Abstract
Rate coefficients at ambient temperature and atmospheric pressure for the reaction of ozone with 2-methoxypropene (2-MPE) and 2-ethoxypropene (2-EPE) were determined in an evacuable 100 L Teflon reaction chamber using absolute and relative rate methods. The product experiments were carried out using a 50 L Teflon reaction chamber in conjunction with FTIR as the detection technique. The rate coefficients (k in units of cm3 molecule−1 s−1) obtained are 1.18 ± 0.13 × 10−17 and 1.89 ± 0.23 × 10−17 for reactions with 2-MPE and 2-EPE, respectively. The effects of the alkoxy group on the gas-phase reactivity of alkyl vinyl ethers toward ozone are compared and discussed. The major ozonolysis products are methyl acetate, formaldehyde and CO2 for 2-MPE, and ethyl acetate, formaldehyde and CO2 for 2-EPE. Possible mechanisms for the two vinyl ethers are proposed based on the observed reaction products. Additionally, atmospheric lifetimes of 32 h and 21 h for 2-MPE and 2-EPE were estimated based on the measured rate constants and the ambient tropospheric concentration of ozone, respectively. The obtained values of the lifetimes indicate that the reaction with ozone is an important loss process for these vinyl ethers in the atmosphere, especially in polluted areas.
1. Introduction
Recent studies suggest that oxygenated volatile organic compounds (OVOCs) are among the most important intermediates in atmospheric chemical processes and highly influence the atmospheric oxidation capacity [1,2]. These compounds are emitted directly into the atmosphere from natural and anthropogenic sources, but also form in situ from the atmospheric oxidation of hydrocarbons. The natural sources are mainly from biogenic emissions, while the anthropogenic sources include emissions from industries, lubricating oils, coatings or intermediate products for the synthesis of flavors, fragrances and medicines [2,3,4]. OVOCs can cause adverse health effects, but also undergo complex chemical reactions in the atmosphere and indirectly affect the environment [5,6,7].
To reduce the damaging effects of these solvents on the environment, some new friendly solvents such as unsaturated ethers have been recommended for use [4,8,9,10,11,12,13,14,15,16]. In particular, 2-methoxypropene (CH2=C(CH3)OCH3, 2-MPE) is commonly used as intermediate in the synthesis of vitamins A and E, and as a protective group for adjacent hydroxyls and 1,2-diols groups in various synthesis [2,17]. Meanwhile, 2-ethoxypropene (CH2=C(CH3)OC2H5, 2-EPE) has gradually been used as intermediate in pharmaceutical industry to replace 2-MPE. The use of these vinyl ethers has gradually increased their emissions into the atmosphere and it has become necessary to precisely evaluate their environmental burden. When released into the air, the photolysis or photochemical oxidation of vinyl ethers occur with OH radicals and O3 in the daytime, with NO3 and O3 in the nighttime and Cl atoms in coastal areas and marine environments [1,2,6,18,19,20]. These reactions may lead to the formation of harmful compounds such as photochemical oxidants which are involved in photo-chemical smog events [2].
To date, relevant kinetic and mechanistic studies have mostly focused on the simplest vinyl ethers. Reactions of methyl vinyl ether (MVE), ethyl vinyl ether (EVE), n-propyl vinyl ether (n-PVE), n-butyl vinyl ether (n-BVE), iso-butyl vinyl ether (i-BVE) and tert-butyl vinyl ether (t-BVE) with O3, OH and NO3 have extensively been reported in the literature [1,3,18,21,22,23,24,25,26,27,28,29,30,31,32]. However, only few studies of the reaction of 2-MPE with OH, NO3 and Cl were reported recently [2]. The rate coefficient of the ozonolysis of EVE was first determined to be around 1.5 × 10−16 cm3 molecule−1 s−1 by using ozone ultraviolet photometry (UV) [23]. Several studies reported similar values using different experimental methods [1,18,29,30]. The kinetics of the reaction of PVE with O3 was investigated and rate coefficients in the range 2.3–2.4 × 10−16 cm3 molecule−1 s−1 were reported [1,30,31]. These rate coefficients are very close to the rate coefficients of the O3 reactions with n-BVE, i-BVE and t-BVE, 2.3 × 10−16, 2.3 × 10−16 and 2.4 × 10−16 cm3 molecule−1 s−1, respectively, determined by chemiluminescence analysis (CL) [1,30,31]. The FTIR method was also used to determine rate constants of 2.6 × 10−16, 2.9 × 10−16 and 5.3 × 10−16 cm3 molecule−1 s−1, for O3 reactions with n-BVE, i-BVE and t-BVE, respectively [32]. This further indicates that the kinetics of vinyl ethers is weakly sensitive to alkyl substitution on the carbon chain. The products of most of the above-mentioned reactions were detected and results indicated that alkyl formates and formaldehyde were the most abundant, with approximately 80% and 20% yields, respectively, while hydroperoxymethyl formate, CO2 and CO were the minor products [25,28,30].
O3 + CH2=C(CH3)OCH3 → Products (k1)
O3 + CH2=C(CH3)OCH2CH3 → Products (k2)
In the present work, we report the kinetic study on the O3 reactions with 2-MPE and 2-EPE, and suggest possible mechanisms. In the above equations, k1 and k2 are the reaction rate coefficients. Experiments were carried out at ~298 K and a total pressure of ~760 torr using absolute rate and relative rate methods with ozone analyzer, gas chromatography with flame ionization detector (GC-FID) and FTIR as detection techniques. Some of the reaction products were characterized to propose the degradation mechanisms for the studied compounds in the atmosphere. Kinetic and mechanism studies are fundamental to determine the fate of these two species and to assess their influence on health and the environment. Furthermore, the kinetic results obtained are presented and discussed in accordance with the influence of the alkoxy group (−OR) and the −CH3 substituent on the reactivity of the studied unsaturated ethers, compared to other parent compounds.
2. Experiments
2.1. Materials
The chemicals used in the experiments were obtained commercially and were used as received without further purification: 2-MPE (TCI, >95.0%), 2-EPE (Adamas, >98%), methyl acetate (Aladdin, >99.5%), ethyl acetate (Adamas, >99.8%), cyclohexene (TCI, >99.0%), cyclohexane (Aladdin, >99.9%), and N2 (> 99.999%). Ozone was generated by high voltage discharge in a flow of pure O2 (Jinan Deyang Special Gas CO., LTD, Jinan, China, 99.999%) using an ozone generator and saved in a Teflon reactor temporarily. 2-MPE is prone to photochemical reactions and is difficult to preserve. 2-MPE (TCI, > 95.0%) was chosen in the study for its suitability to undergo GC analysis and its ability to contain the stabilizer.
