Histone Modifications as Individual-Specific Epigenetic Regulators: Opportunities for Forensic Genetics and Postmortem Analysis
Abstract
1. Introduction
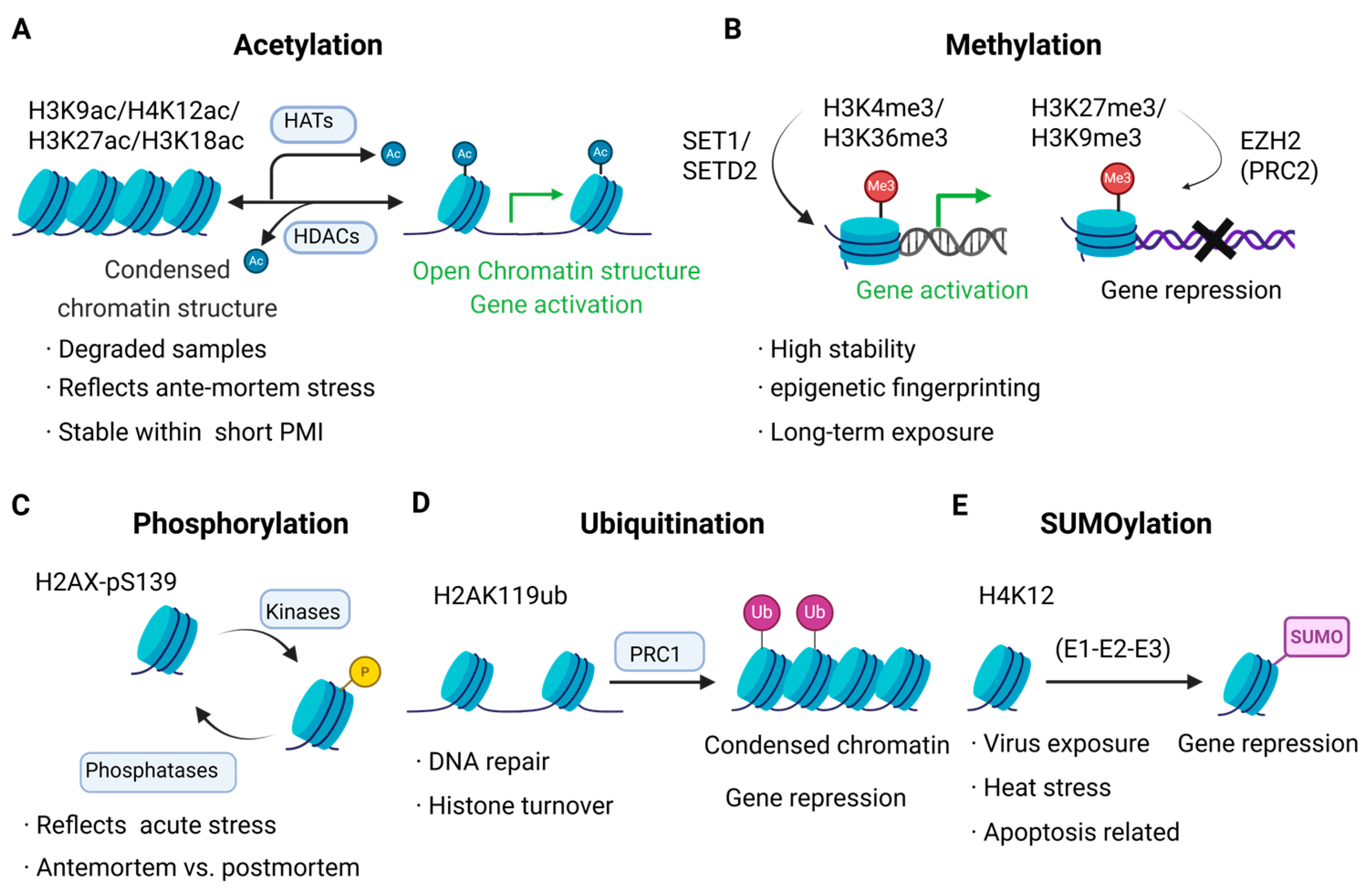
2. Types and Biological Functions of Histone Modifications
2.1. Acetylation
2.2. Methylation
2.3. Phosphorylation
2.4. Ubiquitination and SUMOylation
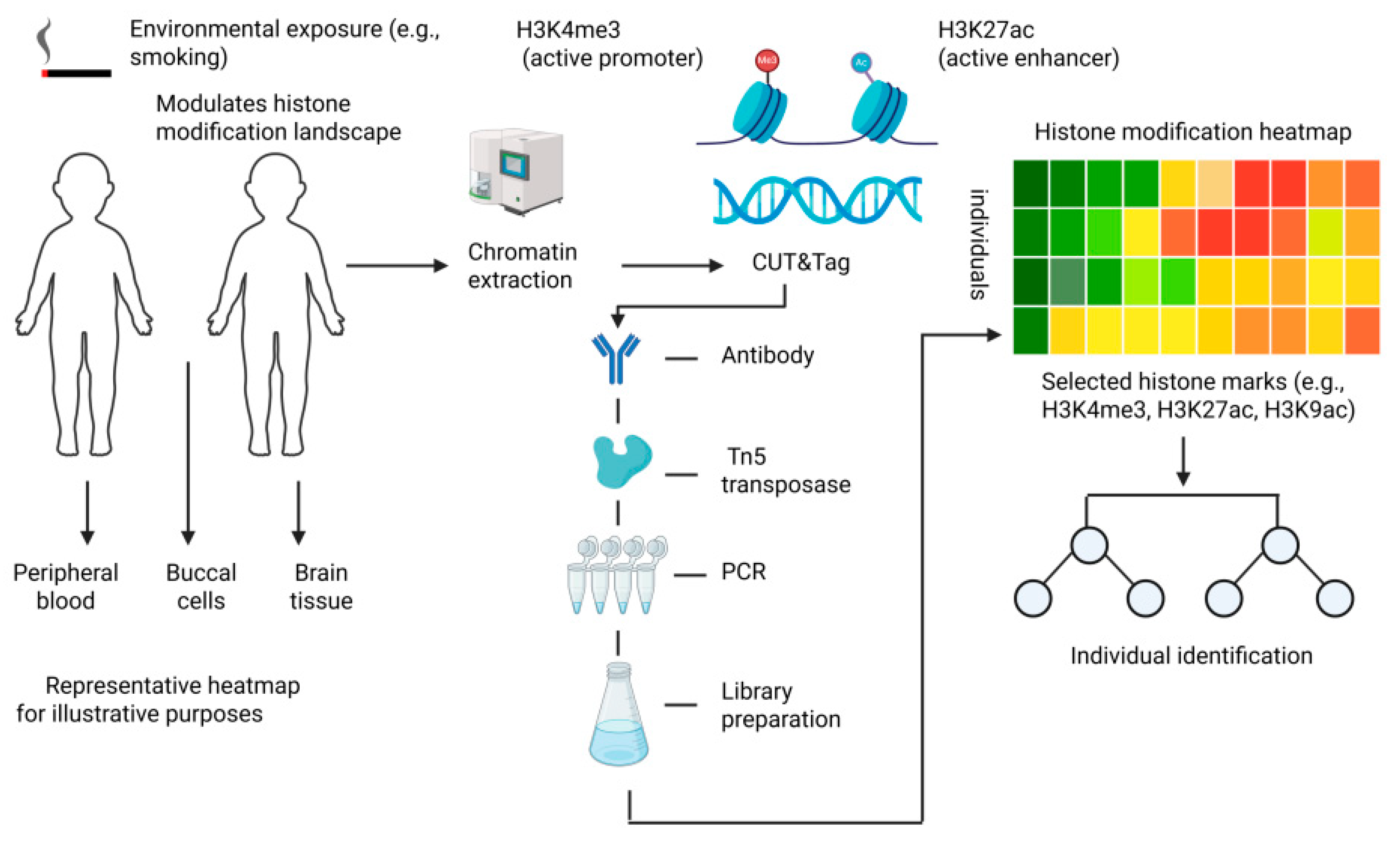
3. Techniques for Detecting Histone Modifications
3.1. Chromatin Immunoprecipitation Sequencing (ChIP-Seq) and Cleavage Under Targets and Tagmentation (CUT&Tag)
3.2. Mass Spectrometry (MS)
3.3. Emerging Single-Molecule and Multi-Omics Platforms
4. Challenges in Forensic Analysis of Degraded Samples and the Role of Histone Modifications
4.1. Characteristics of Degraded Biological Evidence and Forensic Challenges
4.2. Structural Stability of Histones and Nucleosomes
4.3. Forensic Applications of Histone Modifications in Degraded Samples
4.3.1. Epigenetic Fingerprinting for Individual Identification
4.3.2. Molecular Estimation of Sample Degradation
4.3.3. Expanded Analysis of Challenging Substrates
4.3.4. Insights into Cause of Death and Forensic Pathology
5. The Forensic Potential of Histone Modifications in Differentiating Monozygotic Twins
5.1. Challenges and Emerging Strategies in Monozygotic Twin Identification
- (1)
- Somatic Mutation Profiling via High-Throughput Sequencing
- (2)
- Epigenetic Divergence as a Forensic Tool
5.2. Evidence of Histone Modification Differences Between Monozygotic Twins
- (1)
- Tissue-Specific Divergence
- (2)
- Chromatin Accessibility and Active Histone Marks
- (3)
- Immune and Disease-Related Epigenetic Remodeling
6. Histone Modifications and Postmortem Interval (PMI) Estimation
6.1. Current Status and Challenges in PMI Estimation
6.2. Postmortem Dynamics of Histone Modifications
6.2.1. Stability and Changes in the Short-Term PMI
6.2.2. Degradation Patterns in the Mid-to-Long-Term PMI
6.2.3. Tissue-Specific Variation in Histone Decay
6.3. Application Prospects and Future Directions
7. Emerging Case-Relevant Evidence and Forensic Implementation Prospects
7.1. Histone Modifications in Postmortem Human Tissues
7.2. Degraded Bone and Skeletal Remains
7.3. PMI Modeling in Animal Studies
7.4. Standardization and Validation Pathways
8. Conclusions and Future Perspectives
8.1. Technical Limitations and Solutions
8.2. Data Interpretation and Judicial Admissibility
8.3. Ethical and Privacy Considerations
8.4. Future Directions
8.5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Rosenberg, N.A. Record-matching of STR profiles with fragmentary genomic SNP data. Eur. J. Hum. Genet. 2023, 31, 1283–1290. [Google Scholar] [CrossRef]
- Roman, M.G.; Gutierrez, R.; Houston, R. Massively parallel sequencing of Cannabis sativa chloroplast hotspots for forensic typing. J. Cannabis Res. 2022, 4, 13. [Google Scholar] [CrossRef]
- Andersen, M.M.; Kampmann, M.L.; Jepsen, A.H.; Morling, N.; Eriksen, P.S.; Børsting, C.; Andersen, J.D. Shotgun DNA sequencing for human identification: Dynamic SNP selection and likelihood ratio calculations accounting for errors. Forensic Sci. Int. Genet. 2025, 74, 103146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chen, X.; Liu, Z.; Liu, Q.; Song, A.; Bao, G.; Wei, G.; Zhang, S.; Lu, J.; Wu, Y. Identification of the perpetrator among identical twins using next-generation sequencing technology: A case report. Forensic Sci. Int. Genet. 2020, 44, 102167. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, R.; Zhang, S.; Bian, Y.; Lu, D.; Li, C. Differentiating between monozygotic twins through next-generation mitochondrial genome sequencing. Anal. Biochem. 2015, 490, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, S.; Liu, H.; Pan, M.; Dong, H. The Role of Protein Degradation in Estimation Postmortem Interval and Confirmation of Cause of Death in Forensic Pathology: A Literature Review. Int. J. Mol. Sci. 2024, 25, 1659. [Google Scholar] [CrossRef]
- Bauer, M.; Gramlich, I.; Polzin, S.; Patzelt, D. Quantification of mRNA degradation as possible indicator of postmortem interval—A pilot study. Leg. Med. 2003, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef] [PubMed]
- Zaib, S.; Rana, N.; Khan, I. Histone Modifications and their Role in Epigenetics of Cancer. Curr. Med. Chem. 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Secco, L.; Palumbi, S.; Padalino, P.; Grosso, E.; Perilli, M.; Casonato, M.; Cecchetto, G.; Viel, G. “Omics” and Postmortem Interval Estimation: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1034. [Google Scholar] [CrossRef]
- Sabeeha Hasnain, S.E. Forensic Epigenetic Analysis: The Path Ahead. Med. Princ. Pract. 2019, 28, 301–308. [Google Scholar] [CrossRef]
- Gerra, M.C.; Dallabona, C.; Cecchi, R. Epigenetic analyses in forensic medicine: Future and challenges. Int. J. Leg. Med. 2024, 138, 701–719. [Google Scholar] [CrossRef]
- Jarmasz, J.S.; Stirton, H.; Davie, J.R.; Del Bigio, M.R. DNA methylation and histone post-translational modification stability in post-mortem brain tissue. Clin. Epigenet. 2019, 11, 5. [Google Scholar] [CrossRef]
- Vcelkova, T.; Reiter, W.; Zylka, M.; Hollenstein, D.M.; Schuckert, S.; Hartl, M.; Seiser, C. GSE1 links the HDAC1/CoREST co-repressor complex to DNA damage. Nucleic Acids Res. 2023, 51, 11748–11769. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.; Wyrick, J.J.; Parra, M.A. DNA Repair in Nucleosomes: Insights from Histone Modifications and Mutants. Int. J. Mol. Sci. 2024, 25, 4393. [Google Scholar] [CrossRef] [PubMed]
