Abstract
Aerosol pH governs many important atmospheric processes that occur in the marine boundary layer, including regulating halogen and sulfur chemistries, and nutrient fertilization of surface ocean waters. In this study, we investigated the acidity of PM1 over the eastern North Atlantic during the Aerosol and Cloud Experiments in Eastern North Atlantic (ACE-ENA) aircraft campaign. The ISORROPIA-II thermodynamic model was used to predict PM1 pH and water. We first investigated the sensitivities of PM1 pH and water predictions to gas-phase NH3 and HNO3 concentrations. Our sensitivity analysis indicated that even though NH3 and HNO3 were present at very low concentrations in the eastern North Atlantic during the campaign, PM1 pH calculations can still be sensitive to NH3 concentrations. Specifically, NH3 was needed to constrain the pH of populations of PM1 that had low mass concentrations of NH4+ and non-volatile cations (NVCs). We next assumed that gas-phase NH3 and HNO3 concentrations during the campaign were 0.15 and 0.09 µg m−3, respectively, based on previous measurements conducted in the eastern North Atlantic. Using the assumption that PM1 were internally mixed (i.e., bulk PM1), we determined that PM1 pH ranged from 0.3–8.6, with a mean pH of 5.0 ± 2.3. The pH depended on both and . was controlled primarily by the NVCs/SO42− molar ratio, while was controlled by the SO42− mass concentration and RH. Changes in pH with altitude were driven primarily by changes in SO42−. Since aerosols in marine atmospheres are rarely internally mixed, the scenario where non-sea salt species and sea-salt species were present in two separate aerosol modes in the PM1 (i.e., completely externally mixed) was also considered. Smaller pH values were predicted for the aerosol mode comprised only of non-sea salt species compared to the bulk PM1 (difference of around 1 unit on average). This was due to the exclusion of sea-salt species (especially hygroscopic alkaline NVCs) in this aerosol mode, which led to increases in values and decreases in values. This result demonstrated that assumptions of aerosol mixing states can impact aerosol pH predictions substantially, which will have important implications for evaluating the nature and magnitude of pH-dependent atmospheric processes that occur in the marine boundary layer.
1. Introduction
Aerosol acidity is an important property that governs many atmospheric processes that transform the mass concentration and composition of atmospheric aerosols, which in turn have important implications for air quality, climate, and human and ecosystem health. Examples of these atmospheric processes include enhancing the formation of secondary organic aerosols (SOA) through acid-catalyzed reactions during the oxidation of volatile organic compounds (VOCs) [1,2,3], controlling the gas-aerosol partitioning of atmospheric semi-volatile basic and acidic species (e.g., ammonia (NH3), hydrochloric acid (HCl), nitric acid (HNO3)) [4,5,6], regulating the water solubilities of trace metals and nutrient species in aerosols [7,8,9], and modulating halogen and sulfur chemistries in the marine boundary layer [10,11]. pH is the parameter commonly used to characterize the acidity of atmospheric aerosols. Aerosol pH is defined as the negative logarithm of the molality-based hydronium ion (H3O+) activity in the aerosol aqueous phase [12]. Water-soluble ions play important roles in affecting aerosol acidity because they control the balance of ions in the aerosol aqueous phase and regulate the water uptake properties (i.e., hygroscopicity) of aerosols. Water-soluble inorganic ions can comprise a substantial fraction of the dry aerosol mass (25% to 75%), with the main components typically being ammonium (NH4+), sulfate (SO42−), and nitrate (NO3−) [13]. Depending on the location, significant mass concentrations of chloride (Cl−) and non-volatile cations (NVCs) such as sodium (Na+), calcium (Ca2+), potassium (K+), and magnesium (Mg2+) can also be present in atmospheric aerosols. This is especially the case for aerosols found in marine atmospheres and/or areas affected by severe dust events [14]. Fresh dust and sea salts are naturally alkaline due to the presence of inorganic carbonates [11]. However, dust and sea salts can become acidified during atmospheric aging due to the uptake of acidic gases (e.g., HNO3) [15,16,17]. The mixing characteristics of dust and sea salts within the aerosol will also change during atmospheric aging. For example, NaCl in fresh sea salts are typically capped by small quantities of other inorganic species, whereas aged sea salts can be completely encased within large quantities of other inorganic and organic species [18]. NVCs are usually present in larger concentrations in PM2.5 than in PM1, which results in PM2.5 typically being less acidic than PM1 [7]. Water-soluble organic species present in the aerosol can also impact the aerosol pH by changing the H3O+ activity in the aqueous phase and/or by diluting the aqueous phase with aerosol water associated with the organic fraction. However, Battaglia Jr. et al. (2019) showed that water-soluble inorganic ions alone adequately constrain the aerosol pH under conditions where liquid–liquid phase separation is not expected to occur [19].
Due to the complex physicochemical properties of atmospheric aerosols, there are few analytical methods that can directly measure the pH of atmospheric aerosols [14,20]. Thermodynamic equilibrium models (e.g., E-AIM [21], ISORROPIA-II [22], EQUISOLV II [23]) currently provide the most reliable estimates of aerosol pH. Thermodynamic equilibrium models calculate the aerosol water content and pH based on model inputs of meteorological data, gas and aerosol measurements [14]. All particle-phase species are assumed to be internally mixed within the aerosol, so one value of pH represents the aerosol population. PM1 NH4+, SO42−, and NO3− ions are usually measured in ambient studies, thus their concentrations are often included in thermodynamic calculations of aerosol pH and water. In contrast, the concentrations of Cl− and NVCs are sometimes excluded from thermodynamic calculations since they are seldom included in aerosol composition measurements [24]. This is due, in part, to the small contributions of these species to the overall aerosol mass in most locations. The omission of NVCs from thermodynamic calculations can result in erroneously predicted aerosol ammonium-to-sulfate (NH4+/SO42−) molar ratios and pH. Using datasets from southeastern USA during the SOAS campaign and from northeastern USA during the WINTER campaign, Guo et al. (2018) showed that the omission of NVCs in ISORROPIA-II calculations resulted in the overprediction of NH4+/SO42− molar ratios in fine aerosols [25]. This was due to NH4+ ions replacing the excluded NVCs in the thermodynamic calculations. The aerosol pH values for the SOAS and WINTER field campaigns only increased by 0.1 to 0.5 units when NVCs were included in thermodynamic calculations. However, this small increase in aerosol pH values can affect the predicted gas-aerosol partitioning of semi-volatile species (e.g., NH4+–NH3, NO3−–HNO3, Cl−–HCl) due to their nonlinear sensitivity to aerosol pH [5,6]. It should be noted that the concentrations of NVCs in the SOAS and WINTER datasets were very low (i.e., close to or below the limits of detection of aerosol composition measurements) compared to the concentrations of NH4+, SO42−, and NO3− ions. It is likely that aerosol pH will only be insensitive to the model exclusion of NVCs in datasets from environments where NVC concentrations are low.
Previous studies have shown that gas-phase NH3 measurements serve as important constraints on aerosol pH in thermodynamic calculations of aerosols in populated continental locations with anthropogenic emissions of NH3 from agriculture, traffic, and industry [26,27]. This is because not including gas-phase NH3 concentrations in thermodynamic calculations can result in pH predictions that are too low since the thermodynamic model will partition a fraction of the particle-phase NH4+ to the gas phase to make up for the missing gas-phase NH3, which will result in a prediction of a more acidic aerosol. However, NH3 is not commonly measured in field campaigns, especially in studies that are held in remote marine locations [28]. Satellite observations and model simulations have shown that outside of polluted air masses originating from continental and/or biomass burning regions, NH3 mixing ratios over the Atlantic and Pacific Oceans are typically in the parts per trillion (ppt) range [28,29]. This is in line with previous NH3 measurements conducted over the northeast Atlantic Ocean during the ASTEX/MAGE campaign where the measured NH3 mixing ratios ranged from <25 ppt to 710 ppt [30]. The low NH3 concentrations in remote marine regions is attributed to low oceanic NHx emissions and the short lifetime of NH3 [28,31,32,33]. Thus, NH3 concentrations in remote marine locations are substantially lower than those in continental locations. Given that alkaline NVCs are expected to comprise a significant mass fraction of aerosols in remote marine atmospheres, it is currently unclear whether NH3 is an important neutralizing agent of PM1 acidity and/or serves as a key constraint on aerosol pH in thermodynamic calculations of aerosols in remote marine atmospheres.
