Abstract
Sediments and invertebrates were sampled from 9 stormwater retention ponds (SWRPs) and 11 natural, shallow lakes in Denmark. Samples were analyzed for 13 polycyclic aromatic hydrocarbons (PAHs). The SWRPs received urban and highway runoff from various types of drainage areas and the lakes were located in areas of various land uses. Comparing PAHs in the sediments of the SWRPs and the lakes, it was found that levels of total PAH were similar in the two aquatic systems, with median values of 0.94 and 0.63 mg·(kg·DM)−1 in sediments of SWRPs and lakes, respectively. However, the SWRP sediments tended to have higher concentrations of high-molecular-weight PAHs than the lakes. A similar pattern was seen for PAHs accumulated in invertebrates where the median of total PAH was 2.8 and 2.1 mg·(kg·DM)−1 for SWRPs and lakes, respectively. Principal component analysis on the PAH distribution in the sediments and invertebrates showed that ponds receiving highway runoff clustered with lakes in forests and farmland. The same was the case for some of the ponds receiving runoff from residential areas. Overall, results showed that sediment PAH levels in all SWRPs receiving runoff from highways were similar to the levels found in some of the investigated natural, shallow lakes, as were the sediment PAH levels from some of the residential SWRPs. Furthermore, there was no systematic trend that one type of water body exceeded environmental quality standards (EQS) values more often than others. Together this indicates that at least some SWRPs can sustain an invertebrate ecosystem without the organisms experiencing higher bioaccumulation of PAHs then what is the case in shallow lakes of the same region.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are common contaminants in aquatic and terrestrial environments. Several of them are perceived as environmentally problematic, as they have been shown to be carcinogenic and tend to bioaccumulate, while some also can cause endocrine disruption and have tissue-specific toxicity [1,2]. Consequently, several PAHs are viewed as priority pollutants, for example by the European Union [3]. For some of these, the EU Water Framework Directive gives environmental quality standards (EQS) for water, and also for some biota. Other examples are the standards put forward by the Helsinki Commission (HELCOM) for the Baltic Sea or the Canadian sediment quality guidelines, setting standards for marine and freshwater sediments [4,5].
PAHs are ubiquitous in the urban atmosphere and are introduced to the environment by natural and anthropogenic combustion processes [6,7]. Some of the major natural sources of PAHs in the atmosphere are forest and prairie fires and volcanic eruptions. In modern times, however, the dominant emission sources are various anthropogenic activities: combustion of fossil fuels, incineration of domestic waste, residential heating, industrial processes like asphalt and aluminum production and oil refining [8,9]. PAHs are emitted to the atmosphere and transported over long distances as gases and aerosols [10,11]. They are hydrophobic, adhere to air-borne particles and reach top-soils, roads and urban surfaces mainly by atmospheric deposition. Both dry and wet deposition processes are of concern, where especially wet deposition efficiently conveys airborne particles to the ground, causing diffuse contamination over whole landscapes [12,13]. Local sources include vehicular exhaust emissions, asphalt leaching, particles from tire abrasion and lubricating oils [14,15,16].
As stormwater runs off impervious surfaces such as roads, parking lots, driveways and roofs, it washes off many contaminants, including PAHs that, to a large extent, are attached to deposition particles [13,17]. In densely urbanized areas, the percentage of impervious surfaces is high, generating rapid transfer of contaminants to downstream aquatic environments [17]. This causes hotspots where the stormwater runoff is discharged; concentrations of individual PAHs have been reported at levels exceeding environmental quality standards set by the European Union and that of other agencies [3,18,19]. Furthermore, PAH concentrations in urban streams have been found to correlate with the degree of urbanization of the catchment area [20].
Different land uses in a drainage area influence the concentration and composition of PAHs in the soil and sediment of the receiving environment. A study on urban soils found the highest concentrations of PAHs in soils bordering streets with moderate to heavy traffic, followed by soils close to residential streets and open spaces [21]. Treatment of stormwater runoff prior to discharge is therefore often needed. Detention tanks [22,23] and stormwater retention ponds (SWRPs) are common and often preferred techniques to meet this objective. These latter comprise a permanent pool of water, overlaid by a dynamic storage volume. The stored water discharges slowly to the receiving water body until the water level of the permanent pool is reached. Hereby, SWRPs mitigate emission of contaminants and reduce hydraulic impacts, such as downstream flooding and erosion of receiving waterways [24]. In the SWRPs, the stormwater runoff is retained between storm events for a duration sufficient to permit biological, chemical and physical treatment processes to occur, with sedimentation being the dominant removal process [25]. A well-designed SWRP, hence, can efficiently remove pollutants that have a tendency to bind to particles, for example PAHs.
In many ways, SWRPs closely resemble natural, shallow lakes and, in time, they are often colonized by plants [26], which makes them attractive habitats for water-dwelling wildlife [27,28,29]. However, when pollutants such as PAHs accumulate in SWRP sediments, they become available for bioconcentration and bioaccumulation in flora and fauna [30,31,32]. The objective of this study is to assess the levels of PAHs in sediments and invertebrates from SWRPs compared to those of natural, shallow lakes, and to evaluate if these PAH levels can be expected to affect the quality of the habitats which SWRPs can sustain.
2. Materials and Methods
2.1. Sampling Sites Description and Sample Collection
The study involved the sampling of sediments and invertebrates from 20 SWRPs and lakes in Denmark (Figure 1). A total of 9 SWRPs and 11 small and shallow lakes not receiving stormwater runoff from constructed surfaces were sampled from April to July 2010 (Table 1). The regional monthly average precipitation during this period was 52.1 mm·month−1, while the climate normal for the region is 51.8 mm·month−1. All sampling sites—SWRPs as well as lakes—maintained a permanent water pool all year round. The maximum water depth of all SWRPs was between 0.5 and 1.5 m. In order to cover a wide range of PAH loads, the SWRPs were selected so that a range of typical drainage area characteristics were encompassed. Similarly, the lakes were chosen to cover typical uses of the surrounding land. The SWRPs were grouped into three categories corresponding to the land use characteristics of the drainage area; highway, industrial or residential. The shallow lakes were rural and situated in plantation forests, natural regenerated forests, or farmlands, except for one lake located in an urban area, surrounded by industry. The 20 sampling sites were thus chosen to include the widest possible variety of PAH loads, in order to cover how urbanization affects bioaccumulation in SWRPs. The rural lakes situated in forests are surmised to be pristine and less affected by anthropogenic pollutants, while SWRPs receiving runoff from highways and industrial catchments would be more affected. One of the SWRPs (PI1) received runoff from a truck centre and filling station, and the hypothesis was that this pond would have higher PAH levels than the other SWRPs.
