Integrated Transcriptomic and Metabolomic Analyses of the Response of Lutein Accumulation in Marigold Petals to Light Intensity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Intensity Treatment
2.2. Paraffin Section
2.3. RNA Extraction and Quantitative Real-Time PCR
2.4. Determination of Total Lutein Content
2.5. Transcriptome Analysis
2.6. Targeted Metabolomic Analysis of Carotenoids
2.7. Transcriptomic and Metabolomic Integrative Analysis
3. Results
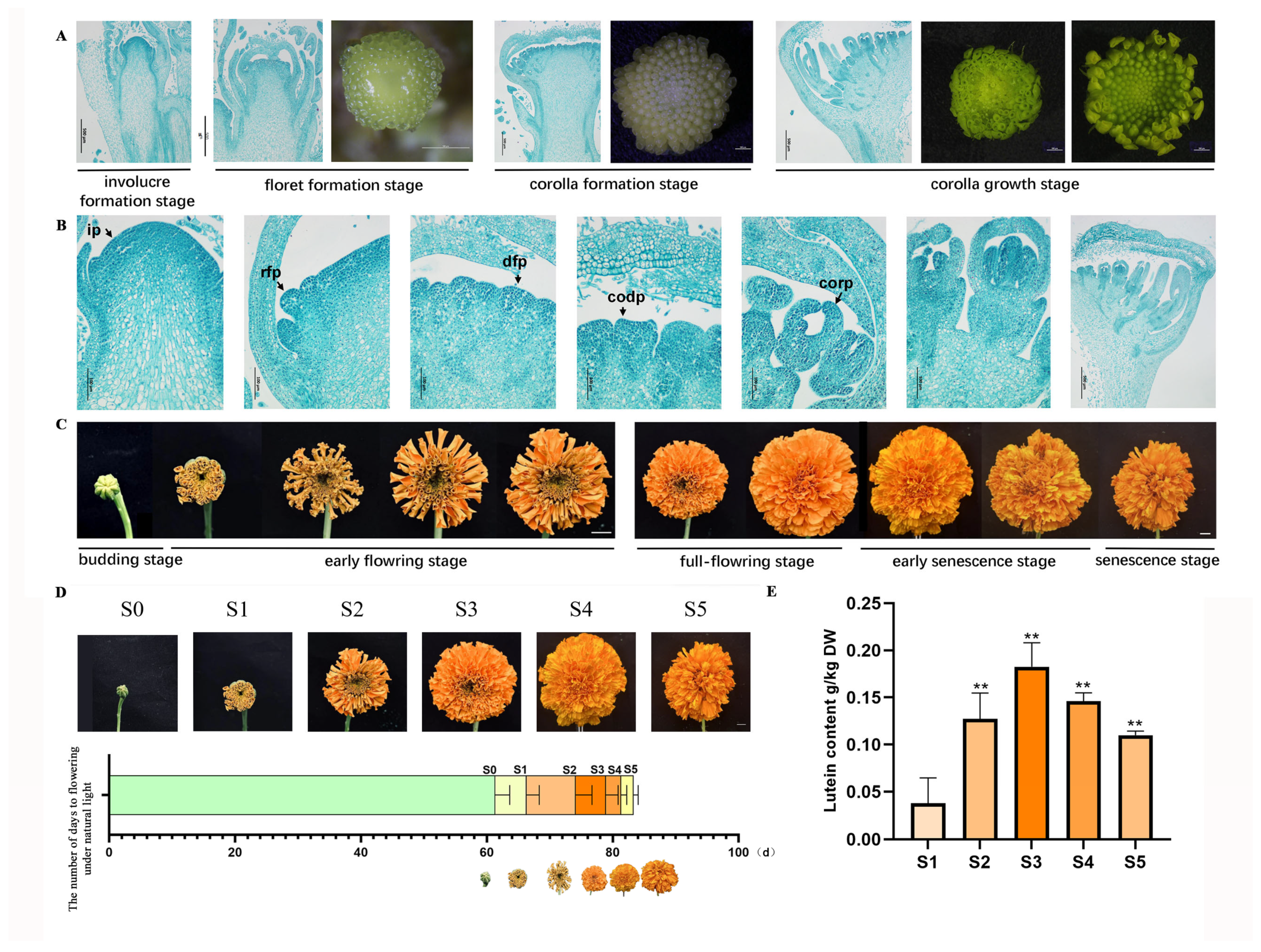
3.1. Flower Organ Development of Marigold
3.2. Marigolds Reached Their Highest Lutein Content at the Full-Flowering Stage, S3
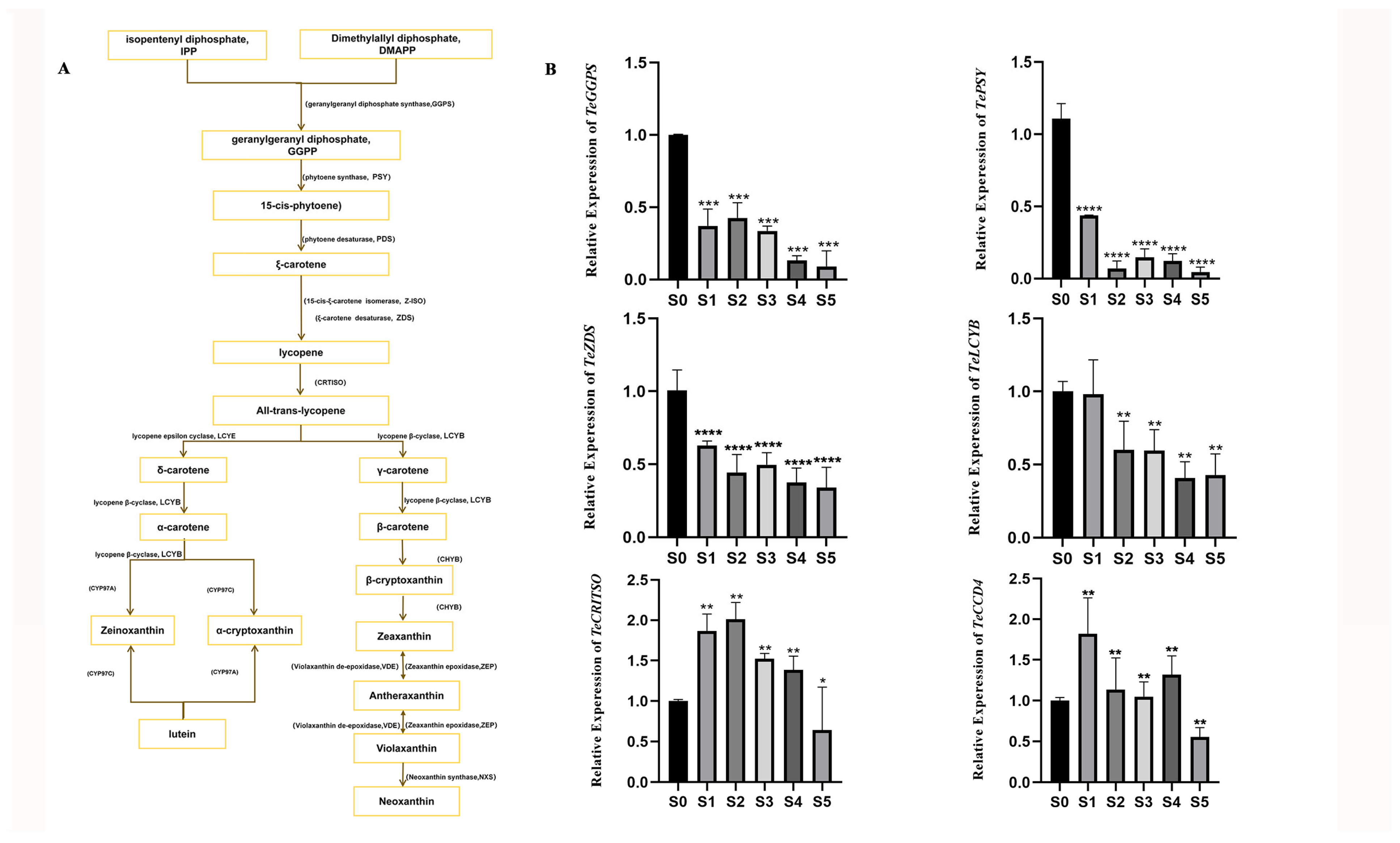
3.3. Expression Levels of Lutein Biosynthesis Pathway Genes at Different Developmental Stages of Marigold
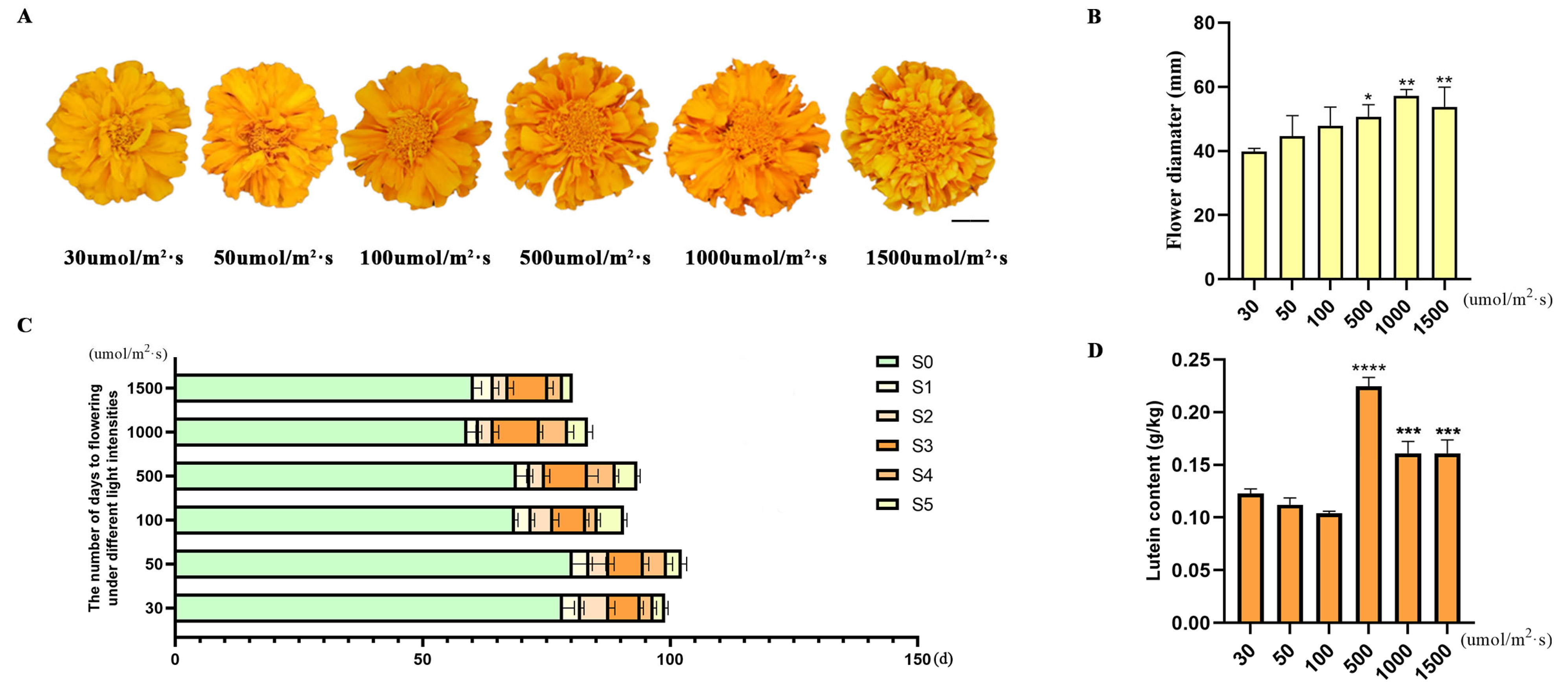
3.4. Determination of Lutein Content Under Different Light Intensity Treatments
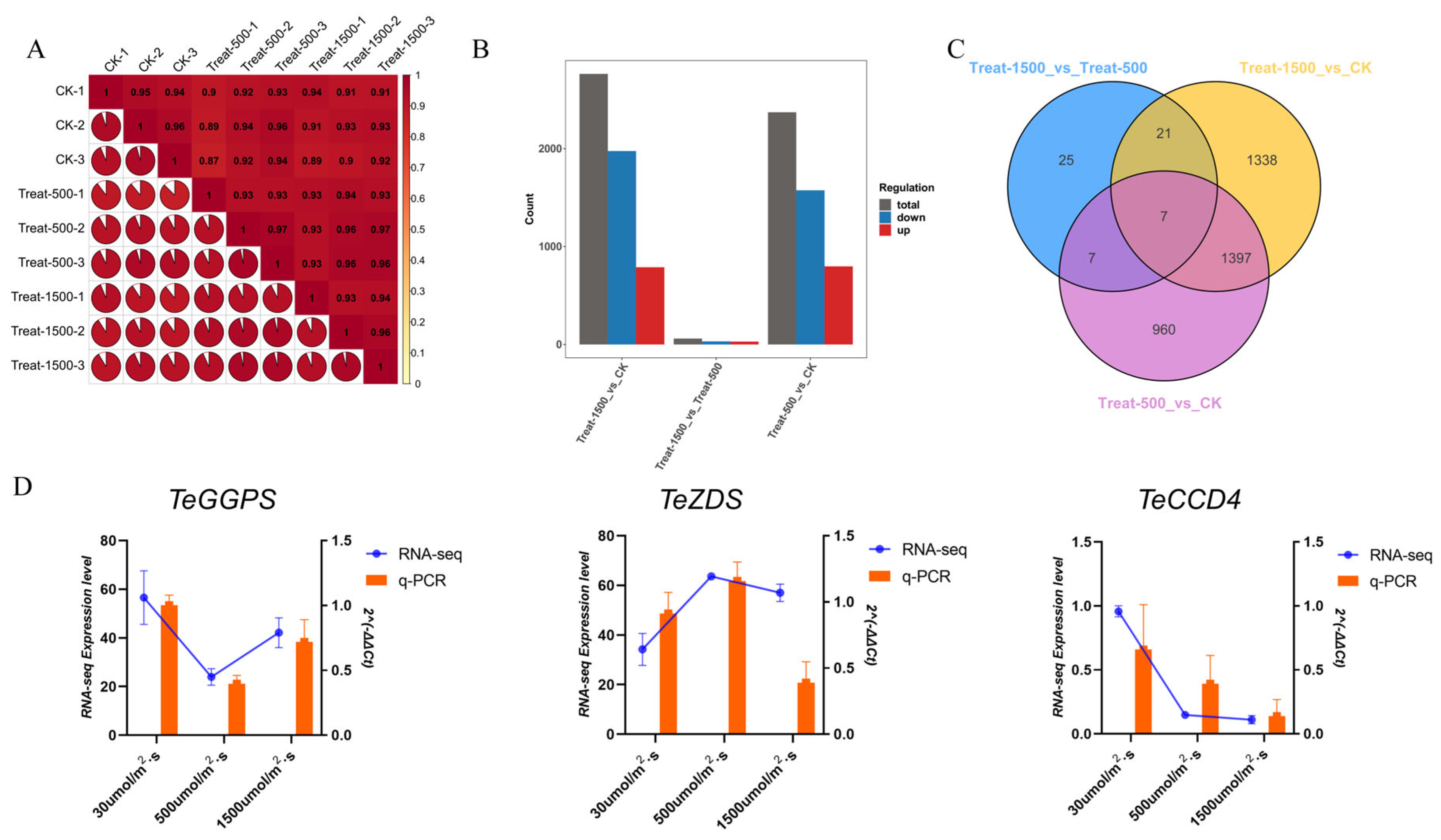
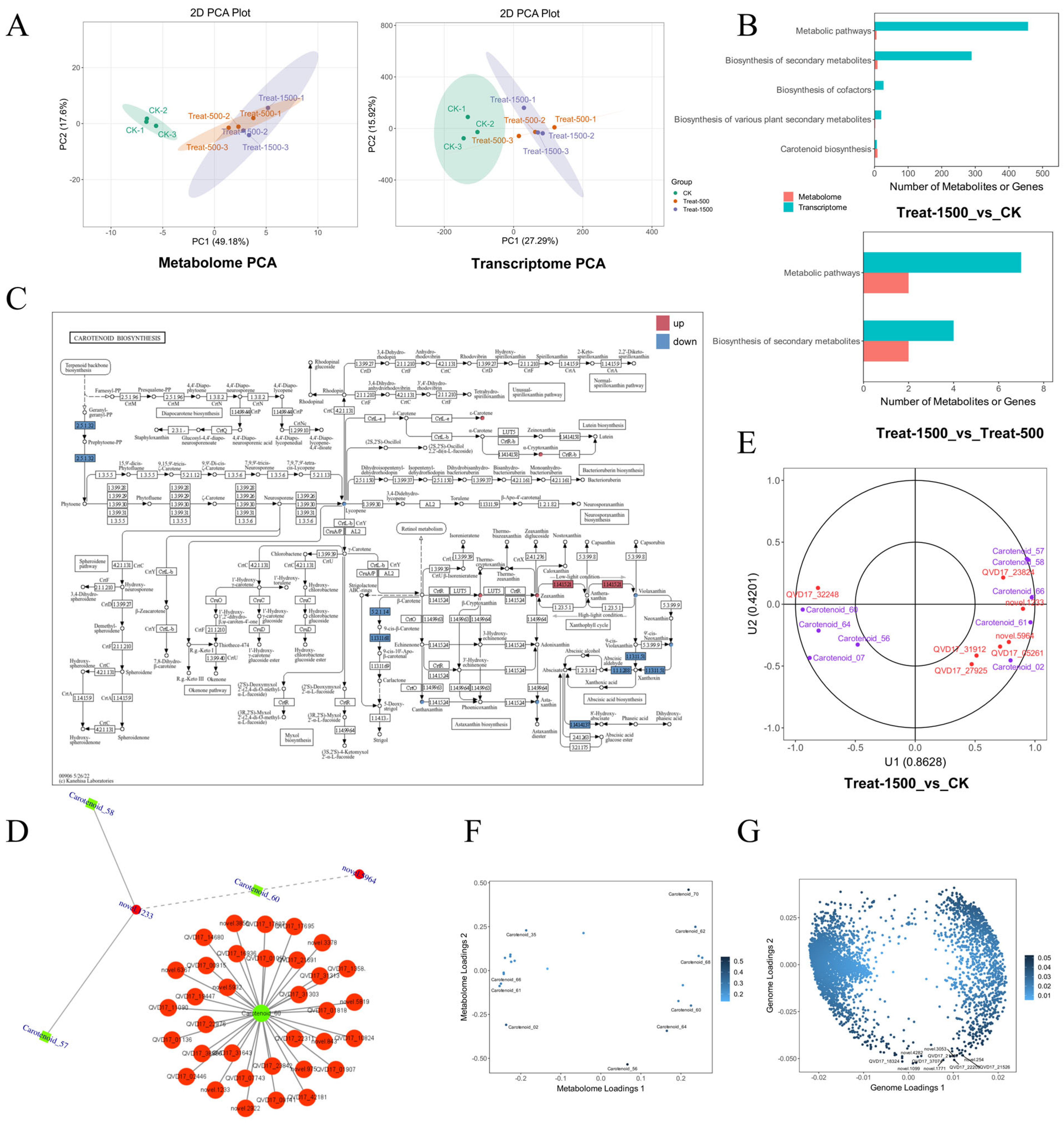
3.5. Transcriptome Analysis of Lutein Response to Different Light Intensities
3.5.1. Overview of Transcriptome Data
