Development of a Refrigerant-Free Cryotrap Unit for Pre-Concentration of Biogenic Volatile Organic Compounds in Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Standard Gas Preparation
2.1.2. Sample Pre-Concentration
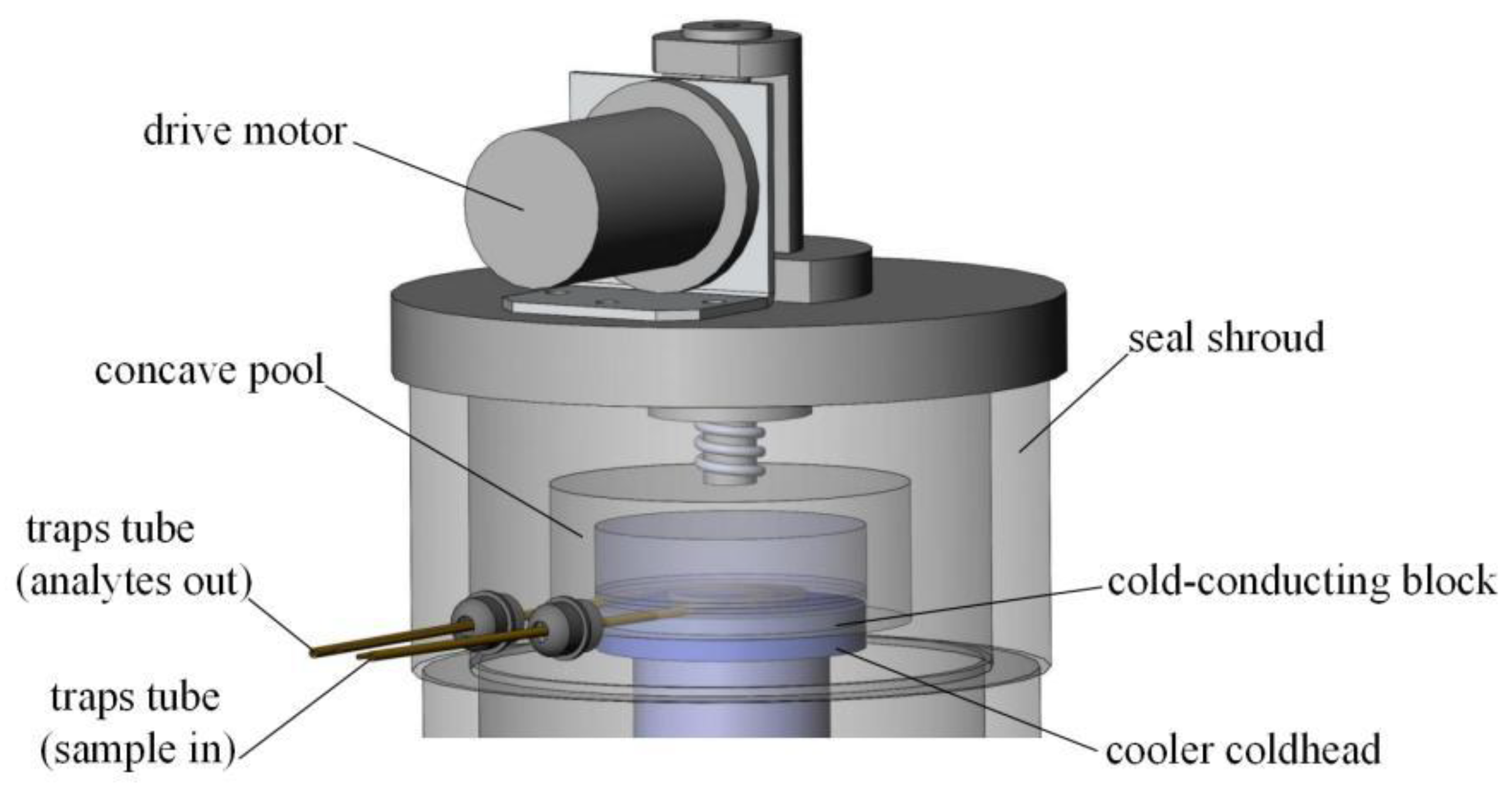
2.1.3. Enrichment Trap and Cooling Technique
2.2. GC-MS/FID Conditions
3. Results and Discussion
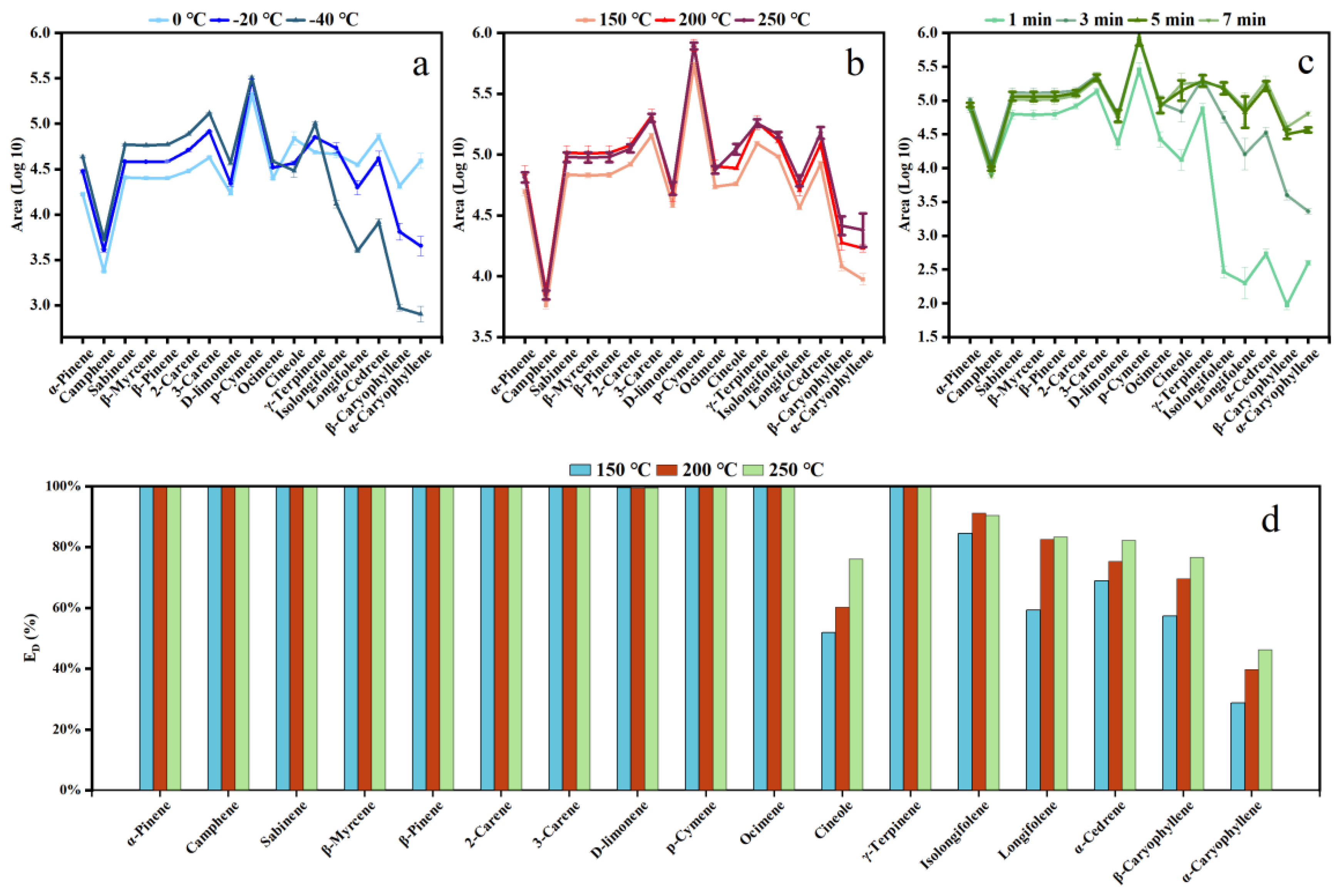
3.1. Optimization of Pre-Concentration Unit Conditions
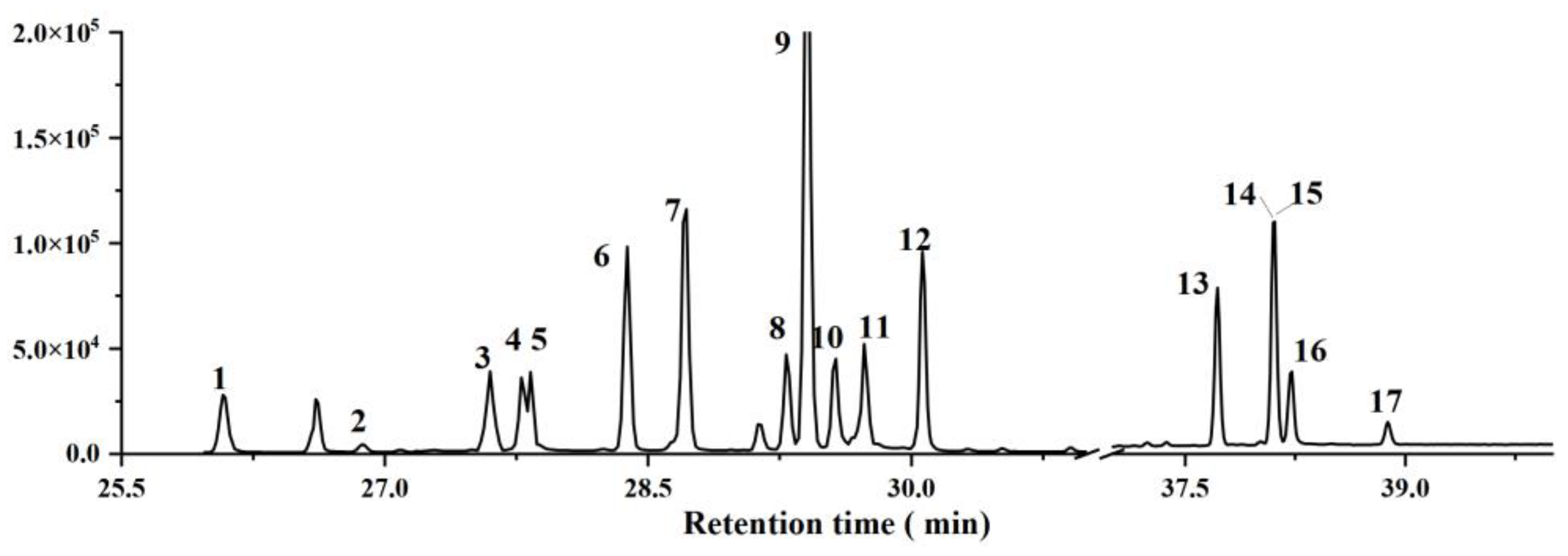
3.2. Overall Performance
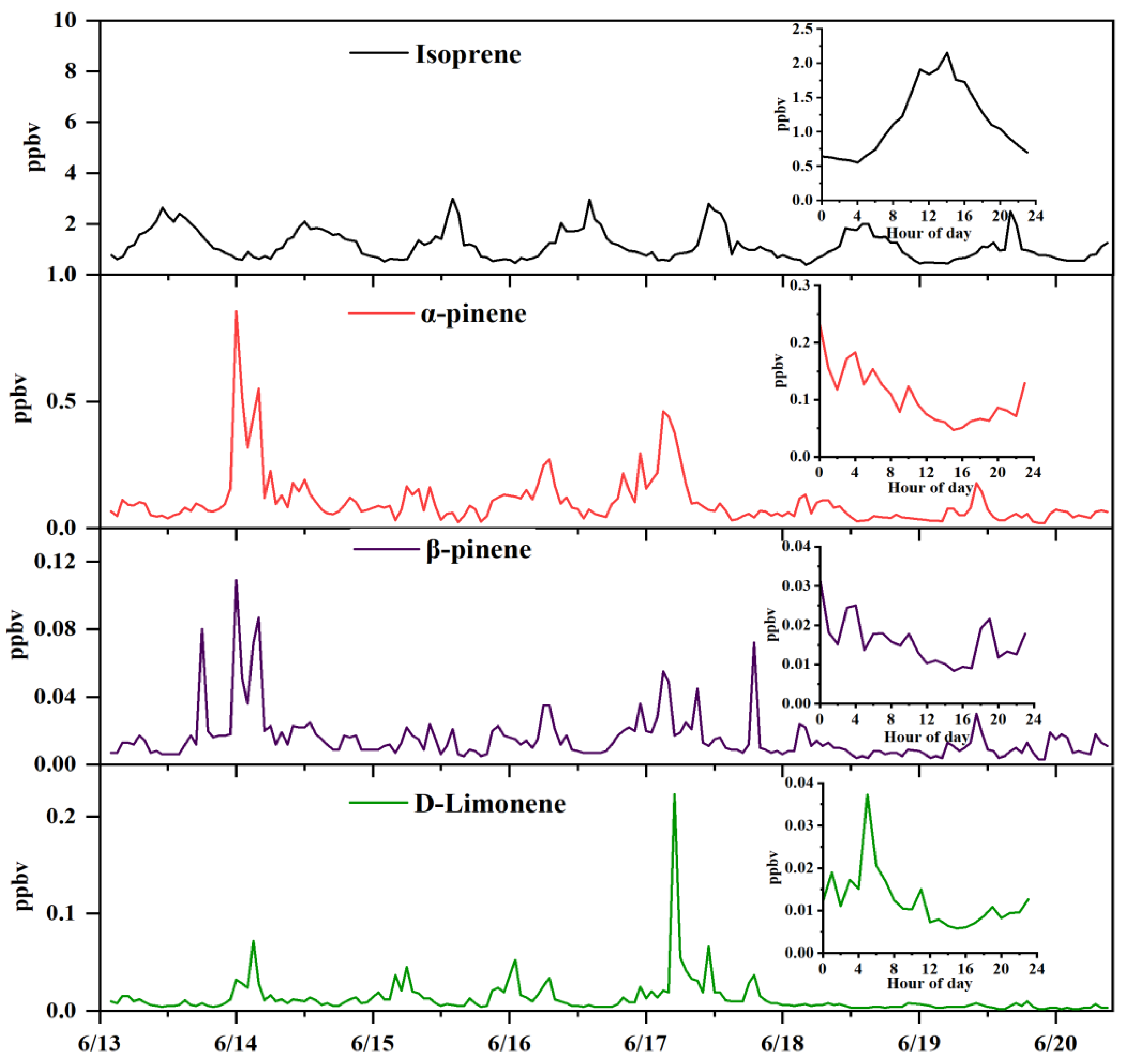
3.3. Real Sample Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic voc emissions calculated by the megan model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Penuelas, J.; Staudt, M. Bvocs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A. Biological and chemical diversity of biogenic volatile organic emissions into the atmosphere. ISRN Atmos. Sci. 2013, 2013, 1–27. [Google Scholar] [CrossRef]
- Wolfe, G.M.; Thornton, J.A.; McKay, M.; Goldstein, A.H. Forest-atmosphere exchange of ozone: Sensitivity to very reactive biogenic voc emissions and implications for in-canopy photochemistry. Atmos. Chem. Phys. 2011, 11, 7875–7891. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Wang, W.J.; Liu, Y.; Wang, M.; Lu, S.H. Evaluation of biogenic isoprene emissions and their contribution to ozone formation by ground-based measurements in beijing, china. Sci. Total Environ. 2018, 627, 1485–1494. [Google Scholar] [CrossRef]
- Lou, C.; Jiang, F.; Tian, X.; Zou, Q.; Zheng, Y.; Shen, Y.; Feng, S.; Chen, J.; Zhang, L.; Jia, M.; et al. Modeling the biogenic isoprene emission and its impact on ozone pollution in zhejiang province, china. Sci. Total. Environ 2023, 865, 161212. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kohno, N.; Ramasamy, S.; Sato, K.; Morino, Y.; Kajii, Y. Investigation of oh-reactivity budget in the isoprene, α-Pinene and m-xylene oxidation with oh under high nox conditions. Atmos. Environ. 2022, 271, 118916. [Google Scholar] [CrossRef]
- Li, C.L.; Liu, Y.F.; Cheng, B.F.; Zhang, Y.P.; Liu, X.G.; Qu, Y.; An, J.L.; Kong, L.W. A comprehensive investigation on volatile organic compounds (vocs) in 2018 in beijing, china: Characteristics, sources and behaviours in response to o3 formation. Sci. Total Environ 2022, 806, 150247. [Google Scholar] [CrossRef]
- Chen, X.; Gong, D.C.; Lin, Y.J.; Xu, Q.; Wang, Y.J.; Liu, S.W.; Li, Q.Q.; Ma, F.Y.; Li, J.Y.; Deng, S.; et al. Emission characteristics of biogenic volatile organic compounds in a subtropical pristine forest of southern china. J. Environ. Sci. 2025, 148, 665–682. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.X.; Qi, Q.; Zhang, H.L. Evaluating the impacts of ground-level o3 on crops in china. Curr. Pollut. Rep. 2021, 7, 565–578. [Google Scholar] [CrossRef]
