Seasonal Variation in Short-Term Ambient Air Pollutants and ST-Elevation Myocardial Infarction Admissions: An Innovative Exploration of Air Pollution’s Health Consequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographic Study Region
2.2. Demographic Characteristics of Patients
2.3. Air Pollutants and Meteorological Data
2.4. Statistical Methods
3. Results
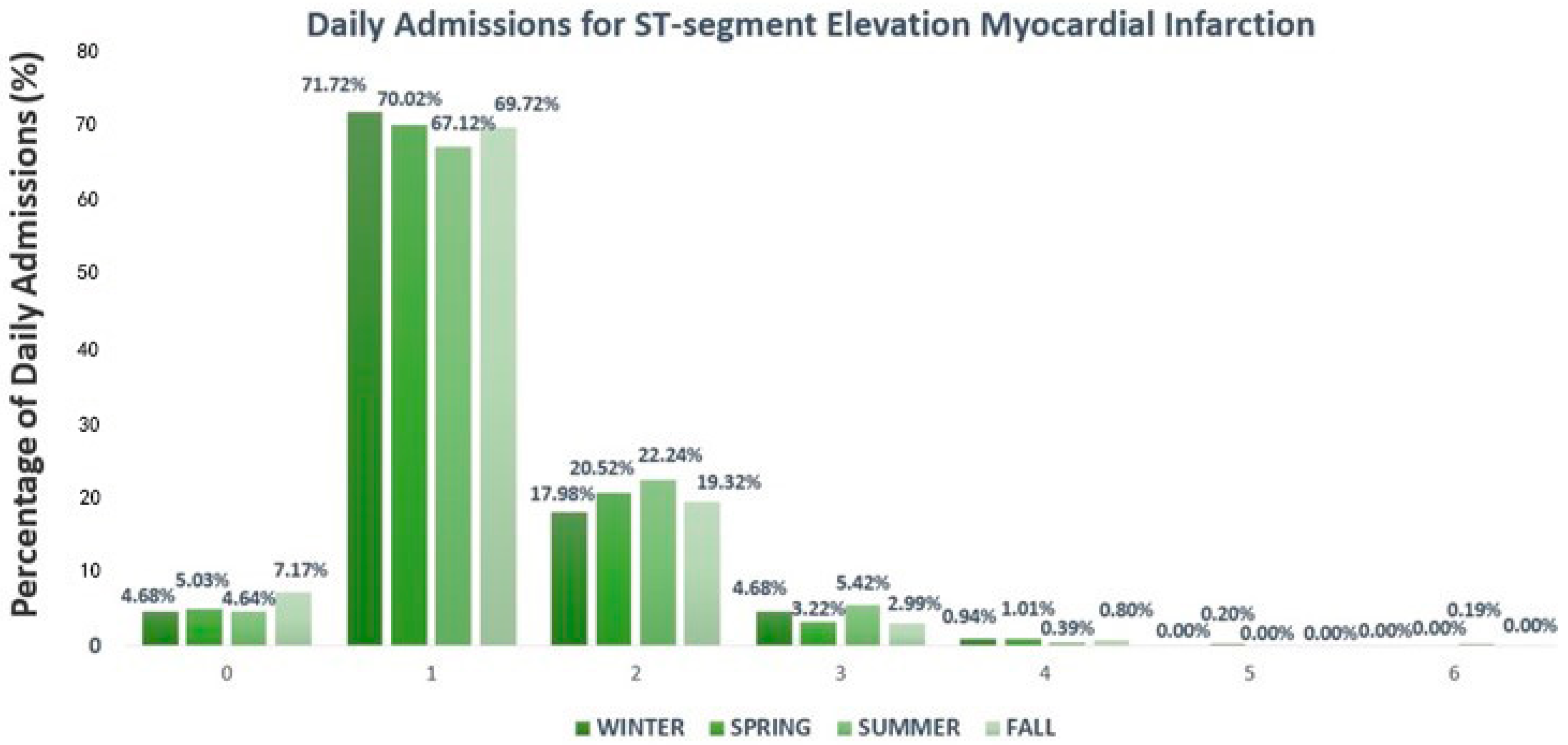
3.1. Clinical and Demographic Features of Patients Hospitalized for ST-Segment Elevation Acute Coronary Syndrome
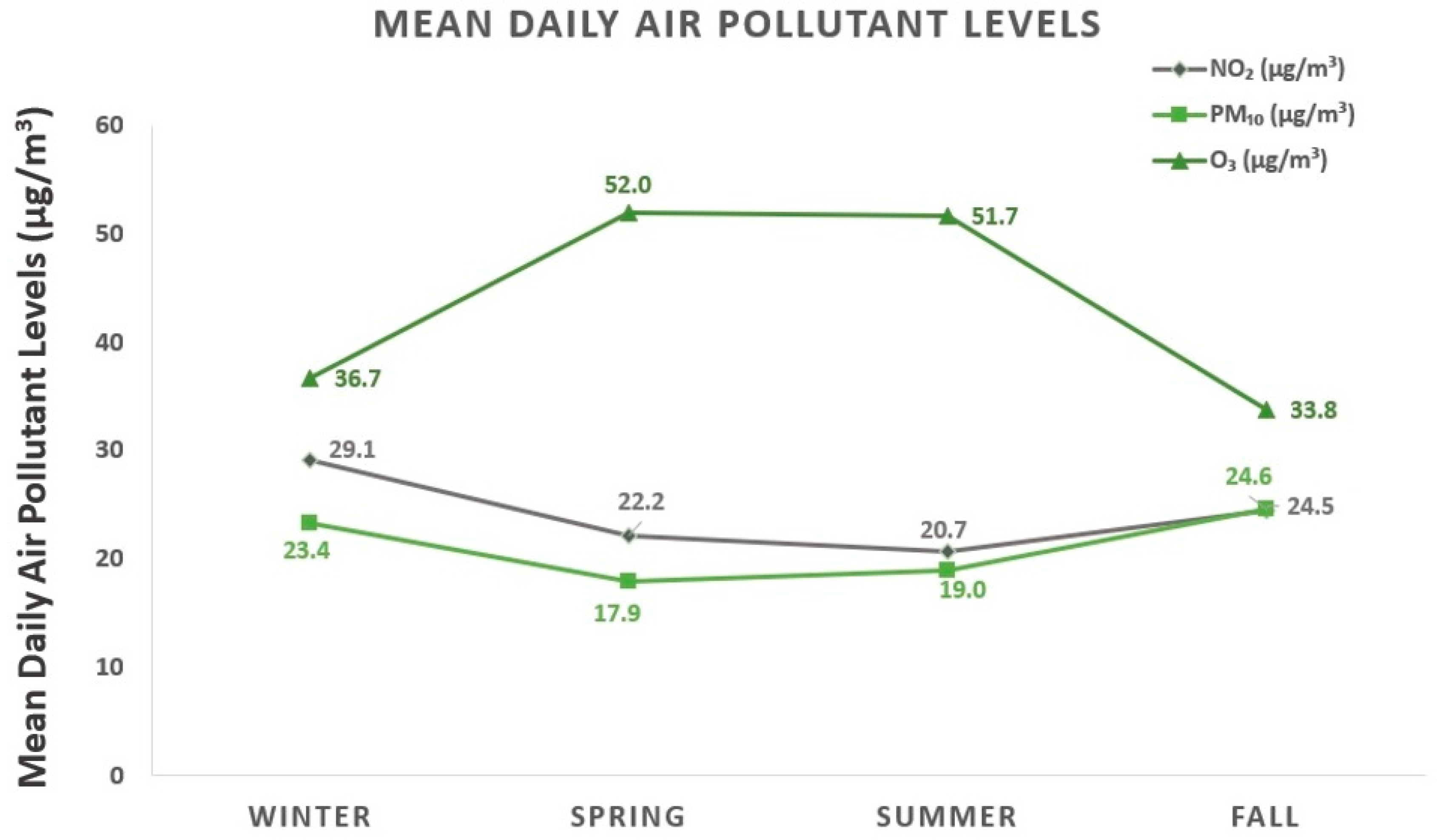
