Abstract
Salt Lakes, having a salt concentration higher than that of seawater and hosting unique extremophiles, are predominantly located in drought-prone zones worldwide, accumulating diverse salts and continuously emitting salt dust or aerosols. However, knowledge on emission, chemical composition, and health impacts of Salt Lake aerosols under climate change is scarce. This review delves into the intricate dynamics of Salt Lake aerosols in the context of climate change, pointing out that, as global warming develops and weather patterns shift, Salt Lakes undergo notable changes in water levels, salinity, and overall hydrological balance, leading to a significant alteration of Salt Lake aerosols in generation and emission patterns, physicochemical characteristics, and transportation. Linked to rising temperatures and intensified evaporation, a marked increase will occur in aerosol emissions from breaking waves on the Salt Lake surface and in saline dust emission from dry lakebeds. The hygroscopic nature of these aerosols, coupled with the emission of sulfate aerosols, will impart light-scattering properties and a cooling effect. The rising temperature and wind speed; increase in extreme weather in regard to the number of events; and blooms of aquatic microorganisms, phytoplankton, and artemia salina in and around Salt Lakes, will lead to the release of more organic substances or biogenic compounds, which contribute to the alteration of saline aerosols in regard to their quantitative and chemical composition. Although the inhalation of saline aerosols from Salt Lakes and fine salt particles suspended in the air due to salt dust storms raises potential health concerns, particularly causing respiratory and cardiovascular disease and leading to eye and skin discomfort, rock salt aerosol therapy is proved to be a good treatment and rehabilitation method for the prevention and treatment of pneumoconiosis and chronic obstructive pulmonary disease (COPD). It is implied that the Salt Lake aerosols, at a certain exposure concentration, likely can delay the pathogenesis of silicosis by regulating oxidative stress and reducing interstitial fibrosis of the lungs. It emphasizes the interconnectedness of climate changes, chemical composition, and health aspects, advocating for a comprehensive and practical approach to address the challenges faced by Salt Lake aerosols in an ever-changing global climate.
1. Introduction
On Earth’s surface, two main types of saltwater exist: marine waters, constituting the vast oceans, and epicontinental (inland surface) Salt Lakes. Salt Lakes are permanent or temporary bodies of water with a salinity level exceeding 3 g L−1, disconnected from the marine environment. These lakes are found in various conditions, including extreme temperatures, and are especially common in drylands, particularly arid and semi-arid regions with low precipitation and vulnerable ecosystems [1]. The term “Salt Lake aerosols” refers to microscopic particles suspended in the air, originating from these Salt Lakes and their water bodies. These particles form through pathways like water spray [2], wind erosion [3], and anthropogenic processes [4]. Saline lakes and playas [5,6] are significant, dynamic sources of inland salt aerosol particles, often mixed with mineral dust, both externally and internally. These particles can undergo long-distance transport [7,8,9,10]; participate in atmospheric heterogeneous chemistry; and influence radiative balance [11], precipitation patterns [12], and climate systems [13], playing active roles in aerosol and cloud formations [14] due to their high hygroscopicity [15] and contributing to the formation of secondary aerosols, which degrade air quality.
Semi-arid and arid climate zones are expected to experience significantly reduced net precipitation and runoff due to global warming. Climate projections suggest an increase of 2.5 °C–3.7 °C in the average global surface temperature by the end of the twenty-first century [16]. This temperature rise, with an annual rate of 0.02 to 0.0325, could lead to a significant increase in evaporation—by about 190 mm, rising from 1300 mm in 1990–2010 to 1490 mm in 2070–2090. This increase is attributed to a temperature rise ranging from 1.7 °C to 3.2 °C [17]. Consequently, lakes and rivers in drylands will undergo changes in both water yield and water quality in response to considerable global shifts in temperature and precipitation patterns due to climate change. Such changes may profoundly affect the structure, functioning, and biodiversity of inland aquatic ecosystems. Inland Salt Lakes are particularly vulnerable, facing significant impacts. Climate change exerts profound and multifaceted effects on these lakes, altering their physical, chemical, and ecological characteristics. The ecosystems of Salt Lakes are especially susceptible to disturbances caused by climate change and global warming, further exacerbated by unsustainable human activities [18]. The impact is particularly pronounced in instances of desiccation [19,20]. Globally, the warming associated with climate change has been linked to reduced primary production, leading to diminished nutrient input into surface waters from mixing [21]. The extensive consequences of such disruptions are well-documented in cases like the Aral Sea, Lake Urmia [22], and other similar instances [23].
As global temperatures rise and weather patterns shift, Salt Lakes undergo notable changes in water levels, salinity, and overall hydrological balance. In recent decades, water bodies like Lake Eyre, Lake Mead, Lake Poopó, the Dead Sea, and the Aral Sea have experienced substantial shrinkage, primarily due to drought and human activities [24]. Additionally, the emissions and transportation of aerosols derived from Salt Lakes are significantly altered. Factors such as climate change, increased lake water temperature, and enhanced evaporation tend to amplify aerosol emissions. The drying of Salt Lakes has led to the occurrence of salt-dust storms, increased emission of aerosols, and soil degradation, all of which negatively impact human health and the environment [25,26].
Despite the vast extent of dry regions globally, comprising about one-third of the total land area, and the numerous Salt Lakes within these areas, limnologists and others interested in inland freshwater have largely overlooked Salt Lakes until recently. This oversight may stem from misunderstandings about their distribution, global volume, and value, all of which have been underestimated. Recognizing their pervasive influence on atmospheric processes, this body of work serves as a foundation for our broader exploration into aerosol dynamics over Salt Lakes, as there has been limited research on aerosol emissions from dry Salt Lakes, and studies on emissions from wet Salt Lakes are notably absent. Regarding the climate change makes great influences on Salt Lakes, as well as their aerosol emission, we conduct this comprehensive review, aiming to recognize the importance of Salt Lake aerosols and address their up-to-date progress on emission; components; physicochemical characteristics; and multifaceted impacts on climate, air quality, human health, and environmental processes.
This review article set out with the following objectives: Firstly, to identify major Salt Lakes around the globe and assess their importance from environmental, health, and social perspectives. Secondly, to conduct a comprehensive examination of changes in Salt Lake aerosol emissions under varying climatic conditions. Furthermore, the review aims to characterize the chemical composition changes of Salt Lake aerosols, with a focus on shifts in size distribution and elemental components. A crucial goal of this article is to evaluate the health implications of these climate-induced changes in Salt Lake aerosols, considering both immediate and long-term effects on respiratory and cardiovascular health. The outcomes of this review are expected to provide valuable insights into the role of Salt Lakes in ecosystem sustainability and health. Additionally, this review explores the diverse conditions of different Salt Lakes and their aerosols, offering future predictions regarding their ecosystems in light of the challenges posed by global warming.
2. Global Distribution of Salt Lakes
A Salt Lake, or saline lake, is a landlocked body of water with a salt concentration that is significantly higher than that of most lakes, typically defined as at least 3000 milligrams of salt per liter. These salts include primarily sodium chloride, as well as sodium sulfate, sodium nitrate, and sodium carbonate. Often, Salt Lakes have a salt concentration higher than that of seawater. To form a Salt Lake, two essential criteria must be met. First, the lake must be part of a closed (endorheic) drainage system. Second, there needs to be a balance between hydrological inputs, which include surface and subsurface inflows, as well as precipitation over the lake, and outputs such as evaporation and seepage into sediments. This balance is especially pertinent in arid and semi-arid regions. Figure 1 illustrates the general relationship between solute concentrations in lake waters and inflow concentrations, highlighting the delicate equilibrium between evaporation and precipitation, assuming no solute loss through seepage. This balance, denoted as 1, is represented by the following equation:
where 1 represents the balance, (v)evap is the average volume of water lost by evaporation, (v)prec is the average volume of water gained from precipitation, and (v)infll is the average volume of water flowing into the lake. The greater the net evaporation, the more concentrated the lake solutes become, with the relationship being hyperbolic. The major ions found in Salt Lakes are the same as those in fresh waters, namely Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and CO32−/HCO3−, but their ionic proportions differ [27].
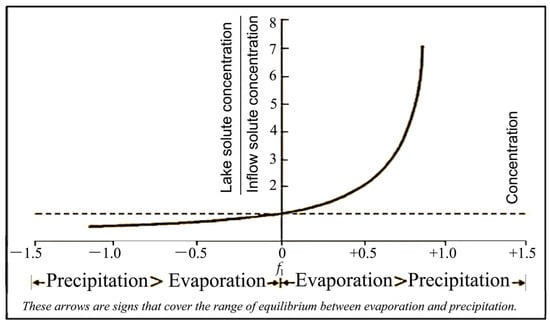
Figure 1.
The relationship between lake salinity and inflow salinity.
It is important to note that Salt Lakes are widespread and found in both cool/cold and warm/hot dry regions on all continents, including Antarctica, as shown in Figure 2A. Global estimates suggest that the volume of freshwater lakes is approximately 105,000 km3, while the volume of inland saline water is estimated at around 85,000 km3 [28]. Notably, Salt Lakes display diverse features, including the Caspian Sea, the largest lake globally, with an area of 371,000 km2 and a salinity of 12–13 g per liter (g L−1). These lakes can also be found at extreme altitudes, over 3000 m above sea level, in regions such as the Altiplano of South America and Tibet. Additionally, the Dead Sea, located approximately 400 m below sea level, is recognized as the lowest lake on Earth [29]. The salinity of saline lakes varies widely, ranging from 3 ppt (parts per thousand) to more than 300 ppt, and these lakes are present in equatorial regions and across both hemispheres (Table 1). In arid Central Asia, various types of lakes are mainly located in the central and western parts. China’s arid land is dotted with more than 700 lakes, predominantly in Xinjiang. Kazakhstan is home to 48,262 lakes, of which 45,248 are smaller than 1 km2, collectively accounting for 40% of the total area of the lake region [30]. Hypersaline lakes (Figure 2B) are unique water bodies that lack natural outlets, constituting approximately 23% of the total lake area on our planet [31,32]. These lakes are typically small and shallow, exhibit dynamic variations in water parameters, and are prone to frequent drying. Found across various regions globally, especially in arid and desert areas, they have been critical in meeting water supply needs through well utilization.
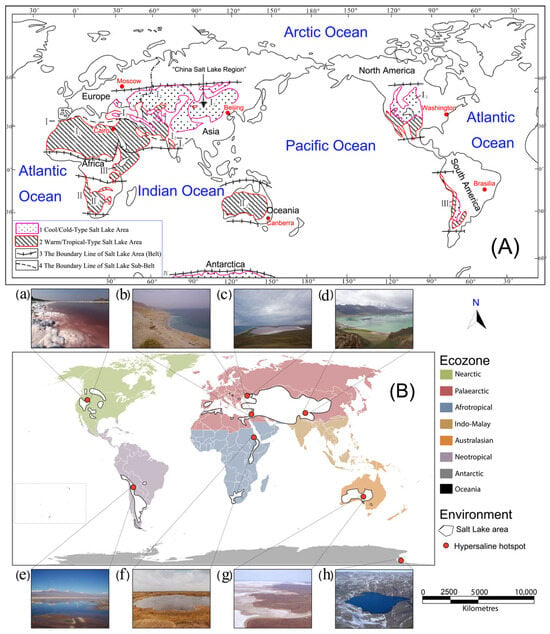
Figure 2.
