Ammonia and Nitrous Oxide Emissions from Dairy Cows on Straw-Based Litter Systems
Abstract
:1. Introduction
2. Materials and Methods
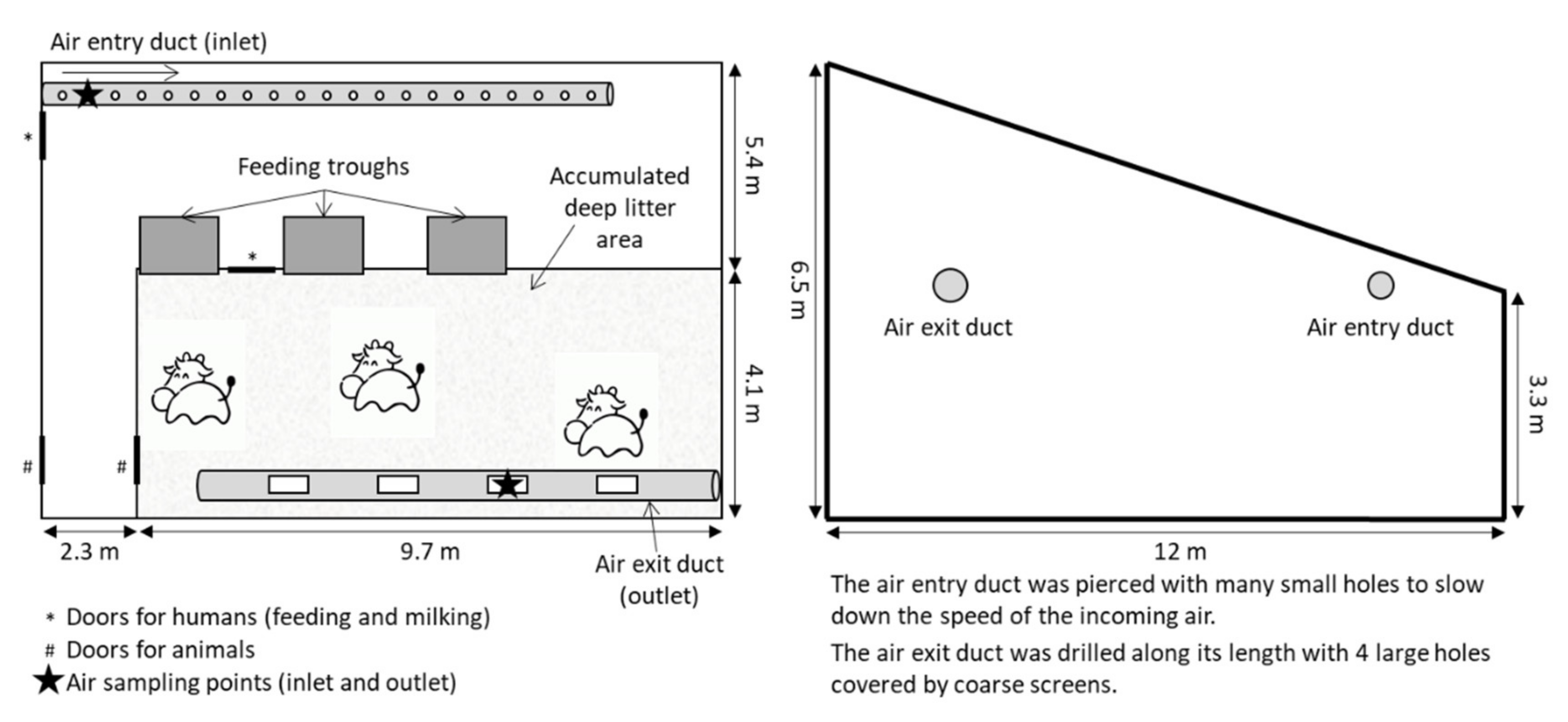
2.1. Ammonia and Nitrous Oxide Emissions in the Barn from Dairy Cows on Straw-Based Deep Litter
2.1.1. Animals and Treatments
2.1.2. Gas Emission Measurements
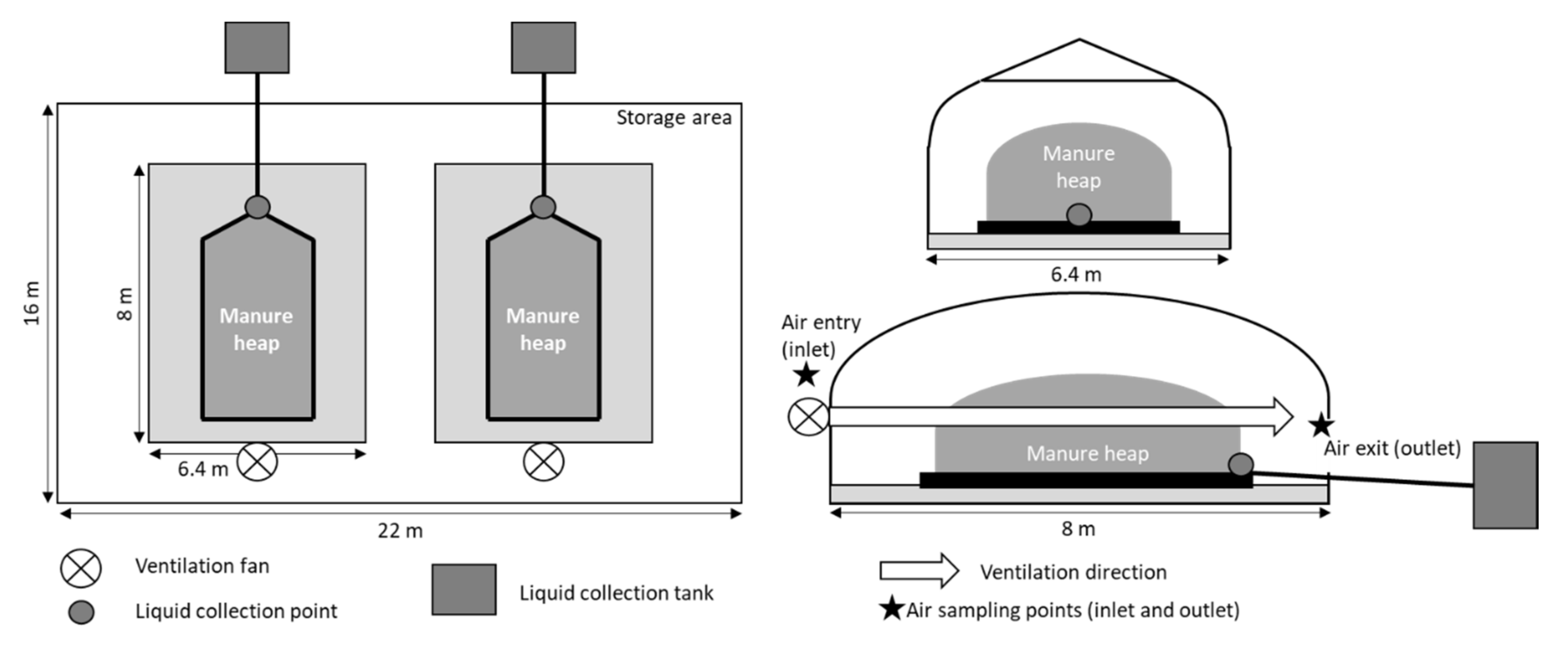
2.2. Ammonia and Nitrous Oxide Emissions during Storage of the Solid Manure
2.3. Data Analysis
3. Results
3.1. Animal Production
3.2. Manure Production and Storage Conditions
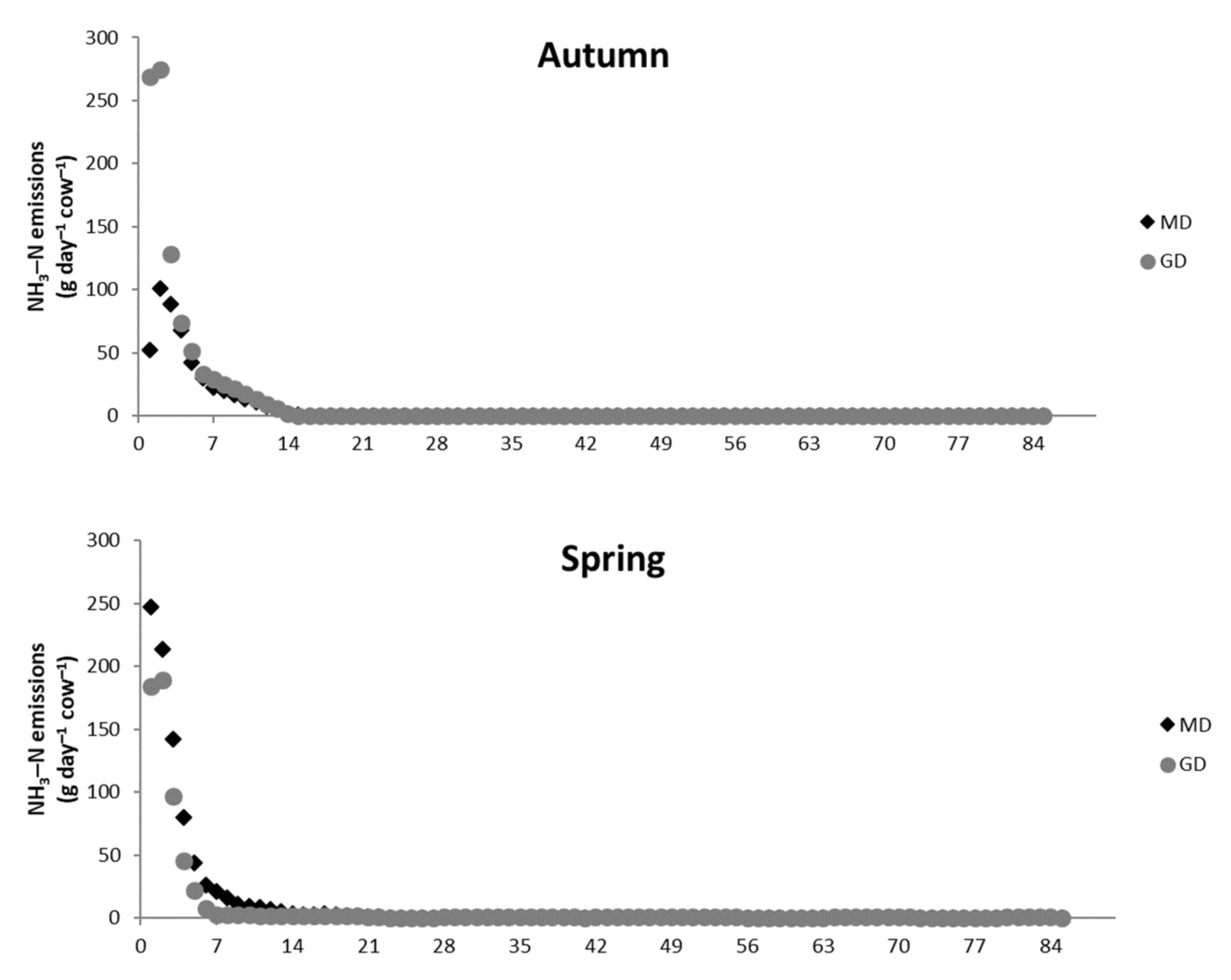
3.3. Ammonia Emissions in the Barn and Storage Phases
3.4. Nitrous Oxide Emissions in the Barn and Storage Phases
4. Discussion
4.1. Ammonia Emissions Associated with N Excretion in the Barn and Temperature during Storage
4.2. Nitrous Oxide Emissions Were Negligible in the Barn and Were Influenced by Temperature and Rainfall during Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinfeld, H.; Mooney, H.A.; Schneider, F.; Neville, L.E. Livestock in a Changing Landscape. Volume 1: Drivers, Consequences, and Responses; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Jeppsson, K.-H. Volatilization of Ammonia in Deep-litter Systems with Different Bedding Materials for Young Cattle. J. Agric. Eng. Res. 1999, 73, 49–57. [Google Scholar] [CrossRef]