The acidity of aerosols in environments with intensive anthropogenic activities have received the most attention. Pye et al. (2020) complied these studies and reported that the mean pH of fine aerosols ranged from around 1 to 6 [14]. NH4+, SO42−, and NO3− ions are the dominant water-soluble inorganic ions in fine aerosols in most areas with intensive anthropogenic activities; thus, aerosol pH and water are usually regulated by these three ions in these environments. Locations with very high levels of acidic sulfate (e.g., southeastern USA, southeast Asia) had fine aerosol pH values of less than 2 [14,34,35]. Higher PM2.5 pH values (around 2 to 3) were reported for Los Angeles during the CalNex campaign due to higher aerosol water concentrations, which was a result of higher total NO3− concentrations and NO3−–HNO3 partitioning [5]. Locations close to areas with intensive agricultural activities that emitted high levels of NH3 (e.g., parts of mainland China and Europe) had fine pH values as high as 6 [14,36,37]. Higher fine aerosol pH values (around 4 to 6) were reported for urban areas impacted by sea salt (e.g., San Paulo, Hawaii) and dust (e.g., Inner Mongolia, Po Valley) due to the neutralizing effect of NVCs [14,38,39,40,41].
There have been comparatively few studies on the acidity of aerosols in remote marine atmospheres. Keene and Savoie (1998) used a Cl phase partitioning model to estimate that aerosols had pH values ranging from the mid-2s to the mid-3s at Bermuda under moderately polluted conditions [42]. Fridlind and Jacobson (2000) applied the EQISOLV II thermodynamic model to estimate that aerosols over the minimally polluted Southern Ocean had pH values that ranged from 0 to 5 [4]. The acidic nature of aerosols observed in these studies were explained by the rapid titration of fresh sea salt alkalinity by acids scavenged from the gas phase or formed via chemical reactions occurring within the aerosol [4,43,44]. Recently, Nault et al. (2021) used the E-AIM thermodynamic model to estimate that PM1 over the Pacific, Southern, Atlantic, and Artic Oceans had pH values ranging from −1 to 3 [28]. It should be noted that NVCs were excluded from thermodynamic calculations performed by Nault et al. (2021), and this resulted in lower estimated aerosol pH values compared to estimates that included accumulation-mode NVCs in thermodynamic calculations. Given the important role that aerosol acidity plays in many atmospheric processes that occur in the remote marine boundary layer (e.g., halogen and sulfur chemistries), it is important to have a strong understanding of the factors that control aerosol pH and water (e.g., roles of NH3 and NVCs, aerosol mixing characteristics) in different remote marine atmospheres.
In this paper, we used the PM1 dataset obtained with a particle-into-liquid sampler (PILS) during the summer 2017 flights of the Aerosol and Cloud Experiments in Eastern North Atlantic (ACE-ENA) campaign to characterize the acidity of PM1 over the eastern North Atlantic. Measurements were carried out in the Azores archipelago in the eastern North Atlantic during the ACE-ENA campaign [45]. Although the ACE-ENA campaign had two intense operating periods (IOP) that occurred during summer 2017 (June to July) and winter 2018 (January to February), the PM1 dataset obtained during the winter IOP was not used because the mass concentrations of many water-soluble aerosol constituents essential for aerosol acidity predictions were very low (i.e., close to or below the limits of detection of aerosol composition measurements). The composition and properties of aerosols in the eastern North Atlantic are known to be influenced by a variety of natural processes, including sea spray aerosol production, the entrainment of aerosols from the free troposphere, new particle formation, and the processing of aerosols inside and outside of clouds [46,47,48]. Aerosols in the eastern North Atlantic are also subjected to occasional anthropogenic influences caused by local pollution from the Azorean islands and polluted air masses originating from North America and northern Europe [30,46,47,48,49,50,51]. These factors make the eastern North Atlantic an ideal location for investigations of pH and water content of PM1 in a remote marine environment where anthropogenic influences are minor. Since each flight consisted of different vertical profiles, we will also provide insights into how PM1 acidity changes with altitude in the eastern North Atlantic.
2. Materials and Methods
The ACE-ENA campaign was a multi-investigator study that included measurements onboard the DOE G-1 research aircraft [45]. The G-1 was operated out of the Lajes airport on Terceira Island. A total of 20 flights occurred during the summer IOP. Each flight comprised of four to six vertical profiles, which allowed measurements as a function of altitude to be obtained. The payload onboard the G-1 included instruments used to measure meteorological parameters, trace gases, aerosols, and cloud properties. Details about the flight paths and the list of instruments onboard the G-1 can be found in Zawadowicz et al. (2021) and Wang et al. (2021) [46,47].
2.1. PILS Sampling and Offline Ion Chromatography Analysis
A PILS was used to measure the water-soluble ions in PM1. The PILS is an aerosol collection device that continuously collects ambient aerosols into water [52,53]. In the PILS, aerosols were mixed with water vapor at around 100 °C produced from heated ultrapure deionized water. The resulting droplets were impacted onto a plate, thus providing a liquid sample with aerosols dissolved in it. During each flight, the PILS continuously sampled from an isokinetic inlet at a flowrate of 15 L/min. The size-cut was provided by a non-rotating MOUDI impactor with 50% transmission efficiency of 1 µm (aerodynamic diameter) at 1 atm ambient pressure [54]. Upstream of the PILS were two honeycomb denuders coated with sodium carbonate and phosphorous acid to remove acidic and basic gases, respectively. The PILS was connected to a Bretchel fraction collector to collect liquid samples for offline ion chromatography (IC) analysis [53]. The liquid sample was pushed into the fraction collector vials at a flowrate of 0.65 mL/min by a peristaltic pump to collect ~1.2 mL of sample per vial every 2 min. The fraction collector system holds 721.5 mL polypropylene vials (Microsolv Technology Corporation, Leland, NC, USA) per carousel. During each flight, carousels were pre-loaded and manually switched out once all the vials in that carousel had been filled. Blank samples were also collected on each flight by diverting the sampled air through a High Efficiency Particulate-Free Air (HEPA) filter (Pall Corp., Port Washington, NY, USA) before being introduced into the PILS. All the data were background corrected. After each flight, all the vials were removed from the carousels and capped with solid caps (Microsolv Technology Corporation, Leland, NC, USA). The capped vails were stored refrigerated until analyzed. IC analysis of the samples began in the field and were completed back at Colorado State University (CSU) following the end of the intensive.
Each vial was brought back to room temperature and then analyzed for anions and cations. For each analysis, 300 µL aliquots were transferred to polypropylene vials. Anions were measured using a potassium hydroxide gradient provided by an eluent generator at a flowrate of 0.015 mL/min. The complete run time was 65 min with an injection volume of 35 µL. The cations were determined using a Dionex DX-500 IC with a gradient pump, conductivity detector, and self-regenerating cation suppressor. A Dionex CS-12A analytical column (3 mm × 150 mm) using an eluent of 20 mM methanesulfonic acid at a flowrate of 0.5 mL/min was used. The injection volume and run time were 190 µL and 17 min, respectively. In the discussions presented below, we focused on PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+ inorganic ions measured by the PILS-fraction collector system.
2.2. Thermodynamic Calculations
The thermodynamic equilibrium model ISORROPIA-II was used to determine the equilibrium phase-partitioning and composition of an NH4+–SO42−–NO3−–Cl−–Na+–Ca2+–K+–Mg2+–water aerosol [22]. The aerosol pH calculated in this study used the molal definition consistent with the pHF definition by Pye et al. (2020) [14]:
where (µg m−3) is the hydronium ion concentration per volume of air, and (µg m−3) is the bulk aerosol water concentration. For simplicity, H3O+ is denoted here as H+ even though we acknowledge that the unhydrated hydrogen ion is rare in aqueous solutions. is the sum of the bulk aerosol water concentrations associated with inorganic and organic species (i.e., . The concentrations of organic species were low during the summer IOP [47], thus we expect to be low. Previous studies have also shown that the effects of to the aerosol pH is not significant [19,34]. Thus, we report aerosol pH only considering . and are outputs of the ISORROPIA-II model.
ISORROPIA-II was run in “forward” mode based on the assumptions that the aerosol was internally mixed, that it existed in a “metastable” equilibrium state (i.e., the aerosols only existed in liquid form), and that the aerosol was in thermodynamic equilibrium with the gas phase. Discussions about how the assumption of aerosols being internally mixed affects predictions of aerosol pH and water will be presented below. In “forward” mode, the model uses the input of the total concentration of a species (i.e., gas + particle) to calculate the gas-aerosol equilibrium partitioning concentrations. The water-soluble inorganic NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+ concentrations measured by the PILS-fraction collector system and the meteorological parameters measured onboard the G-1 were used as model inputs. Gas-phase inorganic measurements were not available for the ACE-ENA campaign. Thus, we used gas-phase NH3 and HNO3 concentrations previously measured in the eastern North Atlantic in our thermodynamic calculations [30]. Discussions about how the inclusions of gas-phase NH3 and HNO3 concentrations in the thermodynamic calculations impact the predictions of aerosol pH and water will be presented below. In “reverse” mode, the model uses the input of only the particle-phase concentration of a species to calculate the gas-aerosol equilibrium partitioning concentrations. “Reverse” mode was not used in this study because this mode is known to be very sensitive to measurement errors, which can cause large errors in the predicted aerosol pH [55].