- Churkina, G.; Kuik, F.; Bonn, B.; Lauer, A.; Grote, R.; Tomiak, K.; Butler, T.M. Effect of voc emissions from vegetation on air quality in berlin during a heatwave. Environ. Sci. Technol. 2017, 51, 6120–6130. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.J.; Nelson, B.S.; Swift, S.J.; Budisulistiorini, S.H.; Drysdale, W.S.; Vaughan, A.R.; Newland, M.J.; Hopkins, J.R.; Cash, J.M.; Langford, B.; et al. Biogenic and anthropogenic sources of isoprene and monoterpenes and their secondary organic aerosol in delhi, india. Atmos. Chem. Phys. 2023, 23, 61–83. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipila, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef]
- Liu, S.; Xing, J.; Zhang, H.L.; Ding, D.; Zhang, F.F.; Zhao, B.; Sahu, S.K.; Wang, S.X. Climate-driven trends of biogenic volatile organic compound emissions and their impacts on summertime ozone and secondary organic aerosol in china in the 2050s. Atmos. Environ. 2019, 218, 117020. [Google Scholar] [CrossRef]
- Cao, J.; Situ, S.P.; Hao, Y.F.; Xie, S.D.; Li, L.Y. Enhanced summertime ozone and soa from biogenic volatile organic compound (bvoc) emissions due to vegetation biomass variability during 1981–2018 in china. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Chen, T.Y.; Jang, M. Secondary organic aerosol formation from photooxidation of a mixture of dimethyl sulfide and isoprene. Atmos. Environ. 2012, 46, 271–278. [Google Scholar] [CrossRef]
- Yanez-Serrano, A.M.; Bourtsoukidis, E.; Alves, E.G.; Bauwens, M.; Stavrakou, T.; Llusia, J.; Filella, I.; Guenther, A.; Williams, J.; Artaxo, P.; et al. Amazonian biogenic volatile organic compounds under global change. Glob. Chang. Biol. 2020, 26, 4722–4751. [Google Scholar] [CrossRef]
- Praplan, A.P.; Tykkä, T.; Schallhart, S.; Tarvainen, V.; Bäck, J.; Hellén, H. Oh reactivity from the emissions of different tree species: Investigating the missing reactivity in a boreal forest. Biogeosciences 2020, 17, 4681–4705. [Google Scholar] [CrossRef]
- Berndt, T.; Mentler, B.; Scholz, W.; Fischer, L.; Herrmann, H.; Kulmala, M.; Hansel, A. Accretion product formation from ozonolysis and oh radical reaction of alpha-Pinene: Mechanistic insight and the influence of isoprene and ethylene. Environ. Sci.Technol. 2018, 52, 11069–11077. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.; Kato, S.; Nakashima, Y.; Kajii, Y. A novel fast gas chromatography method for higher time resolution measurements of speciated monoterpenes in air. Atmos. Meas. Tech. 2014, 7, 1259–1275. [Google Scholar] [CrossRef]