- Gac, A.; Béline, F.; Bioteau, T.; Maguet, K. A French inventory of gaseous emissions (CH4, N2O, NH3) from livestock manure management using a mass-flow approach. Livest. Sci. 2007, 112, 252–260. [Google Scholar] [CrossRef]
- Mazur, K.; Roman, K.; Wardal, W.J.; Borek, K.; Barwicki, J.; Kierończyk, M. Emission of harmful gases from animal production in Poland. Environ. Monit. Assess. 2021, 193, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schrade, S.; Keck, M. Emissions d’Ammoniac dans les Stabulations Libres de Vaches Laitières avec aire d’Exercice Extérieure: Moins de Pertes en Hiver. 2011. Available online: https://api.open-ressources.fr/files/aHR0cHM6Ly9hcGkuem90ZXJvLm9yZy9ncm91cHMvMzM2MTk3L2l0ZW1zLzhFVUFXVFRLL2ZpbGUvdmlldw==/YXBwbGljYXRpb24vcGRm (accessed on 26 January 2022).
- Webb, J.; Sommer, S.G.; Kupper, T.; Groenestein, K.; Hutchings, N.J.; Eurich-Menden, B.; Rodhe, L.; Misselbrook, T.H.; Amon, B. Emissions of Ammonia, Nitrous Oxide and Methane During the Management of Solid Manures. 2011. Available online: https://link.springer.com/chapter/10.1007/978-94-007-1905-7_4 (accessed on 26 January 2022). [CrossRef]