- Procopio, N.; Bonicelli, A. From flesh to bones: Multi-omics approaches in forensic science. Proteomics 2024, 24, 2200335. [Google Scholar] [CrossRef] [PubMed]
- Vidaki, A.; Kayser, M. Recent progress, methods and perspectives in forensic epigenetics. Forensic Sci. Int. Genet. 2018, 37, 180–195. [Google Scholar] [CrossRef]
- Huang, H.S.; Matevossian, A.; Jiang, Y.; Akbarian, S. Chromatin immunoprecipitation in postmortem brain. J. Neurosci. Methods 2006, 156, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, J.; Langa, P.; Deptula, M.; Zieliński, J.; Sachadyn, P.; Wardowska, A.; Pikuła, M. Transcriptional activity of epigenetic remodeling genes declines in keratinocytes after in vitro expansion. Adv. Med. Sci. 2019, 64, 274–279. [Google Scholar] [CrossRef]
- Rintisch, C.; Heinig, M.; Bauerfeind, A.; Schafer, S.; Mieth, C.; Patone, G.; Hummel, O.; Chen, W.; Cook, S.; Cuppen, E.; et al. Natural variation of histone modification and its impact on gene expression in the rat genome. Genome Res. 2014, 24, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Artus, C.; Boujrad, H.; Bouharrour, A.; Brunelle, M.N.; Hoos, S.; Yuste, V.J.; Lenormand, P.; Rousselle, J.C.; Namane, A.; England, P.; et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010, 29, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Gorkin, D.U.; Barozzi, I.; Zhao, Y.; Zhang, Y.; Huang, H.; Lee, A.Y.; Li, B.; Chiou, J.; Wildberg, A.; Ding, B.; et al. An atlas of dynamic chromatin landscapes in mouse fetal development. Nature 2020, 583, 744–751. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, S.; Sun, Y.; Zheng, S.; Zong, L.; Li, P. Cut&tag: A powerful epigenetic tool for chromatin profiling. Epigenetics 2024, 19, 2293411. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Dai, Y.; Yang, L.; Zhang, S. Genome-wide identification and expression analysis of histone deacetylase and histone acetyltransferase genes in response to drought in poplars. BMC Genom. 2024, 25, 657. [Google Scholar] [CrossRef]
- Ma, M.; Fei, X.; Jiang, D.; Chen, H.; Xie, X.; Wang, Z.; Huang, Q. Research Progress on the Mechanism of Histone Deacetylases in Ferroptosis of Glioma. Oncol. Rev. 2024, 18, 1432131. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Mousavi, N.; Yang, X.J. Analysis of Lysine Acetylation and Acetylation-like Acylation In Vitro and In Vivo. Curr. Protoc. 2023, 3, e738. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Sun, X.; Hu, W.; Miao, Q.R. The Role of Histone Protein Acetylation in Regulating Endothelial Function. Front. Cell Dev. Biol. 2021, 9, 672447. [Google Scholar] [CrossRef]
- Oishi, T.; Hatazawa, S.; Kujirai, T.; Kato, J.; Kobayashi, Y.; Ogasawara, M.; Akatsu, M.; Ehara, H.; Sekine, S.I.; Hayashi, G.; et al. Contributions of histone tail clipping and acetylation in nucleosome transcription by RNA polymerase II. Nucleic Acids Res. 2023, 51, 10364–10374. [Google Scholar] [CrossRef] [PubMed]
- English, D.M.; Lee, S.N.; Sabat, K.A.; Baker, I.M.; Pham, T.K.; Collins, M.O.; Cowley, S.M. Rapid degradation of histone deacetylase 1 (HDAC1) reveals essential roles in both gene repression and active transcription. Nucleic Acids Res. 2025, 53, gkae1223. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Lou, A.; Lou, A.; Wang, Z.; Zhang, D.; Shen, Q.W. Histone acetyltransferase inhibitors antagonize AMP-activated protein kinase in postmortem glycolysis. Asian-Australas. J. Anim. Sci. 2017, 30, 857–864. [Google Scholar] [CrossRef]
- Koshi-Mano, K.; Mano, T.; Morishima, M.; Murayama, S.; Tamaoka, A.; Tsuji, S.; Toda, T.; Iwata, A. Neuron-specific analysis of histone modifications with post-mortem brains. Sci. Rep. 2020, 10, 3767. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Hsu, Y.J.; Fujiwara, Y.; Kim, J.; Mao, X.; Yuan, G.-C.; Orkin, S.H. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 2008, 32, 491–502. [Google Scholar] [CrossRef]
- Mauser, R.; Kungulovski, G.; Keup, C.; Reinhardt, R.; Jeltsch, A. Application of dual reading domains as novel reagents in chromatin biology reveals a new H3K9me3 and H3K36me2/3 bivalent chromatin state. Epigenet. Chromatin 2017, 10, 45. [Google Scholar] [CrossRef]
- Shan, Y.; Liang, Z.; Xing, Q.; Zhang, T.; Wang, B.; Tian, S.; Huang, W.; Zhang, Y.; Yao, J.; Zhu, Y.; et al. PRC2 specifies ectoderm lineages and maintains pluripotency in primed but not naive ESCs. Nat. Commun. 2017, 8, 672. [Google Scholar] [CrossRef]
- Zhong, J.; Ye, Z.; Clark, C.R.; Lenz, S.W.; Nguyen, J.H.; Yan, H.; Robertson, K.D.; Farrugia, G.; Zhang, Z.; Ordog, T.; et al. Enhanced and controlled chromatin extraction from FFPE tissues and the application to ChIP-seq. BMC Genom. 2019, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Basova, L.; Lindsey, A.; McGovern, A.M.; Ellis, R.J.; Marcondes, M.C.G. Detection of H3K4me3 Identifies NeuroHIV Signatures, Genomic Effects of Methamphetamine and Addiction Pathways in Postmortem HIV+ Brain Specimens that Are Not Amenable to Transcriptome Analysis. Viruses 2021, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Huang, Y.; Xu, Q.; Zhou, L.-J.; Shang, Z.-F.; Huang, B.; Wang, Y.; Liu, X.-D.; Wu, D.-C.; Zhou, P.-K. DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression. BMC Mol. Biol. 2010, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Skladanowski, A.; Bozko, P.; Basu, A. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Simon, M.; Seluanov, A.; Gorbunova, V. DNA damage and repair in age-related inflammation. Nat. Rev. Immunol. 2023, 23, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA damage response revisited: The p53 family and its regulators provide endless cancer therapy opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef]
