Abstract
The atmosphere’s fine particulate matter (PM2.5) can enter the liver through the circulatory system, leading to hepatic inflammation and fibrosis. As a non-flavonoid polyphenolic compound, resveratrol (RES) has anti-oxidant, anti-inflammatory and hepatoprotective effects, but the molecular mechanisms of liver fibrosis induced by PM2.5 exposure are still limited. In this study, we established an in vitro cell model to investigate the intervention effect of RES with different concentrations (5 and 20 μmol/mL) on mouse hepatic stellate cells (mHSCs) injury induced by PM2.5 (100 μg/mL). We determined the cell viability in mHSCs after treatment with PM2.5 or/and RES for 24 h. We investigated the intracellular oxidative stress by detecting the changes in reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD) and lactate dehydrogenase (LDH) levels. We also measured the protein expressions of fibrosis-related genes (α-SMA, Collagen I and Collagen III) and key genes (SIRT1, NF-κB, NLRP3, Cleaved-Caspase1, IL-1β) in the NLRP3 pathway in mHSCs exposed to PM2.5 with or without RES. The results showed that (1) PM2.5 has cytotoxic effects on mHSCs, whereas RES (5 μmol/L and 20 μmol/L) inhibited PM2.5-induced cytotoxicity and LDH leakage; (2) RES effectively reduces ROS and MDA production caused by PM2.5 while concurrently enhancing SOD levels, thereby improving cellular anti-oxidant capacity; (3) the expression of α-SMA, Collagen I and Collagen III were notably downregulated in the PM2.5 plus RES treatment group compared to the PM2.5-exposed group; (4) RES significantly increased SIRT1 expression and decreased the expression of NF-κB, NLRP3, Cleaved-Caspase1 and IL-1β in mHSCs exposure to PM2.5 compared to the PM2.5 group. These results demonstrate that RES can up-regulate SIRT1 and mitigate PM2.5-induced fibrosis by suppressing oxidative stress in mHSCs and the SIRT1/NF–κB/NLRP3 pathway activated by PM2.5.
1. Introduction
Fine particulate matter (PM2.5) is a common air pollutant with a complex composition that easily adsorbs toxic substances, posing a threat to the cardiovascular, respiratory, nervous and reproductive systems [1,2,3]. PM2.5 particles can traverse the air–blood barrier, and once they enter the circulation, their detrimental effects extend far beyond the lungs, reaching various organs, such as the liver, throughout the body. Empirical research has revealed that PM2.5 infiltrates the liver via the bloodstream, instigating a cascade of pathological alterations, including hepatic inflammation, oxidative stress, steatosis, and liver fibrosis [4,5,6], exerting profound impacts on liver function. A meta-analysis indicated that long-term exposure to PM2.5 was associated with an increased risk of chronic liver disease [7]. Studies have also pointed to a significant relationship between exposure to PM2.5 and the incidence of non-alcoholic fatty liver disease (NAFLD) [8]. A prospective cohort study has shown that long-term exposure to PM2.5 positively correlates with the risk of NAFLD. An increase of 1 μg/m3 in PM2.5 concentration above 23.5 μg/m3 was associated with a hazard ratio (HR) of 1.06 (95% CI: 1.04–1.09) for NAFLD identified by the Fatty Liver Index (FLI), and an HR of 1.05 (95% CI: 1.03–1.07) for NAFLD identified by the Hepatic Steatosis Index (HSI) [9]. Prolonged exposure to PM2.5 induces inflammation and oxidative stress, promoting lipid accumulation in the liver and ultimately increasing the risk of NAFLD [4]. These studies suggested that PM2.5 is a risk factor for liver diseases.
Protective agents significantly mitigate the hepatotoxicity induced by PM2.5 exposure, as research has shown that loquat leaf can alleviate PM2.5-induced NAFLD [10]. Resveratrol (RES) is a non-flavonoid phenolic substance mainly found in grapes, mulberries, pine trees and Japanese knotweed [11]. RES, characterized by its distinctive anti-oxidant, anti-aging, cardio-protective, neuroprotective and anti-fibrotic properties [12,13,14,15], has emerged as a focal point of investigation for numerous researchers. Scientific studies have definitively demonstrated that RES can inhibit the proliferation of rat hepatic stellate cells, manifesting a pronounced anti-fibrotic effect on the liver [16]. Studying the inhibitory impact of protective agents on the occurrence and development of liver fibrosis is of great significance for treating liver diseases. However, there are few reports on the resistant effects of RES against liver fibrosis caused by PM2.5 exposure. This study investigated the antagonistic effects of RES on PM2.5-induced liver fibrosis.
2. Materials and Methods
2.1. PM2.5 Collection and Preparation
The sampling location for PM2.5 is on the rooftop of a campus building at Shanxi University (Taiyuan, China). Taiyuan is a typical resource-based city, and its PM2.5 concentration in the air during the winter heating period was relatively high among Chinese cities. PM2.5 samples were collected using a medium-volume atmospheric sampler (ADS-2062E, AMAE Co., Ltd., Shenzhen, China) with quartz filter membranes (Whatman, Kent, UK) during the winter of 2019. We cut the membranes into small pieces, immersed them in ultrapure water, subjected the mixture to ultrasonication, and then filtered it through six layers of gauze to collect the PM2.5-containing solution. The PM2.5 dry powder was obtained by vacuum freeze-drying. A flow chart for PM2.5 preparation is shown in Figure S1. PM2.5 suspensions of different concentrations using sterilized phosphate buffer saline (PBS) were stored at 4 °C until further experiment.
2.2. mHSC Culture
Mouse hepatic stellate cells (mHSCs) were purchased from Mingzhou Biotechnology Co., Ltd. (Ningbo, China). The cells were cultured in high-sugar DMEM culture with 10% fetal bovine serum (Gibco, Fitzroy North, Australia) and 1% penicillin/streptomycin in a humidified incubator with 5% CO2 at 37 °C. When the cells reached a density of 80–90%, they were washed twice with sterile PBS at 37 °C, digested with 0.25% trypsin for subculturing, and seeded into new culture bottles. The animal cell study protocol was approved by the Animal Ethics Committee of Shanxi University, China (Approval No. SXULL-2021019).
