Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Preparation and Phenotypic Analysis of Human ADSCs
2.3. Analysis of Immunosuppressive Activity by ADSCs in Stimulated Mouse T Cells
2.4. Preparation of T Cells and Td-PBMCs for Allogeneic–antigen Stimulation
2.5. Allogeneic–Antigen Stimulation
2.6. Enzyme-Linked ImmunoSpot (ELISPOT) Analysis
2.7. Treatment with Blocking- and Neutralizing-Antibodies
2.8. Statistics
3. Results
3.1. IFN-γ or Combined Cytokines Increased HLA-ABC Expression on the Surface of ADSCs, but not the Expression of Co-Stimulatory Molecules or NKG2DL
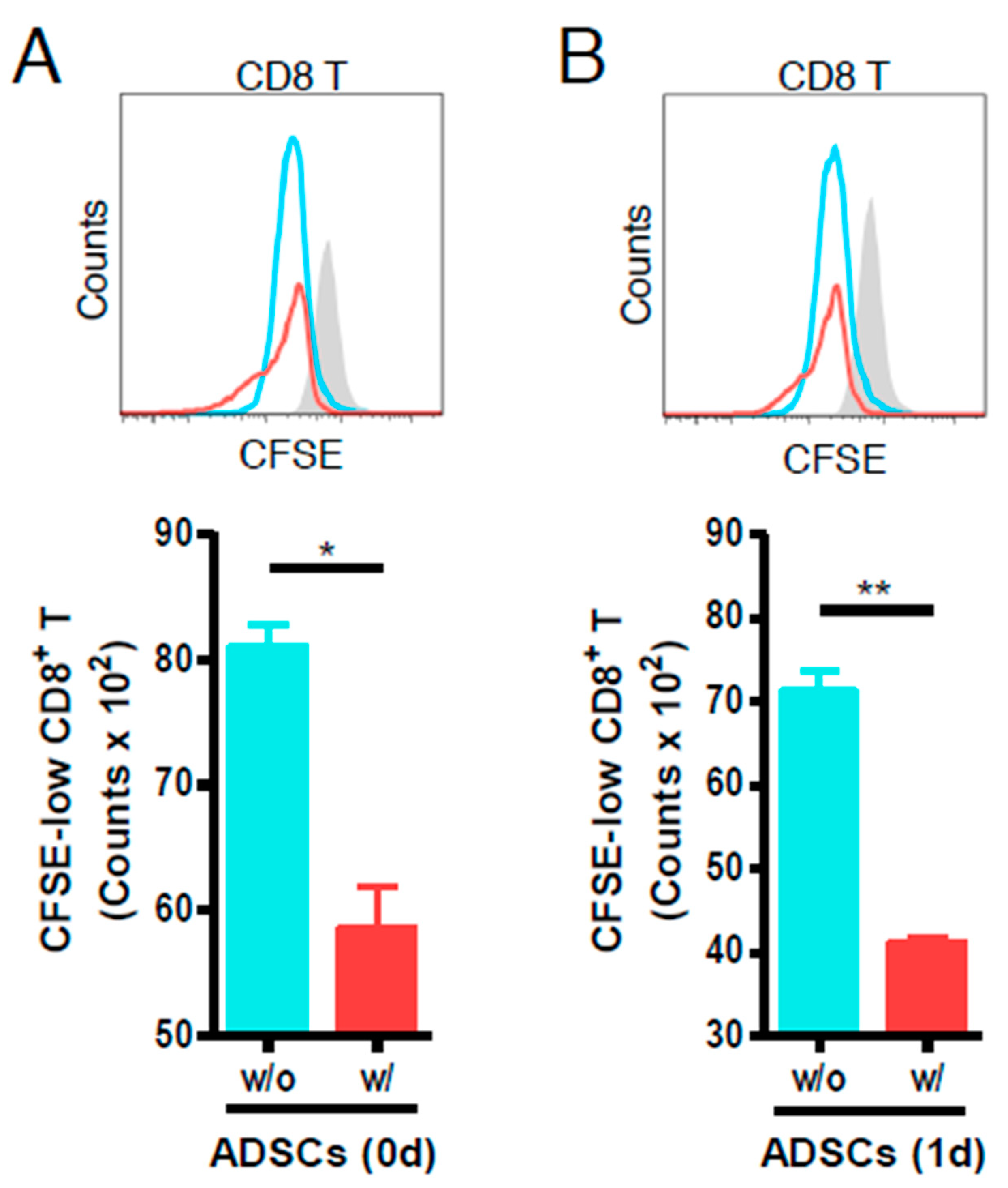
3.2. ADSCs Reduce the Proliferation of Anti-CD3- and Anti-CD28-Stimulated Mouse CD8 T Cells
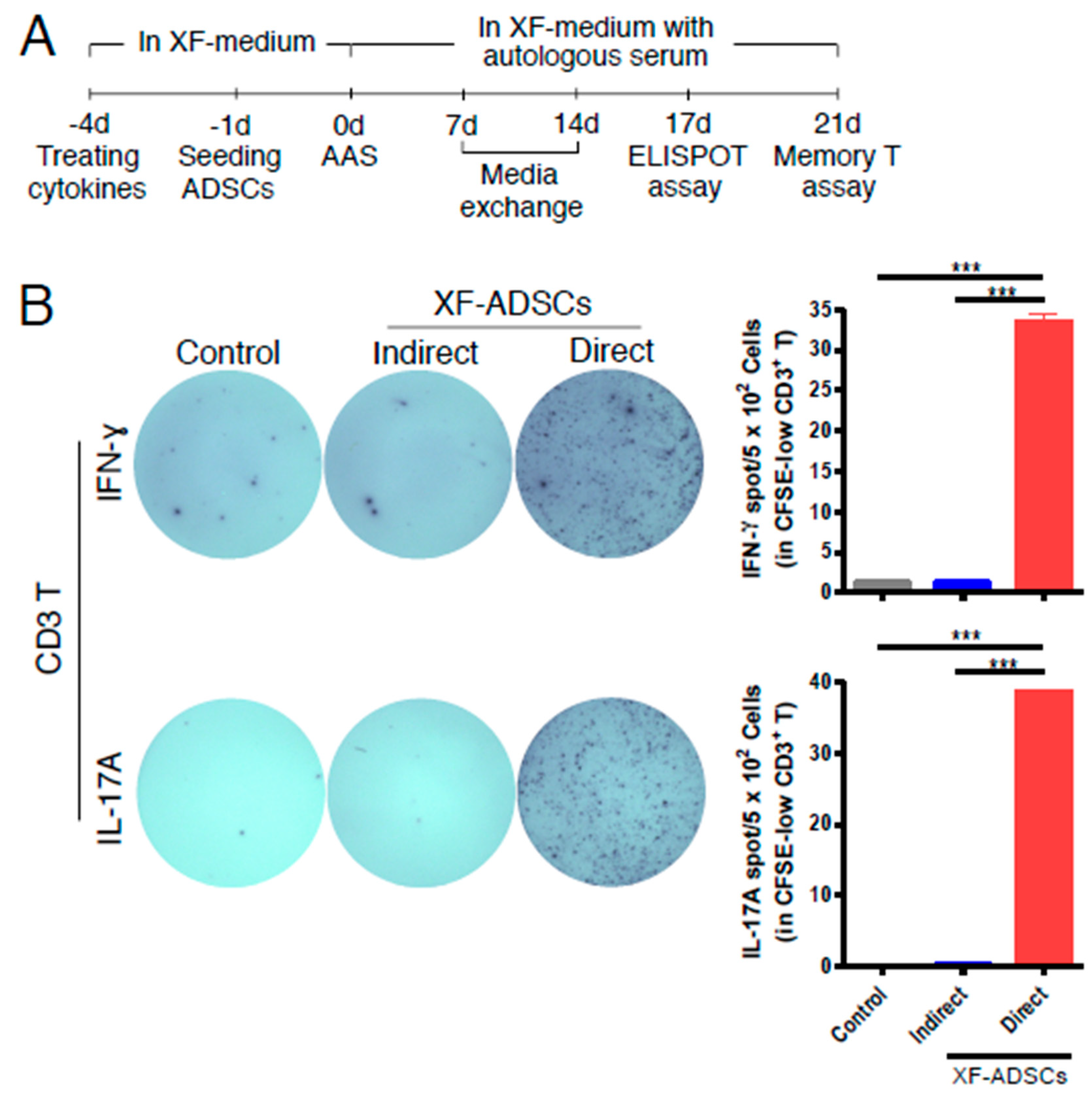
3.3. XF-ADSCs Induce IFN-γ and IL-17A Release by Alloreactive CD3 T Cells in Allogeneic–antigen Stimulation Primarily Via the Direct Pathway
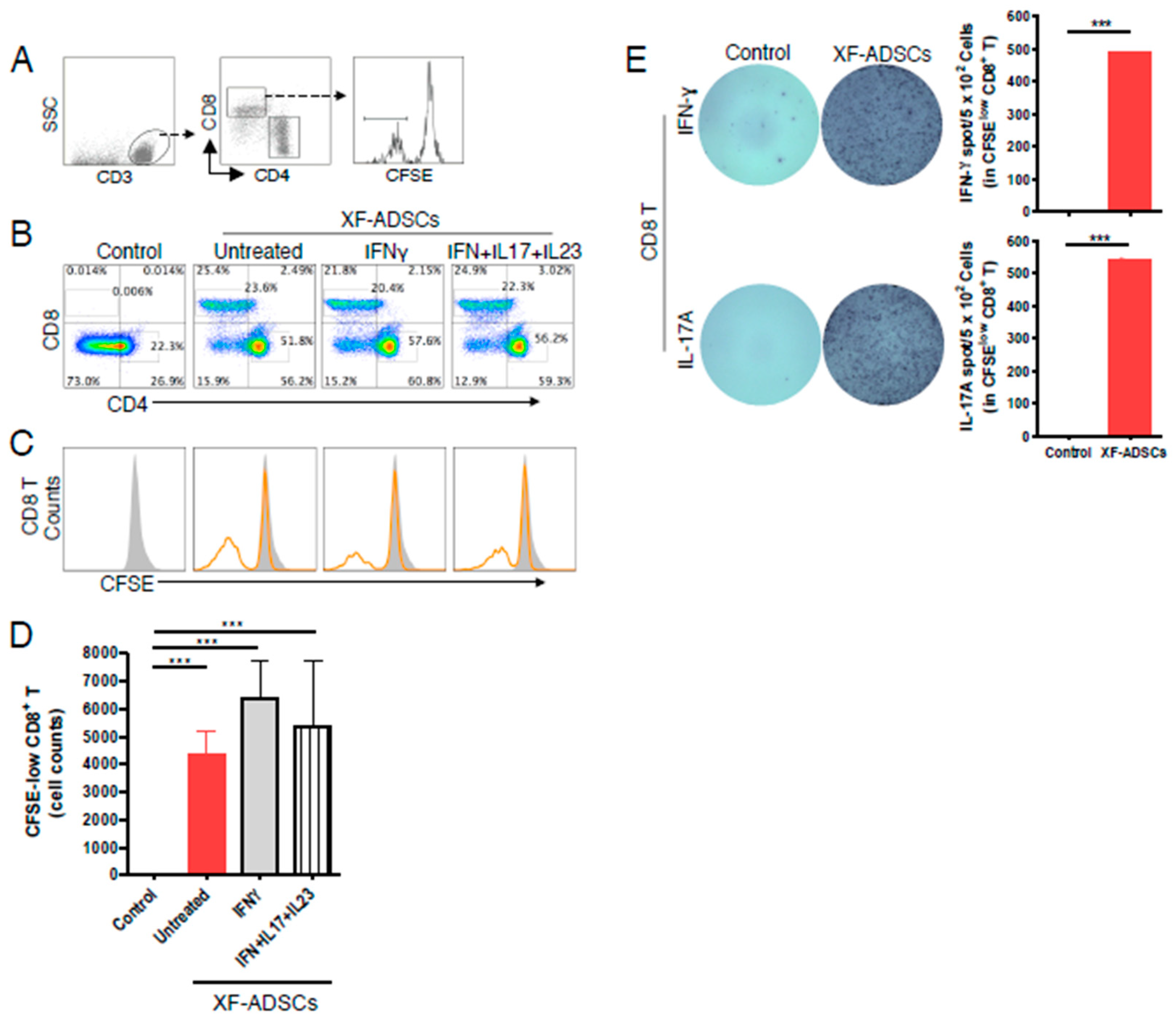
3.4. XF-ADSCs Elicit Significant Production of CFSE-Low CD8 T Cells in Allogeneic–Antigen Stimulation
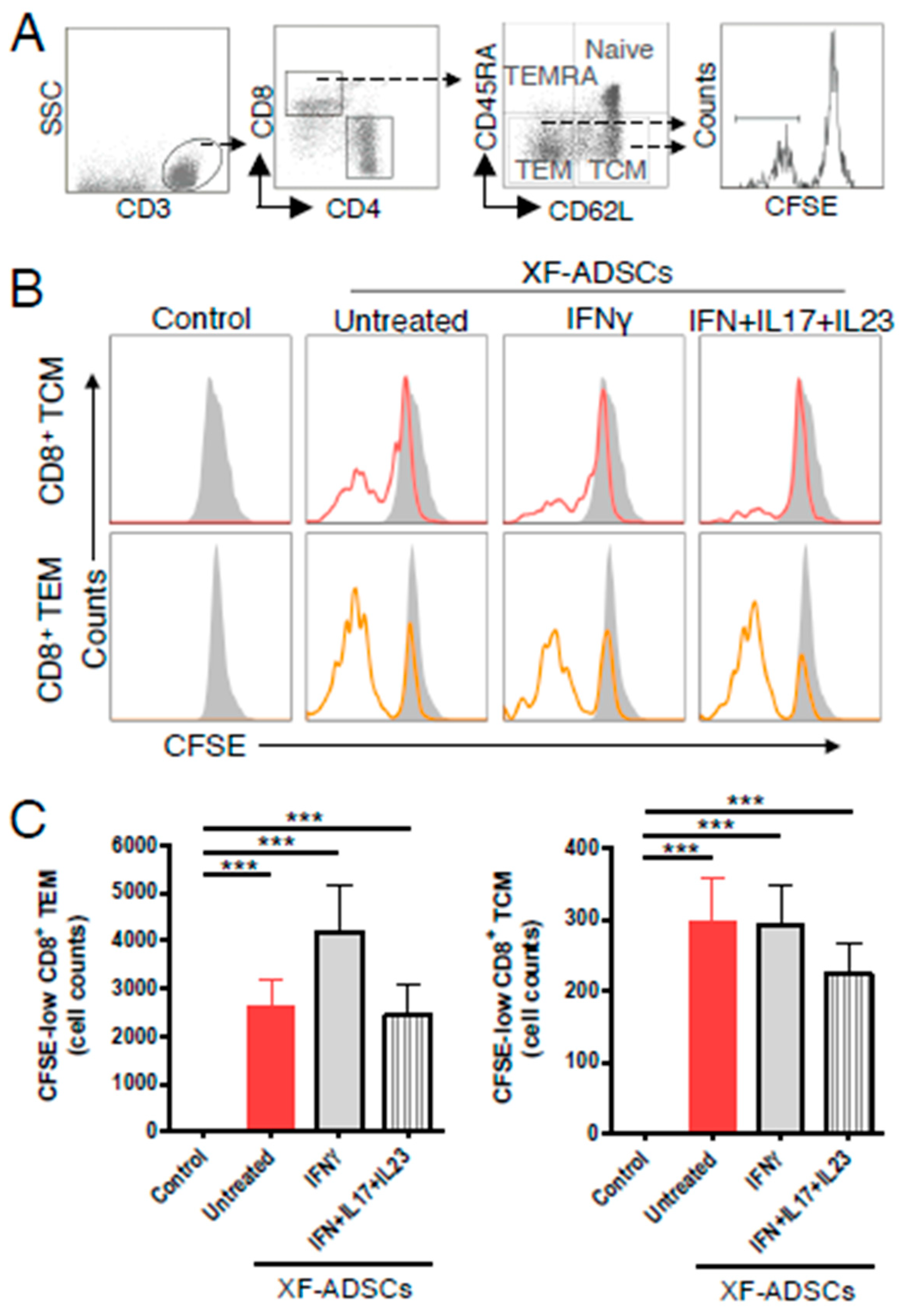
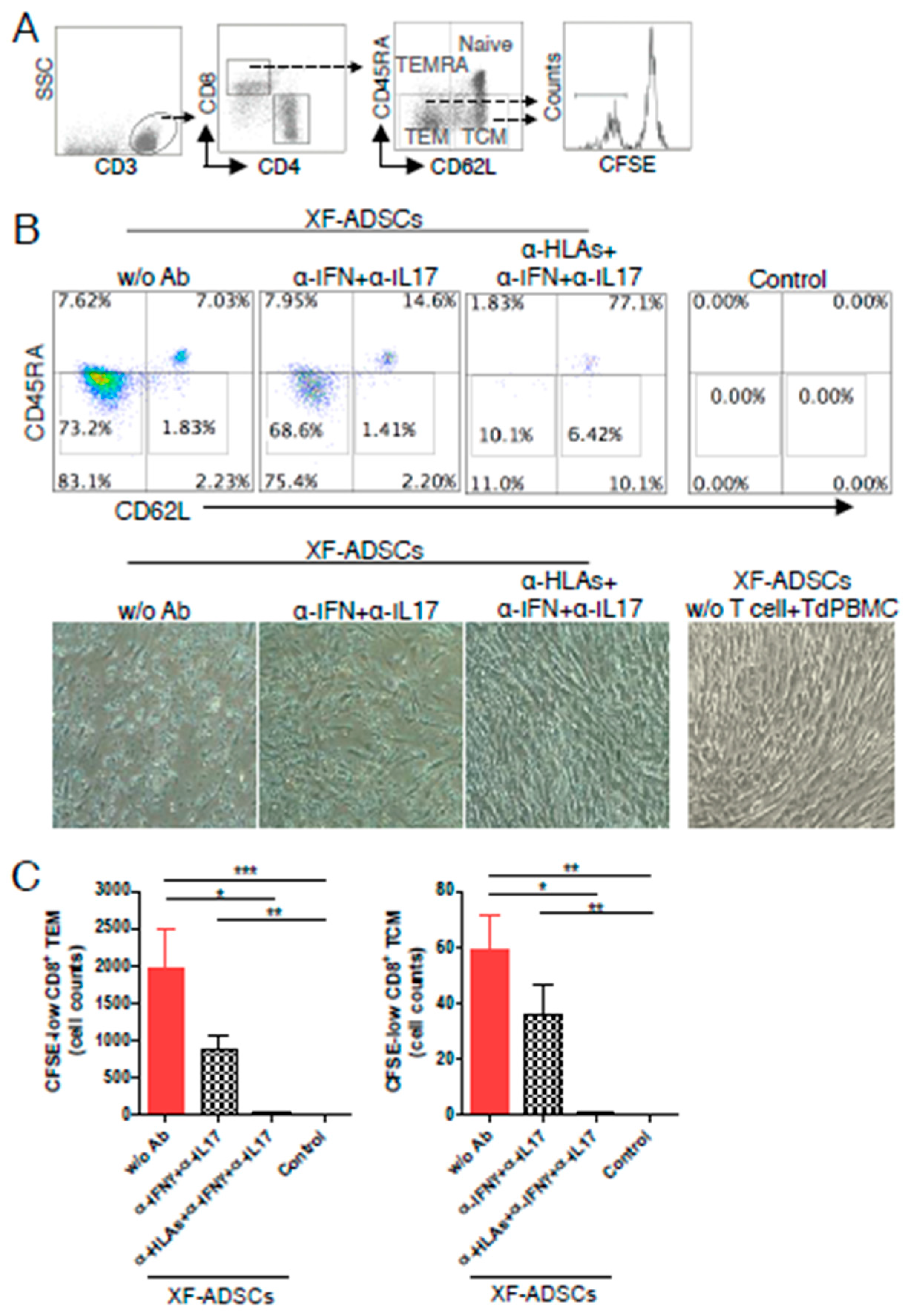
3.5. XF-ADSCs Cause Significant Production of CFSE-Low Memory-CD8 T Cells in Allogeneic–antigen Stimulation
3.6. HLA-Blocking Antibodies Inhibit the Production of Alloreactive Memory-CD8 T Cell in Allogeneic–Antigen Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| XF-ADSCs | Allogeneic ADSCs cultured in xenofree medium |
| TCM | central memory T cells |
| TEM | effector memory T cells |
| Td-PBMCs | T cell-depleted PBMCs |
| AAS | allogeneic–antigen stimulation |
| DPBS | Dulbecco’s phosphate-buffered saline |
| PBMCs | peripheral blood mononuclear cells |
References
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577. [Google Scholar] [CrossRef]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [CrossRef]