(A) Global distribution of Salt Lakes (shaded areas using black lines and dots). (B) Worldwide Salt Lake areas with the main hypersaline hotspots. (a) Great Salt Lake (Utah, USA), (b) Dead Sea (Israel), (c) Crimean Salt Lake (Crimea), (d) Dangxiong Co Salt Lake (Tibet, China), (e) Laguna Puilar, Salar de Atacama (Chile), (f) Gaet’ale Pond (Ethiopia), (g) Kati Thanda-Lake Eyre (Australia), and (h) Deep Lake (Antarctica). Oceania is illustrated in black within the rectangle at the bottom left corner of the map (adapted from Mattia Saccò 2021 [33]).
Salt Lakes possess significant aesthetic, cultural, economic, recreational, scientific, conservation, and ecological values [34]. Historically, they have played essential roles in various human activities and served multiple purposes. These lakes have been utilized for salt production and serve as critical water sources in arid regions where groundwater resources are limited. Additionally, they have contributed to food provision and climate regulation, supported avifauna populations, attracted tourism, and provided visually appealing landscapes [35]. A key species found in these environments is the brine shrimp, Artemia salina, which is an effective initial food source for many aquacultural organisms [36,37]. Artemia plays a vital role in regulating the hydromineral regime of Salt Lakes. As a filter-feeding crustacean, it participates in the transformation and utilization of various minerals and types of organic matter in these basins. It serves as a crucial link in the food chain and in the biogenic migration of elements, including xenobiotics. These roles underscore the value of Salt Lakes to human communities. Salt Lakes are unique and extraordinary aquatic ecosystems, characterized by both abiotic and biotic parameters that are susceptible to environmental changes. These changes, influenced by factors such as air and water temperature, oxygen concentration, pH, and chemical composition, can occur on both annual and daily scales. Since Salt Lake aerosols are closely linked with the water quality, it is important to gain knowledge of the Salt Lakes’ distribution, location, function, and abiotic and biotic parameters for a better understanding the Salt Lake aerosols’ physicochemical characteristics and environmental behaviors.
Besides from the representative Salt Lakes in the world, as listed in Table 1, more attention has been paid to a colorful Salt Lake, which shows red, pink, orange, blue, green, and cyan in summer, autumn, and even winter (Figure 3). Nestled within the vibrant tapestry of China’s landscape, the Yuncheng Salt Lake, with an area of around 132 km2, stands as a captivating natural wonder. It is renowned for its long history, with a mining history of more than 4600 years, and unique and special characteristics such as different separated pools with different colors (Figure 3b), creating distinctive and mesmerizing scenery. Located in the middle inland of China, it lies close to the Zhongtiao Mountain (Figure 3a), and a strong south wind blows over the water surface year by year, leading to high evaporation and a large amount of Salt Lake aerosols generated. The Yuncheng Salt Lake is particularly known for its abundant salt reserves, which form expansive crystalline fields that shimmer under the radiant sunlight. Beyond its economic significance, the lake is ecologically important, providing a habitat for a diverse range of bird species (Figure 3c) and exhibiting remarkable resilience to environmental challenges. The algae community in the lake, particularly the halophilic algae, distinguishes it from freshwater and marine systems. The secretions produced by the growth and reproduction of these halophilic algae in high-salt environments contribute significantly to the lake’s colorful appearance. Also, they contribute to the algal toxins, which are likely contained in the Salt Lake aerosols and influence human health. With global warming, great changes will occur regardless of the water quality or water yield of the lake. Under this condition, it is important to characterize Salt Lake aerosols from different colors of pools in quantity and components and assess their roles played in climate change, the atmospheric environment, and human health.
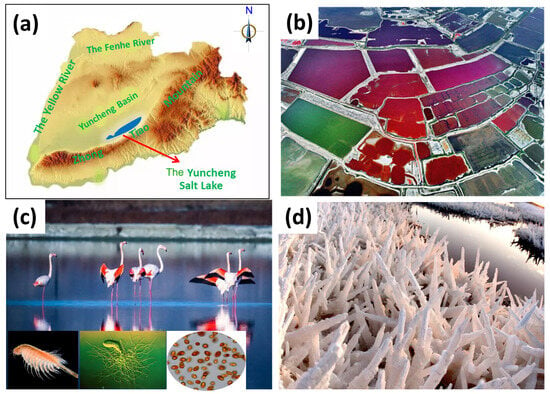
Figure 3.
The ethereal beauty of the Yuncheng Salt Lake (located in Shanxi Province, China), combined with its ecological and economic value, establishes it as a site of both natural wonder and cultural significance. (a) Location of Yuncheng Salt Lake. (b) Color pools due to different salinity and algal growth. (c) Salt Lake biodiversity. (d) Salt crystallization and accumulation under low temperature.

Table 1.
Major characteristics of representative Salt Lakes in the world.
Table 1.
Major characteristics of representative Salt Lakes in the world.
| Lake | Country | Area (km2) | Maximum Depth (m) | Salinity (%) | References |
|---|---|---|---|---|---|
| Caspian Sea | Azerbaijan, Iran, Russia, Turkmenistan, Kazakhstan, | 371,000 | 1015 | 15 | Pervov et al. (2003) [38] |
| Urmia Lake | Iran | 5800 | 16 | 300 | Babkin (2003) [39] |
| Aral Sea | Kazakhstan, Uzbekistan | 8550 | 28 | 100 | Micklin (2007) [40] |
| Dead Sea | Israel, Jordan, Palestine | 940 | 320 | 340 | Yechieli et al. (1998) [41] |
| Balkhash | China, Kazakhstan | 17,000 | 27 | 7 | Williams (1996) [29] |
| Dabuxun | China | 184 | 0.4 | 360 | Yu et al. (2001) [42] |
| Qinghai | China | 4278 | 26 | 14 | Lister et al. (1991) [43] |
| Van | Turkey | 3570 | 450 | 22 | Kempe et al. (1991) [44] |
| Great Salt Lake | United States | 4660 | 14 | 250 | Wurtsbaugh & Berry (1990) [45] |
| Salton Sea | United States | 891 | 12 | 33 | Williams (1996) [29] |
| Mono Lake | United States | 158 | 46 | 95 | Williams (1996) [29] |
| Mar Chiquita | Argentina | 5770 | 8.6 | 360 | Reati et al. (1996) [46] |
| Salar de Uyuni | Bolivia | 10,500 | - | 271 | Schmidt (2010) [47] |
| Natron | Tanzania | 1040 | 0.5 | 12 | Schagerl (2016) [48] |
| Assal | Djibouti | 54 | 40 | 277 | Schagerl (2016) [48] |
| Nakuru | Kenya | 42 | 4.6 | 62 | Jirsa et al. (2013) [49] |
| Bogoria | Kenya | 33 | 9 | 36 | Jirsa et al. (2013) [49] |
| Eyre | Australia | 8430 | 5.7 | 310 | Jankowski & Jacobson (1989) [50] |
| Corangamite | Australia | 233 | 4.9 | 50 | Williams (1996) [29] |
| Tyrrell | Australia | 300 | 0.5 | 160 | Heidelberg et al. (2013) [51] |
| Elton | Russia | 155 | 0.6 | 300 | Argaman et al. (2012) [52] |
| Gallocanta | Spain | 6 | 0.1 | 37 | Pearson et al. (2008) [53] |
| Chiprana | Spain | 0.3 | 5.6 | 73 | Vila et al. (2002) [54] |
| Fuente de Piedra | Spain | 14 | 0.5 | 220 | García & Niell (1993) [55] |
| Pétrola Lake | Spain | 2 | 2 | 50 | Valiente et al. (2018) [56] |
3. Production Mechanism of Salt Lake Aerosols
Salt Lake aerosols are produced through various processes, including lake-water spray, evaporation-induced salt crystallization, wind-driven dust emissions, microbial activities, chemical reactions, and anthropogenic influences. The primary mechanism involves the breaking of wind-driven waves at the lake surface. As the wind blows across the water, it generates waves. When these waves reach a critical size, they break and release tiny droplets into the air, which become airborne particles and form aerosols (Figure 4a). The generation of Salt Lake spray aerosols (LSAs) is governed by a mechanism akin to that of sea spray aerosols (SSAs) in saltwater [57]. LSAs form through the entrainment of air bubbles by breaking water waves, followed by the bursting of these bubbles at the water’s surface (Figure 4b) [58]. LSAs were first identified above the surface of the Laurentian Great Lakes in North America during an aircraft sampling campaign in the summer of 2009 [59]. This discovery has since sparked increased research interest for different types of lakes [60,61].
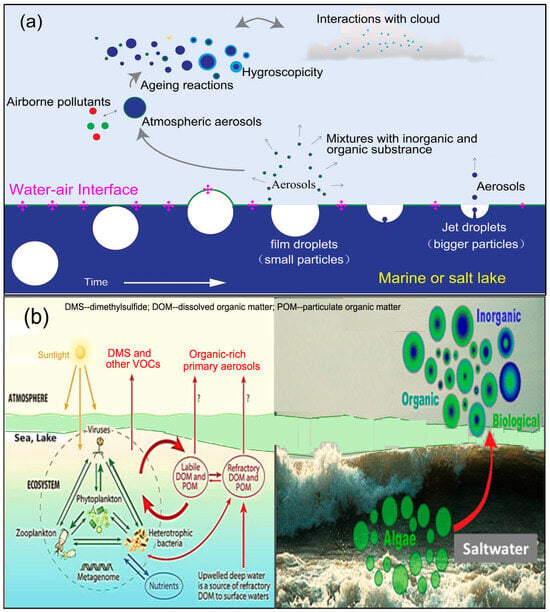
Figure 4.
Mechanism of Salt Lake aerosol generation: (a) aerosols generated from film and jet droplets; (b) possible organic and inorganic components of Salt Lake aerosols.
Apart from the Salt Lake spray aerosols, salt-dust storms are a significant source of aerosols from desiccated surfaces and dried bottoms of Salt Lakes, playing a crucial role in the Earth’s energy balance and hydrological cycle by altering solar and terrestrial radiation [62,63]. During dust storms, the salt-containing particles can transport far away from their sources, making great impacts on air quality, vegetation growth, soil salinization, local climate, and human health [64]. Studies examining the physicochemical characteristics of atmospheric particles in Beijing during Asian dust storms showed that the particles enriched with S, Cl, and Na were likely from the surface soils of dried Salt Lakes and saline soils enriched with chloride and sulfate, demonstrating that, besides deserts, the surface soils from dry Salt Lakes and saline soils of arid and semi-arid areas were also sources of particulates in dust storms [65]. These halite-containing particles from dry Salt Lakes and saline soils of arid and semi-arid areas would have global climate impacts during long-range transport [66,67] and contribute to airborne PM2.5 and PM10 pollution in cities [68]. Characterizations of bulk aerosols and individual particles in dust storms even showed that nearly all the particles from dry Salt Lakes and saline soils experienced an ageing process through heterogeneous reactions, morphometric modifications, and compositional transformations during long-range transport [69,70,71,72]. Cahill et al. [73] reported that the Owens Lake (dry) lakebed is likely the largest single source of PM10 in the United States, with estimates suggesting emissions of 0.9 × 10⁶ to 8 × 10⁶ metric tons per year [74]. Wind gusts exceeding 40 m s−1 can propel tons of sediment across the playa, resulting in PM10 levels as high as 40,620 µg m−3 during a 2 h sampling period. Ebinur Lake has seen a sharp reduction in inflow due to the expansion of irrigated agriculture and the damming of surface runoff, leading to a significant decrease in the lake area to a range of 500–1200 km2. The now dried lakebed, exceeding 500 km2, expels around 4.8 × 10⁶ tons of dust annually [75], becoming a significant source of aerosol emission. The Aral Sea is another notable example, annually releasing approximately 1.0 × 10⁶ tons of saline dust. In short, it is suggested that one globally significant source of saline dust is dry lakebeds (i.e., playas), which emit high mass concentrations of saline dust to the atmosphere compared to their small spatial extents.
