Abstract
Background/Objectives: Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare disease, in which multiple genetic and environmental factors may contribute. This study aimed to identify potential genetic determinants in patients with CTEPH and to compare their occurrence to a control group, which included patients with pulmonary embolism who had not developed CTEPH. Methods: Tier 1 and 2 genes related to coagulation, fibrinolysis and platelet disorders—as recommended by the International Society on Thrombosis and Haemostasis—and genes associated with vascular conditions were analyzed in n = 15 patients with CTEPH and n = 17 controls using next-generation sequencing. Non-synonymous, rare variants were collected and interpreted. Results: As expected, no single gene or variant was consistently present among CTEPH patients. Instead, individuals carried different mutations and combinations of variants. We identified several variants that were not found in the control group. Candidate variants were detected in F12, F13A1, F13B, F5, KNG1, SERPIND1, THBD, ADAMTS13, VWF, STIM1, ETV6, THPO, MPL, SERPINA1, ENG, RASA1, ACVRL1, GDF2, NFE2, SOX17 and RNF213. We did not detect exclusive variants in FGA, CPB2, and BMPR2 although they were suggested as candidates in previous studies. Elevated factor VIII and von Willebrand factor in CTEPH could not be explained by mutations in VWF and F8. Conclusions: Our study supports the hypothesis of heterogeneous genetic background in CTEPH, involving multiple pathways such as coagulation, altered fibrinolysis and impaired angiogenesis. These results provide a basis for more detailed investigations into specific genes and variants potentially associated with CTEPH in larger cohorts.
1. Introduction
Pulmonary embolism (PE) is a life-threatening thromboembolic disorder and a leading cause of cardiovascular morbidity and mortality worldwide [,]. The incidence of acute PE is estimated to be 39–115 cases per 100,000 people annually, with significant variation based on geographical and population-specific factors. Although thrombolytic therapy and anticoagulation are effective in resolving most PE cases, a subset of patients experiences incomplete thrombus resolution, leading to chronic thromboembolic pulmonary hypertension (CTEPH) [,]. This progressive disorder, which occurs in approximately 2–4% of PE survivors, is associated with increased pulmonary vascular resistance, right ventricular dysfunction, and ultimately right heart failure if left untreated [,,,]. The diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) is established in patients who present with persistent pulmonary hypertension (mean pulmonary artery pressure ≥ 20 mmHg, pulmonary artery wedge pressure ≤ 15 mmHg, and pulmonary vascular resistance > 2 Wood units) confirmed by right heart catheterization, in combination with imaging evidence of chronic thromboembolic obstruction, such as mismatched perfusion defects on ventilation/perfusion (V/Q) scanning, or organized thromboembolic lesions visualized on pulmonary angiography, CT pulmonary angiography (CTPA), or MR angiography []. Diagnosis of CTEPH requires that these findings persist despite effective anticoagulation therapy for at least three months in order to exclude subacute or resolving thromboembolic disease.
CTEPH is a complex and multifactorial condition, with acquired and genetic risk factors contributing to its pathogenesis [,,,]. Acquired risk factors include previous deep vein thrombosis (DVT), malignancy, splenectomy, functional asplenia, chronic inflammatory diseases and antiphospholipid syndrome. However, not all individuals with these risk factors will suffer from CTEPH, suggesting that genetic predisposition may also play a critical role in determining disease susceptibility. The presence of genetic susceptibility is also supported by familial clustering studies []. As thrombophilic risk factors were presented in a high proportion of individuals suffering from CTEPH, research into genes related to thrombotic disorders has been conducted. Several candidate genes have been identified in relation to thrombus formation and fibrinolysis, each of which may contribute to the persistence of thrombi and subsequent vascular remodeling, leading to the pathologic condition of CTEPH []. Results of genetic studies executed so far, however, are rather heterogeneous. Although some studies have suggested that variations in SERPINC1, a gene encoding antithrombin (AT), may increase the risk of PE and CTEPH due to impaired anticoagulant activity, classical hereditary thrombophilia risk factors (i.e., causative mutations in SERPINC1, PROC, PROS1 and presence of factor V Leiden and prothrombin 20210A) seemed not to be independent risk factors of CTEPH [,]. Mutations in FGA and FGB, which encode alpha and beta chains of fibrinogen, respectively, have been associated with altered clot structure and resistance to fibrinolysis, potentially predisposing individuals to persistent thrombotic occlusions and the development of CTEPH [,,]. Platelet abnormalities, especially their hyperreactivity in the background of CTEPH, are also plausible hypotheses []. Impairment in fibrinolysis would be a logical explanation for why thrombi are not dissolved after PE, leading to organization of pulmonary thrombi; however, no clear conclusions can be drawn from the studies executed so far [,].
As microvascular remodeling is suggested to have a major role in CTEPH development [,], mutations in genes related to vascular development and signaling, being linked to abnormal vascular remodeling and impaired endothelial barrier function, are also targets of research. This hypothesis is strengthened by the observation that many patients with a definitive CTEPH diagnosis have no history of symptomatic PE; however, they may have had a subclinical thromboembolic event [].
It is still unknown why CTEPH develops only in a minority of patients after PE and which factors contribute to each other, leading to this unique condition. Development of CTEPH after PE is still unpredictable and we are not able to select cases with a high CTEPH risk, although it would be of utmost importance from the point of view of patient management. Research into this field is difficult due to the several potentially contributing pathways and the low number of CTEPH cases even at large clinical centers. Several risk factors seen in CTEPH overlap with PE and venous thromboembolism, making finding predisposing factors for CTEPH even more difficult [,]. To better separate risk factors of CTEPH from those of thrombosis, it would be straightforward to compare the findings to a group of PE patients without the development of CTEPH.
High-throughput genetic methods, like next-generation sequencing (NGS), are powerful tools for identifying genetic variants associated with a certain phenotype. By focusing on protein-coding regions of the genome, whole-exome sequencing (WES) enables the detection of mutations that may disrupt key physiological pathways related to thrombosis and vascular homeostasis.
Our aim was to identify genes and their variations with a plausible role in the development of CTEPH, by using next-generation sequencing (NGS). This study aimed to analyze the differences between PE patients who develop CTEPH and those who do not in order to identify and interpret variants in association with thrombus formation, persistence, impaired fibrinolysis, and endothelial dysfunction.
2. Materials and Methods
2.1. Study Design and Population
An open-label, non-randomized, prospective observational study was conducted at the Department of Cardiology, University of Debrecen. Following approval of the study protocol, patients were prospectively enrolled after providing written informed consent. Prior to enrollment, all participants received detailed information regarding the investigational nature of the study, including potential risks and anticipated benefits.
Patients included in the study (n = 15) were diagnosed with CTEPH and met the established diagnostic criteria (please see above). The suspicion of pulmonary hypertension was raised based on echocardiographic findings and was subsequently confirmed by right heart catheterization using a Swan–Ganz catheter and pulmonary angioplasty. All patients enrolled in the study underwent balloon pulmonary angioplasty (BPA). Patient enrollment commenced in 2022, with follow-up continuing through 2025. Comprehensive demographic and clinical data—including vital status, hospital admissions, comorbid conditions, treatment history, and follow-up outcomes—were extracted from hospital records. The control group consisted of 17 patients with a history of pulmonary embolism in whom echocardiography performed 3 to 6 months after the acute event excluded the development of pulmonary hypertension.
2.2. Laboratory Testing of CTEPH and PE Patients
Blood samples were collected in 3.2% Na-citrate anticoagulated tubes (Greiner, Kremsmunster, Austria). Laboratory testing included screening tests of coagulation and fibrinogen detection by the Clauss method on BCS-XP coagulometer (Siemens, Marburg, Germany). Coagulation factor VIII was measured by the chromogenic method (Siemens FVIII Chromogenic Assay), von Willebrand factor antigen was measured by the Innovance VWF assay (Siemens), and plasminogen and alpha2-plasmin inhibitor were detected by Berichrom plasminogen and Berichrom alpha2-antiplasmin, respectively (Siemens). Protein C (PC), protein S (PS) and antithrombin (AT) were measured by Berichrom Protein C, Innovance free PS Ag and Innovance Antithrombin, respectively, on a BCS-XP coagulometer. Lupus anticoagulant testing was executed according to the current guidelines by the ISTH, using diluted Russel’s viper venom time and lupus anticoagulant-sensitive APTT (Werfen, Milan, Italy and Diagnostica Stago, Asnieres, France, respectively) []. Anticardiolipin and anti-beta2 glycoprotein I IgG and IgM antibodies were detected by chemiluminescent immunoassays on a Bioflash analyzer (Werfen) []. Coagulation FXIII activity was determined by the modified optimized kinetic spectrophotometric ammonia-release assay on a Sysmex CS2500 coagulometer (Siemens) by using Technoclone FXIII reagent (Technoclone, Vienna, Austria). Markers of a pro-thrombotic state, thrombin–antithrombin complex (TAT) and prothrombin fragment 1+2 (PF1+2), were detected by ELISA (Enzygnost TAT micro and Enzygnost F1+2, respectively; both were purchased from Siemens). D-dimer was measured by HemosIL D-dimer HS500 on an ACL-TOP coagulometer (Werfen). PAI-1 antigen was measured by Technozym PAI-1 antigen ELISA (Technoclone), and tPA was detected by Human Tissue Type Plasminogen Activator ELISA kit (Abcam, Cambridge, UK). TFPI concentration was measured by ELISA from Invitrogen (Thermo Fisher, Waltham, MA, USA).