- Saler, M.; Caliogna, L.; Botta, L.; Benazzo, F.; Riva, F.; Gastaldi, G. hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro. Int. J Mol. Sci. 2017, 18, 2699. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.Z. Control of Cross Talk between Angiogenesis and Inflammation by Mesenchymal Stem Cells for the Treatment of Ocular Surface Diseases. Stem Cells Int. 2016, 2016, 7961816. [Google Scholar] [CrossRef]
- Sangiorgi, B.; Panepucci, R.A. Modulation of Immunoregulatory Properties of Mesenchymal Stromal Cells by Toll-Like Receptors: Potential Applications on GVHD. Stem Cells Int. 2016, 2016, 9434250. [Google Scholar] [CrossRef]
- Zou, W.; Liu, G.; Zhang, J. Secretome from bone marrow mesenchymal stem cells: A promising, cell-free therapy for allergic rhinitis. Med. Hypotheses 2018, 121, 124–126. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ma, R.; Yin, Y.; Lan, T.; Yu, M.; Ming, Y. Mesenchymal Stem Cells in Renal Fibrosis: The Flame of Cytotherapy. Stem Cells Int. 2019, 2019, 8387350. [Google Scholar] [CrossRef] [PubMed]
- Huaman, O.; Bahamonde, J.; Cahuascanco, B.; Jervis, M.; Palomino, J.; Torres, C.G.; Peralta, O.A. Immunomodulatory and immunogenic properties of mesenchymal stem cells derived from bovine fetal bone marrow and adipose tissue. Res. Vet. Sci. 2019, 124, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Tapp, H.; Hanley, E.N., Jr.; Patt, J.C.; Gruber, H.E. Adipose-derived stem cells: Characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. (Maywood) 2009, 234, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol 2007, 149, 353–363. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Berrih-Aknin, S.; Miller, A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun. Rev. 2011, 10, 410–415. [Google Scholar] [CrossRef]
- Griffin, M.D.; Ritter, T.; Mahon, B.P. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum. Gene Ther. 2010, 21, 1641–1655. [Google Scholar] [CrossRef]
