Abstract
Heart failure (HF) remains a leading cause of morbidity and mortality worldwide, driven by diverse pathophysiological mechanisms. Among its major risk factors, obesity has emerged as a lobal public health concern affecting individuals across all age groups. The rising prevalence of obesity significantly increases the risk of cardiovascular complications, including the development and progression of HF. MicroRNAs (miRNAs), small non-coding RNA molecules, have garnered attention for their regulatory roles in cardiovascular disease, particularly through post-transcriptional modulation of gene expression. This review highlights the involvement of miRNAs in key pathological processes observed in the obese heart, including cardiac remodeling, apoptosis, angiogenesis, inflammation, mitochondrial dysfunction, and myocardial lipotoxicity. Understanding how specific miRNAs and their targets contribute to HF in the context of obesity may inform the development of novel RNA-based therapeutic strategies for cardiometabolic disease.
1. Introduction
HF is a significant global health problem, affecting over 56 million adults worldwide. Conditions such as coronary heart disease (CHD), diabetes, obesity, and smoking collectively account for 52% of HF cases []. Among these, accumulating evidence points to obesity, particularly elevated body mass index (BMI) and fluctuations in BMI, as a major contributor to HF by increasing cardiovascular stress, exacerbating the cardiac hemodynamic load, and promoting structural and functional changes [,]. These obesity-driven changes result in higher rates of cardiovascular-related mortality [,,,]. For instance, findings from the Framingham Heart Study and others have shown that a one-unit increase in BMI increases the risk of HF by 5% in men and 7% in women [,,].
Importantly, regional adipose distribution, rather than total body adiposity, may play an essential role in the progression of cardiovascular disease (CVD) to HF. Visceral adipose depots, such as intra-abdominal (VAT) [,] and the epicardial adipose tissue (EAT), a unique fat depot located between the myocardium and the visceral layer of the epicardium, have been implicated in HF development []. However, the precise mechanisms linking obesity-associated fat depots to HF pathophysiology are not yet entirely understood.
Over the past 50 years, global obesity rates have nearly tripled, fueling a widespread epidemic []. The significant rise in obesity is the leading cause of the rapid expansion of diabetes mellitus (DM) [] and contributes directly to CVD-associated morbidity and mortality worldwide []. Excessive accumulation of adipose tissue can lead to multifactorial cardiovascular complications, such as atherosclerosis [], hypertension [], and left ventricular (LV) systolic and diastolic dysfunction [], all of which can progress into the development of heart failure (HF).
The two main types of heart failure are heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) [,]. HFrEF is defined by an LVEF of less than 40% and is characterized by impaired systolic function, where the heart muscle cannot contract effectively to pump blood. In contrast, HFpEF is defined by an LVEF of 50% or greater and is primarily associated with diastolic dysfunction, in which the heart muscle maintains contractility but has impaired relaxation and filling capacity []. A recent study suggested that obesity and DM confer a greater risk of HFpEF compared to HFrEF, particularly in women []. Approximately half of individuals affected by obesity experience HFpEF, with patients who have DM exhibiting a more substantial disease burden []. This is evidenced by advanced cardiac imaging techniques, such as myocardial strain analysis, which reveal subclinical myocardial dysfunction in patients with DM [].
MicroRNAs (miRNAs) are essential regulators of multiple biological processes. These small (∼20–22 nt), single-stranded, non-coding RNAs are evolutionarily conserved and regulate gene expression at the post-transcriptional level. MiRNAs are critical in maintaining cellular and systemic homeostasis, making them pivotal in understanding HF pathophysiology. They bind to target mRNAs through base complementarity with the 3′ UTR region, leading to inhibition of their translation or the promotion of mRNA degradation. More than 2000 miRNAs have been identified in human eukaryotic cells [], with functions spanning both physiological and pathophysiological conditions.
Accumulating studies reveal that miRNA dysregulation may impact cardiovascular disease states and they play distinct roles in both HFpEF and HFrEF []. For example, in patients with chronic HF, heart and muscle-specific miRNAs, known as myomiRs, positively correlate with indices of myocardial damage, including cardiac troponin levels []. In patients with DM and HFpEF, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin resulted in significant downregulation of miR-126, miR-342-3p, and miR-638. Conversely, miR-21 and miR-92 were significantly upregulated in the blood following empagliflozin treatment [].
Here, we provide an overview of the role of miRNAs in modulating various aspects of HF in the context of obesity, including cardiac remodeling, inflammation, angiogenesis, apoptosis, mitochondrial dysfunction, and lipotoxicity (Figure 1). Elucidating the role of miRNAs in HF offers new insights into the pathobiology underlying the increased risk of developing HF associated with DM and obesity. This, in turn, may provide novel opportunities for therapeutic intervention.
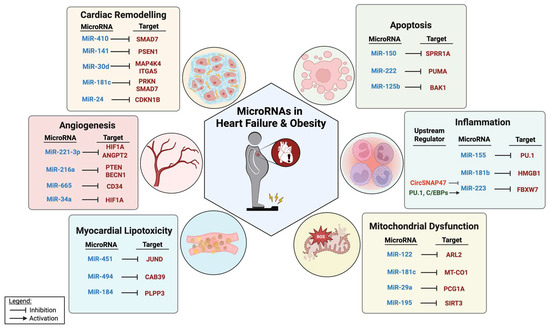
Figure 1.
MiRNAs and their targets in pathological processes relevant to HF in the context of obesity.
2. Cardiac Remodeling
Cardiac remodeling commonly refers to changes in cardiac size, shape, and function, including cardiac hypertrophy and fibrosis, in response to injury or pathological stress. Obesity is closely linked to cardiac remodeling, primarily due to the increased hemodynamic demand on the heart. This elevated demand leads to LV enlargement and dysfunction, ultimately progressing to HF. The underlying mechanisms of this progression involve pathological processes such as myocardial hypertrophy and cardiac fibrosis [].
Myocardial hypertrophy refers to the abnormal enlargement of heart muscle cells in response to increased workload, while cardiac fibrosis involves the excessive deposition of extracellular matrix components, which stiffens the heart muscle and impairs its function []. Recent studies have highlighted the significant role of miRNAs in cardiac remodeling by regulating gene expression and cellular processes in the heart.
Several miRNAs, such as miR-1 [,] miR-133a [,,], miR-30d-5p [], miR-208a [] miR-541-5p [], miR-217 [], miR-155 [], miR-199b-5p [], and miR-378-3p [] have been identified as key regulators of cardiac remodeling. Notably, certain miRNAs have been identified as upregulated in the plasma of obese individuals, where they show strong correlations with the molecular pathways involved in obesity-induced HF. These miRNAs likely contribute to the pathophysiology of obesity-related cardiac dysfunction by modulating key genes involved in hypertrophy and fibrosis. For example, in a rat model of diet-induced obesity (DIO), miR-410-5p was significantly upregulated in cardiac tissue, where it targeted and inhibited SMAD7 expression, leading to increased SMAD2 phosphorylation and subsequent activation of fibrosis. Conversely, the neutralization of miR-410-5p in obese rats mitigated cardiac remodeling by decreasing fibrosis. Interestingly, tissue-specific overexpression of miR-410-5p in kidneys or adipose tissue resulted in increased miR-410-5p expression in the heart and in circulating exosomes, suggesting a potential systemic role of miR-410-5p in obesity-associated cardiac dysfunction [].
MiR-141-3p and miR-144-3p were found to be significantly increased in the myocardium of obese rats, with opposing roles in obesity-associated cardiac remodeling. The inhibition of miR-141-3p or overexpression of miR-144-3p reduced palmitate-induced cardiac hypertrophy and fibrosis, while the overexpression of miR-141-3p or inhibition of miR-144-3p exacerbated cardiac dysfunction []. In support of these findings, miR-141-3p has been shown to target presenilin 1 (PSEN1) and inhibit the Notch1/PTEN/AKT pathway. Blocking this pathway reversed miR-141-3p's effects on palmitate-induced cardiomyocyte apoptosis []. Interestingly, treatment with the SGLT2 inhibitor ipragliflozin reduced both miR-144-3p expression and cardiac hypertrophy in Dahl-sensitive obese rats, suggesting potential tissue- or disease-specific effects of miR-144-3p, such as having protective effects during early remodeling but contributing to maladaptive responses in later stages or under different metabolic conditions [].
MiR-30d-5p was significantly elevated in patients with HF [] and obesity []. In murine models of ischemic HF, the overexpression of miR-30d-5p improved cardiac function by reducing myocardial fibrosis and attenuating apoptosis through targeting the mitogen-associate protein kinase 4 (MAP4K4) pathway. MiR-30d-5p is abundantly expressed in cardiomyocytes, with its levels significantly increasing in response to various stressors. Furthermore, it is highly concentrated in extracellular vesicles (EVs) released by cardiomyocytes, suggesting a role in intercellular communication. In studies using αMHC-MerCreMer-RosaTmG reporter mice, it has been demonstrated that miR-30d-5p can transfer from cardiomyocytes to fibroblasts, facilitating the crosstalk between these two cell types and thereby contributing to cardiac remodeling. Mechanistically, miR-30d-5p released from cardiomyocytes in EVs inhibited fibroblast proliferation and activation by directly targeting integrin α5, and thereby mediating paracrine signaling to cardiac fibroblasts [].
MiR-181c-5p, an miRNA that has been linked to cardiovascular complications [,,], was shown to be significantly increased in the blood of patients with DM and HFpEF. MiR-181c has been shown to target and decrease the expression of Parkin RBR 3 ubiquitin protein ligase (PRKN) and SMAD7, thereby enhancing the pro-fibrotic response and contributing to adverse cardiac remodeling []. Additionally, the overexpression of miR-181c has been linked to increased reactive oxygen species (ROS) production and elevated mitochondrial calcium levels, both of which are associated with oxidative stress leading to HF. In experimental models, mice deficient in miR-181c/d exhibited reduced cardiac hypertrophy compared to wild-type controls when subjected to high-fat diet (HFD) [].
