Abstract
Members of the acyl-CoA-binding protein (ACBP) gene family play vital roles in diverse processes related to lipid metabolism, growth and development, and environmental response. Plant ACBP genes have been well-studied in a variety of species including Arabidopsis, soybean, rice and maize. However, the identification and functions of ACBP genes in cotton remain to be elucidated. In this study, a total of 11 GaACBP, 12 GrACBP, 20 GbACBP, and 19 GhACBP genes were identified in the genomes of Gossypium arboreum, Gossypium raimondii, Gossypium babardense, and Gossypium hirsutum, respectively, and grouped into four clades. Forty-nine duplicated gene pairs were identified in Gossypium ACBP genes, and almost all of which have undergone purifying selection during the long evolutionary process. In addition, expression analyses showed that most of the GhACBP genes were highly expressed in the developing embryos. Furthermore, GhACBP1 and GhACBP2 were induced by salt and drought stress based on a real-time quantitative PCR (RT-qPCR) assay, indicating that these genes may play an important role in salt- and drought-stress tolerance. This study will provide a basic resource for further functional analysis of the ACBP gene family in cotton.
1. Introduction
Plant acyl-CoA-binding proteins (ACBPs) comprise a highly conserved family that bind to a variety of acyl-coenzyme A esters with high specificity and affinity through an acyl-CoA-binding (ACB) domain [1]. Based on molecular weight, subcellular localizations, and the presence of a kelch motif or ankyrin repeats domain, plant ACBPs are often categorized into four classes, namely small ACBPs, ankyrin-ACBPs, large ACBPs, and kelch-ACBPs [2,3]. The 10 kDa protein encoded by BnACBP, the first plant ACBP gene, was discovered in Brassica napus [4]. Subsequent research showed that BnACBP can bind both C16:0-CoA and C18:1-CoA esters [5]. Many ACBP genes have now been found in a variety of plant species, but the number of ACBP genes in different species varies considerably. Six ACBP genes have been found in Arabidopsis thaliana [6], 6 in Oryza sativa [7], 9 in Zea mays [8], 11 in Glycine max [2], 15 in Arachis hypogaea [3], and 19 in B. napus [9]. However, little information on the ACBP gene family has been reported in cotton to date.
Extensive studies show that plant ACBP genes are involved in a wide range of cellular processes including modulation of lipid metabolism, regulation of gene expression, and regulation of plant growth and development [1,2,3]. For example, overexpression of BnACBP altered the fatty acid composition in Arabidopsis developing seeds [10]. AtACBP1 and AtACBP2 have been shown to be involved in lipid transfer. Simultaneous mutation of AtACBP1 and AtACBP2 results in embryo lethality [11]. Furthermore, increasing evidences suggest that plant ACBP genes can be essential for specific environmental conditions such as drought, high salinity or low temperature. AtACBP2 is induced by drought and ABA. Overexpression of AtACBP2 confers transgenic Arabidopsis enhanced drought tolerance [12].
As a major cash crop, cotton not only provides natural fiber for the textile industry, but is also an important source of vegetable oil and protein. The allotetraploid G. hirsutum (2n = 4x = 52, AD1) and G. barbadense (2n = 4x = 52, AD2), which are cultivated worldwide for their economic value, evolved from an occasional hybridization between the A- and D-genome ancestors, followed by chromosome doubling. The diploid G. arboreum (2n = 2x = 26, A2) and G. raimondii (2n = 2x = 26, D5) are closely related to the A- and D-genome ancestors, respectively [13]. Although some ACBP genes have been well-characterized in A. thaliana, particularly with regard to the regulation of lipid metabolism and stress tolerance [11,12], the function of ACBP genes in cotton has not been identified to date. In this study, we performed a genome-wide characterization of the ACBP gene family in four cotton species. The phylogenetic relationships, gene structure, conserved motifs, gene duplication, cis-acting regulatory elements, and expression patterns in different tissues and in response to drought and high salinity were comprehensively analyzed. Our results will provide the basic resource for further functional analysis of the ACBP gene family.