2.2. Methods
2.2.1. Chamber Description
The experiments were carried out in a 100 L Teflon chamber. An inlet and an outlet made of Teflon were designed for the injection and detection of reactants and samplings. The chamber could be evacuated by a vacuum pump (2XZ-4, Zhejiang Huangyan). The atmospheric pressure was maintained in the chamber at all times through an adjustable frame, and all experiments were performed at room temperature (298 ± 2 K). Prior to each experiment, the chamber was cleaned by purging with purified dry air at least three times, after which residual hydrocarbons and ozone could not be detected in the reactor. The bath gases were zero air in kinetic experiments and N2 in product experiments. The zero air generator (111-D3N, Thermo Scientific, Waltham, MA, USA) supplied the dry air. The mass flow controller (D08-8C/ZM, Beijing Sevenstar Electron Corporation, Beijing, China) with a flow accumulator recorded the volume of purified air or N2 in the reactor in kinetic experiments. The chamber was connected to the GC-FID or ozone analyzer in the kinetic experiments and FTIR spectroscopy analytical system in the product studies. Before experiments, homogeneous mixing of the reagents and ozone in the chamber was obtained by repeated pumping–injecting processes with a 100 mL gas syringe. The reagents were injected into the chamber by a microsyringe while the samples were injected into the GC by a 100 mL gas syringe.
2.2.2. Kinetic Studies
Absolute Measurements
Rate coefficients for the gas phase reactions of 2-MPE and 2-EPE with ozone were determined using both absolute rate and relative rate methods. The absolute measurement is based on measuring the loss of ozone in the presence of a known excess concentration of the substrate compounds (2-MPE and 2-EPE). The concentration of ozone was controlled by the following processes:
where kL and k1 are the rate constants of wall loss of O3 (R3) and the O3 reaction with the substrate (R4), respectively.
O3 + Wall → Loss of O3 (kL)
O3 + Substrate → Products (k1)
O3 + Impurities → Products
According to Reaction (R3), the decay of the background ozone is first order reaction, which means that the relationship between ln([O3]0/[O3]) and time is a linear relationship. [O3]0 represents the initial concentration of ozone and [O3] represents the residual one. In the ozone decay experiments, the rate constant of the ozone decay was obtained to be 4.44 ± 0.28 × 10−6 s−1 after 8 h, which is about two orders of magnitude lower than the pseudo-first-order reaction rate constants in this work. Thus, the ozone loss caused by wall effect in our experiments is negligible. The rate constants of the decay of 2-MPE and 2-EPE were also detected in the order of 10−6 s−1. The purities of all compounds were higher than 95% and the side reaction of ozone with impurities was expected to be insignificant. Hence, loss of ozone either at the wall or by reaction with impurities in the substrate compounds was unlikely to be important. Additionally, with the large ratio condition of initial concentrations, [Substrate]0/[O3]0 > 50, the substrate concentration permanently remained a constant during the reaction. Reaction (R4) can thereby be seen as pseudo-first-order reaction, and the following rate equation could be obtained:
where k1 is the rate coefficient for reaction (R4).
Previous studies have shown that OH radicals are generally formed in the reactions of ozone with alkenes under atmospheric conditions [33,34,35,36]. Considering that OH reacts with these compounds several orders of magnitude faster than O3 does, this would result in error in the rate constant determinations. There is much evidence that the interaction of ozone with unsaturated compounds in the gas phase produces significant amounts of OH radicals [36,37,38,39,40]. To prevent the impact of OH on the rate constant determination, high concentrations of cyclohexane were added into the reaction system as OH scavenger. Ozone was generated by high voltage discharge in a flow of pure O2 using an ozone generator and saved in a Teflon reactor temporarily. The reactants and cyclohexane were well mixed for around 30 min prior to adding O3 to the system. The concentration of ozone was monitored by ozone analyzer (Model 49i, Thermo Electron Corporation, Waltham, MA, USA) with a 1-min interval and 0.7 L/min. During the experiments, ozone concentrations were constantly recorded until the concentration in the reactor was lower than 30% of the initial value. The measurement continued for 10–30 min. Typical initial reactants mixing ratios were: [Substrate]0 = 2–20 ppm, [O3]0 = 100–200 ppb, and [Cyclohexane]0 = 300–2000 ppm (1 ppm = 2.46 × 1013 molecule cm−3 at 298 K and 760 Torr total pressure). The secondary reactions of the oxidant with primary reaction products could be considered as negligible, taking into account the sufficient excess of the reactant over the oxidant [41].
Relative Measurements
The relative rate method was chosen to compare the rate coefficients measured by the absolute method. This method relies on the assumption that both compounds in the reaction mixture, the substrate and the reference compound, are removed solely by their reactions with ozone:
where ks and kR are the rate constants for reactions (R6) and (R7), respectively. The decays of the substrate and the reference compounds are governed by the following rate equations:
O3 + Substrate → Products (kS)
O3 + Reference → Products (kR)
Integration and rearrangement of Equations (2) and (3) leads to the expression:
where [Substrate]0 and [Substrate]t are the concentrations of 2-MPE and 2-EPE at time t = 0 and time = t. [Reference]0 and [Reference]t are the concentrations of the reference compound at time t = 0 and time = t. A plot of ln([Substrate]0/[Substrate]t) versus ln([Reference]0/[Reference]t) should be a straight line passing through the origin and whose slope gives the ratio of rate coefficients kS/kR. Each reaction studied was measured relative to the reactions of different reference standards.
Three factors were considered in the choice of the reference compounds: (i) the rate constants for reactions with ozone should be well known; (ii) the ratio of the rate constant of the ozonolysis of the reference compound to that of the substrate should be within an appropriate range, which is 1/10–10; and (iii) the wall loss of the reference compounds should be negligible to minimize errors. Based on these features, furan, 2-methylfuran, isoprene, cyclohexene, 1-methylcyclohexene, propene and cis-2-butene were chosen as potential reference compounds. After testing, only the peaks of the chromatogram of cyclohexene were clearly separated from those of reactants, products and OH scavenger. We then used cyclohexene as reference compound, similar to previous studies [7,18,32]. Initial concentrations of vinyl ethers ranged from 40 to 80 ppm, those of cyclohexene ranged from 40 to 60 ppm, and 3000 ppm of the cyclohexane was added into the reactor to scavenge OH radicals. Ozone decay experiments mentioned in absolute measurements showed that the ozone loss caused by wall-loss can be neglected. The reactants were well mixed for around 30 min prior to adding O3 to the system. Then, approximately 4 ppm of ozone was added to the reaction mixture at the start of the reaction. During the oxidation reactions processes, eight further additions of ozone were added into the reactor in the same manner. The loss of substrate and formation of products were monitored after each ozone addition. Some studies indicated that reaction rate with near-zero intercept means the concomitant secondary reaction is negligible [41,42]. The measurements were repeated three times under different initial concentration ratios of the reactants and reference compounds. The reaction products, detected every 30 min, were similar. Thus, the results of relative measurements showed that the secondary reaction in these reactions can be neglected.