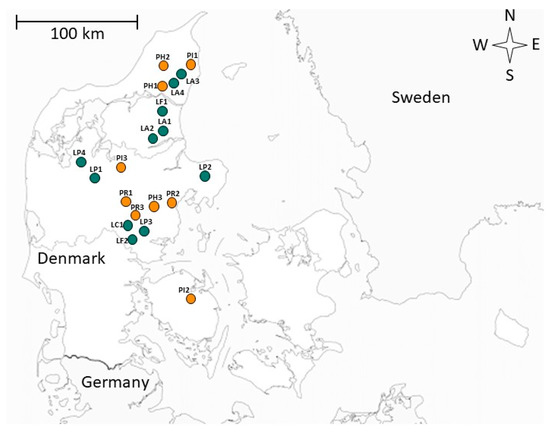
Figure 1.
Sampling locations in Denmark. Orange and green markings indicate stormwater retention ponds (SWRPs) and natural, shallow lakes, respectively. Letter and number identification can be found in Table 1.

Table 1.
Classification of SWRPs and natural, shallow lakes. Invertebrates taxa collected and analyzed for PAHs.
For all sampling sites, sediment samples were collected as intact cores by means of 5-cm-diameter PVC cores. Three SWRP sediments were obtained mid-way between the inlet and the outlet, at 0.5–1.0 m of water depth. Lake sediment cores were taken at 1.0 m water depth, close to the shore: three samples at different locations in the lakes. The uppermost 5 cm of each core was immediately transferred into Rilsan bags, thoroughly mixed and kept on ice during transport to the laboratory. This procedure was repeated three times per sampling sites. One of the SWRPs, PI3, could not be sampled for sediments, as a heavy storm prior to the sampling campaign had raised the water level and made it impossible to obtain sediments. Invertebrate samples were nonetheless collected from PI3. The three sediment subsamples were pooled and analyzed as one sample.
Invertebrate samples were collected using a dip net with 1 mm mesh size. The banks and the macrophyte beds in the littoral zone were continuously swept for 6 h to ensure that sufficient material would be collected for PAH analysis. The samples were kept on ice and transported to the laboratory in 2.5 L containers. Within 24 h, the invertebrates were classified and sorted according to taxa. Most of the taxa were found in only a limited number of the SWRPs or lakes. The following taxa were selected and analyzed for PAHs: damselflies (Coenagrionidae), dragonflies (Libellula sp. and Aeschnae sp.), snails (Planorbidae, Bithyniidae, and Lymnaeidae) and leeches (Hirundinidae) (Table 1). A total of 35 invertebrate samples were collected. As not all taxa were found in each water body, the invertebrates were grouped by taxa and type of water body (SWRPs and lakes). The taxa grouped into 4 samples of damselflies collected from the SWRPs and 6 from the lakes; 8 samples of dragonflies from the SWRPs and 12 from the lakes; 9 samples of snails from the SWRPs and 2 from lakes; and 2 samples of leeches from ponds and 2 from lakes. Some of the samples of the taxa originated from the same SWRP or lake; for example, all 8 dragonflies were collected from only 4 lakes. Further details on which taxa were collected in which SWRP or lake can be found in Stephansen et al. [33].
Sediment and invertebrate samples were frozen and stored at −18 °C prior to freeze-drying (ALPHA 1-2 LD plus, Martin Christ, Germany) in a vacuum at −55 °C for 48 h. Hereafter, the samples were homogenized, following a procedure further described in Stephansen et al. [34]. All samples were handled only with glass materials, in order to avoid PAH contamination from plastic materials. All glass materials used for sampling, processing, extraction and analysis were muffled before use at 500 °C for at least 4 h to ensure the required cleanness. The campaign did not include water samples, as pollutant concentrations in the water phase of SWRPs are known to fluctuate to such a degree that grab samples cannot not be expected to be representative [24].
2.2. Chemical Analysis
PAHs were extracted using microwave-assisted extraction (MAE). For sediments, approximately 2 g dry freeze-dried matter was used, while 0.15–2.0 g was used for invertebrates. The extraction procedure recommended by the US Environmental Protection Agency, EPA Method 3546, which suggests MAE with 1:1 mixture of hexane and acetone, was applied [35]. The subsamples were transferred to Teflon vessels (50 mL capacity) and 15 mL concentrated solvent was added; 1:1 hexane:acetone (Sigma Aldrich, Chromasolv for HPLC; Merck for analysis). Prior to extraction, the samples were spiked with an acetonic solution of recovery standards of perdeuterated PAHs: Anthracene-d10 (Supelco Analytical, 2000 µg·mL−1) and Chrysene-d12 (Supelco Analytical, 2000 µg·mL−1). Extraction conditions for the microwave oven (Multiwave 3000, Anton Paar, Graz, Austria) followed recommendations by EPA method 3546, meaning 10 min at 100 °C and high pressure [35]. After irradiation, the vessels were cooled before decanting the extracts into glass bottles. The extraction was then repeated on the same subsamples with additional 15 mL concentrated solvent. The two supernatants were combined and subsequently filtered through two separate glass columns to remove water, color and impurities. The first column contained 5 g sodium sulphate (Merck, pro analysis) and the second contained 5 g aluminum oxide (Merck, 90 active neutral). Both materials had been activated at 400 °C for 4 h. The columns were plugged with clean fiberglass. Hexane:acetone (10 mL) was used to prewash the filters. 30 mL of extracted sample were then filtered over the sodium sulphate column, along with a small additional amount of hexane:acetone that was used to clean the sample bottles. To flush the column, 12 mL of hexane:acetone was used. The resulting treated sample was then evaporated to 1 mL by nitrogen blow-down. The procedure was repeated for the aluminum oxide column, here flushed with 12 mL ethyl acetate. Finally, the sample was reduced to 0.5 mL by nitrogen blow-down. For PAH quantification an internal standard, triphenylamine (Sigma Aldrich, 98%), was added to the final sample extracts. Hereafter, the extracts were adjusted to 1 mL with ethyl acetate and stored at −18 °C until PAH quantification.
