Abstract
Volatile organic compounds (VOCs) pose significant risks to human health and environmental quality, prompting stringent regulations on their emissions from various industrial processes. Among VOCs, d-limonene stands out due to its low threshold and contribution to malodorous emissions. While biofiltration presents a promising approach for VOC removal, including d-limonene, a comprehensive understanding of its performance and kinetics is lacking. This study aims to comprehensively assess the performance of a lab-scale biotrickling filter in treating gas-phase d-limonene. The experimental results indicate that the biotrickling filter efficiently removed d-limonene, achieving a critical loading rate of 19.4 g m−3 h−1 and a maximum elimination capacity of 31.8 g m−3 h−1 (correspondingly, up to 85% removal) at the condition of 94.2 s of EBRT. Microbial activity played a significant role in biotrickling filter performance, with a strong linear correlation being observed between CO2 production and substrate consumption. The Michaelis–Menten model was employed to represent enzyme-catalyzed reactions, suggesting no inhibition during biotrickling filter operation.
1. Introduction
Volatile organic compounds (VOCs) are widely recognized for their adverse impacts on human health due to their inherent toxicity, serving as precursor pollutants for the secondary formation of organic aerosol and ozone upon release into the atmosphere [1,2,3,4,5]. The emissions of VOCs, primarily originating from various industrial processes, are subject to increasingly stringent regulations worldwide [6].
Of all the VOCs, d-limonene has a relatively low threshold of 0.05 to 7.3 mg/m3 [7,8,9], giving rise to environmental concerns associated with the generation of malodorous emission. This compound is generated from both anthropogenic sources, including sewage treatment plants, composting processes, landfill gas treatment processes, and biogas production processes, and natural sources such as urban forests and street trees [10,11,12,13]. Gaseous limonene concentrations from wastewater treatment plants were reported to reach up to 232.6 μg/m3 [14]. Zarra et al. [15] found the highest concentration of limonene to be 111.21 μg/m3 in the sludge treatment plant. Yuya et al. [12] examined the concentrations of limonene in landfills in China and Japan, which ranged from 0.25 to 259 mg/m3. Nie et al. [10] investigated ozone formation potential from the sewage sludge composting plant, revealing a propene-equivalent concentration of limonene ranging from 32.47 μg/m3 to 1565.28 μg/m3. Terpenes including limonene can contribute to indoor secondary pollution by generating compounds such as formaldehyde, even in the presence of ozone at a low concentration ranging from 17.2 to 21.4 ppb [16].
In comparison to other treatment processes such as adsorption, absorption, and catalytic oxidation, biological methods prove effective and economical for biodegradable odorants and organic compounds. A biotrickling filter is a well-established biological process, and is particularly effective for removing VOCs, including d-limonene [3,17]. In the biotrickling filter, polluted air passed through a microbial-rich packed bed, facilitating the transfer of contaminants to microorganisms for subsequent biodegradation [18]. Compared to conventional biofiltration, biotrickling filters can effectively control excessive biomass accumulation and enhance the efficiency of biofiltration through regulating the pH, temperature, moisture content, and other parameters [1,17,19,20,21,22,23]. This approach represents a reliable means for addressing odorous and hazardous pollutants, ultimately resulting in the formation of odorless and harmless products. In particular, hydrophobic VOCs control processes using biotrickling filters are becoming more widely used [24,25,26,27,28,29]. Hong et al. [30] reported that the removal efficiency of methyl isobuthyl ketone in a tubular-type biotrickling filter ranged from 70% to 99% at a maximum elimination capacity (ECmax) of about 200 g/m3·h. And Chen et al. [25] reported that the removal efficiency of toluene in a tubular biofilter ranged from 52.2% to 99% at a maximum elimination capacity (ECmax) of 83.0 g/m3·h.