The expression of miR-24 was shown to be increased in humans with obesity [] and in murine cardiac tissue with pressure overload-induced hypertrophy. The overexpression of miR-24 resulted in the direct targeting and decreased expression of p27, a cyclin-dependent kinase inhibitor (CDKI) that regulates cell cycle, in neonatal rat ventricular myocytes (NRVM) in vitro and in cardiac tissue in vivo. This interaction promoted cell cycle progression into the S phase, thereby contributing to the development of cardiac hypertrophy by promoting myocyte growth []. Whether the dual regulation of miR-24 by obesity and pressure-overload induced hypertrophy exacerbate the progression of cardiac remodeling into overt HF remains to be studied.
Although several studies to date have demonstrated that miRNAs regulate cardiac remodeling by targeting genes involved in cardiac hypertrophy and fibrosis, miRNA profiling in the context of obesity and HF could provide valuable insights into the molecular mechanisms driving disease progression. Understanding how miRNA expression changes in human plasma and cardiac tissue in response to obesity, and the mechanistic impact on cardiac remodeling during the progression into HF, may inform the development of targeted therapeutic approaches.
3. Angiogenesis
Coronary angiogenesis plays a crucial role in maintaining cardiac vascularization and perfusion in response to pathological and physiological conditions. Impaired coronary angiogenesis, particularly reduced capillarization, is strongly associated with pathological cardiac hypertrophy and ventricular dysfunction.
Microvascular dysfunction and increased metabolic demand in obese patients not only lead to inadequate myocardial perfusion and hypoxia [] but also exacerbates cardiac dysfunction in pressure overload-induced cardiac hypertrophy, accelerating the onset of HF. Conversely, pro-angiogenic stimulation has been shown to delay HF progression, highlighting its importance in preserving cardiac function [,,,].
Endothelial cells (ECs) located at the inner most layer of all blood and lymphatic vessels [], are key mediators of angiogenesis through their regulation of EC proliferation and migration. Obesity-induced endothelial dysfunction is driven by several factors, including dysregulated endothelial nitric oxide (NO) production, a vasodilator that modulates EC survival, proliferation, and migration [,,,]. Additionally, increased endoplasmic reticulum (ER) stress [], elevated circulating free fatty acid levels [,,], and the activation of pro-inflammatory pathways [,,,] exacerbate endothelial stress, ultimately impairing vascular function.
MicroRNAs have been identified as key regulators of EC function in obesity. However, their role in modulating angiogenesis in HF within the context of obesity remains poorly understood. Notably, miR-221-3p expression has been negatively correlated with cardiac function in patients with HF while in vivo neutralization of miR-221-3p promoted cardiac angiogenesis in a murine model of TAC-induced HF through the targeted regulation of hypoxia-inducible factor-1 α (HIF-1α) []. Additionally, miR-221-3p expression correlated positively with BMI and waist circumference, while negatively associating with insulin sensitivity. Its expression was reduced in the plasma of pediatric patients following weight loss [], implicating that the obesity-associated regulation of miR-221-3p may play an important role in the modulation of angiogenesis in HF.
Genetic deletion of miR-216a-5p, a pro-angiogenic microRNA upregulated in obese women [], has been linked to impaired cardiac microvascular EC function. In a TAC model of chronic pressure overload-induced HF, miR-216a-5p deletion resulted in capillary rarefaction, reduced myocardial oxygenation, and accelerated HF onset. This effect was mediated through the dysregulation of EC-angiogenic functions and direct targeting of phosphatase and tensin homolog (PTEN) and beclin-1 (BECN1). Although the role of PTEN in ECs remains insufficiently characterized, knockout of miR-216a-5p in mice exhibited reduced PTEN expression and decreased capillary density. Furthermore, in vitro neutralization of miR-216a-5p led to increased BECN1 levels and enhanced autophagy. Together, these findings demonstrate that miR-216a-5p is essential for maintaining angiogenesis, particularly under conditions of pressure overload and myocardial infarction-induced HF. With studies showing its upregulation in obese humans [], miR-216-5p may be an important positive regulator of EC angiogenic functions in the myocardium under obesity.
MiR-34a was shown to mediate capillary rarefaction by targeting the HIF-1α/VEGF signaling axis in a murine model of right ventricular failure (RVF) and was negatively correlated with capillary density in patients with RVF []. The expression of miR-34a was significantly increased in patients with impaired glucose tolerance, prediabetes, and metabolic syndrome [], suggesting the possible involvement of miR-34a in the etiology of obesity-associated angiogenic dysfunction in HF.
MiR-665-3p levels are elevated in cardiac ECs of patients with HF. The inhibition of miR-665-3p or the overexpression of its target, CD34, in a mouse model of TAC improved EC proliferation, angiogenesis, and cardiac function. Furthermore, TAC upregulates the transcription factor Sp1, which has been shown to regulate miR-665-3p expression in human umbilical vein endothelial cells (HUVECs) []. Notably, miR-665-3p expression was significantly increased in the liver and hepatic cells of mice with DIO []. However, its role in HF in the context of obesity needs to validated in prospective studies in obese patient cohorts with HFpEF or HFrEF.
Finally, elevated miR-204 levels in ECs contribute to endothelial dysfunction by suppressing SIRT1 expression, leading to increased endothelial endoplasmic reticulum (ER) stress, reduced Cav1 (caveolin-1) levels, and impaired vasorelaxation []. Moreover, HFD increased vascular miR-204 expression while decreasing Cav1. Given Cav1’s protective role in shielding cardiac ECs from hemodynamic stress, these findings suggest a potential anti-angiogenic role of miR-204 in heart disease and obesity []. However, whether Cav1 is a direct target of miR-204 remains unclear. Interestingly, miR-204 exhibits a contrasting role under cardiac stress. In a mouse model of TAC-induced HF, miR-204 was found to be upregulated in response to cardiac stress, where it facilitated cardioprotection by modulating Apelin signaling and preventing hypertrophy [].
These findings highlight the dual nature of angiogenic miRNAs in HF: some contribute to endothelial dysfunction while others offer protective effects under stress conditions. Precision-based therapies that selectively target these miRNAs could help restore vascular homeostasis and delay HF progression, particularly in patients with obesity-related cardiovascular risk. Further research into the context-dependent roles of these miRNAs will be essential for developing targeted interventions that enhance LV angiogenesis.
4. Apoptosis
In end-stage HF, loss of cardiomyocytes occurs alongside fibrosis and cellular hypertrophy. Apoptosis, a programmed form of cell death, is a key mechanism contributing to cardiomyocyte loss, with its rate increasing as HF progresses. In contrast, myocyte size, volume, and sarcomere number expand in pathologic hypertrophy. The rate of apoptosis typically rises with age and is exacerbated by pressure overload, gradually leading to HF as cumulative myocyte loss impairs cardiac function []. Obesity-associated oxidative stress, elevated levels of pro-inflammatory cytokines, such as TNF alpha, norepinephrine, and angiotensin II [], along with dysregulated leptin signaling [,], further promote cardiomyocyte apoptosis. Obesity also activates FAS and mitochondria-dependent apoptosis, potentially accelerating of HF progression [,].
Several miRNAs that were dysregulated in obesity were also shown to modulate cardiac apoptotic pathways in murine models of pressure overload-induced hypertrophy. For example, miR-150-5p knockout mice subjected to myocardial infarction (MI) developed worsened cardiac remodeling and increased apoptosis, ultimately leading to HF. MiR-150-5p directly targets the pro-apoptotic factor small proline-rich protein 1A (SPRR1A), inhibiting its activity. Heart tissue from patients with HFrEF has shown increased SPRR1A expression [], while it was also upregulated in murine myocardium post-TAC []. In support of these findings, the overexpression of miR-150-5p in murine heart conferred protection against cardiac hypertrophy and fibrosis following TAC []. While in vivo studies have demonstrated the cardioprotective role of miR-150-5p, its expression was significantly downregulated in patients with HF, serving as a predictive biomarker correlated with established HF parameters, such as N-terminal pro-B-type natriuretic peptide (NT-proBNP) [,]. Interestingly, the expression of miR-150-5p was also downregulated in the serum of obese individuals compared to those with normal BMI [,], while an opposing trend was observed in the pediatric population with increased miR-150-5p expression in the circulation of obese children with high hepatic fat and serum triglyceride [,], which could be due to differences in lipid metabolic regulation and inflammatory responses in children and adolescents, highlighting the complexity of miRNA regulation. Whether overexpression of miR-150-5p can mitigate apoptosis in the setting of obesity and help with the progression of HF remains to be determined.
Similarly, miRNA-222-3p expression was increased in a murine model of pressure overload-induced hypertrophy, where cardiac-specific overexpression following TAC improved cardiac function. Transcriptomic profiling revealed that miR-222-3p targets the p53-up-regulated modulator of apoptosis (PUMA), a pro-apoptotic gene []. Notably, PUMA-deficient mice that underwent TAC maintained cardiac function similar to the sham group lasting up to four weeks []. In Dahl salt-sensitive (DS) obese rats, treatment with the SGLT2 inhibitor ipragliflozin effectively reduced cardiac hypertrophy and concurrently suppressed miR-222-3p expression []. Additionally, miR-222-3p expression was found to be lower in myocardial biopsies from patients with aortic stenosis and dilated cardiomyopathy (DCM), indicating its dysregulation in pressure overload and hypertrophic conditions []. Interestingly, miR-222-3p expression was elevated in morbidly obese individuals [], and its increased expression persisted even after weight loss []. This suggests a potential link between obesity, cardiac stress, and apoptotic regulation, which may play a role in the adaptive response to pressure overload-induced hypertrophy.
Conversely, miR-125b-5p expression was downregulated in the plasma of obese humans [] and in murine cardiac tissue subjected to TAC. Increased miR-125b-5p expression has been shown to suppress apoptosis by reducing the levels of anti-apoptotic genes, such as caspase-3 and Bax, while elevating the pro-apoptotic factor BCL2 through targeting Bcl-2 homologous antagonist/killer 1 (BAK1) []. These findings highlight a critical interplay between obesity and apoptosis in cardiac pathology, where dysregulated microRNA expression may contribute to HF. Together, these results underscore the potential of microRNAs as biomarkers and therapeutic targets in obesity-associated cardiac dysfunction, particularly in the context of pressure overload-induced hypertrophy and HF.