The quantitative determination of PAHs was conducted by gas chromatography (GC) coupled with a mass-selective detector (MS) (Trace GC Ultra, Thermo Scientific, Waltham, MA, USA; DSQII mass-selective detector, Thermo Scientific, Waltham, MA, USA ) using a TR-1MS column, 30 m × 0.25 mm id, with film thickness of 0.25 µm. Extracts (10 µL) were injected by use of an auto-sampler (TriPlus, Thermo Scientific, Waltham, MA, USA) into splitless mode. The inlet and x-line were maintained at 280 °C. The GC-MS was operated applying the following program: The initial column temperature was 40 °C for 3 min. Hereafter, the temperature was ramped as follows: (1) ramp at rate 25.0 °C min−1 to 140 °C; (2) ramp at rate 10.0 °C min−1 to 240 °C; (3) ramp at rate 5.0 °C min−1 to 300 °C, where it stayed for 3 min. The temperature of the ion source was fixed at 200 °C. Helium, the carrier gas, flowed at a constant rate of 1.7 mL·min−1. A total of 13 PAHs (US-EPA recommended priority pollutants, EPA 525 Mix A) were detected by SIM mode (selected ion monitoring): acenaphthylene (ACY), fluorene (FL), phenanthrene (PHEN), anthracene (ANT), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHY), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DBA), indeno(1,2,3-cd)pyrene (INP) and benzo(g,h,i)perylene (BPY). The area response factors of PAHs, relative to the internal standard (triphenylamine), were determined for three PAH-concentrations (0.10, 1.0, 10.0 μg·mL−1). The PAH standards for quantification were prepared in acetone from a commercial PAH solution (Supelco Analytical, EPA 525 PAH Mix-B, 500 μg·mL−1) that contained the 13 PAHs. The internal standard was added to the PAH standards in the same concentration as for the collected samples (50 ng·mL−1).
The samples were processed in batches of 16, containing 1 procedural blank and 2 samples of certified reference material (Fluka Analytical, BNAs Clay Loam 2–CRM131-100G, Lot: 013243). The concentrations of the 13 certified reference PAHs were measured; however, BbF and BkF could not be separated chromatographically from each other. The same was the case for INP and BPY. These were therefore not validated in correlation with the certified reference material. For ACY and BaP, all measured reference samples had concentrations within the prediction intervals. For FL, PHEN, ANT, CHY, and BPY, 62%–77% of the measurements were inside the prediction intervals, and lastly, PYR and BaA had 54% of measured concentrations within the prediction intervals. Regarding recovery of added standards, 89% of the Anthracene-d10 determinations were between 60%–140% of the concentration added, while 82% of the Chrysene-d12 determinations were within that interval. No recovery corrections were performed.
The limits of detection (LOD) were defined specifically for each individual PAH as the lowest concentrations that provided chromatographic peaks with a signal-to-noise ratio equal to or greater than 3. To exclude the possibility that a high content of lipids and proteins in invertebrates interfered with the so-defined LOD as well as the recovery rate, a matrix consisting of fish flour was spiked with the 13 PAHs. The samples were extracted and analyzed following the same procedure as for invertebrates. These validation runs showed that the LOD, as well as the recovery rate, was unaffected by the applied matrix.
2.3. Data Analysis
Principal component analysis (PCA) was used to analyze sediment and invertebrate data separately, looking for internal structures of the data that could explain variance within the data set. The PCA finds the hypothetical variables, termed components, that explain the most of the variances within the data, which reduces the multivariate data input to few explanatory variables. PCA was performed with PC-ORD, MjM Software Design, Gleneden Beach, OR, USA [36]. Scatter plots and loading plots for the two data sets are displayed in the results. Scatter plots show all the samples plotted in the coordinate system defined by principal component 1 and 2, whereas loading plots show to what degree the different PAH compound define the difference and similarities between the samples.
3. Results and Discussion
3.1. Sediments
The sediments from the SWRPs and the small, shallow lakes contained PAH at a wide range of concentrations (Figure 2). The PAH levels in the SWRP were similar to what has been reported for comparable systems [37,38,39], and the concentrations in the lakes were in the upper range of what has been reported for freshwater sediments [40,41]. There was some variation from site to site in the PAH concentrations in both types of sediments. Comparing the sum of the 13 PAHs, the median values in the sediments from the SWRPs (0.75 mg·(kg·DM)−1) and the lakes (0.69 mg·(kg·DM)−1) were close (see lower right corner in Figure 1). A Mann–Whitney test, applied because the data were not normal distributed, showed that, but for BPY, the difference between the medians was not statistically significant (p > 0.05). Considering the calculated median value of the individual PAHs, it was seen that some had accumulated to a higher degree in the SWRP sediments, compared to that of the lakes (see the dotted lines in Figure 2). PHEN and PYR occurred at the highest concentrations in both systems, followed by CHY and BbF/BkF. ACY and ANT were present at the lowest concentrations. The relative differences between the PAH concentrations of the SWRP sediments and the lake sediments were found to be most pronounced for BPY, BaP, CHY, INP/DBA and BbF/BkF. IN particular, the BPY concentrations in SWRP sediments were more than four-fold higher than in lake sediments. For ACY, ANT, and PYR, the median concentrations in the SWRP sediments and the lake sediments were almost identical, whereas for FL and PHEN, the median concentrations were slightly higher in the lake sediments. Only for BPY, the difference between the median concentration of all SWRPs versus the median concentration of all lakes was statistically significant (Mann–Whitney, p = 0.018). The reason for some PAHs accumulating to a higher degree in the sediments of the SWRPs is not known, but could be caused by differences in loads on the two types of water bodies. It could, though, also be caused by differences in biodegradation of the PAHs as it was the PAHs of highest molecular weight (PYR, BaA, CHY, BbF/BkF, BaP, INP/DBA and BPY), which had higher concentrations in the SWRP sediments (Figure 2). The difference in median concentration of these substances in sediments of SWRPs and lakes increased continuously with the molecular weight of the PAHs where PYR had 34% higher concentrations in sediments of SWRPs than lakes, while BPY had 415% higher concentrations. This is likely due to the high-molecular weight PAH being degraded more slowly than low-molecular weight PAHs [42,43]. Another cause could be that the pathways of PAHs to SWRPs and lakes differ, as the main pathway to SWRPs probably is surface runoff from impervious surfaces, while this is not the case for lakes.
