Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Ovarian Cancer Patient Study
2.2. Basophil Phenotyping
2.3. Basophil Activation Test (BAT)
2.4. Flow Cytometric and Statistical Analyses
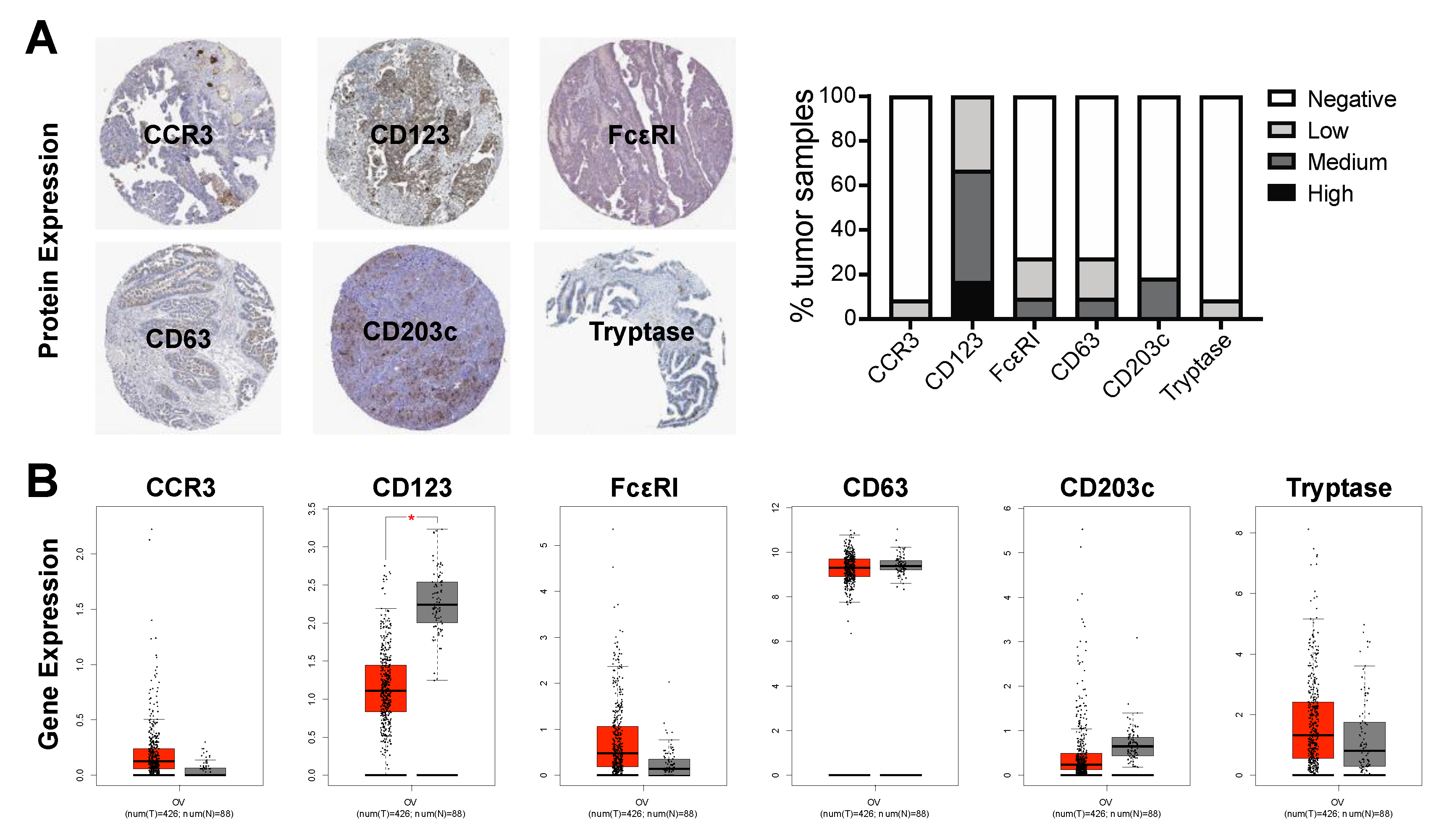
2.5. Basophil Marker Expression in Ovarian Cancer Tumors
2.6. Survival Analyses
3. Results
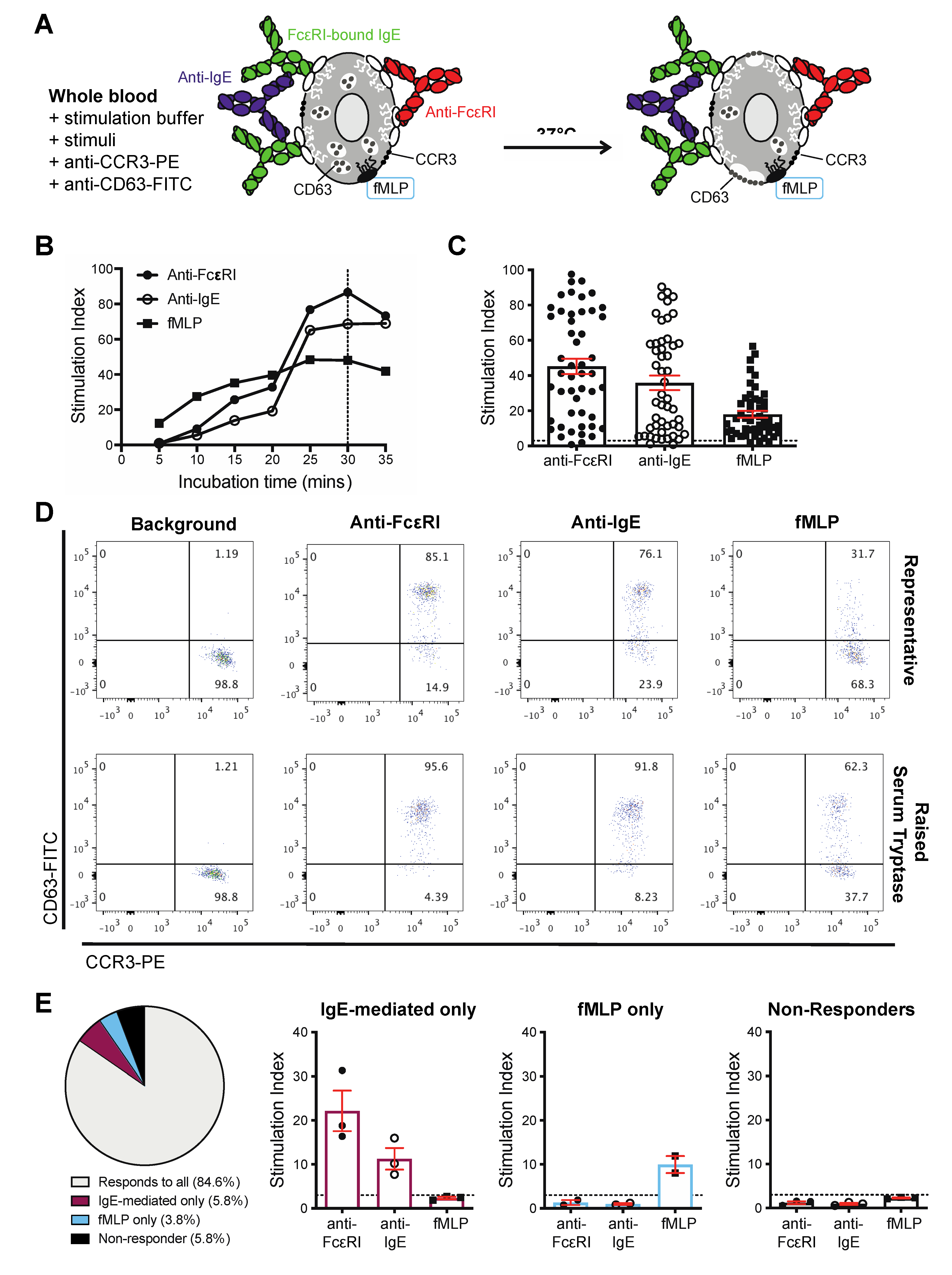
3.1. Basophils Are Detectable in the Blood of Cancer Patients
3.2. Basophils from Cancer Patients Can Be Activated by IgE and Non-IgE-Mediated Triggers Ex Vivo
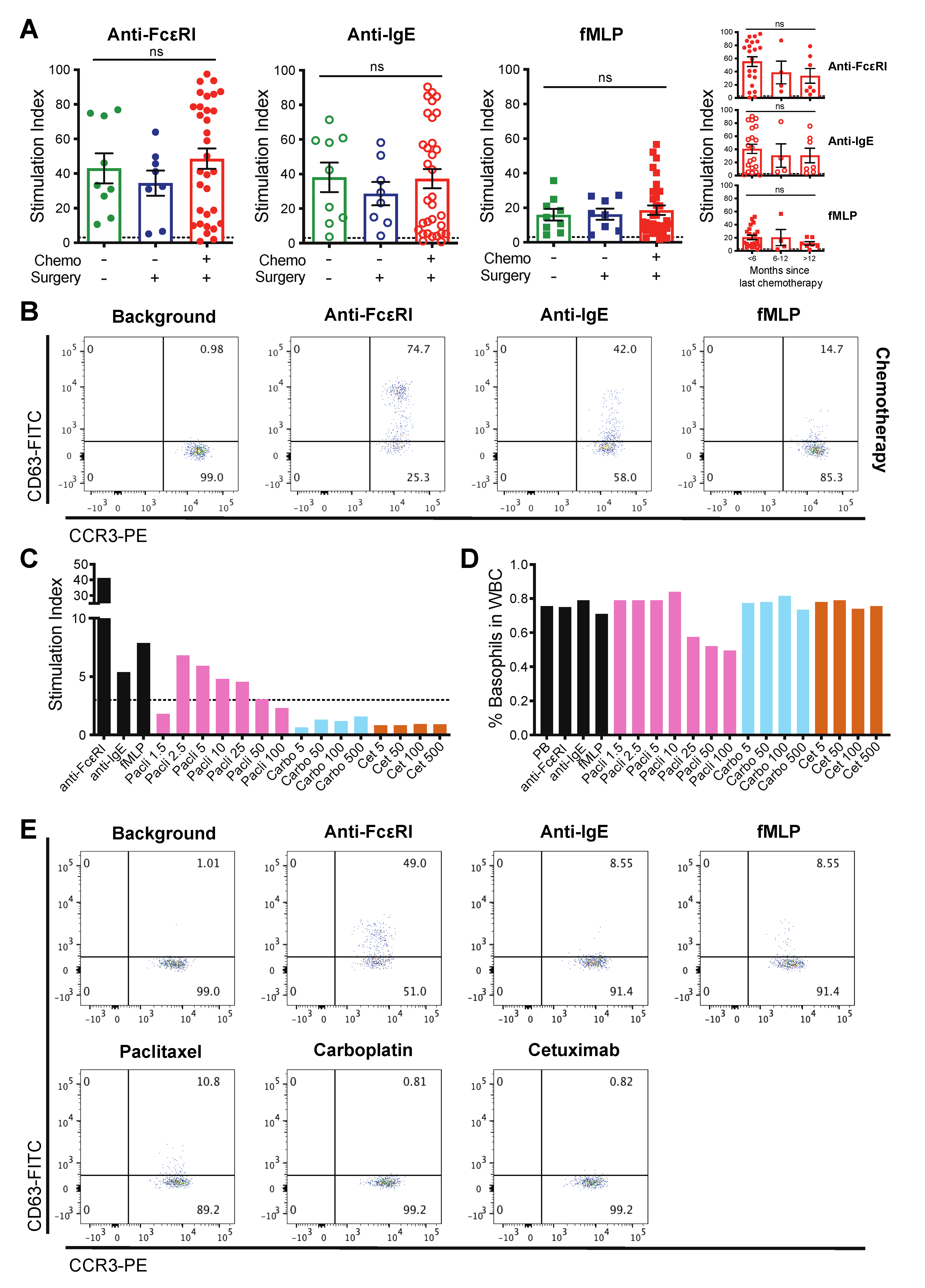
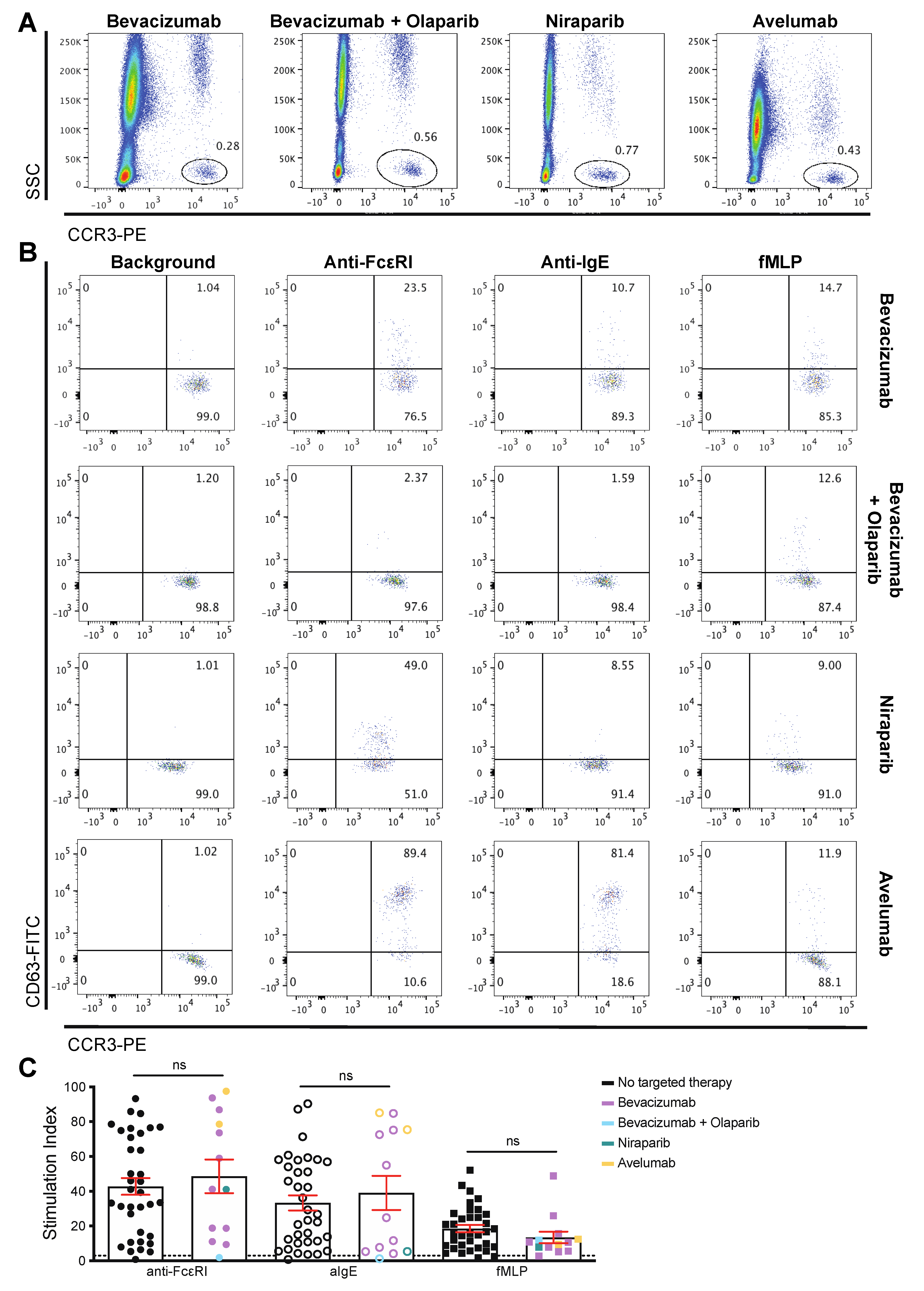
3.3. Patient-Derived Basophils Can Be Activated Ex Vivo Irrespective of Prior Therapy
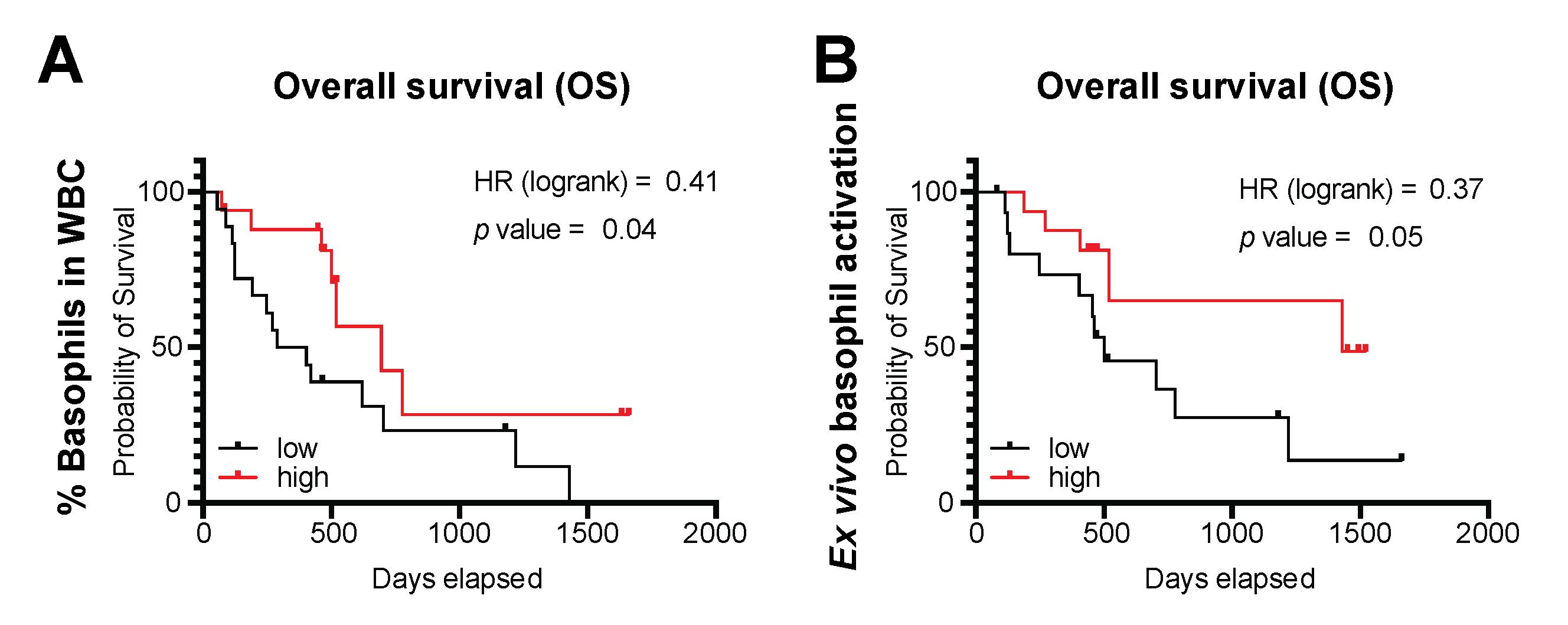
3.4. Basophils and Their Activation Are Associated with Survival Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Monte, L.; Wormann, S.; Brunetto, E.; Heltai, S.; Magliacane, G.; Reni, M.; Paganoni, A.M.; Recalde, H.; Mondino, A.; Falconi, M.; et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016, 76, 1792–1803. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef]
- Rigoni, A.; Colombo, M.P.; Pucillo, C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin Immunol. 2018, 35, 29–34. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tuting, T.; Rudensky, A.Y.; Hammerling, G.J. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef]
- Halloy, J.-L. Use of Basophil Activation Test in a Case of Oxaliplatin Hypersensitivity. J. Aller. Ther. 2011, 2. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hirai, H.; Yamaguchi, N.; Kobayashi, N.; Sugimoto, H.; Tabata, T.; Okuda, M. Carboplatin-induced severe hypersensitivity reaction: Role of IgE-dependent basophil activation and FcepsilonRI. Cancer Sci. 2014, 105, 1472–1479. [Google Scholar] [CrossRef]
- Wilson, J.M.; Platts-Mills, T.A.E. IgE to galactose-alpha-1,3-galactose and the alpha-Gal syndrome: Insights from basophil activation testing. J. Allergy Clin. Immunol. 2019, 143, 101–103. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Hagendorens, M.M.; Aerts, N.E.; De Clerck, L.S.; Stevens, W.J. Basophil activation test by flow cytometry: Present and future applications in allergology. Cytom. B Clin. Cytom. 2008, 74, 201–210. [Google Scholar] [CrossRef]
