Abstract
The abiotic reduction of NO3− to NO2−—coupled with the oxidation of labile organic materials such as citric acid, syringic acid and natural organic matter (NOM) and NH4+ through the goethite-mediated Fe(III)/Fe(II) cycle under anaerobic condition—was investigated at pH values of 4 and 7. The concentrations of the produced Fe2+ and NO2− were monitored. At a pH of 4, concentrations of Fe2+ increased, except for citric acid; no NO2− was detected. The reason why it was not detected is unclear. A possible reaction was the adsorption of NO2− onto goethite at pH < point of zero charge (pzc) of goethite (6.42) due to electrical attractive force. The maximum production of NO2− at a pH of 7 was in the order of citric acid >> syringic acid > NOM. However, Fe2+ was not detected at this pH even though Fe2+ should be required for NO2− production. To better understand of these phenomena, the adsorptive removal of Fe2+ and NO2− onto goethite was experimentally investigated. More than 90% of the produced Fe2+ and NO2− could be removed rapidly by adsorption onto the surface of goethite at pH 7 and 4, respectively. In addition, the reaction of Fe2+ with NO3− appeared to determine the overall reaction rate of the Fe(III)/Fe(II) cycle because of its relatively slow reaction rate. Using these results, we conclude that NO2− can be produced from NO3− reduction through Fe(III)/Fe(II) cycle with labile organic materials and ammonium at a pH of 7; especially, Fe(III)/Fe(II) cycle with citric acid results the maximum NO2− production higher than 600 μM for a long time (over 200 h) and then disappeared. But, the reasons for its disappearance were not addressed in this study.
1. Introduction
Nitrogen has many oxidation states and is important for biomolecules such as enzymes and nucleic acids. Ammonium (NH4+) and nitrate (NO3−) are two major nitrogen sources in surface and subsurface water systems because they are the final reduced and oxidized nitrogen under anaerobic and aerobic conditions, respectively and nitrite (NO2−) is frequently found under specific anaerobic conditions. Nitrogen contamination in surface water and groundwater has mostly been caused by ammonia and nitrate worldwide [1]. Among these nitrogen compounds, NO3− contamination is generally derived from the oxidation of NH4+ through nitrification by autotrophic bacteria. Ammonia has mainly come from the discharge of agricultural fertilizer and the mineralization of organic nitrogen under aerobic and anaerobic conditions [2,3,4].
A soil horizon is a layer parallel to the soil surface. The O horizons are dominated by undecomposed or partially decomposed organic materials such as leaves, needles, moss and lichens and, in the A horizons which formed below O horizon, humidification of the organics occurs under anaerobic conditions. In addition, Fe(III) with a high concentration frequently becomes a major electron acceptor in soils and sediments [5,6]. In these conditions, ferric (Fe(III)) and ferrous (Fe(II)) cycle which is the reduction of Fe(III) to Fe(II) and the sequential oxidation of Fe(II) to Fe(III) has been an important way of involving the biotic or abiotic redox of organic or inorganic materials in anaerobic soils and sediments [6]. Abiotic reactions always occur independently regardless of biotic reactions under certain conditions and differ from biotic reactions in important ways for carbon and nitrogen cycles in nature. In nature, abiotic reactions have been an important role for the nutrient cycling and the change of environmental conditions because biologic processes does not correspond all of these abiotic reactions and abiotic reactions can occur anywhere under appropriate conditions [7].
Fe(III)/Fe(II) cycle plays a particularly important role in abiotic nitrogen oxidation or reduction in forest and wetland soils because iron is the most abundant metal in the soil [6,8]. Davidson et al. [8] suggested the iron-mediated rapid abiotic immobilization of NO3− and transient generation and sequential disappearance of NO2− via Fe(III)/Fe(II) cycle. Zhu-Barker et al. [9] also reported that abiotic reactions between redox-active metals including iron and manganese, organics and reactive intermediates in the nitrogen cycle including nitrite (NO2−) can be significant and then reduce NO2− to N2O at low O2. As such, NO2− is often transiently produced as an intermediate product during abiotic reactions related to N cycle. Factors affecting the concentration of NO2− generated from abiotic NO3− reduction are pH, low O2, NO3− concentration and the type of reduced materials.
In both biotic and abiotic processes, various reduced organic or inorganic materials are required in order to reduce Fe(III) to Fe(II). Lignin is the most abundant natural compound; syringic acid (C9H10O5) is a representative lignin monomer in soil that reacts easily with iron [10]. Citric acid (H3C6H5O7) is also found in a variety of plants, fruits, leaves, roots and in milk, and acts as a chelating agent for heavy metals such as iron, lead and cadmium. Meanwhile goethite (FeOOH), hematite (Fe2O3) and magnetite (Fe3O4) are typical Fe3+ containing soils and the concentration of Fe(III) frequently exceeds that of other electron acceptors in soils. Therefore, an environment suitable for the Fe-mediated redox cycles can be created in anaerobic soils and sediments [11,12,13]. However, better understanding of the relationship between the NO2− transient production during this process and reduced materials is necessary.