3. Results and Discussion
Conditions in the eastern North Atlantic were clean during the summer IOP. Zawadowicz et al. (2021), reported that the mass concentrations of non-refractory PM1 organics, NH4+, SO42−, and NO3− measured by an Aerodyne High-Resolution Time-of-Flight Aerosol Mass Spectrometer during the summer IOP were low, with the mean mass concentrations of these species ranging from 0.01–0.55 µg m−3 [47]. Low mass concentrations of water-soluble inorganic species were also measured by the PILS-fraction collector system (Table S1). These low mass concentrations were unsurprising since the sampling area was far from anthropogenic pollution sources. This provided us with an ideal opportunity to investigate PM1 pH and water in a clean marine environment where anthropogenic influences were minor.
Thermodynamic calculations were performed for periods where the RH was between 35% and 95%. Periods where the RH was below 35% were excluded because the aerosols were less likely to be in a liquid state, which would lead to high uncertainties in pH predictions due to uncertain activity coefficients associated with highly concentrated solutions under these low RH conditions [56,57,58]. Periods where the RH was above 95% were also excluded because the exponential growth in aerosol water with RH would introduce large pH uncertainties [58]. We used the mass concentrations of water-soluble PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+ measured by the PILS-fraction collector system. For the dataset used for the thermodynamic calculations, NVCs (i.e., Na+, Ca2+, K+, and Mg2+) comprised 36.3 ± 16.6% of the PM1 inorganic mass concentration (which we defined here as the sum of NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+ mass concentrations). However, there were time periods where NVCs comprised less than 20% of the PM1 inorganic mass concentration.
In the first part of this paper, we assumed that all the water-soluble inorganic ions were internally mixed in the aerosol, and explored the sensitivity of PM1 pH and water to gas-phase NH3 and HNO3 concentrations (Section 3.1), and the key factors that strongly influenced predictions of PM1 pH and water (Section 3.2). In the second part of this paper, we investigated how assumptions regarding how sea salt species and NVCs mixed in aerosols (i.e., internal vs. external mixtures) will impact predictions of PM1 pH and water (Section 3.3).
3.1. Sensitivity of PM1 pH and Water to the Inclusion of Gas-Phase NH3 and HNO3 Concentrations to ISORROPIA-II
Murphy et al. (2017) previously showed that gas-phase NH3 and HNO3 measurements can serve as important constraints on aerosol pH in thermodynamic calculations of aerosols in populated continental locations with anthropogenic emissions [26]. Gas-phase inorganic measurements were not available for the ACE-ENA campaign. However, gas-phase measurements conducted several years ago during the ASTEX/MAGE study indicated that NH3 and HNO3 concentrations are usually low in the eastern North Atlantic during the summer. During the ASTEX/MAGE study, NH3 mixing ratios ranged from <25 ppt to 710 ppt with a study-averaged mixing ratio of around 200 ppt (around 0.15 µg m−3), while HNO3 mixing ratios ranged from <8 ppt to 164 ppt with a study-averaged mixing ratio of around 28 ppt (around 0.09 µg m−3) [30]. In this section, we explore the sensitivities of PM1 pH and water predictions to gas-phase NH3 and HNO3 concentrations. This will allow us to determine the effects of excluding gas-phase NH3 and HNO3 concentrations in aerosol pH calculations for clean remote environments that have substantially lower concentrations of gas-phase NH3 and HNO3 compared to populated continental locations. The sensitivities were assessed by perturbing the ISORROPIA-II input concentrations of total ammonium (TA = NH3 + NH4+) and total nitrate (TN = HNO3 + NO3−). Different concentrations of NH3 and HNO3 were added into the system, and the responses in PM1 pH and water were quantified. The overall goal of these sensitivity tests is to determine the conditions where gas-phase NH3 and HNO3 serve as critical constraints on aerosol pH in thermodynamic calculations of aerosols measured during the ACE-ENA campaign.
In our NH3 sensitivity analysis, we added 0.01, 0.12, 0.15, and 0.29 µg m−3 of NH3 into the system. The median and mean NH3 concentrations measured during the ASTEX/MAGE study were 0.12 and 0.15 µg m−3, respectively [30]. The addition of 0.12 and 0.15 µg m−3 of NH3 into the system resulted in average NH3/TA mass ratios of 0.61 ± 0.18 and 0.65 ± 0.17, respectively. It was determined that 0.01 and 0.29 µg m−3 were the 25th and 75th percentile NH3 concentrations measured during the ASTEX/MAGE study, respectively [30]. The addition of 0.01 and 0.29 µg m−3 of NH3 into the system resulted in average NH3/TA mass ratios of 0.16 ± 0.14 and 0.77 ± 0.13, respectively. Based on the average NH3/TA mass ratios, the addition of 0.01 µg m−3 of NH3 into the system assumes that majority of the TA species is in the aerosol phase in the form of NH4+ ions. In contrast, the addition of 0.12, 0.15, and 0.29 µg m−3 of NH3 into the system assumes that the majority of the TA species is in the gas phase as NH3. Figure 1a compares the different median and mean pH values with the 10th, 25th, 75th, and 90th percentile values calculated for the different NH3 concentrations added into the system. The median pH did not change substantially for the range of NH3 concentrations added (differed by a maximum of 0.05 units). However, the mean pH increased by approximately 0.4 units upon the addition of ≥0.12 µg m−3 of NH3 into the system. In addition, the 10th percentile pH value increased by approximately 1.8 units when ≥0.12 µg m−3 of NH3 was added. This was because a subset of PM1 in the dataset had low mass concentrations of NH4+ and NVCs. The addition of 0.12 µg m−3 of NH3 into the system resulted in a decrease in for this subset of PM1 due to NH3–NH4+ gas-aerosol partitioning. This was demonstrated by the noticeable decrease in the 10th percentile value (1.7 × 10−4 µg m−3 to 3.2 × 10−5 µg m−3) when 0.12 µg m−3 of NH3 was added into the system (Figure S1a). Adding more NH3 into the system did not change the pH of this subset of PM1 substantially, as demonstrated by the somewhat similar mean pH values (differed by a maximum of 0.1 units) and 10th percentile pH values (differed by a maximum of 0.2 units) obtained when 0.12 vs. 0.15 and 0.29 µg m−3 of NH3 were added into the system. This was because aerosol pH was weakly sensitive to a wide NH3 concentration range due to pH buffering caused by the partitioning of NH3 between the gas and aerosol phases [59]. The addition of 0.01 to 0.29 µg m−3 of NH3 into the system did not result in significant changes in (Figure S1b).
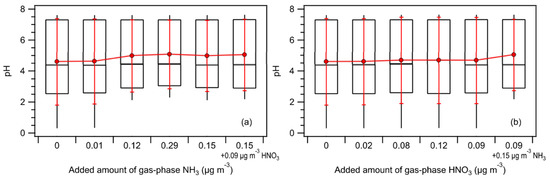
Figure 1.
Box plots depicting the median pH values calculated by ISORROPIA-II for the different concentrations of gas-phase (a) NH3, and (b) HNO3 added into the system. Standard box-and-whisker plots are shown, with the 90th and 10th percentile data indicated by black error bars. The top and bottom of the box are the interquartile ranges (75th and 25th percentile) centered around the median value (50th percentile). The red symbols denote the means, and the red error bars are 1 standard deviation.