2. Materials and Methods
2.1. Identification of Cotton ACBP Genes
The genome data of G. arboreum (CRI, v1.0) [13], G. raimondii (JGI, v2.0) [14], G. barbadense (HAU, v2.0) [15], and G. hirsutum (HAU, v1.1) [15] were downloaded from the CottonFGD database (https://cottonfgd.net/, (accessed on 17 September 2022)) [16]. The Hidden Markov Model (HMM) profile of the ACBP domain (PF00887) retrieved from the InterPro database (https://www.ebi.ac.uk/interpro/entry/pfam/PF00887/, (accessed on 17 September 2022)) [17] was used as a query to search against the protein sequences of the four Gossypium species with an e-value < 1 × 10−10. The resulting hits were further validated in the presence of ACBP domain by the SMART tool (http://smart.embl.de/, (accessed on 19 September 2022)) [18]. The molecular weight (MW) and theoretical isoelectric point (pI) of each ACBP protein were computed using Compute pI/Mw tool (https://web.expasy.org/compute_pi/, (accessed on 19 September 2022)).
2.2. Phylogenic, Gene Structure, and Conserved Motif Analyses
The ACBP domain sequences of ACBP genes identified in four Gossypium genomes and previously reported in Arabidopsis and rice [7] were used for phylogenetic analysis. A maximum likelihood (ML) tree was constructed by MEGA 11 [19] using 1000 bootstrap replicates. The substitution model was Jones–Taylor–Thornton (JTT). The exon–intron structures were retrieved from the GFF files of four Gossypium genomes. The ten conserved motifs were identified using MEME online software (http://meme-suite.org/tools/meme, (accessed on 20 September 2022)) [20]. The phylogenetic tree, gene structure, and conserved motifs were illustrated by TBtools [21].
2.3. Chromosomal Mapping and Gene Duplication Analyses
The chromosomal location information of ACBP genes was extracted from GFF gene annotation files and then visualized with the MapChart software [22]. The duplication pattern for each ACBP gene was analyzed by the method reported in the pear sugar transporter family genes [23]. For example, the 70,199 genes from the G. hirsutum genome were aligned using an all-vs-all local BLAST search with an e-value < 1 × 10−10. MCScanX software [24] was used to analyze the BLAST outputs and detect gene synteny and collinearity. Collinear gene pairs on two segmental loci were considered segmental duplication, whereas tandem duplications were characterized as adjacent homologous genes on a single chromosome without an intervening gene.
2.4. Promoter Analysis for Cis-Acting Regulatory Elements
The promoter sequences of GhACBP genes (1500 bp upstream) were retrieved from G. hirsutum genome sequences [15]. The cis-acting regulatory elements in the promoter region of each GhACBP gene were predicted using the PlantCARE online tool (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 23 September 2022)) [25].
2.5. Expression Profile Analysis of GhACBP Genes
The expression profiles of GhACBP genes in different tissues were determined based on public transcriptome data of genotypes TM-1, 11-0509 and Emian22 [26,27,28]. Transcript levels were calculated as fragments per kilobase of exon model per million mapped fragments (FPKM). The heat maps were visualized using the TBtools software (v1.098769) [21]. Furthermore, the ankyrin-ACBPs clade genes were selected to analyze their response to abiotic stress by RT-qPCR.
Healthy seeds of the G. hirsutum cultivar Lumian451 were grown in commercial soil at 28 °C with a photoperiod of 16 h light/8 h dark. Two-week-old seedlings were gently uprooted, rinsed, and cultivated in Hoagland’s solution for two days. These seedlings were then randomly divided into three groups for 200 mM NaCl, 15% PEG6000 and 100 μM ABA, respectively. Total RNA was isolated from the leaves. RT-qPCR was carried out as described by Chen et al. (2022) [29]. The experiments were biologically repeated three times, and the relative expression levels of the GhACBP genes were calculated based on the 2-ΔΔCt method [30]. The RT-qPCR primers are listed in Table S1.