- Parisis, N.; Dans, P.D.; Jbara, M.; Singh, B.; Schausi-Tiffoche, D.; Molina-Serrano, D.; Brun-Heath, I.; Hendrychová, D.; Maity, S.K.; Buitrago, D.; et al. Histone H3 serine-57 is a CHK1 substrate whose phosphorylation affects DNA repair. Nat. Commun. 2023, 14, 5104. [Google Scholar] [CrossRef]
- Dibitetto, D.; Liptay, M.; Vivalda, F.; Dogan, H.; Gogola, E.; Fernández, M.G.; Duarte, A.; Schmid, J.A.; Decollogny, M.; Francica, P.; et al. H2AX promotes replication fork degradation and chemosensitivity in BRCA-deficient tumours. Nat. Commun. 2024, 15, 4430. [Google Scholar] [CrossRef]
- Tanaka, T.; Halicka, D.; Traganos, F.; Darzynkiewicz, Z. Cytometric analysis of DNA damage: Phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods Mol. Biol. 2009, 523, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.S.; Stope, M.B.; Bekeschus, S.; Lorenzini, A. H2A.X Phosphorylation in Oxidative Stress and Risk Assessment in Plasma Medicine. Oxid. Med. Cell. Longev. 2021, 2021, 2060986. [Google Scholar] [CrossRef] [PubMed]
- Revet, I.; Feeney, L.; Bruguera, S.; Wilson, W.; Dong, T.K.; Oh, D.H.; Dankort, D.; Cleaver, J.E. Functional relevance of the histone gammaH2Ax in the response to DNA damaging agents. Proc. Natl. Acad. Sci. USA 2011, 108, 8663–8667. [Google Scholar] [CrossRef]
- Takano, S.; Shibamoto, Y.; Wang, Z.; Kondo, T.; Hashimoto, S.; Kawai, T.; Hiwatashi, A. Optimal timing of a gammaH2AX analysis to predict cellular lethal damage in cultured tumor cell lines after exposure to diagnostic and therapeutic radiation doses. J. Radiat. Res. 2023, 64, 317–327. [Google Scholar] [CrossRef]
- Ghate, N.B.; Nadkarni, K.S.; Barik, G.K.; Tat, S.S.; Sahay, O.; Santra, M.K. Histone ubiquitination: Role in genome integrity and chromatin organization. Biochim. Biophys. Acta Gene Regul. Mech. 2024, 1867, 195044. [Google Scholar] [CrossRef]
- Sekiguchi, M.; Matsushita, N. DNA Damage Response Regulation by Histone Ubiquitination. Int. J. Mol. Sci. 2022, 23, 8187. [Google Scholar] [CrossRef]
- Ryu, H.Y.; Hochstrasser, M. Histone sumoylation and chromatin dynamics. Nucleic Acids Res. 2021, 49, 6043–6052. [Google Scholar] [CrossRef]
- Schmid, J.A.; Berti, M.; Walser, F.; Raso, M.C.; Schmid, F.; Krietsch, J.; Stoy, H.; Zwicky, K.; Ursich, S.; Freire, R.; et al. Histone Ubiquitination by the DNA Damage Response Is Required for Efficient DNA Replication in Unperturbed S Phase. Mol. Cell 2018, 71, 897–910.e8. [Google Scholar] [CrossRef]
- Han, D.; Chen, C.; Xia, S.; Liu, J.; Shu, J.; Nguyen, V.; Lai, J.; Cui, Y.; Yang, C. Chromatin-associated SUMOylation controls the transcriptional switch between plant development and heat stress responses. Plant Commun. 2021, 2, 100091. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, F.; Sebastian, R.M.; Moore, C.L.; Mulder, M.P.; Ovaa, H.; Shoulders, M.D.; Vertegaal, A.C. SUMOylation and the HSF1-Regulated Chaperone Network Converge to Promote Proteostasis in Response to Heat Shock. Cell Rep. 2019, 26, 236–249.e4. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Finley, D.; Vogel, C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat. Struct. Mol. Biol. 2015, 22, 116–123. [Google Scholar] [CrossRef]
- Ouararhni, K.; Mietton, F.; Sabir, J.; Ibrahim, A.; Molla, A.; Albheyri, R.S.; Zari, A.T.; Bahieldin, A.; Menoni, H.; Bronner, C.; et al. Identification of a novel DNA oxidative damage repair pathway, requiring the ubiquitination of the histone variant macroH2A1.1. BMC Biol. 2024, 22, 188. [Google Scholar] [CrossRef]
- Zhang, S.; Ishida, Y.; Ishigami, A.; Nosaka, M.; Kuninaka, Y.; Hata, S.; Yamamoto, H.; Hashizume, Y.; Matsuki, J.; Yasuda, H.; et al. Forensic Application of Epidermal Ubiquitin Expression to Determination of Wound Vitality in Human Compressed Neck Skin. Front. Med. 2022, 9, 867365. [Google Scholar] [CrossRef]
- Madel, M.B.; Niederstatter, H.; Parson, W. TriXY-Homogeneous genetic sexing of highly degraded forensic samples including hair shafts. Forensic Sci. Int. Genet. 2016, 25, 166–174. [Google Scholar] [CrossRef]
- Hanssen, E.N.; Lyle, R.; Egeland, T.; Gill, P. Degradation in forensic trace DNA samples explored by massively parallel sequencing. Forensic Sci. Int. Genet. 2017, 27, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.N.; Yang, Y.D.; Li, S.J.; Zhang, X.-J.; Fang, X.-D.; Yan, J.-W.; Cong, B. Whole genome nucleosome sequencing identifies novel types of forensic markers in degraded DNA samples. Sci. Rep. 2016, 6, 26101. [Google Scholar] [CrossRef]
- Fernandez-Rojas, M.; Fuller, C.N.; Valadares, T.L.; Tose, L.V.; Willetts, M.; Park, M.A.; Bhanu, N.V.; Garcia, B.A.; Fernandez-Lima, F. Histone Modification Screening using Liquid Chromatography, Trapped Ion Mobility Spectrometry, and Time-Of-Flight Mass Spectrometry. J. Vis. Exp. 2024, 203, e65589. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Wu, Z.; Xu, X.; Jiang, Z.; Qi, S.; Liu, Z.; Wen, L.; Tang, F. scNanoSeq-CUT&Tag: A single-cell long-read CUT&Tag sequencing method forefficient chromatin modification profiling within individual cells. Nat. Methods 2024, 21, 2044–2057. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y. Profiling chromatin regulatory landscape: Insights into the development of ChIP-seq and ATAC-seq. Mol. Biomed. 2020, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wu, J.; Li, P.; Zhang, Y.; Peng, Y.; Liu, R.; Du, W.; Kang, Y.; Sun, J.; Wu, J.; et al. 2cChIP-seq and 2cMeDIP-seq: The Carrier-Assisted Methods for Epigenomic Profiling of Small Cell Numbers or Single Cells. Int. J. Mol. Sci. 2022, 23, 13984. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Okur, H.S.; Wu, S.J.; Codomo, C.A.; Pledger, E.S.; Bryson, T.D.; Henikoff, J.G.; Ahmad, K.; Henikoff, S. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun. 2019, 10, 1930. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G.; Kaya-Okur, H.S.; Ahmad, K. Efficient chromatin accessibility mapping in situ by nucleosome-tethered tagmentation. Elife 2020, 9, e63274. [Google Scholar] [CrossRef] [PubMed]
