Abstract
Nitrate transporter 2 (NRT2) proteins play vital roles in both nitrate (NO3−) uptake and translocation as well as abiotic stress responses in plants. However, little is known about the NRT2 gene family in Brassica rapa. In this study, 14 NRT2s were identified in the B. rapa genome. The BrNRT2 family members contain the PLN00028 and MATE_like superfamily domains. Cis-element analysis indicated that regulatory elements related to stress responses are abundant in the promoter sequences of BrNRT2 genes. BrNRT2.3 expression was increased after drought stress, and BrNRT2.1 and BrNRT2.8 expression were significantly upregulated after salt stress. Furthermore, protein interaction predictions suggested that homologs of BrNRT2.3, BrNRT2.1, and BrNRT2.8 in Arabidopsis thaliana may interact with the known stress-regulating proteins AtNRT1.1, AtNRT1.5, and AtNRT1.8. In conclusion, we suggest that BrNRT2.1, BrNRT2.3, and BrNRT2.8 have the greatest potential for inducing abiotic stress tolerance. Our findings will aid future studies of the biological functions of BrNRT2 family genes.
1. Introduction
Nitrogen is an important element for the growth and development of plants, as it plays a significant role in shaping the composition of proteins and nucleic acids. Furthermore, nitrogen is a key factor affecting both the yield and quality of crops. Within the soil environment, the forms of nitrogen that plants utilize include ammonium nitrogen (NH4+) and nitrate nitrogen (NO3−). Nitrate is the primary nitrogen source for most dryland crops, including wheat, soybean, and Chinese cabbage. Its uptake and transport are facilitated by nitrate transport proteins. Nitrate transporter 2 (NRT2) is part of the NITRATE/NITRITE PORTER (NNP) family, which in turn belongs to the MAJOR FACILITATOR SUPERFAMILY (MFS). The structure of the NRT2 protein generally includes 500–600 amino acids (aa) and contains 12 transmembrane helical segments [1]. This protein was first discovered and characterized in Aspergillus nidulans [2]. Molecular cloning of the NRT2 gene in higher plants was first reported in barley [3]. NRT2 family members are categorized as high-affinity transport system (HATS), and most of the family members need to bind to the molecular chaperone nitrate-assimilation-related 2 (NAR2 or NRT3) to activate their high-affinity transport activity, which is important when there is a low concentration of exogenous nitrate; NRT2 family members, thus, play a key role in plant growth and development.
Several studies have reported the functions and evolutionary history of NRT2 genes in different plant species. NRT2s are responsible for nitrate absorption and transport in plants. In A. thaliana, seven members of the NRT2 family have been identified [4]. AtNRT2.1 performs a key dual role in controlling root development with external NO3− availability [5], and AtNRT2.2 and AtNRT2.1 synergistically regulate NO3− uptake and the dynamic response of plants to changes in the environmental nitrogen content [6]. AtNRT2.4 plays a dual role in the shoots and roots of nitrogen-starved plants [7]. AtNRT2.5 is necessary to support the growth of nitrogen-starved mature plants by working with AtNRT2.1, AtNRT2.2, and AtNRT2.4, which ensures the efficient nitrate uptake, and by facilitating nitrate loading into the phloem during nitrate remobilization [8]. AtNRT2.7 is mainly responsible for the loading of nitrate in seed vacuoles and plays a role in NO3− storage [9]. In bread wheat (Triticum aestivum L.), TaNRT2.1 is involved in nitrate uptake at the post-flowering stage [10]. In chrysanthemum (Chrysanthemum morifolium), CmNRT2.1 is a nitrate-inducible gene, and its activity can affect nitrate uptake [11]. In pineapple (Ananas comosus), AcNRT2.2 is highly expressed in the roots, suggesting that AcNRT2.2 might have a significant impact on alleviating nitrate deficiency [12].
NRT2s also play a role in mediating resistance to biotic and abiotic stress. In A. thaliana, AtNRT2.1 has been shown to regulate root hydraulic conductivity and plasma membrane aquaporin activity [13], suggesting that it may enhance plant drought resistance and other processes related to root hydraulic conductivity. Additionally, AtNRT2.1 works as a significant contributor to Cd uptake by modulating nitrate uptake in high-affinity nitrate systems [14], and NRT2.1 can also affect plant disease resistance by downregulating biotic stress defense mechanisms and promoting abiotic stress responses [15]. AtNRT2.6 expression is induced after inoculation of A. thaliana with the phytopathogenic bacterium Erwinia amylovora. The reduced expression of NRT2.6 can reduce pathogen tolerance, and its activity is likely linked to ROS production under biotic and abiotic stress [16]. In B. napus (Brassica napus L.), a total of seventeen members were identified. BnNRT2.1a, BnNRT2.5s, and BnNRT2.7s were found to be involved in the response to waterlogging stress. BnNRT2.7s plays an important role under P- and K-deficient conditions [17]. In tomato (Solanum lycopersicum), four NRT2 members have been identified to positively affect the response of plants to drought and salt stress [18].
In addition, NRT2s also regulate the transport of auxin to the root system of plants to participate in nitrate-dependent root elongation [19]. They also play a role in regulating the control of cytokinins [20] and are involved in the biosynthesis and signal transduction of ethylene [21]. Their significance is further supported by their contribution to the root morphogenesis of A. thaliana [5]. They enhance the pH-buffering capacity of plants [22] and promote the uptake of manganese [23] and phosphorus in rice [24].
Chinese cabbage (B. rapa) is a biennial herb of the genus Brassica in the Cruciferae family that is widely grown in Asia. It thrives in mild climates and soils with abundant moisture and nitrogen fertilizer. However, B. rapa exhibits low nitrogen utilization and frequently faces challenges such as drought and salt stress, which significantly hinders its yield and quality [25,26]. An increasing number of studies indicate that processes involved in nitrogen assimilation, metabolism, and transport are intimately tied to drought and salt stress in plants. For example, the adverse effects of drought stress on Malus prunifolia can be mitigated when the nitrogen supply is robust [27]. In the case of oil palm, reactive nitrogen metabolic activities and nitrate assimilation processes contribute to the response of plants to drought stress [28]. Additional studies on sorghum and tomato revealed that the introduction of exogenous nitrogen could notably alleviate the intake of Na+ and bolster the K+ content in these plants [29,30]. There is, thus, a need to clarify the roles and molecular mechanisms of related genes. This knowledge is important for enhancing the yield and quality of B. rapa and strengthening its resilience to drought and salt stress. In this study, we obtained basic information on BrNRT2 genes and their expression profiles after drought stress and salt stress treatments. The insights gained from this study will contribute to future studies aimed at clarifying the functions of NRT2 genes.