5. Inflammation
Inflammation plays a crucial role in the development and progression of HF through various pathways, including the activation of pro-inflammatory cytokines and adhesion molecules, which trigger the migration of macrophages, neutrophils, monocytes, and lymphocytes. This process is accompanied by impaired microvascular dilation, fibroblast, myofibroblast proliferation, and capillary rarefaction [], thereby contributing to the systolic and diastolic dysfunction in HF, especially in the setting of HFpEF [].
Several strategies have been explored to reduce inflammation in chronic HF, focusing on targeting TNF-α due to its pivotal role in HF pathogenesis [,,]. However, clinical trials of TNF-α receptor antagonists in HF have reported excess mortality, suggesting that TNF-α receptor antagonism has negative cardiovascular effects. Postulated reasons for these setbacks include the bidirectional role of inflammatory cytokines in HF pathogenesis, potential adverse effects of high doses, and limited impact on myocardial inflammation. Therefore, targeting inflammatory markers in HF remains challenging, and current strategies to regulate cytokine imbalance may not be optimal.
Understanding the contribution of miRNAs to inflammation in HF, especially in the setting of obesity, may offer more etiology-specific therapeutic candidates. The expression of miR-181b was significantly increased in the white adipose tissue (WAT) of obese mice and humans [], while its expression was decreased in the serum and heart tissue of obese mice with cardiac hypertrophy and LV dysfunction []. Additionally, miR-181b levels inversely correlated with the levels of high-sensitivity C-reactive protein, a key inflammatory marker [], further highlighting its potential role in modulating inflammation-related cardiac dysfunction. In a rat model of HF, miR-181b expression was downregulated in both the early and advanced stages of the disease. Early HF was associated with cardiac hypertrophy and reduced miR-181b expression, while advanced HF exhibited increased levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), which further reduced miR-181b expression in the cardiac tissue of rats treated with isoproterenol. In vitro studies demonstrated that miR-181b overexpression could inhibit LPS-induced inflammatory responses in neonatal cardiomyocytes, indicating a regulatory role for miR-181b in inflammation in HF []. In a rat model of sepsis, the overexpression of miR-181b reduced inflammation and cardiomyocyte apoptosis by targeting high-mobility group box-1 protein (HMGB1) and thereby reducing cardiac injury []. Importantly, its expression is increased with methotrexate (MTX), an immunosuppressive drug, decreasing the endothelial inflammation in obese mice [].
MiR-155 also plays a crucial role in obesity and HF pathogenesis by regulating inflammatory responses. Studies have indicated that the expression of miR-155 was strongly upregulated in human and mouse models of myocarditis, and its expression was primarily detected in infiltrating macrophages and T lymphocytes. The inhibition of miR-155 reduced the cardiac infiltration of monocyte-derived macrophages, the activation of T lymphocytes, and myocardial damage, while improving cardiac function by targeting an inhibitor of dendritic cell antigen presentation to T cells []. Additionally, miR-155 expression was increased in the subcutaneous adipose tissue of obese individuals compared to those with normal BMI and positively correlated with TNF-α expression, possibly by targeting PPAR-γ []. On the other hand, research has also explored the role of miR-155 in the obesity paradox, which suggests that individuals with a higher BMI may have better outcomes in cardiovascular disease despite obesity being a known risk factor for heart disease. ApoE knockout (KO) mice, a standard model of atherosclerosis, exhibited increased miR-155 expression in the mice aorta after 3 weeks on HFD and 6 hours of lipopolysaccharide (LPS) stimulation. This upregulation promoted atherogenesis by inducing EC activation and enhancing pro-inflammatory macrophage responses. Furthermore, an ApoE/miR-155 double KO mouse model provided significant insights into the complex interplay among obesity, atherosclerosis, and non-alcoholic fatty liver disease (NAFLD), where, despite increased adipogenesis, these mice showed a reduction in atherosclerotic lesions []. Concurrently, they developed NAFLD and enhanced adipogenesis. These observations underscore the pivotal role of miR-155 in modulating inflammation and lipid metabolism, thereby influencing the pathogenesis of both cardiovascular and metabolic diseases. Further investigations are warranted to elucidate the underlying mechanisms of this paradox, particularly focusing on the interconnections between inflammatory pathways and obesity-related cardiovascular conditions.
MiR-223-3p has been extensively studied in HF and is recognized for its multifaceted roles in disease progression. In HF, the expression patterns of miR-223-3p vary depending on the stage of the disease. In individuals with early-stage HF, particularly those with concurrent diabetes, miR-223-3p expression was downregulated in the heart []. In contrast, patients with end-stage HF showed significantly elevated levels of miR-223-3p expression, suggesting that this increase may be associated with advanced stages of HF []. An upstream regulator of miR-223-3p, the circular RNA known as circSnap47, plays a role in HF progression by targeting the miR-223/MAPK signaling axis []. Other upstream regulators of miR-223-3p, including the transcription factors PU.1 and C/EBPs [], may even work synergistically to enhance inflammation, as they are targeted by the NFκB signaling axis [,]. This regulatory influence of circSNAP47 on miR-223-3p suggests it could be a critical factor in the worsening of HF. In obesity, miR-223-3p exhibits differential expression in various types of adipose tissue. In one study, miR-223-3p was significantly upregulated in human visceral adipose tissue [], often associated with obesity-related inflammation. Conversely, miR-223-5p was markedly reduced in subcutaneous adipose tissue following weight loss from gastric bypass surgery []. While it is not fully understood if miR-223-3p directly impacts HF in response to the low-grade inflammation commonly seen with obesity, its ability to target a key component in inflammatory processes, namely FBXW7, a subunit of the ubiquitin protein ligase complex called SKP1-cullin-F-box and a well-known suppressor of Toll-like receptor 4 (TLR4) signaling in macrophages [,], indicated that miR-223-3p might help modulate the inflammation in HF in the context of obesity.
In a murine HF model achieved by coronary artery ligation, adenoviral overexpression of miRNA-130a improved LV ejection fraction by reducing TNF-α expression in the myocardium while also decreasing the expression of endothelin-1 (ET-1) in plasma []. Furthermore, the profiling of umbilical cord serum miRNAs associated with obesity identified hepatic exosome-derived miR-130a-3p as a regulator of energy metabolism in adipose tissue, where its expression was increased in the circulation of children with an increased risk for obesity [].
Finally, the inhibition of miR-146a was shown to improve cardiac function, enhance hemodynamics, and reduce heart remodeling in myocardial infarction (MI)-induced HF []. It regulates cholesterol metabolism and helps control chronic inflammation in response to an atherogenic diet by limiting proinflammatory signaling in endothelial and bone marrow-derived cells []. MiR-146a-deficient mice fed HFD exhibited increased weight gain, adiposity, and hepatic steatosis compared to the wild-type control, suggesting a protective role of miR-146a against DIO [].
The interplay among miR-155, miR-130a, miR-181b, miR-146a, and miR-223-3p underscores the importance of miRNAs in the regulation of inflammation in both obesity and HF. For example, miR-181b appears to have anti-inflammatory properties, and its upregulation could help mitigate HF progression. Meanwhile, miR-155, which is associated with obesity-related inflammation, might serve as a potential biomarker or target for interventions aimed at reducing cardiovascular risk in obese individuals. Moreover, such interplay also underscores the complexity of HF pathogenesis, necessitating a cautious approach in developing miRNA-based therapies. Given that some miRNAs, such as miR-155, may have paradoxical effects, the modulation of these pathways may yield unintended consequences due to their pleiotropic effects. Developing effective, etiology-specific therapies will require a deeper understanding of microRNA signaling dynamics to ensure targeted interventions that mitigate inflammation without compromising essential immune or metabolic functions.
6. Lipotoxicity
Obesity-associated excessive lipid accumulation in non-adipose cells of the cardiovascular system can lead to cell death, a phenomenon known as lipotoxicity []. In the heart, lipotoxicity impairs the heart’s metabolic flexibility, exacerbating oxidative stress and inflammation, ultimately increasing the risk of HF [,,]. Among the key regulators of lipotoxicity, several miRNAs have emerged as critical post-transcriptional modulators of lipid metabolism and cellular stress responses in the heart.
One such miRNA, miR-494-3p, was downregulated in the myocardium of mice with DIO. Notably, the overexpression of miR-494-3p reduced triglyceride uptake, oxidative stress, and apoptosis by modulating the JunD/PPARγ axis in neonatal rat ventricular myocytes (NRVMs) exposed to palmitate. Mechanistically, Ago2 immunoprecipitation and 3'- UTR luciferase assays in NRVMs confirmed JunD as a direct target of miR-494-3p. Interestingly, JunD expression was significantly elevated in the myocardium of obese patients, correlating with reduced miR-494-3p levels, increased myocardial triglyceride accumulation, upregulated PPARγ expression, and left ventricular dysfunction [] suggesting that miR-494-3p plays a protective role against obesity-induced lipotoxicity by regulating key metabolic pathways.
Similarly, elevated miR-451 levels were detected in neonatal rat cardiac myocytes (NRCMs) treated with palmitate, as well as in the heart tissues of DIO mice. Furthermore, neutralizing miR-451 in NRCMs improved palmitate-induced lipotoxicity. Supporting these in vitro findings, the cardiac-specific deletion of miR-451 alleviated HFD-induced cardiac hypertrophy and improved contractile function by targeting calcium-binding protein 39 (CAB39), a scaffold protein of liver kinase B1 (LKB1) and an upstream kinase of AMP-activated protein kinase (AMPK). This regulation was also associated with a reduction in mammalian target of rapamycin (mTOR) expression [], suggesting miR-451’s potential involvement in metabolic stress responses.
While miR-451 appears to exacerbate lipotoxicity, miR-184 expression has been shown to be elevated in the plasma of patients with obesity [], with its expression significantly reduced in the myocardium of mice fed a HFD for 24 weeks or in vitro in H9C2 cardiomyoblasts treated with palmitic acid. Mechanistically, miR-184 targeted and reduced the expression of phosphatidic acid phosphatase (PLPP3), which decreased diacylglycerol (DAG), a precursor for triglyceride biosynthesis, and increased insulin sensitivity. This was associated with increased glucose consumption, enhanced AKT phosphorylation, and improved insulin signaling in cardiomyocytes []. Whether these effects are tissue-specific, and how they may be leveraged for therapeutic interventions in obesity-associated cardiac dysfunction remains to be elucidated.