The reactants concentrations in the chamber were measured by GC-FID (7890B, Agilent Technologies), using a DB-VRX (60 m × 0.25 mm id × 1.4 μm film thickness, Agilent Technologies) capillary column. The chromatographic conditions used for the analysis were as follows: injector 250 °C, detector 300 °C, column temperature 100 °C, detection time 7 min. Nitrogen was used as the carrier gas at a constant flow of 0.8 mL min−1.
2.2.3. Product Study
The experiment was performed using a 50 L Teflon reaction chamber (with N2 as carrier gas) in conjunction with FTIR absorption spectroscopy as the detection technique. A total path length of 20 cm glass cell equipped with CaF2 windows was used to measure the spectra at room temperature within the spectral range 4000–400 cm−1. The IR spectra were recorded every 30 min by co-adding 128 interferograms with a spectral resolution of 1 cm−1 using a Bruker Vertex 70 FTIR spectrometer fitted with KBr beam splitter and a DLaTGS detector.
Contrary to kinetic studies, the reaction in the product experiment was conducted without OH scavenger as its effect was shown to be negligible in such studies [28]. The concentrations of the substrates were both around 100 ppm. Prior to the experiments, the infrared spectra of each compound were measured before to ensure that the peaks would not be overlapped and that possible products would not interfere with the reactant peaks. According to this, the samples used were monitored in the IR at the following absorption frequencies for the reactions: 1098 cm−1 and 1296 cm−1 for the ozonolysis of 2-MPE, and 1098 and 1291 cm−1 for the ozonolysis of 2-EPE. In the beginning of the experiments, known quantities of each compound were introduced separately into the chamber. After adding ozone for 2 h, the final changes of the infrared spectra of the reactants and products could be obtained. Theoretical calculations at the MP2/aug-cc-pVTZ level of theory were carried out to determine the IR band position of formaldehyde.
3. Results and Discussion
3.1. Kinetic Studies
Absolute measurements. As described above, six runs were conducted in the case of different initial substrate concentrations under pseudo-first-order conditions. In all experiments, the decline of O3 concentration was obtained as a function of reaction time and the ln([O3]0/[O3]) values were plotted for different reaction times (see Figure S1 (1,2)). The experimental condition details are listed in Table S1. As shown in Figure S1, straight lines were obtained for all pseudo-first-order plots. All lines have excellent correlation coefficients (>0.998). Besides, it was evidenced that the ozonolysis of the main products detected hereinafter do nto likely compete with the parent reaction. This demonstrates that Equation (1) is suitable for the kinetic study in this work. This is in line with a previous study based on absolute measurements [29]. From six decay rates of O3 related to different substrate concentrations and using Equation (1), kS was obtained through linear least-squares analysis of the plot of decay rates of O3 versus 2-MPE and 2-EPE concentrations (see Figure 1). The rate constants obtained were 1.18 ± 0.13 × 10−17 and 1.89 ± 0.23 × 10−17 cm3 molecule−1 s−1 for 2-MPE and 2-EPE reactions, respectively. The measurement error is mainly associated with the following. (1) Instrument: The area repeatability is <1% relative standard deviation (RSD) for the Agilent 7890B. The uncertainty is ±10% when the linear dynamic range is more than 107 for the FID detector. In our experiments, the peak intensity is in the range 105–106, which is less than the maximum range of FID. Hence, we consider 1% error for the instrument. (2) Wall loss: The wall loss was less than 3% over the course of 8 h. (3) Other possible errors include the flow measurement in the chamber, the temperature, and the transfer from the chamber to GC. With the compilation of these errors, the uncertainty in the VOC concentration should have at least 10% systematic error on each data point.
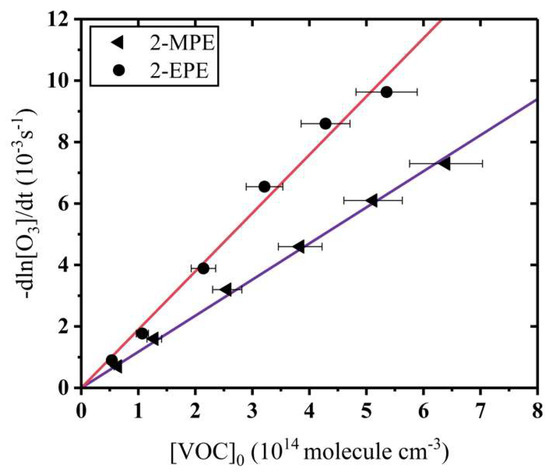
Figure 1.
Plots of -dln[O3]/dt against [2-MPE]0 (◄), and [2-EPE]0 (●). The [2-MPE]0 and [2-EPE]0 represent the concentrations of 2-methoxypropene and 2-ethoxypropene, respectively.
Relative measurements. Relative rate coefficients for the reactions of O3 with the substrates were determined by comparing their rates of decay with the rate constant of ozonolysis of cyclohexene, 7.40 ± 0.50 × 10−17 cm3 molecule−1 s−1 [43]. The typical logarithmic plots of the relative decrease in the form of Equation (4) for the reactions of O3 with vinyl ethers in the air are shown in Figure 2. As expected for relative rate plots of the kinetic data, straight lines with a zero intercept were obtained, showing in all cases a good linear relationship. This linear relationship further suggested that the heterogeneous or secondary reactions were insignificant. In the experiments, the measurement was repeated three times under different initial concentration ratios of the vinyl ethers and reference compounds. For clarity, only one example for each unsaturated vinyl ether and reference compound combination is shown. The rate constants ratio, kS/kR, and the obtained rate constants are summarized in Table 1. The final rate constants for the reaction with O3 were 1.18 ± 0.16 × 10−17 cm3 molecule−1 s−1 for 2-MPE and 1.78 ± 0.24 × 10−17 cm3 molecule−1 s−1 for 2-EPE, taken as an average of all values, in each case. The total errors for the rate coefficients quoted in Table 1 combine twice the standard deviation from the least-squares analysis of slope values and error transmission from uncertainties on the rate constants of the reactions of O3 with reference compounds. The relative rate values were in reasonable agreement with those of absolute measurements, which indicates that both methods are reliable. To the best of our knowledge, the rate coefficients for the O3 reactions with 2-MPE and 2-EPE have not been reported previously in the literature, making the present work the first kinetic study of this kind. Therefore, direct comparison with the literature data cannot be made. Comparison is possible only with reported reactions of other unsaturated compounds as listed in Table 2.