- Chen, J.G.; Tang, J.; Yu, X.X. Environmental and physiological controls on diurnal and seasonal patterns of biogenic volatile organic compound emissions from five dominant woody species under field conditions. Environ. Pollut. 2020, 259, 113955. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schallhart, S.; Tykkä, T.; Räsänen, M.; Merbold, L.; Hellén, H.; Pellikka, P. Biogenic volatile organic compounds in different ecosystems in southern kenya. Atmos. Environ. 2021, 246, 118064. [Google Scholar] [CrossRef]
- Tzitzikalaki, E.; Kalivitis, N.; Kouvarakis, G.; Mihalopoulos, N.; Kanakidou, M. Seasonal and diurnal variability of monoterpenes in the eastern mediterranean atmosphere. Atmosphere 2023, 14, 392. [Google Scholar] [CrossRef]
- Hellén, H.; Schallhart, S.; Praplan, A.P.; Tykkä, T.; Aurela, M.; Lohila, A.; Hakola, H. Sesquiterpenes dominate monoterpenes in northern wetland emissions. Atmos. Chem. Phys. 2020, 20, 7021–7034. [Google Scholar] [CrossRef]
- Wu, J.; Long, J.Y.; Liu, H.X.; Sun, G.P.; Li, J.; Xu, L.J.; Xu, C.Y. Biogenic volatile organic compounds from 14 landscape woody species: Tree species selection in the construction of urban greenspace with forest healthcare effects. J. Environ. Manag. 2021, 300, 113761. [Google Scholar] [CrossRef]
- McGlynn, D.F.; Barry, L.E.R.; Lerdau, M.T.; Pusede, S.E.; Isaacman-VanWertz, G. Measurement report: Variability in the composition of biogenic volatile organic compounds in a southeastern us forest and their role in atmospheric reactivity. Atmos. Chem. Phys. 2021, 21, 15755–15770. [Google Scholar] [CrossRef]
- Helin, A.; Hakola, H.; Hellén, H. Optimisation of a thermal desorption–gas chromatography–mass spectrometry method for the analysis of monoterpenes, sesquiterpenes and diterpenes. Atmos. Meas. Tech. 2020, 13, 3543–3560. [Google Scholar] [CrossRef]
- Mermet, K.; Sauvage, S.; Dusanter, S.; Salameh, T.; Léonardis, T.; Flaud, P.-M.; Perraudin, É.; Villenave, É.; Locoge, N. Optimization of a gas chromatographic unit for measuring biogenic volatile organic compounds in ambient air. Atmos. Meas. Tech. 2019, 12, 6153–6171. [Google Scholar] [CrossRef]
- Marcillo, A.; Jakimovska, V.; Widdig, A.; Birkemeyer, C. Comparison of two common adsorption materials for thermal desorption gas chromatography—Mass spectrometry of biogenic volatile organic compounds. J. Chromatogr. A 2017, 1514, 16–28. [Google Scholar] [CrossRef]