3. Results
3.1. Identification and Phylogenetic Analysis of Gossypium ACBP Genes
To mine ACBP genes in cotton, a genome-wide identification was carried out using HMMER searches with the ACBP domain (PF00887) as a query in the protein database of four Gossypium species, G. arboreum, G. raimondii, G. barbadense, and G. hirsutum. A total of 62 non-redundant ACBP genes were identified, including 11, 12, 20, and 19 in G. arboreum, G. raimondii, G. barbadense, and G. hirsutum, respectively. Based on previous reports in Arabidopsis and rice, the ACBP genes of G. arboreum, G. raimondii, G. barbadense, and G. hirsutum were named GaACBP1 to GaACBP11, GrACBP1 to GrACBP12, GbACBP1 to GbACBP20, and GhACBP1 to GhACBP19, respectively. The detailed information of the Gossypium ACBP genes, including gene locus, chromosome location, exon number, sequence length, MW, and pI are listed in Table 1. The amino acid length of the Gossypium ACBP proteins ranged from 85 (GrACBP11) to 679 (GbACBP9 and GbACBP10), with corresponding MWs varying from 9.59 (GrACBP11) to 74.61 (GbACBP9) kDa. Their pI ranged from 3.98 (GrACBP2) to 9.48 (GbACBP16) (Table 1).

Table 1.
Identification of ACBP genes in four Gossypium species.
To reveal the evolutionary relationship of the ACBP genes in cotton, a total of 74 ACBP genes, including 62 Gossypium ACBP genes, 6 AtACBP genes from A. thaliana and 6 OsACBP genes from O. sativa, were used to construct an ML phylogenetic tree using the MEGA11 software [19]. As shown in Figure 1 and Figure S1, all ACBP genes were classified into four distinct clades, namely small ACBPs, large ACBPs, ankyrin-ACBPs, and kelch-ACBPs (Figure S1). The number of Gossypium ACBP genes in the four clades was 30, 7, 6, and 19, respectively. Each Gossypium gene of the kelch-ACBPs clade contains one ACBP domain in the N-terminus and three kelch domains in the C-terminus. In addition to the ACBP domain, all members of the ankyrin-ACBPs clade contain a C-terminal ankyrin repeat. The ACBP genes of small ACBPs and large ACBP clades contain only one ACBP domain, but they differ in the location of the ACBP domain (Figure S2).
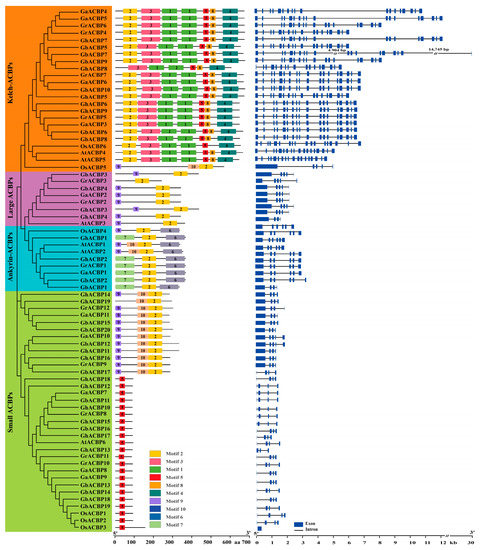
Figure 1.
The phylogenetic tree, gene structures, and conserved motifs of cotton ACBP genes. The ML phylogenetic tree was developed using MEGA11 with 1000 bootstrap replicates. The boxes with different colors indicate different conserved motifs (Figure S3). The blue boxes and black lines represent exons and introns, respectively.
3.2. Gene Structure and Conserved Motif Analysis of Cotton ACBP Genes
The number of exons in Gossypium ACBP genes ranged from 3 to 19 (Figure 1). We found that Gossypium ACBP genes in the same clade had similar gene structures. Specifically, all members of the ankyrin-ACBPs and large ACBPs clades had six and five exons, respectively. In addition, most genes of the small ACBPs clade had 4 exons, and most members of the kelch-ACBPs clade had 18 exons.