2.3. Assessment of Cytotoxicity
The cell suspension was seeded into a 96-well plate at a density of 1.0 × 104 cells/well and incubated overnight to allow for cell attachment. RES was weighed and dissolved in DMSO to form different concentration solutions. Different concentrations of resveratrol solution were added to the cell culture medium in the dish to keep a final concentration of DMSO less than 0.1% in the culture medium. Then, the cytotoxicity test in the mHSCs after different concentrations of PM2.5 (0, 1, 5, 10, 25, 50, 100, 200, 250, 500 μg/mL) and RES (0, 1, 2.5, 5, 10, 20, 50, 100 and 200 μmol/mL) was performed according to the previous study [17] and the instructions of the CCK8 assay kit (Beyotime Biotechnology, Shanghai, China). In brief, we prepared a mixture solution by combining cell culture medium and CCK-8 solution at a volume ratio of 10:1. Following the exposure period, we removed the culture medium and added 110 µL of the above-mixed solution to each well. After incubating the 96-well plate in a cell incubator for 3 h, we took the plate from the incubator. Then, we measured the absorbance values of the reaction solution at 450 nm using a Multifunctional Microplate Reader (Thermo Scientific Varioskan Flash, Waltham, MA, USA).
2.4. Experimental Grouping
The experimental concentrations of PM2.5 and RES were determined based on the cytotoxicity test by the CCK-8 assay kit. Based on the cell viability results and previous studies [18,19,20], PM2.5 suspensions with 100 μg/mL and RES concentrations with 5 and 20 μmol/mL were selected for subsequent cell experiments.
We divided them into six groups, including the control group (Control, PBS), RES low-dose group (RES/L, 5 μmol/mL), RES high-dose group (RES/H, 20 μmol/mL), PM2.5 group (100 μg/mL), PM2.5 + RES low-dose group (PM2.5 + RES/L), and PM2.5 + RES high-dose group (PM2.5 + RES/H).
2.5. Measurement of ROS, SOD, MDA, LDH and IL-1β Levels in the Cells
mHSCs were seeded in 96-well plates at a density of 1.0 × 104 cells/well. After the cells confluence reached 80–90%, they were treated with Control, RES/L, RES/H, PM2.5, PM2.5 + RES/L and PM2.5 + RES/H suspensions for 24 h. Cell membrane damage was evaluated using the lactic dehydrogenase (LDH) cytotoxicity assay kit (Beyotime Biotechnology, Shanghai, China). The ROS assay kit (Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China) utilized the fluorescent probe DCFH-DA to detect reactive oxygen species (ROS). DCFH-DA is non-fluorescent by itself and, upon entering the cell, is hydrolyzed by intracellular esterases to generate DCFH. ROS within the cell can then oxidize the non-fluorescent DCFH to produce the fluorescent compound DCF. By measuring the fluorescence of DCF, the level of intracellular ROS can be determined. The superoxide dismutase (SOD) activity was determined using the xanthine oxidase method (hydroxylamine method) with a specific SOD assay kit (Nanjing Jiancheng Biological Engineering Research Institute, China). The malondialdehyde (MDA) assay kit (Nanjing Jiancheng Biological Engineering Research Institute, China) employs a colorimetric approach based on the reaction between MDA and thiobarbituric acid (TBA), which yields a red product. We strictly followed the procedures outlined in the kit manual.
Acute inflammatory response was measured using the IL-1β assay kits (Beyotime Institute of Biotechnology, China). All experiment steps were according to the manufacturer’s protocols.
2.6. Western Blot
mHSCs (5 × 105 cells/well) were seeded in 6 cm culture dishes. After treatment with different groups of suspensions for 24 h, we added a protein lysis buffer containing phenylmethanesulfonyl fluoride (PMSF) (100:1). The primary components of the protein lysis buffer include 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, as well as various inhibitors such as sodium pyrophosphate, β-glycerophosphate, EDTA, Na3VO4 and leupeptin. All cells in the culture dishes were scraped off with a cell scraper and then centrifuged at 12,000 rpm for 15 min at 4 °C to collect the supernatant. Following the instructions of the BCA kit, we measured the protein concentration of all samples, normalized the protein samples, added a loading buffer and prepared the protein loading samples. Then, the protein samples were transferred to nitrocellulose membranes via an SDS-PAGE gel experiment. The membrane was blocked with a blocking solution, followed by incubation with primary antibodies specific to SIRT1, NF-κB, NLRP3, Caspase1, α-SMA, Collagen I, Collagen III and β-actin overnight at 4 °C. The next day, the membrane was washed three times with PBST, followed by incubation with fluorescently labeled goat anti-rabbit secondary antibody (Yeasen Biotech, Shanghai, China, 1:20,000) for 1.5 h, and then washed four times with PBST. Finally, protein expression was detected using the Odyssey system. β-actin antibody was purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) The remaining antibodies were all purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China) The primary antibody for β-actin was diluted at a ratio of 1:3000, while the remaining primary antibodies were diluted at 1:100.
2.7. Statistical Analysis
The results were expressed as mean ± SD (n = 4). By using SPSS 19.0 software, the data with homogeneity of variance were evaluated using analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test. p < 0.05 was considered to be statistically significant.
3. Results
3.1. mHSC Viability Results Provide a Dosage Basis of PM2.5 and RES
As shown in Figure 1A, after 24 h of exposure, PM2.5 exhibited significant toxicity to mHSCs at higher concentrations (≥100 μg/mL) than the control group (p < 0.05 or p < 0.01), with no apparent toxicity at lower concentrations (1–50 μg/mL). Based on the results of the CCK8 experiment, PM2.5 suspensions with 100 μg/mL were selected for subsequent cell experiments.
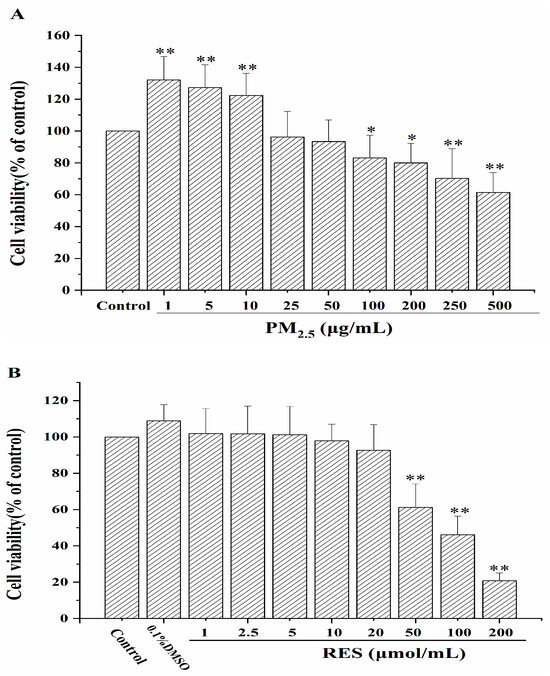
Figure 1.