- Lee, J.H.; Batterman, S.A.; Jia, C.; Chernyak, S. Ozone artifacts and carbonyl measurements using tenax gr, tenax ta, carbopack b, and carbopack x adsorbents. J. Air Waste Manag. Assoc. 2006, 56, 1503–1517. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, Z.; Cai, S.; Wang, S.; Lu, H. Analysis of volatile organic compounds using cryogen-free thermal modulation based comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometry. J. Chromatogr. A 2019, 1587, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Ho, K.-F.; Lee, S.C.; Nichol, J.E. Biogenic volatile organic compounds (bvoc) in ambient air over hong kong: Analytical methodology and field measurement. Int. J. Environ. Anal. Chem. 2010, 90, 988–999. [Google Scholar] [CrossRef]
- Barkley, C.S.; Zhou, Y.; Troop, D.; Wang, Y.L.; Little, W.C.; Wingenter, O.W.; Russo, R.S.; Varner, R.K.; Talbot, R. Development of a cryogen-free concentration system for measurements of volatile organic compounds. Anal. Chem. 2005, 77, 6989–6998. [Google Scholar]
- Wang, M.; Zeng, L.M.; Lu, S.H.; Shao, M.; Liu, X.L.; Yu, X.N.; Chen, W.; Yuan, B.; Zhang, Q.; Hu, M.; et al. Development and validation of a cryogen-free automatic gas chromatograph system (gc-ms/fid) for online measurements of volatile organic compounds. Anal. Methods 2014, 6, 9424–9434. [Google Scholar] [CrossRef]
- Obersteiner, F. A versatile, refrigerant- and cryogen-free cryofocusing–thermodesorption unit for pre-concentration of traces gases in air. Atmos. Meas. Tech. 2016, 9, 5265–5279. [Google Scholar] [CrossRef]
- Ghorbanian, K.; Karimi, M. Design and optimization of a heat driven thermoacoustic refrigerator. Appl. Therm. Eng. 2014, 62, 653–661. [Google Scholar] [CrossRef]
- Dai, W.; Luo, E.C.; Hu, J.Y.; Ling, H. A heat-driven thermoacoustic cooler capable of reaching liquid nitrogen temperature. Appl. Phys. Lett. 2005, 86, 224103. [Google Scholar] [CrossRef]
- Karimi, M.; Ghorbanian, K.; Gholamrezaei, M. Energy and exergy analyses of an integrated gas turbine thermoacoustic engine. Proc. IMechE. Part A J. Power Energy 2011, 225, 389–402. [Google Scholar] [CrossRef]
- Xu, J.Y.; Luo, E.C.; Hochgreb, S. A thermoacoustic combined cooling, heating, and power (cchp) system for waste heat and lng cold energy recovery. Energy 2021, 227, 120341. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, D.S.; Sun, J.; Wang, Y.H.; Yao, D.; Zhao, S.M.; Yu, X.N.; Zeng, L.M.; Zhang, R.J.; Zhang, H.; et al. Ambient volatile organic compounds in a suburban site between beijing and tianjin: Concentration levels, source apportionment and health risk assessment. Sci. Total Environ. 2019, 695, 133889. [Google Scholar] [CrossRef]