This work, therefore, aims to verify the kinetics of the production of NO2− from abiotic reduction of NO3− in the Fe(III)/Fe(II) cycle under anaerobic conditions with NH4+ and several labile organic materials such as citric acid, syringic acid and natural organic matter (NOM) at pH 4 and 7, assuming the conditions as the forest and wetland soils which have generally acidic pH value (about 4) due to anaerobic acidification of organic materials by anaerobic bacteria [14]. With assumption that the pH did not affect the Fe(III)/Fe(II) cycle, relationship between the production of Fe2+ and NO2− was also investigated. In this study, the Fe2+ profile was also observed during the Fe(III)/Fe(II) cycle as well as NO2− produced by reacting Fe2+ with NO3− under anaerobic conditions.
The reaction formula for the oxidation of citric acid, syringic acid and ammonia used in this study and the reduction of Fe3+ to Fe2+ is as shown in Equations (1)–(3), respectively and the reaction of Fe2+ with NO3− to produce NO2− is presented in Equation (4).
The overall reactions for syringic acid, citric acid and ammonium with goethite and NO3− present in Equations (5)–(7).
2. Materials and Methods
2.1. Labile Reduced Materials
Citric acid (Sigma-Aldrich, >99% St. Louis, MO, USA), syringic acid (Sigma-Aldrich, >99% St. Louis, MO, USA), dissolved NOM and ammonium chloride (NH4Cl, Sigma-Aldrich, >98% St. Louis, MO, USA) were used as labile reduced materials. The dissolved NOM was collected from riparian groundwater in an O-horizon layer near a wetland located in Maryland, USA. The water was collected and stored in polypropylene bottles in the refrigerator. Before use, the water was filtered with 0.2-μm pore size syringe filter. The organic carbon concentrations of NOM measured by TOC analyzer was about 150–240 mg/L (12.5 to 20 mM of TOC). The TOC concentration of NOM was measured for each experiment and the final TOC concentration of NOM was adjusted to be 1 mM.
2.2. Synthesis of Goethite
As an Fe(III) source, goethite was synthesized by a modification of the Atkinson Method [15]. First, 400 mL of a 2.5 M KOH solution was mixed with 1650 mL of prefiltered 0.15 M Fe(NO3)3 solution in a plastic container and stirred vigorously. The mixture was aged for 48 h at 80 °C and then centrifuged to separate the precipitate from the solution. The precipitate was dispersed in 0.01 M HNO3 solution and centrifuged again. This step was repeated 10 times and rinsed three times with oxygen-free ultrapure water which had been purged with N2 gas for 24 h to be deoxygenated. The synthesized goethite concentration was 89.4 g/L and the goethite was stored in ultrapure water to minimize oxygen exposure. The mineralogical composition of the synthesized goethite after being freeze-dried by a Freeze Dryer (FDU-2100, Eyela, Japan) was identified by X-ray powder diffraction (D/Max-2500, Rigaku, Japan); the results are shown in Figure 1. The determined d-spacing values of the goethite are compared with those determined by prior researchers in Table 1. The XRD pattern and d-spacing values were very similar to those of goethite synthesized by other researchers [16]. The point of zero charge (pzc) of the synthesized goethite was measured by the point of zero salt effect (PZSE) method [17]; as shown in Figure 2, the resulting pzc was approximately 6.4.
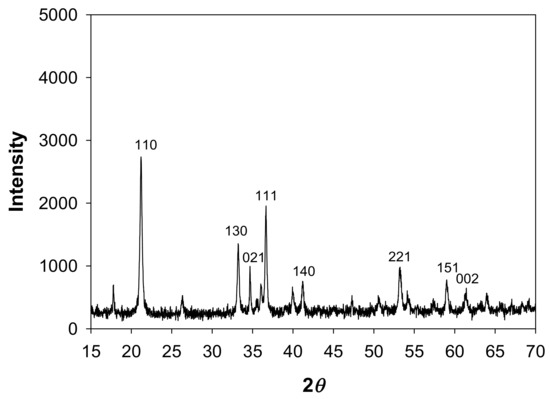
Figure 1.
X-ray diffraction (XRD) of synthesized goethite.

Table 1.
Comparison of d-spacing of the synthesized goethite in this study with other reference [16].
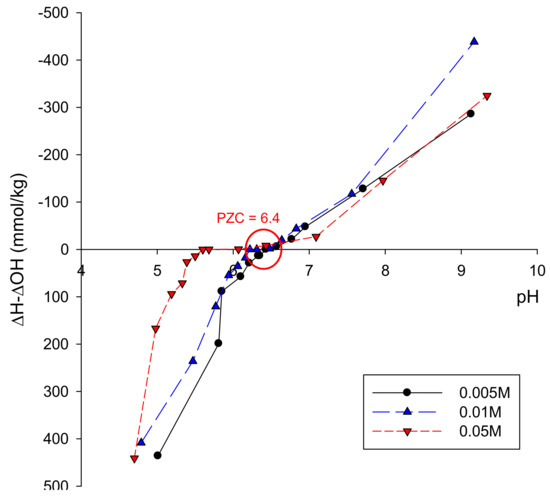
Figure 2.