In summary, obesity-related metabolic cardiomyopathy and HF primarily arise from the accumulation of lipotoxic by-products within cardiac cells, leading to myocyte apoptosis and impaired contractility. Lipotoxicity, driven by an excessive buildup of triglycerides, free fatty acids, and other lipid intermediates, causes significant cardiac damage by generating reactive oxygen species (ROS) and activating apoptotic pathways. Studies have suggested that mitigating cardiac lipotoxicity may help alleviate obesity-associated heart dysfunction.
Understanding the mechanisms by which microRNAs influence lipid biosynthesis, lipid metabolism, and the accumulation of these harmful products in the heart is critical. Despite growing interest in the role of microRNAs in lipotoxicity, it is still unclear which metabolic pathways they most strongly regulate, which lipid products are the most harmful, and how lipotoxicity interacts with inflammation and glucose metabolism. In addition, miRNAs have emerged as potential prognostic biomarkers of lipotoxicity and lipid metabolism, particularly in ischemic heart disease and HF in the obese population. This is because changes in lipid metabolism and cellular fuel usage often precede more recognizable alterations, such as cardiac remodeling and functional impairments, which are typically detected through imaging and other diagnostic methods.
7. Mitochondrial Dysfunction
Maintaining mitochondrial homeostasis for endogenous energy production is essential for proper cardiac function. The dysregulation of miRNAs and their involvement in mitochondrial dysfunction has garnered significant attention, especially in the context of HF and cardiomyopathies. This highlights the critical role of mitochondrial function in cardiac health []. Furthermore, the association between obesity, as a metabolic disorder, and mitochondrial dysfunction has been extensively investigated. Research has particularly emphasized how mitochondrial dysfunction impairs various organs in the context of obesity []. However, a gap remains in understanding the specific role of miRNAs linking mitochondrial dysfunction to obesity-related cardiac dysfunction.
One such miRNA, miR-195, has been strongly implicated in mitochondrial dysfunction and plays a role in obesity and HF by targeting mitochondrial health and metabolic function. miR-195 expression is significantly increased in a murine TAC model and in human HF [,,]. Elevated miR-195 levels are associated with the hyperacetylation of mitochondrial enzymes, resulting from its downregulation and direct targeting of sirtuin 3 (SIRT3), a mitochondrial deacetylase. SIRT3 helps protect mitochondrial enzymes from acetylation, preserving their activity and stability. By directly targeting SIRT3, miR-195 increased protein acetylation, leading to diminished mitochondrial enzymatic activity, impaired energy production, and exacerbated mitochondrial dysfunction in HF []. Beyond its role in HF, elevated miR-195 levels have also been linked to metabolic syndrome, where its expression was correlated with increased BMI [,] and hypertension [,]. These findings suggest that miR-195 may serve as a potential biomarker for metabolic and mitochondrial health.
MiR-194 expression was significantly elevated in the circulation of age-matched patients with a BMI greater than 28 compared to lean individuals with a BMI below 23. This correlated with higher levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) and reduced ejection fraction (EF), both of which are key indicators of LV dysfunction []. To explore the functional impact of miR-194, a mouse model of HFD-induced obesity was used, where the systemic neutralization of miR-194 using a viral sponge enhanced mitochondrial activity. This resulted in increased ATP production and basal oxygen consumption of cardiac tissue, ultimately resulting in better EF and reduced cardiac hypertrophy []. Additionally, in an isoproterenol-induced cardiac hypertrophy model, calcineurin A (CnA) was identified as a direct target of miR-194 in cardiac myocytes [] further supporting miR-194’s role in mitochondrial and cardiac dysfunction.
An exosomal microRNA, miR-122, previously identified as being secreted by the liver, has been linked to obesity-related cardiomyopathy. Wang and colleagues demonstrated that miR-122 expression was elevated in the plasma samples of patients with obesity, showing a significant positive correlation with cardiac dysfunction. Mechanistically, miR-122 directly targets the mitochondrial protein ARL-2 (ADP-ribosylation factor-like 2), a regulatory GTPase. The dysregulation of this interaction leads to mitochondrial impairment and abnormal cardiac remodeling, as evidenced by in vitro studies on primary cardiomyocytes and in vivo mouse models with DIO [].
MiR-181c is a microRNA classified as a MitomiR (mitochondrial microRNA), a term that describes microRNAs translocated to the mitochondria upon maturation in the cytoplasm. Elevated levels of miR-181c have been observed in heart samples from patients with cardiac dysfunction, and its presence was also increased in the mitochondrial fraction of cardiac tissue obtained from mice with DIO, where it was associated with myocardial lipidosis. In addition to its role in regulating lipidosis in the myocardium, miR-181c can alter lipid metabolism and biosynthesis in the liver under DIO conditions []. In a whole-body genetic knockout mouse model of miR-181c/d, cardioprotective effects were demonstrated in DIO by reducing ROS production and limiting mitochondrial calcium accumulation. Mechanistically, miR-181c directly binds to the 3′-UTR of mt-COX1, indirectly downregulating the mitochondrial Sp1/MICU1 axis that controls calcium influx [,,].
In human plasma samples with obesity, increased miR-29a expression was correlated with cardiac dysfunction. The treatment of primary cardiomyocytes with circulating human exosomal miR-29a induced mitochondrial dysfunction, while systemic neutralization of miR-29a restored mitochondrial function, improved ATP production, and reduced IκB phosphorylation in cardiac tissue, thus improving the cardiac function of mice with DIO []. Additionally, miR-29 genetic deficiency in a double KO mice model (miR-29a/b1−/−) was associated with the development of HFpEF. In these miR-29a-deficient mice, the authors reported systemic hypertension, a metabolic shift from fatty acid utilization to glucose utilization, along with mitochondrial abnormalities in cardiac tissue []. These effects are likely mediated through previously reported direct targets of miR-29a, PGC1α [], a crucial regulator of mitochondrial biogenesis and function.
Excessive caloric intake, increased mitochondrial substrate load, or mitochondrial dysfunction that impairs the effective dissipation of the proton gradient can lead to elevated ROS production. This results in cellular damage, mtDNA mutations, and apoptosis. Perturbations in cardiac energy metabolism represent some of the earliest changes observed in the myocardium during the progression of metabolic syndrome-related heart dysfunction. Despite these insights, the identification of miRNAs regulated by these early metabolic disturbances remains underexplored. A deeper understanding of these miRNAs could significantly enhance their diagnostic utility in heart disease, especially as early biomarkers of metabolic disruptions. However, significant gaps persist in our understanding of miRNAs and their connection to obesity-related HF. In particular, the functional roles of mitochondria-associated miRNAs and their interactions with nuclear-encoded miRNAs require further investigation to elucidate their contributions to obesity-related cardiac dysfunction. Emerging evidence indicates that miRNAs may regulate critical processes, such as mitochondrial fusion–fission dynamics, mitochondrial metabolism, calcium homeostasis, and other pathways involved in mitochondrial dysfunction. Nevertheless, many of these mechanisms, including the regulation of mitophagy and mitochondrial dynamics by miRNAs, remain poorly characterized. There is a pressing need for well-designed preclinical and clinical studies that leverage advanced methodologies capable of isolating mitochondrial genetic material with minimal contamination. Such approaches could provide crucial insights into miRNA-mediated changes in the mitochondrial genetic landscape and its functional implications. Addressing these gaps will be crucial for advancing our understanding of how obesity-induced mitochondrial dysfunction contributes to cardiac disease and for identifying novel therapeutic and diagnostic opportunities that target mitochondrial miRNAs.
8. Conclusions and Perspectives
MicroRNAs are present in various biological fluids and exhibit remarkable stability, making them easily extractable from biological samples, such as blood []. They are differentially regulated both during the progression of many disease states and in response to various HF therapies and can simultaneously target multiple genes and pathways (Table 1), providing a potentially valuable non-invasive means for accurate diagnosis, prognosis, treatment guidance, and treatment response evaluation []. Despite challenges in targeted delivery to tissues and specific cell types, advances in RNA chemistry and delivery technologies, particularly nanoparticle systems, have facilitated the transition of microRNA-based approaches into clinical settings [].

Table 1.
Key microRNAs (miRNAs) implicated in heart failure (HF), their targets, and mechanisms of action. This table summarizes miRNAs that play significant roles in HF and highlights their validated or potential utility as therapeutic targets. Each miRNA is associated with specific molecular targets and contributes to HF progression through distinct pathophysiological mechanisms, including cardiac hypertrophy, fibrosis, angiogenesis, apoptosis, and inflammation. * = Potential target, # = targeted by approved pharmacological agents.
With a growing understanding of the roles various microRNAs play in diseases, engineered microRNA therapeutics hold promise for revolutionizing therapeutic strategies by targeting multiple proteins and enzymes involved in disease progression, rather than targeting them individually [,,,].
Achieving targeted delivery to specific cell types or tissues is crucial for the effective development of miRNA-based therapeutics. One approach involves conjugating miRNA modulators with peptides or antibodies to facilitate their homing to particular cell types []. Alternatively, lipid-based formulations can encapsulate antimiRs or miRNA mimics, enhancing their cell specificity []. In the absence of highly specific delivery methods, device-based approaches, like stents or catheters, local injections, and ectopic delivery, can help overcome some delivery obstacles. Given the established role of cardiac catheterization in clinical practice, such approaches could be highly feasible in HF. The currently reported delivery techniques for antisense oligonucleotides already include inhalation for lung delivery, enema formulations for gut delivery, intraventricular or intrathecal administration for brain delivery, and direct intraocular delivery for ocular conditions. In addition to conventional protocols, additional methods for administering miRNA-based drugs are being investigated. An encouraging strategy involves incorporating miRNA therapeutics into biodegradable 3D matrices, which can be surgically implanted into affected tissues []. Further advancements are anticipated, as 3D matrices are also tailored for delivering various nucleic acid-based therapies and conventional drugs through different routes, such as edible or injectable carriers [].
