobically treated urban wastewater. Sep. Sci. Technol. 2017, 52, 1412–1422. [Google Scholar] [CrossRef]
- Alvarez, L.H.; Cervantes, F.J. Assessing the impact of alumina nanoparticles in an anaerobic consortium: Methanogenic and humus reducing activity. Appl. Microbiol. Biotechnol. 2012, 95, 1323–1331. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- García-Gómez, C.; Drogui, P.; Zaviska, F.; Seyhi, B.; Gortáres-Moroyoqui, P.; Buelna, G.; Neira-Sáenz, C.; Estrada-alvarado, M.; Ulloa-Mercado, R.G. Experimental design methodology applied to electrochemical oxidation of carbamazepine using Ti/PbO2 and Ti/BDD electrodes. J. Electroanal. Chem. 2014, 732, 1–10. [Google Scholar] [CrossRef]
- Ong, S.-A.; Toorisaka, E.; Hirata, M.; Hano, T. Decolorization of azo dye (Orange II) in a sequential UASB–SBR system. Sep. Purif. Technol. 2005, 42, 297–302. [Google Scholar] [CrossRef]
- Olivo-Alanis, D.; Garcia-Reyes, R.B.; Alvarez, L.H.; Garcia-Gonzalez, A. Mechanism of anaerobic bio-reduction of azo dye assisted with lawsone-immobilized activated carbon. J. Hazard. Mater. 2018, 347, 423–430. [Google Scholar] [CrossRef]
- Hu, T.L. Kinetics of azoreductase and assessment of toxicity of metabolic products from azo dyes by Pseudomonas luteola. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2001, 43, 261–269. [Google Scholar] [CrossRef]
- Alvarez, L.H.; Valdez-Espinoza, R.; García-Reyes, R.B.; Olivo-Alanis, D.; Garza-González, M.T.; Meza-Escalante, E.R.; Gortáres-Moroyoqui, P. Decolorization and biogas production by an anaerobic consortium: Effect of different azo dyes and quinoid redox mediators. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2015, 72, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Bras, R.; Gomes, A.; Ferra, M.I.; Pinheiro, H.M.; Goncalves, I.C. Monoazo and diazo dye decolourisation studies in a methanogenic UASB reactor. J. Biotechnol. 2005, 115, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Faouzi, M.; Cañizares, P.; Gadri, A.; Lobato, J.; Nasr, B.; Paz, R.; Rodrigo, M.A.; Saez, C. Advanced oxidation processes for the treatment of wastes polluted with azoic dyes. Electrochim. Acta 2006, 52, 325–331. [Google Scholar] [CrossRef]
- Faouzi Elahmadi, M.; Bensalah, N.; Gadri, A. Treatment of aqueous wastes contaminated with Congo Red dye by electrochemical oxidation and ozonation processes. J. Hazard. Mater. 2009, 168, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Efficient treatment for textile wastewater through sequential electrocoagulation, electrochemical oxidation and adsorption processes: Optimization and toxicity assessment. J. Electroanal. Chem. 2020, 878, 114578. [Google Scholar] [CrossRef]
- Zaied, M.; Bellakhal, N. Electrocoagulation treatment of black liquor from paper industry. J. Hazard. Mater. 2009, 163, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Pajootan, E.; Arami, M.; Mahmoodi, N.M. Binary system dye removal by electrocoagulation from synthetic and real colored wastewaters. J. Taiwan Inst. Chem. Eng. 2012, 43, 282–290. [Google Scholar] [CrossRef]
- Wei, M.-C.; Wang, K.-S.; Huang, C.-L.; Chiang, C.-W.; Chang, T.-J.; Lee, S.-S.; Chang, S.-H. Improvement of textile dye removal by electrocoagulation with low-cost steel wool cathode reactor. Chem. Eng. J. 2012, 192, 37–44. [Google Scholar] [CrossRef]
- Aravind, P.; Subramanyan, V.; Ferro, S.; Gopalakrishnan, R. Eco-friendly and facile integrated biological-cum-photo assisted electrooxidation process for degradation of textile wastewater. Water Res. 2016, 93, 230–241. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Qudah, Y.; Assirey, E. Combined biological wastewater treatment with electrocoagulation as a post-polishing process: A review. Sep. Sci. Technol. 2020, 55, 2334–2352. [Google Scholar] [CrossRef]
- Telke, A.; Joshi, S.; Jadhav, S.; Shah, D.; Govindwar, S. Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation 2009, 21, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Drogui, P. Electrochemical removal of microcystin-LR from aqueous solution in the presence of natural organic pollutants. J. Environ. Manag. 2013, 114, 253–260. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).