- Witkowska, D.; Korczyński, M.; Koziel, J.A.; Sowińska, J.; Chojnowski, B. The Effect of Dairy Cattle Housing Systems on the Concentrations and Emissions of Gaseous Mixtures in Barns Determined by Fourier-Transform Infrared Spectroscopy. Ann. Anim. Sci. 2020, 20, 1487–1507. [Google Scholar] [CrossRef]
- Petersen, S.; Blanchard, M.; Chadwick, D.; del Prado, A.; Edouard, N.; Mosquera, J.; Sommer, S.G. Manure management for greenhouse gas mitigation. Animal 2013, 7, 266–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Hanajima, D.; Toyoda, S.; Yoshida, N.; Morioka, R.; Osada, T. Microbiology of nitrogen cycle in animal manure compost. Microb. Biotechnol. 2011, 4, 700–709. [Google Scholar] [CrossRef]
- Petersen, S.O.; Lind, A.-M.; Sommer, S.G. Nitrogen and organic matter losses during storage of cattle and pig manure. J. Agric. Sci. 1998, 130, 69–79. [Google Scholar] [CrossRef]
- Shan, N.; Li, H.; Li, J.-Z.; Ng, E.L.; Ma, Y.; Wang, L.-G.; Chen, Q. A major pathway for carbon and nitrogen losses — Gas emissions during storage of solid pig manure in China. J. Integr. Agric. 2019, 18, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Külling, D.R.; Menzi, H.; Kröber, T.F.; Neftel, A.; Sutter, F.; Lischer, P.; Kreuzer, M. Emissions of ammonia, nitrous oxide and methane from different types of dairy manure during storage as affected by dietary protein content. J. Agric. Sci. 2001, 137, 235–250. [Google Scholar] [CrossRef]