In our HNO3 sensitivity analysis, we added 0.02, 0.08, 0.09, and 0.12 µg m−3 of HNO3 into the system. The median and mean HNO3 concentrations measured during the ASTEX/MAGE study were 0.08 and 0.09 µg m−3, respectively [30]. The addition of 0.08 and 0.09 µg m−3 of HNO3 into the system resulted in average HNO3/TN mass ratios of 0.75 ± 0.15 and 0.77 ± 0.14, respectively. It was determined that 0.02 and 0.12 µg m−3 were the 25th and 75th percentile HNO3 concentrations measured during the ASTEX/MAGE study, respectively [30]. The addition of 0.02 and 0.12 µg m−3 of HNO3 into the system resulted in average HNO3/TN mass ratios of 0.48 ± 0.19 and 0.81 ± 0.12, respectively. Figure 1b compares the different median and mean pH values with the 10th, 25th, 75th, and 90th percentile values calculated for the different HNO3 concentrations added into the system. Unlike the addition of NH3 into the system, adding HNO3 into the system did not lead to noticeable changes in the calculated pH values. After the addition of up to 0.12 µg m−3 of HNO3 into the system, the median and mean pH values only differed by 0.29 and 0.1 units, respectively. These small changes in the pH values were due to the weak sensitivities of and to the range of HNO3 concentrations added into the system. There were no significant changes in , while the median and mean values only increased by a maximum of 0.22 and 0.25 µg m−3, respectively (Figure S2). The increase in can be attributed to the thermodynamic model partitioning a fraction of HNO3 from the gas phase to the aerosol phase, and the hygroscopicity of particle-phase NO3− salts.
Overall, our sensitivity analysis demonstrated that even though NH3 (and HNO3) may be present at very low concentrations in clean marine atmospheres (relative to continental locations with anthropogenic emissions), aerosol pH calculations will be sensitive to NH3 concentrations under some conditions. This will especially be the case for populations of aerosols that have low mass concentrations of NVCs where TA will act as the main neutralizing agent of aerosol acidity. For pH predictions of PM1 during the summer IOP, we observed that not including NH3 concentrations into TA in aerosol pH calculations will lead to the overestimation of aerosol acidity for populations of aerosols that have low mass concentrations of NH4+ and NVCs. This is because NH3–NH4+ gas-aerosol partitioning calculated in thermodynamic models will be derived solely on the measured particle-phase NH4+ mass concentrations. A fraction of this NH4+ will be partitioned into the gas phase as NH3 in the thermodynamic model, thus leading to the release of more particle-phase H+, which will result in the prediction of a more acidic aerosol. In this study, for PM1 populations with low mass concentrations of NH4+ and NVCs, adding 0.15 µg m−3 of NH3 into the system generally resulted in the predicted pH increasing by approximately 2 units. Hence, NH3 measurements can serve as important constraints on the pH of aerosols found in clean marine atmospheres.
3.2. Factors That Influence PM1 pH and Water
For the rest of the discussion carried out in this paper, we assumed that gas-phase NH3 and HNO3 concentrations during the summer IOP were 0.15 and 0.09 µg m−3, respectively [30]. These were the mean concentrations measured during the ASTEX/MAGE study. Under these conditions, the predicted PM1 pH ranged from 0.3 to 8.6, with a mean pH of 5.0 ± 2.3. Figure 1 shows the different median and mean pH values with the 10th, 25th, 75th, and 90th percentile values calculated when 0.15 µg m−3 of NH3 and 0.09 µg m−3 of HNO3 were included in the thermodynamic calculations.
The dependence of PM1 pH on is shown in Figure 2. An increase in generally led to a noticeable decrease in PM1 pH. was observed to depend mainly on the total molar concentration of NVCs (i.e., NVCs = Na+ + Ca2+ + K+ + Mg2+) relative to the SO42− molar concentration in the aerosol. The values were grouped based on their NVCs/SO42− molar ratios, and their statistics are shown as a function of the NVCs/SO42− molar ratio in Figure 3. decreased substantially when the NVCs/SO42− molar ratio increased from 0 to 3. In addition, when the amount of SO42− was low, even small amounts of NVCs impacted significantly. However, when the amount of SO42− was high (comprised at least 30% of the mole fraction of the sum of PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+), small amounts of the NVCs (up to 30% of the mole fraction of the sum of PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+) did not reduce substantially. Previous studies have reported that the pH of aerosols in other marine and coastal regions was similarly impacted by the NVCs/SO42− molar ratio. During the WINTER campaign, the aerosol pH increased by more than 2 units when the SO42− mass concentration decreased and the NaCl mass concentration increased during flights made over or near coastal regions in northeastern USA [6]. Fine aerosols over the Southern Ocean became less acidic when the Na+/SO42− ratio increased [4]. The relationship between aerosol acidity and the NVCs/SO42− molar ratio can be explained by the non-volatile nature of alkaline NVCs present in aerosols. The NVCs preferentially and irreversibly neutralized SO42− in the aerosol over NH3, which reduced the concentration of H+ in the aerosol.
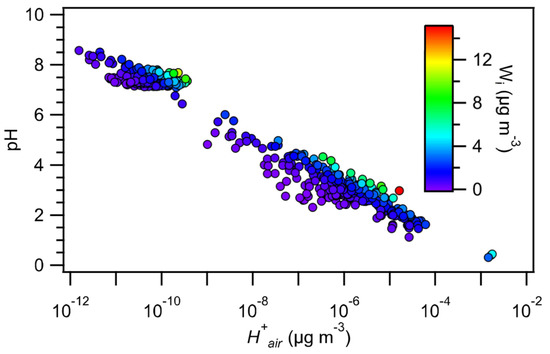
Figure 2.
PM1 pH versus colored by . Gas-phase NH3 and HNO3 concentrations were assumed to be 0.15 and 0.09 µg m−3, respectively, in the ISORROPIA-II calculations.
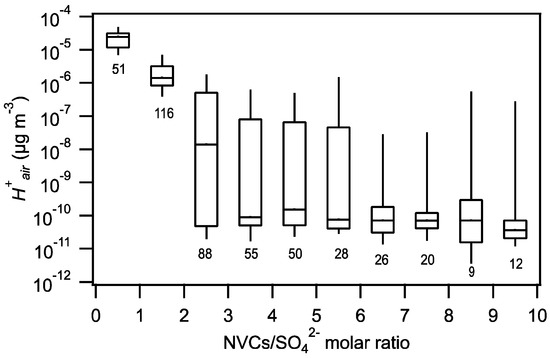
Figure 3.
Box plot of versus the NVCs/SO42− molar ratio. The box plot was generated by segregating the data into ten equally-spaced NVCs/SO42− molar ratio bins. Standard box-and-whisker plots are shown, with the 90th and 10th percentile data indicated by black error bars. The top and bottom of the box are the interquartile ranges (75th and 25th percentile) centered around the median value (50th percentile). The number of points considered for each bin are also shown.
The influence of on PM1 pH was also evident in Figure 2. For any given value, smaller pH values were predicted for smaller values. Since was predicted by ISORROPIA-II from the mass concentrations of water-soluble inorganic species, one would expect to be dependent on RH and the mass concentrations of hygroscopic inorganic species. Although NVCs are known hygroscopic species that can elevate , no obvious relationship was observed between the NVCs mass loadings and for PM1. SO42− was the major anion species with high hygroscopicity in PM1. In general, increased with the SO42− mass concentration and RH (Figure S3). No obvious relationships were observed between and NO3− and Cl−, and this was likely due to the low mass concentrations of NO3− and Cl−.
To determine the major factors that influence PM1 pH during the summer IOP, we performed a series of sensitivity tests of PM1 pH to major water-soluble inorganic ions (i.e., SO42−, Na+, Ca2+, K+, and Mg2+), (TA = NH3 + NH4+), and meteorological conditions (i.e., RH and temperature). In each sensitivity test, we evaluated how the variable affected the PM1 pH by inputting the real-time measured value of this variable and the mean values of the other parameters into ISORROPIA-II. The relative standard deviation (RSD) of the re-calculated pH values reflected the degree of sensitivity that variations in the variable had on aerosol acidity. The larger the RSD was, the greater the impact the variable had on the aerosol acidity (and vice versa). This method was previously utilized by Ding et al. (2019) and Wang et al. (2020) to determine the major factors that influence aerosol pH in different parts of China [36,60].
Table 1 shows the results of the PM1 pH sensitivity tests. PM1 pH was found to be most sensitive to SO42− and Ca2+. Elevated SO42− levels were essential for the increase of and (Table S2), thus it played a key role in influencing aerosol acidity. Elevated Ca2+ levels could reduce substantially to increase the PM1 pH (Table S2), especially in cases where the mass concentration of SO42− in the aerosol was low. In the ISORROPIA-II output, Ca2+ existed primarily as CaSO4, which is a marginally water-soluble species [22]. PM1 pH was insensitive to variations in TA, and this could be due to the high mean values of NVCs used in the sensitivity test. As discussed in Section 3.1, variations in TA will mostly affect the pH of PM1 populations that have low mass concentrations of NVCs. Although PM1 pH was somewhat insensitive to variations in meteorological conditions (i.e., RH and temperature), RH impacted substantially (Table S2). Elevated RH conditions could enhance water uptake, which will lead to an increase in (Figure S3). Consequently, high levels of can promote the gas-to-aerosol partitioning of NH3 and HNO3.