The effects of PM2.5 and RES on mHSCs cell viability. (A) The impact of PM2.5 at different concentrations on mHSCs viability. (B) The influence of RES at various concentrations on mHSCs viability (n = 4, compared with control group, * p < 0.05, ** p < 0.01).
From Figure 1B, after treating mHSCs with different concentrations of RES (1, 2.5, 5, 10, 20, 50, 100, 200 μmol/mL) for 24 h, RES at concentrations below 20 μmol/mL exhibited no cytotoxic effects on mHSCs. In this study, we selected RES concentrations of 5 and 20 μmol/mL for subsequent experiments.
3.2. Effect of RES on Oxidative Stress and Cytotoxicity Induced by PM2.5
From Figure 2A, compared to the control group, the PM2.5 group showed a significant increase in cytoplasmic LDH activity (p < 0.01). Following RES intervention, there was a considerable reduction in cytoplasmic LDH release compared to the PM2.5 group, with higher concentrations of RES exhibiting a greater attenuation of PM2.5-induced LDH activity enhancement.
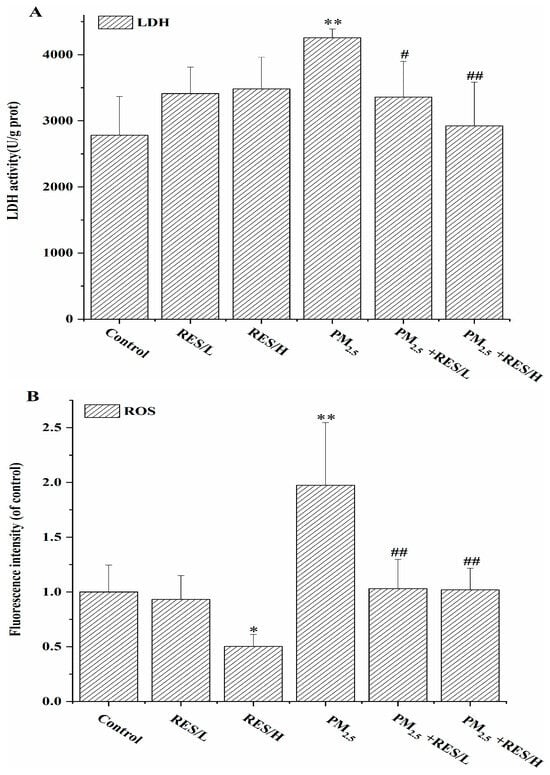
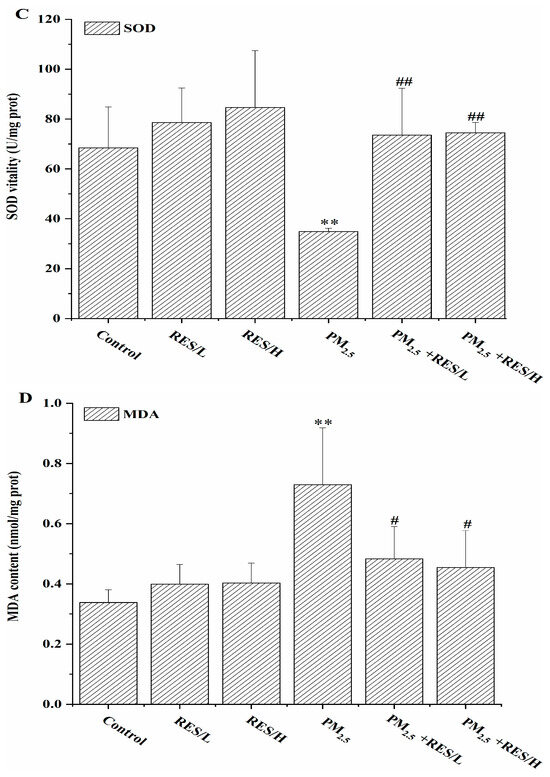
Figure 2.
The effects of RES on PM2.5-induced alterations in cell LDH and oxidative stress. (A) LDH activity, (B) ROS levels, (C) SOD activity, (D) MDA content (n = 4, compared with the control group, * p < 0.05, ** p < 0.01; compared with PM2.5 group, # p < 0.05, ## p < 0.01).
As shown in Figure 2B–D, compared to the control group, the PM2.5 group exhibited significantly increased intracellular ROS levels and MDA content, with significantly decreased SOD activity, suggesting that PM2.5 exposure causes cellular oxidative damage. However, with RES intervention, intracellular ROS levels and MDA content were significantly reduced compared to the PM2.5 group (p < 0.05 or p < 0.01), and SOD levels were significantly increased (p < 0.01), indicating that RES can reduce ROS generated by PM2.5 and enhance cellular anti-oxidant enzyme activity, improving the cell’s anti-oxidant capacity.
3.3. Effect of RES on PM2.5-Induced Fibrosis Biomarker Level Change in mHSCs
The Western blot results showed that after PM2.5 exposure, the expression of liver fibrosis marker proteins α-SMA, Collagen I and Collagen III significantly increased (Figure 3, p < 0.01). Compared to the PM2.5 group, RES intervention under two concentrations significantly downregulated the expression of three fibrosis-related proteins (p < 0.05).
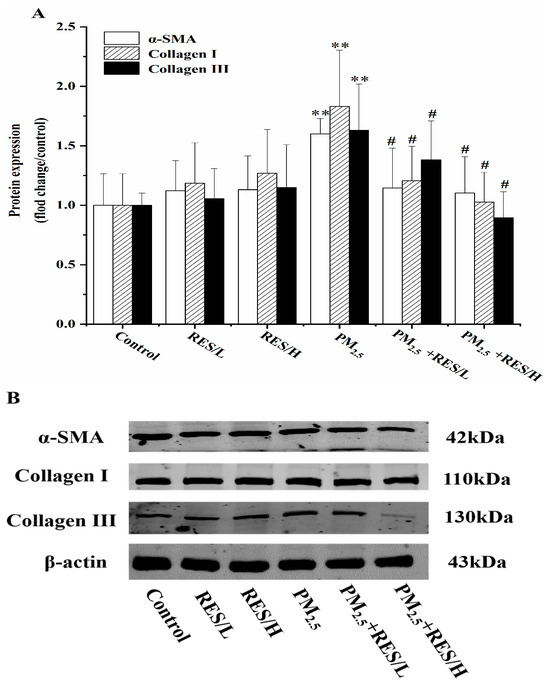
Figure 3.