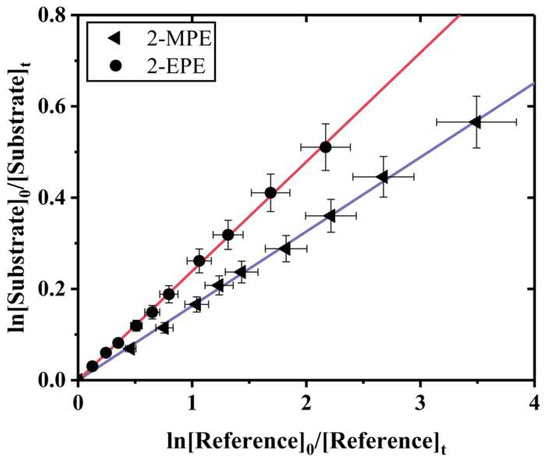
Figure 2.
Relative rate plots for O3 reactions with 2-MPE (◄) and 2-EPE (●), using cyclohexene as reference compound.

Table 1.
Rate coefficient ratios (kS/kR) and rate coefficients (kS) for the reactions of O3 with 2-MPE and 2-EPE.

Table 2.
Comparison of the rate coefficients (in cm3 molecule−1 s−1) measured in the present work at 298 K for the ozonolysis of selected vinyl ethers with values for other vinyl ethers and alkenes reported in the literature at the same temperature.
Comparison of rate coefficient. It can be observed from the data in Table 2 that the rate coefficients of O3 reactions with linear alkyl vinyl ethers are substantially larger than those of corresponding alkenes, except for 2-MPE and 2-EPE reactions for which the rate constants are only slightly larger than those of 2-methylpropene and 2-methylbutene reactions. The remarkably large rate coefficients of linear alkyl vinyl ethers can be attributed to the strong electron donating inductive effect from the −OR functional group adjacent to the C=C double bond, which does not exist in alkenes. The alkoxy group increases the charge density on the C=C bond and favors the addition of electrophilic radicals [32,52,53,54]. This phenomenon was also found in the reactions of 2-MPE with OH, NO3 and Cl [2]. Besides the activating effect of the alkoxy group in alkyl vinyl ethers compared to corresponding alkenes, the effects of chain length of alkyl vinyl ethers can also be compared. It is seen that, for two consecutive alkyl vinyl ethers, the rate constants for the reaction with ozone rarely increase by a factor of up to 2. For example, we found that the rate constant for the reaction with 2-EPE is larger than that of the reaction with 2-MPE only by a factor of 1.6. This is higher than the factor of 1.2 difference between rate constants of O3 reactions with EVE and PVE determined using the same technique [18,30]. This difference gets smaller as the chain length increases. A possible explanation for this is that the alkyl group next to the C=C double bond likely hinders the addition of ozone to the reactive double bond of 2-MPE and 2-EPE from forming 1,2,3-trioxolane in the primary ozonide, thus counteracting the inductive stabilization effect and resulting in a lowering of the rate coefficient. With the length of the alkyl group increasing, the activating effect of the alkoxy group (−OR) becomes more predominant and the rate coefficients increase.
3.2. Product Study
The IR spectra obtained during the oxidation of 2-MPE are shown in Figure 3 along with that of the identified products. The peaks of intermediates in the reaction of 2-MPE with O3, monitored by the FTIR, are shown in the Supplementary Materials (Figure S3). Possible products bands were determined to be 1247 and 1778 cm−1 for methyl acetate (CH3C(O)OCH3), 1247 and 1769 cm−1 for ethyl acetate (CH3C(O)OCH2CH3), and 2349 cm−1 for CO2. In laboratory studies, formaldehyde gas (without water) is produced by thermal depolymerization of trioxymethylene. This process is complicated, and formaldehyde gas is not readily available. According to the literature, the IR peaks of formaldehyde are 1167, 1249, 1500, 1745, 2782 and 2843 cm−1 [2,28,55,56]. Theoretical calculations predict these peaks at 1198, 1266, 1541, 1754, 2971, and 3044 cm−1, respectively. To avoid overlapping with parent bands or other product bands, 1745 cm−1 was chosen as the feature for formaldehyde. After a reaction time of 2 h and full consumption of 2-MPE, the main products detected were CO2, methyl acetate and formaldehyde. A reaction mechanism is proposed for this reaction, based on the reaction products identified and previous mechanism studies of unsaturated compounds [2,25,28,42].
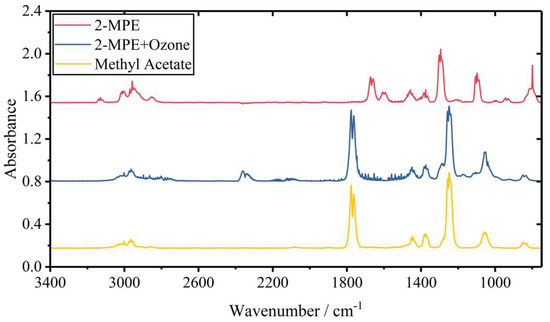
Figure 3.
Infrared spectra of the detected products in the reaction of 2-MPE with O3. The top line indicates the spectrum of 2-MPE at t = 0 h, the middle line represents the spectrum of 2-MPE at t = 2 h and the bottom line is the standard methyl acetate.