Table 1.
Sensitivity of PM1 pH to SO42−, TA (NH3 + NH4+), Na+, Ca2+, K+, Mg2+, RH, and temperature. The larger the relative standard deviation (RSD) was, the greater the impact the variable had on pH.
Figure 4a shows the vertical distribution of PM1 pH from 0 to 2500 m altitude. The majority of the PM1 pH values reported here were for PM1 below 1 km due to the RH range (35–95%) chosen for thermodynamic calculations. RH, temperature, and aerosol mass concentrations decreased with altitude. The 250 m altitude-binned statistics showed a somewhat uniform PM1 pH range of 2 to 7.5 for altitudes below 1.5 km, and slightly lower PM1 pH ranges for altitudes above 1.5 km. The 250 m altitude-binned median and mean PM1 pH values increased with altitude. This can be attributed primarily to the decrease in with altitude, which was likely caused by the decrease in the molar ratio of SO42− with altitude (Figure 4b). Here, we defined the SO42− molar ratio as the SO42− molar concentration divided by the sum of the molar concentrations of PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+.
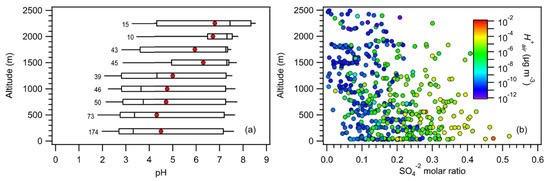
Figure 4.
(a) Vertical profile of PM1 pH (250 m altitude-binned) from 0 to 2500 m altitude. Standard box-and-whisker plots are shown, with the 90th and 10th percentile data indicated by black error bars. The top and bottom of the box are the interquartile ranges (75th and 25th percentile) centered around the median value (50th percentile). The red symbols denote mean pH values. The number of points considered for each bin are also shown. (b) Vertical profile of the SO42− molar ratio (colored by ).
Crustals were treated explicitly in our above ISORROPIA-II analysis. Previous studies that used the E-AIM thermodynamic model to predict aerosol acidity sometimes treated crustals as mole-equivalent Na+ (i.e., Ca2+ = 2Na+, Mg2+ = 2Na+, and K+ = Na+) because E-AIM cannot explicitly treat crustal species. The mole-equivalent Na+ treatment does not impact aerosol pH predictions significantly (difference of less than 1 unit) in areas with low levels of NVCs. We examined the impact that treating crustals as mole-equivalent Na+ will have on pH predictions of ACE-ENA PM1 where NVC concentrations were substantial. Higher mean and median pH values were predicted (approximately 1.0 units and 3.1 units higher, respectively) when Ca2+, Mg2+, and K+ were treated as mole-equivalent Na+ (Figure S4). Differences in the predicted pH values were due to substantial differences in predicted and values between the two treatments, which were caused by the non-ideality of divalent ions (i.e., Ca2+ and Mg2+) and the different hygroscopicity behaviors of Na+ salts vs. Ca2+, Mg2+, and K+ salts. In particular, values were noticeably higher in the mole-equivalent Na+ treatment (approximately 1.0 µg m−3 higher on average). This was due to the formation of marginally soluble CaSO4 in calculations where crustals were explicitly treated [22]. CaSO4 does not significantly contribute to water uptake [56], which will lead to smaller predicted values in these calculations. Overall, our analysis emphasizes the need to treat crustals explicitly in thermodynamic calculations of PM1 in marine atmospheres (or any other environments with substantial mass concentrations of NVCs). Not doing so will lead to erroneous predictions of aerosol pH, which has important implications for gas-aerosol partitioning predictions.
3.3. Internal Mixture vs. Mixture with Two Modes
Our analysis up to this point assumed that all the water-soluble inorganic ions were internally mixed in the aerosol. Thus, pH predictions presented above were obtained using the total PM1 NH4+, SO42−, NO3−, Cl−, Na+, Ca2+, K+, and Mg2+ mass concentrations measured by the PILS-fraction collector system for thermodynamic calculations. However, aerosols that are close to source regions are likely externally mixed. Gas-aerosol interactions, interactions between particle-phase species, and atmospheric aging during transport will make aerosols more homogeneous, eventually leading to internally mixed aerosols [61]. Particle-phase species in marine atmospheres have traditionally been divided into two groups: those that originate from sea salt, and those that originate from non-sea salt (nss) sources. In relatively clean marine atmospheres like the eastern North Atlantic, sea–salt species (especially the NVCs) are expected to contribute a significant mass fraction of aerosols. Sea salts are known to be naturally alkaline [11]. In addition, sea salts are mainly present in the coarse mode, with a tail extending into the fine mode [62]. Hence, non-sea salt species are often not well mixed with sea-salt species in fine aerosols due to their different sources and sizes.
In this section, we investigate how assumptions regarding how sea-salt species and NVCs were mixed in aerosols (i.e., internal vs. external mixtures) will impact predictions of PM1 pH and water. We consider the scenario that non-sea salt species and sea-salt species were present in two separate aerosol modes in the PM1 (i.e., completely externally mixed). The mode consisting of non-sea salt species was assumed to be internally mixed. Again, we assumed that gas-phase NH3 and HNO3 concentrations were 0.15 and 0.09 µg m−3, respectively. NH3, HNO3, and water vapor were assumed to still equilibrate between these two modes due to the short equilibrating timescales for PM1 [56,63,64]. Assuming that all the measured Na+ and Cl− in PM1 were exclusively from sea salt, mass concentrations of non-sea salt K+, Mg2+, Ca2+, and SO42− can be calculated using the following equations [65]:
For instances where the calculated mass concentration of the non-sea salt species were a negative value, the negative calculated value was substituted with 0 µg m−3. This treatment of negative calculated non-sea salt mass concentrations only affected 2% of the dataset. Non-sea salt NVCs comprised 28.9% ± 15.9% of the PM1 non-sea salt inorganic mass concentration. Thermodynamic calculations were performed to predict the pH of the aerosol mode containing non-sea salt species. These results were then compared to those presented in Section 3.2, which provided insights into how the assumption that non-sea salt species are internally mixed with sea-salt species in the aerosol will impact pH predictions.
Figure 5 compares the median and mean pH values with the 10th, 25th, 75th, and 90th percentile values calculated for internally mixed aerosols vs. the aerosol mode containing only non-sea salt species. For the aerosol mode comprised only of non-sea salt species, ISORROPIA-II predicted smaller median and mean pH values compared to those predicted using the internally mixed aerosol assumption (differed by 1.8 and 0.8 units, respectively). The differences in the predicted pH values were due to substantial differences in predicted and values (Figure S5). On average, higher values were predicted for the aerosol mode containing only non-sea salt species compared to internally mixed aerosols (3.20 × 10−5 vs. 1.23 × 10−5 µg m−3). This was unsurprising since the aerosol mode containing only non-sea salt species had lower mass concentrations of alkaline NVCs after the exclusion of sea-salt NVCs in the thermodynamic calculations. In addition, smaller values were predicted for the aerosol mode containing only non-sea salt species (1.7 vs. 0.9 µg m−3) due to the lower mass concentrations of hygroscopic NVC species.
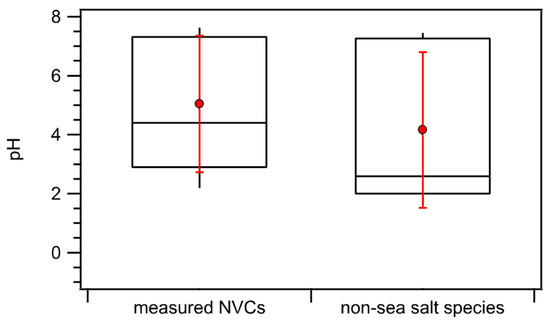
Figure 5.
Box plots depicting the median pH values calculated by ISORROPIA-II for internally mixed aerosols vs. the aerosol mode containing only non-sea salt species. Standard box-and-whisker plots are shown, with the 90th and 10th percentile data indicated by black error bars. The top and bottom of the box are the interquartile ranges (75th and 25th percentile) centered around the median value (50th percentile). The red symbols denote the means, and the red error bars are 1 standard deviation.