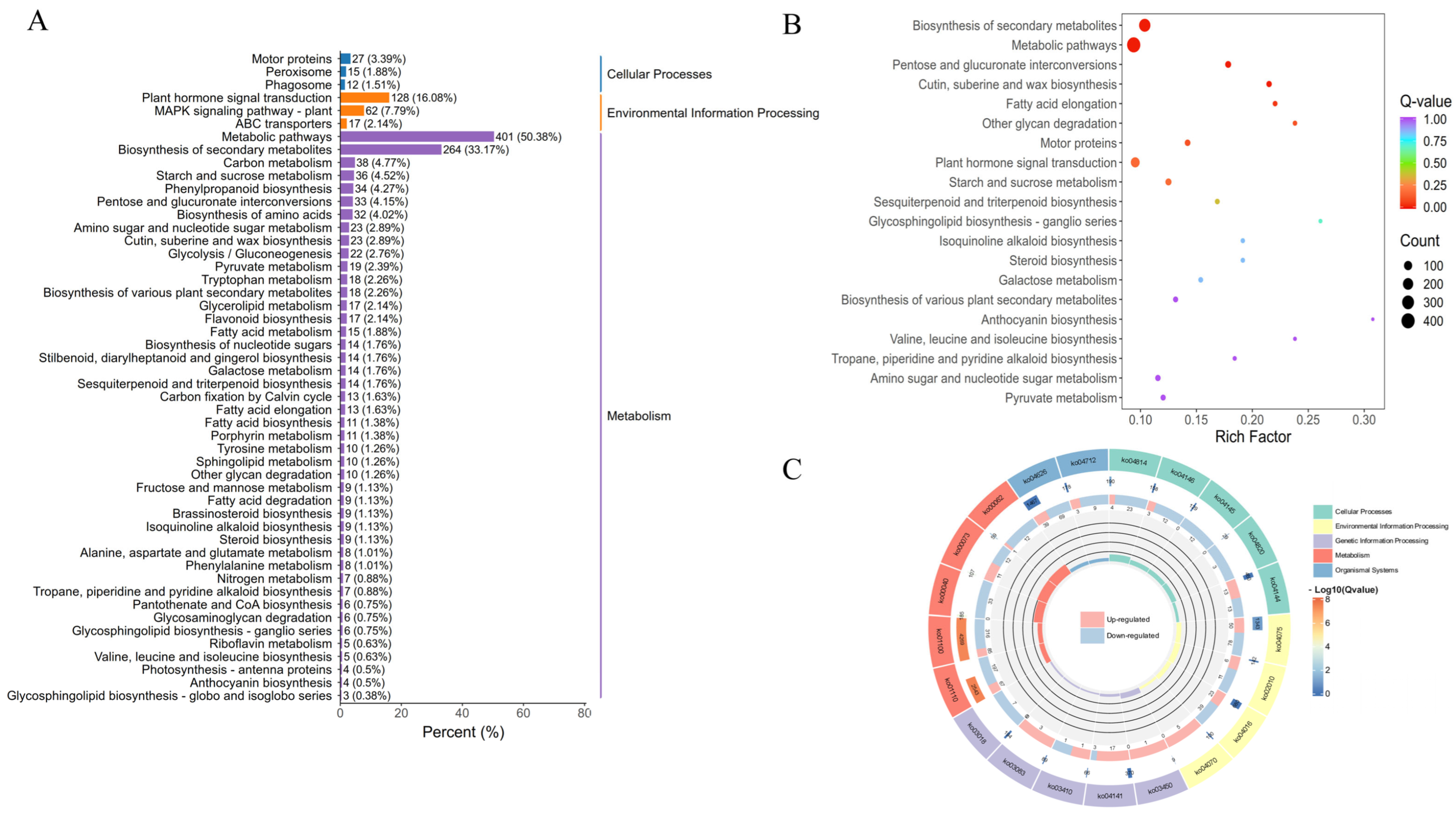
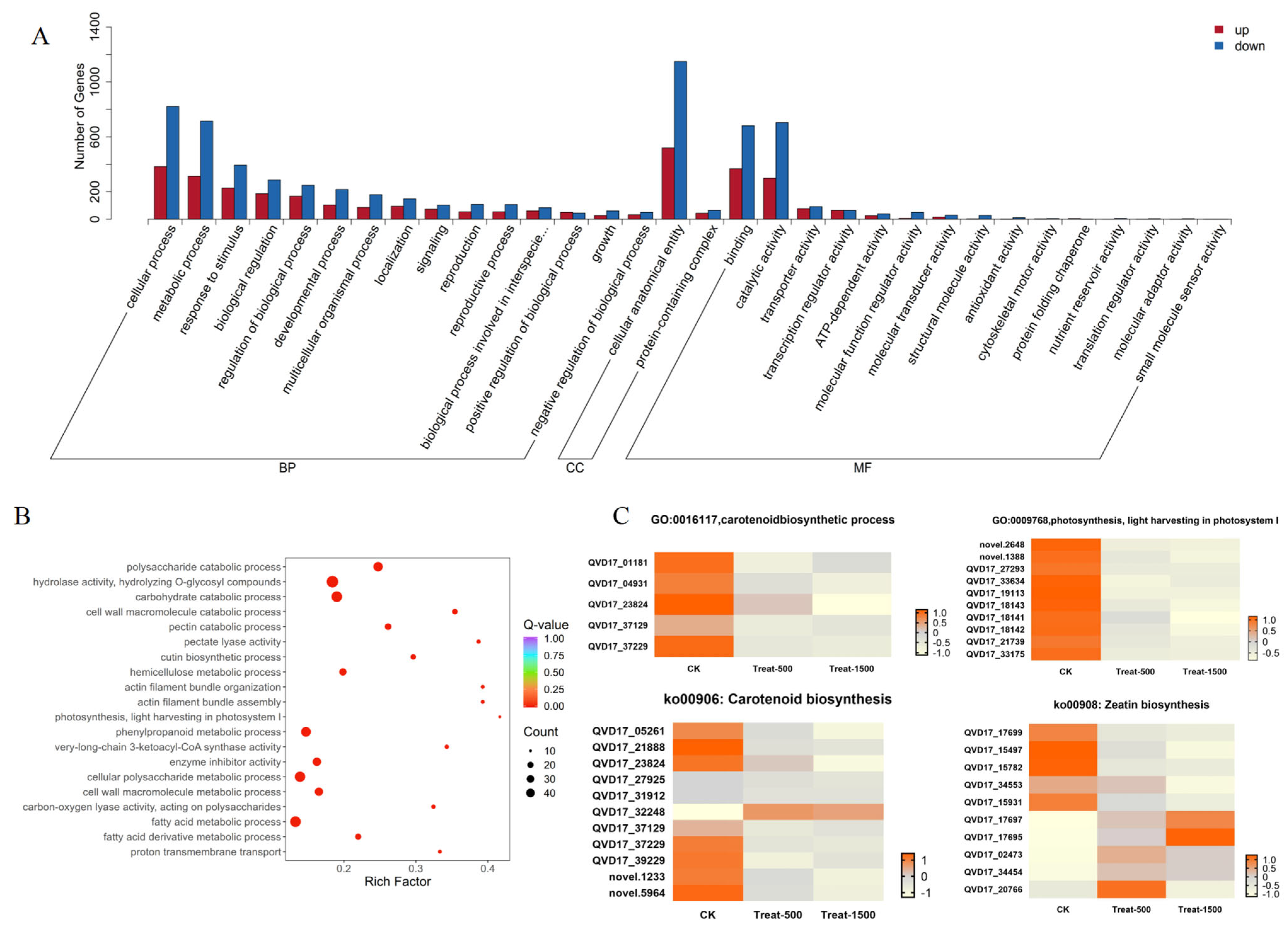
3.5.2. DEGs Enrichment Analysis
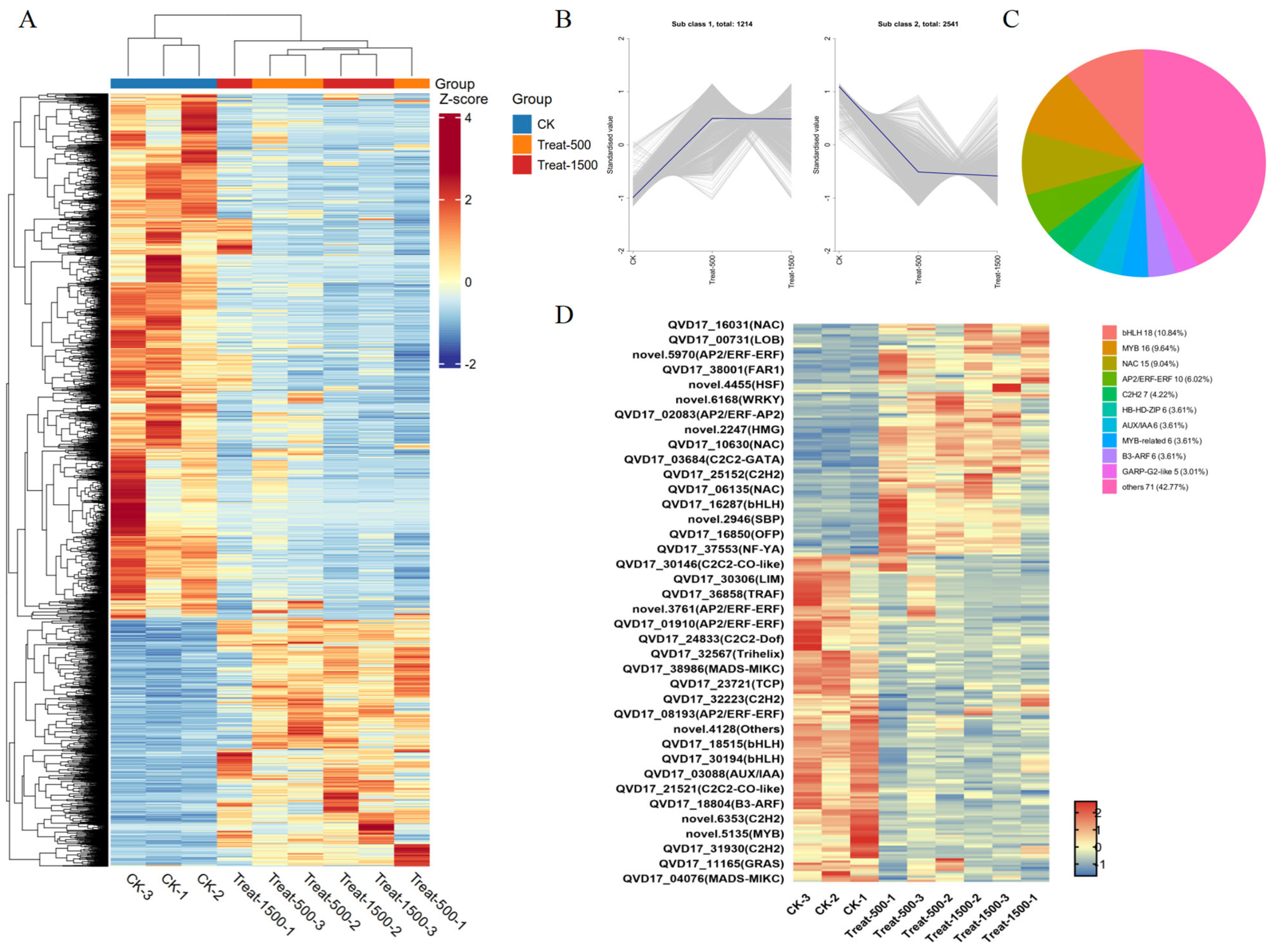
3.5.3. Transcription Factor Analysis of Differentially Expressed Genes
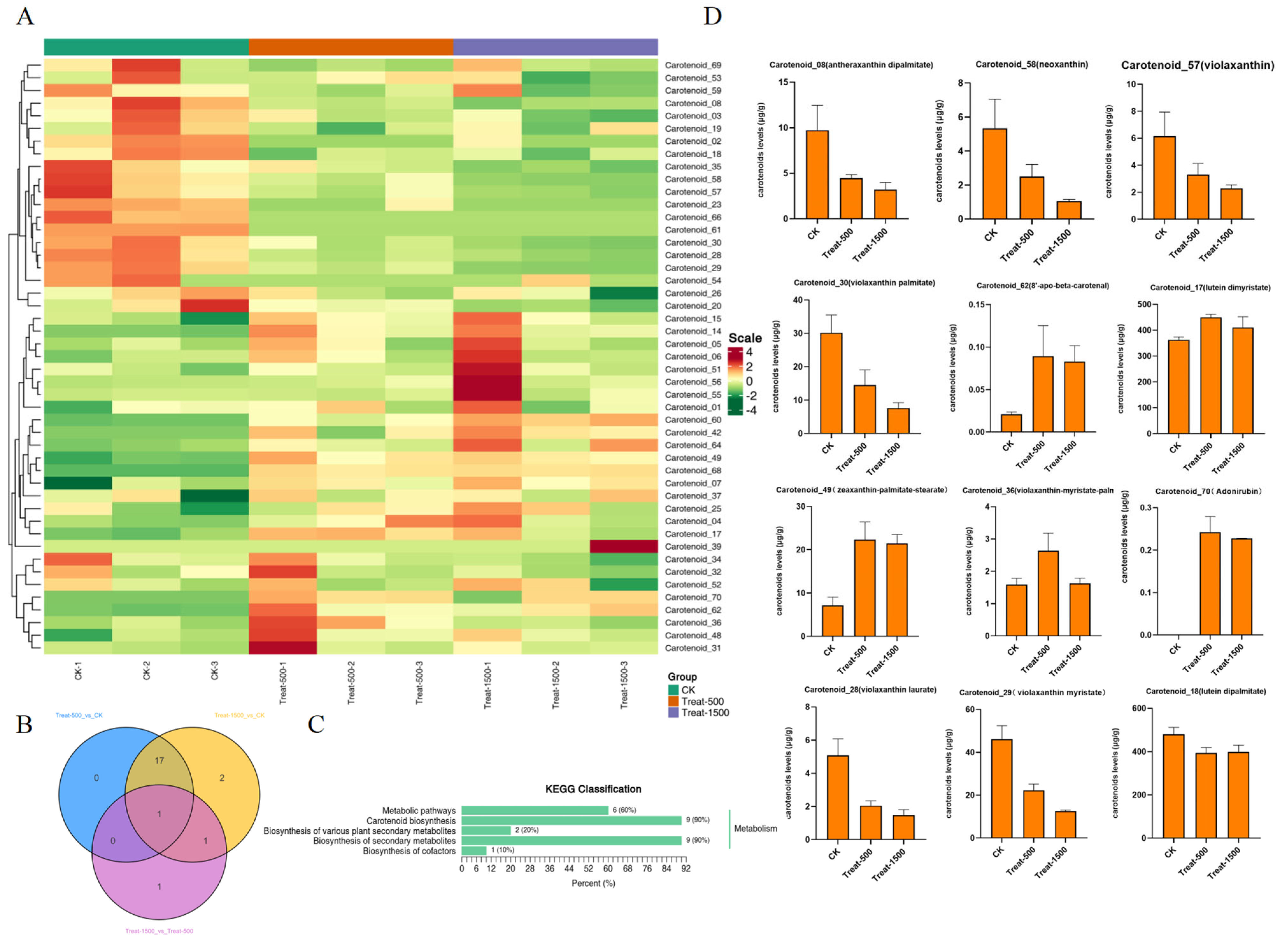
3.6. Metabolomic Analysis of Lutein in Response to Different Light Intensities
3.7. Integrated Analysis of Transcriptome and Metabolome
4. Discussion
4.1. The Full-Flowering Stage (S3) Is a Critical Period for Lutein Accumulation
4.2. 500 μmol·m−2·s−1 Is the Optimal Condition for Promoting Lutein Accumulation
4.3. Integrated Multi-Omics Analysis Untangles the Complex Network of Light Intensity in Regulating Lutein Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoshi, Y.; Praphruet, R.; Hironaka, K. Genome Size Determination and Chromosome Characterization of Two Marigold Species (Asteraceae). Cytologia 2019, 84, 37–42. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cong, R.C.; Wang, M.L.; Dong, A.X.; Xin, H.B.; Yi, M.F.; Guo, H. Development of SSR Molecular Markers Based on Transcriptome Sequencing of Tagetes erecta. Acta Hortic. Sin. 