As can be seen in Scheme 1, the reaction of 2-MPE with O3 pursued by O3 electrophilic addition on the double bond and accordingly led to the formation of the primary ozonide, which has been proved to form in similar reactions in our previous study [57]. Primary ozonide could rapidly decompose by two pathways: the first one is decomposition to CH3C(O)OCH3 and a Criegee intermediate ([CH2OO]*), and the second is the formation of another Criegee intermediate ([CH3OC(CH3)OO]*) and HCHO. The rates of these two pathways were rapid and the products were barely detected by matrix isolation technique [57]. Both Criegee intermediates could undergo collisional stabilization and thus are formed in either excited or stabilized forms, resulting in a total of four decomposition channels for the reaction. The excited forms were proposed to have a number of different decomposition and/or rearrangement pathways. [CH2OO]* could form dioxirane and rapidly recombine to excited formic acid, therewith decomposing to OH and some small molecules such as CO2 and CO. HCOOH and formaldehyde are both reaction partners for Criegee biradicals in Reactions (3c), (3d), (5a) and (5d) in Scheme 1. These paths can become important sinks if a sufficient amount of Criegee biradicals and formaldehyde have been formed. Reactions (5a) and (5b) may form additional methyl acetate in the presence of HCHO and H2O and lead to higher yield than from Reactions (1a) and (1b) alone. This assumption could be verified by the intense IR vibration of methyl acetate in Figure 3. The branching ratio of the decomposition of the primary ozonide is usually close to 1:1 for n-alkyl substituted alkenes. In contrast, bulky or branched-chain substituted alkenes would preferably lead to the formation of the longer carbonyl (such as methyl formate) and the shorter Criegee biradical (i.e., [CH2OO]*) [25]. This rule implies that the effect of the methoxy substitution is similar to that of a bulky or branched substituent. Similar results were observed in previous studies of the ozonolysis of linear vinyl ethers, where Reactions (1a) and (1b) could account for 60–80% of the total decomposition of the primary ozonide [18,25,28,29]. However, no direct evidence of the branching ratios for the formation of the excited or stabilized Criegee biradicals could be inferred from the present study. In conclusion, the four types of Criegee intermediates formed in the ozonolysis of 2-MPE can undergo a variety of further reactions, and all possible pathways are summarized in Scheme 1.
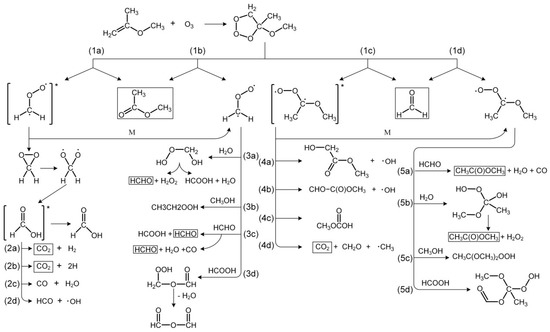
Scheme 1.
Proposed mechanism for the reaction of 2-MPE with O3.
For the reaction of 2-EPE with ozone, the main products observed were CO2 (2349 cm−1), ethyl acetate (1247 and 1769 cm−1) and formaldehyde (1745 cm−1) (these peaks are shown in Figure S2), and, likewise, a similar mechanism to the mechanism of the 2-MPE + O3 reaction could be speculated (see Scheme S1). As shown in Scheme S1, the primary ozonide rapidly decomposes to [CH2OO]*, ethyl acetate ([CH3CH2OC(CH3)OO]*), and formaldehyde. The excited Criegee intermediates can be stabilized, or decomposed to OH and some small molecules such as CO2 and CO. Besides, it could also be observed the strong absorption bands of ethyl acetate in the IR spectrum, which illustrates that Reactions (6a) and (6b) are the dominant channels. The observed products, esters and aldehydes, can be explained by a mechanism proceeding mainly by O3-addition to the C=C double bond. A similar addition mechanism was also observed in the reaction of 2-MPE with OH [2]. Comparing the reaction of different vinyl ethers with O3, OH, NO3 and Cl from different studies, a general mechanism based on initial radical addition could be proposed, as the main products observed from these studies were esters and aldehydes [2,18,25,28,58]. This mechanism could be extended to other unsaturated functionalized compounds like unsaturated alcohols and unsaturated aldehydes, for the initial mechanism step is the oxidant addition to the C=C double bond [29,54]. However, the following steps depend on the nature of the functional group present in the compound.
The calculation of rate coefficients from the structure–reactivity relationship (SAR) method [59,60,61,62,63,64] does not apply to 2-MPE and 2-EPE since the rate coefficient, 5.68 × 10−17 cm3 molecule−1 s−1 at 298 K, for the reaction of O3 with both vinyl ethers is higher than the values determined experimentally in this work. The difference between the experimental values and those calculated may be due to the hindrance of the alkyl group next to the C=C double bond.
4. Atmospheric Implications
Oxidation, photolysis, and wet/dry deposition of volatile organic compounds are their main atmospheric transformation processes. Since vinyl ethers do not absorb in the tropospheric actinic region, photolysis is a negligible process in their transformation [22]. It could also be postulated that wet deposition is a negligible loss process because of their low Henry’s law constants [32]. Meanwhile, due to the existence of the alkene moiety in their structures, vinyl ethers show high reactivity toward OH radicals, ozone, and NO3 radicals. Lifetimes of 2-MPE based on its reactions with OH and NO3 have been reported in a previous study to be 2.44 and 0.023 h, respectively [2]. To our knowledge, no lifetime for 2-EPE has been reported previously.
Based on rate constants observed in this work, the estimated atmospheric lifetimes (τ) of 2-MPE and 2-EPE considering their reactions with ozone were calculated using the relationship τ = 1/k[X], where [X] represents the typical concentration of ozone and k is the reaction rate constant. With a 24-h average concentration for ozone of 7 × 1011 molecule cm−3 [65], the tropospheric lifetimes of 2-MPE and 2-EPE were estimated to be 32 h and 21 h, respectively. At this O3 concentration, the lifetime of 2-MPE is not as important as the lifetimes based on reactions with OH and NO3. However, in some urban areas where O3 concentration levels reach 4.57 × 1012 molecule cm−3, the lifetime of 2-MPE can get as low as 5 h while that of 2-EPE could become 3 h. Under these conditions, the ozonolysis of 2-MPE and 2-EPE would play a relatively important role in the atmospheric oxidation processes. Combination of the current results with those previously published on the reactions of vinyl ethers with other main atmospheric oxidants provides a comprehensive set of kinetic data to evaluate their importance in atmospheric chemistry.