- Li, Y.F.; Gao, R.; Xue, L.K.; Wu, Z.H.; Yang, X.; Gao, J.; Ren, L.H.; Li, H.; Ren, Y.Q.; Li, G.; et al. Ambient volatile organic compounds at wudang mountain in central china: Characteristics, sources and implications to ozone formation. Atmos. Res. 2021, 250, 105359. [Google Scholar] [CrossRef]
- Wu, Y.J.; Fan, X.L.; Liu, Y.; Zhang, J.Q.; Wang, H.; Sun, L.N.; Fang, T.G.; Mao, H.J.; Hu, J.; Wu, L.; et al. Source apportionment of vocs based on photochemical loss in summer at a suburban site in beijing. Atmos. Environ. 2023, 293, 119459. [Google Scholar] [CrossRef]
- Faiola, C.L.; Erickson, M.H.; Fricaud, V.L.; Jobson, B.T.; VanReken, T.M. Quantification of biogenic volatile organic compounds with a flame ionization detector using the effective carbon number concept. Atmos. Meas. Tech. 2012, 5, 1911–1923. [Google Scholar] [CrossRef]
- Bouvier-Brown, N.C.; Goldstein, A.H.; Gilman, J.B.; Kuster, W.C.; de Gouw, J.A. In-situ ambient quantification of monoterpenes, sesquiterpenes, and related oxygenated compounds during bearpex 2007: Implications for gas- and particle-phase chemistry. Atmos. Chem. Phys. 2009, 9, 5505–5518. [Google Scholar] [CrossRef]
- Gong, D.C.; Wang, H.; Zhang, S.Y.; Wang, Y.; Liu, S.C.; Guo, H.; Shao, M.; He, C.R.; Chen, D.H.; He, L.Y.; et al. Low-level summertime isoprene observed at a forested mountaintop site in southern china: Implications for strong regional atmospheric oxidative capacity. Atmos. Chem. Phys. 2018, 18, 14417–14432. [Google Scholar] [CrossRef]
- Hellén, H.; Kuronen, P.; Hakola, H. Heated stainless steel tube for ozone removal in the ambient air measurements of mono- and sesquiterpenes. Atmos. Environ. 2012, 57, 35–40. [Google Scholar] [CrossRef]
- Li, H.J.; Xiao, Q.; Han, C.M.; Liu, L.Y.; Fan, X.F.; Chen, N.; Pan, Y.P. On-line monitoring of vocs in ambient air based on cryo-trapping-gc-ms-fid. Environ. Monitoring in China 2017, 33, 101–110. [Google Scholar]
- Hellén, H.; Praplan, A.P.; Tykkä, T.; Ylivinkka, I.; Vakkari, V.; Bäck, J.; Petäjä, T.; Kulmala, M.; Hakola, H. Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of a boreal forest. Atmos. Chem. Phys. 2018, 18, 13839–13863. [Google Scholar] [CrossRef]
- Sheu, R.; Marcotte, A.; Khare, P.; Charan, S.; Ditto, J.C.; Gentner, D.R. Advances in offline approaches for chemically speciated measurements of trace gas-phase organic compounds via adsorbent tubes in an integrated sampling-to-analysis system. J. Chromatogr. A 2018, 1575, 80–90. [Google Scholar] [CrossRef]
- Zeng, J.q.; Song, W.; Zhang, Y.L.; Mu, Z.b.; Pang, W.H.; Zhang, H.N.; Wang, X.M. Emissions of isoprenoids from dominant tree species in subtropical china. Front. For. Glob. Change 2022, 5, 1089676. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wen, S.; Lv, H.X.; Wang, X.M.; Sheng, G.; Fu, J.M. Diurnal variations of vocs and relative contribution to ozone in forest of guangzhou. Environ. Sci. Technol. 2009, 32, 94–98. [Google Scholar]