3.2. Characteristics of Air Pollutants and Weather Factors
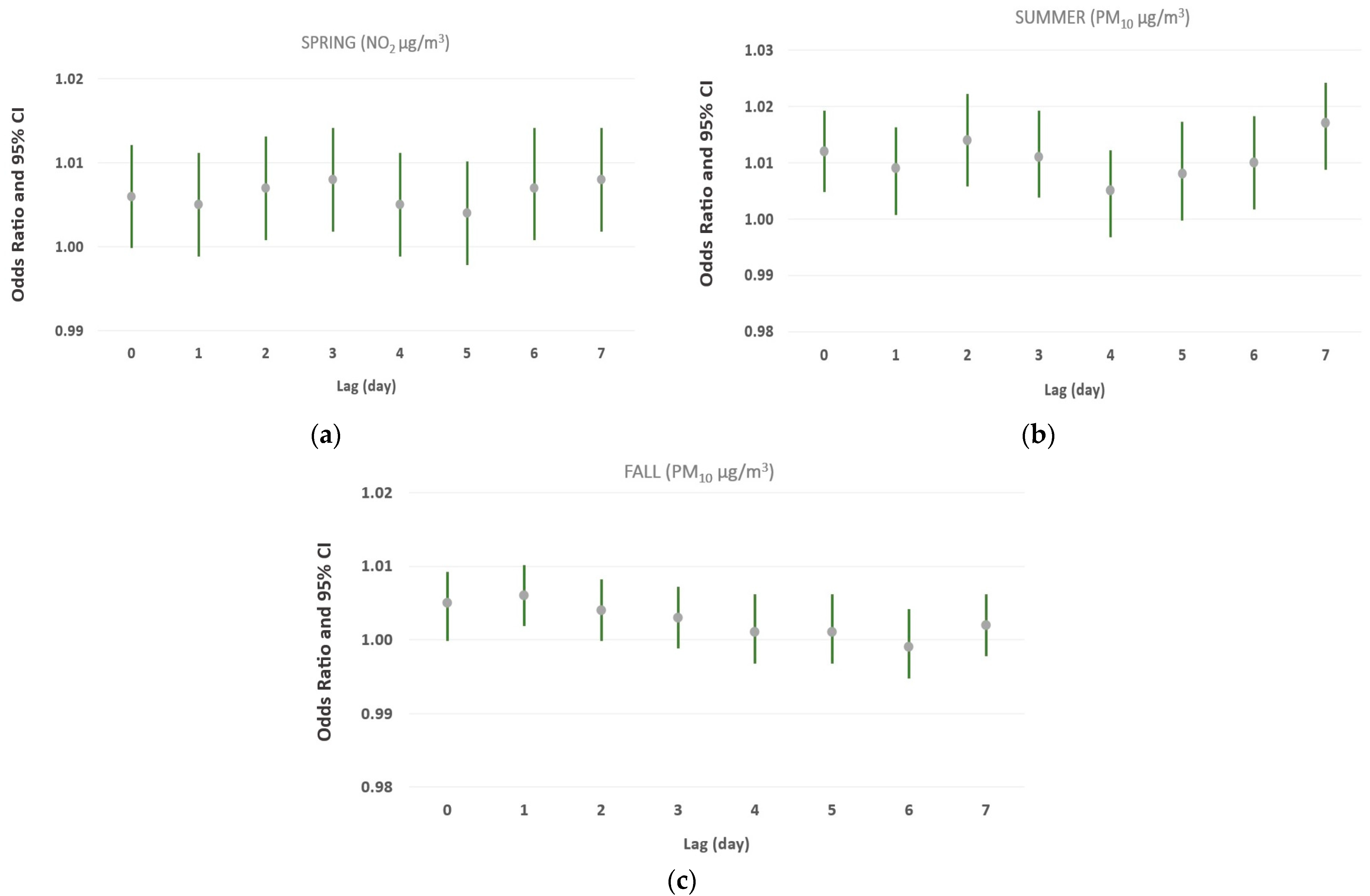
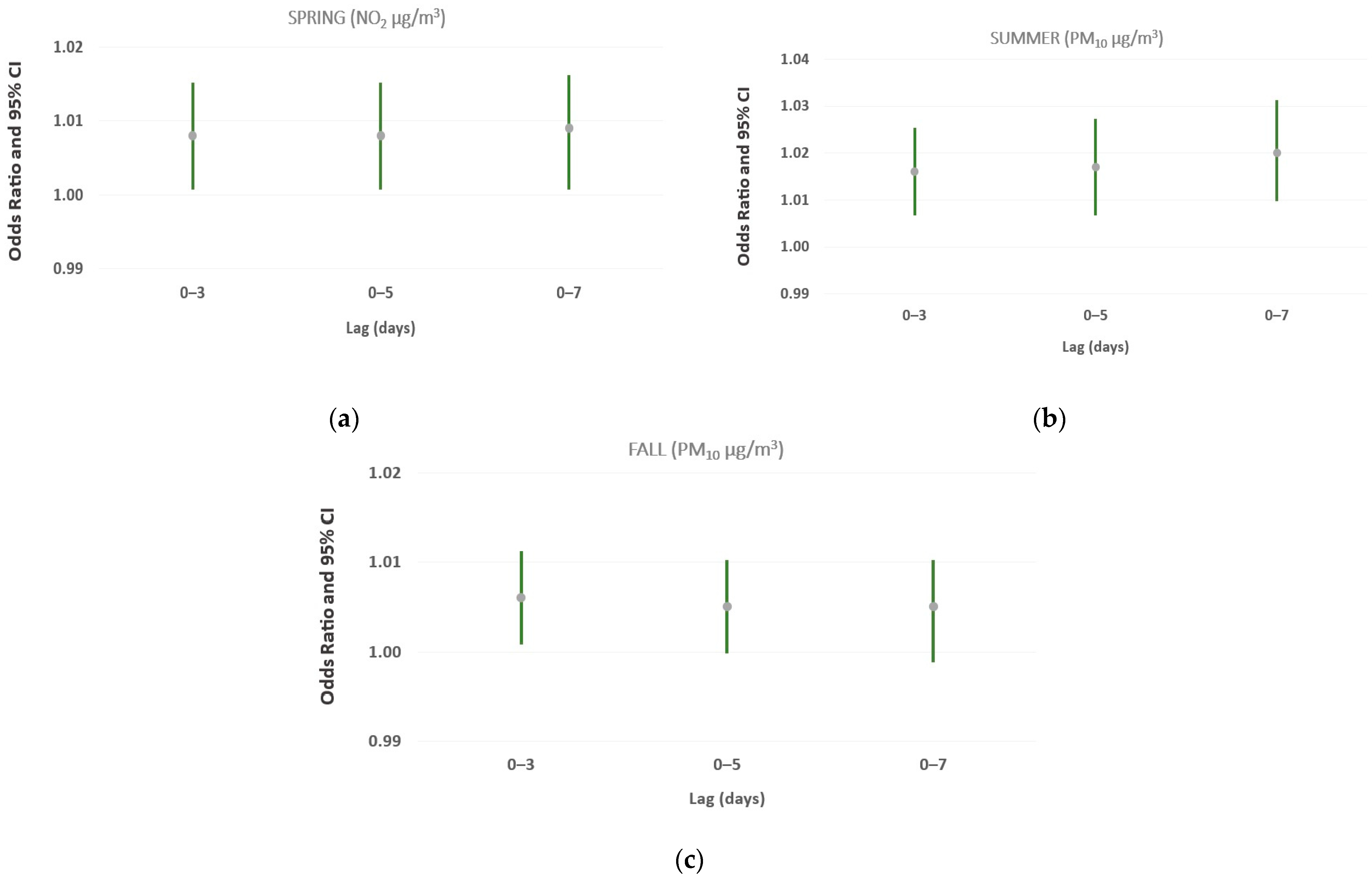
3.3. Analysis of the Effects of Brief Increments in Air Pollutant Levels (≥10 μg/m3) over Different Single and Cumulative Lag Days
4. Discussion
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959, Erratum in Eur. Heart J. 2015, 36, 794. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210, Erratum in Lancet 2017, 389, 156. [Google Scholar] [CrossRef] [PubMed]