2. Materials and Methods
2.1. Identification, Physicochemical Characterization, Chromosomal Localization, and Subcellular Localization of BrNRT2s
In this study, the whole genome sequence of A. thaliana was downloaded from EnsemblPlants (release 56) (http://plants.ensembl.org/, accessed on 1 May 2023) to extract the NRT2 protein sequence of A. thaliana via TBtools (v1.120) [31]. B. rapa NRT2 (BrNRT2) genes were predicted from the B. rapa genome via the BLASTP tool with the A. thaliana NRT2 (AtNRT2) aa sequences as input, and candidate genes were then further identified by conserved structural domain analysis. TBtools (v1.120) combined with Expasy (v3.0) (https://www.expasy.org/, accessed on 1 May 2023) [32] was used to analyze the physicochemical properties of the BrNRT2 genes, such as isoelectric point (pI), protein molecular weight (MW), and amino acid length (aa). Subcellular localization was predicted using WoLF PSORT (v0.2) [33] (http://www.genscript.com/wolf-psort.html, accessed on 1 May 2023). We then extracted the location information of BrNRT2 family members from the gene structure annotation information file of B. rapa and mapped their positions on chromosomes using TBtools (v1.120).
2.2. Phylogenetic Tree Construction and Collinearity Analysis
The aa sequences of BrNRT2s, AtNRT2s, OsNRT2s, PtNRT2s, and HvNRT2s were aligned using the MUSCLE algorithm in MEGA X (v11.0.13), and a phylogenetic tree was constructed using the maximum likelihood method (model: LG + G) in MEGA X with 1000 bootstrap replicates to ensure the accuracy and reliability of our results [34]. The phylogenetic tree was visualized using the online software iTOL (v6.8) [35] (https://itol.embl.de/, accessed on 1 May 2023). To explore the genetic homology across different plant species, we downloaded the genomic and annotation information files of Arabidopsis, soybean, and potato from the EnsemblPlants database (release 56) (http://plants.ensembl.org/, accessed on 1 May 2023). The MCScanX (Multiple Collinearity Scan toolkit) plug-in in TBtools (v1.120) was used to analyze interspecies collinearity.
2.3. Gene Structure and Conserved Domain Analysis
The structure of BrNRT2s was visualized using the Visualize Gene Structure program of TBtools (v1.120) [31].
We analyzed the conserved domains of BrNRT2s using the Web CD-Search tools (v3.18) on the NCBI website (https://www.ncbi.nlm.nih.gov/cdd, accessed on 1 May 2023) [36], and we used TBtools (v1.120) to visualize the results. The conserved motifs of the identified BrNRT2s were analyzed using the online tool MEME (v5.5.1) (http://meme-suite.org/tools/meme, accessed on 1 May 2023), and the predicted number of motifs was set to 10 [37].
2.4. Cis-Regulatory Elements, and GO Enrichment Analysis
TBtools (v1.120) was used to extract the 2.0 kb promoter sequence upstream of the start codon of BrNRT2s. The characteristics of the cis-regulatory elements were predicted using the PlantCARE (v1) (http:/bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 3 May 2023) [38] with the default parameters.
In addition, to analyze NRT2s based on their functional similarity, we performed GO enrichment analysis using the online DAVID database (v2023q1) [39] (https://david.ncifcrf.gov/, accessed on 3 May 2023), and the GO annotation data were processed and graphically demonstrated using bioinformatics (http://www.bioinformatics.com.cn/, accessed on 3 May 2023).
2.5. Transcriptome Data Expression, Stress Treatments, Total RNA Extraction, and qRT-PCR
Transcriptome data for the tissue-specific expression of B. rapa (GSE43245) and drought-sensitive plants under drought stress (GSE73963) were downloaded from the Brassicaceae Database (v3.0) (http://www.brassicadb.cn/, accessed on 6 May 2023). Heatmaps of gene expression profiles were prepared using TBtools (v1.120).
The first-generation hybrid cultivar of B. rapa with stable self-incompatibility was used for stress treatments. Plump seeds were seeded in MS Modified Medium (with vitamins, Sucrose, Agar) (PM10121-307, Coolaber, Beijing, China) and cultivated in a plant incubator (16 h light/8 h dark photoperiod at 25 °C, light intensity 2000 lx). When the seedlings were 4 weeks old, seedlings with similar growth conditions were subjected to stress treatments. These seedlings were immersed in a 150 mmol·L−1 NaCl solution prepared with half-strength Hoagland nutrient solution (pH = 5.8) to simulate salt stress; they were also immersed in 15% PEG6000 to simulate drought conditions, whereas normal hydroponic seedlings were used as the control (the hydroponic pots had a volume of 10 L). All stress treatments were administered for 0, 4, 6, and 12 h for a total of 4 treatments. Each treatment consisted of three biological replicates. The leaves and roots of B. rapa under drought and salt stress were sampled and used for RNA extraction and qRT-PCR analysis.
Total RNA was extracted using the SteadyPure Universal RNA Extraction Kit (Accuratel Biology, Hunan, China). The qRT-PCR primer sequences (Table S1) were designed using the qPrimerDB-qPCR Primer Database (v2.0) (https://biodb.swu.edu.cn/qprimerdb/, accessed on 11 May 2023) [40] and synthesized by Qingdao PrimeTech Zixi Biotechnology Co. Ltd. The qRT-PCR reactions were performed on a QuantStudio 3 system (Applied Biosystems, Waltham, MA, USA) with ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China), and BraActin2 (accession: Bra037560) was used as the internal reference gene. The reactions were performed with three technical replicates. The relative expression levels of BrNRT2 genes were analyzed using the 2−∆∆CT method and graphed using Excel 2016.
2.6. NRT2 Protein Secondary Structure, Tertiary Structure, Protein Transmembrane Domains, and Protein Interaction Network Analysis
For protein secondary structure analysis, we utilized the PRABI tool (v5.0.5) (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html, accessed on 12 May 2023), and the results were analyzed and plotted using Excel 2016. SWISS-MODEL (v1.0) was used to (https://swissmodel.expasy.org/, accessed on 12 May 2023) [41] predict tertiary structure of BrNRT2 proteins.
TMHMM Server v2.0 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0, accessed on 12 May 2023) was used to predict the transmembrane domains of BrNRT2 proteins [42].
The STRING website (v11.5) (https://cn.string-db.org/, accessed on 12 May 2023) was used to predict protein–protein interaction (PPI) relationships [43]. In this study, we selected “A. thaliana” as the focal organism and used the “single protein by sequence” method. Specifically, we searched for A. thaliana NRT2 proteins in the STRING database that were similar to BrNRT2.1, BrNRT2.3, and BrNRT2.8 aa sequences using the BLASTP method. From this analysis, we selected the most similar proteins to establish a comprehensive protein interaction network diagram.