2.3. Genetic Testing of CTEPH and PE Patients
DNA was isolated from peripheral blood leukocytes by QIAmp DNA Blood Mini kit (Qiagen, Hilden, Germany). After isolation, the purity of DNA was checked by NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). DNA concentration was determined by Qubit® dsDNA HS Assay Kits on a Qubit Fluorometer (Thermo Fisher), then DNA was diluted to yield 200 ng DNA in 30 μL. Library preparation for clinical exome sequencing was executed using the Clinical exome solution v3 kit (SOPHiA GENETICS, Lausanne, Switzerland) according to the manufacturer’s instructions. Quality control analysis of the library pools was performed by Agilent Fragment Analyzer capillary electrophoresis (Agilent, Santa Clara, CA, USA). Next-generation sequencing (NGS) was performed on an Illumina NextSeq 500 instrument (Illumina, San Diego, CA, USA) with the NextSeq 500/550 Mid Output Kit v2.5 (300 Cycles).
Factor V Leiden (rs6025) and prothrombin 20210A (rs1799963) polymorphisms were detected by a LightCycler 480 instrument (Roche, Basel, Switzerland) by using real-time PCR followed by melting curve analysis with in-house-designed primers (TIB® MOLBIOL, Berlin, Germany) and probes (Roche). Primers were as follows: FII forward: 5′-CCG CTG GTA TCA AAT GGG-3′; FII reverse: 5′-CCA CTA GTA TTA CTG GCT CTT CCT G-3′; FV forward: 5′-TAATCTGTAAGAGCAGA XT CC-3′, where X = BODYPY630/650 NHS ester; FV reverse: 5′-TGTTATCACACTGGTGCTAA-3′. Probes were as follows: FII anchor probe: 5′-X TCC CAG TGC TAT TCA TGG GC Y-3′, where X = BODYPY630/650, Y = 3Phos; FII sensor probe: 5′-CTC AGC GAG CCT CAA TG X-3′, where X = 6FAM; and FV sensor probe: 5′-AATACCTGTATTCCTCGCCTGTC X-3′, where X = 6FAM. The PCR procedure was executed using the Genotyping Master kit (Roche).
2.4. Data Analysis and Statistics
Continuous variables are reported as either mean ± standard deviation (SD) or median with range, depending on the underlying distribution. Categorical variables are presented as absolute frequencies and percentages. The normality of data distributions was assessed using the Kolmogorov–Smirnov test. Student’s t-test or the Mann–Whitney U-test was carried out in order to compare the values between two groups. In the case of categorical variables, chi-square test was used. A two-tailed p-value of <0.05 was considered indicative of statistical significance. All analyses were performed using IBM SPSS Statistics, version 29.
Bioinformatical analysis of NGS data was executed by SOPHiA DDM software v6.4, and annotation was performed using the hg38 reference genome. Two virtual gene panels were generated: The first one included Tier 1 genes associated with coagulation, fibrinolysis and platelets, as recommended recently by the International Society on Thrombosis and Haemostasis (ISTH, https://www.isth.org/page/GinTh_GeneLists (accessed on 25 July 2025)). The second group of genes was selected based on their association with vascular conditions (please see below). Variants captured by the software were described according to the American College of Medical Genetics and Genomics (ACMG) recommendations and were classified into pathogenic, likely pathogenic, VUS, likely benign and benign according to its built-in algorithm, which uses data from different genetic databases and in silico prediction software (PolyPhen2, SIFT, MutPred, Mutation Taster, v6.4) []. Selected variants were also checked manually in the available genetic databases, Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk), Online Mendelian Inheritance in Men (OMIM, https://www.omim.org/) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/ (accessed between 1 August 2025 and 25 September 2025)), for classification. Allele frequency data of variants were obtained from the gnomAD and 1000 genomes databases (https://gnomad.broadinstitute.org/ and https://www.internationalgenome.org/).
2.5. Ethical Statement
The study was approved by the Regional Scientific and Ethical Committee of the University of Debrecen, Clinical Center, as well as by the National Scientific and Research Ethics Committee of Hungary (Approval No: RKEB/IKEB: 6153-2022). Written informed consent was obtained from all participants prior to inclusion, in accordance with the principles of the Declaration of Helsinki.
3. Results
3.1. Characteristics of the Patients with CTEPH
The CTEPH cohort included both male and female patients (10 males, 5 females), all of whom were of white (Caucasian) ethnicity. Their demographic and laboratory characteristics are demonstrated in Table 1. The mean age at diagnosis showed a wide distribution, and their body mass index showed a wide range from normal weight to severe obesity, with the highest BMI value of 34.29. At the time of enrollment, most patients (n = 10) were classified as New York Heart Association (NYHA) functional class II or III, indicative of moderate functional limitation, while a smaller proportion (n = 3) presented in class IV. The mean distance achieved on the 6 min walk test (6MWT) was 336.1 ± 164.7 m (range: 42–616 m), reflecting substantial variability in exercise capacity. The average baseline level of N-terminal pro-brain natriuretic peptide (NT-proBNP) was 2694.3 ± 2407.2 pg/mL (range: 82–7133), consistent with variable degrees of right ventricular (RV) dysfunction. Left ventricular function was preserved in all patients.

Table 1.
Demographic and laboratory characteristics of CTEPH patients and PE patients without the development of CTEPH.
Systolic pulmonary artery pressures, as measured by echocardiography and Swan–Ganz catheterization, averaged 77.2 ± 23.5 mmHg and 72.9 ± 22.6 mmHg, respectively. Right heart catheterization further confirmed elevated pulmonary vascular resistance (mean PVR: 720.3 ± 373.5 dyn · s · cm−5) and increased mean pulmonary artery pressure (mPAP: 44.0 ± 9.1 mmHg). Pulmonary capillary wedge pressure (PCWP) and right atrial pressure (RAP) were 11.7 ± 3.3 mmHg and 7.6 ± 4.2 mmHg, respectively. Cardiac output (CO) and cardiac index (CI) were reduced (median CO: 3.82, range 2.72–8.53 L/min; CI: 2.03, range 1.66–4.04 L/min/m2), consistent with compromised right ventricular performance.
Medical history revealed a high thromboembolic burden, as six patients had a documented history of pulmonary embolism (PE), and two were found to have established inherited thrombophilia (one patient homozygous for the prothrombin gene 20210A mutation, rs1799963, and one patient heterozygous for factor V Leiden, rs6025, Supplementary Table S1). No AT, PC and PS deficiencies were registered. One patient had lupus anticoagulant and another one had moderate elevation of anti-beta 2 glycoprotein I IgG (133.2 CU). An elevated factor VIII (FVIII) level above 200 IU/dL was observed in one patient, while elevated von Willebrand factor antigen (vWF:Ag) above 200 IU/dL was measured in five patients. No patient had a history of splenectomy. Electrocardiographic findings commonly included right bundle branch block (RBBB) and patterns of RV strain; atrial fibrillation was observed in a minority of patients. There were no cases of provoked PE in our study population—neither in the CTEPH group nor in the control group—with no transient risk factors such as surgery, trauma, or immobilization identified. No patients with hemoglobinopathies, including sickle cell disease (SCD), were found in our cohort. During follow-up, six patients died, including three from non-cardiovascular causes—COVID-19, pneumonia, and septic shock.
3.2. Characteristics of Patients with PE Without the Development of CTEPH
The baseline demographic and anthropometric characteristics are shown in Table 1. The mean body mass index ranged from normal weight to severe obesity (BMI 44.6). In this group, three patients stopped anticoagulation therapy 6 months after the acute event, while three patients were on long-term rivaroxaban, nine patients were on apixaban and two patients were on dabigatran at the time of investigation. Among PE patients, there were two FV Leiden heterozygotes and two patients carried the prothrombin 20210A allele in heterozygous form. No classical AT, PC and PS deficiencies were registered; however, one patient was a heterozygous carrier of the PS Heerlen polymorphism (rs121918472, c.1501T > C, p.Ser501Pro) with a free PS antigen level of 63%, which is considered a mild risk factor for thrombosis []. One patient had lupus anticoagulant and none of the PE patients had elevated antiphospholipid antibody values. An elevated vWF:Ag level exceeding 200 IU/dL was detected in one patient; her FVIII activity was 190 IU/dL.