- Dharma, S.; Andriantoro, H.; Dakota, I.; Purnawan, I.; Pratama, V.; Isnanijah, H.; Yamin, M.; Bagus, T.; Hartono, B.; Ratnaningsih, E.; et al. Organisation of reperfusion therapy for STEMI in a developing country. Open Heart 2015, 2, e000240. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Goldberg, R.J.; Gore, J.M.; Fox, K.A.; Eagle, K.A.; Flather, M.D.; Sadiq, I.; Kasper, R.; Rushton-Mellor, S.K.; Anderson, F.A.; et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am. J. Cardiol. 2002, 90, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, T.C.; Cătălina, A.G.; Katalin, B.; Imre, B.; Radu, C.; Mircea, C.; Elvira, C.; Dan, M.D.; Dan, D.; Dan, D.; et al. RO-STEMI. The First Romanian Registry for ST-elevation Myocardial Infarction 1997–2009—Final Report; Amaltea Medical Publishing House: Bucharest, Romania, 2010; p. 44. ISBN 978-973-162-068-8. [Google Scholar]
- World Health Organization. Air Pollution Costs European Economies US$ 1.6 Trillion a Year in Diseases and Deaths, New WHO Study Says. 28 April 2015. Available online: https://www.who.int/europe/news/item/28-04-2015-air-pollution-costs-european-economies-us-1-6-trillion-a-year-in-diseases-and-deaths-new-who-study-says (accessed on 1 March 2024).