4. Chemical Composition of Salt Lake Aerosols
Endorheic saline lakes are common features in arid and semi-arid regions. On the one hand, the Salt Lake spray aerosols (LSAs) are generated through the entrainment of air bubbles by breaking water waves, followed by the bursting of these bubbles at the water’s surface; on the other hand, the saline aerosols can be formed from the dry lakebed due to desiccated Salt Lakes and the saline soils near them. Lake desiccation is associated with increased water salinity, as well as the exposure of easily erodible sandy soil that is bare of vegetation, which promotes increased primary aerosol emissions. Thus, resuspension becomes the predominant mechanism to emit saline and crustal emissions aloft above the lakebed, leading to nearly 60% of PM10 [7]. Whether for the LSAs or the resuspended saline dust, their chemical composition depends on the characteristics of the lake and the source of emission. In a study on the spatial distribution of water-soluble ion concentrations in wet deposition samples around Lake Urmia, the world’s second largest hypersaline lake, it was found that the most dominant ions are as follows: Ca2+ > Cl− > SO42− > Na+ > NO3−. Meanwhile, organic acids and methanesulfonate (MSA) contributed negligibly to the total ion concentrations [76]. A principal component analysis (PCA) and correlation coefficient (CC) analyses showed that a majority of ions throughout the region were associated with salt and crustal particles comprising Cl−, Br−, SO42−, Na+, Ca2+, and Mg2+, with the minority of ions (e.g., NH4+ and NO3−) stemming from anthropogenic emissions. Concurrent measurements of water-soluble ions in aerosol samples were also taken at two sites in the north and southeast of Lake Urmia from January 2013 to September 2013 [77]. Particulate matters (PMs) were measured using a high-volume sampler and HAZ-DUST EPAM-5000 particulate air monitors. The results, supported by backward trajectories and correlation analysis, indicated that the concentrations of SO42−, organic carbons (OCs), As, Pb, and Zn were at their maximum levels. Another investigation of aerosol properties near Lake Eyre in Central Australia, a dominant mineral dust source in the southern hemisphere, also highlighted the dominance of SO42−, Ca2+, Na+, and Cl−; in the aerosols, implying that halite or reacted halite particles were included [78]. Previous research at Qinghai Lake, the largest saline lake in China, primarily focused on paleoclimate and paleoenvironmental information derived from lake sediments [79,80] and water chemistry [81]. A study conducted on Qinghai Lake by Zhang et al. [82] explored the chemical composition of PM2.5 and total suspended particulate (TSP) samples from June to September 2010, showing that the predominant anions and cations in both PM2.5 and TSP samples were SO42− and Ca2+, followed by NO3−, Na+, K+, and Cl−. Therein, the recorded mass concentrations were 21.27 ± 10.70 µg/m3 for PM2.5 and 41.47 ± 20.25 µg/m3 for TSP, with a mean PM2.5/TSP ratio of 0.51. It is implied that the water-soluble ions in PM2.5 and TSP were not only affected by lakewater but by acid gases from anthropogenic sources. Concentrations of the major ions in different Salt Lake aerosols are shown in Table 2.

Table 2.
Concentration of major ions (μg m−3) in different Salt Lake aerosols.
The typical NaCl- and Na2SO4-containing aerosols were identified from the individual particle samples collected over the Yuncheng Salt Lake (Figure 5). Through SEM-EDX, not only the morphology and chemical compositions of NaCl- and Na2SO4-containing particles were illustrated, but also the elemental atomic concentrations were obtained using Monte Carlo Simulations by a modified CASINO Monte Carlo program [83,84]. The quantitative elemental composition analysis by low-Z particle EPMA revealed the atomic concentrations of a NaCl-containing particle as C 58.52%, Cl 21.96%, Na 17.48%, O 1.77%, and of a Na2SO4-containing particles as O 52.03%, Na 23.74%, S 14.48%, Mg 5.21%, and C 4.50% (Figure 5B). It is noted that a high concentration of C is included in the NaCl-containing particles, and dark shades encircle particles in the secondary electron images (Figure 5A,B), suggesting that a large amount of organic substance is included in the Salt Lake aerosols.
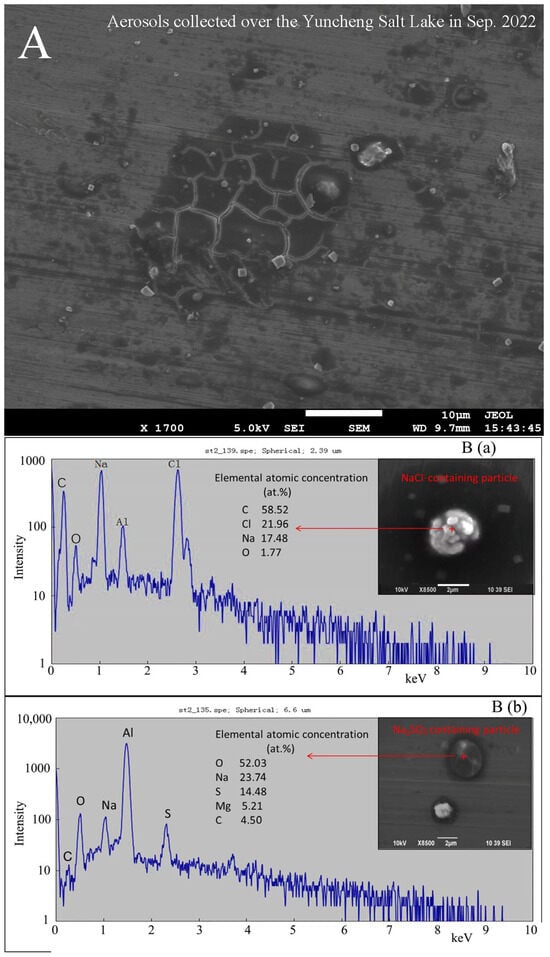
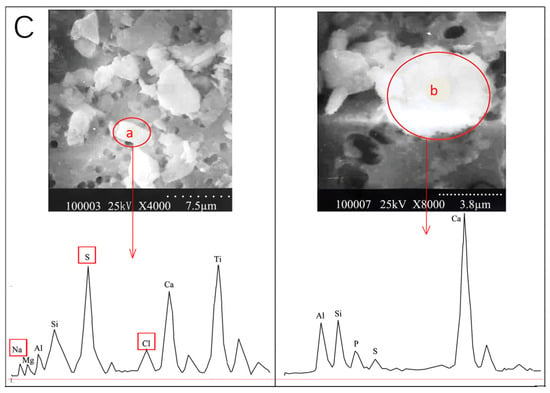
Figure 5.
A few examples of morphology and chemical composition of Salt Lake aerosols (SLAs). (A) The secondary electron images (SEIs) of Salt Lake aerosols collected over the Yuncheng Salt Lake, Shanxi Province, China, in September 2022. (B) The typical SEIs by SEM-EDX and elemental atomic concentrations of (a) a NaCl-containing particle; (b) a Na2SO4-containing particle, in Yuncheng Salt Lake aerosols collected on Al foil in September 2022 by the authors. The high aluminum peak in the EDX spectrum is attributed to the Al foil, used to collect the atmospheric aerosols. (C) The SEIs and EDX spectra of atmospheric aerosols collected during a dust storm episode: (a) a Na-, S-, and Cl-rich particle, likely from dried salt-lakes and saline soils; and (b) a common dust particle (Zhang et al., 2009 [7]).
Regarding the fact that Salt Lake aerosols can be transported to the downwind area in the atmosphere, desiccated Salt Lakes and saline soils become the primary contributors to salt dust storms in arid regions. A comparative study was conducted on individual particles derived from a significant super dust storm (DS) with non-dust storm (NDS) aerosols in urban Beijing and a desert region located at Duolun, China [7]. A particle analysis, particularly through a Positive Matrix Factorization (PMF) analysis, revealed that the major components in (Na + S + Cl)-rich particles were Na2SO4 and NaCl, constituting 9% of the total particles and demonstrating a significant positive correlation between S and Cl. The identification of (Na + S + Cl)-containing particles was confirmed through both energy-dispersive X-ray spectroscopy (EDX) and scanning electron microscopy (SEM) images (Figure 5(Ca), compared with the common dust particles shown in Figure 5(Cb)), suggesting that the S-, Cl-, and Na-rich particles during the dust storm were likely not of desert origin but rather from dried Salt Lakes and saline soils in northern or northwest China [7].
5. Impact of Climate Change on Emissions and Chemical Composition of Salt Lake Aerosols
5.1. Impact of Climate Change on Salt Lake Aerosol Emissions
The changing climate significantly influences aerosol emissions from Salt Lakes, ushering in a new era of environmental dynamics and atmospheric composition. These effects extend to emissions, transport, and atmospheric chemistry, impacting tropospheric ozone and aerosol levels. Biogenic emissions of NOx and hydrocarbons are temperature-sensitive. Additionally, emissions of dimethyl sulfide (DMS), organic matter, and mineral dust are all affected by changes in surface winds [85]. Temperature influences the emissions of precursors, the rates of chemical reactions, and the partitioning of semi-volatile species between gases and aerosols, thereby impacting PM2.5 concentrations. As temperatures rise, ammonium and nitrate tend to partition to the gas phase, affecting nitrate aerosol levels. Higher temperatures may also accelerate gas-phase reactions and increase oxidants, influencing sulfate levels and the formation of non-sea-salt sulfate (nss-SO42−) aerosols [86,87]. In these scenarios, aerosols are expected to contain a significant amount of PM10, largely due to salt crusts, salt particles, and windblown dust. This coarse particulate matter may include fragments of salt, mineral particles, and other debris, especially under conditions of increased temperature and evaporation (Figure 6).
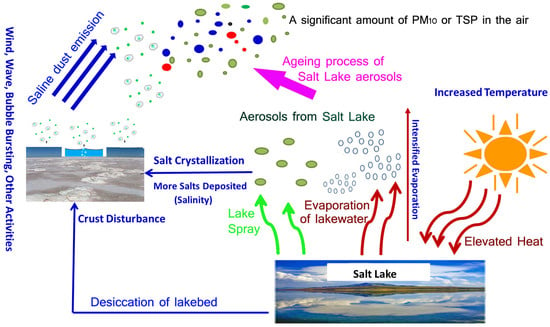
Figure 6.
Emission pathways of Salt Lake aerosols.
Wind stress and surface temperature are the primary influencers of Salt Lake aerosol emission [88,89]. A notable study by Slade et al. [59] in 2009 explored substantial particle generation from the surface of the Great Lakes, specifically over Lake Michigan. This involved vertical profiling of submicron particle size distributions. The study revealed significant increases in concentrations of ultrafine particles, with their source identified at or near the lake surface. The observed correlation between Aitken mode aerosol concentration and temperature (Figure 7A) follows an exponential pattern. This suggests that the particle formation observed is likely attributed to breaking waves along the lake surface, influenced by both temperature and wind speed. Another study, which was focused on the Western Antarctic Peninsula (WAP), analyzed satellite-retrieved marine aerosols over a 12-year period, using proxies such as the coarse-mode aerosol optical depth (AODC) and marine aerosol optical depth (MAOD) across open ocean regions [90]. The study found that the MAOD showed strong correlations with wind speed, indicating a relationship between wind-driven aerosol production and open ocean conditions. Additionally, a significant correlation with wind speed and a weak but notable correlation with sea surface temperature (SST) were observed. These findings contribute to a better understanding of marine aerosol dynamics in the unique environmental conditions of the Western Antarctic Peninsula. Furthermore, a statistical analysis of a global dataset of airborne sea salt aerosol (SSA) measurements, collected at 150 to 200 m above the ocean surface during the NASA Atmospheric Tomography Mission, revealed that incorporating SST significantly improves the predictability of observed SSA concentrations compared to using wind speed alone. These results underscore the importance of including SST in SSA source functions in global models to enhance our understanding of the atmospheric burden of SSA [91].