- Wang, J.; Zhang, Y.L.; Xiao, S.X.; Wu, Z.F.; Wang, X.M. Ozone formation at a suburban site in the pearl river delta region, china: Role of biogenic volatile organic compounds. Atmosphere 2023, 14, 609. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Zhang, Y.; Li, Y.; Zhang, W.; Wang, X.; Bi, F.; Zhang, H.; Gao, J.; Chai, F.; et al. Atmospheric isoprene and monoterpenes in a typical urban area of beijing: Pollution characterization, chemical reactivity and source identification. J. Environ. Sci. 2018, 71, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Q.Y.; Greenberg, J.; Guenther, A.; Li, J.Y.; Cao, J.J.; Wang, J.; Liao, H.; Wang, Q.Y.; Zhang, Q. Impacts of biogenic and anthropogenic emissions on summertime ozone formation in the guanzhong basin, china. Atmos. Chem. Phys. 2018, 18, 7489–7507. [Google Scholar] [CrossRef]
- Guo, H.; Ling, Z.H.; Simpson, I.J.; Blake, D.R.; Wang, D.W. Observations of isoprene, methacrolein (mac) and methyl vinyl ketone (mvk) at a mountain site in hong kong. J. Geophys. Res. 2012, 117, D19303. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Dominutti, P.; Pedrosa, G.S.; Coelho, M.S.; Nogueira, T.; Borbon, A.; Souza, S.R.; Fornaro, A. Isoprene in urban atlantic forests: Variability, origin, and implications on the air quality of a subtropical megacity. Sci. Total Environ 2022, 824, 153728. [Google Scholar] [CrossRef] [PubMed]
- Hellén, H.; Tykkä, T.; Hakola, H. Importance of monoterpenes and isoprene in urban air in northern europe. Atmos. Environ. 2012, 59, 59–66. [Google Scholar] [CrossRef]
- Li, H.Y.; Canagaratna, M.R.; Riva, M.; Rantala, P.; Zhang, Y.J.; Thomas, S.; Heikkinen, L.; Flaud, P.-M.; Villenave, E.; Perraudin, E.; et al. Atmospheric organic vapors in two european pine forests measured by a vocus ptr-tof: Insights into monoterpene and sesquiterpene oxidation processes. Atmos. Chem. Phys. 2021, 21, 4123–4147. [Google Scholar] [CrossRef]
- Yee, L.D.; Isaacman-VanWertz, G.; Wernis, R.A.; Meng, M.; Rivera, V.; Kreisberg, N.M.; Hering, S.V.; Bering, M.S.; Glasius, M.; Upshur, M.A.; et al. Observations of sesquiterpenes and their oxidation products in central amazonia during the wet and dry seasons. Atmos. Chem. Phys. 2018, 18, 10433–10457. [Google Scholar] [CrossRef]
- Zou, Y.; Deng, X.J.; Zhu, D.; Gong, D.C.; Wang, H.; Li, F.; Tan, H.B.; Deng, T.; Mai, B.R.; Liu, X.T.; et al. Characteristics of 1 year of observational data of vocs, nox and o3 at a suburban site in guangzhou, china. Atmos. Chem. Phys. 2015, 15, 6625–6636. [Google Scholar] [CrossRef]