MiRNAs hold potential as diagnostic or prognostic tools due to their ability to reflect origin, and other pathological factors through their expression profiles. They serve as precise molecular markers owing to their stability and resistance to RNase degradation, likely due to their small size. There is a growing availability of miRNA markers for diagnosing various cardiovascular disease states. For example, in murine models of autoimmune and viral myocarditis, circulating mmu-miR-721 has been shown to be significantly elevated. Plasma levels of its human homolog hsa-miR-Chr8:96 successfully distinguished myocarditis from myocardial infarction and from healthy individuals across various cohorts []. MiR-19b-3p has emerged as a promising prognostic biomarker of acute HF. Elevated levels of circulating miR-19b-3p at the onset of HF have been associated with poorer outcomes, including increased mortality and higher rates of hospital readmission. Its expression was correlated with markers of LV hypertrophy and fibrosis, indicating its potential role in cardiac remodeling processes []. Such ongoing research highlights the potential for microRNAs to serve as valuable biomarkers and offer additional diagnostic or prognostic benefits beyond the standard markers, which are sensitive but lack specificity for HF, such as brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP). This is particularly useful for the non-invasive diagnostic detection of HF in patients with DM and obesity [,].
AntimiR therapies have demonstrated promising results after the positive outcomes observed in a few Phase I and Phase II clinical trials. The miR-29 family (consisting of miR-29a/b/c) is known to reduce expression levels in fibrotic conditions, where it acts to inhibit the accumulation of extracellular matrix []. MRG-110, a mixer of LNA and DNA antagomiR molecules, modified with phosphorothioate internucleotide bridges, targets miR-92, aiming to address cardiovascular diseases, such as HF. By inhibiting miR-92a, MRG-110 was designed to stimulate angiogenesis as a potential treatment approach in a Phase I trial. In a clinical Phase 1b study, cardiac miR-132-3p levels were shown to increase in patients with HF, while CDR132L, an antisense oligonucleotide specific to miR-132 attenuated HF in preclinical models. The study, conducted on a small group of patients, demonstrated high efficacy, significant decreases in cardiac fibrosis, and improved cardiac function, providing encouraging preclinical observations in humans []. Increased levels of low-density lipoprotein cholesterol (LDL-C) are a major risk factor for obesity-induced HF. Recently, FDA-approved siRNA-based therapy inclisiran showed efficacy in reducing circulating LDL-C by targeting PCSK9. PCSK9 binds to LDL receptors, inducing conformational changes that inhibit receptor recycling, thereby reducing LDL receptors on the cell surface. This results in elevated LDL-C levels in the blood, contributing to hypercholesterolemia. Mechanistically, inclisiran, which is designed with triantennary GalNAc, specifically targets asialoglycoprotein receptors (ASGPRs) on hepatocytes, facilitating its uptake into liver cells, where it degrades PCSK9 mRNA [].
The effectiveness of miRNA therapeutics depends on the route of administration and successful intracellular delivery to avoid systemic side effects beyond the targeted cells and tissues. One notable example of this was a synthetic miR-34a mimic that led to severe immune-related side effects and was terminated from clinical trials []. Despite obstacles to integrating therapeutic miRNAs into clinical practice, these challenges are identifiable. Novel approaches that target miRNAs during the development of HF may pave the way for preventative strategies and targeted therapies.
Author Contributions
Conceptualization, B.I.; methodology, B.I., P.S., F.B. and S.V.; software, P.S., F.B., S.V. and G.F.G.; validation, P.S., F.B. and S.V.; formal analysis, B.I., P.S., F.B. and S.V.; investigation, B.I., P.S., F.B. and S.V.; resources, B.I.; data curation, B.I., P.S., F.B. and S.V.; writing—original draft preparation, B.I., P.S., F.B., S.V., G.F.G. and R.M.B.; writing—review and editing, B.I., P.S., F.B., S.V., G.F.G. and R.M.B.; visualization, B.I.; supervision, B.I.; project administration, B.I.; funding acquisition, B.I. and R.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by 1R01HL149999 to B.I. and by 1R01HL162919 to R.M.B.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
None declared.
Abbreviations
| AMPK | AMP-activated protein kinase |
| ARL-2 | ADP-ribosylation factor-like 2 |
| ASGPRs | Asialoglycoprotein receptors |
| BECN1 | Beclin-1 |
| BNP | Brain natriuretic peptide |
| CAB39 | Calcium-binding protein 39 |
| CDKI | Cyclin-dependent kinase inhibitor |
| Cav1 | Caveolin-1 |
| DAG | Diacylglycerol |
| FAS | Fas cell surface death receptor |
| GTPase | Guanosine triphosphatase |
| HCMEC | Human cardiac microvascular endothelial cells |
| HMGB1 | High-mobility group box-1 protein |
| HIF-1α | Hypoxia-inducible factor-1 α |
| LDL-C | Low-density lipoprotein cholesterol |
| LKB1 | Liver kinase B1 |
| LPS | Lipopolysaccharide |
| MAP4K4 | Mitogen-activated protein kinase 4 |
| mTOR | Mammalian target of rapamycin |
| NO | Nitric oxide |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| NMVM | Neonatal mouse ventricular myocyte |
| NRVM | Neonatal rat ventricular myocytes |
| NRCM | Neonatal rat cardiomyocytes |
| NRCF | Neonatal rat cardiac fibroblasts |
| PUMA | p53-upregulated modulator of apoptosis |
| PRKN | Parkin RBR 3 ubiquitin protein ligase |
| PTEN | Phosphatase and tensin homolog |
| PLPP3 | Phosphatidic acid phosphatase |
| PSEN1 | Presenilin 1 |
| SGLT2 | Sodium-glucose cotransporter 2 |
| SIRT3 | Sirtuin 3 |
| SPRR1A | Small proline-rich protein 1A |
| TLR4 | Toll-like receptor 4 |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Flores, M.; Aguilar Salinas, C.; Piché, M.E.; Auclair, A.; Poirier, P. Effect of bariatric surgery on heart failure. Expert. Rev. Cardiovasc. Ther. 2017, 15, 567–579. [Google Scholar] [CrossRef]
- Corrada, M.M.; Kawas, C.H.; Mozaffar, F.; Paganini-Hill, A. Association of body mass index and weight change with all-cause mortality in the elderly. Am. J. Epidemiol. 2006, 163, 938–949. [Google Scholar] [CrossRef]
- Ishida, Y.; Maeda, K.; Murotani, K.; Shimizu, A.; Ueshima, J.; Nagano, A.; Sonoi, N.; Inoue, T.; Mori, N. Association of body mass index and weight change with death in patients with advanced cancer. Nutrition 2023, 115, 112152. [Google Scholar] [CrossRef]
- Almuwaqqat, Z.; Hui, Q.; Liu, C.; Zhou, J.J.; Voight, B.F.; Ho, Y.L.; Posner, D.C.; Vassy, J.L.; Gaziano, J.M.; Cho, K.; et al. Long-Term Body Mass Index Variability and Adverse Cardiovascular Outcomes. JAMA Netw. Open 2024, 7, e243062. [Google Scholar] [CrossRef]
- Karolina, D.S.; Tavintharan, S.; Armugam, A.; Sepramaniam, S.; Pek, S.L.; Wong, M.T.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E2271–E2276. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Hu, G.; Jousilahti, P.; Antikainen, R.; Katzmarzyk, P.T.; Tuomilehto, J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation 2010, 121, 237–244. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Nicklas, B.J.; Brubaker, P.H.; Hundley, W.G.; Brinkley, T.E.; Upadhya, B.; Becton, J.T.; Nelson, M.D.; Chen, H.; Kitzman, D.W. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- (NCD-RisC), N.R.F.C. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Boyko, E.J.; Magliano, D.J.; Karuranga, S.; Piemonte, L.; Riley, P.; Saeedi, P.; Sun, H. (Eds.) International Diabetes Federation, 10th ed.; IDF Diabetes Atlas: Brussels, Belgium, 2021; p. 135. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuniga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Abebe, T.B.; Gebreyohannes, E.A.; Tefera, Y.G.; Abegaz, T.M. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: A retrospective cohort study. BMC Cardiovasc. Disord. 2016, 16, 232. [Google Scholar] [CrossRef]