- Hemmings, O.; Kwok, M.; McKendry, R.; Santos, A.F. Basophil Activation Test: Old and New Applications in Allergy. Curr. Allergy Asthma Rep. 2018, 18, 77. [Google Scholar] [CrossRef]
- Hoffmann, H.J.; Knol, E.F.; Ferrer, M.; Mayorga, L.; Sabato, V.; Santos, A.F.; Eberlein, B.; Nopp, A.; MacGlashan, D. Pros and Cons of Clinical Basophil Testing (BAT). Curr. Allergy Asthma Rep. 2016, 16, 56. [Google Scholar] [CrossRef]
- Moneret-Vautrin, D.A.; Sainte-Laudy, J.; Kanny, G.; Fremont, S. Human basophil activation measured by CD63 expression and LTC4 release in IgE-mediated food allergy. Ann. Allergy Asthma Immunol. 1999, 82, 33–40. [Google Scholar] [CrossRef]
- Ocmant, A.; Mulier, S.; Hanssens, L.; Goldman, M.; Casimir, G.; Mascart, F.; Schandene, L. Basophil activation tests for the diagnosis of food allergy in children. Clin. Exp. Allergy 2009, 39, 1234–1245. [Google Scholar] [CrossRef]
- Santos, A.F.; Douiri, A.; Becares, N.; Wu, S.Y.; Stephens, A.; Radulovic, S.; Chan, S.M.; Fox, A.T.; Du Toit, G.; Turcanu, V.; et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J. Allergy Clin. Immunol. 2014, 134, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Shreffler, W.G. Road map for the clinical application of the basophil activation test in food allergy. Clin. Exp. Allergy 2017, 47, 1115–1124. [Google Scholar] [CrossRef]
- Sato, S.; Tachimoto, H.; Shukuya, A.; Kurosaka, N.; Yanagida, N.; Utsunomiya, T.; Iguchi, M.; Komata, T.; Imai, T.; Tomikawa, M.; et al. Basophil activation marker CD203c is useful in the diagnosis of hen’s egg and cow’s milk allergies in children. Int. Arch. Allergy Immunol. 2010, 152, 54–61. [Google Scholar] [CrossRef]
- Bokanovic, D.; Arzt-Gradwohl, L.; Schwarz, I.; Schrautzer, C.; Laipold, K.; Aberer, W.; Binder, B.; Sturm, G.J. Possible utility of basophil activation test in dual honeybee and vespid sensitization. J. Allergy Clin. Immunol. Pract. 2020, 8, 392–394. [Google Scholar] [CrossRef]