- Ceredig, R. A look at the interface between mesenchymal stromal cells and the immune system. Immunol. Cell Biol. 2013, 91, 3–4. [Google Scholar] [CrossRef]
- Haworth, R.; Sharpe, M. The issue of immunology in stem cell therapies: A pharmaceutical perspective. Regen. Med. 2015, 10, 231–234. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, M.; Storb, R.; Georges, G.E.; Golubev, L.; Nikitine, A.; Hwang, B.; Nash, R.A.; Torok-Storb, B. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biol. Blood Marrow Transplant 2011, 17, 214–225. [Google Scholar] [CrossRef]
- Sivanathan, K.N.; Gronthos, S.; Rojas-Canales, D.; Thierry, B.; Coates, P.T. Interferon-gamma modification of mesenchymal stem cells: Implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation. Stem Cell Rev. 2014, 10, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.L.; Chagastelles, P.C.; Sesterheim, P.; Pranke, P. In Vivo Immunogenic Response to Allogeneic Mesenchymal Stem Cells and the Role of Preactivated Mesenchymal Stem Cells Cotransplanted with Allogeneic Islets. Stem Cells Int. 2017, 2017, 9824698. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Julian, S.; Arnalich-Montiel, F.; Jaumandreu, L.; Leal, M.; Casado, A.; Garcia-Tunon, I.; Hernandez-Jimenez, E.; Lopez-Collazo, E.; De Miguel, M.P. Adipose-derived mesenchymal stem cell administration does not improve corneal graft survival outcome. PLoS ONE 2015, 10, e0117945. [Google Scholar] [CrossRef]
- Badillo, A.T.; Beggs, K.J.; Javazon, E.H.; Tebbets, J.C.; Flake, A.W. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol. Blood Marrow Transplant. 2007, 13, 412–422. [Google Scholar] [CrossRef]