RES can inhibit PM2.5-induced fibrosis in mHSCs. (A) Protein expression (fold change/control) of fibrosis-related protein expression(/control) and (B) protein bands of α-SMA, Collagen I and Collagen III (n = 4, compared with the control group, ** p < 0.01; compared with PM2.5 group, # p < 0.05).
3.4. The Effects of RES Activate SIRT1 on PM2.5-Induced NF-κB/NLRP3 Pathway
Compared to the control group, PM2.5 suppresses the protein expression of SIRT1 (Figure 4A,D, p < 0.05), while high-dose RES significantly increases SIRT1 expression (p < 0.01). After the addition of RES (5 and 20 µmol/mL), SIRT1 expression significantly rises compared to the PM2.5 group (p < 0.05 or p < 0.01). These results indicate that RES can activate SIRT1, playing an anti-oxidative role during PM2.5-induced oxidative damage in the body.
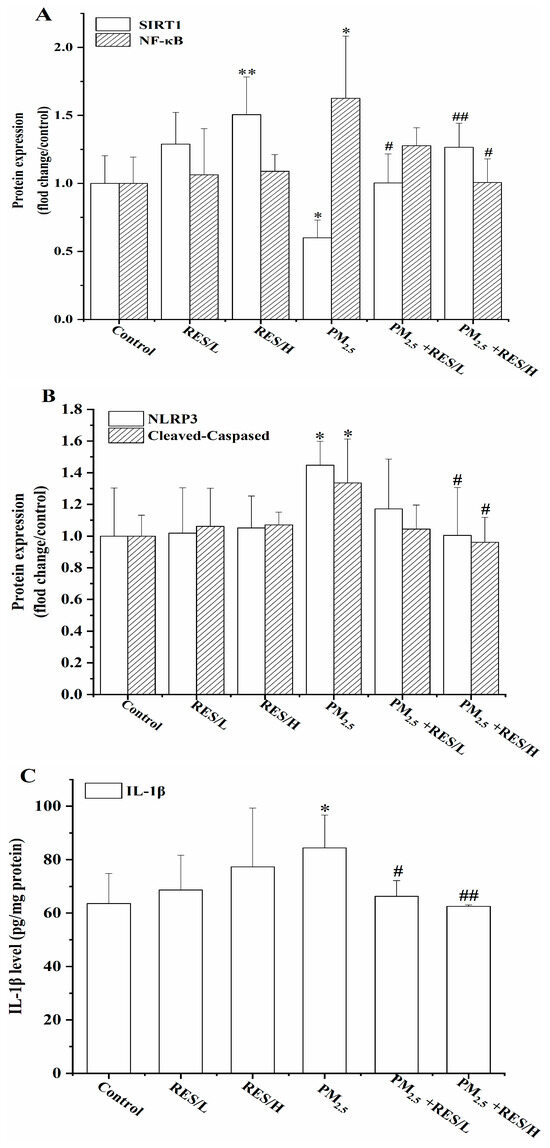
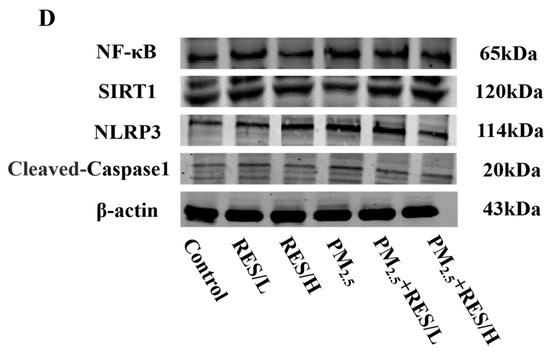
Figure 4.
RES can activate SIRT1 to inhibit PM2.5-induced activation of NF-κB/NLRP3. (A) Protein expression (fold change/control) of SIRT1 and NF-κB, (B) protein expression (fold change/control) of NLPR3, Caspase1, (C) IL-1β level and (D) protein bands of SIRT1, NF-κB, NLPR3 and Caspase1 (n = 4, compared with control group, * p < 0.05, ** p < 0.01; compared with PM2.5 group, # p < 0.05, ## p < 0.01).
Figure 4 also shows that PM2.5 exposure leads to the upregulation of NF-κB, NLRP3, Cleaved-Caspase1 and IL-1β protein expression, significantly higher than the control group (p < 0.05). However, after RES intervention (20 µmol/mL), the expression of NF-κB, NLRP3 and Cleaved-Caspase1 significantly reduced in mHSCs relative to the PM2.5 group (p < 0.05 or p < 0.01). Two concentrations of RES treatment significantly decreased IL-1β protein expression in the cells compared with the PM2.5 group (p < 0.05 or p < 0.01).
4. Discussion
Atmospheric PM2.5 is widely distributed in the environment and harms human health. The liver is an active organ central to metabolism and detoxification for exogenous chemicals. Many research studies indicate that PM2.5 has toxic effects on the liver [7,8], including triggering liver cancer and NAFLD. The toxicological mechanisms involve liver pathology injury, inflammation, oxidative stress, liver fibrosis and abnormal lipid metabolism induced by prolonged exposure to PM2.5 [4,6,21]. Finding active substances or protective agents to reduce liver damage caused by PM2.5 pollution and protect people’s health is significant. In this study, we used the mHSC cell model to investigate the liver fibrosis mechanism of PM2.5 by measuring oxidative stress biomarkers, inflammatory factors and fibrosis-related genes. We also explored the protective effects of RES against liver fibrosis in mHSCs caused by PM2.5.
First, our study demonstrates that PM2.5 exhibited significant cytotoxicity and oxidative stress to mHSCs when the concentration exceeds 100 μg/mL, along with decreased cell survival, elevated ROS and LDH levels and changed oxidative stress biomarker levels. LDH release is a mark when the plasma membrane is damaged. Interestingly, RES could inhibit such responses incurred by PM2.5.