- Abdin, A.; Bohm, M.; Shahim, B.; Karlstrom, P.; Kulenthiran, S.; Skouri, H.; Lund, L.H. Heart failure with preserved ejection fraction epidemiology, pathophysiology, diagnosis and treatment strategies. Int. J. Cardiol. 2024, 412, 132304. [Google Scholar] [CrossRef]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Prausmuller, S.; Weidenhammer, A.; Heitzinger, G.; Spinka, G.; Goliasch, G.; Arfsten, H.; Abdel Mawgoud, R.; Gabler, C.; Strunk, G.; Hengstenberg, C.; et al. Obesity in heart failure with preserved ejection fraction with and without diabetes: Risk factor or innocent bystander? Eur. J. Prev. Cardiol. 2023, 30, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Sanders, P.; Marwick, T.H. Subclinical Myocardial Impairment in Metabolic Diseases. JACC Cardiovasc. Imaging 2017, 10, 692–703. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Akat, K.M.; Moore-McGriff, D.; Morozov, P.; Brown, M.; Gogakos, T.; Correa Da Rosa, J.; Mihailovic, A.; Sauer, M.; Ji, R.; Ramarathnam, A.; et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc. Natl. Acad. Sci. USA 2014, 111, 11151–11156. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Marzocco, S.; De Gennaro, S.; Famiglietti, M.; Macina, G.; et al. Empagliflozin Improves the MicroRNA Signature of Endothelial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Diabetes. J. Pharmacol. Exp. Ther. 2023, 384, 116–122. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Bostick, B.P. Effects of Obesity on Cardiovascular Hemodynamics, Cardiac Morphology, and Ventricular Function. Curr. Obes. Rep. 2016, 5, 424–434. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, L.; Zhang, S.; Zhang, Y.; Lei, S. MicroRNA-1 suppresses cardiac hypertrophy by targeting nuclear factor of activated T cells cytoplasmic 3. Mol. Med. Rep. 2015, 12, 8282–8288. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, Y.; Ren, J. IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: Role of microRNA-1 and microRNA-133a. J. Cell Mol. Med. 2012, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Drawnel, F.M.; Wachten, D.; Molkentin, J.D.; Maillet, M.; Aronsen, J.M.; Swift, F.; Sjaastad, I.; Liu, N.; Catalucci, D.; Mikoshiba, K.; et al. Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J. Cell Biol. 2012, 199, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, X.; Yang, X.; Chang, J. NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1340–H1347. [Google Scholar] [CrossRef]
- Care, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Li, J.; Sha, Z.; Zhu, X.; Xu, W.; Yuan, W.; Yang, T.; Jin, B.; Yan, Y.; Chen, R.; Wang, S.; et al. Targeting miR-30d reverses pathological cardiac hypertrophy. EBioMedicine 2022, 81, 104108. [Google Scholar] [CrossRef]
- Huang, X.H.; Li, J.L.; Li, X.Y.; Wang, S.X.; Jiao, Z.H.; Li, S.Q.; Liu, J.; Ding, J. miR-208a in Cardiac Hypertrophy and Remodeling. Front. Cardiovasc. Med. 2021, 8, 773314. [Google Scholar] [CrossRef]
- Liu, F.; Li, N.; Long, B.; Fan, Y.Y.; Liu, C.Y.; Zhou, Q.Y.; Murtaza, I.; Wang, K.; Li, P.F. Cardiac hypertrophy is negatively regulated by miR-541. Cell Death Dis. 2014, 5, e1171. [Google Scholar] [CrossRef]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids 2018, 12, 254–266. [Google Scholar] [CrossRef]
- Seok, H.Y.; Chen, J.; Kataoka, M.; Huang, Z.P.; Ding, J.; Yan, J.; Hu, X.; Wang, D.Z. Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ. Res. 2014, 114, 1585–1595. [Google Scholar] [CrossRef]
- da Costa Martins, P.A.; Salic, K.; Gladka, M.M.; Armand, A.S.; Leptidis, S.; el Azzouzi, H.; Hansen, A.; Coenen-de Roo, C.J.; Bierhuizen, M.F.; van der Nagel, R.; et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat. Cell Biol. 2010, 12, 1220–1227. [Google Scholar] [CrossRef]
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 2013, 127, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Zhu, M.; Ma, Y.C.; Xiao, F.; Yu, X.; Xu, L.; Ma, L.Q.; Yang, J.; Dong, J.Z. MicroRNA-410-5p exacerbates high-fat diet-induced cardiac remodeling in mice in an endocrine fashion. Sci. Rep. 2018, 8, 8780. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xin, X.; Yu, H.; Bao, Y.; Jia, P.; Wu, N.; Jia, D. microRNA Expression Profiles in Myocardium of High-Fat Diet-Induced Obesity Rat. Diabetes Metab. Syndr. Obes. 2020, 13, 1147–1159. [Google Scholar] [CrossRef]
- Xin, X.; Duan, L.; Yang, H.; Yu, H.; Bao, Y.; Jia, D.; Wu, N.; Qiao, Y. miR-141-3p regulates saturated fatty acid-induced cardiomyocyte apoptosis through Notch1/PTEN/AKT pathway via targeting PSEN1. Environ. Toxicol. 2022, 37, 741–753. [Google Scholar] [CrossRef]
- Takasu, T. The Role of SGLT2 Inhibitor Ipragliflozin on Cardiac Hypertrophy and microRNA Expression Profiles in a Non-diabetic Rat Model of Cardiomyopathy. Biol. Pharm. Bull. 2022, 45, 1321–1331. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Garufi, G.; Pasarica, M.; Seyhan, A.A. Elevated and Correlated Expressions of miR-24, miR-30d, miR-146a, and SFRP-4 in Human Abdominal Adipose Tissue Play a Role in Adiposity and Insulin Resistance. Int. J. Endocrinol. 2018, 2018, 7351902. [Google Scholar] [CrossRef]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef]
- Morrison, K.R.; Solly, E.L.; Shemesh, T.; Psaltis, P.J.; Nicholls, S.J.; Brown, A.; Bursill, C.A.; Tan, J.T.M. Elevated HDL-bound miR-181c-5p level is associated with diabetic vascular complications in Australian Aboriginal people. Diabetologia 2021, 64, 1402–1411. [Google Scholar] [CrossRef]
- Solly, E.L.; Psaltis, P.J.; Bursill, C.A.; Tan, J.T.M. The Role of miR-181c in Mechanisms of Diabetes-Impaired Angiogenesis: An Emerging Therapeutic Target for Diabetic Vascular Complications. Front. Pharmacol. 2021, 12, 718679. [Google Scholar] [CrossRef]
- Frias Fde, T.; de Mendonca, M.; Martins, A.R.; Gindro, A.F.; Cogliati, B.; Curi, R.; Rodrigues, A.C. MyomiRs as Markers of Insulin Resistance and Decreased Myogenesis in Skeletal Muscle of Diet-Induced Obese Mice. Front. Endocrinol. 2016, 7, 76. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Mone, P.; Avvisato, R.; Varzideh, F.; De Gennaro, S.; Salemme, L.; Macina, G.; Kansakar, U.; Cioppa, A.; Frullone, S.; et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: Validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech. Ageing Dev. 2023, 212, 111818. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.; Kaur, P.; Ashok, D.; Kohr, M.; Biswas, R.; O’Rourke, B.; Steenbergen, C.; Das, S. Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity. J. Mol. Cell Cardiol. 2020, 144, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, M.; Liu, R.F.; Zhang, J.S.; Xu, M. Cardiac Hypertrophy is Positively Regulated by MicroRNA-24 in Rats. Chin. Med. J. 2018, 131, 1333–1341. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, K.; Wang, L.Y.; Jiang, J.G. The role of endothelial cell in cardiac hypertrophy: Focusing on angiogenesis and intercellular crosstalk. Biomed. Pharmacother. 2023, 163, 114799. [Google Scholar] [CrossRef]
- Walsh, K.; Shiojima, I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J. Clin. Investig. 2007, 117, 3176–3179. [Google Scholar] [CrossRef]
- Luxan, G.; Dimmeler, S. The vasculature: A therapeutic target in heart failure? Cardiovasc. Res. 2022, 118, 53–64. [Google Scholar] [CrossRef]
- Friehs, I.; Margossian, R.E.; Moran, A.M.; Cao-Danh, H.; Moses, M.A.; del Nido, P.J. Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis. Basic. Res. Cardiol. 2006, 101, 204–213. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Cooke, J.P. NO and angiogenesis. Atheroscler. Suppl. 2003, 4, 53–60. [Google Scholar] [CrossRef]
- Murohara, T.; Witzenbichler, B.; Spyridopoulos, I.; Asahara, T.; Ding, B.; Sullivan, A.; Losordo, D.W.; Isner, J.M. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.F.; Fishman, M.C.; et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef]
- Demirel-Yalciner, T.; Sozen, E.; Ozer, N.K. Endoplasmic Reticulum Stress and miRNA Impairment in Aging and Age-Related Diseases. Front. Aging 2021, 2, 790702. [Google Scholar] [CrossRef]
- Chinen, I.; Shimabukuro, M.; Yamakawa, K.; Higa, N.; Matsuzaki, T.; Noguchi, K.; Ueda, S.; Sakanashi, M.; Takasu, N. Vascular lipotoxicity: Endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology 2007, 148, 160–165. [Google Scholar] [CrossRef]
- Mao, Y.; Luo, W.; Zhang, L.; Wu, W.; Yuan, L.; Xu, H.; Song, J.; Fujiwara, K.; Abe, J.-i.; LeMaire, S.A.; et al. Correction to: STING-IRF3 Triggers Endothelial Inflammation in Response to Free Fatty Acid-Induced Mitochondrial Damage in Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e60. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Bullo, M.; Garcia-Lorda, P.; Megias, I.; Salas-Salvado, J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes. Res. 2003, 11, 525–531. [Google Scholar] [CrossRef]
- Yudkin, J.S. Adipose tissue, insulin action and vascular disease: Inflammatory signals. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. 3), S25–S28. [Google Scholar] [CrossRef]
- Tesauro, M.; Schinzari, F.; Rovella, V.; Melina, D.; Mores, N.; Barini, A.; Mettimano, M.; Lauro, D.; Iantorno, M.; Quon, M.J.; et al. Tumor necrosis factor-α antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care 2008, 31, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, C.; Fan, J.; Hou, Z.; Han, Y. MiR-221-3p targets Hif-1alpha to inhibit angiogenesis in heart failure. Lab. Investig. 2021, 101, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodriguez, A.; Assmann, T.S.; Alonso-Pedrero, L.; Azcona-Sanjulian, M.C.; Milagro, F.I.; Marti, A. Circulating miRNAs in girls with abdominal obesity: miR-221-3p as a biomarker of response to weight loss interventions. Pediatr. Obes. 2022, 17, e12910. [Google Scholar] [CrossRef]
- Vonhogen, I.G.C.; Mohseni, Z.; Winkens, B.; Xiao, K.; Thum, T.; Calore, M.; da Costa Martins, P.A.; de Windt, L.J.; Spaanderman, M.E.A.; Ghossein-Doha, C. Circulating miR-216a as a biomarker of metabolic alterations and obesity in women. Noncoding RNA Res. 2020, 5, 144–152. [Google Scholar] [CrossRef]
- Juni, R.P.; Kocken, J.M.M.; Abreu, R.C.; Ottaviani, L.; Davalan, T.; Duygu, B.; Poels, E.M.; Vasilevich, A.; Hegenbarth, J.C.; Appari, M.; et al. MicroRNA-216a is essential for cardiac angiogenesis. Mol. Ther. 2023, 31, 1807–1828. [Google Scholar] [CrossRef]