- Bartosovic, M.; Kabbe, M.; Castelo-Branco, G. Single-cell CUT&Tag profiles histone modifications and transcription factors in complex tissues. Nat. Biotechnol. 2021, 39, 825–835. [Google Scholar] [CrossRef]
- Janssens, D.H.; Greene, J.E.; Wu, S.J.; Codomo, C.A.; Minot, S.S.; Furlan, S.N.; Ahmad, K.; Henikoff, S. Scalable single-cell profiling of chromatin modifications with sciCUT&Tag. Nat. Protoc. 2024, 19, 83–112. [Google Scholar] [CrossRef]
- Noberini, R.; Robusti, G.; Bonaldi, T. Mass spectrometry-based characterization of histones in clinical samples: Applications, progress, and challenges. FEBS J. 2022, 289, 1191–1213. [Google Scholar] [CrossRef]
- Lahiri, S.; Sun, N.; Buck, A.; Imhof, A.; Walch, A. MALDI imaging mass spectrometry as a novel tool for detecting histone modifications in clinical tissue samples. Expert Rev. Proteom. 2016, 13, 275–284. [Google Scholar] [CrossRef]
- Sidoli, S.; Bhanu, N.V.; Karch, K.R.; Wang, X.; Garcia, B.A. Complete Workflow for Analysis of Histone Post-translational Modifications Using Bottom-up Mass Spectrometry: From Histone Extraction to Data Analysis. J. Vis. Exp. 2016, 111, 54112. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Liu, L.; Chen, Y. Simultaneous detection of site-specific histone methylations and acetylation assisted by single template oriented molecularly imprinted polymers. Analyst 2020, 145, 1376–1383. [Google Scholar] [CrossRef]
- Merkley, E.D.; Wunschel, D.S.; Wahl, K.L.; Jarman, K.H. Applications and challenges of forensic proteomics. Forensic Sci. Int. 2019, 297, 350–363. [Google Scholar] [CrossRef]
- Whiteaker, J.R.; Paulovich, A.G. Peptide immunoaffinity enrichment coupled with mass spectrometry for peptide and protein quantification. Clin. Lab. Med. 2011, 31, 385–396. [Google Scholar] [CrossRef]
- Li, J.; Van Vranken, J.G.; Pontano, V.L.; Vaites, L.P.; Schweppe, D.K.; Huttlin, E.L.; Etienne, C.; Nandhikonda, P.; Viner, R.; Robitaille, A.M.; et al. TMTpro reagents: A set of isobaric labeling mass tags enables simultaneous proteome-wide measurements across 16 samples. Nat. Methods 2020, 17, 399–404. [Google Scholar] [CrossRef]
- Yue, X.; Xie, Z.; Li, M.; Wang, K.; Li, X.; Zhang, X.; Yan, J.; Yin, Y. Simultaneous profiling of histone modifications and DNA methylation via nanopore sequencing. Nat. Commun. 2022, 13, 7939. [Google Scholar] [CrossRef] [PubMed]
- Bartosovic, M.; Castelo-Branco, G. Multimodal chromatin profiling using nanobody-based single-cell CUT&Tag. Nat. Biotechnol. 2023, 41, 794–805. [Google Scholar] [CrossRef]
- Naqvi, S.Z.; Ahmed, U.; Daga, S.S.; Daga, S.S.; Rawat, P.; Singhal, G.; Patil, B. Examining the Impact of Environmental Variables on DNA Extraction Efficiency in Forensic Blood Samples. Period. Mineral. 2024, 93, 440. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, D.; Zhang, L.; Wang, T.; Yan, J. Environmental microbiota from substrate may interfere with microbiome-based identification of forensically relevant body fluids: A pilot study. Forensic Sci. Int. Genet. 2025, 74, 103170. [Google Scholar] [CrossRef]
- Morozova, I.; Flegontov, P.; Mikheyev, A.S.; Bruskin, S.; Asgharian, H.; Ponomarenko, P.; Klyuchnikov, V.; ArunKumar, G.; Prokhortchouk, E.; Gankin, Y.; et al. Toward high-resolution population genomics using archaeological samples. DNA Res. 2016, 23, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Shiga, M.; Asari, M.; Takahashi, Y.; Isozaki, S.; Hoshina, C.; Mori, K.; Namba, R.; Okuda, K.; Shimizu, K. DNA methylation-based age estimation and quantification of the degradation levels of bisulfite-converted DNA. Leg. Med. 2024, 67, 102336. [Google Scholar] [CrossRef]
- Shen, C.H.; Allan, J. MNase Digestion Protection Patterns of the Linker DNA in Chromatosomes. Cells 2021, 10, 2239. [Google Scholar] [CrossRef]
- Cole, H.A.; Cui, F.; Ocampo, J.; Burke, T.L.; Nikitina, T.; Nagarajavel, V.; Kotomura, N.; Zhurkin, V.B.; Clark, D.J. Novel nucleosomal particles containing core histones and linker DNA but no histone H1. Nucleic Acids Res. 2016, 44, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wernig-Zorc, S.; Kugler, F.; Schmutterer, L.; Räß, P.; Hausmann, C.; Holzinger, S.; Längst, G.; Schwartz, U. nucMACC: An MNase-seq pipeline to identify structurally altered nucleosomes in the genome. Sci. Adv. 2024, 10, eadm9740. [Google Scholar] [CrossRef]
- Dulka, K.; Lajko, N.; Nacsa, K.; Gulya, K. Opposite and Differently Altered Postmortem Changes in H3 and H3K9me3 Patterns in the Rat Frontal Cortex and Hippocampus. Epigenomes 2024, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Jarmasz, J.S.; Stirton, H.; Basalah, D.; Davie, J.R.; Clarren, S.K.; Astley, S.J.; Del Bigio, M.R. Global DNA Methylation and Histone Posttranslational Modifications in Human and Nonhuman Primate Brain in Association with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2019, 43, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Robusti, G.; Vai, A.; Bonaldi, T.; Noberini, R. Investigating pathological epigenetic aberrations by epi-proteomics. Clin. Epigenet. 2022, 14, 145. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Furlan, S.N.; Mihalas, A.B.; Kaya-Okur, H.S.; Feroze, A.H.; Emerson, S.N.; Zheng, Y.; Carson, K.; Cimino, P.J.; Keene, C.D.; et al. Single-cell CUT&Tag analysis of chromatin modifications in differentiation and tumor progression. Nat. Biotechnol. 2021, 39, 819–824. [Google Scholar] [CrossRef]