Excessively generated ROS can induce oxidative stress and directly impair cell membranes, instigating an irreversible cell demise process. MDA is a product of LPO, while SOD is one of the anti-oxidant enzymes. They are important biomarkers of oxidative stress [22]. Levels of SOD and MDA in liver tissue can reflect the extent of lipid peroxidation and the ability to eliminate free radicals [23]. As the primary end product of lipid peroxidation, MDA can directly activate Kupffer cells and hepatic stellate cells, ultimately leading to liver cell damage. SOD is an essential anti-oxidant enzyme capable of reducing the attack of oxygen free radicals on cells [24]. Oxidative stress, a critical intermediary mechanism for such detrimental effects, is pivotal in the pathogenesis of liver fibrosis triggered by PM2.5 exposure. Qiu et al. found that PM2.5 may induce mitochondrial autophagy by increasing ROS and activating the PINK1/Parkin signaling pathway, which activates HSCs and contributes to liver fibrosis [5]. Xin et al. found that exposure to PM2.5 for eight weeks enhanced oxidative stress (SOD and MDA) in the liver of rats [25]. These research findings support our results.
As a natural polyphenol plant compound, RES possesses anti-inflammatory properties, protects cardiovascular and cerebral vessels and exhibits an anti-fibrotic effect [26]. Studies have found that RES can scavenge ROS produced by the body, demonstrating anti-oxidant capability and protective effects against damage to multiple organs [12,27]. Ahmad et al. found a significant restoration of levels of oxidative damage biomarkers (MDA, SOD, protein carbonyls and membrane-bound ATPases) to inhibit HSC activation in N’-nitrosodimethylamine-induced liver fibrosis [28]. Bujanda et al. demonstrated that RES significantly decreased MDA and nitric oxide synthase levels in the rats, while increasing the activities of SOD, glutathione peroxidase (GSH-Px) and catalase, thereby greatly lessening NAFLD [29]. Polydatin, a glucoside form of RES, can inhibit the production of 4-Hydroxynonenal (4-HNE) in the liver and the expression of NADPH oxidase 4 (NOX4). It alleviates chronic liver injury and fibrosis by suppressing oxidative stress and inflammation [30]. We found that after PM2.5 stimulation, intracellular ROS levels and MDA content increased while SOD activity decreased. Of note, RES intervention alleviated the cytotoxic effect to a certain extent and combatted lipid peroxidation, exerting a positive anti-oxidant function.
Second, we focused on the PM2.5-caused liver fibrosis in mHSCs and the RES-induced anti-fibrotic effect. Liver fibrosis refers to the process in which the liver excessively produces an extracellular matrix after chronic injury, destroying normal liver tissue structure and forming fibrotic scars [31]. Although liver fibrosis is reversible, without timely intervention or treatment, liver fibrosis will gradually develop into liver cirrhosis with a high mortality rate [32]. Liver fibrosis is a reversible damage repair process based on the transdifferentiation of human HSCs [33]. The expression of α-SMA is intimately linked to the fibrotic disease process [34]. In the context of liver fibrosis, quiescent HSCs respond to injury cues by undergoing a marked phenotypic shift towards a myofibroblast-like state [33]. During this transition, HSCs become highly activated, producing an abundance of extracellular matrix components and significantly upregulating the expression of both α-SMA and Collagen I [35]. The excessive generation and deposition of these two molecules form the principal scaffold of the fibrotic scar tissue. The expression levels and deposition of Collagen I, due to their strong correlation with the extent of fibrosis and the pace of disease progression, are widely employed as critical yardsticks for gauging the depth and velocity of the fibrotic process [35,36]. Concurrently, Collagen III assumes a non-negligible role in the early stages of liver fibrosis. The dynamic changes in Collagen III expression serve as crucial indicators of the initiation and evolving trajectory of the fibrotic process [37]. Taken together, the expression regulation of α-SMA, Collagen I and Collagen III collectively constitutes pivotal biological markers within the fibrotic pathology of the liver. Their dynamic fluctuations contribute to a deeper understanding of fibrosis development mechanisms, offering valuable leads for diagnosis and therapeutic strategies.
Activated human HSCs differentiate into fibroblasts or myofibroblasts, expressing a large amount of α-SMA [38]. Meanwhile, the proliferative and differentiating abilities of human HSCs are enhanced, producing large amounts of collagen, resulting in imbalanced degradation and deposition of the extracellular matrix. Fibrous tissue proliferation and deposition, mainly composed of type I and III collagen fibers in the portal vein area and hepatic lobules, trigger the occurrence and development of liver fibrosis [33,38]. Previous research demonstrated that exposure to PM2.5 upregulated myofibroblast markers Collagen I and α-SMA, activating HSCs and causing liver fibrosis [5]. An in vivo study revealed the anti-fibrotic effect of RES on dimethylnitrosamine (DMN)-induced liver fibrosis in rats due to its anti-oxidant properties (glutathione levels elevation and MDA content decline) [39]. In this study, PM2.5 exposure activated the protein expression of fibrotic markers (α-SMA, Collagen I and Collagen III). However, after RES intervention, the protein expression of fibrotic markers decreased. It suggested that RES intervention can significantly alleviate PM2.5-induced liver cell fibrosis.
Third, we have investigated the inflammatory factor changes in mHSCs exposure to PM2.5 and RES in this study. The NLRP3 inflammasome is a large multimeric protein complex composed of the NOD-like receptor NLRP3, the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC) and the effector molecule pro-Caspase1 [40]. It plays a key role in liver injury and the development of fibrosis [41]. The inflammasome can participate in the formation of liver fibrosis by activating hepatic stellate cells [42]. The NLRP3 inflammasome is an upstream signaling factor that activates Caspase-1 and controls IL-1β release. Various external stimuli can activate the NLRP3 inflammasome, promoting the activation of pro-Caspase1 and the release of downstream factors like IL-1β [43]. IL-1β is a major pro-inflammatory component produced by activation of NLRP3 inflammasome. Our research showed that NLRP3 was activated in mHSCs by PM2.5, along with elevated levels of Caspase1 and IL-1β, which confirmed that NLRP3–Caspase-1–IL-1β pathway activation was involved in the occurrence and development of inflammation and Caspase1 and IL-1β expression mediated by NLRP3 was related to fibrosis in liver cells [44]. A study aimed at elucidating the potential mechanisms underlying liver inflammation during type 2 diabetes mellitus (T2DM) established a diabetic rat model through a 20-week high-fructose diet [45]. The results demonstrated a significant upregulation of NLRP3 inflammasome components in the livers of diabetic rats, with histopathological examination revealing hepatic fibrosis. Treatment with RES significantly attenuated the expression of the NLRP3 inflammasome. This indicated that RES can alleviate liver inflammation in diabetic rats by decreasing NLRP3 inflammasome levels [45]. Another study utilized carbon tetrachloride (CCL4) to establish a liver fibrosis model in BALB/c mice to investigate whether resveratrol exerts regulatory effects on liver fibrosis in these mice through the NLRP3 inflammasome. The results showed that resveratrol can modulate CCL4-induced liver fibrosis by influencing the activation of the NLRP3 inflammasome and its downstream gene expression [46]. In this study, with RES intervention, NLRP3, Caspase1 and IL-1β expression decreased, suggesting that RES can alleviate liver fibrosis responses in mHSCs by inhibiting inflammation mediated by the NLRP3–Caspase-1–IL-1β signaling pathway.