3.3. Comparison of Laboratory Parameters Between CTEPH Patients and PE Patients
Among parameters reflecting coagulation, CTEPH patients had significantly elevated FVIII and vWF:Ag levels as compared to controls (Table 1). Fibrinogen concentration did not differ between the two groups. D-dimer was also not different; however, parameters reflecting pro-thrombotic states (TAT complex and PF1+2) were significantly higher in patients with PE without the development of CTEPH, suggesting a continuously higher level of coagulation activation, which was not so pronounced in CTEPH patients. TFPI levels were not different between the two groups. By investigating factors involved in fibrinolysis, plasminogen and alpha2-PI levels were significantly lower in CTEPH individuals; however, on the contrary, tPA was significantly higher. There was no difference in PAI-1 and FXIII levels. These results suggest—although the sample size is very low—that the lower plasminogen level is associated with higher tPA activity and with lower alpha2-PI activity in CTEPH patients, resulting in an altered balance in fibrinolysis. This observation, however, does not allow us to draw a clinically meaningful conclusion, since none of these laboratory parameters fell out of the corresponding reference intervals and no extreme values were detected. Elevation in FVIII and vWF:Ag levels in CTEPH was more pronounced, suggesting the role of endothelial activation in this disease.
3.4. Investigation of Genes Associated with Hemostasis and Thrombosis in CTEPH and PE Patients
First, we established a virtual gene panel including Tier 1 genes as recommended by the SSC Subcommittee on OMICS in Thrombosis and Hemostasis of the ISTH, (https://www.isth.org/page/GinTh_GeneLists, accessed on 2 November 2025). This gene list currently contains 109 genes associated with coagulation, fibrinolysis and platelet disorders. By this search, n = 397 different variants were found within CTEPH patients. After excluding common variants with higher allele frequency values than 0.05, as based on 1000genomes and/or gnomAD data, n = 134 different variants remained for further analysis (Figure 1). We found n = 87 non-synonymous variants, among which n = 55 variants were related to platelet-associated genes, while n = 32 variants were related to coagulation or fibrinolysis-related genes including the von Willebrand factor gene (VWF) and ADAMTS13. Most variants were missense mutations (76%) caused by single-nucleotide exchanges at coding regions. All variants were detected in heterozygous form in CTEPH patients, except for a missense variant in PIGA (located at chromosome X), which was found in a male patient (c.55C > T, p.Arg19Trp) as hemizygous.
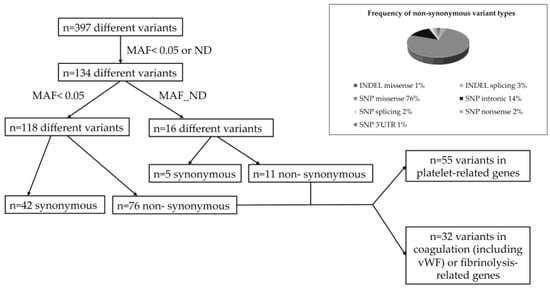
Figure 1.
Algorithm of variant detection in CTEPH patients. Frequency of different non-synonymous variants. MAF, minor allele frequency; ND, non-determined; vWF, von Willebrand factor; INDEL, insertion or deletion; SNP, single-nucleotide substitution.
Among genes related to coagulation, variants were found within clotting factor genes F10, F12, F13A1, F13B, F5 and F8 (Table 2). Among these, F10 p.Met336Val, F12 p.Leu140Val, F13A1 p.Tyr205Phe, F13B intronic mutations and F5 p.Met1811Leu and p.Met2148Thr were not found in the control group. By investigating genes encoding proteins involved in fibrinolysis, one variant was found in the KNG1 gene encoding high-molecular-weight kininogen (p.Arg412*), which was absent from the control group. PLG (encoding plasminogen) p.Val291Met was also found in one CTEPH patient; however, the plasminogen level was normal in that individual (P12, plasminogen 101 IU/dL) and it is not associated with plasminogen deficiency according to the latest curated databases. SERPINE1 p.Val17Ile was detected only in one CTEPH patient (P7), whose PAI-1 antigen concentration was below the lower limit of the reference interval (4.1 ng/mL, reference interval 7–43 ng/mL), rather characteristic of a mild PAI-1 deficiency. Among genes encoding proteins serving as natural anticoagulants, an intronic variant in SERPIND1 encoding heparin cofactor II was found in P2 and it was absent from the control group. We found mutations in the gene encoding thrombomodulin (THBD) and in the gene encoding PC (PROC), which were also absent from the PE group. The PC level in patient 6 with the mutation c.-21-37G > A in PROC, however, was normal (84 IU/dL) and this variant seems not to be causative of PC disorders. Two variants were detected in ADAMTS13; one of them (p.Gln1174*) was not found in the control group. Finally, seven different variants were described in VWF, among which five mutations were not present in the controls.

Table 2.
Variants in genes related to coagulation and fibrinolysis (Tier 1 genes of the ISTH, panel 1) in CTEPH patients.
Concerning platelet-associated genes, no gene or variant was found, which was potentially relevant from the point of view of CTEPH or any thrombotic phenotype (Supplementary Tables S2 and S3). Genes included in the Tier 1 ISTH database for platelet disorders are rather associated with thrombocytopenia, or platelet function disorders with a bleeding phenotype, mainly if mutations are carried in homozygous form. However, there were some potential exceptions. One CTEPH patient (P10) was a carrier of a STIM1 mutation (c.1859+1G > A, rs118128831), suggesting a splicing defect. This variant was not found in the control group. The gene is associated with the autosomal dominant Stormorken syndrome with functional asplenia, thrombocytopenia and Howell–Jolly bodies, which are features also described in association with CTEPH. Our patient with a STIM1 mutation had mild thrombocytopenia with large platelets but he had no Howell–Jolly bodies in his blood smear. A variant of the THPO gene (c.889A > G, p.Thr297Ala, rs530613857) was found in another CTEPH patient (P9). An ETV6 mutation (c.602T > C, p.Leu201Pro, rs145477191) was found in P11. These variants were also absent from the controls.
An additional 11 genes considered as Tier 2 genes according to ISTH recommendation were also investigated. Among them NFE2, MAST2, APOLD1 and SERPINA1 were potentially interesting because of their association with clonal hematopoetic regulation, venous thromboembolism, endothelial cell signaling and alpha1-antitrypsin, respectively. By the investigation of rare, non-synonymous variants within these genes, we detected a SERPINA1 c.863A > T (p.Glu288Val) variant (allele frequency 0.023) in patient P4 and a NFE2 c.518A > G (p.Asp173Gly) variant (allele frequency unknown) in patient P12. These mutations are considered as likely pathogenic and VUS, respectively, in association with the corresponding diseases according to recent clinical genetic databases. These variants were not detected in the control group.
3.5. Investigation of Genes Associated with Vascular Diseases in CTEPH and in PE Patients
Our second virtual gene panel consisted of genes which have been associated with vascular diseases or involved in vascular development, angiogenesis, or thrombotic phenotype, based on literature data and clinical databases (Supplementary Table S4). The following genes were investigated: ENG, ACVRL1, BMPR2, RASA1, GDF2, SMAD4, SOX17, CAV1, KCNK3, RNF213, SMAD9, SLC2A10, KDR, CPB2 and HRG. Variant screening was performed for ISTH genes, and variants with MAF above 0.05 and synonymous ones were excluded. By this search, we collected n = 15 different missense or splicing variants (Table 3). Most variants were carried by one patient each, and only RASA1 p.Ala99Val, KDR p.Cys482Arg and RNF213 p.Leu4283Ile were carried by two CTEPH patients each; however, these mutations were also detected in the control group. There were exclusive variants in RASA1, ENG, GDF2, SOX17, ACVRL1 and RNF213, which were not found in PE patients without CTEPH.

Table 3.
Variants in genes related to vascular disorders/conditions (panel 2) in CTEPH patients.
Although variants in genes BMPR2 and KDR were also found in PE patients without CTEPH, they might have significance in CTEPH, not directly in its development but rather in its severity and extension, which is well demonstrated by our patients’ clinical histories. All three patients (P1, P4 and P6) had severe and extensive CTEPH, with a total of 13 (out of which only 4 were operable), 14 (out of which only 6 were operable in multiple BPA sessions), and 11 (out of which all were dilated by BPA, but in multiple BPA sessions) segmental pulmonary arteries affected, respectively.