- World Health Organization. Ambient (Outdoor) Air Pollution. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 1 March 2024).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Markandya, A.; Sampedro, J.; Smith, S.J.; Van Dingenen, R.; Pizarro-Irizar, C.; Arto, I.; González-Eguino, M. Health co-benefits from air pollution and mitigation costs of the Paris Agreement: A modelling study. Lancet Planet. Health 2018, 2, e126–e133. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Pawankar, R.; Vitale, C.; Lanza, M.; Molino, A.; Stanziola, A.; Sanduzzi, A.; Vatrella, A.; D’Amato, M. Climate Change and Air Pollution: Effects on Respiratory Allergy. Allergy Asthma Immunol. Res. 2016, 8, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Brunekreef, B.; Fischer, P.; van Wijnen, J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology 2001, 12, 355–357. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Fan, C.; Xu, R.; Wang, Y.; Xu, C.; Xie, S.; Zhang, H.; Cui, X.; Peng, Z.; et al. Short-Term Exposure to Ambient Air Pollution and Mortality From Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 271–281. [Google Scholar] [CrossRef]
- Nuvolone, D.; Balzi, D.; Chini, M.; Scala, D.; Giovannini, F.; Barchielli, A. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: Results of the cardiovascular risk and air pollution in Tuscany (RISCAT) study. Am. J. Epidemiol. 2011, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Ruidavets, J.B.; Cournot, M.; Cassadou, S.; Giroux, M.; Meybeck, M.; Ferrières, J. Ozone air pollution is associated with acute myocardial infarction. Circulation 2005, 111, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Khaniabadi, Y.O.; Daryanoosh, S.M.; Hopke, P.K.; Ferrante, M.; De Marco, A.; Sicard, P.; Oliveri Conti, G.; Goudarzi, G.; Basiri, H.; Mohammadi, M.J.; et al. Acute myocardial infarction and COPD attributed to ambient SO2 in Iran. Environ. Res. 2017, 156, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Hajat, S.; Armstrong, B.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. The effects of hourly differences in air pollution on the risk of myocardial infarction: Case crossover analysis of the MINAP database. BMJ 2011, 343, d5531. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, V.; Faustini, A.; Stafoggia, M.; Cattani, G.; Marconi, A.; Perucci, C.A.; Forastiere, F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 2010, 21, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, S.E.; Liao, N.S.; Liu, X.; Van Den Eeden, S.K.; Sidney, S. Long-Term PM2.5 Exposure and Risks of Ischemic Heart Disease and Stroke Events: Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e016890. [Google Scholar] [CrossRef]
- Montone, R.A.; Camilli, M.; Russo, M.; Termite, C.; La Vecchia, G.; Iannaccone, G.; Rinaldi, R.; Gurgoglione, F.; Del Buono, M.G.; Sanna, T.; et al. Air Pollution and Coronary Plaque Vulnerability and Instability: An Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2022, 15, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Hanigan, I.C.; Rolfe, M.I.; Knibbs, L.D.; Salimi, F.; Cowie, C.T.; Heyworth, J.; Marks, G.B.; Guo, Y.; Cope, M.; Bauman, A.; et al. All-cause mortality and long-term exposure to low level air pollution in the ‘45 and up study’ cohort, Sydney, Australia, 2006-2015. Environ Int. 2019, 126, 762–770. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Statistics. Available online: https://insse.ro/cms/en/content/websites-regionalcounty-statistics-offices (accessed on 11 April 2023).
- National Meteorological Administration. Available online: https://www.meteoromania.ro/clima/clima-romaniei/ (accessed on 2 March 2024).