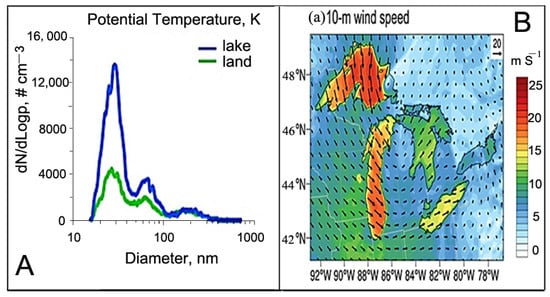
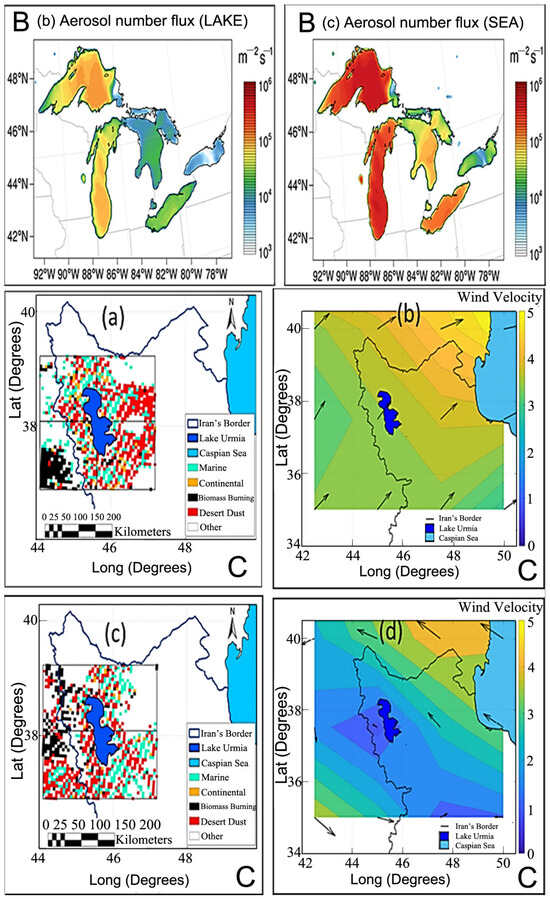
Figure 7.
Aerosol generation under temperature ((A), adapted from [59]). (B) Lake spray aerosol emission flux under wind speed (a–c) (adapted from [92]). (C) Aerosol types and wind velocity in March 2016 (a,b) and June 2016 (c,d) (adapted from [93]).
Similar to the process of generating SSAs in saltwater, Salt Lake spray aerosols can be generated through the entrainment of air bubbles by breaking water waves, followed by the subsequent bursting of these bubbles on the water surface. Harb et al. [92] conducted an experiment to develop a Lake Spray Source Function (LSSF) based on measurements of foam area and the corresponding emission flux of lake spray aerosols in a Marine Aerosol Reference Tank (MART). For comparative purposes, a Sea Spray Source Function (SSSF) was also developed. These functions were then integrated into the Community Multiscale Air Quality (CMAQ) model to simulate particle emissions from the surface of the Great Lakes from 10 November to 30, 2016. The model outcomes indicated that the lake spray aerosol emission flux from the Great Lakes could reach approximately 105 m−2 s−1 during high wind-speed episodes (Figure 7(Ba–c)). Although these emissions increased the total aerosol number concentrations in the region by up to 1.65% on average, their impact was more substantial on coarse-mode aerosols, with a potential 19-fold increase in certain areas. The model showed that aerosol loading was primarily concentrated near the source region but could be transported up to 1000 km inland. Above the lakes, the lake spray aerosol particles could reach the cloud layer, causing increases of up to 3% and 98% in total and coarse-mode particle concentrations, respectively. This study is significant in quantifying lake spray aerosol emissions and understanding their impact on regional aerosol loading and cloud layers.
Many researchers have cited salt aerosol emissions from dried lakebeds influenced by wind effects. A case study on Lake Urmia, which is undergoing rapid drying, exemplifies this wind erosion effect [93]. Threshold values for aerosol optical depth (AOD) and the Ångström exponent were used to classify particle types over the Lake Urmia basin (Figure 7(Ca–d)), revealing distinct monthly patterns for desert dust and marine aerosol types. The relevance of dust events and background conditions in the basin highlights the need for comprehensive assessments with observations and potentially different threshold values [94]. From 2000 to 2021, dust storms increased the AOD550 above average around Urmia Lake. The vertical profile of aerosols indicated the most significant contribution to total aerosol loading over the lake at altitudes of 1.5–3 km, 1.5–4 km, 1.5–5 km, and 1.5–3 km during the winter, spring, summer, and autumn seasons, respectively [95]. Taking the example of the Salton Sea in California as a natural experiment that is shrinking, it is observed that each 0.3048 m decline in lake elevation corresponds to a 0.28 μg m−3 (2.6%) increase in PM2.5 concentrations [96].
The increasing temperatures, wind speeds, and heightened evaporation associated with climate change are contributing to a more arid environment. This aridity facilitates wind action, which becomes a prominent factor in the enhanced production of aerosols from Salt Lakes. As the salinity levels in the lakes rise, more salt-containing fine particles will be formed and entrained into the air. In the Salt Lake region, the dust aerosols, often containing salts and other minerals, become a significant component of the overall Salt Lake aerosol load. In addition, changes in the Salt Lake ecosystem due to climate shifts can impact biological processes therein. This will lead to modifications in the release of biogenic compounds from algae, bacteria, and other microorganisms present in the water, which further contribute to changes in the size, number, and chemical composition of Salt Lake aerosols.
5.2. Impact of Climate Change on Chemical Composition of Salt Lake Aerosols
Climate change intricately links the chemical composition of Salt Lake aerosols to the complex dynamics within these unique ecosystems. Rising temperatures and altered precipitation patterns influence evaporation rates, impacting the concentration of dissolved salts and subsequently shaping the aerosol composition. Extensive studies conducted on saline lakes revealed a dominance of Na, Cl, and sulfate ions in larger lakes [97,98,99], implying that the Salt Lake aerosols mainly consisted of NaCl and Na2SO4. Fluctuations in the water levels and quality of saline waters lead to noticeable changes in the aerosol’s chemical composition, potentially reflecting regional climatic shifts [100]. The elemental composition of particulate matter in the southeast and north of Lake Urmia identifies the lake’s crustal soil as the primary source of NaCl; BaCl2; and elements such as Al, Ti, Ca, P, Mn, K, F, and Si [101]. However, direct evidence of Lake Urmia’s involvement in regional dust events is limited, partly due to the absence of air pollution monitoring stations near the lake.
Saline dust storms represent a unique phenomenon, transporting significant quantities of saline, alkaline, and potentially harmful particulate matter from dried lakebeds to neighboring regions [102]. Strong winds carry large-scale aerosol particles predominantly towards the downwind direction, impacting the atmospheric chemistry over the downwind region. Wind speeds exceeding 8 m s−1 result in particle emission rates of approximately 106 m−2 s−1. In the Great Lakes region, the composition of these large-scale aerosol particles is mainly dominated by CaCO3, which accounts for about 58% of the mass. These particles also include components such as CaSO4, Ca(NO3)2, Na2SO4, NaCl, NaNO3, and organic carbon (OC) [103]. Less than 24% of the mass fraction comprises elements with anthropogenic origins [103]. Given that cations and other inorganic species constitute the majority of large-scale aerosol emissions, they can potentially alter the thermodynamic equilibrium of the local aerosol population.
Wind erosion significantly influences sediment emission and their chemical composition from lake surfaces. A study conducted on QeHan Lake (114°45′–115°04′ E, 43°22′–43°29′ N), a closed Salt Lake located in the northern part of the Otindag sandy land region on the Inner Mongolia Plateau, examined aerosol particle emission and their chemical composition under wind erosion from two dried surfaces (permanently and intermittently dried surfaces) [104]. It was found that the proportion of salt in the dust was higher for the intermittently dried surfaces than for the permanently dried surfaces. The dust content varied linearly with the height of the intermittently dried surface and exponentially with the height of the permanently dried surface. The salt dust particles collected from both surfaces were below PM10 in size, and the unit mass concentration of each ion (mainly Na+, Cl−, and SO42−) in the salt dust was also higher for the intermittently dried surface than for the permanently dried surface. The results indicate that salt dust emission is a key factor in salt dust aerosol emission, particularly under the influence of wind speed.
As reported by Qingmin Meng [105], climate changes, especially local warming and extreme weather including both precipitation and temperature, drive the dynamics of the Great Salt Lake (GSL) surface levels. The annual increasing rate of 0.0313 in temperature from 1971 to 2016 could result in more than 40% loss of its total water storage each year. It can be deduced that climate change significantly impacts the chemical composition of Salt Lake aerosols through various interconnected processes. Changes in temperature and precipitation patterns alter evaporation rates, influencing the concentration of salts and dissolved substances in Salt Lakes, and thereby shaping the aerosols’ composition during evaporation. Shifts in salinity, resulting from climate-induced variations in freshwater input and evaporation, affect the types and concentrations of salts in aerosols. Additionally, climate-driven changes in wind patterns, soil moisture, and vegetation cover can influence dust emissions from surrounding areas, introducing new chemical components to Salt Lake aerosols. Furthermore, the impact of climate change on microbial activity within Salt Lake ecosystems can lead to the production of organic compounds and gases, further contributing to aerosol composition. These complex interactions underscore the interrelated nature of climate change, hydrology, and aerosol chemistry in Salt Lake environments.
6. Health Effects of Salt Lake Aerosols
The inhalation of aerosols from Salt Lakes, formed from fine salt particles suspended in the air, raises potential health concerns, particularly for respiratory and cardiovascular well-being. Originating from dry lakebeds or Salt Lakes, these aerosols can cause respiratory irritation and, in high concentrations, may lead to eye and skin discomfort. Research focusing on air pollution from Salt Lakes’ saline flow resources has gained prominence. A notable example is Lake Urmia, which has seen a significant increase in aerosol pollution over the past decade [106]. This concern is primarily linked to the extraction of deposited salts from the exposed saline flows on the lakebed. Alizadeh et al. [107] reported an inverse correlation between fluctuations in the water level of Lake Urmia and aerosol pollution. They observed an increase in the mean aerosol optical depth from 2010 to 2019, reaching 0.42, and a significant post-2013 surge in the annual mean PM10 concentration (this value is 15 µg m−3, from WHO global air quality guidelines [108]), peaking at approximately 876.13 μg m−3 in December 2015. It means that the decrease in the water level in Salt Lake might lead to an increase in ambient particulate matter, likely making hazardous effects on local people’s health. Harmful algal blooms (HABs), when whipped onto land by winds, can spray aerosolized toxins directly into the upper respiratory tract. Backer et al. [109] documented brevetoxins in nasal–pharyngeal swabs from people exposed to red tides. This highlights the potential health implications of inhaling aerosolized HAB toxins during such events (Figure 8). It is estimated that about 15% of asthma cases worldwide could be attributed to inhaling aerosolized HAB toxins in coastal areas, while a 2012 red tide outbreak in Florida was associated with approximately 11,000 hospitalizations and 4000 emergency room visits.