3.6. Combination of Panel 1 and 2 Gene Variants in CTEPH Patients
We collected the different variants detected in virtual panels 1 and 2 according to CTEPH patients and investigated the combined occurrence of these mutations (Table 4 and Figure 2). The combination of the different variants was rather heterogeneous and in most cases, more than one suspected variant was detected. While there were several variants also found in the control group, we could identify potentially interesting rare variants exclusively detected in CTEPH patients. In most patients, variants related to vascular disorders and those associated with coagulation and fibrinolysis were combined, suggesting the possibility of their additive or synergistic effect.

Table 4.
Combination of gene variants with plausible association with CTEPH and/or thrombotic phenotype in Tier 1 and 2 ISTH genes and panel 2 genes in CTEPH patients.
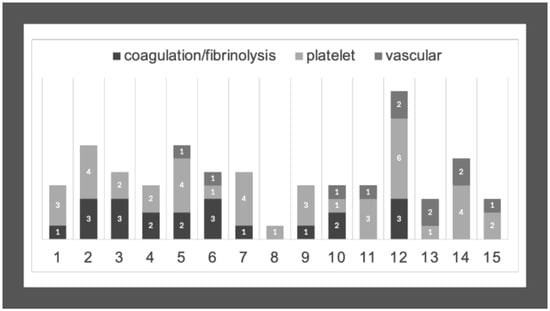
Figure 2.
Combined occurrence of variants in CTEPH patients. Numbers on the X-axis represent CTEPH patients from P1 to P15. Numbers on the columns represent the number of the detected variants in genes associated with coagulation/fibrinolysis, platelet disorders, or vascular conditions.
4. Discussion
Despite advances in our understanding of CTEPH genetics, significant knowledge gaps remain regarding the contribution of different genes and variants and the interplay between genetic susceptibility and environmental factors. As expected, no single gene or variant was consequently detected in our CTEPH patients, and no variant was found that could be directly associated with the disease. Our main goal was to investigate rare variants in genes related to thrombosis, hemostasis, and vascular disorders in order to identify potentially interesting ones that are worthy of further study. We compared the presence of the detected variants in CTEPH patients to those in individuals with PE, but without the development of CTEPH. Then we investigated whether the variants found exclusively in the CTEPH group might be associated with the disease, based on clinical genetic databases and/or literature data. It was not surprising that most of the variants were considered as VUS for the corresponding disease in the available databases, and no literature data were available in the context of CTEPH.
Among genes associated with coagulation, only genes encoding different fibrinogen chains and F5 (due to FV Leiden mutation, p.Arg534Gln) have been associated with CTEPH in previous studies [,]. In our cohort, no fibrinogen variants were detected. The FV Leiden mutation was found in one CTEPH patient in our cohort; however, it was also detected in two individuals in the PE (control) group. The presence of FV Leiden was shown to confer a 3-fold risk of early-onset CTEPH in a European study and it represented a risk for CTEPH partly shared with acute PE in a recent large genome-wide association study [,]. FV Leiden was not found in idiopathic pulmonary hypertension in that study. Our results support the role of this polymorphism in PE and CTEPH (frequency of FV Leiden is n = 3/32, 10% in the combined group of CTEPH and PE). We have found two other F5 mutations, p.Met1811Leu and p.Met2148Thr, which were present only in CTEPH patients. While the variant p.Met1811Leu has been reported as a variant of uncertain significance (VUS) for FV-related disorders, p.Met2148Thr has been considered as benign not only from the point of view of bleeding, but also from the point of view of thrombophilia [].
Mutations in F10 usually cause FX deficiency and bleeding symptoms mostly in homozygous patients, and no association with thrombotic disorders has been published so far. Therefore, it is not likely that F10 p.Met336Val, found in CTEPH, may be a risk factor for either CTEPH or thrombosis; however, this variant has not been analyzed in vitro yet. F12 p.Leu140Val was identified in deep vein thrombosis and also in hereditary angioedema (HAE); however, its pathogenicity has not been clarified in these disorders yet [] and it seems to be benign in relation to FXII deficiency and severe HAE. However, as coagulation FXII has a role in fibrinolysis and in complement activation rather than in coagulation, this variant may be a candidate for further investigation in a larger cohort of CTEPH patients. Factor XIII is a heterotetrameric molecule with two subunits A and B, where subunit A is the active enzyme transglutaminase and subunit B is a carrier molecule []. The involvement of FXIII in thrombotic disorders has been widely investigated with the identification of certain polymorphisms with potential roles in arterial or venous thrombosis. Among them, F13A1 p.Tyr205Phe, found in one of our patients (P5), has been previously associated with arterial and venous thrombosis, although it was not confirmed as a risk factor for ischemic stroke in a meta-analysis []. The patient with this mutation had FXIII activity within the reference interval (131 IU/dL); however, its role in modifying fibrin cross-linking and fibrinolysis cannot be ruled out. Intronic variants in the gene encoding the B subunit of FXIII with unknown allele frequency data were found in a single patient with CTEPH (P3), suggesting a linkage among these alterations. There are still conflicting results of the association of F13B polymorphisms with thrombotic diseases; moreover, their association with CTEPH remains unclear [,].
The systemic fibrinolysis pathway is typically considered not to be affected in CTEPH, but imbalances in local expression of enzymes involved in fibrinolysis may play a role [,]. Among genes associated with fibrinolysis, the KNG1 variant that leads to a stop codon and suggests the presence of a truncated protein was found in a CTEPH patient and it was absent from controls. This variant (p.Arg412*) was annotated in a large study including patients with venous thrombosis and—since HMWK encoded by this gene is a protein involved in fibrinolysis regulation and in inflammatory processes—it is worthy of further investigation []. The SERPINE1 p.Val17Ile variant may be related to lower secretory dynamics of PAI-1 and to lower PAI-1 levels, as it was also seen in our patient; therefore, the association of this variant with CTEPH is unlikely [].
Among genes encoding proteins serving as natural anticoagulants, SERPINC1, PROC and PROS1 were investigated in detail earlier, with inconclusive results []. In our study, no relevant variants were found in these genes. Instead, an intronic variant was found in SERPIND1 (c.1309-3C > T), a gene encoding heparin cofactor II, a serin protease inhibitor with a rapid thrombin inhibitory effect in the presence of negatively charged glycosaminoglycans like heparan sulfate, dermatan sulfate, and chondroitin sulfate []. Heparin cofactor II seems to be an important factor in the case of atherosclerotic diseases and it seems to prevent vascular restenosis, especially after coronary interventions []. Being a natural thrombin inhibitor, whose function is strongly related to vascular wall properties, its role in CTEPH is plausible. It is a question, however, whether this variant has any structural or functional consequences on the protein. This issue is worthy of further investigation, and the role of heparin cofactor II in CTEPH is suggested to be examined in more detail. The PROC variant that we found in this study is considered a likely benign mutation according to the clinical databases, which does not influence PC levels, as also seen in our patient. Moreover, it is unlikely that this c.-21-37G > A variant upstream of the coding region of PC would have any effect on the fibrinolysis-regulatory, cytoprotective, and anti-inflammatory functions of PC. The mutation we found in THBD (p.Pro501Leu) is an already-described variant with uncertain significance in the context of thrombomodulin-related disorders; however, as thrombomodulin not only regulates thrombus formation but also complement factor I-induced C3b inactivation, its association with CTEPH may be relevant [,].
Von Willebrand factor and related proteins have been associated with the development of CTEPH in several studies. Elevated levels of factor VIII and von Willebrand factor were found in patients with CTEPH, suggesting their role in its development; however, this finding also might be a marker of chronic inflammation and endothelial dysfunction in this disease []. Although elevated FVIII and vWF levels were described in our CTEPH patients, as compared to controls, no clearly causative mutations for this phenotype were identified in their corresponding genes. There were several VWF mutations in our CTEPH patients, among which two variants (p.Arg854Gln and p.Tyr1584Cys) are clearly associated with vWD and bleeding phenotype according to clinical databases, and they are not candidates as CTEPH risk factors [,]. The other three mutations (p.Thr1951Ala, p.Arg1399His and p.Thr1054Met) are not considered as vWD-causing ones; however, they may modify vWF levels (or function) and their contribution to thrombotic phenotype cannot be excluded []. Our patients carrying these variants all had rather elevated vWF:Ag and vWF:Ac levels. There is no data about ADAMTS13 p.Gln1174Term mutations in the available databases. It potentially leads to a truncated protein, and may be associated with a thrombotic phenotype with the risk of multiple microvascular thrombus formation.