- Erdmann, S.M.; Sachs, B.; Kwiecien, R.; Moll-Slodowy, S.; Sauer, I.; Merk, H.F. The basophil activation test in wasp venom allergy: Sensitivity, specificity and monitoring specific immunotherapy. Allergy 2004, 59, 1102–1109. [Google Scholar] [CrossRef]
- Sturm, G.J.; Bohm, E.; Trummer, M.; Weiglhofer, I.; Heinemann, A.; Aberer, W. The CD63 basophil activation test in Hymenoptera venom allergy: A prospective study. Allergy 2004, 59, 1110–1117. [Google Scholar] [CrossRef]
- Aranda, A.; Mayorga, C.; Ariza, A.; Dona, I.; Rosado, A.; Blanca-Lopez, N.; Andreu, I.; Torres, M.J. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy 2011, 66, 247–254. [Google Scholar] [CrossRef]
- Dewachter, P.; Chollet-Martin, S.; Mouton-Faivre, C.; de Chaisemartin, L.; Nicaise-Roland, P. Comparison of Basophil Activation Test and Skin Testing Performances in NMBA Allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 1681–1689. [Google Scholar] [CrossRef]
- Fernandez, T.D.; Ariza, A.; Palomares, F.; Montanez, M.I.; Salas, M.; Martin-Serrano, A.; Fernandez, R.; Ruiz, A.; Blanca, M.; Mayorga, C.; et al. Hypersensitivity to fluoroquinolones: The expression of basophil activation markers depends on the clinical entity and the culprit fluoroquinolone. Med. (Baltim.) 2016, 95, e3679. [Google Scholar] [CrossRef]
- Gomez, E.; Blanca-Lopez, N.; Torres, M.J.; Requena, G.; Rondon, C.; Canto, G.; Blanca, M.; Mayorga, C. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: Value of basophil activation test in the identification of patients. Clin. Exp. Allergy 2009, 39, 1217–1224. [Google Scholar] [CrossRef]
- Laguna, J.J.; Bogas, G.; Salas, M.; Mayorga, C.; Dionicio, J.; Gonzalez-Mendiola, R.; Ariza, A.; Fernandez-Santamaria, R.; Olazabal, I.; Dona, I.; et al. The Basophil Activation Test Can Be of Value for Diagnosing Immediate Allergic Reactions to Omeprazole. J. Allergy Clin. Immunol. Pract. 2018, 6, 1628–1636. [Google Scholar] [CrossRef]
- Salas, M.; Fernandez-Santamaria, R.; Mayorga, C.; Barrionuevo, E.; Ariza, A.; Posadas, T.; Laguna, J.J.; Montanez, M.I.; Molina, N.; Fernandez, T.D.; et al. Use of the Basophil Activation Test May Reduce the Need for Drug Provocation in Amoxicillin-Clavulanic Allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 1010–1018. [Google Scholar] [CrossRef]