5. Conclusions
We report a series of chamber experiment studies on the reactions of ozone with two vinyl ethers, 2-MPE and 2-EPE. The experiments were performed based on absolute method with ozone analyzer, and relative method with GC-FID and FTIR. At room temperature and atmospheric pressure, the following rate coefficients (in units of cm3 molecule−1 s−1) have been obtained: 1.18 ± 0.13 × 10−17 and 1.89 ± 0.23 × 10−17 for 2-MPE and 2-EPE, respectively. The effects of the alkoxy groups between the branched chain and linear chain of the alkyl vinyl ethers are discussed. Comparing the rate coefficients of the ozonolysis of different unsaturated ethers led to the following observation: the alkyl group next to the C=C double bond can hinder the addition of ozone to the C=C bond of 2-MPE and 2-EPE from forming 1,2,3-trioxolane in the primary ozonide. Meanwhile, the inhibition of alkyl group becomes weaker with the increase of hydrophobic chain.
The main products are methyl acetate, formaldehyde and CO2 for 2-MPE, and ethyl acetate, formaldehyde and CO2 for 2-EPE, and reaction mechanisms were proposed from the observed products. The mechanism of the ozonolysis of these two unsaturated ethers follows the Criegee mechanism, from which four decomposition channels for the reaction can be obtained. From the tendency of forming the longer carbonyl and the shorter Criegee biradical, the methoxy substitution plays a similar role to that of a bulky or branched substituent. Future work should include more functionalized ethylenic compounds such as unsaturated alcohols and unsaturated aldehydes, which would be useful and informative to assess their environmental impact and their role in atmospheric chemistry.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4433/9/10/401/s1, Figure S1: Plots of ln([O3]0/[O3]) versus reaction time for different initial 2-MPE (1) and 2-EPE (2) concentrations: [2-MPE]0 = 6.68 × 1013 molecule cm−3 (a); [2-MPE]0 = 13.36 × 1013 molecule cm−3 (b); [2-MPE]0 = 26.72 × 1013 molecule cm−3 (c); [2-MPE]0 = 40.07 × 1013 molecule cm−3 (d); [2-MPE]0 = 53.43 × 1013 molecule cm−3 (e); [2-MPE]0 = 66.79 × 1013 molecule cm−3 (f); [2-EPE]0 = 5.59 × 1013 molecule cm−3 (g); [2-EPE]0 = 11.18 × 1013 molecule cm−3 (h); [2-EPE]0 = 22.37 × 1013 molecule cm−3 (i); [2-EPE]0 = 33.55 × 1013 molecule cm−3 (j); [2-EPE]0 = 44.73 × 1013 molecule cm−3 (k); and [2-EPE]0 = 55.91 × 1013 molecule cm−3 (l). Figure S2: IR spectra of the detected products in the reaction of 2-EPE with O3. The violet line (top) indicates the spectrum of 2-EPE at t = 0 h; the green line (middle) represents the spectrum of 2-EPE at t = 2 h; and the brown line (bottom) is the standard ethyl acetate. Figure S3: The 2-MPE with O3 reaction process monitored by FTIR spectra. Scheme S1: Proposed mechanism for the reaction of 2-EPE with O3. Table S1: The experimental conditions and results from the reactions of O3 with 2-MPE and 2-EPE using the absolute method at 298 ± 2 K in 1 atm of air.
Author Contributions
Experimental design, C.L. and L.D.; Experimental implementation, C.L. and X.J.; Data analysis, C.L. and N.T.T.; Draft preparation, C.L.; and Review and editing, N.T.T., W.W. and L.D.
Funding
This research was funded by National Natural Science Foundation of China grant number 91644214, Shandong Natural Science Fund for Distinguished Young Scholars grant number JQ201705 and Postdoctoral Science Foundation of China grant number 2017M612276.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, S.; Barnes, I.; Zhu, T.; Bejan, I.; Benter, T. Kinetic study of the gas-phase reactions of OH and NO3 radicals and O3 with selected vinyl ethers. J. Phys. Chem. A 2006, 110, 7386–7392. [Google Scholar] [CrossRef] [PubMed]
- Taccone, R.A.; Moreno, A.; Colmenar, I.; Salgado, S.; Martín, M.P.; Cabañas, B. Kinetic study of the OH, NO3 radicals and Cl atom initiated atmospheric photo-oxidation of iso-propenyl methyl ether. Atmos. Environ. 2016, 127, 80–89. [Google Scholar] [CrossRef]
- Peirone, S.A.; Aranguren Abrate, J.P.; Taccone, R.A.; Cometto, P.M.; Lane, S.I. Kinetic study of the OH-initiated photo-oxidation of four unsaturated (allyl and vinyl) ethers under simulated atmospheric conditions. Atmos. Environ. 2011, 45, 5325–5331. [Google Scholar] [CrossRef]
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric chemistry of oxygenated volatile organic compounds: Impacts on air quality and climate. Chem. Rev. 2015, 115, 3984–4014. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Mellouki, A.; Le Bras, G.; Sidebottom, H. Kinetics and mechanisms of the oxidation of oxygenated organic compounds in the gas phase. Chem. Rev. 2003, 103, 5077–5096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S. Atmospheric Oxidation of Vinyl Ethers. Ph.D. Thesis, University of Wuppertal, North Rhine-Westphalia, Germany, April 2007. [Google Scholar]
- Cavalli, F. Atmospheric oxidation of selected alcohols and esters. Ph.D. Thesis, University of Wuppertal, North Rhine-Westphalia, Germany, December 2001. [Google Scholar]
- Aschmann, S.M.; Atkinson, R. Rate constants for the gas-phase reactions of selected dibasic esters with the OH radical. Int. J. Chem. Kin. 1998, 30, 471–474. [Google Scholar] [CrossRef]
- Aschmann, S.M.; Atkinson, R. Kinetics of the gas-phase reactions of the OH radical with selected glycol ethers, glycols, and alcohols. Int. J. Chem. Kin. 1998, 30, 533–540. [Google Scholar] [CrossRef]
- Atkinson, R. Product studies of gas-phase reactions of organic compounds. Pure Appl. Chem. 1998, 70, 1335–1343. [Google Scholar] [CrossRef]
