Highlights
What are the main findings?
- Limbal epithelial stem cells (LESCs) are central regulators of corneal immune and (lymph)angiogenic privilege, integrating niche-derived biochemical and mechanical cues to balance regeneration with avascularity.
- There are key stem cell markers that identify functionally distinct limbal cell subsets, with specialised epithelial cells supporting repair-associated angiogenic responses and matching stromal cells exerting strong anti-inflammatory and anti-(lymph)angiogenic effects.
What are the implications of the main findings?
- Loss or dysfunction of the limbal niche disrupts immune and vascular privilege, driving limbal stem cell deficiency (LSCD), chronic inflammation, neovascularization, and vision loss.
- Future LSCD therapies should combine epithelial regeneration with stabilization of immune and vascular privilege, leveraging stem cell populations or their paracrine signals to achieve durable ocular surface restoration.
Abstract
The cornea maintains transparency by preserving immune and (lymph)angiogenic privilege through active suppression of inflammation and vascular invasion, a process centrally regulated by limbal epithelial stem cells (LESCs) located at the corneoscleral junction. Beyond renewing the corneal epithelium, LESCs maintain immune and vascular balance via extracellular matrix interactions and paracrine signalling, exerting predominantly anti-inflammatory and anti-(lymph)angiogenic effects in vivo. Disruption of the limbal niche by trauma, UV exposure, or genetic disorders such as aniridia leads to limbal stem cell deficiency (LSCD), chronic inflammation, loss of corneal avascularity, and vision loss. The identification of ABCB5 as a key LESC marker has clarified functional limbal subsets, highlighting ABCB5+ epithelial cells as mediators of repair, remodelling, and immune suppression, and positioning them as promising therapeutic targets for treatments that restore both epithelial integrity and corneal immune privilege.
1. Introduction
The cornea is the major refractive element of the eye. To properly focus light into the eye, the cornea must efficiently transmit light through itself by remaining transparent [1,2]. The refraction and transparency of the cornea is maintained through precise arrangement of collagen in the corneal stroma [3]. The stromal cells that maintain this collagen matrix also match their own refractive index to the cornea’s by regulating their cytoplasmic content [4].
Loss of corneal transparency is often a downstream consequence of two failures: breakdown of immune privilege and escape from (lymph)angiogenic privilege. Clinically, once afferent lymphatic routes and efferent blood vessels invade the cornea, antigen trafficking and leukocyte recruitment accelerate, rejection risk rises in case of subsequent corneas transplantation, and vision declines, explaining why the cornea’s exceptional graft success has long been linked to its immune-privileged status and avascularity [5,6].
This is a dynamic balance maintained by reduced antigen presentation, FasL-mediated deletion of infiltrating effectors, anterior chamber-associated immune deviation ACAID, and endogenous vascular inhibitors, including soluble VEGF receptors (sVEGFR-2/-3) that neutralize VEGF-C/-D to suppress lymphangiogenesis, thereby limiting both edema-inducing lymphatic flux and the priming arm of alloimmunity. When this network is perturbed by inflammation, hypoxia, or injury, the cornea’s angiogenic balance is disrupted, allowing neovessels to breach the limbal barrier [7,8,9,10].
The region where this balance physically evident and most relevant is the limbus, an oblique boundary where the cornea meets the sclera that encircles the entire cornea [11]. At the limbus, LESCs occupy a niche adjacent to dense vascular and immune circuits yet normally sustain an avascular, immune-regulated surface. In this context, several putative LESC markers, including ABCB5, have been associated with regenerative potential and with modulation of the local microenvironment. Available evidence suggests that LESC integrate extracellular matrix and mechanotransductive cues, as well as paracrine signaling programs, that collectively influence leukocyte activity and vascular growth [12,13]. This review synthesizes the following key features of the corneal limbus: 1—how LESCs and their microenvironment preserve immune and lymphangiogenic privilege in health; 2—how common sources of corneal pathological stress (e.g., UV, inflammation) lead to LSCD and neovascularization; and 3—the emerging relevance of ABCB5-expressing cell populations in corneal repair.
2. Cornea Anatomy
The cornea is the clear, dome-shaped front part of the eye. It contributes the majority of the eye’s total refractive power, making its shape and clarity critical for sharp vision. Anatomically, the human cornea consists of five layers, each distinct in its function for the maintenance of optical clarity and mechanical integrity (Figure 1). The epithelium consists of 5 to 7 layers of cells in humans and 2 to 3 layers in mice [14,15]. It comprises non-keratinized stratified squamous cells and acts as a physical barrier against environmental pathogens and trauma. Also, the epithelium contributes to the total corneal refractive power by maintaining a smooth optical surface. This layer undergoes continuous regeneration driven by LESC, which proliferate and migrate centripetally before differentiating and being shed from the surface. Beneath the epithelium, Bowman’s layer, which is an acellular, non-regenerating collagenous matrix situated between the epithelial basement membrane and the anterior stroma. In the central human cornea, Bowman’s layer measures approximately 10 μm in thickness and consists of randomly oriented 20 nm collagen fibrils with an undulating epithelial base. Scanning electron microscopy reveals a meshwork-like structure on its epithelial side, and it is clearly demarcated from the underlying substantia propria [16]. Functionally, Bowman’s layer contributes to corneal rigidity, tensile strength, and surface smoothness [17]. Furthermore, stromal nerve branches traverse this layer to form the sub-basal nerve plexus, located between Bowman’s layer and the basal epithelium, which extends fine perpendicular nerve terminals to innervate the anterior epithelial layers [18].
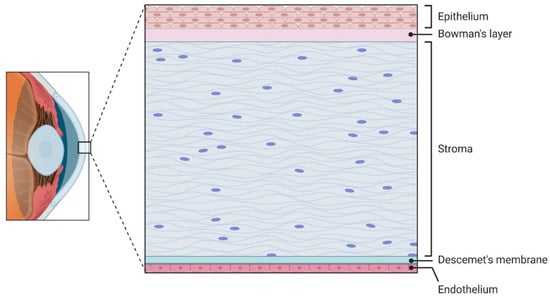
Figure 1.
Cross-section of the corneal tissue layers, specifically in the central cornea. Stratified epithelium sits atop the acellular Bowman’s layer. The stroma makes up the bulk of the corneal volume with its highly organized collagen ECM. The acellular Descemet’s membrane separates the stroma from the innermost cell layer, the single-cell thick endothelium.
The corneal stroma makes up an estimated 80–85% of the total corneal thickness volume. It forms the primary structural scaffold of the cornea, and is essential for its transparency and mechanical strength [14]. The stroma consists of homogenous collagen fibrils of diameter 30 nm [19]. These fibrils are orthogonally arranged and organized into lamellae that run across the corneal surface. Collagen type I is the most abundant collagen type present, making up a reported 75% of the corneal collagen. Collagen type V is reported to be a consistent 20% of the total fibrillar collagen synthesized [20], and Collagen type III is reported to be present in quantities as low as 2%, but increases during wound healing and inflammation [21]. In the anterior stroma, the lamellae are thinner and more interwoven, providing enhanced resistance to shear forces and helping maintain anterior curvature. In contrast, the posterior stromal lamellae are thicker and more regularly parallel, contributing to overall tensile strength [22]. Interspersed between these lamellae are keratocytes, specialized fibroblast-like cells that maintain the extracellular matrix by secreting collagen and glycosaminoglycans (GAGs). Keratocytes also perform essential roles in wound healing responses [14]. The stroma is rich in GAGs, particularly keratan sulfate, chondroitin sulfate, and dermatan sulfate, which regulate hydration and precise interfibrillar spacing, both are critical features for the maintenance of the corneal shape and volume [22,23].
Furthermore, recent findings highlight the existence of corneal stromal stem cells (CSSCs depicted in Figure 2), especially concentrated in the anterior limbal stroma. CSSCs exhibit mesenchymal markers (e.g., CD73, CD90, CD105) and modulate keratocyte and immune cells activity through paracrine immunomodulatory and matrix-regulating signals supporting stromal homeostasis and transparency [24,25].
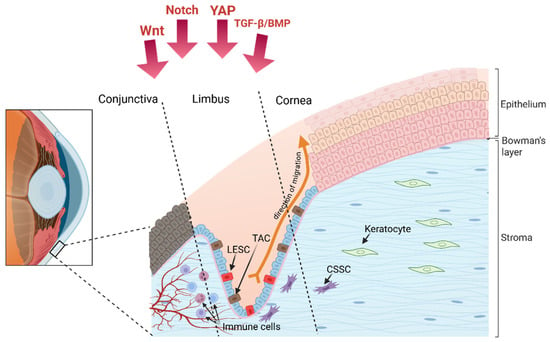
Figure 2.
The limbus as a stem cell niche, with the Limbal Epithelial Stem Cell (LESC) and its descendent Transit-Amplifying Cell (TAC) that divide and migrate away from the limbus and into the central to form the corneal epithelium. Adjacent to the epithelial cells are the Corneal Stromal Stem Cells (CSSC), these are the cells that divide and differentiate to maintain the stromal keratocyte population. Under the limbal crypt are the blood vessels, lymphatic vessels, and immune cells; the presence of each of these components is regulated by the production of several important growth factor, some of which are shown here as Wnt Notch, YAP, and TGF-β/BMP.
Beneath the stroma lies Descemet’s membrane (DM), a distinct, acellular basement membrane critical for corneal structure and transparency. It consists of three distinct zones: an interfacial matrix that is anchored to the stroma, an anterior banded layer (2–4 μm) formed during fetal development, and a posterior non-banded layer that develops after birth and thickens with age (~1 μm per decade) [17,26]. DM is primarily composed of collagen types IV and VIII (the latter forming a hexagonal lattice), as well as laminin, fibronectin, and various proteoglycans [17,27]. the DM is the support for endothelial cell adhesion, regulates corneal hydration, and maintains curvature and biomechanical stability [17,28].
The innermost layer of the cornea, the endothelium, is a single layer of hexagonal cells responsible for regulating stromal hydration through active ion transport mechanisms, often described as a pump-leak system [29,30]. By maintaining precise stromal hydration, the endothelium preserves corneal transparency and prevents swelling, which would otherwise impair vision. Human endothelial cells exhibit limited regenerative capacity, compensating for cell loss mainly via enlargement and migration rather than division [31]. Consequently, endothelial dysfunction is a leading indication for corneal transplantation in industrialized countries [32]. Recent advances focus on ex vivo expansion and cell injection therapies to restore endothelial function [33].
3. The Corneal Limbus
3.1. Limbal Anatomy
The limbus is a narrow, ring-shaped transition zone measuring approximately 1.5 to 2 mm in width, located at the corneoscleral junction and completely encircling the peripheral cornea [34,35]. Anteriorly, it is defined by the termination of Bowman’s layer, while posteriorly it corresponds to the peripheral edge of Descemet’s membrane and aligns with the scleral spur [36,37]. Structurally, the limbus serves as a bridge between the transparent, avascular cornea and the opaque, vascularized sclera. The corneal stroma transitions peripherally into the scleral stroma, creating a continuous connective tissue [34]. The limbal stroma blends with adjacent stromal tissues and contains radial fibrovascular ridges known as the palisades of Vogt [38,39]. These palisades are innervated and vascularized structures rich in melanocytes and antigen-presenting cells, forming a specialized microenvironment [40,41]. From its inner to outer aspects, the limbus is adjacent to several critical structures, including the trabecular meshwork, scleral spur, Schlemm’s canal, episclera, conjunctival stroma, and conjunctival epithelium [37]. Unlike the avascular cornea, the limbus is highly vascularized, receiving its blood supply primarily from the anterior ciliary arteries and containing a dense network of blood and lymphatic vessels that facilitate metabolic exchange and immune cell presence [37,40]. Importantly, the palisades of Vogt serve as the main niche for LESCs, and within and beneath them, limbal epithelial crypts form epithelial extensions particularly enriched in LESCs that maintain corneal epithelial renewal [34,38,42,43]. Collectively, these anatomical and functional features establish the limbus as an essential zone for ocular surface homeostasis. It should be noted that the palisades of Vogt are absent in some species, including mice, and are instead predominantly found in primates and pigs. This species-specific difference in limbal architecture complicates direct comparison between mouse models and the human limbal stem cell niche [44,45].