- Torres, M.J.; Padial, A.; Mayorga, C.; Fernandez, T.; Sanchez-Sabate, E.; Cornejo-Garcia, J.A.; Antunez, C.; Blanca, M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin. Exp. Allergy 2004, 34, 1768–1775. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.; Galvao, V.R.; Picard, M.; Caiado, J.; Castells, M.C. Basophil Activation Test is a Relevant Biomarker of the Outcome of Rapid Desensitization in Platinum Compounds-Allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 728–736. [Google Scholar] [CrossRef]
- Iwamoto, T.; Sugimoto, H.; Tabata, T.; Okuda, M. Clinical Utility of Basophil CD203c as a Biomarker for Predicting the Timing of Hypersensitivity Reaction in Carboplatin Rechallenge: Three Case Reports. Clin. Ther. 2016, 38, 1537–1541. [Google Scholar] [CrossRef]
- Iwamoto, T.; Yuta, A.; Tabata, T.; Sugimoto, H.; Gabazza, E.C.; Hirai, H.; Kojima, S.; Okuda, M. Evaluation of basophil CD203c as a predictor of carboplatin-related hypersensitivity reaction in patients with gynecologic cancer. Biol. Pharm. Bull. 2012, 35, 1487–1495. [Google Scholar] [CrossRef]
- Ornelas, C.; Caiado, J.; Campos Melo, A.; Pereira Barbosa, M.; Castells, M.C.; Pereira Dos Santos, M.C. The Contribution of the Basophil Activation Test to the Diagnosis of Hypersensitivity Reactions to Oxaliplatin. Int. Arch. Allergy Immunol. 2018, 177, 274–280. [Google Scholar] [CrossRef]
- Mukai, K.; Gaudenzio, N.; Gupta, S.; Vivanco, N.; Bendall, S.C.; Maecker, H.T.; Chinthrajah, R.S.; Tsai, M.; Nadeau, K.C.; Galli, S.J. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 h before analysis. J. Allergy Clin. Immunol. 2017, 139, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.J.; Kranzelbinder, B.; Sturm, E.M.; Heinemann, A.; Groselj-Strele, A.; Aberer, W. The basophil activation test in the diagnosis of allergy: Technical issues and critical factors. Allergy 2009, 64, 1319–1326. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lanczky, A.; Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, O.V.; Gentinetta, T.; Fux, M.; Ducrest, S.; Pichler, W.J.; Dahinden, C.A. Robust expression of CCR3 as a single basophil selection marker in flow cytometry. Allergy 2011, 66, 85–91. [Google Scholar] [CrossRef]