Point of zero charge (pzc) of the synthesized goethite.
2.3. Fe(III)/Fe(II) Cycling Experiment
2.3.1. Effect of pH and Reduced Material
Next, 11.2 mL of stock suspension containing 89.4 g/L goethite, 20 mL of 100-mM pH buffer solutions, 20 mL of 1-mM reduced carbon stock solutions or 10-mM ammonium stock solutions, 20 mL of 1-mM nitrate stock solution, and 20 mL of 10-mM NaCl stock solution for ionic strength was added into a 250 mL bottle and then milli-Q water was filled to 200 mL of the final volume. For pH buffer, 10-mM MOPs and 10-mM butyric acid was used for pH 7 and 4, respectively. The final concentrations of the reduced carbon, ammonium and nitrate were all about 0.1 mM. After adding goethite and solutions, the bottle was shaken on a magnetic stirrer in an anaerobic glove box (Coy Lab, Grass Lake, MI, USA). Sampling was conducted at a predetermined time schedule in the glove box under strictly anaerobic conditions. The collected samples were filtered with a syringe filter in the glove box and then analyzed for water quality including the concentrations of Fe2+, NO2−, NO3− and TOC.
To estimate the reaction rate of the second step of the Fe(III)/Fe(II) cycle in which Fe2+ and NO3− react to produce Fe3+ and NO2−. The concentration of Fe2+ and NO2− was monitored at predetermined sampling times. To do so, 20 mL of 2-mM Fe2+ and 20 mL of 20-mM NO3− stock solutions were added to a 250 mL amber bottle that was then filled to 200 mL with ultrapure water. The final concentrations of Fe2+ and NO3− in the solution were 0.1 and 1.0 mM, respectively. The solution was mixed well with a magnetic stirrer and sampled by a syringe with a syringe filter with a pore size of 0.2 μm at predetermined times in the anaerobic glove box. Fe2+ and NO2− concentrations in the filtered samples were analyzed immediately. All experiments were executed in duplicate.
2.3.2. The Fate of Fe2+, NO2− and Goethite at pH Values of 4 and 7
As Fe2+ and NO2− are the target materials, their stability and final concentrations were investigated at pH values of 4 and 7. To do so, 1 g (dry base) of the synthesized goethite solution, pH buffers (butyric acid for a pH of 4 and MOPS for a pH of 7), and 2 mL of 2-mM Fe2+ or NO2− stock solution was added to a 250 mL bottle that was then filled to 200 mL with ultrapure water. Therefore, the final concentration of Fe2+ or NO2− was 0.1 mM. The bottle was mixed on a magnetic stirrer and sampled by a syringe with a syringe filter with a pore size of 0.2 μm at predetermined times in the anaerobic glove box.
2.4. Analytic Methods
The pH was measured immediately after sampling and filtration in the glove box. The filtered samples were diluted 25 times with ultrapure water. Then 0.5 mL of the diluted samples were analyzed. The concentration of syringic and citric acids in samples were measured using capillary electrophoresis (CE) (P/ACE MDQ, Beckman Coulter, Brea, CA, USA) with diode-array UV-visible detector with buffer solutions consisting of 25-mM phosphate and 0.5-mM tetradecyltrimethylammonium bromide (TTAB, Sigma-Aldrich, >99%, pH 7) for syringic acid and 25-mM ortho-P plus pyro-P (pH 9.5) and 0.5-mM TTAB for citric acid. The total organic carbon (TOC) content of the NOM was measured using a Dohrmann Phoenix 2000 UV-persulfate oxidation TOC analyzer (Tekmar-Dohrmann, Mason, OH, USA). TOC concentrations of syringic and citric acids were calculated by multiplying the concentrations measured via CE by the number of carbon atoms present in syringic acid or citric acid. The NO2− concentration was measured with a UV-Vis spectrophotometer (Shimadzu UV-1800, Kyoto, Japan) at 543 nm by diazotized sulfanilamide with the N-(1-naphthyl)-ethylenediamine dihydrochloride (NED) method [18]. The Fe2+ concentration was analyzed with the UV-Vis spectrophotometer at 562 nm by the ferrozine method [19]. The detection limits for NO2− and Fe2+ were 0.024 μM and 0.046 μM, respectively. The total iron concentration was measured by atomic absorption spectroscopy (Perkin Elmer AAnalyst 100 Waltham, MA, USA).