- Huo, Z.; Zhang, S.; Su, G.; Cai, Y.; Chen, R.; Jiang, M.; Yang, D.; Zhang, S.; Xiong, Y.; Zhang, X. Immunohistochemical Profiling of Histone Modification Biomarkers Identifies Subtype-Specific Epigenetic Signatures and Potential Drug Targets in Breast Cancer. Int. J. Mol. Sci. 2025, 26, 770. [Google Scholar] [CrossRef]
- Anink-Groenen, L.C.; Maarleveld, T.R.; Verschure, P.J.; Bruggeman, F.J. Mechanistic stochastic model of histone modification pattern formation. Epigenet. Chromatin 2014, 7, 30. [Google Scholar] [CrossRef]
- Menon, G.; Howard, M. Investigating Histone Modification Dynamics by Mechanistic Computational Modeling. In Histone Methyltransferases: Methods and Protocols; Methods in Molecular Biology; Springer Nature: New York, NY, USA, 2022; Volume 2529, pp. 441–473. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef]
- Yi, S.J.; Lee, H.; Lee, J.; Lee, K.; Kim, J.; Kim, Y.; Park, J.-I.; Kim, K. Bone Remodeling: Histone Modifications as Fate Determinants of Bone Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3147. [Google Scholar] [CrossRef]
- Johnson, A.B.; Denko, N.; Barton, M.C. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 2008, 640, 174–179. [Google Scholar] [CrossRef]
- Svobodova, K.A.; Legartova, S.; Krejci, J.; Kovaříková, A.S. H3K9me3 and H4K20me3 represent the epigenetic landscape for 53BP1 binding to DNA lesions. Aging 2018, 10, 2585–2605. [Google Scholar] [CrossRef] [PubMed]
- Abini-Agbomson, S.; Gretarsson, K.; Shih, R.M.; Hsieh, L.; Lou, T.; De Ioannes, P.; Vasilyev, N.; Lee, R.; Wang, M.; Simon, M.D.; et al. Catalytic and non-catalytic mechanisms of histone H4 lysine 20 methyltransferase SUV420H1. Mol. Cell 2023, 83, 2872–2883. [Google Scholar] [CrossRef]
- Perez, M.; Gomez, M.; Castellar-Lopez, J.; Araos, P.; Mendoza-Torres, E.; Bolívar, S. Epigenetic modifications in cardiac fibrosis: Recent evidence of new pharmacological targets. Front. Mol. Biosci. 2025, 12, 1583446. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; He, Z.; Rauf, A.; Kalkhoran, S.B.; Heiestad, C.M.; Stensløkken, K.-O.; Parish, C.R.; Soehnlein, O.; Arjun, S.; Davidson, S.M.; et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2022, 118, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Z.; Shliaha, P.V.; Miele, M.; Hendrickson, R.C.; Jiang, X.; Helin, K. H3K4me3 regulates RNA polymerase II promoter-proximal pause-release. Nature 2023, 615, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, A.; Perez-Rodriguez, D.; Fernandez-Pereira, C.; Prieto-González, J.M.; Agís-Balboa, R.C. Genomic Factors and Therapeutic Approaches in HIV-Associated Neurocognitive Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 14364. [Google Scholar] [CrossRef]
- Day, C.A.; Grigore, F.; Hakkim, F.L.; Paul, S.; Langfald, A.; Weberg, M.; Fadness, S.; Schwab, P.; Sepaniac, L.; Stumpff, J.; et al. The histone H3.3 K27M mutation suppresses Ser31phosphorylation and mitotic fidelity, which can directly drive gliomagenesis. Curr. Biol. 2025, 35, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Lee, Y.; Masuda, T.; Ozono, K.; Iwatani, Y.; Watanabe, M.; Okada, Y.; Sakai, N. Functional landscape of genome-wide postzygotic somatic mutations between monozygotic twins. DNA Res. 2024, 31, dsae028. [Google Scholar] [CrossRef] [PubMed]
- Hwa, H.L.; Lin, C.Y.; Yu, Y.J.; Linacre, A.; Lee, J.C.-I. DNA identification of monozygotic twins. Forensic Sci. Int. Genet. 2024, 69, 102998. [Google Scholar] [CrossRef]
- Morimoto, Y.; Ono, S.; Imamura, A.; Okazaki, Y.; Kinoshita, A.; Mishima, H.; Nakane, H.; Ozawa, H.; Yoshiura, K.-I.; Kurotaki, N. Deep sequencing reveals variations in somatic cell mosaic mutations between monozygotic twins with discordant psychiatric disease. Hum. Genome Var. 2017, 4, 17032. [Google Scholar] [CrossRef]
- Nishioka, M.; Bundo, M.; Ueda, J.; Yoshikawa, A.; Nishimura, F.; Sasaki, T.; Kakiuchi, C.; Kasai, K.; Kato, T.; Iwamoto, K. Identification of somatic mutations in monozygotic twins discordant for psychiatric disorders. NPJ Schizophr. 2018, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Aourangzaib, M.; Chandra, M.; Maham, R.; Naz, A.; Malathi, H.; Qadeer, S.; Mateen, R.M.; Parveen, R. Solving the twin paradox-forensic strategies to identify the identical twins. Forensic Sci. Int. 2024, 363, 112205. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, S.; Son, H.; Kim, D.; Bin Kim, I.; Kim, M.-H.; Sim, N.S.; Kim, D.S.; Ha, Y.-J.; Lee, J.; et al. Analysis of low-level somatic mosaicism reveals stage and tissue-specific mutational features in human development. PLoS Genet. 2022, 18, e1010404. [Google Scholar] [CrossRef] [PubMed]
- Krawczak, M.; Budowle, B.; Weber-Lehmann, J.; Rolf, B.; Weir, B. Distinguishing genetically between the germlines of male monozygotic twins. PLoS Genet. 2018, 14, e1007756. [Google Scholar] [CrossRef]
- Rolf, B.; Krawczak, M. The germlines of male monozygotic (MZ) twins: Very similar, but not identical. Forensic Sci. Int. Genet. 2021, 50, 102408. [Google Scholar] [CrossRef]