We analyzed the motif distribution of the 62 Gossypium ACBP proteins and found that each Gossypium ACBP protein had different conserved motifs ranging from 1 to 7 (Figure 1). Among the 10 conserved motifs, motif 2 is present in 42 Gossypium ACBP proteins, followed by motif 5 in 37 Gossypium ACBP proteins. Notably, members of different clades showed unique distribution patterns of conserved motifs. Specifically, all Gossypium ACBP proteins in large ACBPs clade contained motifs 2 and 9, members in ankyrin-ACBPs clade all had motifs 2, 6, and 7, and all proteins except GhACBP8 (missing motif 2) in kelch-ACBPs clade had motifs 1, 2, 3, 4, 5, and 8. In the small ACBPs clade, 18 Gossypium ACBP proteins had only motif 5, and the remaining 12 members except GhACBP19 (missing motif 9) contained motifs 2, 9, and 10 (Figure 1). In conclusion, Gossypium ACBP genes in the same clade generally have similar gene structures and motif distribution patterns.
3.3. Genomic Localization and Gene Duplication Analysis of Cotton ACBP Genes
According to the sequenced genome data, the 62 Gossypium ACBP genes were physically anchored to 35 specific chromosomes in four Gossypium species (Figure 2). Specifically, a total of 11 GaACBP genes were mapped to six chromosomes of G. arboreum. Chromosome 11 contained five GaACBP genes, chromosome 13 contained two GaACBP genes, and chromosomes 6, 7, 9, and 12 contained only one GaACBP gene each. Similarly, the 12 GrACBP genes were located on 6 chromosomes, with five GrACBP genes on chromosome 7, three on chromosome 13, and one each on chromosomes 1, 6, 8, and 10. Of the 19 GhACBP genes identified in G. hirsutum, 10 members were anchored to six chromosomes on the A-subgenome, and 9 GhACBP genes were mapped to five chromosomes on the D-subgenome. Chromosomes A11 and D11 contained five GhACBP genes each, while the remaining nine chromosomes contained only one GhACBP gene each. In G. babardense, 20 GbACBP genes were mapped to 12 chromosomes with 5 GbACBP genes on chromosome A11, 4 on chromosome D11, 2 on chromosome D13, and 1 each on chromosomes A06, A07, A09, A12, A13, D06, D07, D09, and D12. In addition, 44 ACBP genes (70.97%) were distributed at both ends of chromosomes, such as GaACBP2 at the top of chromosome Chr06 and GbACBP1 at the bottom of chromosome A09. In conclusion, the Gossypium ACBP genes were unevenly distributed on their chromosomes, with most ACBP genes located at both ends of the chromosomes.

Figure 2.
Chromosomal mapping of the Gossypium ACBP genes. Bars with different colors denote the chromosomes of four Gossypium species, respectively. The scale bar on the left indicates the chromosomal lengths (Mb). Only chromosomes with ACBP genes are shown in the figure.
To reveal the expansion of Gossypium ACBP genes, gene duplication analysis was performed in the four Gossypium species using the MCScanX program [24] and the coding sequences of all genes, and the details of duplicated pairs are listed in Table 2. A total of 6, 8, 18, and 17 pairs of duplicated ACBP genes were detected in G. arboreum, G. raimondii, G. barbadense, and G. hirsutum, respectively. Specifically, 17 pairs of GhACBP genes, involving 16 GhACBP genes, were segmental duplications, and 2 pairs (GhACBP12/GhACBP13 and GhACBP17/GhACBP18) were tandem duplications within the G. hirsutum genome (Table 2 and Figure S4). Similarly, segmentally duplicated ACBP genes were dominant in G. arboreum, G. raimondii, and G. barbadense. These results suggest that segmental duplication played a more important role in the expansion of the Gossypium ACBP genes than tandem duplication. Furthermore, all duplication pairs, except GhACBP3/GhACBP4 and GhACBP12/GhACBP17, had Ka/Ks values less than 1, ranging from 0.194 to 0.801, indicating that the vast majority of ACBP genes in Gossypium species have been subjected to purifying selection during the long evolutionary process.