- Liang, L.; Cai, Y.; Lyu, B.; Zhang, D.; Chu, S.; Jing, H.; Rahimi, K.; Tong, Z. Air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in Beijing: A time-series study. Respir. Res. 2022, 23, 81. [Google Scholar] [CrossRef]
- Argacha, J.F.; Collart, P.; Wauters, A.; Kayaert, P.; Lochy, S.; Schoors, D.; Sonck, J.; de Vos, T.; Forton, M.; Brasseur, O.; et al. Air pollution and ST-elevation myocardial infarction: A case-crossover study of the Belgian STEMI registry 2009–2013. Int. J. Cardiol. 2016, 223, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sahlén, A.; Ljungman, P.; Erlinge, D.; Chan, M.Y.; Yap, J.; Hausenloy, D.J.; Yeo, K.K.; Jernberg, T. Air pollution in relation to very short-term risk of ST-segment elevation myocardial infarction: Case-crossover analysis of SWEDEHEART. Int. J. Cardiol. 2019, 275, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Mantovani, K.C.; Nascimento, L.F.; Moreira, D.S.; Vieira, L.C.; Vargas, N.P. Air pollutants and hospital admissions due to cardiovascular diseases in São José do Rio Preto, Brazil. Cien Saude Colet. 2016, 21, 509–515. [Google Scholar] [CrossRef][Green Version]
- Wong, T.W.; Lau, T.S.; Yu, T.S.; Neller, A.; Wong, S.L.; Tam, W.; Pang, S.W. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup. Environ. Med. 1999, 56, 679–683. [Google Scholar] [CrossRef]
- Yang, S.; Lee, S.P.; Park, J.B.; Lee, H.; Kang, S.H.; Lee, S.E.; Kim, J.B.; Choi, S.Y.; Kim, Y.J.; Chang, H.J. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1355–1364. [Google Scholar] [CrossRef]
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Cardiovasc. Res. 2020, 116, 279–294. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation; ESC Heart Failure Association. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef]
- Mills, N.L.; Törnqvist, H.; Gonzalez, M.C.; Vink, E.; Robinson, S.D.; Söderberg, S.; Boon, N.A.; Donaldson, K.; Sandström, T.; Blomberg, A.; et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N. Engl. J. Med. 2007, 357, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Rückerl, R.; Greven, S.; Ljungman, P.; Aalto, P.; Antoniades, C.; Bellander, T.; Berglind, N.; Chrysohoou, C.; Forastiere, F.; Jacquemin, B.; et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007, 115, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.B.; Duncan, K.E.; Jardim, M.; Schmitt, M.T.; Rappold, A.G.; Diaz-Sanchez, D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 2012, 126, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. The effect of particulate air pollution on emergency admissions for myocardial infarction: A multicity case-crossover analysis. Environ. Health Perspect. 2005, 113, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Koken, P.J.; Piver, W.T.; Ye, F.; Elixhauser, A.; Olsen, L.M.; Portier, C.J. Temperature, air pollution, and hospitalization for cardiovascular diseases among elderly people in Denver. Environ. Health Perspect. 2003, 111, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- D’Ippoliti, D.; Forastiere, F.; Ancona, C.; Agabiti, N.; Fusco, D.; Michelozzi, P.; Perucci, C.A. Air pollution and myocardial infarction in Rome: A case-crossover analysis. Epidemiology 2003, 14, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Williams, G.M.; Schwartz, J.; Best, T.L.; Neller, A.H.; Petroeschevsky, A.L.; Simpson, R.W. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006, 114, 1018–1023. [Google Scholar] [CrossRef]
- Lanki, T.; Pekkanen, J.; Aalto, P.; Elosua, R.; Berglind, N.; D’Ippoliti, D.; Kulmala, M.; Nyberg, F.; Peters, A.; Picciotto, S.; et al. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: The HEAPSS study. Occup. Environ. Med. 2006, 63, 844–851. [Google Scholar] [CrossRef]
| STEMI (n = 2570) | |
|---|---|
| Gender | |