- Hannon, E.; Knox, O.; Sugden, K.; Burrage, J.; Wong, C.C.Y.; Belsky, D.W.; Corcoran, D.L.; Arseneault, L.; Moffitt, T.E.; Caspi, A.; et al. Characterizing genetic and environmental influences on variable DNA methylation using monozygotic and dizygotic twins. PLoS Genet. 2018, 14, e1007544. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.L.; Walker, M.; Arat, S.; Ananda, G.; Petkova, P.; Powers, N.R.; Tian, H.; Spruce, C.; Ji, B.; Rausch, D.; et al. Tissue-Specific Trans Regulation of the Mouse Epigenome. Genetics 2019, 211, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.D.; Fischer, D.K.; Zhang, S.; Bond, A.M.; Czarnecki, K.S.; Woolf, M.T.; Song, H.; Heller, E.A. Cell-type specific profiling of histone post-translational modifications in the adult mouse striatum. Nat. Commun. 2022, 13, 7720. [Google Scholar] [CrossRef] [PubMed]
- Matlik, K.; Govek, E.E.; Hatten, M.E. Histone bivalency in CNS development. Genes Dev. 2025, 39, 428–444. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.-J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Signal, B.; Phipps, A.J.; Giles, K.A.; Huskins, S.N.; Mercer, T.R.; Robinson, M.D.; Woodhouse, A.; Taberlay, P.C. Ageing-Related Changes to H3K4me3, H3K27ac, and H3K27me3 in Purified Mouse Neurons. Cells 2024, 13, 1393. [Google Scholar] [CrossRef]
- Vecellio, M.; Paraboschi, E.M.; Ceribelli, A.; Isailovic, N.; Motta, F.; Cardamone, G.; Robusto, M.; Asselta, R.; Brescianini, S.; Sacrini, F.; et al. DNA Methylation Signature in Monozygotic Twins Discordant for Psoriatic Disease. Front. Cell Dev. Biol. 2021, 9, 778677. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, S.; Isozaki, M.; Yao, A.; Higashino, Y.; Yamauchi, T.; Kidoguchi, M.; Kawajiri, S.; Tsunetoshi, K.; Neish, H.; Imoto, H.; et al. Cross-tissue correlations of genome-wide DNA methylation in Japanese live human brain and blood, saliva, and buccal epithelial tissues. Transl. Psychiatry 2023, 13, 72. [Google Scholar] [CrossRef]
- Edgar, R.D.; Jones, M.J.; Meaney, M.J.; Turecki, G.; Kobor, M.S. BECon: A tool for interpreting DNA methylation findings from blood in the context of brain. Transl. Psychiatry 2017, 7, e1187. [Google Scholar] [CrossRef]
- Tu, J.; Li, W.; Hansbro, P.M.; Yan, Q.; Bai, X.; Donovan, C.; Kim, R.Y.; Galvao, I.; Das, A.; Yang, C.; et al. Smoking and tetramer tryptase accelerate intervertebral disc degeneration by inducing METTL14-mediated DIXDC1 m(6) modification. Mol. Ther. 2023, 31, 2524–2542. [Google Scholar] [CrossRef]
- Glass, K.; Thibault, D.; Guo, F.; Mitchel, J.A.; Pham, B.; Qiu, W.; Li, Y.; Jiang, Z.; Castaldi, P.J.; Silverman, E.K.; et al. Integrative epigenomic analysis in differentiated human primary bronchial epithelial cells exposed to cigarette smoke. Sci. Rep. 2018, 8, 12750. [Google Scholar] [CrossRef] [PubMed]
- Surace, A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.Y.; Lee, S.Y.; Kim, S.-Y.; Park, J.-L.; Lee, S.D. DNA methylome profiling of blood to identify individuals in a pair of monozygotic twins. Genes Genom. 2023, 45, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Nau, A.M.; Ditto, P.; Steadman, D.W.; Mockus, A. Identifying factors that help improve existing decomposition-based PMI estimation methods. J. Forensic Sci. 2025, 70, 1249–1260. [Google Scholar] [CrossRef]
- Stigter, H.; Krap, T.; Duijst, W.L.J.M. Estimation of the post-mortem interval; added value of mechanical excitability of human skeletal muscle. J. Forensic Leg. Med. 2024, 103, 102664. [Google Scholar] [CrossRef]
- Kurup, S.; Bharathi, M.; Narayan, G.; Suvvari, T.K. Estimation of Time Since Death From Potassium Levels in Vitreous Humor in Cases of Unnatural Death: A Facility-Based Cross-Sectional Study. Cureus 2023, 15, e39572. [Google Scholar] [CrossRef]
- Kaul, M.; Kumar, K.; Kaul, A.K.; Chanana, A.; Kumar, A. Digestive Status of Stomach contents—An indicator of Time Since Death. J. Dent. Med. Sci. 2017, 16, 26–35. [Google Scholar] [CrossRef]
- Baek, I.S.; Oh, H.S.; Kim, Y.R.; Kang, M.-G.; Jung, J.-B.; Park, S.-H. Medical-Legal Entomology in Action: Evaluation of Insect-Based Post-Mortem Interval Estimation in South Korean Death Investigations. Insects 2025, 16, 231. [Google Scholar] [CrossRef]
- Pittner, S.; Merold, V.; Anders, S.; Lohner, L.; Amendt, J.; Klinger, M.; Hausmann, R.; Kissling, S.; Monticelli, F.; Geissenberger, J.; et al. A standard protocol for the analysis of postmortem muscle protein degradation: Process optimization and considerations for the application in forensic PMI estimation. Int. J. Leg. Med. 2022, 136, 1913–1923. [Google Scholar] [CrossRef]
- Zissler, A.; Stoiber, W.; Steinbacher, P.; Geissenberger, J.; Monticelli, F.C.; Pittner, S. Postmortem Protein Degradation as a Tool to Estimate the PMI: A Systematic Review. Diagnostics 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pozhitkov, A.E.; Neme, R.; Domazet-Loso, T.; Leroux, B.G.; Soni, S.; Tautz, D.; Noble, P.A. Tracing the dynamics of gene transcripts after organismal death. Open Biol. 2017, 7, 160267. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrog, B. Histone modifications as regulators of life and death in Saccharomyces cerevisiae. Microb. Cell 2015, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Javaid, N.; Choi, S. Acetylation- and Methylation-Related Epigenetic Proteins in the Context of Their Targets. Genes 2017, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Rouaux, C.; Jokic, N.; Mbebi, C.; Boutillier, S.; Loeffler, J.; Boutillier, A. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 2003, 22, 6537–6549. [Google Scholar] [CrossRef] [PubMed]