ISTH-recommended platelet-related genes are basically not relevant from the point of view of CTEPH, as they are mainly associated with bleeding phenotype with thrombocytopenia and/or platelet functional defects. Based on the results of our study, only four platelet-related genes are worthy of consideration. The STIM1 gene associated with autosomal-dominant Stormorken syndrome with functional asplenia may be a candidate for further studies []. ETV6 encodes a transcriptional repressor, and its variants may be associated with impaired hematopoiesis and clonal abnormalities, whose features were suggested as risk factors for CTEPH []. THPO variants might be associated with increased thrombopoietin level; thus, recurrent thrombotic episodes may be present in carriers []. Finally, variants of the MPL gene are associated with thrombocytosis and an abnormal function of thrombopoietin receptor, which makes this gene and its variants considerable factors to be investigated [].
Vascular genes, especially those involved in hereditary hemorrhagic telangiectasia (HHT) and primary pulmonary hypertension, have been suggested to serve as risk factors for CTEPH development in previous studies [,]. We found variants in ENG (p.Gly191Asp, p.Thr5Met and p.Pro131Leu), which encodes endoglin, a component of the transforming growth factor-beta receptor complex, and it is an important vascular endothelium-associated glycoprotein. Mutations in ENG are mainly responsible for HHT1 [] and the abovementioned variants are considered as benign from the point of view of this disease. We do not know whether they have any effect on CTEPH development yet. ACVRL1 c.1378-216C > T is a likely benign mutation in the context of HHT2, and GDF2 p.Val211Met is a VUS in association with HHT5; however, their effect on CTEPH is not known. RASA1 is responsible for capillary malformation–arteriovenous malformation syndrome, a phenotype closely related to HHT (OMIM 608354). p.Gly89Arg, which was found in one of our CTEPH patients, is an undescribed variant and is potentially worthy of investigation. We described three variants in RNF213, the ring finger protein 213-encoding gene (c.2656-5A > G, p.Thr4638 and p.Gln2184Arg), which were not detected in the controls. As this gene was also suggested to be associated with CTEPH and a variant p.Arg4810Lys was found in patients with bad prognosis, our variants are also worthy of research []. SOX17 is associated with primary pulmonary hypertension (type 7) and its role in CTEPH may be plausible []. Variants p.Ala33Asp and p.Met270Leu are considered as likely benign for PPH type 7; however, their behavior in CTEPH is not known. The KDR gene encodes a growth factor receptor tyrosine kinase (VEGFR-2), to which vascular endothelial growth factor (VEGF) binds with high affinity, and it is involved in angiogenesis []. High VEGFR-2 expression might be associated with pulmonary hypertension []. Therefore, it is plausible that variants in KDR might play a role in CTEPH. In our cohort, two patients were carriers of a single variant, p.Cys482Arg; however, this was also detected in two control individuals. The role of genetic variants of bone morphogenic protein type II receptor (BMPR2) and angiotensin-converting enzyme (ACE) in CTEPH was suggested in previous studies; however, their significance is still controversial [,]. We did not find mutations in ACE in our cohort, and the mutation in BMPR2 (p.Ser775Asn) that was detected in one CTEPH patient was also found in a control subject. Based on the clinical findings of our patients carrying the BMPR2 and KDR variants, we hypothesize that these genes might play a role not directly in the development of CTEPH but rather in the progression and severity of the disease.
To summarize the results of our study, based on variant interpretations, the following genes may be candidates for more detailed research from the point of view of CTEPH development: F12, F13A1, F13B, F5, KNG1, SERPIND1, THBD, ADAMTS13, VWF, STIM1, ETV6, THPO, MPL, SERPINA1, ENG, RASA1, ACVRL1, GDF2, NFE2, SOX17 and RNF213; among these, some were also suggested previously by others [,].
We are aware of the small sample size in our study; however, due to the rarity of the disease, it was not possible to collect a larger number of patients. This small sample size, however, does not allow us to draw firm conclusions on the roles of these variants in this disease. It is known that sample size is critical from the point of view of precision and reliability of research findings. A small sample size may lead to inconclusive results, whereas a too-large sample may detect statistically significant but clinically negligible effects []. This is also the case in genetic studies, where a small sample size has low power to detect the effects of rare variants individually and can lead to an overestimation of their significance []. With this consideration, we cannot state clearly whether the variants found in our cohort have an impact on CTEPH; we rather give an overview of potential candidates for further investigations.
We could not support some previous findings in association with CTEPH in our cohort. First, we did not find alterations in genes encoding fibrinogen. According to a recent meta-analysis, the fibrinogen alpha p.Thr312Ala (FGA rs6050) polymorphism is positively associated with susceptibility to venous thromboembolism and CTEPH []. Moreover, five fibrinogen heterozygous gene mutations have been previously discovered in CTEPH compared to healthy controls in FGA and FGB genes, resulting in a disorganized fibrin structure and fibrinolytic resistance; however, these variants were not detected in our cohort either []. We also did not find mutations in CPB2 (TAFI), and the mutation found in BMPR2 in a CTEPH patient was also detected in the control group [,]. We did not detect the RNF213 p.Arg4810Lys variant in our patients; however, we identified other, potentially interesting rare mutations within this gene [].
Our study strengthens the hypothesis of heterogeneous genetic background and multifactorial nature of CTEPH, where not only gene–gene but also gene–environment interactions may contribute. The interaction between genetic variants and inflammatory pathways, for example, is an area of ongoing research, as chronic inflammation has been proposed as a contributing factor to thrombus persistence and vascular remodeling []. Moreover, gene–environment interactions involving anticoagulant therapy, lifestyle factors, and comorbidities such as obesity and metabolic syndrome may further modulate disease risk []. There may be an interplay between several pathways including thrombosis, altered fibrinolysis, defective angiogenesis, and inflammation, which may be inherited or acquired []. Recent studies utilizing WES have revealed novel genetic associations with PE and CTEPH, highlighting the potential for precision medicine approaches in identifying at-risk individuals [,]. Based on these studies, it is also suggested that a complex interaction between thrombotic–fibrinolytic processes, vascular remodeling, and pulmonary vasculature lesions may exist behind the pathogenesis of CTEPH, in which the contribution of different factors has not been elucidated yet.
These complex interactions are difficult to identify, and clinical studies focusing on distinct areas, like most genetic studies, do not have the chance to shed light on them. Different factors may have only minor effects on the development of the disease, and it is almost impossible to collect all pieces of the puzzle. We can not draw a clear conclusion, but rather may have suggestions for further investigations including larger clinical studies and in vitro experiments to confirm the contribution of a certain factor to the disease.
At our university center, most patients with acute PE are managed and followed using NOACs, reflecting this guideline-driven shift. However, in patients diagnosed with CTEPH, the anticoagulation regimen is typically converted to vitamin K antagonists (VKAs) after diagnosis, as VKAs remain the standard of care supported by stronger evidence in this specific population. These patients are followed by the experts of the CTEPH team. This practice is also endorsed by current CTEPH management recommendations []. Lifelong anticoagulation is indicated for all patients with confirmed CTEPH, and this approach was uniformly applied in our study.
Our study has several limitations. First, the sample size is rather small; however, as CTEPH is a rare disease, it is hard to collect a large number of patients in a single center. We focused only on rare variants in genes involved in thrombosis and hemostasis and in vascular diseases and did not investigate others involved in potentially relevant mechanisms, like inflammation, hematopoiesis, or intracellular signaling. We did not have the chance to examine the role of epigenetic factors, like the role of microRNAs in CTEPH or gene expression profile, or factors of clonal hematopoiesis; however, some studies have already described a potentially causative nature of these [,,,]. We could not study gene–dose effects—which would have been strenghtened genotype–phenotype association—in case of the identified variants, because all patients were heterozygous carriers of the identified mutations. We did not perform in silico analysis of the detected variants in this study; we only used the built-in prediction tools in the software that we applied for NGS data analysis. We did not have the chance to recruit a validation cohort and perform the validation in an independent sample. Despite limitations, our study has the advantage of comparing CTEPH individuals with those with PE but without CTEPH and our results may serve as a starting point for more detailed investigations of certain genes and variants with potential association with CTEPH in larger cohorts.
5. Conclusions
By investigation of rare mutations in genes involved in thrombosis and hemostasis and in vascular diseases, several potential candidate variants were identified, whose roles in CTEPH are worthy of further studies. We could not identify a single variant with a higher frequency in our small cohort, and most of the patients carried more than one mutation, suggesting the complex genetic background of the disease. Without further pieces of evidence, their role in CTEPH, of course, remains an assumption.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes16111336/s1. Table S1: Established thrombotic risk factors and/or abnormal values in parameters related to thrombosis in CTEPH patients and in PE patients without the development of CTEPH; Table S2: Variants related to platelet defects and detected in CTEPH patients; Table S3: Tier 1 platelet-dependent genes according to the ISTH recommendation, in which variants were found in CTEPH patients; Table S4: Genes associated with vascular diseases and/or development and included in virtual gene panel 2; Table S5: Combination of variants in Tier 1 and 2 ISTH genes and panel 2 genes in our CTEPH patients.