Table 2.
Duplicated ACBP genes in four Gossypium species.
3.4. Cis-Acting Regulatory Analysis of GhACBP Genes’ Promoters
We identified a number of cis-acting regulatory elements from the 1500 bp upstream regions of the GhACBP genes. Apart from eukaryotic basal regulatory elements such as TATA-box and CAAT-box, eight and nine regulatory elements related to phytohormone responsiveness and environmental stress responsiveness, respectively, were identified in the 19 GhACBP genes (Figure 3). Each GhACBP gene contains at least two phytohormone-responsive elements and two stress-responsive elements. In addition, the cis-acting elements for ABA response (ABRE), ethylene response (ERE), anoxic response (ARE), and drought response (MYB, MYB-like, and MYC) are present in most GhACBP genes, whereas the element for GA response (TATC-box) is present in GhACBP1, GhACBP6, and GhACBP12. Interestingly, the divergence of regulatory elements occurred in all duplicated GhACBP genes. For example, only one out of five cis-acting elements for hormone response was shared by the GhACBP1/GhACBP2 duplicate pair (Figure 3). These results suggest that GhACBP genes may be differentially regulated by different transcription factors.

Figure 3.
Cis-acting regulatory elements in response to phytohormone and stress identified in the promoter regions of GhACBP genes.
3.5. Expression Pattern Analysis of GhACBP Genes
According to the transcriptomic data of upland cotton genotypes TM-1, 11-0509, and Emian22 [26,27,28], the expression profiles of GhACBP genes in different tissues or developmental stages were analyzed and visualized by a heat map (Figure 4). As shown, GhACBP genes were differentially expressed in Leaf, Root, Stem, and Ovules. GhACBP12, GhACBP13, GhACBP17, and GhACBP18 were highly expressed in all tissues. In contrast, GhACBP10 and GhACBP15 were not expressed in all detected tissues. Notably, 14 of the 17 expressed GhACBP genes had their highest expression levels in the Ovule (1~20 dpa, days post anther), while GhACBP11, GhACBP14, and GhACBP19 were highly expressed in the Leaf (Figure 4). In addition, GhACBP genes had similar expression patterns in developing embryos of two cotton genotypes 11-0509 and Emian22 with remarkably different seed oil content. In particular, GhACBP12, GhACBP13, GhACBP17, and GhACBP18 had significantly higher expression levels in embryos at 10 and 20 dpa compared to other GhACBP genes (Figure 4).

Figure 4.
Expression profiles of 19 GhACBP genes in different tissues and developmental stages.
Previous studies have shown that drought or salt treatment induces expression of the ankyrin-ACBPs clade genes [12,31]. We evaluated the expression patterns of GhACBP1 and GhACBP2 after exposure to 15% PEG6000, 200 mM NaCl, and 100 μM ABA, respectively. As shown in Figure 5, the expression levels of GhACBP1 and GhACBP2 were significantly altered under drought, salt, and ABA treatments. Furthermore, GhACBP1 is more sensitive to drought stress than GhACBP2. In addition, the expression of GhACBP2 increased remarkably at 1h after salt treatment, decreased significantly at 3h, then increased at 6h and finally peaked at 12h (Figure 5).
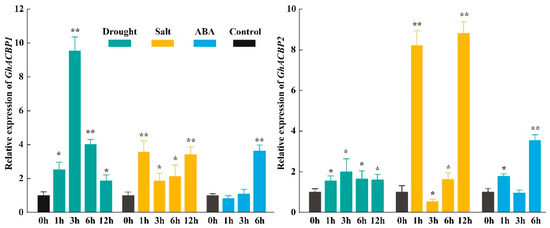
Figure 5.
Expression patterns of GhACBP1 and GhACBP2 under drought, salt, and ABA treatments determined by RT-qPCR. The standard deviation is indicated by the error bars, and “*” (t-test, p ≤ 0.05) and “**” (p ≤ 0.01) indicate significant differences between the treatment and control.