- Eberlein, B.; Leon Suarez, I.; Darsow, U.; Rueff, F.; Behrendt, H.; Ring, J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin. Exp. Allergy 2010, 40, 411–418. [Google Scholar] [CrossRef]
- Frezzolini, A.; Cadoni, S.; De Pita, O. Usefulness of the CD63 basophil activation test in detecting Anisakis hypersensitivity in patients with chronic urticaria: Diagnosis and follow-up. Clin. Exp. Derm. 2010, 35, 765–770. [Google Scholar] [CrossRef]
- Netchiporouk, E.; Moreau, L.; Rahme, E.; Maurer, M.; Lejtenyi, D.; Ben-Shoshan, M. Positive CD63 Basophil Activation Tests Are Common in Children with Chronic Spontaneous Urticaria and Linked to High Disease Activity. Int. Arch. Allergy Immunol. 2016, 171, 81–88. [Google Scholar] [CrossRef]
- Sharma, M.; Das, M.; Stephen-Victor, E.; Galeotti, C.; Karnam, A.; Maddur, M.S.; Bruneval, P.; Kaveri, S.V.; Bayry, J. Regulatory T cells induce activation rather than suppression of human basophils. Sci. Immunol. 2018, 3, eaan0829. [Google Scholar] [CrossRef]
- MacGlashan, D., Jr. Expression of CD203c and CD63 in human basophils: Relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin. Exp. Allergy 2010, 40, 1365–1377. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Mertens, C.H.; Hagendorens, M.M.; Stevens, W.J.; De Clerck, L.S. Analyzing histamine release by flow cytometry (HistaFlow): A novel instrument to study the degranulation patterns of basophils. J. Immunol. Methods 2012, 375, 30–38. [Google Scholar] [CrossRef]
- Knol, E.F.; Mul, F.P.; Jansen, H.; Calafat, J.; Roos, D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J. Allergy Clin. Immunol. 1991, 88, 328–338. [Google Scholar] [CrossRef]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D.; et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef]
- Commins, S.P.; James, H.R.; Stevens, W.; Pochan, S.L.; Land, M.H.; King, C.; Mozzicato, S.; Platts-Mills, T.A. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J. Allergy Clin. Immunol. 2014, 134, 108–115. [Google Scholar] [CrossRef]