- Krosel, M.; Gabathuler, M.; Moser, L.; Maciukiewicz, M.; Züllig, T.; Seifritz, T.; Tomšič, M.; Distler, O.; Ospelt, C.; Klein, K. The histone acetyl transferases CBP and p300 regulate stress response pathways in synovial fibroblasts at transcriptional and functional levels. Sci. Rep. 2023, 13, 17112. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Maheu, M.; Lopez, J.P.; Vaillancourt, K.; Cruceanu, C.; Gross, J.A.; Arnovitz, M.; Mechawar, N.; Turecki, G. Effects of postmortem interval on biomolecule integrity in the brain. J. Neuropathol. Exp. Neurol. 2015, 74, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Olney, K.C.; Gibson, K.A.; Cadiz, M.P.; Rahimzadeh, N.; Swarup, V.; Fryer, J.D. Postmortem Interval Leads to Loss of Disease-Specific Signatures in Brain Tissue. eNeuro 2025, 12, ENEURO.0505-24.2025. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Huerta, P.A.; Coliboro-Dannich, V.; Castro, A.F.; Barbaro, A. Evaluation of DNA in Human Teeth-Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review. Genes 2025, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, L.K.; Ransome, Y.; Ekstrom, T.J.; Karlsson, O. Evaluation of Post-Mortem Effects on Global Brain DNA Methylation and Hydroxymethylation. Basic Clin. Pharmacol. Toxicol. 2018, 122, 208–213. [Google Scholar] [CrossRef]
- Tomita, Y.; Nihira, M.; Ohno, Y.; Sato, S. Ultrastructural changes during in situ early postmortem autolysis in kidney, pancreas, liver, heart and skeletal muscle of rats. Leg. Med. 2004, 6, 25–31. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Munoz-Aguirre, M.; Reverter, F.; Sa Godinho, C.P.; Sousa, A.; Amadoz, A.; Sodaei, R.; Hidalgo, M.R.; Pervouchine, D.; Carbonell-Caballero, J.; et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat. Commun. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Montanari, E.; Giorgetti, R.; Busardo, F.P.; Giorgetti, A.; Tagliabracci, A.; Alessandrini, F. Suitability of miRNA assessment in postmortem interval estimation. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1774–1787. [Google Scholar] [CrossRef]
- Rashid, B.; Destrade, M.; Gilchrist, M.D. Influence of preservation temperature on the measured mechanical properties of brain tissue. J. Biomech. 2013, 46, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Garces-Parra, C.; Saldivia, P.; Hernandez, M.; Uribe, E.; Román, J.; Torrejón, M.; Gutiérrez, J.L.; Cabrera-Vives, G.; García-Robles, M.d.L.Á.; Aguilar, W.; et al. Enhancing late postmortem interval prediction: A pilot study integrating proteomics and machine learning to distinguish human bone remains over 15 years. Biol. Res. 2024, 57, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.J.; Liu, M.F.; Li, N.; Dang, L.-H.; An, G.-S.; Lu, X.-J.; Wang, L.-L.; Du, Q.-X.; Cao, J.; et al. Multi-omics integration strategy in the post-mortem interval of forensic science. Talanta 2024, 268 Pt 1, 125249. [Google Scholar] [CrossRef] [PubMed]
- Bonicelli, A.; Mickleburgh, H.L.; Chighine, A.; Locci, E.; Wescott, D.J.; Procopio, N. The ‘ForensOMICS’ approach for postmortem interval estimation from human bone by integrating metabolomics, lipidomics, and proteomics. Elife 2022, 11, e83658. [Google Scholar] [CrossRef]
- Jehanno, C.; Flouriot, G.; Le Goff, P.; Michel, D. A model of dynamic stability of H3K9me3 heterochromatin to explain the resistance to reprogramming of differentiated cells. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 184–195. [Google Scholar] [CrossRef]
- Duong, V.; Park, J.; Lim, H.; Lee, H. Proteomics in Forensic Analysis: Applications for Human Samples. Appl. Sci. 2021, 8, 3393. [Google Scholar] [CrossRef]
- Lahiri, S.; Sun, N.; Solis-Mezarino, V.; Fedisch, A.; Ninkovic, J.; Feuchtinger, A.; Götz, M.; Walch, A. In situ detection of histone variants and modifications in mouse brain using imaging mass spectrometry. Proteomics 2016, 16, 437–447. [Google Scholar] [CrossRef]
- Onofri, M.; Alessandrini, F.; Aneli, S.; Buscemi, L.; Chierto, E.; Fabbri, M.; Fattorini, P.; Garofano, P.; Gentile, F.; Presciuttini, S.; et al. A Ge.F.I. Collaborative Study: Evaluating Reproducibility and Accuracy of a DNA-Methylation-Based Age-Predictive Assay for Routine Implementation in Forensic Casework. Electrophoresis 2025, 46, 76–91. [Google Scholar] [CrossRef]
- Maas, S.; Vidaki, A.; Teumer, A.; Costeira, R.; Wilson, R.; van Dongen, J.; Beekman, M.; Völker, U.; Grabe, H.J.; Kunze, S.; et al. Validating biomarkers and models for epigenetic inference of alcohol consumption from blood. Clin. Epigenet. 2021, 13, 198. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G.; Ahmad, K.; Paranal, R.M.; Janssens, D.H.; Russell, Z.R.; Szulzewsky, F.; Kugel, S.; Holland, E.C. Epigenomic analysis of formalin-fixed paraffin-embedded samples by CUT&Tag. Nat. Commun. 2023, 14, 5930. [Google Scholar] [CrossRef]
- Kaneko, S.; Mitsuyama, T.; Shiraishi, K.; Ikawa, N.; Shozu, K.; Dozen, A.; Machino, H.; Asada, K.; Komatsu, M.; Kukita, A.; et al. Genome-Wide Chromatin Analysis of FFPE Tissues Using a Dual-Arm Robot with Clinical Potential. Cancers 2021, 13, 2126. [Google Scholar] [CrossRef]
- Amatori, S.; Persico, G.; Paolicelli, C.; Hillje, R.; Sahnane, N.; Corini, F.; Furlan, D.; Luzi, L.; Minucci, S.; Giorgio, M.; et al. Epigenomic profiling of archived FFPE tissues by enhanced PAT-ChIP (EPAT-ChIP) technology. Clin. Epigenet. 2018, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Amatori, S.; Ballarini, M.; Faversani, A.; Belloni, E.; Fusar, F.; Bosari, S.; Pelicci, P.G.; Minucci, S.; Fanelli, M. PAT-ChIP coupled with laser microdissection allows the study of chromatin in selected cell populations from paraffin-embedded patient samples. Epigenet. Chromatin 2014, 7, 18. [Google Scholar] [CrossRef]