3.2. ECM Composition and Anatomical Characteristics of the Limbal Niche
The extracellular matrix (ECM) of the limbal niche is fundamental for maintaining limbal epithelial stem cell (LESC) function, providing both structural support and biochemical cues essential for stemness. Unlike the central cornea, the limbal epithelial basement membrane reportedly lacks the long form of type XII collagen but is enriched in type IV collagen as well as laminin isoforms α2 and β2, which improve cell adhesion and selective cell signalling within the niche [41,46]. Additional components, including vitronectin, fibronectin, and tenascin-C, further distinguish the limbal ECM from the corneal stroma [34,47]. Tenascin-C is specifically expressed in the limbus in the same region as ABCG2+/p63+ basal epithelial cell clusters, indicating a key role in modulating cell adhesion and maintaining an undifferentiated stem/progenitor cell state [41,48].
Fibronectin, another important ECM glycoprotein, contributes to cell adhesion, migration, and wound healing. In rabbit models, it has been shown to promote self-renewal of LESCs, suggesting a supportive role in niche dynamics, though human-specific evidence is still evolving.
Hyaluronan (HA), a high-molecular-weight glycosaminoglycan synthesized by hyaluronan synthases (HAS1, HAS2, HAS3), is a major constituent of the limbal ECM. In murine models, HA is localized almost exclusively to the limbus, absent in the central cornea, with weaker staining in the perilimbal conjunctiva [49,50]. Disruption or downregulation of HAS enzymes, particularly HAS2, leads to decreased epithelial stratification, altered basal cell morphology, delayed wound healing, and loss of CK15+ cells, reflecting HA’s essential role in maintaining LESC function and preventing conjunctivalization [50]. Following corneal injury, HA expression transiently expands into the central cornea, accompanying a progenitor-like phenotype to promote epithelial repair. In inflammation, limbal HA can also promote lymphangiogenesis [49,50]. Collectively, these specialized ECM components create a unique and highly regulated microenvironment that preserves the regenerative capacity of limbal stem cells and safeguards corneal clarity and integrity [41].
Tenascin-C, fibronectin, and hyaluronan (HA) in the limbal ECM support LESC stemness which in turn regulate immune and vascular responses. Tenascin-C limits endothelial cell migration, [51], fibronectin promotes an anti-inflammatory environment [52], and HA helps suppress local inflammation [53]. Together, they contribute to the cornea’s immune and (lymph)angiogenic privilege.
3.3. Structure and Cellular Components of the Limbal Niche
In humans, LESCs reside within specialized anatomical microenvironments, including the palisades of Vogt, limbal crypts, and limbal lacunae, forming a highly structured niche that protects them from mechanical and UV-induced stress while providing vascular, neural, and extracellular matrix (ECM) support [42,54,55,56]. The human central cornea lacks a resident stem cell population, reinforcing the limbus as the principal stem cell reservoir of regenerative capacity [57,58].
The limbal niche does not only offer structural shelter but also actively regulates LESC behavior through autocrine and paracrine signals, as well as mechanical cues and specific ECM interactions [59,60,61,62]. The migratory maintenance of the corneal epithelium can be described by the X-Y-Z hypothesis, which describes corneal epithelial renewal as occurring in along three axes. LESCs proliferate and generate transit-amplifying cells TACs in the X axis, which migrate centripetally along a Y axis to replace terminally differentiated corneal epithelial cells that are shed from the surface along a Z axis [63,64].
Transcriptome and proteome analyses confirm that these niche components contribute to a microenvironment that supports both symmetric and asymmetric division of LESCs [65,66,67].
The LESC niche is highly cellularly diverse: limbal melanocytes protect LESCs from UV-induced damage and regulate their quiescence; limbal stromal cells, including keratocytes and CSSCs, modulate ECM composition and secrete paracrine factors supporting LESC maintenance [68,69]. Immune cells, particularly dendritic cells and macrophages, are also found adjacent to limbal crypts, contributing to niche immune protection [70,71]. Neural elements within the limbal niche contribute to LESC regulation through trophic signalling and cytokine gradients [72]. The limbal region is supported by a capillary bed branching from the episcleral arterial circle, providing nourishment to the limbal population [73,74]. The metabolic activity of the central cornea is comparatively lower and doesn’t require the arterial supply and venous drainage of the limbus. Instead, metabolic requirements are met by the delivery of glucose and oxygen from the aqueous humour and tear fluid [75].
3.4. LESC Markers
LESCs are characterized by the expression of a specific set of molecular markers, distinguishing them from transit-amplifying cells (TACs) and terminally differentiated corneal epithelial cells (See Table 1). Classic limbal stem cell markers include nuclear p63α, particularly the N-terminally truncated ΔNp63α isoform, which plays a critical role in maintaining the proliferative potential of limbal stem cells and their ability to migrate into the cornea [76,77,78]. In the absence of an attached limbus, p63α is absent from the corneal epithelium [79].
CK14 and CK15 are co-expressed in the basal limbal epithelium and serve as markers of undifferentiated progenitor epithelial cells, often used to identify the limbal epithelial stem cell (LESC) population [80,81]. ABCG2, a member of the ATP-binding cassette transporter family, is a marker of clonogenic stem cells, functioning as a side population determinant and contributing to cellular protection [82]. PAX6 regulates corneal epithelial development by establishing limbal stem cell identity during development and regulating gene expression profile of LESCs to retain non-keratinized epithelial cell properties [83]. Haploinsufficiency in Pax6 disrupts clonal expansion, epithelial maturation, and K12 expression, leading to defects analogous to human aniridia-related keratopathy [84,85,86,87]. Ksander and colleagues identified ABCB5, an ATP-binding cassette transporter, as a key marker of LESCs. ABCB5+ cells in the limbus demonstrate high clonogenic potential and the ability to support long-term corneal epithelial regeneration, confirming their identity as stem cells [12]. Supporting this, Vattulainen et al. showed that ABCB5+/∆Np63α+ cells derived from pluripotent stem cells exhibit LESC-like characteristics and enhanced wound healing capacity [12,88]. The co-expression of ABCB5 with established markers such as ∆Np63α and CK15 further validates its role in identifying functional LESC populations.
Other cell surface markers proposed include CD200 and CD109. CD200 appears on a small quiescent population capable of holoclone formation, and CD109 associated with more proliferative progenitors. Both were co-expressed with ∆Np63α, though the functional hierarchy among these subpopulations remains unclear [58,89].


Table 1.
Summary of selected molecular markers associated with human corneal limbal stem cells (LSCs). The table lists markers commonly used to characterize limbal epithelial stem cell populations, including proteins positively associated with stemness as well as markers that are downregulated or absent in LSC-rich regions and instead associated with epithelial differentiation. Marker type, biological role, and key literature references are provided to support interpretation and experimental use.
Table 1.
Summary of selected molecular markers associated with human corneal limbal stem cells (LSCs). The table lists markers commonly used to characterize limbal epithelial stem cell populations, including proteins positively associated with stemness as well as markers that are downregulated or absent in LSC-rich regions and instead associated with epithelial differentiation. Marker type, biological role, and key literature references are provided to support interpretation and experimental use.
| Marker | Type | Role | References |
|---|---|---|---|
| ΔNp63α | Transcription factor | Highly expressed in basal limbal epithelial cells | [79] |
| ABCG2 | ATP-binding cassette transporter | Marker of small basal limbal cell clusters | [82,90] |
| ABCB5 | ATP-binding cassette transporter | Marks a slowly cycling population with regenerative capacity | [12,88,91] |
| CK14 | Cytokeratin (intermediate filament) | Expressed in undifferentiated epithelial cells in limbal basal layer. | [90,92] |
| CK15 | Cytokeratin | Co-expressed with stem/progenitor populations in limbus; lower in differentiated cells. | [80,90] |
| Integrin α9 | Cell adhesion receptor | Enriched in basal limbus; interacts with limbal ECM components | [90,93] |
| Integrin β1 | Cell adhesion receptor | Basal limbal expression, linked to epithelial progenitor identity. | [94] |
| N-cadherin | Cell adhesion molecule | Adhesion-linked stemness marker identified in basal limbal cells | [95] |
| Frizzled-7 (Fzd7) | Wnt receptor | Associated with canonical and non-canonical Wnt signalling in LESC | [96] |
| PAX6 | Transcription factor | Master regulator of ocular identity; expressed in limbal progenitors. | [83] |
| BCAM | Basal Cell Adhesion Molecule | Mediates adhesion to laminin, associated with basal progenitor cells | [97] |
| CD200 | Cell surface receptor | Immunoregulatory molecule; associated with quiescent limbal stem | [58,89] |
| CD109 | Cell surface receptor | GPI-anchored protein; regulates TGF-β signaling | [92] |
| CK32 | Cytokeratin | Expressed by cells in the limbal crypts | [98] |
3.5. LESC Regulatory Pathways
The healthy LESC niche is tightly regulated signalling cascades, including Wnt (canonical and non-canonical), Notch, TGF-β/BMP, Sonic hedgehog (SHH), and YAP/TAZ-Hippo, shape their fate (Figure 3). Activation or inhibition of these pathways is orchestrated through interactions with niche components such as extracellular matrix proteins, limbal mesenchymal cells, feeder cells, and human amniotic membrane (HAM) [99].
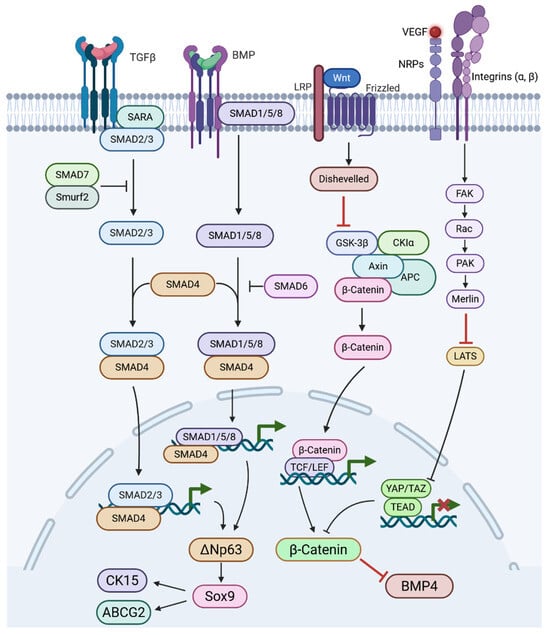
Figure 3.
Diagram of Wnt, YAP, TGF-β, and BMP signalling that lead to the regulation of stem cell markers ΔNp63, Sox9, ABCG2, CK15, BMP4, and β-catenin. TGF-β binds its receptor, Smad2/3 are phosphorylated, the Smad complex enters the nucleus, and TGF-β/Smad activity regulates ΔNp63. BMP binds to type II BMP receptors and receptor-activated Smads (Smad1/5/8) are phosphorylated, Smad1/5/8 form a complex with Smad4 and translocate to the nucleus to regulates ΔNp63 in limbal epithelial stem cells. Wnt proteins bind to the Frizzled receptor and co-receptor LRP, this inhibits the β-catenin destruction complex, allowing β-catenin to enter the nucleus and partners with TCF/LEF transcription factors. Mechanical cues can prevent YAP translocation to the nucleus and allows β-Catenin, ABCG2, and CK15 to be highly expressed via ΔNp63 activation.