- Mehlich, J.; Fischer, J.; Hilger, C.; Swiontek, K.; Morisset, M.; Codreanu-Morel, F.; Schiener, M.; Blank, S.; Ollert, M.; Darsow, U.; et al. The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J. Allergy Clin. Immunol. 2019, 143, 182–189. [Google Scholar] [CrossRef]
- Bax, H.J.; Josephs, D.H.; Pellizzari, G.; Spicer, J.F.; Montes, A.; Karagiannis, S.N. Therapeutic targets and new directions for antibodies developed for ovarian cancer. MAbs 2016, 8, 1437–1455. [Google Scholar] [CrossRef]
- (FDA), U.S. Food & Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0169lbl.pdf,electronic (accessed on 6 July 2020).
- (eMC), Electronic Medicine Compendium. Available online: https://www.medicines.org.uk/emc/product/3885/smpc (accessed on 6 July 2020).
- Sloane, D.; Govindarajulu, U.; Harrow-Mortelliti, J.; Barry, W.; Hsu, F.I.; Hong, D.; Laidlaw, T.; Palis, R.; Legere, H.; Bunyavanich, S.; et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J. Allergy Clin. Immunol. Pr. 2016, 4, 497–504. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef]
- Gonzalez-Martin, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- The Human Protein Atlas. CCR3, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000183625-CCR3/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- The Human Protein Atlas. IL3RA, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000185291-IL3RA/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- The Human Protein Atlas. FCER1A, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000179639-FCER1A/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- The Human Protein Atlas. CD63, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000135404-CD63/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- The Human Protein Atlas. ENPP3, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000154269-ENPP3/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- The Human Protein Atlas. TPSAB1, Ovarian Cancer Protein Expression. Available online: https://www.proteinatlas.org/ENSG00000172236-TPSAB1/pathology/ovarian+cancer#ihc (accessed on 6 July 2020).