- Chang, S.H.; Park, C.G. Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines. Exp Mol. Med. 2019, 51, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Wang, F.; Heng, B.C.; Cai, Z.; Zhou, J.; Liu, H. Understanding the immunological mechanism of mesenchymal stem cells in allogeneic transplantation: From the aspect of Major Histocompatibility Complex class I. Stem Cells Dev. 2019. [Google Scholar] [CrossRef]
- Kiernan, C.H.; Wolvius, E.B.; Brama, P.A.J.; Farrell, E. The Immune Response to Allogeneic Differentiated Mesenchymal Stem Cells in the Context of Bone Tissue Engineering. Tissue Eng. Part B Rev. 2018, 24, 75–83. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Yang, X.F.; Chen, T.; Ren, L.W.; Yang, L.; Qi, H.; Li, F.R. Immunogenicity of insulin-producing cells derived from human umbilical cord mesenchymal stem cells. Exp. Ther. Med. 2017, 13, 1456–1464. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Kojima, Y.; Ikarashi, S.; Seino, S.; Watanabe, Y.; Kawata, Y.; Terai, S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm. Regen. 2017, 37, 16. [Google Scholar] [CrossRef] [PubMed]
- Shelton, M.W.; Walp, L.A.; Basler, J.T.; Uchiyama, K.; Hanto, D.W. Mediation of skin allograft rejection in scid mice by CD4+ and CD8+ T cells. Transplantation 1992, 54, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.J.; Goto, R. Mechanisms of rejection: Current perspectives. Transplantation 2012, 93, 1–10. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L.; Baker, A. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Morelli, A.E.; Thomson, A.W. Role of dendritic cells in the immune response against allografts. Curr. Opin. Nephrol. Hypertens. 2000, 9, 607–613. [Google Scholar] [CrossRef]
- Valujskikh, A.; Li, X.C. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J. Am. Soc. Nephrol. 2007, 18, 2252–2261. [Google Scholar] [CrossRef]