Author Contributions
Conceptualization, Z.B., G.K. and T.S.; methodology, É.M., S.B., P.E.B. and Z.B.; software, S.B.; validation, Z.B.; data curation, G.K., L.B., K.R., T.S., A.P. and J.B.; writing—original draft preparation, Z.B. and G.K.; writing—review and editing, T.S.; funding acquisition, Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hungarian Scientific Research Fund (OTKA K-139293). The APC was funded by University of Debrecen. This work was supported by the University of Debrecen Program for Scientific Publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Scientific and Ethical Committee at the University of Debrecen, Clinical Center, and by the National Scientific of Ethical Committee of Hungary (RKEB/IKEB: 6153-2022, 8 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| 6MWT | 6-Minute Walk Test |
| ACMG | American College of Medical Genetics and Genomics |
| ACE | Angiotensin-Converting Enzyme |
| ACVRL1 | Activin A Receptor-Like Type 1 |
| ADAMTS13 | A Disintegrin And Metalloproteinase with Thrombospondin Motifs 13 |
| APTT | Activated Partial Thromboplastin Time |
| AT | Antithrombin |
| BMI | Body Mass Index |
| BMPR2 | Bone Morphogenetic Protein Receptor Type 2 |
| BPA | Balloon Pulmonary Angioplasty |
| CAV1 | Caveolin 1 |
| CI | Cardiac Index |
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| CTPA | Computed Tomography Pulmonary Angiography |
| CO | Cardiac Output |
| CPB2 | Carboxypeptidase B2 |
| CT | Computed Tomography |
| DVT | Deep Vein Thrombosis |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ENG | Endoglin |
| ETV6 | Variant Transcription Factor 6 |
| F10 | Coagulation Factor X |
| F12 | Coagulation Factor XII |
| F13A1 | Coagulation Factor XIII A Chain |
| F13B | Coagulation Factor XIII B Chain |
| F5 | Coagulation Factor V |
| F8 | Coagulation Factor VIII |
| FGA | Fibrinogen Alpha Chain |
| FGB | Fibrinogen Beta Chain |
| FGG | Fibrinogen Gamma Chain |
| FVIII | Factor VIII |
| FXIII | Factor XIII |
| GDF2 | Growth Differentiation Factor 2 (also known as BMP9) |
| GnomAD | Genome Aggregation Database |
| HAE | Hereditary Angioedema |
| HGMD | Human Gene Mutation Database |
| HRG | Histidine-Rich Glycoprotein |
| HMWK | High-Molecular-Weight Kininogen |
| IBM SPSS | International Business Machines—Statistical Package for the Social Sciences |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| ISTH | International Society on Thrombosis and Haemostasis |
| KCNK3 | Potassium Channel Subfamily K Member 3 |
| KNG1 | Kininogen 1 |
| LA | Lupus Anticoagulant |
| mPAP | Mean Pulmonary Artery Pressure |
| MAF | Minor Allele Frequency |
| MAST2 | Microtubule-Associated Serine/Threonine Kinase 2 |
| MPL | Myeloproliferative Leukemia Protein |
| MR | Magnetic Resonance |
| NFE2 | Nuclear Factor, Erythroid 2 |
| NGS | Next-Generation Sequencing |
| NT-proBNP | N-terminal pro–B-type Natriuretic Peptide |
| NYHA | New York Heart Association (Functional Classification) |
| OMIM | Online Mendelian Inheritance in Man |
| PAI-1 | Plasminogen Activator Inhibitor Type 1 |
| PC | Protein C |
| PCR | Polymerase Chain Reaction |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PE | Pulmonary Embolism |
| PF1+2 | Prothrombin Fragment 1+2 |
| PLG | Plasminogen |
| PROC | Protein C Gene |
| PROS1 | Protein S Gene |
| PS | Protein S |
| PVR | Pulmonary Vascular Resistance |
| RAP | Right Atrial Pressure |
| RASA1 | RAS p21 Protein Activator 1 |
| RNF213 | Ring Finger Protein 213 |
| RBBB | Right Bundle Branch Block |
| SD | Standard Deviation |
| SERPINA1 | Serpin Family A Member 1 (Alpha-1 Antitrypsin) |
| SERPINC1 | Serpin Family C Member 1 (Antithrombin) |
| SERPIND1 | Serpin Family D Member 1 (Heparin Cofactor II) |
| SERPINE1 | Serpin Family E Member 1 (Plasminogen Activator Inhibitor Type 1) |
| SLC2A10 | Solute Carrier Family 2 Member 10 |
| SMAD4 | Mothers Against Decapentaplegic Homolog 4 |
| SMAD9 | Mothers Against Decapentaplegic Homolog 9 |
| SOX17 | SRY-Box Transcription Factor 17 |
| STIM1 | Stromal Interaction Molecule 1 |
| TFPI | Tissue Factor Pathway Inhibitor |
| THBD | Thrombomodulin |
| THPO | Thrombopoietin |
| TAT | Thrombin–Antithrombin Complex |
| tPA | Tissue-Type Plasminogen Activator |
| V/Q | Ventilation/Perfusion (Scan) |
| vWF or VWF | von Willebrand Factor |
| vWF:Ag | von Willebrand Factor Antigen |
| VUS | Variant of Uncertain Significance |
| WES | Whole-Exome Sequencing |
References
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef]
- Medrek, S.; Safdar, Z. Epidemiology and Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension: Risk Factors and Mechanisms. Methodist. Debakey Cardiovasc. J. 2016, 12, 195–198. [Google Scholar] [CrossRef]
- Lang, I.M.; Madani, M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014, 130, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.A.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.M.; Meyer, K.; Vizza, C.D.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef]
- Marchetta, S.; Verbelen, T.; Claessen, G.; Quarck, R.; Delcroix, M.; Godinas, L. A Comprehensive Assessment of Right Ventricular Function in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 12, 47. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Jais, X.; Jevnikar, M.; Boucly, A.; Weatherald, J.; Brenot, P.; Planche, O.; Parent, F.; Savale, L.; Fadel, E.; et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J. Heart Lung Transpl. 2019, 38, 833–842. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension: Results of a Multicenter Registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef] [PubMed]
- Kolodzey, G.; Peter, A.; Darago, A.; Balogh, L.; Bereczky, Z.; Barta, J.; Csanadi, Z.; Szuk, T. Assessment of Right Ventricular Pressure in Chronic Thromboembolic Pulmonary Hypertension: Comparison of Diagnostic Modalities and Balloon Pulmonary Angioplasty Outcomes. Diagnostics 2025, 15, 2050. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schafers, H.J.; Jansa, P.; Lindner, J.; Simkova, I.; Martischnig, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2009, 33, 325–331. [Google Scholar] [CrossRef]
- Wilkens, H.; Konstantinides, S.; Lang, I.M.; Bunck, A.C.; Gerges, M.; Gerhardt, F.; Grgic, A.; Grohe, C.; Guth, S.; Held, M.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Updated Recommendations from the Cologne Consensus Conference 2018. Int. J. Cardiol. 2018, 272S, 69–78. [Google Scholar] [CrossRef]
- Lang, I.; Kerr, K. Risk factors for chronic thromboembolic pulmonary hypertension. Proc. Am. Thorac. Soc. 2006, 3, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.W.; Allen-Brady, K.; Brown, L.M.; Elliott, C.G.; Cannon-Albright, L.A. Chronic Thromboembolic Pulmonary Hypertension Cases Cluster in Families. Chest 2019, 155, 384–390. [Google Scholar] [CrossRef]
- Colorio, C.C.; Martinuzzo, M.E.; Forastiero, R.R.; Pombo, G.; Adamczuk, Y.; Carreras, L.O. Thrombophilic factors in chronic thromboembolic pulmonary hypertension. Blood Coagul. Fibrinolysis. 2001, 12, 427–432. [Google Scholar] [CrossRef]
- Noiri, J.I.; Tsuji, A.; Ueda, J.; Miyata, T.; Neki, R.; Ida, K.; Kugo, K.; Osawa, R.; Kimura, T.; Asano, R.; et al. Familial onset of venous thromboembolism due to inherited antithrombin deficiency with a novel gene variant (p.Arg14Gly). J. Cardiol. Cases 2024, 30, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; D’Armini, A.M.; Morsolini, M.; Snijder, R.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef]