It should be noted that the range of predicted pH values for the aerosol mode containing only non-sea salt species was higher than those reported by Nault et al. for PM1 over the Pacific, Southern, Atlantic, and Artic Oceans (pH 1–3) [28]. This was because Nault et al. (2021) excluded all the NVCs (i.e., non-sea salt NVCs and sea-salt NVCs) from their thermodynamic calculations. The authors showed that PM1 NVC mass concentrations were low during their field campaign, and thus would not impact their PM1 pH predictions substantially. In contrast, we observed that the non-sea salt NVCs constituted a significant mass fraction of the PM1 non-sea salt inorganic mass concentration during the ACE-ENA campaign (an average of 28.9% ± 15.9%), and thus they were included in our thermodynamic calculations for the aerosol mode containing only non-sea salt species.
Overall, our results showed that assumptions of the mixing characteristics of aerosols (i.e., internally vs. externally mixed) can significantly impact predicted pH values for fine aerosols in marine atmospheres. Despite these differences, the pH values of the aerosol mode containing only non-sea salt species demonstrated similar trends as those of the internally mixed aerosols. The pH of the aerosol mode containing only non-sea salt species depended strongly on , where an increase in generally led to a noticeable decrease in pH. Similarly, was observed to depend mainly on the total molar concentration of non-sea salt NVCs relative to the molar concentration of non-sea salt SO42− in the aerosol mode. In addition, the pH values of the aerosol mode containing only non-sea salt species also increased with altitude due to the decrease in the molar ratio of non-sea salt SO42− in the aerosol mode (i.e., non-sea salt SO42− molar concentration divided by the sum of the molar concentrations of non-sea salt species) with altitude.
4. Conclusions
The pH of aerosols in remote marine atmospheres influences many important atmospheric processes that occur in the marine boundary layer, including regulating halogen and sulfur chemistries, and nutrient fertilization of surface ocean waters [11,14]. However, the scarcity and limitations (e.g., use of ion balance and molar ratio to determine aerosol pH) of currently available data of aerosol acidity in remote marine atmospheres hinders our understanding of these processes. In this study, we used ISORROPIA-II to predict the acidity of PM1 over the eastern North Atlantic during the summer IOP of the ACE-ENA campaign. Conditions over the eastern North Atlantic were clean during the summer IOP, as reflected by the low mass concentrations of water-soluble species in PM1 measured by the PILS-fraction collector system. We first assessed the sensitivities of PM1 pH and water predictions to a range of gas-phase NH3 and HNO3 concentrations. Our sensitivity analysis revealed that aerosol pH calculations are sensitive to NH3 concentrations even though NH3 may be present at very low concentrations in clean marine atmospheres. Not including NH3 concentrations in aerosol pH calculations will lead to the overestimation of aerosol acidity for populations of aerosols that have low mass concentrations of NH4+ and NVCs. Thus, NH3 measurements serve as important constraints on pH calculations of aerosols in clean marine atmospheres.
Gas-phase NH3 and HNO3 concentrations measured previously in the eastern North Atlantic were used to constrain the aerosol pH calculations. Using the assumption that aerosols were internally mixed (i.e., bulk PM1), we determined that PM1 pH ranged from 0.3 to 8.6, with a mean pH of 5.0 ± 2.3. The pH depended on both and . was controlled primarily by the total molar concentration of NVCs relative to the SO42− molar concentration, while was controlled by the SO42− mass concentration and RH. Overall, the pH was most sensitive to changes in PM1 SO42− and Ca2+ mass concentrations. Elevated SO42− levels were essential for the increase of and . Elevated Ca2+ levels could lead to substantial reduction of , especially in cases where the mass concentration of SO42− in the aerosol was low. Our analysis also indicated that PM1 acidity decreased with altitude. This was due to the decrease in with altitude, which was likely caused by the decrease in the molar ratio of acidic SO42− with altitude.
Since aerosols in marine atmospheres are rarely internally mixed, we also considered the scenario where non-sea salt species and sea-salt species were present in two separate aerosol modes (i.e., completely externally mixed) in the PM1. We showed that smaller pH values would be predicted for the aerosol mode comprised only of non-sea salt species (difference of around 1 unit on average). This was due to the exclusion of sea-salt species (especially hygroscopic alkaline NVCs), which generally led to increases in values and decreases in values. This analysis indicated that assumptions of aerosol mixing states can impact aerosol pH predictions substantially. Given the non-linear response of many marine boundary layer atmospheric processes to aerosol pH, further assessment of the possible effects of aerosol mixing states on aerosol pH should be carried out for other marine atmospheres that are chemically different from the eastern North Atlantic conditions evaluated in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/atmos12081040/s1. Figure S1: Box plots depicting the median (a) , and (b) values calculated by ISORROPIA-II for the different concentrations of gas-phase NH3 added into the system. Figure S2: Box plots depicting the median (a) , and (b) values calculated by ISORROPIA-II for the different concentrations of gas-phase HNO3 added into the system. Figure S3: Box plots of vs. (a) SO42−, and (b) RH. Figure S4: Box plots depicting the median (a) pH, (b) , and (c) values calculated by ISORROPIA-II where crustals were treated explicitly vs. as mole-equivalent Na+. Figure S5: Box plots depicting the median (a) , and (b) values calculated by ISORROPIA-II for internally mixed aerosols vs. the aerosol mode containing only non-sea salt species. Table S1: Average mass concentrations of chemical species measured by the PILS-fraction collector system. Table S2: Sensitivity of and to SO42−, TA (NH3 + NH4+), Na+, Ca2+, K+, Mg2+, RH, and temperature.
Author Contributions
Data curation: A.P.S. and R.J.W.; formal analysis and investigation: J.Y. and T.N.; writing—original draft: T.N.; writing—review and editing: T.N., J.Y., J.W., R.J.W. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
T.N. and J.Y. were supported by startup funds from the City University of Hong Kong (project number 9610409). J.W. was supported by the Atmospheric System Research (ASR) program as part of the DOE Office of Biological and Environmental Research under awards No. DE-SC0020259. A.P.S. and R.J.W. were supported by the Department of Energy (DOE) under contract DOE 333890.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets used in this paper are publicly available on the ARM website (arm.gov/data, accessed on 12 August 2021).
Acknowledgments