- Zhang, Q.; Lakkis, F.G. Memory T Cell Migration. Front. Immunol. 2015, 6, 504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Kloc, M.; Ghobrial, R.M. Memory T cells in transplantation - progress and challenges. Curr. Opin. Organ Transplant 2013, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brook, M.O.; Carvalho-Gaspar, M.; Zhang, J.; Ramon, H.E.; Sayegh, M.H.; Wood, K.J.; Turka, L.A.; Jones, N.D. Allograft rejection mediated by memory T cells is resistant to regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 19954–19959. [Google Scholar] [CrossRef] [PubMed]
- Auchincloss, H. No tolerance for depletion. Nat. Med. 2004, 10, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cianflone, D.; Vecchio, V.; Banfi, M.; Vermi, A.C.; De Metrio, M.; Grigore, L.; Pellegatta, F.; Pirillo, A.; Garlaschelli, K.; et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. J. Am. Heart Assoc. 2012, 1, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R. Dual personality of memory T cells. Nature 1999, 401, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Gebhardt, T.; Carbone, F.R.; Heath, W.R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol 2013, 31, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Calabresi, P.A. Novel therapies for memory cells in autoimmune diseases. Clin. Exp. Immunol. 2015, 180, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.H.; Frassle, S.P.; Sommermeyer, D.; Buchholz, V.R.; Riddell, S.R. Role of memory T cell subsets for adoptive immunotherapy. Semin. Immunol. 2016, 28, 28–34. [Google Scholar] [CrossRef]
- Chahroudi, A.; Silvestri, G.; Lichterfeld, M. T memory stem cells and HIV: A long-term relationship. Curr. HIV/AIDS Rep. 2015, 12, 33–40. [Google Scholar] [CrossRef]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Alagesan, S.; Griffin, M.D. Autologous and allogeneic mesenchymal stem cells in organ transplantation: What do we know about their safety and efficacy? Curr. Opin. Organ Transplant 2014, 19, 65–72. [Google Scholar] [CrossRef]