- Opitz, I.; Kirschner, M.B. Molecular Research in Chronic Thromboembolic Pulmonary Hypertension. Int. J. Mol. Sci. 2019, 20, 784. [Google Scholar] [CrossRef]
- Li, J.F.; Lin, Y.; Yang, Y.H.; Gan, H.L.; Liang, Y.; Liu, J.; Yang, S.Q.; Zhang, W.J.; Cui, N.; Zhao, L.; et al. Fibrinogen Aalpha Thr312Ala polymorphism specifically contributes to chronic thromboembolic pulmonary hypertension by increasing fibrin resistance. PLoS ONE 2013, 8, e69635. [Google Scholar] [CrossRef]
- Morris, T.A.; Marsh, J.J.; Chiles, P.G.; Magana, M.M.; Liang, N.C.; Soler, X.; Desantis, D.J.; Ngo, D.; Woods, V.L., Jr. High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood 2009, 114, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Remkova, A.; Simkova, I.; Valkovicova, T. Platelet abnormalities in chronic thromboembolic pulmonary hypertension. Int. J. Clin. Exp. Med. 2015, 8, 9700–9707. [Google Scholar]
- Lang, I.M.; Marsh, J.J.; Olman, M.A.; Moser, K.M.; Loskutoff, D.J.; Schleef, R.R. Expression of type 1 plasminogen activator inhibitor in chronic pulmonary thromboemboli. Circulation 1994, 89, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, N.; Satoh, K.; Satoh, T.; Sugimura, K.; Tatebe, S.; Yamamoto, S.; Aoki, T.; Miura, M.; Miyata, S.; Kawamura, T.; et al. Thrombin-Activatable Fibrinolysis Inhibitor in Chronic Thromboembolic Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1293–1301. [Google Scholar] [CrossRef]
- Simonneau, G.; Dorfmuller, P.; Guignabert, C.; Mercier, O.; Humbert, M. Chronic thromboembolic pulmonary hypertension: The magic of pathophysiology. Ann. Cardiothorac. Surg. 2022, 11, 106–119. [Google Scholar] [CrossRef]
- Dorfmuller, P.; Gunther, S.; Ghigna, M.R.; Thomas de Montpreville, V.; Boulate, D.; Paul, J.F.; Jais, X.; Decante, B.; Simonneau, G.; Dartevelle, P.; et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: A role for pulmonary veins and systemic vasculature. Eur. Respir. J. 2014, 44, 1275–1288. [Google Scholar] [CrossRef]
- Yan, L.; Li, X.; Liu, Z.; Zhao, Z.; Luo, Q.; Zhao, Q.; Jin, Q.; Yu, X.; Zhang, Y. Research progress on the pathogenesis of CTEPH. Heart Fail. Rev. 2019, 24, 1031–1040. [Google Scholar] [CrossRef]
- Ghani, H.; Pepke-Zaba, J. Chronic Thromboembolic Pulmonary Hypertension: A Review of the Multifaceted Pathobiology. Biomedicines 2023, 12, 46. [Google Scholar] [CrossRef]
- Dodson, M.W.; Cirulis, M.M.; Elliott, C.G. Analysis of family histories suggests shared genetic risk for chronic thromboembolic pulmonary hypertension and venous thromboembolism. Pulm. Circ. 2022, 12, e12170. [Google Scholar] [CrossRef]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M.; Pierangeli, S.S.; de Laat, B.; Tripodi, A.; Atsumi, T.; Ortel, T.L.; Subcommittee on Lupus Anticoagulant/Phospholipid/Dependent Antibodies. Testing for antiphospholipid antibodies with solid phase assays: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Suchon, P.; Germain, M.; Delluc, A.; Smadja, D.; Jouven, X.; Gyorgy, B.; Saut, N.; Ibrahim, M.; Deleuze, J.F.; Alessi, M.C.; et al. Protein S Heerlen mutation heterozygosity is associated with venous thrombosis risk. Sci. Rep. 2017, 7, 45507. [Google Scholar] [CrossRef]
- Curran, J.M.; Fatah-Ardalani, K.; Tornvall, P.; Humphries, S.E.; Green, F.R. A hypothesis to explain the reported association of the alpha-fibrinogen A312 allele with thromboembolic disease. Thromb. Haemost. 2001, 85, 1122–1123. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Szydlo, R.; Gibbs, S.; Laffan, M. Hereditary and acquired thrombotic risk factors for chronic thromboembolic pulmonary hypertension. Blood Coagul. Fibrinolysis. 2010, 21, 201–206. [Google Scholar] [CrossRef]
- Kido, K.; Shimizu, M.; Shiga, T.; Hashiguchi, M.; Jalil, B.; Caccamo, M.; Sokos, G. Meta-Analysis Comparing Direct Oral Anticoagulants Versus Vitamin K Antagonists in Patients With Chronic Thromboembolic Pulmonary Hypertension. Am. J. Cardiol. 2024, 210, 172–176. [Google Scholar] [CrossRef]
- Liley, J.; Newnham, M.; Bleda, M.; Bunclark, K.; Auger, W.; Barbera, J.A.; Bogaard, H.; Delcroix, M.; Fernandes, T.M.; Howard, L.; et al. Shared and Distinct Genomics of Chronic Thromboembolic Pulmonary Hypertension and Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2024, 209, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Scanavini, D.; Girelli, D.; Lunghi, B.; Martinelli, N.; Legnani, C.; Pinotti, M.; Palareti, G.; Bernardi, F. Modulation of factor V levels in plasma by polymorphisms in the C2 domain. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Wang, M.; Yu, J.; Martinelli, I.; Yu, F.; Passamonti, S.M.; Consonni, D.; Pappalardo, E.; Menegatti, M.; Scherer, S.E.; et al. Identification of genetic risk variants for deep vein thrombosis by multiplexed next-generation sequencing of 186 hemostatic/pro-inflammatory genes. BMC Med. Genom. 2012, 5, 7. [Google Scholar] [CrossRef]
- Bereczky, Z.; Muszbek, L. Factor XIII and venous thromboembolism. Semin. Thromb. Hemost. 2011, 37, 305–314. [Google Scholar] [CrossRef]
- Wei, L.K.; Griffiths, L.R.; Kooi, C.W.; Irene, L. Meta-Analysis of Factor V, Factor VII, Factor XII, and Factor XIII-A Gene Polymorphisms and Ischemic Stroke. Medicina 2019, 55, 101. [Google Scholar] [CrossRef]
- Mezei, Z.A.; Katona, E.; Kallai, J.; Bereczky, Z.; Somodi, L.; Molnar, E.; Kovacs, B.; Miklos, T.; Ajzner, E.; Muszbek, L. Factor XIII levels and factor XIII B subunit polymorphisms in patients with venous thromboembolism. Thromb. Res. 2017, 158, 93–97. [Google Scholar] [CrossRef]
- Balogh, L.; Katona, E.; Mezei, Z.A.; Kallai, J.; Gindele, R.; Edes, I.; Muszbek, L.; Papp, Z.; Bereczky, Z. Effect of factor XIII levels and polymorphisms on the risk of myocardial infarction in young patients. Mol. Cell Biochem. 2018, 448, 199–209. [Google Scholar] [CrossRef]
- Jankun, J.; Skrzypczak-Jankun, E. Val17Ile single nucleotide polymorphisms similarly as Ala15Thr could be related to the lower secretory dynamics of PAI-1 secretion: Theoretical evidence. Curr. Mol. Med. 2011, 11, 512–516. [Google Scholar] [CrossRef]
- Borsi, E.; Potre, C.; Ionita, I.; Samfireag, M.; Secosan, C.; Potre, O. Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Systematic Review of Prevalence, Clinical Phenotype, and Surgical Outcomes. Biomedicines 2025, 13, 2215. [Google Scholar] [CrossRef]
- Baglin, T.P.; Carrell, R.W.; Church, F.C.; Esmon, C.T.; Huntington, J.A. Crystal structures of native and thrombin-complexed heparin cofactor II reveal a multistep allosteric mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 11079–11084. [Google Scholar] [CrossRef]
- Takamori, N.; Azuma, H.; Kato, M.; Hashizume, S.; Aihara, K.; Akaike, M.; Tamura, K.; Matsumoto, T. High plasma heparin cofactor II activity is associated with reduced incidence of in-stent restenosis after percutaneous coronary intervention. Circulation 2004, 109, 481–486. [Google Scholar] [CrossRef]
- Delvaeye, M.; Noris, M.; De Vriese, A.; Esmon, C.T.; Esmon, N.L.; Ferrell, G.; Del-Favero, J.; Plaisance, S.; Claes, B.; Lambrechts, D.; et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009, 361, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Maga, T.; Meyer, N.C.; Wang, K.; Thomas, C.P.; Nester, C.M.; Smith, R.J. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2014, 25, 55–64. [Google Scholar] [CrossRef]