2018, 45, 159–167. (In Chinese) [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and Lutein Ester Content in Different Types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Xin, H.B.; Ji, F.F.; Wu, J.; Zhang, S.; Yi, C.; Zhao, S.; Cong, R.; Zhao, L.; Zhang, H.; Zhang, Z. Chromosome-Scale Genome Assembly of Marigold (Tagetes erecta L.): An Ornamental Plant and Feedstock for Industrial Lutein Production. Hortic. Plant J. 2023, 9, 1119–1130. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-Lopez, U.; Farre, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally Important Carotenoids as Consumer Products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein Nanocrystals as Antioxidant Formulation for Oral and Dermal Delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Gombač, Z.; Črnivec, I.G.O.; Skrt, M.; Istenič, K.; Knafelj, A.K.; Pravst, I.; Ulrih, N.P. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods 2021, 10, 500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moehs, C.P.; Tian, L.; Osteryoung, K.W.; Dellapenna, D. Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol. Biol. 2001, 45, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; De Vita, P.; Mastrangelo, A.M.; Pecchioni, N.; Houston, K.; et al. The Carotenoid Biosynthetic and Catabolic Genes in Wheat and Their Association with Yellow Pigments. BMC Genom. 2017, 18, 122–128. [Google Scholar] [CrossRef]
- Bruno, M.; Beyer, P.; Al-Babili, S. The Potato Carotenoid Cleavage Dioxygenase 4 Catalyzes a Single Cleavage of β-Ionone Ring-Containing Carotenes and Non-Epoxidated Xanthophylls. Arch. Biochem. Biophys. 2015, 572, 126–133. [Google Scholar] [CrossRef]
- Giorio, G.; Yildirim, A.; Stigliani, A.L.; D’Ambrosio, C. Elevation of Lutein Content in Tomato: A Biochemical Tug-of-War Between Lycopene Cyclases. Metab. Eng. 2013, 20, 167–169. [Google Scholar] [CrossRef]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of Three Members of the Arabidopsis Carotenoid Cleavage Dioxygenase Family Demonstrates the Divergent Roles of This Multifunctional Enzyme Family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhang, H.; Chen, X.; Liang, F.; Qin, H.; Zhang, Y.; Cong, R.; Xin, H.; Zhang, Z. Carotenoid Metabolite and Transcriptome Dynamics Underlying Flower Color in Marigold (Tagetes erecta L.). Sci. Rep. 2020, 10, 16835. [Google Scholar] [CrossRef] [PubMed]