- Ben Azouna, N.; Jenhani, F.; Regaya, Z.; Berraeis, L.; Ben Othman, T.; Ducrocq, E.; Domenech, J. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: Comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res. Ther. 2012, 3, 6. [Google Scholar] [CrossRef]
- Julavijitphong, S.; Wichitwiengrat, S.; Tirawanchai, N.; Ruangvutilert, P.; Vantanasiri, C.; Phermthai, T. A xeno-free culture method that enhances Wharton’s jelly mesenchymal stromal cell culture efficiency over traditional animal serum-supplemented cultures. Cytotherapy 2014, 16, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Kim, K.J.; Kim, M.K.; Lee, S.J.; Ryu, Y.H.; Seo, B.F.; Oh, D.Y.; Ahn, S.T.; Lee, H.Y.; Rhie, J.W. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs 2014, 199, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kruisbeek, A.M.; Shevach, E.; Thornton, A.M. Proliferative assays for T cell function. Curr. Protoc. Immunol. 2004, 3. [Google Scholar] [CrossRef]
- Marino, E.; Grey, S.T. B cells as effectors and regulators of autoimmunity. Autoimmunity 2012, 45, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Chatterjea, A.; LaPointe, V.L.; Alblas, J.; Chatterjea, S.; van Blitterswijk, C.A.; de Boer, J. Suppression of the immune system as a critical step for bone formation from allogeneic osteoprogenitors implanted in rats. J. Cell. Mol. Med. 2014, 18, 134–142. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Buja, L.M.; Vela, D. Immunologic and inflammatory reactions to exogenous stem cells implications for experimental studies and clinical trials for myocardial repair. J. Am. Coll. Cardiol. 2010, 56, 1693–1700. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Burgess, S.J.; Maasho, K.; Masilamani, M.; Narayanan, S.; Borrego, F.; Coligan, J.E. The NKG2D receptor: Immunobiology and clinical implications. Immunol. Res. 2008, 40, 18–34. [Google Scholar] [CrossRef]