3.5.1. Wnt Signalling
Canonical and non-canonical Wnt signalling play critical roles in LESC proliferation and maintenance through distinct yet interconnected mechanisms. This regulation is supported by Several Wnt ligands, Wnt2, Wnt6, Wnt11, and Wnt16b, and their receptors are more highly expressed in the limbus, where LESCs are located, suggesting region-specific regulation of Wnt activity [100,101]. Supporting the involvement of canonical signalling, β-catenin shows nuclear localization in basal limbal cells, where TCF4 is also co-expressed with stemness markers p63 and ABCG2 (Figure 3). Within this context, canonical Wnt signalling promotes LESC proliferation through the β-catenin/TCF4/survivin axis [101,102]. Activators like lithium chloride enhance colony formation [101,103], while inhibitors (e.g., IC15, XAV939) impair stemness and promote differentiation [104,105]. In parallel, non-canonical Wnt/PCP signalling, mediated by Wnt11 and Frizzled-7 (Fzd7), drives proliferation via syndecan-4/fibronectin/ROCK in rabbit and human LESCs [100,106]. MicroRNAs like miR-103/107 modulate both arms: they suppress canonical Wnt3a, activate PCP via JNK phosphorylation, and reduce YAP1 and Sox9, factors involved in stemness and Ca2+-dependent signalling [107]. Sox9, downstream of Wnt/Ca2+, can suppress β-catenin, indicating antagonism between pathways [108]. Moreover, the HC-HA/PTX3 peptide promotes LESC quiescence by activating Wnt/PCP and BMP pathways in limbal fibroblasts [109]. Thus, a dynamic balance between canonical and non-canonical Wnt pathways, modulated by niche cues, underlies LESC fate and regenerative potential.
3.5.2. Notch Signalling
Notch signalling is crucial for regulating the asymmetric division of limbal stem cells (LESCs) and corneal epithelial stratification. Notch components are broadly expressed in the cornea and limbus [110,111]. The intermittent presence of NICD, HES1, and HEY1 in limbal tissue suggests that Notch activation occurs during corneal regeneration [104]. Genetic knock out or pharmacological inhibition of Notch enhances LESC traits while suppressing epithelial differentiation, suggesting its suppression supports stemness [104,112]. Studies showed that the Notch ligand form matters: immobilised Jagged1 activates Notch [113], while soluble Jagged1 inhibits it [114]. Also, Notch’s effects depend on crosstalk with Wnt, NF-κB, and YAP/TAZ pathways [112,115,116]. Altogether, evidence indicates that Notch signalling inhibition promotes LESC maintenance and proper corneal regeneration, though Notch’s role is nuanced and context-dependent.
3.5.3. TGFβ/BMP Signalling
Transforming Growth Factor β/Bone Morphogenic Protein (TGFβ/BMP) signalling counteracts Wnt signalling and activates canonical pathways through ligand binding to type II receptors, which phosphorylate type I receptors, leading to Smad2/3 or Smad1/5/8 activation and nuclear translocation with Smad4 to drive gene expression [117]. TGFβ receptors are more highly expressed in the basal limbal epithelium than in the central cornea [118,119], and BMP4 is upregulated in the human limbus [120]. In vitro, BMP4 and phosphorylated Smad1/5/8 are enriched in LESCs cultured with limbal niche cells, while BMP inhibition activates Wnt/β-catenin signalling, increases colony-forming efficiency, and K12 expression, suggesting BMP-Wnt balance regulates LESC proliferation [105]. Human amniotic membrane (HAM), a substrate used to culture transplantable LESCs, provides TGFβ and other factors that promote proliferation and anti-inflammatory effects [121,122]. TGFβ1 in HAM may induce MMP-9, aiding ECM remodelling and epithelial outgrowth from the limbal explant [123,124]. However, excess TGFβ1 can trigger EMT in LESCs, which is counteracted by Smad7 [125].
3.5.4. Sonic Hedgehog Signalling
SHH signalling promotes LESC proliferation and prevents their terminal differentiation. SHH is active in the limbal basal epithelium and signals through the Patched–Smoothened–Gli axis to upregulate cyclin D1, driving cell cycle progression [126]. In human and rabbit LESCs, SHH activation increases Sox9 expression, which suppresses both stemness and differentiation markers while promoting progenitor proliferation [108]. SHH inhibition reduces colony-forming efficiency and blocks the proliferative effect of a 44–amino acid peptide derived from pigment epithelium-derived factor (PEDF), which activates LESCs via SHH/Gli1/Gli3 signalling [127].
3.6. Mechanotransduction and ECM Regulation of LESCs
LESCs detect mechanical signals, such as blinking and tear flow, through mechanotransduction, where ECM-derived forces are converted into intracellular responses via integrins and adhesion complexes [128,129].
The soft niche environment suppresses YAP/TAZ nuclear localisation and drives LESC, proliferation, stratification, and β-catenin–driven stemness (Figure 3) [130,131]. In contrast, stiffer substrates promote nuclear YAP/TAZ and BMP4 activation, driving differentiation and migration. In vivo, cytoplasmic YAP predominates in the limbus, reflecting this mechanical regulation [132].
Chemical injury increases niche stiffness and depletes LESCs, while collagenase-induced softening can restore stem cell markers in vitro and in animal models [23,133]. Integrins (α3β1, α6β4) activate Wnt/β-catenin via integrin-linked kinase (ILK), supporting stemness [134]. N-cadherin also modulates β-catenin signalling in response to mechanical cues [95].
ECM proteins are major contributors of the limbus’ mechanical properties. Laminin-521 and Laminin-511 promote LESC proliferation through integrins, while Laminin-332 maintains undifferentiated markers [135]. Collagen synthesis (e.g., via ascorbic acid) enhances stemness independent of antioxidant effects [136,137]. Fibronectin activates non-canonical Wnt/PCP signalling via Frizzled-7 and Syndecan-4, enhancing self-renewal [106,138]. SPARC boosts ABCG2 and p63 through MAPK/JNK signalling, further reinforcing progenitor identity [139,140].
4. Corneal Privilege
LESCs are closely linked to both immune privilege and blood/lymphatic vessel privilege because they maintain the epithelial barrier and release factors that suppress inflammation and pathological vessel growth.
4.1. Immune Privilege
The cornea maintains its immune privilege through a combination of active and passive mechanisms that act across the three darts of an immune reflex arc [141,142]. Corneal avascularity limits immune cell trafficking and antigen exposure, thereby contributing to immune privilege while preserving the capacity for immune surveillance [143]. This avascularity is not a passive state but is actively sustained by anti-(lymph)angiogenic factors, ensuring that even after injury, such as refractive laser surgery, the cornea remains largely free of neovascularization [5].
Functionally, immune privilege is enhanced by reduced expression of major histocompatibility complex (MHC) class I molecules and a paucity of MHC class II-positive antigen-presenting cells within the corneal tissue [144]. This limited antigen presentation capacity dampens immune activation. In addition, the corneal epithelium expresses CD95 ligand (FasL), which can induce apoptosis in Fas-expressing immune cells, further contributing to immune tolerance [145,146]. Physiologically, anterior chamber-associated immune deviation (ACAID) induces systemic tolerance by generating regulatory T cells that suppress delayed-type hypersensitivity while preserving non-complement-fixing antibody production [147].
Beyond structural and cellular mechanisms, soluble immunomodulatory factors within the aqueous humour and corneal microenvironment, such as transforming growth factor-beta (TGF-β) and alpha-melanocyte-stimulating hormone (α-MSH), actively suppress inflammatory responses [148]. The interplay between these soluble mediators and structural barriers creates a tightly regulated microenvironment, where angiogenic and immune pathways are intricately linked. Notably, pro-angiogenic mediators like vascular endothelial growth factor (VEGF) have dual roles, influencing both vascular growth and immune cell recruitment [149].
4.2. Lymphangiogenic Privilege
The cornea lymph-angiogenic privilege is essential for maintaining optical transparency and immune homeostasis. Owing to its normally vessel-free status, the cornea is a widely used in vivo model for studying the biology of angiogenesis and lymphangiogenesis [5,150,151]. Pathological vascularisation of the mouse cornea can be induced by placing intra-stromal sutures in the cornea. These sutures will cause a chronic inflammation that will drive vessel to invade into the normally avascular cornea (Figure 4).
Pathological vascularization in the cornea is largely driven by vascular endothelial growth factor (VEGF) family members, which activate VEGF receptors on endothelial cells of the limbal arcade [152,153]. Triggers such as inflammation and hypoxia increase VEGF release, but under physiological conditions, the cornea resists vascular invasion through a threshold-based buffering system capable of neutralizing low concentrations of proangiogenic factors [10].
A range of endogenous anti-angiogenic mediators supports the maintenance of this vessel-free state. The endogenous antiangiogenic factors that play a role in angiogenic privilege can be categorized into various groups: Endostatin and its analogs (including endostatin, arrests, and tumstatin), plasminogen/serine protease inhibitors (such as angiostatin and pigment epithelium-derived factor [PEDF]), thrombospondin-1 and -2 (TSP-1 and -2), as well as soluble VEGF receptors (sVEGFRs) [5,154,155,156,157,158]. Among these, TSP-1 is of particular importance in maintaining corneal avascularity [159]. In parallel, the corneal epithelium contributes actively through the expression of soluble VEGF receptors (sVEGFR-1/2/3) [8,9,154]. Functionally, sVEGFR-1 sequesters VEGF-A and additionally suppresses VEGFR-1/-2 signalling by heterodimerization (Ambati et al., 2006) [154], whereas sVEGFR-2 and sVEGFR-3 act as specific inhibitors of lymphangiogenesis by neutralizing VEGF-C and VEGF-D [8,9].
Further layers of inhibition derive from immunoregulatory factors in the aqueous humor, including α-MSH and VIP, which display both anti-angiogenic and anti-lymphangiogenic activity [160]. Under hypoxic conditions, VEGF-A, VEGF-C, and VEGF-D are normally upregulated [161]; however, the cornea counterbalances this with the expression of inhibitory PAS domain protein (IPAS), which antagonizes Hypoxia-inducible factor 1-alpha (HIF-1α) signalling [162]. Collectively, these overlapping mechanisms buffer minor pro-(lymph)angiogenic stimuli to maintain corneal transparencywhilst allowing neovascular response. While anti-hemangiogenic pathways are comparatively well defined, several endogenous inhibitors of lymphangiogenesis have also been identified in recent years, including by our group [159,163,164,165,166].
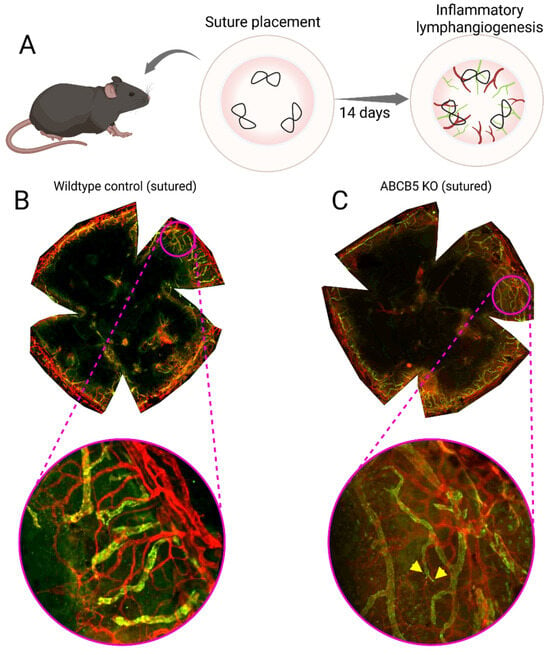

Figure 4.
Mouse model of inflammatory lymphangiogenesis using three intrastromal, figure-eight sutures to induce chronic inflammation in the cornea over the course of 2 weeks (A). Flat mount of a wildtype sutured murine cornea (B); the central cornea is now vascularized after two weeks of chronic inflammation, with the vessels clearly extending across the limbus. Flat mount of a ACBC5 KO sutured murine cornea (C), the central cornea is vascularized and there is a greater amount of lymphatic vessel sprouts (labelled with yellow wedges) compared to the wildtype sutured animal [167], the vessels clearly traverse the limbus. Blood vessels are stained red and lymphatic vessels are stained green.