- Chan, Y.C.; Ramadani, F.; Santos, A.F.; Pillai, P.; Ohm-Laursen, L.; Harper, C.E.; Fang, C.; Dodev, T.S.; Wu, S.Y.; Ying, S.; et al. "Auto-anti-IgE": Naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J. Allergy Clin. Immunol. 2014, 134, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Karnam, A.; Das, M.; Kaveri, S.V.; Bayry, J. Acid Stripping of Surface IgE Antibodies Bound to FcepsilonRI is Unsuitable for the Functional Assays that Require Long-Term Culture of Basophils and Entire Removal of Surface IgE. Int. J. Mol. Sci. 2020, 21, 510. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Stephen-Victor, E.; Karnam, A.; Das, M.; Gilardin, L.; Maddur, M.S.; Wymann, S.; Vonarburg, C.; Chevailler, A.; Dimitrov, J.D.; et al. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J. Allergy Clin. Immunol. 2019, 144, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, F.; Buschor, P.; Hobi, G.; Brigger, D.; Dahinden, C.A.; Villiger, P.M.; Eggel, A. IL-3 but not monomeric IgE regulates FcepsilonRI levels and cell survival in primary human basophils. Cell Death Dis. 2018, 9, 510. [Google Scholar] [CrossRef]
- Panaszek, B.; Pawlowicz, R.; Grzegrzolka, J.; Obojski, A. Autoreactive IgE in Chronic Spontaneous/Idiopathic Urticaria and Basophil/Mastocyte Priming Phenomenon, as a Feature of Autoimmune Nature of the Syndrome. Arch. Immunol Exp. 2017, 65, 137–143. [Google Scholar] [CrossRef]
- Eberlein, B.; Hann, R.; Eyerich, S.; Pennino, D.; Ring, J.; Schmidt-Weber, C.B.; Buters, J. Optimizing of the basophil activation test: Comparison of different basophil identification markers. Cytom. B Clin. Cytom. 2015, 88, 183–189. [Google Scholar] [CrossRef]
- Shelley, W.B.; Parnes, H.M. The Absolute Basophil Count. JAMA 1965, 192, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, L. The effect of corticotrophin and corticosteroids on the basophil and eosinophil granulocytes. Acta Haematol. 1963, 29, 157–165. [Google Scholar] [CrossRef]
- Dunsky, E.H.; Zweiman, B.; Fischler, E.; Levy, D.A. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J. Allergy Clin. Immunol. 1979, 63, 426–432. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Gillis, S.; MacGlashan, D.W., Jr. A comparative study of releasing and nonreleasing human basophils: Nonreleasing basophils lack an early component of the signal transduction pathway that follows IgE cross-linking. J. Allergy Clin. Immunol. 1990, 85, 1020–1029. [Google Scholar] [CrossRef]
- MacGlashan, D.W., Jr. Relationship between spleen tyrosine kinase and phosphatidylinositol 5’ phosphatase expression and secretion from human basophils in the general population. J. Allergy Clin. Immunol. 2007, 119, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.S.; Bloom, K.A.; Nowak-Wegrzyn, A.H.; Shreffler, W.G.; Masilamani, M.; Sampson, H.A. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J. Allergy Clin. Immunol. 2013, 131, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Haeberli, G.; Bronnimann, M.; Hunziker, T.; Muller, U. Elevated basal serum tryptase and hymenoptera venom allergy: Relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin. Exp. Allergy 2003, 33, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.T.; Ewan, P.W.; Diwakar, L.; Durham, S.R.; Frew, A.J.; Leech, S.C.; Nasser, S.M. Diagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clin. Exp. Allergy 2011, 41, 1201–1220. [Google Scholar] [CrossRef] [PubMed]
- Ludolph-Hauser, D.; Rueff, F.; Fries, C.; Schopf, P.; Przybilla, B. Constitutively raised serum concentrations of mast-cell tryptase and severe anaphylactic reactions to Hymenoptera stings. Lancet 2001, 357, 361–362. [Google Scholar] [CrossRef]
- Potier, A.; Lavigne, C.; Chappard, D.; Verret, J.L.; Chevailler, A.; Nicolie, B.; Drouet, M. Cutaneous manifestations in Hymenoptera and Diptera anaphylaxis: Relationship with basal serum tryptase. Clin. Exp. Allergy 2009, 39, 717–725. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Yavuz, S.T.; Buyuktiryaki, B.; Cavkaytar, O.; Yilmaz, E.A.; Tuncer, A.; Sackesen, C. Serum basal tryptase may be a good marker for predicting the risk of anaphylaxis in children with food allergy. Allergy 2014, 69, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, D.K.; Sharma, H.; Myles, J.; Jiang, Y.; Torretti, B.; Schwartz, L.B.; McMorris, M.; Akin, C. Do Baseline Serum Total Tryptase Levels Predict Severity Of Food Allergy Reactions? J. Allergy Clin. Immunol. 2011, 127, AB180. [Google Scholar] [CrossRef]
- Seitz, C.S.; Brockow, K.; Hain, J.; Trautmann, A. Non-steroidal anti-inflammatory drug hypersensitivity: Association with elevated basal serum tryptase? Allergy Asthma Clin. Immunol. 2014, 10, 19. [Google Scholar] [CrossRef]
- Beeh, K.M.; Ksoll, M.; Buhl, R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur. Respir. J. 2000, 16, 609–614. [Google Scholar] [CrossRef]