3. Results
3.1. Identification, Physicochemical Characterization, Chromosomal Localization, and Subcellular Localization of BrNRT2s
A total of 14 BrNRT2 genes were screened from the B. rapa genome, and they were unequally distributed on 6 of the 10 chromosomes of B. rapa (Figure 1). There were two BrNRT2 genes on chromosomes 1, 2, 8, 9, and 10. Four genes were mapped to chromosome 6. They were named BrNRT2.1~BrNRT2.14 (Table 1) according to their sequences on the chromosomes. To investigate the physicochemical properties of these BrNRT2 genes, we used ExPASy online software and found that the isoelectric point (pI) of the proteins ranged from 6.89 (BrNRT2.7) to 9.15 (BrNRT2.4). The number of basic proteins (12) was significantly greater than the number of acidic proteins (2). The molecular weights of the proteins ranged from 52,132.17 Da (BrNRT2.3) to 62,619.10 Da (BrNRT2.9). The lengths of the BrNRT2 proteins ranged from 484 aa (BrNRT2.3) to 575 aa (BrNRT2.9). The subcellular localization analysis of BrNRT2s showed that all BrNRT2 proteins were distributed in the plasma membrane (plas), suggesting that these proteins might perform their functions on the plasma membrane and that they potentially mediate nitrate transport.
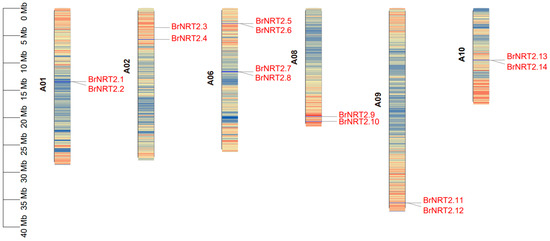
Figure 1.
Chromosomal localization of BrNRT2s. The chromosomal position of each NRT2 gene was mapped according to the B. rapa genome. The number of chromosomes is listed on the left side of each chromosome. Each chromosome shows gene density.

Table 1.
Information of NRT2 family genes in B. rapa.
3.2. Phylogenetic Tree Construction and Collinearity Analysis
To better understand the evolutionary features among NRT2 family members, a phylogenetic tree based on sequence similarity was created for the NRT2 family genes from diverse plant species using the maximum likelihood method (Figure 2A). BrNRT2 genes were classified into four subfamilies: BrNRT2.1, BrNRT2.2, BrNRT2.4, BrNRT2.7, BrNRT2.8, and BrNRT2.14 belong to the same subfamily; BrNRT2.5, BrNRT2.6, BrNRT2.10, BrNRT2.11, BrNRT2.12, and BrNRT2.13 belong to the same subfamily; BrNRT2.3 belongs to a separate subfamily; and BrNRT2.9 belongs to another subfamily. BrNRT2.3 and AtNRT2.7, BrNRT2.9 and AtNRT2.5, and BrNRT2.13 and AtNRT2.4 are directly homologous. Most BrNRT2 genes were found to have a covalent relationship with AtNRT2 genes.
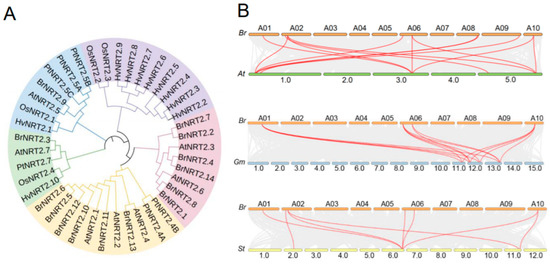
Figure 2.
Phylogenetic tree and collinearity analysis of BrNRT2s. (A) A phylogenetic tree of NRT2 proteins from different plants. Different colors represent different branches; B. rapa (Br), A. thaliana (At), Oryza sativa (Os), Populus trichocarpa (Pt), Hordeum vulgare (Hv). (B) Collinearity analysis of BrNRT2 members. The red lines indicate BrNRT2 gene members with collinearity, and the gray lines indicate other genes; Glycine max (Gm), Solanum tuberosum (St).
To gain further insights into the origin and evolution of BrNRT2 genes, we examined the syntenic relationships between B. rapa and three other plants (A. thaliana, G. max, and S. tuberosum). There were nine collinear pairs of BrNRT2 genes and AtNRT2 genes. There were six collinear pairs of BrNRT2 genes and GmNRT2 genes and six collinear pairs of BrNRT2 genes and StNRT2 genes. These observations suggest that B. rapa and A. thaliana are evolutionarily closely related and functionally similar; these findings aid exploration of the functions of BrNRT2s (Figure 2B).
3.3. Gene Structure and Conserved Domain Analysis
To explore the structure and function of BrNRT2 genes, we visualized and analyzed the exon–intron structure of BrNRT2 genes. The gene structure analysis (Figure 3A) showed that BrNRT2.1, BrNRT2.3, and BrNRT2.8 had one intron and two exons. BrNRT2.10, BrNRT2.11, BrNRT2.12, and BrNRT2.14 had two introns and three exons. BrNRT2.2, BrNRT2.4, BrNRT2.5, BrNRT2.6, BrNRT2.7, and BrNRT2.13 had three introns and four exons. The gene structure of BrNRT2.9 is significantly different from other family members, with 6 introns and 7 exons.
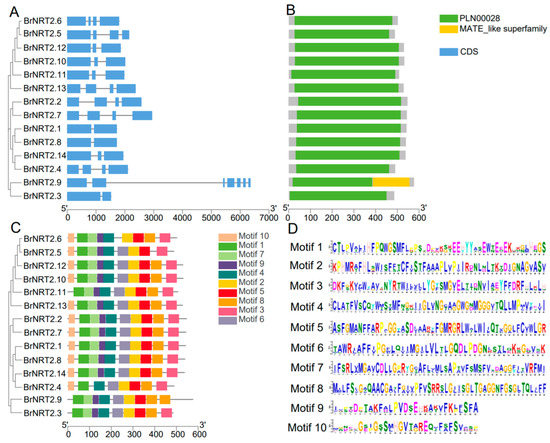
Figure 3.
Gene structure and conserved domain analysis of BrNRT2s. (A) Analysis of gene structure. The introns and exons are shown as black lines and blue boxes, respectively. (B) Analysis of conserved domains of BrNRT2 genes. (C) Analysis of the conserved motifs of BrNRT2 genes. The length of each motif is also shown. (D) Motif sequence.
To predict the conserved domains of NRT2 proteins in B. rapa (Figure 3B), we utilized NCBI-CDD and identified two functional domains present in this gene family: PLN00028 and MATE_like superfamily. BrNRT2.9 has a unique combination of PLN00028 and MATE_Like superfamily domains, suggesting that this gene potentially has special functions. The other 13 family members only have PLN00028 domain.