Terpene-like volatile organic compounds are typically characterized as being hydrophobic. In particular, limonene is highly hydrophobic, with a solubility in water of 13.8 mg/L at 25 °C and a Henry’s constant of 0.0111 atm·m3/mol at 25 °C. In a study conducted by van Groenestijn and Liu [31], alpha-pinene exhibited removal efficiencies ranging from 50% to 90% in a biofilter, with an inlet loading of 24 to 38 g/m3·h. Cabeza et al. [32] reported that in the biofilter system, alpha-pinene removal was less than 60% at a higher inlet loading of 180 g/m3·h. Another study by Viswanathan et al. [33] demonstrated that applying limonene and beta-pinene to a compost biofilter resulted in 85% and 45% removal, respectively, at an inlet loading rate of 55 g/m3·h. Fatih and Mark [13] reported that a biotrickling filter for limonene and methane showed 83% removal at an inlet loading of 35.5 g/m3·h. While several studies have reported on terpene removal in biofilters, the overall performance, including kinetics and microbial activity, remains incompletely understood. Therefore, further study should be conducted to comprehensively assess the performance of terpene removal, focusing on kinetics and microbial activity. A comprehensive analysis of the activity and kinetics of microorganisms within a biotrickling filter is required to ascertain the operational parameters, including the influent load, gas flow rate, and backwash conditions, for the biofilter operation. Moreover, it is essential to conduct further research into the scientific and engineering fundamentals of biotrickling filters, with a particular focus on the removal of malodorous compounds, including limonene. This study presents a comprehensive application of both stoichiometric and kinetic analyses to elucidate the biological reactions involved in d-limonene treatment. This methodological approach facilitates a deeper understanding of microbial activity and substrate kinetics, which has been insufficiently addressed in prior research.
The objective of this study is to comprehensively assess the performance of a lab-scale biotrickling filter in treating gas-phase d-limonene. The evaluation involves kinetic and stoichiometric analyses to elucidate the characteristics of the biological reactions occurring in the biotrickling filter. Specifically, the study aims to determine the biotrickling filter’s critical loading rate and maximum elimination capacity (ECmax) and to understand the biological behavior through the application of two biokinetic models (Michaelis–Menten model and Haldane model). Additionally, the carbon mass balance over six months operation of the biotrickling filter was established to analyze carbon utilization and the biological reaction.
2. Materials and Methods
2.1. Biofilter Setup
The laboratory-scale biotrickling filter was setup as shown in Figure 1. It consisted of 7 cylindrical PMMA (Polymethyl methacrylate) parts with an internal diameter of 100 mm, a total length of 1500 mm, and a total volume of 8.25 L (a packed bed volume of 4.71 L). Polyurethane foam (PUF) was used as the packing materials for this experiment because it has advantages of high surface area, good gas permeability, and efficient moisture distribution, and it performs better than ceramic or activated carbon carriers [34] (Juntai Plastic, Hangzhou, China). The PUF was cut into a cubic structure of 15 mm × 15 mm × 15 mm, a packing depth of 600 mm, and a packing volume of 4.71 L. The pore size and porosity of PUF were 1–2 mm and 96–97.5%, respectively. A mineral salts medium (MSM) tank was placed to supply moisture and nutrients to the biotrickling filter. The air was consistently supplied through mass flow controllers (MFC), while d-limonene was provided as the sole carbon source through a syringe pump. Table 1 presents the specific operating conditions for each reactor. Backwashing was performed when the compression of more than 15% of the bed volume was observed with the naked eye.
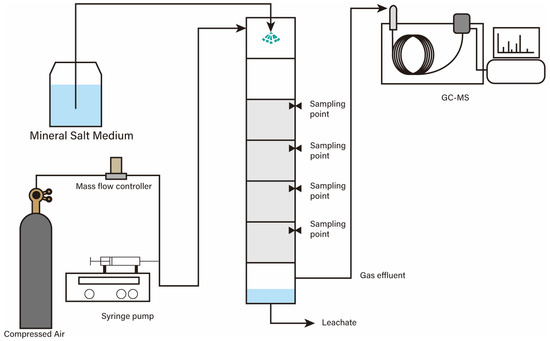
Figure 1.
Schematic diagram of biotrickling filter setup in this study.

Table 1.
Experimental conditions for the biotrickling filter.