The authors thank the G-1 flight and ground crews for supporting the ACE-ENA campaign.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eddingsaas, N.C.; VanderVelde, D.G.; Wennberg, P.O. Kinetics and Products of the Acid-Catalyzed Ring-Opening of Atmospherically Relevant Butyl Epoxy Alcohols. J. Phys. Chem. A 2010, 114, 8106–8113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.S.; Czoschke, N.M.; Lee, S.; Kamens, R.M. Heterogeneous Atmospheric Aerosol Production by Acid-Catalyzed Particle-Phase Reactions. Science 2002, 298, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Chan, A.W.H.; Eddingsaas, N.C.; Chan, M.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive Intermediates Revealed in Secondary Organic Aerosol Formation from Isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridlind, A.M.; Jacobson, M.Z. A Study of Gas-Aerosol Equilibrium and Aerosol pH in the Remote Marine Boundary Layer during the First Aerosol Characterization Experiment (ACE 1). J. Geophys. Res. Atmos. 2000, 105, 17325–17340. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Froyd, K.D.; Roberts, J.M.; Veres, P.R.; Hayes, P.L.; Jimenez, J.L.; Nenes, A.; Weber, R.J. Fine Particle pH and Gas-Particle Phase Partitioning of Inorganic Species in Pasadena, California, during the 2010 CalNex Campaign. Atmos. Chem. Phys. 2017, 17, 5703–5719. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Sullivan, A.P.; Campuzano-Jost, P.; Schroder, J.C.; Lopez-Hilfiker, F.D.; Dibb, J.E.; Jimenez, J.L.; Thornton, J.A.; Brown, S.S.; Nenes, A.; et al. Fine Particle pH and the Partitioning of Nitric Acid during Winter in the Northeastern United States. J. Geophys. Res. Atmos. 2016, 121, 10355–10376. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Longo, A.F.; Feng, Y.; Lai, B.; Landing, W.M.; Shelley, R.U.; Nenes, A.; Mihalopoulos, N.; Violaki, K.; Ingall, E.D. Influence of Atmospheric Processes on the Solubility and Composition of Iron in Saharan Dust. Environ. Sci. Technol. 2016, 50, 6912–6920. [Google Scholar] [CrossRef]
- Meskhidze, N.; Chameides, W.L.; Nenes, A.; Chen, G. Iron Mobilization in Mineral Dust: Can Anthropogenic SO2 Emissions Affect Ocean Productivity? Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef] [Green Version]
- Katoshevski, D.; Nenes, A.; Seinfeld, J.H. A Study of Processes that Govern the Maintenance of Aerosols in the Marine Boundary Layer. J. Aerosol Sci. 1999, 30, 503–532. [Google Scholar] [CrossRef]
- Keene, W.C.; Sander, R.; Pszenny, A.A.P.; Vogt, R.; Crutzen, P.J.; Galloway, J.N. Aerosol pH in the Marine Boundary Layer: A Review and Model Evaluation. J. Aerosol Sci. 1998, 29, 339–356. [Google Scholar] [CrossRef]
- Buck, R.P.; Rondinini, S.; Covington, A.K.; Baucke, F.G.K.; Brett, C.M.A.; Camoes, M.F.; Milton, M.J.T.; Mussini, T.; Naumann, R.; Pratt, K.W.; et al. Measurement of pH. Definition, Standards, and Procedures. Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar] [CrossRef] [Green Version]
- Heintzenberg, J. Fine Particles in the Global Troposphere A Review. Tellus Ser. B Chem. Phys. Meteorol. 1989, 41, 149–160. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Nenes, A.; Alexander, B.; Ault, A.P.; Barth, M.C.; Clegg, S.L.; Collett, J.L.; Fahey, K.M.; Hennigan, C.J.; Herrmann, H.; et al. The Acidity of Atmospheric Particles and Clouds. Atmos. Chem. Phys. 2020, 20, 4809–4888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondy, A.L.; Wang, B.; Laskin, A.; Craig, R.L.; Nhliziyo, M.V.; Bertman, S.B.; Pratt, K.A.; Shepson, P.B.; Ault, A.P. Inland Sea Spray Aerosol Transport and Incomplete Chloride Depletion: Varying Degrees of Reactive Processing Observed during SOAS. Environ. Sci. Technol. 2017, 51, 9533–9542. [Google Scholar] [CrossRef]
- Laskin, A.; Iedema, M.J.; Cowin, J.P. Quantitative Time-Resolved Monitoring of Nitrate Formation in Sea Salt Particles Using a CCSEM/EDX Single Particle Analysis. Environ. Sci. Technol. 2002, 36, 4948–4955. [Google Scholar] [CrossRef]
- Laskin, A.; Moffet, R.C.; Gilles, M.K.; Fast, J.D.; Zaveri, R.A.; Wang, B.; Nigge, P.; Shutthanandan, J. Tropospheric Chemistry of Internally Mixed Sea Salt and Organic Particles: Surprising Reactivity of NaCl with Weak Organic Acids. J. Geophys. Res. Atmos. 2012, 117. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Yang, X.; Li, W.; Xue, L.; Wang, T.; Chen, J.; Wang, W. Mixed Chloride Aerosols and their Atmospheric Implications: A Review. Aerosol Air Qual. Res. 2017, 17, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, M.A., Jr.; Weber, R.J.; Nenes, A.; Hennigan, C.J. Effects of Water-Soluble Organic Carbon on Aerosol pH. Atmos. Chem. Phys. 2019, 19, 14607–14620. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.A.; Ott, E.-J.E.; Marak, K.E. Role of pH in Aerosol Processes and Measurement Challenges. J. Phys. Chem. A 2019, 123, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wexler, A.S.; Clegg, S.L. Atmospheric Aerosol Models for Systems Including the Ions H+, NH4+, Na+, SO42−, NO3−, Cl−, Br−, and H2O. J. Geophys. Res. Atmos. 2002, 107, ACH 14-11–ACH 14-14. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A Computationally Efficient Thermodynamic Equilibrium Model for K+-Ca2+-Mg2+-Nh4+-Na+-SO42−-NO3−-Cl−-H2O Aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.Z. Fundamentals of Atmospheric Modeling, 2nd ed.; Cambridge University Press: New York, NY, USA, 2005; p. 820. [Google Scholar]
- Xu, J.; Song, S.; Harrison, R.M.; Song, C.; Wei, L.; Zhang, Q.; Sun, Y.; Lei, L.; Zhang, C.; Yao, X.; et al. An Interlaboratory Comparison of Aerosol Inorganic Ion Measurements by Ion Chromatography: Implications for Aerosol pH Estimate. Atmos. Meas. Tech. 2020, 13, 6325–6341. [Google Scholar] [CrossRef]
- Guo, H.Y.; Nenes, A.; Weber, R.J. The Underappreciated Role of Nonvolatile Cations in Aerosol Ammonium-Sulfate Molar ratios. Atmos. Chem. Phys. 2018, 18, 17307–17323. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.G.; Gregoire, P.K.; Tevlin, A.G.; Wentworth, G.R.; Ellis, R.A.; Markovic, M.Z.; VandenBoer, T.C. Observational Constraints on Particle Acidity using Measurements and Modelling of Particles and Gases. Faraday Discuss. 2017, 200, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Su, H.; Wang, S.; Andreae, M.O.; Pöschl, U.; Cheng, Y. Multiphase Buffer Theory Explains Contrasts in Atmospheric Aerosol Acidity. Science 2020, 369, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Nault, B.A.; Campuzano-Jost, P.; Day, D.A.; Jo, D.S.; Schroder, J.C.; Allen, H.M.; Bahreini, R.; Bian, H.; Blake, D.R.; Chin, M.; et al. Chemical Transport Models Often Underestimate Inorganic Aerosol Acidity in Remote Regions of the Atmosphere. Commun. Earth Environ. 2021, 2, 93. [Google Scholar] [CrossRef]
- Bian, H.; Chin, M.; Hauglustaine, D.A.; Schulz, M.; Myhre, G.; Bauer, S.E.; Lund, M.T.; Karydis, V.A.; Kucsera, T.L.; Pan, X.; et al. Investigation of Global Particulate Nitrate from the AeroCom Phase III Experiment. Atmos. Chem. Phys. 2017, 17, 12911–12940. [Google Scholar] [CrossRef] [Green Version]
- Huebert, B.J.; Zhuang, L.; Howell, S.; Noone, K.; Noone, B. Sulfate, Nitrate, Methane Sulfonate, Chloride, Ammonium, and Sodium Measurements from Ship, Island, and Aircraft During the Atlantic Stratocumulus Transition Experiment/Marine Aerosol Gas Exchange. J. Geophys. Res. Atmos. 1996, 101, 4413–4423. [Google Scholar] [CrossRef]
- Paulot, F.; Jacob, D.J.; Johnson, M.T.; Bell, T.G.; Baker, A.R.; Keene, W.C.; Lima, I.D.; Doney, S.C.; Stock, C.A. Global Oceanic Emission of Ammonia: Constraints from Seawater and Atmospheric Observations. Glob. Biogeochem. Cycles 2015, 29, 1165–1178. [Google Scholar] [CrossRef] [Green Version]
- Paulot, F.; Stock, C.; John, J.G.; Zadeh, N.; Horowitz, L.W. Ocean Ammonia Outgassing: Modulation by CO2 and Anthropogenic Nitrogen Deposition. J. Adv. Modeling Earth Syst. 2020, 12, e2019MS002026. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Lowe, D.; Derwent, R.G.; Foulds, A.; Chhantyal-Pun, R.; McFiggans, G.; Orr-Ewing, A.J.; Percival, C.J.; Shallcross, D.E. Global and Regional Model Simulations of Atmospheric Ammonia. Atmos. Res. 2020, 234, 104702. [Google Scholar] [CrossRef]