| male | 1871 (72.80%) |
| female | 699 (27.20%) |
| Age (years) | 61.35 ± 12.17 |
| Cardiovascular risk factors | |
| Arterial hypertension | 1589 (61.82%) |
| Hypercholesterolemia | 570 (22.18%) |
| Diabetes mellitus | 573 (22.29%) |
| Types of Coronary Artery Disease | |
| Single-vessel lesion | 1162 (45.21%) |
| Multi-vessel lesion | 1408 (54.79%) |
| Type of treatment | |
| Fibrinolysis | 677 (26.30%) |
| Interventional approach (balloon angioplasty or PCI primary) | 2320 (90.27%) |
| Surgical approach | 48 (1.87%) |
| Conservative | 202 (7.86%) |
| Study Days (n = 1096) | Mean ± SD | Frequency Distribution | ||||
|---|---|---|---|---|---|---|
| Air Pollutants (μg/m3) | Minimum | P25 | P50 | P75 | Maximum | |
| Winter | ||||||
| NO2 | 29.1 ± 15.5 | 5.3 | 18.8 | 26.0 | 35.6 | 121.8 |
| PM10 | 23.4 ± 14.3 | 0.6 | 12.7 | 20.7 | 30.9 | 83.5 |
| O3 | 36.7 ± 19.6 | 5.0 | 21.8 | 33.5 | 47.3 | 127.9 |
| Spring | ||||||
| NO2 | 22.2 ± 10.1 | 4.6 | 14.6 | 21.4 | 29.2 | 70.1 |
| PM10 | 17.9 ± 10.8 | 2.2 | 9.8 | 15.4 | 23.6 | 106.1 |
| O3 | 52.0 ± 17.5 | 13.2 | 39.1 | 52.3 | 62.0 | 141.7 |
| Summer | ||||||
| NO2 | 20.7 ± 10.4 | 1.8 | 13.5 | 17.8 | 26.7 | 53.9 |
| PM10 | 19.0 ± 9.2 | 1.5 | 12.7 | 17.7 | 23.5 | 61.1 |
| O3 | 51.7 ± 15.4 | 11.7 | 40.5 | 51.7 | 61.8 | 95.8 |
| Fall | ||||||
| NO2 | 24.5 ± 13.7 | 0.8 | 16.2 | 22.3 | 29.9 | 88.0 |
| PM10 | 24.6 ± 16.5 | 1.8 | 13.9 | 20.5 | 30.7 | 114.7 |
| O3 | 33.8 ± 17.2 | 5.0 | 20.4 | 30.7 | 44.7 | 88.8 |
| Meteorological factors | ||||||
| Winter | ||||||
| Temperature (°C) | 3.5 ± 4.4 | −9.1 | 0.4 | 3.6 | 6.7 | 15.9 |
| Relative Humidity (%) | 79.3 ± 13.6 | 39.0 | 72.0 | 81.0 | 90.0 | 100.0 |
| Spring | ||||||
| Temperature (°C) | 12.9 ± 5.8 | −0.5 | 9.1 | 13.0 | 16.5 | 26.9 |
| Relative Humidity (%) | 65.8 ± 14.6 | 34.0 | 55.0 | 64.0 | 78.0 | 98.0 |
| Summer | ||||||
| Temperature (°C) | 22.0 ± 3.8 | 8.5 | 19.3 | 22.2 | 24.7 | 30.0 |
| Relative Humidity (%) | 66.5 ± 11.8 | 34.0 | 58.0 | 65.0 | 75.0 | 98.0 |
| Fall | ||||||
| Temperature (°C) | 12.1 ± 5.4 | −1.8 | 7.8 | 12.3 | 16.4 | 24.5 |
| Relative Humidity (%) | 76.4 ± 11.7 | 41.0 | 68.0 | 76.0 | 86.0 | 99.0 |
| NO2 (μg/m3) | PM10 (μg/m3) | |
|---|---|---|
| Cumulative Lag Days | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Gender Subgroups | ||
| Male | ||
| Winter | ||
| 0–3 | 1.001 (0.996–1.006) | 0.998 (0.991–1.004) |
| 0–5 | 1.003 (0.997–1.009) | 0.997 (0.990–1.004) |
| 0–7 | 1.004 (0.998–1.010) | 0.997 (0.989–1.004) |
| Spring | ||
| 0–3 | 1.008 (1.002–1.014) * | 0.995 (0.985–1.005) |
| 0–5 | 1.010 (1.003–1.017) * | 0.993 (0.982–1.004) |
| 0–7 | 1.010 (1.003–1.018) * | 0.992 (0.980–1.004) |
| Summer | ||
| 0–3 | 1.005 (0.998–1.011) | 1.017 (1.007–1.027) * |
| 0–5 | 1.006 (0.998–1.014) | 1.017 (1.005–1.029) * |
| 0–7 | 1.007 (0.998–1.015) | 1.021 (1.008–1.033) * |
| Fall | ||
| 0–3 | 1.001 (0.994–1.007) | 1.006 (1.000–1.012) * |
| 0–5 | 1.001 (0.994–1.008) | 1.006 (0.999–1.012) |
| 0–7 | 1.002 (0.994–1.009) | 1.005 (0.999–1.011) |
| Female | ||
| Winter | ||
| 0–3 | 1.004 (0.994–1.014) | 0.997 (0.987–1.007) |
| 0–5 | 1.003 (0.993–1.013) | 0.996 (0.986–1.007) |
| 0–7 | 1.003 (0.993–1.013) | 0.996 (0.985–1.008) |
| Spring | ||
| 0–3 | 1.010 (0.999–1.022) | 1.004 (0.988–1.020) |
| 0–5 | 1.011 (0.999–1.023) | 1.004 (0.987–1.021) |
| 0–7 | 1.012 (0.999–1.025) | 1.002 (0.984–1.019) |
| Summer | ||
| 0–3 | 1.007 (0.994–1.021) | 1.016 (0.997–1.035) |
| 0–5 | 1.007 (0.993–1.020) | 1.016 (0.996–1.036) |
| 0–7 | 1.008 (0.994–1.022) | 1.020 (0.999–1.041) |
| Fall | ||
| 0–3 | 1.001 (0.991–1.012) | 1.006 (0.998–1.014) |
| 0–5 | 1.003 (0.992–1.013) | 1.004 (0.996–1.012) |
| 0–7 | 1.004 (0.993–1.014) | 1.003 (0.994–1.012) |
| Age Subgroups | ||