2.2. Microbials Inoculation and Nutrient Medium
The microbial inoculum for the biotrickling filter was prepared using activated sludge from the aeration tank in the wastewater treatment plant located in Uijeongbu City, Republic of Korea. The supernatant of the activated sludge was acclimated to d-limonene as the sole carbon source through aeration for one week. To facilitate microbial growth, nitrogen and phosphorus were supplied in the form of NaNO3 and NaH2PO4·H2O, respectively, according to the d-limonene concentration. The ratio of COD:N:P was maintained at 100:5:1, and NaHCO3 was used as a buffer solution. Trace elements were supplied for microbial growth, including FeCl3, MgCl2, CaCl2, KHSO4, CoCl2, CuCl2, MnCl2, and ZnCl2. All chemicals were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
2.3. Analysis Methods
2.3.1. Biotrickling Filter Performance
Gas samples were collected from the sampling point of the biotrickling filter through gas-tight syringes. D-limonene was analyzed using gas chromatography-mass spectrometry (GC-MS, YL-6500GC, YoungIn, Anyang City, Republic of Korea) equipped with a DB-5MS column (30 m × 0.25 mm × 0.25 μm). The temperature of the injector, ion source, and oven were set to 250, 200, and 50 to 125 °C, respectively. The method detection limit of d-limonene analyzed using GC-MS was 1.26 ppb with 98.5% accuracy and 9.2% RSD repeatability. CO2 was analyzed using GC-FID with a methanizer (YL-6100GC, YoungIn, Anyang City, Republic of Korea) equipped with a Porapak N column (1.83 m × 2 mm, mesh 80/100). The injector, detector, and oven temperature were set to 120, 250, and 50 °C, respectively. Helium gas was used as the carrier gas at a flow rate of 20 mL/min for GC-FID with a methanizer and 1 mL/min for GC-MS, respectively. Liquid samples were collected from the effluent of the biofilter for volatile suspended solids (VSS) and total organic carbon (TOC) analyses. The activity of the microbial in the biofilter was indirectly assessed via analyzing the amount of VSS. The total organic carbon was measured using a TOC analyzer (TOC-L, Shimadzu, Kyoto, Japan).
2.3.2. Microbial Kinetic
In order to understand the dynamic behavior of microorganisms and their biochemical reactions in the biotrickling filter, two microbial kinetics, i.e., the Michaelis–Menten model and Haldane model, were employed to offer distinct perspectives on enzyme–substrate interactions and microbial growth. The Michaelis–Menten model is a microkinetic representation of enzyme-catalyzed reactions, which can be rearranged for applications in biotrickling filters as follows [35]:
where Cln is the logarithmic average concentrations of the biotrickling filter, ECmax is the maximal elimination capacity, and KS is the saturation constant.
The Haldane model is an extension of the classical Michaelis–Menten equation, which into account the inhibitory effects of a substance on the enzyme activity. This equation can be applied to a biotrickling filter as follows [36]:
where EC* is the maximal EC in the absence of inhibition, KS′ is the saturation constant, and KI is the inhibition constant of the Haldane model.
2.3.3. Mass Balance Analysis
The following mass balance equation can describe the behavior of the substrate of the biotrickling filter:
where Cremoved is the removed carbon amount of d-limonene, is the CO2 of effluent gas, CVSS is the VSS of the effluent liquid, CTOC is the total organic carbon of the effluent liquid, and Cunknoun is the unrecovered carbon, and all terms can be converted into carbon mole. A typical cellular composition for a heterogeneous microorganism in the biotrickling filter can be represented as C5H7O2N [37].
The overall reaction for the aerobic biodegradation of d-limonene in the biotrickling filter under steady state conditions without the accumulation of intermediate products and nitrate used as nitrogen sources can be thus given by the following equation:
The overall reaction for the aerobic biodegradation of d-limonene can be determined using the following half reaction (half-reaction of an electron receptor, Ra; an electron donor, Rd; and a cell synthesis, Rc) because the biological activity could be due to cell synthesis and energy production.
The fraction of electrons used for cell synthesis and energy generation in the overall reaction can be expressed as fs and fe, respectively. The overall reaction equation taking into account the electron fraction is as follows:
The assumptions of this stoichiometric interpretation are as follows: The d-limonene was supplied in a steady state. There was no endogenous respiration and denitrification reaction involving microorganisms. The intermediate step of d-limonene degradation was not considered. There was no inhibition by oxygen or the substrate.
3. Results and Discussion
3.1. Biotrickling Filter Performance
Figure 2 shows the performance of the biotrickling filter with respect to the d-limonene inlet concentration and the removal efficiency (RE). The biotrickling filter was started with an influent d-limonene concentration of 14 ppmv, with a corresponding loading rate of 3.1 g m−3 h−1. The inlet loading was then stepwise increased as planned in Table 1. Although the overall performance was unstable during the acclimation period, the biotrickling filter reached an RE up to 95%. The RE then remained above 99% until a loading rate of 23.8 g m−3 h−1. The corresponding pH in the effluent liquid from the biotrickling filter remained stable at around 8.0 during these loading rates. However, when the loading rate exceeded 23.8 g m−3 h−1, the RE dropped to below 85%. On day 148, a bed compression was observed, which may have been caused by the overgrowth of biomass in the biotrickling filter bed. However, no significant pressure drop was found during operation. It should be noted that PUF cubic media is preferred due to its low pressure drop property. The impact of biomass overgrowth on performance may vary depending on the condition of the biotrickling filter, including the packing media [31].