4. Discussion
Since the discovery of the first plant ACBP gene, BnACBP, in 1994, plant ACBP genes have been studied for nearly three decades and have been functionally implicated in many physiological processes, such as fatty-acid metabolism, growth and development, and stress tolerance [3]. In this study, we performed a systematic analysis of the cotton ACBP genes to investigate their potential functions in oil accumulation and abiotic stress response. A total of 62 ACBP genes were identified in the four Gossypium genomes, including 19 GhACBP genes in G. hirsutum (Table 1). Based on phylogenetic analysis, the cotton ACBP genes were classified into four distinct clades, namely small ACBPs, ankyrin-ACBPs, large ACBPs and kelch-ACBPs (Figure 1), which is consistent with the results reported in rice [7], maize [8], and soybean [2]. In particular, the small ACBPs and kelch-ACBPs clades have expanded in cotton compared to those in Arabidopsis. For example, the two diploid species, G. arboreum and G. raimondii, each contain 5 AtACBP6 paralogs, whereas the two tetraploid species, G. hirsutum and G. barbadense, each contain 10 AtACBP6 paralogs (Figure 1). Furthermore, we analyzed the ACBP gene duplication in the four cotton genomes and identified 49 duplicated ACBP gene pairs, including 43 segmental duplicates and 6 tandem duplicates (Table 2). In addition, 47 of the 49 duplicated gene pairs had undergone purifying selection during evolution based on the Ka/Ks analysis. These results suggest that segmental duplication and purifying selection may have played an important role in the evolution of the ACBP gene family in cotton.
Accumulating evidence showed that many ACBP genes were found to be involved in lipid metabolism [1,3]. AtACBP6, the small ACBP gene, expressed in all tissues of Arabidopsis. Ectopic expression of AtACBP6 altered erucic acid levels in transgenic oilseed rape seeds [32]. BnACBP6, the AtACBP6 ortholog, has been shown to be expressed in all tissues and to a greater extent in developing embryos and flowers [4,33]. Overexpression of BnACBP6 significantly increased 18:2 and 18:3 levels and decreased 20:1, 16:0, and 18:0 levels in transgenic Arabidopsis seed oil [10]. In this study, the small ACBPs clade contains 10 GhACBP genes (GhACBP10–GhACBP19). Expression analysis shows that GhACBP12, GhACBP13, GhACBP17, and GhACBP18 are highly expressed in 10 and 20 dpa embryos (Figure 4), suggesting that these four GhACBP genes may play a crucial role in seed oil accumulation and relate with the oil contents in cotton. Recently, ACBP6 (GhACBP13 in this study) was shown to be highly expressed in developing cotton embryos. Overexpression of G. barbadense ACBP6 significantly increased oil content in transgenic yeast. In addition, the expression level of ACBP6 in G. barbadense acc. 3–79 (33.79% seed oil content), was remarkably higher than that in G. hirsutum cv. Emian22 (24.97%) during almost the entire seed development process, suggesting that ACBP6 may play a decisive role in the accumulation of high oil content [28]. Based on amino acid alignment, GhACBP13 shares 79.55% and 80.90% sequence identity with the AtACBP6 and BnACBP6, respectively, indicating that GhACBP13, like AtACBP6 and BnACBP6, may influence the fatty-acid composition in cotton seeds.