- Ito, A.; Shimura, H.; Nitahara, A.; Tomiyama, K.; Ito, M.; Kanekura, T.; Okumura, K.; Yagita, H.; Kawai, K. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int. Immunol. 2008, 20, 1343–1349. [Google Scholar] [CrossRef]
- Zhuo, M.; Fujiki, M.; Wang, M.; Piard-Ruster, K.; Wai, L.E.; Wei, L.; Martinez, O.M.; Krams, S.M. Identification of the rat NKG2D ligands, RAE1L and RRLT, and their role in allograft rejection. Eur. J. Immunol. 2010, 40, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Aranami, T.; Yamamura, T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS). Allergol. Int. 2008, 57, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, S.Q.; Zhao, Y.M.; Yu, S.; Ge, L.H.; Xu, B.H. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol. Med. Rep. 2018, 18, 4969–4977. [Google Scholar] [CrossRef] [PubMed]
- Sudres, M.; Norol, F.; Trenado, A.; Gregoire, S.; Charlotte, F.; Levacher, B.; Lataillade, J.J.; Bourin, P.; Holy, X.; Vernant, J.P.; et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 2006, 176, 7761–7767. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Popp, F.C.; Koehl, G.E.; Piso, P.; Schlitt, H.J.; Geissler, E.K.; Dahlke, M.H. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation 2006, 81, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Lewis, C.J.; Madsen, J.C. Organ Transplantation: A Clinical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Lin, C.M.; Gill, R.G. Direct and indirect allograft recognition: Pathways dictating graft rejection mechanisms. Curr. Opin. Organ. Transplant 2016, 21, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.D.; Clarkson, M.R.; Yagita, H.; Turka, L.A.; Sayegh, M.H.; Li, X.C. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J. Immunol. 