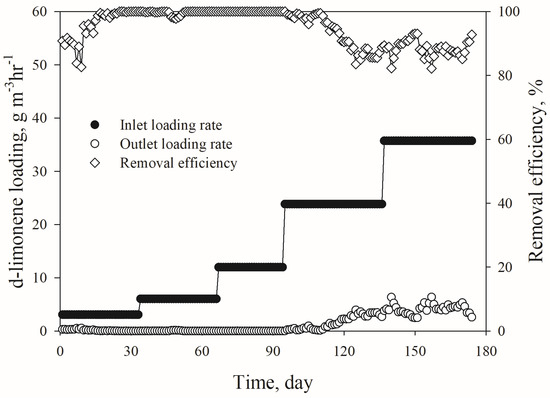
Figure 2.
Biotrickling filter performance with respect to d-limonene removal.
Figure 3 presents the elimination capacity (EC) of the biotrickling filter as a function of the loading rate of d-limonene. This confirms that relatively high loading rates above 23.8 g m−3 h−1 lead to the substantial deteriorating of the biotrickling filter performance. The critical loading rate was found to be about 19.4 g m−3 h−1, and the maximum elimination capacity (ECmax) was 31.8 g m−3 h−1 under the given condition of biotrickling filter operation.
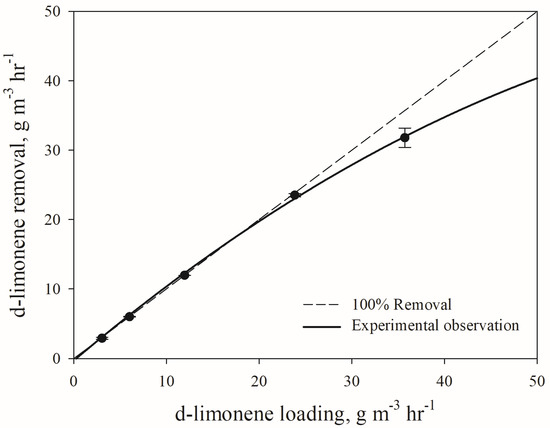
Figure 3.
D-limonene removal capacity of d-limonene with respect to its loading rate.
3.2. Kinetic Analysis
Microorganisms play a significant role in the biotrickling filter performance because the removal capability is mostly dependent on the microbial activity [38]. It is therefore necessary to understand the biochemical reaction and the removal behavior of the substrate in the biotrickling filter. Different models have been studied to evaluate microbial kinetics and substrate degradation [35,36,39]. In addition, several studies have been carried out to analyze the mass balance through measuring the biomass growth and CO2 production in the biotrickling filter, as well as assessing the operating characteristics of the biotrickling filter [40,41].
Figure 4 shows the elimination capacity of the loading of the biotrickling filter using the logarithmic mean of the substrate concentration and microbial activity in the biotrickling filter, as demonstrated in the Michaelis–Menten and Haldane models. The Michaelis–Menten and the Haldane models (denoted as ECmax and EC* for each model) provided maximum removal efficiencies of 32.0 g m−3 h−1 and 26.0 g m−3 h−1, respectively, whereas the experimentally observed value was 31.8 g m−3 h−1. It can be deduced from Figure 4 that there was no substrate inhibition during the biotrickling filter run. The reduced performance may be due to limitations in mass transfer. As limonene is highly hydrophobic, some research is investigating the addition of surfactants or the use of fungi to improve the removal of hydrophobic compounds. Other possible causes of reduced performance could be the saturation of microbial capacity or changes in the microbial community. This is beyond the scope of this study, but is worth considering for future studies. Therefore, further investigation is necessary to determine the reason for the observed decrease in the removal efficiency with an increase in d-limonene loading.