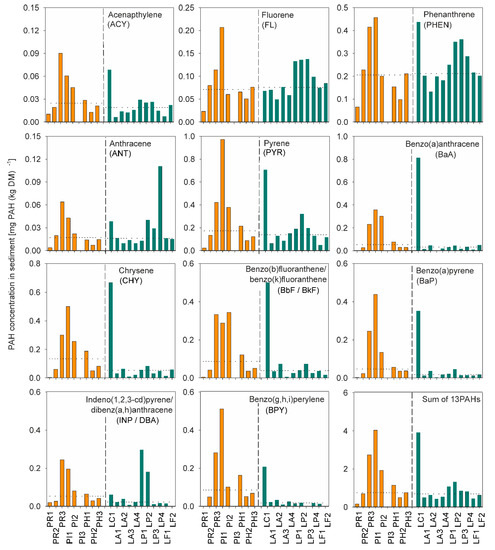
Figure 2.
Polycyclic aromatic hydrocarbons (PAH) concentrations in sediments of the SWRPs (orange bars) and the natural, shallow lakes (green bars). The dotted lines represent the overall median concentrations in the SWRPs versus the lakes. No sediment sample was obtained in PI3, because of a raised water level in the sampling period, due to heavy precipitation.
Within the group of SWRPs, PR3 and PI1 had accumulated the highest PAH concentrations in the sediments. The pond PI1 received stormwater runoff from a truck center and filling station near the coastal city of Frederikshavn, which has ferry service to both Sweden and Norway. Consequently, trucks stopping overnight often occupy the catchment area of the pond. Visual observations made during sampling, e.g., the presence of an oil film on the pond surface, malodor and gas bubbles in the pond sediments, indicated a generally level of organic pollutants in the incoming stormwater. The pond PR3, on the other hand, had no such obvious source of pollutants. It received stormwater runoff from a small village without industrial activity. However, being an urbanized area without district heating means that alternative residential heating consisting of oil-fired boilers, automatic stokers and wood burning stoves are widely used and potential PAH emission sources. Furthermore, such installations sometimes might burn inappropriate domestic waste, which could give off additional amounts of PAHs.
Of the 11 lakes, the city lake, LC1, had the highest concentrations of PAHs, except for FL, ANT, and INP/DBA. LC1 sediments had even higher concentrations of BaA, CHY and BbF/BkF than any of the SWRPs. Based on historical aerial photos, the lake has been dated to be more than 100 years old [44]. It is not connected to the public sewage or stormwater system, and the lake most likely has a high water-retention time. Industrial and commercial activities have, over recent decades, developed around the lake, increasing the risk of uncontrolled discharges of pollutants, which could subsequently have accumulated in the lake sediments.
Results are inconsistent with the expectation of relatively high levels of PAH in SWRPs that receive runoff from highways. The logic underlying the expectation remains plausible, as traffic-related sources, such as vehicle emissions, wear and tear from asphalt, tires, and vehicles have been reported as major sources of PAHs [15,45], and previous studies have shown that road runoff in Denmark contained 2.9–90 µg·L−1 of total PAH [46]. These are quite high concentrations compared to other studies; Tromp et al. [47] found PAH levels in Dutch runoff from highways to be 2.4 µg·L−1. Similarly, Vollertsen et al. [25] found total PAH concentrations (flow weighted average over one full year of monitoring) in Norwegian runoff to be 1.8 µg·L−1. Part of the explanation for the differences could be the regulation of PAH in tires sold on the European market from January 2010 (restriction number 50 in Annex XVII of REACH limits).
Some investigations indicate that 11%–17% of the PAH content in the road runoff was dissolved, and the rest was particle-bound [48], and therefore after sedimentation would end up in the SWRP sediments. Of the particle-bound fraction, El-Mufleh et al. [49] found that roughly half of the PAH was associated with particles lighter than 1.9 g·cm−3, a density that contributed the main part of the organic matter in the investigated samples. A screening study of sediments from 70 Danish SWRPs found that 83% of the samples had total PAH concentrations below 4 mg·(kg·DM)−1, and hence were to be treated as unpolluted soil with respect to this contaminant [39]. Similar to the results of the present study (Figure 2), Grauert et al. [39] concluded that the SWRPs did not have markedly higher PAH levels than the natural lakes included in their survey.
For several of the investigated PAHs, there have been put forward EQS values for sediments. Comparing, for example, values from Canada, Norway and HELCOM [4,5,50], it is seen that both SWRPs and lakes commonly exceeded EQS values for specific PAHs (Table 2). For example, for fluorene, where Canada has an EQS of 21.2 µg·(kg·DM)−1 and Norway one of 150 µg·(kg·DM)−1, all SWRPs and lakes exceeded the Canadian EQS value, while one SWRP exceeded the Norwegian one. For phenanthrene, where Canada has an EQS of 41.9 µg·(kg·DM)−1 and Norway one of 780 µg·(kg·DM)−1, all SWRPs and lakes exceeded the Canadian EQS value while, while no water body exceeded the Norwegian one. For anthracene, where Canada has an EQS of 46.9 µg·(kg·DM)−1 and Norway one of 4.6 µg·(kg·DM)−1, one SWRP and one lake exceeded the Canadian EQS value while, while all water bodies but one SWRP exceeded the Norwegian one. HELCOM sets EQS for anthracene to 24 µg·(kg·DM)−1, which was exceeded in 2 out of 8 SWRPs and 4 out of 11 lakes. A similar pattern is seen for other PAHs were EQS values have been defined. All in all, there was no systematic trend that one type of water body exceeded EQS values more commonly than others.