- Seidizadeh, O.; Peyvandi, F.; Mannucci, P.M. Von Willebrand disease type 2N: An update. J. Thromb. Haemost. 2021, 19, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, P.A.; Tijet, N.; Haberichter, S.L.; Flood, V.H.; Ross, J.; Notley, C.; Rawley, O.; Montgomery, R.R.; Zimmerman Project, I.; James, P.D.; et al. The common VWF variant p.Y1584C: Detailed pathogenic examination of an enigmatic sequence change. J. Thromb. Haemost. 2024, 22, 666–675. [Google Scholar] [CrossRef]
- Sadler, B.; Christopherson, P.A.; Haller, G.; Montgomery, R.R.; Di Paola, J. von Willebrand factor antigen levels are associated with burden of rare nonsynonymous variants in the VWF gene. Blood 2021, 137, 3277–3283. [Google Scholar] [CrossRef]
- Lafabrie, E.; Vrdoljak Pazur, M.; Laporte, J.; Bohm, J. STIM1 in-frame deletion of eight amino acids in a patient with moderate tubular aggregate myopathy/Stormorken syndrome. J. Med. Genet. 2025, 62, 381–387. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Churpek, J.E.; Keel, S.B.; Walsh, T.; Lee, M.K.; Loeb, K.R.; Gulsuner, S.; Pritchard, C.C.; Sanchez-Bonilla, M.; Delrow, J.J.; et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015, 47, 180–185. [Google Scholar] [CrossRef]
- Graziano, C.; Carone, S.; Panza, E.; Marino, F.; Magini, P.; Romeo, G.; Pession, A.; Seri, M. Association of hereditary thrombocythemia and distal limb defects with a thrombopoietin gene mutation. Blood 2009, 114, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Moliterno, A.R.; Williams, D.M.; Gutierrez-Alamillo, L.I.; Salvatori, R.; Ingersoll, R.G.; Spivak, J.L. Mpl Baltimore: A thrombopoietin receptor polymorphism associated with thrombocytosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11444–11447. [Google Scholar] [CrossRef]
- Xi, Q.; Liu, Z.; Zhao, Z.; Luo, Q.; Huang, Z. High Frequency of Pulmonary Hypertension-Causing Gene Mutation in Chinese Patients with Chronic Thromboembolic Pulmonary Hypertension. PLoS ONE 2016, 11, e0147396. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Simeoni, I.; Downes, K.; Frazer, Z.C.; Megy, K.; Bernabeu-Herrero, M.E.; Shurr, A.; Brimley, J.; Patel, D.; Kell, L.; et al. Mutational and phenotypic characterization of hereditary hemorrhagic telangiectasia. Blood 2020, 136, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Asano, R.; Ishibashi, T.; Endo, H.; Fujisaki, S.; Takano, R.; Akao, M.; Nishi, N.; Hayashi, H.; Kotoku, A.; et al. Balloon Pulmonary Angioplasty in Heterozygous RNF213 p.Arg4810Lys Variant Carriers Diagnosed With Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2025, 14, e039002. [Google Scholar] [CrossRef]
- Sun, W.; Wu, T.; Zhou, Z.; Jiang, D.; Wei, T.Y.; Han, S.Y.; Shyy, J.; Li, G.; Shi, R. SOX17 Regulates Nestin/p16(INK4a) Axis to Mitigate Endothelial Senescence in Pulmonary Arterial Hypertension. Res. Sq. 2025, 1–27. [Google Scholar] [CrossRef]
- Shah, F.H.; Nam, Y.S.; Bang, J.Y.; Hwang, I.S.; Kim, D.H.; Ki, M.; Lee, H.W. Targeting vascular endothelial growth receptor-2 (VEGFR-2): Structural biology, functional insights, and therapeutic resistance. Arch. Pharm. Res. 2025, 48, 404–425. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, K.; Zeng, L.; He, J.; Gao, X.; Gu, X.; Chen, X.; Jing Li, J.; Wang, M.; Wu, D.; et al. Targeting VEGF-A/VEGFR2 Y949 Signaling-Mediated Vascular Permeability Alleviates Hypoxic Pulmonary Hypertension. Circulation 2022, 146, 1855–1881. [Google Scholar] [CrossRef]
- Feng, Y.X.; Liu, D.; Sun, M.L.; Jiang, X.; Sun, N.; Mao, Y.M.; Jing, Z.C. BMPR2 germline mutation in chronic thromboembolic pulmonary hypertension. Lung 2014, 192, 625–627. [Google Scholar] [CrossRef]
- Heeneman, S.; Sluimer, J.C.; Daemen, M.J. Angiotensin-converting enzyme and vascular remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, N.; Satoh, K.; Satoh, T.; Shimizu, T.; Saito, S.; Sugimura, K.; Tatebe, S.; Yamamoto, S.; Aoki, T.; Kikuchi, N.; et al. Identification of the Novel Variants in Patients With Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e015902. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafo, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef]
- Lee, S.; Abecasis, G.R.; Boehnke, M.; Lin, X. Rare-variant association analysis: Study designs and statistical tests. Am. J. Hum. Genet. 2014, 95, 5–23. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, H.; Zhang, Y.; Wei, Z.; Xia, L.; Yang, J. Association of Fibrinogen Aalpha Thr312Ala (rs6050) Polymorphism with Venous Thrombosis and Chronic Thromboembolic Pulmonary Hypertension: A Meta-Analysis. Clin. Appl. Thromb. Hemost. 2025, 31, 10760296251314476. [Google Scholar] [CrossRef]
- Marsh, J.J.; Chiles, P.G.; Liang, N.C.; Morris, T.A. Chronic thromboembolic pulmonary hypertension-associated dysfibrinogenemias exhibit disorganized fibrin structure. Thromb. Res. 2013, 132, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Szamalek-Hoegel, J.; Hersberger, M.; Fischler, M.; Garcia, J.S.; Huber, L.C.; Grunig, E.; Janssen, B.; Speich, R. Sequence variants in BMPR2 and genes involved in the serotonin and nitric oxide pathways in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Relation to clinical parameters and comparison with left heart disease. Respiration 2010, 79, 279–287. [Google Scholar] [CrossRef]
- Kurakula, K.; Smolders, V.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef]
- Martin, K.A.; Cuttica, M.J. Chronic thromboembolic pulmonary hypertension: Anticoagulation and beyond. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 478–484. [Google Scholar] [CrossRef]
- Sacks, R.S.; Remillard, C.V.; Agange, N.; Auger, W.R.; Thistlethwaite, P.A.; Yuan, J.X. Molecular biology of chronic thromboembolic pulmonary hypertension. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 265–276. [Google Scholar] [CrossRef]
- Morris, T.A. Why acute pulmonary embolism becomes chronic thromboembolic pulmonary hypertension: Clinical and genetic insights. Curr. Opin. Pulm. Med. 2013, 19, 422–429. [Google Scholar] [CrossRef]
- Wiedenroth, C.B.; Jenkins, D.; Brenot, P.; Lang, I.M.; Matsubara, H.; Pepke-Zaba, J.; Channick, R.; Jais, X.; Simonneau, G.; Delcroix, M.; et al. Management of chronic thromboembolic pulmonary hypertension. J. Heart Lung Transpl. 2025, 44, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nakajima, T.; Tanabe, N.; Hinohara, K.; Sakao, S.; Kasahara, Y.; Tatsumi, K.; Inoue, Y.; Kimura, A. Susceptibility to chronic thromboembolic pulmonary hypertension may be conferred by miR-759 via its targeted interaction with polymorphic fibrinogen alpha gene. Hum. Genet. 2010, 128, 443–452. [Google Scholar] [CrossRef]
- Gu, S.; Su, P.; Yan, J.; Zhang, X.; An, X.; Gao, J.; Xin, R.; Liu, Y. Comparison of gene expression profiles and related pathways in chronic thromboembolic pulmonary hypertension. Int. J. Mol. Med. 2014, 33, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Kenneweg, F.; Hobohm, L.; Bang, C.; Gupta, S.K.; Xiao, K.; Thum, S.; Ten Cate, V.; Rapp, S.; Hasenfuss, G.; Wild, P.; et al. Circulating miR-let7a levels predict future diagnosis of chronic thromboembolic pulmonary hypertension. Sci. Rep. 2024, 14, 4514. [Google Scholar] [CrossRef] [PubMed]
- Momoi, M.; Katsumata, Y.; Kunimoto, H.; Inami, T.; Miya, F.; Anzai, A.; Goto, S.; Miura, A.; Shinya, Y.; Hiraide, T.; et al. Clonal Hematopoiesis in Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2024, 13, e035498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).