Plant ACBP genes, particularly the ankyrin-ACBPs clade, have been implicated in several stress responses, including salt stress and drought stress [7,8,12]. In Arabidopsis, the ankyrin-ACBPs clade consists of two members, AtACBP1 and AtACBP2. AtACBP1 was induced by NaCl and mannitol treatments. Transgenic Arabidopsis plants overexpressing AtACBP1 showed reduced tolerance to salt and mannitol treatments, whereas the acbp1 mutant exhibited the opposite phenotype [31]. AtACBP2 was induced by drought and ABA. Overexpression of AtACBP2 increased drought tolerance and enhanced sensitivity to ABA treatment in Arabidopsis [12]. In soybean, GmACBP3 and GmACBP4 belong to the ankyrin-ACBPs clade and share 98.02% amino acid sequence identity [2]. Under salt treatment, the transcript levels of GmACBP3, GmACBP4, and their alternatively spliced isoforms were all increased in soybean roots [34]. Transgenic soybean hairy roots and Arabidopsis overexpressing of GmACBP3 or GmACBP4 were more sensitive to salt stress compared to wild plants, while transgenic plants overexpressing the alternatively spliced isoforms were more salt-tolerant [34]. In this study, the ankyrin-ACBPs clade contains two GhACBP genes, GhACBP1 and GhACBP2, which are induced by salt and drought stresses (Figure 5). GhACBP1 shares 97.55%, 66.19%, 65.83%, 65.74%, and 65.73% sequence identity with GhACBP2, AtACBP1, AtACBP2, GmACBP3, and GmACBP4, respectively. The high similarity of amino acid sequences and expression patterns under abiotic stress indicates that the functions of the ankyrin-ACBPs clade genes are highly conserved. However, further studies are needed to validate the function of GhACBP1 and GhACBP2 in abiotic stress tolerance.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1,Table S1: The primers used for RT-qPCR. Figure S1: The ML phylogenetic tree of cotton ACBP genes. Figure S2: The conserved domains identified in cotton ACBP proteins. Figure S3: The consensus sequences of the 10 conserved motifs predicted in GhACBP proteins. Figure S4: Circos diagram of the ACBP duplication pairs in G. hirsutum and G. barbadense.
Author Contributions
Conceptualization, Z.L.; data curation, Y.C., M.F. and H.L.; formal analysis, Y.C. and M.F.; investigation, Y.C., M.F. and L.W.; methodology, Z.L. and Y.C.; software, M.F.; visualization, H.L. and L.W.; writing—original draft, Y.C. and Z.L.; writing—review andediting, R.L. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation Project of Agricultural Science and Technology of Shandong Academy of Agricultural Science (CXGC2023A05), and the Seed-Industrialized Development Program in Shandong Province (2020LZGC002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raboanatahiry, N.; Wang, B.; Yu, L.; Li, M. Functional and structural diversity of acyl-CoA binding proteins in oil crops. Front. Genet. 2018, 9, 182. [Google Scholar] [CrossRef]
- Azlan, N.S.; Guo, Z.H.; Yung, W.S.; Wang, Z.; Lam, H.M.; Lung, S.C.; Chye, M.L. In silico analysis of acyl-CoA-binding protein expression in soybean. Front. Plant Sci. 2021, 12, 646938. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Lin, L.; Xie, H.; Zheng, Y.; Wan, X. Genome-wide identification of acyl-CoA binding proteins and possible functional prediction in legumes. Front. Genet. 2023, 13, 1057160. [Google Scholar] [CrossRef]
- Hills, M.J.; Dann, R.; Lydiate, D.; Sharpe, A. Molecular cloning of a cDNA from Brassica napus L. for a homologue of acyl-CoA-binding protein. Plant Mol. Biol. 1994, 25, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Johnson, P.; Rawsthorne, S.; Hills, M.J. Expression and properties of acyl-CoA binding protein from Brassica napus. Plant Physiol. Biochem. 1998, 36, 629–635. [Google Scholar] [CrossRef]
- Xiao, S.; Chye, M.L. An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physiol. Bioch. 2009, 47, 479–484. [Google Scholar] [CrossRef]