4.3. The Role of LESCs in Lymphangiogenic Privilege
Corneal cells play a pivotal role in sustaining the corneal lymph (angiogenic) privilege. Early studies demonstrated that differentiated corneal epithelium exerts strong anti-angiogenic properties [10,168,169,170]. Anatomically, the limbus represents the vascularized junction between the avascular cornea and the conjunctiva and functions as a physical barrier against the ingrowth of blood and lymphatic vessels. Within this specialized microenvironment, the limbal niche, a complex interplay of fibroblasts, melanocytes, immune cells, and a unique extracellular matrix enriched in collagen IV α1/α2 chains, laminin α5, β2, and γ1 chains, nidogen-1/-2, and thrombospondin-4, supports the maintenance of resident LESCs. These LESCs are located at the basal layers of the limbal epithelium, concentrated within stromal crypts of the palisades of Vogt [41], and provide the continuous renewal of the corneal epithelium, replacing cells shed through physiological turnover or injury [54].
The first identification of vascular endothelial growth factor (VEGF) in the cornea came from immunolocalization studies by van Setten, who described its presence in the basal epithelial layer [171]. Subsequent work in a rat model linked LESC deficiency to VEGF induction and inflammation, where VEGF upregulation coincided with corneal neovascularization [172]. Following injury, infiltrating leukocytes further contribute to VEGF secretion [173,174]. Specifically, VEGF-A165 and VEGF-A189 mRNA were strongly induced after corneal cautery, while VEGF transcripts localized to neutrophils and macrophages [173]. In addition, VEGF, TGF-α, and TGF-β1 were detected within the basal epithelium, endothelial cells of newly formed vessels, and infiltrating immune cells such as T lymphocytes and macrophages [143]. Despite this, the precise mechanisms by which the limbal niche, and in particular its stem cells, safeguard corneal avascularity remain incompletely understood. However, in LESC deficiency multiple soluble factors, including cytokines, chemokines, and growth factors, drive pathological neovascularization [175].
Addressing this question, Veréb and colleagues used genome-wide microarray profiling to compare ex vivo cultured putative LESCs with differentiated corneal epithelial cells. They showed that LESCs exhibited a more pro-angiogenic transcriptional profile [176]. Upregulated genes included Fibronectin, SERPINE1, MMP9, and F3, while anti-angiogenic regulators such as PLG, TIMP-1, FOXO4, and TGFBR1 were downregulated. Several cytokines and growth factors with strong angiogenic potential, IL-1β, CXCL10, TGF-β1, VEGF-A, IL-6, and IL-8, were significantly upregulated in LESCs, together with EDN1, EREG, and BMP2. In contrast, differentiated epithelial cells displayed a relatively anti-angiogenic profile, with downregulation of FGF1, IL-17F, TGF-β2, and c-Kit ligand [176].
These findings are consistent with our own results showing that UVA/UVB-induced LESC differentiation, marked by loss of stem cell marker expression and reduced clonogenic potential, led to downregulation of pro-angiogenic cytokines and acquisition of an anti-angiogenic paracrine effect on vascular endothelial cells [54,177,178].
The concept of LESCs displaying a relatively pro-angiogenic transcriptional profile is counterintuitive, particularly given their clinical application in transplantation for limbal stem cell deficiency (LSCD), a condition frequently associated with chronic inflammation and pathological neovascularization [179]. To reconcile this paradox, several groups have highlighted the immunoregulatory properties of LESCs within the damaged niche. These cells promote an anti-inflammatory microenvironment in the transplanted wound bed, thereby counteracting angiogenic drive.
In vitro, mouse LESCs dose-dependently suppressed lymphocyte proliferation and reduced pro-inflammatory cytokine release. In mixed lymphocyte reactions, they were more immunosuppressive than mesenchymal stem cells or natural regulatory T cells. They also showed higher expression of Fas ligand and the anti-apoptotic genes Mcl-1, XIAP, and survivin. Overall, freshly isolated adult LESCs display strong immunomodulatory and anti-apoptotic properties [180].
Such immunoregulatory activity indirectly contributes to the maintenance of corneal avascularity, since infiltrating leukocytes and macrophages are a major source of pro-angiogenic factors that drive hemangiogenesis and lymphangiogenesis [149].
4.4. The ABCB5+ Limbal Epithelial Cell Population: Dual Regulators of Corneal Angiogenesis and Immune Privilege
Our recent work using ABCB5 as a selective marker has provided further mechanistic insight. ABCB5 is a transmembrane ATP-binding cassette transporter that identifies LESCs and stromal progenitors, allowing their functional characterization and isolation. In our first study, we showed that ABCB5+ LESCs regulate both developmental and inflammatory (lymph)angiogenesis in the murine cornea, demonstrating their active participation in vessel remodeling under homeostatic and stress conditions [167]. These cells were capable of modulating endothelial cell behavior through paracrine mechanisms, suggesting that they contribute to a finely tuned balance between pro- and anti-angiogenic signalling in the limbal niche.
Notably, in a follow-up study, we identified a distinct stromal ABCB5+ population with strong anti-inflammatory and anti-(lymph)angiogenic properties. These stromal cells secreted factors that inhibited the proliferation, migration, and tube formation of blood and lymphatic endothelial cells in vitro, highlighting their role in maintaining corneal angiogenic privilege [25]. Notably, these stromal ABCB5+ cells also expressed immunomodulatory molecules, suggesting that their paracrine activity helps suppress inflammation-driven neovascularization. Together, these studies show that ABCB5+ cells in different compartments of the limbus have context-dependent, complementary functions: epithelial ABCB5+ LESCs can promote angiogenic processes when required for repair or development, whereas stromal ABCB5+ progenitors act to reinforce the anti-angiogenic and anti-lymphangiogenic microenvironment.
5. Tip of the Balance: Limbal Niche Disruption and Loss of Privilege
The corneal limbal niche delicately maintains a balance that preserves corneal transparency, avascularity, and regenerative capacity (Figure 5). Disruption of this equilibrium, through injury, genetic defects, or environmental stressors, can lead to LSCD, inflammation, and corneal (lymph)angiogenesis. Once this “tip of the balance” occurs, the cornea becomes susceptible to chronic epithelial defects, conjunctivalization, neovascularization, and severe vision impairment [35,54].
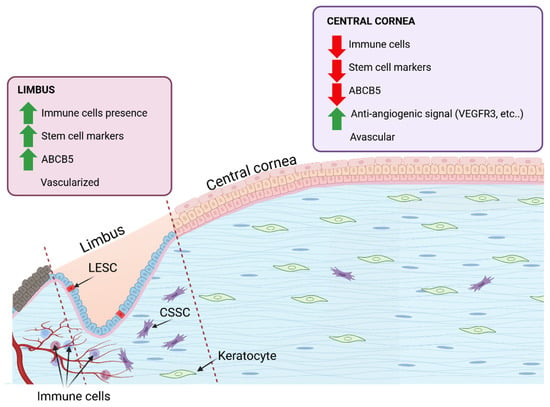
Figure 5.
The corneal limbus and corneal centre provide different environmental cues. These cues influence the behaviour of the cells housed within and the overall tissue functionality. At the limbus, partial vascularisation provides greater oxygen and nutrient access, while the avascular central cornea depends on diffusion, both passive and active, for sustenance. The presence of immune cells at the limbus is partly mediated by the stem cells that immuno-interact. The lower presence of stem cells in the central cornea means a reduced immune cell interactivity, as long as there is no injury.
Lymphatic vessels contribute to immune surveillance and regulation in limbal region, particularly when they are recruited during injury or inflammation. Insights from skin reveal that the resident stem cells reshape the adjacent lymphatic vessels to better regulate their own activity [181]. There is a dynamic remodeling of the lymphatic vessels to support stem cells, with transient increases in lymphatic vessel caliber when stem cells are activated [182]. These findings underline the dynamic communication between stem cells and lymphatic system. In the cornea, lymphatic vessels similarly support limbal stem-cell function via Prox1-mediated paracrine signaling, and by draining inflammatory cytokines or immune cells that would otherwise impair stem cell maintenance [183].
5.1. LSCD Leading to Inflammation and Neovascularization
LSCD occurs when LESCs are depleted or their niche is severely compromised, resulting in impaired maintenance of the corneal epithelium. This promotes conjunctival epithelial ingrowth (conjunctivalization), persistent epithelial defects, chronic stromal inflammation, and neovascularization [184,185]. Loss of LESCs also permits pro-angiogenic factors such as VEGF-A to override anti-angiogenic mechanisms, further promoting vascular invasion into the normally avascular cornea [54,149].
Several conditions can lead to LSCD by severely damaging the LESCs or limbal niche [186]. These include ocular cicatricial pemphigoid (OCP), Stevens-Johnson syndrome (SJS), thermal or chemical burns, contact lens use, ultraviolet (UV) irradiation, multiple eye surgeries, the use of chemotherapeutic drugs like 5-FU (Fluorouracil) and MMC (Mitomycin C), and congenital aniridia [54,187,188].
5.2. Trauma
Severe ocular surface trauma, particularly chemical and thermal burns, is a leading cause of limbal stem cell deficiency (LSCD). Alkali burns rapidly penetrate ocular tissues, resulting in extensive loss of limbal stem cells, disruption of the stem cell niche, and persistent activation of inflammatory and angiogenic pathways [35,72,189]. This process triggers the release of cytokines, chemokines, and matrix metalloproteinases, which promote stromal remodelling, neovascularization, and conjunctivalisation [190]. In contrast, thermal burns induce similar pathological changes through protein denaturation, ischemic injury, and sustained inflammatory responses. These mechanisms compromise limbal barrier integrity and drive progressive corneal opacification [188,189]. Both types of burns eliminate the stem cell pool and destabilize the microenvironment required for epithelial regeneration, resulting in a chronic disease state characteristic of burn-induced LSCD.
5.3. Ultraviolet Irradiation in Limbal Stem Cell Deficiency
UV radiation directly damages ocular structures and can lead to partial or total blindness. The cornea is particularly susceptible due to its transparency and curvature, which amplify UV intensity up to 20-fold at the nasal limbus, where LESCs reside. This region is the site of pterygium, a noncancerous, often bilateral vascularized corneal growth that invades the limbal barrier, alters epithelial morphology, activates fibroblasts, and promotes inflammation, neovascularization, and extracellular matrix remodeling [54,151,191].
Short-term UVA and UVB exposure differentially affect the limbal niche. UVA irradiation reduces LESC marker expression and colony-forming efficiency while creating an anti-inflammatory and antilymphangiogenic micromilieu. In contrast, UVB triggers proinflammatory and macrophage-recruiting cytokines (TNFα, MCP1, IFN-γ), promoting immune cell infiltration, neovascularization, and niche dysfunction [177,178]. Limbal fibroblasts are key mediators in this process: UV-irradiated fibroblasts lose their ability to maintain LESC phenotype and alter paracrine signalling, shifting the balance between pro- and antiangiogenic responses depending on the type of UV exposure.
Mechanistic studies reinforce these findings: Repeated UVB exposure reduces ABCB5, a key stem cell marker, and activates epithelial–mesenchymal transition (EMT) and fibrosis, driving LESCs toward a scar-forming phenotype, as also observed in pterygium tissue [192]. Short bursts of UVB induce persistent DNA damage that only partially responds to repair mechanisms, highlighting the ongoing vulnerability of LESCs [193].
Clinically, pterygium acts as a visible example of UV-induced LSCD. This noncancerous, often bilateral, vascular growth invades the limbal barrier, alters the shape and behaviour of epithelial cells, activates fibroblasts, and results in increased inflammation, new vessel formation, and remodelling of surrounding tissue. Overall, these UV-driven mechanisms emphasise the importance of protective strategies, such as UV-blocking contact lenses, which laboratory studies have shown can help preserve stem cell health, reduce DNA damage, and maintain normal cell function [54,151,191].
5.4. Aniridia
Aniridia is a rare congenital eye disease with a prevalence of 1 in 40,000–100,000 births. Most cases result from heterozygous mutations in the PAX6 gene, inherited in an autosomal dominant pattern (≈70%) or arising as de novo mutations (≈30%), with occasional reports of paternal mosaicism [194,195]. Clinical features include iris hypoplasia, foveal hypoplasia, cataract, glaucoma, and aniridia-associated keratopathy (AAK), which leads to corneal conjunctivalization and neovascularization [194,196]. However, LESC proliferation is not fully lost, and stromal–epithelial interactions appear crucial in disease progression [86,197]. More recent studies show that niche dysfunction plays a central role, with loss of palisades of Vogt, basement membrane irregularities, inflammatory infiltration, extracellular matrix changes, abnormal melanocytes, and disturbed Wnt/β-catenin and retinoic acid signalling, leading to epithelial maldifferentiation and PAX6 mislocalization [198]. Also, recent paper showed that ARK corneas display fetal-like signalling with increased Wnt/β-catenin, SHH, and mTOR activity, alongside reduced Notch1 due to elevated inhibitors [199]. These findings suggest that both intrinsic PAX6-related defects and extrinsic niche breakdown drive AAK progression.