Table 2.
Environmental quality standards from Canada, Norway and the Helsinki Commission (HELCOM) for the Baltic Sea [4,5,50].
3.2. Invertebrates
Somewhat similar to what was seen for PAHs in sediment, there was a slight tendency towards high-molecular-weight PAHs occurring at higher concentrations in the invertebrates of the SWRPs, compared to the lakes (Figure 3). While this was less pronounced than for the sediments, the high-molecular weight PHAs all had higher median concentrations in SWRP invertebrates than lake invertebrates, ranging from 12% higher concentrations for PYR to 315% for BPY. However, for BaP, INP/DBA and BPY most samples were below detection limits and the low-molecular compound ACY was higher in the SWRP invertebrates than lake invertebrates, hence limiting the validity of this conclusion.
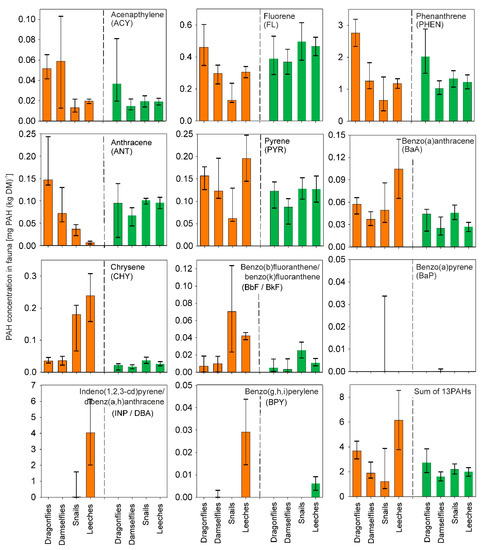
Figure 3.
Median PAH concentrations in invertebrates in the SWRPs (orange bars) and the natural, shallow lakes (green bars). Nomenclature: damselfly (median concentration calculated on 4 pond samples/6 lake samples); dragonfly (8 pond samples/12 lake samples); snails (9 pond samples/2 lake samples); leech (2 pond samples/2 lake samples).
Few studies have investigated PAH concentrations in invertebrates from systems similar to the ones of the present study. Nevertheless, the levels of PAH in the biota of the studied water bodies were generally some orders of magnitude higher than Heintzman et al. [51] found for adult odonates from an urban area in the USA, substantially higher than Yoon et al. [52] found for fish and invertebrates in a tidal zone of the Arabian Gulf affected by urban activities and an order of magnitude higher than Viñas et al. [53] found for wild mussels in an estuary of Spain. However, the PAH levels were quite similar to what was found for benthic invertebrates in Kongsfjorden, Svalbard [54], and also to what Thuy et al. [55] reported as common for various bivalves in the ocean. The concentrations in the invertebrates of the present study were furthermore quite below potential bioaccumulation levels reported for high-exposure scenarios for arctic and temperate marine invertebrates reported by Szczybelski et al. [56]. A PCA of invertebrates from the SWRPs (18 biota samples distributed on 4 taxa, Table 1) and the natural, shallow lakes (19 biota samples distributed on 4 taxa) showed that PC1 accounted for 30.1% of the total variance, whereas PC2 accounted for 19.7% (Figure 4). PCA revealed that all invertebrates collected in the natural, shallow lakes clustered together on the PC1-axis, while they were more spread out on the PC2-axis. Many of the invertebrate samples caught in the SWRPs also clustered together with invertebrates of the lakes. However, some invertebrates from SWRPs were scattered towards the lower end of the PC1 axis, and towards the upper end of the PC2 axis. These differences were mainly caused by higher concentrations of CHY, BbF/BkF, INP/DBA and PYR in the invertebrate samples. Of the eight invertebrate taxa outside the main cluster, three originated from the SWRP, PI1, and five were identified as snail species.
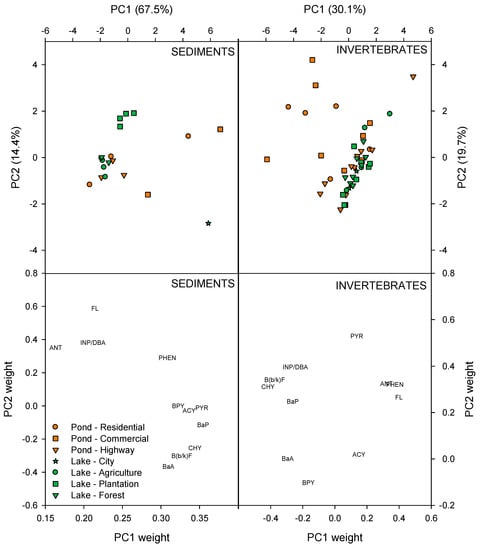
Figure 4.
Principal component analysis of PAHs in sediments and invertebrates of the SWRPs and the natural, shallow lakes.
Like for sediments, there have been put forward EQS values for biota, albeit to a lesser extent (Table 2). The European Union and HELCOM has set EQS for benzo(a)pyrene to 5 µg·(kg·DM)−1 [3,4]. Benzo(a)pyrene was not detected in any invertebrates; however, these EQS values were below the LOD for this substance in the present study, and it cannot hence be determined if this value actually was exceeded or not. The Norwegian EPA has set EQS benzo(a)atracene to 304 µg·(kg·DM)−1 [50], which also was not exceeded in any biota sample. Taking the scarcity of data from similar water bodies and the specific EQS values into account, it seems that the PAH concentrations in the invertebrates of the SWRPs, as well as the natural lakes, were not especially high or problematic for the exposed fauna.