- Edouard, N.; Charpiot, A.; Robin, P.; Lorinquer, E.; Dollé, J.-B.; Faverdin, P. Influence of diet and manure management on ammonia and greenhouse gas emissions from dairy barns. Animal 2019, 13, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Groot, J.C.; Zhang, K.; Schulte, R.P. Effects of housing system, measurement methods and environmental factors on estimating ammonia and methane emission rates in dairy barns: A meta-analysis. Biosyst. Eng. 2021, 205, 64–75. [Google Scholar] [CrossRef]
- Sanchis, E.; Calvet, S.; del Prado, A.; Estellés, F. A meta-analysis of environmental factor effects on ammonia emissions from dairy cattle houses. Biosyst. Eng. 2018, 178, 176–183. [Google Scholar] [CrossRef]
- Agabriel, J. Alimentation des Bovins, Ovins et Caprins—Besoins des Animaux—Valeur des Aliments—Tables INRA 2007; Editions Quae: Paris, France, 2007; Available online: http://www.civamad53.org/wp-content/uploads/2020/12/Tables-INRA.pdf (accessed on 26 January 2022).
- Hassouna, M.; Robin, P.; Charpiot, A.; Edouard, N.; Méda, B. Infrared photoacoustic spectroscopy in animal houses: Effect of non-compensated interferences on ammonia, nitrous oxide and methane air concentrations. Biosyst. Eng. 2013, 114, 318–326. [Google Scholar] [CrossRef]