- Crespo-Lessmann, A.; Curto, E.; Mateus, E.; Soto, L.; Garcia-Moral, A.; Torrejon, M.; Belda, A.; Giner, J.; Ramos-Barbon, D.; Plaza, V. Total and specific immunoglobulin E in induced sputum in allergic and non-allergic asthma. PLoS ONE 2020, 15, e0228045. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.J.; Khiabany, A.; Stavraka, C.; Pellizzari, G.; Chan Wah Hak, C.; Robinson, A.; Ilieva, K.M.; Woodman, N.; Naceur-Lombardelli, C.; Gillett, C.; et al. Basophil activation test in cancer patient blood evaluating potential hypersensitivity to an anti-tumor IgE therapeutic candidate. Allergy 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.F.; Basu, B.; Montes, A.; Banerji, U.; Kristeleit, R.; Veal, G.J.; Corrigan, C.; Till, S.J.; Nintos, G.; Brier, T.; et al. Phase 1 trial of MOv18, a first-in-class IgE antibody therapy for cancer. Aacr Annu. Meet. 2020, VPO.CT01. [Google Scholar]
- Knight, B.; Rassam, D.; Liao, S.; Ewesuedo, R. A phase I pharmacokinetics study comparing PF-06439535 (a potential biosimilar) with bevacizumab in healthy male volunteers. Cancer Chemother. Pharm. 2016, 77, 839–846. [Google Scholar] [CrossRef]
- Kloover, J.S.; den Bakker, M.A.; Gelderblom, H.; van Meerbeeck, J.P. Fatal outcome of a hypersensitivity reaction to paclitaxel: A critical review of premedication regimens. Br. J. Cancer 2004, 90, 304–305. [Google Scholar] [CrossRef]
- Dye, D.; Watkins, J. Suspected anaphylactic reaction to Cremophor EL. Br. Med. J. 1980, 280, 1353. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of complement-activating particles in aqueous solutions of Taxol: Possible role in hypersensitivity reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]
- Weiszhar, Z.; Czucz, J.; Revesz, C.; Rosivall, L.; Szebeni, J.; Rozsnyay, Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur. J. Pharm. Sci. 2012, 45, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.G.; Gao, J.L.; Lyu, G.Y.; Su, J.; Zhang, Q.I.; Ji, X.; Yan, J.Z.; Qiu, Q.L.; Zhang, Y.L.; et al. Low local blood perfusion, high white blood cell and high platelet count are associated with primary tumor growth and lung metastasis in a 4T1 mouse breast cancer metastasis model. Oncol. Lett. 2015, 10, 754–760. [Google Scholar] [CrossRef]
- Gooch, J.L.; Lee, A.V.; Yee, D. Interleukin 4 inhibits growth and induces apoptosis in human breast cancer cells. Cancer Res. 1998, 58, 4199–4205. [Google Scholar] [PubMed]
- Wei, Y.; Zhang, X.; Wang, G.; Zhou, Y.; Luo, M.; Wang, S.; Hong, C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage-colorectal cancer. Asia Pac. J. Clin. Oncol. 2018, 14, e243–e251. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Gambardella, A.R.; Mattei, F.; Mancini, J.; Schiavoni, G.; Varricchi, G. Basophils in Tumor Microenvironment and Surroundings. Adv. Exp. Med. Biol. 2020, 1224, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Martinel Lamas, D.J.; Croci, M.; Carabajal, E.; Crescenti, E.J.; Sambuco, L.; Massari, N.A.; Bergoc, R.M.; Rivera, E.S.; Medina, V.A. Therapeutic potential of histamine H(4) receptor agonists in triple-negative human breast cancer experimental model. Br. J. Pharm. 2013, 170, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Denzel, A.; Maus, U.A.; Rodriguez Gomez, M.; Moll, C.; Niedermeier, M.; Winter, C.; Maus, R.; Hollingshead, S.; Briles, D.E.; Kunz-Schughart, L.A.; et al. Basophils enhance immunological memory responses. Nat. Immunol. 2008, 9, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Merluzzi, S.; Betto, E.; Ceccaroni, A.A.; Magris, R.; Giunta, M.; Mion, F. Mast cells, basophils and B cell connection network. Mol. Immunol. 2015, 63, 94–103. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharm. 2016, 778, 146–151. [Google Scholar] [CrossRef]
- Crivellato, E.; Travan, L.; Ribatti, D. Mast cells and basophils: A potential link in promoting angiogenesis during allergic inflammation. Int. Arch. Allergy Immunol. 2010, 151, 89–97. [Google Scholar] [CrossRef]
- Heneberg, P. Mast cells and basophils: Trojan horses of conventional lin- stem/progenitor cell isolates. Curr. Pharm. Des. 2011, 17, 3753–3771. [Google Scholar] [CrossRef]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bax, H.J.; Chauhan, J.; Stavraka, C.; Khiabany, A.; Nakamura, M.; Pellizzari, G.; Ilieva, K.M.; Lombardi, S.; Gould, H.J.; Corrigan, C.J.; et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells 2020, 9, 1631. https://doi.org/10.3390/cells9071631
Bax HJ, Chauhan J, Stavraka C, Khiabany A, Nakamura M, Pellizzari G, Ilieva KM, Lombardi S, Gould HJ, Corrigan CJ, et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells. 2020; 9(7):1631. https://doi.org/10.3390/cells9071631
Chicago/Turabian StyleBax, Heather J., Jitesh Chauhan, Chara Stavraka, Atousa Khiabany, Mano Nakamura, Giulia Pellizzari, Kristina M. Ilieva, Sara Lombardi, Hannah J. Gould, Christopher J. Corrigan, and et al. 2020. "Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome" Cells 9, no. 7: 1631. https://doi.org/10.3390/cells9071631
APA StyleBax, H. J., Chauhan, J., Stavraka, C., Khiabany, A., Nakamura, M., Pellizzari, G., Ilieva, K. M., Lombardi, S., Gould, H. J., Corrigan, C. J., Till, S. J., Katugampola, S., Jones, P. S., Barton, C., Winship, A., Ghosh, S., Montes, A., Josephs, D. H., Spicer, J. F., & Karagiannis, S. N. (2020). Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells, 9(7), 1631. https://doi.org/10.3390/cells9071631