- Atkinson, R. Gas-phase degradation of organic compounds in the troposphere. Pure Appl. Chem. 1998, 70, 1327–1334. [Google Scholar] [CrossRef]
- Aschmann, S.M.; Atkinson, R. Atmospheric chemistry of 1-methyl-2-pyrrolidinone. Atmos. Environ. 1999, 33, 591–599. [Google Scholar] [CrossRef]
- Aschmann, S.M.; Atkinson, R. Products of the gas-phase reactions of the OH radical with n-butyl methyl ether and 2-isopropoxyethanol: Reactions of ROC(Ȯ)<radicals. Int. J. Chem. Kin. 1999, 31, 501–513. [Google Scholar]
- Aschmann, S.M.; Arey, J.; Atkinson, R. Atmospheric chemistry of selected hydroxy-carbonyls. J. Phys. Chem. 2000, 104, 3998–4003. [Google Scholar] [CrossRef]
- Lewis, A.C.; Carslaw, N.; Marriott, P.J.; Kinghorn, R.M.; Morrison, P.; Lee, A.L.; Bartle, K.D.; Pilling, M.J. A larger pool of ozone-forming carbon compounds in urban atmospheres. Nature 2000, 405, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Agre, B.; Taber, A.; Beregovykh, V.; Klebanova, F.; Nekrasov, N.; Sobolev, O.; Kalechits, I. Kinetics of the catalytic synthesis of 2-methoxypropene. Pharm. Chem. J. 1983, 17, 221–225. [Google Scholar] [CrossRef]
- Thiault, G.; Thévenet, R.; Mellouki, A.; Le Bras, G. OH and O3-initiated oxidation of ethyl vinyl ether. Phys. Chem. Chem. Phys. 2002, 4, 613–619. [Google Scholar] [CrossRef]
- He, M.; Wang, H.; Sun, X.; Zhang, Q.; Wang, W. Theoretical study of OH-initiated atmospheric oxidation for propyl vinyl ether. J. Theor. Comput. Chem. 2009, 8, 261–277. [Google Scholar] [CrossRef]
- Wang, L.; Ge, M.; Wang, W. Kinetic study of the reactions of chlorine atoms with ethyl vinyl ether and propyl vinyl ether. Chem. Phys. Lett. 2009, 473, 30–33. [Google Scholar] [CrossRef]
- Perry, R.A.; Atkinson, R.; Pitts, J.N. Rate constants for the reaction of OH radicals with dimethyl ether and vinyl methyl ether over the temperature range 299–427 K. J. Chem. Phys. 1977, 67, 611–614. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosjean, D. The gas phase reaction of unsaturated oxygenates with ozone: Carbonyl products and comparison with the alkene-ozone reaction. J. Atmos. Chem. 1997, 27, 271–289. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosjean, D. Rate constants for the gas-phase reaction of ozone with unsaturated oxygenates. Int. J. Chem. Kinet. 1998, 30, 21–29. [Google Scholar] [CrossRef]
- Grosjean, E.; Grosjean, D. The reaction of unsaturated aliphatic oxygenates with ozone. J. Atmos. Chem. 1999, 32, 205–232. [Google Scholar] [CrossRef]
- Klotz, B.; Barnes, I.; Imamura, T. Product study of the gas-phase reactions of O3, OH and NO3 radicals with methyl vinyl ether. Phys. Chem. Chem. Phys. 2004, 6, 1725–1734. [Google Scholar] [CrossRef]
- Scarfogliero, M.; Picquet-Varrault, B.; Salce, J.; Durand-Jolibois, R.; Doussin, J.-F. Kinetic and mechanistic study of the gas-phase reactions of a series of vinyl ethers with the nitrate radical. J. Phys. Chem. A 2006, 110, 11074–11081. [Google Scholar] [CrossRef] [PubMed]
- Thiault, G.; Mellouki, A. Rate constants for the reaction of OH radicals with n-propyl, n-butyl, iso-butyl and tert-butyl vinyl ethers. Atmos. Environ. 2006, 40, 5566–5573. [Google Scholar] [CrossRef]
- Zhou, S.; Barnes, I.; Zhu, T.; Klotz, B.; Albu, M.; Bejan, I.; Benter, T. Product study of the OH, NO3, and O3 initiated atmospheric photooxidation of propyl vinyl ether. Environ. Sci. Technol. 2006, 40, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Al Mulla, I.; Viera, L.; Morris, R.; Sidebottom, H.; Treacy, J.; Mellouki, A. Kinetics and mechanisms for the reactions of ozone with unsaturated oxygenated compounds. ChemPhysChem 2010, 11, 4069–4078. [Google Scholar] [CrossRef] [PubMed]
- Mellouki, A. Atmospheric Fate of Unsaturated Ethers. In Environmental Simulation Chambers: Application to Atmospheric Chemical Processes; Barnes, I., Rudzinski, K.J., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 163–169. [Google Scholar]
- Al Mulla, I.A.S. Kinetic and mechanisms for the atmospheric degradation of unsaturated oxygen containing compounds. Ph.D. Thesis, National University of Ireland, Dublin, Ireland, April 2006. [Google Scholar]
- Zhou, S.; Barnes, I.; Zhu, T.; Benter, T. Kinetic study of gas-phase reactions of OH and NO3 radicals and O3 with iso-butyl and tert-butyl vinyl ethers. J. Phys. Chem. A 2012, 116, 8885–8892. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Carter, W.P.L. Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions. Chem. Rev. 1984, 84, 437–470. [Google Scholar] [CrossRef]
- Atkinson, R. Gas-Phase Tropospheric Chemistry of Volatile Organic Compounds: 1. Alkanes and Alkenes. J. Phys. Chem. Ref. Data 1997, 26, 215–290. [Google Scholar] [CrossRef]
- Johnson, D.; Marston, G. The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem. Soc. Rev. 2008, 37, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.M.; Kroll, J.H.; Anderson, J.G.; Demerjian, K.L. Direct observation of OH production from the ozonolysis of olefins. Geophys. Res. Lett. 1998, 25, 59–62. [Google Scholar] [CrossRef]
- Atkinson, R.; Aschmann, S.M.; Arey, J.; Shorees, B. Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J. Geophys. Res.: Atmos. 1992, 97, 6065–6073. [Google Scholar] [CrossRef]
- Grosjean, D.; Grosjean, E.; Williams, E.L. Rate constants for the gas-phase reactions of ozone with unsaturated alcohols, esters, and carbonyls. Int. J. Chem. Kinet. 1993, 25, 783–794. [Google Scholar] [CrossRef]
- Paulson, S.E.; Orlando, J.J. The reactions of ozone with alkenes: An important source of HOx in the boundary layer. Geophys. Res. Lett. 1996, 23, 3727–3730. [Google Scholar] [CrossRef]