- Hassouna, M.; Eglin, T.; Cellier, P.; Colomb, V.; Cohan, J.-P.; Decuq, C.; Delabuis, M.; Edouard, N.; Espagnol, S.; Eugene, M.; et al. Measuring Emissions from Livestock Farming: Greenhouse Gases, Ammonia and Nitrogen Oxides; INRA-ADEME: Paris, France, 2016; Available online: https://hal.archives-ouvertes.fr/hal-01567208/document (accessed on 26 January 2022).
- Baptista, F.; Bailey, B.; Randall, J.; Meneses, J. Greenhouse Ventilation Rate: Theory and Measurement with Tracer Gas Techniques. J. Agric. Eng. Res. 1999, 72, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Demmers, T.G.M.; Phillips, V.R.; Short, L.S.; Burgess, L.R.; Hoxey, R.P.; Wathes, C.M. Validation of ventilation rate measurement methods and the ammonia emission from naturally ventilated dairy and beef buildings in the United Kingdom. J. Agric. Eng. Res. 2001, 79, 107–116. [Google Scholar] [CrossRef]
- Lorinquer, E.; Charpiot, A.; Raynal, J.; Dollé, J.B.; Robin, P.; Hassouna, M.; Oudart, D.; Planchais, J.; Guiziou, F.; Loyon, L.; et al. Ammonia and Greenhouse Gas Emissions from Cattle Farm Yard Manure; ADEME: Angers, France, 2015; Available online: https://www.ademe.fr/sites/default/files/assets/documents/ges-fumiers-bovins-rapport-201512.pdf (accessed on 26 January 2022).