- Meng, W.; Su, Y.C.F.; Saunders, R.M.K.; Chye, M.L. The rice acyl-CoA-binding protein gene family: Phylogeny, expression and functional analysis. New Phytol. 2011, 189, 1170–1184. [Google Scholar] [CrossRef]
- Zhu, J.; Li, W.; Zhou, Y.; Pei, L.; Liu, J.; Xia, X.; Che, R.; Li, H. Molecular characterization, expression and functional analysis of acyl-CoA-binding protein gene family in maize (Zea mays). BMC Plant Biol. 2021, 21, 94. [Google Scholar] [CrossRef]
- Raboanatahiry, N.H.; Yin, Y.; Chen, L.; Li, M. Genome-wide identification and phylogenic analysis of kelch motif containing ACBP in Brassica napus. BMC Genom. 2015, 16, 512. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, O.P.; Nykiforuk, C.L.; Moloney, M.M.; Stahl, U.; Banas, A.; Stymne, S.; Weselake, R.J. A 10-kDa acyl-CoA-binding protein (ACBP) from Brassica napus enhances acyl exchange between acyl-CoA and phosphatidylcholine. Plant Biotechnol. J. 2009, 7, 602–610. [Google Scholar] [CrossRef]
- Chen, Q.F.; Xiao, S.; Qi, W.; Mishra, G.; Ma, J.; Wang, M.; Chye, M.L. The Arabidopsis acbp1acbp2 double mutant lacking acyl-CoA-binding proteins ACBP1 and ACBP2 is embryo lethal. New Phytol. 2010, 186, 843–855. [Google Scholar] [CrossRef]
- Du, Z.Y.; Chen, M.X.; Chen, Q.E.; Xiao, S.; Chye, M.L. Overexpression of Arabidopsis acyl-CoA-binding protein ACBP2 enhances drought tolerance. Plant Cell Environ. 2013, 36, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Huang, G.; He, S.; Yang, Z.; Sun, G.; Ma, X.; Li, N.; Zhang, X.; Sun, J.; Liu, M.; et al. Resequencing of 243 diploid cotton accessions based on an updated A genome identifies the genetic basis of key agronomic traits. Nat. Genet. 2018, 50, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef]
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, D1, D458–D460. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Fu, M.; Chen, Y.; Li, H.; Wang, L.; Liu, R.; Liu, Z. Genome-wide identification and expression analyses of the cotton AGO genes and their potential roles in fiber development and stress response. Genes 2022, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Huang, Y.; Cui, Y.; Hua, J. Gene network of oil accumulation reveals expression profiles in developing embryos and fatty acid composition in upland cotton. J. Plant Physiol. 2018, 228, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Le, Y.; Zhang, R.; Li, X.; Lin, Z. A global survey of the gene network and key genes for oil accumulation in cultivated tetraploid cottons. Plant Biotechnol. J. 2021, 19, 1170–1182. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, M.; Li, H.; Wang, L.; Liu, R.; Liu, Z. Genome-wide characterization of the UDP-glycosyltransferase gene family reveals their potential roles in leaf senescence in cotton. Int. J. Biol. Macromol. 2022, 222, 2648–2660. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, M.X.; Hu, T.H.; Xue, Y.; Zhu, F.Y.; Du, Z.Y.; Lo, C.; Chye, M.L. Arabidopsis acyl-coenzyme-A-binding protein ACBP1 interacts with AREB1 and mediates salt and osmotic signaling in seed germination and seedling growth. Environ. Exp. Bot. 2018, 156, 130–140. [Google Scholar] [CrossRef]
- Enikeev, A.G.; Mishutina, U.O. Physiological effects of rapeseed transformation with the acb gene as affected by the genetic vector structure. Russ. J. Plant Physiol. 2005, 52, 668–671. [Google Scholar] [CrossRef]
- Engeseth, N.J.; Pacovsky, R.S.; Newman, T.; Ohlrogge, J.B. Characterization of an acyl-CoA binding protein from Arabidopsis thaliana. Arch. Biochem. Biophys. 1996, 331, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lung, S.C.; Lai, S.H.; Wang, H.; Zhang, X.; Liu, A.; Guo, Z.H.; Lam, H.M.; Chye, M.L. Oxylipin signaling in salt-stressed soybean is modulated by ligand-dependent interaction of class II acyl-CoA-binding proteins with lipoxygenase. Plant Cell 2022, 34, 1117–1143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).