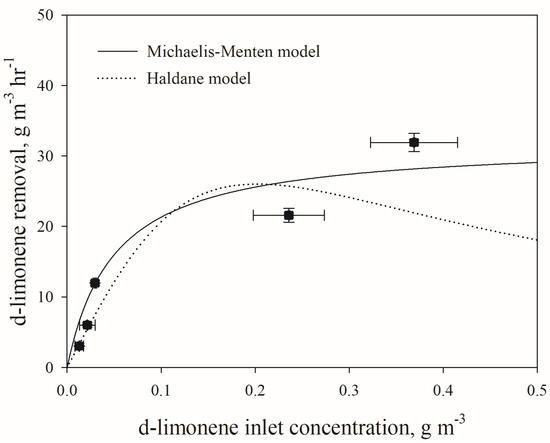
Figure 4.
Elimination capacity according to the logarithmic average of d-limonene concentration for the biotrickling filter.
It can be deduced that oxygen transfer to the biofilm was inhibited during the biotrickling filter operation. To prevent biofilter failure, excess oxygen must be allowed to pass through the entire biofilm as an electron acceptor. The maximum practical concentration of d-limonene in the biofilm at the air–biofilm interface can be estimated from the maximum equilibrium concentration of oxygen in the biofilm, as suggested by Williamson and McCarty [42].
where S is a concentration of d-limonene and O2 (8.3 mg L−1 of water solubility at 25 °C and 1 atm) at the biofilm surface, υ is a stoichiometric reaction coefficient for d-limonene and O2, MW is the molecular weight of d-limonene and O2, and D is the diffusion coefficient of d-limonene and O2. The diffusion coefficient can be calculated through the Wilke-Chang equation [41]. The ratio of Doxygen/Dsubstrate was 3.03 for d-limonene. The maximum practical concentration of d-limonene (Ssubstrate) in the biotrickling filter can be calculated as 1698 ppmv. At the given experimental conditions, the d-limonene loading rate corresponded to 360.83 g m−3·h−1. Since the observed value of the elimination capacity (ECmax) was 31.8 g m−3 h−1, it can be speculated that oxygen was not limited in the biotrickling filter bed in this study.
Another possible reason for the decrease in the removal efficiency with an increase in d-limonene loading is that d-limonene has a low water solubility (7.57 mg L−1, 25 °C) [7], which limited the mass transfer between the gas and biofilm. This, in consequence, would inhibit the performance of the biotrickling filter [36]. It should be noted that the effectiveness of the biotrickling filter may be correlated with their physical and chemical properties of VOCs [41].
3.3. CO2 Generation and Biomass Yield
The biotrickling filter essentially operates as an aerobic biological process, with CO2 production serving as a useful indicator of the biological activity resulting from the aerobic oxidation of organic matters and endogenous respiration. In a biotrickling filter, biomass can accumulate and sometime endogenous respiration may occur [41]. Therefore, the carbon mass balance can be expressed as a CO2 production rate, which was suggested by [41,43], as follow:
where RCO2 is the CO2 production rate, Rsub is the substrate consumption rate, Rbiomass is the biomass production rate, and Rendo is the endogenous respiration rate of microbes. Moreover, this equation can be interpreted by the biomass yield coefficient (Y, mole C as biomass/mole C as the substrate) as follows:
Figure 5 shows the CO2 production rate as a function of consumed d-limonene. The regression line in Figure 5 represents a term of (1 − Y) in Equation (14), while the y-intercept represents the endogenous respiration rate. The observed biomass yield coefficients (mole biomass/mole carbon as the substrate) were 0.437, which corresponds to 0.363 g biomass/g substrate (dry weight basis) if a molecular formula for biomass C5H7O2N is considered. The endogenous respiration rate was low (0.013), indicating that endogenous microbial activity was less significant during the operation period. This observation is consistent with those found in Section 3.2, where we assumed that the oxygen inhibition did not occur during the operation period.
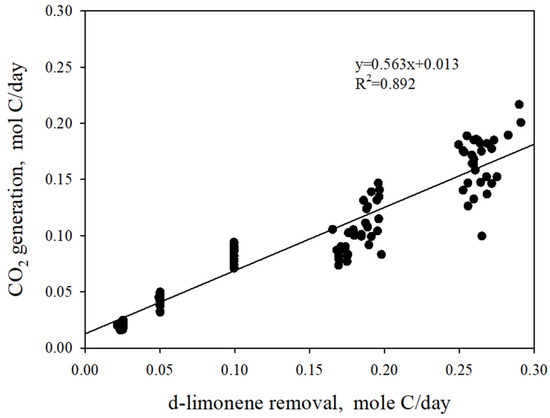
Figure 5.