6. Perspective: Future Directions in LSCD Therapy with a Focus ABCB5+ Cells Role in Anti-Inflammatory and Anti-Angiogenic Strategies and Present Challenges
Building upon current therapeutic approaches, future directions in LSCD treatment increasingly emphasize strategies that not only restore epithelial integrity but also stabilize the underlying niche. Over the past three decades, surgical transplantation of limbal tissue, whether as autografts, allografts, or ex vivo expanded LESCs, has established proof-of-principle that regeneration of a functional ocular surface is possible [200,201,202]. Yet these interventions are constrained by major challenges: autologous grafts are not an option in bilateral disease, allografts require systemic immunosuppression with attendant risks, and the long-term persistence of transplanted cells remains uncertain. Evidence suggests that their benefit may instead rely on indirect effects, such as reactivating residual host stem cells or modulating the limbal microenvironment, rather than durable epithelial replacement [98].
Substrate selection has played a pivotal role in shaping therapeutic outcomes. Amniotic membrane provides not only a physical carrier but also intrinsic anti-inflammatory and anti-angiogenic effects. However, its biological variability and dependence on processing methods prompted a search for alternatives such as fibrin gels, temperature-responsive polymers, or synthetic scaffolds, which aim to deliver more standardized and reproducible culture systems [188]. At the same time, regulatory authorities have classified LESC-based interventions as advanced therapy medicinal products, underscoring the need to develop xenobiotic-free and GMP-compliant protocols to ensure both safety and reproducibility [179,188,203].
As these clinical and regulatory foundations were established, research has increasingly shifted toward understanding the pathophysiology of LSCD at the level of the niche. The failure to maintain corneal avascularity and immune privilege is now seen as central to disease progression. Loss of LESCs compromises the limbal barrier, resulting in conjunctivalisation, chronic inflammation, and hem- and lymphangiogenesis [54]. These insights have redirected attention toward strategies that combine epithelial regeneration with anti-inflammatory and anti-angiogenic modulation. In this regard, ABCB5+ epithelial and stromal subpopulations have emerged as particularly promising, since they not only retain clonogenic and regenerative potential but also exert immunomodulatory and anti-(lymph)angiogenic effects, suggesting a dual mechanism of action that stabilizes the niche while restoring epithelial integrity [25,179].
Parallel efforts have emphasized the importance of standardized potency assays capable of predicting therapeutic efficacy. Macrophage suppression has been identified as a critical functional marker, reflecting the central role of macrophages in orchestrating VEGF-driven angiogenesis and inflammatory cascades (Cursiefen et al., 2004; Sadeghi et al., 2024) [149,204]. Such assays not only bridge basic research with clinical translation but also align cell-based approaches with molecular therapies targeting VEGF and related angiogenic pathways already validated in corneal neovascular disease [205,206].
These developments point toward a future in which LSCD therapy moves beyond simple stem cell replacement. The integration of transplantation with adjunctive immunomodulatory and anti-angiogenic interventions offers a path to more durable restoration of ocular surface homeostasis. Protecting and reinforcing the limbal niche, whether through the direct action of transplanted stem cells, the immunoregulatory properties of specialized subpopulations, or pharmacological inhibition of proangiogenic signalling, is likely to prove essential for long-term success. In this integrative model, surgery, cell therapy, and targeted molecular modulation converge, offering not only epithelial repair but also stabilization of the corneal microenvironment. As highlighted across recent Publications in the field, the next generation of therapies will be defined by their ability to combine these complementary strategies, uniting regenerative approaches with immune and vascular control, to achieve lasting restoration of vision in patients with limb stem cell deficiency.
Experimental studies reveal the immune-regulating role of LESCs. When expanded ex vivo, LESCs express several genes linked to angiogenesis, including fibronectin, MMP9, IL-6, and VEGFA [176]. This profile raised questions about whether LESCs might paradoxically promote angiogenesis. However, when studied in their native context, they behave differently: freshly isolated cells suppress T-cell proliferation and dampen inflammatory cytokines, sometimes more strongly than mesenchymal stem cells or regulatory T cells [180]. By restraining immune activation, they indirectly reduce VEGF signalling and help preserve avascularity [149]. The contrast between culture and in vivo data highlights a key point: the function of LESCs depends on their microenvironment, and flexibility may be central to their physiological role.
The fragility of this system is evident when it is challenged. Ultraviolet radiation provides a striking example. Short-term UVA exposure weakens stemness but promotes an anti-inflammatory and antilymphangiogenic setting, whereas UVB induces cytokines such as TNFα, MCP-1, and IFN-γ, triggering immune infiltration, neovascularization, and fibrosis [177,178]. Repeated UVB stress also reduces ABCB5 expression and pushes cells toward epithelial–mesenchymal transition, changes linked to pterygium development [192,193]. Similar collapse of privilege follows chemical burns, autoimmune inflammation, or congenital defects like aniridia [189,198]. Taken together, these examples show how easily the limbal barrier can be tipped, and they reinforce the idea that maintaining privilege is an ongoing, active process.
The discovery of ABCB5 as a marker of limbal progenitors has helped to clarify the picture. Epithelial ABCB5+ cells can support developmental or injury-associated angiogenesis, suggesting a transient role in repair [12,167]. Stromal ABCB5+ cells, in contrast, secrete factors that strongly inhibit endothelial proliferation, migration, and tube formation, while also suppressing inflammatory pathways [25]. This points to a cooperative system: epithelial subsets allow controlled vascular responses during healing, whereas stromal subsets reinforce immune and lymphangiogenic privilege under steady conditions. It seems likely that the success of the niche lies in this division of labor, balancing the competing demands of regeneration and avascularity.
These insights carry obvious weight for therapy. In limbal stem cell deficiency, the breakdown of cornea immune and angiogenic privilege is as damaging as the loss of renewal. Conjunctival ingrowth, chronic inflammation, and neovascularization drive progressive vision loss [188]. Transplantation of limbal tissue or ex vivo expanded cells has shown that the surface can be resurfaced [200,201], but these approaches are limited by bilateral disease, reliance on systemic immunosuppression, and uncertain long-term survival of transplanted cells. It has been suggested that the therapeutic success of LESC transplantation may rely less on long-term engraftment of donor cells and more on their ability to influence the host environment through paracrine mechanisms [98]. From this perspective, treatments that fail to address the immune and vascular aspects of limbal niche dysfunction are unlikely to achieve lasting restoration.
ABCB5+ subsets provide a framework for moving forward. Stromal ABCB5+ cells, with their potent immunomodulatory and anti-(lymph)angiogenic activity, could be used either directly in cell therapy or through their secreted products. Epithelial ABCB5+ cells remain indispensable for restoring the epithelial barrier, but their benefit is likely greater when paired with stromal subsets that reinforce privilege. The combination of both populations offers a more holistic therapeutic concept, one that addresses not only the surface defect but also the microenvironment that sustains long-term clarity. This will also help to address the global unmet need for options to promote graft survival in vascularized high-risk transplants [207].
Looking ahead, several challenges remain. The molecular signals that determine whether LESCs adopt a regenerative or immunomodulatory role are still poorly defined. Pathways including Wnt, Notch, and TGFβ/BMP are implicated [101,105], but their hierarchy and interactions remain uncertain. Standardized tests, such as macrophage suppression or inhibition of lymphatic endothelial growth, could bridge laboratory findings with clinical outcomes [149,204]. Advances in biomaterials are another promising direction. Scaffolds enriched with laminin-511/521 or collagen IV support limbal stemness and may provide more physiological carriers for transplantation [23,135].
7. Conclusions
Altogether, the evolving view of LESCs highlights them as both regenerative and regulatory cells, capable of adapting to context and balancing the competing needs of healing and vascular restraint. The identification of two ABCB5+ subsets, in the epithelia and stroma, now offers tools to isolate and study these roles with greater precision. This suggests the future of LSCD therapy lies in integrated strategies that couple epithelial regeneration with reinforcement of immune and vascular privilege. Paracrine or molecule-based therapies represent a more feasible strategy for sustained ocular surface regeneration, offering superior standardization and delivery compared to cell- or scaffold-based approaches. Clinically, approaches that combine epithelial repair with restoration of immune and lymphangiogenic privilege, using cell-based or paracrine strategies, are likely to be required for durable treatment of LSCD.