Activation of the NF-κB/NLRP3 signaling pathway is responsible for oxidative stress, inflammatory response and liver cell fibrosis [47]. NF-κB, as a crucial nuclear transcription factor, can activate NLRP3 and induce the transcriptional expression of NLRP3 and pro-IL-1β [48]. NF-κB plays a vital role in the development of liver fibrosis [49] and it regulates pro-inflammatory cytokine (IL-6, IL-1β and TNF-α) levels in livers of NAFLD model rats [50]. Furthermore, a review paper has shown that RES can promote the expression of anti-oxidant enzymes (SOD, CAT and glutathione) through the NF-κB pathway, enhancing liver anti-oxidant and anti-inflammatory capacity, reducing oxidative stress and thus effectively alleviating liver fibrosis [51]. In this study, RES intervention could significantly downregulate the expression of NF-κB/NLRP3 signaling pathway-related proteins and increase SOD activity, suggesting that RES can reduce the expression of fibrosis-related genes (α-SMA, Collagen I and Collagen III) by inhibiting oxidative stress and inflammation mediated by NF-κB/NLRP3 signaling pathway.
Finally, our study showed that PM2.5 regulates NF-κB expression via SIRT1, whereas SIRT1 exerts protective effects against PM2.5-induced liver damage in mHSCs. SIRT1 plays a vital role in the regulation of NF-kB. As a pivotal regulatory factor, SIRT1 not only plays a significant role in modulating inflammatory responses within the organism but also intricately engages in vital physiological processes governing energy balance and metabolic homeostasis, such as glucose and lipid metabolism [52]. In the process of regulating inflammatory responses, one central aspect of SIRT1′s mechanism of action lies in its effective suppression of inflammation-related signaling pathways. SIRT1′s inhibitory effect on the NF-κB signaling cascade highlights the critical juncture it occupies within the network of inflammatory control. A study revealed that allyl isothiocyanate significantly ameliorates hepatic inflammation by activating SIRT1 and inhibiting the NF-κB pathway [53]. Peng et al.’s study found that SIRT1 upregulating in chronic obstructive pulmonary disease can inhibit the activation of the NLRP3 inflammasome, thereby suppressing inflammatory responses [54]. Moreover, SIRT1 can regulate acetylation modification of the NF-κB p65 subunit, reducing NF-κB expression and modulating the progression of inflammatory responses [55]. Yan et al. reported that RES can inhibit the activity of the NF-κB pathway in a liver fibrosis model, improving fibrosis conditions [54]. Research indicates that RES targets a protein family of Sirtuins (SIRTs) [56]. As an agonist of SIRT1, it activates SIRT1, which modulates the activity of anti-oxidant enzymes by negatively regulating the expression of nuclear factor-κB (NF-κB), thereby alleviating oxidative stress and inflammation in the liver [57]. This may represent a key mechanism by which RES mitigates liver injury induced by PM2.5. In this study, RES relieved PM2.5-induced inflammation and the expression of fibrosis-related genes in mHSCs in vitro by activating SIRT1 activation and inhibiting NF-κB signaling pathways, consistent with the previous research [58]. It suggests that SIRT1 exerts a positive regulating role in NF-κB signaling pathways.
In a mHSC model, we proposed the possible mechanisms of RES-relieved liver fibrosis against PM2.5 through the SIRT1–NF-κB–NLRP3–Caspase1–IL-1β pathway. After exposure to PM2.5, ROS and MDA levels increased while SOD activity decreased in mHSCs. NF-κB, NLRP3, Caspase1 and IL-1β expressions were upregulated, whereas SIRT1 was downregulated. RES inhibited the SIRT1/NF-κB pathway, reducing oxidative stress and inflammation, and then regulated the NLRP3 pathway, decreasing the expression of fibrosis-related genes in mHSCs.
RES’s application has some limitations due to its low bioavailability [59]. Scientists are also actively exploring strategies to enhance RES’s bioavailability, focusing on synergetic interactions, pro-drug designs and formulation improvements to increase its bioaccessibility [59]. This will facilitate the better application of resveratrol in treating various diseases. Furthermore, reinforcing fundamental research on resveratrol, such as investigating the optimal dosage and elucidating the mechanisms behind its anti-oxidative, anti-inflammatory and anti-fibrotic effects on the liver, is essential and will actively promote its utilization. Researchers should drive the translation of resveratrol from basic research to clinical application, furnishing ample and compelling clinical evidence for its efficacy in treating liver diseases.
5. Conclusions
The present study indicated that PM2.5 significantly increased the expression of fibrotic proteins and oxidative stress in the mHSCs. At the same time, RES downregulated the expression of fibrosis-related proteins, reduced ROS levels, decreased lipid peroxidation and increased the activity of anti-oxidant enzymes. Our study showed that RES is potentially protective against liver injury induced by PM2.5, and its regulatory mechanism is associated with the SIRT1/NF-κB/NLRP3 pathway. However, the specific mechanism of SIRT1 on NLRP3 and how NLRP3 activates downstream factors to regulate HSC cell fibrosis require further in-depth investigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/atmos15050588/s1, Figure S1: A flow chart for PM2.5 preparation.
Author Contributions
Conceptualization, R.L. and M.Z.; methodology, M.Z. and S.C.; validation, L.B., S.C. and W.C.; formal analysis, M.Z.; data curation, L.B., S.C. and W.C.; writing—original draft preparation, S.C.; writing—review and editing, R.L.; supervision, R.L.; project administration, R.L.; funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (22176116).