We predicted 10 conserved motifs of BrNRT2 genes (Figure 3C,D). All BrNRT2 genes contained Motif 1, Motif 2, Motif 4, and Motif 5, indicating that these are the core motifs of BrNRT2s. BrNRT2.9 contained a duplicate Motif 8. BrNRT2.1, BrNRT2.2, BrNRT2.7, BrNRT2.8, BrNRT2.10, BrNRT2.12, BrNRT2.13, and BrNRT2.14 all had 10 Motifs; Motif 7 and Motif 9 were absent in BrNRT2.4, Motif 8 was absent in BrNRT2.5, Motif 10 was absent in BrNRT2.3 and BrNRT2.11, and Motif 6 was absent in BrNRT2.6.
3.4. Cis-Elements Analysis
To further investigate the mechanism of the BrNRT2 genes in response to abiotic stress, the 2 kb promoter sequences upstream of the start codon of BrNRT2s were analyzed (Figure 4). Each member of the BrNRT2 family contains abundant light-responsive elements, which emphasizes the key role of light signal regulation in the growth and development of B. rapa. Additionally, all members contain phytohormone response elements; approximately 90% of the BrNRT2 genes contain ABA elements, and approximately 85% of BrNRT2 genes contain methyl jasmonate (MeJA)-responsive elements. With the exception of BrNRT2.7 and BrNRT2.12, all members contained cis-regulatory elements related to abiotic stress, including low-temperature-responsive elements, drought-inducibility elements, defense- and stress-responsive elements, and anaerobic induction elements. These results indicate that members of the BrNRT2 gene family play a fundamental role in enhancing abiotic stress tolerance in plants.
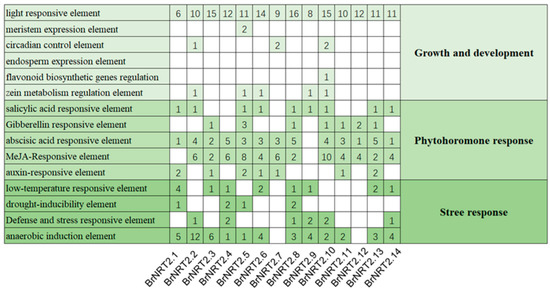
Figure 4.
Predicted cis-elements in BrNRT2 promoters. The figure shows the number of cis-elements contained in the BrNRT2 promoters.
3.5. GO Enrichment Analysis
GO analysis on the BrNRT2 genes contributed to determining their functions. GO analysis showed that BrNRT2 genes were enriched in biological process (BP), cellular component (CC), and molecular function (MF) (Figure 5; Table S2). Based on a previous study, nitrate transporters play an essential role in drought and salt tolerance [18]. The three enriched terms in the BP category were nitrate transport (GO:0015706), cellular response to nitrate (GO:0071249), and nitrate assimilation (GO:0042128). This prompted us to further characterize the expression changes of BrNRT2 family members under drought and salt stress conditions. The three enriched terms in the CC category were plant-type vacuole membrane (GO:0009705), plasma membrane (GO:0005886), and integral component of membrane (GO:0016021). The GO–MF enrichment results revealed one enriched term: nitrate transmembrane transporter activity (GO:0015112). Two BrNRT2 genes were enriched in the nitrate assimilation term; nine genes were enriched in nitrate transport and cellular response to nitrate, albeit with a small p-value but high confidence; and nine genes were enriched in plant-type vacuole membrane, plasma membrane, integral component of membrane, and nitrate transmembrane transporter activity.
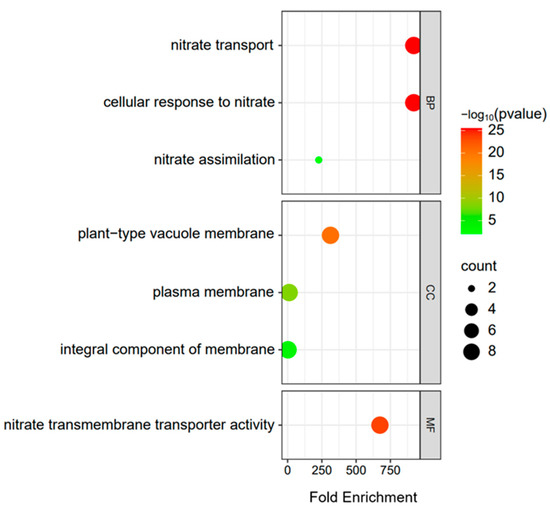
Figure 5.
GO enrichment analysis of BrNRT2s. The size and color of the dot bubbles indicate the number of genes and p-value, respectively.
3.6. Gene Expression Analysis
3.6.1. Tissue-Specific Expression
To investigate the expression of the BrNRT2 genes in various tissues of B. rapa, we obtained tissue-specific transcriptome data from the Brassicaceae Database. The transcriptome data of BrNRT2s extracted from five different tissues (root, stem, flower, leaf, and silique) (Figure 6; Table S3) revealed that the expression levels of BrNRT2.1, BrNRT2.5, BrNRT2.6, BrNRT2.8, BrNRT2.10, BrNRT2.11, BrNRT2.12, and BrNRT2.13 were significantly higher in the roots than in the other tissues, indicating that these genes may play an important role in nitrate uptake from the soil and in the response to root-associated stresses. BrNRT2.4 and BrNRT2.14 were only highly expressed in the stems. These genes, which were specifically expressed in a single tissue, may play a role in loading and discharging nitrate. BrNRT2.2 and BrNRT2.7 were highly expressed in stem and flower tissues. BrNRT2.3 was highly expressed in stem and leaf tissues, and BrNRT2.9 was highly expressed in root, stem, and leaf tissues, suggesting that these genes may play a role in the long-distance transport of nitrate from the roots to the aboveground parts. The expression of all genes in the callus was not significant. These tissue-specific expression differences among the BrNRT2 genes suggest that they play distinct roles in different stages of plant development.
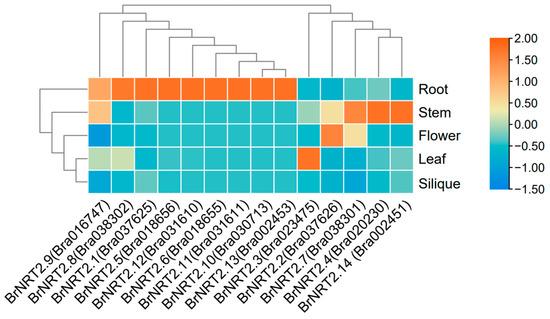
Figure 6.
Expression patterns of BrNRT2s in different tissues. The abundance of each gene was determined using TPM.