- Thomas, S.P.; Haws, S.A.; Borth, L.E.; Denu, J.M. A practical guide for analysis of histone post-translational modifications by mass spectrometry: Best practices and pitfalls. Methods 2020, 184, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, S.; Raubenheimer, K.; Melton, P.E.; Huang, R.C. Machine learning and clinical epigenetics: A review of challenges for diagnosis and classification. Clin. Epigenet. 2020, 12, 51. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.; Wen, J. Predicting gene expression from histone modifications with self-attention based neural networks and transfer learning. Front. Genet. 2022, 13, 1081842. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Teo, Y.V.; Evans, S.A.; Neretti, N.; Sedivy, J.M. Regulation of Cellular Senescence by Polycomb Chromatin Modifiers through Distinct DNA Damage- and Histone Methylation-Dependent Pathways. Cell Rep. 2018, 22, 3480–3492. [Google Scholar] [CrossRef]
- Ramirez, M.A.; Smerlak, M.; Traulsen, A.; Jost, J. Diversity enables the jump towards cooperation for the Traveler’s Dilemma. Sci. Rep. 2023, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Dupras, C.; Song, L.; Saulnier, K.M.; Joly, Y. Epigenetic Discrimination: Emerging Applications of Epigenetics Pointing to the Limitations of Policies Against Genetic Discrimination. Front. Genet. 2018, 9, 202. [Google Scholar] [CrossRef]
- Vidaki, A.; Kalamara, V.; Carnero-Montoro, E.; Spector, T.D.; Bell, J.T.; Kayser, M. Investigating the Epigenetic Discrimination of Identical Twins Using Buccal Swabs, Saliva, and Cigarette Butts in the Forensic Setting. Genes 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W.; Evans, B.J.; Hazel, J.W.; A Rothstein, M. The law of genetic privacy: Applications, implications, and limitations. J. Law Biosci. 2019, 6, 1–36. [Google Scholar] [CrossRef] [PubMed]


| Forensic Application | Challenges | Advantages of Histone Modifications | Representative Studies |
|---|---|---|---|
| Analysis of Degraded Biological Samples | Severe DNA fragmentation, STR amplification failure, and susceptibility to environmental contamination and microbial interference, leading to failure of conventional genotyping methods | Histones coexist with DNA in chromatin and are protected by stable nucleosomal structures; certain histone modification sites show high stability and can serve as alternative molecular markers | Acetylation marks such as H3K9ac, which reflect cellular activity and degradation status, were reported by Jarmasz et al. [13] to remain detectable up to 72 h postmortem, indicating short-term stability. |
| Monozygotic Twin Individual Identification | Identical nuclear genomic sequences; conventional STR genotyping fails to distinguish between genetically identical individuals | Epigenetic modifications are influenced by environment, behavior, disease, and lifestyle, leading to inter-individual variation with temporal and tissue specificity; useful for assessing exposure history and physiological status | Histone marks such as H3K4me3 and H3K27me3 show stable differences between monozygotic twins [100,101]. ChIP-seq has been successfully applied to detect such differences in peripheral blood samples [63,64]. |
| Postmortem Interval (PMI) Estimation | Conventional indicators have large margins of error; individual variation and environmental influences complicate decomposition rate and PMI estimation | Certain histone modifications (e.g., methylation and phosphorylation) exhibit time-dependent dynamic changes postmortem and have the potential to function as “epigenetic molecular clocks” for PMI estimation | H3K27me3 and γ-H2AX modifications demonstrate reproducible patterns of signal decay or elevation across different PMI stages, supporting their utility in molecular timing systems for death estimation [47,102]. |
| Limitation | Proposed Solution | Implementation Considerations |
|---|---|---|
| Low-Input Sensitivity | Single-cell CUT&Tag [65,66] | High cost; expertise required |
| Workflow Standardization | Forensic histone consortium; PAT-ChIP validation [156,157] | Cross-laboratory coordination |
| Modification Stability | Kinetic decay modeling [91,92] | Extensive data collection needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, L.; Lin, M.; Shen, C.; Reheman, A. Histone Modifications as Individual-Specific Epigenetic Regulators: Opportunities for Forensic Genetics and Postmortem Analysis. Genes 2025, 16, 940. https://doi.org/10.3390/genes16080940
Yang S, Chen L, Lin M, Shen C, Reheman A. Histone Modifications as Individual-Specific Epigenetic Regulators: Opportunities for Forensic Genetics and Postmortem Analysis. Genes. 2025; 16(8):940. https://doi.org/10.3390/genes16080940
Chicago/Turabian StyleYang, Sheng, Liqin Chen, Miaofang Lin, Chengwan Shen, and Aikebaier Reheman. 2025. "Histone Modifications as Individual-Specific Epigenetic Regulators: Opportunities for Forensic Genetics and Postmortem Analysis" Genes 16, no. 8: 940. https://doi.org/10.3390/genes16080940
APA StyleYang, S., Chen, L., Lin, M., Shen, C., & Reheman, A. (2025). Histone Modifications as Individual-Specific Epigenetic Regulators: Opportunities for Forensic Genetics and Postmortem Analysis. Genes, 16(8), 940. https://doi.org/10.3390/genes16080940