3. Results
3.1. Effect of Labile Organic Materials on Nitrite and Ferrous Production
The resulting profiles of NO2− and Fe2+ production from the Fe(III)/Fe(II) reaction cycle with labile organic materials of NOM, syringic acid and citric acid are shown in Figure 3. When using NOM as the organic material and at a pH of 4 (Figure 3a), the concentration of Fe2+ increased almost linearly with time whereas that of NO2− was almost zero. At a pH of 7 (Figure 3b), a peak NO2− concentration of approximately 4 μM was observed at about 120 h of reaction time before the concentration decreased but no Fe2+ was detected. A similar pattern was exhibited for syringic acid; Fe2+ and not NO2− was observed at a pH of 4 (Figure 3c), whereas NO2−, but not Fe2+ was observed at a pH of 7 (Figure 3d). Moreover, the concentration of syringic acid declined faster than did the concentration of NOM. When citric acid was used, however, a very different pattern was seen. No Fe2+ was detected at a pH of 4, even though the concentration of citric acid decreased with time. At a pH of 7, the concentration of NO2− increased to approximately 600 μM at 320 h, which was the highest concentration of NO2− produced in this experiment. This indicates that citric acid was the best organic carbon source for the Fe(III)/Fe(II) cycle among the compounds studied.
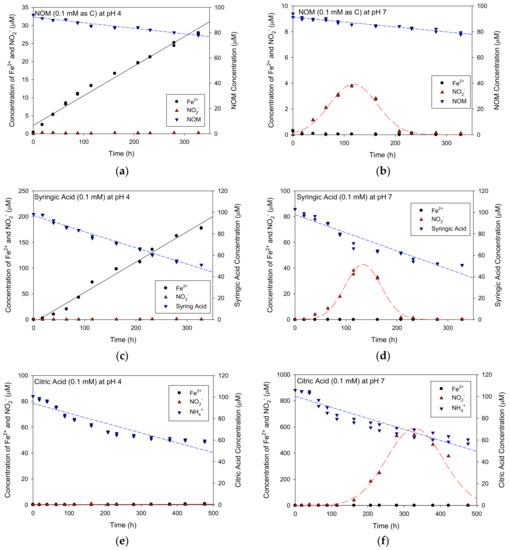
Figure 3.
Fe2+ and NO2− concentrations via time throughout Fe(II)/Fe(III) cycle with natural organic matter (NOM), syringic acid and citric acid at pH 4 and 7: (a) for NOM at pH 4; (b) for NOM at pH 7; (c) for syringic acid at pH 4; (d) for syringic acid at pH 7; (e) for citric acid at pH 4; (f) for citric acid at pH 7.
At a pH of 4 (i.e., in Figure 3a,c,e), the kinetics of Fe2+ production were nearly linear; the fitted slope, representing the production rate of Fe2+ (μM/h) when using NOM, syringic acid and citric acid was 0.0925, 0.5612 and 0.0010 μM/h, respectively, indicating that the syringic acid was the most favorable organic material for Fe2+ production. If Fe2+ is exposed to oxygen, it rapidly oxidizes to Fe3+ [20]; the linear increase in Fe2+ concentration seen here thus indicates that this reaction occurred under anaerobic conditions. However, no concentration of Fe2+ was detected when citric acid was used as reduced organic carbon compound. Citric acid can be combined easily with Fe2+ and Fe3+ as chelating agents to produce iron-citrate, which may have then been filtered out during the sampling step. As a result, no Fe2+ was produced when citric acid was used and syringic acid had the highest production rate of Fe2+. Meanwhile, no NO2− was detected at this pH. Moreover, even if NO2− was produced. This was likely due to the positive surface charge of goethite at pH < pzc, where the NO2− can be combined with the surface of goethite, due to electrical attraction.
At a pH of 7 (i.e., in Figure 3b,d,f), no Fe2+ was detected, due to its attachment onto the negative surface charge of goethite at pH > pzc. The highest peak concentration of NO2− was found when using citric acid (600 μM), followed by syringic acid (40 μM) and then NOM (4 μM), indicating that citric acid was the best reactivity compared to other substances due to high affinity of citrate to Fe2+ and Fe3+ for use as a chelating agent and its relatively simple molecular structure compared to syringic acid. And then the NO2− peaks decreased over time.
The TOC was then analyzed as a common organic unit to compare the Fe2+ or NO2− production kinetics for the varied reduced organic carbon compounds. The TOC concentrations of syringic acid and citric acid were calculated by multiplying their concentrations with the carbon number of the acids (μM). Therefore, the TOC-normalized Fe2+ and NO2− concentrations or concentration per unit carbon, could be calculated by dividing the observed Fe2+ and NO2− concentration by the carbon number of the syringic and citric acids. The resulting TOC-normalized Fe2+ and NO2− production kinetics are shown in Figure 4a,b at pH values of 4 and 7, respectively. At a pH of 4—as in Figure 3a,c,e above—the concentration of Fe2+ increased linearly with time and fitted well to a linear model when NOM or syringic acid were used, but not for citric acid. The slope, representing the TOC-based Fe2+ production rate in μM/h, was highest when NOM was used, followed by syringic acid, and then citric acid. Thus, the carbon in NOM was utilized most easily. Furthermore, the TOC-based NO2− production at a pH of 7 also depended on the carbon type. The maximum TOC-based NO2− production (approximately 90 μM) appeared at about 320 h for citric acid, in spite of no detection of Fe2+ at pH 4, followed by syringic acid (4.2 μM) and NOM (3.8 μM).