CO2 production as a function of d-limonene consumption (CO2 production rate and d-limonene consumption rate were calculated on a carbon-mole basis).
Figure 5 also highlights a strong linear correlation between CO2 production and substrate consumption. This observation indicates that the biological activity within the biotrickling filter was consistently maintained under pseudo-steady state conditions, regardless of the variations in substrate inlet loadings applied.
Figure 5 provides information on carbon recovery as CO2 at different substrate loadings, from which fe (the fraction of energy generation) and fs (the fraction of cell synthesis) can be derived from Equation (11). As a result, the stoichiometric reaction in the biotrickling filter (Equation (15)) represents the biological response at a critical substrate loading. In general, the biological response of a biofilter can vary depending on the depth of the reactor and the duration of the operation. However, the stoichiometric equation for the biological degradation of d-limonene using the biotrickling filter obtained in this study can be useful for a comprehensive understanding of the substrate-dependent biological response.
4. Conclusions
This study presents the performance and kinetics of a biotrickling filter system for the removal of gas-phase d-limonen. The specific conclusions and recommendations that can be drawn from this study include the following:
- (1)
- The biotrickling filter with concurrent gas–liquid downflow demonstrated the efficient removal of d-limonene, with a critical loading rate of 19.4 g m−3 h−1 and a maximum elimination capacity of 31.8 g m−3 h−1. Loading rates above this threshold resulted in a substantial deterioration of the biotrickling filter performance, possibly due to bed compression caused by biomass overgrowth.
- (2)
- Microbial activity plays a significant role in biotrickling filter performance, with biochemical reactions and substrate degradation being key factors. Michaelis–Menten and Haldane kinetic models have been studied to understand microbial kinetics and substrate degradation, providing insights into the biological response of the biotrickling filter to varying substrate concentrations. The Michalis–Menten model was employed to represent the enzyme-catalyzed reactions in this biotrickling filter, given that no inhibition was observed during the biotrickling filter operation.
- (3)
- The study observed a strong linear correlation between CO2 production and substrate consumption, with the observed biomass yield coefficients being 0.437 mole biomass/mole carbon as the substrate. The stoichiometric equation for the biological reaction provided a comprehensive understanding of the substrate-dependent biological response in the biotrickling filter.
Author Contributions
Conceptualization, D.K.; methodology, Y.C. and D.K.; software, Y.C.; validation, Y.C. and D.K.; formal analysis, Y.C.; investigation, Y.C.; resources, D.K.; data curation, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, D.K.; visualization, Y.C.; supervision, D.K.; project administration, D.K.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Seoul National University of Science and Technology.
Data Availability Statement
All data supporting the conclusions of this article are included in this manuscript.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Yang, C. Volatile organic compound removal via biofiltration: Influences, challenges, and strategies. Chem. Eng. J. 2023, 471, 144420. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lv, Y.H.; Wang, C.; Jiang, G.Y.; Han, M.F.; Deng, J.G.; Hsi, H.C. Microbial community evolution and functional trade-offs of biofilm in odor treatment biofilters. Water Res. 2023, 235, 119917. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Li, X.; Yang, C. Enhanced Removal of Hydrophobic Short-Chain n-Alkanes from Gas Streams in Biotrickling Filters in Presence of Surfactant. Environ. Sci. Technol. 2022, 56, 10349–10360. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsi, M.; Khabiri, B.; Buelna, G.; Jones, J.P.; Heitz, M. Air biofilters for a mixture of organic gaseous pollutants: An approach for industrial applications. Crit. Rev. Biotechnol. 2023, 43, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, G.; Lens, P.N.L.; He, Y.; Qie, L.; Shen, X.; Chen, J.; Cheng, Z.; Chen, D. Enhanced removal of mixed VOCs with different hydrophobicities by Tween 20 in a biotrickling filter: Kinetic analysis and biofilm characteristics. J. Hazard. Mater. 