Author Contributions
Conceptualization, M.N.; writing—original draft preparation, B.M.; writing—review and editing, T.V., C.C. and M.N.; supervision, M.N.; funding acquisition, C.C. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
SFB1607/A05 + A03 https://www.sfb1607.de/projekt/a05 (accessed on 10 December 2025) DFG FOR2240 (MN, CC; www.for2240.de, (accessed on 10 December 2025)). CMMC (https://www.cmmc-uni-koeln.de/research/research-areas-projects/research-area-a/notara-assoc-rg-47 and https://www.cmmc-uni-koeln.de/research/new-funding-period-1/fp-2020-2022-ipfp-projects/fp-2020-2022-notara-maria-cursiefen-claus-a-09 (accessed on 10 December 2025)). DFG, under Germany’s Excellence Strategy—EXC 2030—390661388. EU RestoreVision (CC; https://restorevision-project.eu/people/univ-prof-dr-med-claus-cursiefen-febo-farvo/, (accessed on 10 December 2025)). STEM-CORE MSCA (MN, CC; https://www.ru.nl/en/research/research-news/stem-core-new-eu-programme-to-train-the-next-generation-eye-therapy-experts, (accessed on 10 December 2025)).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qazi, Y.; Wong, G.; Monson, B.; Stringham, J.; Ambati, B.K. Corneal transparency: Genesis, maintenance and dysfunction. Brain Res. Bull. 2010, 81, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C.; Lewis, P.N.; Morgan, S.R.; Hayes, S. Structural control of corneal transparency, refractive power and dynamics. Eye 2025, 39, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.J.; White, N.; Albon, J.; Knupp, C.; Kamma-Lorger, C.S.; Meek, K.M. Measuring the Refractive Index of Bovine Corneal Stromal Cells Using Quantitative Phase Imaging. Biophys. J. 2015, 109, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. In Food Allergy: Molecular Basis and Clinical Practice; Chemical Immunology and Allergy; KARGER: Basel, Switzerland, 2007; Volume 92, pp. 50–57. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The immune privilege of corneal grafts. J. Leukoc. Biol. 2003, 74, 167–171. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Corneal transplantation and immune privilege. Int. Rev. Immunol. 2013, 32, 57–67. [Google Scholar] [CrossRef]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Vanbuskirk, E.M. The Anatomy of the Limbus. Eye 1989, 3, 101–108. [Google Scholar] [CrossRef]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.; Wang, J.; Wray, B.; Patel, P.; Yang, W.; Peng, H.; Lavker, R.M. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3570–3583. [Google Scholar] [CrossRef]
- Hayashi, S.; Osawa, T.; Tohyama, K. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 2002, 254, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Bowman’s layer in the cornea- structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef]
- Meek, K.M.; Leonard, D.W. Ultrastructure of the corneal stroma: A comparative study. Biophys. J. 1993, 64, 273–280. [Google Scholar] [CrossRef]
- Mclaughlin, J.S.; Linsenmayer, T.F.; Birk, D.E. Type v collagen synthesis and deposition by chicken embryo corneal fibroblasts in vitro. J. Cell Sci. 1989, 94, 371–379. [Google Scholar] [CrossRef]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Veréb, Z.; Póliska, S.; Albert, R.; Olstad, O.K.; Boratkó, A.; Csortos, C.; Moe, M.C.; Facskó, A.; Petrovski, G. Role of Human Corneal Stroma-Derived Mesenchymal-Like Stem Cells in Corneal Immunity and Wound Healing. Sci. Rep. 2016, 6, 26227. [Google Scholar] [CrossRef] [PubMed]
- Meshko, B.; Volatier, T.L.A.; Mann, J.; Kluth, M.A.; Ganss, C.; Frank, M.H.; Frank, N.Y.; Ksander, B.R.; Cursiefen, C.; Notara, M. Anti-Inflammatory and Anti-(Lymph)angiogenic Properties of an ABCB5+ Limbal Mesenchymal Stem Cell Population. Int. J. Mol. Sci. 2024, 25, 9702. [Google Scholar] [CrossRef]
- Jeng, B.H.; Meisler, D.M. A combined technique for surgical repair of Descemet’s membrane detachments. Ophthalmic Surg. Lasers Imaging Retin. 2006, 37, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Fitch, J.M.; Birk, D.E.; Linsenmayer, C.; Linsenmayer, T.F. The spatial organization of Descemet’s membrane-associated type IV collagen in the avian cornea. J. Cell Biol. 1990, 110, 1457–1468. [Google Scholar] [CrossRef]
- Ali, M.; Raghunathan, V.; Li, J.Y.; Murphy, C.J.; Thomasy, S.M. Biomechanical relationships between the corneal endothelium and Descemet’s membrane. Exp. Eye Res. 2016, 152, 57–70. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Dikstein, S.; Maurice, D.M. The active control of corneal hydration. Isr. J. Med. Sci. 1972, 8, 1523–1528. [Google Scholar]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Smeringaiova, I.; Utheim, T.P.; Jirsova, K. Ex vivo expansion and characterization of human corneal endothelium for transplantation: A review. Stem Cell Res. Ther. 2021, 12, 554. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Azuara-Blanco, A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef]
- Van Buskirk, E.M. Anatomic correlates of changing aqueous outflow facility in excised human eyes. Investig. Ophthalmol. Vis. Sci. 1982, 22, 625–632. [Google Scholar]
- van der Merwe, E.L.; Kidson, S.H. Advances in imaging the blood and aqueous vessels of the ocular limbus. Exp. Eye Res. 2010, 91, 118–126. [Google Scholar] [CrossRef]
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Dua, H.S.; Shanmuganathan, V.A.; Powell-Richards, A.O.; Tighe, P.J.; Joseph, A. Limbal epithelial crypts: A novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005, 89, 529–532. [Google Scholar] [CrossRef]
- Shanmuganathan, V.A.; Foster, T.; Kulkarni, B.B.; Hopkinson, A.; Gray, T.; Powe, D.G.; Lowe, J.; Dua, H.S. Morphological characteristics of the limbal epithelial crypt. Br. J. Ophthalmol. 2007, 91, 514–519. [Google Scholar] [CrossRef]
- Townsend, W.M. The limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1991, 89, 721–756. [Google Scholar] [PubMed]
- Guo, Z.H.; Zeng, Y.M.; Lin, J.S. Dynamic spatiotemporal expression pattern of limbal stem cell putative biomarkers during mouse development. Exp. Eye Res. 2020, 192, 107915. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Michael, A.F.; Sun, T.T.; Kenney, M.C. Human corneal basement membrane heterogeneity: Topographical differences in the expression of type IV collagen and laminin isoforms. Lab. Investig. 1995, 72, 461–473. [Google Scholar] [PubMed]
- Yeung, A.M.; Schlötzer-Schrehardt, U.; Kulkarni, B.; Tint, N.L.; Hopkinson, A.; Dua, H.S. Limbal epithelial crypt: A model for corneal epithelial maintenance and novel limbal regional variations. Arch. Ophthalmol. 2008, 126, 665–669. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Soleymani, S.; Harounian, A.; Bhakta, B.; Troyanovsky, S.M.; Brunken, W.J.; Pellegrini, G.; Ljubimov, A.V. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol. Vis. 2011, 17, 2177–2190. [Google Scholar]
- Gesteira, T.F.; Sun, M.; Coulson-Thomas, Y.M.; Yamaguchi, Y.; Yeh, L.K.; Hascall, V.; Coulson-Thomas, V.J. Hyaluronan Rich Microenvironment in the Limbal Stem Cell Niche Regulates Limbal Stem Cell Differentiation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4407–4421. [Google Scholar] [CrossRef]
- Sun, M.; Puri, S.; Mutoji, K.N.; Coulson-Thomas, Y.M.; Hascall, V.C.; Jackson, D.G.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan Derived From the Limbus is a Key Regulator of Corneal Lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1050–1062. [Google Scholar] [CrossRef]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell. Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef]
- Barbariga, M.; Vallone, F.; Mosca, E.; Bignami, F.; Magagnotti, C.; Fonteyne, P.; Chiappori, F.; Milanesi, L.; Rama, P.; Andolfo, A.; et al. The role of extracellular matrix in mouse and human corneal neovascularization. Sci. Rep. 2019, 9, 14272. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.F.; Marshall, P.L.; Wang, E.; Kaber, G.; Bollyky, P.L. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 2019, 78-79, 292–313. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 8620172. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Radtke, F. Corneal epithelial stem cells and their niche at a glance. J. Cell Sci. 2017, 130, 1021–1025. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Ramirez-Miranda, A.; Deng, S.X. Limbal lacuna: A novel limbal structure detected by in vivo laser scanning confocal microscopy. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, e129–e131. [Google Scholar] [CrossRef]
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 1999, 145, 769–782. [Google Scholar] [CrossRef]
- Bojic, S.; Hallam, D.; Alcada, N.; Ghareeb, A.; Queen, R.; Pervinder, S.; Buck, H.; Amitai Lange, A.; Figueiredo, G.; Rooney, P.; et al. CD200 Expression Marks a Population of Quiescent Limbal Epithelial Stem Cells with Holoclone Forming Ability. Stem Cells 2018, 36, 1723–1735. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef]
- Mei, H.; Gonzalez, S.; Deng, S.X. Extracellular Matrix is an Important Component of Limbal Stem Cell Niche. J. Funct. Biomater. 2012, 3, 879–894. [Google Scholar] [CrossRef]
- Stepp, M.A.; Zieske, J.D. The corneal epithelial stem cell niche. Ocul. Surf. 2005, 3, 15–26. [Google Scholar] [CrossRef]
- Polisetti, N.; Zenkel, M.; Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 2016, 34, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.B.; Tighe, P.J.; Mohammed, I.; Yeung, A.M.; Powe, D.G.; Hopkinson, A.; Shanmuganathan, V.A.; Dua, H.S. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genom. 2010, 11, 526. [Google Scholar] [CrossRef]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef]
- Ordonez, P.; Di Girolamo, N. Limbal epithelial stem cells: Role of the niche microenvironment. Stem Cells 2012, 30, 100–107. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Armer, H.E.; Levis, H.J.; Shortt, A.J.; Tuft, S.; Daniels, J.T. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS ONE 2014, 9, e94283. [Google Scholar] [CrossRef]
- Kureshi, A.K.; Dziasko, M.; Funderburgh, J.L.; Daniels, J.T. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Sci. Rep. 2015, 5, 16186. [Google Scholar] [CrossRef]
- Vantrappen, L.; Geboes, K.; Missotten, L.; Maudgal, P.C.; Desmet, V. Lymphocytes and Langerhans cells in the normal human cornea. Investig. Ophthalmol. Vis. Sci. 1985, 26, 220–225. [Google Scholar]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Munro, P.M.; Khaw, P.T.; Tuft, S.J.; Daniels, J.T. Characterization of the limbal epithelial stem cell niche: Novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 2007, 25, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.A. Patterns of blood flow in episcleral vessels studied by low-dose fluorescein videoangiography. Eye 1988, 2, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.G.; Young, R.D. Scleral structure, organisation and disease. A review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.A. The circulation of the human limbus. Eye 1989, 3, 121–127. [Google Scholar] [CrossRef]
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C.; et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–718. [Google Scholar] [CrossRef]
- Westfall, M.D.; Mays, D.J.; Sniezek, J.C.; Pietenpol, J.A. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol. Cell. Biol. 2003, 23, 2264–2276. [Google Scholar] [CrossRef]
- McKeon, F. p63 and the epithelial stem cell: More than status quo? Genes. Dev. 2004, 18, 465–469. [Google Scholar] [CrossRef]
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of ΔNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523–9528. [Google Scholar] [CrossRef]
- Yoshida, S.; Shimmura, S.; Kawakita, T.; Miyashita, H.; Den, S.; Shimazaki, J.; Tsubota, K. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4780–4786. [Google Scholar] [CrossRef]
- Zhao, B.; Allinson, S.L.; Ma, A.; Bentley, A.J.; Martin, F.L.; Fullwood, N.J. Targeted cornea limbal stem/progenitor cell transfection in an organ culture model. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3395–3401. [Google Scholar] [CrossRef]
- de Paiva, C.S.; Chen, Z.; Corrales, R.M.; Pflugfelder, S.C.; Li, D.Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005, 23, 63–73. [Google Scholar] [CrossRef]
- Kitazawa, K.; Hikichi, T.; Nakamura, T.; Sotozono, C.; Kinoshita, S.; Masui, S. PAX6 regulates human corneal epithelium cell identity. Exp. Eye Res. 2017, 154, 30–38. [Google Scholar] [CrossRef]