Institutional Review Board Statement
The animal cell study protocol was approved by the Animal Ethics Committee of Shanxi University, China (Approval No. SXULL-2021019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and cardiovascular health risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin suppresses PM2.5-induced neuroinflammation by regulating Akt Phosphorylation in BV-2 Microglial cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, Y.J.; Huang, H.; Cui, L.X.; Li, F.Q. PM2.5 exposure induces reproductive injury through IRE1/JNK/autophagy signaling in male rats. Ecotoxicol. Environ. Saf. 2021, 211, 111924. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Ge, C.X.; Qin, Y.T.; Gu, T.T.; Lou, D.S.; Li, Q.; Hu, L.F.; Feng, J.; Huang, P.; Tan, J. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 2019, 130, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.N.; Wang, G.H.; Zhou, F.; Hao, J.J.; Tian, L.; Guan, L.F.; Geng, X.K.; Ding, Y.C.; Wu, H.W.; Zhang, K.Z. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 2019, 167, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.X.; Tan, J.; Zhong, S.Y.; Lai, L.L.; Chen, G.; Zhao, J.J.; Yi, C.; Wang, L.Y.; Zhou, L.W.; Tang, T.T.; et al. Nrf2 mitigates prolonged PM2.5 exposure-triggered liver inflammation by positively regulating SIKE activity: Protection by Juglanin. Redox Biol. 2020, 36, 101645. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Xia, H.; Zhao, Q.; Sun, G.J.; Cai, Y.Y. Long-term exposure to fine particulate matter and the risk of chronic liver diseases: A Meta-Analysis of observational studies. Int. J. Environ. Res. Public Health 2022, 19, 10305. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.F.; Sun, X.; Chen, X.; Wang, N.N. The influence of PM2.5 exposure on non-alcoholic fatty liver disease. Life Sci. 2021, 270, 119135. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, Q.; Zhou, Q.; Cao, W.; Yu, S.; Zhan, S.; Sun, F. Long-term exposure to air pollution, habitual physical activity and risk of non-alcoholic fatty liver disease: A prospective cohort study. Ecotoxicol. Environ. Saf. 2022, 15, 113440. [Google Scholar] [CrossRef]
- Jian, T.Y.; Ding, X.Q.; Wu, Y.X.; Ren, B.R.; Li, W.L.; Lv, H.; Chen, J. Hepatoprotective effect of loquat leaf flavonoids in PM2.5-induced non-alcoholic fatty liver disease via regulation of IRs-1/Akt and CYP2E1/JNK Pathways. Int. J. Mol. Sci. 2018, 19, 3005. [Google Scholar] [CrossRef]
- Du, C.; Ren, Y.J.; Wang, Q.W.; Jin, L. Synthesis and Anti-tumor Activities of resveratrol Derivatives on Cervical Cancer HeLa Cells. Chin. J. Org. Chem. 2013, 33, 1279–1283. [Google Scholar] [CrossRef][Green Version]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J.H. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Kumar, V.; Singh, A.K.; Kashyap, M.P.; Khanna, V.K.; Siddiqui, M.A.; Pant, A.B. Trans-Resveratrol protects ischemic PC12 Cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem. Neurosci. 2013, 4, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.W.; Qin, C.X.; Woodman, O.L. Effects of resveratrol and flavonols on cardiovascular function: Physiological mechanisms. BioFactors 2010, 36, 350–359. [Google Scholar] [CrossRef]
- Bishayee, A.; Darvesh, A.S.; Politis, T.; McGory, R. Resveratrol and liver disease: From bench to bedside and community. Liver Int. 2010, 30, 1103–1114. [Google Scholar] [CrossRef]
- Vairappan, B.; Sundhar, M.; Srinivas, B.H. Resveratrol restores neuronal tight junction proteins through correction of ammonia and inflammation in CCl4-induced cirrhotic mice. Mol. Neurobiol. 2019, 56, 4718–4729. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, L.; Li, X.; Liu, R.; Zhang, L.; Xu, Y. REDD1 (regulated in development and DNA damage-1)/autophagy inhibition ameliorates fine particulate matter (PM2.5) -induced inflammation and apoptosis in BEAS-2B cells. Bioengineered 2021, 12, 1403–1414. [Google Scholar] [CrossRef]
- Jin, X.T.; Su, R.J.; Li, R.J.; Song, L.; Chen, M.L.; Cheng, L.; Li, Z.Y. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Li, Z.P.; Yue, J.W.; Xu, M.; Zhang, Y.H.; Yung, K.K.L.; Li, R.J. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef]
- He, Y.G.; Zhang, Y.D.; Zhang, G.B.; Li, L.; He, Y.F.; Xi, J.K.; Zheng, H. Role of zinc in resveratrol-induced mitochondrial cardioprotection. Chin. J. New Drugs 2016, 25, 928–932+948. [Google Scholar]
- Jeong, S.; Park, S.A.; Park, I.; Kim, P.; Cho, N.H.; Hyun, J.W.; Hyun, Y.M. PM2.5 exposure in the respiratory system induces distinct inflammatory signaling in the lung and the liver of mice. J. Immunol. Res. 2019, 2019, 3486841. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, J.J.; Mo, W.B. Effects of dendrobium officinale flavonoid on oxidative stress and autophagy in the liver of an exhaustive exercise rat model. Chin. J. Tissue Eng. Res. 2022, 26, 3212–3219. [Google Scholar]
- Ma, W.W.; Zhang, S.S.; Li, Y.; Chen, T.S.; Yang, Q.; Feng, X. Adiponectin alleviates non-alcoholic fatty liver injury via regulating oxidative stress in liver cells. Minerva Med. 2022, 113, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.P.; Qin, L.; Nong, R.N.; Liu, D.H.; Wang, J.J.; Chen, Y.; Wang, X.Y. Effects of Isodon ternifolia on NLRP3/Caspase-1/GSDMD Signaling Pathway in rats with hepatic fibrosis induced by CCl4. Pharmacol. Clin. Chin. Mater. Med. 2021, 37, 96–101. [Google Scholar]
- Xin, S.; Qu, J.; Xu, N.; Xu, B. PM2.5 inhalation aggravates inflammation, oxidative stress, and apoptosis in nonalcoholic fatty liver disease. Environ. Dis. 2019, 4, 62–68. [Google Scholar]
- Yao, Q.C.; Wu, Q.C.; Xu, X.Y.; Xing, Y.X.; Liang, J.; Lin, Q.Q.; Huang, M.Q.; Chen, Y.L.; Lin, B.; Chen, W.F. Resveratrol ameliorates systemic sclerosis via suppression of fibrosis and inflammation through activation of SIRT1/mTOR signaling. Drug Des. Dev. Ther. 2020, 14, 5337–5348. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, R. Resveratrol mitigate structural changes and hepatic stellate cell activation in N’-nitrosodimethylamine-induced liver fibrosis via restraining oxidative damage. Chem. Biol. Interact. 2014, 221, 1–12. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef]