- Yang, W.Q.; Yu, Q.Q.; Pei, C.L.; Liao, C.H.; Liu, J.J.; Zhang, J.P.; Zhang, Y.L.; Qiu, X.N.; Zhang, T.; Zhang, Y.B.; et al. Characteristics of volatile organic compounds in the pearl river delta region, china: Chemical reactivity, source, and emission regions. Atmosphere 2021, 13, 9. [Google Scholar] [CrossRef]
- Meng, Y.; Song, J.W.; Zeng, L.W.; Zhang, Y.Y.; Zhao, Y.; Liu, X.F.; Guo, H.; Zhong, L.J.; Ou, Y.B.; Zhou, Y.; et al. Ambient volatile organic compounds at a receptor site in the pearl river delta region: Variations, source apportionment and effects on ozone formation. J. Environ. Sci 2022, 111, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, H.L.; Jing, S.G.; Gao, Y.Q.; Peng, Y.R.; Lou, S.R.; Cheng, T.T.; Tao, S.K.; Li, L.; Li, Y.J.; et al. Characteristics and sources of volatile organic compounds (vocs) in shanghai during summer: Implications of regional transport. Atmos. Environ. 2019, 215, 116902. [Google Scholar] [CrossRef]
- Hui, L.R.; Liu, X.G.; Tan, Q.W.; Feng, M.; An, J.L.; Qu, Y.; Zhang, Y.H.; Deng, Y.J.; Zhai, R.X.; Wang, Z. Voc characteristics, chemical reactivity and sources in urban wuhan, central china. Atmos. Environ. 2020, 224, 117340. [Google Scholar] [CrossRef]


| Compounds | Evaporation Point (°C) | Freezing Point (°C) | Retention Time (min) | Quantifier m/z | Qualifier m/z |
|---|---|---|---|---|---|
| Isoprene | 34.1 | −146.0 | 8.8 | 67 | 68 |
| α-Pinene | 156.1 | −62.5 | 26.1 | 93 | 91 |
| Camphene | 159.5 | 52.0 | 26.9 | 93 | 121 |
| Sabinene | 163.7 | - | 27.6 | 93 | 91 |
| β-Myrcene | 167.0 | −10.0 | 27.8 | 93 | 69 |
| β-Pinene | 166.0 | −61.0 | 27.8 | 93 | 69 |
| 2-Carene | 167.5 | 25.0 | 28.4 | 93 | 121, 138 |
| 3-Carene | 170.2 | 25.0 | 28.7 | 93 | 91, 79 |
| D-limonene | 176.5 | −74.0 | 29.3 | 93 | 93, 67 |
| p-Cymene | 177.1 | −67.9 | 29.4 | 119 | 134, 91 |
| Ocimene | 184.1 | −27.0 | 29.6 | 93 | 91, 79 |
| Cineole | 176.4 | 1.50 | 29.7 | 81 | 108, 71 |
| γ-Terpinene | 182.0 | −59.0 | 30.1 | 93 | 91, 136 |
| Isolongifolene | 256.0 | - | 37.7 | 161 | 105, 91 |
| Longifolene | 257.8 | - | 38.1 | 161 | 94, 91 |
| α-Cedrene | 262.0 | - | 38.1 | 119 | 93, 105 |
| β-Caryophyllene | 264.0 | <25.0 | 38.2 | 93 | 133, 91 |
| α-Caryophyllene | 268.0 | <25.0 | 38.9 | 93 | 80, 121 |
| Order | Compound | Internal Standard | Linear Range (ppbv) | Calibration Equation | R2 | DL (ppbv) | Repeatability | Recovery | Memory Effect |
|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | IS-3 a | 0.022~2.63 | y = 14766x − 2005 | 0.989 | 0.006 | 3.8% | 85.2% | 0.1% |