| Young Adults (20–44 age) | ||
| Winter | ||
| 0–3 | 1.009 (0.992–1.026) | 0.986 (0.968–1.004) |
| 0–5 | 1.009 (0.992–1.027) | 0.982 (0.963–1.001) |
| 0–7 | 1.009 (0.992–1.027) | 0.981 (0.962–1.001) |
| Spring | ||
| 0–3 | 1.008 (0.986–1.029) | 0.987 (0.945–1.031) |
| 0–5 | 1.008 (0.986–1.030) | 0.996 (0.953–1.041) |
| 0–7 | 1.011 (0.988–1.034) | 1.006 (0.959–1.056) |
| Summer | ||
| 0–3 | 1.007 (0.981–1.034) | 1.027 (0.997–1.059) |
| 0–5 | 1.009 (0.983–1.035) | 1.027 (0.994–1.062) |
| 0–7 | 1.012 (0.987–1.038) | 1.032 (0.997–1.069) |
| Fall | ||
| 0–3 | 1.008 (0.987–1.029) | 1.001 (0.985–1.018) |
| 0–5 | 1.009 (0.986–1.032) | 0.999 (0.983–1.015) |
| 0–7 | 1.009 (0.986–1.033) | 0.998 (0.981–1.014) |
| Middle-Aged Adults (45–64 age) | ||
| Winter | ||
| 0–3 | 1.004 (0.997–1.011) | 0.997 (0.988–1.005) |
| 0–5 | 1.004 (0.996–1.011) | 0.996 (0.988–1.005) |
| 0–7 | 1.005 (0.997–1.013) | 0.996 (0.987–1.005) |
| Spring | ||
| 0–3 | 1.009 (1.001–1.017) * | 0.992 (0.980–1.004) |
| 0–5 | 1.010 (1.001–1.018) * | 0.993 (0.980–1.005) |
| 0–7 | 1.010 (1.002–1.019) * | 0.994 (0.980–1.007) |
| Summer | ||
| 0–3 | 1.003 (0.994–1.013) | 1.014 (1.000–1.027) * |
| 0–5 | 1.005 (0.995–1.016) | 1.015 (1.000–1.030) * |
| 0–7 | 1.006 (0.996–1.017) | 1.020 (1.004–1.036) * |
| Fall | ||
| 0–3 | 0.998 (0.990–1.007) | 1.006 (0.999–1.013) |
| 0–5 | 0.998 (0.989–1.007) | 1.006 (0.998–1.013) |
| 0–7 | 0.999 (0.990–1.008) | 1.006 (0.998–1.013) |
| Older Adults (≥65 age) | ||
| Winter | ||
| 0–3 | 1.002 (0.994–1.009) | 1.000 (0.992–1.008) |
| 0–5 | 1.001 (0.994–1.009) | 1.001 (0.992–1.009) |
| 0–7 | 1.002 (0.994–1.009) | 1.001 (0.992–1.010) |
| Spring | ||
| 0–3 | 1.012 (1.003–1.021) * | 1.004 (0.991–1.016) |
| 0–5 | 1.012 (1.002–1.022) * | 1.002 (0.988–1.016) |
| 0–7 | 1.011 (1.001–1.022) * | 0.997 (0.982–1.012) |
| Summer | ||
| 0–3 | 1.006 (0.996–1.016) | 1.017 (1.004–1.030) * |
| 0–5 | 1.006 (0.995–1.016) | 1.016 (1.001–1.031) * |
| 0–7 | 1.006 (0.996–1.017) | 1.019 (1.003–1.035) * |
| Fall | ||
| 0–3 | 1.002 (0.994–1.011) | 1.007 (1.000–1.014) * |
| 0–5 | 1.003 (0.994–1.011) | 1.006 (0.999–1.014) |
| 0–7 | 1.004 (0.995–1.013) | 1.005 (0.997–1.013) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, A.-A.; Şoşdean, R.; Lazăr, M.-A.; Simonescu, M.; Luca, S.-A.; Dima, C.N.; Frişan, A.-C.; Gaiţă, D.; Mornoş, C. Seasonal Variation in Short-Term Ambient Air Pollutants and ST-Elevation Myocardial Infarction Admissions: An Innovative Exploration of Air Pollution’s Health Consequences. Atmosphere 2024, 15, 590. https://doi.org/10.3390/atmos15050590
Rus A-A, Şoşdean R, Lazăr M-A, Simonescu M, Luca S-A, Dima CN, Frişan A-C, Gaiţă D, Mornoş C. Seasonal Variation in Short-Term Ambient Air Pollutants and ST-Elevation Myocardial Infarction Admissions: An Innovative Exploration of Air Pollution’s Health Consequences. Atmosphere. 2024; 15(5):590. https://doi.org/10.3390/atmos15050590
Chicago/Turabian StyleRus, Andreea-Alexandra, Raluca Şoşdean, Mihai-Andrei Lazăr, Marius Simonescu, Silvia-Ana Luca, Ciprian Nicuşor Dima, Alexandra-Cătălina Frişan, Dan Gaiţă, and Cristian Mornoş. 2024. "Seasonal Variation in Short-Term Ambient Air Pollutants and ST-Elevation Myocardial Infarction Admissions: An Innovative Exploration of Air Pollution’s Health Consequences" Atmosphere 15, no. 5: 590. https://doi.org/10.3390/atmos15050590
APA StyleRus, A.-A., Şoşdean, R., Lazăr, M.-A., Simonescu, M., Luca, S.-A., Dima, C. N., Frişan, A.-C., Gaiţă, D., & Mornoş, C. (2024). Seasonal Variation in Short-Term Ambient Air Pollutants and ST-Elevation Myocardial Infarction Admissions: An Innovative Exploration of Air Pollution’s Health Consequences. Atmosphere, 15(5), 590. https://doi.org/10.3390/atmos15050590