2023, 450, 131063. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Kraakman, N.B.; Muñoz, R.; Lebrero, R. A comparative analysis of odour treatment technologies in wastewater treatment plants. Environ. Sci. Technol. 2011, 45, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.; Ladefoged, O.; Søborg, I. Evaluation of Health Hazards by Exposure to d-Limonene and Proposal of a Health-Based Quality Criterion for Ambient Air; The Danish Environmental Protection Agency, Environmental Project: Copenhagen, Denmark, 2013; pp. 1–36. [Google Scholar]
- Padrayuttawat, A.; Yoshizawa, T.; Tamura, H.; Tokunaga, T. Optical isomers and odor thresholds of volatile constituents in Citrus sudachi. Food Sci. Technol. Int. Tokyo 1997, 3, 402–408. [Google Scholar] [CrossRef]
- Program, N.T. NTP toxicology and carcinogenesis studies of d-limonene (CAS No. 5989-27-5) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 1990, 347, 1–165. [Google Scholar]
- Nie, E.; Zheng, G.; Gao, D.; Chen, T.; Yang, J.; Wang, Y.; Wang, X. Emission characteristics of VOCs and potential ozone formation from a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 659, 664–672. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Z.; Kännaste, A.; Guo, M.; Zhou, G.; Niinemets, Ü. Isoprenoid and aromatic compound emissions in relation to leaf structure, plant growth form and species ecology in 45 East-Asian urban subtropical woody species. Urban For. Urban Green. 2020, 53, 126705. [Google Scholar] [CrossRef]
- Takuwa, Y.; Matsumoto, T.; Oshita, K.; Takaoka, M.; Morisawa, S.; Takeda, N. Characterization of trace constituents in landfill gas and a comparison of sites in Asia. J. Mater. Cycles Waste Manag. 2009, 11, 305–311. [Google Scholar] [CrossRef]
- Hosoglu, F.; Fitch, M.W. Abatement of synthetic landfill gas including limonene by biotrickling filter and membrane biofiltration. J. Environ. Sci. Health Part A 2012, 47, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Godayol, A.; Marce, R.M.; Borrull, F.; Anticó, E.; Sanchez, J.M. Development of a method for the monitoring of odor-causing compounds in atmospheres surrounding wastewater treatment plants. J. Sep. Sci. 2013, 36, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Zarra, T.; Reiser, M.; Naddeo, V.; Belgiorno, V.; Kranert, M. Odour emissions characterization from wastewater treatment plants by different measurement methods. Chem. Eng. Trans. 2014, 40, 37–42. [Google Scholar]
- Huang, H.-L.; Tsai, T.-J.; Hsu, N.-Y.; Lee, C.-C.; Wu, P.-C.; Su, H.-J. Effects of essential oils on the formation of formaldehyde and secondary organic aerosols in an aromatherapy environment. Build. Environ. 2012, 57, 120–125. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Dai, M.; Wu, S.; Li, X.; Yang, C. Chemical structure of hydrocarbons significantly affects removal performance and microbial responses in gas biotrickling filters. Bioresour. Technol. 2024, 398, 130480. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Rene, E.R.; Malhautier, L.; Rocher, J.; Bayle, S.; Veiga, M.C.; Kennes, C. One-stage biotrickling filter for the removal of a mixture of volatile pollutants from air: Performance and microbial community analysis. Bioresour. Technol. 2013, 138, 245–252. [Google Scholar] [CrossRef]
- Danila, V.; Zagorskis, A.; Januševičius, T. Effects of water content and irrigation of packing materials on the performance of biofilters and biotrickling filters: A review. Processes 2022, 10, 1304. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Wang, S.; Yang, C.; Chen, H.; Zhu, Y.; Yu, L.e.; Li, J.; Kazemian, H. Performance promotion and its mechanism for n-hexane removal in a lab-scale biotrickling filter with reticular polyurethane sponge under intermittent spraying mode. Process Saf. Environ. Prot. 2021, 152, 654–662. [Google Scholar] [CrossRef]
- Montes, M.; Veiga, M.C.; Kennes, C. Two-liquid-phase mesophilic and thermophilic biotrickling filters for the biodegradation of α-pinene. Bioresour. Technol. 2010, 101, 9493–9499. [Google Scholar] [CrossRef]