| 2 | Camphene | IS-4 b | 0.022~2.63 | y = 1736x − 56 | 0.999 | 0.007 | 2.2% | 92.8% | 1.6% |
| 3 | Sabinene | IS-4 | 0.022~2.63 | y = 20215x − 3487 | 0.974 | 0.006 | 3.5% | 88.2% | 0.1% |
| 4 | β-Myrcene | IS-4 | 0.022~2.63 | y = 20051x − 3509 | 0.973 | 0.007 | 4.0% | 89.7% | 0.1% |
| 5 | β-Pinene | IS-4 | 0.022~2.63 | y = 20194x − 3466 | 0.974 | 0.008 | 3.7% | 88.2% | 0.1% |
| 6 | 2-Carene | IS-4 | 0.022~2.63 | y = 25584x − 4126 | 0.984 | 0.009 | 3.0% | 91.3% | 0.1% |
| 7 | 3-Carene | IS-4 | 0.022~2.63 | y = 47647x − 7274 | 0.988 | 0.007 | 2.7% | 92.8% | 0.1% |
| 8 | D-limonene | IS-4 | 0.022~2.63 | y = 14587x − 2606 | 0.977 | 0.008 | 5.3% | 92.8% | 1.0% |
| 9 | p-Cymene | IS-4 | 0.023~2.75 | y = 185674x − 23335 | 0.995 | 0.006 | 2.0% | 88.6% | 0.1% |
| 10 | Ocimene | IS-4 | 0.018~2.10 | y = 19435x − 2559 | 0.983 | 0.007 | 2.9% | 93.2% | 0.1% |
| 11 | Cineole | IS-4 | 0.016~1.85 | y = 42588x − 6874 | 0.999 | 0.006 | 0.9% | 81.8% | 0.1% |
| 12 | γ-Terpinene | IS-4 | 0.022~2.63 | y = 39384x − 5649 | 0.987 | 0.005 | 4.9% | 86.7% | 0.1% |
| 13 | Isolongifolene | IS-4 | 0.018~2.10 | y = 39203x -2170 | 0.998 | 0.007 | 2.3% | 85.6% | 0.8% |
| 14 | Longifolene | IS-4 | 0.015~1.75 | y = 20635x − 1156 | 0.997 | 0.006 | 1.6% | 85.6% | 1.0% |
| 15 | α-Cedrene | IS-4 | 0.015~1.75 | y = 51656x − 3322 | 0.996 | 0.008 | 3.2% | 84.4% | 1.0% |
| 16 | β-Caryophyllene | IS-4 | 0.015~1.75 | y = 8529x − 892 | 0.988 | 0.007 | 5.2% | 86.1% | 0.6% |
| 17 | α-Caryophyllene | IS-4 | 0.017~2.00 | y = 6580x − 1005 | 0.975 | 0.009 | 5.8% | 86.1% | 0.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Gong, D.; Li, Q.; Liu, S.; Deng, S.; Wang, H.; Li, H.; Wang, B. Development of a Refrigerant-Free Cryotrap Unit for Pre-Concentration of Biogenic Volatile Organic Compounds in Air. Atmosphere 2024, 15, 587. https://doi.org/10.3390/atmos15050587
Ding X, Gong D, Li Q, Liu S, Deng S, Wang H, Li H, Wang B. Development of a Refrigerant-Free Cryotrap Unit for Pre-Concentration of Biogenic Volatile Organic Compounds in Air. Atmosphere. 2024; 15(5):587. https://doi.org/10.3390/atmos15050587
Chicago/Turabian StyleDing, Xiaoxiao, Daocheng Gong, Qinqin Li, Shiwei Liu, Shuo Deng, Hao Wang, Hongjie Li, and Boguang Wang. 2024. "Development of a Refrigerant-Free Cryotrap Unit for Pre-Concentration of Biogenic Volatile Organic Compounds in Air" Atmosphere 15, no. 5: 587. https://doi.org/10.3390/atmos15050587
APA StyleDing, X., Gong, D., Li, Q., Liu, S., Deng, S., Wang, H., Li, H., & Wang, B. (2024). Development of a Refrigerant-Free Cryotrap Unit for Pre-Concentration of Biogenic Volatile Organic Compounds in Air. Atmosphere, 15(5), 587. https://doi.org/10.3390/atmos15050587