- Paulson, S.E.; Chung, M.; Sen, A.D.; Orzechowska, G. Measurement of OH radical formation from the reaction of ozone with several biogenic alkenes. J. Geophys. Res.: Atmos. 1998, 103, 25533–25539. [Google Scholar] [CrossRef]
- Zogka, A.G.; Mellouki, A.; Romanias, M.N.; Bedjanian, Y.; Idir, M.; Grosselin, B.; Daele, V. Atmospheric Chemistry of 1-Methoxy 2-Propyl Acetate: UV Absorption Cross Sections, Rate Coefficients, and Products of Its Reactions with OH Radicals and CI Atoms. J. Phys. Chem. A 2016, 120, 9049–9062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Wang, W.; Ge, M. Gas-phase reaction of two unsaturated ketones with atomic Cl and O3: Kinetics and products. Phys. Chem. Chem. Phys. 2015, 17, 12000–12012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, Y.; Cazaunau, M.; Daële, V.; Hu, Y.; Chen, J.; Mellouki, A. Rate coefficients for the reaction of ozone with 2- and 3-carene. Chem. Phys. Lett. 2015, 621, 71–77. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Y.; Jia, L. Arrhenius parameters for the gas-phase reactions of O3 with two butenes and two methyl-substituted butenes over the temperature range of 295–351 K. Int. J. Chem. Kinet. 2011, 43, 238–246. [Google Scholar] [CrossRef]
- Wegener, R.; Brauers, T.; Koppmann, R.; Bares, S.R.; Rohrer, F.; Tillmann, R.; Wahner, A.; Hansel, A.; Wisthaler, A. Simulation chamber investigation of the reactions of ozone with short-chained alkenes. J. Geophys. Res.: Atmos. 2007, 112, D13301. [Google Scholar] [CrossRef]
- Avzianova, E.V.; Ariya, P.A. Temperature-dependent kinetic study for ozonolysis of selected tropospheric alkenes. Int. J. Chem. Kinet. 2002, 34, 678–684. [Google Scholar] [CrossRef]
- Mason, S.A.; Arey, J.; Atkinson, R. Rate constants for the gas-phase reactions of NO3 radicals and O3 with C6-C14 1-alkenes and 2-methyl-1-alkenes at 296 ± 2 K. J. Phys. Chem. A 2009, 113, 5649–5656. [Google Scholar] [CrossRef] [PubMed]
- Duncianu, M.; Olariu, R.I.; Riffault, V.; Visez, N.; Tomas, A.; Coddeville, P. Development of a new flow reactor for kinetic studies. Application to the ozonolysis of a series of alkenes. J. Phys. Chem. A 2012, 116, 6169–6179. [Google Scholar] [CrossRef] [PubMed]
- Leather, K.E.; Mcgillen, M.R.; Percival, C.J. Temperature-dependent ozonolysis kinetics of selected alkenes in the gas phase: An experimental and structure-activity relationship (SAR) study. Phys. Chem. Chem. Phys. 2010, 12, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Neeb, P.; Moortgat, G.K. Formation of OH Radicals in the Gas-Phase Reaction of Propene, Isobutene, and Isoprene with O3: Yields and Mechanistic Implications. J. Phys. Chem. A 1999, 103, 9003–9012. [Google Scholar] [CrossRef]
- Grosjean, D.; Grosjean, E. Rate constants for the gas-phase reaction of ozone with 1,1-disubstituted alkenes. Int. J. Chem. Kinet. 1996, 28, 911–918. [Google Scholar] [CrossRef]
- Pfrang, C.; King, M.D.; Braeckevelt, M.; Canosa-Mas, C.E.; Wayne, R.P. Gas-phase rate coefficients for reactions of NO3, OH, O3 and O(3P) with unsaturated alcohols and ethers: Correlations and structure–activity relations (SARs). Atmos. Environ. 2008, 42, 3018–3034. [Google Scholar] [CrossRef]
- Gai, Y.; Ge, M.; Wang, W. Kinetics of the gas-phase reactions of some unsaturated alcohols with Cl atoms and O3. Atmos. Environ. 2011, 45, 53–59. [Google Scholar] [CrossRef]
- Gibilisco, R.G.; Blanco, M.B.; Bejan, I.; Barnes, I.; Wiesen, P.; Teruel, M.A. Atmospheric sink of (E)-3-hexen-1-ol, (Z)-3-hepten-1-ol, and (Z)-3-octen-1-ol: Rate coefficients and mechanisms of the OH-radical initiated degradation. Environ. Sci. Technol. 2015, 49, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Nakanaga, T.; Kondo, S.; Saëki, S. Infrared band intensities of formaldehyde and formaldehyde-d2. J. Chem. Phys. 1982, 76, 3860–3865. [Google Scholar] [CrossRef]
- Wohar, M.M.; Jagodzinski, P.W. Infrared spectra of H2CO, H213CO, D2CO, and D213CO and anomalous values in vibrational force fields. J. Mol. Spectrosc. 1991, 148, 13–19. [Google Scholar] [CrossRef]
- Lv, C.; Du, L.; Tang, S.; Tsona, N.T.; Liu, S.; Zhao, H.; Wang, W. Matrix isolation study of the early intermediates in the ozonolysis of selected vinyl ethers. RSC Adv. 2017, 7, 19162–19168. [Google Scholar] [CrossRef]
- Colmenar, I.; Martín, P.; Cabañas, B.; Salgado, S.; Tapia, A.; Martínez, E. Reaction products and mechanisms for the reaction of n-butyl vinyl ether with the oxidants OH and Cl: Atmospheric implications. Atmos. Environ. 2015, 122, 282–290. [Google Scholar] [CrossRef]
- Biermann, H.W.; Mac Leod, H.; Atkinson, R.; Winer, A.M.; Pitts, J.N. Kinetics of the gas-phase reactions of the hydroxyl radical with naphthalene, phenanthrene, and anthracene. Environ. Sci. Technol. 1985, 19, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1986, 86, 69–201. [Google Scholar] [CrossRef]
- Atkinson, R. Estimations of OH radical rate constants from H-atom abstraction from CH and OH bonds over the temperature range 250–1000 K. Int. J. Chem. Kinet. 1986, 18, 555–568. [Google Scholar] [CrossRef]
- Atkinson, R. A structure-activity relationship for the estimation of rate constants for the gas-phase reactions of OH radicals with organic compounds. Int. J. Chem. Kinet. 1987, 19, 799–828. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics of the gas-phase reactions of a series of organosilicon compounds with hydroxyl and nitrate (NO3) radicals and ozone at 297 ± 2 K. Environ. Sci. Technol. 1991, 25, 863–866. [Google Scholar] [CrossRef]
- Kwok, E.S.; Atkinson, R. Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update. Atmos. Environ. 1995, 29, 1685–1695. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).