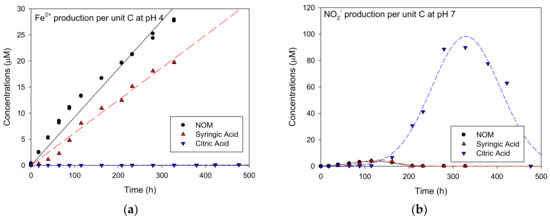
Figure 4.
The total organic carbon (TOC)-normalized Fe2+ and NO2− concentrations via time at (a) pH 4 and (b) pH 7.
The production of NO2− at a pH of 4 thus remained unclear. If Fe2+ produced by goethite reduction was reacted consecutively with NO3− to produce NO2−, it would be difficult to increase the Fe2+ concentration linearly. Furthermore, NO2− is likely to be re-adsorbed onto goethite, as identified in Section 3.3. However, with an initial concentration of NO3− of 1000 μM, it is difficult to detect the minor variation in concentration by reaction. Therefore, NO3− is not an indicator to determine whether the concentration decreases due to the reaction.
3.2. Possibility of Ammonium Used as a Reduced Material for Nitrite and Ferrous Production
The resulting concentration variation of Fe2+ and NO2− when NH4+ was used as a reduced material is shown in Figure 5. Even with an initial NH4+ concentration of 1000 μM, the Fe2+ or NO2− production for NH4+ was very low when compared with other organic materials used in the prior section. The production rate of Fe2+ was 0.0281 μM/h at a pH of 4 (Figure 5a) and the maximum production of NO2− was approximately 2.2 μM and then NO2− concentrations decreased at a pH of 7 (Figure 5b). This is in accordance with the hypothesis presented by Clement et al. [5] that coupling the NH4+ oxidation to NO2− with Fe3+ reduction under anaerobic conditions in sediments is thermodynamically feasible. However, from Figure 5, it was difficult to directly analyze whether ammonia influenced nitrite production. To analyze the effect of NH4+ on NO2− production, it was assumed in this study that it was also generated at pH 7 (Figure 5b) at the same level as the Fe2+ concentration generated at pH 4 (Figure 5a). Comparing Figure 5a,b, about 6 μM of Fe2+ concentration was found at the same time as the observed time (about 210 h) for the highest NO2− concentration (approximately 2.3 μM). At that time, the NO2−/Fe2+ concentration ratio was 0.38 which was lower than 0.5. The reason for this low ratio was thought to be that the portion in which the generated NO2− was converted into another nitrogen compound increased. Therefore, it is necessary to analyze the linear relationship between NO2− and Fe2+ in the initial time with low NO2− conversion rate, which is described in discussion.
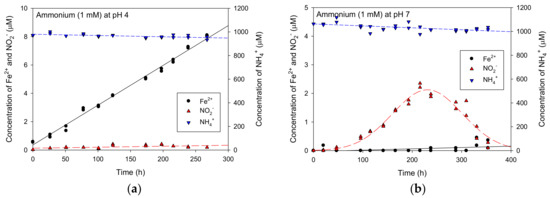
Figure 5.
The Fe2+ and NO2− production kinetics for NH4+ used as a reduced material at (a) pH 4 and (b) pH 7.
3.3. Effect of pH on Fe2+ and NO2− Adsorption onto Goethite
The adsorption of the produced Fe2+ onto goethite was then investigated at pH values of 4 and 7; the resulting concentration variation is shown in Figure 6, where the initial concentrations of Fe2+ and goethite were 100 μM and 1 g, respectively. At a pH of 4, the concentration of Fe2+ produced by Fe3+ reduction remained nearly stable, due to the repulsive forces driven by the positive charges of both of them. On the other hand, the Fe2+ was almost entirely adsorbed within 1 min at a pH of 7. Thus, even if Fe2+ was produced by Fe(III)/Fe(II) cycle, it was not detected in the solution because it was adsorbed onto the goethite quickly, due to the negative charge on the goethite surface at pH < pzc. This explains why no Fe2+ was detected in the results presented in Section 3.1 at a pH of 7 (Figure 3b,d,f).
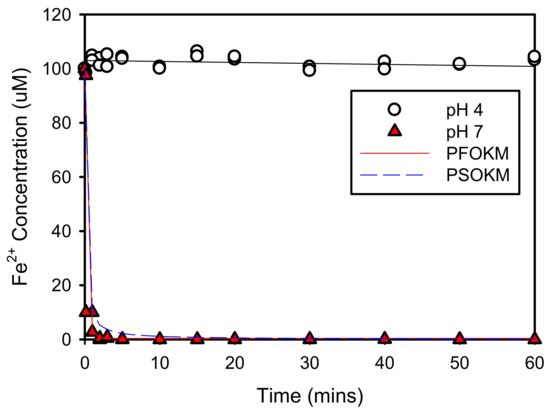
Figure 6.