- Pereira, J.; Misselbrook, T.H.; Chadwick, D.R.; Coutinho, J.; Trindade, H. Effects of temperature and dairy cattle excreta characteristics on potential ammonia and greenhouse gas emissions from housing: A laboratory study. Biosyst. Eng. 2012, 112, 138–150. [Google Scholar] [CrossRef]
- Gustafsson, A.; Palmquist, D. Diurnal Variation of Rumen Ammonia, Serum Urea, and Milk Urea in Dairy Cows at High and Low Yields. J. Dairy Sci. 1993, 76, 475–484. [Google Scholar] [CrossRef]
- Burgos, S.; Fadel, J.G.; Depeters, E.J. Prediction of Ammonia Emission from Dairy Cattle Manure Based on Milk Urea Nitrogen: Relation of Milk Urea Nitrogen to Urine Urea Nitrogen Excretion. J. Dairy Sci. 2007, 90, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Mathot, M.; Lambert, R.; Stilmant, D.; Decruyenaere, V. Carbon, nitrogen, phosphorus and potassium flows and losses from solid and semi-solid manures produced by beef cattle in deep litter barns and tied stalls. Agric. Syst. 2019, 178, 102735. [Google Scholar] [CrossRef]
- Bussink, D.; Oenema, O. Ammonia volatilization from dairy farming systems in temperate areas: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 19–33. [Google Scholar] [CrossRef]
- Sommer, S. Effect of composting on nutrient loss and nitrogen availability of cattle deep litter. Eur. J. Agron. 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Aguerre, M.; Wattiaux, M.; Hunt, T.; Lobos, N. Effect of nitrogen content and additional straw on changes in chemical composition, volatile losses, and ammonia emissions from dairy manure during long-term storage. J. Dairy Sci. 2012, 95, 3454–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillat, J.-M.; Robin, P.; Hassouna, M.; Leterme, P. Predicting ammonia and carbon dioxide emissions from carbon and nitrogen biodegradability during animal waste composting. Atmos. Environ. 2005, 39, 6833–6842. [Google Scholar] [CrossRef] [Green Version]
- Mathot, M.; Decruyenaere, V.; Lambert, R.; Stilmant, D. Deep litter removal frequency rate influences on greenhouse gas emissions from barns for beef heifers and from manure stores. Agric. Ecosyst. Environ. 2016, 233, 94–105. [Google Scholar] [CrossRef]