3.6.2. Effect of Drought Stress on the Expression of BrNRT2s
Drought stress can negatively affect B. rapa production. To gain a preliminary understanding of the function of BrNRT2 genes in response to drought stress, we obtained the transcriptome sequencing data of drought-sensitive (DS) B. rapa from the Brassicaceae Database (Figure 7A) and used RNA-seq to detect the expression levels of BrNRT2 genes after drought stress (Figure 7B). Three genes (BrNRT2.1, BrNRT2.3, and BrNRT2.10) with high expression levels in B. rapa were selected for further qRT-PCR detection (Figure 7C). The relative expression of BrNRT2.1 peaked after 4 h of drought treatment and then decreased. The relative expression of BrNRT2.3 was upregulated significantly at all time points, and its expression was 4–7-fold higher under drought stress relative to the CK treatment. The relative expression of BrNRT2.10 initially increased and then decreased, reaching a maximum after 4 h of drought treatment.
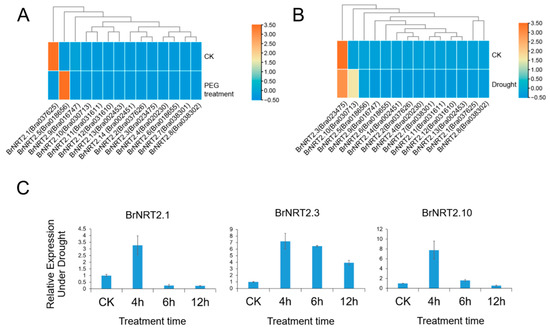
Figure 7.
Expression analysis of BrNRT2s under drought stress. (A) Analysis of transcriptome data of NRT2 genes under drought stress in drought-sensitive B. rapa obtained from the Brassicaceae Database. (B) Heatmap of expression patterns of BrNRT2 genes under drought stress. (C) The relative expression levels of BrNRT2 genes under drought stress were analyzed using qRT-PCR.
3.6.3. Effect of Salt Stress on the Expression of BrNRT2s
Salt stress can also negatively affect the yield and quality of B. rapa. Based on the results of the RNA-seq assay of the NRT2 genes in B. rapa following exposure to salt stress (Figure 8A), we selected three genes (BrNRT2.1, BrNRT2.3, and BrNRT2.8) with high expression levels in B. rapa for qRT-PCR detection (Figure 8B). The relative expression levels of BrNRT2.1 and BrNRT2.8 were significantly upregulated at each time point after treatment, and this was consistent with the results of the RNA-seq analysis. The relative expression of BrNRT2.3 first increased and then decreased, reaching a peak after 4 h of salt treatment. Overall, the pronounced changes in the expression of NRT2 members under salt stress suggest that there was a relationship between plant responses to salt stress and nitrogen transportation.
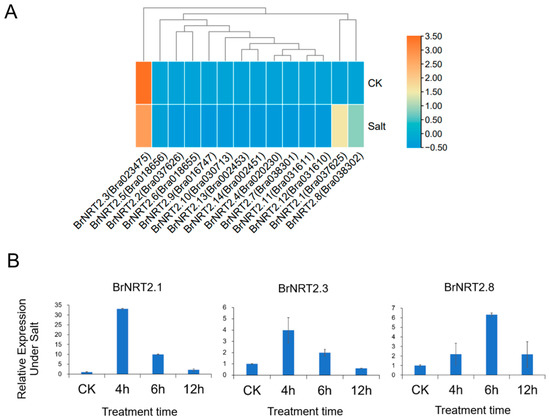
Figure 8.
Expression analysis of BrNRT2s under salt stress. (A) Heatmap of expression patterns of BrNRT2 genes under salt stress. (B) The relative expression levels of BrNRT2 genes under salt stress were analyzed using qRT-PCR.
3.7. Protein Secondary Structure and Tertiary Structure Prediction of BrNRT2s
The structure of proteins is inextricably linked to their biological functions; thus, study of the structure of NRT2 proteins can provide important insights into their functions. In this study, we analyzed the predicted protein secondary structures of BrNRT2s and found that all members had α-helixes, random coil components, extended strands, and β-turns. The α-helixes accounted for the highest proportion of the protein secondary structures of BrNRT2s, and the β-turns accounted for the lowest proportion of protein secondary structures (Figure 9A). The different proportions of protein secondary structures in different family members might be related to the diverse roles of BrNRT2 genes in cell metabolism. The SWISS-MODEL analysis revealed that members of the same subgroup exhibit high structural similarity in protein tertiary structure (Figure 9B), indicating that they maintained homologous structures during the evolutionary process; this provides basic information that could aid subsequent studies of the functions of BrNRT2 proteins.
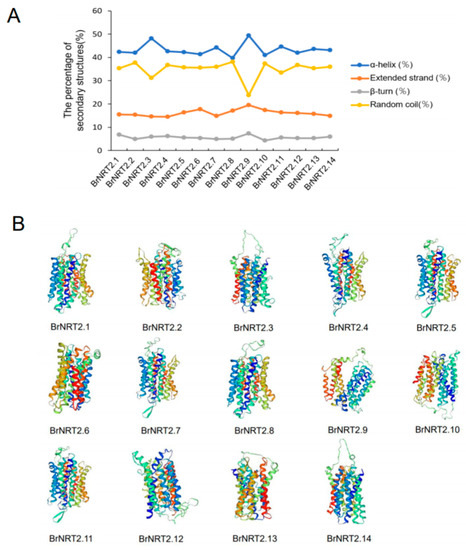
Figure 9.
Protein secondary and tertiary structure prediction of BrNRT2s. (A) Prediction of the secondary structure of BrNRT2 proteins. Different colors indicate different secondary structures. (B) Prediction of the tertiary structures of BrNRT2 proteins. Different colors indicate different subunits.
3.8. Prediction of BrNRT2 Protein Transmembrane Domains
We used TMHMM to predict the transmembrane domains of NRT2 proteins in B. rapa (Figure 10). Only the BrNRT2.9 protein contained 13 transmembrane domains, and the remaining 13 BrNRT2 proteins had 8–11 transmembrane domains, suggesting that BrNRT2.9 can respond more quickly to environmental changes and has a stronger nitrate transport function. Additionally, the number of aa and their positions in each transmembrane domain are similar, indicating that they have similar structures and functions.
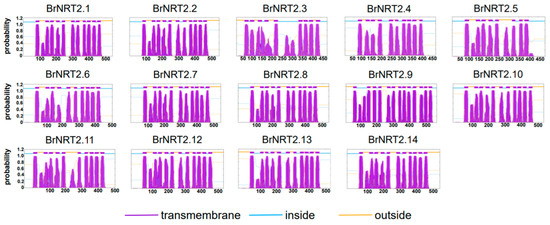
Figure 10.
Prediction of BrNRT2 protein transmembrane domains. The gene names are listed above, the x-axis shows the length of the protein sequence, and the y-axis shows the probability.