- Pinnola, A.; Gerotto, C.; Morosinotto, T.; Bassi, R.; Alboresi, A. Zeaxanthin Binds to Light-Harvesting Complex Stress-Related Protein to Enhance Nonphotochemical Quenching in Physcomitrella patens. Plant Cell 2013, 25, 3519–3534. [Google Scholar] [CrossRef]
- Stange, C. Carotenoids in Nature. Subcell. Biochem. 2016, 79, 3–61. [Google Scholar]
- Ma, R.J.; Zhao, X.R.; Xie, Y.P.; Ho, S.H.; Chen, J.F. Enhancing Lutein Productivity of Chlamydomonas sp. via High-Intensity Light Exposure with Corresponding Carotenogenic Genes Expression Profiles. Bioresour. Technol. 2019, 275, 416–420. [Google Scholar] [CrossRef]
- Schüler, L.M.; Santos, T.; Pereira, H.; Duarte, P.; Gangadhar, K.N.; Florindo, C.; Schulze, P.S.C.; Barreira, L.; Varela, J.C.S. Improved Production of Lutein and β-Carotene by Thermal and Light Intensity Upshifts in the Marine Microalga Tetraselmis sp. CTP4. Algal Res. 2020, 45, 101732. [Google Scholar] [CrossRef]