3.3. PAHs in Sediments and Invertebrates
The levels of PAHs in the sediments and invertebrates of the SWRPs were, in general, close to the levels found in the lakes. Especially the SWRPs receiving runoff from highways and to some degree the ponds receiving runoff from residential areas were, in this respect, not distinguishable from the main body of the studied lakes. Still, some of the SWRPs, as well as the city lake, were clearly distinguishable, indicating that land use is a factor for accumulation of PAHs in both sediments and invertebrates of SWRPs. Even though the absolute differences in concentration levels were not large, some of the individual PAHs in some of the SWRPs and lakes were occasionally significantly higher than the median. For substances where the median concentrations in sediments of SWRPs and lakes were similar, there was furthermore a tendency that concentrations in the biota were also similar between the two types of water bodies (ACY, FL, PHEN, ANT, PYR and BaA). For substances where there tended to be higher levels in the SWRP sediments compared to the lakes, this was also reflected in the biota (CHY, BbF/BkF, INP/DBA and BPY). It hence seems that PAH levels in the sediments to some degree were reflected by concentrations in the invertebrate population of the water bodies.
The median level of PAH in the sediments of all water bodies was 0.75 mg·(kg·DM)−1, which places them in the upper range of what has been reported for lake sediments around the world [41]. However, the concentrations were still one to two orders of magnitudes below what was reported for the most polluted natural lakes. At the same time, PAH levels in the invertebrates were in the upper range of what has been reported for other aquatic environments [55]; however, they seemed not to exceed EQS values. All in all, the obtained data on PAH in sediments and biota indicate this pollutant would not cause a deterioration of the aquatic habitats in SWRPs.
4. Conclusions
The concentration of total PAH in sediments and invertebrates from 9 SWRPs and the 11 lakes was comparable, indicating that the PAH load on the two types of water bodies led to somewhat similar concentration levels in their sediments and invertebrates. The levels in the SWRPs were similar to what has been previously reported for such water bodies by other studies, while lakes were in the upper range of reported concentrations. However, when looking at individual PAHs, differences in the levels of high molecular weight PAHs could be identified, while the low molecular weight PAHs were similar in concentration. When doing a PCA analysis on the individual PAHs in the sediments, the SWRPs receiving highway runoff clustered with lakes in forests and farmlands. A similar phenomenon was seen for some of the SWRPs receiving runoff from residential areas. Many of the invertebrate taxa caught in SWRPs receiving highway runoff also clustered with those from natural, shallow lakes.
The observation that PAH levels in the sediment and biota of the SWRPs were rather comparable to the studied lakes could indicate that these SWRPs were subject to a pollution pressure from PAHs equivalent to that of the natural, shallow lakes. This seems especially to be the case for SWRPs receiving highway runoff, which indicates that technical water bodies managing stormwater runoff from at least some types of drainage areas can sustain invertebrate ecosystems without the biota therein being subjected to a higher degree of PAH bioaccumulation than natural, shallow lakes of the same region. Whether or not this is still the case, or if the PAH concentrations have been even further reduced since the sampling of 2010 due to for example the out-phasing of PAHs in tires, cleaner combustion engines, and a general trend towards better environmental standards is an important perspective to address in future studies.
Author Contributions
Conceptualization, D.A.S., A.H.N. and H.B.; methodology, D.A.S., C.A.A. and A.H.N.; software, D.A.S., M.L.F. and A.H.N.; validation, D.A.S., C.A.A., M.L.F. and A.H.N.; formal analysis, D.A.S.; investigation, D.A.S.; resources, H.B. and M.L.F.; data curation, D.A.S.; writing—original draft preparation, D.A.S.; writing—review and editing, D.A.S., C.A.A., H.B., M.L.F. and A.H.N.; visualization, D.A.S.; supervision, A.H.N.; project administration, D.A.S.; funding acquisition, D.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collins, J.F.; Brown, J.P.; Alexeeff, G.V.; Salmon, A.G. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- EU. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2013, 226, 1–17. [Google Scholar]
- HELCOM. OUTCOME of the 50th Meeting of the Heads of Delegation Heads of Delegation. Baltic Marine Environment Protection Commission, Heads of Delegation, Laulasmaa, Estonia, 15–16 June 2016. Available online: https://portal.helcom.fi/meetings/hod%2050-2016-327/default.aspx (accessed on 20 April 2020).
- CCME. Polycyclic aromatic hydrocarbons (PAHs). Canadian Sediment Quality Guidelines for the Protection of Aquatic Life. Canadian Environmental Quality Guidelines, Canadian Council of Ministers of the Environment. 1999. Available online: http://ceqg-rcqe.ccme.ca/download/en/243 (accessed on 20 April 2020).