2006, 176, 1394–1401. [Google Scholar] [CrossRef]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Yu, Z.; Wenyan, T.; Xuewen, S.; Baixiang, D.; Qian, W.; Zhaoyan, W.; Yinxiang, Y.; Suqing, Q.; Zuo, L. Immunological effects of the intraparenchymal administration of allogeneic and autologous adipose-derived mesenchymal stem cells after the acute phase of middle cerebral artery occlusion in rats. J. Transl. Med. 2018, 16, 339. [Google Scholar] [CrossRef]

| Antibody Target | Antibody Name (Clone) | Antibody Type | Manufacturer |
|---|---|---|---|
| ADSC-surface marker | CD13 (WM15) | Monoclonal | BioLegend |
| CD44 (IM7) | Monoclonal | eBioscience | |
| CD73 (AD2) | Monoclonal | eBioscience | |
| CD90 (5E10) | Monoclonal | eBioscience | |
| CD105 (MJ7/18) | Monoclonal | eBioscience | |
| Co-stimulatory molecule | CD80 (2D10.4) | Monoclonal | eBioscience |
| CD86 (IT2.2) | Monoclonal | eBioscience | |
| Human leukocyte antigen | HLA-ABC (W6/32) | Monoclonal | BioLegend |
| HLA-DR (L243) | Monoclonal | BioLegend | |
| NKG2DL | MIC-A (159227) | Monoclonal | R&D Systems |
| MIC-B (236511) | Monoclonal | R&D Systems | |
| ULBP-1 (170818) | Monoclonal | R&D Systems | |
| ULBP-2/5/6 (165903) | Monoclonal | R&D Systems |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-H.; Kim, H.J.; Park, C.-G. Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens. Cells 2020, 9, 1246. https://doi.org/10.3390/cells9051246
Chang S-H, Kim HJ, Park C-G. Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens. Cells. 2020; 9(5):1246. https://doi.org/10.3390/cells9051246
Chicago/Turabian StyleChang, Sung-Ho, Hyun Je Kim, and Chung-Gyu Park. 2020. "Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens" Cells 9, no. 5: 1246. https://doi.org/10.3390/cells9051246
APA StyleChang, S.-H., Kim, H. J., & Park, C.-G. (2020). Allogeneic ADSCs Induce the Production of Alloreactive Memory-CD8 T Cells through HLA-ABC Antigens. Cells, 9(5), 1246. https://doi.org/10.3390/cells9051246