- Collinson, J.M.; Chanas, S.A.; Hill, R.E.; West, J.D. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/−) mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1101–1108. [Google Scholar] [CrossRef]
- Collinson, J.M.; Quinn, J.C.; Hill, R.E.; West, J.D. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev. Biol. 2003, 255, 303–312. [Google Scholar] [CrossRef]
- Ramaesh, T.; Collinson, J.M.; Ramaesh, K.; Kaufman, M.H.; West, J.D.; Dhillon, B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1871–1878. [Google Scholar] [CrossRef]
- Ouyang, J.; Shen, Y.C.; Yeh, L.K.; Li, W.; Coyle, B.M.; Liu, C.Y.; Fini, M.E. Pax6 overexpression suppresses cell proliferation and retards the cell cycle in corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2397–2407. [Google Scholar] [CrossRef]
- Vattulainen, M.; Ilmarinen, T.; Viheriälä, T.; Jokinen, V.; Skottman, H. Corneal epithelial differentiation of human pluripotent stem cells generates ABCB5 and increment Np63α cells with limbal cell characteristics and high wound healing capacity. Stem Cell Res. Ther. 2021, 12, 609. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Katayama, T.; Quantock, A.J.; Nishida, K. CD200 facilitates the isolation of corneal epithelial cells derived from human pluripotent stem cells. Sci. Rep. 2018, 8, 16550. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Kim, S.; Li, J.M.; Gao, Q.; Choi, J.; Bian, F.; Hu, J.; Zhang, Y.; Li, J.; Lu, R.; et al. Single-cell transcriptomics identifies limbal stem cell population and cell types mapping its differentiation trajectory in limbal basal epithelium of human cornea. Ocul. Surf. 2021, 20, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.C.; Sung, M.; Brown, K.D.; Contet, J.; Belluschi, S.; Hamel, R.; Moreno-Moral, A.; Dos Santos, R.L.; Gough, J.; Polo, J.M.; et al. Single nuclei transcriptomics of the in situ human limbal stem cell niche. Sci. Rep. 2024, 14, 6749. [Google Scholar] [CrossRef] [PubMed]
- Catala, P.; Groen, N.; Dehnen, J.A.; Soares, E.; van Velthoven, A.J.H.; Nuijts, R.; Dickman, M.M.; LaPointe, V.L.S. Single cell transcriptomics reveals the heterogeneity of the human cornea to identify novel markers of the limbus and stroma. Sci. Rep. 2021, 11, 21727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; de Paiva, C.S.; Luo, L.; Kretzer, F.L.; Pflugfelder, S.C.; Li, D.Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 2004, 22, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Chen, Z.; Song, X.J.; de Paiva, C.S.; Kim, H.S.; Pflugfelder, S.C. Partial enrichment of a population of human limbal epithelial cells with putative stem cell properties based on collagen type IV adhesiveness. Exp. Eye Res. 2005, 80, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Yamato, M.; Sugiyama, H.; Sumide, T.; Yang, J.; Okano, T.; Tano, Y.; Nishida, K. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells 2007, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Ruiz, M.; Gonzalez, S.; Tseng, C.H.; Bourges, J.L.; Behar-Cohen, F.; Deng, S.X. Single mRNA detection of Wnt signaling pathway in the human limbus. Exp. Eye Res. 2023, 229, 109337. [Google Scholar] [CrossRef]
- Sasamoto, Y.; Lee, C.A.A.; Wilson, B.J.; Buerger, F.; Martin, G.; Mishra, A.; Kiritoshi, S.; Tran, J.; Gonzalez, G.; Hildebrandt, F.; et al. Limbal BCAM expression identifies a proliferative progenitor population capable of holoclone formation and corneal differentiation. Cell Rep. 2022, 40, 111166. [Google Scholar] [CrossRef]
- Notara, M.; Daniels, J.T. Biological principals and clinical potentials of limbal epithelial stem cells. Cell Tissue Res. 2008, 331, 135–143. [Google Scholar] [CrossRef]
- Robertson, S.Y.T.; Roberts, J.S.; Deng, S.X. Regulation of Limbal Epithelial Stem Cells: Importance of the Niche. Int. J. Mol. Sci. 2021, 22, 11975. [Google Scholar] [CrossRef]
- Mei, H.; Nakatsu, M.N.; Baclagon, E.R.; Deng, S.X. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells 2014, 32, 938–945. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Ding, Z.; Ng, M.Y.; Truong, T.T.; Yu, F.; Deng, S.X. Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4734–4741. [Google Scholar] [CrossRef]
- Lu, R.; Bian, F.; Zhang, X.; Qi, H.; Chuang, E.Y.; Pflugfelder, S.C.; Li, D.Q. The β-catenin/Tcf4/survivin signaling maintains a less differentiated phenotype and high proliferative capacity of human corneal epithelial progenitor cells. Int. J. Biochem. Cell Biol. 2011, 43, 751–759. [Google Scholar] [CrossRef]
- Zhang, C.; Mei, H.; Robertson, S.Y.T.; Lee, H.J.; Deng, S.X.; Zheng, J.J. A Small-Molecule Wnt Mimic Improves Human Limbal Stem Cell Ex Vivo Expansion. iScience 2020, 23, 101075. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Oh, D.; Baclagon, E.R.; Zheng, J.J.; Deng, S.X. Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Investig. Ophthalmol. Vis. Sci. 2019, 60, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Chen, S.Y.; Zhu, Y.T.; Tseng, S.C. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem Cell Res. 2014, 12, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tian, C.; Fan, T.; Xu, B. Fibronectin regulates the self-renewal of rabbit limbal epithelial stem cells by stimulating the Wnt11/Fzd7/ROCK non-canonical Wnt pathway. Exp. Eye Res. 2019, 185, 107681. [Google Scholar] [CrossRef]
- Peng, H.; Park, J.K.; Katsnelson, J.; Kaplan, N.; Yang, W.; Getsios, S.; Lavker, R.M. microRNA-103/107 Family Regulates Multiple Epithelial Stem Cell Characteristics. Stem Cells 2015, 33, 1642–1656. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Zenkel, M.; Polisetti, N.; Sock, E.; Wegner, M.; Kruse, F.E.; Schlötzer-Schrehardt, U. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep. 2018, 8, 10268. [Google Scholar] [CrossRef]
- Chen, S.Y.; Han, B.; Zhu, Y.T.; Mahabole, M.; Huang, J.; Beebe, D.C.; Tseng, S.C. HC-HA/PTX3 Purified from Amniotic Membrane Promotes BMP Signaling in Limbal Niche Cells to Maintain Quiescence of Limbal Epithelial Progenitor/Stem Cells. Stem Cells 2015, 33, 3341–3355. [Google Scholar] [CrossRef]
- Ma, A.; Boulton, M.; Zhao, B.; Connon, C.; Cai, J.; Albon, J. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3576–3585. [Google Scholar] [CrossRef]
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344. [Google Scholar]
- González, S.; Halabi, M.; Ju, D.; Tsai, M.; Deng, S.X. Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells 2020, 9, 1945. [Google Scholar] [CrossRef]
- Small, D.; Kovalenko, D.; Kacer, D.; Liaw, L.; Landriscina, M.; Di Serio, C.; Prudovsky, I.; Maciag, T. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J. Biol. Chem. 2001, 276, 32022–32030. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Odermatt, P.D.; Azzolin, L.; Hohnel, S.; Wagner, E.F.; Fantner, G.E.; Lutolf, M.P.; Barrandon, Y.; Piccolo, S.; Radtke, F. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 2016, 18, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tsuchiya, K.; Watanabe, M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J. Gastroenterol. 2007, 42, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Qin, J.Z.; Chaturvedi, V.; Denning, M.F.; Bonish, B.; Miele, L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002, 9, 842–855. [Google Scholar] [CrossRef]
- Yuan, G.; Zhan, Y.; Gou, X.; Chen, Y.; Yang, G. TGF-β signaling inhibits canonical BMP signaling pathway during palate development. Cell Tissue Res. 2018, 371, 283–291. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zieske, J.D. Transforming growth factor-beta receptor expression in human cornea. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1922–1928. [Google Scholar]
- Zieske, J.D.; Hutcheon, A.E.; Guo, X.; Chung, E.H.; Joyce, N.C. TGF-beta receptor types I and II are differentially expressed during corneal epithelial wound repair. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1465–1471. [Google Scholar]
- Nakatsu, M.N.; Vartanyan, L.; Vu, D.M.; Ng, M.Y.; Li, X.; Deng, S.X. Preferential biological processes in the human limbus by differential gene profiling. PLoS ONE 2013, 8, e61833. [Google Scholar] [CrossRef]
- Grueterich, M.; Espana, E.M.; Tseng, S.C. Ex vivo expansion of limbal epithelial stem cells: Amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 2003, 48, 631–646. [Google Scholar] [CrossRef]
- Grueterich, M.; Tseng, S.C. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch. Ophthalmol. 2002, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Luo, L.; Pflugfelder, S.C.; Li, D.Q. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Hsieh, H.L.; Hsiao, L.D.; Yang, C.M. PI3-K/Akt/JNK/NF-κB is essential for MMP-9 expression and outgrowth in human limbal epithelial cells on intact amniotic membrane. Stem Cell Res. 2012, 9, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Espana, E.M.; Higa, K.; Kato, N.; Li, W.; Tseng, S.C. Activation of Smad-mediated TGF-β signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells. J. Cell. Physiol. 2013, 228, 225–234. [Google Scholar] [CrossRef]
- Saika, S.; Muragaki, Y.; Okada, Y.; Miyamoto, T.; Ohnishi, Y.; Ooshima, A.; Kao, W.W. Sonic hedgehog expression and role in healing corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2577–2585. [Google Scholar] [CrossRef]
- Fan, N.W.; Ho, T.C.; Wu, C.W.; Tsao, Y.P. Pigment epithelium-derived factor peptide promotes limbal stem cell proliferation through hedgehog pathway. J. Cell. Mol. Med. 2019, 23, 4759–4769. [Google Scholar] [CrossRef]
- Gipson, I.K. The epithelial basement membrane zone of the limbus. Eye 1989, 3, 132–140. [Google Scholar] [CrossRef]
- Masterton, S.; Ahearne, M. Mechanobiology of the corneal epithelium. Exp. Eye Res. 2018, 177, 122–129. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef]
- Lepert, G.; Gouveia, R.M.; Connon, C.J.; Paterson, C. Assessing corneal biomechanics with Brillouin spectro-microscopy. Faraday Discuss. 2016, 187, 415–428. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Dreier, B.; Morgan, J.T.; Tuyen, B.C.; Rose, B.W.; Reilly, C.M.; Russell, P.; Murphy, C.J. Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells. PLoS ONE 2014, 9, e109811. [Google Scholar] [CrossRef]
- Fatima, A.; Iftekhar, G.; Sangwan, V.S.; Vemuganti, G.K. Ocular surface changes in limbal stem cell deficiency caused by chemical injury: A histologic study of excised pannus from recipients of cultured corneal epithelium. Eye 2008, 22, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Morgner, J.; Ghatak, S.; Jakobi, T.; Dieterich, C.; Aumailley, M.; Wickström, S.A. Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat. Commun. 2015, 6, 8198. [Google Scholar] [CrossRef]
- Polisetti, N.; Sorokin, L.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511 and -521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci. Rep. 2017, 7, 5152. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lan, J.; Liu, D.; Backman, L.J.; Zhang, W.; Zhou, Q.; Danielson, P. Ascorbic Acid Promotes the Stemness of Corneal Epithelial Stem/Progenitor Cells and Accelerates Epithelial Wound Healing in the Cornea. Stem Cells Transl. Med. 2017, 6, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; Backman, L.J.; Zhou, Q.; Danielson, P. Ciliary Neurotrophic Factor Promotes the Migration of Corneal Epithelial Stem/progenitor Cells by Up-regulation of MMPs through the Phosphorylation of Akt. Sci. Rep. 2016, 6, 25870. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.Y.; Li, C.Y.; Wu, J.Y.; Zhang, Y.T.; Pang, K.P.; Wei, Y.; Du, L.Q.; Liu, M.; Wu, X.Y. SPARC promotes self-renewal of limbal epithelial stem cells and ocular surface restoration through JNK and p38-MAPK signaling pathways. Stem Cells 2020, 38, 134–145. [Google Scholar] [CrossRef]
- Lee, M.H.; Padmashali, R.; Koria, P.; Andreadis, S.T. JNK regulates binding of alpha-catenin to adherens junctions and cell-cell adhesion. FASEB J. 2011, 25, 613–623. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: Therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 2003, 3, 879–889. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Ocular immune privilege and ocular melanoma: Parallel universes or immunological plagiarism? Front. Immunol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Cursiefen, C.; Rummelt, C.; Küchle, M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea 2000, 19, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. See no evil, hear no evil, do no evil: The lessons of immune privilege. Nat. Immunol. 2006, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Griffith, T.S.; Brunner, T.; Fletcher, S.M.; Green, D.R.; Ferguson, T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270, 1189–1192. [Google Scholar] [CrossRef]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef]