- Boyd, T.J.; Montgomery, M.T.; Steele, J.K.; Pohlman, J.W.; Reatherford, S.R.; Spargo, B.J.; Smith, D.C. Dissolved oxygen saturation control PAH biodegradation in freshwater estuary sediments. Microb. Ecol. 2005, 49, 226–235. [Google Scholar] [CrossRef]
- Maletic, S.P.; Beljin, J.M.; Roncevic, S.D.; Grgic, M.G.; Dalmacija, B.D. State of the art and future challenges for polycyclic aromatic hydrocarbons is sediments: Sources, fate, bioavailability and remediation techniques. J. Hazard. Mater. 2019, 365, 467–482. [Google Scholar] [CrossRef]
- Baek, S.O.; Field, R.A.; Goldstone, M.E.; Kirk, P.W.; Lester, J.N.; Perry, R. A review of atmospheric polycyclic aromatic hydrocarbons: Sources, fate and behaviour. Water Air Soil Pollut. 1991, 60, 279–300. [Google Scholar] [CrossRef]
- Heim, S.; Schwarzbauer, S. Pollution history revealed by sedimentary records: A review. Environ. Chem. Lett. 2013, 11, 255–270. [Google Scholar] [CrossRef]
- Manoli, E.; Samara, C. Polycyclic aromatic hydrocarbons in natural waters: Sources, occurrence and analysis. Trends Anal. Chem. 1999, 18, 417–428. [Google Scholar] [CrossRef]
- Shen, H.Z.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.F.; Wang, B.; Zhang, Y.Y.; Chen, Y.C.; Lu, Y.; et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef]
- Schwarz, K.; Gocht, T.; Grathwohl, P. Transport of polycyclic hydrocarbons in highly vulnerable karst system. Environ. Pollut. 2011, 159, 133–139. [Google Scholar] [CrossRef]
- Aryal, R.; Furumai, H.; Nakajima, F.; Beecham, S. Variation in PAH pattern in road runoff. Water Sci. Tecnol. 2013, 67, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.S.; Hoffmann, E.J.; Quinn, J.G. Source of petroleum hydrocarbons in urban runoff. Water Air Soil Pollut. 1990, 52, 1–21. [Google Scholar] [CrossRef]
- Dunbar, C.J.; Lin, C.I.; Vergucht, I.; Wong, J.; Durant, J.L. Estimating the contributions of mobile sources of PAH to urban air using real-time PAH monitoring. Sci. Total Environ. 2001, 279, 1–19. [Google Scholar] [CrossRef]
- Demir, T.; Yenisoy-Karakaş, S.; Karakaş, D. PAHs, elemental and organic carbons in a highway tunnel atmosphere and road dust: Discrimination of diesel and gasoline emissions. Building Environ. 2019, 160, 106166. [Google Scholar] [CrossRef]
- Aryal, R.K.; Furumai, H.; Nakajima, F.; Boller, M. Characteristics of particle-associated PAHs in a first flush of a highway runoff. Water Sci. Technol. 2006, 53, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Lei, Y.D.; Wania, F. Transport of polycyclic hydrocarbons and pesticides during snowmelt within an urban watershed. Water Res. 2011, 45, 1147–1156. [Google Scholar] [CrossRef]
- Kessler, S.; Bierl, R.; Meyer, B.; Krein, A. Chemical effects of a near-to-nature detention pond on a small urban headwater. Limnologica 2017, 62, 118–125. [Google Scholar] [CrossRef]
- Bryant, W.L., Jr.; Goodbred, S.L. The response of hydrophobic organics and potential toxicity in streams to urbanization of watersheds in six metropolitan areas of the United States. Environ. Monit. Assess. 2009, 157, 419–447. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Ma, P.; Rowden, J.; Mielke, H.D.; Gonzales, C.; Powell, E. Sources and distribution of polycyclic aromatic hydrocarbons in urban soils: Case studies of Detroit and New Orleans. Soil Sediment Contam. 2008, 17, 547–563. [Google Scholar] [CrossRef]
- Todeschini, S.; Papiri, S.; Ciaponi, C. Performance of stormwater detention tanks for urban drainage systems in northern Italy. J. Environ. Manag. 2012, 101, 33–45. [Google Scholar] [CrossRef]
- Calabró, P.S.; Viviani, G. Simulation of the operation of the detention tanks. Water Res. 2006, 40, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Hvitved-Jacobsen, T.; Vollertsen, J.; Nielsen, A.H. Urban and Highway Stormwater Pollution; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Vollertsen, J.; Åstebøl, S.O.; Coward, J.E.; Fageraas, T.; Nielsen, A.H.; Hvitved-Jacobsen, T. Performance and modeling of a highway wet detention pond designed for cold climate. Water Qual. Res. J. 2009, 44, 253–262. [Google Scholar] [CrossRef]
- Snodgrass, J.W.; Casey, R.E.; Joseph, D.; Simon, J.A. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Sci. Total Environ. 2008, 359, 145–155. [Google Scholar]
- Casey, R.E.; Simon, J.A.; Atueyi, S.; Snodgrass, J.W.; Karouna-Renier, N.; Sparling, D.W. Temporal trends of trace metals in sediment and invertebrates from stormwater management ponds. Water Air Soil Pollut. 2006, 178, 69–77. [Google Scholar] [CrossRef]
- Brand, A.B.; Snodgrass, J.W. Value of artificial habitats for amphibian reproduction in altered landscapes. Conserv. Biol. 2010, 24, 295–301. [Google Scholar] [CrossRef]
- Hamer, A.J.; Smith, P.J.; McDonnell, M.J. The importance of habitat design and aquatic connectivity in amphibian use of urban stormwater retention ponds. Urban Ecosyst. 2012, 15, 451–471. [Google Scholar] [CrossRef]
- Xu, F.; Wu, W.; Wang, J.; Qin, N.; Wang, Y.; He, Q.; He, W.; Tao, S. Residual levels and health risk of polycyclic aromatic hydrocarbons in freshwater from Lake Small Bai-Yang-Dian, Northern China. Ecol. Model. 2011, 222, 275–286. [Google Scholar] [CrossRef]
- Rose, A.; Ken, D.; Kehinde, O.; Babajide, A. Bioaccumulation of polycyclic aromatic hydrocarbons in fish and invertebrates of Lagos Lagoon, Nigeria. J. Emerg. Trends Eng. Appl. Sci. 2012, 3, 287–295. [Google Scholar]
- Sun, Z.; Sokolova, E.; Brittain, J.E.; Saltveit, S.J.; Rauch, S.; Meland, S. Impact of environmental factors on aquatic biodiversity in roadside stormwater ponds. Sci. Rep. 2019, 9, 5994. [Google Scholar] [CrossRef]
- Stephansen, D.A.; Nielsen, A.H.; Hvitved-Jacobsen, T.; Pedersen, M.L.; Vollertsen, J. Invertebrates in stormwater wet detention ponds—Sediment accumulation and bioaccumulation of heavy metals have no effect on biodiversity and community structure. Sci. Total Environ. 2016, 566–567, 1579–1587. [Google Scholar] [CrossRef]
- Stephansen, D.A.; Nielsen, A.H.; Hvitved-Jacobsen, T.; Arias, C.A.; Brix, H.; Vollertsen, J. Distribution of metals in fauna, flora and sediments of wet detention ponds and natural shallow lakes. Ecol. Eng. 2014, 66, 43–51. [Google Scholar] [CrossRef]
- US EPA. Method 3546: Microwave extraction. In: SW-846 “Test Methods for Evaluating Solid Waste, Physical/Chemical Methods” 2007. Available online: http://www.epa.gov/osw/hazard/testmethods/sw846/ (accessed on 15 January 2010).