- Reddy, S.; Hu, D.Q.; Zhao, M.; Ichimura, S.; Barnes, E.A.; Cornfield, D.N.; Alejandre Alcazar, M.A.; Spiekerkoetter, E.; Fajardo, G.; Bernstein, D. MicroRNA-34a-Dependent Attenuation of Angiogenesis in Right Ventricular Failure. J. Am. Heart Assoc. 2024, 13, e029427. [Google Scholar] [CrossRef]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ba-Ssalamah, A.; de Gier, C.; Valent, I.; Item, C.B.; Greber-Platzer, S.; Zeyda, M. Circulating microRNAs 34a, 122, and 192 are linked to obesity-associated inflammation and metabolic disease in pediatric patients. Int. J. Obes. 2021, 45, 1763–1772. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Nie, X.; Yin, Z.; Zhao, Y.; Zhang, X.; Yuan, S.; Li, Y.; Chen, C.; Wang, D.W. MiR-665 aggravates heart failure via suppressing CD34-mediated coronary microvessel angiogenesis. Aging 2018, 10, 2459–2479. [Google Scholar] [CrossRef]
- Yu, Y.; Tian, T.; Tan, S.; Wu, P.; Guo, Y.; Li, M.; Huang, M. MicroRNA-665-3p exacerbates nonalcoholic fatty liver disease in mice. Bioengineered 2022, 13, 2927–2942. [Google Scholar] [CrossRef]
- Kassan, M.; Vikram, A.; Kim, Y.R.; Li, Q.; Kassan, A.; Patel, H.H.; Kumar, S.; Gabani, M.; Liu, J.; Jacobs, J.S.; et al. Sirtuin1 protects endothelial Caveolin-1 expression and preserves endothelial function via suppressing miR-204 and endoplasmic reticulum stress. Sci. Rep. 2017, 7, 42265. [Google Scholar] [CrossRef]
- Cheng, J.P.; Mendoza-Topaz, C.; Howard, G.; Chadwick, J.; Shvets, E.; Cowburn, A.S.; Dunmore, B.J.; Crosby, A.; Morrell, N.W.; Nichols, B.J. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J. Cell Biol. 2015, 211, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Kim, Y.R.; Jacobs, J.S.; Yoon, J.Y.; Li, Q.; Cai, A.; Shankaiahgari, H.; London, B.; Irani, K.; Vikram, A. The microRNA-204-5p inhibits APJ signalling and confers resistance to cardiac hypertrophy and dysfunction. Clin. Transl. Med. 2022, 12, e693. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, G.; Abbi, R.; Quaini, F.; Kajstura, J.; Cheng, W.; Nitahara, J.A.; Quaini, E.; Di Loreto, C.; Beltrami, C.A.; Krajewski, S.; et al. Apoptosis in the failing human heart. N. Engl. J. Med. 1997, 336, 1131–1141. [Google Scholar] [CrossRef]
- Haudek, S.B.; Taffet, G.E.; Schneider, M.D.; Mann, D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Investig. 2007, 117, 2692–2701. [Google Scholar] [CrossRef]
- Bray, G.A.; York, D.A. Clinical review 90: Leptin and clinical medicine: A new piece in the puzzle of obesity. J. Clin. Endocrinol. Metab. 1997, 82, 2771–2776. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Lee, S.D.; Tzang, B.S.; Kuo, W.W.; Lin, Y.M.; Yang, A.L.; Chen, S.H.; Tsai, F.J.; Wu, F.L.; Lu, M.C.; Huang, C.Y. Cardiac fas receptor-dependent apoptotic pathway in obese Zucker rats. Obesity 2007, 15, 2407–2415. [Google Scholar] [CrossRef]
- Ing, D.J.; Zang, J.; Dzau, V.J.; Webster, K.A.; Bishopric, N.H. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ. Res. 1999, 84, 21–33. [Google Scholar] [CrossRef]
- Aonuma, T.; Moukette, B.; Kawaguchi, S.; Barupala, N.P.; Sepulveda, M.N.; Corr, C.; Tang, Y.; Liangpunsakul, S.; Payne, R.M.; Willis, M.S.; et al. Cardiomyocyte microRNA-150 confers cardiac protection and directly represses proapoptotic small proline-rich protein 1A. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Pradervand, S.; Yasukawa, H.; Muller, O.G.; Kjekshus, H.; Nakamura, T.; St Amand, T.R.; Yajima, T.; Matsumura, K.; Duplain, H.; Iwatate, M.; et al. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004, 23, 4517–4525. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhang, Y.; Zhu, X.; Zhang, R.; Guan, L.; Tang, Q.; Jiang, H.; Huang, C.; Huang, H. MicroRNA-150 Protects Against Pressure Overload-Induced Cardiac Hypertrophy. J. Cell Biochem. 2015, 116, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Conserva, F.; Passantino, A.; Iacoviello, M.; Lagioia, R.; Gesualdo, L. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: A genome-wide prospective study. J. Heart Lung Transplant. 2017, 36, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Meese, E.; Saleh, M.A.; Keller, A.; Abdul-Khaliq, H.; Raedle-Hurst, T. Micro-RNA 150-5p predicts overt heart failure in patients with univentricular hearts. PLoS ONE 2019, 14, e0223606. [Google Scholar] [CrossRef]
- Nunez Lopez, Y.O.; Garufi, G.; Seyhan, A.A. Altered levels of circulating cytokines and microRNAs in lean and obese individuals with prediabetes and type 2 diabetes. Mol. Biosyst. 2016, 13, 106–121. [Google Scholar] [CrossRef]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Lin, H.; Mercer, K.E.; Ou, X.; Mansfield, K.; Buchmann, R.; Borsheim, E.; Tas, E. Circulating microRNAs Are Associated With Metabolic Markers in Adolescents With Hepatosteatosis. Front. Endocrinol. 2022, 13, 856973. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Hastings, M.H.; Xiao, C.; Damilano, F.; Platt, C.; Lerchenmuller, C.; Zhu, H.; Wei, X.P.; Yeri, A.; et al. miR-222 inhibits pathological cardiac hypertrophy and heart failure. Cardiovasc. Res. 2024, 120, 262–272. [Google Scholar] [CrossRef]
- Mandl, A.; Huong Pham, L.; Toth, K.; Zambetti, G.; Erhardt, P. Puma deletion delays cardiac dysfunction in murine heart failure models through attenuation of apoptosis. Circulation 2011, 124, 31–39. [Google Scholar] [CrossRef]
- Verjans, R.; Peters, T.; Beaumont, F.J.; van Leeuwen, R.; van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload-Induced Heart Failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef]
- Ortega, F.J.; Mercader, J.M.; Catalan, V.; Moreno-Navarrete, J.M.; Pueyo, N.; Sabater, M.; Gomez-Ambrosi, J.; Anglada, R.; Fernandez-Formoso, J.A.; Ricart, W.; et al. Targeting the circulating microRNA signature of obesity. Clin. Chem. 2013, 59, 781–792. [Google Scholar] [CrossRef]
- Hess, A.L.; Larsen, L.H.; Udesen, P.B.; Sanz, Y.; Larsen, T.M.; Dalgaard, L.T. Levels of Circulating miR-122 are Associated with Weight Loss and Metabolic Syndrome. Obesity 2020, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mao, S.; Liu, X.; Li, S.; Zhou, H.; Gu, Y.; Liu, W.; Fu, L.; Liao, C.; Wang, P. MiR-125b inhibits cardiomyocyte apoptosis by targeting BAK1 in heart failure. Mol. Med. 2021, 27, 72. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Deswal, A.; Bozkurt, B.; Seta, Y.; Parilti-Eiswirth, S.; Hayes, F.A.; Blosch, C.; Mann, D.L. Safety and efficacy of a soluble P75 tumor necrosis factor receptor (Enbrel, etanercept) in patients with advanced heart failure. Circulation 1999, 99, 3224–3226. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 2002, 86, 123–130. [Google Scholar] [CrossRef]
- Gullestad, L.; Aass, H.; Fjeld, J.G.; Wikeby, L.; Andreassen, A.K.; Ihlen, H.; Simonsen, S.; Kjekshus, J.; Nitter-Hauge, S.; Ueland, T.; et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 2001, 103, 220–225. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Copier, C.U.; Leon, L.; Fernandez, M.; Contador, D.; Calligaris, S.D. Circulating miR-19b and miR-181b are potential biomarkers for diabetic cardiomyopathy. Sci. Rep. 2017, 7, 13514. [Google Scholar] [CrossRef]
- Yang, H.; Shan, L.; Gao, Y.; Li, L.; Xu, G.; Wang, B.; Yin, X.; Gao, C.; Liu, J.; Yang, W. MicroRNA-181b Serves as a Circulating Biomarker and Regulates Inflammation in Heart Failure. Dis. Markers 2021, 2021, 4572282. [Google Scholar] [CrossRef]
- Ling, L.; Zhi, L.; Wang, H.; Deng, Y.; Gu, C. MicroRNA-181b Inhibits Inflammatory Response and Reduces Myocardial Injury in Sepsis by Downregulating HMGB1. Inflammation 2021, 44, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Haemmig, S.; Zhou, H.; Perez-Cremades, D.; Sun, X.; Chen, L.; Li, J.; Haneo-Mejia, J.; Yang, T.; Hollan, I.; et al. Methotrexate attenuates vascular inflammation through an adenosine-microRNA-dependent pathway. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef]
- Tryggestad, J.B.; Teague, A.M.; Sparling, D.P.; Jiang, S.; Chernausek, S.D. Macrophage-Derived microRNA-155 Increases in Obesity and Influences Adipocyte Metabolism by Targeting Peroxisome Proliferator-Activated Receptor γ. Obesity 2019, 27, 1856–1864. [Google Scholar] [CrossRef]
- Virtue, A.; Johnson, C.; Lopez-Pastraña, J.; Shao, Y.; Fu, H.; Li, X.; Li, Y.F.; Yin, Y.; Mai, J.; Rizzo, V.; et al. MicroRNA-155 Deficiency Leads to Decreased Atherosclerosis, Increased White Adipose Tissue Obesity, and Non-alcoholic Fatty Liver Disease: A NOVEL MOUSE MODEL OF OBESITY PARADOX. J. Biol. Chem. 2017, 292, 1267–1287. [Google Scholar] [CrossRef]
- Greco, S.; Fasanaro, P.; Castelvecchio, S.; D’Alessandra, Y.; Arcelli, D.; Di Donato, M.; Malavazos, A.; Capogrossi, M.C.; Menicanti, L.; Martelli, F. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes 2012, 61, 1633–1641. [Google Scholar] [CrossRef]
- Barsanti, C.; Trivella, M.G.; D’Aurizio, R.; El Baroudi, M.; Baumgart, M.; Groth, M.; Caruso, R.; Verde, A.; Botta, L.; Cozzi, L.; et al. Differential regulation of microRNAs in end-stage failing hearts is associated with left ventricular assist device unloading. Biomed. Res. Int. 2015, 2015, 592512. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, L.; Zhang, J.; Liu, N.; Zhao, P. A novel identified circular RNA, circSnap47, promotes heart failure progression via regulation of miR-223-3p/MAPK axis. Mol. Cell Biochem. 2023, 478, 459–469. [Google Scholar] [CrossRef]