3.9. Analysis of the Protein Interaction Network of BrNRT2s
Proteins seldom perform their functions alone but interact with other proteins present in their surroundings to accomplish their biological activities [44]. Therefore, understanding protein interactions is necessary for elucidating the mechanisms underlying cellular functions. B. rapa and A. thaliana are closely related, and the functions of the AtNRT2 genes have been intensively studied. Therefore, we could predict the function of corresponding homologous genes in B. rapa through a PPI analysis of the AtNRT2 genes, which would help further clarify the functions of BrNRT2 family members. We found that the relative expression of BrNRT2.3 was significantly upregulated after drought stress, and the relative expression of BrNRT2.1 and BrNRT2.8 was significantly upregulated after salt stress according to analysis of the qRT-PCR data. To clarify the interaction network of BrNRT2 proteins, integrated network maps of the homologous genes of BrNRT2.1, BrNRT2.3, and BrNRT2.8 and their interacting proteins were constructed based on the resources and algorithms available in the STRING database to identify their functions as well as physical interactions. The homolog of BrNRT2.3 in A. thaliana was AtNRT2.7, and the predicted protein interactions (Figure 11A) revealed strong interactions of AtNRT2.7 with nitrate reductase (NIA), nitrite reductase 1 (NIR1), and nitrate transporter 1 (NRT1). The homolog of BrNRT2.1 and BrNRT2.8 in A. thaliana was AtNRT2.6. AtNRT2.6 expresses high-affinity nitrate transporter proteins and sequentially interacts with nitrate reductase 1 (NIA1) to participate in nitrate assimilation; it also strongly interacted with nitrite reductase 1 (NIR1), nitrate transporter 1 (NRT1), and ammonium transporter (AMT) (Figure 11B). Some members of the NRT1 family have been shown to enhance drought and salt stress tolerance in plants. These findings suggest that BrNRT2s may interact with proteins in different families to respond to abiotic stress.
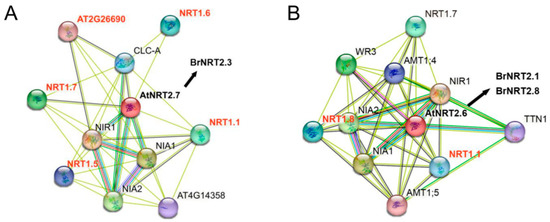
Figure 11.
Predicted interaction network of NRT2s. (A) AtNRT2.7 (BrNRT2.3 homologous gene) PPIs. (B) AtNRT2.6 (BrNRT2.1 and BrNRT2.8 homologous gene) PPIs. The colored nodes represent the query proteins. The filled nodes represent proteins with known or predicted 3D structures. The edges represent protein associations. Different colored lines between the nodes represent the type of evidence for the interaction.
4. Discussion
Nitrate is an essential element for plant growth, as it serves as a key nutrient in the nitrogen assimilation pathway and as a vital signal for plant development [45]. NRT2 family members are categorized as the high-affinity transport system (HATS) and are known to play key roles in nitrate uptake, transport, and response to biotic and abiotic stresses in various plant species. However, the NRT2 gene family have not been well studied in B. rapa. In this study, we analyzed 14 NRT2 genes from B. rapa, including their physicochemical properties, structural features, phylogenetic relationships, cis-elements, GO enrichment, expression patterns under abiotic stress, protein structures, and protein interactions. These results aided the analysis of their gene functions.
Phylogenetic relationships and collinearity analysis indicated close evolutionary relationships between BrNRT2s and AtNRT2s. Therefore, we could predict the gene functions of BrNRT2s based on the gene functions of AtNRT2s. BrNRT2.1, BrNRT2.3, and BrNRT2.8 only contained one intron; other BrNRT2 genes had more introns. Previous studies have suggested that the expression levels of genes with fewer introns can rapidly change in response to stress [46]. Moreover, conserved domain and motif analyses were carried out to clarify the relationship between the various members and their potential functions. All NRT2 family members have PLN00028 domain, indicating that the NRT2 subfamily might function through the PLN00028 domain. Motif 1, Motif 2, Motif 4, and Motif 5 are present in all BrNRT2 members, suggesting that they play an essential role in mediating nitrate transport.
Promoters are the regulatory centers of gene transcription, and an in-depth study of promoters can help clarify the mechanisms underlying the regulation of gene transcription. Analysis of the cis-elements in the promoter regions of BrNRT2 genes revealed abundant light-responsive elements, suggesting that the expression of BrNRT2 genes might be closely related to the regulation of light. In addition, many phytohormone-responsive elements and stress-responsive elements have been identified, suggesting that BrNRT2 genes may be involved in growth and developmental activities and stress responses in B. rapa. GO analysis of the BrNRT2 genes revealed that the enriched terms in BP were mainly related to nitrate transport, cellular response to nitrate, and nitrate assimilation. Analysis of tissue-specific data showed that BrNRT2 genes were mainly expressed in the roots, which highlights their importance in nitrate uptake and transport.
An increasing number of studies have shown that nitrogen uptake, transport, and assimilation are related to drought and salt tolerance in plants [27,29]. Therefore, the expression of BrNRT2 genes has been observed under drought and salt stress conditions. The qRT-PCR results showed that the relative expression of BrNRT2.3 was significantly upregulated after drought stress. Gene structure analysis showed that BrNRT2.3 contains only one intron, and the expression level of this gene can change rapidly in response to stress. Cis-element analysis showed that BrNRT2.3 contains ABA-responsive elements and MeJA-responsive elements. ABA is a critical hormone that regulates water status and stomatal movement. Under drought conditions, ABA production and accumulation in plant guard cells induce the closure of the stomata to conserve water [47]. MeJA can induce the synthesis of defensive compounds that improve drought resistance by altering various biochemical characteristics of plants, such as increasing the concentration of organic osmoprotectants and oxidase activities [48,49]. BrNRT2.3 is mainly expressed in the stem and leaf tissues, suggesting that these genes might play a role in the long-distance transport of nitrate from the roots to the aboveground parts. Protein–protein interaction predictions also suggest that the BrNRT2.3 homolog AtNRT2.7 might interact with AtNRT1.1, AtNRT1.5, AtNRT1.6, AtNRT1.7, and AT2G26690. AtNRT1.1 (CHL1) can regulate stomatal opening; a previous study showed that chl1 mutant plants are drought tolerant because of their ability to reduce water loss [50]. AtNRT1.5 mediates the redistribution of nitrate to the root system and promotes the expression of stress-response-related genes, which enhances salt, drought, and cadmium stress tolerance. The redistribution of nitrate within plants serves as a signal that links diverse stress cues to extensive physiological adjustments, which ultimately enhances their stress tolerance [51]. AtNRT1.6, AtNRT1.7, and AT2G26690 all belong to the PTR2/POT transporter family, and PTR2 in A. thaliana is negatively regulated by ABI4 and promotes water uptake during early seed germination [52]. Similarly, BrNRT2.3 may help respond to drought stress by interacting with genes in these families. Therefore, we speculate that when plants initially experience water deficits, their main response is to limit water loss and enhance water uptake; however, when drought persists, BrNRT2.3 might mediate the rapid response to changes in the external environment and cope with drought stress through the hormone signaling pathway or by interacting with other proteins.