- Li, R.; Li, J.; Huang, Y.; Li, H.; Yan, S.; Lin, J.; Chen, Y.; Wu, L.; Liu, B.; Wang, G.; et al. Polydatin attenuates diet-induced nonalcoholic steatohepatitis and fibrosis in mice. Int. J. Biol. Sci. 2018, 14, 1411–1425. [Google Scholar] [CrossRef]
- Campana, L.; Iredale, J.P. Regression of Liver Fibrosis. Semin. Liver Dis. 2017, 37, 1–10. [Google Scholar] [PubMed]
- Lin, L.; Zhou, F.; Shen, S.; Zhang, T. Fighting liver fibrosis with naturally occurring antioxidants. Planta Med. 2018, 84, 1318–1333. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y.J. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Chen, H.; Yuan, Q.; Wang, J.; Niu, M.; Hou, L.; Gu, J.; Zhang, J. MyD88 in hepatic stellate cells enhances liver fibrosis via promoting macrophage M1 polarization. Cell Death Dis. 2022, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Cao, C.; Hu, X.; Du, K.; Zhang, J.; Li, M.; Li, B.; Lin, H.; Zhang, A.; Li, Y.; et al. Kaempferol attenuates carbon tetrachloride (CCl4)-induced hepatic fibrosis by promoting ASIC1a degradation and suppression of the ASIC1a-mediated ERS. Phytomedicine 2023, 121, 155125. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Xie, J.; Jiang, X.; Xiao, B.; Hu, X.; Xiang, J. Aspirin attenuates liver fibrosis by suppressing TGF-β1/Smad signaling. Mol. Med. Rep. 2022, 25, 181. [Google Scholar] [CrossRef]
- Attallah, A.M.; Mosa, T.E.; Omran, M.M.; Abo-Zeid, M.M.; El-Dosoky, I.; Shaker, Y.M. Immunodetection of collagen types I, II, III, and IV for differentiation of liver fibrosis stages in patients with chronic HCV. J. Immunoass. Immunochem. 2007, 28, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 91491. [Google Scholar] [CrossRef]
- Abdu, S.B.; Al-Bogami, F.M. Influence of resveratrol on liver fibrosis induced by dimethylnitrosamine in male rats. Saudi J. Biol. Sci. 2019, 26, 201–209. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef]
- Wu, X.Q.; Dong, L.; Lin, X.H.; Li, J. Relevance of the NLRP3 inflammasome in the pathogenesis of chronic liver disease. Front. Immunol. 2017, 8, 1728. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Ghani, A.; Mehal, W.Z. Inflammasome biology in fibrogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Wang, Z.H.; Liu, X.; Xiao, H.; Liu, Y.C.; Wang, J.Q.; Chen, C.L.; Wang, X.; Liu, W.; Xiang, Z. Therapeutic effect of Yiyi Fuzi Baijiang formula on TNBS-induced ulcerative colitis via metabolism and Th17/Treg cell balance. J. Ethnopharmacol. 2023, 309, 116301. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Wei, W. Role of NLRP3/Caspase-1/IL-1β signaling pathway in liver fibrosis. Chin. Arch. Tradit. Chin. Med. 2022, 40, 75–79. [Google Scholar]
- Rai, R.C.; Bagul, P.K.; Banerjee, S.K. NLRP3 inflammasome drives inflammation in high fructose fed diabetic rat liver: Effect of resveratrol and metformin. Life Sci. 2020, 253, 117727. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Resveratrol Regulates NLRP3 Inflammasome and Its Role in Liver Fibrosis Mice. Master’s Thesis, Guilin Medical University, Guilin, China, 2021. [Google Scholar]
- Wang, W.; Hu, C.G.; Liang, W.L. Study on Inhibitory Effect of Danggui Shaoyao San combined with TLR4 inhibitor on rat liver fibrosis and regulation of NF-κB/NLRP3 pathway. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Med. 2023, 25, 1147–1154. [Google Scholar]
- Scheiblich, H.; Schlütter, A.; Golenbock, D.T.; Latz, E.; Martinez-Martinez, P.; Heneka, M.T. Activation of the NLRP3 inflammasome in microglia: The role of ceramide. J. Neurochem. 2017, 143, 534–550. [Google Scholar] [CrossRef]
- Huo, S.M.; Li, B.; Du, J.Y.; Zhang, X.L.; Zhang, J.; Wang, Q.; Song, M.; Li, Y. Dibutyl phthalate induces liver fibrosis via p38MAPK/NF-κB/NLRP3-mediated pyroptosis. Sci. Total Environ. 2023, 897, 165500. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yan, X.; Zhu, L.; Lin, M.; Lyu, D.; Liao, J.; Chen, F. Mechanic study of Qushi Kaiyu decoction on non-alcoholic fatty liver disease model rats based on the inhibition of TLR4/NF-ĸB pathway. TMR Integr. Med. 2023, 7, e23019. [Google Scholar] [CrossRef]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The Role of resveratrol in liver disease: A comprehensive review from in vitro to clinical trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Y.L.; Wu, Y.C. The role of sirt1 in ischemic stroke: Pathogenesis and therapeutic strategies. Front. Neurosci. 2018, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, J.; Wan, X.Y.; Yi, C.; Xu, C.F.; Feng, Z.M.; Zeng, H.; Lin, Y.M.; Ma, H.; Xu, P.; et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J. Gastroenterol. 2019, 25, 5120–5133. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.Y.; Zhang, W.X.; Qiao, J.F.; He, B.M. Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1β in rats with COPD. Int. Immunopharmacol. 2018, 62, 23–28. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Yao, H.W.; McBurney, M.W.; Gu, W.; Guarente, L.; Rahman, I.; Brookes, P.S. SIRT1-mediated acute cardioprotection. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1506. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Chhonker, S.K.; Naik, R.A.; Koiri, R.K. Modulation of antioxidant enzymes, SIRT1 and NF-κB by resveratrol and nicotinamide in alcohol-aflatoxin B1-induced hepatocellular carcinoma. J. Biochem. Mol. Toxicol. 2021, 35, e22625. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.D.; Jiang, H.X.; Luo, W.; Hu, B.L.; Yu, B.; Li, F.; Fu, Y.J. Resveratrol can improve liver fibrosis by inhibiting the NF-κB pathway in liver macrophages. Chin. J. Gastroenterol. Hepatol. 2020, 29, 576–580. [Google Scholar]
- Shu, X.H. Resveratrol and its bioavailability. J. Dalian Med. Univ. 2018, 40, 193–197. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).