The Aral Sea region experiences strong, salty winds about 10 times a year, leading to the erosion of the dried lake bottom and the dispersion of large quantities of dust in the surrounding area. As Glantz (1999) reported [110], inhaling airborne dust has triggered various health problems, ranging from respiratory and skin issues to serious pulmonary diseases. The escalating levels of salt and inorganic substances in the lake have severe environmental impacts. Examining the effects of Salt Lake aerosols on Artemia parthenogenetica, a small crustacean thriving in Siberian lakes, is crucial for understanding the ecological impacts of elevated salinity levels [111]. A study investigating 27 populations of Artemia delves into the relationship between aerosol-induced salinity variations, the reproductive biology of Artemia females, and various biometric parameters [112]. With salinity levels ranging from 50 to 265 g L−1 in the study lakes, the research reveals intriguing patterns in shrimp biomass, cyst numbers, and brood characteristics across different salinity gradients of 70–144 g L−1, shedding light on the intricate ecological dynamics of this unique crustacean in response to aerosol-induced changes in its habitat.
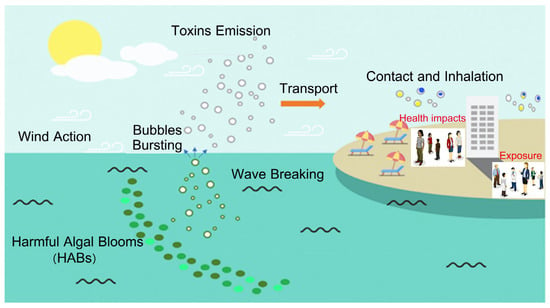
Figure 8.
Aerosolized toxins emission and health implications (adapted from study of Lim et al., 2023 [111]).
Though salt dust storms from Salt Lakes have deleterious effects on nearby populations, such as those living near the Aral Sea, some studies suggest potential health benefits of Salt Lake aerosols, particularly in children [113]. Numerous studies have indicated that salt-containing aerosols can damage the respiratory system, cardiovascular system, lungs, liver, etc. However, there is contrasting evidence, including a study from the Aral Sea region showing that children living nearby have a lower chance of suffering from lung function injuries [113]. In China, there is direct evidence that Salt Lake aerosols are beneficial for lung therapy, and the positive effects of salt vapor therapy have been discussed in various studies. In the Occupational Disease Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China, the clinical efficacy and safety of salt rock aerosol in the treatment of occupational pneumoconiosis were analyzed from 1 July 2018 to March 2010 [114]. A total of 120 patients with occupational pneumoconiosis who were treated were randomly selected as the main subjects. Twenty patients were in each group. The same basic treatment was given separately, and the treatment group applied conventional intervention as a basis for the treatment of rock salt aerosol, twice a day for 30 min. The results showed that not only the percentage improvement of the above indicators in the treatment group was significantly higher than that of the control group (p < 0.05), but also the cure time was significantly short compared with the control group: (13.6 ± 2.1) days vs. (20.4 ± 2.2) days, suggesting that, in the treatment options of occupational pneumoconiosis, the salt rock aerosol therapy is a non-drug, safe, and nontoxic method. In another study, a total of 452 subjects from six hospitals were divided based on the multilevel hierarchical random design [115]. The patients in the treatment group received “conventional comprehensive treatment + rock salt aerosol therapy”. Rock salt aerosol therapy showed more of a significant effect compared with the routine method. The clinical symptoms tend to be stable after two weeks of treatment with rock salt aerosol therapy. The curative effect increases with the extension of the treatment time. Two-to-four weeks for one course of treatment can improve the curative effect. It is concluded that rock salt aerosol therapy can effectively improve the quality of life of pneumoconiosis patients, suggesting that it is a good treatment and rehabilitation method for the prevention and treatment of pneumoconiosis; thus, it is worthy of clinical application. Hu and Li (2021) [116] reported that, compared with the routine clinical treatment, a routine clinical treatment such as bronchodilator combined with rock salt aerosol could significantly improve the forced expiratory volume in the first second (FEV1), the ratio of FEV1/Forced Vital Capacity (FVC), and other lung function indexes, and the clinical total effective rate reached 95.0%, implying that the bronchodilator combined with rock salt aerosol has outstanding clinical effect on the patients with pneumoconiosis and chronic obstructive pulmonary disease (COPD). A toxicological experiment indicated that the rock salt aerosol intervention can delay the pathogenesis of silicosis by improving the inflammatory response, regulating oxidative stress, and reducing interstitial fibrosis of lungs [117]. This suggests, in the future, the need for a broader discussion on the health effects of Salt Lake aerosols, incorporating large-scale epidemiological studies and conducting detailed investigations. Different therapies, such as Halotherapy and Inhaled Hypertonic Saline, are commonly used for treating respiratory disorders. Halotherapy involves inhaling salty air to clear mucus, which is a thick and sticky material in the lungs, nose, and other parts of the body, causing breathing problems. Inhaling salty aerosols reduces mucus thickness because the moisture in Salt Lake aerosols helps in thinning mucus (Figure 9). Inhaled Hypertonic Saline, a therapy involving the inhalation of a saline solution with a higher salt concentration than typically found in the body, is commonly used to manage certain respiratory conditions, particularly cystic fibrosis (CF) in younger children. It is a well-established therapy for individuals with CF.
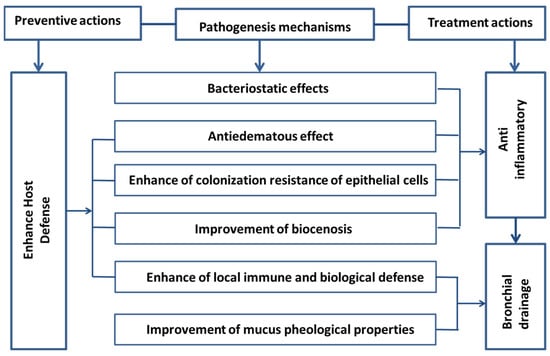
Figure 9.
Some possible health benefits of salt aerosols.
7. Interactions between Salt Lake Aerosols and Climate Change
Rising temperatures, shifting precipitation patterns, and increased evaporation associated with climate change can disrupt the delicate balance of Salt Lake ecosystems. Mitigation strategies aimed at tackling climate change, such as reducing greenhouse gas emissions and promoting sustainable practices, indirectly affect the dynamics of Salt Lake aerosols. Preserving the health of Salt Lake ecosystems necessitates adaptive measures and sustainable water management practices. The feedback loops between Salt Lake aerosols and the global climate system underscore the need for a comprehensive and interdisciplinary approach to understand and address these interdependencies. A potentially significant atmospheric change injurious to Salt Lakes involves the decrease in ozone concentration in the atmosphere’s upper strata [29]. This decrease is crucial, as it leads to increased penetration of ultraviolet radiation, which can adversely affect biota if excessive. Ultraviolet radiation (UV-B) is rapidly absorbed in the upper layers of water in lakes, but in shallow lakes, such as many temporary Salt Lakes, absorption may be minimal before impacting the biota. Lake Cantara South, a shallow Salt Lake in South Australia, is one example of a lake likely to be affected in this manner.
Lake-surface water temperature and evaporation are closely interlinked and contribute to increased aerosol emissions. The surface water temperature of European lakes has risen by +0.32 °C per decade from 1973 to 2014 [118]. Projections suggest that the anticipated rise in air temperature could lead to an 8% net increase in evaporation rates in Australian reservoirs by 2040, compared to the 1991–2010 average [17]. Additionally, regional climate models predict a potential 10% reduction in water supply from Brazilian reservoirs by 2100 due to increased evaporation [119]. In a study examining the influential factors of climate change, namely evaporation and lake surface temperature, on the saline Lake Qarun, Elaf et al. [120] used data from climate reanalysis and remote sensing datasets, specifically ERA5 and MODIS11A1. This study revealed that the minimum annual evaporation rate was 143.5 cm year−1 in 1983, while the maximum evaporation reached 187 cm year−1 in 2016, averaging an increase of 35 mm per decade. The researchers developed a model that offers a reliable and less time-consuming approach for estimating lake evaporation compared to traditional methods and field estimates. Additionally, the study noted an increase in the lake’s surface temperature over the 19-year period, with an average rise of 0.4 °C per decade. In the past two decades, the lake’s surface temperature fluctuated between approximately 31.9 °C and 33.9 °C, with the minimum value recorded in 2003 and the maximum in 2018. These variations in lake surface temperature and evaporation might be attributed to the impacts of climate change.
The dynamics of aerosols play a crucial role in atmospheric processes. While marine aerosols have received considerable attention, there are intriguing parallels with aerosols derived from Salt Lakes. Saline aerosols can act as seeds for cloud droplet production, altering cloud properties and influencing solar radiation [121]. Similarly, Salt Lake aerosols, which include salt particles, possess characteristics that can contribute to cloud dynamics and radiative effects in a manner analogous to marine aerosols. Globally, increases in tropospheric chlorine (Cl) and bromine (Br) concentrations (20–40%) were observed, leading to a decrease in ozone levels (−3 to −6%) and a subsequent decrease in the hydroxyl radical (·OH) concentration (−3 to −5%), resulting in an extended methane lifetime (3–6%). While the study’s findings suggest that the chemistry of Salt Lake aerosols has a minor impact on total radiative forcing compared to sea salt aerosols (approximately 2%), it may have potential implications for surface ozone pollution in the Salt Lake regions [122]. The primary objective of such geoengineering methods is to reduce temperatures by reflecting sunlight away from the Earth. The release of halogens from sea salt particles and Salt Lake aerosols is an important aspect to consider. Examining Salt Lake aerosols, their composition, and their potential impacts on atmospheric chemistry could provide valuable insights into their role as contributors to aerosol-induced alterations in the Earth’s energy balance and atmospheric composition.
Sulfate aerosols, which result from the oxidation of dimethyl sulfoniopropionate (DMSP), a compound produced by various species of phytoplankton, play a significant role in influencing atmospheric radiation effects, as highlighted in a study [123] (Figure 10). These aerosols contribute to cloud albedo by becoming a component of clouds and serving as cloud condensation nuclei (CCN). A study investigating the influence of dimethyl sulfide (DMS) emissions at higher altitudes of 12 km and under varying boundary conditions focused on contrasting seasons, specifically winter (February) and summer (July). The study found that DMS emissions lead to sulfate enhancements across the entire oceanic expanse at different altitudes, as shown in Figure 10a,b. In February, DMS emissions elevate surface-layer sulfate levels by approximately 0.2 μg m−3, while in July, this enhancement increases to about 0.4 µg m−3. Notably, these emissions consistently yield higher sulfate levels in summer compared to winter. The impact of DMS-induced sulfate enhancements is most pronounced at the surface and gradually diminishes with altitude, reaching approximately 10–20% at around 5 km. As a result, DMS emissions augment sulfate concentrations in the surface layer (Figure 10c) and have an impact aloft; however, the effect is more restrained in the lower troposphere. DMS was found to increase mean annual sulfate concentration over both seawater and land, with a larger impact observed over seawater [124].
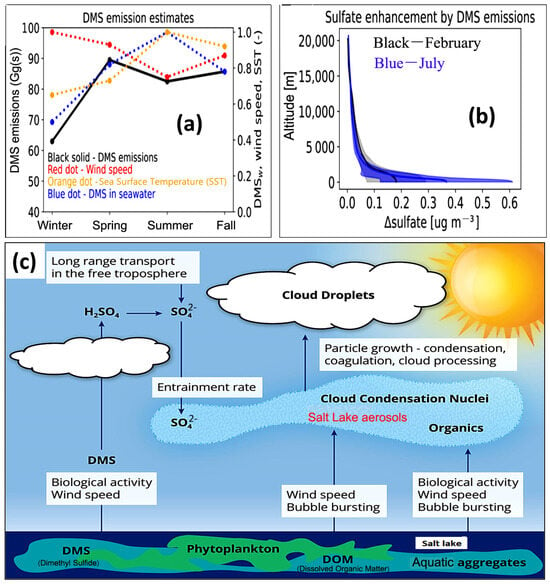
Figure 10.