In addition, we found that BrNRT2.1 and BrNRT2.8 may play a role in regulating salt stress. Both BrNRT2.1 and BrNRT2.8 contain only one intron, and the expression levels of these genes can change rapidly in response to stress. Cis-element analysis revealed that BrNRT2.1 contains ABA-responsive elements and auxin-responsive elements, and BrNRT2.8 contains MeJA-responsive elements, gibberellin-responsive elements, and ABA-responsive elements; all four hormones play a regulatory role in the response to salt stress. Auxin affects gene expression through a series of functionally distinct transcription factors, including DNA-binding auxin response factors (ARFs). Different ARFs regulate the soluble sugar content and maintain the chlorophyll content to promote the adaptation of plants to salt stress [53]. In rapeseed (Brassica napus L. cv. Talaye), the exogenous application of MeJA increased the soluble sugar level, relative water content, and photosynthetic rate to counteract the inhibitory effect of NaCl [54]. In sorghum, the exogenous application of gibberellins (GAs) can alleviate salt-stress-induced cell wall thickening by increasing the cellulose and hemicellulose content of root cells, allowing the rapid entry of water into root cells, and altering the dynamic balance of endogenous hormones in cells, thereby mitigating the effects of salt stress on germination and seedling growth [55]. Under high-salt conditions, ABA can stimulate short-term responses such as stomatal closure, thereby maintaining the water balance and mediating long-term growth responses by regulating the expression of stress-response genes [56]. Tissue-specific analysis showed that BrNRT2.1 and BrNRT2.8 are highly expressed in the roots, indicating that these genes may play an important role in the uptake of nitrate from the soil and in coping with root-associated stresses. Both the transcriptome data and qRT-PCR results showed that the relative expression of BrNRT2.1 and BrNRT2.8 was significantly elevated under salt stress. The predicted protein interactions also indicated that the BrNRT2.1 and BrNRT2.8 homolog AtNRT2.6 may interact with NRT1.1 and NRT1.8. Previous studies have shown that inhibition of NRT1.1 expression in plants decreases their root Cl− uptake and reduces NH4+-conferred salt hypersensitivity [57]. Under stress conditions, NRT1.8 affects the stress tolerance of plants by regulating the partitioning of nitrate between the roots and aboveground parts [58]. These analyses revealed that BrNRT2.1 and BrNRT2.8 might regulate salt stress responses through hormone–protein interactions.
The protein secondary structure analyses revealed that all members of the BrNRT2 family have the highest proportion of α-helixes, and β-turns account for the lowest proportion of secondary structures. Members of the same subgroup exhibit high structural similarity in protein tertiary structure, indicating that they maintained homologous structures during the evolutionary process. The predicted transmembrane structure of NRT2 proteins in B. rapa revealed that only the BrNRT2.9 protein exhibits 13 transmembrane domains and can respond rapidly to environmental changes with enhanced nitrate transport. This provides basic information for subsequent in-depth studies of the functions of BrNRT2 proteins.
Based on a comprehensive analysis of sequence features, cis-elements, expression profiles, protein interaction, and existing published data, we conclude that BrNRT2.3 most likely regulates drought stress, whereas BrNRT2.1 and BrNRT2.8 most likely regulate salt stress. However, the specific underlying regulatory mechanisms require further investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14081564/s1, Table S1: The primers used in the qRT-PCR; Table S2: Gene Ontology annotation of BrNRT2 genes; Table S3: Expression of BrNRT2 genes in different tissues.
Author Contributions
J.H. and Q.D. planned the project and designed the experiments; B.L., X.W. and Y.L. performed the bioinformatics analysis and experiments with the help of C.Z. and Y.C.; B.L., X.W. and Y.L. analyzed the data and wrote the original manuscript; J.H. and Q.D. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Program of Shandong Province Science Foundation (ZR2020KC017) and Shandong Provincial Natural Science Foundation (ZR2022MC021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article/Supplementary Materials.
Acknowledgments
We thank Zhilong Bao’s lab for their help with the qRT-PCR experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, H.; Gao, Y.; Tai, Y.; Liu, C.; Qu, D.; Tang, R.; Wang, Y. Research Advances in Elucidating the Function and Molecular Mechanism of the Nitrate Transporter 2 (NRT2) Proteins in Plants. Chin. Bull. Bot. 2022. (In Chinese) [Google Scholar] [CrossRef]
- Brownlee, A.G.; Arst, H.N., Jr. Nitrate uptake in Aspergillus nidulans and involvement of the third gene of the nitrate assimilation gene cluster. J. Bacteriol. 1983, 155, 1138–1146. [Google Scholar] [CrossRef]
- Trueman, L.J.; Richardson, A.; Forde, B.G. Molecular cloning of higher plant homologues of the high-affinity nitrate transporters of Chlamydomonas reinhardtii and Aspergillus nidulans. Gene 1996, 175, 223–231. [Google Scholar] [CrossRef]
- Okamoto, M.; Vidmar, J.J.; Glass, A.D. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: Responses to nitrate provision. Plant Cell Physiol. 2003, 44, 304–317. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef]
- Cerezo, M.; Tillard, P.; Filleur, S.; Munos, S.; Daniel-Vedele, F.; Gojon, A. Major Alterations of the Regulation of Root NO3− Uptake Are Associated with the Mutation of Nrt2.1 and Nrt2.2 Genes in Arabidopsis. Plant Physiol. 2001, 127, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Brehaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell. 2012, 24, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
- David, L.C.; Dechorgnat, J.; Berquin, P.; Routaboul, J.M.; Debeaujon, I.; Daniel-Vedele, F.; Ferrario-Mery, S. Proanthocyanidin oxidation of Arabidopsis seeds is altered in mutant of the high-affinity nitrate transporter NRT2.7. J. Exp. Bot. 2014, 65, 885–893. [Google Scholar] [CrossRef]
- Taulemesse, F.; Le Gouis, J.; Gouache, D.; Gibon, Y.; Allard, V. Post-flowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1. PLoS ONE 2015, 10, e0120291. [Google Scholar] [CrossRef]