| Autumn | Spring | |||
|---|---|---|---|---|
| MD | GD | MD | GD | |
| Ingredients (g kg–1 DM) | ||||
| Fresh forage | — | 660 | — | 1000 |
| Hay | — | 340 | — | — |
| Maize silage | 715 | — | 731 | — |
| Mix concentrate 1 | 70 | — | 86 | — |
| Soybean meal | 205 | — | 172 | — |
| Minerals | 10 | — | 11 | — |
| Nutrients | ||||
| DM (g k–1) | 370 ± 18 | 202 ± 53 | 402 | 164 |
| NDF (g kg–1 DM) | 453 ± 2 | 551 ± 25 | 362 | 507 |
| CP (g kg–1 DM) | 153 ± 5 | 179 ± 24 | 149 | 178 |
| Nutritive value | ||||
| PDIN (g kg–1 DM) 2 | 103 | 117 | 99 | 116 |
| PDIE (g kg–1 DM) 3 | 97 | 97 | 97 | 99 |
| NEL (MJ kg–1 DM) 4 | 6.76 | 6.33 | 7.11 | 6.61 |
| (PDIN–PDIE) NEL–1 | 0.89 | 3.16 | 0.28 | 2.57 |
| Autumn | Spring | |||
|---|---|---|---|---|
| MD | GD | MD | GD | |
| Intake | ||||
| DM (kg cow–1 day–1) | 19.9 ± 1.9 | 13.1 ± 1.4 | 20.0 ± 1.7 | 15.7 ± 2.2 |
| Water (L cow–1 day–1) | 69.4 ± 7.9 | 40.5 ± 15.6 | 83.1 ± 9.1 | 43.5 ± 14.7 |
| N (g cow–1 day–1) | 495 ± 43 | 410 ± 79 | 482 ± 32 | 446 ± 88 |
| PDIN (g cow–1 day–1) 1 | 2045 ± 188 | 1532 ± 277 | 1979 ± 138 | 1803 ± 362 |
| PDIE (g cow–1 day–1) 2 | 1925 ± 176 | 1275 ± 154 | 1938 ± 151 | 1545 ± 222 |
| NEL (MJ cow–1 day–1) 3 | 141.5 ± 12.8 | 82.5 ± 9.2 | 142.3 ± 12.1 | 103.8 ± 14.9 |
| Production | ||||
| Milk (kg cow–1 day–1) | 23.4 ± 2.1 | 13.4 ± 1.8 | 28.4 ± 5.6 | 26.0 ± 3.5 |
| Milk N (g–1 cow–1 day–1) | 151 ± 11 | 80 ± 6 | 155 ± 11 | 132 ± 13 |
| Milk urea N (mg dL–1) | 8.8 ± 1.5 | 17.6 ± 4.9 | 7.6 ± 1.7 | 8.1 ± 1.4 |
| NUE, % 4 | 30.9 ± 5.8 | 20.5 ± 4.9 | 32.2 ± 1.5 | 30.6 ± 6.5 |
| Autumn | Spring | |||
|---|---|---|---|---|
| MD | GD | MD | GD | |
| Initial amount (kg fresh matter cow–1) | 1443 | 1983 | 1680 | 2360 |
| Initial amount (kg DM cow–1) | 383 | 472 | 464 | 548 |
| Initial composition (g kg DM–1) | ||||
| Organic matter | 900 | 875 | 890 | 834 |
| Carbon | 450 | 517 | 420 | 400 |
| Nitrogen | 17.2 | 18.3 | 19.4 | 19.1 |
| Solid manure temperature | ||||