- McCune, B.; Mefford, M.J. PC-ORD Multivariate Analysis of Ecological Data; Version 6; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Bentzen, T.R.; Larsen, T. Heavy metal and PAH concentrations in highway runoff deposits fractionated on settling velocities. J. Environ. Eng. 2009, 135, 1244–1247. [Google Scholar] [CrossRef]
- Istenič, D.; Arias, C.A.; Matamoros, V.; Vollertsen, J.; Brix, H. Elimination and accumulation of polycyclic aromatic hydrocarbons in urban stormwater wet detention ponds. Water Sci. Technol. 2011, 64, 818–825. [Google Scholar] [CrossRef]
- Grauert, M.; Larsen, M.; Mollerup, M. Quality of sediment in detention basins—Mapping of the Danish national road network. Procedia Soc. Behav. Sci. 2012, 48, 393–402. [Google Scholar] [CrossRef][Green Version]
- Valentyne, A.; Crawford, K.; Cook, T.; Mathewson, P.D. Polycyclic aromatic hydrocarbon contamination and source profiling in watersheds serving three small Wisconsin, USA cities. Sci. Total Environ. 2018, 627, 1453–1463. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Anthropogenic PAHs in lake sediments: A literature review (2002–2018). Environ Sci. Process. Impacts 2018, 20, 1649–1666. [Google Scholar] [CrossRef]
- Larsen, S.B.; Karakashev, D.; Angelidaki, I.; Schmidt, J.E. Ex-situ bioremediation of polycyclic aromatic hydrocarbons in sewage sludge. J. Hazard. Mater. 2009, 164, 1568–1572. [Google Scholar] [CrossRef]
- Milić, J.; Avdalović, J.; Šolević-Knudsen, T.; Gojgić-Cvijović, G.; Jednak, T.; Vrvić, M.M. Initial microbial degradation of polycyclic aromatic hydrocarbons. Chem. Ind. Chem. Eng. Q. 2016, 22, 293–299. [Google Scholar] [CrossRef]
- Arealinformation, Cartographical Information and Orthophotos for Denmark with Regards to e.g., Landscape Characteristics, Nature Types, Road Systems and City Areas. Available online: https://arealinformation.miljoeportal.dk/html5/index.html?viewer=distribution (accessed on 3 July 2020).
- Markiewicz, A.; Bjorklund, K.; Eriksson, E.; Kalmykova, Y.; Stromvall, A.; Siopi, A. Emissions of organic pollutants from traffic and roads: Priority pollutants selection and substance flow analysis. Sci. Total Environ. 2017, 580, 1162–1174. [Google Scholar] [CrossRef]
- Miljøstyrelsen. Miljøfremmede stoffer i overfladeafstrømning fra befæstede arealer. Miljøprojekt nr. 355. Environmental pollutants in stormwater runoff from impervious urban catchments. Environmental project nr. 355, Danish Environmental Agency; Danish Environmental Agency: Odense, Denmark, 1997. [Google Scholar]
- Tromp, K.; Lima, A.T.; Barendregt, A.; Verhoeven, J.T.A. Retention of heavy metals and poly-aromatic hydrocarbons from road water in constructed wetland and the effect of de-icing. J. Hazard. Mater. 2012, 203, 290–298. [Google Scholar] [CrossRef]
- Stotz, G. Investigations of the properties of the surface water run-off from Federal Highways in the FRG. Sci. Total Environ. 1987, 59, 329–337. [Google Scholar] [CrossRef]
- El-Mufleh, A.; Béchet, B.; Basile-Doelsch, I.; Geffroy-Rodier, C.; Gaudin, A.; Ruban, V. Distribution of PAHs and trace metals in urban stormwater sediments: Combination of density fractionation, mineralogy and microanalysis. Environ. Sci. Pollut. Res. 2014, 21, 9764–9776. [Google Scholar] [CrossRef] [PubMed]
- Miljødirektoratet. Miljødirektoratet. Grenseverdier for klassifisering av vann, sediment og biota. Miljødirektoratet 2016, M-608, 26. [Google Scholar]
- Heintzman, L.J.; Anderson, T.A.; Carr, D.L.; McIntyre, N.E. Local and landscape influences on PAH contamination in urban stormwater. Landsc. Urban Plan. 2015, 142, 29–37. [Google Scholar] [CrossRef]
- Yoon, S.J.; Hong, S.; Kim, T.; Lee, J.; Kwon, B.O.; Allam, A.A.; Al-khedhairy, A.A.; Khim, J.S. Occurrence and bioaccumulation of persistent toxic substances in sediments and biota from intertidal zone of Abu Ali Island, Arabian Gulf. Mar. Pollut. Bull. 2019, 144, 243–252. [Google Scholar] [CrossRef]
- Viñas, L.; Franco, A.; Blanco, X.; Bargiela, J.; Soriano, J.A.; Perez-Fernandez, B.; Gonzalez, J.J. Temporal and spatial changes of PAH concentrations in Mytilus galloprovincialis from Ria de Vigo (NW Spain). Environ. Sci. Pollut. Res. 2012, 19, 529–539. [Google Scholar] [CrossRef]
- Szczybelski, A.S.; van den Heuvel-Greve, M.J.; Kampen, T.; Wang, C.; van den Brink, N.W.; Koelmans, A.A. Bioaccumulation of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and hexachlorobenzene by three Arctic benthic species from Kongsfjorden (Svalbard, Norway). Mar. Pollut. Bull. 2016, 112, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Thuy, H.T.T.; Loan, T.T.C.; Phuong, T.H. The potential accumulation of polycyclic aromatic hydrocarbons in phytoplankton and bivalves in Can Gio coastal wetland, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 17240–17249. [Google Scholar] [CrossRef]
- Szczybelski, A.S.; Diepens, N.J.; van den Heuvel-Greve, M.J.; van den Brink, N.W.; Koelmans, A.A. Bioaccumulation of polycyclic aromatic hydrocarbons by arctic and temperate benthic species. Environ. Toxicol. Chem. 2019, 38, 883–895. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).