Fe2+ adsorption onto goethite at pH 4 and 7.
The resulting adsorption of NO2− onto goethite at pH values of 4 and 7 is presented in Figure 7. Unlike Fe2+, the NO2− concentration decreased to less than 5 μM within 60 min at a pH value of 4; whereas at a pH value of 7, the NO2− concentration remained constant. At a pH value of 7, both NO2− and goethite have negative charges at pH > pzc of goethite, thus accounting for the lack of adsorption.
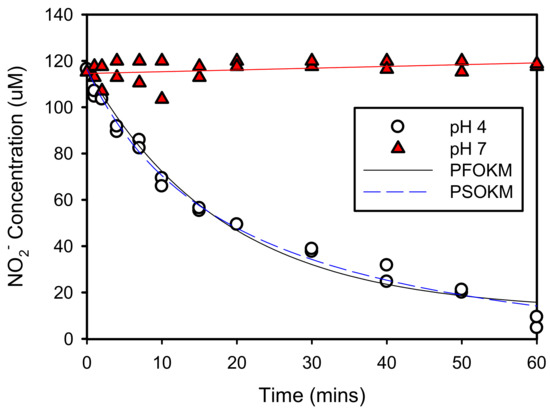
Figure 7.
NO2− adsorption onto goethite at pH 4 and 7.
To analysis the adsorption kinetics of Fe2+ at a pH of 4 and NO2− at a pH of 7, the Lagergren pseudo-first-order kinetic model (PFOKM) [21] and the pseudo-second-order kinetic model (PSOKM) [21,22] were used. The equations for PFOKM and PSOKM are presented at Equations (8) and (9), respectively.
where k1 and k2 are the rate constant of PFOKM (h−1) and PSOKM (h−1), respectively. C0 and C(t) denote the solute concentrations in the solution (μmol/L) at time 0 and time t (h), respectively. The qe is the equilibrium concentration in the solid phase (μmol/kg), respectively. W and V are the weight of goethite (kg) and solution volume (L). Then, the equilibrium concentration, Ce, of solute in solution can be calculated by Equation (10).
Table 2 shows the PFOKM and PSOKM parameters for the adsorption of Fe2+ and NO2− onto goethite at pH values of 7 and 4, respectively. Of the two models, PFOKM (R2 = 0.8491 and 0.9823 for Fe2+ and NO2−, respectively) was more accurately interpreted than PSOKM (R2 = 0.8147 and 0.9773, respectively). Therefore, according to PFOKM analyses, the equilibrium concentrations of Fe2+ and NO2− were 0.395 and 11.90 μmol/L, respectively. This means that Fe2+ and NO2− are removed by 99.6% and 89.8% by adsorption. Moreover, the amount of adsorbed solute on goethite was 19.92 mmol/kg for Fe2+ and 20.9 mmol/kg for NO2−.

Table 2.
Pseudo first order kinetic model (PFOKM) and pseudo second order kinetic model (PSOKM) parameters for the sorption of Fe2+ at pH 7 and NO2− at pH 4.
Overall, the adsorption of Fe2+ and NO2− onto the surface of goethite depends on the surface charge of the goethite, which is dependent on the pH and a very fast adsorption rate at a pH of 4. Therefore, even if NO2− is produced, it may not be detected because of its rapid adsorption onto the goethite. Fe2+ generated from the Fe(III) reduction was mostly adsorbed onto goethite at a pH of 7 and about 90% of NO2− generated from NO3− reduction was also adsorbed at a pH of 4.
3.4. The Reaction of Fe2+ and NO3− to Produce NO2−
The kinetics of Fe2+ removal and NO2− production are shown in Figure 8. Overall, the NO2− concentration as the Fe2+ concentration decreased, indicating that the reaction occurred successfully, although the Fe2+ decreased more rapidly than NO2− was produced. Considering the two reaction rates as a first-order reaction (C = C0 e−kt for Fe2+ and C = C0 (1 − e−kt) for NO2−), a high coefficient of determination (R2 > 0.947) was obtained (Table 3). Although the resulting reaction rate was relatively slow compared to the adsorption rates of Fe2+ and NO2− onto goethite, approximately 80% of Fe2+ was removed within 140 h, indicating that the overall rate of reaction depends upon the rate of the reaction between Fe2+ and NO3−.