- Guo, H.; Xu, L.; Bougiatioti, A.; Cerully, K.M.; Capps, S.L.; Hite, J.R., Jr.; Carlton, A.G.; Lee, S.H.; Bergin, M.H.; Ng, N.L.; et al. Fine-Particle Water and pH in the Southeastern United States. Atmos. Chem. Phys. 2015, 15, 5211–5228. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Lau, A.K.H.; Yu, J.Z. A Study of Acidity on PM2.5 in Hong Kong Using Online Ionic Chemical Composition Measurements. Atmos. Environ. 2011, 45, 7081–7088. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, L.L.; Li, Y.Q.; Wang, C.; Wang, W.S.; Yin, S.S.; Zhang, R.Q. Effect of Ammonia on Fine-Particle pH in Agricultural Regions of China: Comparison between Urban and Rural Sites. Atmos. Chem. Phys. 2020, 20, 2719–2734. [Google Scholar] [CrossRef] [Green Version]
- Kakavas, S.; Patoulias, D.; Zakoura, M.; Nenes, A.; Pandis, S.N. Size-Resolved Aerosol pH Over Europe During Summer. Atmos. Chem. Phys. 2021, 21, 799–811. [Google Scholar] [CrossRef]
- Vieira-Filho, M.; Pedrotti, J.J.; Fornaro, A. Water-Soluble Ions Species of Size-Resolved Aerosols: Implications for the Atmospheric Acidity in Sao Paulo Megacity, Brazil. Atmos. Res. 2016, 181, 281–287. [Google Scholar] [CrossRef]
- Pszenny, A.A.P.; Moldanov, J.; Keene, W.C.; Sander, R.; Maben, J.R.; Martinez, M.; Crutzen, P.J.; Perner, D.; Prinn, R.G. Halogen Cycling and Aerosol pH in the Hawaiian Marine Boundary Layer. Atmos. Chem. Phys. 2004, 4, 147–168. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ding, J.; Xu, J.; Wen, J.; Han, J.; Wang, K.; Shi, G.; Feng, Y.; Ivey, C.E.; Wang, Y.; et al. Aerosols in an Arid Environment: The Role of Aerosol Water Content, Particulate Acidity, Precursors, and Relative Humidity on Secondary Inorganic Aerosols. Sci. Total Environ. 2019, 646, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squizzato, S.; Masiol, M.; Brunelli, A.; Pistollato, S.; Tarabotti, E.; Rampazzo, G.; Pavoni, B. Factors Determining the Formation of Secondary Inorganic Aerosol: A Case Study in the Po Valley (Italy). Atmos. Chem. Phys. 2013, 13, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Keene, W.C.; Savoie, D.L. The pH of Deliquesced Sea-Salt Aerosol in Polluted Marine Air. Geophys. Res. Lett. 1998, 25, 2181–2184. [Google Scholar] [CrossRef]
- Chameides, W.L.; Stelson, A.W. Aqueous-Phase Chemical Processes in Deliquescent Sea-Salt Aerosols: A Mechanism that Couples the Atmospheric Cycles of S and Sea Salt. J. Geophys. Res. Atmos. 1992, 97, 20565–20580. [Google Scholar] [CrossRef]
- Erickson, D.J.; Seuzaret, C.; Keene, W.C.; Gong, S.L. A General Circulation Model Based Calculation of HCl and ClNO2 Production from Sea Salt Dechlorination: Reactive Chlorine Emissions Inventory. J. Geophys. Res. Atmos. 1999, 104, 8347–8372. [Google Scholar] [CrossRef]
- Wang, J.; Wood, R.; Jensen, M.P.; Chiu, J.C.; Liu, Y.; Lamer, K.; Desai, N.; Giangrande, S.E.; Knopf, D.A.; Kollias, P.; et al. Aerosol and Cloud Experiments in the Eastern North Atlantic (ACE-ENA). Bull. Am. Meteorol. Soc. 2021, 1–51. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, G.; Jensen, M.; Knopf, D.; Laskin, A.; Matthews, A.; Mechem, D.; Mei, F.; Moffet, R.; Sedlacek, A.; et al. Vertical Profiles of Trace Gas and Aerosol Properties over the Eastern North Atlantic: Variations with Season and Synoptic Condition. Atmos. Chem. Phys. Discuss. 2021, 2021, 1–39. [Google Scholar] [CrossRef]
- Zawadowicz, M.A.; Suski, K.; Liu, J.; Pekour, M.; Fast, J.; Mei, F.; Sedlacek, A.J.; Springston, S.; Wang, Y.; Zaveri, R.A.; et al. Aircraft Measurements of Aerosol and Trace Gas Chemistry in the Eastern North Atlantic. Atmos. Chem. Phys. 2021, 21, 7983–8002. [Google Scholar] [CrossRef]
- Zheng, G.J.; Wang, Y.; Aiken, A.C.; Gallo, F.; Jensen, M.P.; Kollias, P.; Kuang, C.G.; Luke, E.; Springston, S.; Uin, J.; et al. Marine Boundary Layer Aerosol in the Eastern North Atlantic: Seasonal Variations and Key Controlling Processes. Atmos. Chem. Phys. 2018, 18, 17615–17635. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.; Wyant, M.; Bretherton, C.S.; Remillard, J.; Kollias, P.; Fletcher, J.; Stemmler, J.; de Szoeke, S.; Yuter, S.; Miller, M.; et al. Clouds, Aerosols, and Precipitation in the Marine Boundary Layer: An Arm Mobile Facility Deployment. Bull. Am. Meteorol. Soc. 2015, 96, 419–439. [Google Scholar] [CrossRef] [Green Version]
- Parrish, D.D.; Trainer, M.; Holloway, J.S.; Yee, J.E.; Warshawsky, M.S.; Fehsenfeld, F.C.; Forbes, G.L.; Moody, J.L. Relationships Between Ozone and Carbon Monoxide at Surface Sites in the North Atlantic Region. J. Geophys. Res. Atmos. 1998, 103, 13357–13376. [Google Scholar] [CrossRef]
- Raes, F.; Bates, T.; McGovern, F.; Van Liedekerke, M. The 2nd Aerosol Characterization Experiment (ACE-2): General Overview and Main Results. Tellus Ser. B Chem. Phys. Meteorol. 2000, 52, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Orsini, D.A.; Ma, Y.L.; Sullivan, A.; Sierau, B.; Baumann, K.; Weber, R.J. Refinements to the Particle-into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition. Atmos. Environ. 2003, 37, 1243–1259. [Google Scholar] [CrossRef]
- Sorooshian, A.; Brechtel, F.J.; Ma, Y.; Weber, R.J.; Corless, A.; Flagan, R.C.; Seinfeld, J.H. Modeling and Characterization of a Particle-into-Liquid Sampler (PILS). Aerosol Sci. Technol. 2006, 40, 396–409. [Google Scholar] [CrossRef] [Green Version]
- Marple, V.A.; Rubow, K.L.; Behm, S.M. A Microorifice Uniform Deposit Impactor (MOUDI): Description, Calibration, and Use. Aerosol Sci. Technol. 1991, 14, 434–446. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Izumi, J.; Sullivan, A.P.; Weber, R.J.; Nenes, A. A Critical Evaluation of Proxy Methods Used to Estimate the Acidity of Atmospheric Particles. Atmos. Chem. Phys. 2015, 15, 2775–2790. [Google Scholar] [CrossRef] [Green Version]
- Fountoukis, C.; Nenes, A.; Sullivan, A.; Weber, R.; Van Reken, T.; Fischer, M.; Matias, E.; Moya, M.; Farmer, D.; Cohen, R.C. Thermodynamic Characterization of Mexico City Aerosol during MILAGRO 2006. Atmos. Chem. Phys. 2009, 9, 2141–2156. [Google Scholar] [CrossRef] [Green Version]
- Bertram, A.K.; Martin, S.T.; Hanna, S.J.; Smith, M.L.; Bodsworth, A.; Chen, Q.; Kuwata, M.; Liu, A.; You, Y.; Zorn, S.R. Predicting the Relative Humidities of Liquid-Liquid Phase Separation, Efflorescence, and Deliquescence of Mixed Particles of Ammonium Sulfate, Organic Material, and Water Using the Organic-to-Sulfate Mass Ratio of the Particle and the Oxygen-to-Carbon Elemental Ratio of the Organic Component. Atmos. Chem. Phys. 2011, 11, 10995–11006. [Google Scholar] [CrossRef] [Green Version]
- Malm, W.C.; Day, D.E. Estimates of Aerosol Species Scattering Characteristics as a Function of Relative Humidity. Atmos. Environ. 2001, 35, 2845–2860. [Google Scholar] [CrossRef]
- Weber, R.J.; Guo, H.Y.; Russell, A.G.; Nenes, A. High Aerosol Acidity Despite Declining Atmospheric Sulfate Concentrations over the Past 15 Years. Nat. Geosci. 2016, 9, 282–285. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, P.S.; Su, J.; Dong, Q.; Du, X.; Zhang, Y.F. Aerosol pH and Its Driving Factors in Beijing. Atmos. Chem. Phys. 2019, 19, 7939–7954. [Google Scholar] [CrossRef] [Green Version]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Whitby, K.T. The Physical Characteristics of Sulfur Aerosols. Atmos. Environ. 1978, 12, 135–159. [Google Scholar] [CrossRef]
- Cruz, C.N.; Dassios, K.G.; Pandis, S.N. The Effect of Dioctyl Phthalate Films on the Ammonium Nitrate Aerosol Evaporation Rate. Atmos. Environ. 2000, 34, 3897–3905. [Google Scholar] [CrossRef]
- Dassios, K.G.; Pandis, S.N. The Mass Accommodation Coefficient of Ammonium Nitrate Aerosol. Atmos. Environ. 1999, 33, 2993–3003. [Google Scholar] [CrossRef]
- Dickson, A.G.; Goyet, C. DOE: Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water; United States Department of Education: Washington, DC, USA, 1994. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).