(a,b) Dimethylsulfide emission and sulfate enhancement. (c) Salt Lake aerosols as cloud albedo agent (Modified from Sarwar et al., 2023 [124]).
8. A Compendium of Findings and Future Recommendations
The conclusion of this review article highlights several key points regarding Salt Lake aerosols. These unique lakes, characterized by a salt concentration exceeding 3.5%, are mainly located in drought-prone zones worldwide and accumulate diverse salts, including sodium, chloride, and sulfate. Notably, lakes such as the Caspian Sea, Owens Lake, and QeHan West Lake are significant contributors to aerosol emissions, with Owens Lake being particularly recognized for its role in wind-driven dust emission in the USA. The exposure and drying of saline flows, as exemplified by Lake Urmia, lead to substantial aerosol pollution, a phenomenon exacerbated by climate change-induced alterations in precipitation patterns and evaporation rates. The hygroscopic nature of these aerosols, coupled with the emission of sulfate aerosols, imparts light-scattering properties and a cooling effect. In addition, Salt Lake aerosols can be generated from the breaking of wind-driven waves at the lake surface. Their morphology and chemical composition are closely related to both abiotic and biotic components of lake water. Apart from inorganic ions, these include organic substances and microcystins produced by certain cyanobacteria or green alga species and artemia salina. Sometimes, harmful algal blooms can occur in some Salt Lakes, leading to algal toxins produced and transported.
The review highlights a marked increase in aerosol emissions linked to rising temperatures and intensified evaporation, impacting Salt Lake ecosystems. Dust emission from dry lakebeds has become a primary source of aerosols. Rising temperatures and changing precipitation patterns can influence the salinity of Salt Lakes, thereby affecting aerosol composition. Higher salinity levels can lead to the formation of fine particles that can be entrained into the air. Additionally, changes in lake ecosystems may affect biological processes, releasing biogenic compounds from algae, bacteria, and other microorganisms, which further contribute to the aerosol’s chemical composition. The inhalation of Salt Lake aerosols derived from lake water or from fine salt particles suspended in the air during salt-dust storms raises potential health concerns, particularly regarding respiratory and cardiovascular disease and eye and skin discomfort. However, the rock-salt aerosol therapy is thought to be a good treatment and rehabilitation method for the prevention and treatment of pneumoconiosis and COPD since it can effectively improve the quality of life of pneumoconiosis patients and can delay the pathogenesis of silicosis by regulating oxidative stress and reducing interstitial fibrosis of lungs. It means that the effects of Salt Lake aerosols on health need further observing and researching.
In conclusion, our review contributes to the growing body of knowledge surrounding global Salt Lake aerosols under climate change. It emphasizes the interconnectedness of climate changes, chemical composition, and health aspects, advocating for a comprehensive and practical approach to address the challenges faced by Salt Lake aerosols in an ever-changing global climate.
Author Contributions
Conceptualization, H.G. and M.S.A.; methodology, Q.Z.; validation, D.G.; writing—original draft preparation, M.S.A.; writing—review and editing, R.H.M.G. and A.F.L.G.; visualization, Y.Y.; supervision, project administration, and funding acquisition, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Foreign Experts Project (G2022004013L) funded from State Administration of Foreign Experts Affairs of the Ministry of Science and Technology, China; and by the Applied Basic Research Project of Shanxi Province (No. 202203021222017). Also, it was financially supported by the open fund from the MOE Key Laboratory of Coal Environmental Pathogenicity and Prevention, Shanxi Medical University, China (MEKLCEPP/SXMU-202302); and by the open fund provided from the Yuncheng Salt Lake Ecological Protection and Development Center, China (01140123015001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Daizhou Zhang from the Prefectural University of Kumamoto, Japan, for his help in the organization of the manuscript and thank Chul-Un Ro from Inha University, Korea, for his help in editing and checking the revised manuscript.
Conflicts of Interest
There is no conflict of interest for this study.
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and Soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Grythe, H.; Ström, J.; Krejci, R.; Quinn, P.; Stohl, A. A review of sea-spray aerosol source functions using a large global set of sea salt aerosol concentration measurements. Atmos. Chem. Phys. 2014, 14, 1277–1297. [Google Scholar] [CrossRef]
- Wang, X.; Hua, T.; Zhang, C.; Lang, L.; Wang, H. Aeolian salts in Gobi deserts of the western region of Inner Mongolia: Gone with the dust aerosols. Atmos. Res. 2012, 118, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C.; et al. A review of biomass burning: Emissions and impacts on air quality, health and climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hua, T.; Zhang, C.; Qian, G.; Luo, W. Salts in the clay playas of China’s arid regions: Gone with the wind. Environ. Earth Sci. 2012, 68, 623–631. [Google Scholar] [CrossRef]
- Gill, T.E. Eolian sediments generated by anthropogenic disturbance of playas: Human impacts on the geomorphic system and geomorphic impacts on the human system. Geomorphology 1996, 17, 207–228. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, G.; Yuan, H.; Rahn, K.A.; Wang, Z.; An, Z. Aerosol particles from dried salt-lakes and saline soils carried on dust storms over Beijing. Terr. Atmos. Ocean. Sci. 2009, 20, 619–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abuduwaili, J.; Gabchenko, M.V.; Junrong, X. Eolian transport of salts—A case study in the area of Lake Ebinur (Xinjiang, Northwest China). J. Arid. Environ. 2008, 72, 1843–1852. [Google Scholar] [CrossRef]
- Prospero, J.M. Environmental characterization of global sources of atmospheric soil dust identified with the NIMBUS 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev. Geophys. 2002, 40, 2-1–2-31. [Google Scholar] [CrossRef]
- Gaston, C.J.; Pratt, K.A.; Suski, K.J.; May, N.W.; Gill, T.E.; Prather, K.A. Laboratory studies of the cloud droplet activation properties and corresponding chemistry of saline playa dust. Environ. Sci. Technol. 2017, 51, 1348–1356. [Google Scholar] [CrossRef]
- Ramanathan, V.C.P.J.; Crutzen, P.J.; Kiehl, J.T.; Rosenfeld, D. Aerosols, climate, and the hydrological cycle. Science 2001, 294, 2119–2124. [Google Scholar] [CrossRef]
- Lai, H.W.; Chen, H.W.; Kukulies, J.; Ou, T.; Chen, D. Regionalization of seasonal precipitation over the Tibetan Plateau and associated large-scale atmospheric systems. J. Clim. 2021, 34, 2635–2651. [Google Scholar] [CrossRef]
- Chen, S.; Xue, L.; Yau, M.K. Impact of aerosols and turbulence on cloud droplet growth: An in-cloud seeding case study using a parcel–DNS (direct numerical simulation) approach. Atmos. Chem. Phys. 2020, 20, 10111–10124. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Froyd, K.D.; Hoose, C.; Jensen, E.J.; Diao, M.; Zondlo, M.A.; Murphy, D.M. Clarifying the dominant sources and mechanisms of cirrus cloud formation. Science 2013, 340, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change—The Physical Science Basis; Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Helfer, F.; Lemckert, C.; Zhang, H. Impacts of climate change on temperature and evaporation from a large reservoir in Australia. J. Hydrol. 2012, 475, 365–378. [Google Scholar] [CrossRef]
- Hussein, A.M.; Al-Zubaidi, A.; Naje, A.S.; Al-Ridah, Z.A.; Chabuck, A.; Ali, I.M. A statistical technique for modelling dissolved oxygen in salt lakes. Cogent. Eng. 2021, 8, 1875533. [Google Scholar] [CrossRef]
- Obianyo, J.I. Effect of salinity on evaporation and the water cycle. Emerg. Sci. J. 2019, 3, 255–262. [Google Scholar] [CrossRef]
- Irwandi, H.; Rosid, M.S.; Mart, T. The effects of ENSO, climate change and human activities on the water level of Lake Toba, Indonesia: A critical literature review. Geosci. Lett. 2021, 8, 21. [Google Scholar] [CrossRef]
- Patti, B.; Fiorenti, F.; Fortibuoni, T.; Somarakis, S.; García-Lafuente, J. Editorial: Impacts of environmental variability related to climate change on biological resources in the Mediterranean. Front. Mar. Sci. 2022, 9, 1059424. [Google Scholar] [CrossRef]
- Mojtahedi, A.; Dadashzadeh, M.; Azizkhani, M.; Mohammadian, A.; Almasi, R. Assessing climate and human activity effects on lake characteristics using spatio temporal satellite data and an emotional neural network. Environ. Earth Sci. 2022, 81, 61. [Google Scholar] [CrossRef]
- Tusupova, K.; Peder Hjorth, A.; Morave, M. Drying lakes: A review on the applied restoration strategies and health conditions in contiguous areas. Water 2020, 12, 749. [Google Scholar] [CrossRef]
- Izdebski, A.; Pickett, J.; Roberts, N.; Waliszewski, T. The environmental, archaeological and historical evidence for regional climatic changes and their societal impacts in the Eastern Mediterranean in Late Antiquity. Quat. Sci. Rev. 2015, 136, 189–208. [Google Scholar] [CrossRef]
- Satgé, F.; Espinoza, R.; Zolá, R.; Roig, H.; Timouk, F.; Molina, J.; Garnier, J.; Calmant, S.; Seyler, F.; Bonnet, M.P. Role of climate variability and human activity on Poopó Lake droughts between 1990 and 2015 assessed using remote sensing data. Remote Sens. 2017, 9, 12. [Google Scholar] [CrossRef]
- Farebrother, W.; Hesse, P.P.; Chang, H.C.; Jones, C. Dry lake beds as sources of dust in Australia during the Late Quarternary: A volumetric approach based on lake bed and deflated dune volumes. Quat. Sci. Rev. 2017, 161, 81–98. [Google Scholar] [CrossRef]
- Hammer, U.T. Saline Lake Ecosystems of the World; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1986; p. 616. [Google Scholar]
- Shiklomanov, I.A. Global water resources. Nat. Resour. 1990, 26, 34–43. [Google Scholar]