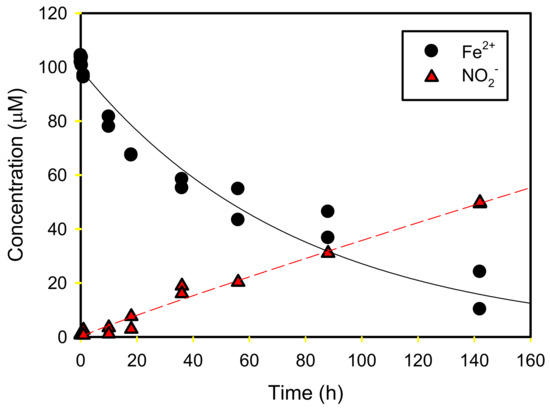
Figure 8.
Fe2+ and NO2− concentration produced from NO3− reduction.

Table 3.
First-order model parameters for Fe2+ removal and NO2− production.
4. Discussion
From Section 3.3, different situation of the removal of Fe2+ and NO2− may be involved to the pH values. However, there was no evidence that goethite-mediated Fe(III)/Fe(II) cycle was affected by the pH value in this study. If the pH only controls the adsorptive ability of goethite, the goethite-mediated Fe(III)/Fe(II) cycle can occur regardless of pH changes. The adsorption of NO2− and Fe2+ onto goethite at pH values of 4 and 7 was the main reason for no detection of them as mentioned in Section 3.3. The concentrations of Fe2+ also could not be estimated at a pH of 7 even though NO2− was observed from the reaction of Fe2+ and NO3−. Therefore, it was assumed that the amount of Fe2+ produced in a pH of 7 was equal to that in a pH of 4 and the amount of NO2− produced through Fe(III)/Fe(II) cycle in a pH of was equal to that in a pH of 7.
The relationships between TOC-normalized Fe2+ production at pH 4 and TOC-normalized NO2− production at pH 7 for NOM, syringic acid, citric acid and ammonium were shown in Figure 9. For NOM, syringic acid and ammonium, TOC-normalized NO2− production increased linearly with TOC-normalized Fe2+ production (0.963 < R2 < 0.983) in the relatively low concentration range of TOC-normalized Fe2+ production before reaching the peak concentrations. The linearly increase with high R2 values indicates that it was indirect evidence that Fe(III)/Fe(II) cycle successfully occurred at both pH 4 and 7 with constant rate. The ratios of TOC-normalized Fe2+ production and TOC-normalized NO2− production for NOM, syringic acid and ammonium were 0.2690, 0.4764 and 0.5585, respectively.
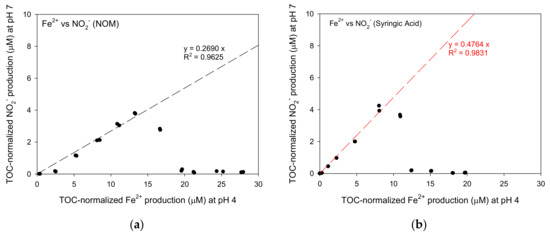
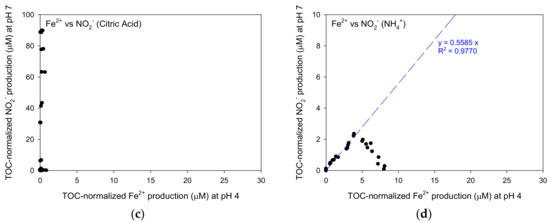
Figure 9.
The relationship between TOC-based Fe2+ concentrations at a pH of 4 and NO2− concentrations at a pH of 7: (a) NOM, (b) syringic acid, (c) citric acid and (d) ammonium.
The theoretical Fe2+ productions per mole of syringic acid, citric acid and ammonium are 20, 36 and 8 moles, respectively, as shown in the stoichiometry of the Equations (1)–(3). In addition, the NO2− production per mole of syringic acid, citric acid and ammonium through overall reactions are 10, 18 and 4 moles of NO2− produced, respectively. Therefore, the theoretical ratio of NO2− production per Fe2+ production calculated considering overall equations are 0.5, 0.5 and 0.67 mole NO2−/mole Fe2+, respectively. If these values were compared to the experimental results presented in Figure 9, the theoretical ratios for syringic acid and ammonium were slightly higher than the experimental ratios: 0.5 > 0.4764 for syringic acid and 0.67 > 0.5585 for ammonium. Therefore, the assumption that the Fe(III)/Fe(II) cycle occurred regardless the pH seemed to be right. Even though the Fe2+ concentration produced at a pH of 4 was equal to that at a pH of 7, the difference between the experimental Fe2+ production and the theoretical Fe2+ production was still unknown because some of them can be converted quickly or adsorbed onto the goethite again due to the instability of NO2−.