- Fukao, T.; Fukuda, Y.; Kiga, K.; Sharif, J.; Hino, K.; Enomoto, Y.; Kawamura, A.; Nakamura, K.; Takeuchi, T.; Tanabe, M. An evolutionarily conserved mechanism for microRNA-223 expression revealed by microRNA gene profiling. Cell 2007, 129, 617–631. [Google Scholar] [CrossRef]
- Etzrodt, M.; Ahmed, N.; Hoppe, P.S.; Loeffler, D.; Skylaki, S.; Hilsenbeck, O.; Kokkaliaris, K.D.; Kaltenbach, H.M.; Stelling, J.; Nerlov, C.; et al. Inflammatory signals directly instruct PU.1 in HSCs via TNF. Blood 2019, 133, 816–819. [Google Scholar] [CrossRef]
- Poli, V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998, 273, 29279–29282. [Google Scholar] [CrossRef] [PubMed]
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962. [Google Scholar] [CrossRef] [PubMed]
- Macartney-Coxson, D.; Danielson, K.; Clapham, J.; Benton, M.C.; Johnston, A.; Jones, A.; Shaw, O.; Hagan, R.D.; Hoffman, E.P.; Hayes, M.; et al. MicroRNA Profiling in Adipose Before and After Weight Loss Highlights the Role of miR-223-3p and the NLRP3 Inflammasome. Obesity 2020, 28, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Guo, M.; Hao, M. MiR-223-3p affects myocardial inflammation and apoptosis following myocardial infarction via targeting FBXW7. J. Thorac. Dis. 2022, 14, 1146–1156. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Du, J.Y.; Wang, D.D.; Yang, X. miRNA-130a improves cardiac function by down-regulating TNF-α expression in a rat model of heart failure. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8454–8461. [Google Scholar] [CrossRef]
- Takatani, R.; Yoshioka, Y.; Takahashi, T.; Watanabe, M.; Hisada, A.; Yamamoto, M.; Sakurai, K.; Takatani, T.; Shimojo, N.; Hamada, H.; et al. Investigation of umbilical cord serum miRNAs associated with childhood obesity: A pilot study from a birth cohort study. J. Diabetes Investig. 2022, 13, 1740–1744. [Google Scholar] [CrossRef]
- He, J.; Lu, Y.; Song, X.; Gong, X.; Li, Y. Inhibition of microRNA-146a attenuated heart failure in myocardial infarction rats. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Nelson, M.C.; Lee, S.H.; Voth, W.; Alexander, M.; Hu, R.; Wallace, J.; Petersen, C.; Panic, V.; Villanueva, C.J.; et al. Anti-inflammatory microRNA-146a protects mice from diet-induced metabolic disease. PLoS Genet. 2019, 15, e1007970. [Google Scholar] [CrossRef]
- Sletten, A.C.; Peterson, L.R.; Schaffer, J.E. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J. Intern. Med. 2018, 284, 478–491. [Google Scholar] [CrossRef]
- Trivedi, P.C.; Bartlett, J.J.; Perez, L.J.; Brunt, K.R.; Legare, J.F.; Hassan, A.; Kienesberger, P.C.; Pulinilkunnil, T. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim. Biophys. Acta 2016, 1861, 1893–1910. [Google Scholar] [CrossRef]
- Cerf, M.E. Cardiac Glucolipotoxicity and Cardiovascular Outcomes. Medicina 2018, 54. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, A.; Fisman, E.Z. Impaired glucose metabolism in patients with heart failure: Pathophysiology and possible treatment strategies. Am. J. Cardiovasc. Drugs 2004, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Akhmedov, A.; Melina, G.; Mohammed, S.A.; Othman, A.; Ambrosini, S.; Wijnen, W.J.; Sada, L.; Ciavarella, G.M.; Liberale, L.; et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur. Heart J. 2019, 40, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288. [Google Scholar] [CrossRef]
- Lu, G.; Gao, H.; Dong, Z.; Jiang, S.; Hu, R.; Wang, C. Change Profiles and Functional Targets of MicroRNAs in Type 2 Diabetes Mellitus Patients with Obesity. Diabetes Metab. J. 2023, 47, 559–570. [Google Scholar] [CrossRef]
- Chang, W.; Fa, H.; Xiao, D.; Wang, J. MicroRNA-184 alleviates insulin resistance in cardiac myocytes and high fat diet-induced cardiac dysfunction in mice through the LPP3/DAG pathway. Mol. Cell Endocrinol. 2020, 508, 110793. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Wang, S.Q.; Chen, Y.; Fu, L.Y.; Xu, Y.N.; Li, L.; Tao, L.; Shen, X.C. MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases. Front. Pharmacol. 2021, 12, 663322. [Google Scholar] [CrossRef]
- Murri, M.; El Azzouzi, H. MicroRNAs as regulators of mitochondrial dysfunction and obesity. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H291–H302. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef]
- Busk, P.K.; Cirera, S. MicroRNA profiling in early hypertrophic growth of the left ventricle in rats. Biochem. Biophys. Res. Commun. 2010, 396, 989–993. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Liao, X.; Castillero, E.; Kennel, P.J.; Brunjes, D.L.; Franz, M.; Mobius-Winkler, S.; Drosatos, K.; George, I.; et al. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation 2018, 137, 2052–2067. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Jeong, H.J.; Park, S.Y.; Lee, W. Saturated fatty acid-induced miR-195 impairs insulin signaling and glycogen metabolism in HepG2 cells. FEBS Lett. 2014, 588, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Boersma, G.J.; Johnson, M.D.; Velasquez, F.C.; Dunkerly-Eyring, B.; O’Brien, S.; Yamaguchi, A.; Steenbergen, C.; Tamashiro, K.L.K.; Das, S. Role of miR-181c in Diet-induced obesity through regulation of lipid synthesis in liver. PLoS ONE 2021, 16, e0256973. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Pan, Y.; Zhou, Y. Exosomal microRNA-194 causes cardiac injury and mitochondrial dysfunction in obese mice. Biochem. Biophys. Res. Commun. 2018, 503, 3174–3179. [Google Scholar] [CrossRef]
- Wang, J.; An, Y.; Lin, J.; Tang, G. MicroRNA-194 inhibits isoproterenol-induced chronic cardiac hypertrophy via targeting CnA/NFATc2 signaling in H9c2 cells. Ann. Transl. Med. 2022, 10, 780. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, P.; Liu, J.; Xie, X. Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondrial ADP-ribosylation factor-like 2. Clin. Sci. 2019, 133, 1871–1881. [Google Scholar] [CrossRef]
- Das, S.; Bedja, D.; Campbell, N.; Dunkerly, B.; Chenna, V.; Maitra, A.; Steenbergen, C. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS ONE 2014, 9, e96820. [Google Scholar] [CrossRef]
- Li, F.; Zhang, K.; Xu, T.; Du, W.; Yu, B.; Liu, Y.; Nie, H. Exosomal microRNA-29a mediates cardiac dysfunction and mitochondrial inactivity in obesity-related cardiomyopathy. Endocrine 2019, 63, 480–488. [Google Scholar] [CrossRef]
- Caravia, X.M.; Fanjul, V.; Oliver, E.; Roiz-Valle, D.; Moran-Alvarez, A.; Desdin-Mico, G.; Mittelbrunn, M.; Cabo, R.; Vega, J.A.; Rodriguez, F.; et al. The microRNA-29/PGC1alpha regulatory axis is critical for metabolic control of cardiac function. PLoS Biol. 2018, 16, e2006247. [Google Scholar] [CrossRef]
- Liang, J.; Liu, C.; Qiao, A.; Cui, Y.; Zhang, H.; Cui, A.; Zhang, S.; Yang, Y.; Xiao, X.; Chen, Y.; et al. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. J. Hepatol. 2013, 58, 535–542. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Somlo, G.; Yu, Y.; Palomares, M.R.; Li, A.X.; Zhou, W.; Chow, A.; Yen, Y.; Rossi, J.J.; Gao, H.; et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J. Transl. Med. 2012, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Bassot, A.; Dragic, H.; Haddad, S.A.; Moindrot, L.; Odouard, S.; Corlazzoli, F.; Marinari, E.; Bomane, A.; Brassens, A.; Marteyn, A.; et al. Identification of a miRNA multi-targeting therapeutic strategy in glioblastoma. Cell Death Dis. 2023, 14, 630. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, W.; Lu, L.; Han, R. MicroRNAs with Multiple Targets of Immune Checkpoints, as a Potential Sensitizer for Immune Checkpoint Inhibitors in Breast Cancer Treatment. Cancers 2023, 15. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, Y.E.; Chen, J.; Ma, P.X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 2016, 7, 10376. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Trivedi, R. 3D Printed Bioconstructs: Regenerative Modulation for Genetic Expression. Stem Cell Rev. Rep. 2021, 17, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Fromm, B.; Patil, A.H.; Halushka, M.K. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2022, 387, 1912. [Google Scholar] [CrossRef]
- Su, Y.; Sun, Y.; Tang, Y.; Li, H.; Wang, X.; Pan, X.; Liu, W.; Zhang, X.; Zhang, F.; Xu, Y.; et al. Circulating miR-19b-3p as a Novel Prognostic Biomarker for Acute Heart Failure. J. Am. Heart Assoc. 2021, 10, e022304. [Google Scholar] [CrossRef]
- Wang, H.; Cai, J. The role of microRNAs in heart failure. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2019–2030. [Google Scholar] [CrossRef]
- Horita, M.; Farquharson, C.; Stephen, L.A. The role of miR-29 family in disease. J. Cell Biochem. 2021, 122, 696–715. [Google Scholar] [CrossRef]
- Taubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Bajaj, A.; Brousseau, M.E.; Taub, P.R. Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran. J. Am. Heart Assoc. 2024, 13, e032031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).