| Entire storage period (°C) | 29.9 ± 11.9 | 37.9 ± 15.5 | 57.2 ± 10.1 | 53.4 ± 7.6 |
| First week of storage (°C) | 46.6 ± 12.4 | 54.3 ± 11.8 | 75.4 ± 4.3 | 61.0 ± 8.3 |
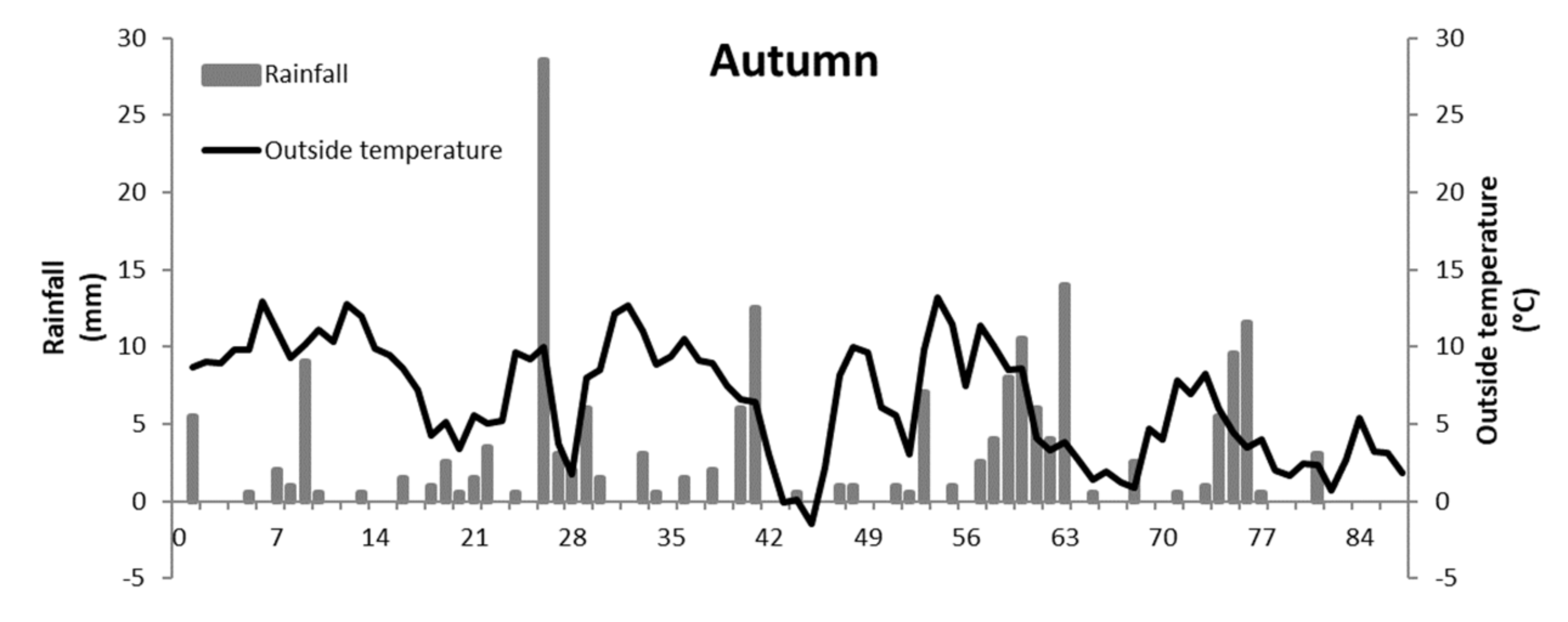
| Weather conditions | ||||
| Mean temperature (°C) | 6.6 ± 3.5 | 16.9 ± 3.2 | ||
| Mean relative humidity (%) | 87.1 ± 5.9 | 71.2 ± 6.1 | ||
| Cumulative rainfall (mm) | 262 | 111 | ||
| Autumn | Spring | |||
|---|---|---|---|---|
| NH3–N | MD | GD | MD | GD |
| g cow–1 period–1 | ||||
| Barn, 28 days | 410 | 489 | 340 | 318 |
| Storage, 85 days | 498 | 964 | 869 | 614 |
| Total, 113 days | 908 | 1453 | 1209 | 932 |
| % of N intake 1 | ||||
| Barn | 3.0 | 4.2 | 2.5 | 2.5 |
| Storage | 3.6 | 8.4 | 6.4 | 4.9 |
| Total | 6.6 | 12.6 | 8.9 | 7.4 |
| Autumn | Spring | |||
|---|---|---|---|---|
| N2O–N | MD | GD | MD | GD |
| g cow–1 period–1 | ||||
| Barn, 28 days | — | — | — | — |
| Storage, 85 days | 92 | 100 | 99 | 157 |
| Total, 113 days | 92 | 100 | 99 | 157 |
| % of N intake 1 | ||||
| Barn | — | — | — | — |
| Storage | 0.66 | 0.87 | 0.73 | 1.26 |
| Total | 0.66 | 0.87 | 0.73 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, J.G.R.; Lorinquer, E.; Robin, P.; Ribeiro-Filho, H.M.N.; Edouard, N. Ammonia and Nitrous Oxide Emissions from Dairy Cows on Straw-Based Litter Systems. Atmosphere 2022, 13, 283. https://doi.org/10.3390/atmos13020283
Almeida JGR, Lorinquer E, Robin P, Ribeiro-Filho HMN, Edouard N. Ammonia and Nitrous Oxide Emissions from Dairy Cows on Straw-Based Litter Systems. Atmosphere. 2022; 13(2):283. https://doi.org/10.3390/atmos13020283
Chicago/Turabian StyleAlmeida, João G. R., Elise Lorinquer, Paul Robin, Henrique M. N. Ribeiro-Filho, and Nadège Edouard. 2022. "Ammonia and Nitrous Oxide Emissions from Dairy Cows on Straw-Based Litter Systems" Atmosphere 13, no. 2: 283. https://doi.org/10.3390/atmos13020283
APA StyleAlmeida, J. G. R., Lorinquer, E., Robin, P., Ribeiro-Filho, H. M. N., & Edouard, N. (2022). Ammonia and Nitrous Oxide Emissions from Dairy Cows on Straw-Based Litter Systems. Atmosphere, 13(2), 283. https://doi.org/10.3390/atmos13020283