- Rybarczyk, P. Removal of volatile organic compounds (VOCs) from air: Focus on biotrickling filtration and process modeling. Processes 2022, 10, 2531. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Szulczyński, B.; Gospodarek, M.; Gębicki, J. Effects of n-butanol presence, inlet loading, empty bed residence time and starvation periods on the performance of a biotrickling filter removing cyclohexane vapors from air. Chem. Pap. 2020, 74, 1039–1047. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Yang, C. Interactive effects of dual short-chain n-alkanes on removal performances and microbial responses of biotrickling filters. Chem. Eng. J. 2023, 461, 141747. [Google Scholar] [CrossRef]
- López de León, L.R.; Deaton, K.E.; Deshusses, M.A. Miniaturized biotrickling filters and capillary microbioreactors for process intensification of VOC treatment with intended application to indoor air. Environ. Sci. Technol. 2018, 53, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Chheda, D.; Sorial, G.A. Evaluation of co-metabolic removal of trichloroethylene in a biotrickling filter under acidic conditions. J. Environ. Sci. 2017, 57, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Lebrero, R.; Quijano, G.; Pérez, R.; Figueroa-González, I.; García-Encina, P.A.; Muñoz, R. Methane abatement in a gas-recycling biotrickling filter: Evaluating innovative operational strategies to overcome mass transfer limitations. Chem. Eng. J. 2014, 253, 385–393. [Google Scholar] [CrossRef]
- Marycz, M.; Rodríguez, Y.; Gębicki, J.; Muñoz, R. Systematic comparison of a biotrickling filter and a conventional filter for the removal of a mixture of hydrophobic VOCs by Candida subhashii. Chemosphere 2022, 306, 135608. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, Y.; Peng, L.; Ni, J.; Guo, Y.; Ji, J.; Jiang, B.; Yu, G. Long-term MIBK removal in a tubular biofilter: Effects of organic loading rates and gas empty bed residence times. Process Saf. Environ. Prot. 2018, 119, 87–95. [Google Scholar] [CrossRef]
- Van Groenestijn, J.; Liu, J. Removal of alpha-pinene from gases using biofilters containing fungi. Atmos. Environ. 2002, 36, 5501–5508. [Google Scholar] [CrossRef]
- Cabeza, I.; López, R.; Giraldez, I.; Stuetz, R.; Díaz, M. Biofiltration of α-pinene vapours using municipal solid waste (MSW)–Pruning residues (P) composts as packing materials. Chem. Eng. J. 2013, 233, 149–158. [Google Scholar] [CrossRef]
- Viswanathan, S.; Neerackal, G.; Buyuksonmez, F. Removal of beta-pinene and limonene using compost biofilter. J. Air Waste Manag. Assoc. 2013, 63, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Woiski, C.; Winkler, F.; Engesser, K.-H.; Dobslaw, C. Prevention of clogging in a polyurethane foam packed biotrickling filter treating emissions of 2-butoxyethanol. J. Clean. Prod. 2018, 200, 609–621. [Google Scholar] [CrossRef]
- Ramirez, A.A.; Bénard, S.; Giroir-Fendler, A.; Jones, J.P.; Heitz, M. Kinetics of microbial growth and biodegradation of methanol and toluene in biofilters and an analysis of the energetic indicators. J. Biotechnol. 2008, 138, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, L.; Wang, Y.; Liu, J. Effects of substrate fluctuation on the performance, microbial community and metabolic function of a biofilter for gaseous dichloromethane treatment. Chemosphere 2020, 249, 126185. [Google Scholar] [CrossRef] [PubMed]
- Comeau, Y. Microbial metabolism. Biol. WastewaterTreat. Princ. Model. Des. Cap 2008, 2, 9–32. [Google Scholar]
- Zhu, R.; Li, S.; Bao, X.; Dumont, É. Comparison of biological H2S removal characteristics between a composite packing material with and without functional microorganisms. Sci. Rep. 2017, 7, 42241. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Fernández, A.; Revah, S.; Moreno-Casas, P.; Scott, F. Biofiltration of volatile organic compounds using fungi and its conceptual and mathematical modeling. Biotechnol. Adv. 2018, 36, 1079–1093. [Google Scholar] [CrossRef]
- Bordoloi, A.; Gostomski, P.A. Fate of degraded pollutants in waste gas biofiltration: An overview of carbon end-points. Biotechnol. Adv. 2019, 37, 579–588. [Google Scholar] [CrossRef]
- Kim, D.; Sorial, G.A. Nitrogen utilization and biomass yield in trickle bed air biofilters. J. Hazard. Mater. 2010, 182, 358–362. [Google Scholar] [CrossRef]
- Williamson, K.; McCarty, P.L. A model of substrate utilization by bacterial films. J. (Water Pollut. Control Fed.) 1976, 48, 9–24. [Google Scholar] [PubMed]
- Diks, R.; Ottengraf, S.; Vrijlnad, S. The existence of a biological equilibrium in a trickling filter for waste gas purification. Biotechnol. Bioeng. 1994, 44, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).