- Das, P.R.; Del Moro, D.S.; Givens, S.R.; Armstrong, S.P.; Walters, K.J. Propagation Light Intensity Influences Yield, Morphology, and Phytochemistry of Purple-Leaf Butterhead Lettuce (Lactuca sativa). J. Agric. Food Res. 2024, 16, 101210. [Google Scholar] [CrossRef]
- Akemi, O.; Masaya, K.; Takehiko, S.; Kenji, N.; Sanae, K.; Masayasu, N. Molecular Basis of Carotenoid Accumulation in Horticultural Crops. Hortic. J. 2019, 88, 135–149. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.D. Transcriptome Analysis and Molecular Mechanism of Carotenoid Synthesis Pathway of Marigold. PhD. Thesis, Hefei University of Technology, Hefei, China, 2019. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Mayne, S.T.; Sies, H. Carotenoids in Health and Disease; CRC Press Inc.: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bartley, E.G.; Scolnik, P.A. Plant Carotenoids: Pigments for Photoprotection, Visual Attraction and Human Health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Hing, C.F.; Wu, C.F.; Lin, C.L.; Chen, B.H. Determination of Carotenoids and Their Esters in Fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef]
- Geyer, R.; Peacock, A.D.; White, D.C.; Lytle, C.; Van, G.J. Atmospheric Pressure Chemical Ionization and Atmospheric Pressure Photoionization for Simultaneous Mass Spectrometric Analysis of Microbial Respiratory Ubiquinones and Menaquinones. J. Mass Spectrom. 2004, 39, 922–929. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Del Villar-Martínez, A.A.; García-Saucedo, P.A.; Carabez-Trejo, A.; Cruz-Hernández, A.; Paredes-Lópeza, O. Carotenogenic gene expression and ultrastructural changes during development in marigold. J. Plant Physiol. 2005, 162, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H.; Wang, W.; Fan, C.; Yuan, G.; Zhou, R.; Lu, J.; Liu, J.; Wang, C. RcOST1L phosphorylates RcPIF4 for proteasomal degradation to promote flowering in rose. New Phytol. 2024, 243, 1387–1405. [Google Scholar] [CrossRef] [PubMed]
- Maragò, E.; Michelozzi, M.; Calamai, L.; Camangi, F.; Sebastiani, L. Antioxidant properties, sensory characteristics and volatile compounds profile of apple juices from ancient Tuscany (Italy) apple varieties. Eur. J. Hortic. Sci. 2016, 81, 255–262. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams III, W.W. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Qiu, H.; Xue, M.; Aiwaili, P. Integrated Transcriptomic and Metabolomic Analyses of the Response of Lutein Accumulation in Marigold Petals to Light Intensity. Genes 2025, 16, 1350. https://doi.org/10.3390/genes16111350
Zhang H, Qiu H, Xue M, Aiwaili P. Integrated Transcriptomic and Metabolomic Analyses of the Response of Lutein Accumulation in Marigold Petals to Light Intensity. Genes. 2025; 16(11):1350. https://doi.org/10.3390/genes16111350
Chicago/Turabian StyleZhang, Haimin, Hong Qiu, Meng Xue, and Palinuer Aiwaili. 2025. "Integrated Transcriptomic and Metabolomic Analyses of the Response of Lutein Accumulation in Marigold Petals to Light Intensity" Genes 16, no. 11: 1350. https://doi.org/10.3390/genes16111350
APA StyleZhang, H., Qiu, H., Xue, M., & Aiwaili, P. (2025). Integrated Transcriptomic and Metabolomic Analyses of the Response of Lutein Accumulation in Marigold Petals to Light Intensity. Genes, 16(11), 1350. https://doi.org/10.3390/genes16111350