5. Conclusions
In this study, the transient generation of NO2− by the abiotic reaction of goethite-mediated Fe(III)/Fe(II) cycle with labile materials and NO3– reduction was investigated under anaerobic conditions in the soil. The main reaction was the abiotic Fe(III)/Fe(II) cycle, which was conducted at pH values of 4 (acidic condition) and 7 (neutral condition). The production of NO2− through the Fe(III)/Fe(II) cycle was greatly influenced by the relationship between the pH and pzc due to the change in the surface charge of goethite according to pzc. It was confirmed that NO2− is produced in a neutral environment and not in an acidic environment. On the TOC basis, citric acid was the best labile organic carbon; using NOM and syringic acid demonstrated similar performances. Using NH4+ demonstrated a similar phenomenon, but with a very low production of NO2−. The adsorption of the produced Fe2+ and NO2− onto goethite was also dependent on the relationship between the pH and pzc. The reaction rates of Fe2+ and NO3− were relatively slow, which had a significant impact on the Fe(III)/Fe(II) cycle reaction rates. According to stoichiometric analyses, the reaction through Fe(III)/Fe(II) cycle was not affected by the pH. Consequently, the best environment to form NO2− is likely an anaerobic zone containing a large amount of organic matter at a neutral pH, such as the organic horizon of wetland subsoil.
Author Contributions
Conceptualization, H.C. and S.O.; methodology, S.O.; software, S.O.; formal analysis, H.C. and S.O.; investigation, H.C. and S.O.; data curation, H.C. and S.O.; writing—original draft preparation, S.O.; writing—review and editing, H.C. and S.O.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Kyungpook National University Research Fund, 2017.
Acknowledgments
The authors are grateful to the Kyungpook National University for supporting this research
Conflicts of Interest
The authors declare no conflict of interest.
References
- Umezawa, Y.; Hosono, T.; Onodera, S.; Siringan, F.; Buapeng, S.; Delinom, R.; Yoshimizu, C.; Tayasu, I.; Nagata, T.; Taniguchi, M. Sources of nitrate and ammonium contamination in groundwater under developing Asian megacities. Sci. Total Environ. 2008, 404, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, T.; You, X.; Gao, M.R. Nutrient release from weathering of purplish rocks in the Sichuan Basin, China. Pedosphere 2008, 18, 257–264. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soil 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Buss, S.R.; Herbert, A.W.; Morgan, P.; Thornton, S.F.; Smith, J.W.N. A review of ammonium attenuation in soil and groundwater. Q. J. Eng. Geol. Hydroge. 2004, 37, 347–359. [Google Scholar] [CrossRef]
- Clement, J.C.; Shrestha, J.; Ehrenfeld, J.G.; Jaffe, P.R. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol. Biochem. 2005, 37, 2323–2328. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Emerson, D.; Megonigal, J.P. Life at the energetic edge: Kinetics of circumneutral iron oxidation by lithotrophic iron oxidizing bacteria isolated from the wetland-plant rhizosphere. Appl. Environ. Microbiol. 2002, 68, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.A. The abiotic nitrogen cycle. ACS Earth Space Chem. 2017, 1, 411–421. [Google Scholar] [CrossRef]
- Davidson, E.A.; Chorover, J.; Dail, D.B. A mechanism of abiotic immobilization of nitrate in forest ecosystems: The ferrous wheel hypothesis. Glob. Chang. Biol. 2003, 9, 228–236. [Google Scholar] [CrossRef]
- Zhu-Barker, X.; Cavazos, A.R.; Ostrom, N.E.; Horwath, W.R.; Glass, J.B. The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 2015, 126, 251–267. [Google Scholar] [CrossRef]
- Davidson, E.A.; Dail, D.B.; Chorover, J. Iron interference in the quantification of nitrate in soil extracts and its effect on hypothesized abiotic immobilization of nitrate. Biogeochemistry 2008, 90, 65–73. [Google Scholar] [CrossRef]
- Giles, M.; Morley, N.; Baggs, E.M.; Daniell, T.J. Soil nitrate reducing processes-drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 2012, 3, 407. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 1986, 51, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, E.; Bouwman, L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modelling of global annual emissions. Nutr. Cycl. Agroecosyst. 2006, 74, 207–228. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Olson-Rutz, K. Soil pH and organic matter. In Nutrient Management; Montana State University: Bozeman, MT, USA, 2017; p. 4449-8. [Google Scholar]
- Atkinson, R.J.; Posner, A.M.; Quirk, J.P. Crystal nucleation and growth in hydrolysing iron(III) chloride solutions. Clays Clay Mineral. 1977, 25, 49–56. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, R.; Park, H.S.; Jeon, B.H. Photocatalytic efficiency of iron oxide nanoparticles for the degradation of priority pollutant anthracene. Geosyst. Eng. 2016, 20, 21–27. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.I.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis Part 3 Chemical Methods, 1st ed.; Soil Science Society of America, Inc., American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 1244–1248. [Google Scholar]
- Shinn, M.B. Colorimetric method for determination of nitrite. Ind. Eng. Chem. Anal. Ed. 1941, 13, 33–35. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine-a new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Perez-Guzman, L.; Bogner, K.R.; Lower, B.H. Earth’s ferrous wheel. Nat. Edu. Knowl. 2010, 3, 32. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Gosset, T.; Trancart, J.L.; Thevenot, D.R. Batch metal removal by peat. Kinetics and thermodynamics. Water Res. 1986, 20, 21–26. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).