- Clahsen, T.; Hadrian, K.; Notara, M.; Schlereth, S.L.; Howaldt, A.; Prokosch, V.; Volatier, T.; Hos, D.; Schroedl, F.; Kaser-Eichberger, A.; et al. The novel role of lymphatic vessels in the pathogenesis of ocular diseases. Prog. Retin. Eye Res. 2023, 96, 101157. [Google Scholar] [CrossRef]
- Joukov, V.; Sorsa, T.; Kumar, V.; Jeltsch, M.; Claesson-Welsh, L.; Cao, Y.; Saksela, O.; Kalkkinen, N.; Alitalo, K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997, 16, 3898–3911. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The lymphatic vasculature in disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar]
- Clahsen, T.; Büttner, C.; Hatami, N.; Reis, A.; Cursiefen, C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. J. Clin. Med. 2020, 9, 479. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1117–1124. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6554–6560. [Google Scholar] [CrossRef]
- Schito, L. Hypoxia-Dependent Angiogenesis and Lymphangiogenesis in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 71–85. [Google Scholar] [CrossRef]
- Makino, Y.; Cao, R.; Svensson, K.; Bertilsson, G.; Asman, M.; Tanaka, H.; Cao, Y.; Berkenstam, A.; Poellinger, L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001, 414, 550–554. [Google Scholar] [CrossRef]
- Büttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase Is a Novel Endogenous Regulator of Developmental and Inflammatory Lymphangiogenesis. Am. J. Pathol. 2019, 189, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Hatami, N.; Büttner, C.; Bock, F.; Simfors, S.; Musial, G.; Reis, A.; Cursiefen, C.; Clahsen, T. Cystathionine β-synthase as novel endogenous regulator of lymphangiogenesis via modulating VEGF receptor 2 and 3. Commun. Biol. 2022, 5, 950. [Google Scholar] [CrossRef] [PubMed]
- Regenfuß, B.; Dreisow, M.L.; Hos, D.; Masli, S.; Bock, F.; Cursiefen, C. The Naïve Murine Cornea as a Model System to Identify Novel Endogenous Regulators of Lymphangiogenesis: TRAIL and rtPA. Lymphat. Res. Biol. 2015, 13, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Heishi, T.; Hosaka, T.; Suzuki, Y.; Miyashita, H.; Oike, Y.; Takahashi, T.; Nakamura, T.; Arioka, S.; Mitsuda, Y.; Takakura, T.; et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol. 2010, 176, 1950–1958. [Google Scholar] [CrossRef]
- Meshko, B.; Volatier, T.L.A.; Hadrian, K.; Deng, S.; Hou, Y.; Kluth, M.A.; Ganss, C.; Frank, M.H.; Frank, N.Y.; Ksander, B.; et al. ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation. Cells 2023, 12, 1731. [Google Scholar] [CrossRef]
- Kaminska, G.M.; Niederkorn, J.Y. Spontaneous corneal neovascularization in nude mice. Local imbalance between angiogenic and anti-angiogenic factors. Investig. Ophthalmol. Vis. Sci. 1993, 34, 222–230. [Google Scholar]
- Kamiński, M.; Kamińska, G. Inhibition of lymphocyte-induced angiogenesis by enzymatically isolated rabbit cornea cells. Arch. Immunol. Ther. Exp. 1978, 26, 1079–1082. [Google Scholar]
- Sekiyama, E.; Nakamura, T.; Kawasaki, S.; Sogabe, H.; Kinoshita, S. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp. Eye Res. 2006, 83, 741–746. [Google Scholar] [CrossRef]
- van Setten, G.B. Vascular endothelial growth factor (VEGF) in normal human corneal epithelium: Detection and physiological importance. Acta Ophthalmol. Scand. 1997, 75, 649–652. [Google Scholar] [CrossRef]
- Amano, S.; Rohan, R.; Kuroki, M.; Tolentino, M.; Adamis, A.P. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 1998, 39, 18–22. [Google Scholar]
- Edelman, J.L.; Castro, M.R.; Wen, Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1112–1123. [Google Scholar]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Stechschulte, S.U.; Kirchhof, B.; Dartt, D.A.; Fong, G.H.; Rudge, J.; Wiegand, S.J.; Yancopoulos, G.D.; et al. VEGF-dependent conjunctivalization of the corneal surface. Investig. Ophthalmol. Vis. Sci. 2003, 44, 117–123. [Google Scholar] [CrossRef]
- Ma, D.H.; Chen, J.K.; Zhang, F.; Lin, K.Y.; Yao, J.Y.; Yu, J.S. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006, 25, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Veréb, Z.; Albert, R.; Póliska, S.; Olstad, O.K.; Akhtar, S.; Moe, M.C.; Petrovski, G. Comparison of upstream regulators in human ex vivo cultured cornea limbal epithelial stem cells and differentiated corneal epithelial cells. BMC Genom. 2013, 14, 900. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-Term Ultraviolet A Irradiation Leads to Dysfunction of the Limbal Niche Cells and an Antilymphangiogenic and Anti-inflammatory Micromilieu. Investig. Ophthalmol. Vis. Sci. 2016, 57, 928–939. [Google Scholar] [CrossRef]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015, 15, 643–654. [Google Scholar] [CrossRef]
- Norrick, A.; Esterlechner, J.; Niebergall-Roth, E.; Dehio, U.; Sadeghi, S.; Schröder, H.M.; Ballikaya, S.; Stemler, N.; Ganss, C.; Dieter, K.; et al. Process development and safety evaluation of ABCB5(+) limbal stem cells as advanced-therapy medicinal product to treat limbal stem cell deficiency. Stem Cell Res. Ther. 2021, 12, 194. [Google Scholar] [CrossRef]
- Holan, V.; Pokorna, K.; Prochazkova, J.; Krulova, M.; Zajicova, A. Immunoregulatory properties of mouse limbal stem cells. J. Immunol. 2010, 184, 2124–2129. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Yang, H.; Baksh, S.C.; Miao, Y.; Levorse, J.; Kataru, R.P.; Liu, X.; de la Cruz-Racelis, J.; Mehrara, B.J.; Fuchs, E. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 2019, 366, 1218–1225. [Google Scholar] [CrossRef]
- Pena-Jimenez, D.; Fontenete, S.; Megias, D.; Fustero-Torre, C.; Grana-Castro, O.; Castellana, D.; Loewe, R.; Perez-Moreno, M. Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J. 2019, 38, e101688. [Google Scholar] [CrossRef]
- Wang, A.; Li, Z.; Jiang, Y.; Chen, M.; Yu, H.; Li, Z.; Sun, S.; Bai, G.; Wang, Q.; Huang, Y.; et al. Prox1 Protein in Corneal Limbal Lymphatic Vessels Maintains Limbal Stem Cell Stemness and Regulates Corneal Injury Repair. Investig. Ophthalmol. Vis. Sci. 2025, 66, 81. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Ocular lymphatics: State-of-the-art review. Lymphology 2009, 42, 66–76. [Google Scholar] [PubMed]
- Ahmad, S. Concise review: Limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl. Med. 2012, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.; Chen, H.C.; Lai, J.Y.; Sun, C.C.; Wang, S.F.; Lin, K.K.; Chen, J.K. Matrix revolution: Molecular mechanism for inflammatory corneal neovascularization and restoration of corneal avascularity by epithelial stem cell transplantation. Ocul. Surf. 2009, 7, 128–144. [Google Scholar] [CrossRef]
- Masood, F.; Chang, J.H.; Akbar, A.; Song, A.; Hu, W.Y.; Azar, D.T.; Rosenblatt, M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells 2022, 11, 3247. [Google Scholar] [CrossRef]
- Notara, M.; Alatza, A.; Gilfillan, J.; Harris, A.R.; Levis, H.J.; Schrader, S.; Vernon, A.; Daniels, J.T. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp. Eye Res. 2010, 90, 188–195. [Google Scholar] [CrossRef]
- Moshirfar, M.; Masud, M.; Harvey, D.H.; Payne, C.; Bruce, E.; Ronquillo, Y.C.; Hoopes, P.C. The Multifold Etiologies of Limbal Stem Cell Deficiency: A Comprehensive Review on the Etiologies and Additional Treatment Options for Limbal Stem Cell Deficiency. J. Clin. Med. 2023, 12, 4418. [Google Scholar] [CrossRef]
- Kethiri, A.R.; Raju, E.; Bokara, K.K.; Mishra, D.K.; Basu, S.; Rao, C.M.; Sangwan, V.S.; Singh, V. Inflammation, vascularization and goblet cell differences in LSCD: Validating animal models of corneal alkali burns. Exp. Eye Res. 2019, 185, 107665. [Google Scholar] [CrossRef]
- Notara, M.; Behboudifard, S.; Kluth, M.A.; Maßlo, C.; Ganss, C.; Frank, M.H.; Schumacher, B.; Cursiefen, C. UV light-blocking contact lenses protect against short-term UVB-induced limbal stem cell niche damage and inflammation. Sci. Rep. 2018, 8, 12564. [Google Scholar] [CrossRef]
- Domdey, M.; Kluth, M.A.; Maßlo, C.; Ganss, C.; Frank, M.H.; Frank, N.Y.; Coroneo, M.T.; Cursiefen, C.; Notara, M. Consecutive dosing of UVB irradiation induces loss of ABCB5 expression and activation of EMT and fibrosis proteins in limbal epithelial cells similar to pterygium epithelium. Stem Cell Res. 2022, 64, 102936. [Google Scholar] [CrossRef] [PubMed]
- Volatier, T.; Schumacher, B.; Meshko, B.; Hadrian, K.; Cursiefen, C.; Notara, M. Short-Term UVB Irradiation Leads to Persistent DNA Damage in Limbal Epithelial Stem Cells, Partially Reversed by DNA Repairing Enzymes. Biology 2023, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, M.; Hanson, I.; van Heyningen, V. Aniridia. Eur. J. Hum. Genet. 2012, 20, 1011–1017. [Google Scholar] [CrossRef]
- Hill, R.E.; Favor, J.; Hogan, B.L.; Ton, C.C.; Saunders, G.F.; Hanson, I.M.; Prosser, J.; Jordan, T.; Hastie, N.D.; van Heyningen, V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 1991, 354, 522–525. [Google Scholar] [CrossRef] [PubMed]
- van Velthoven, A.J.H.; Utheim, T.P.; Notara, M.; Bremond-Gignac, D.; Figueiredo, F.C.; Skottman, H.; Aberdam, D.; Daniels, J.T.; Ferrari, G.; Grupcheva, C.; et al. Future directions in managing aniridia-associated keratopathy. Surv. Ophthalmol. 2023, 68, 940–956. [Google Scholar] [CrossRef]
- Ramaesh, T.; Ramaesh, K.; Martin Collinson, J.; Chanas, S.A.; Dhillon, B.; West, J.D. Developmental and cellular factors underlying corneal epithelial dysgenesis in the Pax6+/- mouse model of aniridia. Exp. Eye Res. 2005, 81, 224–235. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Latta, L.; Gießl, A.; Zenkel, M.; Fries, F.N.; Käsmann-Kellner, B.; Kruse, F.E.; Seitz, B. Dysfunction of the limbal epithelial stem cell niche in aniridia-associated keratopathy. Ocul. Surf. 2021, 21, 160–173. [Google Scholar] [CrossRef]
- Vicente, A.; Sloniecka, M.; Liu, J.X.; Byström, B.; Pedrosa Domellöf, F. Aniridia-related keratopathy relevant cell signaling pathways in human fetal corneas. Histochem. Cell Biol. 2022, 158, 169–180. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef]
- Daniels, J.T.; Notara, M.; Shortt, A.J.; Secker, G.; Harris, A.; Tuft, S.J. Limbal epithelial stem cell therapy. Expert Opin. Biol. Ther. 2007, 7, 1–3. [Google Scholar] [CrossRef]
- Schlereth, S.L.; Hos, D.; Matthaei, M.; Hamrah, P.; Schmetterer, L.; O’Leary, O.; Ullmer, C.; Horstmann, J.; Bock, F.; Wacker, K.; et al. New Technologies in Clinical Trials in Corneal Diseases and Limbal Stem Cell Deficiency: Review from the European Vision Institute Special Interest Focus Group Meeting. Ophthalmic Res. 2021, 64, 145–167. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nimtz, L.; Niebergall-Roth, E.; Norrick, A.; Hägele, S.; Vollmer, L.; Esterlechner, J.; Frank, M.H.; Ganss, C.; Scharffetter-Kochanek, K.; et al. Potency assay to predict the anti-inflammatory capacity of a cell therapy product for macrophage-driven diseases: Overcoming the challenges of assay development and validation. Cytotherapy 2024, 26, 512–523. [Google Scholar] [CrossRef]
- Bock, F.; Maruyama, K.; Regenfuss, B.; Hos, D.; Steven, P.; Heindl, L.M.; Cursiefen, C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog. Retin. Eye Res. 2013, 34, 89–124. [Google Scholar] [CrossRef]
- Drzyzga, Ł.; Śpiewak, D.; Dorecka, M.; Wyględowska-Promieńska, D. Available Therapeutic Options for Corneal Neovascularization: A Review. Int. J. Mol. Sci. 2024, 25, 5479. [Google Scholar] [CrossRef]
- Wiedemann, J.; Hos, D.; Limburg, E.; Zettelmeyer, U.; Schiller, P.; Franklin, J.; Bachmann, B.; Bohringer, D.; Dietrich-Ntoukas, T.; Fuchsluger, T.A.; et al. UV light-mediated corneal crosslinking as (lymph)angioregressive pretreatment to promote graft survival after subsequent high-risk corneal transplantation (CrossCornealVision): Protocol for a multicenter, randomized controlled trial. Trials 2024, 25, 169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.