- Williams, W.D. The largest, highest and lowest lakes of the world: Saline lakes. Verhandlungen Int. Ver. Limnol. 1996, 26, 61–79. [Google Scholar] [CrossRef]
- Abuduwaili, J.; Issanova, G.; Saparov, G. Hydrology and Limnology of Central Asia; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2021. [Google Scholar]
- Wurtsbaugh, W.A.; Miller, C.; Null, S.E.; DeRose, R.J.; Wilcock, P.; Hahnenberger, F.; Howe, M.; Moore, J. Decline of the world saline lakes. Nat. Geosci. 2017, 10, 816–821. [Google Scholar] [CrossRef]
- Jellison, R.; Williams, W.D.B.; Timms, B.; Aladin, N.V. Salt lakes: Values, threats, and future. In Aquatic Ecosystems: Trends and Global Prospects; Cambridge University Press: Cambridge, UK, 2008; pp. 94–110. [Google Scholar]
- Saccò, M.; White, N.E.; Harrod, C.; Salazar, G.; Aguilar, P.; Cubillos, C.F.; Meredith, K.; Baxter, B.K.; Oren, A.; Anufriieva, E.; et al. Salt to conserve: A review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. 2021, 96, 2828–2850. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.D. Conservation of salt lakes. Hydrobiologia 1993, 267, 291–306. [Google Scholar] [CrossRef]
- Ioannidou, I.; Manolaki, P.; Litskas, V.D.; Vogiatzakis, I.N. Temporary salt lakes: Ecosystem services shift in a Ramsar Site Over a 50-Year Period. Front. Ecol. Evol. 2021, 9, 662107. [Google Scholar] [CrossRef]
- Wooldridge, J.B.; Adams, K.M.; Fernandes, M. Biotic responses to extreme hypersalinity in an arid zone estuary. S. Afr. J. Bot. 2016, 107, 160–169. [Google Scholar] [CrossRef]
- Shadkam, F.; Ludwig, T.H.; van Vliet, A.; Pastor, G.; Kabat, P. Preserving the world second largest hypersaline lake under future irrigation and climate change. Sci. Total Environ. 2016, 559, 317–325. [Google Scholar] [CrossRef]
- Pervov, A.G.; Andrianov, A.P.; Efremov, R.V.; Desyatov, A.V.; Baranov, A.E. A new solution for the Caspian Sea desalination: Low-pressure membranes. Desalination 2003, 157, 377–384. [Google Scholar] [CrossRef]
- Babkin, V.I. The earth and its physical features. In World Water Resources at the Beginning of the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2003; pp. 1–17. [Google Scholar]
- Micklin, P. The Aral Sea disaster. Annu. Rev. Earth. Planet. Sci. 2007, 35, 47–72. [Google Scholar] [CrossRef]
- Yechieli, Y.; Gavrieli, I.; Berkowitz, B.; Ronen, D. Will the Dead Sea die? Geology 1998, 26, 755–758. [Google Scholar] [CrossRef]
- Yu, G.; Harrison, S.P.; Xue, B. Lake Status Records from China: Data Base Documentation; Max Planck Institute for Biogeochemistry: Jena, Germany, 2001; pp. 1–243. [Google Scholar]
- Lister, G.S.; Kelts, K.; Zao, C.K.; Yu, J.Q.; Niessen, F. Lake Qinghai, China: Closed-basin like levels and the oxygen isotope record for ostracoda since the latest Pleistocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 84, 141–162. [Google Scholar] [CrossRef]
- Kempe, S.; Kazmierczak, J.; Landmann, G.; Konuk, T.; Reimer, A.; Lipp, A. Largest known microbialites discovered in Lake Van, Turkey. Nature 1991, 349, 605–608. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Berry, T.S. Cascading effects of decreased salinity on the plankton chemistry, and physics of the Great Salt Lake (Utah). Can. J. Fish. Aquat. Sci. 1990, 47, 100–109. [Google Scholar] [CrossRef]
- Reati, G.J.; Florín, M.; Fernández, G.J.; Montes, C. The Laguna de Mar Chiquita (Córdoba, Argentina): A little known, secularly fluctuating, Saline lake. Int. J. Salt Lake Res. 1996, 5, 187–219. [Google Scholar] [CrossRef]
- Schmidt, N. Hydrological and hydrochemical investigations at the Salar de Uyuni (Bolivia) with regard to the extraction of lithium. FOG-Freib. Online Geosci. 2010, 26. [Google Scholar] [CrossRef]
- Schagerl, M. Soda Lakes of East Africa; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Jirsa, F.; Gruber, M.; Stojanovic, A.; Omondi, S.O.; Mader, D.; Körner, W.; Schagerl, M. Major and trace element geochemisttry of Lake Bogoria and Lake Nakuru, Kenya, during extreme draught. Geochemistry 2013, 73, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Jacobson, G. Hydrochemical evolution of regional groundwaters to playa brines in central Australia. J. Hydrol. 1989, 108, 123–173. [Google Scholar] [CrossRef]
- Heidelberg, K.B.; Nelson, W.C.; Holm, J.B.; Eisenkolb, N.; Andrade, K.; Emerson, J.B. Characterization of eukaryotic microbial diversity in hypersaline Lake Tyrrell, Australia. Front. Microbiol. 2013, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Argaman, E.; Keesstra, S.D.; Zeiliguer, A. Monitoring the impact of surface albedo on a saline lake in SW Russia. Land Degrad. Dev. 2012, 23, 398–408. [Google Scholar] [CrossRef]
- Pearson, E.J.; Juggins, S.; Farrimond, P. Distribution and significance of longchain alkenones as salinity and temperature indicators in Spanish saline lake sediments. Geochim. Cosmochim. Acta 2008, 72, 4035–4046. [Google Scholar] [CrossRef]
- Vila, X.; Guyoneaud, R.; Cristina, X.P.; Figueras, J.B.; Abella, C.A. Green sulfur bacteria from hypersaline Chiprana Lake (Monegros, Spain): Habitat description and phylogenetic relationship of isolated strains. Photosynth. Res. 2002, 71, 165–172. [Google Scholar] [CrossRef]
- García, C.M.; Niell, F.X. Seasonal change in a saline temporary lake (Fuentede Piedra, southern Spain). Hydrobiologia 1993, 267, 211–223. [Google Scholar] [CrossRef]
- Valiente, N.; Carrey, R.; Otero, N.; Soler, A.; Sanz, D.; Muñoz-Martín, A.; Gómez-Alday, J.J. A multi-isotopic approach to investigate the influence of land use on nitrate removal in a highly saline lake-aquifer system. Sci. Total Environ. 2018, 631, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.R.; Schwartz, S.E. Sea Salt Aerosol Production: Mechanisms, Methods, Measurements and Models; A Critical Review, Geophysical Monograph; American Geophysical Union: Washington, DC, USA, 2004. [Google Scholar]
- May, N.W.; Olson, N.E.; Panas, M. Aerosol emissions from great lakes harmful algal blooms. Environ. Sci. Technol. 2018, 52, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Slade, J.H.; VanReken, T.M.; Mwaniki, G.R.; Bertman, S.; Stirm, B.; Shepson, P.B. Aerosol production from the surface of the Great Lakes. Geophys. Res. Lett. 2010, 37, L18807. [Google Scholar] [CrossRef]
- Axson, J.L.; May, N.W.; Colón-Bernal, I.D.; Pratt, K.A.; Ault, A.P. Lake spray aerosol: A chemical signature from individual ambient particles. Environ. Sci. Technol. 2016, 50, 9835–9845. [Google Scholar] [CrossRef]
- Olson, N.E.; May, N.W.; Kirpes, R.M.; Watson, A.E.; Hajny, K.D.; Slade, J.H.; Shepson, P.B.; Stirm, B.H.; Pratt, K.A.; Ault, A.P. Lake spray aerosol incorporated into Great Lakes Clouds. ACS Earth Space Chem. 2019, 3, 2765–2774. [Google Scholar] [CrossRef]
- Che, H.; Xia, X.; Zhu, J.; Li, Z.; Dubovik, O.; Holben, B.; Goloub, P.; Chen, H.; Estellés, V.; Cuevas-Agulló, E.; et al. Column aerosol optical properties and aerosol radiative forcing during a serious haze-fog month over North China Plain in 2013 based on ground-based sunphotometer measurements. Atmos. Chem. Phys. 2014, 14, 2125–2138. [Google Scholar] [CrossRef]
- Liu, Y.; Sato, Y.; Jia, R.; Xie, Y.; Huang, J.; Nakajima, T. Modeling study on the transport of summer dust and anthropogenic aerosols over the Tibetan Plateau. Atmos. Chem. Phys. 2015, 15, 12581–12594. [Google Scholar] [CrossRef]
- Yuan, H.; Zhuang, G.; Rahn, K.A.; Zhang, X.; Li, Y. Composition and mixing of individual particles in dust and nondust conditions of north China, spring 2002. J. Geophys. Res. 2006, 111, D20208. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, S.; Shen, Z.; Mei, F.; Xi, X.; Liu, L.; Zhou, Z.; Wang, D.; Wang, Y.; Cheng, Y. Characterization of soil dust aerosol in China and its transport and distribution during 2001 ACE-Asia: 1. Network observations. J. Geophys. Res. 2003, 2108, ACH 3/1–ACH 3/13. [Google Scholar] [CrossRef]
- Zhuang, G.; Guo, J.; Yuan, H.; Zhao, C. The compositions, sources, and size distribution of the dust storm from China in spring of 2000 and its impact on the global environment. Chin. Sci. Bull. 2001, 46, 895–900. [Google Scholar] [CrossRef]
- Zhuang, G.; Guo, J.; Yuan, H.; Zhang, X. Coupling and feedback between iron and sulphur in air-sea exchange. Chin. Sci. Bull. 2003, 48, 1080–1086. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, X.Y.; Arimoto, R.; Cao, J.J.; Shen, Z.X. The transport path ways and sources of PM10 pollution in Beijing during spring 2001, 2002 and 2003. Geo Phys. Res. Lett. 2004, 31, L14110. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, G.; Yuan, H.; Zhang, X.Y. The physic-chemical characteristics and its source analysis of the 2002 super dust storm in Beijing. Chin. Sci. Bull. 2004, 9, 291–298. [Google Scholar] [CrossRef]
- Gao, Y.; Anderson, J.R. Characteristics of Chinese aerosols determined by individual particle analysis. J. Geophys. Res. 2001, 106, 18037–18045. [Google Scholar] [CrossRef]
- Whittaker, A.G.; Jones, T.P.; Shao, L.; Shi, Z.; Bérubé, K.A.; Richards, R.J. Mineral dust in urban air: Beijing, China. Mineral. Mag. 2003, 67, 173–182. [Google Scholar] [CrossRef]
- Shi, Z.; Shao, L.; Jone, T.P.; Whittaker, A.J.; Lu, S.; Bérubé, K.A.; He, T.; Richards, R.J. Characterization of air borne individual particles collected in an urban area, a satellite city and a clean air area in Beijing, 2001. Atmos. Environ. 2003, 37, 4097–4108. [Google Scholar] [CrossRef]
- Cahill, T.A.; Gill, T.E.; Reid, J.S.; Gearhart, E.A.; Gillette, D.A. Saltating particles, playa crusts and dust aerosols at Owens (dry) lake, California. Earth. Surf. Process. Landf. 1994, 21, 621–639. [Google Scholar] [CrossRef]
- Gill, T.E.; Gillette, D.A. ‘Owens Lake: A natural laboratory for aridification, playa desiccation and desert dust. Geol. Soc. Am. Bull. 1991, 23, 462. [Google Scholar]
- Abuduwaili, J.; Guijin, M. Analysis on the dust storms and their disasters in the lakebed region of Ebinur Lake, Xinjiang. Arid Land Geography. 2002, 25, 149–154, (In Chinese, with English abstract). [Google Scholar]
- Hesam, A.-B.; Parisa, R.; Joseph, S.S.; Alberto, C.-R.; Mojtaba, A.A.; Armin, S. Is there a relationship between Lake Urmia saline lakebed emissions and wet deposition composition in the caucasus region? ACS Earth Space Chem. 2021, 5, 2970–2985. [Google Scholar]