- Gu, C.; Song, A.; Zhang, X.; Wang, H.; Li, T.; Chen, Y.; Jiang, J.; Chen, F.; Chen, S. Cloning of chrysanthemum high-affinity nitrate transporter family (CmNRT2) and characterization of CmNRT2.1. Sci. Rep. 2016, 6, 23462. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.; Hu, B.; Priyadarshani, S.V.G.N.; Hou, Z.; Ojolo, S.P.; Xiong, J.; Zhao, H.; Qin, Y. Characterization and the Expression Analysis of Nitrate Transporter (NRT) Gene Family in Pineapple. Trop. Plant Biol. 2018, 11, 177–191. [Google Scholar] [CrossRef]
- Li, G.; Tillard, P.; Gojon, A.; Maurel, C. Dual regulation of root hydraulic conductivity and plasma membrane aquaporins by plant nitrate accumulation and high-affinity nitrate transporter NRT2.1. Plant Cell Physiol. 2016, 57, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chen, M.; Cao, Z. NRT2.1, a major contributor to cadmium uptake controlled by high-affinity nitrate transporters. Ecotoxicol. Environ. Saf. 2021, 218, 112269. [Google Scholar] [CrossRef] [PubMed]
- Camanes, G.; Pastor, V.; Cerezo, M.; Garcia-Andrade, J.; Vicedo, B.; Garcia-Agustin, P.; Flors, V. A deletion in NRT2.1 attenuates Pseudomonas syringae-induced hormonal perturbation, resulting in primed plant defenses. Plant Physiol. 2012, 158, 1054–1066. [Google Scholar] [CrossRef]
- Dechorgnat, J.; Patrit, O.; Krapp, A.; Fagard, M.; Daniel-Vedele, F. Characterization of the Nrt2.6 gene in Arabidopsis thaliana: A link with plant response to biotic and abiotic stress. PLoS ONE 2012, 7, e42491. [Google Scholar] [CrossRef]
- Tong, J.; Walk, T.C.; Han, P.; Chen, L.; Shen, X.; Li, Y.; Gu, C.; Xie, L.; Hu, X.; Liao, X.; et al. Genome-wide identification and analysis of high-affinity nitrate transporter 2 (NRT2) family genes in rapeseed (Brassica napus L.) and their responses to various stresses. BMC Plant Biol. 2020, 20, 464. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Cetin, D. Genome-wide identification and characterization of high-affinity nitrate transporter 2 (NRT2) gene family in tomato (Solanum lycopersicum) and their transcriptional responses to drought and salinity stresses. J. Plant Physiol. 2022, 272, 153684. [Google Scholar] [CrossRef]
- Naz, M.; Luo, B.; Guo, X.; Li, B.; Chen, J.; Fan, X. Overexpression of Nitrate Transporter OsNRT2.1 Enhances Nitrate-Dependent Root Elongation. Genes 2019, 10, 290. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Zheng, D.; Han, X.; An, Y.I.; Guo, H.; Xia, X.; Yin, W. The nitrate transporter NRT2.1 functions in the ethylene response to nitrate deficiency in Arabidopsis. Plant Cell Environ. 2013, 36, 1328–1337. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123. [Google Scholar] [CrossRef]
- Luo, B.; Chen, J.; Zhu, L.; Liu, S.; Li, B.; Lu, H.; Ye, G.; Xu, G.; Fan, X. Overexpression of a High-Affinity Nitrate Transporter OsNRT2.1 Increases Yield and Manganese Accumulation in Rice Under Alternating Wet and Dry Condition. Front. Plant Sci. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Feng, H.; Li, B.; Zhi, Y.; Chen, J.; Li, R.; Xia, X.; Xu, G.; Fan, X. Overexpression of the nitrate transporter, OsNRT2.3b, improves rice phosphorus uptake and translocation. Plant Cell Rep. 2017, 36, 1287–1296. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Yang, W.; Pan, Q.; Li, C.; Sun, Q.; Zeng, Q.; Li, B.; Zhang, L. Comparative Metabolic Study of Two Contrasting Chinese Cabbage Genotypes under Mild and Severe Drought Stress. Int. J. Mol. Sci. 2022, 23, 5947. [Google Scholar] [CrossRef]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrao, S.; Tester, M. Salt stress under the scalpel—Dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Zhou, K.; Sun, T.; Hu, L.; Li, C.; Ma, F. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]
- Wang, L.; Lee, M.; Ye, B.; Yue, G.H. Genes, pathways and networks responding to drought stress in oil palm roots. Sci. Rep. 2020, 10, 21303. [Google Scholar] [CrossRef]
- de Souza Miranda, R.; Gomes-Filho, E.; Prisco, J.T.; Alvarez-Pizarro, J.C. Ammonium improves tolerance to salinity stress in Sorghum bicolor plants. Plant Growth Regul. 2015, 78, 121–131. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Prasad, S.M. Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol. Biochem. 2016, 109, 72–83. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Rombauts, S.; Dehais, P.; Van Montagu, M.; Rouze, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Lu, K.; Li, T.; He, J.; Chang, W.; Zhang, R.; Liu, M.; Yu, M.; Fan, Y.; Ma, J.; Sun, W.; et al. qPrimerDB: A thermodynamics-based gene-specific qPCR primer database for 147 organisms. Nucleic Acids Res. 2018, 46, D1229–D1236. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, P.; Luo, J.; Jiang, Y. Secreted protein prediction system combining CJ-SPHMM, TMHMM, and PSORT. Mamm. Genome 2003, 14, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.; Saha, S.; Singh, H. Prediction of protein-protein interaction using graph neural networks. Sci. Rep. 2022, 12, 8360. [Google Scholar] [CrossRef]
- Orsel, M.; Krapp, A.; Daniel-Vedele, F. Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol. 2002, 129, 886–896. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bahler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Guo, F.Q.; Young, J.; Crawford, N.M. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell 2003, 15, 107–117. [Google Scholar] [CrossRef]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Choi, M.G.; Kim, E.J.; Song, J.Y.; Choi, S.B.; Cho, S.W.; Park, C.S.; Kang, C.S.; Park, Y.I. Peptide transporter2 (PTR2) enhances water uptake during early seed germination in Arabidopsis thaliana. Plant Mol. Biol. 2020, 102, 615–624. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant 2022, 174, e13714. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabala, L.; Huang, Y.; Bie, Z. An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zhu, Y.X.; Fang, X.Z.; Ye, J.Y.; Du, W.X.; Zhu, Q.Y.; Lin, X.Y.; Jin, C.W. Ammonium aggravates salt stress in plants by entrapping them in a chloride over-accumulation state in an NRT1.1-dependent manner. Sci. Total Environ. 2020, 746, 141244. [Google Scholar] [CrossRef]
- Li, J.Y.; Fu, Y.L.; Pike, S.M.; Bao, J.; Tian, W.; Zhang, Y.; Chen, C.Z.; Zhang, Y.; Li, H.M.; Huang, J.; et al. The Arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell. 2010, 22, 1633–1646. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).