- Gholampour, A.; Nabizadeh, R.; Hassanvand, M.S.; Taghipour, H.; Nazmara, S.; Mahvi, A.H. Characterization of saline dust emission resulted from Urmia Lake drying. J. Environ. Health. Sci. Eng. 2015, 28, 82. [Google Scholar] [CrossRef] [PubMed]
- Radhi, M.; Box, M.A.; Box, G.P.; Mitchell, R.M.; Cohen, D.D.; Stelcer, E.; Keywood, M.D. Size-resolved mass and chemical properties of dust aerosols from Australia’s Lake Eyre Basin. Atmos. Environ. 2010, 44, 3519–3528. [Google Scholar] [CrossRef]
- Liu, X.Q.; Shen, J.; Wang, S.M.; Yang, X.D.; Tong, G.B.; Zhang, E.L. A 16000-year pollen record of Qinghai Lake and its paleoclimate and paleoenvironment. Chin. Sci. Bull. 2002, 47, 1931–1937. [Google Scholar] [CrossRef]
- Xu, H.; Ai, L.; Tan, L.C.; An, Z.S. Stable isotopes in bulk carbonates and organic matter in recent sediments of Lake Qinghai and their climatic implications. Chem. Geol. 2006, 235, 262–275. [Google Scholar] [CrossRef]
- Henderson, A.C.G.; Holmes, J.A.; Zhang, J.W.; Leng, M.J.; Carvalho, L.R. A carbon- and oxygen-isotope record of recent environmental change from Qinghai Lake, NE Tibetan Plateau. Chin. Sci. Bull. 2003, 48, 1463–1468. [Google Scholar]
- Zhang, N.; Cao, J.; Liu, S.; Zhao, Z.; Xu, H.; Xiao, S. Chemical composition and sources of PM2.5 and TSP collected at Qinghai Lake. Atmos. Res. 2014, 138, 213–222. [Google Scholar] [CrossRef]
- Geng, H.; Hwang, H.; Liu, X.; Dong, S.; Ro, C.U. Investigation of aged aerosols in size-resolved Asian dust storm particles transported from Beijing, China, to Incheon, Korea, using low-Z particle EPMA. Atmos. Chem. Phys. 2014, 14, 3307–3323. [Google Scholar] [CrossRef]
- Yoo, H.; Wu, L.; Geng, H.; Ro, C.-U. Physicochemical and temporal characteristics of individual atmospheric aerosol particles in urban Seoul during KORUS-AQ campaign: Insights from single-particle analysis. Atmos. Chem. Phys. 2024, 24, 853–867. [Google Scholar] [CrossRef]
- Bopp, L.; Boucher, O.; Aumont, O.; Belviso, S.; Dufresne, J.L.; Pham, M.; Monfray, P. Will marine dimethylsulfide emissions amplify or alleviate global warming? A model study. Can. J. Fish. Aquat. Sci. 2004, 61, 826–835. [Google Scholar] [CrossRef]
- Dawson, J.P.; Adams, P.J.; Pandis, S.N. Sensitivity of PM2.5 to climate in the eastern U.S.: A modeling case study. Atmos. Chem. Phys. 2007, 7, 4295–4309. [Google Scholar] [CrossRef]
- Liao, H.; Chen, W.T.; Seinfeld, J.H. Role of climate change in global predictions of future tropospheric ozone and aerosols. J. Geophys. Res. 2006, 111, 12304. [Google Scholar] [CrossRef]
- Barthel, S.; Tegen, I.; Wolke, R. Do new sea spray aerosol source function functions improve the results of a regional aerosol model. Atmos. Environ. 2019, 198, 265–278. [Google Scholar] [CrossRef]
- Textor, C.; Schulz, M.; Guibert, S.; Kinne, S.; Balkanski, Y.; Bauer, S.; Berntsen, T.; Berglen, T.; Boucher, O.; Chin, M.; et al. Analysis and quantification of the diversities of aerosol life cycles within AeroCom. Atmos. Chem. Phys. 2006, 6, 1777–1813. [Google Scholar] [CrossRef]
- Dasarathy, S.; Russell, L.M.; Rodier, S.D.; Bowman, J.S. Wind-driven and seasonal effects on marine aerosol production in the Bellingshausen Sea, Antarctica. Geophys. Res. Lett. 2023, 50, e2022GL099723. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.C.; Fryod, K.D.; Schill, G.P. Sea spray aerosol concentration modulated by sea surface temperature. Proc. Natl. Acad. Sci. USA 2021, 118, e2020583118. [Google Scholar] [CrossRef] [PubMed]
- Harb, C.; Foroutan, H. Experimental development of a lake spray source function and its model implementation for Great Lakes surface emissions. Atmos. Chem. Phys. 2022, 22, 11759–11779. [Google Scholar] [CrossRef]
- Moghim, S.; Ramezanpoor, R. Characterization of aerosol types over Lake Urmia Basin. E3S Web Conf. 2019, 99, 01006. [Google Scholar] [CrossRef]
- Toledano, C.; Cachorro, V.E.; Berjon, A.; de Frutos, A.M.; Sorribas, M.; de la Morena, B.A.; Goloub, P. Aerosol optical depth and angstrom exponent climatology at El Arenosillo AERONET site (Huelva, Spain). Q. J. R. Meteorol. Soc. 2007, 133, 795–807. [Google Scholar] [CrossRef]
- Abadi, A.R.S.; Hamzeh, N.H.; Karim, S.; Christian, O.; Chandra, D.U. Long-Term Investigation of Aerosols in the Urmia Lake Region in the Middle East by Ground-Based and Satellite Data in 2000–2021. Remote Sen. 2022, 14, 3827. [Google Scholar] [CrossRef]
- Jones, B.A.; Fleck, J. Shrinking lakes, air pollution, and human health: Evidence from California’s Salton Sea. Sci. Total Environ. 2020, 712, 136490. [Google Scholar] [CrossRef] [PubMed]
- Esenov, S.E.; Shlygin, E.D. Geology of USSR. V. 20. Central Kazakhstan. Geological Specification; Nedra: Moscow, Russia, 1972. [Google Scholar]
- Bragina, T.M.; Bragin, E.A. The Most Important Wetlands of Northern Kazakhstan (In the Kostanay and Western Parts of the North Kazakhstan Region); WWF Russia Nature Conservation Publication Series: Moscow, Russia, 2002. [Google Scholar]
- Boros, E.; Jurecska, L.; Tatár, E.; Vörös, L.; Kolpakova, M. Chemical composition and trophic state of shallow saline steppe lakes in central Asia (North Kazakhstan). Environ Monit Assess. 2017, 189, 546. [Google Scholar] [CrossRef]
- Li, Z.; Williams, A.l.; Rood, M.J. Influence of soluble surfactant properties on the activation of aerosol particles containing inorganic solutes. J. Atmos. Sci. 1998, 55, 1859–1866. [Google Scholar] [CrossRef]
- Gholampour, A.; Nabizadeh, R.; Hassanvand, M.S.; Nazmara, S.; Mahvi, A.M. Elemental composition of particulate matters around Urmia Lake, Iran. Toxicol. Environ. Chem. 2017, 99, 17–31. [Google Scholar] [CrossRef]
- Abuduwaili, J.; Liu, D.W.; Wu, G.Y. Saline dust storms and their ecological impacts in arid regions. J. Arid Land 2010, 2, 144–150. [Google Scholar] [CrossRef]
- May, N.W.; Axson, J.L.; Watson, A.; Pratt, K.A.; Ault, A.P. Lake sprays aerosol generation: A method for producing representative particles from freshwater wave breaking. Atmos. Meas. Tech. 2016, 9, 4311–4325. [Google Scholar] [CrossRef]
- Qi, S.; Ren, X.; Dang, X.; Meng, Z. Mechanisms of dust emissions from lakes during different drying stages in semi-arid grassland in northern China. Front. Environ. Sci. 2023, 10, 2022. [Google Scholar] [CrossRef]
- Meng, Q. Climate change and extreme weather drive the declines of saline lakes: A showcase of the Great Salt Lake. Climate 2019, 7, 19. [Google Scholar] [CrossRef]
- Ghale, Y.A.G.; Tayanc, M.; Unal, A. Dried bottom of Urmia Lake as a new source of dust in northwestern Iran: Understanding the impacts on local and regional air quality. Atmos. Environ. 2021, 262, 118635. [Google Scholar] [CrossRef]
- Alizadeh, F.; Hamzehpour, N.; Mola, A.; Abasiyan, S.; Rahmati, M.T. Wind erodibility in the newly emerged surfaces of Urmia Playa Lake and adjacent agricultural lands and its determining factors. Catena 2020, 194, 10467. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574594/ (accessed on 5 February 2024).
- Backer, L.C.; Carmichael, W.; Kirkpatrick, B. Recreational exposure to low concentrations of microsystins during an algal bloom in a small lake. Mar. Drugs 2008, 6, 389–406. [Google Scholar] [CrossRef]
- Glantz, M.H. Creeping Environmental Problems and Sustainable Development in the Aral Sea Basin; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Lim, C.C.; Yoon, J.; Reynolds, K.; Geral, L.B.; Ault, A.P.; Heo, S.; Bell, M.L. Harmful algal bloom aerosols and human health. Ebio Med. 2023, 93, 104604. [Google Scholar] [CrossRef] [PubMed]
- Litvinenko, L.I.; Kozlov, A.V.; Kovalenko, A.I.; Bauer, D.S. Salinity of water as a factor to determine the development of the brine shrimp Artemia populations in Siberian lakes. Hydrobiologia 2007, 576, 95–101. [Google Scholar] [CrossRef]
- Asselman, J.; Acker, E.V.; Rijcke, M.D.; Tilleman, L.; Nieuwerbugh, F.V.; Mees, J.; Jansen, C.R. Positive human health effects of sea spray aerosol: Molecular evidence from exposed lung cell lines. bioRxiv 2018, 397141. [Google Scholar] [CrossRef]
- Er, W.; Hou, T.; Bao, Z. Research of clinical efficacy and safety of salt rock aerosol in the treatment of occupational pneumoconiosis. Xinjiang Med. J. 2019, 49, 804–806. (In Chinese) [Google Scholar]
- Chen, C.; Zhang, Q.; Luo, W.; Liu, Z.; Xu, H.; Wang, Y.; Chen, G.; Cuo, X.; Ming, Y.; Zhang, X.; et al. Effect of rock salt aerosol therapy on quality of life of patients with pneumoconiosis: A multicenter, randomized, double-blind clinical trial. Park. J. Pharm. Sci. 2022, 35, 441–445. [Google Scholar]
- Hu, X.; Li, L. Short-term and long-term efficacy evaluation of bronchodilators combined with rock salt aerosol in patients with pneumoconiosis complicated with COPD. J. Hunan Normal Univ. Med. Sci. 2021, 18, 149–152. (In Chinese) [Google Scholar]
- Wang, S.; Zhao, X.; Xu, Q.; Li, X.; Zhang, J.; Hao, X.; Guo, L.; Liu, H. The effect of rock salt aerosol on the prevention of silicosis in rats. Chin. Occup. Med. 2020, 47, 147–153. [Google Scholar]
- Woolway, R.I.; Weyhenmeyer, G.A.; Schmid, M.; Dokulil, M.T.; de Eyto, E.; Maberly, S.C.; Merchant, C.J. Substantial increase in minimum lake surface temperatures under climate chang. Clim. Change 2019, 155, 81–94. [Google Scholar] [CrossRef]
- Althoff, D.; Rodrigues, L.N.; da Silva, D.D. Impacts of climate change on the evaporation and availability of water in small reservoirs in the Brazilian savannah. Clim. Chang. 2020, 159, 215–232. [Google Scholar] [CrossRef]
- Elaf, S.; Hamad, S.; Adhm, M.Y.; Mohammad, S.E.; Yasmeen, D.; Yehya, E.I. Climate change effect on water temperature and evaporation in Lake Qarun. Water Sci. 2021, 37, 1–10. [Google Scholar]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- Horowitz, H.M.; Holmes, C.; Wright, A.; Sherwen, T.; Wang, X.; Evans, M.; Alexander, B. Effects of sea salt aerosol emissions form marine cloud brightening on atmospheric chemistry: Implications for radiative forcing. Geophy Res. Lett. 2020, 47, e2019GL085838. [Google Scholar] [CrossRef]
- Stefels, J.; Steinke, M.; Turner, S.; Malin, G.; Belviso, S. Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 2007, 83, 245–275. [Google Scholar] [CrossRef]
- Sarwar, G.; Kang, D.; Henderson, B.H.; Hogrefe, C.; Appel, W.; Mathur, R. Examining the impact of dimethyl sulfide emissions on atmospheric sulfate over the Continental U.S. J. Atmos. 2023, 14, 660. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).